Chapter 105 Hemobilia and bilhemia
Overview
Hemorrhage into the biliary tract occurs when disease or trauma produces an abnormal communication between blood vessels and bile ducts, termed hemobilia (Sandblom, 1948), from the Greek haima (“blood”) and the Latin bilis (“bile”); the term bilhemia describes the rare condition of bile in the bloodstream. Causes of hemobilia include iatrogenic and accidental trauma, gallstones, inflammation, vascular disorders, and neoplasms. With the increasing use of invasive diagnostic and therapeutic procedures that involve the biliary tract, iatrogenic trauma has become the predominant etiology of hemobilia (see Chapters 28, 50D, 102, and 104).
Major, profuse hemobilia is a rare and sometimes life-threatening complication of liver or biliary tract disease or trauma. Minor hemobilia occurs more frequently but is rarely of long-lasting clinical significance. Although often difficult to recognize, hemobilia should be in the differential diagnosis of upper gastrointestinal (UGI) bleeding, especially in patients with a recent or remote history of liver trauma or biliary tree instrumentation (see Chapters 19, 28, 50D, and 102).
With the advent of interventional radiologic approaches, treatment of symptomatic hemobilia has shifted from surgical therapy to selective transcatheter hepatic arterial embolization (see Chapter 104). Surgical therapy via partial hepatectomy, hepatic artery ligation, or a combination of both procedures is rarely necessary and is reserved for hepatic arterial branches that cannot be safely or adequately embolized, for anatomic or technical reasons, or in patients who have large intrahepatic hematomas or sepsis.
History
Because of its obscure appearance and apparent rarity, hemorrhage into the biliary tract, although first described in the seventeenth century, was not called hemobilia until more recently. In the first detailed description of hepatic anatomy from 1654, Francis Glisson (Fig. 105.1) discussed the possibility of hemorrhage into the biliary tract when describing the case of a man who died from presumed GI hemorrhage following a stab wound to the liver suffered in a duel: “I believe that if the liver is injured by a contusion, it may lead to blood leaving the body by way of vomit or the stool for there is no doubt that the biliary tract takes unto itself (to the great good of the patient) some of the blood issuing into the liver and leads it down to the intestines. From there it is either impelled upwards through reverse peristalsis or downwards the normal way” (Glisson, 1654).
Over a century elapsed before the subject of hemorrhage into the biliary tract was brought up again. In Morgagni’s Epistles (1765), the founder of clinical pathology noted, in the section on the causes of dilation of the biliary tract, that abscesses in the liver and the voiding of sharp gallstones could lead to bleeding through the biliary ducts (Morgagni, 1765). In 1777, Antoine Portal presented a case in which he made the diagnosis before the death of the patient and confirmed it at autopsy. In this early treatise, Portal drew attention to the difficulty of finding the source of hemorrhage in the biliary tract, “when they are slight in quantity and occur but seldom,” and to the risk of mistakenly tracing them to a healthy organ, a mistake that has been made repeatedly in the history of hemobilia (Portal, 1813).
The first published case in North America was by a Boston surgeon who reported on the clinical and pathologic consequences of an “aneurysm of the hepatic artery bursting into the hepatic duct,” the first direct observation of an abnormal communication between the blood vessels and biliary ducts (Jackson, 1834; see Chapter 104). In 1871, the German surgeon Quincke further characterized biliary tract hemorrhage by establishing three cardinal symptoms—GI hemorrhage, biliary colic, and jaundice—commonly referred to as Quincke’s triad (Quincke, 1871). Hemobilia formerly was regarded as a medical curiosity, but it is now being diagnosed with increasing frequency, owing to more widespread knowledge of the syndrome and to improved diagnostic means (Sandblom, 1972).
Etiology
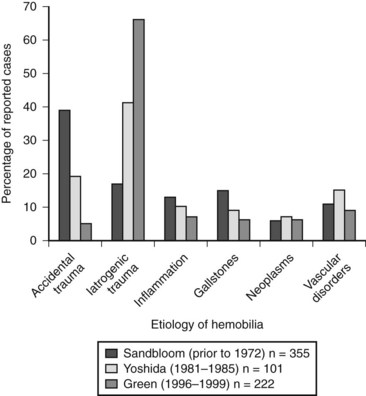
Common causes of abnormal vascular and biliary communication giving rise to hemobilia include trauma (accidental and iatrogenic), gallstones, neoplasms, inflammatory processes, and vascular disorders. Other less common causes include nematodes, blood coagulation defects, choledochal cysts, pancreatitis, and portal hypertension. Figure 105.2 illustrates the three largest reported case series of hemobilia and demonstrates a shift in etiology over the course of time from accidental to iatrogenic trauma. In one of the first publications devoted solely to hemobilia, Sandblom reported on 355 cases reported prior to 1972, with the largest number secondary to accidental trauma (Sandblom, 1972). Over the ensuing 30 years, iatrogenic trauma surpassed all other causes of hemobilia, and today it is responsible for the majority of cases (Green et al, 2001; Yoshida et al, 1987). The increase in hemobilia caused by iatrogenic trauma parallels the advent and increasing use of both diagnostic and therapeutic instrumentation of the biliary tract.
Iatrogenic Trauma
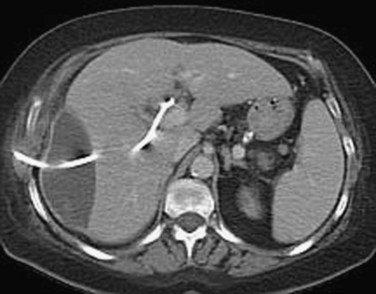
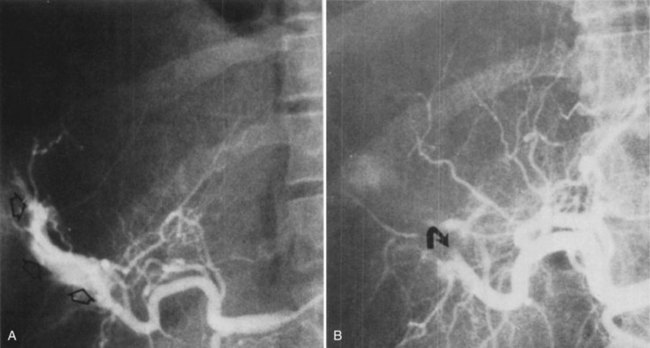
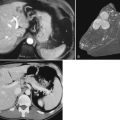
To an increasing extent, biliary hemorrhage is reported as a complication of diagnostic and therapeutic procedures on the liver or the bile ducts and is now responsible for the majority of reported cases of hemobilia (Fig. 105.3). The incidence of clinically significant hemobilia following percutaneous liver biopsy is between 0.01% and 0.2% (Piccinino et al, 1986; Seeff et al, 2010; see Chapter 20). Hemobilia arises secondary to unintentional laceration of a hepatic artery during the procedure and results in subsequent communication with the biliary tree (Fig. 105.4). Previous studies demonstrated that the risk of hemobilia following percutaneous liver biopsy may be greater in patients with chronic liver disease because of the presence of ascites, coagulopathy, and platelet dysfunction; the risk may also be higher following liver transplantation (Jabbour et al, 1995; Piccinino et al, 1986). In a recent study 2740 percutaneous liver biopsies were performed on patients with advanced chronic liver disease (cirrhosis), and it was determined that only an elevated international normalized ratio (INR) above 1.3 and a platelet count below 60,000 was associated with an increased risk of periprocedural hemobilia (Seeff et al, 2010).
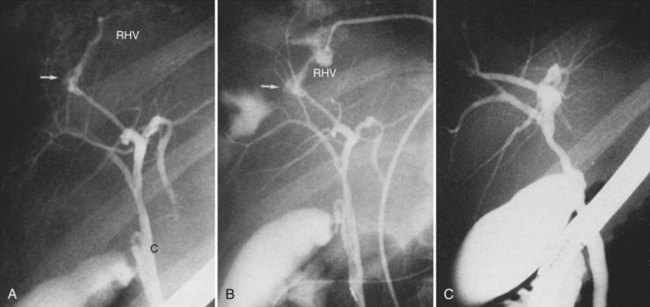
Percutaneous transhepatic biliary drainage (PTBD) and percutaneous transhepatic cholangiography (PTC) are associated with a significantly higher rate of hemobilia than percutaneous liver biopsy because of the proximity of the hepatic vasculature to the biliary tree (Fig. 105.5; see Chapters 28 and 50D). In a series of 1397 patients undergoing PTBD or PTC, the incidence of clinically significant hemobilia was 1.9% (Fidelman et al, 2008). Patients undergoing PTBD had a 3.7-fold higher incidence of hemobilia compared with patients undergoing PTC, and they had a delayed presentation of hemobilia; presumably this was due to multiple catheter exchanges and a tamponade effect in PTBD patients. In this series, only large-bore needles were associated with hemobilia.
Other studies have demonstrated that use of a 21-gauge needle to access the biliary tree during PTC or PTBD, rather than an 18-gauge needle, is associated with a reduced incidence of hemobilia (Burke et al, 2003; Dousset et al, 1997; Harbin et al, 1980). In addition, correction of coagulopathy should reduce the development of clinically significant hemobilia (Hines et al, 1972). Another study concluded that neither a left-sided or right-sided hepatic approach to either PTBD or PTC is specifically associated with a higher incidence of hemobilia (Rivera-Sanfeliz et al, 2004). Other potential risk factors include cirrhosis, ascites, and absence of biliary dilation; extensive hepatic metastases do not appear to be predictive of hemobilia.
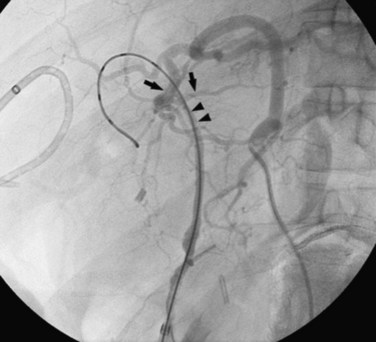
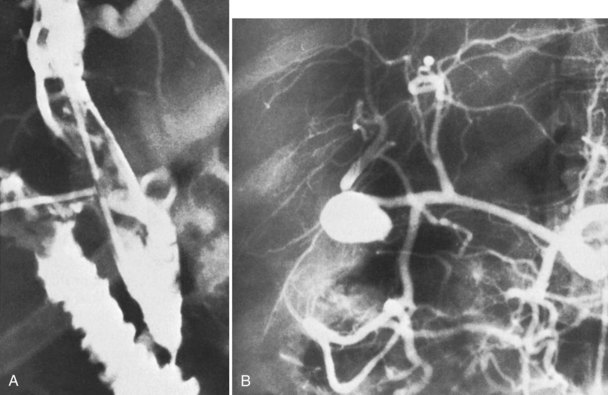
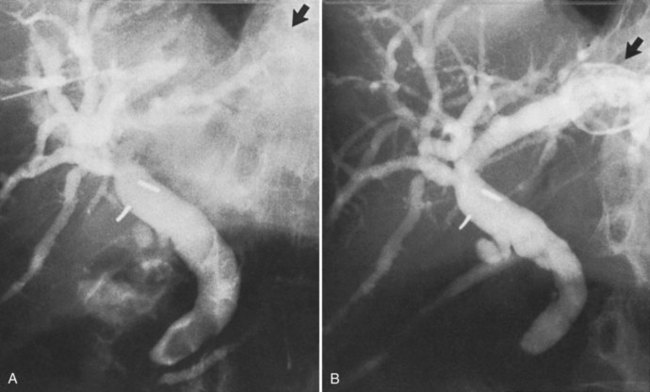
Surgery of the biliary tree may also lead to hemobilia as a result of injury of the hepatic artery by suture, dissection, diathermy, clip migration, erosion and subsequent arteriobiliary fistula, or pseudoaneurysm eroding into the extrahepatic bile duct (Fig. 105.6). The development of a hepatic artery pseudoaneurysm may precede the clinical manifestations of hemobilia by several weeks because of a delayed presentation; therefore, a high clinical suspicion and accurate history taking is often needed to accurately diagnose hemobilia (see Chapter 19). The advent of laparoscopic cholecystectomy for treatment of cholelithiasis and cholecystitis was originally feared to have produced an increased incidence of hemobilia secondary to vascular injury within the porta hepatis, specifically to the right hepatic artery (see Chapter 34). Stewart and colleagues (1995) reported five cases of hemobilia following laparoscopic cholecystectomy, and four of these were the result of traumatic aneurysm of the right hepatic artery; however, a large registry of 77,604 cases of patients undergoing laparoscopic cholecystectomy demonstrated that significant vascular injury was seen in only 0.16% (Deziel et al, 1993). It appears that fears of increased incidence of hemobilia following laparoscopic cholecystectomy were unfounded, overstated, and most likely secondary to the learning curve of laparoscopic surgery; however, the presentation of UGI bleeding from an unknown source weeks after laparoscopic cholecystectomy should heighten the suspicion of a potential injury to the right hepatic artery and resultant hemobilia.
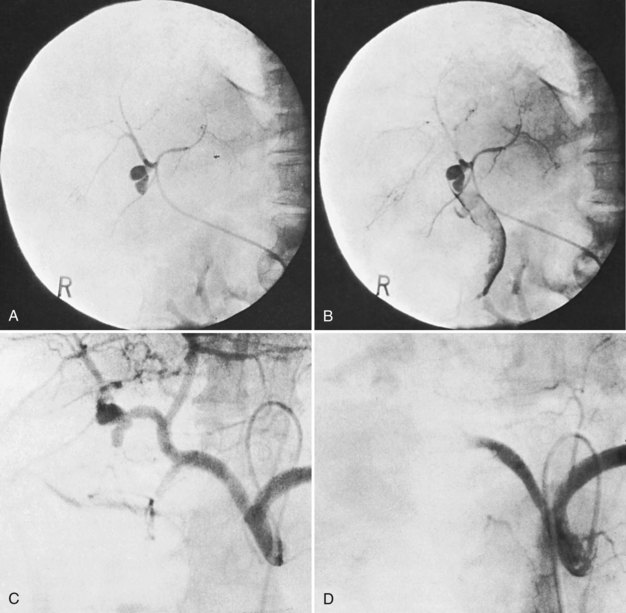
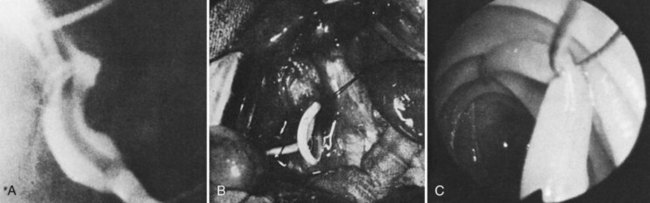
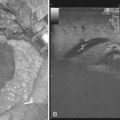
Surgical procedures and exploratory instrumentation of the intrahepatic ducts may damage their walls, and the risk of hemorrhage is great. Minor bleeding constantly accompanies such procedures, but the bleeding generally ceases spontaneously, and the resulting clots promptly dissolve. Forceful instrumentation during exploration of the biliary tree and extraction of stones can result significant hemorrhage (Fig. 105.7). Bleeding from a T-tube occasionally can be caused by local erosion of the mucosa from tube, but if an arteriogram is done, it frequently exposes another source: a pseudoaneurysm that originates from an intraductal lesion, caused by instrumentation (see Fig. 105.7). As in other kinds of traumatic hemobilia, sometimes a considerable interval passes between the trauma and the bleeding episode.
Accidental Trauma
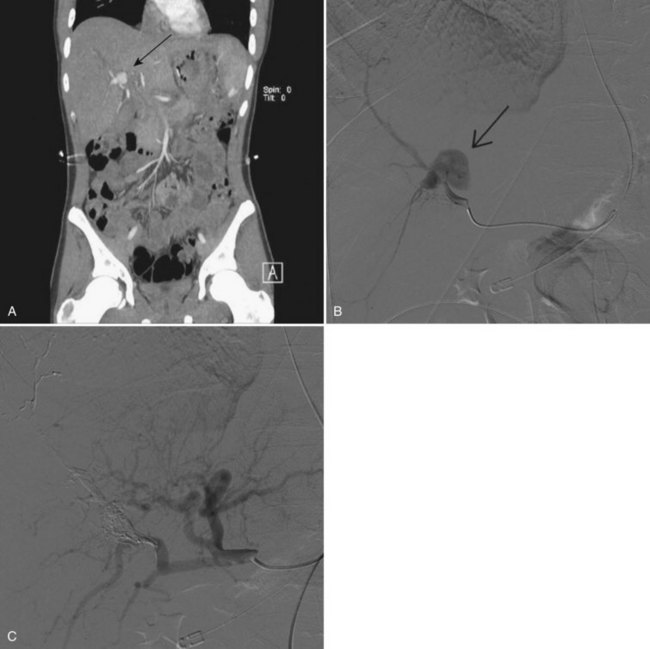
The liver is one of the organs most commonly injured by blunt trauma, with injuries detected in almost 25% of patients on routine computed tomography (CT) scan (Matthes et al, 2003; see Chapter 102). Nonsurgical management of blunt liver trauma in hemodynamically stable patients has become the standard treatment and is successful in 50% to 80% of patients (Carrillo et al, 1998; Forlee et al, 2004). The incidence of hemobilia following major liver injury has been reported to be as high as 3% in one series (Merrell & Schneider, 1991), but in other reports, the overall incidence is less than 0.2% (Parks et al, 1999). Other accidental traumas that result in hemobilia include injuries to the gallbladder, which is the organ injured in up to 2% of blunt abdominal trauma cases (Erb et al, 1994).
The clinical manifestations of blunt trauma to the liver are dependent on the anatomic site of damage. Tearing or injury to the liver capsule without deep parenchymal injury typically results in intraperitoneal hemorrhage that is most often managed with emergent operative exploration. Deep parenchymal injury or a central rupture may leave a large cavity, into which damaged bile ducts and blood vessels drain; the resulting biloma or hematoma may continue to expand, liver healing is impaired, and subsequent necrosis of the cavity wall may cause erosion of adjacent structures or formation of an intrahepatic artery pseudoaneurysm (Fig. 105.8).
If operation for blunt liver trauma is necessary, careful exploration of central injuries should be performed to ensure adequate hemostasis and ligation of biliary radicles before using packs to control bleeding. Indiscriminate use of deep parenchymal sutures should be avoided to prevent further injury to biliary radicles and vessels, potentially creating a central cavity that predisposes to the development of postoperative hemobilia. The conservative management of liver injuries has not resulted in an increase in the incidence of hemobilia following accidental trauma (Carrillo et al, 1998). In a series of 135 patients with severe liver injuries managed nonoperatively, only two developed hemobilia (Carrillo et al, 1999).
Vascular Disorders
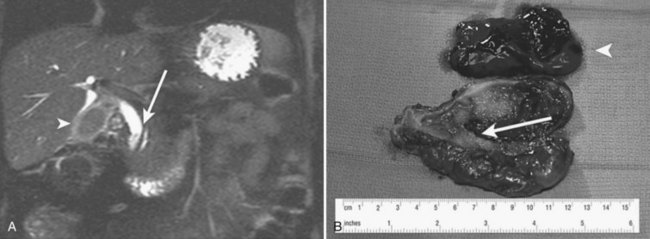
Vascular disorders account for only about 10% of cases of clinically significant hemobilia (see Chapter 19; Green et al, 2001; Yoshida et al, 1987). Hepatic artery aneurysms are the most common primary vascular disorder to produce hemobilia. Other less common causes include angiodysplasia, arteriovenous malformations, and hemangiomas (Vishnevsky et al, 1991). The frequency of true aneurysms (Ryan et al, 2002) of the hepatic artery or its branches (Maralcan et al, 2003; Morioka et al, 2004) rupturing into the biliary tract is diminishing with the disappearance of mycotic aneurysms, leaving only aneurysms of atherosclerotic origin or those associated with polyarteritis nodosa (Dutta et al, 2004; Yazici et al, 1997), fibromuscular dysplasia (Shussman et al, 2008), or trauma. When an aneurysm only leaks, it might give rise to inconspicuous hemobilia; when it ruptures into the biliary tract, the symptoms are generally clinically significant, with exsanguinating hemorrhage and intense biliary colic. Sometimes, vascular lesions associated with arterial hypertension can result in hemobilia; the structure usually affected is the gallbladder, and the disorder is known as apoplexy of the gallbladder (Parekh & Corvera, 2010; Fig. 105.9).
Neoplasms
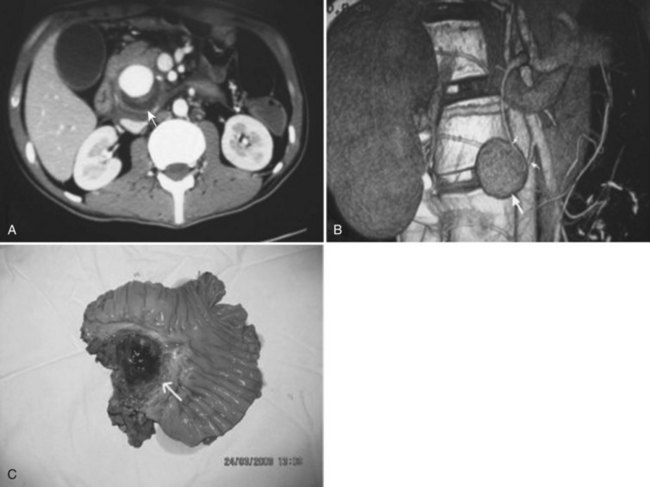
Neoplasms account for only about 5% of all cases of major hemobilia (Green et al, 2001; Sandblom, 1972; Yoshida et al, 1987). Primary and secondary malignant neoplasms of the liver, bile ducts, gallbladder, and pancreas can all lead to hemobilia when the tumors invade the biliary system. The rate of blood loss is rarely rapid and in most instances produces the slow onset of microcytic anemia. Malignant tumors are three times more likely to cause hemobilia than benign tumors, and liver metastases hardly ever cause hemobilia; similarly, hemobilia is not a frequent sequela of primary cancers in the liver (Kitagawa et al, 1999), although it has been described from metastases in such rare locations as the gallbladder wall (Kubota et al, 2000) or from melanoma in the ductal mucosa. In cases of hemobilia caused by malignant tumors in the gallbladder (Hernandez-Castillo et al, 2002), the bleeding is most marked if the tumor grows in a polypoid fashion (Strauss, 1929). In the bile ducts, benign adenoma (Teter, 1954), polyps, and polyposis have a protracted course of hemobilia. Treatment of hepatic tumors, both primary and metastatic, with either chemotherapy (Verset et al, 2010) or locoregional therapy (Goto et al, 2010) has also been reported to result in major hemobilia.
Gallstones
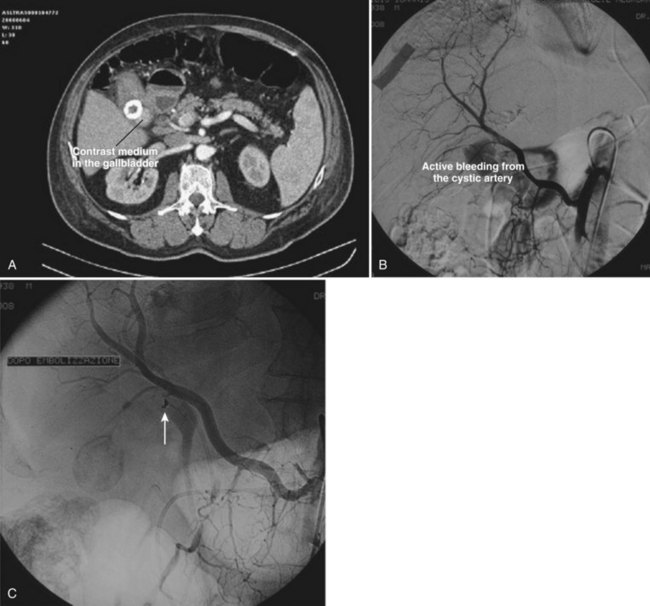
In gallstone disease, microscopic hemorrhage can be demonstrated in one of four cases with stones in the gallbladder and in one of three with stones in the common duct (Gad, 1962). Macroscopic hemobilia is rare, constituting less than 10% of all major hemobilia cases reported (Green et al, 2001; Sandblom, 1972; Yoshida et al, 1987). It generally occurs when large stones erode the cystic artery or penetrate into an adjacent viscus or vessel (Ben-Ishay et al, 2010; Contini et al, 2009; Fig. 105.10). The stones may cause profuse, exsanguinating hemobilia, and 11 out of 20 patients in one report bled to death. Hemobilia should be regarded just as seriously but is a much less frequent complication of neglected gallstones (Joo et al, 2003) compared with obstructive jaundice, cholecystitis, and pancreatitis.
Infections and Inflammation
Parasitic infection by various organisms has been reported in association with hemobilia, and it should be considered in patients with a history of travel to endemic regions, including Africa and the Asian Rim (see Chapter 45). Ascaris lumbrocoides, a nematode with a tendency to invade the bile ducts, frequently produces bleeding (Fig. 105.11). In India, 500 cases of biliary ascariasis have been reported (Khurro et al, 1990). Suppuration, but no bleeding, is mentioned. Because blood iron levels were low, and no examination for hematochezia was performed, it may be that many cases were overlooked. In addition, ruptured liver abscess has caused hemobilia in a few cases (Liou et al, 1996).
Clinical Manifestations
The bleeding in hemobilia may originate in the liver parenchyma, intrahepatic biliary tract, or extrahepatic biliary tract, including the gallbladder. Most episodes of significant hemobilia are the result of an abnormal fistulous communication between a hepatic arterial branch and the biliary tree. The clinical manifestations of hemobilia are due to blood loss and formation of clots within the biliary tree. Major hemobilia causes shock and possible death from exsanguination, unless an emergency intervention is performed. Occult or minor hemobilia, if continued, may result in chronic secondary anemia as can be seen with biliary tract neoplasms (Ahmad et al, 2010).
GI bleeding in connection with biliary symptoms should arouse the suspicion of biliary tract hemorrhage. Quincke described a triad of signs and symptoms associated with hemobilia: upper abdominal pain, UGI hemorrhage, and jaundice (Quincke, 1871). The GI bleeding manifests as melena in 90% of cases and as hematemesis in 60%. Biliary colic symptoms are seen in 70% of cases, and jaundice is identified in up to 60% of patients. All three findings are seen in approximately a quarter of patients with hemobilia (Green et al, 2001). In addition to retained blood clots within the biliary tree, which produce the signs and symptoms of obstructive jaundice, patients may be seen initially with ascending cholangitis and accompanying high fevers and rigors (Kurisu et al, 2005).
The fate of blood in the biliary tree depends on a variety of factors, including the rate, the source (arterial or venous), the timing (continuous or intermittent), and the frequency (solitary or recurrent) of the bleeding. A sudden onset of massive bleeding, as in a ruptured hepatic artery aneurysm, may produce such a rapid volume of blood that the initial clinical presentation is hematemesis or melena. The formation of clots depends on the quality and quantity of the bleeding, and their fate depends on whether they are dissolved, expelled into the intestine, or retained in the biliary tract (Sandblom & Mirkovich, 1979). When clots are retained, they act like calculi and cause symptoms of biliary colic when passed (Clancy & Warren, 1997), jaundice when retained (Prata Martins et al, 2008), cholecystitis if lodged in the gallbladder (Parekh & Corvera, 2010), and pancreatitis if they obstruct a common channel above the sphincter (Alis et al, 2010). Only rarely do clots remain in the biliary tree long enough to be encrusted and form stones (Luzuy et al, 1987; Olsen, 1982).
Diagnosis
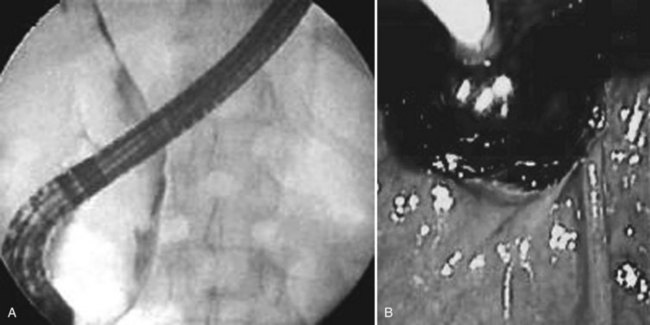
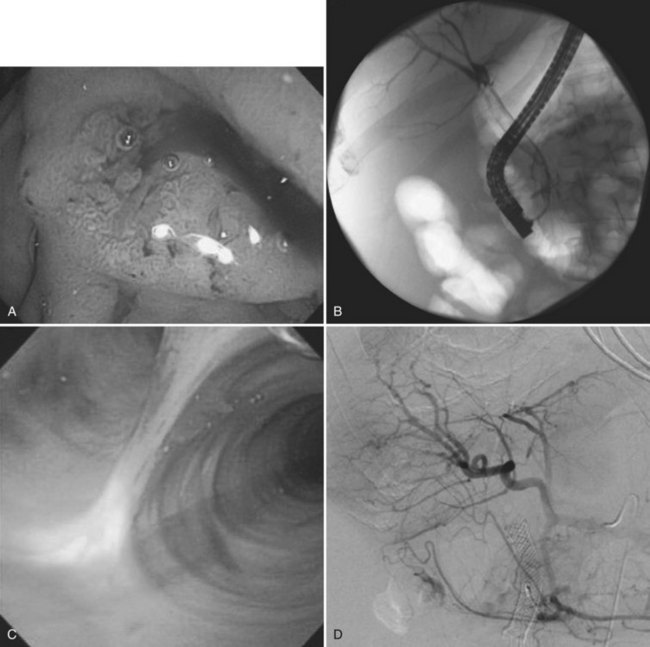
A high index of suspicion is necessary to accurately diagnose and treat hemobilia. The actual workup of a patient with suspected hemobilia is ultimately dependent on the presentation and suspected etiology. It is important to remember that hemobilia may have a delayed presentation and may recur repeatedly over months, even years (Ahmad et al, 2010). For patients who come to medical attention with UGI bleeding, esophagogastroduodenoscopy (EGD) is the initial diagnostic test of choice. Although blood or clot at the ampulla of Vater is the sine qua non of hemobilia (Fig. 105.12), about 10% of upper endoscopies will be nondiagnostic and will necessitate additional diagnostic tests to confirm the diagnosis (Yoshida et al, 1987).
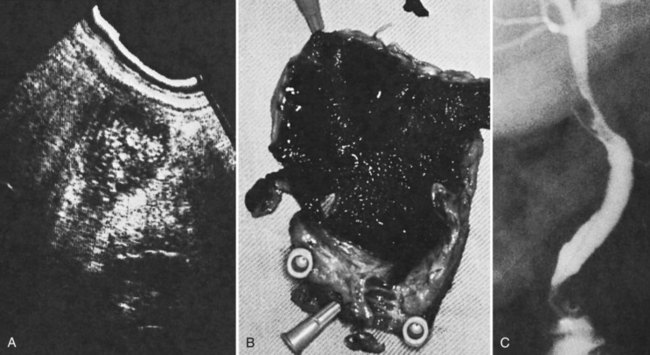
Other imaging modalities employed include endoscopic retrograde cholangiopancreatography (ERCP), which sometimes reveals clots in the bile ducts (Guitron-Cantu et al, 2002; Hendriks et al, 2009; Jornod et al, 1999; see Chapter 18 and Fig. 105.12). A lesion often first appears on ultrasonography (Trakarnsanga, et al, 2010) or CT (Burns & Slakey, 2009); these imaging modalities may show even small traumatic lesions, if a hematoma is produced, but not if there is only an arteriobiliary fistula. The ultrasound appearance of intracholecystic blood produces less distinctive shadows (or defects) than stones, and the shadows often are interpreted as being due to gravel or inspissated bile (Fig. 105.13). Magnetic resonance imaging (MRI) also can show active bleeding to the biliary tract, and MR cholangiopancreatography (MRCP) can improve visualization of the biliary tract and gallbladder (Tseng et al, 2001; see Chapter 17).
The best way to verify a suspected diagnosis of hemobilia is by selective arteriography of the hepatic artery (see Chapter 19), which reveals the source of major bleeding in most cases (Xu et al, 2005; Yao & Arnell, 2010); hemobilia may also be demonstrated by extravasation; by displacement of the vessels around a liver mass; or by filling of a true aneurysm (see Fig. 105.7). When caught at the right moment, contrast material may be seen traveling down the hepatic duct, proving the existence of a communication with the biliary tract (see Fig. 105.6; Kelley et al, 1983), and in some cases, the artery also opens into the portal system (Gurakuqi et al, 2008). The arteriographic catheter should not be withdrawn until it has been decided whether embolization of the feeding artery should be considered as treatment. Arteriography is of special value in discovering central liver lesions, which may be difficult or impossible to localize during exploratory laparotomy. Although selective angiography is considered the gold standard for diagnosis in hemobilia, care must be taken in interpreting arteriograms; they may appear normal if the study is performed when there is no active bleeding.
Cholangiography done postoperatively through a T-tube by means of ERCP or through diagnostic percutaneous liver puncture (Fig. 105.14) may reveal a lesion by contrast filling of a cavity or by demonstration of clots, which appear as defects in the contrast filling (Millbourn, 1951).
Treatment
Surgical Intervention
In 1903, Kehr first demonstrated that ligation of the proper hepatic artery could be successfully used in hemobilia in a case of communication between a hepatic artery aneurysm and the cystic duct. The predominant initial treatment method for hemobilia, surgical intervention in the form of surgical resection or arterial ligation, has declined in popularity with the advent of nonoperative approaches (see Chapters 19, 28, and 102; Green et al, 2001; Sandblom, 1972; Yoshida et al, 1987); however, surgical intervention still has a role in cases where transarterial embolization (TAE) has failed, or where the etiology demands an operative approach. Cholecystectomy done for gallstones and surgical resection for intrahepatic or extrahepatic neoplasms are suitable approaches in patients who are hemodynamically stable with signs and symptoms of hemobilia. The surgical approach involves ligation of the bleeding vessel and/or excision of the pseudoaneurysm. If the bleeding vessel is not appropriately identified intraoperatively, ligation of the right or left hepatic artery can be done with little concern for perioperative necrosis, provided free flow of the respective portal vein is demonstrated to ensure adequate oxygenation of liver parenchyma.
Arterial Embolization (See Chapters 19 and 28)
Since the description of the first successful TAE of a hemobilia-producing right hepatic pseudoaneurysm in 1976 by Walter and colleagues, nonoperative embolization has become the preferred approach for the management of hemobilia (Fig. 105.15). The goal of TAE is to decrease blood flow to the abnormal communication between the vessel and biliary tree by placement of the catheter at the periphery of the bleeding vessel and embolization with occluding materials, such as absorbable gelatin sponge, coils, polyvinyl alcohol particles, cyanoacrylate, or microballoons. As a result of the selective embolization of the bleeding vessel, oftentimes a segmental branch of the hepatic artery, the risk of hepatic necrosis is minimal, especially if the portal vein is patent. It is important to perform selective embolization, because occlusion of a major hepatic arterial branch not only allows the possibility of rebleeding, owing to revascularization, it also makes impossible repeat embolization, which may be necessary. TAE successfully manages hemobilia in 75% to 100% of cases with little to no periprocedural morbidity (Lygidakis et al, 1991; Srivastava et al, 2006; Vaughan et al, 1984).
Biliary Tract Management
To address the issue, the initial procedure should be cholangiography, which may identify a biliary-venous fistula or a biliary-arterial fistula. Portal venous fistulas are easily treated by upsizing the biliary stent; that usually will be sufficient to tamponade the fistula tract (see Chapter 50D). If arterial hemorrhage is suspected, the patient should proceed to diagnostic angiography and TAE. If a bleeding site is not identified with the biliary drainage catheter in place, it may be necessary to remove the catheter prior to performing the arteriogram.
Bilhemia
Bilhemia denotes the reverse of hemobilia, with bile entering the bloodstream through a communication with the hepatic veins (Sandblom, 2000; Turk et al, 2010) or with branches of the portal vein (Lagagne et al, 1988). The reversed flow is due to inversion of the pressure gradient (François et al, 1994) caused by high intraductal biliary pressure, generally resulting from a peripheral obstruction or low venous pressure. In a large series of cases of obstructive jaundice, the pressure in the bile ducts was invariably higher than in the hepatic veins (Wiechel, 1964). In five instances, gallstones eroded into the portal vein. The first case was reported in 1559, preceding the description of hemobilia by more than a century, at the necropsy of Saint Ignatius of Loyola, with three gallstones found in the portal vein.
Portal vein bilhemia was recognized relatively recently. The first recent case to be reported was caused by liver biopsy, described by Brown and Walsh in 1952; a second description was provided 15 years later by Mehta and Rubenstone (1967). The condition, which was named by Clemens and Wittrin in 1975, is rare but dangerous. Of the 50 cases reported in the literature, half were fatal. The cause of the pathologic passage was often accidental: blunt trauma in 25 cases and iatrogenic injury or manipulation during operation in 3 cases (Koehler et al, 1978). The symptoms are a rapidly increasing jaundice with elevated direct bilirubin without increase of liver enzymes. Septicemia has occurred through bilhemia with infected bile (Morettin & Dodd, 1972). Selective arteriography is nondiagnostic in bilhemia, whereas scintigraphy has been useful (François et al, 1994). ERCP, in which the contrast material follows the direction of the biliary flow, is the best means to show the fistula (Singh et al, 2007; Sugiyama et al, 2000; Fig. 105.16).
Treatment aims at release of the ductal obstruction, sometimes through endoscopic sphincterotomy or, with a higher location, through a percutaneous transhepatic drain. This procedure sometimes causes the fistula to close or at least gives temporary relief (Sears et al, 1997); more lasting effect can be obtained with endostenting (Gable et al, 1997; Weintraub et al, 2006). For definitive cure, the affected liver may have to be resected (Clemens & Wittrin, 1975), or the fistula can be occluded via angiography or ERCP (Struyven et al, 1982).
Hemosuccus Pancreaticus
Hemosuccus pancreaticus, defined as hemorrhage into the pancreatic duct and through the ampulla of Vater into the GI tract, is a rare cause of intermittent UGI bleeding. First described by Lower and Farrell in 1931, the term was not popularized until 1970 by Sandblom. It is typically caused by the rupture of a pseudoaneurysm from the celiac trunk vasculature into the pancreatic duct (Fig. 105.17) secondary to acute and/or chronic pancreatitis (Sakorafas et al, 2000), although rupture of primary celiac trunk aneurysms (Massani et al, 2009) and rupture of vessels secondary to cystic neoplasms of the pancreas have also been described (Shinzeki et al, 2010; see Chapter 104).
Similar to the diagnosis of hemobilia, a high degree of clinical suspicion is needed to diagnose hemosuccus pancreaticus. Although there is no named triad, the combination of abdominal pain, hyperamylasemia, acute upper GI bleeding, and a history of acute and/or chronic pancreatitis should raise clinical suspicion of the diagnosis. The clinical sign of abdominal pain is present in most cases secondary to raised intraductal pressure from hemorrhage into the pancreatic duct, and it is accompanied by elevated serum amylase (Sakorafas et al, 2000).
Upper endoscopy is the preferred first diagnostic test, as the diagnosis of hemosuccus pancreaticus is so rare that other sources of UGI bleeding need to be ruled out. If the hemorrhage is active, blood can be seen emanating from the ampullary papilla at the time of upper endoscopy; however, because the bleeding can be intermittent in nature, a negative upper endoscopy does not preclude the diagnosis. Axial imaging with CT can often aid in the diagnosis of a pseudoaneurysm of the celiac trunk artery. ERCP can also be of a diagnostic help in unclear cases, as cholangiography can demonstrate the filling of the pancreatic duct with clots (Toyoki et al, 2008). Similar to hemobilia, selective angiography is the optimal imaging modality for suspected hemosuccus pancreaticus. The angiogram can demonstrate findings of a pseudoaneurysm of the celiac trunk vessels, and in rare instances, it can demonstrate a connection between the vessel and the pancreatic duct (Arnaud et al, 1994).
Two treatment options are available for hemosuccus pancreaticus: surgery and angiographic embolization. Following resuscitation and hemodynamic stabilization, the initial treatment should be angiographic embolization of the aneurysm with particles, as mentioned previously (see Chapters 19 and 104). Definite treatment of the bleeding using this technique is successful in 67% to 100% of cases (Lermite et al, 2007). Surgical treatment is generally reserved for uncontrolled hemorrhage or failure of angiographic embolization.
Ahmad SS, et al. Cholangiocarcinoma presenting as hemobilia and recurrent iron-deficiency anemia: a case report. J Med Case Reports. 2010;4:133.
Alis H, et al. Case report: acute pancreatitis caused by postcholecystectomic hemobilia. BMC Gastroenterol. 2010;10:75.
Arnaud JP, et al. Pancreatoduodenectomy for hemosuccus pancreaticus in silent chronic pancreatitis. Arch Surg. 1994;129(3):333-334.
Ben-Ishay O, et al. Gallbladder ulcer erosion into the cystic artery: a rare cause of upper gastrointestinal bleeding: case report. World J Emerg Surg. 2010;5:8.
Brown CY, Walsh GC. Fatal bile embolism following liver biopsy. Treat Serv Bull. 1952;7:445-450.
Burke DR, et al. Society of Interventional Radiology Standards of Practice Committee: quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14:S243-S246.
Burns L, Slakey DP. Laparoscopic management of massive hemobilia from an intrahepatic aneurysm. JSLS. 2009;13:60-63.
Carrillo EH, et al. Non-operative management of blunt hepatic trauma. Br J Surg. 1998;85:461-468.
Carrillo EH, et al. Interventional techniques are useful adjuncts in nonoperative management of hepatic injuries. J Trauma. 1999;46:619-622.
Clancy TE, Warren RL. Endoscopic treatment of biliary colic resulting from hemobilia after nonoperative management of blunt hepatic injury: case report and review of the literature. J Trauma. 1997;43:527-529.
Clemens M, Wittrin G, 1975: Bilhämie und Hämobilie nach Reitunfall. Taglich Nordw Dtsch Chirurg Vortrag, 1976, 166.
Contini S, et al. Gallbladder ulcer eroding the cystic artery: a rare cause of hemobilia. Am J Surg. 2009;198:e17-e19.
Deziel DJ, et al. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165(1):9-14.
Dousset B, et al. Selective surgical indications for iatrogenic hemobilia. Surgery. 1997;121:37-41.
Dutta U, et al. Hemobilia as presenting manifestation of polyarteritis nodosa. Indian J Gastroenterol. 2004;23:71.
Erb RE, Mirvis SE, Shanmuganathan K. Gallbladder injury secondary to blunt trauma: CT fndings. J Comput Assist Tomogr. 1994;18:778-784.
Fidelman N, et al. Hepatic arterial injuries after percutaneous biliary interventions in the era of laparoscopic surgery and liver transplantation: experience with 930 patients. Radiology. 2008;247:880-886.
Forlee MV, et al. Haemobilia after penetrating and blunt liver injury: treatment with selective hepatic artery embolisation. Injury. 2004;35:23.
François D, et al. Hepatobiliary scintigraphy in a patient with bilhaemia. Eur J Nucl Med. 1994;20:1020.
Gable DR, et al. Endoscopic treatment of posttraumatic “bilhemia”: case report. J Trauma. 1997;43:534-536.
Gad P. Okkult blodning ved galdesten. Nordisk Medicin. 1962;68:1069.
Glisson F. Anatomia Hepatis. London: O. Pullein; 1654.
Goto E, et al. Hemorrhagic complications of percutaneous radiofrequency ablation for liver tumors. J Clin Gastroenterol. 2010;44:374-380.
Green MHA, et al. Haemobilia. Br J Surg. 2001;88:773-786.
Guitron-Cantu A, et al. [Endoscopic diagnosis and treatment of non-traumatic bilhemia: a case report]. Rev Gastroenterol Mexico. 2002;67:259.
Gurakuqi GC, et al. Fatal hemobilia resulting from an iatrogenic arteriobiliary fistula as a rare complication of transjugular liver biopsy. Eur J Gastroenterol Hepatol. 2008;20:83-86.
Harbin WP, Mueller PR, Ferrucci JTJr. Transhepatic cholangiography: complications and use patterns of the fine needle technique: a multi-institutional survey. Radiology. 1980;135:15-22.
Hendriks MP, Wanten GJ, Drenth JP. Management of hemobilia and pancreatitis after liver biopsy: a key role for endoscopic retrograde cholangiopancreaticography. Liver Transpl. 2009;15:1653-1654.
Hernandez-Castillo E, et al. Haemobilia and gallbladder carcinoma. Dig Liver Dis. 2002;34:681.
Hines C, et al. Percutaneous transhepatic cholangiography. Dig Dis. 1972;17:868.
Jabbour N, et al. Arterioportal fstula following liver biopsy: three cases occurring in liver transplant recipients. Dig Dis Sci. 1995;40:1041-1044.
Jackson JBS. Aneurysm of the hepatic artery bursting into the hepatic duct. Med Mag. Boston. 1834;3:115.
Joo YE, et al. Hemobilia caused by liver abscess due to intrahepatic duct stones. J Gastroenterol. 2003;38:507.
Jornod P, et al. Hemobilia, a rare cause of acute pancreatitis after percutaneous liver biopsy: diagnosis and treatment by endoscopic retrograde cholangiopancreatography. Am J Gastroenterol. 1999;94:3051-3054.
Kehr H. Der erste Fall von erfolgreicher Unterbindung der Art: Hepatica propria wegen Aneurysma. Munch Med Wochenschr. 1903;50:1861-1867.
Kelley CJ, et al. Non-surgical management of post-cholecystectomy hemobilia. Br J Surg. 1983;70:502.
Khurro M, et al. Hepatobiliary and pancreatic ascariasis in India. Lancet. 1990;335:1503.
Kitagawa K, et al. Selective transcatheter hepatic arterial chemoembolization for hemobilia from hepatocellular carcinoma: report of three cases. J Vasc Interv Radiol. 1999;10:1357.
Koehler LS, et al. A complication of percutaneous cholangiography resulting in the hypoxia and death of an anesthetized patient. Anesthesiology. 1978;49:210.
Kubota H, et al. A patient with undifferentiated carcinoma of gallbladder presenting with hemobilia. J Gastroenterol. 2000;35:63.
Kurisu A, et al. Rupture of a pancreaticoduodenal artery aneurysm into the common bile duct resulting in fatal suppurative cholangitis: report of a case. Surg Today. 2005;35:94-96.
Lagagne PM, et al. Bilioportal fistula as a complication of choledochoduodenostomy. Surgery. 1988;103:125.
Lermite E, et al. Diagnosis and treatment of hemosuccus pancreaticus: development of endovascular management. Pancreas. 2007;34:229-232.
Liou T-C, et al. Liver abscess concomitant with hemobilia due to rupture of hepatic artery aneurysm. Hepatogastroenterology. 1996;43:241.
Lower WE, Farrell JT. Aneurysm of the splenic artery: report of a case and review of the literature. Arch Surg. 1931;23:182-190.
Luzuy F, et al. Biliary calculi caused by hemobilia. Surgery. 1987;102:886.
Lygidakis NJ, Okazaki M, Damtsios G. Iatrogenic hemobilia: how to approach it. Hepatogastroenterology. 1991;38:454-457.
Maralcan G, et al. Hemobilia in a patient with multiple intrahepatic, hepatic artery aneurysms. Acta Chir Belg. 2003;103:113.
Massani M, et al. Hemosuccus pancreaticus due to primary splenic artery aneurysm: a diagnostic and therapeutic challenge. JOP. 2009;10:48-52.
Matthes G, et al. Blunt liver injuries in polytrauma: results from a cohort study with the regular use of whole-body helical computed tomography. World J Surg. 2003;27:1124-1130.
Mehta S, Rubenstone AI. Pulmonary bile thromboemboli. A report of two cases. Am J Clin Pathol. 1967;47:490-496.
Merrell SW, Schneider PD. Hemobilia—evolution of current diagnosis and treatment. West J Med. 1991;155:621-625.
Millbourn E. Cholangiografiskt pånavisade blodkoagel i gallväuagarna i samband med choldochusingrepp. Nord Med. 1951;45:103.
Morettin L, Dodd G. Percutaneous transhepatic cholangiography. Dig Dis. 1972;17:831.
Morgagni S. Liber III. Epist. 1765;XXXVII:82.
Morioka D, et al. Hemobilia caused by pseudoaneurysm of the cystic artery. J Gastroenterol Hepatol. 2004;19:724.
Olsen WR. Late complications of central liver injuries. Surgery. 1982;92:733.
Parekh J, Corvera CU. Hemorrhagic cholecystitis. Arch Surg. 2010;145:202-204.
Parks RW, Chrysos E, Diamond T. Management of liver trauma. Br J Surg. 1999;86:1121-1135.
Piccinino F, et al. Complications following percutaneous liver biopsy: a multicentre retrospective study on 68 276 biopsies. J Hepatol. 1986;2:165-173.
Portal A, 1813: Observations sur la nature et le traitment des maladies du foie. Manuscript.
Prata Martins F, et al. Obstructive jaundice caused by hemobilia after liver biopsy. Endoscopy. 2008;40:E265-E266.
Quincke H. Ein Fall con Aneurysma der Leberarterise. Berliner Klinische Wochenschrift. 1871;8:349.
Rivera-Sanfeliz GM, et al. Incidence of important hemobilia following transhepatic biliary drainage: left-sided versus right-sided approaches. Cardiovasc Intervent Radiol. 2004;27:137-139.
Ryan MF, et al. Hemobilia due to idiopathic hepatic artery aneurysm: case report. Can Assoc Radiol J. 2002;53:149.
Sakorafas GH, et al. Hemosuccus pancreaticus complicating chronic pancreatitis: an obscure cause of upper gastrointestinal bleeding. Langenbecks Arch Surg. 2000;385:124-128.
Sandblom P. Hemorrhage into the biliary tract following trauma: traumatic hemobilia. Surgery. 1948;94:271.
Sandblom P. Gastrointestinal hemorrhage through the pancreatic duct. Ann Surg. 1970;171:61-66.
Sandblom P. Hemobilia (biliary tract hemorrhage): history, pathology, diagnosis, treatment. Springfield, IL: Charles C. Thomas; 1972.
Sandblom P, Mirkovitch V. Minor hemobilia: clinical significance and pathophysiological background. Ann Surg. 1979;190:254-264.
Sandblom P. Fatal bilhemia. Surgery. 2000;127:354.
Sears R, et al. Endoscopic diagnosis and therapy of a case of bilhemia after percutaneous liver biopsy. Gastrointest Endosc. 1997;46:276.
Seeff LB, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877-883.
Shinzeki M, et al. Mucinous cystic neoplasm of the pancreas presenting with hemosuccus pancreaticus: report of a case. Surg Today. 2010;40:470-473.
Shussman N, et al. Hemobilia due to hepatic artery aneurysm as the presenting sign of fibro-muscular dysplasia. World J Gastroenterol. 2008;14:1797-1799.
Singh V, et al. Endoscopic management of traumatic hepatobiliary injuries. J Gastroenterol Hepatol. 2007;22:1205-1209.
Srivastava DN, et al. Transcatheter arterial embolization in the management of hemobilia. Abdom Imaging. 2006;31:439-448.
Stewart BT, et al. Postcholecystectomy hemobilia: enjoying a renaissance in the laparoscopic era? Aust N Z J Surg. 1995;65:185-188.
Strauss A. Über Verblutung aus den Gallenwegen. Unfallheil Mschr. 1929;36:438.
Struyven J, et al. Post-traumatic bilhemia: diagnosis and catheter therapy. AJR Am J Roentgenol. 1982;138:746.
Sugiyama M, et al. Endoscopic biliary stenting for treatment of persistent biliary fistula after blunt hepatic injury. Gastrointest Endosc. 2000;51:42-44.
Teter LF. Massive hemorrhage from benign adenoma of the biliary duct causing death. J Mich Med Soc. 1954;53:62.
Toyoki Y, et al. Hemosuccus pancreaticus: problems and pitfalls in diagnosis and treatment. World J Gastroenterol. 2008;14:2776-2779.
Trakarnsanga A, et al. Massive hemobilia from a ruptured hepatic artery aneurysm detected by endoscopic ultrasound (EUS) and successfully treated. Endoscopy. 2010;42:E340-E341.
Tseng JH, et al. Icteric-type hepatoma: magnetic resonance imaging and magnetic resonance cholangiographic features. Abdom Imaging. 2001;26:171.
Turk E, et al. I. Bilhemia, an unusual complication after blunt liver trauma in a child: case report and review of the literature. Eur J Pediatr Surg. 2010;20:212-214.
Vaughan R, et al. Treatment of hemobilia by transcatheter vascular occlusion. Eur J Radiol. 1984;4:183-189.
Verset G, et al. Fatal hemobilia in advanced hepatocellular carcinoma invading biliary tract after treatment with sorafenib and biliary stenting. Ann Oncol. 2010;21:1381-1382.
Vishnevsky VA, et al. Surgical treatment of giant cavernous hemangioma liver. HPB Surg. 1991;4:69-78.
Walter JF, Paaso BT, Cannon WB. Successful transcatheter embolic control of massive hematobilia secondary to liver biopsy. AJR Am J Roentgenol. 1976;127:847-849.
Weintraub JL, et al. Treatment of a biliary-venous fistula following percutaneous biopsy in a pediatric living related liver transplant patient. Pediatr Radiol. 2006;36:555-557.
Wiechel KL. Percutaneous transhepatic cholangiography. Acta Chir Scand. 1964;Suppl 24:330.
Xu ZB, et al. Evaluation of selective hepatic angiography and embolization in patients with massive hemobilia. Hepatobiliary Pancreat Dis Int. 2005;4:254-258.
Yao CA, Arnell TD. Hepatic artery pseudoaneurysm following laparoscopic cholecystectomy. Am J Surg. 2010;199:e10-e11.
Yazici Z, et al. Polyarteritis nodosa presenting with hemobilia and intestinal hemorrhage. Eur Radiol. 1997;7:1059-1061.
Yoshida J, Donahue PE, Nyhus LM. Hemobilia: review of recent experience with a worldwide problem. Am J Gastroenterol. 1987;82:448-453.