Hemifacial Microsomia
Evaluation and Treatment
• Considerations during Infancy and into Early Childhood
• Dysmorphology Associated with Hemifacial Microsomia
• Facial Growth Potential with Hemifacial Microsomia
• Classification of Temporomandibular Joint–Mandibular Malformation
• Staging of Skeletal Reconstruction: Timing and Techniques
• Consideration of First-Stage Mandibular Reconstruction during the Mixed Dentition
• Facial Soft-Tissue Reconstruction
Hemifacial microsomia (HFM) is a craniofacial malformation that results in varying degrees of hypoplasia of the structures within the first and second branchial archs.34,35,38,53,62,65,66,81,84,174,227 Congenital hypoplasia is generally unilateral, although bilateral (asymmetric) involvement occurs in 5% to 15% of patients. Most cases of this condition are sporadic, but there are rare familial causes that exhibit autosomal dominant inheritance. The term hemifacial microsomia was first used by Gorlin and Pindborg during the early 1960s, although the first recorded cases may have been those of Canton in 1861 and Von Arlt in 1881.62 Many terms have been used for this malformation, thus indicating the wide spectrum of anomalies observed and emphasized by authors from various disciplines. In addition to HFM, the malformation has been called craniofacial microsomia, oculo–auriculo–vertebral dysplasia, first and second branchial arch syndrome, lateral facial dysplasia, unilateral oto–mandibular dysostosis, facio–auriculo–vertebral sequence, and oculo–auriculo–vertebral spectrum.
Inheritance Pattern
The occurrence of HFM has been estimated to range from 1 in 3500 to 1 in 26,550 live births. Cohen suggests that the birth prevalence is likely to be around 1 in 5600 live births.62,26 Morrison and colleagues stated a prevalence rate of conditions on the oculo–auriculo–vertebral spectrum of 1 in 45,000 in Northern Ireland.26
Kelberman and colleagues performed a genome-wide search for linkage in two families with features of hemifacial microsomia.105 In one of these families, the data were highly suggestive of linkage to a region of approximately 10.7 cM on chromosome 14q32, with a maximum multipoint LOD score of 3.00 between microsatellite markers D14S987 and D14S65. Linkage to this region was excluded in the second family, thereby suggesting genetic heterogeneity. With the use of an animal model.
Poswillo demonstrated that early vascular disruption with expanding hematoma formation in utero resulted in the destruction of differentiating tissues in the region of the ear and the jaw.214–218 The degree of local destruction appeared to be related to the severity of tissue damage caused by the hematoma. The constellation of anomalies seen in patients with HFM suggests an origin that occurs at about 30 to 45 days’ gestation. Naora and colleagues described a transgenic mouse line that carried an autosomal dominant insertion mutation that resulted in facial anomalies that resemble HFM, including microtia and abnormal occlusion.164
Engiz and colleagues reviewed the clinical and laboratory findings of 31 individuals with Goldenhar syndrome (15 boys and 16 girls) between the ages of 1 day and 16 years.47 The characteristic features were preauricular skin tags (90%), microtia (52%), hemifacial microsomia (77%), and epibulbar dermoids (39%). Vertebral anomalies were noted in 70%, cardiac malformations were found in 39%, genitourinary anomaly were noted in 23%, and various central nervous system malformations were found in 47%.
Several classification systems for HFM have focused on one or more fundamental anatomic features of this anomaly. In 1991, Vento and colleagues described the OMENS classification for HFM. This system substratifies each of five anatomic manifestations of HFM in accordance with their dysmorphic severity on a scale from 0 to 3. The five manifestations each constitute one letter of the OMENS acronym: orbital asymmetry; mandibular hypoplasia; ear deformity; nerve dysfunction; and soft-tissue deficiency. Scoring was done on the basis of conventional radiographs (e.g., posteroanterior, lateral, submental, panoramic), physical examinations, and photographs. Although the OMENS classification does allow for the objective cataloguing of a range of the abnormalities that constitute the spectrum of HFM, it falls short in terms of providing effective communication when determining the timing and technique that are best suited for the reconstruction of each component of the anomaly (i.e., maxillo-mandibular, orbito–zygomatic, soft tissues, external ear). It also is of limited value with regard to overall patient management.276
At the present time, HFM should be regarded as a nonspecific symptom complex that is etiologically and pathogenetically heterogeneous.* Extreme variability of expression is the characteristic finding.
Considerations during Infancy and into Early Childhood
At the time of birth, for a child with HFM, concerns will center on the adequacy of the airway; swallowing and feeding; hearing; vision; the presence of other associated malformations; and family unit psychosocial issues.3,16,27,28,56,57,112,125,131,165,175,193,196,197,199,207,208
The airway may be compromised as a result of 1) maxillary hypoplasia with choanal (nasal) obstruction 2) mandibular micrognathia with a retropositioned tongue obstructing the oropharyngeal/hypopharyngeal spaces 3) laryngomalacia/tracheomalacia or 4) neuromotor involvement.3,16,27,28,57,195 Depending on the severity of these malformations, a spectrum of airway problems may be present and necessitate treatment that ranges from special infant positioning with an extended hospital stay to mandibular osteotomies with advancement; on rare occasions, immediate or delayed tracheostomy may be required.
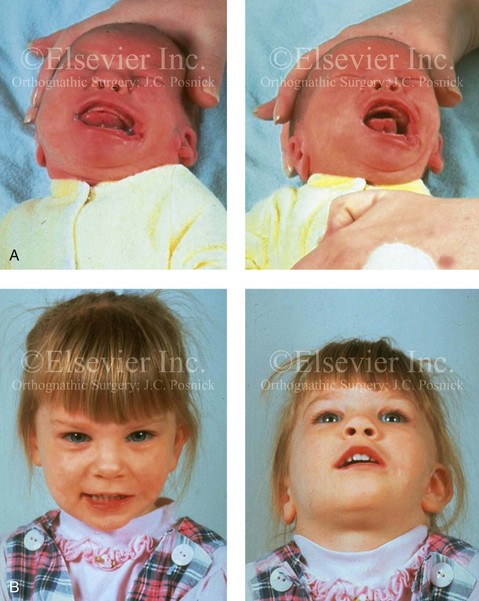
When a cleft (of the lip, palate, or both) or macrostomia is present, the timing of correction of these anomalies is generally similar to that used for the patient with non-syndromic cleft lip and palate (Fig. 28-1).
Dysmorphology Associated with Hemifacial Microsomia
Facial Soft Tissues
From a clinical perspective, the soft-tissue deficiencies associated with HFM can be considered to affect four anatomic regions of the head and neck: 1) the external ear; 2) the eyelid–adnexal structures; 3) the preauricular–cheek–lip soft tissues; and 4) the temporal fossa (Figs. 28-2 and 28-3).19,51,85,98,101,106,130,133,129,228,251 The soft tissues within each region that may be deficient and dysmorphic include the bulk of the cutaneous and subcutaneous tissue, the volume of fat, the muscles of mastication and facial expression, the cranial nerves, and the parotid and submandibular glands.
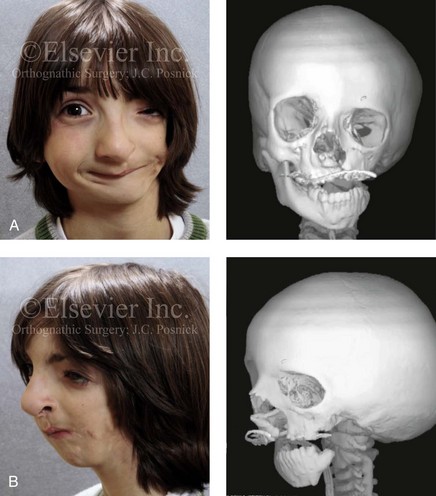
Figure 28-2 A 9-year-old boy who was born with left-sided hemifacial microsomia and a unilateral cleft lip and palate. He underwent lip and palate repair during early childhood at another institution. He is congenitally missing the left external ear and external auditory canal. The soft tissues of the eyelids and the cheek region are also deficient. The skeletal malformation involves the upper facial skeleton (i.e., the left zygomatic complex, the left orbit, and the left squamous temporal bone) and the lower facial skeleton (i.e., the maxilla and the mandible). He has a type III glenoid fossa mandibular malformation. A, Frontal facial and computed tomography scan views. B, Oblique facial and computed tomography scan views.
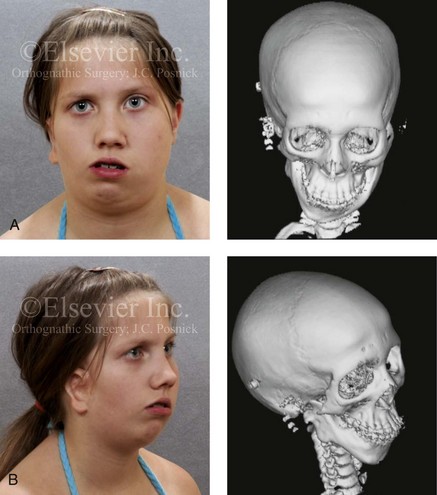
Figure 28-3 A 14-year-old girl who was born with right-sided hemifacial microsomia, including microtia and an absent external auditory canal. There is soft-tissue deficiency on the right side of the face. The skeletal malformations involve the right zygomatic complex as well as the mandible and the maxilla. There is a Type IIB mandibular malformation. The patient had previously undergone right ear reconstruction and the placement of a bone-anchored hearing aid (BAHA) at another institution. A, Frontal facial and computed tomography scan views. B, Oblique facial and computed tomography scan views.
According to the research of Kane and colleagues, in patients with HFM, the extent of hypoplasia of specific muscles of mastication frequently predicts the extent of dysplasia of the osseous origin and insertion of those muscles.98 If the temporalis muscle is hypoplastic, a deficiency of the coronoid process will be present. When the masseter muscle is hypoplastic, the gonial region of the mandible will also be deficient. When the lateral pterygoid muscle is deficient, the condylar head is deficient or absent.
Abnormalities of the external ear are a consistent finding and range from anotia to a mildly dysmorphic ear. Farkas and James were unable to demonstrate a direct relationship between the degree of microtia and the extent of skeletal deformity.51
The External Auditory Canal, the Middle- and Inner-Ear Structures, and Audiologic Findings
A narrow external auditory canal is frequently found in patients with mild external ear deformity, whereas atretic canals are expected in more severe cases.17,18,223,280 At times, a small external ear with normal middle-ear architecture is seen. Audiometry will delineate the nature of the hearing loss, which likely includes conductive and, less frequently, sensorineural loss in 15% of patients. Hypoplasia or agenesis of the ossicles may occur. In a comprehensive study, Caldarelli and colleagues used air and bone conduction audiometry and temporal bone tomography to evaluate 57 patients with hemifacial microsomia.17,18 The authors were unable to correlate the degree of auricular (i.e., external ear) deformity with hearing function. Focused temporal bone CT scans offer the best documentation of the middle-ear structures. Assessment of the unaffected and presumed normal ear is an essential part of the evaluation.
Maxillomandibular Region
A variable degree of hypoplasia of the skeletal structures within the first and second branchial arches will occur. As a result, the anteroposterior, transverse, and vertical dimensions of the face are diminished on the affected side, with secondary deformities on the contralateral side. This is especially true in the maxillomandibular region (see Figs. 28-2 and 28-3).134
Facial Growth Potential with Hemifacial Microsomia
The potential for longitudinal facial growth in a child who is born with HFM is an important factor when considering the timing and techniques of reconstruction.96,97,114,115,133,144–147,152–154,168,194,202–204,225,226,230–232 An additional pivotal consideration for the maxillofacial reconstruction of the individual with HFM is the integrity of the glenoid fossa–condyle–ascending ramus of the mandible (Fig. 28-4). Some authors speculate that a reduced mandibular growth potential secondarily leads to the observed maxillary deficiency on the ipsilateral side with progressive canting of the occlusal plane. Those who advocate mandibular surgery during the mixed dentition often do so with the belief that it will effectively correct the lower jaw malformation and that normal ongoing mandibular growth will continue with the prevention of secondary maxillary growth deformities in an otherwise normal upper jaw. There are objective clinical and radiographic studies in the literature that review the issue of the progression of deformity with HFM during growth.87–89,194,225,226
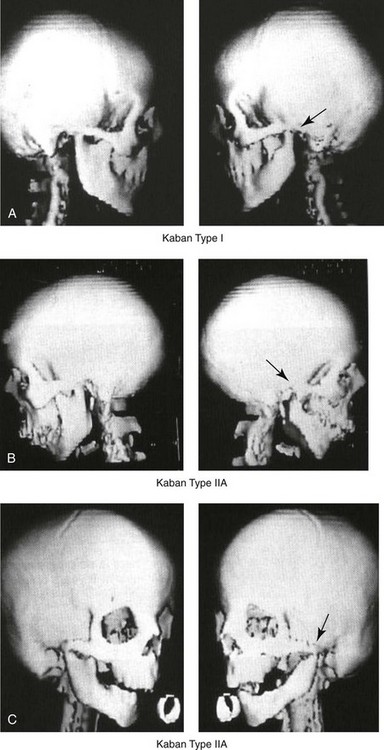
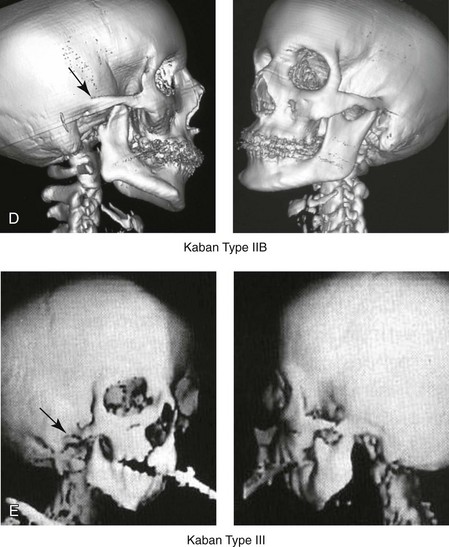
Figure 28-4 Kaban and colleagues described a classification system to define the degree of glenoid fossa–mandibular malformation observed in patients with hemifacial microsomia. Three-dimensional craniofacial computed tomography (CT) scans illustrate this classification system (see text for complete description). A, CT scans of a Type I mandibular malformation. B and C, CT scans of a Type IIA mandibular malformation. D, CT scans of a Type IIB mandibular malformation. E, CT scans of a Type III glenoid fossa–mandibular malformation in a patient who also has a unilateral cleft lip and palate.
Rune and associates placed metallic implants in the jaws to study the facial growth of 11 patients with HFM.225,226 They collected and studied serial roentgen stereophotogrammetry images from each patient. They found that, in five patients, the cant of the occlusal plane seemed to “slightly” increase; in the remaining six patients, the occlusal plane asymmetry either remained the same or improved with time. The authors also stated that the pattern of mandibular displacement showed no correlation with the severity of the presenting deformity. They concluded that the data “do not support the claim that the asymmetry of the jaws is invariably increased in time because of growth disparity between the affected and the unaffected sides.”225,226
Polley and associates completed a longitudinal radiographic cephalometric study of the maxillofacial region in patients with HFM.194 Twenty-six patients were included in the study and were followed longitudinally. Five patients (19%) had a Pruzansky grade I mandibular deformity, 14 patients (54%) had a Pruzansky grade II deformity, and 7 patient (27%) had a Pruzansky grade III deformity. The average age at the time that the initial cephalometric records for all patients were obtained was 3.5 years (range, 0.7 to 9.2 years), and the average age at the time that the final cephalometric records were obtained was 16.7 years (range, 10.1 to 22.5 years). None of the study patients had undergone surgical or orthopedic jaw manipulation during the time of the study, although nine patients underwent fixed appliance orthodontic treatment. Vertical and horizontal skeletal mandibular asymmetry was evaluated with posteroanterior cephalometry. The authors found that the growth of the affected side in patients with HFM parallels that of the contralateral side. The degree of mandibular asymmetry in the study patients remained relatively constant throughout craniofacial development. The mandibular skeletal deformity as measured in the study subjects did not progress with time. The growth rate of the affected side was similar to that of the contralateral side in each subject, irrespective of the degree of presenting mandibular deformity on the involved side. In the study group, subsequent growth occurred in both mandibular rami in each patient. This was true for all patients, irrespective of the Pruzansky grade and the side of the mandible that was affected.
Ongkosuwito and colleagues studied mandibular growth in 84 consecutive patients with a confirmed diagnosis of unilateral HFM. The mandibular malformation for each subject was categorized into one of four grades on the basis of the Pruzansky/Kaban classification. The malformations were then regrouped to reflect those with a functional glenoid fossa and condyle (Type I and Type IIA) and those without (Type IIB and Type III). The study groups were compared with a normal age-matched Danish control group. For each subject, an orthopantomogram was obtained and used to perform measurements of ramal height (i.e., the distance measured in millimeters between the condylion and the gonion) on each side. The data confirmed that patients with HFM start with a shorter ramal height and end up with a shorter ramal height, although growth is the same in both patients with HFM (regardless of the extent of malformation) and controls. In patients with HFM, the ramal height is smaller on both sides, which means that growth is characterized not simply by unilateral underdevelopment but by a complex three-dimensional phenomenon.161A
According to the independent findings of Polley and colleagues, Rune and colleagues, Meazzini and colleagues, Ongkosuwito and colleagues, the mandibular asymmetry associated with HFM is not progressive. In these studies, progressive canting of the maxillary plane was not routinely observed (Figs. 28-5 through 28-7).

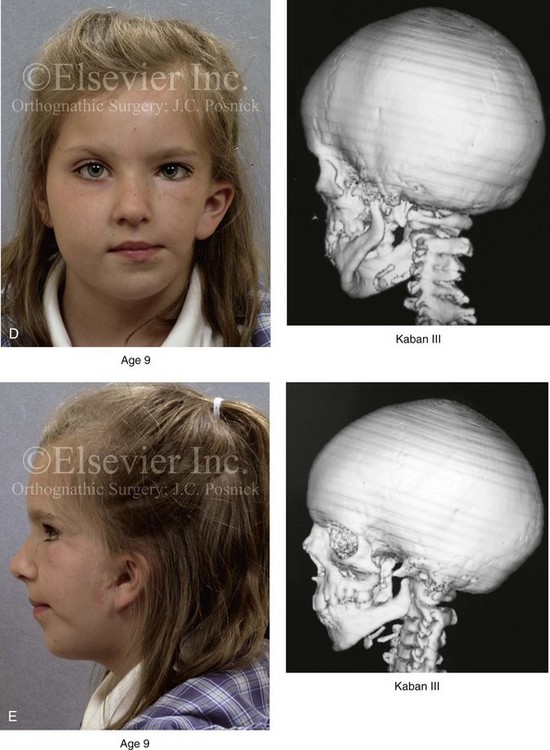
Figure 28-5 A girl who was born with left-sided hemifacial microsomia is followed up longitudinally at 5, 9, and 12 years of age without treatment intervention. There is a Type III glenoid fossa–mandibular malformation. No progression of the deformity has occurred. A, Frontal facial views at 5, 9, and 12 years of age. B, Oblique facial views at 5, 9, and 12 years of age. C, Profile views at 5, 9, and 12 years of age. D and E, Facial and computed tomography scan views at 9 years of age confirmed the extent of skeletal malformations involving the zygomatic–orbital complex, the maxilla, and the mandible.
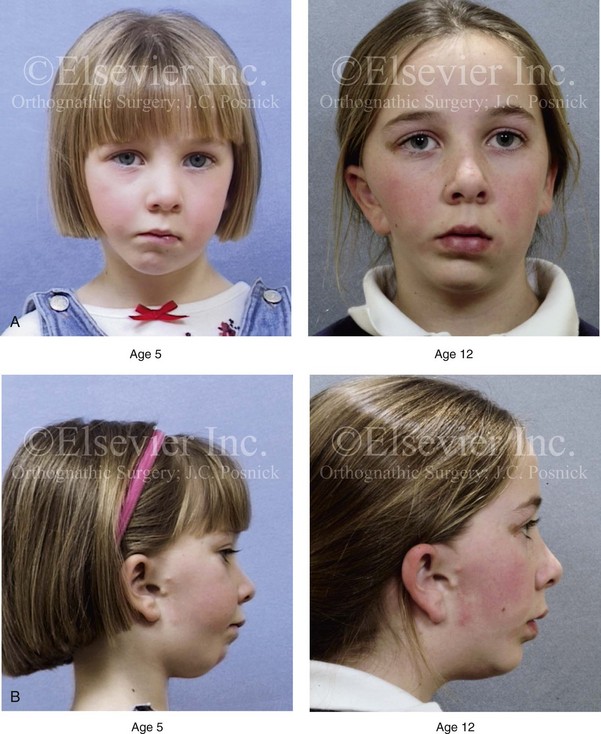
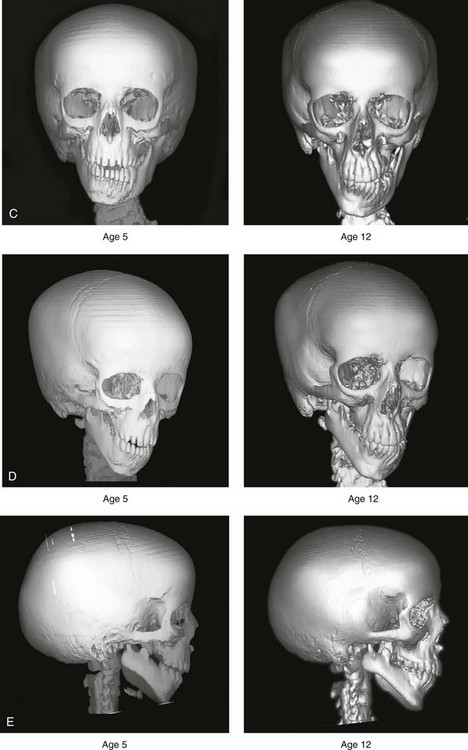
Figure 28-6 A girl who was born with right-sided hemifacial microsomia is followed up longitudinally at 5 and 12 years of age without treatment intervention. There is a Type IIB–mandibular malformation. No progression of the deformity is demonstrated. A, Frontal facial views in repose at 5 and 12 years of age. B, Profile views at 5 and 12 years of age. C, D, and E, Computed tomography scan views at 5 and 12 years of age that confirm no progression of the skeletal malformation.
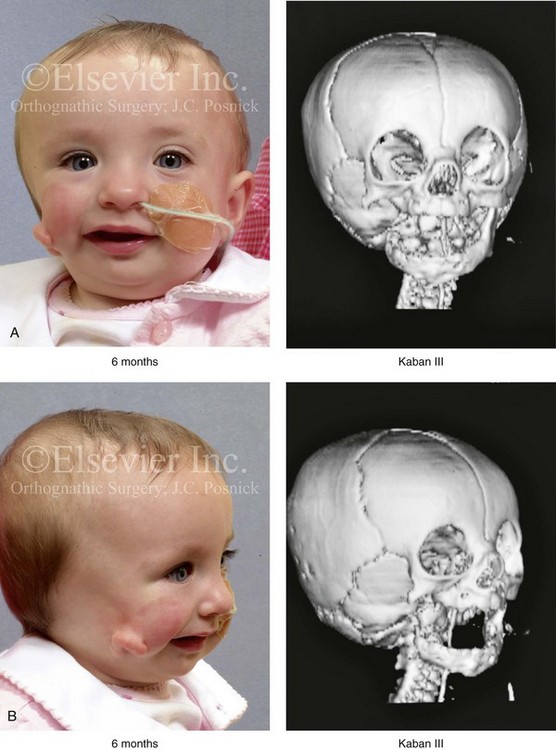
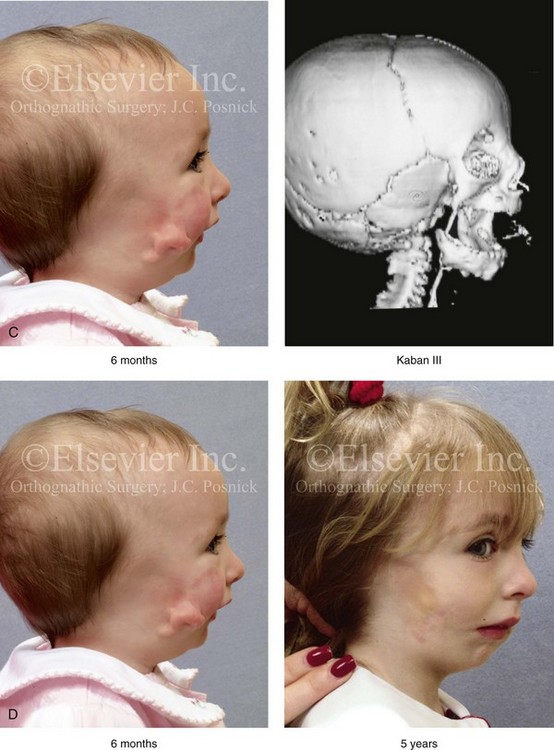
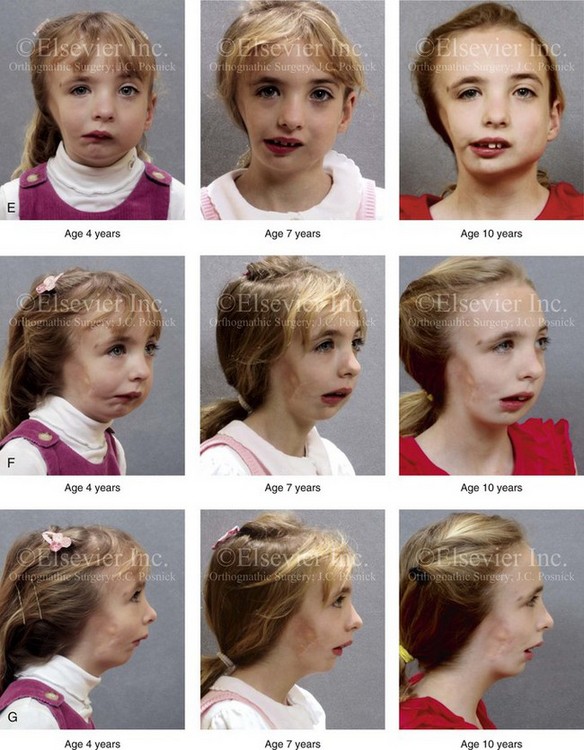
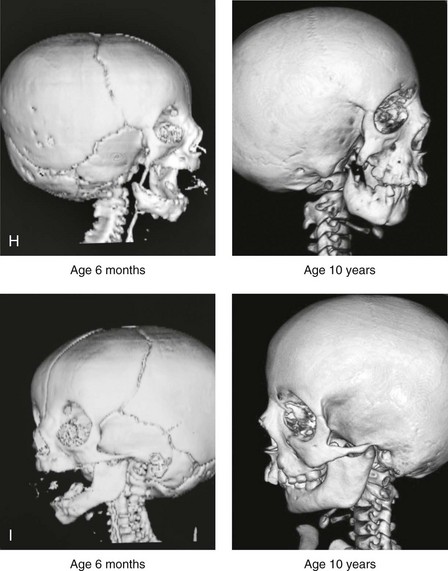
Figure 28-7 A child who was born with hemifacial microsomia of the right side. The extent of the soft-tissue and skeletal deficiencies of the structures within the first and second branchial arches is shown. There is a Type III glenoid fossa–mandibular malformation. The only treatment intervention was the excision of displaced redundant auricular tissue in the right cheek region. No progression of the skeletal malformation occurred between 6 months and 10 years of age. A, Frontal facial and computed tomography (CT) scan views at 6 months of age. B, Oblique facial and CT scan views at 6 months of age. C, Right profile and CT scan views at 6 months of age. D, Profile views at 6 months and 5 years of age. The excision of right cheek redundant soft tissue was carried out at 1 year of age with facial nerve preservation. E, Frontal views at 4 years, 7 years, and 10 years of age without treatment intervention. F, Oblique facial views at 4 years, 7 years, and 10 years of age without treatment intervention. G, Profile views at 4 years, 7 years, and 10 years of age without treatment intervention. H and I, Left and right CT scan profile views at 6 months and 10 years of age.
An alternative perspective is presented by Kaban and colleagues.92,93,95 Their research suggests to them that, as a result of ongoing growth after birth in patients with HFM, there is progressive canting measured at the pyriform rims, the occlusal plane, and the intergonial angles, especially with the Type IIB and III mandibular malformations. The authors recommend that mandibular reconstruction during the mixed dentition be considered to correct the lower jaw deformity in anticipation of normal ongoing longitudinal growth; to prevent secondary maxillary deformities (in an otherwise normal maxilla); and to alleviate psychosocial issues that would otherwise occur during childhood. I believe that Kaban and colleagues’ rationale for first-stage mandibular reconstruction during the mixed dentition in children with HFM are overly optimistic.
1. In perspective, even if a small degree of asymmetric jaw growth occurs during childhood in the patient with HFM, significant progressive dysfunction (i.e., in the areas of chewing, breathing, speech, lip control, or swallowing) that would justify a first-stage mandibular procedure is not generally seen.
2. The effectiveness of a first-stage mandibular procedure during the mixed dentition as a definitive long-term maxillofacial reconstruction has not been documented. Not only would a successful initial lengthening and derotation of the mandible be required, but the fabrication and then the periodic modification of an effective removable acrylic splint to create and maintain an ipsilateral posterior open bite (to allow for the supra-eruption of the maxillary posterior teeth) would be needed. The necessity of constant clinician monitoring and splint modification in conjunction with ongoing orthodontic treatment and rigorous patient compliance would be essential. This requires an experienced and dedicated orthodontist, a persistent surgical coordinator, and a zealous patient and family. All of the treating clinicians and the family must be in close geographic proximity and without socioeconomic barriers to treatment. Unfortunately, this is not a combination of events that is readily achievable.
3. Unfortunately, the costochondral graft construction of the neocondyle–ascending ramus required for the treatment of HFM Type IIB and III mandibular deformities carried out during the mixed dentition will result in a significant percentage of poorly growing mandibles (i.e., overgrowth or undergrowth). At minimum, a second definitive orthognathic reconstruction will be required (see the section to follow in this chapter about costochondral grafting).
4. Researchers have documented that, when a distraction osteogenesis (DO) approach is used as a first-stage mandibular procedure for HFM during the mixed dentition, symmetric lower jaw growth does not occur. The resulting residual mandibular asymmetry and deformity will, at minimum, require later definitive orthognathic reconstruction. In addition, the completion of a mandibular ramus–body osteotomy and DO during the mixed dentition is documented to result in the destruction of ipsilateral developing permanent molars in a significant percentage of children who have undergone such an operation.
Classification of Temporomandibular Joint–Mandibular Malformation
The extent of temporomandibular joint (TMJ)–mandibular deficiency is an important factor when considering the timing of and techniques for reconstruction.108,118,119,249 Imprecise definitions of the degrees of TMJ–mandibular anomalies have resulted in miscommunication among clinicians and in the literature. In an article published during the late 1960s, Pruzansky classified the presenting mandibular anomalies of HFM into three grades (Type I through Type III) in accordance with the extent of hypoplasia of the mandibular condyle and ramus.220 Kaban and colleagues refined the Pruzansky classification to further clarify the degree of glenoid fossa–condyle–ascending ramus malformation observed in patients with HFM.94,157 The Kaban classification provides an excellent starting point for defining the reconstruction required in each individual patient (see Fig. 28-4).
A Type I mandible has only a minimal degree of hypoplasia of the glenoid fossa, the condyle, and the ascending ramus (see Fig. 28-4, A). All of the skeletal components are present, all of the masticatory muscles are present, and function is within normal limits. There is a degree of asymmetric mandibular retrognathia, with a shift of the chin off of the facial midline. A limited degree of maxillary cant can usually be documented. Note: With Type I malformations, the condyle and the glenoid fossa are fully intact. There is no need to construct a new condyle or a new glenoid fossa.
A Type IIA mandible has a moderate degree of glenoid fossa and condyle–ascending ramus hypoplasia (see Fig. 28-4, B and C). By definition, the extent of hypoplasia results in the TMJ complex being located anteriorly and medially as compared with the normal side; however, joint function remains satisfactory. The masticatory muscles have a variable degree of hypoplasia. The mandible is retrognathic, the chin is shifted to the ipsilateral side, and an anterior open bite is frequently seen. A moderate maxillary cant is usually present. With a Type IIA malformation, despite significant hypoplasia, a decision to retain the TMJ complex (i.e., the condyle and the glenoid fossa) as part of the maxillomandibular reconstruction has been made.
The Type IIB mandible involves severe hypoplasia of the condyle–ascending ramus (see Fig. 28-4, D). The glenoid fossa has an anterior and medial location, but its placement is adequate for the construction of a neocondyle. The hypoplastic condyle, if it is present at all, is rudimentary and not substantial enough to seat into the glenoid fossa. There is not a consistent “posterior stop” to the mandible against the glenoid fossa. The mandible functions with rotation but not with a condylar component in the glenoid fossa, and there is little or no translational jaw movement. Vertical mouth opening is present (albeit reduced), with a shift of the chin to the ipsilateral side. The extent of mandibular dysmorphology, asymmetric retrognathia, and anterior open bite is marked. Variable but definite deficiencies of the masticatory muscles are present. There is usually significant hypoplasia of the ipsilateral maxilla, with obvious canting. By definition, with a Type IIB mandibular deficiency and deformity, the condylar remnant is too far displaced and rudimentary to be useful. Construction of a neocondyle–ascending ramus into the current acceptable glenoid fossa is required.
The Type III mandible is free-floating on the ipsilateral side, with no “posterior stop” of the lower jaw against the skull base on the affected side (see Fig. 28-4, E). The condyle and all or part of the ramus on the ipsilateral side are absent. The disc, the TMJ capsule, and the glenoid fossa are also not developed. Mandibular dysmorphology is severe and includes ipsilateral retrognathia and decreased posterior (ramus) facial vertical height. The masticatory muscles are severely hypoplastic, and the lateral pterygoid remnant is not attached to the mandibular structures. There is significant hypoplasia of the maxilla, with obvious canting. Occasionally, a tracheostomy is required shortly after birth to manage the airway compromise that results from a combination of factors, including (but generally not limited to) the degree of mandibular hypoplasia. By definition, with a Type III glenoid fossa–mandibular malformation, the eventual construction of both a neo–glenoid fossa and a neocondyle ascending ramus will be required to achieve successful maxillofacial reconstruction.
Staging of Skeletal Reconstruction: Timing and Techniques
The facial rehabilitation of the patient with HFM must address unique and specific components of the malformation that may include the following: 1) the zygomatic and orbital region; 2) the maxillomandibular regions; 3) the facial soft tissues; 4) the external ear; 5) the auditory canal; and 6) the middle-ear structures.*
Some clinicians have speculated that there is a reduced facial growth potential in patients with HFM which then leads to a progression of the primary malformation and secondary deformities of the maxillofacial skeleton. This assumption has led some to advocate early mandibular reconstruction to prevent progression. This author agrees with Rune and colleagues,225,226 Polley and colleagues,194 Meazzini et al.136A and Ongkosuwito and colleagues161A that the observed facial asymmetry in patients with HFM is not progressive with ongoing growth (see the section earlier in this chapter about facial growth potential with HFM) (Figs. 28-5 through 28-7).203,209–212,233,234
Zygomatic and Orbital Reconstruction
The benefits of thoughtfully timed and meticulously executed orbito–zygomatic reconstruction for the patient with HFM are often underappreciated.209–213 When upper face (i.e., orbito–zygomatic) reconstruction is needed, it is carried out through a coronal scalp incision. Correction of the upper face skeletal deformity may require the intracranial disassembly and reconstruction of the dysmorphic ipsilateral anterior cranial vault; the squamous portion of the temporal bone; and the lateral orbit regions with complete construction of the zygomatic complex, including the glenoid fossa. The use of full- and split-thickness cranial bone grafts is required (Fig. 28-8).
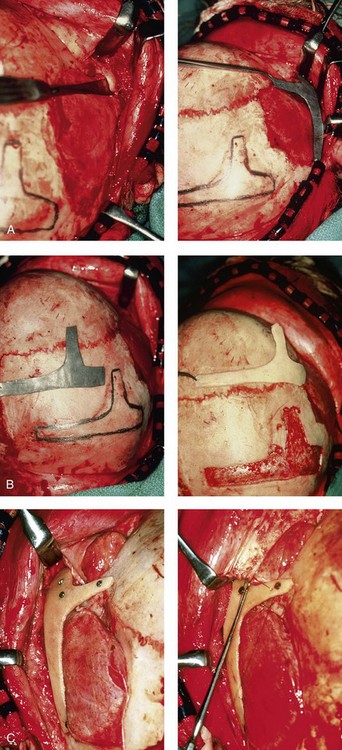
Figure 28-8 Intraoperative views of a 7-year-old child demonstrate the technique of zygomatic–orbital reconstruction as described in this chapter. When working through a coronal scalp incision, the zygomatic and orbital structures are exposed. A full-thickness cranial bone graft is harvested, and a zygomatic complex is crafted from this. The crafted zygoma is inset and stabilized with microplates and screws. The orbital defects are reconstructed as indicated with cranial bone graft, and the orbital rim is reshaped with a rotary drill. A lateral canthopexy is completed through the coronal incision and fixed to the new frontozygomatic suture region. A, View of the dissected rudimentary zygomatic remnant. A template of the proposed zygomatic complex had been made. B, The template is taken to the temporoparietal region of the skull, and its outline is marked out for craniotomy. Through a craniotomy, a full-thickness cranial bone graft is harvested for zygomatic complex reconstruction. C, The full-thickness cranial bone graft is fixed in place with microscrews. The lateral canthus is identified through the coronal incision, and a 28-gauge stainless steel wire is passed through it. The lateral canthus is pexied to the new frontozygomatic region. From Posnick JC, Goldstein JA, Waitzman AA: Surgical correction of the Treacher-Collins malar deficiency: quantitative CT scan analysis of long-term results, Plast Reconstr Surg 92:12-22, 1993.
This author believes that the reconstruction of the cranial vault, malar, and orbital deficiencies before the age of 7 years is not indicated unless functional disability warrants it.213 After the patient reaches this age, cranio–orbitozygomatic skeletal development is nearly mature. When indicated, an adult-sized anterior cranial vault, orbit, and zygomatic complex may be constructed and matched with the contralateral normal side with little concern about how future growth will alter the initial results achieved. Donor site skull reconstruction is also simpler after the age of 7 years than it is in younger children, because the presence of bicortical cranial bone will facilitate the splitting of the inner and outer tables for efficient reconstruction. Artificial bone material may also be used to reconstruct the graft donor site of the posterior skull (see Chapter 27).
Other surgeons have recommended variations in the methods and timing of the reconstruction of the upper face skeletal anomalies associated with HFM.31 Posnick and colleagues confirmed that the construction of the complete zygomatic complex with full-thickness autogenous cranial bone maintains volume and shape better than onlay grafts, which have been universally disappointing. Over time, onlay autogenous bone grafts that have been placed in the craniofacial region (e.g., supraorbital ridge, zygoma, anterior maxilla, angle of mandible, chin) from all tried donor sites (e.g., skull, hip, rib) have demonstrated significant and unpredictable resorption. Furthermore, no commensurate growth (i.e., volume expansion) of the graft with the underlying bone (e.g., the zygoma) should be expected. Some feel that, even if the onlay graft partially resorbs, at least something has been gained; however, this philosophy should be abandoned, because surface irregularities result in secondary deformities that further detract from the baseline malformation.
Maxillomandibular Reconstruction
An essential consideration when choosing the timing and techniques for maxillomandibular reconstruction in a patient with HFM is an understanding of the presenting TMJ–mandibular anatomy.2,6,7,20,25,30–32,43,46,59,96,97,180,209–212,226 The classification of the TMJ–mandibular malformation, which was described earlier in this chapter, is clinically relevant and facilitates communication.94,157 In the patient with HFM with either a Type I or Type IIA malformation, the basic maxillomandibular skeletal asymmetry and dysmorphology that requires reconstruction include the following: 1) degrees of altered facial height (i.e., decreased posterior facial height on the ipsilateral side); 2) diminished horizontal projection (i.e., deficiency more prominent on the ipsilateral side); and 3) decreased facial width (i.e. deficiency on the ipsilateral side). These malformations frequently result in canting (i.e., roll orientation) of the pyriform apertures, the maxilla, and the gonial angles; the shifting of the maxillary, mandibular, and chin midlines off of the facial midline (i.e. yaw orientation); clockwise rotation of the occlusal plane (i.e., pitch orientation); and an asymmetric class II malocclusion often with anterior open-bite malocclusion. According to the classification described, a patient who presents with a Type IIB malformation requires the construction of a neocondyle. For the individual with a Type III malformation in addition to this, there is a need for the construction of a neo–glenoid fossa.
Type I and IIA mandibular malformations are best reconstructed after all of the permanent teeth have erupted and orthodontic goals have been reached (see Fig. 28-4, A, B, and C). Surgical objectives can be met by making use of sagittal split ramus osteotomies of the mandible in combination with Le Fort I osteotomy (often in segments) and osseous genioplasty. This combination represents standard techniques, and it does not require bone grafts. The success of reconstruction is dependent on approximating mirror-image symmetry and Euclidian proportions of the skeleton through orthognathic procedures.
For Type IIB mandibular malformations, costochondral graft reconstruction of the deficient condyle ascending ramus at the time of skeletal maturity remains this surgeon’s preferred approach in most cases (see Fig. 28-4, D), despite its limitations (see the section to follow concerning condylar reconstruction with the use of costochondral graft). A sagittal split ramus osteotomy is completed on the contralateral side to derotate the distal mandible. This is combined with a Le Fort I osteotomy (often in segments) and an osseous genioplasty.
A ramus osteotomy of the contralateral side of the mandible is completed first to control repositioning of the distal mandible. The distal mandible is then secured to the maxilla via intermaxillary fixation through a custom designed acrylic splint to create the preferred reorientation of the lower jaw (see Chapter 14). It may be necessary to resect the ipsilateral coronoid process before repositioning the distal mandible. The contralateral ramus osteotomy is then rigidly fixed with bicortical screws. The ipsilateral proximal mandible is reconstructed with the autogenous costochondral graft. Harvesting the rib graft from the contralateral chest wall provides the best contour for mandibular reconstruction. The fixation of the rib graft to the native distal mandible is accomplished with a titanium miniplate and 2.0-mm or 2.3-mm screws. The fixation plate extends from the graft forward along the inferior border of the body of the mandible (fig. 28-15). It is generally necessary to recontour (i.e., with a bur on a rotary drill) the outer cortex of the distal mandible before onlaying the graft. Graft placement and fixation is often carried out through an extraoral Risdon neck incision. The avoidance of intraoral incisions on the ipsilateral mandibular ramus region during this procedure may minimize the incidence of infection. The effective seating of the neocondyle in the glenoid fossa is a critical step for successful reconstruction. In some patients, the extent of associated soft-tissue and skeletal deficiency will favor use of a vascularized fibula composite flap over the use of a costochondral graft.
The Type III glenoid fossa–mandibular malformation requires the surgical construction of the congenitally missing parts (see Fig. 28-4, E). The mandibular reconstruction is generally carried out as previously described for the Type IIB deformity. When the glenoid fossa requires construction (i.e., with a Type III malformation), so will the zygomatic complex. The glenoid fossa–zygoma and orbital reconstruction are best carried out as a separate operation when the patient is at least 7 years old (see the previous section about orbito–zygomatic reconstruction) and before mandibular reconstruction. With regard to the mandibular reconstruction, the deficiency of both the condyle-ascending ramus and the associated soft tissues may benefit from a vascularized fibula composite flap rather than a costochondral graft.
Consideration of First-Stage Mandibular Reconstruction during the Mixed Dentition
Personal Perspective Concerning First-Stage Mandibular Reconstruction during Childhood
The maxillofacial malformations in patients with HFM may be severe enough that, for airway or psychosocial reasons, the clinician feels compelled to recommend first-stage mandibular reconstruction during the mixed detention (i.e., between the ages of 7 and 11 years). Reported options for first-stage mandibular reconstruction in patients with HFM generally include 1) a ramus/body osteotomy followed by DO carried out over time for Type I and Type IIA malformations or 2) costochondral grafting with the immediate construction of a neocondyle ascending ramus for Type IIB and Type III malformations. In either case, derotation of the mandible to position the chin in the facial midline with the creation of an ipsilateral posterior open bite is usually considered the objective. The reconstructive option selected (i.e., costochondral graft versus DO) should be based on the presenting malformation and then on the following: 1) the three-dimensional morphologic results believed to be achievable; 2) the anticipated ongoing growth potential after surgery; 3) the differences in perioperative morbidity among the techniques; and 4) the overall burden of treatment to the patient, the family, the clinicians, and the health care system.209–212
Interestingly, most published reports of first-stage mandibular reconstruction in patients with HFM do not fully clarify the details of the presenting dysmorphology of the native glenoid fossa and condylar components before surgery (Figure 28-9). As stated, for DO to be effective, a functional glenoid fossa and an adequate condyle must be present (i.e., in the presence of Type I or Type IIA malformation). Unfortunately, a high incidence of bony and fibrous ankylosis has been reported in conjunction with mandibular DO in HFM.49 In addition, the consistent occurance of “undergrowth” after mandibular DO reconstruction carried out during the mixed dentition was confirmed by Meazzini and colleagues.143 Those authors conducted a comparison study of long-term follow up until the completion of facial growth of two homogenous samples of children with HFM. The experimental group was treated with mandibular DO during the deciduous or early mixed dentition in an attempt to correct the mandibular deformity. The control group was not subjected to any treatment until adulthood. The experimental group included children (n = 14) who underwent mandibular ramus osteotomies with DO (mean age, 5.9 years) with a mean follow up of 11.2 years. With the use of quantitative measurements on serial panorex radiographs, the DO group was compared with the control group (n = 8). The study results document that facial proportions in patients with HFM are maintained throughout growth when no treatment is undertaken. Unfortunately, after ramus osteotomies with DO, the mandibular disproportions returned to their original level of asymmetry during growth. The authors concluded the following: 1) HFM does not progress with regard to the degree of facial asymmetry or deformity when the patient is left to mature naturally and 2) early intervention with mandibular ramus osteotomies and DO does not effectively reduce long-term facial asymmetry in the patient with HFM.
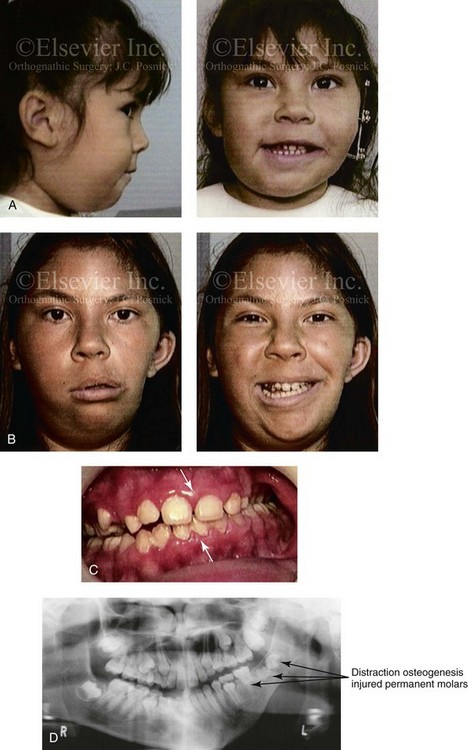
Figure 28-9 A Hispanic girl who was born with a mild form of left-sided hemifacial microsomia. The mandibular malformation was Type I. At another institution, she underwent a mandibular distraction procedure when she was 4 years old with an external device; the goals were to “derotate” the mandible, to shift the chin to the midline, and to create a posterior open bite. An acrylic splint was then used in an attempt to hypererupt the maxillary molars over time. The patient is shown at the time of procedure and then 7 years later after her referral to this surgeon. Unfortunately, the procedure carried out did not improve the occlusion or correct the facial asymmetry. During the process, the left mandibular permanent molars (i.e., the first, second, and third molars) were injured, and secondary deformities of the mandible also resulted. A, The child is shown when she was 4 years old, during the mandibular distraction. B, She is also shown at 7 years of age note the distortion and deformity of the mandible and the facial scarring. C, Residual malocclusion as a result of the external distraction. D, Panorex radiograph taken when the child was 11 years old (i.e., 7 years after the distraction) that illustrates the injuries to the first, second, and third permanent molars on the distracted side.
Ongoing problems when choosing the costochondral graft option to be carried out during the mixed dentition for the Type IIB or III malformation include overgrowth, undergrowth, ankylosis, and resorption of the mandible (Fig. 28-10). The overgrowth concerns are diminished but not eliminated when the costochondral graft procedure is carried out in a child who is in the late mixed dentition stage (i.e., 9 to 11 years old) and in whom only a minimum amount of cartilage (i.e., ≈2 mm) is left at the articulating surface of the graft (see section to follow concerning condylar reconstruction with costochondral graft).





Figure 28-10 A newborn with left-sided hemifacial microsomia. She had a good airway as well as swallowing and chewing abilities. She was treated at another institution until she was referred to this surgeon when she was 15 years old. A, B, and C, Facial and computed tomography (CT) scan views taken during infancy that indicate microtia with an absent external auditory canal, hypoplasia and clefting through the zygomatic arch, and mandibular malformation (Type IIB) with a shortened posterior facial height and a rudimentary condyle. D, Frontal and CT scan views taken when the patient was 3 years old indicate no progression of the malformation. E, The same child when she was 5 years old just before undergoing a mandibular procedure at another institution. At surgery the mandible was derotated to shift the chin to the midline with the creation of a posterior open bite on the ipsilateral side. A costochondral graft was harvested and used to reconstruct the left condyle ascending ramus. An acrylic splint was then worn for several years in an attempt to “grow” the left maxilla and relieve the posterior open bite. The external ear was later reconstructed, but with suboptimal results. F, G, H, and I, Facial and CT scan views of the patient when she was 15 years old, at the time of referral to this surgeon (i.e., 10 years after costochondral grafting) and without other interventions. There has been extensive overgrowth of the costochondral graft previously placed on the left side of the mandible. This has negatively altered (distorted) the growth of the whole mandible and the maxilla, and it has also resulted in limited mandibular mobility. There is gross distortion of the entire face, with negative effects on the airway, speech articulation, chewing ability, lip closure/posture, and self-esteem. J, K, L, and M, Comparable CT scan views are shown when the patient was 3 years old (i.e., before rib grafting) and at the age of 15 years (i.e., 11 years after rib grafting).
If a first-stage mandibular procedure is carried out during the mixed dentition for the Type IIB or III malformation, then the need for definitive mandibular reconstruction in conjunction with a Le Fort I maxillary osteotomy and an osseous genioplasty at the time of skeletal maturity should be anticipated.3,16,28,27 The idea of carrying out a mixed dentition mandibular reconstruction to avoid definitive maxillomandibular orthognathic reconstruction during adulthood is generally unrealistic.
1. Cant correction of the maxilla with the asymmetric intrusion of the anterior maxilla to establish a more normal upper lip–to–tooth relationship in repose and during smiling as well as to improve lip competence (i.e., roll orientation)
2. Correction of the maxillary dental midline to the facial midline with control of the yaw orientation to achieve an improved cheek region appearance and a better angle of mandibular symmetry
3. Either extrusion or intrusion of the posterior maxilla on each side, depending on the extent of counterclockwise rotation required to achieve adequate horizontal projection of the mandible and chin (i.e., pitch orientation)
4. Widening of the maxilla (i.e., segmentation) to correct crossbites and to add facial fullness
5. Horizontal advancement to improve midface projection and to open the upper airway
6. Bone grafting of the zygomatic buttress on the ipsilateral side to improve facial symmetry
7. Provision of access for intranasal procedures (e.g., septoplasty, inferior turbinate reduction, recontouring of the pyriform rims and the nasal floor).
Osteodistraction Reconstruction of the Mandible during Childhood
Since 1992, DO has been used as a technique in an attempt to correct mandibular morphology in young patients with HFM.* In 2002, a literature overview was published by Mommaerts and colleagues to address the long-term results of early DO among patients with HFM.148 It was concluded that no convincing evidence supported the benefits of DO over other techniques for the treatment of HFM, and DO was not found to offer advantages with regard to the limiting of complications. In 2009, Nagy and colleagues published a critical and exhaustive literature review in search of evidence of long-term stability after early distraction osteogenesis of the mandible in patients with HFM.163 Eighty-nine relevant articles were reviewed. Only 13 studies were found to have sufficient scientific merit to be included. The authors concluded the following:
1. The majority of the studies reported unstable results after DO with regard to facial symmetry that especially affected ramus height.
2. Clinicians frequently hypothesized that the volume of the soft tissues of the affected side would increase after DO in patients with HFM. Interestingly, the only published study that used a volumetric evaluation showed no postsurgical improvement of the soft-tissue deficiency on the affected side after DO reconstruction.
3. Even after accepting the shortcomings of certain study designs and evaluation methods, none of the published studies demonstrated the convincing long-term stability of the mandibular dimensions after DO mandibular elongation.
4. Relapse in regeneration after DO was seen in the majority of the studies that used reliable evaluation methods.
5. Repeated DO was reported to be necessary when pursuing attempts to maintain facial symmetry during growth in the patient with HFM. Therefore, any gains in mandibular dimensions after early DO were not stable, whether as a result of relapse or growth impairment.
6. In published reports, most children with HFM were still rated as “unattractive” after surgery and showed no “improvement in self-esteem.” Children with HFM have an elevated risk for childhood psychosocial difficulties.
7. If early DO is the treatment that is selected to be carried out during childhood for HFM, the patient and family must accept that the procedure will have to be repeated later during the patient’s life.
Costochondral Graft Reconstruction of the Mandible during Childhood
There are several basic theories regarding the mechanism of how mandibular growth occurs:
1. One theory of mandibular growth involves chondroblastic proliferation (i.e., cartilage formation with subsequent osseous replacement). Linear growth is primarily thought to be the result of condylar cartilage proliferation pushing the mandible forward and downward.
2. A second theory is the functional matrix theory, which explains mandibular growth primarily as a result of facial soft-tissue growth with subsequent projection (or pulling) of the mandible downward and forward in response to the forces of function. The condyle (and the condylar cartilage) is thought to merely secondarily react to the stimulating functional matrix forces that draw the mandible downward and forward. This is the theory behind the use of a Herbst appliance to “grow” a small mandible.
3. A third line of thinking is that both theories have merit. This explanation holds that a condylar growth center (i.e., chondroblastic proliferation) is present but that it is at least somewhat influenced by the physiologic function (i.e., functional matrix) of the mandible.
When the condyle and the components of the ascending ramus are hypoplastic or absent in patients with conditions such as HFM, surgeons have selected varied donor sources to replace the missing parts.* Autogenous replacements for the condyle have included 1) the metatarsal bones 2) the proximal head of the fibula 3) the costochondral junction of the rib (CCJ/rib) and 4) the sternoclavicular joint.
Clinical experience indicates that the CCJ/rib graft that is placed in the child or young teenager is prone to excessive growth. Although some would argue that overgrowth of the mandible and the need for additional surgical correction is a relatively minor consequence, experienced clinicians have found the secondary distortions in the maxillomandibular complex difficult to correct in a satisfactory way as compared with what could be achieved by single-stage reconstruction at the time of early skeletal maturity (Fig. 28-11). Unless pressing functional issues occur during childhood (e.g., obstructive sleep apnea), the advantages of a single-stage approach at jaw growth maturity to achieve favorable long-term function and aesthetic enhancement are difficult to ignore (Figs. 28-12 through 28-16).



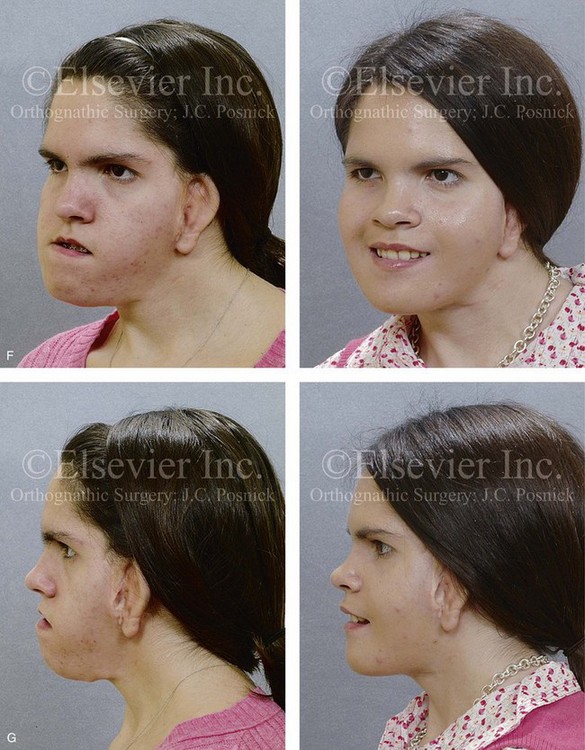

Figure 28-11 The 15-year-old girl who was born with hemifacial microsomia on the left side of the face and who was shown in Fig. 28-10 is shown at the time of referral to this surgeon. It has now been 10 years since she underwent left mandibular reconstruction with a costochondral graft. The mandibular opening was limited to approximately 23 mm but without bony ankylosis. The physical findings were the result of both the original malformation and the surgical intervention earlier during her life. She then underwent a combined orthodontic and surgical approach with this surgeon. A, Facial and computed tomography scan views taken when the patient was 15 years old indicate the extent of deformity and asymmetry. Articulated dental cast indicates analytic model planning to derotate the maxillomandibular complex (i.e., roll orientation correction). B, Facial views in repose before and after reconstruction. C, Frontal views with smile before and after reconstruction. D, Right oblique facial views before and after reconstruction. E, Right profile views before and after reconstruction. F, Left oblique facial views before and after reconstruction. G, Left profile views before and after reconstruction. H, Occlusal views before and after reconstruction. I, Lateral cephalometric radiographs before and after reconstruction.





Figure 28-12 A child who was born with right-sided hemifacial microsomia (Type IIA mandible) was followed up longitudinally without skeletal interventions until jaw maturity. No progression of the malformation occurred with growth. The patient had nasal obstruction and forced mouth breathing, and she developed a long face growth pattern. There is clockwise rotation of the maxillomandibular complex with a retrognathic profile (i.e., pitch orientation). The chin is also vertically long and retrusive. There is severe canting of the maxillomandibular complex (i.e., roll orientation). The patient was referred to this surgeon and agreed to further orthodontic treatment and orthognathic surgery. Her procedures included maxillary Le Fort I osteotomy (vertical intrusion, counterclockwise rotation, and correction of cant); bilateral sagittal split ramus osteotomies (correction of asymmetry, counterclockwise rotation, and horizontal advancement); osseous genioplasty (vertical reduction and horizontal advancement); and septoplasty, inferior turbinate reduction, and nasal floor recontouring. A, Frontal views from 6 months to 14 years of age. B, Frontal views in repose before and after reconstruction. C, Frontal views with smile before and after reconstruction. D, Profile views before and after reconstruction. E, Worm’s-eye views before and after reconstruction. F, Occlusal views before and after reconstruction. G and H, Articulated dental casts indicate analytic model planning. I, Lateral cephalometric radiographs before and after reconstruction.
C,D, From Posnick JC: Hemifacial microsomia: evaluation and staging of reconstruction, J Oral Maxillofac Surg 56:646, 1998.
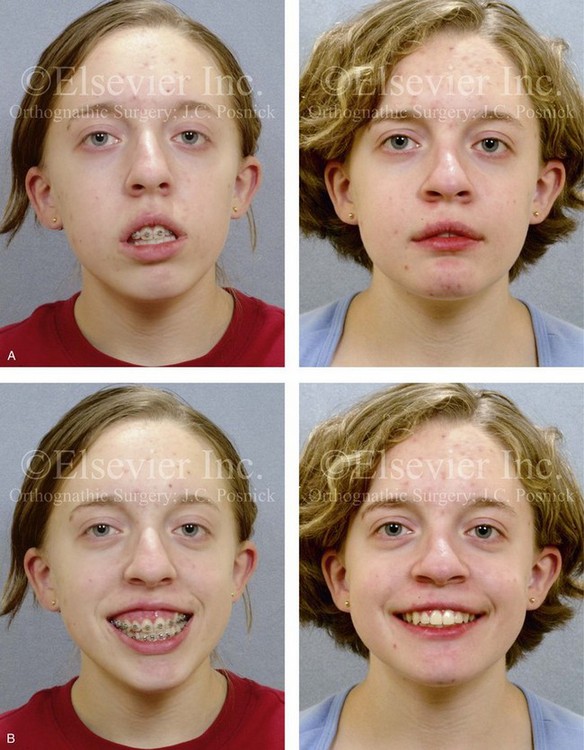
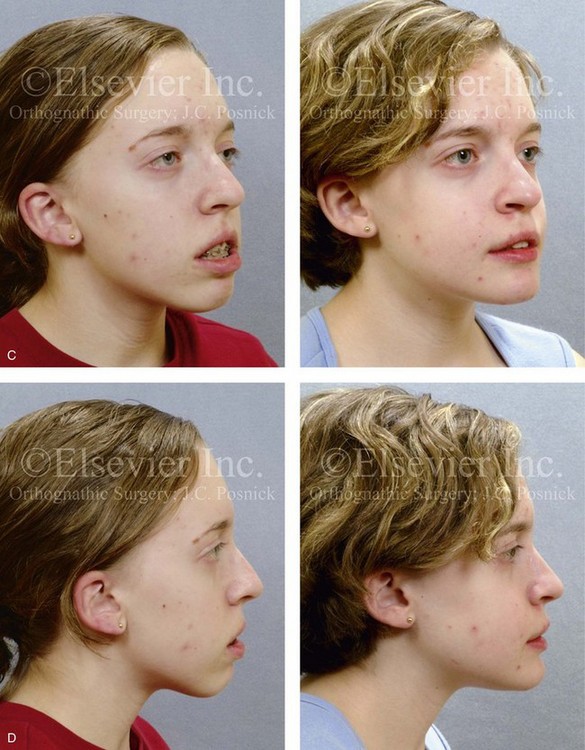

Figure 28-13 A teenage girl who was born with a mild form of left-sided hemifacial microsomia. She presented to this surgeon as a teenager with a lifelong history of difficulty breathing through the nose, a long lower anterior facial height (i.e., gummy smile and lip incompetence), facial asymmetry, and retrusive jaws in profile. Her external ears were also malformed. She agreed to a combined orthodontic and surgical approach. The patient’s procedures included maxillary Le Fort I osteotomy in segments (horizontal advancement, vertical intrusion, cant correction, and transverse widening); bilateral sagittal split ramus osteotomies (correction of asymmetry and horizontal advancement); osseous genioplasty (vertical shortening and horizontal advancement); and septoplasty, inferior turbinate reduction, and recontouring of the nasal floor. A, Frontal views in repose before and after reconstruction. B, Frontal views with smile before and after reconstruction. C, Oblique facial views before and after reconstruction. D, Profile views before and after reconstruction. E, Occlusal views with orthodontics in progress and after treatment. F, Articulated dental casts that indicate analytic model planning. G, Lateral cephalometric radiographs before and 1 year after reconstruction.
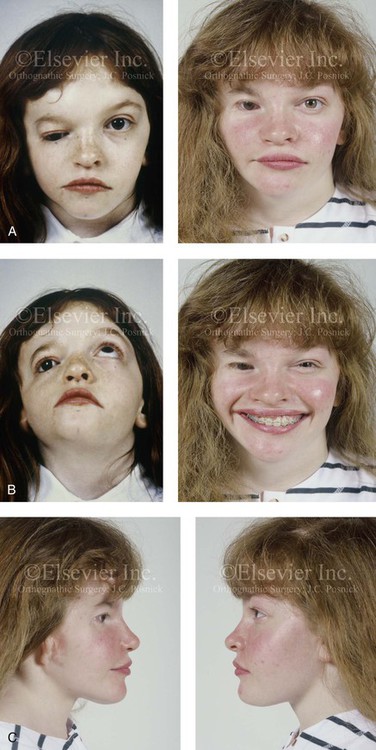
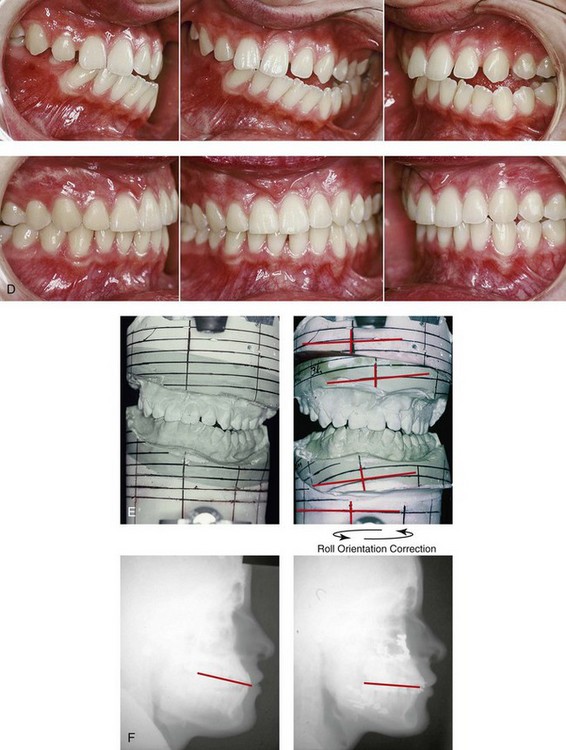

Figure 28-14 A girl who was born with a severe form of hemifacial microsomia (right side) that included extensive involvement of the upper facial skeleton (including micro-ophthalmia, lower facial skeleton issues (Type IIA mandibular malformation), and the external ear. She had undergone multiple intracranial cranio–orbital procedures, with limited success. She was referred to this surgeon as a teenager, and she underwent two additional reconstructive procedures. The first included cranio–orbito–zygomatic reconstruction through a coronal scalp incision (i.e., an intracranial approach) that required osteotomies of the cranial vault, the orbit, and the zygoma; this included autogenous cranial bone grafting. The second required orthognathic surgery, including a Le Fort I osteotomy (cant correction and horizontal advancement), bilateral sagittal split ramus osteotomies (asymmetry correction and horizontal advancement), and an osseous genioplasty (horizontal advancement) in combination with orthodontic treatment. The patient is shown before and after the two described procedures. A, Frontal views in repose before and after reconstruction. B, Worm’s-eye view before surgery and frontal view with smile after reconstruction. C, Right and left profile views after reconstruction. D, Occlusal views before and after reconstruction. E, Articulated dental casts that indicate model planning. F, Lateral cephalometric radiographs before and after reconstruction. G, Intraoperative views of upper orbits before and after redo cranio-orbit zygomatic reconstruction. H, Intraoperative views of cranial vault before and after redo cranio-orbito-zygomatic reconstruction. A,B,D, From Posnick JC: Hemifacial microsomia: evaluation and staging of reconstruction, J Oral Maxillofac Surg 56:648, 1998.


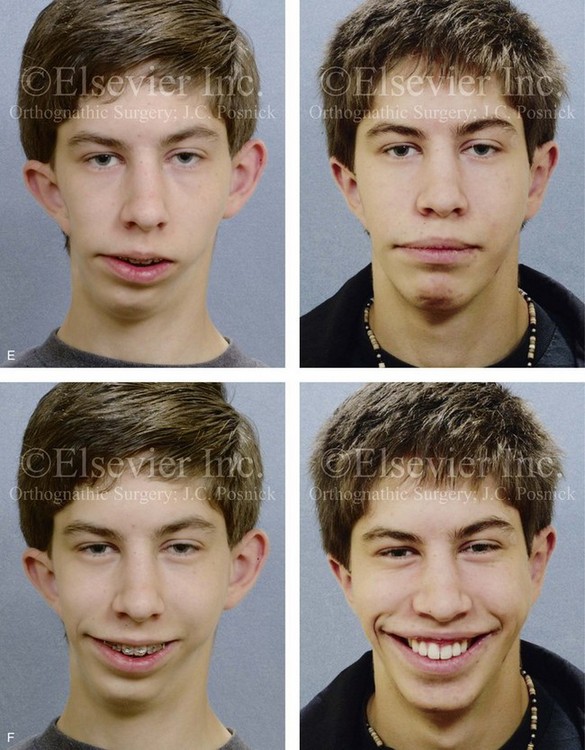


Figure 28-15 A boy who was born with hemifacial microsomia involving the right side of face. There was also a Type IIB mandibular malformation. The patient was followed longitudinally without treatment intervention until he approached skeletal maturity. Facial views demonstrate no progression of the malformation. After the patient was in the adult dentition and his orthodontic decompensation was complete, he underwent single-stage reconstruction that included Le Fort I osteotomy in segments (cant correction, horizontal advancement arch expansion, and vertical adjustment); left-sided sagittal split ramus osteotomy; right mandibular reconstruction with a costochondral graft (horizontal advancement and asymmetry correction); osseous genioplasty (asymmetry correction and horizontal advancement); and septoplasty, inferior turbinate reduction, and bilateral otoplasty (i.e., ear set-back). A, Frontal facial views in repose from the ages of 11 to 14 without treatment intervention indicate no progression of deformity. B, Illustrations of the presenting skeletal deformities and of the reconstruction that was carried out. Part B modified from an original illustration by Bill Winn. C and D, Articulated dental casts that indicate analytic model planning with the use of the “mandible first” technique (see Chapter 14). E, Frontal views in repose before and after reconstruction. F, Frontal views with smile before and after reconstruction. G, Left oblique facial views before and after reconstruction. H, Right oblique facial views before and after reconstruction. I, Occlusal views with orthodontics in progress and after reconstruction. J, Frontal facial and computed tomography scan views after reconstruction. K, Profile computed tomography scan views before and after reconstruction that indicate mandibular malformation and costochondral graft reconstruction.
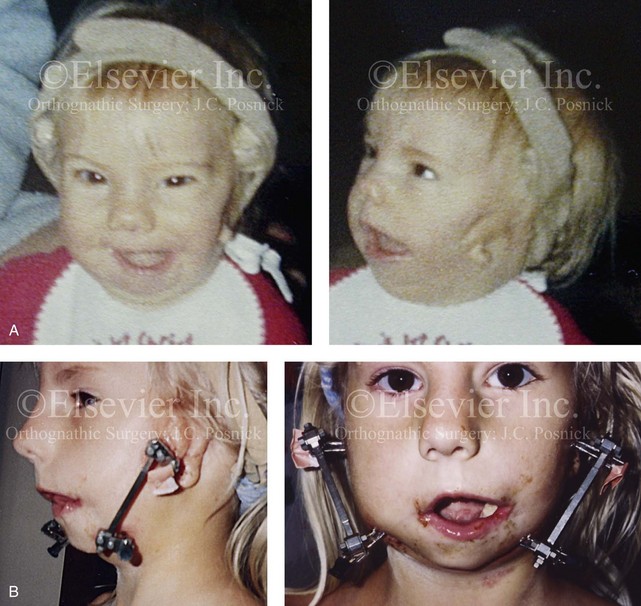
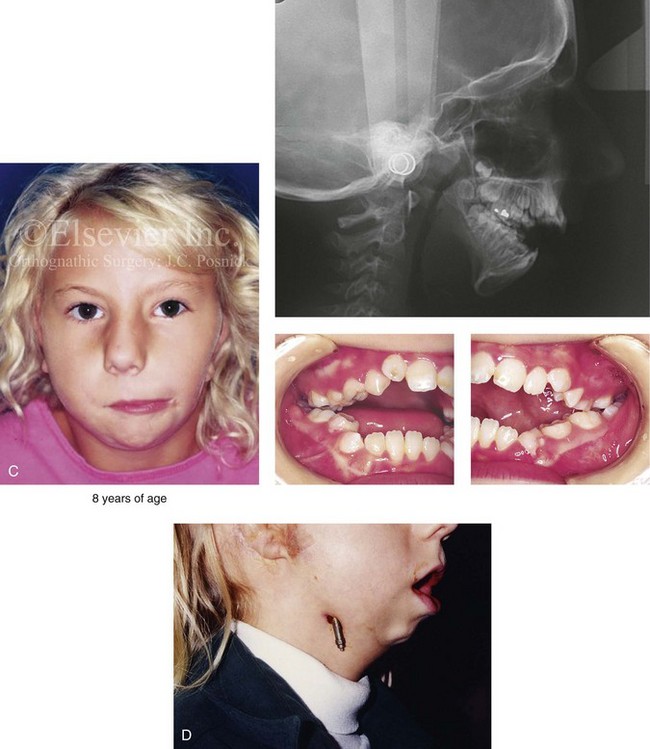
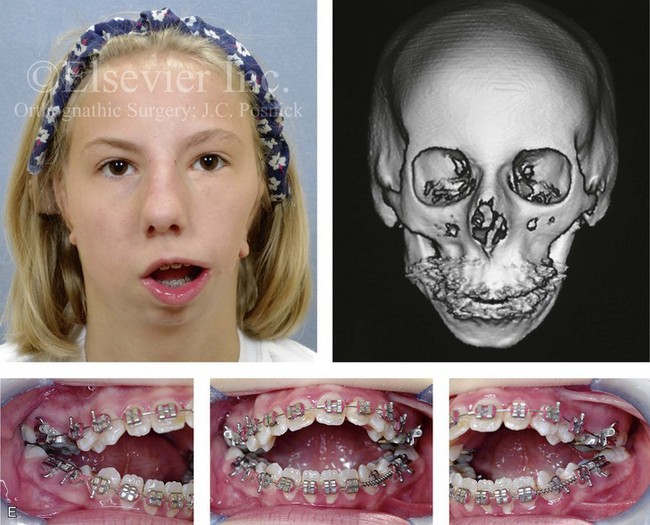



Figure 28-16 A newborn with a bilateral form of hemifacial microsomia that is more severe on the left side than the right and that involves the facial soft tissues and the jaws. The upper facial skeleton and the adnexal structures were essentially normal. The patient had an adequate airway and feeding ability at the time of birth and throughout childhood. At another institution when she was 7 years old, she underwent bilateral external distraction of the mandible. The procedure was unsuccessful for improving the mandibular dysmorphology or the occlusion. When she was 11 years of age, at another institution, the patient underwent a second mandibular distraction procedure with an internal device. This was also unsuccessful for improving the mandibular malformation or malocclusion. The patient had also undergone bilateral external ear reconstruction with a suboptimal result, and she wore external hearing aids. She presented to this surgeon when she was 15 years old with orthodontics in progress including 4 bicuspid extractions. She had a lifelong history of obstructed nasal breathing and deformities of the maxilla and the mandible. There was a marked Class II anterior open-bite malocclusion. An assessment was carried out that included an evaluation of the cervical spine, a complete computed tomography scan, and evaluation by a speech pathologist and an otolaryngologist. Reconstruction was executed and included septoplasty and inferior turbinate reduction; Le Fort I osteotomy (horizontal advancement, vertical adjustment, and counterclockwise rotation); bilateral ramus osteotomies (horizontal advancement and counterclockwise rotation) with interpositional grafting; and osseous genioplasty (vertical shortening and horizontal advancement). A, Facial views at 1 year of age, before any intervention. B, Facial views at 7 years of age, with bilateral mandibular distractors in place. C, Facial views at 8 years of age (i.e., 1 year after mandibular distraction) that indicate residual malocclusion and deformities of the maxilla and the mandible. D, Facial view at 11 years of age with bilateral mandibular distractions in place. E, Facial view, computed tomography scan, and occlusal views at 15 years of age at the time of referral to this surgeon. F, Profile and computed tomography scan views at time of referral to this surgeon. G, Articulated dental casts that indicate analytic model planning with the “mandible first” technique (see Chapter 14). H, Facial views with smile before and after reconstruction. I, Oblique facial views before and after reconstruction. J, Occlusal views with orthodontics in progress and after reconstruction. K, Lateral cephalometric radiographs before and after reconstruction.
Peltomaki and colleagues used a rat model to analyze the dynamic histology that occurred with the growth of the CCJ/rib.183–192 The observed histologic findings are dependent on the amount of cartilage that remains on the autogenous CCJ at the time of transplant. One of the initial findings of these authors was that “costochondral grafts do not adapt to the new environment, but rather preserve their inherent growth potential.” In subsequent studies, Peltomaki and colleagues continued to explore the idea of whether the intrinsic growth of the CCJ/rib may also be influenced by the interaction of hormonal factors and masticatory movements.183–192 It is now clear that variations in mandibular growth after the use of a CCJ/rib graft in both humans and experimental animal model simulations likely vary in accordance with the amount of cartilage used in the graft.
Osteodistraction of the Residual Mandibular Deformity after Costochondral Graft Placement during Childhood
Wan and colleagues reported their findings for the reconstruction of Type IIB and III mandibular malformations in patients with HFM.279 Their basic approach was to reconstruct the ipsilateral mandible with a CCJ/rib graft (N = 30 grafts, 27 patients) during the mixed dentition (mean age, 9.9 years ± 4.1 years). As a result of a high failure rate, this was followed by DO treatment of the residual deficient mandible. CCJ/rib graft failure that required regrafting occurred in 7 out of 30 cases (23%). Undergrowth that required DO during the mixed dentition occurred in 17 cases (57%). Overgrowth with severe distortion that required surgical correction occurred in 3 of the 30 cases (10%). For the majority of study patients who later required CCJ/rib graft DO, the complication rate was exceedingly high. In addition, all study patients (N = 27) required definitive orthognathic surgery at growth maturity, despite earlier procedures having been carried out.
Corcoran and colleagues presented a series of 8 patients with HFM who underwent mixed dentition costochondral reconstruction and who then submitted to DO.33 They found a 62.5% complication rate. Stelnicki and colleagues similarly reported on 9 patients who underwent mixed dentition CCJ/rib graft reconstruction and then required distraction.252A They reported a 33% rate of fibrous nonunion as well as other significant complications and limited ultimate success. On the basis of current published studies, it must be concluded that first-stage mandibular reconstruction with the use of CCJ/rib grafts during the mixed dentition for patients with Type IIB and Type III HFM deformities is fraught with complications and achieves limited success. The theory that a mandible reconstructed in this way can be reliably “distracted’ later if it does not grow normally has proved to be false.
Facial Soft-Tissue Reconstruction
For many patients, the extent of soft-tissue hypoplasia in the preauricular–cheek region will be distinctly noticeable at conversational distance, even after effective skeletal reconstruction.61,86,89,91,117,123,124,149,204,205,244,245,258,268,269 This results from multilevel hypoplasia of the subcutaneous tissue; the fat; the muscles of mastication and facial expression; and the parotid gland. For these patients, a well-designed soft-tissue “free” vascularized composite flap should be considered. When meticulously designed and expertly executed, the transfer of soft-tissue flaps from other body regions (with microvascular reanastomosis) may be the alternative of choice. Parascapular free flaps have become the workhorse for the reestablishment of facial soft-tissue volume since this process was first described by Dos Santos.40 Siebert and colleagues found that the parascapular flap allows for the reasonable correction of contour defects and yields improved facial aesthetic results in selected patients with HMF while minimizing scarring by providing vascularized soft tissue in the subcutaneous plane.244,245 Fortunately, hypoplasia of the centrally located soft tissues of the face (i.e., the nose, the medial aspects of the upper and lower lips, and the submental region) is not seen in patients with HFM. The soft-tissue reconstruction requires an experienced microvascular surgeon working closely with a maxillofacial and a auricular surgeon to coordinate the flap inset; to minimize recipient-site morbidity; and to coordinate skeletal and soft-tissue reconstruction. The timing of soft-tissue preauricular–cheek region reconstruction is generally planned to follow the skeletal reconstruction.
Dermal fat grafts, autogenous fat injections, and other forms of soft-tissue augmentation have gained in popularity. The results of autogenous fat injection are variable and considered technique sensitive, but are generally not harmful and clearly promising. Tanna and colleagues investigated the use of serial autologous fat grafting to restore soft-tissue contour in patients with HFM.259 Patients with moderate to severe HFM were divided into two groups. Group I included those who were undergoing microvascular free-flap reconstruction (i.e., inframammary extended circumflex scapular flaps, n = 10). Group II included patients who were undergoing multiple staged autogenous fat grafting (n = 21). The two patient groups had similar OMENS scores (2.4 and 2.3, respectively) and similar pre-reconstruction facial symmetry scores (74% and 75%, respectively). During the final evaluation, facial symmetry scores were 121% for the microvascular free-flap group and 99% for the fat-grafting group. Furthermore, no statistically significant difference between the microvascular and fat graft groups with regard to either patient or physician rating of overall satisfaction was noted.67,107,259
External Ear Reconstruction
In the hands of a few experts, surgical reconstruction of the external ear can be satisfactorily achieved with a staged approach.8,10–15,54,260,261 The methods described by Brent continue to represent the reference standard for auricular reconstruction.10–15 The successful placement of a well-sculpted autogenous cartilage framework is the foundation for a sound auricle repair. Brent prefers to wait until the patient is at least 6 years old, when rib cartilage is adequate for the reconstruction in most children. The synchondrotic region of the sixth and seventh ribs will then provide an ample cartilage block to use to form a framework for the ear. With the use of a prefabricated template of the contralateral ear and preoperatively determined measurements of the face, the ear’s position and size are chosen, and a small preauricular incision is made. Other stages of auricular construction include lobule transposition, the detachment of the ear with a skin graft, hairline management, and tragus construction. Other options for external ear reconstruction include the use of alloplastic and homologous frameworks. Alloplastic materials currently in use include silicone or Medpor (Porex Surgical, Inc, College Park, Ga). These foreign substances are more susceptible to infection, soft-tissue wound dehiscence, and minor trauma, even decades after reconstruction. Techniques of tissue engineering with bovine cartilage cells continue to be tested; these cells can be grown in the laboratory and seeded on a synthetic biodegradable ear template that is then implanted beneath the skin of an immunocompetent mouse. Unfortunately, until tissue engineering evolves beyond the currently encountered immunogenic problems, sculpted autogenous rib cartilage will remain the material of choice for the surgical repair of the ear.
External Auditory Canal and Middle-Ear Reconstruction
Occasionally, neurosensory hearing loss is present in the patient with HFM, but hearing loss is generally attributable to external auditory canal stenosis or atresia, hypoplasia of the middle-ear cavity, or ankylotic or missing ossicles.17,18,223,280 Ankylotic or non-functioning ossicles limit hearing conduction to the same extent that would occur if the ossicles were absent. Generally, attempts to reconstruct the external auditory canal and the bones of the middle ear for patients with HFM are not carried out. As long as adequate hearing is present in the contralateral ear, clinical problems generally relate only to the patient’s ability to locate the origin of the sound. For most patients, attempts to restore middle-ear function involve residual conductive hearing loss to the extent that “stereo” hearing is rarely achieved.
Bone-Anchored Hearing Aid Option
Colquitt and colleagues completed a systematic review and economic evaluation of BAHAs for people who are bilaterally deaf. 29 Previous prospective studies of adults or children with bilateral hearing loss were eligible for review. Twelve studies were included; there were seven cohort pre-post studies and five cross-sectional audiologic comparison studies. Comparisons were made between BAHAs and the following: 1) conventional hearing aids, which are also known as air-conduction hearing aids (ACHAs) 2) bone-conduction hearing aids (BCHAs) and 3) unaided hearing and ear surgery. The study also looked at 4) unilateral versus bilateral BAHAs. Outcomes reviewed included hearing measures, validated measures of quality of life, adverse events, and measures of cost-effectiveness.
Janssen and colleagues completed a systematic review of bilateral BAHAs for bilateral permanent conductive hearing loss.88 This was a review of the literature from between 1977 and 2011, and it included studies in which subjects of any age had permanent conductive hearing loss and bilateral implanted BAHAs. Eleven studies met the criteria for data analysis. Bilateral BAHAs were found to provide audiologic benefit as compared with unilateral BAHAs (i.e., improved thresholds for tones, speech in quiet and in noise, improved localization/lateralization, and patient’s perceived subjective benefit). Disadvantages of bilateral BAHAs included 1) listening in noise in some condition 2) presumed additional cost and 3) presumed increasing adverse event risk. The authors concluded that bilateral BAHAs provided additional objective and subjective benefit as compared with unilateral BAHAs.
References
1. Altug-Atac, AT, Grayson, BH, McCarthy, JG. Comparison of skeletal and soft tissue changes following unilateral mandibular distraction osteogenesis. Plast Reconstr Surg. 2008; 121:1751.
2. Anderson, PJ, McLean, NR, David, DJ. Modified costochondral graft osteotomy in hemifacial microsomia. Br J Plast Surg. 2003; 56(4):414–415.
3. Aoe, T, Kohchi, T, Mizuguchi, T. Respiratory induced cyanosis plethysmography and pulse oximeter in the assessment of upper airway patency in a child with Goldenhar syndrome. Can J Anesth. 1990; 37:369.
4. Baas, EM, Horsthuis, RBG, Lange, JD. Subjective alveolar nerve function after bilateral sagittal split osteotomy or distraction osteogenesis of mandible. J Oral Maxillofac Surg. 2012; 70:910–918.
5. Baek, SH, Kim, S. The determinants of successful distraction osteogenesis of the mandible in hemifacial microsomia from longitudinal results. J Craniofac Surg. 2005; 16(4):549–558.
6. Batra, P, Ryan, FS, Witherow, H, Calvert, ML. Long-term results of mandibular distraction. J Indian Soc Pedod Prev Dent. 2006; 24:30.
7. Beichman, K. Response of muscles to altered skeletal morphology and functional rehabilitation of severely malformed mandibles in hemifacial microsomia [thesis]. San Francisco, Calif: University of California School of Dentistry; 1990.
8. Bennun, RD, Mulliken, JB, Kaban, LB, Murray, JE. Microtia: A microform of hemifacial microsomia. Plast Reconstr Surg. 1985; 76(6):859–865.
9. Bergmann, C, Zerres, K, Peschgens, T, et al. Overlap between VACTERL and hemifacial microsomia illustrating a spectrum of malformations seen in axial mesodermal dysplasia complex (AMDC). Am J Med Genet A. 2003; 121A(2):151–155.
10. Brent, B. The correction of microtia with autogenous cartilage grafts: I. The classic deformity. Plast Reconstr Surg. 1980; 66:11.
11. Brent, B. The correction of microtia with autogenous cartilage grafts: II. Typical and complex deformities. Plast Reconstr Surg. 1980; 66:13.
12. Brent, B. Auricular repair using autogenous rib cartilage grafts: Two decades of experience with 600 cases. Plast Reconstr Surg. 1992; 90:355.
13. Brent, B. Advances in ear reconstruction with autogenous rib cartilage grafts: Personal experience with 1200 cases. Plast Reconstr Surg. 1999; 104:319.
14. Brent, B. The team approach to treating the microtia atresia patient. Otolaryngol Clin North Am. 2000; 33:1353.
15. Brent, B. Microtia repair with rib cartilage grafts: a review of personal experience with 1000 cases. Clin Plast Surg. 2002; 29:257.
16. Burstein, FD, Cohen, SR, Scott, PH, et al. Surgical therapy for severe refractory sleep apnea in infants and children: Application of the airway zone concept. Plast Reconstr Surg. 1995; 96:34.
17. Caldarelli, DD, Hutchinson, JC, Gould, HJ. Hemifacial microsomia: Priorities and sequence of comprehensive otologic management. Cleft Palate J. 1980; 17:111.
18. Caldarelli, DD, Hutchinson, JG, Jr., Pruzansky, S, et al. A comparison of microtia and temporal bone anomalies in hemifacial microsomia and mandibulofacial dysostosis. Cleft Palate J. 1980; 17:103.
19. Canton, E. Arrest of development of the left ramus of the lower jaw, combined with malformation of the external ear. Trans Pathol Soc Lond. 1861; 12:237.
20. Carlotti, AE, Jr. A variable treatment alternative for hemifacial microsomia: Case report. J Maxillofac Surg. 1981; 9:176.
21. Carls, FR, Sailer, HF. Seven years clinical experience with mandibular distraction in children. Craniomaxillofac Surg. 1998; 26:197.
22. Carlson, DS. Growth of a costochondral graft in the rat temporomandibular joint [discussion]. J Oral Maxillofac Surg. 1992; 50:857.
23. Cascone, P, Gennaro, P, Spuntarelli, G, Lannetti, G. Mandibular distraction: Evolution of treatment protocols in hemifacial microsomia. J Craniofac Surg. 2005; 16(4):563–571.
24. Cavaliere, CM, Buchman, SR. Mandibular distraction in the absence of an ascending ramus and condyle. J Craniofac Surg. 2002; 13(4):527–532.
25. Choung, PH, Nam, IW, Kim, KS. Vascularized cranial bone grafts for mandibular and maxillary reconstruction: The parietal osteofascial flap. J Craniomaxillofac Surg. 1989; 19:85.
26. Cohen, MM, Jr. Variability versus “incidental findings” in the first and second branchial arch syndrome: Unilateral variants with anophthalmia. Birth Defects Orig Artic Ser. 1989; 7:103.
27. Cohen, SR, Holmes, Re, Machado, L, Magit, A. Surgical strategies in the treatment of complex obstructive sleep apnea in children. Paediatr Respir Rev. 2002; 3:25.
28. Cohen, SR, Levitt, CA, Simms, C, Burstein, FD. Airway disorders in hemifacial microsomia. Plast Reconstr Surg. 1999; 103(1):27–33.
29. Colquitt, JL, Jones, J, Harris, P, Loveman, E, et al. Bone anchored hearing aids (BAHAs) for people who are bilaterally deaf; a systematic review and economic evaluation. Health Technol Assess. 2011; 15:1–200.
30. Converse, JM, Coccaro, PJ, Becker, M, Wood-Smith, D. On hemifacial microsomia: The first and second branchial arch syndrome. Plast Reconstr Surg. 1973; 51(3):268–279.
31. Converse, JM, Horowitz, SL, Coccaro, PJ, et al. The corrective treatment of the skeletal asymmetry in hemifacial microsomia. Plast Reconstr Surg. 1973; 52:221.
32. Converse, JM, Shapiro, HH. Treatment of developmental malformations of the jaws. Plast Reconstr Surg. 1952; 19:173.
33. Corcoran, J, Hubli, EH, Salyer, KE. Distraction osteogenesis of costochondral neomandibles: A clinical experience. Plast Reconstr Surg. 1997; 100:311–315.
34. Cousley, RR. A comparison of two classification system for hemifacial microsomia. Br J Oral Maxillofac Surg. 1993; 31(2):78–82.
35. Cousley, RR, Calvert, ML. Current concepts in the understanding and management of hemifacial microsomia. Br J Plast Surg. 1997; 50(7):536–551.
36. Cranin, AN, Gallo, L. Hemifacial microsomia with an edentulous mandible: Forme fruste or a new syndrome? Oral Surg Oral Med Oral Pathol. 1990; 70(1):29–33.
37. Daniels, S, Ellis, E, III., Carlson, DS. Histological analysis of costochondral and sternoclavicular grafts in the TMJ of the juvenile monkey. J Oral Maxillofac Surg. 1987; 45:675–682.
38. David, DJ, Mahatumarat, C, Cooter, RD. Hemifacial microsomia: A multisystem classification. Plast Reconstr Surg. 1987; 80:525.
39. Diner, PA, Tomat, C, Soupre, V, et al. Intraoral mandibular distraction: Indications, technique and long-term results. Ann Acad Med Singapore. 1999; 25:634.
40. Dos Santos, LF. The vascular anatomy and dissection of the free scapular flap. Plast Reconstr Surg. 1982; 73(4):599–603.
41. Duncan, PA, Shapiro, LR. Interrelationships of the hemifacial microsomia-VATER, VATER and sirenomelia phenotypes. Am J Med Genet. 1993; 47(1):75–84.
42. Dyggve, HV, Mikkelsen, M. Partial deletion of the short arms of a chromosome of the 4–5 group (Denver). Arch Dis Child. 1965; 40:82.
43. Edgerton, MT, Marsh, JL. Surgical treatment of hemifacial microsomia: First and second branchial arch syndrome. Plast Reconstr Surg. 1977; 59:653.
44. Ellis, E, III., Carlson, DS. Histological comparison of the costochondral, sternoclavicular, and temporomandibular joints during growth in Macaca mulatta. J Oral Maxillofac Surg. 1986; 44:312–321.
45. Ellis, E, III., Carlson, DS, Schneiderman, ED. Growth of the mandible following replacement of the mandibular condyle: An experimental investigation in Macaca mulatta. J Oral Maxillofac Surg. 2002; 60:1461–1470.
46. Ellis, E, III., Johnson, DG, Hayward, JR. Use of orthognathic surgery simulating instrument in the presurgical evaluation of facial asymmetry. J Oral Maxillofac Surg. 1984; 42:805.
47. Engiz, O, Balci, S, Unsal, M, et al. 31 cases with oculoauriculovertebral dysplasia (Goldenhar syndrome): Clinical, neuroradiologic, audiologic and cytogenetic findings. Genet Couns. 2007; 18(3):277–288.
48. Entin, MA. Reconstruction in congenital deformity of the temporomandibular component. Plast Reconstr Surg. 1958; 21:461.
49. Fan, K, Andrews, BT, Liao, E, et al. Protection of the temporomandibular joint during syndromic neonatal mandibular distraction using condylar unloading. Plast Reconstr Surg. 2012; 129:1151–1161.
50. Fan, WS, Mulliken, JB, Padwa, BL. An association between hemifacial microsomia and facial clefting. J Oral Maxillofac Surg. 2005; 63(3):330–334.
51. Farkas, LG, James, JS. Anthropometry of the face in lateral facial dysplasia: The unilateral form. Cleft Palate J. 1977; 14:193.
52. Figueroa, AA, Friede, H. Craniovertebral malformations in hemifacial microsomia. J Craniofac Genet Dev Biol Suppl. 1985; 1:167–178.
53. Figueroa, AA, Pruzansky, S. The external ear, mandible and other components of hemifacial microsomia. J Maxillofac Surg. 1982; 10:200.
54. Firmin, F. Microtia: Reconstruction by Brent’s technique. Ann Chir Plast Esthet. 1992; 37:119.
55. Fisher, E, Staffenberg, DA, McCarthy, JG, et al. Histopathologic and biochemical changes in the muscles affected by distraction osteogenesis of the mandible. Plast Reconstr Surg. 1997; 99:366.
56. Funayama, E, Igawa, HH, Nishizawa, N, et al. Velopharyngeal insufficiency in hemifacial microsomia: Analysis of correlated factors. Otolaryngol Head Neck Surg. 2007; 136(1):33–37.
57. Gallagher, DM, Hyler, RL, Epker, BN. Hemifacial microsomia: An anesthetic airway problem. Oral Surg Oral Med Oral Pathol. 1980; 49:2.
58. Garcia-Cimbrelo, E, Olsen, B, Ruiz-Yague, M, et al. Ilizarov technique: Results and difficulties. Clin Orthop. 1992; 283:116.
59. Gateno, J, Xia, JJ, Teichgraeber, JF, et al. Clinical feasibility of computer-aided surgical simulation (CASS) in the treatment of complex cranio-maxillofacial deformities. J Oral Maxillofac Surg. 2007; 65(4):728–734.
60. Glahn, M, Winther, JE. Metatarsal transplants as replacement for lost mandibular condyle (3-year follow-up). Scand J Plast Reconstr Surg. 1967; 1:97.
61. Goldsmith, D, Sharzer, L, Berkman, MD. Microvascular groin flaps in the treatment of hemifacial microsomia. Cleft Palate Craniofac J. 1992; 29:44.
62. Gorlin, RJ, Jue, KL, Jacobsen, U, et al. Oculoauriculovertebral dysplasia. J Pediatr. 1963; 63:991.
63. Gosain, AK. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2001; 107:278.
64. Gosain, AK, McCarthy, JG, Pinto, RS. Cervicovertebral anomalies and basilar impression in Goldenhar syndrome. Plast Reconstr Surg. 1994; 93:498.
65. Gougoutas AJ, Singh DJ, Low DW, Bartlett: Hemifacial microsomia: Clinical features and pictographic representations of the OMENS classification system. 120(7):112–120, 2007.
66. Grabb, WC. The first and second branchial arch syndrome. Plast Reconstr Surg. 1965; 36:485.
67. Grahovac, TL, Rubin, JP. Discussion: An analysis of the experiences of 62 patients with moderate complications after full face fat injection for augmentation. Plast Reconstr Surg. 2012; 129:1369.
68. Grayson, BH, McCormick, S, Santiago, PE, McCarthy, JG. Vector of device placement and trajectory of mandibular distraction. J Craniofac Surg. 1997; 8:473.
69. Greenberg, F, et al, Chromosome abnormalities associated with facio-auriculo-vertebral dysplasia. 1987.
70. Gripp, L, Husgen, W, Luhr, HG, et al. Hemifacial microsomia: Extraoral appliance for the early treatment of an infant. J Orofac Orthop. 1997; 58(6):352–360.
71. Gursoy, S, Hukki, J, Hurmerinta, K. Five-year follow-up of mandibular distraction osteogenesis on the dentofacial structures of syndromic children. Orthod Craniofac Res. 2008; 11:57.
72. Guyette, TW, Polley, JW, Figueroa, AA, Cohen, M. Mandibular distraction osteogenesis: Effects on articulation and velopharyngeal function. J Craniofac Surg. 1996; 7:186.
73. Guyuron, B, Lasa, CI, Jr. Unpredictable growth pattern of costochondral grafts. Plast Reconstr Surg. 1992; 90:880.
74. Hagino, H, Sawaki, Y, Ueda, M. The fate of developing teeth in mandibular lengthening by distraction: An experimental study. J Craniomaxillofac Surg. 2001; 29:94.
75. Hartsfield, JK. Review of the etiologic heterogeneity of the oculo-auriculo-vertebral spectrum (hemifacial microsomia). Orthod Craniofac Res. 2007; 10(3):121–128.
76. Harvold, E. Centric relation: A study of pressure and tension systems in bone modeling and mandibular positioning. Dent Clin North Am. 1975; 19:473.
77. Harvold E, Chierici G, Vargervik K, eds. Treatment of hemifacial microsomia. New York: Alan R. Liss, 1983.
78. Hathout, EH, Elmendorf, E, Bartley, J. Hemifacial microsomia and abnormal chromosome 22. Am J Med Genet. 1998; 76(1):71–73.
79. Hennig, TB, Ellis, E, III., Carlson, DS. Growth of the mandible following replacement of the mandibular condyle with the sternal end of the clavicle: An experimental investigation in Macaca mulatta. J Oral Maxillofac Surg. 1992; 50:1196.
80. Hollier, LH, Kim, JH, Grayson, B, McCarthy, JG. Mandibular growth after distraction in patients under 48 months of age. Plast Reconstr Surg. 1999; 103:1361.
81. Horgan, JE, Padwa, BL, LaBrie, RA, Mulliken, JB. OMENS-Plus: Analysis of craniofacial and extracraniofacial anomalies in hemifacial microsomia. Cleft Palate Craniofac J. 1995; 32(5):405–412.
82. Huisinga-Fisher, CE, Vaandrager, JM, Prahl-Anderson, B. Longitudinal results of mandibular distraction osteogenesis in hemifacial microsomia. J Craniofac Surg. 2003; 14(6):924–933.
83. Huisinga-Fisher, CE, Vaandager, JM, Prahl-Anderson, B, van Ginkel, FC. Masticatory muscle right-left differences in controls and hemifacial microsomia patients. J Craniofac Surg. 2004; 15(1):42–46.
84. Huisinga-Fisher, CE, Zonneveld, FW, Vaandrager, JM, Prahl-Anderson, B. Relationship in hypoplasia between the masticatory muscle and the craniofacial skeleton in hemifacial microsomia, as determined by 3-D CT imaging. J Craniofac Surg. 2001; 12(1):31–40.
85. Hwang, K, Chung, RS. Masks depicting hemifacial microsomia and cleft lip. J Craniofac Surg. 2002; 13(5):721–723.
86. Iñigo, F, Jimenez-Murat, Y, Arroyo, O, et al. Restoration of facial contour in Romberg’s disease and hemifacial microsomia: Experience with 118 cases. Microsurgery. 2000; 20(4):167–172.
87. Jansma, J, Bierman, MW, Becking, AG. Intraoral distraction osteogenesis to lengthen the ascending ramus: Experience with seven patients. Br J Oral Maxillofac Surg. 2004; 45:526.
88. Janssen, RM, Hong, P, Chadha, NK. Bilateral bone-anchored hearing aids for bilateral permanent conductive hearing loss: A systematic review. Otolaryngol Head Neck Surg. 2012; 147:412–422.
89. Ji, Y, Li, T, Shamburger, S, et al. Microsurgical anterolateral thigh fasciocutaneous flap for facial contour correction in patients with hemifacial microsomia. Microsurgery. 2002; 22(1):34–38.
90. Johnston, MC, Bronsky, PT. Animal models for human craniofacial malformations. J Craniofac Genet Dev Biol. 1991; 11:277.
91. Jurkiewicz, MJ, Nahai, F. The omentum: Its use as a free vascularized graft for reconstruction of the head and neck. Ann Plast Surg. 1982; 9:756.
92. Kaban, LB, Moses, MH, Mulliken, JB. Correction of hemifacial microsomia in the growing child: A follow-up study. Cleft Palate J. 1986; 23(Suppl 1):50.
93. Kaban, LB, Moses, MH, Mulliken, JB. Surgical correction of hemifacial microsomia in the growing child. Plast Reconstr Surg. 1988; 82:9.
94. Kaban, LB, Mulliken, JB, Murray, JE. Three-dimensional approach to analysis and treatment of hemifacial microsomia. Cleft Palate J. 1981; 18:90.
95. Kaban, LB, Padwa, BL, Mulliken, JB. Surgical correction of mandibular hypoplasia in hemifacial microsomia: The case for treatment in early childhood. J Oral Maxillofac Surg. 1998; 56(5):628–638.
96. Kamiji, T, Ohmori, K, Takada, H. Clinical experiences with patients with facial bone deformities associated with hemifacial microsomia. J Craniofac Surg. 1992; 2:181.
97. Kan, EY, Doyle, A, de Chalain, TB. Morphological variability of inferior alveolar nerve in low-grade craniofacial microsomia. J Craniofac Surg. 2002; 13(1):53–58.
98. Kane, AA, Lo, LL, Christensen, D, et al. Relationship between bone and muscles of mastication in hemifacial microsomia. Plast Reconstr Surg. 1997; 99:990.
99. Kaplan, RG. Induced condylar growth in a patient with hemifacial microsomia. Angle Orthod. 1989; 59(2):85–90.
100. Karp, NS, Thorne, CHM, McCarthy, JG, et al. Bone lengthening in the craniofacial skeleton. Ann Plast Surg. 1990; 24:231.
101. Kaye, CI, Rollnick, BR, Pruzansky, S. Malformations of the auricle: Isolated and in syndromes: IV. Cumulative pedigree data. Birth Defects Orig Artic Ser. 1979; 15:163.
102. Kearns, G, Kaban, LB, Padwa, B, et al. Progression of facial asymmetry in patients with hemifacial microsomia. J Oral Maxillofac Surg. 1997; 55(Suppl):48.
103. Kearns, GJ, Padwa, BL, Mulliken, JB, Kaban, LB. Progression of facial asymmetry in hemifacial microsomia. Plast Reconstr Surg. 2000; 105(2):493–498.
104. Keegan, CE, Mulliken, JB, Wu, BL, Korf, BR. Townes-Brocks syndrome versus expanded spectrum hemifacial microsomia: Review of eight patients and further evidence of a “hot spot” for mutation in the SALL1 gene. Genet Med. 2001; 3(4):310–313.
105. Kelberman, D, Tyson, J, Chandler, DC, et al. Hemifacial microsomia: Progress in understanding the genetic basis of a complex malformation syndrome. Hum Genet. 2001; 109(6):638–645.
106. Keogh, IJ, Troulis, MJ, Monroy, AA, et al. Isolated microtia as a marker for unsuspected hemifacial microsomia. Arch Otolaryngol Head Neck Surg. 2007; 133(10):997–1001.
107. Kim, SM, Kim, YS, Hong, JW, et al. An analysis of the experiences of 62 patients with moderate complications after full face fat injection for augmentation. Plast Reconstr Surg. 2012; 129:1359.
108. Kitai, N, Murakami, S, Takashima, M, et al. Evaluation of temporomandibular joint in patients with hemifacial microsomia. Cleft Palate Craniofac J. 2004; 41(2):157–162.
109. Knowles, CC. Cephalometric treatment, planning and analysis of maxillary growth following bone grafting to the ramus in hemifacial microsomia. Dent Pract Dent Rec. 1966; 17:28.
110. Ko, EW, Hung, KF, Huang, CS, Chen, PK. Correction of facial asymmetry with multiplanar mandible distraction: A one-year follow-up study. Cleft Palate Craniofac J. 2004; 41(1):5–12.
111. Kofod, T, Norhold, SE, Pedersen, TK, Jensen, J. Unilateral mandibular ramus elongation by intraoral distraction osteogenesis. J Craniofac Surg. 2005; 16:247.
112. Krucylak, CP, Schreiner, MS. Orotracheal intubation of an infant with hemifacial microsomia using a modified lighted stylet. Anesthesiology. 1992; 77:826.
113. Kulewicz, M, Cudzilo, D, Hortis-Dzierzbicka, M, et al. Distraction osteogenesis in the treatment of hemifacial microsomia. Med Wieku Rozwoj. 2004; 8:761.
114. Kunz, C, Brauchli, L, Moehle, T, et al. Theoretical considerations for the surgical correction of mandibular deformity in hemifacial microsomia patients using multifocal distraction osteogenesis. J Oral Maxillofac Surg. 2003; 61(3):364–368.
115. Kusnoto, B, Figueroa, AA, Polley, JW. A longitudinal three-dimension evaluation of the growth pattern in hemifacial microsomia treated by mandibular distraction osteogenesis: A preliminary report. J Craniofac Surg. 1999; 10:480.
116. Lai, G, Zhiyong, Z, Zang, M, et al. Restoration of facial symmetry in hemifacial microsomia with mandibular outer cortex bone grafting combined with distraction osteogenesis. Plast Reconstr Surg. 2011; 127:1997–2004.
117. LaRossa, D, Whitaker, L, Dabb, R, et al. The use of microvascular free flaps for soft tissue augmentation of the face in children with hemifacial microsomia. Cleft Palate J. 1980; 17:138.
118. Lauritzen, C, Munro, IR, Ross, RB. Classification and treatment of hemifacial microsomia. Scand J Plast Reconstr Surg. 1985; 19:33.
119. Lawson, K, Waterhouse, N, Gault, DT, et al. Is hemifacial microsomia linked to multiple maternities? Br J Plast Surg. 2002; 55(6):474–478.
120. Link, JO, Hoffman, DC, Laskin, DM. Hyperplasia of a costochondral graft in an adult. J Oral Maxillofac Surg. 1993; 51:1392–1394.
121. Longacre, JJ, deStefano, GA, Holmstrand, K. The early versus the late reconstruction of congenital hypoplasias of the facial skeleton and skull. Plast Reconstr Surg. 1961; 27:489.
122. Longacre, JJ, deStefano, GA, Holmstrand, KE. The surgical management of the first and second branchial arch syndromes. Plast Reconstr Surg. 1963; 31:507.
123. Longaker, MT, Siebert, JW. Microsurgical correction of bilateral facial contour deformities. Plast Reconstr Surg. 1996; 98:951.
124. Longaker, MT, Siebert, JW. Microsurgical correction of facial contour in congenital craniofacial malformations: The marriage of the hard and soft tissue. Plast Reconstr Surg. 1996; 98:942.
125. Luce, EA, McGibbon, B, Hoopes, JE. Velopharyngeal insufficiency in hemifacial microsomia. Plast Reconstr Surg. 1977; 30(4):602–606.
126. MacIntosh, RB, A current spectrum of costochondral grafting. Surgical correction of dentofacial deformities. Bell, WH, eds. Surgical correction of dentofacial deformities; Vol III. WB Saunders, Philadelphia, 1985:355–410.
127. MacIntosh, RB, Costochondral grafting. Modern practice in orthognathic and reconstructive surgery. Bell, WH, eds. Modern practice in orthognathic and reconstructive surgery; Vol II. WB Saunders, Philadelphia, 1992:873–949.
128. MacIntosh, RB, Henry, FA. A spectrum of application of autogenous costochondral grafts. J Maxillofac Surg. 1977; 5:257–267.
129. MacQuillian, A, Biarda, FU, Grobbelaar, A. The incidence of anterior belly of digastrics agenesis in patients with hemifacial microsomia. Plast Reconstr Surg. 2010; 126:1285–1290.
130. MacQuillan, A, Vesely, M, Harrison, D, Grobbelaar, A. Reanimation options in patients with hemifacial microsomia and marginal mandibular nerve palsy. Plast Reconstr Surg. 2003; 112(7):1962–1963.
131. Maris, CL, Endriga, MC, Omnell, ML, Speltz, ML. Psychosocial adjustment in twin pairs with and without hemifacial microsomia. Cleft Palate Craniofac J. 1999; 36:43.
132. Marquez, IM, Fish, LC, Stella, JP. Two-year follow-up of distraction osteogenesis: Its effect on mandibular ramus height in hemifacial microsomia. Am J Orthod Dentofacial Orthop. 2000; 117:130.
133. Marsh, JL, Baca, D, Vannier, MW. Facial musculoskeletal asymmetry in hemifacial microsomia. Cleft Palate J. 1989; 26:292.
134. Maruko, E, Hayes, C, Evans, CA, et al. Hypodontia in hemifacial microsomia. Cleft Palate Craniofac J. 2001; 38(1):15–19.
135. Mathog, RH, Leonard, MS. Surgical correction of Goldenhar syndrome. Laryngoscope. 1980; 90:1137–1147.
136. Matsumoto, K, Nakanishi, H, Koizumi, Y, et al. Occlusal difficulties after simultaneous mandibular and maxillary distraction in an adult case of hemifacial microsomia. J Craniofac Surg. 2004; 15(3):464–468.
137. McCarthy, JG, Grayson, BH, Coccaro, PJ, et al, Craniofacial microsomia. Plastic Surgery. McCarthy, J, eds. Plastic Surgery; Vol 4. WB Saunders, Philadelphia, 1990:3054–3100.
138. McCarthy, JG, Schreiber, J, Karp, N, et al. Lengthening of the human mandible by gradual distraction. Plast Reconstr Surg. 1992; 89:1.
139. McCarthy, JG, Stelnicki, EJ, Grayson, BH. Distraction osteogenesis of the mandible: A ten-year experience. Semin Orthod. 1999; 5:3.
140. McCarthy, JG, Stelnicki, EJ, Mehrara, BJ, Longaker, MT. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2001; 107:1812.
141. McNamara, JA, Carlson, DS, Ribbens, KA. The effect of surgical intervention on craniofacial growth. Ann Arbor, Mich: University of Michigan, Center for Human Growth and Development; 1982.
142. Meazzini, MC, Caprioglio, A, Garattini, L, Poggio, CE. Hemimandibular hypoplasia successfully treated with functional appliances: Is it truly hemifacial microsomia? Cleft Palate Craniofac J. 2008; 45(1):50–56.
143. Meazzini, MC, Mazzoleni, F, Bozzetti, A, Brusati, R. Comparison of mandibular vertical growth in hemifacial microsomia patients treated with early distraction or not treated: Follow up till the completion of growth. J Craniomaxillofac Surg. 2012; 40:105–111.
144. Meazzini, MC, Mazzoleni, F, Gabriele, C, Bozzetti, A. Mandibular distraction osteogenesis in hemifacial microsmia: Long-term follow-up. J Craniomaxillofacial Surg. 2005; 33(6):370–376.
145. Melsen, B, Bjerregaard, J, Bundgaard, M. The effect of treatment with functional appliance on a pathologic growth pattern of the condyle. Am J Orthod Dentofacial Orthop. 1986; 90:503.
146. Molina, F. Mandibular distraction: Surgical refinements and long-term results. Clin Plast Surg. 2004; 31:443.
147. Molina, F, Ortiz-Monasterio, F. Mandibular elongation and remodeling by distraction: A farewell to major osteotomies. Plast Reconstr Surg. 1995; 96:825.
148. Mommaerts, MY, Nagy, K. Is early osteodistraction a solution for the ascending ramus compartment in hemifacial microsomia? A literature study. J Craniomaxillofac Surg. 2002; 30(4):201–207.
149. Mordick, TG, Larossa, D, Whitaker, L. Soft tissue reconstruction of the face: A comparison of dermal–fat grafting and vascularized tissue transfer. Ann Plast Surg. 1992; 29:390.
150. Morovic, CG, Monasterio, L. Distraction osteogenesis for obstructive apneas in patients with congenital craniofacial malformations. Plast Reconstr Surg. 2000; 105:2321.
151. Moses, JJ, Lo, HH. The use of asymmetric yaw in the correction of lateral facial defects in hemifacial microsomia deformities: A case report. Int J Adult Orthodon Orthognath Surg. 1992; 7(4):229–234.
152. Moss, ML. The functional matrix. In: Kraus BS, Riedel RA, eds. Vistas of orthodontics. Philadelphia: Lea and Febiger; 1962:85–98.
153. Moss, ML. The primacy of functional matrices in orofacial growth. Dent Pract. 1968; 19:65–73.
154. Moss, ML, Salentijn, L. The primary role of functional matrices in facial growth. Am J Orthod. 1969; 55:566–577.
155. Moulin-Romsee, C, Verdonck, A, Schoenaers, J, Carels, C. Treatment of hemifacial microsomia in a growing child: The importance of co-operation between the orthodontist and the maxillofacial surgeon. J Orthod. 2004; 31(3):190–200.
156. Mulliken, JB, Ferraro, NF, Vento, AR. A retrospective analysis of growth of the constructed condyle–ramus in children with hemifacial microsomia. Cleft Palate J. 1989; 26:312.
157. Mulliken, JB, Kaban, LB. Analysis and treatment of hemifacial microsomia in childhood. Clin Plast Surg. 1987; 14:91.
158. Munro, IR. One-stage reconstruction of the temporomandibular joint in hemifacial microsomia. Plast Reconstr Surg. 1980; 66:699.
159. Munro, IR, Phillips, JH, Griffin, G. Growth after construction of the temporomandibular joint in children with hemifacial microsomia. Cleft Palate J. 1989; 26:303.
160. Murray, JE, Kaban, LB, Mulliken, JB. Analysis and treatment of hemifacial microsomia. Plast Reconstr Surg. 1984; 74:186.
161. Murray, JE, Kaban, LB, Mulliken, JB, et al. Analysis and treatment of hemifacial microsomia. J Craniofac Surg. 1985; 33:377.
Ongkosuwito, EM, van Vooren, J, van Neck, JW, et al. Changes of mandibular ramal height, during growth in unilateral hemifacial microsomia patients and unaffected controls. J Craniomaxillofac Surg. 2013; 41:92–97.
162. Murray, JE, Mulliken, JB, Kaban, IB, et al. Twenty-year experience in maxillofacial surgery: An evaluation of early surgery on growth function and body image. Ann Surg. 1979; 190:320.
163. Nagy, K, Kuijpers-Jagtman, AM, Mommaerts, MY. No evidence for long-term effectiveness of early osteodistraction in hemifacial microsomia. Plast Reconstr Surg. 2009; 124:2061–2071.
164. Naora, H, Kimura, M, Otani, H, et al. Transgenic mouse model of hemifacial microsomia: Cloning and characterization of insertional mutation region on chromosome 10. Genomics. 1994; 23(3):515–519.
165. Nargozian, C, Ririe, DG, Bennun, RD, Mulliken, JB. Hemifacial microsomia: Anatomical prediction of difficult intubation. Paediatr Anaesth. 1999; 9(5):393–398.
166. Obwegeser, HL. Zur korrektur der dysostosis ostomandibularis. Schweiz Monatschr Zahnhlkd. 1970; 80:331–340.
167. Obwegeser, HL. Correction of skeletal anomalies of otomandibular dysostosis. J Maxillofac Surg. 1974; 2:73–92.
168. Obwegeser, HL, Lello, GE, Sailer, HF, Otomandibular dysostosis. Surgical correction of dentofacial deformities. Bell, WH, eds. Surgical correction of dentofacial deformities; Vol III. WB Saunders, Philadelphia, 1985:639–661.
169. Oeltomaki, T, Vahatalo, K, Ronning, O. The effects of a unilateral costochondral graft on the growth of the marmoset mandible. J Oral Maxillofac Surg. 2002; 60(11):1307–1314.
170. Ortiz-Monasterio, F. Early mandibular and maxillary osteotomies for the correction of hemifacial microsomia: A preliminary report. Clin Plast Surg. 1982; 9:509.
171. Ortiz-Monasterio, F, Molina, F, Andrade, L, et al. Simultaneous mandibular and maxillary distraction in hemifacial microsomia in adults: Avoiding occlusal disorders. Plast Reconstr Surg. 1997; 100:852.
172. Ousterhout, DK, Vargervik, K. Surgical treatment of the jaw deformities in hemifacial microsomia. Aust N Z J Surg. 1987; 57(2):77–87.
173. Ow, AT, Cheung, LK. Meta-analysis of mandibular distraction osteogenesis: Clinical applications and functional outcomes. Plast Reconstr Surg. 2008; 121:54.
174. Padwa, BL, Bruneteau, RJ, Mulliken, JB. Association between “plagiocephaly” and hemifacial microsomia. Am J Med Genet. 1993; 47(8):1202–1207.
175. Padwa, BL, Evans, CA, Pillemer, FC. Psychosocial adjustment in children with hemifacial microsomia and other craniofacial deformities. Cleft Palate Craniofac J. 1991; 28:354.
176. Padwa, BL, Kaiser, MO, Kaban, LB. Occlusal cant in the facial plane as a reflection of facial asymmetry. J Oral Maxillofac Surg. 1997; 55:811.
177. Padwa, BL, Mulliken, JB, Maghen, A, Kaban, LB. Midfacial growth after costochondral graft construction of the mandibular ramus in hemifacial microsomia. J Oral Maxillofac Surg. 1998; 56:122.
178. Padwa, BL, Mulliken, JB, Maghen, BA, et al. Midfacial growth after costochondral graft reconstruction of the mandibular ramus in hemifacial microsomia. J Oral Maxillofac Surg. 1997; 55:1144.
179. Padwa, BL, Zaragoza, SM, Sonis, AL. Proximal segment displacement in mandibular distraction osteogenesis. J Craniofac Surg. 2002; 13(2):293–296.
180. Paeng JY, Lee JH, Kim MJ: Condyle as the point of rotation for 3-D planning of distraction osteogenesis for hemifacial microsomia. J Craniomaxillofac Surg 35(2):91–102.
181. Pashayan, H, Pinsky, L, Fraser, FC. Hemifacial microsomia-oculo-auriculo-vertebral dysplasia: A patient with overlapping features. J Med Genet. 1970; 7(2):185–188.
182. Patterson, AR, Brady, G, Loukota, RA. Distraction of the mandibular ramus in hemifacial microsomia with a defect of the glenoid fossa. Br J Oral Maxillofac Surg. 2007; 45(7):599–600.
183. Peltomaki, T. Growth of a costochondral graft in the rat temporomandibular joint. J Oral Maxillofac Surg. 1992; 50(8):851–857.
184. Peltomaki, T. Histologic structure of human costochondral junction. Plast Reconstr Surg. 1994; 94(5):585–588.
185. Peltomaki, T, Isotupa, K. The costochondral graft: A solution or a source of facial asymmetry in growing children: A case report. Proc Finn Dent Soc. 1991; 87(1):167–176.
186. Peltomaki, T, Hakkinen, L. Growth of the ribs at the costochondral junction in the rat. J Anat. 1992; 181:259–264.
187. Peltomaki, T, Kylamarkula, S, Vinkka-Puhakka, H, et al. Tissue-separating capacity of growth cartilages. Eur J Orthod. 1997; 19(5):473–481.
188. Peltomaki, T, Quevedo, LA, Jeldes, G, Ronning, O. Histology of surgically removed overgrown osteochondral rib grafts. J Craniomaxillofac Surg. 2002; 30(6):355–360.
189. Peltomaki, T, Ronning, O. Interrelationship between size and tissue-separating potential of costochondral transplants. Eur J Orthod. 1991; 13(6):459–465.
190. Peltomaki, T, Ronning, O. Costochondral graft as replacement of a dysplastic mandibular condyle [comment]. Plast Reconstr Surg. 1993; 92(5):981–983.
191. Peltomaki, T, Ronning, O. Growth of costochondral fragments transplanted from mature to young isogeneic rats. Cleft Palate Craniofac J. 1993; 30(2):159–163.
192. Peltomaki, T, Vahatalo, K, Ronning, O. The effect of a unilateral costochondral graft on the growth of the marmoset mandible. J Oral Maxillofac Surg. 2002; 60(11):1307–1314.
193. Perkins, JA, Sie, KC, Milczuk, H, et al. Airway management in children with craniofacial anomalies. Cleft Palate Craniofac J. 1997; 34:135.
194. Perrott, DH. Clinical and computed tomographic findings in costochondral grafts replacing the mandibular condyle [discussion]. J Oral Maxillofac Surg. 1996; 54:1400.
195. Perrot, DH, Umeda, H, Kaban, LB. Costochondral graft construction/reconstruction of the ramus/condyle unit: Long-term follow-up. Int J Oral Maxillofac Surg. 1994; 23:321–328.
196. Pertschuk, MJ, Whitaker, LA. Psychosocial adjustment and craniofacial malformations in childhood. Plast Reconstr Surg. 1985; 75:177.
197. Phillips, J, Whitaker, LA. The social effects of craniofacial deformity and its correction. Cleft Palate J. 1979; 16:7.
198. Pilai, RR, Singh, IJ. Hemifacial microsomia with Goldenhar syndrome: Report case. Dent Dig. 1970; 76(9):382–385.
199. Pillemer, FG, Cook, KV. The psychosocial adjustment of pediatric craniofacial patients after surgery. Cleft Palate J. 1989; 26:201.
200. Politi, M, Sembronio, S, Robiony, M, Costa, F. The floating bone technique of the vertical ramus in hemifacial microsomia: Case report. Int J Adult Orthodon Orthognath Surg. 2002; 17(3):223–229.
201. Politis, C, Fossion, E, Bossuyt, M. The use of costochondral grafts in arthroplasty of the temporomandibular joint. J Craniomaxillofac Surg. 1987; 15:345–354.
202. Polley, JW, Figueroa, AA. Distraction osteogenesis: Its application in severe mandibular deformities in hemifacial microsomia. J Craniofac Surg. 1997; 8(5):422–430.
203. Polley, JW, Figueroa, AA, Jein-Wein Liou, E, et al. Longitudinal analysis of mandibular asymmetry in hemifacial microsomia. Plast Reconstr Surg. 1997; 99:328.
204. Poole, MD. A composite flap for early treatment of hemifacial microsomia. Br J Plast Surg. 1989; 42(2):163–172.
205. Poole, MD. Hemifacial microsomia. World J Surg. 1989; 13:396.
206. Poon, CC, Meata, JG, Heggie, AA. Hemifacial microsomia: Use of the OMENS-Plus classification at the Royal Children’s Hospital of Melbourne. Plast Reconstr Surg. 2003; 111(3):1011–1018.
207. Pope, AW, Speltz, ML. Research on psychosocial issues of children with craniofacial anomalies: Progress and challenges. Cleft Palate Craniofac J. 1997; 34:371.
208. Pope, AW, Ward, J. Self-perceived facial appearance and psychosocial adjustment in preadolescents with craniofacial anomalies. Cleft Palate Craniofac J. 1997; 34:396.
209. Posnick, JC. Discussion: Midface growth after costochondral graft reconstruction of the mandibular ramus in hemifacial microsomia. J Oral Maxillofac Surg. 1998; 56:127–128.
210. Posnick, JC. Surgical correction of mandibular hypoplasia in hemifacial microsomia: A personal perspective. J Oral Maxillofac Surg. 1998; 56(5):639–650.
211. Posnick, JC, Hemifacial microsomia: Evaluation and treatment. Craniofacial and Maxillofacial Surgery in Children and Young Adults. Posnick, JC, eds. Craniofacial and Maxillofacial Surgery in Children and Young Adults; Vol 20. WB Saunders Co, Philadelphia, 2000:419–445.
212. Posnick, JC, Fantuzzo, J, Orchin, J. Deliberate operative rotation of the maxillo-mandibular complex to alter the A-point to B-point relationship for enhanced facial esthetics. J Oral Maxillofac Surg. 2006; 64:1687–1695.
213. Posnick, JC, Goldstein, JA, Waitzman, A. Surgical correction of the Treacher Collins malar deficiency: Quantitative CT scan analysis of long-term results. Plast Reconstr Surg. 1993; 92:12.
214. Poswillo, DE. The pathogenesis of the first and second branchial arch syndrome. Oral Surg. 1973; 35:302–328.
215. Poswillo, DE. Otomandibular deformity: Pathogenesis as a guide to reconstruction. J Maxillofac Surg. 1974; 2:64–72.
216. Poswillo, DE. Hemorrhage in development of the face. Birth Defects Orig Artic Ser. 1975; 11(7):61–81.
217. Poswillo, DE. The embryological basis of craniofacial dysplasia. Postgrad Med J. 1977; 53:517.
218. Poswillo, DE. Biologic approach to temporomandibular reconstruction. In: Whitaker LA, Randall P, eds. Symposium on reconstruction of jaw deformity. St. Louis: Mosby; 1978:139–145.
219. Preston, CB, Losken, HW, Evans, WG. Restitution of facial form in a patient with hemifacial microsomia: A case report. Angle Orthod. 1985; 55:197.
220. Pruzansky, S. Not all dwarfed mandibles are alike. Birth Defects. 1969; 1:120.
221. Rachmiel, A, Aizeenbud, D, Eleftheriou, S, et al. Extraoral vs. intraoral distraction osteogenesis in the treatment of hemifacial microsomia. Ann Plast Surg. 2000; 45(4):386–394.
222. Rachmiel, A, Manor, R, Peled, M, Laufer, D. Intraoral distraction osteogenesis of the mandible in hemifacial microsomia. J Oral Maxillofac Surg. 2001; 59(7):728–733.
223. Rahbar, R, Robson, CD, Mulliken, JB, et al. Craniofacial, temporal bone, and audiologic abnormalities in the spectrum of hemifacial microsomia. Arch Otolaryngol Head Neck Surg. 2001; 127(3):265–271.
224. Raustia, A, Pernu, H, Pyhtinen, J, Oikarinen, K. Clinical and computed tomographic findings in costochondral grafts replacing the mandibular condyle. J Oral Maxillofac Surg. 1996; 54:1393–1400.
225. Regev, E, Jensen, JN, McCarthy, JG, et al. Removal of mandibular tooth follicles before distraction osteogenesis. Plast Reconstr Surg. 2004; 113:1910.
226. Robinson, M, Stoughton, D. Surgical-orthodontic treatment of a case of hemifacial microsomia. Am J Orthod. 1970; 57(3):287–292.
227. Rodgers, SF, Eppley, BL, Nelson, CL, Sadove, AM. Hemifacial microsomia: Assessment of classification system. J Craniofac Surg. 1991; 2(3):114–126.
228. Rogers, GF, Mulliken, JB. Repair of transverse facial cleft in hemifacial microsomia: Long-term anthropometric evaluation of commissural symmetry. Plast Reconstr Surg. 2007; 120(3):728–737.
229. Rollinck, BR. Oculoauriculovertebral anomaly: Variability and causal heterogeneity. Am J Med Genet. 1988; 4(Suppl):41.
230. Rollnick, BR, Kaye, CI. Hemifacial microsomia and variants: Pedigree data. Am J Med Genet. 1983; 15:233.
231. Ross, RB. Lateral facial dysplasia (first and second branchial arch syndrome, hemifacial microsomia). Birth Defects. 1975; 11:51.
232. Rubio-Bueno, P, Padron, A, Villa, E, Diaz-Gonzales, FJ. Distraction osteogenesis of the ascending ramus for mandibular hypoplasia using extraoral or intraoral devices: A report of 8 cases. J Oral Maxillofac Surg. 2000; 58:593.
233. Rune, B, Sarnas, KV, Selvik, G, et al. Roentgen stereometry with the aid of metallic implants in hemifacial microsomia. Am J Orthod. 1983; 84:231.
234. Rune, B, Selvik, G, Sarnas, KV, et al. Growth in hemifacial microsomia studied with the aid of roentgen stereophotogrammetry and metallic implants. Cleft Palate J. 1981; 18:128.
235. Samman, N, Cheung, LK, Tiderman, H. Overgrowth of a costochondral graft in an adult male. Int J Oral Maxillofac Surg. 1995; 24:333–335.
236. Sandhu, S, Kaur, T. Hemifacial microsomia: A case report and review. Ind J Dent Res. 2002; 13(2):82–86.
237. Santoh, K, Suzuki, H, Uemura, T, Hosaka, Y. Maxillo-mandibular distraction osteogenesis for hemifacial microsomia in children. Ann Plast Surg. 2002; 49(6):572–578.
238. Sarnas, KV, Pancherz, H, Rune, B, et al. Hemifacial microsomia treated with the Herbst appliance: Report of a case analyzed by means of roentgen stereometry and metallic implants. Am J Orthod. 1982; 82:68.
239. Sarnas, KV, Rune, B, Aberg, M. Maxillary and mandibular displacement in hemifacial microsomia: A longitudinal, roentgen stereometric study of 21 patients with the aid of metallic implants. Cleft Palate Craniofac J. 2004; 41(3):290–303.
240. Sarnas, KV, Rune, B, Selvik, G, Jacobsson, S. Hemifacial microsomia. Plast Reconstr Surg. 1985; 75(6):928–929.
241. Scolozzi, P, Herzog, G, Jaques, B. Simultaneous maxillo-mandibular distraction osteogenesis in hemifacial microsomia: A new technique using two distractors. Plast Reconstr Surg. 2006; 117(5):1530–1541.
242. Shetye, PR, Grayson, BH, Mackool, RJ, McCarthy, JG. Long-term stability and growth following unilateral mandibular distraction in growing children with craniofacial microsomia. Plast Reconstr Surg. 2006; 118:985.
243. Sidiropoulou, S, Antoniades, K, Kolokithas, G. Orthopedically induced condylar growth in a patient with hemifacial microsomia. Cleft Palate Craniofac J. 2003; 40(6):645–650.
244. Siebert, JW, Goesel, A, Longaker, MT. Microsurgical correction of facial asymmetry in 60 consecutive cases. Plast Reconstr Surg. 1996; 97:354.
245. Siebert, JW, Longaker, MT. Microsurgical correction of facial asymmetry in hemifacial microsomia: Operative techniques. Plast Reconstr Surg. 1994; 1:93.
246. Silla Freitas, R, Tolazzi, ARD, Alonso, N, Cruz, GA. Evaluation of molar teeth and buds in patients submitted to mandibular distraction: Long-term results. Plast Reconstr Surg. 2010; 121:1335–1342.
247. Singer, SL, Haan, E, Slee, J, Goldblatt, J. Familial hemifacial microsomia due to autosomal dominant inheritance: Case reports. Aust Dent J. 1994; 39(5):287–291.
248. Singh, A, Malhotra, G, Singh, GP, et al. Goldenhar syndrome: A case report. Acta Chir Plast. 1994; 36:111.
249. Singh, DJ, Bartlett, SP. Congenital mandibular hypoplasia: Analysis and classification. J Craniofac Surg. 2005; 16:291.
250. Snyder, CC, Levine, GA, Swanson, HM, et al. Mandibular lengthening by gradual distraction. Plast Reconstr Surg. 1973; 51:506.
251. Steinbacher, D, Gougoutas, A, Bartlett, SP. An analysis of mandibular volume in hemifacial microsomia. Plast Reconstr Surg. 2011; 127:2407.
252. Steinberg, B, Fattahi, T. Distraction osteogenesis in management of pediatric airway: Evidence to support its use. J Oral Maxillofac Surg. 2005; 63:1206.
Stelnicki, EJ, Hollier, L, Lee, C, Lin, WY, Grayson, B, McCarthy, JG. Distraction osteogenesis of costochondral bone grafts in the mandible. Plast Reconstr Surg. 2002; 109:925–933.
253. Stringer, DE, Steed, DL, Johnson, RP, et al. Correction of hemifacial microsomia. J Oral Surg. 1981; 39:35.
254. Stucki-Mccormick, SU. Reconstruction of mandibular condyle using transport distraction osteogenesis. J Craniofac Surg. 1997; 8:48.
255. Sulik, KK. Craniofacial defects from genetic and teratogen-induced deficiencies in presomite embryos. Birth Defects. 1984; 20:79.
256. Swanson, LT, Murray, JE. Mandibular reconstruction in hemifacial microsomia. In: Tanzer RC, Edgerton MT, eds. Symposium on reconstruction of the auricle. St. Louis: CV Mosby; 1974:270.
257. Takashima, M, Kitai, N, Mori, Y, et al. Mandibular distraction osteogenesis using an intraoral device and bite plate for a case of hemifacial microsomia. Cleft Palate Craniofac J. 2003; 40(4):437–445.
258. Takushima, A, Harii, K, Asato, H, Yamada, A. Neurovascular free-muscle transfer to treat facial paralysis associated with hemifacial microsomia. Plast Reconstr Surg. 2002; 109(4):1219–1227.
259. Tanna, N, Wan, DC, Kawamoto, HK, Bradley, JP. Craniofacial microsomia soft-tissue reconstruction comparison: Inframammary extended circumflex scapular flap versus serial fat grafting. Plast Reconstr Surg. 2011; 127:802.
260. Tanzer, RC. Total reconstruction of the external ear. Plast Reconstr Surg. 1959; 23:1.
261. Tanzer, RC. Total reconstruction of the auricle: The evolution of a plan of treatment. Plast Reconstr Surg. 1971; 47:523.
262. Tharanon, W, Sinn, DP. Mandibular distraction osteogenesis with multidirectional extraoral distraction device in hemifacial microsomia patients: Three-dimensional treatment planning, prediction tracings, and case outcomes. J Craniofac Surg. 1999; 10(3):202–213.
263. Thomas, P. Goldenhar syndrome and hemifacial microsomia: Observations on three patients. Eur J Pediatr. 1980; 133(3):287–292.
264. Tiner, BD, Quaroni, AL. Facial asymmetries in hemifacial microsomia, Goldenhar syndrome, and Treacher Collins syndrome. Atlas Oral Maxillofac Surg Clin North Am. 1996; 4(1):37–52.
265. Trahar, M, Sheffield, R, Kawamoto, H, et al. Cephalometric evaluation of the craniofacial complex in patients treated with an intraoral distraction osteogenesis device: A preliminary report. Am J Orthod Dentofacial Orthop. 2003; 124:639.
266. Troulis, MJ, Everett, P, Seldin, EB, et al. Development of a three-dimensional treatment planning system based on computed tomographic data. Int J Oral Maxillofac Surg. 2002; 31(4):349–357.
267. Tsirikos, AL, McMaster, MJ. Goldenhar-associated conditions (hemifacial microsomia) and congenital deformities of the spine. Spine. 2006; 31(13):E400–E407.
268. Tweed, AE, Manktelow, RT, Zuker, RM. Facial contour reconstruction with free flaps. Ann Plast Surg. 1984; 12:313.
269. Upton, J, Mulliken, JB, Hicks, PD, et al. Restoration of facial contour using free vascularized omental transfer. Plast Reconstr Surg. 1980; 66:500.
270. Vargervik, K. Sequence and timing of treatment phases in hemifacial microsomias. In: Harvold EP, ed. Treatment of hemifacial microsomia. New York: Alan R. Liss; 1983:133–137.
271. Vargervik, K. Treatment of hemifacial microsomia in patients without a functioning temporomandibular articulation. In: Harvold EP, ed. Treatment of hemifacial microsomia. New York: Alan R. Liss; 1983:207–242.
272. Vargervik, K. Discussion of relationship between bone and muscles of mastication in hemifacial microsomia. Plast Reconstr Surg. 1997; 99:990.
273. Vargervik, K. Mandibular malformations: Growth characteristics and management in hemifacial microsomia and Nager syndrome. Acta Odontol Scand. 1998; 56(6):331–338.
274. Vargervik, K, Miller, AJ. Neuromuscular patterns in hemifacial microsomia. Am J Orthod. 1984; 86:33.
275. Vargervik, K, Ousterhout, DK, Farias, M. Factors affecting long-term results in hemifacial microsomia. Cleft Palate J. 1986; 23(Suppl I):53.
276. Vento, AR, LaBrie, RA, Mulliken, JB. The OMENS classification of hemifacial microsomia. Cleft Palate Craniofac J. 1991; 28:68–76.
277. Vilkki, SK, Hukki, J, Nietosvaara, Y, et al. Microvascular temporomandibular joint and mandibular ramus reconstruction in hemifacial microsomia. J Craniofac Surg. 2002; 13(6):809–815.
278. Vu, HL, Panchal, J, Levine, N. Combined simultaneous distraction osteogenesis of the maxilla and mandible using a single distraction device in hemifacial microsomia. J Craniofac Surg. 2001; 12(3):253–258.
279. Wan, D, Taub, P, Allam, KA, et al. Distraction osteogenesis of costocartilaginous rib grafts and treatment algorithm for severely hypoplastic mandibles. Plast Reconstr Surg. 2011; 127:2005–2013.
280. Wan, J, Meara, JG, Kovanlikaya, A, et al. Clinical, radiological, and audiological relationships in hemifacial microsomia. Ann Plast Surg. 2003; 51(2):161–166.
281. Werler, MM, Sheehan, JE, Hayes, C, et al. Vasoactive exposures, vascular events, and hemifacial microsomia. Birth Defects Res A Clin Mol Teratol. 2004; 70(6):389–395.
282. Werler, MM, Sheehan, JE, Hayes, C, et al. Demographic and reproductive factors associated with hemifacial microsomia. Cleft Palate Craniofac J. 41(5), 2004. [494–450].
283. Wiens, JL, Forte, RA, Weins, JP. The use of distraction osteogenesis to treat hemifacial microsomia: A clinical report. J Prosthet Dent. 2003; 89(1):11–14.
284. Wijbenga, JG, Verlinden, CR, Jansma, J, et al. Long lasting neurosensory disturbance following advancement of the retrognathic mandible: Distraction osteogenesis versus bilateral sagittal split osteotomy. Int J Oral Maxillofac Surg. 2009; 38:719.
285. Williamson, EH. Mandibular growth following a costochondral transplant in the treatment of hemifacial microsomia. Facial Orthop Temporomandibular Arthrol. 1988; 5:3.
286. Yasui, N, Kojimoto, H, Shimizu, H, Shimomura, Y. The effect of distraction upon bone, muscle, and periosteum. Orthop Clin North Am. 1991; 22:563.
287. Zhou, YQ, Mu, XZ, Ren, W, Yu, ZY. Surgical treatment of hemifacial microsomia [Chinese]. Zhonghua Wai Ke Za Zhi. 2006; 44(11):754–756.
*References 9, 26, 36, 41, 42, 47, 50, 52, 64, 69, 75, 78, 83, 90, 104–106, 119, 164, 181, 198, 206, 214–218, 229–231, 247, 248, 255, 263, 264, 267, 276, 281, 282
*References 70, 76, 77, 92–95, 99, 109, 135, 137, 142, 145, 151, 157, 166–168, 170, 172, 236, 243, 253, 256, 266, 270–275, 277, 287
*References 1, 4–6, 21, 23, 34, 39, 55, 58, 63, 68, 71, 72, 74, 79, 80, 82, 87, 100, 109–111, 113–116, 132, 136, 138–140, 144, 146–148, 150, 171, 173, 179, 182, 200, 202, 221, 222, 225, 232, 237, 241, 242, 246, 250, 252, 254, 257, 262, 265, 278, 279, 283, 284, 286
*References 2, 22, 37, 44, 45, 48, 60, 73, 118, 120, 126–128, 156, 158, 159, 160–162, 169, 177, 178, 183–192, 194, 195, 201, 209–211, 219, 224, 235, 285