157 Hematology and Oncology in Children
 Hematology
Hematology
Anemia
A normal decrease in the hemoglobin (Hb) level is observed during the first weeks of life because of a limited release of erythropoietin. For this reason, the normal range of Hb concentration changes with age: 18.5 ± 2.0 g/dL (mean ± 2 standard deviations) during the first week of life, 11.5 ± 1.2 g/dL at 2 months, 12.0 ± 0.7 g/dL at 12 months, 13.5 ± 1.0 g/dL at 9 years, and 14.0 ± 1.0 g/dL after 12 years of age.1 Based on these ranges, anemia is observed in 33% of patients on admission to PICU, and an additional 41% become anemic during their PICU stay.2
The small total blood volume of neonates and children (e.g., about 240 mL in a 3-kg patient) makes blood loss from phlebotomy or procedures a major cause of anemia in PICU.2 Other causes include hemorrhage, hemolysis (immunologic, infectious, microangiopathic, or toxic), and decreased production (invasion of the bone marrow, side effect of therapy, nutritional deficiency, blunted production of erythropoietin in response to hypoxia).3 Causes quite specific to pediatric practice include congenital anemias (e.g., sickle cell disease, thalassemia, Blackfan-Diamond disease), glucose-6-phosphate dehydrogenase deficiency, and metabolic disorders. Sickle cell disease merits specific comment.
Sickle Cell Disease
Many types of abnormal Hb are observed in sickle cell disease. However, only Hb SS (homozygous sickle cell Hb), Hb SC, and Hb S-β-thalassemia can cause severe clinical problems. Hypoxemia, acidosis, polycythemia, infection, and a high proportion of abnormal Hb concentration are the main risk factors for sickle cell disease complications. The following sickle cell crises can be life threatening: acute chest syndrome, stroke, acute splenic sequestration, aplastic crisis, and infection. General management always includes optimization of oxygenation, adequate analgesia, and treatment of the precipitating cause of the crisis.4 Hyperhydration is also recommended in most instances, but fluid requirements must be adapted with caution in patients with respiratory symptoms or pulmonary hypertension. Red blood cell (RBC) transfusion is a cornerstone of therapy, aiming to decrease abnormal Hb while maintaining hematocrit below 35%. An exchange transfusion should be considered in severe cases, especially in acute chest syndrome or stroke.
Acute chest syndrome is a leading cause of morbidity and mortality in sickle cell disease.5,6 It is defined by the development of a new pulmonary alveolar infiltrate involving at least one complete lung segment, accompanied by fever, chest pain, tachypnea, cough, and hypoxia. Severe forms are analogous to acute respiratory distress syndrome. Acute chest syndrome results from intricate mechanisms including pulmonary infection (mostly by atypical bacteria and virus), fat embolization, and local vaso-occlusion.6 Perturbation of nitric oxide metabolism7 and hypercoagulability have also been demonstrated.6 Growing evidence suggests that pulmonary hypertension play a crucial role during sickle cell disease evolution, which may lead to abrupt severe right heart failure.6 Besides general management and empirical antibiotic therapy, covering atypical bacteria and Streptococcus pneumoniae, incentive spirometry is encouraged. Mechanical ventilation may be required to improve oxygenation; noninvasive ventilation can be successful.8 Owing to disturbance of nitric oxide metabolism, inhaled nitric oxide has been used in small trials or case reports, but its efficacy remains to be validated.9
Red Blood Cell Transfusion
The management of anemia in critically ill patients is discussed in Chapters 19 and 150; this includes prevention of blood loss, transfusion of blood products, and administration of folic acid and iron. Prophylactic erythropoietin use has not been evaluated in large pediatric trials, but its utility appears questionable insofar as most RBC transfusions are received during the first few days after admission.2,10
The risks and benefits of RBC transfusion are not similar in adults and children. Necrotizing enterocolitis in neonates11 or erythrocyte alloimmunization in young girls (up to 8% of patients)12 are significant problems in pediatric patients. RBC transfusion to neonates increases the ratio of adult to fetal Hb, which decreases the affinity of blood for oxygen.13 Nevertheless, RBCs improve oxygen transport in critically ill children,14–17 although the improvement in clinically significant outcomes remains to be determined. Large variation in transfusion practice was observed among pediatric intensivists,18,19 reflecting the unidentified Hb threshold with the best risk/benefit ratio in critically ill children. Maintaining Hb above 5.0 g/dL in hospitalized pediatric patients decreases the risk of death.20,21 In a large randomized clinical trial enrolling 637 patients in 19 PICUs, Lacroix et al.22 demonstrated that a transfusion strategy to maintain Hb above 7 g/dL was as safe as a strategy to maintain Hb above 9.5 g/dL in stable critically ill children. While the number of transfusions was much lower in the restrictive transfusion group, no difference was observed in mortality rate or occurrence of new organ dysfunction.22 These findings were not different in three planned a priori subgroup analyses of patients in sepsis,23 in postsurgical patients,24 or in patients admitted following cardiac surgery.25 These data and the cohesiveness of all subgroup analyses strongly support limiting RBC transfusion to patients with Hb below 7.0 g/dL in stable conditions. More data are required to identify a threshold in patients with cardiorespiratory instability.
Transfusion in the neonatal period, in children with immunodeficiency, or transfusion using blood donated by family members are situations at higher risk of transfusion-associated graft-versus-host disease26; irradiated packed RBCs should be used in these conditions.
Hemorrhagic Disorders
Disseminated intravascular coagulation (DIC) is the most frequent hemorrhagic disorder observed in the PICU. Its causes, pathophysiology, and treatment are similar to those in adults (see Chapter 21), even though purpura fulminans is more frequent in the PICU.
Thrombocytopenia in critically ill patients is related most often to sepsis, DIC, multiple organ dysfunction syndrome, or is drug-induced (see Chapter 20). Heparin-induced thrombocytopenia must also be considered. Immune-mediated thrombocytopenia in newborns can be secondary to alloimmunization or maternal disease (e.g., maternal lupus erythematosus). Idiopathic thrombocytopenic purpura is frequent in children, but it rarely causes severe bleeding. Patients with hemolytic uremic syndrome and thrombotic thrombocytopenic purpura mostly require PICU admission because of renal failure or central nervous system involvement, but significant hemorrhage can occur. In these conditions, platelet transfusion can accelerate microangiopathy and should therefore be avoided unless a significant bleeding is present.
Thrombosis and Emboli
Elsewhere in this book, there are chapters on pulmonary emboli (Chapter 62), thromboembolic diseases (Chapter 153), and their prophylaxis. Most thromboses observed in pediatric critically ill patients are acquired during the PICU stay. Catheter-related thrombosis is common, appearing rapidly after catheter insertion.27 Heparin-coated catheters may prevent catheter-related thrombosis,28 but their cost/benefit ratio and the risk of heparin-induced thrombopenia remains to be determined. DIC, allergy to heparin, prothrombic states (e.g., G20210A prothrombin gene mutation, factor V Leiden, anticardiolipin antibody, antithrombin III, or protein C deficiency), and blood flow stasis are common risk factors for thrombosis in children. The incidence of deep vein thrombosis is lower with peripherally inserted central catheters (PICC lines) than with centrally inserted catheters.29
Cerebral venous sinus thrombosis and renal vein thrombosis are more frequent in children than in adults. Symptoms of cerebral venous sinus thrombosis include seizures, headache, coma, paresis, cranial nerve palsies, and increased intracranial pressure. Head and neck infections, connective tissue disorders, or prothrombic states are frequently associated.30 Symptoms of neonatal renal vein thrombosis are acute renal insufficiency, hematuria, and hypernephrosis.
 Oncology
Oncology
Cancer in Children
Respiratory System
Causes of respiratory dysfunction in cancer patients include those observed in patients without cancer (see Chapters 58 and 72). However, many specific diseases merit comments.
Pediatric cancer patients frequently develop acute respiratory failure resulting from infections due to unusual pathogens and from noninfectious mechanisms: pulmonary edema, local treatment toxicity, graft-versus-host disease, bronchiolitis obliterans, alveolar hemorrhage. Invasive investigations should be discussed, such as bronchoalveolar lavage or lung biopsy, because identification of an atypical pathogen or a noninfectious cause can facilitate management. However, the risk of severe complications (death, barotrauma, hemorrhage) is high in the most severe patients,31,32 and empirical treatment is frequently considered in the absence of diagnostic maneuvers.
Noninvasive mechanical ventilation is an effective means of preventing endotracheal intubation in pediatric immunocompromised patients with acute respiratory failure.33 Patients who require intubation and invasive ventilation have a higher risk of death33,34; a lung-protective strategy should be applied (see Chapter 58).
Cardiovascular System
All types of shock can be observed in patients with malignancy, but sepsis is the leading cause of shock in pediatric cancer patients (see Chapter 130). Hemodynamic failure can also result from heart obstruction (e.g., mass in the auricula), extrinsic compression, pericardial effusion (inflammatory, hemorrhagic, or septic), infection or inflammation of cardiac structures (endocarditis, myocarditis, pericarditis), fibrotic restrictive myocarditis, or dysrhythmia induced by cardiac electrical system inflammation. Congestive heart failure may also be caused by chemotherapy, especially anthracycline and cyclophosphamide.
Digestive System
Typhlitis is an inflammation of the caecum and surrounding tissue, reported in 10% of leukemic patients at postmortem examination.35 Usual clinical signs of abdominal inflammation can be absent in neutropenic patients; therefore, typhlitis must be feared in all patients with suspicion of infection. Abdominal computed tomography is a good diagnostic test for this condition. Secondary sepsis or bleeding is the usual cause of death.
Diarrhea is also frequent in this population. Clostridium difficile colitis must be suspected in patients who have recently received antibiotics (see Chapter 146). Gastroenteritis may be caused by unusual organisms such as Cryptosporidium.
Hepatic dysfunction can be caused by viral infection, drug toxicity (methotrexate), or veno-occlusive disease resulting from chemotherapy or radiation therapy. Severe hepatic veno-occlusive disease has a high mortality rate and should be aggressively managed. Treatment includes reduction of weight gain (diuretics, close fluid and electrolytes monitoring) and appropriate nutrition. Thrombolytic therapy or anticoagulation (antithrombin III) have been proposed but need to be evaluated.36 Defibrotide also seems to be a promising adjunctive therapy which is under evaluation.37,38
Most cases of pancreatitis result from cytotoxic reactions to chemotherapy.
Metabolic Problems
Lactic acidosis in critically ill patients is usually a consequence of cardiovascular dysfunction, sepsis, or multiple organ dysfunction. However, cancer with rapid and large turnover of malignant cells (e.g., leukemia, lymphoma) can be associated with lactic acidosis.39,40
Hematologic Problems
The proportion of cancer patients receiving chemotherapy who present with a significant hemorrhage is about 10%.41 Hemorrhagic risk is the consequence of multiple mechanisms: coagulopathy, thrombocytopenia, and treatment-induced tissue fragility.
Bone marrow failure is extremely frequent in patients with cancer. It is an expected side effect of cytotoxic and radiation therapies, but it can also result from the cancer itself, infection, and many other causes. A reactive hemophagocytic syndrome can also occur in patients with severe multiple organ dysfunction syndrome.42
The risk of infections particularly increases if the neutrophil count is lower than 1000/mm3. Various colony-stimulating factors (CSFs) such as granulocyte-macrophage CSF, granulocyte CSF, and macrophage-granulocyte inducer are frequently used to shorten neutropenia. However, the usefulness of these treatments on the number of transfusions required, incidence of infection, and survival rate remains to be established.43,44
Infectious Problems
Children with cancer must always be considered immunodeficient. Community-acquired pneumonia (Chapter 66), nosocomial pneumonia (Chapter 67), infections in the immunocompromised patient (Chapters 68 and 137), vascular catheter–related infections (Chapter 128), and prevention as well as control of nosocomial infection (Chapter 126) are of considerable importance in these patients.
Outcome and Ethical Considerations
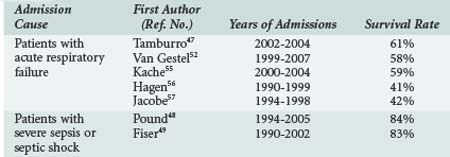
The current consensus among PICU caregivers is that intensive care is inappropriate if the chance of short-term survival is poor because the patient is in the late stage of a chronic disease.45,46 However, over the last 2 decades, the outcomes of cancer patients requiring intensive care has largely improved (Table 157-1).47 Oncologic patients admitted to PICU for severe sepsis have a survival rate similar to patients without cancer.48,49 The mortality rate remains high when the admission cause is acute respiratory failure, but more than half of patients will survive. The mortality risk is higher in patients with a history of hematopoietic stem cell transplant.47 Various scores or indicators of severity at admission have been correlated with mortality, but no index is powerful enough to permit a decision on the futility of a PICU admission if the oncologic disease itself is controlled. Usually these patients should be admitted to PICU, and full efforts should be made in support of a curative goal. However, it is important to reevaluate the survival possibility after a few days of this “ICU trial,”50 because mortality is highly correlated with the number of persistent organ dysfunctions after several days,50–53 and the goal of care may sometimes be redirected to comfort care.
TABLE 157-1 Outcomes of Patients Admitted to the Pediatric Intensive Care Unit with Malignancy or Hematopoietic Stem Cell Transplant
End-of-life decisions (see Chapter 217) about patients with cancer or bone marrow transplant must be addressed by a multidisciplinary team including the patient when capable, family members, nurses, oncologists, and intensivists. It must be based on the chance of recovery from the acute disease, chance of survival from the underlying disease, quality of life before the acute problem, and the wishes of the patient and parents.54
Key Points
Bateman ST, Lacroix J, Boven K, et al. Anemia, blood loss and blood transfusion in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26-33.
Lacroix J, Hébert PC, Hutchison JH, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619.
Jenkins TL. Sickle cell anemia in the pediatric intensive care unit: novel approaches for managing life-threatening complications. AACN Clin Issues. 2002;13:154-168.
Piastra M, De Luca D, Pietrini D, et al. Noninvasive pressure-support ventilation in immunocompromised children with ARDS: a feasibility study. Intensive Care Med. 2009;35:1420-1427.
Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996-2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9:270-277.
1 Nathan DG, Orkin SH. Nathan and Oski’s hematology of infancy and childhood, 5th ed. Philadelphia: W.B. Saunders Co.; 1998.
2 Bateman ST, Lacroix J, Boven K, et al. Anemia, blood loss and blood transfusion in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26-33.
3 Krafte-Jacobs B, Bock GH. Circulating erythropoietin and interleukin-6 concentrations increase in critically ill children with sepsis and septic shock. Crit Care Med. 1996;24:1455-1459.
4 Jacob E, Miaskowski C, Savedra M, Beyer JE, Treadwell M, Styles L. Management of vaso-occlusive pain in children with sickle cell disease. J Pediatr Hematol Oncol. 2003;25:307-311.
5 Jenkins TL. Sickle cell anemia in the pediatric intensive care unit: novel approaches for managing life-threatening complications. AACN Clin Issues. May 2002;13(2):154-168.
6 Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. Nov 20 2008;359(21):2254-2265.
7 Sullivan KJ, Kissoon N, Gauger C. Nitric oxide metabolism and the acute chest syndrome of sickle cell anemia. Pediatr Crit Care Med. Mar 2008;9(2):159-168.
8 Essouri S, Chevret L, Durand P, Haas V, Fauroux B, Devictor D. Noninvasive positive pressure ventilation: five years of experience in a pediatric intensive care unit. Pediatr Crit Care Med. Jul 2006;7(4):329-334.
9 Ataga KI. Novel therapies in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2009:54-61.
10 Liet JM, Paranon S, Baraton L, Dejode JM, Rozé JC. Is a preventive treatment by erythropoietin relevant to reduce red blood cell transfusion in PICU? Pediatr Crit Care Med. 2006;7:541-544.
11 McGrady GA, Rettig PJ, Istre GR, Jason JM, Holman RC, Evatt BI. An outbreak of necrotizing enterocolitis association with transfusion of packed red blood cells. Am J Epidemiol. 1987;126:1165-1172.
12 Experts Working Group. Guidelines for red blood cell and plasma transfusions for adults and children. Can Med Assoc J. 1997;156(Suppl 11):S1-24.
13 De Halleux V, Truttmann A, Gagnon C, Bard H. The effect of blood transfusion on the hemoglobin oxygen dissociation curve of very early preterm infants during the first week of life. Semin Perinatol. 2002;26:411-415.
14 Beekman RH, Tuuri DT. Acute hemodynamic effects of increasing hemoglobin concentration in children with a right to left ventricular shunt and relative anemia. J Am Coll Cardiol. 1985;5:357-362.
15 Lucking SE, Williams TM, Chaten FC, Metz RI, Mickell JJ. Dependence of oxygen consumption on oxygen delivery in children with hyperdynamic septic shock and low oxygen extraction. Crit Care Med. 1990;18:1316-1319.
16 Mink RB, Pollack MM. Effect of blood transfusion on oxygen consumption in pediatric septic shock. Crit Care Med. 1990;18:1087-1091.
17 Seear M, Wensley D, MacNab A. Oxygen consumption-oxygen delivery relationship in children. J Pediatr. 1993;123:208-214.
18 Goodman AM, Pollack MM, Patel KM, Luban NLC. Pediatric red blood cell transfusions increase resource use. J Pediatr. 2003;142:123-127.
19 Laverdière C, Gauvin F, Hébert PC, et al. Survey of transfusion practices in pediatric intensive care units. Pediatr Crit Care Med. 2002;3(2):335-340.
20 English M, Ahmed M, Ngando C, Berkley J, Ross A. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet. 2002;359:494-495.
21 Lackritz EM, Campbell CC, Ruebush TK, et al. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1992;340:524-528.
22 Lacroix J, Hébert PC, Hutchison JH, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609-1619.
23 Karam O, Tucci M, Ducruet T, Hume HA, Lacroix J, Gauvin F, Canadian Critical Care Trials Group and the PALISI Network. Red blood cell transfusion thresholds in pediatric patients with sepsis. Pediatr Crit Care Med. 2010 Nov 4. [Epub ahead of print]
24 Rouette J, Trottier H, Ducruet T, Beaunoyer M, Lacroix J, Tucci M. Red blood cell transfusion threshold in postsurgical pediatric intensive care patients: a randomized clinical trial. Ann Surg. Mar 2010;251(3):421-427.
25 Willems A, Harrington K, Lacroix J, et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med. Feb 2010;38(2):649-656.
26 Parshuram C, Doyle J, Lau W, Shemie SD. Transfusion-associated graft versus host disease. Pediatr Crit Care Med. 2002;3:57-62.
27 Beck C, Dubois J, Grignon A, Lacroix J, David M. Incidence and risk factors of catheter-related deep vein thrombosis in a pediatric intensive care unit: A prospective study. J Pediatr. 1998;133:237-241.
28 Pierce CM, Wade A, Mok Q. Heparin-bonded central venous lines reduce thrombotic and infective complications in critically ill children. Intensive Care Med. 2000;26:967-972.
29 Dubois J, Rypens F, Garel L, David M, Lacroix J, Gauvin F. Incidence of deep vein thrombosis related to peripherally inserted central catheters in children and adolescents. CMAJ. Nov 6 2007;177(10):1185-1190.
30 deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417-423.
31 Davies L, Dolgin S, Kattan M. Morbidity and mortality of open lung biopsy in children. Pediatrics. 1997;99:660-664.
32 Kornecki A, Shemie SD. Open lung biopsy in children with respiratory failure. Crit Care Med. 2001;29:1247-1250.
33 Piastra M, De Luca D, Pietrini D, et al. Noninvasive pressure-support ventilation in immunocompromised children with ARDS: a feasibility study. Intensive Care Med. Aug 2009;35(8):1420-1427.
34 Schiller O, Schonfeld T, Yaniv I, Stein J, Kadmon G, Nahum E. Bi-level positive airway pressure ventilation in pediatric oncology patients with acute respiratory failure. J Intensive Care Med. Nov-Dec 2009;24(6):383-388.
35 Steinberg D, Gold J, Brodin A. Necrotizing enterocolitis in leukemia. Arch Intern Med. 1973;131:538-544.
36 Haussmann U, Fischer J, Eber S, Scherer F, Seger R, Gungor T. Hepatic veno-occlusive disease in pediatric stem cell transplantation: impact of pre-emptive antithrombin III replacement and combined antithrombin III/defibrotide therapy. Haematologica. Jun 2006;91(6):795-800.
37 Bulley SR, Strahm B, Doyle J, Dupuis LL. Defibrotide for the treatment of hepatic veno-occlusive disease in children. Pediatr Blood Cancer. Jun 15 2007;48(7):700-704.
38 Richardson PG, Soiffer RJ, Antin JH, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multi-organ failure post stem cell transplantation: a multi-center, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16:1005-1017. Epub 2010 Feb 16
39 Ali AA, Flombaum CD, Brochstein JA, Gillio AP, Bussel JB, Boulad F. Lactic acidosis and renal enlargement at diagnosis and relapse of acute lymphoblastic leukemia. J Pediatr. 1994;125:584-586.
40 Robson WL, Leung AK. Lactic acidosis in a patient with acute lymphoblastic leukemia. J Pediatr. 1995;126:1021-1022.
41 Belt RJ, Leite C, Haas CD, Stephens RL. Incidence of hemorrhagic complications in patients with cancer. JAMA. 1978;239:2571-2574.
42 Gauvin F, Toledano B, Champagne J, Lacroix J. Reactive hemophagocytic syndrome presenting as a component of multiple organ system failure. Crit Care Med. 2000;28:3341-3345.
43 Cairo MS, Agosti J, Ellis R, et al. A randomized, double-blind, placebo-controlled trial of prophylactic recombinant human granulocyte-macrophage colony-stimulating factor to reduce nosocomial infections in very low birth weight neonates. J Pediatr. 1999;134:64-70.
44 Gruson D, Hilbert G, Vargas F, et al. Impact of colony-stimulating factor therapy on clinical outcome and frequency rate of nosocomial infections in intensive care unit neutropenic patients. Crit Care Med. 2000;28:3155-3160.
45 Randolph AG, Zollo MB, Wigton RS, Yeh TS. Factors explaining variability among caregivers in the intent to restrict life-support interventions in a pediatric intensive care unit. Crit Care Med. 1997;25:435-439.
46 Randolph AG, Zollo MB, Egger MJ, Guyatt GH, Nelson RM, Stidham GL. Variability in physician opinion on limiting pediatric life support. Pediatrics. 1999;103:e807-e808.
47 Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996-2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9(3):270-277.
48 Pound CM, Johnston DL, Armstrong R, Gaboury I, Menon K. The morbidity and mortality of pediatric oncology patients presenting to the intensive care unit with septic shock. Pediatr Blood Cancer. 2008;51(5):584-588.
49 Fiser RT, West NK, Bush AJ, Sillos EM, Schmidt JE, Tamburro RF. Outcome of severe sepsis in pediatric oncology patients. Pediatr Crit Care Med. Sep 2005;6(5):531-536.
50 Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. Mar 2007;35(3):808-814.
51 Diaz MA, Vicent MG, Prudencio M, et al. Predicting factors for admission to an intensive care unit and clinical outcome in pediatric patients receiving hematopoietic stem cell transplantation. Haematologica. 2002;87:292-298.
52 van Gestel JP, Bollen CW, Bierings MB, Boelens JJ, Wulffraat NM, van Vught AJ. Survival in a recent cohort of mechanically ventilated pediatric allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. Dec 2008;14(12):1385-1393.
53 Keenan HT, Bratton SL, Martin LD, Crawford SW, Weiss NS. Outcome of children who require mechanical ventilatory support after bone marrow transplantation. Crit Care Med. 2000;28:830-835.
54 Blot F, Azoulay E. Insuffisance respiratoire aiguë chez l’immunodéprimé: critères d’admission en réanimation. In: Française SdRdL, editor. Actualités en réanimation et urgences. Paris: Elsiever; 2003:76-88.
55 Kache S, Weiss IK, Moore TB. Changing outcomes for children requiring intensive care following hematopoietic stem cell transplantation. Pediatr Transplant. May 2006;10(3):299-303.
56 Hagen SA, Craig DM, Martin PL, et al. Mechanically ventilated pediatric stem cell transplant recipients: Effect of cord blood transplant and organ dysfunction on outcome. Pediatr Crit Care Med. 2003;4:206-213.
57 Jacobe SJ, Hassan A, Veys P, Mok Q. Outcome of children requiring admission to an intensive care unit after bone marrow transplantation. Crit Care Med. 2003;31:1299-1305.

