CHAPTER 10 Hematologic/immunologic disorders
General hematology assessment
Observation
• General: Chills, night sweats, altered mental status, confusion, restlessness, vertigo, visual changes
• Pain: Painful lymph nodes, painful joints, sore throat, sinusitis, headaches, abdominal pain
• Respiratory: Shortness of breath, exertional or chronic dyspnea, cough, hemoptysis, orthopnea
• Cardiovascular: Activity intolerance, dizziness, palpitations, chest discomfort, painful legs, swollen legs
• Skin: Unusual bruising, itching, paleness, jaundice, grayness, ulcers, difficulty stopping bleeding from small cuts
• Musculoskeletal: Swollen and/or tender joints, sore muscles, weakness
• Gastrointestinal: Decreased appetite, feeling of fullness, hematemesis, melena, black stools, “coffee grounds” stomach secretions, weight loss, diarrhea, constipation
• Genitourinary: Cystitis, hematuria, heavy menstrual periods, enlarged groin lymph nodes
History
Patients at risk of hematologic or immunologic problems may report having the following disorders:
• Infections: Recent or recurrent; prior blood transfusion
• Allergies: Foods, beverages, medications, plants, animals, birds, fish, detergents, household cleaners, fragrances, seasonal patterns of respiratory symptoms
• Immunologic compromise: Cancer, human immunodeficiency virus (HIV), liver or renal disorders; prior splenectomy, diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, Hashimoto thyroiditis
• Healing: Prolonged bleeding or delayed healing with prior surgeries, including dental procedures
• Presence of foreign bodies: Prosthetic heart valves, inferior vena caval (IVC) filter, implantable defibrillators or other cardiac devices, vascular access devices
• Social history: Multiple sexual partners, excessive alcohol consumption, exposure to chemicals or radiation
• Medications, including over-the-counter medications: Aspirin, aspirin-containing drugs, nonsteroidal anti-inflammatory drugs (NSAIDs); glucocorticoids/steroids, anticoagulants, platelet inhibitors (e.g., clopidogrel), chemotherapy, hormone therapy, oral contraceptives
Commonly Reviewed Components of the Complete Blood Count (CBC)
| Parameters | Significance | Normal Values |
|---|---|---|
| Hemoglobin (Hgb) | Protein in red blood cells containing iron which carries oxygen to tissues | 14–18 g/dL (males) 12–16 g/dL (female) |
| Hematocrit (Hct) | The percentage of red blood cells in the bloodstream. When the Hct is too low, those with anemia may experience fatigue. | 42%–52% (male) 37%–47% (female) |
| White blood cells (WBCs) | Cells of the immune system that protect the body from bacterial, fungal, and viral infections. Incidence of infection increases when WBCs are decreased. | 5000–10,000/mm3 |
| Absolute neutrophil count (ANC) | The number of neutrophils (mature white cells) in the blood. Neutrophils are a type of WBC that help fight infection. When ANC decreases, the patient is neutropenic and more prone to infection. Risk of infection increases when the ANC falls below 2000 and the greatest risk is below 500—a “right shift” on the WBC differential. | 2000/mm3 and above |
| WBC differential | Measures the percentage of each type of WBC in the total WBC count—a “left shift.” Indicates a large percentage of WBCs are neutrophils; indicates the bone marrow has been stimulated by a severe infection to produce neutrophils to fight the infection. Bands are immature neutrophils. “Right shift”: Indicates a small percentage of WBCs are neutrophils, putting the patient at higher risk for an infection; neutropenia. Eosinophilia: Increased eosinophils indicate an allergic reaction is present. Monocytes and lymphocytes: Act as “backup” to the neutrophils. Percentages increase during infection when oncology patients begin bone marrow recovery. If levels do not rise and then fall in a normal pattern, this can be a indication the patient has a poor prognosis for recovery. |
Neutrophils: 50%–62% Bands: 3%–6% Monocytes: 3%–7% Basophils: 0%–1% Eosinophils: 0%–3% Lymphocytes: 25%–40% |
| Platelets (thrombocytes) | Cells that form the matrix on which blood clots are formed | 150,000–400,000/mm3 |
Anaphylactic shock
Pathophysiology
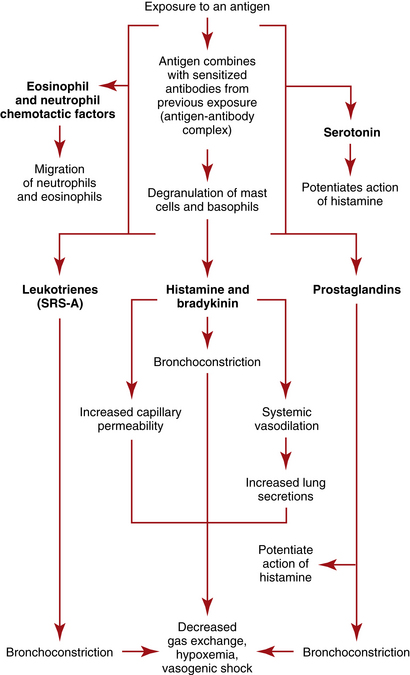
Anaphylaxis (anaphylactic shock) is a potentially life-threatening condition resulting from an exaggerated or hypersensitive response to an antigen or allergen. The classic presentation occurs in a sensitized person (i.e., someone who has been exposed previously to the same antigen), within 1 to 20 minutes of exposure to the antigenic substance, most often drugs, foods, insect stings or bites, antisera, and blood products. The hypersensitive response results in airway inflammation that causes obstruction and respiratory distress, which can lead to respiratory arrest, with a relative hypovolemia caused by massive vasodilation. Fluids shift from the vasculature into interstitial spaces, creating a false hypovolemia or vasogenic (vasodilated) shock, which progresses to end-organ dysfunction secondary to tissue hypoxia from poor perfusion.
The antigen-antibody complexes activate production of prostaglandins and leukotrienes, which are termed slow-reacting substances of anaphylaxis (SRSA)—chemical mediators that produce systemic effects with potentially deleterious results, including profound shock. The leukotrienes produce severe bronchoconstriction and cause venule dilation and increased vascular permeability. The prostaglandins exaggerate bronchoconstriction and potentiate the effects of histamine on vascular permeability and pulmonary secretions. Kinins contribute to bronchoconstriction, vasodilation, and increased vascular permeability. Eosinophilic chemotactic factor of anaphylaxis (ECFA) is then released to attract eosinophils, which work to neutralize mediators such as histamine, but the amount of neutralization is ineffective in reversing the anaphylaxis. (See Figure 10-1 for a depiction of the pathophysiologic process of anaphylaxis.)
Assessment: anaphylactic shock
Goal of system assessment
• Assess the symptoms: Assess for ineffective breathing patterns, impaired gas exchange and airway obstruction, along with altered tissue perfusion related to vasodilation and third spaced intravascular fluids. Symptoms vary with means of antigen entry.
• Classify severity of reaction: Should be determined following initial assessment. Treatment must begin immediately; prior to when diagnostic test results are available. Shock may progress rapidly to circulatory collapse and cardiopulmonary arrest if improperly managed.
• Evaluate effectiveness of prior treatments: Determine patient’s prior treatment regime if asthmatic; classify which “step” of treatment has been needed to control symptoms; patient may need to move to the next step of treatment to maintain control (see Acute Asthma Exacerbation, p 354).
Vital signs
• Pulse oximetry: Oxygen saturation is decreased from patient’s baseline value.
• Tachycardia (heart rate [HR] greater than 140 beats/min [bpm]) and tachypnea (respiratory rate [RR] greater than 40 breaths/min)
• Hypotension may be present; hypotension is exacerbated by underlying vasodilation coupled with increased capillary permeability prompting third spacing of intravascular fluids.
Observation (see table 10-1)
• Red to purple discoloration of face and body; “extreme flushing” with swelling of lips, eyelids, and face due to angioedema
• Tears coming from eyes with strained facial expression
• Severe reactions render patients unable to speak due to breathlessness
• Use of accessory muscles; fatigued, with or without diaphoresis
• Chest expansion may be decreased or restricted.
• Altered level of consciousness (LOC) (confusion, disorientation, agitation)
• Agitation is more commonly associated with hypoxemia while somnolence is associated with hypercarbia (elevated carbon dioxide level).
• General early indicators (occurring within seconds to minutes) include uneasiness, lightheadedness, tingling feeling, flushing, and pruritus.
• General late indicators (occurring within minutes) include rapid progression of urticaria involving large areas of skin; angioedema (tissue swelling; more commonly the eyes, lips, tongue, hands, feet, and genitalia); cough, hoarseness, dyspnea, and respiratory distress; lightheadedness or syncope; and abdominal cramps, diarrhea, and vomiting
• Ingestion of antigen: Cramping, diarrhea, nausea, and vomiting may precede systemic shock symptoms.
• Inhalation of antigen: Cough, hoarseness, wheezing, dyspnea
• Allergic reaction: Edema, urticaria, itching at the site of a bee sting or drug injection
| System | Effects | Cause |
|---|---|---|
| Neurologic | Apprehension; headache; confusion; decreased LOC progressing to coma | Vasodilation; hypoperfusion; cerebral hypoxia or cerebral edema occurring with interstitial fluid shifts |
| Respiratory | Dyspnea progressing to air hunger and complete respiratory obstruction; hoarseness; noisy breathing; high-pitched, “barking” cough; wheezes; crackles; rhonchi; decreasing breath sounds; pulmonary edema (some patients) | Laryngeal edema; bronchoconstriction; increased pulmonary secretions |
| Cardiovascular | Decreased BP leading to profound hypotension; increased HR; decreased amplitude of peripheral pulses; palpitations and dysrhythmias (atrial tachycardias, premature atrial beats, atrial fibrillation, premature ventricular beats progressing to ventricular tachycardia, or ventricular fibrillation); lymphadenopathy | Increased vascular permeability; systemic vasodilation; decreased cardiac output with decreased circulating volume; reflex increase in HR; vasogenic shock |
| Renal | Increased or decreased urine output; incontinence | Decreased renal perfusion; smooth muscle contraction of urinary tract |
| Gastrointestinal | Nausea, vomiting, diarrhea, abdominal cramping | Smooth muscle contraction of GI tract; increased mucus secretion |
| Cutaneous | Urticaria; angioedema (hands, lips, face, feet, genitalia); itching; erythema; flushing; cyanosis | Histamine-induced disruption of cutaneous vasculature; vasodilation, increased capillary permeability; decreased oxygen saturation |
BP, Blood pressure; HR, heart rate; GI, gastrointestinal; LOC, level of consciousness.
Percussion
| The diagnosis of anaphylaxis is based on presenting signs and symptoms. Treatment should be initiated before laboratory results are available. | ||
| Test | Purpose | Abnormal Findings |
| Arterial blood gas analysis (ABG) | Assess for abnormal gas exchange or compensation for metabolic derangements. Initially, PaO2 is normal and then decreases as the ventilation-perfusion mismatch becomes more severe. | pH changes: Acidosis may reflect respiratory failure; alkalosis may reflect tachypnea; Carbon dioxide: Elevated CO2 reflects respiratory failure; decreased CO2 reflects tachypnea; rising PCO2 is an ominous sign, since it signals severe hypoventilation which can lead to respiratory arrest. Hypoxemia: PaO2 <80 mm Hg Oxygen saturation: SaO2 <92% |
| Complete blood count (CBC) with WBC differential | WBC differential evaluates the strength of the immune system’s response to the trigger of response | Eosinophils: Increased in patients not receiving corticosteroids; indicative of magnitude of inflammatory response Hematocrit (Hct): May be increased from hypovolemia and hemoconcentration |
| Tryptase level | Assesses for this chemical mediator released by mast cells following anaphylaxis | Increases within 1 hour following anaphylaxis and remains elevated for 4–6 hours |
| IgE levels | Used to confirm origin of the reaction is an allergic response | Levels are elevated if allergic response is present. |
| 12-Lead electrocardiogram (ECG) | To detect dysrhythmias reflective of myocardial ischemia | Ischemic changes (ST depression) may be present as shock progresses. |
Collaborative management (see figure 10-2)
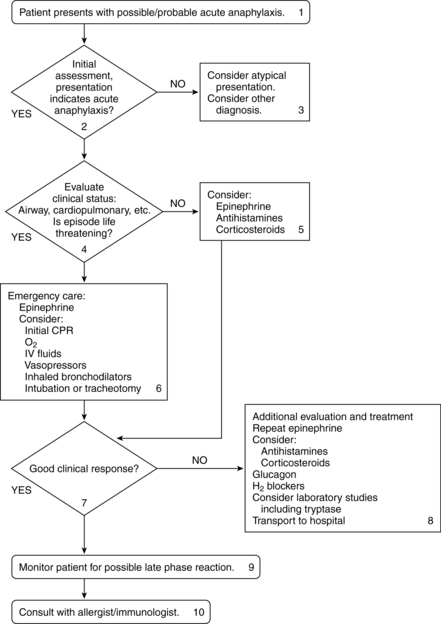
Figure 10-2 Algorithm for the treatment of acute anaphylaxis.
(From Nicklas RA, et al: The diagnosis and management of anaphylaxis. J Allergy Clin Immunol 101(6 Pt 2):S465–S528, 1998.)
Care priorities
2. Provide supplemental oxygen:
Administered to support ventilation and aerobic metabolism. Amount and method of oxygen administration are guided by arterial blood gas (ABG) results. Often initiated at 6 L/min via nasal cannula or oxygen mask.
3. Manage vasodilation and increased capillary permeability
• Standard adult dose: 0.2 to 0.5 mg (0.2 to 0.5 ml of a 1:1000 solution) given intramuscularly (IM) or subcutaneously (SC)
• Alternate initial dose: 0.1 mg (0.1 ml of a 1:1000 solution) may be administered.
• Repeat dosage: may be repeated every 10 to 15 minutes as needed
• IV dosage: preferred if patient is in shock and/or has severe airway obstruction: Initial dose of 0.1 to 0.25 mg (1.0 to 2.5 ml of 1:10,000 solution) over 5 to 10 minutes. The dose may be increased to 0.3 to 0.5 mg. Repeat every 5 to 15 minutes as needed.
• IV infusion: after initial dose, an IV drip of epinephrine 1.0 mg in 250 ml D5W may be infused at 1 mcg/min and increased to 4 mcg/min (or more) as needed to achieve desired response.
• Endotracheal (ET) tube: 1.0 to 2.5 ml of 1:10,000 solution (1 mg epinephrine in 10-ml solution) into ET tube, followed by use of manual resuscitation (Ambu) bag. May repeat if needed.
Used if fluid replacement does not increase blood pressure (BP). Drugs are titrated for the desired response (see Appendix 6). Usual dosages are as follows:
• Dopamine hydrochloride: Effects are dose dependent. Increases cardiac contractility at 5 to 10 mcg/kg/min and systemic vascular resistance at 10 to 20 mcg/kg/min. Consider switching to norepinephrine if dose exceeds 20 mcg/kg/min.
• Norepinephrine: Initial dosage is 2 to 8 mcg/min and can be increased to achieve the necessary BP.
• Phenylephrine: Usual dosage is 40 to 60 mcg/min. Doses exceeding 200 mcg/min have been used.
CARE PLANS FOR ANAPHYLAXIS AND ANAPHYLACTIC SHOCK
1. Assess continuously for obstructed airway and increased respiratory effort: Note increased pulmonary secretions, cough, expiratory wheezing, SOB, and dyspnea. Suction as needed. Caution: An oral airway provides airway support only as far as the posterior pharynx. If laryngeal edema is present, the oral airway cannot relieve symptoms because the obstruction is below the oral airway. If ET intubation is attempted and is not possible due to laryngeal edema, prepare for tracheostomy or cricothyroidotomy.
2. Monitor for decreased breath sounds or changes in wheezing at frequent intervals: Absent breath sounds in a distressed patient may indicate impending respiratory arrest. Identify patient requiring actual/potential airway insertion. Consult physician and prepare for ET intubation if lingual edema is present and/or respiratory distress continues.
3. Position patient for comfort and to promote optimal gas exchange: High Fowler’s position, with the patient leaning forward and elbows propped on the over-the-bed table to promote maximal chest excursion, may reduce use of accessory muscles and diaphoresis due to work of breathing. May not be possible with severe hypotension, as further decrease in BP may result.
1. Monitor for signs of increasing hypoxia at frequent intervals: Restlessness, agitation, and personality changes are indicative of severe reaction. Cyanosis of the lips (central) and nail beds (peripheral) are late indicators of hypoxia, but may be difficult to see with severe angioedema.
2. Monitor for signs of hypercapnia at frequent intervals: Confusion, listlessness, and somnolence are indicative of respiratory failure.
3. Provide medications to abate allergic response: Administer epinephrine and IV and inhaled bronchodilators as appropriate to attain control of deterioration.
Respiratory Status: Ventilation
1. Monitor FIO2 to ensure that oxygen is within prescribed concentrations. If patient does not retain carbon dioxide, 100% nonrebreather mask may be used to provide maximal oxygen support. If the patient retains CO2 and is unrelieved by positioning, lower-dose oxygen, bronchodilators and steroids, intubation, and mechanical ventilation may be necessary sooner than in patients who are able to receive higher doses of oxygen by mask.
2. Monitor ABGs when continuous pulse oximetry values or patient assessment reflects progressive hypoxemia or development of hypercapnia. Monitor and report ABG values with increasing PaCO2 (greater than 50 mm Hg) or decreasing PaO2 (less than 60 mm Hg) indicative of impending respiratory failure.
3. Administer antihistamines as prescribed.
4. Administer glucocorticoids as prescribed.
5. Position patient to alleviate dyspnea (assist patient to sitting position if BP is stable).
6. Stay with patient to promote safety and reduce fear. Use calm, reassuring approach.
![]() Respiratory Monitoring; Emotional Support; Medication Administration, Medication Management
Respiratory Monitoring; Emotional Support; Medication Administration, Medication Management
Circulation Status; Tissue Perfusion: Cardiac; Vital Signs
1. Assess for physical and hemodynamic indicators of decreased cardiac output:
2. Monitor for dysrhythmias, such as atrial tachycardias, PVCs, ventricular tachycardia, and ventricular fibrillation, which may signal hypoxemia or occur as side effects of drugs such as aminophylline or epinephrine.
4. Administer epinephrine as prescribed. Observe for therapeutic effects as evidenced by increased SVR, increased CO/CI, increased ScVO2, increased arterial BP and MAP, stronger peripheral pulses, warming of extremities, and increased urine output.
5. Administer fluid replacement therapy as prescribed, using a large-bore IV catheter. Colloids and crystalloids may be given together.
Altered tissue perfusion: peripheral, renal, and cerebral
related to hypovolemia secondary to fluid shift from the vascular space to the interstitial space
Tissue Perfusion: Abdominal Organs, Tissue Perfusion: Peripheral; Tissue Perfusion: Cerebral
1. ![]() Assess peripheral pulses. Report decreased amplitude of pulses.
Assess peripheral pulses. Report decreased amplitude of pulses.
2. Assess capillary refill. Delayed capillary refill (greater than 2 seconds) is likely with edema and decreased vascular volume.
3. Assess degree of peripheral edema.
4. Assess color and warmth of extremities. Report presence of coolness and pallor.
5. Monitor BP at frequent intervals. Be alert for indicators of hypotension such as BP readings greater than 20 mm Hg below patient’s normal pressure, dizziness, restlessness, altered mentation, and decreased urinary output.
6. Monitor urine output hourly. Continuous CO monitoring using a noninvasive system or a pulmonary artery catheter may be needed to guide fluid resuscitation.
7. Observe for indicators of decreased cerebral perfusion such as anxiety, restlessness, confusion, and decreased LOC.
HIGH ALERT!
Changes in LOC may signal either decreased cerebral perfusion (tissue hypoxia) or increasing intracranial pressure (IICP) caused by interstitial swelling from capillary permeability.
8. Administer fluid and pharmacologic agents as prescribed (see previous nursing diagnosis).
![]() Hemodynamic Regulation; Anaphylaxis Management; Medication Administration, Medication Management
Hemodynamic Regulation; Anaphylaxis Management; Medication Administration, Medication Management
related to urticaria and angioedema secondary to allergic response
Tissue Integrity: Skin and Mucous Membranes
1. Assess patient for urticaria (hives) and itching of hands, feet, neck, and genitalia.
2. Administer antihistamines as prescribed to relieve itching.
3. Discourage patient from scratching the skin. If unavoidable, teach patient to use pads of fingertips rather than nails.
4. Apply cool washcloths or covered ice as a soothing measure to irritated and edematous areas.
![]() Environmental Management: Comfort; Medication Administration
Environmental Management: Comfort; Medication Administration
Deficient knowledge illness care: severe hypersensitivity reaction, its causes, and its symptoms
related to no prior exposure or incomplete understanding
1. Provide information about the antigenic agent that caused the anaphylaxis, including ways to avoid it in the future.
2. Explain need for wearing a medical-alert identification tag or bracelet to identify the allergy.
3. Give information about anaphylaxis emergency treatment kits. Teach patient self-administration technique and the importance of prompt treatment.
4. Stress the importance of seeking treatment immediately if symptoms of allergy occur, including flushing, warmth, itching, anxiety, and hives.
5. Explain the importance of identifying and checking all over-the-counter (OTC) medications for the presence of potential allergens.
![]() Health Education; Risk Identification; Teaching: Prescribed Medication
Health Education; Risk Identification; Teaching: Prescribed Medication
Profound anemia and hemolytic crisis
Pathophysiology
Anemia
Anemia reflects a reduction in total body hemoglobin (Hgb) concentration and is common in critically ill patients. By the third day in an intensive care unit (ICU), 95% of patients have reduced Hgb concentrations. As the Hgb decreases, the oxygen-carrying capacity of the blood is reduced, resulting in tissue hypoxia unless compensatory mechanisms are adequate to assist the body with oxygen delivery. Anemia may be classified under one of three functional classes after initial evaluation of the CBC and reticulocyte index. (See Table 10-2 for functional classification.)
| Blood Loss/Hemolysis | Decreased RBC Production | Maturation Disorders |
| Autoimmune diseases Thrombotic Thrombocytopenia Purpura (TTP), Goodpasture’s Syndrome, Systemic Lupus Erythematosus (SLE), Wegener’s Granulomatosis |
Damaged bone marrow: malignancy, lead poisoning, aplastic/hypoplastic anemia, chemotherapy, viruses | Abnormal RBC cytoplasm Phenylketonuria (PKU), G6PD |
| Abnormal hemoglobin Sickle cell disease, Hgb S, C D, E |
Iron deficiency: malignancy, autoimmune disorders | Abnormal RBC nucleus |
| Abnormal RBC membranes Spherocytosis, hemolytic uremic syndrome, paroxysmal nocturnal hematuria | Erythropoietin deficiency: renal failure, malaria, thalassemias | Iron deficiency: dietary, chronic alcoholism |
| Bleeding/hemorrhage Physical trauma to blood (bypass, balloon, valves), antibodies (drug-induced antibodies), endotoxins (malaria, clostridia), GI bleed, trauma, rupture, excess menstruation | Inflammation/infection: chronic inflammatory disease; critical illness | |
| Excessive phlebotomies: lab sampling | Metabolic disturbance: pernicious anemia, hypothyroidism, megaloblastic anemia |
Assessment
Hemolytic crisis
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Red blood cell count (RBCs) | Enumeration of the red cells found in each cubic millimeter of blood | Reduced; in hemolytic crisis, an increased number of premature RBCs (nucleated RBCs) will be present. |
| Hemoglobin (Hgb) | Hemoglobin content of RBCs | Decreased |
| Hematocrit (Hct) | Percentage of RBCs in relation to total blood volume | Decreased |
| Reticulocyte count, reticulocyte index, corrected reticulocyte | RBC precursors; measures how fast RBCs are produced in the bone marrow | Elevated: because of increased bone marrow production of RBCs due to blood loss or RBC destruction; also a sign of marrow recovery after chemotherapy. |
| Mean corpuscular volume (MCV) (subcategory of red cell indices) Macrocytic: MCV >100 mcg3 Microcytic: MCV <80 mcg3 Normocytic: MCV 80–100 mcg3 |
Morphologic classification of RBCs: average size of individual RBCs. Obtained by dividing HCT by total RBC count | Low in microcytic anemia; high in macrocytic anemia |
| Sickle cell test | Indicative of sickle cell anemia (trait, disease) | Presence of Hemoglobin S (Hgb S) |
| Hemoglobin (Hgb) electrophoresis | Screens for abnormal hemoglobins often present in hemolytic anemias Many hemoglobinopathies are interrelated. Disease expression is based on the degree of genetic abnormalities. Various combinations of abnormal hemoglobins are possible |
Hemoglobins A1, A2, and F: Normal Hgb Hemoglobin C: Generally benign; May cause joint pain, splenomegaly and gallstones; may protect against malaria Hemoglobins D and E: Rarely occur “singly”; sometimes present with sickle cell disease or thalassemias Hemoglobin H: Causes premature destruction of RBCs and abnormal binding of O2 to RBCs; causes alpha thallasemia Hemoglobin S: Most common abnormal hemoglobin, occurring in 10% of the African American population; causes sickle cell disease or sickle cell trait |
| Erythrocyte sedimentation rate (ESR), sedimentation rate or Biernacki reaction | Rate at which RBCs precipitate in a period of 1 hour: nonspecific measure of inflammation | Elevated in hemolytic anemia; decreased in sickle cell anemia, polycythemia and congestive heart failure |
| C3 proactivator | Proactivator of complement 3 in the alternate pathway of complement activation | Increased in hemolytic anemia |
| Total iron-binding capacity (TIBC) | Measures the blood’s capacity to bind iron with transferrin; also indirect test of liver function (rarely used for that) TIBC is typically measured along with serum iron to evaluate people suspected of having either iron deficiency or iron overload. |
Normal or reduced, depending on the type of anemia |
| Ferritin | Iron stores: with damage to organs that contain ferritin (especially the liver, spleen, and bone marrow), ferritin levels can become elevated even though the total amount of iron in the body is normal. | Reduced with iron deficiency anemia; normal or elevated with anemia of critical illness; elevated with hemachromatosis |
| Transferrin | Used to determine the cause of anemia, to examine iron metabolism (for example, in iron deficiency anemia) and to determine the iron-carrying capacity of the blood. | Reduced with anemia of chronic inflammation, anemia of critical illness. |
| Transferrin saturation | The iron concentration divided by TIBC—a more useful indicator of iron status than iron or TIBC alone. | Reduced with anemia of chronic inflammation, anemia of critical illness. |
| Folate; folic acid | Measures folic acid in the blood | Reduced with nutritional deficiency leading to megaloblastic anemia. |
| Erythropoietin (EPO, EP) | Measures the amount of a hormone called erythropoietin (EPO) in blood; acts on stem cells in the bone marrow to increase the production of red blood cells; made by cells in the kidney, which release the hormone when oxygen levels are low. | Reduced with renal disease and normal in those who are critically ill who should have an elevated level if anemia of any cause is present. Reticulocyte response to EP has been shown to be reduced in many critically ill patients with elevated EP levels. |
| Vitamin B12 | Measures the amount of vitamin B12 in the blood; used with folic acid test, because a lack of either can cause megaloblastic anemia. | Reduced with pernicious or megaloblastic anemia. |
| Unconjugated bilirubin: free bilirubin, indirect bilirubin | Measures bilirubin that has not been conjugated in the liver. It gives an indirect reaction to the Van Den Bergh test. | Elevated in hemolytic anemia due to liver’s inability to process increasing bilirubin released during hemolysis. |
| Serum lactic dehydrogenase isoenzymes (LDH1 and LDH2 ) | General indicator of the existence and severity of acute or chronic tissue damage and, sometimes, as a monitor of progressive conditions; monitor damage caused by muscle trauma or injury and to help identify hemolytic anemia | Elevated in hemolytic anemia because of their release when an RBC is destroyed. |
| Haptoglobin level | Used to detect and evaluate hemolytic anemia; not to diagnose cause of the hemolysis. Haptoglobin levels should be drawn prior to transfusion. | Decreased in hemolytic anemia due to increased binding of haptoglobin, which facilitates removal of increased Hgb from blood. |
| Peripheral blood smear | Microscopic examination of cells from drop of blood; investigates hematologic problems or parasites such as malaria and filaria | May reveal abnormally shaped RBCs, such as spherocytes. RBC hyperplasia (abnormal number) is present in nearly all cases of chronic hemolysis with intact bone marrow. |
| Bone marrow aspiration | Evaluates bone marrow status; diagnose blood disorders and determine if cancer or infection has spread to the bone marrow. | May reveal abnormal size, shape, or amounts of RBCs, WBCs or platelets |
| Coombs test: Direct antiglobulin test; Indirect antiglobulin test | Detects antibodies that may bind to RBCs and cause premature RBC destruction | Positive in antibody-mediated immunologic hemolysis. |
| Immunoglobulin levels | Measures the level of immunoglobulins, also known as antibodies, in the blood. | Elevated: autoimmune disorders, sickle cell; lower in immunocompromised states. |
| Glucose-6-phosphate dehydrogenase (G6PD) levels | Measures G6PD—enzyme levels are normal in newly produced cells but fall as RBCs age and only deficient cells are destroyed. | Decreased in G6PD deficiency, hemolysis Elevated: MI, liver failure, chronic blood loss, hyperthyroidism |
| Radiologic examinations | X-rays and bone scans Liver/spleen scans |
Decreased density, aseptic necrosis of bones Hepatomegaly, splenomegaly, lesions |
Collaborative management: anemias
Care priorities
4. Elimination of causative factor:
Certain drugs and chemicals, cold temperatures, and stress can worsen many anemias, but most profoundly affected are hemolytic and aplastic anemias. Identifying and removing the causative agent can prevent life-threatening crisis. If the patient is bleeding, the cause of the bleeding must be addressed and the bleeding controlled.
9. Bone marrow transplantation:
CARE PLANS FOR ANEMIAS
related to lack of RBCs; hemoglobin abnormalities
Respiratory Status: Gas Exchange; Tissue Perfusion: Pulmonary
1. Administer supplemental oxygen, using appropriate device (i.e., nasal cannula, face mask/shield, or mechanical ventilation as necessary). Monitor oxygen liter flow. Provide for oxygen when patient is transported.
2. Monitor rate, rhythm, and depth of respirations.
3. Monitor for increased restlessness, anxiety, and air hunger.
4. Monitor oxygen saturation using pulse oximeter continuously. Consult with physician for persistent values less than 90% or, if oxygen saturation is chronically decreased, a sustained drop of greater than 10% of baseline.
5. Note changes in SaO2, SVO2, ScVO2, StO2 (if available) and changes in arterial blood gas (ABG) values, as appropriate.
6. Maintain large-bore (18-gauge) IV catheter(s) in case transfusion or rapid volume expansion is necessary. Administer intravenous fluids to maintain hydration.
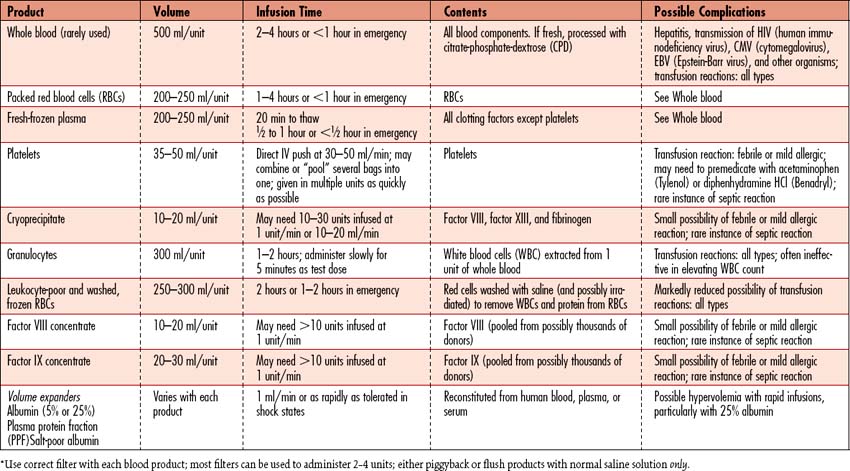
7. Transfuse with packed cells (RBCs) (Table 10-3) as prescribed to facilitate oxygen delivery and assist in volume expansion, and/or implement aggressive strategy to augment RBC production.
8. Describe the purpose of blood product transfusion therapy to the patient and significant others.
9. Carefully evaluate dyspnea and chest pain in patients with sickle cell disease because of the possibility of pulmonary infarction.
![]() Airway Management; Oxygen Therapy; Circulatory Precautions; Cardiac Precautions
Airway Management; Oxygen Therapy; Circulatory Precautions; Cardiac Precautions
related to anemia/lack of oxygen-carrying capacity of the blood
1. Alternate periods of rest and activity to avoid stress that increases oxygen demand.
2. Collaborate with occupational therapy (OT), physical therapy (PT), and/or recreational therapy personnel in planning and monitoring an activity program as appropriate.
3. Determine patient’s physical limitations. Focus on what patient can do, rather than on deficits.
4. Reposition patient slowly while monitoring effects on myocardial and cerebral perfusion.
5. Reduce fear, pain, and anxiety to decrease oxygen demand.
6. Determine causes of fatigue (e.g., treatments, pain, medications).
7. Monitor nutritional intake to ensure adequate energy resources.
8. Teach patient to avoid stressful situations, which can exacerbate symptoms of anemia and precipitate hemolytic crisis in patients with hemolytic anemia.
9. Teach signs of hypoxemia: altered mental status, activity intolerance, SOB, chest pain, and weakness.
10. Teach patient and significant others about the specific anemia affecting the patient.
Risk for impaired skin integrity
![]() related to impaired oxygen transport secondary to chronic anemia
related to impaired oxygen transport secondary to chronic anemia
Patient’s skin remains intact during hospitalization.
Tissue Integrity: Skin and Mucous Membranes
1. Keep extremities warm to promote circulation and help prevent tissue hypoxia.
2. Perform a comprehensive appraisal of peripheral circulation (e.g., check peripheral pulses, edema, capillary refill, color, and temperature of extremity).
3. Monitor for sources of pressure and friction.
4. Monitor for infection, especially of edematous areas.
5. Use a bed cradle to reduce pressure of covers on extremities.
6. Monitor skin and mucous membranes for areas of discoloration and bruising.
7. Monitor skin for rashes, abrasions, excessive dryness, and moisture.
8. Provide adequate nutrition and nutritional supplements as appropriate. Negative nitrogen state or low serum protein or albumin increases the risk for skin breakdown.
9. Teach patient the signs of skin breakdown, since it can occur at any time with chronic anemia.
10. Instruct the patient on the importance of preventing venous stasis.
11. Teach patient about appropriate nutrition as discussed in Nutritional Support, p. 117.
12. Apply appropriate skin-saving dressing (e.g., Duoderm) or initiate aggressive skin care regimen to areas of breakdown.
13. For additional interventions, see Wound and Skin Care, p. 167.
![]() Pressure Ulcer Prevention; Skin Surveillance; Nutrition Management; Circulatory Precautions
Pressure Ulcer Prevention; Skin Surveillance; Nutrition Management; Circulatory Precautions
Collaborative management: hemolytic crisis
Care priorities
4. Transfusions/blood component replacement:
Packed RBCs may be necessary in the management of profound anemia to help increase the blood’s oxygen-carrying capacity. For patients who refuse blood transfusions, aggressive strategies to augment RBC production such as IV iron therapy and subcutaneous administration of erythropoietin may be implemented. These therapies may take up to 7 days or longer to promote significant improvement in the reticulocyte count and Hgb and Hct levels. The oxygen-carrying capacity of banked blood is best when used within 14 days of collection. Blood transfused more than 21 days after collection has been linked to increased mortality rates in the critically ill, especially HIV-positive patients. Benefits must be weighed against risks, particularly in immunosuppressed patients.
Cytapheresis procedure for patients experiencing symptoms of excessive thrombosis, to attempt rapid platelet reduction to decrease clotting before onset of MODS (see Multiple Organ Dysfunction Syndrome in SIRS, Sepsis and MODS, p. 924)
CARE PLANS FOR HEMOLYTIC CRISIS
Ineffective tissue perfusion: peripheral, cardiopulmonary, gastrointestinal, renal, and cerebral
related to interruption of arterial or venous blood flow secondary to formation of microthrombi
Circulatory care: arterial insufficiency
1. Initiate aggressive IV fluid volume replacement as prescribed to prevent deposition of hemolyzed RBCs in the microvasculature.
2. Assess extremities for inadequate peripheral perfusion: amplitude of peripheral pulses, coolness, pallor, and prolonged capillary refill. Use Doppler if unable to palpate pulses.
3. Evaluate chest pain. Note cardiac dysrhythmias and symptoms of decreased cardiac output. Monitor respiratory status for symptoms of heart failure.
4. Monitor vital signs frequently for signs of impending shock: increased HR and RR, increased restlessness and anxiety, and cool and clammy skin, followed by a decrease in BP.
5. Monitor abdomen for signs of decreased perfusion.
6. Keep lower extremities elevated slightly to promote venous blood flow.
7. Monitor ventilation and perfusion: assess ABG values for acidosis (i.e., pH less than 7.35, hypercarbia/CO2 retention [PaCO2 greater than 45 mm Hg]), indicating hypoperfusion, and respiratory insufficiency. Assess for hypoxemia using continuous pulse oximetry and ScVO2 or SVO2 monitoring to detect decreased oxygen saturation. Consult physician or midlevel practitioner for sustained deterioration in status.
8. Monitor urinary output for decrease, which can signal decreased renal perfusion. Consult physician or midlevel practitioner for urine output less than 0.5 ml/kg/hr for 2 consecutive hours.
9. Monitor neurologic status every 2 to 4 hours, using the Glasgow Coma Scale (see Appendix 2).
10. Teach patient and significant others about hemolytic anemia, including the signs of impending hemolytic crisis, rendering information on the following:
1. Monitor patient for signs of discomfort, including increases in HR, BP, and RR. Devise a pain scale with patient, rating discomfort from 0 (no pain) to 10.
2. Perform a comprehensive assessment of pain to include location, characteristics, onset/duration, frequency, quality, intensity or severity of pain, and precipitating factors.
3. Medicate for pain as prescribed. Assess effectiveness of medication using the pain scale. Confer with physician if pain relief is ineffective; devise an alternate plan for analgesia.
4. Recognize that components of chronic and acute pain are present and tolerance may be higher than expected for age and size. During a crisis, exacerbation of pain may be unpredictable due to intermittent vessel occlusion, so both baseline and breakthrough medications will be required to achieve pain relief.
5. If pain medication injections are frequent, consider an IV rather than an intramuscular (IM) route, when possible. While quick-acting, meperidine may not be drug of choice due to impact on renal function over time.
6. Administer adjuvant analgesics and/or medications when needed to potentiate analgesia.
7. Consider continuous infusion (alone or with bolus opioids) to maintain serum levels.
8. Collaborate with the physician if drug, dose, route of administration, or interval changes are indicated, making specific recommendations based on equianalgesic principles.
9. Consider complementary method of pain control such as relaxation techniques: guided imagery, controlled breathing, meditation, and listening to soft, soothing music. Use therapeutic/healing touch to relieve pain if practitioner is trained and patient agrees to participate. Alternatively, consult trained practitioner.
10. Control environmental factors that may add to discomfort (e.g., room temperature, light, noise).
11. Apply warm compresses to joints to increase circulation and thereby improve tissue oxygenation.
12. Apply elastic stockings to promote venous return and enhance circulation.
13. Teach patient to perform isometric or range-of-motion (ROM) exercises to promote circulation.
14. Help allay fears by reassuring patient that pain will decrease as the crisis subsides.
15. Provide emotional support to patient during the crisis episode. Reassure patient that the crisis is time-limited, and enable significant others to be with patient, if possible, during the crisis.
16. Teach patient to assess extremities daily for evidence of tissue breakdown or blood sequestration (i.e., swelling, erythema, tenderness) so that early interventions can be implemented in an attempt to prevent severe pain.
Risk for deficient fluid volume
Fluid Balance; Electrolyte and Acid-Base Balance
1. ![]() Monitor intake and output (I&O) hourly. Consult physician or midlevel practitioner for a urinary output less than 0.5 ml/kg/hr for 4 consecutive hours. Insert urinary catheter if patient is unable to void.
Monitor intake and output (I&O) hourly. Consult physician or midlevel practitioner for a urinary output less than 0.5 ml/kg/hr for 4 consecutive hours. Insert urinary catheter if patient is unable to void.
2. Monitor and document heart rate, rhythm, pulses, and blood pressure.
3. Evaluate efficacy of volume expansion by closely monitoring CVP. Overzealous volume expansion can lead to heart failure and pulmonary edema, with CVP greater than 20% to 25% of normal values.
4. Administer diuretics as prescribed in the well-hydrated patient with urine output less than 0.5 ml/kg/hr.
5. Assess patient for volume depletion, including poor skin turgor, dry mucous membranes, hypotension, tachycardia, and decreasing urine output and CVP.
6. Monitor electrolytes and serum osmolality. A universal increase in electrolytes and osmolality is indicative of dehydration. A universal decrease signals fluid overload.
7. Assess pH (normal range is 7.35 to 7.45) before replacing electrolytes. Acidosis and alkalosis alter electrolyte values. Replace potassium if the pH is outside the normal range.
Additional nursing diagnoses
Uncontrolled pain, bleeding, and complications of hemolysis can be terrifying to the patient and significant others, who may fear that the patient will die. Chronic illness with episodic acute exacerbations may require more individualized intervention when handling long-term illness and its sequelae. See Emotional Support of Patient and Significant Others, p. 200.
Bleeding and thrombotic disorders
Pathophysiology
1. Hemodilution: related to administration of large amounts of retained fluids, IV fluids, multiple medications given in 50- to 100-ml “piggybacks,” or blood/blood products
2. Increased platelet destruction or consumption: includes heparin-induced thrombocytopenia (HIT); antiphospholipid antibody syndrome (APAS) or lupus anticoagulant syndrome; idiopathic thrombocytopenic purpura (ITP); thrombotic thrombocytopenic purpura (TTP); hemolytic-uremic syndrome (HUS); febrile reactions; severe sepsis; hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. Disseminated intravascular coagulation (DIC) presents with a combined coagulopathy and platelet consumption.
3. Platelet sequestration: related to hypersplenism and hyperthermia
4. Decreased production of platelets: caused by alcohol (EtOH) abuse, bone marrow irradiation, bone marrow/stem cell disease, graft-versus-host disease, aplastic anemia, vitamin B12 or folate deficiencies, metastatic carcinoma, some renal diseases, leukemia, and myeloproliferative disorders
Platelet destruction may be mediated by congenital autoimmune or alloimmune disorders, or by acquired immunologic or nonimmunologic mechanisms. Causative or related factors include septicemia, systemic inflammatory response syndrome (SIRS), pulmonary hypertension, extracorporeal circulation, thrombotic disorders, acute transplant rejection, severe allergic reactions, rheumatic disorders, intravascular catheters and prosthetics, fat emboli, acute respiratory distress syndrome (ARDS), and HIV infection. The most significant diagnostic finding ![]() associated with severe thrombocytopenia is presence of petechiae in dependent areas (i.e., back, ankles, posterior thighs of bedridden patients). Larger purpura such as ecchymoses and hematomas may also be present but are nonspecific for diagnosis of platelet disorders. Patients must be assessed for risk of bleeding with thrombocytopenia, considering the severity and cause as well as comorbid factors.
associated with severe thrombocytopenia is presence of petechiae in dependent areas (i.e., back, ankles, posterior thighs of bedridden patients). Larger purpura such as ecchymoses and hematomas may also be present but are nonspecific for diagnosis of platelet disorders. Patients must be assessed for risk of bleeding with thrombocytopenia, considering the severity and cause as well as comorbid factors.
1. Reflex vasoconstriction: Vascular spasm that decreases blood flow to the site of injury
2. Platelet aggregation: Accumulation of platelets that leads to formation of a platelet plug to help support the repair of the injury. If the damage to the vessel is small, the plug is sufficient to seal the injury. If the hole is large, a blood clot is necessary to stop the bleeding.
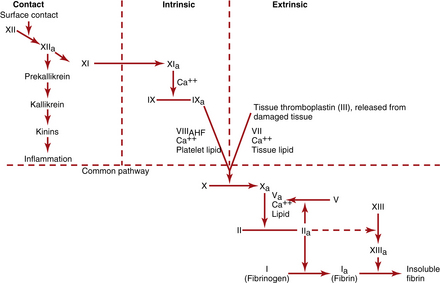
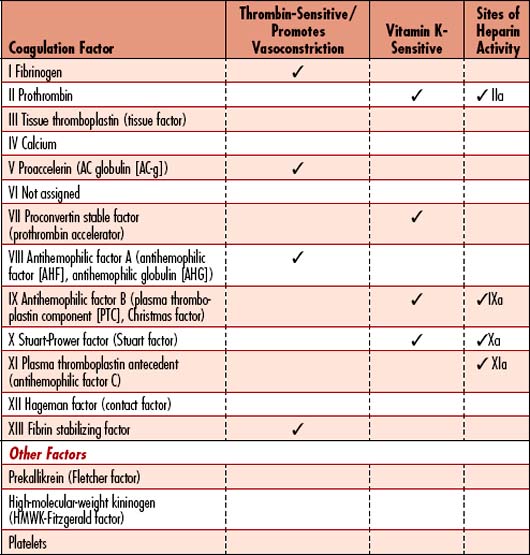
3. Activation of plasma clotting factors: Stimulation of factors that leads to the formation of a fibrin clot. The pathways that initiate clotting factors (Figure 10-3) include the following:
4. Growth of fibrous tissue: Rubbery tissue that completes the clot within approximately 7 to 10 days after injury. This process results in permanent closure of the vessel injury. Both the intrinsic and extrinsic pathways are activated after rupture of a blood vessel. Tissue thromboplastin from the vessel initiates the extrinsic pathway, while contact of factor XII and platelets with the injured vessel wall traumatizes the blood and initiates the intrinsic pathway. The extrinsic pathway is able to form clots in as little as 15 seconds with severe trauma, whereas the intrinsic pathway requires 2 to 6 minutes for clot formation. Both are necessary to maintain clot.
Heparin-induced thrombocytopenia
Pathophysiology
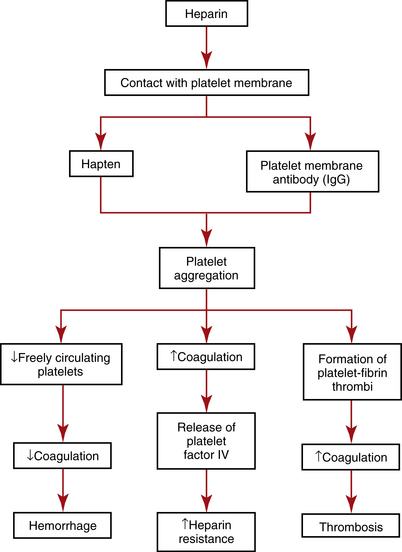
Heparin is the most widely used IV anticoagulant and one of the most frequently prescribed drugs in the United States. Heparin prevents the conversion of fibrinogen to fibrin. Heparin-induced thrombocytopenia (HIT), also called heparin-induced thrombocytopenic thrombosis (HITT), white clot syndrome, or heparin-associated thrombocytopenia (HAT), types I and II, occurs when heparin therapy causes either a mild to moderate (i.e., HAT type I) or severe (i.e., HAT type II) decrease in the number of freely circulating platelets. Platelets in affected patients exhibit unusual aggregation and can result in heparin resistance, arterial and venous thrombosis, and subsequent emboli in extreme cases (Figure 10-4). Depending on the source of the heparin received, HIT is reported in about 5% of all patients receiving heparin. Bovine (beef-based) heparin has been associated with HIT more frequently than other heparins. It is estimated that as many as 50% of patients on heparin may be asymptomatic but generate antibodies to heparin-platelet factor 4 (H-PF4), which increases the risk of HIT on their next exposure to heparin. HIT is not related to the heparin dosage and has been seen in patients receiving low-dose subcutaneous heparin, as well as in patients receiving simple heparin “flushes” to maintain patency of IV lines.
![]() Two types of HIT have been described:
Two types of HIT have been described:
• Mild to moderate, low morbidity: Generally occurs 1 to 2 days after initiation of heparin. It may resolve within 5 days after symptoms begin. Platelets may decrease to levels as low as 100,000/mm3 or may remain in the low-normal range. No treatment is required, and heparin therapy may be continued if the patient is asymptomatic.
• High morbidity (immune-mediated): Generally begins 5 to 7 days after initiation of heparin. Symptoms persist until heparin is discontinued. Platelets decrease to less than 100,000/mm3. Thrombosis with subsequent embolization and bleeding is apparent. Complications may include pulmonary emboli, myocardial infarction, cerebral infarction, and circulatory impairment resulting in limb amputations. Mortality rate is 29%. Overall, 0.6% of all patients receiving heparin therapy develop thromboembolization.
Assessment
Observation
Palpation
• Bone tenderness (especially rib and sternal areas)
• Enlargement of the liver and/or spleen
• Enlargement of the liver and/or spleen
Diagnostic Tests for Heparin-induced Thrombocytopenia
| Test | Purpose | Abnormal Findings |
| Platelet count | Used to diagnose bleeding disorders or bone marrow disease | Mild to moderate: 100,000–150,000/mm3; severe: <100,000/mm3 caused by severe clumping or aggregation of platelets |
| Bleeding time | Measures how quickly blood clots, using platelets, coagulation factors, and small vessel vasospasm | Prolonged if platelets are <100,000/mm3 |
| Platelet antibody screen | Identifies antibodies against platelets | Positive findings because of the presence of immunoglobulin G (IgG) platelet antibodies |
| Coagulation screening (prothrombin time [PT]; partial thromboplastin time [PTT], thromboplastin time) | PT – extrinsic pathway (Coumadin) PTT – intrinsic pathway (Heparin) |
Normal, because the clotting factors that govern these test results are normal |
| Fibrinogen | Measures clot formation ability: may be ordered as a follow-up to an abnormal PT or PTT and/or an episode of prolonged or unexplained bleeding | May be low-normal or low due to increased consumption. Normal is 200–400 mg/dl |
| Fibrin degradation products: FDP, FSP, fibrin split products; fibrin breakdown products | Measures fibrin degradation products (which result from clots dissolving) in blood | Elevated to ≥40 mcg/ml because of fibrinolysis of platelet-fibrin thrombi. Normal is <10 mcg/ml |
| Platelet aggregation | Measures the rate and degree to which platelets form a clump | Results will be >100% (or high value of specific laboratory) because of release of platelet membrane antibody leading to “clumping.” |
| Heparin-induced platelet aggregation | Adds patient platelet-poor plasma (as a source of immunoglobulin) to normal platelet-rich plasma in the presence of heparin to induce platelet aggregation | Reflects abnormal aggregation curve with decrease in the optical density in the aggregometer |
| Serotonin release testing and ELISA heparin PF4 | Detects the presence of antibodies to PF4/heparin/TSP-1 complexes | Help in the differential diagnosis of HIT |
| Bone marrow aspiration | Evaluates bone marrow status; diagnose blood disorders and determine if cancer or infection has spread to the bone marrow. | Normal or increased number of megakaryocytes (platelet precursors), indicative of normal production of platelets or increased response to need for platelets |
Collaborative management
Care priorities
1. Screen preheparin platelet count, and monitor platelets and amount of heparin needed:
A preheparin platelet count is made to establish baseline values. Daily platelet counts should be done for at least the first 4 days of heparin therapy. Subsequent counts are made every 2 days. If increasing amounts of heparin are needed to maintain therapeutic levels (i.e., PTT 40 to 60 seconds), heparin resistance should be suspected, which sometimes precedes HIT (see Figure 10-4).
2. Administer defibrinogenating agents if high morbidity symptoms are present:
Ancrod (Arvin) may be given to reduce the possibility of thrombosis.
3. Prevent pulmonary emboli with a vena caval filter:
If patient experiences thrombosis with decrease or loss of perfusion to an extremity, the physician or midlevel practitioner may consider surgical insertion of a vena caval filter to reduce the risk of pulmonary emboli caused by clot migration from an extremity. See Pulmonary Embolus, p. 396.
5. Consider use of newer anticoagulation agents in those who are difficult to manage:
New medications using recombinant DNA technology focus on each step of the coagulation process; many are under development. These agents are grouped into three stages of coagulation: initiation, propagation, and fibrin formation. Tissue factor pathway inhibitor (TFPI), nematode anticoagulant peptide, and factor VIIa target initiation of coagulation. Soluble thrombomodulin, drotrecogin alfa (activated protein C), protein C concentrate, fondaparinux, and idraparinux inhibit clot propagation. Direct thrombin inhibitors (antithrombins) block fibrin formation for clot completion.
6. Provide platelet transfusions for high morbidity patients who continue to bleed:
May be initiated after heparin therapy is discontinued if bleeding fails to subside.
7. Provide plasma exchange for high morbidity patients who fail to respond to other therapies:
CARE PLANS: HEPARIN-INDUCED THROMBOCYTOPENIA
related to decreased platelet count with risk of bleeding and thromboembolization
1. Assess patient at least every 2 hours for signs of bleeding, including hemoptysis, ecchymosis, petechiae on dependent areas, GI bleeding, hematuria, and bleeding from invasive procedure sites or mucous membranes. Monitor the patient closely for hemorrhage. Note Hgb and Hct levels before and after blood loss, and at least daily as indicated.
2. Assess for signs of internal bleeding including tachycardia and dysrhythmias, tachypnea and hypotension. Sustained increase in HR and RR or ECG changes, such as ST-segment depression or elevation, may precede hypotension.
3. Protect the patient from trauma that may cause bleeding. Do not use rectal temperatures to monitor for fever. Avoid IM injections and venous and arterial punctures as possible until bleeding time normalizes.
4. Perform a comprehensive assessment of peripheral circulation (e.g., check peripheral pulses, edema, capillary refill, and color and temperature of extremities) at least every 2 hours and assess patient for signs of thrombosis, including decreased peripheral pulses, altered sensation in extremities (i.e., paresthesias, numbness), pallor, coolness, cyanosis, or capillary refill time more than 2 seconds. Monitor extremities for areas of heat, pain, redness, or swelling.
5. Maintain adequate hydration to prevent increased blood viscosity and to help prevent constipation.
6. Administer stool softeners to help reduce straining with bowel movements, which may prompt rectal bleeding.
7. Monitor platelet count daily for significant changes. Consult physician or midlevel practitioner for values that remain less than 150,000/mm3 or below patient’s baseline.
8. Monitor heparin dosage carefully. If increasing doses are required to maintain a therapeutic level (PTT 40 to 60 seconds or 2 to 2.5 times patient’s baseline), consult physician or midlevel practitioner regarding possible heparin resistance, an early indicator of HIT. If heparin has been discontinued and new anticoagulants initiated, monitor appropriate values. If a direct thrombin inhibitor (e.g., Argatroban) is used alone, or in combination with warfarin, monitor PT and international normalized ratio (INR).
9. Assess patient’s neurologic status hourly if platelet count decreases to less than 30,000.
10. Monitor for signs of MODS secondary to thrombosis or prolonged hypotension, if patient has hemorrhaged. (See SIRS, Sepsis and MODS, p. 924).
11. Teach patient and significant others about the basic pathophysiology of HIT, and instruct them to report this problem to all subsequent health care providers. Teach patient to wear a medical-alert bracelet to alert health care providers if patient becomes unable to speak.
Deficient fluid volume (or risk for same)
1. Monitor patient for signs of hypovolemia, including increased HR and RR, decreased BP, increased restlessness or fatigue, and decreased urine output.
2. Administer supplemental oxygen if patient is actively bleeding.
3. Maintain accurate I&O record. Weigh daily and monitor trends.
4. Assess for intra-abdominal bleeding: note any abdominal pain, tenderness, guarding, or back pain.
5. Check excretions for occult blood, and observe for blood in emesis, sputum, feces, urine, nasogastric (NG) drainage, and wound drainage as appropriate.
6. Instruct the patient and/or family on the need for blood replacement as appropriate.
7. Replace lost volume with plasma expanders (e.g., albumin, hetastarch) or blood products as indicated. See Table 10-3 for more information.
Immune thrombocytopenia purpura (itp)
Assessment
Risk factors
• Acute ITP: History of antecedent viral infection occurring about 3 weeks before the hemorrhagic episode
• Chronic ITP: Insidious and sometimes associated with autoimmune hemolytic anemia, HIV disease, hemophilia, lymphoma, chronic lymphocytic leukemia, systemic lupus erythematosus, sarcoidosis, high-titer anticardiolipin antibodies, pregnancy, malabsorption and thyrotoxicosis. The cause is often unknown.
Observation
• Petechiae, purpura, bruising on skin and mucous membranes, prolonged bleeding; intracranial hemorrhage occurs in less than 1% of patients if the thrombocytopenia is severe.
• Diminished LOC, indicative of intracranial hemorrhage
• Epistaxis, gingival bleeding, gastrointestinal (GI), genitourinary (GU), gynecologic (GYN) bleeding such as increased menstrual flow
Palpation
| Test | Purpose | Abnormal Findings |
| Platelet count | Used to diagnose bleeding disorders or bone marrow disease | Decreased to 5000–75,000/mm3 (or lower) because of premature destruction. Normal range is 150,000–400,000/mm3. |
| Bleeding time | Measures how quickly blood clots, using platelets, coagulation factors, and small vessel vasospasm | Prolonged if platelets are less than 100,000/mm3 |
| Platelet antibody screen | Identifies antibodies against platelets | Positive findings because of the presence of IgG and IgM antiplatelet antibodies |
| Coagulation screening (prothrombin time [PT]; partial thromboplastin time [PTT]; thromboplastin time) | PT – extrinsic pathway (Coumadin) PTT – intrinsic pathway (Heparin) |
Normal, because these tests measure nonplatelet components of the coagulation pathway. |
| Complete Blood Count (CBC) with Differential | Measures RBCs, WBCs and platelets and the WBC differential | Decreased Hgb and Hct due to insidious blood loss or simultaneous hemolytic anemia (Evans syndrome) Normal WBC count: unless ITP is associated with another disease impacting differential leukocyte count. |
| Capillary fragility test: Rumpel-Leede Capillary-Fragility Test | Method to determine a patient’s hemorrhagic tendency: assesses fragility of capillary walls: used to identify thrombocytopenia. Seldom used in current practice | Will show >1+, which signals that more than 11 petechiae were present in a 2.5-cm radial area on the skin after prolonged application of a BP cuff. Normal is 1+ or <10 petechiae |
| Bone marrow aspiration | Evaluates bone marrow status; diagnose blood disorders and determine if cancer or infection has spread to the bone marrow. | Biopsy will reveal megakaryocytes (platelet precursors) in normal or increased numbers with a “nonbudding” appearance, possibly indicating defective maturation or failure of platelet production |
Collaborative management: immune thrombocytopenic purpura
Care priorities
1. Suppress immune response to reduce platelet destruction
• Corticosteroid therapy: Adrenocorticosteroids (e.g., prednisone 1 to 2 mg/kg/day) are effective in increasing the platelet count in 1 to 3 weeks after initiation of treatment. Effectiveness is attributed to suppression of phagocytic activity of the macrophage system (particularly the spleen), which increases the life span of the antibody-coated platelets. If improvement does not occur within 2 to 3 weeks, excessive doses of steroids are required, or if patient cannot tolerate tapering of steroids, splenectomy should be considered. “Normal” responders are able to have steroid dosage tapered over several weeks until platelets reach a sustained value of 50,000/mm3. Relapse during or after tapering prednisone is a common occurrence.
• IV immunoglobulin (IVIG): Given at 400 mg/kg/day for 2 to 5 consecutive days, resulting in increased platelet count in 60% to 70% of patients. Serum sickness (fever, chills, rash) is not uncommon between 9 to 14 days. It is less effective in patients with longstanding chronic ITP. The platelet level at initiation of treatment and incidence of serum sickness is not necessarily correlated to individual response. Duration of response may be longest in individuals who achieve the highest initial platelet increases.
• Danazol: 400 to 800 mg/day has resulted in complete remission or partial improvement in 60% to 70% of patients in several studies. Use is controversial because other researchers have reported poor results and many untoward side effects.
• Splenectomy: Treatment of choice in cases refractory to corticosteroid therapy. The condition stabilizes in 60% to 70% of patients who undergo splenectomy. The positive results are attributed to the removal of the site of destruction of the antibody-sensitized platelets. Prospective splenectomy candidates should have pneumococcal, meningococcal, and Haemophilus influenzae type B vaccinations before a planned splenectomy, to reduce the risk of postoperative infection with these organisms.
• Immunosuppression: Various immunosuppressive drugs, including azathioprine, cyclophosphamide, methotrexate, vincristine, and cyclosporine, given alone or in combination with prednisone, have been used successfully in limited situations. A trial of immunosuppression therapy may be indicated in patients who fail to respond to splenectomy or in those who are too unstable to be surgical candidates.
• Anti-Rh immunoglobulin: Low dose (200 to 1000 mcg) given IV for 1 to 5 days has been effective in limited studies. Success of treatment is attributed to sensitization of recipient RBCs, which results in low-grade hemolysis and blockade of the platelet destruction by the reticuloendothelial system.
• Colchicine: A small percentage of patients refractory to other treatments may improve with 1.2 mg colchicine daily for 2 weeks or longer. The drug has been used successfully in limited studies.
• Plasmapheresis: Several days of machine-assisted plasma exchange to remove approximately 1 to 1.5 times the total plasma volume per procedure and replace it with a suitable solution (e.g., colloids, crystalloids, plasma). Therapy is reserved for patients with life-threatening hemorrhage unresponsive to other measures. It is costly and of marginal benefit.
• Romiplostim: A promising treatment approved for use in patients with chronic ITP who are refractory to corticosteroids, immunoglobulins, or splenectomy. A thrombopoeitin receptor agonist that stimulates bone marrow megakaryocytes to increase platelet production; 1 mcg/kg is given as a subcutaneous injection once weekly, titrated to a maximum dose of 10 mcg/kg to achieve a platelet count of greater than 50,000. Rare side effects include bone marrow fibrosis and reticulin formation.
• Platelet transfusions: Platelets are given only in cases of life-threatening hemorrhage. The shortened platelet life span renders prophylactic transfusions ineffective.
• Vinca “alkaloid-loaded” platelets: Transfusions of platelets “loaded” with vinblastine may reduce the phagocytic destruction of platelets in patients who fail to respond to other treatments.
CARE PLANS FOR IMMUNE THROMBOCYTOPENIC PURPURA
related to decreased platelet count, resulting in increased risk of bleeding
Decreased intracranial adaptive capacity: (or risk for same)
Risk for deficient fluid volume
![]() Analgesic Administration; Pain Management; Coping Enhancement; Anxiety Reduction
Analgesic Administration; Pain Management; Coping Enhancement; Anxiety Reduction
Disseminated intravascular coagulation
Pathophysiology
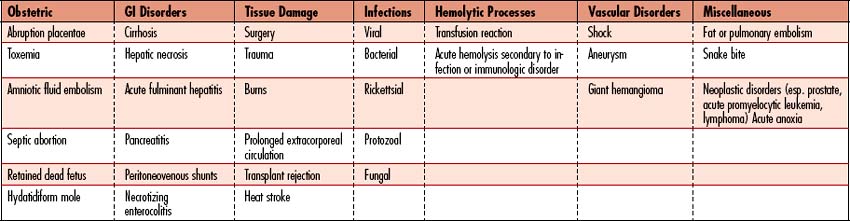
DIC is a syndrome characterized by overstimulation of the normal coagulation cascade, often related to severe sepsis or shock. DIC is a coagulopathy with potential to cause both profuse bleeding and widespread thrombosis leading to MODS. Inherent bodily control of bleeding requires a balance between procoagulants and thrombus formation, along with anticoagulants, inhibitors, and thrombolysis (see Figure 10-3, Table 10-4). The delicate balance may be upset by disease processes (Table 10-5), resulting in a cascade of uncontrolled coagulation and fibrinolysis. The abnormal clotting cascade that develops during DIC is as follows:
• Platelets and coagulation factors are activated by a disease stimulus and are rapidly consumed, particularly factors V and XIII and fibrinogen.
• Thrombin is formed very rapidly, and inherent inhibitors cannot stop the formation of the vast amounts of thrombin generated. Thrombin directly activates fibrinogen.
• Fibrin is deposited throughout the capillary beds of organs and tissues.
• The fibrinolytic system lyses fibrin and impairs thrombin formation.
• FDPs (or FSPs) result from fibrinolysis, which changes platelet aggregation and inhibits fibrin polymerization. See Figure 10-3 for the normal coagulation pathway.
Assessment
Observation
• Bleeding with abrupt onset: From invasive procedure sites and mucosal surfaces (e.g., oral, nasal, tracheal, gastric, urethral, vaginal, rectal); may include hematuria, petechiae, stools or gastric aspirate positive for occult blood, pallor, tachycardia, tachypnea, vertigo, hypotension, ecchymoses (e.g., on palate, gums, skin, conjunctivae), lethargy, irritability, or feeling of impending doom, and possible back pain and abdominal tenderness.
• Petechiae, purpura, ecchymoses, oozing blood, mottling
• Conjunctival hemorrhage and periorbital petechiae
• SOB, ST-segment elevation/depression, T-wave inversion
• Grey Turner sign (flank ecchymoses)
• Hemoptysis, tarry stools, melena
• Decreased responsiveness, confusion, altered mentation, headaches
• Abnormal thrombosis may manifest with extremity pain, diminished pulses, oliguria or anuria, diminished or absent bowel sounds, severe chest pain with SOB (indicative of either myocardial infarction or pulmonary embolism), or paresis or paralysis (indicative of cerebral thrombus)
Auscultation
| Test | Purpose | Abnormal Findings |
|---|---|---|
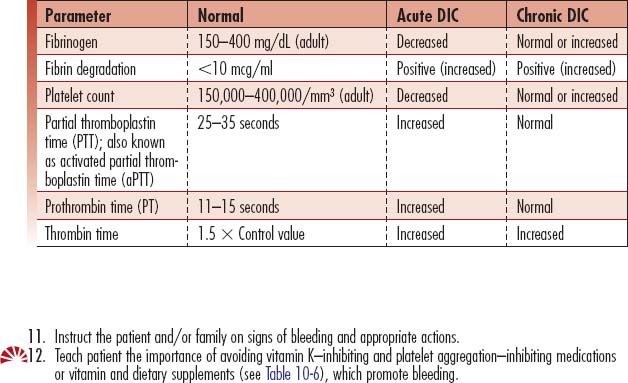
| Fibrin degradation products: FDP, FSP, fibrin split products; fibrin breakdown products | Measures fibrin degradation products (which result from clots dissolving) in blood | Increased (>10 mcg/ml) due to widespread fibrinolysis, which produces FDPs as the end product of clot lysis. Critical value: >40 ng/ml |
| D-dimer assay | Measures cleavage products of fibrin | Increased to >500 due to increased thrombin and plasmin generation. This is a rapid measurement technique, less sensitive than FDPs, and not recommended as a substitute for FDPs and fibrinogen determinations. |
| Fibrinogen | Measures clot formation ability: may be ordered as a follow-up to an abnormal PT or PTT and/or an episode of prolonged or unexplained bleeding | May remain normal or decrease in the early acute phase. As the process continues, fibrinogen levels will decrease. Normal range is 150–400 mg/dl. |
| PTT or activated partial thromboplastin time (aPTT) | Measure of the integrity of the intrinsic and common pathways of the coagulation cascade. The aPTT is the time, in seconds, for patient plasma to clot after the addition of an intrinsic pathway activator, phospholipid and calcium. | Prolonged (>40 seconds) because of activation of the intrinsic pathway, causing consumption of coagulation factors. Critical value: >70 seconds. In chronic DIC the value may be normal (25–35 seconds) or less than normal. |
| Prothrombin time (PT), International Normalized Ratio (INR) | Measure of the integrity of the extrinsic pathway of the coagulation cascade. | Prolonged (>15 seconds) because of activation of the extrinsic pathway, causing consumption of the extrinsic clotting factors. Critical value: >40 seconds. |
| Thrombin time, thrombin clotting time (TCT) | Test of the time it takes for a clot to form, measuring the conversion of fibrinogen to fibrin | Prolonged (>1.5 times the control value or >2 seconds in excess of a 9- to 13-second control value) because of rapid conversion of fibrinogen into fibrin. |
| Antithrombin III (AT-III), functional antithrombin III, antithrombin, activity and antigen | Evaluates whether the total amount of functional antithrombin is normal. Activity will be decreased with both type 1 and type 2 antithrombin deficiencies, so this test can be used as an initial screen for both. If the antithrombin activity is low, then the antithrombin antigen test is performed to determine the quantity of antithrombin present. | Decreased (<50% of control value using a plasma sample, or <80% using functional values) because of rapid consumption of this thrombin inhibitor. The action of AT-III is catalyzed by heparin. |
| Euglobulin clot lysis time (ECLT) | Measures overall fibrinolysis; measures fibrinogen activity via measurement of plasminogen and plasminogen activator, which assist in prevention of fibrin clot formations. | Decreased time is seen with DIC. Normal: lysis in 2–4 hours Critical value: 100% lysis in 1 hour |
| Platelet count | Used to diagnose bleeding disorders or bone marrow disease | Decreased (<140,000/mm3) because of rapid rate of platelet aggregation to form clots during DIC. Aggregation decreases the freely circulating platelets. |
| Alpha2-antiplasmin | Measures alpha2-antiplasmin, which is an inhibitor that regulates the fibrinolytic system primarily by blocking the enzymatic activity of plasmin | Decreased because of rapid consumption due to large amounts of plasmin generated. When all alpha2-antiplasmin is depleted, excessive hyperfibrinolysis (massive, rapid clot lysis) occurs. |
| Protamine sulfate test | Associated with the formation of excessive amounts of thrombin and secondary fibrinolysis. | Results are positive (normal: negative), indicative of presence of fibrin strands. |
| Peripheral blood smear | Microscopic examination of cells from drop of blood; investigates hematologic problems or parasites such as malaria and filaria | For visualization during microscopic examination of schistocytes and burr cells, which indicate the deposition of fibrin in the small blood vessels. |
Collaborative management: disseminated intravascular coagulation
Care priorities
1. Treat the primary cause of the disease:
![]() Aggressively treat the underlying cause. A primary disease promotes the development of DIC. If treatment of the disease fails, the mortality rate of DIC is high. When DIC occurs without apparent cause, the possibility of undiagnosed malignancy (e.g., prostate cancer or APL), a large abdominal aortic aneurysm, a progressive gram-negative bacterial infection, or hepatic cirrhosis should be explored. If the diagnosis is by laboratory tests alone, conservative management is appropriate. Other conditions and medication side effects should be considered (see Table 10-5).
Aggressively treat the underlying cause. A primary disease promotes the development of DIC. If treatment of the disease fails, the mortality rate of DIC is high. When DIC occurs without apparent cause, the possibility of undiagnosed malignancy (e.g., prostate cancer or APL), a large abdominal aortic aneurysm, a progressive gram-negative bacterial infection, or hepatic cirrhosis should be explored. If the diagnosis is by laboratory tests alone, conservative management is appropriate. Other conditions and medication side effects should be considered (see Table 10-5).
2. Manage abnormal clotting with continuous IV heparin therapy:
There are three conditions associated with DIC in which heparin may be effective:
4. Consider use of thrombolytic agents for abnormal clotting:
Use of streptokinase, urokinase, and tissue plasminogen activator (rtPA) is not indicated for patients with thrombosis because these agents may facilitate excessive bleeding.
5. Provide replacement of necessary blood components:
Clotting factors and inhibitors are replaced in the form of fresh-frozen plasma. The PT/INR may be the most accurate parameter(s) for guiding plasma replacement. Patients with markedly decreased fibrinogen levels may be given cryoprecipitate, which contains 5 to 10 times more fibrinogen than plasma contains. Thrombocytopenia in DIC may not be severe. The platelet count is usually more than 50,000/mm3. General replacement therapy guidelines indicate approximately 10 units of cryoprecipitate should be given for every 2 to 3 units of plasma. Platelet transfusions are used if the patient has impaired platelet production and profuse bleeding. AT-III concentrate has been used on a limited basis (see Table 10-3). More recently, DIC is being managed with antithrombin concentrate, activated protein C (APC) (drotrecogin alfa), tissue factor pathway inhibitor (TFPI), and synthetic serine protease inhibitors (e.g., aprotinin).
8. Manage hypotension related to heart failure, as appropriate:
If patient becomes severely hypotensive due to heart failure, the following drugs may be considered: milrinone, dobutamine, dopamine, epinephrine, and nitroprusside (see Appendix 6).
CARE PLANS: DISSEMINATED INTRAVASCULAR COAGULATION
1. Discuss bleeding history with patient or significant others. Assess prior incidences of bleeding from gums, skin, or urine; tarry/bloody stools; bleeding from muscles or into joints; hemoptysis, vomiting of blood, epistaxis, or prolonged bleeding from small wounds or after tooth extraction; or unusual bruising or tendency to bruise easily. Provide soft tooth brush.
2. Question patients about current medications, including over-the-counter (OTC) preparations, since many medications promote bleeding (Table 10-6).
3. Monitor coagulation tests daily. Consult physician or midlevel practitioner for abnormal values (Table 10-7).
4. Monitor closely for increased bleeding, bruising, petechiae, and purpura. Assess for internal bleeding by testing suspicious secretions (i.e., sputum, urine, stool, emesis, gastric drainage) for the presence of blood. Monitor for hemorrhage.
5. Monitor neurologic status (see Glasgow Coma Scale, Appendix 2) every 2 hours by assessing LOC, orientation, pupillary reaction, and movement and strength of extremities. Changes in status can indicate intracranial bleeding.
6. Use alcohol-free mouthwash and swabs for oral care to minimize gingival/gum injury. Use normal saline solution (NSS) or solution of NSS and sodium bicarbonate (500 ml NSS with 15 ml bicarbonate) to irrigate the oral cavity if irritated. Massage gums gently with a sponge-tipped applicator to help remove debris. Do not attempt to remove large clots from the mouth, to avoid profuse bleeding.
7. Use electric rather than safety razor for shaving patient.
8. Refrain from inserting objects into a bleeding orifice. Avoid taking rectal temperatures.
9. Protect the patient from trauma. Avoid unnecessary venipunctures and IM injections.
10. If patient undergoes an invasive procedure, manually hold pressure over the insertion site for 3 to 5 minutes for IV catheters and 10 to 15 minutes for arterial catheters or until bleeding subsides.
11. Instruct the patient and/or family on signs of bleeding and appropriate actions.
12. ![]() Teach patient the importance of avoiding vitamin K–inhibiting and platelet aggregation–inhibiting medications or vitamin and dietary supplements (see Table 10-6), which promote bleeding.
Teach patient the importance of avoiding vitamin K–inhibiting and platelet aggregation–inhibiting medications or vitamin and dietary supplements (see Table 10-6), which promote bleeding.
| Analgesics Nonsteroidal anti-inflammatory agents (NSAIDs) Aspirin (acetylsalicylic acid) Acetaminophen Antipyrine Ibuprofen Indomethacin Fenoprofen Sodium salicylate Antirheumatic agents Oxyphenbutazone Phenylbutazone Hydroxychloroquine Gold salts Antimicrobials Ampicillin Cephalothin Methicillin Penicillin Pentamidine Streptomycin Sulfonamides (antibiotics) Chloramphenicol Isoniazid Nitrofurantoin Rifampin Trimethoprim Anticoagulants Heparin Enoxaparin Dalteparin Thrombolytics Alteplase Reteplase Streptokinase Urokinase Anisoylated plasminogen streptokinase Tenecteplase |
Diuretic Agents Sulfonamide derivatives Acetazolamide Chlorpropamide Chlorothiazide Chlorthalidone Clopamide Diazoxide Furosemide Bumetanide Hydrochlorothiazide Tolbutamide Spironolactone Mercurial diuretics Glycoprotein IIb/IIIa inhibitors Abciximab Eptifibatide Tirofiban Phenothiazines Chlorpromazine Promethazine Trifluoperazine Phosphodiesterase inhibitors Caffeine Dipyridamole Theophyllines Antiplatelet drugs Aspirin (acetylsalicylic acid) Ticlopidine Clopidogrel Prostaglandins I2 D2 E Sedative-hypnotics Benzodiazepines Clonazepam Diazepam Vasodilators Nitroglycerin Nitroprusside |
Other Antihistamines Ethanol Heparin Beta adrenergic blocking agents General anesthetics Local anesthetics Chemotherapeutic agents Vitamin E Estrogens Digitoxin Cimetidine Levodopa Propylthiouracil |
| Salicylates Aspirin and aspirin-combination drugs Other salicylates Coumarins Anisindione Dicumarol Warfarin |
Broad-Spectrum Antibiotics Sulfonamides Triple sulfa Sulfamethoxazole Sulfasalazine Sulfisoxazole Sulfamethoxazole-trimethoprim Clindamycin Gentamicin Neomycin Tobramycin Vancomycin Imipenem Cefamandole Cefoxitin |
Vitamins A E |
![]() Infection Control; Infection Protection; Surveillance: Safety
Infection Control; Infection Protection; Surveillance: Safety
Risk for deficient fluid volume
related to bleeding/hemorrhage
1. Monitor every 2 hours for increases in HR and RR, decreased BP, and decreasing pulse pressure.
2. Measure urinary output every 2 to 4 hours. Consult physician or midlevel practitioner for output less than 0.5 ml/kg/hr.
3. Maintain accurate I&O record. Weigh daily, and monitor trends.
4. Increase measurement of vital signs and urine output to at least every 30 minutes for active bleeding. For profuse bleeding, check vital signs at least every 15 minutes. Inspect invasive procedure sites and dressings for bleeding.
5. Monitor CBC daily for significant alterations in Hct, Hgb, and platelets (Table 10-8).
6. Ensure that patient has typed and cross-matched blood available for transfusion.
7. Monitor coagulation studies (PT, PTT, fibrinogen, FDP/FSP, platelet counts), as appropriate.
8. Assess for signs of impending shock if the following signs are noted: increased HR and RR; decreased BP; or pallor, diaphoresis, cool extremities, delayed capillary refill, decreased pulse amplitude, restlessness, or disorientation.
9. Maintain at least one 18-gauge or larger IV catheter for use during shock management, at which time rapid infusion of blood products or IV fluids may be necessary.
| Parameters | Common Measurement Value | Population |
|---|---|---|
| Red blood cells (RBCs) | 4–5.5 million/mm3 4.5–6.2 million/mm3 |
Adult females Adult males |
| Hemoglobin (Hgb) | 12–16 g/dl 14–18 g/dl |
Adult females Adult males |
| Hematocrit (Hct) Mean corpuscular volume (MCV) Mean cell hemoglobin (MCH) Mean cell hemoglobin concentration (MCHC) White blood cells (WBCs) |
37%–47% 83–93 mcg3 26–34 pg 31%–38% 4,500–11,000/mm3 |
Adult females Adults Adults Adults Adults |
| Differential White Blood Cells (Granulocytes) | ||
| Segmented neutrophils (Segs) Band neutrophils (Bands) Eosinophils (Eos) Basophils (Basos) Monocytes (Monos) Lymphocytes (Lymphs) Platelets |
54%–62% 3%–5% 1%–3% 0–0.75% 3%–7% 25%–33% 150,000–400,000/mm3 |
Adults Adults Adults Adults Adults Adults Adults |
![]() related to blood loss or presence of microthrombi
related to blood loss or presence of microthrombi
1. Assess and document peripheral perfusion every 2 hours, including temperature, sensation, pulses, and movement in extremities. Perform a comprehensive assessment of peripheral circulation (i.e., check peripheral pulses, edema, capillary refill, and color and temperature of extremities).
2. Monitor vital signs frequently. Evaluate chest pain. Document cardiac dysrhythmias.
3. Monitor BP and assess for early signs of perfusion deficit at least every 2 hours, including dizziness, confusion, and decreased urinary output.
4. Monitor for decreased myocardial or pulmonary perfusion as evidenced by chest pain, ST-segment depression or elevation, T-wave inversion, SOB, dyspnea, and decreased oxygen saturation using continuous pulse oximetry.
5. Monitor CVP, observing for both high and low readings. Decreased pressures are indicative of hypovolemia/hemorrhage. Anticipate vasoconstriction with hypovolemia: CO may increase or decrease from normal range of 4 to 7 L/min, depending on cardiac contractility.
6. Monitor GI status by observing tolerance to diet or tube feedings, bowel habits (e.g., constipation, diarrhea), character of stool (e.g., tarry, bloody), and presence or absence of bowel sounds.
Impaired gas exchange (or risk for same)
related to loss of oxygen-carrying capacity through hemorrhage or pulmonary microembolus formation
Respiratory Status: Gas Exchange
1. Assess respiratory status every 2 hours, noting rate, rhythm, depth, and regularity of respirations. Provide supplemental oxygen as appropriate.
2. Monitor for signs of respiratory failure: restlessness, anxiety, and air hunger indicative of hypoxemia; ABG values for increased PaCO2 and decreased pH indicative of hypoventilation; or oxygen saturation less than 95% via pulse oximetry indicative of decreased ventilation. Consult physician or midlevel practitioner if signs of respiratory insufficiency are present.
3. Monitor ScVO2 or SVO2: steady increase or decrease from patient’s normal level may indicate deterioration.
4. Assess lungs for bibasilar crackles (rales), indicative of pulmonary edema.
5. Monitor patient’s respiratory secretions. Institute respiratory therapy treatments as needed.
6. Monitor for pulmonary embolus, including sharp, stabbing chest pain, dyspnea, pallor, cyanosis, pupillary dilation, rapid or irregular pulse, profuse diaphoresis, and anxiety. Assess need for supplemental oxygen, and consult physician immediately. Patients with severe pulmonary emboli may require mechanical ventilation. See Pulmonary Embolus, p. 396.
7. Assess patient for changes in sensorium (i.e., confusion, lethargy, somnolence), indicative of inadequate cerebral oxygenation or CO2 retention (respiratory insufficiency).
related to blood product administration
1. Check blood to be transfused with another professional to ensure the patient receives the correct blood. Verify the following: patient’s name, patient’s birthday and hospital number, blood unit number, blood expiration date, blood group, and blood type.
2. Discuss signs and symptoms of potential reaction with patient and family. Premedicate if there is a history of reaction or antibodies are present.
3. When blood products are being infused, check vital signs every 15 minutes for the first hour. Check patient frequently throughout the first 15 minutes of the transfusion to observe for signs of an acute hemolytic transfusion reaction, including fever, chills, dyspnea, hypotension, flushing, tachycardia, back pain, hematuria, mentation changes, and shock.
4. Observe for transfusion reactions throughout the transfusion and during the 8-hour period afterward. If a transfusion reaction (Table 10-9) occurs, implement the following:
5. If an intravascular hemolytic reaction is confirmed, implement the following:
6. With massive transfusion for severe bleeding, manage hypocalcemia caused by citrated blood, hyperkalemia of uncertain etiology, hypothermia from refrigerated blood, ARDS, coagulopathy, and hemochromatosis (iron overload).
| Type | Symptoms | Time Frame |
|---|---|---|
| Acute intravascular hemolytic | Fever, chills, dyspnea, tachycardia, hypotension, back pain, flushing, hematuria, shock | After start of transfusion within 5–30 minutes |
| Acute extravascular hemolytic | Fever, elevated bilirubin, unusually low posttransfusion hematocrit and hemoglobin | Usually within 8 hours Delayed: 7–10 days |
| Allergic (mild) | Rash, hives pruritus | Within 1 hour |
| Anaphylactic | Dyspnea, shortness of breath, bronchospasms, tachycardia, flushing, hypotension, shock | Within 30 min–1 hour |
| Febrile | Fever, chills | Within 4 hours |
| Hypervolemic | Dyspnea, tachycardia, bibasilar crackles, jugular venous distention, possible hypertension, headache | Within 1–2 hours |
| Septic | Fever, chills, tachycardia, hypotension, vomiting, shock, muscle pain, cardiac arrest | Within 5 minutes–4 hours |
![]() Bleeding Precautions; Bleeding Reduction; Blood Products Administration
Bleeding Precautions; Bleeding Reduction; Blood Products Administration
Additional nursing diagnoses
The uncontrolled bleeding related to DIC can be terrifying to the patient and significant others, who may fear that the patient will die. Refer to nursing diagnoses in Emotional and Spiritual Support of the Patient and Significant Others, p. 200. For patients who manifest activity intolerance, see that nursing diagnosis in Prolonged Immobility, p. 149. For patients with sepsis or septic shock, see SIRS, Sepsis and MODS, p. 924.
Abraham J. Romiplostim for treating thrombocytopenia in chronic idiopathic thrombocytopenia purpura. Commun Oncol. 2008;5(12):651.
American Society of Anesthesiologists. Practice guidelines for perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208.
Circular of information: For the use of human blood and blood componentswww.aabb.org/-Content/About_Blood/Circulars_of_Information/aabb_coi.htm
Gentry CA, Gross KB, Sud B, Drevets DA. Adverse outcomes associated with the use of drotrecogin alfa (activated) in patients with severe sepsis and baseline bleeding precautions. Crit Care Med. 2009;37(1):19–25.
Goldman L, Ausiello D, editors. Cecil Medicine, 23rd ed, Philadelphia, PA: Saunders Elsevier, 2007. chap 164
Gould S, Cimino M, Gerber D. Packed red cell transfusion in the intensive care unit: limitations and consequences. Am J Crit Care. 2007;16(1):39.
Hebert P, Tinmouth A, Corwin H. Controversies in RBCc transfusion in the critically ill. Chest. 2007;131:1583–1590.
Kelton J, Warkentin T. Heparin induced thrombocytopenia: a historical perspective. Blood. 2008;112(12):2607.
Klein H, Spahn D, Carson J. Red blood cell transfusion in clinical practice. Lancet. 2007;370:415–426.
Lim B, Henry D. Stroke syndrome secondary to hypercoagulability of lung cancer. Commun Oncol. 2008;5(11):595.
Marik P, Corwin H. Efficacy of red blood cell transfusion in the critically ill, a systematic review of the literature. Crit Care Med. 2008;36:2667–2674.
McMahon B. Malignancy-associated thrombosis. Oncol Iss. 2009;24(1):20.
Murphy G, Reeves B, Rogers C, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–2552.
Practice guidelines for blood transfusion: a compilation from recent peer-reviewed literature. http://www.redcross.org/www-files/Documents/WorkingWiththeRedCross/practice-guidelinesforbloodtrans.pdf
Reeves B, Murphy G. Increased morality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Anaesthesiol. 2008;21:669–673.
Rome S, Doss D, Miller K, et al. Thromboembolic events associated with novel therapies in patients with multiple myeloma: consensus statement of the IMF nurse leadership board. Clin J Oncol Nursing. 2008;12(3, suppl):21.
Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition -and management of anaphylaxis: summary report, Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397.
Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2008;121(2 suppl):S402-S407.
Simons FE. Anaphylaxis: Recent advances in assessment and treatment. J Allergy Clin Immunol. 2009;124(4):625–636.
Yap C, Lau L, Krishnaswamy M, et al. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86:554–559.