Hematologic and Immunologic Systems
SYSTEMWIDE ELEMENTS
(a) Spongy center of the bones where the hematologic and immunologic cell lines originate and mature before being released into the circulation
(b) Present throughout the bones of the body, although the majority of the cells are produced in the vertebrae, ribs, sternum, pelvis, and proximal epiphyses of the femur and humerus
(a) Located in the upper right quadrant of the abdomen in the peritoneal space below the diaphragm and under the rib cage. The liver receives 27% of the resting cardiac output—approximately 1350 ml of blood flow each minute—via the hepatic artery and portal vein.
(b) Synthesizes various plasma proteins, including clotting factors and albumin. In addition, the liver clears damaged and nonfunctioning red blood cells (RBCs), or erythrocytes, from circulation.
i. Bone marrow (see preceding description)
(a) The thymus is a bilobed lymphoid organ located in the mediastinum below the thyroid. Early in life, lymphocytes released from the bone marrow migrate to the thymus, where they mature into T cells before being released into the circulation.
(b) During fetal development and throughout the first 2 years of life, the thymus grows rapidly. After puberty the thymus slowly involutes as the circulating, long-lived T-cell population is maximized.
iii. The lymph system is a separate vessel system that collects plasma and leukocytes that are not returned to the circulatory system from the tissue capillary beds. This lymph fluid is filtered and returned to the circulatory system, so that appropriate tissue fluid pressures are maintained and edema is prevented. Lymph fluid is propelled along the system by the normal contraction of skeletal muscles.
(a) Lymph fluid is a pale yellow liquid made up of plasma, leukocytes, enzymes, and antibodies; it lacks clotting factors and thus coagulates very slowly
(b) Lymphatic capillaries and vessels are a network of open-ended tubes with one-way valves that collect lymph fluid from the tissues and eventually return it to the venous system via both the right lymphatic duct, which drains into the right subclavian vein, and the thoracic duct, which drains into the left subclavian vein
(c) Lymph nodes are small, flat, bean-shaped patches of tissue located along the length of the lymphatic system that filter microorganisms from the lymph fluid before it is returned to the bloodstream
(1) Lymph nodes can become swollen with white blood cells (WBCs), or leukocytes, that are responding to invading microorganisms if an infectious process is occurring in the area drained by the lymph node
(2) Lymph nodes can also become swollen with metastatic cancer cells that have migrated away from the primary site and become trapped in the network of the lymph node
iv. The spleen is a lymphoid organ located in the upper left quadrant of the abdomen that clears damaged or nonfunctioning RBCs and filters antigens from the blood for evaluation by lymphocytes
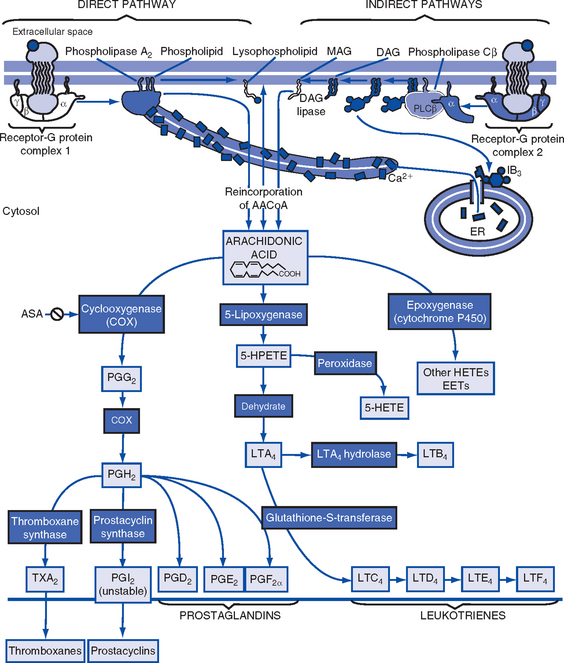
b. Components: See Tables 7-3, 7-4, and 7-5, and Figure 7-3
TABLE 7-4

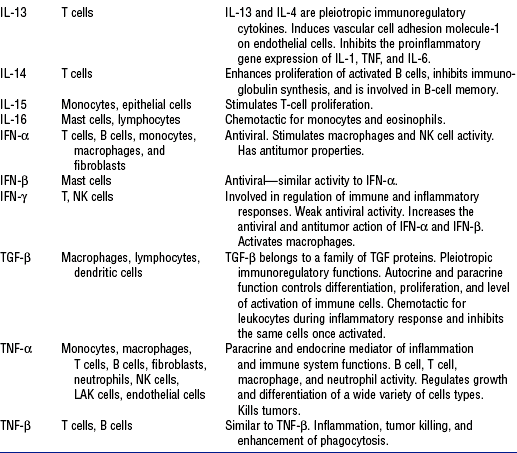
From Rankin JA: Biological mediators of acute inflammation, AACN Clin Issues 15(1):6, 2004.
TABLE 7-5
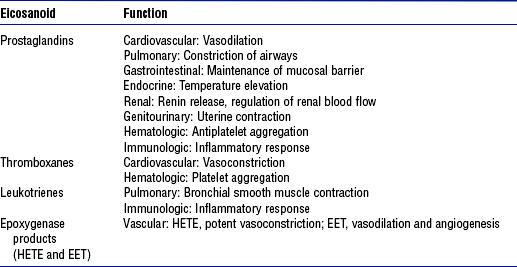
Data from Boron W, Boulpaep EL: Medical physiology, Philadelphia, 2003, Saunders. EET, Cis-epoxyeicosatrienoic acid; HETE, hydroxyeicosatetraenoic acid.
Patient Assessment
i. Many times a hematologic or immunologic problem is identified when the patent seeks medical attention for some other reason
ii. Elements of the medical history indicating a potential or existing hematologic or immunologic problem include the following:
(a) Recent, recurrent, or chronic infections
(b) Cancer or prior treatment for cancer
(c) Human immunodeficiency virus (HIV) infection
(g) Any prolonged bleeding or delayed healing with prior surgeries and/or dental extractions
(h) Receipt of a blood transfusion
(j) Placement of a prosthetic heart valve
(k) Placement of an indwelling venous access device, which indicates that the patient needed long-term venous access
iii. Review of systems with the patient and/or family for signs and symptoms
(a) General: Fatigue, weakness, lethargy, malaise, fever, chills, night sweats, dyspnea, restlessness, apprehension, pain, altered mental status, vertigo, dizziness, confusion
(b) Skin: Pruritus, change in skin color, rash, unusual bruising, ulcers or other lesions
(c) Head and neck: Headache, change in vision, sinus pain, epistaxis, gingival bleeding, sore throat, pain with swallowing, enlarged lymph nodes
(d) Respiratory: Cough, hemoptysis, dyspnea, orthopnea
(e) Cardiovascular: Palpitations, dizziness with position changes
(f) Gastrointestinal: Change in eating habits, anorexia, abdominal fullness, nausea, vomiting, hematemesis, change in bowel habits, hematochezia, melena, pain with defecation, change in weight
(g) Genitourinary: Hematuria, pain with urination, menorrhagia, enlarged inguinal lymph nodes
(h) Musculoskeletal: Swelling of joints, tenderness or pain in the bones or joints
b. Family history indicating a potential hematologic or immunologic problem: Hemophilia, sickle cell anemia, cancer, or death of a relative at a young age for reasons other than trauma
c. Social history and habits that may assist with the diagnosis and treatment of the underlying condition, including the following:
i. Any unusual or excessive exposure to chemicals (e.g., gasoline, benzene, solvents, glues, paints, varnishes) or radiation (e.g., x-rays) at work or in pursuit of a hobby
ii. Any unusual dietary preferences, pica
iii. Excessive alcohol consumption
iv. Sexual preference, number of partners, history of sexually transmitted diseases, current contraceptive method, use of safe sex practices
i. Current medications or a recent change in medication may suggest an underlying hematologic or immunologic problem. Always ask about over-the-counter medication use, because many of these preparations contain aspirin or nonsteroidal antiinflammatory drugs (NSAIDs).
ii. Many medications used to treat nonhematologic and nonimmunologic problems can affect the hematologic and immunologic systems; examples of these drugs are the following:
(a) Analgesics and antiinflammatory drugs
(1) Chloramphenicol (Chloromycetin)
(3) Paraaminosalicylic acid (PAS)
(6) Trimethoprim-sulfamethoxazole (TMP-SMX, Bactrim, Septra)
(e) Antidiabetic agents, such as chlorpropamide
(f) Antineoplastic chemotherapy agents, such as
(g) Antipsychotic agents, such as clozapine (Clozaril)
(h) Antirheumatic agents, such as
(i) Cardiovascular agents, such as
(j) Diuretics, such as chlorothiazide (Diuril)
(l) Immunosuppressives, such as
2. Nursing examination of patient
(a) Temperature: Exceeds 101° F (38.3° C)
(b) Skin: Pallor, jaundice, flushing, rash, petechiae, purpura, ecchymoses, hematomas, urticaria, integrity
(c) Head and neck: Integrity of mucosal membranes, tongue appearance (e.g., smooth, coated), conjunctival bleeding
(d) Chest: Shortness of breath, hemoptysis
(e) Abdomen: Vomiting, hematemesis, hematuria, diarrhea, melena
3. Appraisal of patient characteristics: Patients needing acute or life-saving care for hematologic disorders or for immunologic compromise come to critical care units as a result of their comorbidities or primary complications. The need for critical care may be brief or extended with quick to no recovery. Many hematologic and immunologic disorders are incurable. Occasionally, the focus is anticipated end-of-life care, although in no case is one able to predict with any certainty. Some patient characteristics that the nurse needs to assess for this population are the following:
i. Level 1—Minimally resilient: A frail 27-year-old young man with acquired immunodeficiency syndrome (AIDS) dementia, severe stomatitis, and chronic diarrhea, who is unresponsive to antiretroviral therapy
ii. Level 3—Moderately resilient: A 55-year-old woman with newly diagnosed acute lymphocytic leukemia following her second bone marrow transplant due to failed engraftment of the original donor stem cells
iii. Level 5—Highly resilient: An 18-year-old man with a hemoglobin level of 8.0 g/dl recovering from a hypovolemic hemorrhage related to a table saw accident at his family’s farm
i. Level 1—Highly vulnerable: A 45-year-old man with acute myelogenous leukemia who is neutropenic and has a lung abscess and thrombocytopenia
ii. Level 3—Moderately vulnerable: A 36-year-old man, who is otherwise healthy, experiencing a type I hypersensitivity reaction to poison ivy hidden in the brush he was removing
iii. Level 5—Minimally vulnerable: A petite 40-year-old woman with vitamin B12 deficiency anemia and alcoholism
i. Level 1—Minimally stable: A 78-year-old widowed retired Air Force colonel who develops a viral pneumonia and confusion on the second day after admission for induction chemotherapy for acute lymphocytic leukemia
ii. Level 3—Moderately stable: A 59-year-old housewife diagnosed with thrombocytopenia who has a platelet count of 60,000/mm3
iii. Level 5—Highly stable: A 28-year-old professor with lymphoma recovering in contact isolation from a methicillin-resistant Staphylococcus aureus toenail infection
i. Level 1—Highly complex: A 48-year-old mother of four who develops disseminated intravascular coagulation (DIC) after a bilateral mastectomy and transversus rectus abdominis myocutaneous (TRAM) flap surgical reconstruction
ii. Level 3—Moderately complex: A 19-year-old man with a diagnosed HIV infection that he wishes to be kept confidential from his parents and siblings
iii. Level 5—Minimally complex: A 35-year-old accountant in whom autoimmune thrombocytopenic purpura (ATP) is diagnosed following a prolonged case of the flu
i. Level 1—Few resources: A 47-year-old housekeeper with rheumatoid arthritis who provides the sole income for a family of six and receives a diagnosis of leukemia
ii. Level 3—Moderate resources: A 32-year-old Spanish-speaking migrant worker who contracted hepatitis C after a blood transfusion and has a bilingual sister who is a United States citizen and a social worker
iii. Level 5—Many resources: A 49-year-old chief executive officer of a major computer software company who is hospitalized for maintenance chemotherapy
i. Level 1—No participation: An obese 26-year-old woman in anaphylactic shock with pulmonary edema and oral intubation
ii. Level 3—Moderate level of participation: A 67-year-old salesman who is learning about home care for a Hickman catheter
iii. Level 5—Full participation: A 32-year-old woman with AIDS who is compliant with antiretroviral therapy, exercise, and diet recommendations
g. Participation in decision making
i. Level 1—No participation: A 30-year-old children’s tennis coach who chooses to let God’s will be done after the physician team decides not to render further treatment for advanced cancer of the thoracic spine
ii. Level 3—Moderate level of participation: A 56-year-old Mediterranean man who defers to his wife for decisions about treating his blood disorder
iii. Level 5—Full participation: A 37-year-old kindergarten teacher who asks to know platelet counts before consenting to each platelet transfusion
i. Level 1—Not predictable: An elegant 82-year-old grandmother with newly diagnosed chronic myelocytic leukemia
ii. Level 3—Moderately predictable: A 29-year-old day care worker receiving a second bone marrow transplant (BMT) after complete engraftment of the primary BMT stem cells
iii. Level 5—Highly predictable: A 27-year-old man with cognitive and behavioral challenges from AIDS dementia
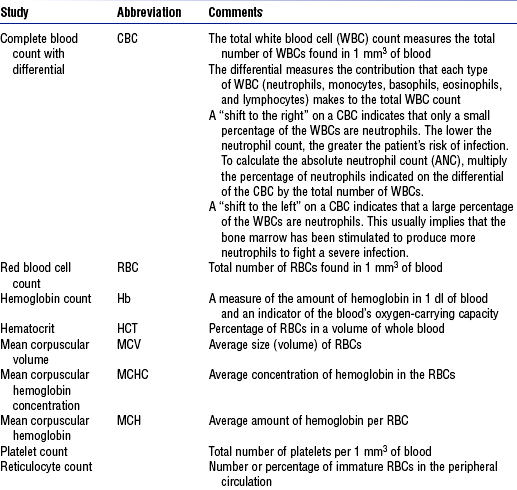
a. Laboratory: See Table 7-7 for normal values
TABLE 7-7
| Laboratory Test | Reference Values | Description |
| (White blood cell (WBC) count | 4500-10,000/mm3 | Total number of leukocytes |
| Differential WBC: | Part of CBC; indicates distribution of five types of leukocytes | |
| Neutrophils | 2500-7000/mm3 | |
| Segments | 2500-6500/mm3 | |
| Bands | 0-500/mm3 | |
| Monocytes | 200-600/mm3 | |
| Basophils | 40-100/mm3 | |
| Eosinophils | 100-300/mm3 | |
| Lymphocytes | 1700-3500/mm3 | |
| Red blood cell (RBC) indices: | Erythrocyte indicators for anemia | |
| RBC count | ||
| Men | 4.6-6.0 million/mm3 | |
| Women | 4.0-5.0 million/mm3 | |
| Mean corpuscular volume (MCV) | 80-98 mm3 | Indicates size of RBC |
| Mean corpuscular hemoglobin (MCH) | 27-31 pg | Indicates weight of hemoglobin in RBC |
| Mean corpuscular hemoglobin concentration (MCHC) | 32%-36% | Hemoglobin per volume RBC |
| RBC distribution width (RDW) | 11.5-14.5 Coulter S | Size (width) difference of RBCs |
| Hemoglobin (Hb) level | Iron composition of RBC for oxygen-carrying capability | |
| Men | 13.5-17 g/dl | |
| Women | 11.2-115 g/dl | |
| Hematocrit (HCT) of blood | Measure of the percentage of the total blood volume that is made up by RBCs | |
| Men | 40%-54% | |
| Women | 36%-46% | |
| Panic value | <15% and >60% | |
| Reticulocyte count | 0.5%-1.5% | Indicator of bone marrow activity |
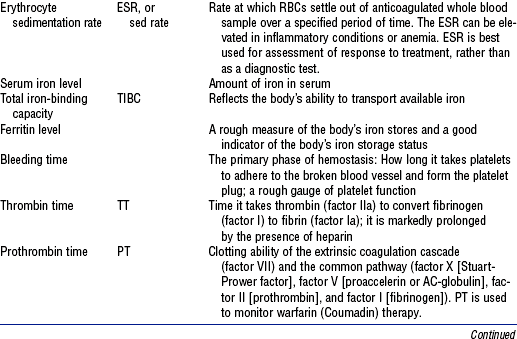
| Erythrocyte sedimentation rate | Rate at which erythrocytes settle (sediment) in unclotted blood | |
| Men | 0-9 mm/hr (Wintrobe method) | |
| Women | 0-15 mm/hr (Wintrobe method) | |
| Serum ferritin level | An indicator of protein stores of iron in the tissues, where 1 ng/ml ferritin = 8 mg stored iron | |
| Men | 15-445 ng/ml | |
| Women | 10-235 ng/ml | |
| Postmenopausal | 15-310 ng/ml | |
| Total iron-binding capacity (TIBC) | 250-450 mg/dl | Total (maximum) iron-binding capacity of transferrin for transport of iron to marrow for hemoglobin synthesis |
| Platelet count (PLT) | 150,000-400,000/mm3 | Measure of thrombocytes available for coagulation of blood |
| Fibrin split products (FSP) | 2-10 mg/ml | Indicator of fibrin degradation products acting as anticoagulant in continuous bleeding associated with hemorrhage |
| Clotting times | 10-13 sec | Measures clotting factor ability |
| Prothrombin time (PT) | ||
| Partial thromboplastin time (PTT) | 60-70 sec | Detects deficiencies in clotting factors |
| International normalized ratio (INR) | 2.5-3.5 | Standard for warfarin-sensitive PT |
Data from Kee JL: Laboratory diagnostic tests with nursing implications, ed 5, Stamford, Conn, 1999, Appleton & Lange.
ii. Sputum culture: Detects and identifies microorganisms in the sputum
(a) Urinalysis can detect gross amounts of blood or protein in the urine
(b) Urine cultures detect and identify microorganisms in the urine
(c) Urine protein electrophoresis determines the levels of proteins excreted in the urine, particularly the levels of immunoglobulins
iv. Stool occult blood test (Hemoccult): Detects microscopic amounts of blood in the stool
i. Spleen ultrasonography is used to estimate the size of the spleen
ii. In a liver-spleen scan, a radioactive tracer is used to evaluate the size as well as the function of the liver and spleen
iii. In a gallium scan, a radioactive tracer is used to detect the presence of malignant tissue, particularly malignant lymphoid tissues
iv. In a lymphangiogram, contrast dye is used to radiologically visualize the lymph system, particularly the size and architecture of lymph nodes
i. Bone marrow biopsy includes aspiration of bone marrow fluid and removal of a needle core biopsy sample of the bone marrow tissue for pathologic examination
ii. In a lymph node biopsy, one or more lymph nodes are removed for pathologic examination
d. Skin tests: Barometers of immune functioning, pointing out hyposensitivities or hypersensitivities to a particular antigen. Examples of allergens used in skin testing are allergenic extracts (e.g., dust, pollen, animal dander); purified protein derivative (PPD) for tuberculin skin tests; mumps virus; Candida albicans; and skin fungi.
Patient Care
1. Susceptibility to infection
i. Immune compromise and/or coagulopathy increases patient risk for opportunistic and host infections
ii. Clinical findings include reports of fever, chills, night sweats, sore throat, cough, malaise, pain with swallowing, pain with urination, pain with defecation, diarrhea, reddened areas, sore areas, and swollen areas (these symptoms may not be present if the patient is neutropenic and unable to mount a WBC response); flushing, lethargy, skin warm to touch; abnormal vital signs—temperature exceeding 101° F (38.3° C), hypotension, tachycardia; WBC count lower than 1500/mm3, absolute neutrophil count (ANC) lower than 500/mm3
i. Vital signs within normal limits for the patient
ii. Absence of signs or symptoms of active infection
iii. Maintenance of the WBC count within an acceptable range
iv. Patient’s and family’s verbalization of an understanding of the underlying pathology and infection prevention
c. Collaborating professionals on health care team
d. Interventions: See Table 7-9
TABLE 7-9
Leukocyte Intervention Activity Bundle
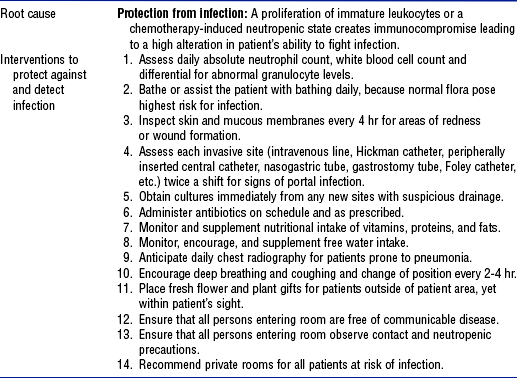
From Schneider S: Interventions for hematologic problems. In Ignatavicius DD, Workman ML, editors: Medical-surgical nursing: critical thinking for collaborative care, ed 4, Philadelphia, 2002, Saunders.
2. Increased risk for hemorrhage
i. Disease and/or treatment creates an altered state of protection against bleeding
ii. Clinical findings include reports of unusual bruising, prolonged bleeding, hematemesis, hemoptysis, hematochezia, melena, hematuria, menorrhagia
i. No evidence of spontaneous bleeding
ii. Maintenance of platelet counts and levels of clotting factors within an acceptable range
iii. Patient’s and family’s verbalization of an understanding of the underlying pathology and bleeding precautions
c. Collaborating professionals on health care team
d. Interventions: See Table 7-10
TABLE 7-10
Erythrocyte Intervention Activity Bundle

From Schneider S: Interventions for hematologic problems. In Ignatavicius DD, Workman ML, editors: Medical-surgical nursing: critical thinking for collaborative care, ed 4, Philadelphia, 2002, Saunders.
3. Impaired respiratory gas transport: See Chapter 2
4. Impaired fluid volume regulation (see also Chapter 5)
i. Fever, vomiting, diarrhea, hemorrhage, and shock deplete body fluid volume
ii. Clinical findings include reports of thirst, sweating, vomiting, polyuria, diarrhea, lightheadedness; pallor, mucosal dryness, loss of skin turgor, decreased venous filling; abnormal vital signs—fever, tachycardia, hypotension, changes in orthostatic vital signs; decreased urine output and concentrated urine with a specific gravity exceeding 1.020; altered mental status
c. Collaborating professionals on health care team
d. Interventions: See Table 7-11
TABLE 7-11
Dehydration Intervention Activity Bundle

From Schneider S: Interventions for hematologic problems. In Ignatavicius DD, Workman ML, editors: Medical-surgical nursing: critical thinking for collaborative care, ed 4, Philadelphia, 2002, Saunders.
i. Administer IV fluids and blood products as prescribed
(a) Transfuse blood products (Table 7-12)
TABLE 7-12
Indications for Treatment with Blood Components
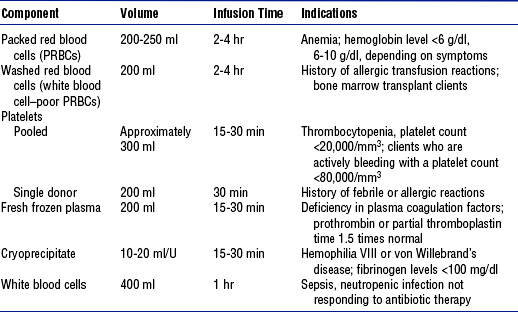
From Schneider S: Interventions for clients with hematologic problems. In Ignatavicius DD, Workman ML, editors: Medical-surgical nursing: critical thinking for collaborative care, ed 4, Philadelphia, 2002, Saunders, chap 40.
(b) Keep in mind special considerations related to blood product administration (Table 7-13 and Box 7-1)
TABLE 7-13
Types of Blood Transfusion Reaction
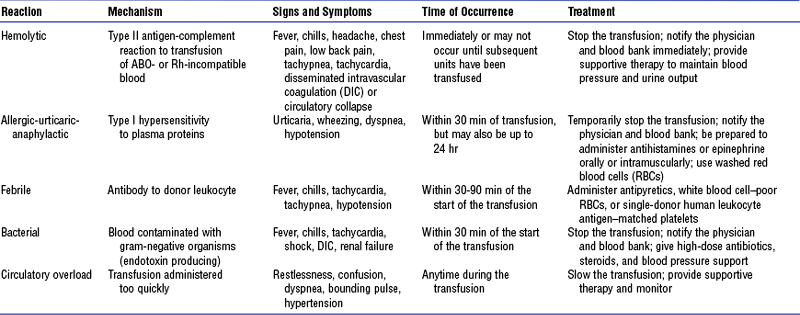
Data from Hankins J, Lonsway RAW, Hedrick D, et al: Infusion therapy in clinical practice, ed 2, St Louis, 2001, Saunders; and Ignatavicius DD, Workman ML, editors: Medical-surgical nursing: critical thinking for collaborative care, ed 4, Philadelphia, 2002, Saunders.
ii. Treat underlying condition as prescribed
iii. Encourage oral fluid intake as the patient’s condition allows
iv. Monitor fluid balance with recording of intake and output and daily weights
i. Potential for activity intolerance related to disease or the treatment of disease
ii. Clinical findings include reports of fatigue, weakness, malaise, inability to sleep, and inability to concentrate; changes in vital signs with activity
i. Vital signs within normal limits for the patient
ii. Patient’s ability to accomplish the activities of daily living without tachycardia or hypotension
c. Collaborating professionals on health care team
d. Interventions: See Table 7-14
TABLE 7-14
Fatigue Intervention Activity Bundle

From Schneider S: Interventions for clients with hematologic problems. In Ignatavicius DD, Workman ML, editors: Medical-surgical nursing: critical thinking for collaborative care, ed 4, Philadelphia, 2002, Saunders; and National Comprehensive Cancer Network: Practice guidelines for cancer related fatigue, 2003, retrieved May 24, 2004, from http://www.nccn.org.
SPECIFIC PATIENT HEALTH PROBLEMS
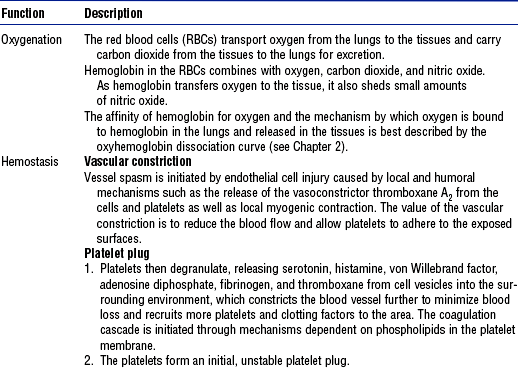
a. Anemia is a reduction in the number of RBCs, the quantity of hemoglobin, or the volume of RBCs. Because the main function of RBCs is oxygenation, anemia results in varying degrees of hypoxia. The body compensates for anemia by increasing cardiac output and respiratory rate, by redistributing blood to sustain blood supply to the brain and heart through a reduction in blood supply to the skin, gut, and kidneys, and by increasing the kidney’s production of erythropoietin to stimulate erythropoiesis.
b. Acute blood loss, such as with arterial rupture, dramatically changes the body’s hemodynamic status and necessitates emergency intervention. With chronic blood loss occurring over weeks or months, such as in slow gastrointestinal bleeding or menorrhagia, the body has time to compensate, and thus the symptoms of chronic blood loss may be more insidious. Although patients with chronic anemia are not usually admitted to the critical care unit, chronic anemia can complicate other medical conditions that do necessitate treatment in a critical care setting.
ii. Chronic inflammatory disease (e.g., rheumatoid arthritis, chronic osteomyelitis)
iv. Bone marrow infiltration with malignant cells
v. Current or recent treatment with antineoplastic chemotherapy
vi. History of radiation therapy to bones where blood cells are made (i.e., vertebrae, ribs, skull, pelvis, femur, or humerus)
vii. Bone marrow transplantation
viii. Dietary deficiencies, particularly in iron, cobalamine (B12), or folate
ii. RBC membrane defects (e.g., hereditary spherocytosis)
(a) Sickle cell anemia is an autosomal recessive genetic disorder found primarily in persons of African descent that results from substitution of the amino acid valine for glutamic acid at position 6 of the β-globin protein; this substitution leads to the production of defective hemoglobin, hemoglobin S (HbS). The deoxygenation of HbS leads to distortion of the RBC into the classic sickle cell shape.
(b) The major consequence of the sickle cell shape is that RBCs are less able to deform and thus obstruct the microcirculation. Sickle-shaped RBCs have a life span of 10 to 20 days (vs. 120 for nonsickled RBCs) and hemolyze rapidly.
(c) Clinical manifestations of sickle cell anemia are commonly divided into vasoocclusive, hematologic, and infectious crises
(1) Vasoocclusive crisis occurs when the microcirculation is occluded by sickled RBCs, which causes ischemic injury to the organ perfused; pain is the most frequent complaint
(2) Hematologic crisis is manifested by sudden exacerbation of anemia with a corresponding drop in hemoglobin level
(3) Infectious crisis is due to a compromised immune system that is susceptible to common infectious agents such as Haemophilus influenzae, Streptococcus pneumoniae, Mycoplasma pneumoniae, Salmonella typhimurium, S. aureus, and Escherichia coli
(d) Treatment for patients with sickle cell anemia includes rest, hydration, supplemental oxygen, analgesia, antibiotic therapy, and blood transfusion as needed
a. Symptoms: Fatigue; dyspnea, especially with exertion; shortness of breath; possible bone pain if the bone marrow is infiltrated with malignant cells; altered mental status (e.g., dizziness, especially when changing position from lying down to sitting or standing; inability to concentrate; confusion)
b. Signs: Pallor, possibly jaundice; possible hepatosplenomegaly with liver disease and some types of malignant disease, tenderness of the liver and spleen with palpation and percussion; tachycardia, hypotension, and orthostatic changes in vital signs
i. Urine: Can test positive for blood
ii. Stool: Can test positive for blood
(a) Hemoglobin level of less than 7 g/dl, hematocrit (HCT) of less than 21%
(b) Other findings vary with the cause of anemia and can include increased reticulocyte count, decreased serum iron level, increased or decreased total iron-binding capacity, decreased ferritin level, increased indirect bilirubin level, positive Coombs’ test result
b. Radiologic: Gastrointestinal series may be obtained to detect the source of bleeding
c. Biopsy: Bone marrow biopsy may be performed to evaluate bone marrow production of RBCs or detect the presence of bone marrow infiltration with malignant cells
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Patients with anemia can have an acute event related to loss of RBCs due to hemorrhage or a chronic disorder related to inadequate production of RBCs and/or hemoglobin. Throughout their course of recovery and discharge, patients with anemia may be expected to have needs in the following areas:
i. Positioning: High Fowler’s position for shortness of breath
ii. Nutrition: Diet or feeding supplement with iron, vitamin B12, and folate may need to be considered
iii. Pharmacology: Patient may need education on taking oral iron preparations with food to prevent peptic ulcers and on treating constipation as a primary side effect
iv. Treatment: Invasive treatment with whole blood or packed RBCs
(a) Mechanism: Due to diminished oxygen-carrying capacity
(b) Management: Place the patient in a position of comfort to ease the work of breathing; oxygen administration and transfusion of packed RBCs may be required
(a) Mechanism: Inadequate circulating hemoglobin decreases oxygen availability to cells, creating decreased energy stores in the body
(b) Management: Conserve the patient’s energy by assisting with nutrition and the activities of daily living, passive range-of-motion exercises; consider providing supplemental oxygen if the patient is out of bed to a chair or is ambulating
Disseminated Intravascular Coagulation
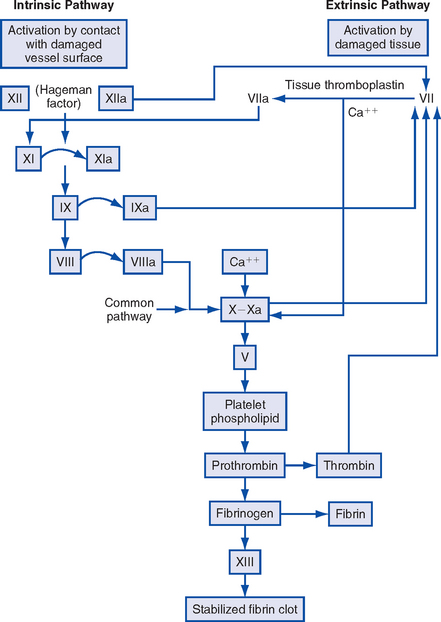
a. DIC is a hypercoagulable state that occurs when the normal coagulation cascade is overstimulated; this results in simultaneous thrombosis and hemorrhage. DIC is always secondary to another pathologic process.
b. Coagulation takes place normally, but it occurs at so many sites in the body that the normal inhibitory mechanisms are overwhelmed. Eventually all available platelets and clotting factors are depleted, and systemic uncontrolled hemorrhage results.
b. Major trauma, crush injuries, burns
d. Acute tumor lysis syndrome (see Acute Leukemia)
e. Obstetric complications, such as abruptio placentae or fetal demise
a. Symptoms: Diagnosis of DIC is based on a constellation of findings, including the sudden onset of a bleeding disorder without a prior history of bleeding or blood coagulation abnormalities
b. Signs: Occurrence of spontaneous bleeding for no obvious reason, a preceding or concurrent pathologic process that is known to precipitate DIC, petechiae, purpura, ecchymoses, hematomas, epistaxis, conjunctival bleeding, spontaneous and/or uncontrollable hemorrhage from multiple unrelated sites, such as sites of venipuncture, tubes, drains, lines, incisions, and wounds
a. Blood: Prolonged PT, decreased fibrinogen level, increased level of fibrin split products, increased levels of D-dimers, and prolonged PTT
b. Radiologic: Findings are usually noncontributory except to identify the underlying pathologic process
b. Reversal of the clotting mechanism
c. Replacement of coagulation components
d. Prevention of clinical sequelae, such as hypovolemic shock, cardiac arrest, organ damage, and limb loss
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Patients with DIC are in a life-threatening clinical situation secondary to overstimulation of the coagulation cascade. Because primary disorders such as sepsis, shock, crushing injury, burns, acute respiratory distress syndrome, or obstetrical complications precipitate DIC, death results in over half of the cases. The best treatment is prevention. Throughout their recovery, patients may be expected to have needs in the following areas:
i. Pharmacology: Patient and family may need education on clotting mechanisms, coagulation components, and oxygen delivery
ii. Psychosocial issues: Patient and/or family may need chaplain or social service support in the event of death, organ failure, loss of limbs, or alteration in self-concept related to compartment syndrome, petechiae, and bruising
iii. Treatments: Oxygen therapy, mechanical ventilation, blood product transfusion, and infusion of anticlotting agents
(a) Mechanism: Thrombocytopenia creates bleeding that is difficult to control
(b) Management: Monitor the patient for a systolic blood pressure of less than 90 mm Hg, heart rate of over 100 beats/min, anxiety, unresponsiveness, diaphoresis, urine output of less than 0.5 ml/kg/hr, platelet count of less than 50,000/mm3, PTT of longer than 40 seconds, and visible uncontrolled bleeding; anticipate transfusions of platelets, fresh frozen plasma, and/or cryoprecipitate
Thrombocytopenia
1. Pathophysiology: The number of platelets available to assist with coagulation is inadequate, which puts the patient at increased risk of hemorrhage. In various hematologic malignancies such as leukemia and lymphoma, the cancerous cells crowd out normal cell lines in the bone marrow, which results in thrombocytopenia as well as neutropenia and anemia. Management includes treating the underlying malignancy with antineoplastic chemotherapy, which can itself also cause pancytopenia. Treatment of either solid tumor or hematologic malignancies with antineoplastic chemotherapy is a major cause of thrombocytopenia. Chemotherapy indiscriminately targets rapidly dividing cells in the bone marrow, including megakaryocytes. Thrombocytopenia can be expected to appear 10 to 14 days after chemotherapeutic treatment; it lasts until the bone marrow is able to replenish its megakaryocyte pool.
a. Decreased platelet production
i. Bone marrow infiltration with malignant cells (e.g., leukemia, multiple myeloma, malignant metastases)
ii. Current or recent treatment with antineoplastic agents
iii. History of radiation therapy to the bones in which blood cells are made
b. Increased platelet destruction
i. DIC: See Disseminated Intravascular Coagulation section
(a) Immune thrombocytopenic purpura (ITP)
(1) Agents known to induce ITP include sulfonamides, thiazide diuretics, chlorpropamide, quinidine, and gold
(2) Patients with HIV infection are at increased risk for development of ITP
iii. Thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS) (see Hypercoagulable Disorders)
c. Sequestration of platelets in the spleen (e.g., with liver disease and portal hypertension)
d. Massive transfusion of RBCs over a short period of time, which can lead to a dilutional thrombocytopenia
a. Symptom: Unexplained bleeding
b. Signs: Pallor, petechiae, purpura, ecchymoses; oozing of blood from venipuncture sites, conjunctival bleeding; bleeding from the oropharynx, gastrointestinal tract, or genitourinary tract and splenomegaly may be present
b. Radiologic: Spleen ultrasonography or liver-spleen scan to determine the size of the spleen
c. Bone marrow biopsy to determine whether adequate numbers of platelets are being made in the bone marrow
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Children with thrombocytopenia usually develop symptoms after a viral infection, and the disorder resolves spontaneously in 90% of cases. Adult ITP and ATP is not as well understood; only 10% to 20% of adult patients experience a spontaneous remission. Patients may be expected to have needs in the following areas:
i. Positioning: Handle with care due to ease of bruising
ii. Transport: May need supplies for spontaneous nose bleeding or oral bleeding; inform team members of risk for bruising
iii. Discharge planning: Patient and family may need education about home environmental risks for injury that may precipitate spontaneous bleeding, need to seek health care for monitoring of platelet counts or if bleeding occurs, and effects of steroid therapy
Hypercoagulable Disorders
a. Hypercoagulable disorders occur when the normal mechanisms of hemostasis involving platelets and clotting factors are disrupted, which results in uncontrolled or inappropriate clotting. Paradoxically, a secondary bleeding disorder develops in many of these patients when their reserves of platelets and clotting factors are depleted.
b. Venous thromboses result from activation of the coagulation cascade caused by venous stasis, ischemia, or infarction. Arterial emboli result when a venous thrombus breaks away from its site of origin and migrates into the arterial vascular system. Pulmonary embolus, myocardial infarction, and thrombotic cerebrovascular accidents can be caused by arterial emboli. Patients are often admitted to critical care units for hemodynamic and neurologic support as well as for thrombolytic therapy with streptokinase, urokinase, or tissue plasminogen activator. Anticoagulation therapy puts these patients at risk for bleeding, although they have an underlying hypercoagulable disorder.
c. TTP appears to be an exaggerated immunologic response to vessel injury that results in extensive thrombus formation and decreased blood flow to the affected site. These patients are critically ill; fever, thrombocytopenia, hemolytic anemia, renal impairment, and neurologic symptoms develop. HUS appears to be a variant of TTP that is seen more commonly in children. Patients with HUS tend to have more severe renal impairment, but fewer neurologic signs and symptoms, than do patients with TTP.
d. DIC is another hypercoagulable disorder (see Disseminated Intravascular Coagulation)
a. Changes in blood flow (e.g., deep vein thrombosis)
b. Changes in circulating blood coagulation factors (e.g., TTP)
a. Symptoms: Tenderness or pain with palpation
b. Signs: Unexplained bleeding, sudden painful swelling of one extremity, and other signs may be present, depending on the organ system involved; temperature exceeding 101° F (38.3° C), petechiae, purpura, ecchymoses, hematomas; circumference of one extremity different from that of the other corresponding extremity; changes in vital signs; with an arterial thrombosis, decreased blood flow in one extremity may be detected by Doppler ultrasonography
i. With venous stasis, laboratory values may be normal until anticoagulation therapy begins
ii. With TTP, RBC levels are decreased; reticulocyte count, bilirubin level, and lactate dehydrogenase levels are increased; fragmented RBCs are seen on peripheral smear
b. Radiologic: Angiography or venography can indicate vessel blockage
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Full recovery from the complications of hypercoagulopathies, such as deep venous thrombosis, polycythemia, and temporary hyperviscosity of blood, can reasonably be expected when the patient is given early thrombolytic therapy combined with watchful collaborative care. Hypercoagulable disorders complicated by comorbidities, poor response to thrombolytics or collaborative care, and septicemia, however, may lead to loss of limbs due to ischemia or life-threatening DIC. Patients may be expected to have needs in the following areas:
i. Positioning: Care in handling the patient due to the ease of bruising and propensity to create an embolus
ii. Skin care: Prevention of complications of immobility and prolonged bed rest, which could lead to tissue alterations and ischemia
iii. Pain management: Patient-controlled analgesia for deep venous thrombosis, joint swelling
iv. Treatments: Thrombolytic therapy and potential blood product administration
Neutropenia
a. Occurs when the total number of neutrophils is abnormally low and puts the patient at increased risk of infection. The longer the patient is neutropenic, the greater the chance of infection. Patients are often admitted to critical care units with a diagnosis such as sepsis or acute leukemia that is complicated by neutropenia.
b. The most common sites of infection seen in neutropenic patients are the lung (pneumonia), blood (septicemia), skin, urinary tract, and gastrointestinal tract (mucositis, esophagitis, perirectal lesions). The major infectious gram-negative bacilli include Klebsiella pneumoniae and E. coli. The major infectious gram-positive cocci include S. aureus, Enterococcus, and Staphylococcus epidermidis. Because affected patients do not have adequate numbers of WBCs to mount an immunologic response, the classical signs of infection may be absent. Fever may be the only sign of infection.
a. Decreased neutrophil production
i. Bone marrow infiltration with malignant cells
ii. Recent history of antineoplastic chemotherapy, especially if high-dose chemotherapy was administered as part of bone marrow transplantation
iii. Any history of radiation therapy to bones in which blood cells are made
iv. Use of certain drugs (e.g., zidovudine, clozapine)
v. Autoimmune disorder (e.g., systemic lupus erythematosus, rheumatoid arthritis)
a. Symptoms: Malaise; reports of fever, chills, and night sweats; sore throat; dyspnea; shortness of breath; abdominal pain, sinus pain, headache; confusion; pain with swallowing, urination, or defecation
b. Signs: Cough, diarrhea, temperature exceeding 101° F (38.3° C), loss of integrity of skin and mucous membranes (especially at IV and central venous catheter sites), lymphadenopathy, tachycardia, hypotension, crackles, and rhonchi
a. Laboratory: ANC lower than 500/microliter
b. Radiologic: Findings are usually noncontributory to the diagnosis of neutropenia, but studies may be indicated to identify the source of infection secondary to neutropenia
5. Goals of care: Absence of infection
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Mortality rate is 18% to 40% in the first 48 hours for patients with an ANC lower than 500/microliter. The goal of therapy is to support the patient until his or her own WBCs are available to fight infection.
8. Evaluation of patient care: Protection successful in avoiding infectious processes
Acute Leukemia
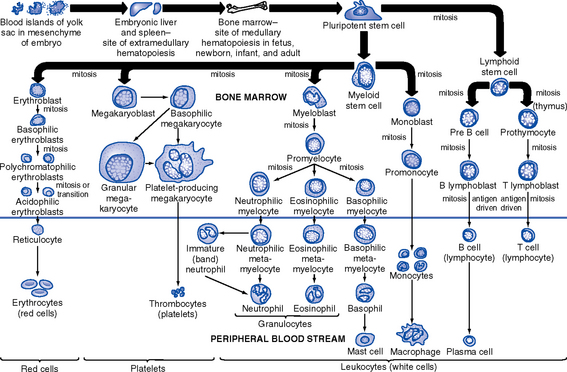
a. Leukemia is a cancer of the WBCs. There are many different types of leukemia, each based on which WBC lineage is affected and how quickly the leukemic clone multiples. Acute nonlymphocytic leukemia (ANLL), also called acute myelogenous leukemia (AML), is a cancer of the granulocyte cell line (Figure 7-1) affecting primarily adults. Acute lymphocytic leukemia (ALL) is a cancer of the lymphocyte cell line (Figure 7-1) affecting primarily children. The leukemic cells themselves are nonfunctional. They crowd out the normal bone marrow cells and thereby induce pancytopenia, manifested as anemia, neutropenia, and thrombocytopenia.
b. Patients with leukemia are usually treated on oncology wards, but two complications of leukemia, overwhelming sepsis and acute tumor lysis syndrome, bring patients to critical care units for hemodynamic support and close observation
a. Largely unknown, probably multifactorial
b. Excessive radiation exposure
c. Previous exposure to certain chemicals (e.g., benzene)
d. Previous exposure to certain drugs (e.g., alkylating chemotherapy agents)
a. Symptoms: Fatigue, malaise, bone pain, headache; reports of fever, chills, night sweats
b. Signs: Pallor, petechial rash, easy bruising, weight loss, temperature exceeding 101° F (38.3° C), flushing, lethargy, petechiae, purpura, ecchymosis, hematomas, possible lymphadenopathy, possible hepatomegaly, possible splenomegaly, tachycardia, and hypotension
i. Total WBC count is very high or very low with primarily immature blast cells seen on the differential
ii. Low RBC and platelet counts are usually also seen because these cell lines are crowded out of bone marrow by leukemic cells
iii. In acute tumor lysis syndrome, uric acid levels exceed 7.2 mg/dl, potassium levels exceed 5.3 mg/dl, PO4 levels exceeds 4.5 mg/dl, and calcium level is less than 8.6 mg/dl
b. Bone marrow biopsy is usually performed to help with the diagnosis and guide treatment
a. Protection against infections
b. Protection against injury from bleeding
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Patient trajectory depends on demographic factors, type of WBC affected, and the maturational pathway from which the abnormal cells arise. Deaths from leukemia account for 4% of cancer deaths in the United States. During the course of treatment to discharge, patients with leukemia may be expected to have needs in the following areas:
i. Pain management: Increased work of the bone marrow causes pain in both ALL and AML
ii. Nutrition: Nausea, vomiting, and diarrhea may indicate a need to manage dietary intake with supplements
iii. Infection control: Because 80% of infections are due to the patient’s own endogenous flora, daily and frequent bath care with an oral examination every 4 hours is essential
iv. Transport: During induction chemotherapy, a patient in profound neutropenia must be protected from infection
v. Discharge planning: Information on prognosis, availability of leukemia support groups, and treatment protocol needs to be shared with the family and patient prior to discharge
vi. Pharmacology: Patient and family may need education about chemotherapy, radiation therapy, and the physiologic markers for leukemia
vii. Psychosocial issues: Social service support for the patient and family may be indicated when the patient’s stay is prolonged (4 to 8 weeks) during induction or consolidation chemotherapy; provision of therapeutic distractions from pain, alopecia, nausea and vomiting, and fatigue can be challenging
viii. Treatments: Chemotherapy, radiation, IV antibiotics, transfusions, bone marrow transplantation
(a) Mechanism: The acute leukemias are initially treated with high-dose chemotherapy, called induction therapy, to induce bone marrow hypoplasia and allow normal cells to repopulate the bone marrow; the patient’s ability to fight off infection is reduced until this repopulation is complete
(b) Management: Monitor for persistent hypotension and hypoperfusion despite adequate fluid resuscitation, IV antibiotic therapy, and contact and neutropenic precautions
(a) Mechanism: Tumor cell destruction creates a metabolic imbalance with rapid serum uptake of intracellular potassium, phosphorus, and nucleic acids
(b) Management: In anticipation of the possibility of acute tumor lysis syndrome, medical management should include aggressive IV hydration, alkalinization of the urine, and allopurinol administration before chemotherapy is initiated. After chemotherapy is started, blood electrolyte levels should be monitored frequently and adjustments to the plan of care rapidly implemented as indicated. Hemodialysis may be necessary to prevent acute tumor lysis syndrome even with aggressive management. For patients with WBC counts exceeding 100,000/mm3, leukapheresis may be performed to remove the leukemic WBCs from the circulation before chemotherapy is initiated. This reduces the risk of acute tumor lysis syndrome.
Bone Marrow Transplantation and Peripheral Blood Stem Cell Transplantation
a. Bone marrow transplantation (BMT) and peripheral blood stem cell transplantation (PBSCT) are procedures performed to reconstitute the hematologic and immunologic systems after patients with malignancies receive dosages of chemotherapy and radiation therapy high enough to permanently kill the bone marrow. BMT is also used in patients with aplastic anemia in an attempt to repopulate the marrow. Harvested marrow or peripheral stem cells are infused into the patient intravenously. Through their innate homing mechanism, the cells travel to the bone marrow and reestablish normal hematopoiesis.
i. In allogeneic BMT, bone marrow from a human leukocyte antigen (HLA)–matched donor is used. Overall, there is a 25% chance that a sibling of a patient needing a BMT will be an HLA match. The chances of finding an HLA-matched unrelated donor (MUD) for a MUD allogeneric transplant are much smaller.
ii. In autologous BMT, or bone marrow rescue, bone marrow from the patient is harvested and preserved before chemotherapy is initiated; the harvested bone marrow is infused after treatment. Using the patient’s own bone marrow eliminates the risk of rejection and graft-versus-host disease (GVHD); however, the risk of cancer recurrence is higher. This type of BMT is the best option for patients with a solid tumor who require high doses of chemotherapy and radiation therapy but who have healthy bone marrow that can be harvested before treatment and returned after therapy. Patients with hematologic malignancies can have their bone marrow harvested during remission, purged of malignant cells, and then returned after high-dose therapy.
iii. In PBSCT, the patient’s bone marrow is stimulated with colony-stimulating factors, and then the patient’s peripheral stem cells are harvested through repeated phereses. After chemotherapy and radiation therapy treatment, the patient’s stem cells are reinfused, as in BMT. As with autologous BMT, the risks of rejection and GVHD are eliminated. In addition, stem cells reengraft more quickly than bone marrow, which reduces the length of neutropenia and risk of infection. Often, PBSCT is performed concurrently with autologous BMT.
2. Etiology and risk factors (i.e., indications for BMT or PBSCT)
a. Administration of dosages of chemotherapy and radiation to treat a malignancy that are toxic to bone marrow
b. Genetic defect (e.g., severe combined immunodeficiency disease)
c. Aplastic syndromes (e.g., aplastic anemia, agranulocytosis)
3. Signs and symptoms during transplantation
a. Symptoms: Malaise, fatigue, weakness, lethargy, reports of chills and night sweats, sore throat, dyspnea, shortness of breath, sinus pain, headache, confusion; pain with swallowing, urination, or defecation
b. Signs: Petechial rash, cough, diarrhea, temperature exceeding 101° F (38.3° C), pallor, jaundice, petechiae, rash, weight changes, loss of integrity of skin and mucous membranes; bleeding from the oropharynx, gastrointestinal tract, or genitourinary tract; hepatomegaly, tachycardia, hypotension, crackles, rhonchi, hyperactive bowel sounds
i. WBC count, HCT, hemoglobin level, and platelet count all can be expected to be low
ii. With renal insufficiency, increased creatinine levels and electrolyte abnormalities
iii. Increased bilirubin level, increased liver enzyme levels, prolonged PT, and prolonged PTT
b. Biopsy: Definitive diagnosis of the complications of transplantation often requires a tissue biopsy; however, biopsy is often contraindicated because of coagulopathies
b. Absence of hemorrhage related to disease or treatment
c. Absence of fluid volume deficit due to fever, diarrhea, hemorrhage, or shock
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: BMT or PBSCT places a client at considerable risk of death from infection
(a) Mechanism: Conditioning chemotherapy and radiation prior to BMT obliterates the patient’s own marrow
(b) Management: Targeted at side effects of conditioning therapy, including profound nausea and vomiting, mucositis, capillary leak syndrome, diarrhea, and bone marrow suppression
(a) Mechanism: Failure of transfused peripheral stem cells and marrow cells to take up residence engrafted to recipient’s bones
(b) Management: Repeat transfusion of peripheral stem cells to prevent certain death
(a) Mechanism: Immunocompetent cells of donated marrow mount an autoimmune response against the recipient’s tissue
(b) Management: Treatment includes administration of immunosuppressive drugs to suppress the transplanted T lymphocytes (with antithymocyte globulin or murine monoclonal antibody to CD3), cyclosporine, and low-dose methotrexate, and hemodynamic support (see also Transplant Rejection)
(a) Mechanism: Occlusion of hepatic circulation by clotting and phlebitis
(b) Management: Supportive treatment with fluid resuscitation and assessment of increased girth, hepatomegaly, ascites, or weight gain
(a) Mechanism: Pancytopenia obliterates the patient’s own ability to fight off infection
(b) Management: Prophylactic use of the antifungal drug fluconazole decreases the incidence of C. albicans infection in BMT patients; prophylactic treatment with acyclovir can decrease the incidence of reactivation of herpes simplex virus and cytomegalovirus in seropositive BMT patients
Transplant Rejection
a. When tissue from one person is transplanted into another person, the immune system of the recipient can recognize the transplanted tissue, or allograft, as foreign. Rejections occur through various mechanisms:
i. Type III, Arthus-type hypersensitivity reaction in the blood vessels of the graft immediately after transplantation
ii. Cytotoxic T lymphocytes (TC) can directly attack the allograft, which results in acute transplant rejection and occurs within days of the transplantation
iii. B lymphocytes can make antibodies against the allograft; these activate the complement pathways and attract platelets. Fibrin accumulates on the transplanted tissue, causing ischemia. In this way the allograft is slowly rejected over many months to years.
b. HLA matching of donor to recipient before transplantation is an attempt to choose a donor whose antigens match the recipient’s as closely as possible so that the recipient’s immune system is not triggered to attack the allograft after the transplantation procedure
c. Allogeneic BMT is fundamentally different from solid organ transplantation. In allogeneic BMT, the immune system itself is being transplanted into a new host. Therefore, it may attack any tissue in the new host, resulting in GVHD (see Bone Marrow Transplantation and Peripheral Blood Stem Cell Transplantation). Because GVHD is usually a limited (albeit serious) problem, the majority of patients undergoing allogeneic BMT can eventually discontinue immunosuppressive therapy. In patients receiving solid organ transplants, the host’s own immune system attacks the donated organ, so recipients must receive lifelong immunosuppressive therapy.
2. Etiology and risk factors: Activation of the immune response against transplanted tissue
a. Symptoms: Malaise, poor appetite, myalgia, tenderness of the allograft
b. Signs: Swelling of the allograft, temperature exceeding 101° F (38.3° C)
4. Diagnostic study findings: Specific to the organ transplanted
a. Nonproliferation of immunocompetent cells
b. Suppression of the activity of helper T cells (TH) and TC cells
Immunosuppression
a. Immunosuppression occurs when some defect in the immunologic system puts the patient at increased risk for infection. The longer the patient is immunosuppressed, the greater the risk of infection. Neutropenia is one form of immunosuppression (see Neutropenia). Although there are primary forms of immune dysfunction, patients are more often admitted to critical care units with immunosuppression as a complication of an underlying disease.
b. Various drugs prescribed to suppress one part of the immune system have untoward effects on other parts of the hematologic and immunologic systems. After organ transplantation, various drugs are used to suppress the immune system and prevent transplant rejection. These drugs act primarily on B cells and T cells and suppress not only the immunologic response to the allograft but also the patient’s ability to fight bacteria, viruses, fungi, and parasites.
b. Genetic (e.g., severe combined immunodeficiency disease)
3. Signs and symptoms: See Neutropenia
4. Diagnostic study findings: Laboratory cultures give positive results for unusual or opportunistic organisms (e.g., Pneumocystis carinii)
5. Goals of care: See Neutropenia and Table 7-9
6. Collaborating professionals on health care team: See Neutropenia
7. Management of patient care: See Neutropenia and Table 7-9
8. Evaluation of patient care: See Neutropenia and Table 7-9
HIV Infection
a. HIV type 1, previously known as human T-lymphotropic virus type 3 (HTLV-3), is a retrovirus that infects cells expressing CD4 on their cell membranes, primarily TH lymphocytes and macrophages. The HIV copies its RNA into the host cell’s DNA and then remains quiescent until the host cell is activated to mount an immunologic response. Activation of the host CD4 cells also initiates replication and production of the HIV RNA, which is released into the circulation. This newly made HIV then infects other cells expressing CD4.
i. The initial stage of HIV infection last 4 to 8 weeks. High levels of virus are in the blood. The patient experiences generalized flulike symptoms.
ii. The virus then enters a latent stage in which it is inactive in infected, resting CD4 cells, replicating only when the host cell is activated for an immune response. Levels of virus are high in the lymph nodes, where CD4 cells reside, but low in the blood. TC cells, which express CD8 and so are not infected by HIV, and B cells attempt to destroy the CD4 cells harboring the virus. However, the TC cells and B cells are crippled without adequate TH support. This latent stage lasts on the average between 2 and 12 years, during which time the patient is asymptomatic. During this time, the number of CD4 cells declines.
iii. During the third stage of HIV infection, the patient begins to experience opportunistic infections. Levels of CD4 cells are usually below 500/mm3 and declining, whereas levels of virus in the blood are increasing. This stage can last 2 to 3 years.
iv. Once the CD4 cell levels drop below 200/mm3, the patient is considered to have AIDS. Virus levels in the blood are high. This stage ends in death, usually within 1 year.
2. Etiology and risk factors: HIV is transmitted via intimate sexual contact, contaminated needles or contaminated blood products, from mother to fetus, and from mother to breast-feeding infant
a. Symptoms and history: Fatigue, night sweats, sore throat, dyspnea, shortness of breath, pain, history of frequent infections, social history of IV drug abuse with shared needles, history of unprotected sexual contact with persons possibly infected with HIV, history of blood transfusion
b. Signs: Weight loss, diarrhea, temperature exceeding 101° F (38.3° C), loss of integrity of skin and mucous membranes, possible cachexia, possible lymphadenopathy, tachycardia, hypotension, crackles, and rhonchi
i. Western blot test result that is positive for HIV (Note: Enzyme-linked immunosorbent assay [ELISA] is a less expensive screening test for HIV antibody. If the ELISA result is positive, a Western blot test should be performed to confirm the findings, because false-positive results do occur with ELISA.)
ii. CD4 lymphocyte counts that are lower than 500/mm3
iii. Nonreactive results on skin test panel
iv. Infection with unusual or opportunistic organisms (e.g., P. carinii)
a. Education about prevention of the spread of HIV infection
b. Maintenance of universal precautions
c. Containment of associated opportunistic diseases
d. Ability of the patient to express fear, grief, and social isolation
a. Anticipated patient trajectory: The time from initial HIV infection to the development of AIDS ranges from months to years depending on demographic characteristics, lifestyle, and interventional factors. Progression to death accelerates with the development of multiple opportunistic infections. Throughout their course of clinical care and discharge, patients may be expected to have needs in the following areas:
i. Nutrition: Avoid fatty foods if chronic diarrhea is present, avoid fruits with peels, maintain good hydration, maintain high protein consumption, encourage consumption of foods that the patient likes, assess for oral lesions and hygiene
ii. Infection control: Education of the patient and the family or significant others may be key in decreasing the spread of infection by sexual contact, IV needle use, and maternal-child transmission; health care workers must use universal precautions
iii. Pharmacology: Education for the patient and the family or significant others may be warranted to describe antiretroviral therapy, prophylactic treatment for opportunistic infections, and use of hematopoiesis-stimulating factors
iv. Psychosocial issues: There is no cure for HIV infection or AIDS; therefore, individual coping ability should be explored
v. Ethical issues: Presence of HIV infection or AIDS raises the concern of intentional spread of infection
(a) Mechanism: HIV invasion of the central nervous system, which occurs in 70% of cases
(b) Management: Initiation of fall precautions and support for cognitive, motor, or behavioral impairment
(a) Mechanism: Suppression of immune responses resulting from infection with HIV
(b) Management: Antiretroviral therapy with zidovudine, didanosine (ddI, Videx), zalcitabine (ddC, Hivid), or stavudine (d4T, Zerit); prophylactic antibiotic therapy with trimethoprim-sulfamethoxazole, aerosolized pentamidine (Pentam), and/or other agents; antiretroviral therapy with agents with HIV-1 protease inhibitors such as saquinavir (Invirase), indinavir (Crixivan), nelfinavir (Viracept), and/or ritonavir (Norvir)
Anaphylactic Hypersensitivity Reactions
a. There are four types of hypersensitivity reaction, classified according to the time between the exposure and the reaction, the immune mechanism involved, and the site of the reaction
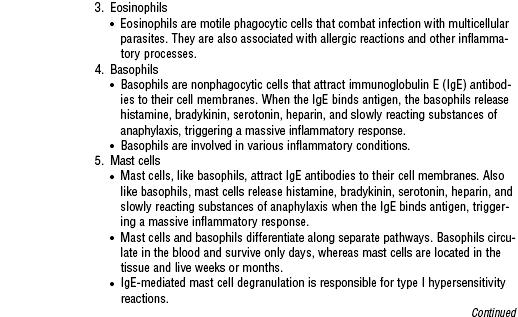
i. Type I immediate hypersensitivity reactions are mediated by immunoglobulin E (IgE) in reaction to common allergens such as dust, pollen, animal dander, insect sting, some foods, and various drugs. These reactions can be local, resulting in local swelling and discomfort, or systemic, resulting in anaphylaxis and possibly in death if not recognized and treated promptly.
ii. Type II immediate hypersensitivity reactions are mediated by antibody and complement. These reactions can occur with a mismatched blood transfusion or as a response to various drugs.
iii. Type III immediate hypersensitivity reactions result in tissue damage caused by precipitation of antigen-antibody immune complexes. These reactions can occur with serum sickness or in response to various drugs.
iv. Type IV delayed hypersensitivity reactions result from migration of immune cells to the site of exposure days after the exposure to the antigen. These reactions can occur in contact dermatitis, measles rash, or tuberculin skin testing or in response to various drugs. Transplanted graft rejection is a type IV hypersensitivity reaction.
b. After a first, sensitizing exposure to a specific allergen, such as an insect sting, in which abnormally large amounts of IgE antibodies are made, subsequent exposures to the same allergen trigger an exaggerated antibody reaction. When the patient comes in contact with the antigen a second time, IgE triggers the release of histamine, heparin, and other cytokines (see Table 7-4) from mast cells, causing bronchiole constriction, peripheral vasoconstriction, and increased vascular permeability, which quickly progress to airway obstruction, pulmonary edema, peripheral edema, hypovolemia, hypotension, shock, and circulatory collapse.
a. Symptoms: Apprehension, dyspnea, restlessness
b. Signs: Urticaria, facial edema, tachypnea, stridor, cyanosis, tachycardia, hypotension, wheezing
4. Diagnostic study findings: Usually noncontributory to the diagnosis of anaphylaxis
6. Collaborating professionals on health care team
a. Anticipated patient trajectory: Patients experiencing an anaphylactic hypersensitivity or anaphylactoid event differ in their clinical course depending on age, medical history, number of prior exposures, and the extent of prior reactions. Throughout their course of recovery, airway and circulatory compromise or collapse may threaten life. Mild hypersensitivity reactions with localized symptoms can be prevented from becoming acute if pharmacologic management is immediate. Patients may be expected to have needs in the following areas:
i. Positioning: Use a chin lift in the supine position or high Fowler’s position to provide a patent airway
ii. Transport: Maintain a patent airway, circulatory support, and cervical neck traction until the patient is hemodynamically stable
iii. Discharge planning: Patient education may be required to reinforce the importance of wearing a medic alert bracelet, the home use of epinephrine, the need to contact health care personnel immediately at the onset of a hypersensitivity reaction, and the importance of avoiding allergens
(a) Mechanism: Antibody response to allergen results in vasodilation and bronchoconstriction
(b) Management: Cardiopulmonary resuscitation, administration of corticosteroids (IV Solu-Cortef or Solu-Medrol) and histamine blockers (diphenhydramine [Benadryl], 25 to 50 mg; epinephrine, 0.2 to 0.5 ml subcutaneously or 0.5 ml of 1:1000 IV)
Guyton, AC, Hall, JE. Textbook of medical physiology, ed 10. Philadelphia: Saunders, 2000.
Janeway, CA, Jr., Travers, P. Immunobiology, ed 3. New York: Garland, 1997.
McCance, KL, Huether, SE. Pathophysiology: the biologic basis for disease in adults and children, ed 4. St Louis: Mosby, 2002.
McMahon, TJ, Exton Stone, A, Bonaventura, J, et al. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J Biol Chem. 2000;275:16738–16745.
Metcalf, D. Cellular hematopoiesis in the twentieth century. Semin Hematol. 1999;36(4 suppl 7):5–12.
Porth, CM. Pathophysiology: concepts of altered health status, ed 6. Philadelphia: Lippincott Williams & Wilkins, 2002.
Deglin, JH, Vallerand, AH. Davis drug guide for nurses, ed 8. Philadelphia: FA Davis, 2003.
Hankins, J, Lonsway, RAW, Hedrick, D, et al. Infusion therapy in clinical practice, ed 2. St Louis: Saunders, 2001.
Ignatavicius DD, Workman ML, eds. Medical-surgical nursing: critical thinking for collaborative care, ed 4, Philadelphia: Saunders, 2002.
Kee, JL. Laboratory and diagnostic tests with nursing implications, ed 5. Stamford, Conn: Appleton & Lange, 1999.
Pagana, KD, Pagana, TJ. Mosby’s diagnostic and laboratory test reference, ed 6. St Louis: Mosby, 2003.
Wilson, BA, Shannon, MT, Stang, CL. Nurse’s drug guide. Upper Saddle River, NJ: Prentice Hall, 2004.
Specific Patient Health Problems
Cook, LS. A simple case of anemia: pathophysiology of a common symptom. J Intraven Nurs. 2000;23(5):271–281.
Goram, AL. Factors and predictors of response with epoetin alfa for chemotherapy-related anemia. J Pharm Technol. 2000;16(6):227–235.
Loney, M, Chernecky, C. Anemia. Oncol Nurs Forum. 2000;27(6):951–966.
Ludwig, H, Strasser, K. Symptomatology of anemia. Semin Oncol. 2001;28(2 suppl 8):7–14.
Pearl, RG, Pohlman, A, Understanding and managing anemia in critically ill patients. Crit Care Nurse 2002;(suppl):1–16.
Sobrero, A, Puglisi, F, Guglielmi, A, et al. Fatigue: a main component of anemia symptomatology. Semin Oncol. 2001;28(2 suppl 8):15–18.
Taher, A, Kazzi, Z. Anemia, sickle cell. retrieved July 12, 2004, from http://www.emedicine.com/emerg/topic26.htm, January 5, 2005.
Volberding, P. Consensus statement: anemia in HIV infection: Current trends, treatment options, and practice strategies. Clin Ther. 2000;22(9):1004–1020.
Dice, RD. Intraoperative disseminated intravenous coagulopathy. Crit Care Nurs Clin North Am. 2000;12(2):175–179.
Levi, M. Pathogenesis and treatment of disseminated intravascular coagulation in the septic patient. J Crit Care. 2001;16(4):167–177.
Levi, M, de Jonge, E. Current management of disseminated intravascular coagulation. Hosp Pract (Off Ed). 2000;35(8):59–66. 92
Maxson, JH. Management of disseminated intravascular coagulation. Crit Care Nurs Clin North Am. 2000;12(3):341–352.
Stillwell SB, ed. Mosby’s critical care nursing reference. St Louis: Mosby, 2002.
Ansani, NT. Heparin-induced thrombocytopenia and thrombosis: a review of pharmacologic therapy. J Pharm Technol. 2001;17(5):189–197.
Argatroban is a new option for patients with heparin-induced thrombocytopenia. Drugs Ther Perspect. 2001;17(19):1–4.
Deitcher, SR. Heparin-induced thrombocytopenia: pathogenesis, management, and prevention. Formulary. 2001;36(1):26–28. 30, 39-41
Doyle, B, Porter, DL. Thrombocytopenia. AACN Clin Issues. 1997;8(3):469–480.
Fukuyama, SN, Itano, J, Thrombocytopenia secondary to myelosuppression. Am J Nurs 1999;(suppl):5–8. 34-36
Horrell, CJ, Rothman, J. The etiology of thrombocytopenia. Dimens Crit Care Nurs. 2001;20(4):10–16.
Nguyen, TN, Gal, P, Ransom, JL, et al. Lepirudin use in a neonate with heparin-induced thrombocytopenia. Ann Pharmacother. 2003;37(2):229–233.
Todisco, M, Casaccia, P, Rossi, N. Severe bleeding symptoms in refractory idiopathic thrombocytopenic purpura: a case successfully treated with melatonin. Am J Ther. 2003;10(2):135–136.
Dickey, TL. The hypercoagulable state as a risk factor for venous thromboembolism, part I. J Am Acad Physician Assist. 2002;15(11):28–30. 32, 35
Gardner, J. Factor V Leiden with deep venous thrombosis. Clin Lab Sci. 2003;16(1):6–9.
Lapointe, LA, Von Rueden, KT. Coagulopathies in trauma patients. AACN Clin Issues. 2002;13(2):192–203.
Meissner, MH, Chandler, WL, Elliott, JS. Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability? J Trauma Injury Infect Crit Care. 2003;54(2):224–231.
Mulroy, JF, De Jong, MJ. Syndromes of hypercoagulability: protein C and protein S deficiencies in acutely ill adults. Am J Nurs. 103(5), 2003. Critical Care Extra, 64KK, 64MM, 64OO
Neufeld, EJ. Coagulation disorders and treatment strategies. Hematol Oncol Clin North Am. 1998;12(6):ix–x. 1141-1144
Subar, M. Clinical evaluation of hypercoagulable states. Clin Geriatr Med. 2001;17(1):57–70.
Spero, JA. Venous thromboembolism and hypercoagulability. Top Emerg Med. 2000;22(3):9–22.
van Cott, EM, Laposata, M. Your lab focus: overview. Algorithms for hypercoagulation testing. Lab Med. 2003;34(3):216–220. 222
Asumang, A, Criswell, J. Neutropenic patients. Care Crit Ill. 1998;14(6):195–198.
Bow, EJ. Management of the febrile neutropenic cancer patient: lessons from 40 years of study. Clin Microbiol Infect. 2005;11(suppl 5):24–29.
Burney, KY. Tips for timely management of febrile neutropenia. Oncol Nurs Forum. 2000;27(4):617–618.
Corey, L, Boeckh, M. Persistent fever in patients with neutropenia. N Engl J Med. 2002;346(4):222–224.
Freifeld, AG, Walsh, TJ, Pizzo, PA. Infections in the cancer patient. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. ed 5. Philadelphia: Lippincott; 1997:2659–2704.
Klastersky, J, Paesmans, M, Rubenstein, EB, et al. The Multinational Association for Supportive Care in Cancer Risk Index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18(16):3038–3051.
Mutnick, AH, Kirby, JT, Jones, RN. CANCER Resistance Surveillance Program: initial results from hematology-oncology centers in North America. Ann Pharmacother. 2003;37(1):47–56.
Reigle, BS, Dienger, MJ. Sepsis and treatment-induced immunosuppression in the patient with cancer: tables/charts. Crit Care Nurs Clin North Am. 2003;15(1):109–118.
Santolaya, ME, Alvarez, AM, Becker, A, et al. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. J Clin Oncol. 2001;19(14):3415–3421.
Soni, S, Radel, E. Neutropenia: striking a balance between caution and alarm. Contemp Pediatr. 2002;19(8):77–78. 82-85
Wilson, BJ. Dietary recommendations for neutropenic patients. Semin Oncol Nurs. 2002;18(1):44–49.
Devine, H, DeMeyer, E. Hematopoietic cell transplantation in the treatment of leukemia. Semin Oncol Nurs. 2003;19(2):118–132.
Dietz-Lovett, K. An overview of acute and chronic leukemias. Dev Support Cancer Care. 1998;2(3):66–72. 103-105
Mackey, HT, Klemm, P, Leukemia: aggressive therapies predispose patients to a host of side effects. Am J Nurs 2000;(suppl):27–31. 52-54
Medoff, E. Oncology today: new horizons: leukemia. RN. 2000;63(9):42–46. 49-50
Murphy-Ende, K, Chernecky, C. Assessing adults with leukemia. Nurse Pract. 2002;27(11):52–56. 49, 59-60
Rodak, BF, Leclair, SJ. The new WHO nomenclature: introduction and myeloid neoplasms. Lab Sci. 2002;15(1):44–54. 60-63
Scheinberg, DA, Maslak, P, Weiss, M. Acute leukemias. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. ed 5. Philadelphia: Lippincott; 1997:2293–2321.
Shannon-Dorcy, K, Wolfe, V. Decision-making in the diagnosis and treatment of leukemia. Semin Oncol Nurs. 2003;19(2):142–149.
Warrell, RP. Metabolic emergencies. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. ed 5. Philadelphia: Lippincott; 1997:2486–2500.
Wujcik, D. Molecular biology of leukemia. Semin Oncol Nurs. 2003;19(2):83–89.
Yoder, LH. Diseases treated with blood cell transplants. Semin Oncol Nurs. 1997;13(3):164–171.
Bone Marrow Transplantation and Peripheral Blood Stem Cell Transplantation
Buchsel, PC, Leum, EW, Randolph, SR. Delayed complications of bone marrow transplantation: an update. Oncol Nurs Forum. 1996;23(8):1267–1291.
Campos de Carvalho, E, Goncalves, PG, Bontempo, APM, et al. Interpersonal needs expressed by patients during bone marrow transplantation. Cancer Nurs. 2000;23(6):462–467.
Centers for Disease Control and Prevention, Infectious Disease Society of America, American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep. 2000;49(RR-10):1–95. 97-125, CE1-7
Cohen, MZ, Ley, CD. Bone marrow transplantation: the battle for hope in the face of fear. Oncol Nurs Forum. 2000;27(3):473–480.
Farquhar, C, Basser, R, Marjoribanks, J, et al, High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer. Cochrane Database of Systematic Reviews 2003;(1). doi:10.1002/14651858.CD003139. Art No: CD003139
Fife, BL, Huster, GA, Cornetta, KG, et al. Longitudinal study of adaptation to the stress of bone marrow transplantation. J Clin Oncol. 2000;18(7):1539–1549.
Hacker, ED. Quantitative measurement of quality of life in adult patients undergoing bone marrow transplant or peripheral blood stem cell transplant: a decade in review. Oncol Nurs Forum. 2003;30(4):613–631.
Hematopoietic stem cell therapy. Hematol Oncol Clin North Am. 1999;13(5):xi–xii. 889-1112
Hematopoietic stem cell transplantation. Curr Opin Hematol. 2002;9(6):477–508.
Keller, C, Thieszen, S. Researchers extending potential for stem cell transplants. Blood Marrow Transplant Newsl. 2003;14(1):2. 4
Maloy, BJ. Hematopoiesis, stem cells, and transplantation: what have we learned for the new millennium? J Intraven Nurs. 2000;23(5):298–303.
Maningo, J. Peripheral blood stem cell transplant: easier than getting blood from a bone. Nursing. 2002;32(12):52–55.
Marena, C, Zecca, M, Carenini, ML, et al. Incidence of, and risk factors for, nosocomial infections among hematopoietic stem cell transplantation recipients, with impact on procedure-related mortality. Infect Control Hosp Epidemiol. 2001;22(8):510–517.
Middleton, R. Changing treatment options in bone marrow transplantation. Transplant Nurses J. 1999;8(2):8–13.
Myer, SA, Oliva, J. Severe aplastic anemia and allogeneic hematopoietic stem cell transplantation. AACN Clin Issues. 2002;13(2):169–191.
Olver, WJ, James, SA, Lennard, A, et al. Nosocomial transmission of Saccharomyces cerevisiae in bone marrow transplant patients. J Hosp Infect. 2002;52(4):268–272.
Rebellato, LM, Dobbs, LJ, Jr. Haploidentical transplantation: importance of histocompatibility testing and chimerism studies in allogeneic bone marrow/stem cell transplantation. J Infus Nurs. 2001;24(5):311–318.
Schmit-Pokorny, K, Franco, T, Frappier, B, et al. The cooperative care model: an innovative approach to deliver blood and marrow stem cell transplant care. Clin J Oncol Nurs. 2003;7(5 pt 1):509–514. 556, 542-543
Schneider, S. Interventions for clients with hematologic problems. In: Ignatavicius DD, Workman ML, eds. Medical-surgical nursing: critical thinking for collaborative care. ed 4. Philadelphia: Saunders; 2002:853–856.
Serody, JS, Shea, TC. Prevention of infections in bone marrow transplant recipients. Infect Dis Clin North Am. 1997;11(2):459–477.
Shivnan, J, Shelton, BK, Onners, BK. Bone marrow transplantation: issues for critical care nurses. AACN Clin Issues. 1996;7(1):95–108. 179-180
Yeager, KA, Webster, J, Crain, M, et al. Implementation of an oral care standard for leukemia and transplantation patients. Cancer Nurs. 2000;23(1):40–48.
Giuliano, KK, Sims, TW. Transplant issues: infections and immunosuppressant drugs. Dimens Crit Care Nurs. 1999;18(2):16–19.
Lake, K. Acute rejection: prevention, diagnosis and management. Dis Manag Digest. 2000;4(4):4–5. 14-15
Morris, RE. New immunosuppressive molecules for control of organ transplant rejection. In: Williams BAH, Sandiford-Guttenbeil DM, eds. Trends in organ transplantation. New York: Springer; 1996:83–94.
NIH tests ways to prevent transplant rejection. Diabetes Dateline. 1999-2000;Winter:1–4.
O’Donnell, M, Parmenter, KL. Transplant medications. Crit Care Nurs Clin North Am. 1996;8(3):253–271.
Poole, P, Greer, E. Immunosuppression in transplantation: a new millennium in care. Crit Care Nurs Clin North Am. 2000;12(3):315–321.
Bush, WW. Overview of transplantation immunology and the pharmacotherapy of adult solid organ transplant recipients: focus on immunosuppression. AACN Clin Issues. 1999;10(2):253–269. 304-306
Cohen, SM. Current immunosuppression in liver transplantation. Am J Ther. 2002;9(2):119–125.
Huizinga, R. Update in immunosuppression. Nephrol Nurs J. 2002;29(3):261–267.
Immunosuppressive therapies: an overview. Dis Manag Digest. 2000;4(5):2–3. 14-15
Poole, P, Greer, E. Immunosuppression in transplantation: a new millennium in care. Crit Care Nurs Clin North Am. 2000;12(3):315–321.
Reigle, BS, Dienger, MJ. Sepsis and treatment-induced immunosuppression in the patient with cancer. Crit Care Nurs Clin North Am. 2003;15(1):109–118.
Wahrenberger, A. Pharmacologic immunosuppression: cure or curse? Crit Care Nurs Q. 1995;17(4):27–36.
Abel, E, Painter, L. Factors that influence adherence to HIV medications: perceptions of women and health care providers. J Assoc Nurses AIDS Care. 2003;14(4):61–69.
Drug resistance guide being pondered by CDC: as HIV drug resistance rises, surveillance needed. AIDS Alert. 2003;18(11):146–147.
Ferri, RS, Adinolfi, A, Orsi, AJ, et al. Treatment of anemia in patients with HIV infection, part 2: guidelines for management of anemia. J Assoc Nurses AIDS Care. 2002;13(1):50–59.
Goldschmidt, RH, Dong, BJ. Treatment of AIDS and HIV-related conditions: 2001. J Am Board Fam Pract. 2001;14(4):283–309.
Jones, SG. Nursing practice for HIV/AIDS fever care: a descriptive study. J Assoc Nurses AIDS Care. 1998;9(5):53–60.
Anaphylactic Type I Hypersensitivity Reactions
Brazil, E, MacNamara, AF. “Not so immediate” hypersensitivity: the danger of biphasic anaphylactic reactions. J Accid Emerg Med. 1998;15(4):252–253.
Carr, BW, Burke, C, Outpatient chemotherapy: hypersensitivity and anaphylaxis: oncology nurses must know how to respond quickly and correctly. Am J Nurs 2001;(suppl):27–30. 49-50
Carroll, P. Anaphylaxis. RN. 2001;64(12):45–50.
Green, LC. Anaphylactic shock and its implication for nurses. Accid Emerg Nurs. 1998;6(2):103–105.
Jurewicz, MA. Anaphylaxis: when the body overreacts. Nursing. 2000;30(7):58–61.
Dimeo, F, Rumberger, BG, Keul, J. Aerobic exercise as therapy for cancer fatigue. Clin J Am Coll Sports Med. 1998;30(4):475–478.
Mock, V, Dow, KH, Meares, CJ, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncol Nurs Forum. 1997;24(6):991–1000.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. retrieved June 1, 2004, from http://www.nccn.org/physician_gls/f_guidelines.html.
Porock, D, Kristjanson, LJ, Tinnelly, K, et al. An exercise intervention for advanced cancer patients experiencing fatigue: a pilot study. J Palliat Care. 2000;16(3):30–36.
Schneider, S. Interventions for hematologic problems. In Ignatavicius DD, Workman ML, eds.: Medical-surgical nursing: critical thinking for collaborative care, ed 4, Philadelphia: Saunders, 2002.