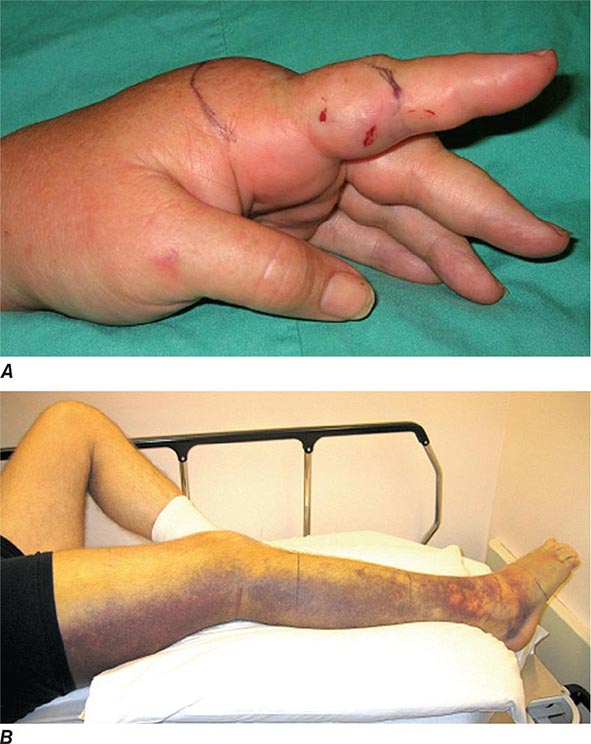
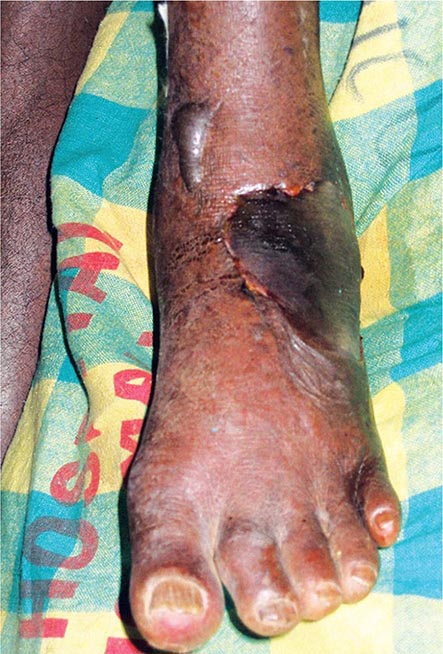
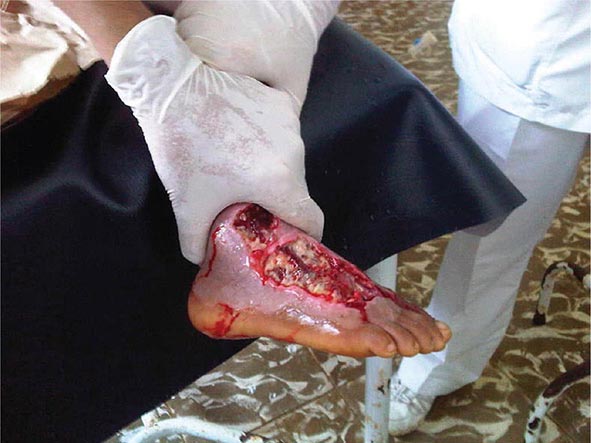
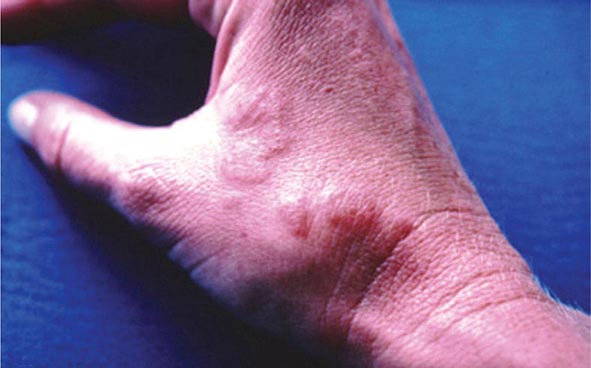
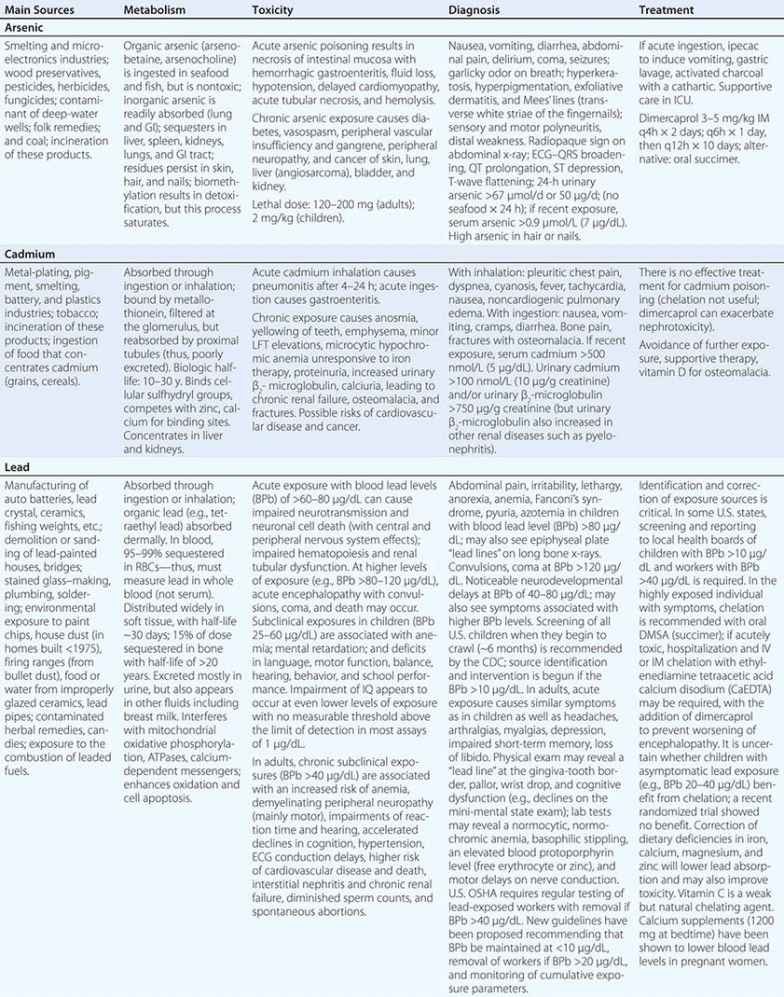
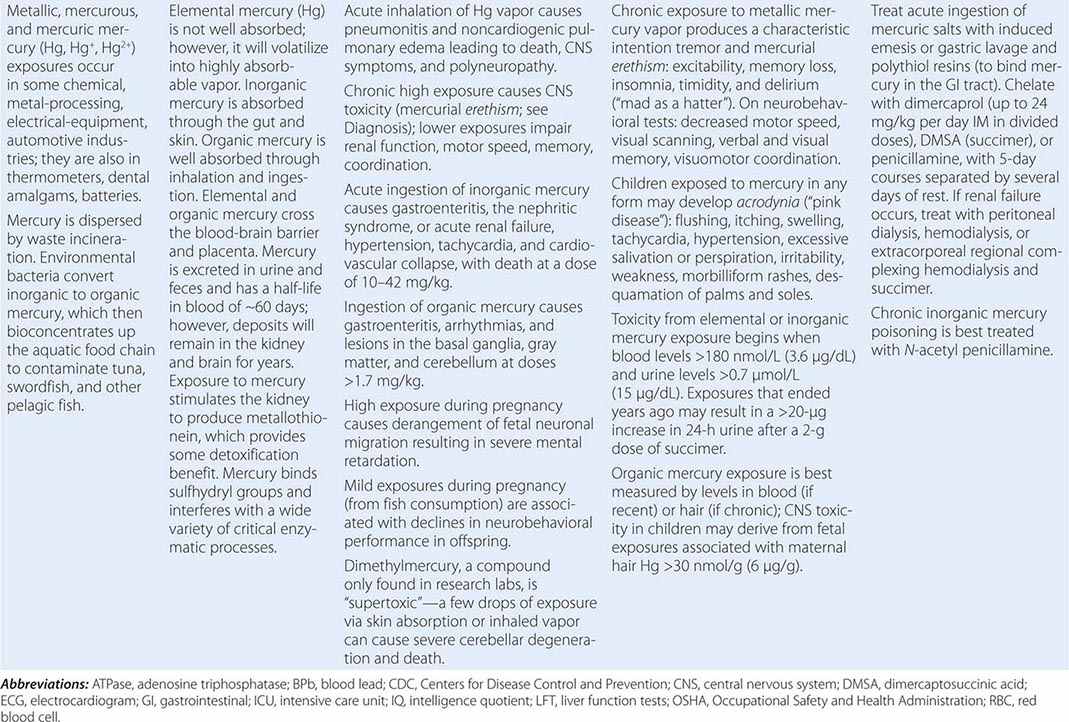
Metals pose a significant threat to health through low-level environmental as well as occupational exposures. One indication of their importance relative to other potential hazards is their ranking by the U.S. Agency for Toxic Substances and Disease Registry, which maintains an updated list of all hazards present in toxic waste sites according to their prevalence and the severity of their toxicity. The first, second, third, and seventh hazards on the list are heavy metals: lead, mercury, arsenic, and cadmium, respectively (http://www.atsdr.cdc.gov/spl/). Specific information pertaining to each of these metals, including sources and metabolism, toxic effects produced, diagnosis, and the appropriate treatment for poisoning, is summarized in Table 472e-1.
|
HEAVY METALS |
Metals are inhaled primarily as dusts and fumes (the latter defined as tiny particles generated by combustion). Metal poisoning can also result from exposure to vapors (e.g., mercury vapor in creating dental amalgams). When metals are ingested in contaminated food or drink or by hand-to-mouth activity (implicated especially in children), their gastrointestinal absorption varies greatly with the specific chemical form of the metal and the nutritional status of the host. Once a metal is absorbed, blood is the main medium for its transport, with the precise kinetics dependent on diffusibility, protein binding, rates of biotransformation, availability of intracellular ligands, and other factors. Some organs (e.g., bone, liver, and kidney) sequester metals in relatively high concentrations for years. Most metals are excreted through renal clearance and gastrointestinal excretion; some proportion is also excreted through salivation, perspiration, exhalation, lactation, skin exfoliation, and loss of hair and nails. The intrinsic stability of metals facilitates tracing and measurement in biologic material, although the clinical significance of the levels measured is not always clear.
Some metals, such as copper and selenium, are essential to normal metabolic function as trace elements (Chap. 96e) but are toxic at high levels of exposure. Others, such as lead and mercury, are xenobiotic and theoretically are capable of exerting toxic effects at any level of exposure. Indeed, much research is currently focused on the contribution of low-level xenobiotic metal exposure to chronic diseases and to subtle changes in health that may have significant public health consequences. Genetic factors, such as polymorphisms that encode for variant enzymes with altered properties in terms of metal binding, transport, and effects, also may modify the impact of metals on health and thereby account, at least in part, for individual susceptibility to metal effects.
The most important component of treatment for metal toxicity is the termination of exposure. Chelating agents are used to bind metals into stable cyclic compounds with relatively low toxicity and to enhance their excretion. The principal chelating agents are dimercaprol (British anti-Lewisite [BAL]), ethylenediamine tetraacetic acid (EDTA), succimer (dimercaptosuccinic acid [DMSA]), and penicillamine; their specific use depends on the metal involved and the clinical circumstances. Activated charcoal does not bind metals and thus is of limited usefulness in cases of acute metal ingestion.
In addition to the information provided in Table 472e-1, several other aspects of exposure, toxicity, or management are worthy of discussion with respect to the four most hazardous toxicants (arsenic, cadmium, lead, and mercury).
Arsenic, even at moderate levels of exposure, has been clearly linked with increased risks for cancer of the skin, bladder, renal pelvis, ureter, kidney, liver, and lung. These risks appear to be modified by smoking, folate and selenium status, genetic traits (such as ability to methylate arsenic), and other factors. Studies in community-based populations are beginning to demonstrate that arsenic exposure is a risk factor for increased coronary heart disease and stroke. Evidence is also emerging that low-level arsenic may cause neurodevelopmental delays in children and possibly diabetes, but the evidence remains uneven.
Serious cadmium poisoning from the contamination of food and water by mining effluents in Japan contributed to the 1946 outbreak of “itai-itai” (“ouch-ouch”) disease, so named because of cadmium-induced bone toxicity that led to painful bone fractures. Modest exposures from environmental contamination have recently been associated in some studies with a lower bone density, a higher incidence of fractures, and a faster decline in height in both men and women, effects that may be related to cadmium’s calciuric effect on the kidney. There is some evidence for synergy between the adverse impacts of cadmium and lead on kidney function. Environmental exposures have also been linked to lower lung function (even after adjusting for smoking cigarettes, which contain cadmium) as well as increased risk of cardiovascular disease and mortality, stroke, and heart failure. Several studies have also raised concerns that cadmium may be carcinogenic and contribute to elevated risks of prostate, breast, and pancreatic cancer. Overall, this growing body of research indicates that cadmium exposure may be contributing significantly to morbidity and mortality rates in the general population.
Advances in our understanding of lead toxicity have recently benefited by the development of K x-ray fluorescence (KXRF) instruments for making safe in vivo measurements of lead levels in bone (which, in turn, reflect cumulative exposure over many years, as opposed to blood lead levels, which mostly reflect recent exposure). Higher bone lead levels measured by KXRF have been linked to increased risk of hypertension and accelerated declines in cognition in both men and women from an urban population. Upon reviewing these studies in conjunction with other epidemiologic and toxicologic studies, a recent federal expert panel concluded that the impact of lead exposure on hypertension and cognition in adults was causal. Prospective studies have also demonstrated that higher bone lead levels are a major risk factor for increased cardiovascular morbidity and mortality rates in both community-based and occupational-exposed populations. Lead exposure at community levels has also been recently associated with increased risks of hearing loss, Parkinson’s disease, and amyotrophic lateral sclerosis. With respect to pregnancy-associated risks, high maternal bone lead levels were found to predict lower birth weight, head circumference, birth length, and neurodevelopmental performance in offspring by age 2 years. In a randomized trial, calcium supplementation (1200 mg daily) was found to significantly reduce the mobilization of lead from maternal bone into blood during pregnancy.
The toxicity of low-level organic mercury exposure (as manifested by neurobehavioral performance) is of increasing concern based on studies of the offspring of mothers who ingested mercury-contaminated fish. With respect to whether the consumption of fish by women during pregnancy is good or bad for offspring neurodevelopment, balancing the trade-offs of the beneficial effects of the omega-3-fatty acids (FAs) in fish versus the adverse effects of mercury contamination in fish has led to some confusion and inconsistency in public health recommendations. Overall, it would appear that it would be best for pregnant women to either limit fish consumption to those species known to be low in mercury contamination but high in omega-3-FAs (such as sardines or mackerel) or to avoid fish and obtain omega-3-FAs through supplements or other dietary sources. Current evidence has not supported the recent contention that ethyl mercury, used as a preservative in multiuse vaccines administered in early childhood, has played a significant role in causing neurodevelopmental problems such as autism. With regard to adults, there is conflicting evidence as to whether mercury exposure is associated with increased risk of hypertension and cardiovascular disease. At this point, conclusions cannot be drawn.
 Heavy metals pose risks to health that are especially burdensome in selected parts of the world. For example, arsenic exposure from natural contamination of shallow tube wells inserted for drinking water is a major environmental problem for millions of residents in parts of Bangladesh and Western India. Contamination was formerly considered only a problem with deep wells; however, the geology of this region allows most residents only a few alternatives for potable drinking water. The combustion of leaded gasoline with resulting contamination of air and soil with lead oxide remains a problem in some countries of Central Asia, Southeast Asia, Africa, and the Middle East. Populations living in the Arctic have been shown to have particularly high exposures to mercury due to long-range transport patterns that concentrate mercury in the polar regions, as well as the traditional dependence of Arctic peoples on the consumption of fish and other wildlife that bioconcentrate methylmercury.
Heavy metals pose risks to health that are especially burdensome in selected parts of the world. For example, arsenic exposure from natural contamination of shallow tube wells inserted for drinking water is a major environmental problem for millions of residents in parts of Bangladesh and Western India. Contamination was formerly considered only a problem with deep wells; however, the geology of this region allows most residents only a few alternatives for potable drinking water. The combustion of leaded gasoline with resulting contamination of air and soil with lead oxide remains a problem in some countries of Central Asia, Southeast Asia, Africa, and the Middle East. Populations living in the Arctic have been shown to have particularly high exposures to mercury due to long-range transport patterns that concentrate mercury in the polar regions, as well as the traditional dependence of Arctic peoples on the consumption of fish and other wildlife that bioconcentrate methylmercury.
A few additional metals deserve brief mention but are not covered in Table 472e-1 because of the relative rarity of their being clinically encountered or the uncertainty regarding their potential toxicities. Aluminum contributes to the encephalopathy in patients with severe renal disease, who are undergoing dialysis (Chap. 424). High levels of aluminum are found in the neurofibrillary tangles in the cerebral cortex and hippocampus of patients with Alzheimer’s disease, as well as in the drinking water and soil of areas with an unusually high incidence of Alzheimer’s. The experimental and epidemiologic evidence for the aluminum–Alzheimer’s disease link remains relatively weak, however, and it cannot be concluded that aluminum is a causal agent or a contributing factor in neurodegenerative disease. Hexavalent chromium is corrosive and sensitizing. Workers in the chromate and chrome pigment production industries have consistently had a greater risk of lung cancer. The introduction of cobalt chloride as a fortifier in beer led to outbreaks of fatal cardiomyopathy among heavy consumers. Occupational exposure (e.g., of miners, dry-battery manufacturers, and arc welders) to manganese can cause a parkinsonian syndrome within 1–2 years, including gait disorders; postural instability; a masked, expressionless face; tremor; and psychiatric symptoms. With the introduction of methylcyclopentadienyl manganese tricarbonyl (MMT) as a gasoline additive, there is concern for the toxic potential of environmental manganese exposure. For example, a recent study found a high prevalence of parkinsonian disorders in a community with risks proportionate to estimated manganese exposures emitted by local ferroalloy industries. Epidemiologic studies have also suggested that manganese may interfere with early childhood neurodevelopment in ways similar to that of lead. Nickel exposure induces an allergic response, and inhalation of nickel compounds with low aqueous solubility (e.g., nickel subsulfide and nickel oxide) in occupational settings is associated with an increased risk of lung cancer. Overexposure to selenium may cause local irritation of the respiratory system and eyes, gastrointestinal irritation, liver inflammation, loss of hair, depigmentation, and peripheral nerve damage. Workers exposed to certain organic forms of tin (particularly trimethyl and triethyl derivatives) have developed psychomotor disturbances, including tremor, convulsions, hallucinations, and psychotic behavior.
Thallium, which is a component of some insecticides, metal alloys, and fireworks, is absorbed through the skin as well as by ingestion and inhalation. Severe poisoning follows a single ingested dose of >1 g or >8 mg/kg. Nausea and vomiting, abdominal pain, and hematemesis precede confusion, psychosis, organic brain syndrome, and coma. Thallium is radiopaque. Induced emesis or gastric lavage is indicated within 4–6 h of acute ingestion; Prussian blue prevents absorption and is given orally at 250 mg/kg in divided doses. Unlike other types of metal poisoning, thallium poisoning may be less severe when activated charcoal is used to interrupt its enterohepatic circulation. Other measures include forced diuresis, treatment with potassium chloride (which promotes renal excretion of thallium), and peritoneal dialysis.
473e |
Poisoning and Drug Overdose |
Poisoning refers to the development of dose-related adverse effects following exposure to chemicals, drugs, or other xenobiotics. To paraphrase Paracelsus, the dose makes the poison. In excessive amounts, substances that are usually innocuous, such as oxygen and water, can cause toxicity. Conversely, in small doses, substances commonly regarded as poisons, such as arsenic and cyanide, can be consumed without ill effect. Although most poisons have predictable dose-related effects, individual responses to a given dose may vary because of genetic polymorphism, enzymatic induction or inhibition in the presence of other xenobiotics, or acquired tolerance. Poisoning may be local (e.g., skin, eyes, or lungs) or systemic depending on the route of exposure, the chemical and physical properties of the poison, and its mechanism of action. The severity and reversibility of poisoning also depend on the functional reserve of the individual or target organ, which is influenced by age and preexisting disease.
EPIDEMIOLOGY
More than 5 million poison exposures occur in the United States each year. Most are acute, are accidental (unintentional), involve a single agent, occur in the home, result in minor or no toxicity, and involve children <6 years of age. Pharmaceuticals are involved in 47% of exposures and in 84% of serious or fatal poisonings. Unintentional exposures can result from the improper use of chemicals at work or play; label misreading; product mislabeling; mistaken identification of unlabeled chemicals; uninformed self-medication; and dosing errors by nurses, pharmacists, physicians, parents, and the elderly. Excluding the recreational use of ethanol, attempted suicide (deliberate self-harm) is the most common reported reason for intentional poisoning. Recreational use of prescribed and over-the-counter drugs for psychotropic or euphoric effects (abuse) or excessive self-dosing (misuse) is increasingly common and may also result in unintentional self-poisoning.
About 20–25% of exposures require bedside health-professional evaluation, and 5% of all exposures require hospitalization. Poisonings account for 5–10% of all ambulance transports, emergency department visits, and intensive care unit admissions. Up to 30% of psychiatric admissions are prompted by attempted suicide via overdosage. Overall, the mortality rate is low: <1% of all exposures. It is much higher (1–2%) among hospitalized patients with intentional (suicidal) overdose, who account for the majority of serious poisonings. Acetaminophen is the pharmaceutical agent most often implicated in fatal poisoning. Overall, carbon monoxide is the leading cause of death from poisoning, but this prominence is not reflected in hospital or poison center statistics because patients with such poisoning are typically dead when discovered and are referred directly to medical examiners.
DIAGNOSIS
Although poisoning can mimic other illnesses, the correct diagnosis can usually be established by the history, physical examination, routine and toxicologic laboratory evaluations, and characteristic clinical course.
HISTORY
The history should include the time, route, duration, and circumstances (location, surrounding events, and intent) of exposure; the name and amount of each drug, chemical, or ingredient involved; the time of onset, nature, and severity of symptoms; the time and type of first-aid measures provided; and the medical and psychiatric history.
In many cases the patient is confused, comatose, unaware of an exposure, or unable or unwilling to admit to one. Suspicious circumstances include unexplained sudden illness in a previously healthy person or a group of healthy people; a history of psychiatric problems (particularly depression); recent changes in health, economic status, or social relationships; and onset of illness during work with chemicals or after ingestion of food, drink (especially ethanol), or medications. When patients become ill soon after arriving from a foreign country or being arrested for criminal activity, “body packing” or “body stuffing” (ingesting or concealing illicit drugs in a body cavity) should be suspected. Relevant information may be available from family, friends, paramedics, police, pharmacists, physicians, and employers, who should be questioned regarding the patient’s habits, hobbies, behavioral changes, available medications, and antecedent events. A search of clothes, belongings, and place of discovery may reveal a suicide note or a container of drugs or chemicals. The imprint code on pills and the label on chemical products may be used to identify the ingredients and potential toxicity of a suspected poison by consulting a reference text, a computerized database, the manufacturer, or a regional poison information center (800-222-1222). Occupational exposures require review of any available material safety data sheet (MSDS) from the worksite. Because of increasing globalization, unfamiliar poisonings may result in local emergency department evaluation. Pharmaceuticals, industrial chemicals, or drugs of abuse from foreign countries may be identified with the assistance of a regional poison center or via the World Wide Web.
PHYSICAL EXAMINATION AND CLINICAL COURSE
The physical examination should focus initially on vital signs, the cardiopulmonary system, and neurologic status. The neurologic examination should include documentation of neuromuscular abnormalities such as dyskinesia, dystonia, fasciculations, myoclonus, rigidity, and tremors. The patient should also be examined for evidence of trauma and underlying illnesses. Focal neurologic findings are uncommon in poisoning, and their presence should prompt evaluation for a structural central nervous system (CNS) lesion. Examination of the eyes (for nystagmus and pupil size and reactivity), abdomen (for bowel activity and bladder size), and skin (for burns, bullae, color, warmth, moisture, pressure sores, and puncture marks) may reveal findings of diagnostic value. When the history is unclear, all orifices should be examined for the presence of chemical burns and drug packets. The odor of breath or vomitus and the color of nails, skin, or urine may provide important diagnostic clues.
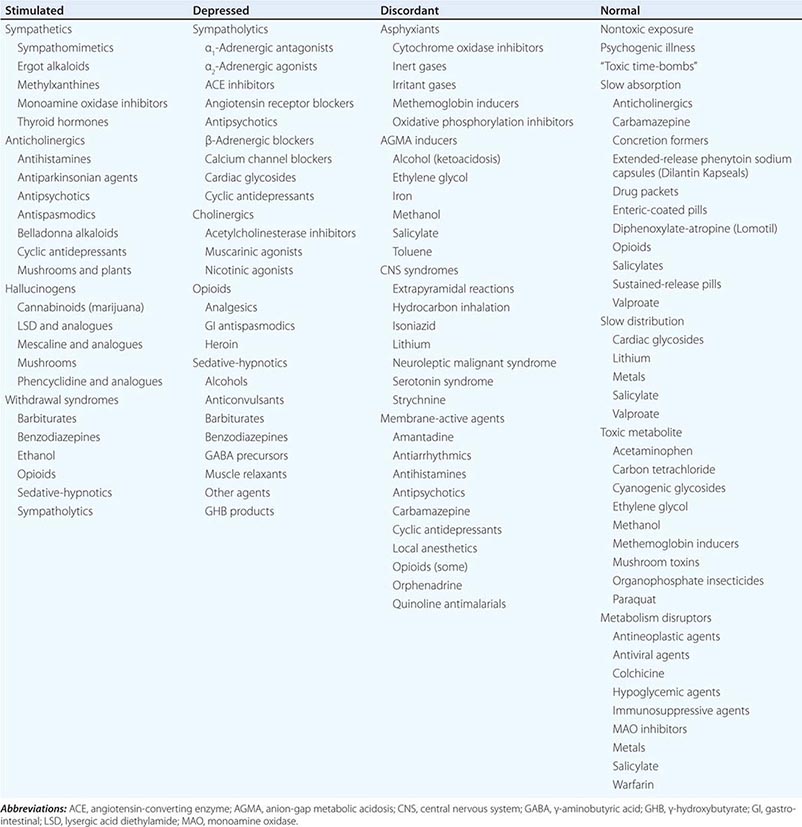
The diagnosis of poisoning in cases of unknown etiology primarily relies on pattern recognition. The first step is to assess the pulse, blood pressure, respiratory rate, temperature, and neurologic status and to characterize the overall physiologic state as stimulated, depressed, discordant, or normal (Table 473e-1). Obtaining a complete set of vital signs and reassessing them frequently are critical. Measuring core temperature is especially important, even in difficult or combative patients, since temperature elevation is the most reliable prognosticator of poor outcome in poisoning or drug withdrawal. The next step is to consider the underlying causes of the physiologic state and to attempt to identify a pathophysiologic pattern or toxic syndrome (toxidrome) based on the observed findings. Assessing the severity of physiologic derangements (Table 473e-2) is useful in this regard and also for monitoring the clinical course and response to treatment. The final step is to attempt to identify the particular agent involved by looking for unique or relatively poison-specific physical or ancillary test abnormalities. Distinguishing among toxidromes on the basis of the physiologic state is summarized next.
|
DIFFERENTIAL DIAGNOSIS OF POISONING BASED ON PHYSIOLOGIC STATE |
|
SEVERITY OF PHYSIOLOGIC STIMULATION AND DEPRESSION IN POISONING AND DRUG WITHDRAWAL |

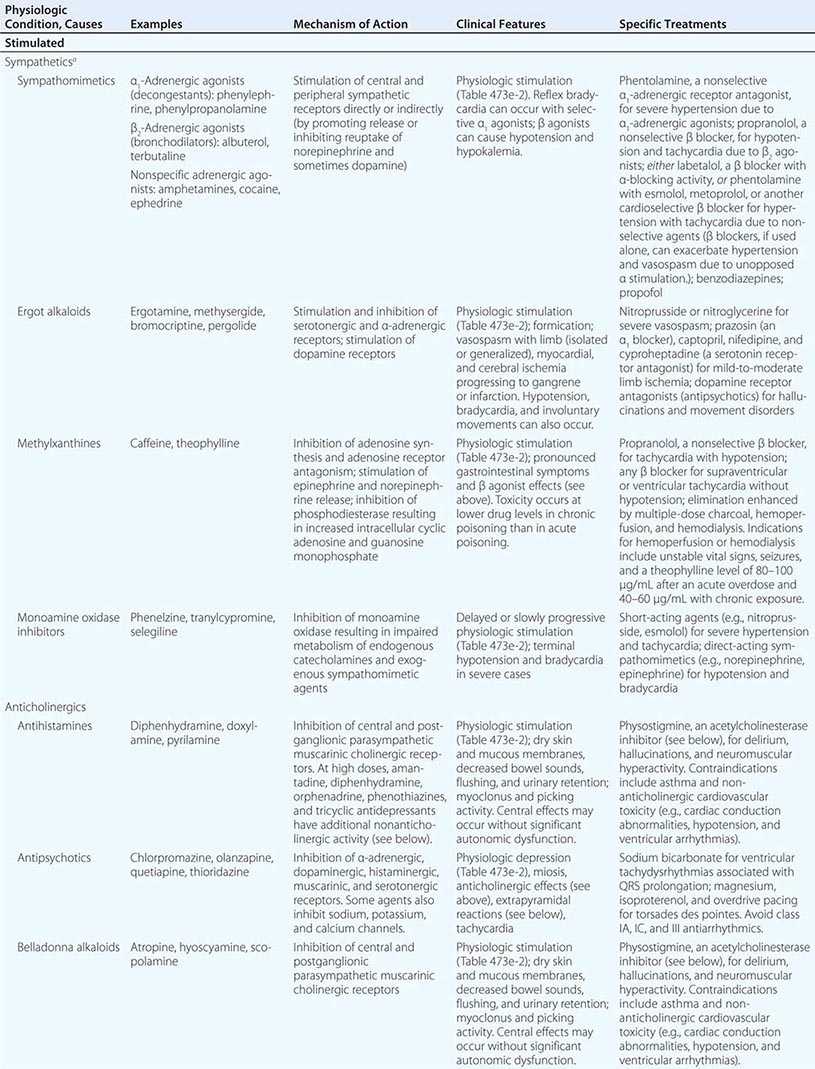
The Stimulated Physiologic State Increased pulse, blood pressure, respiratory rate, temperature, and neuromuscular activity characterize the stimulated physiologic state, which can reflect sympathetic, antimuscarinic (anticholinergic), or hallucinogen poisoning or drug withdrawal (Table 473e-1). Other features are noted in (Table 473e-2). Mydriasis, a characteristic feature of all stimulants, is most marked in antimuscarinic (anticholinergic) poisoning since pupillary reactivity relies on muscarinic control. In sympathetic poisoning (e.g., due to cocaine), pupils are also enlarged, but some reactivity to light remains. The antimuscarinic (anticholinergic) toxidrome is also distinguished by hot, dry, flushed skin; decreased bowel sounds; and urinary retention. Other stimulant syndromes increase sympathetic activity and cause diaphoresis, pallor, and increased bowel activity with varying degrees of nausea, vomiting, abnormal distress, and occasionally diarrhea. The absolute and relative degree of vital-sign changes and neuromuscular hyperactivity can help distinguish among stimulant toxidromes. Since sympathetics stimulate the peripheral nervous system more directly than do hallucinogens or drug withdrawal, markedly increased vital signs and organ ischemia suggest sympathetic poisoning. Findings helpful in suggesting the particular drug or class causing physiologic stimulation include reflex bradycardia from selective α-adrenergic stimulants (e.g., decongestants), hypotension from selective β-adrenergic stimulants (e.g., asthma therapeutics), limb ischemia from ergot alkaloids, rotatory nystagmus from phencyclidine and ketamine (the only physiologic stimulants that cause this finding), and delayed cardiac conduction from high doses of cocaine and some anticholinergic agents (e.g., antihistamines, cyclic antidepressants, and antipsychotics). Seizures suggest a sympathetic etiology, an anticholinergic agent with membrane-active properties (e.g., cyclic antidepressants, orphenadrine, phenothiazines), or a withdrawal syndrome. Close attention to core temperature is critical in patients with grade 4 physiologic stimulation (Table 473e-2).
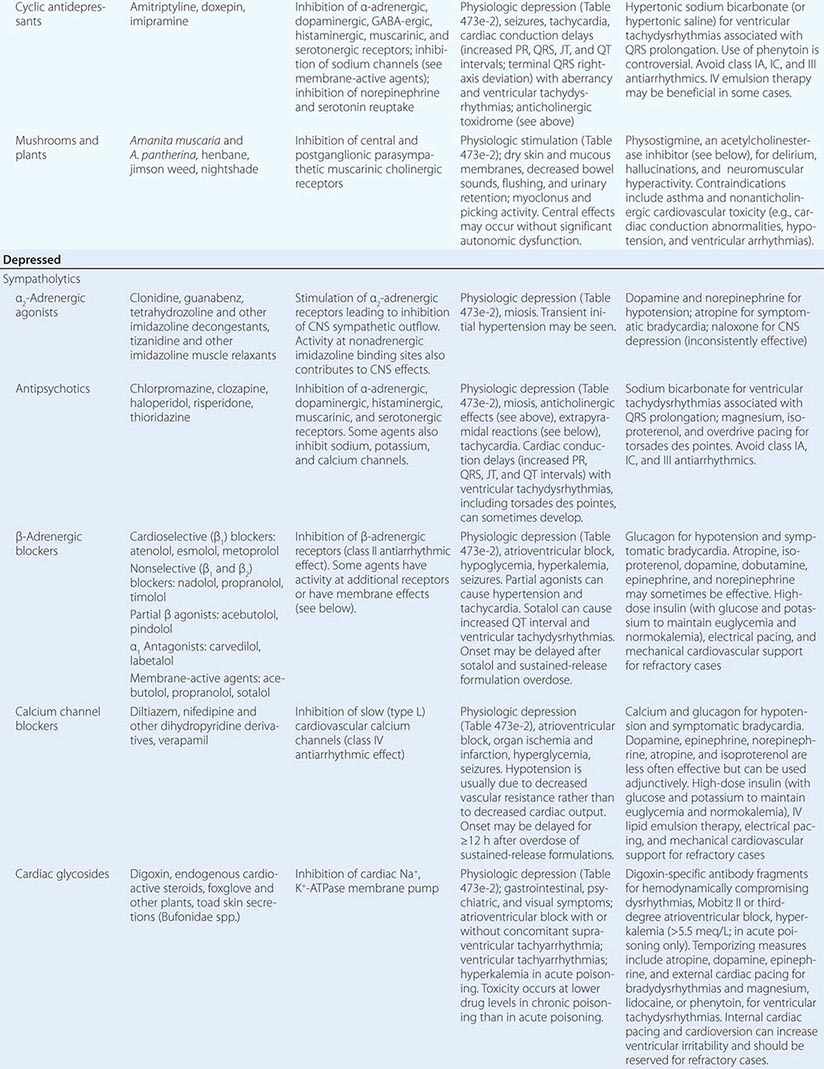
The Depressed Physiologic State Decreased pulse, blood pressure, respiratory rate, temperature, and neuromuscular activity are indicative of the depressed physiologic state caused by “functional” sympatholytics (agents that decrease cardiac function and vascular tone as well as sympathetic activity), cholinergic (muscarinic and nicotinic) agents, opioids, and sedative-hypnotic γ-aminobutyric acid (GABA)-ergic agents (Table 473e-1 and 473e-2). Miosis is also common and is most pronounced in opioid and cholinergic poisoning. Miosis is distinguished from other depressant syndromes by muscarinic and nicotinic signs and symptoms (Table 473e-1). Pronounced cardiovascular depression in the absence of significant CNS depression suggests a direct or peripherally acting sympatholytic. In contrast, in opioid and sedative-hypnotic poisoning, vital-sign changes are secondary to depression of CNS cardiovascular and respiratory centers (or consequent hypoxemia), and significant abnormalities in these parameters do not occur until there is a marked decrease in the level of consciousness (grade 3 or 4 physiologic depression; [Table 473e-2]). Other clues that suggest the cause of physiologic depression include cardiac arrhythmias and conduction disturbances (due to antiarrhythmics, β-adrenergic antagonists, calcium channel blockers, digitalis glycosides, propoxyphene, and cyclic antidepressants), mydriasis (due to tricyclic antidepressants, some antiarrhythmics, meperidine, and diphenoxylate-atropine [Lomotil]), nystagmus (due to sedative-hypnotics), and seizures (due to cholinergic agents, propoxyphene, and cyclic antidepressants).
The Discordant Physiologic State The discordant physiologic state is characterized by mixed vital-sign and neuromuscular abnormalities, as observed in poisoning by asphyxiants, CNS syndromes, membrane-active agents, and anion-gap metabolic acidosis (AGMA) inducers (Table 473e-1). In these conditions, manifestations of physiologic stimulation and physiologic depression occur together or at different times during the clinical course. For example, membrane-active agents can cause simultaneous coma, seizures, hypotension, and tachyarrhythmias. Alternatively, vital signs may be normal while the patient has an altered mental status or is obviously sick or clearly symptomatic. Early, pronounced vital-sign and mental-status changes suggest asphyxiant or membrane-active agent poisoning; the lack of such abnormalities suggests an AGMA inducer; and marked neuromuscular dysfunction without significant vital-sign abnormalities suggests a CNS syndrome.
The Normal Physiologic State A normal physiologic status and physical examination may be due to a nontoxic exposure, psychogenic illness, or poisoning by “toxic time-bombs”: agents that are slowly absorbed, are slowly distributed to their sites of action, require metabolic activation, or disrupt metabolic processes (Table 473e-1). Because so many medications have now been reformulated into a once-a-day preparations for the patient’s convenience and adherence, toxic time-bombs are increasingly common. Diagnosing a nontoxic exposure requires that the identity of the exposure agent be known or that a toxic time-bomb exposure be excluded and the time since exposure exceed the longest known or predicted interval between exposure and peak toxicity. Psychogenic illness (fear of being poisoned, mass hysteria) may also follow a nontoxic exposure and should be considered when symptoms are inconsistent with exposure history. Anxiety reactions resulting from a nontoxic exposure can cause mild physiologic stimulation (Table 473e-2) and be indistinguishable from toxicologic causes without ancillary testing or a suitable period of observation.
LABORATORY ASSESSMENT
Laboratory assessment may be helpful in the differential diagnosis. Increased AGMA is most common in advanced methanol, ethylene glycol, and salicylate intoxication but can occur with any poisoning that results in hepatic, renal, or respiratory failure; seizures; or shock. The serum lactate concentration is more commonly low (less than the anion gap) in the former and high (nearly equal to the anion gap) in the latter. An abnormally low anion gap can be due to elevated blood levels of bromide, calcium, iodine, lithium, or magnesium. An increased osmolal gap—a difference of >10 mmol/L between serum osmolality (measured by freezing-point depression) and osmolality calculated from serum sodium, glucose, and blood urea nitrogen levels—suggests the presence of a low-molecular-weight solute such as acetone; an alcohol (benzyl, ethanol, isopropanol, methanol); a glycol (diethylene, ethylene, propylene); ether (ethyl, glycol); or an “unmeasured” cation (calcium, magnesium) or sugar (glycerol, mannitol, sorbitol). Ketosis suggests acetone, isopropyl alcohol, salicylate poisoning, or alcoholic ketoacidosis. Hypoglycemia may be due to poisoning with β-adrenergic blockers, ethanol, insulin, oral hypoglycemic agents, quinine, and salicylates, whereas hyperglycemia can occur in poisoning with acetone, β-adrenergic agonists, caffeine, calcium channel blockers, iron, theophylline, or N-3-pyridylmethyl-N′-p-nitrophenylurea (PNU [Vacor]). Hypokalemia can be caused by barium, β-adrenergic agonists, caffeine, diuretics, theophylline, or toluene; hyperkalemia suggests poisoning with an α-adrenergic agonist, a β-adrenergic blocker, cardiac glycosides, or fluoride. Hypocalcemia may be seen in ethylene glycol, fluoride, and oxalate poisoning.
The electrocardiogram (ECG) can be useful for rapid diagnostic purposes. Bradycardia and atrioventricular block may occur in patients poisoned by α-adrenergic agonists, antiarrhythmic agents, beta blockers, calcium channel blockers, cholinergic agents (carbamate and organophosphate insecticides), cardiac glycosides, lithium, or tricyclic antidepressants. QRS- and QT-interval prolongation may be caused by hyperkalemia, various antidepressants, and other membrane-active drugs (Table 473e-1). Ventricular tachyarrhythmias may be seen in poisoning with cardiac glycosides, fluorides, membrane-active drugs, methylxanthines, sympathomimetics, antidepressants, and agents that cause hyperkalemia or potentiate the effects of endogenous catecholamines (e.g., chloral hydrate, aliphatic and halogenated hydrocarbons).
Radiologic studies may occasionally be useful. Pulmonary edema (adult respiratory distress syndrome (ARDS) can be caused by poisoning with carbon monoxide, cyanide, an opioid, paraquat, phencyclidine, a sedative-hypnotic, or salicylate; by inhalation of irritant gases, fumes, or vapors (acids and alkali, ammonia, aldehydes, chlorine, hydrogen sulfide, isocyanates, metal oxides, mercury, phosgene, polymers); or by prolonged anoxia, hyperthermia, or shock. Aspiration pneumonia is common in patients with coma, seizures, and petroleum distillate aspiration. The presence of radiopaque densities on abdominal x-rays suggests the ingestion of calcium salts, chloral hydrate, chlorinated hydrocarbons, heavy metals, illicit drug packets, iodinated compounds, potassium salts, enteric-coated tablets, or salicylates.
Toxicologic analysis of urine and blood (and occasionally of gastric contents and chemical samples) can sometimes confirm or rule out suspected poisoning. Interpretation of laboratory data requires knowledge of the qualitative and quantitative tests used for screening and confirmation (enzyme-multiplied, fluorescence polarization, and radio-immunoassays; colorimetric and fluorometric assays; thin-layer, gas-liquid, or high-performance liquid chromatography; gas chromatography; mass spectrometry), their sensitivity (limit of detection) and specificity, the preferred biologic specimen for analysis, and the optimal time of specimen sampling. Personal communication with the hospital laboratory is essential to an understanding of institutional testing capabilities and limitations.
Rapid qualitative hospital-based urine tests for drugs of abuse are only screening tests that cannot confirm the exact identity of the detected substance and should not be considered diagnostic or used for forensic purposes: False-positive and false-negative results are common. A positive screen may result from other pharmaceuticals that interfere with laboratory analysis (e.g., fluoroquinolones commonly cause “false-positive” opiate screens). Confirmatory testing with gas chromatography/mass spectrometry can be requested, but it often takes weeks to obtain a reported result. A negative screening result may mean that the responsible substance is not detectable by the test used or that its concentration is too low for detection at the time of sampling. For instance, recent new drugs of abuse that often result in emergency department evaluation for unexpected complications, such as synthetic cannabinoids (spice), cathinones (bath salts), and opiate substitutes (kratom), are not detectable by hospital-based tests. In cases where a drug concentration is too low to be detected early during clinical evaluation, repeating the test at a later time may yield a positive result. Patients symptomatic from drugs of abuse often require immediate management based on the history, physical examination, and observed toxidrome without laboratory confirmation (e.g., apnea from opioid intoxication). When the patient is asymptomatic or when the clinical picture is consistent with the reported history, qualitative screening is neither clinically useful nor cost-effective. Thus, qualitative drug screens are of greatest value for the evaluation of patients with severe or unexplained toxicities, such as coma, seizures, cardiovascular instability, metabolic or respiratory acidosis, and nonsinus cardiac rhythms. In contrast to qualitative drug screens, quantitative serum tests are useful for evaluation of patients poisoned with acetaminophen (Chap. 361), alcohols (including ethylene glycol and methanol), anticonvulsants, barbiturates, digoxin, heavy metals, iron, lithium, salicylate, and theophylline as well as for the presence of carboxyhemoglobin and methemoglobin. The serum concentration in these cases guides clinical management, and results are often available within an hour.
The response to antidotes is sometimes useful for diagnostic purposes. Resolution of altered mental status and abnormal vital signs within minutes of IV administration of dextrose, naloxone, or flumazenil is virtually diagnostic of hypoglycemia, opioid poisoning, and benzodiazepine intoxication, respectively. The prompt reversal of dystonic (extrapyramidal) signs and symptoms following an IV dose of benztropine or diphenhydramine confirms a drug etiology. Although complete reversal of both central and peripheral manifestations of anticholinergic poisoning by physostigmine is diagnostic of this condition, physostigmine may cause some arousal in patients with CNS depression of any etiology.
|
TREATMENT |
POISONING AND DRUG OVERDOSE |
GENERAL PRINCIPLES
Treatment goals include support of vital signs, prevention of further poison absorption (decontamination), enhancement of poison elimination, administration of specific antidotes, and prevention of reexposure (Table 473e-3). Specific treatment depends on the identity of the poison, the route and amount of exposure, the time of presentation relative to the time of exposure, and the severity of poisoning. Knowledge of the offending agents’ pharmacokinetics and pharmacodynamics is essential.
|
FUNDAMENTALS OF POISONING MANAGEMENT |
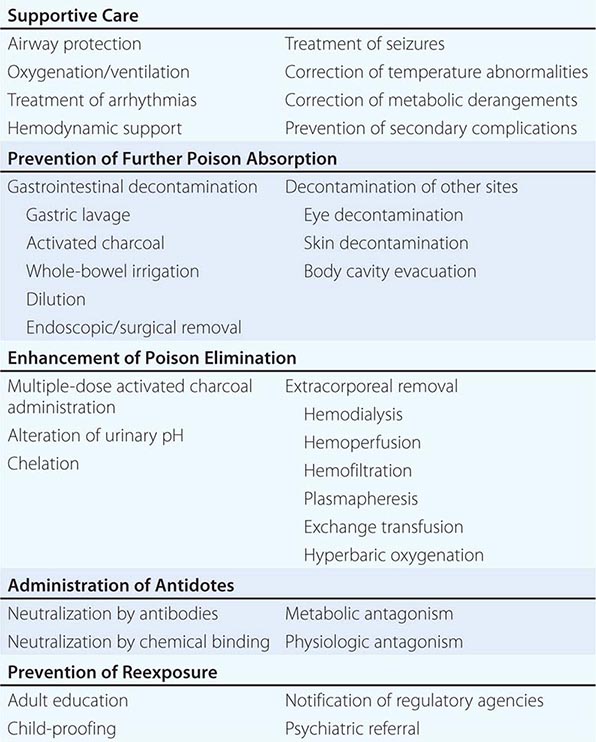
During the pretoxic phase, prior to the onset of poisoning, decontamination is the highest priority, and treatment is based solely on the history. The maximal potential toxicity based on the greatest possible exposure should be assumed. Since decontamination is more effective when accomplished soon after exposure and when the patient is asymptomatic, the initial history and physical examination should be focused and brief. It is also advisable to establish IV access and initiate cardiac monitoring, particularly in patients with potentially serious ingestions or unclear histories.
When an accurate history is not obtainable and a poison causing delayed toxicity (i.e., a toxic time-bomb) or irreversible damage is suspected, blood and urine should be sent for appropriate toxicologic screening and quantitative analysis. During poison absorption and distribution, blood levels may be greater than those in tissue and may not correlate with toxicity. However, high blood levels of agents whose metabolites are more toxic than the parent compound (acetaminophen, ethylene glycol, or methanol) may indicate the need for additional interventions (antidotes, dialysis). Most patients who remain asymptomatic or who become asymptomatic 6 h after ingestion are unlikely to develop subsequent toxicity and can be discharged safely. Longer observation will be necessary for patients who have ingested toxic time-bombs.
During the toxic phase—the interval between the onset of poisoning and its peak effects—management is based primarily on clinical and laboratory findings. Effects after an overdose usually begin sooner, peak later, and last longer than they do after a therapeutic dose. A drug’s published pharmacokinetic profile in standard references such as the Physician’s Desk Reference (PDR) is usually different from its toxicokinetic profile in overdose. Resuscitation and stabilization are the first priority. Symptomatic patients should have an IV line placed and should undergo oxygen saturation determination, cardiac monitoring, and continuous observation. Baseline laboratory, ECG, and x-ray evaluation may also be appropriate. Intravenous glucose (unless the serum level is documented to be normal), naloxone, and thiamine should be considered in patients with altered mental status, particularly those with coma or seizures. Decontamination should also be considered, but it is less likely to be effective during this phase than during the pretoxic phase.
Measures that enhance poison elimination may shorten the duration and severity of the toxic phase. However, they are not without risk, which must be weighed against the potential benefit. Diagnostic certainty (usually via laboratory confirmation) is generally a prerequisite. Intestinal (gut) dialysis with repetitive doses of activated charcoal (see “Multiple-Dose Activated Charcoal,” later) can enhance the elimination of selected poisons such as theophylline or carbamazepine. Urinary alkalinization may enhance the elimination of salicylates and a few other poisons. Chelation therapy can enhance the elimination of selected metals. Extracorporeal elimination methods are effective for many poisons, but their expense and risk make their use reasonable only in patients who would otherwise have an unfavorable outcome.
During the resolution phase of poisoning, supportive care and monitoring should continue until clinical, laboratory, and ECG abnormalities have resolved. Since chemicals are eliminated sooner from the blood than from tissues, blood levels are usually lower than tissue levels during this phase and again may not correlate with toxicity. This discrepancy applies particularly when extracorporeal elimination procedures are used. Redistribution from tissues may cause a rebound increase in the blood level after termination of these procedures. When a metabolite is responsible for toxic effects, continued treatment may be necessary in the absence of clinical toxicity or abnormal laboratory studies.
SUPPORTIVE CARE
The goal of supportive therapy is to maintain physiologic homeostasis until detoxification is accomplished and to prevent and treat secondary complications such as aspiration, bedsores, cerebral and pulmonary edema, pneumonia, rhabdomyolysis, renal failure, sepsis, thromboembolic disease, coagulopathy, and generalized organ dysfunction due to hypoxemia or shock.
Admission to an intensive care unit is indicated for the following: patients with severe poisoning (coma, respiratory depression, hypotension, cardiac conduction abnormalities, cardiac arrhythmias, hypothermia or hyperthermia, seizures); those needing close monitoring, antidotes, or enhanced elimination therapy; those showing progressive clinical deterioration; and those with significant underlying medical problems. Patients with mild to moderate toxicity can be managed on a general medical service, on an intermediate care unit, or in an emergency department observation area, depending on the anticipated duration and level of monitoring needed (intermittent clinical observation versus continuous clinical, cardiac, and respiratory monitoring). Patients who have attempted suicide require continuous observation and measures to prevent self-injury until they are no longer suicidal.
Respiratory Care Endotracheal intubation for protection against the aspiration of gastrointestinal contents is of paramount importance in patients with CNS depression or seizures as this complication can increase morbidity and mortality rates. Mechanical ventilation may be necessary for patients with respiratory depression or hypoxemia and for facilitation of therapeutic sedation or paralysis of patients in order to prevent or treat hyperthermia, acidosis, and rhabdomyolysis associated with neuromuscular hyperactivity. Since clinical assessment of respiratory function can be inaccurate, the need for oxygenation and ventilation is best determined by continuous pulse oximetry or arterial blood-gas analysis. The gag reflex is not a reliable indicator of the need for intubation. A patient with CNS depression may maintain airway patency while being stimulated but not if left alone. Drug-induced pulmonary edema is usually noncardiac rather than cardiac in origin, although profound CNS depression and cardiac conduction abnormalities suggest the latter. Measurement of pulmonary artery pressure may be necessary to establish the cause and direct appropriate therapy. Extracorporeal measures (membrane oxygenation, venoarterial perfusion, cardiopulmonary bypass) and partial liquid (perfluorocarbon) ventilation may be appropriate for severe but reversible respiratory failure.
Cardiovascular Therapy Maintenance of normal tissue perfusion is critical for complete recovery to occur once the offending agent has been eliminated. If hypotension is unresponsive to volume expansion, treatment with norepinephrine, epinephrine, or high-dose dopamine may be necessary. Intraaortic balloon pump counterpulsation and venoarterial or cardiopulmonary perfusion techniques should be considered for severe but reversible cardiac failure. For patients with a return of spontaneous circulation after resuscitative treatment for cardiopulmonary arrest secondary to poisoning, therapeutic hypothermia should be used according to protocol. Bradyarrhythmias associated with hypotension generally should be treated as described in Chaps. 274 and 275. Glucagon, calcium, and high-dose insulin with dextrose may be effective in beta blocker and calcium channel blocker poisoning. Antibody therapy may be indicated for cardiac glycoside poisoning.
Supraventricular tachycardia associated with hypertension and CNS excitation is almost always due to agents that cause generalized physiologic excitation (Table 473e–1). Most cases are mild or moderate in severity and require only observation or nonspecific sedation with a benzodiazepine. In severe cases or those associated with hemodynamic instability, chest pain, or ECG evidence of ischemia, specific therapy is indicated. When the etiology is sympathetic hyperactivity, treatment with a benzodiazepine should be prioritized. Further treatment with a combined alpha and beta blocker (labetalol), a calcium channel blocker (verapamil or diltiazem), or a combination of a beta blocker and a vasodilator (esmolol and nitroprusside) may be considered for cases refractory to high doses of benzodiazepines. Treatment with an α-adrenergic antagonist (phentolamine) alone may sometimes be appropriate. If the cause is anticholinergic poisoning, physostigmine alone can be effective. Supraventricular tachycardia without hypertension is generally secondary to vasodilation or hypovolemia and responds to fluid administration.
For ventricular tachyarrhythmias due to tricyclic antidepressants and other membrane-active agents (Table 473e-1), sodium bicarbonate is indicated, whereas class IA, IC, and III antiarrhythmic agents are contraindicated because of similar electrophysiologic effects. Although lidocaine and phenytoin are historically safe for ventricular tachyarrhythmias of any etiology, sodium bicarbonate should be considered first for any ventricular arrhythmia suspected to have a toxicologic etiology. Intravenous emulsion therapy has shown benefit for treatment of arrhythmias and hemodynamic instability from various membrane-active agents. Beta blockers can be hazardous if the arrhythmia is due to sympathetic hyperactivity. Magnesium sulfate and overdrive pacing (by isoproterenol or a pacemaker) may be useful in patients with torsades des pointes and prolonged QT intervals. Magnesium and anti-digoxin antibodies should be considered in patients with severe cardiac glycoside poisoning. Invasive (esophageal or intracardiac) ECG recording may be necessary to determine the origin (ventricular or supraventricular) of wide-complex tachycardias (Chaps. 276 and 277). If the patient is hemodynamically stable, however, it is reasonable to simply observe him or her rather than to administer another potentially proarrhythmic agent. Arrhythmias may be resistant to drug therapy until underlying acid-base, electrolyte, oxygenation, and temperature derangements are corrected.
Central Nervous System Therapies Neuromuscular hyperactivity and seizures can lead to hyperthermia, lactic acidosis, and rhabdomyolysis and should be treated aggressively. Seizures caused by excessive stimulation of catecholamine receptors (sympathomimetic or hallucinogen poisoning and drug withdrawal) or decreased activity of GABA (isoniazid poisoning) or glycine (strychnine poisoning) receptors are best treated with agents that enhance GABA activity, such as benzodiazepine or barbiturates. Since benzodiazepines and barbiturates act by slightly different mechanisms (the former increases the frequency and the latter increases the duration of chloride channel opening in response to GABA), therapy with both may be effective when neither is effective alone. Seizures caused by isoniazid, which inhibits the synthesis of GABA at several steps by interfering with the cofactor pyridoxine (vitamin B6), may require high doses of supplemental pyridoxine. Seizures resulting from membrane destabilization (beta blocker or cyclic antidepressant poisoning) require GABA enhancers (benzodiazepines first, barbiturates second). Phenytoin is contraindicated in toxicologic seizures: Animal and human data demonstrate worse outcomes after phenytoin loading, especially in theophylline overdose. For poisons with central dopaminergic effects (methamphetamine, phencyclidine) manifested by psychotic behavior, a dopamine receptor antagonist, such as haloperidol, may be useful. In anticholinergic and cyanide poisoning, specific antidotal therapy may be necessary. The treatment of seizures secondary to cerebral ischemia or edema or to metabolic abnormalities should include correction of the underlying cause. Neuromuscular paralysis is indicated in refractory cases. Electroencephalographic monitoring and continuing treatment of seizures are necessary to prevent permanent neurologic damage. Serotonergic receptor overstimulation in serotonin syndrome may be treated with cyproheptadine.
Other Measures Temperature extremes, metabolic abnormalities, hepatic and renal dysfunction, and secondary complications should be treated by standard therapies.
PREVENTION OF POISON ABSORPTION
Gastrointestinal Decontamination Whether or not to perform gastrointestinal decontamination and which procedure to use depends on the time since ingestion; the existing and predicted toxicity of the ingestant; the availability, efficacy, and contraindications of the procedure; and the nature, severity, and risk of complications. The efficacy of all decontamination procedures decreases with time, and data are insufficient to support or exclude a beneficial effect when they are used >1 h after ingestion. The average time from ingestion to presentation for treatment is >1 h for children and >3 h for adults. Most patients will recover from poisoning uneventfully with good supportive care alone, but complications of gastrointestinal decontamination, particularly aspiration, can prolong this process. Hence, gastrointestinal decontamination should be performed selectively, not routinely, in the management of overdose patients. It is clearly unnecessary when predicted toxicity is minimal or the time of expected maximal toxicity has passed without significant effect.
Activated charcoal has comparable or greater efficacy; has fewer contraindications and complications; and is less aversive and invasive than ipecac or gastric lavage. Thus it is the preferred method of gastrointestinal decontamination in most situations. Activated charcoal suspension (in water) is given orally via a cup, straw, or small-bore nasogastric tube. The generally recommended dose is 1 g/kg body weight because of its dosing convenience, although in vitro and in vivo studies have demonstrated that charcoal adsorbs ≥90% of most substances when given in an amount equal to 10 times the weight of the substance. Palatability may be increased by adding a sweetener (sorbitol) or a flavoring agent (cherry, chocolate, or cola syrup) to the suspension. Charcoal adsorbs ingested poisons within the gut lumen, allowing the charcoal-toxin complex to be evacuated with stool. Charged (ionized) chemicals such as mineral acids, alkalis, and highly dissociated salts of cyanide, fluoride, iron, lithium, and other inorganic compounds are not well adsorbed by charcoal. In studies with animals and human volunteers, charcoal decreases the absorption of ingestants by an average of 73% when given within 5 min of ingestant administration, 51% when given at 30 min, and 36% when given at 60 min. For this reason, charcoal given before hospital arrival increases the potential clinical benefit. Side effects of charcoal include nausea, vomiting, and diarrhea or constipation. Charcoal may also prevent the absorption of orally administered therapeutic agents. Complications include mechanical obstruction of the airway, aspiration, vomiting, and bowel obstruction and infarction caused by inspissated charcoal. Charcoal is not recommended for patients who have ingested corrosives because it obscures endoscopy.
Gastric lavage should be considered for life-threatening poisons that cannot be treated effectively with other decontamination, elimination, or antidotal therapies (e.g., colchicine). Gastric lavage is performed by sequentially administering and aspirating ~5 mL of fluid per kilogram of body weight through a no. 40 French orogastric tube (no. 28 French tube for children). Except in infants, for whom normal saline is recommended, tap water is acceptable. The patient should be placed in Trendelenburg and left lateral decubitus positions to prevent aspiration (even if an endotracheal tube is in place). Lavage decreases ingestant absorption by an average of 52% if performed within 5 min of ingestion administration, 26% if performed at 30 min, and 16% if performed at 60 min. Significant amounts of ingested drug are recovered from <10% of patients. Aspiration is a common complication (occurring in up to 10% of patients), especially when lavage is performed improperly. Serious complications (esophageal and gastric perforation, tube misplacement in the trachea) occur in ~1% of patients. For this reason, the physician should personally insert the lavage tube and confirm its placement, and the patient must be cooperative during the procedure. Gastric lavage is contraindicated in corrosive or petroleum distillate ingestions because of the respective risks of gastroesophageal perforation and aspiration pneumonitis. It is also contraindicated in patients with a compromised unprotected airway and those at risk for hemorrhage or perforation due to esophageal or gastric pathology or recent surgery. Finally, gastric lavage is absolutely contraindicated in combative patients or those who refuse, as most published complications involve patient resistance to the procedure.
Syrup of ipecac, an emetogenic agent that was once the substance most commonly used for decontamination, no longer has a role in poisoning management. Even the American Academy of Pediatrics—traditionally the strongest proponent of ipecac—issued a policy statement in 2003 recommending that ipecac should no longer be used in poisoning treatment. Chronic ipecac use (by patients with anorexia nervosa or bulimia) has been reported to cause electrolyte and fluid abnormalities, cardiac toxicity, and myopathy.
Whole-bowel irrigation is performed by administering a bowel-cleansing solution containing electrolytes and polyethylene glycol (Golytely, Colyte) orally or by gastric tube at a rate of 2 L/h (0.5 L/h in children) until rectal effluent is clear. The patient must be in a sitting position. Although data are limited, whole-bowel irrigation appears to be as effective as other decontamination procedures in volunteer studies. It is most appropriate for those who have ingested foreign bodies, packets of illicit drugs, and agents that are poorly adsorbed by charcoal (e.g., heavy metals). This procedure is contraindicated in patients with bowel obstruction, ileus, hemodynamic instability, and compromised unprotected airways.
Cathartics are salts (disodium phosphate, magnesium citrate and sulfate, sodium sulfate) or saccharides (mannitol, sorbitol) that historically have been given with activated charcoal to promote the rectal evacuation of gastrointestinal contents. However, no animal, volunteer, or clinical data have ever demonstrated any decontamination benefit from cathartics. Abdominal cramps, nausea, and occasional vomiting are side effects. Complications of repeated dosing include severe electrolyte disturbances and excessive diarrhea. Cathartics are contraindicated in patients who have ingested corrosives and in those with preexisting diarrhea. Magnesium-containing cathartics should not be used in patients with renal failure.
Dilution (i.e., drinking water, another clear liquid, or milk at a volume of 5 mL/kg of body weight) is recommended only after the ingestion of corrosives (acids, alkali). It may increase the dissolution rate (and hence absorption) of capsules, tablets, and other solid ingestants and should not be used in these circumstances.
Endoscopic or surgical removal of poisons may be useful in rare situations, such as ingestion of a potentially toxic foreign body that fails to transit the gastrointestinal tract, a potentially lethal amount of a heavy metal (arsenic, iron, mercury, thallium), or agents that have coalesced into gastric concretions or bezoars (heavy metals, lithium, salicylates, sustained-release preparations). Patients who become toxic from cocaine due to its leakage from ingested drug packets require immediate surgical intervention.
Decontamination of Other Sites Immediate, copious flushing with water, saline, or another available clear, drinkable liquid is the initial treatment for topical exposures (exceptions include alkali metals, calcium oxide, phosphorus). Saline is preferred for eye irrigation. A triple wash (water, soap, water) may be best for dermal decontamination. Inhalational exposures should be treated initially with fresh air or oxygen. The removal of liquids from body cavities such as the vagina or rectum is best accomplished by irrigation. Solids (drug packets, pills) should be removed manually, preferably under direct visualization.
ENHANCEMENT OF POISON ELIMINATION
Although the elimination of most poisons can be accelerated by therapeutic interventions, the pharmacokinetic efficacy (removal of drug at a rate greater than that accomplished by intrinsic elimination) and clinical benefit (shortened duration of toxicity or improved outcome) of such interventions are often more theoretical than proven. Accordingly, the decision to use such measures should be based on the actual or predicted toxicity and the potential efficacy, cost, and risks of therapy.
Multiple-Dose Activated Charcoal Repetitive oral dosing with charcoal can enhance the elimination of previously absorbed substances by binding them within the gut as they are excreted in the bile, are secreted by gastrointestinal cells, or passively diffuse into the gut lumen (reverse absorption or enterocapillary exsorption). Doses of 0.5–1 g/kg of body weight every 2–4 h, adjusted downward to avoid regurgitation in patients with decreased gastrointestinal motility, are generally recommended. Pharmacokinetic efficacy approaches that of hemodialysis for some agents (e.g., phenobarbital, theophylline). Multiple-dose therapy should be considered only for selected agents (theophylline, phenobarbital, carbamazepine, dapsone, quinine). Complications include intestinal obstruction, pseudo-obstruction, and nonocclusive intestinal infarction in patients with decreased gut motility. Because of electrolyte and fluid shifts, sorbitol and other cathartics are absolutely contraindicated when multiple doses of activated charcoal are administered.
Urinary Alkalinization Ion trapping via alteration of urine pH may prevent the renal reabsorption of poisons that undergo excretion by glomerular filtration and active tubular secretion. Since membranes are more permeable to non-ionized molecules than to their ionized counterparts, acidic (low-pKa) poisons are ionized and trapped in alkaline urine, whereas basic ones become ionized and trapped in acid urine. Urinary alkalinization (producing a urine pH ≥7.5 and a urine output of 3–6 mL/kg of body weight per hour by the addition of sodium bicarbonate to an IV solution) enhances the excretion of chlorophenoxyacetic acid herbicides, chlorpropamide, diflunisal, fluoride, methotrexate, phenobarbital, sulfonamides, and salicylates. Contraindications include congestive heart failure, renal failure, and cerebral edema. Acid-base, fluid, and electrolyte parameters should be monitored carefully. Although acid diuresis may make theoretical sense for some overdoses (amphetamines), it is never indicated and is potentially harmful.
Extracorporeal Removal Hemodialysis, charcoal or resin hemoperfusion, hemofiltration, plasmapheresis, and exchange transfusion are capable of removing any toxin from the bloodstream. Agents most amenable to enhanced elimination by dialysis have low molecular mass (<500 Da), high water solubility, low protein binding, small volumes of distribution (<1 L/kg of body weight), prolonged elimination (long half-life), and high dialysis clearance relative to total-body clearance. Molecular weight, water solubility, and protein binding do not limit the efficacy of the other forms of extracorporeal removal.
Dialysis should be considered in cases of severe poisoning due to carbamazepine, ethylene glycol, isopropyl alcohol, lithium, methanol, theophylline, salicylates, and valproate. Although hemoperfusion may be more effective in removing some of these poisons, it does not correct associated acid-base and electrolyte abnormalities, and most hospitals no longer have hemoperfusion cartridges readily available. Fortunately, recent advances in hemodialysis technology make it as effective as hemoperfusion for removing poisons such as caffeine, carbamazepine, and theophylline. Both techniques require central venous access and systemic anticoagulation and may result in transient hypotension. Hemoperfusion may also cause hemolysis, hypocalcemia, and thrombocytopenia. Peritoneal dialysis and exchange transfusion are less effective but may be used when other procedures are unavailable, contraindicated, or technically difficult (e.g., in infants). Exchange transfusion may be indicated in the treatment of severe arsine- or sodium chlorate–induced hemolysis, methemoglobinemia, and sulfhemoglobinemia. Although hemofiltration can enhance elimination of aminoglycosides, vancomycin, and metal-chelate complexes, the roles of hemofiltration and plasmapheresis in the treatment of poisoning are not yet defined.
Candidates for extracorporeal removal therapies include patients with severe toxicity whose condition deteriorates despite aggressive supportive therapy; those with potentially prolonged, irreversible, or fatal toxicity; those with dangerous blood levels of toxins; those who lack the capacity for self-detoxification because of liver or renal failure; and those with a serious underlying illness or complication that will adversely affect recovery.
Other Techniques The elimination of heavy metals can be enhanced by chelation, and the removal of carbon monoxide can be accelerated by hyperbaric oxygenation.
ADMINISTRATION OF ANTIDOTES
Antidotes counteract the effects of poisons by neutralizing them (e.g., antibody-antigen reactions, chelation, chemical binding) or by antagonizing their physiologic effects (e.g., activation of opposing nervous system activity, provision of a competitive metabolic or receptor substrate). Poisons or conditions with specific antidotes include acetaminophen, anticholinergic agents, anticoagulants, benzodiazepines, beta blockers, calcium channel blockers, carbon monoxide, cardiac glycosides, cholinergic agents, cyanide, drug-induced dystonic reactions, ethylene glycol, fluoride, heavy metals, hypoglycemic agents, isoniazid, membrane-active agents, methemoglobinemia, opioids, sympathomimetics, and a variety of envenomations. Intravenous lipid emulsion has been shown to be a successful antidote for poisoning from various anesthetics and membrane-active agents (e.g., cyclic antidepressants), but the exact mechanism of benefit is still under investigation. Antidotes can significantly reduce morbidity and mortality rates but are potentially toxic if used for inappropriate reasons. Since their safe use requires correct identification of a specific poisoning or syndrome, details of antidotal therapy are discussed with the conditions for which they are indicated (Table 473e-4).
|
PATHOPHYSIOLOGIC FEATURES AND TREATMENT OF SPECIFIC TOXIC SYNDROMES AND POISONINGS |
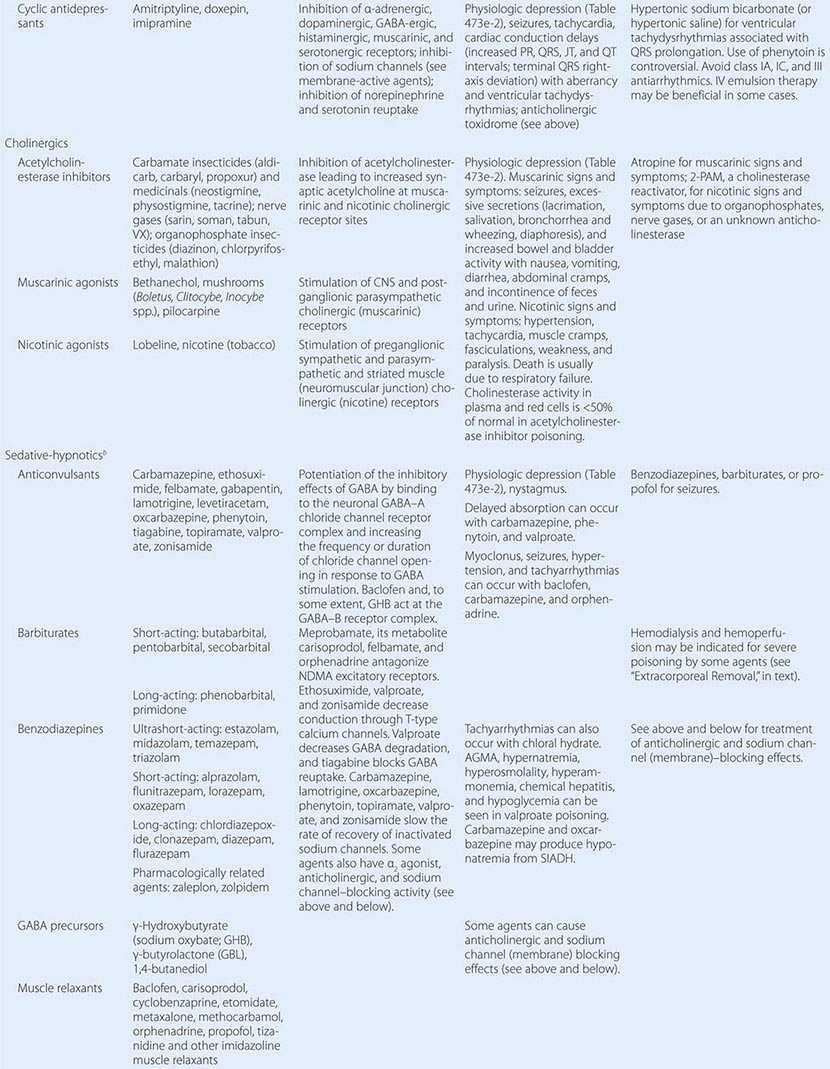
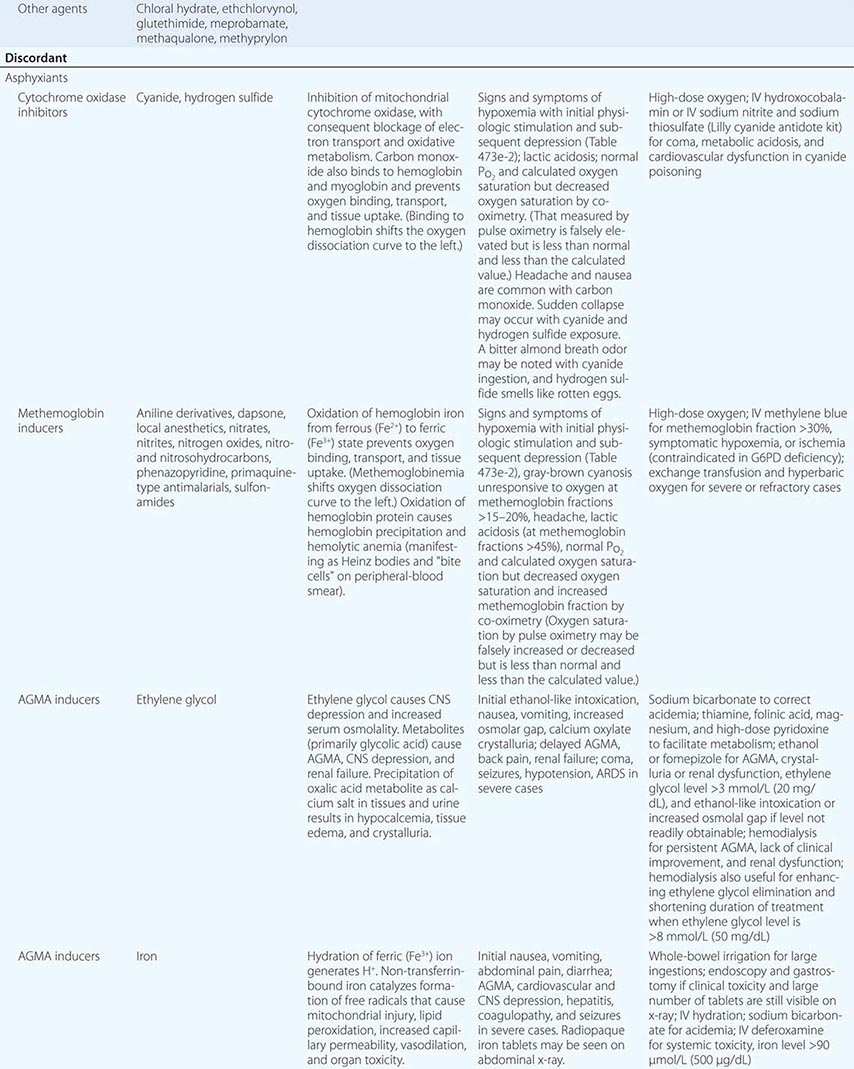
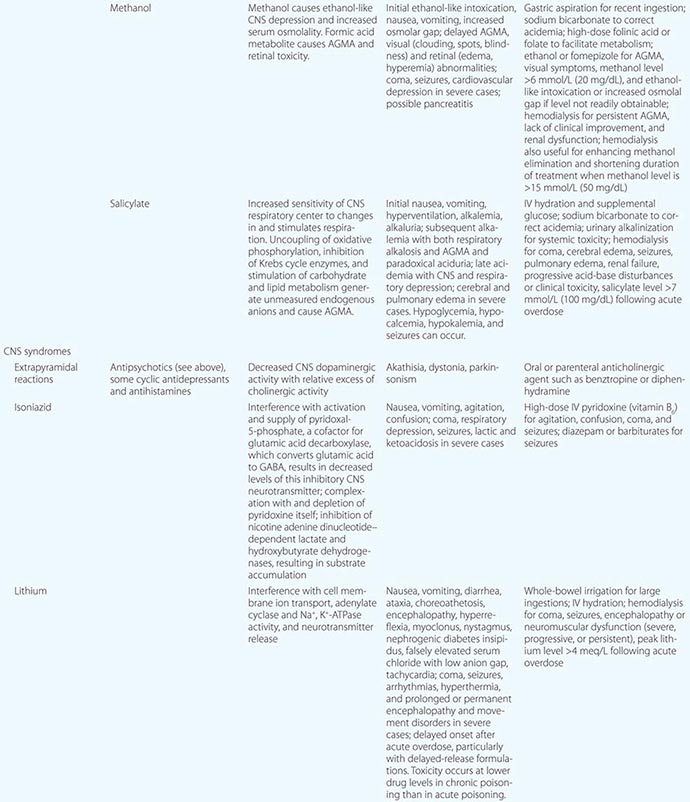

PREVENTION OF REEXPOSURE
Poisoning is a preventable illness. Unfortunately, some adults and children are poison-prone, and recurrences are common. Unintentional polypharmacy poisoning has become especially common among adults with developmental delays, among the growing population of geriatric patients who are prescribed a large number of medications, and among adolescents and young adults experimenting with pharmaceuticals for recreational euphoria. Adults with unintentional exposures should be instructed regarding the safe use of medications and chemicals (according to labeling instructions). Confused patients may need assistance with the administration of their medications. Errors in dosing by health care providers may require educational efforts. Patients should be advised to avoid circumstances that result in chemical exposure or poisoning. Appropriate agencies and health departments should be notified in cases of environmental or workplace exposure. The best approach to young children and patients with intentional overdose (deliberate self-harm or attempted suicide) is to limit their access to poisons. In households where children live or visit, alcoholic beverages, medications, household products (automotive, cleaning, fuel, pet-care, and toiletry products), inedible plants, and vitamins should be kept out of reach or in locked or child-proof cabinets. Depressed or psychotic patients should undergo psychiatric assessment, disposition, and follow-up. They should be given prescriptions for a limited supply of drugs with a limited number of refills and should be monitored for compliance and response to therapy.
SPECIFIC TOXIC SYNDROMES AND POISONINGS
Table 473e-4 summarizes the pathophysiology, clinical features, and treatment of toxidromes and poisonings that are common, produce life-threatening toxicity, or require unique therapeutic interventions. In all cases, treatment should include attention to the general principles discussed above and, in particular, supportive care. Poisonings not covered in this chapter are discussed in specialized texts.
Alcohol, cocaine, hallucinogen, and opioid poisoning and alcohol and opioid withdrawal are discussed in Chaps. 467–469e; nicotine addiction is discussed in Chap. 470; acetaminophen poisoning is discussed in Chap. 361; the neuroleptic malignant syndrome is discussed in Chap. 449; and heavy metal poisoning is discussed in Chap. 472e.
ACKNOWLEDGMENT
The author acknowledges the contributions of Christopher H. Linden and Michael J. Burns to this chapter in previous editions of this text.
474 |
Disorders Caused by Venomous Snakebites and Marine Animal Exposures |
This chapter outlines general principles for the evaluation and management of victims of envenomation and poisoning by venomous snakes and marine animals. Because the incidence of serious bites and stings is relatively low in developed nations, there is a paucity of relevant clinical research; as a result, therapeutic decision making often is based on anecdotal information.
VENOMOUS SNAKEBITE
EPIDEMIOLOGY
The venomous snakes of the world belong to the families Viperidae (subfamily Viperinae: Old World vipers; subfamily Crotalinae: New World and Asian pit vipers), Elapidae (including cobras, coral snakes, sea snakes, kraits, and all Australian venomous snakes), Lamprophiidae (subfamily Atractaspidinae: burrowing asps), and Colubridae (a large family in which most species are nonvenomous and only a few are dangerously toxic to humans). Most snakebites occur in developing countries with temperate and tropical climates in which populations subsist on agriculture and fishing. Recent estimates indicate that somewhere between 1.2 million and 5.5 million snakebites occur worldwide each year, with 421,000–1,841,000 envenomations and 20,000–94,000 deaths. Such wide-ranging estimates reflect the challenges of collecting accurate data in the regions most affected by venomous snakes; many victims do not seek hospital treatment, and reporting and record keeping are generally poor.
SNAKE ANATOMY/IDENTIFICATION
The typical snake venom delivery apparatus consists of bilateral venom glands situated below and behind the eyes and connected by ducts to hollow anterior maxillary fangs. In viperids (vipers and pit vipers), these fangs are long and highly mobile; they are retracted against the roof of the mouth when the snake is at rest and brought to an upright position for a strike. In elapids, the fangs are smaller and are relatively fixed in an erect position. Approximately 20% of pit viper bites and higher percentages of other snakebites (up to 75% for sea snakes) are “dry” bites, meaning no venom is released. Significant envenomation probably occurs in ~50% of all venomous snakebites.
Differentiation of venomous from nonvenomous snake species can be difficult. Viperids are characterized by somewhat triangular heads (a feature shared with many harmless snakes), elliptical pupils (also seen in some nonvenomous snakes, such as boas and pythons), enlarged maxillary fangs, and, in pit vipers, heat-sensing pits (foveal organs) on each side of the head that assist with locating prey and aiming strikes. The New World rattlesnakes possess a series of interlocking keratin plates (the rattle) on the tip of the tail that emits a buzzing sound when the snake rapidly vibrates its tail; this sound serves as a warning signal to perceived threats. Identifying venomous snakes by color pattern is notoriously misleading, as many harmless snakes have color patterns that closely mimic those of venomous snakes found in the same region.
VENOMS AND CLINICAL MANIFESTATIONS
Snake venoms are highly variable and complex mixtures of enzymes, low-molecular-weight polypeptides, glycoproteins, and other constituents. Among the deleterious components are hemorrhagins that promote vascular leakage and cause both local and systemic bleeding. Proteolytic enzymes cause local tissue necrosis, affect the coagulation pathway at various steps, and impair organ function. Hyaluronidases promote the spread of venom through connective tissue. Myocardial depressant factors reduce cardiac output, and bradykinins cause vasodilation and hypotension. Neurotoxins act either pre- or postsynaptically to block transmission at the neuromuscular junction, causing muscle paralysis. Most snake venoms have multisystem effects on their victims.
After a venomous snakebite, the time to symptom onset and clinical presentation can be quite variable and depend on the species involved, the anatomic location of the bite, and the amount of venom injected. Envenomations by most viperids and some elapids with necrotizing venoms cause progressive local pain, swelling, ecchymosis (Fig. 474-1), and (over a period of hours to days) hemorrhagic or serum-filled vesicles and bullae. In serious bites, tissue loss can be significant (Figs. 474-2 and 474-3). Systemic findings are extremely variable and can include tachycardia or bradycardia, hypotension, generalized weakness, changes in taste, mouth numbness, muscle fasciculations, pulmonary edema, renal dysfunction, and spontaneous hemorrhage (from essentially any anatomic site). Envenomations by neurotoxic elapids such as kraits (Bungarus species), many Australian elapids (e.g., death adders [Acanthophis species] and tiger snakes [Notechis species]), some cobras (Naja species), and some viperids (e.g., the South American rattlesnake [Crotalus durissus] and some Indian Russell’s vipers [Daboia russelii]) cause neurologic dysfunction. Early findings may consist of nausea and vomiting, headache, paresthesias or numbness, and altered mental status. Victims may develop cranial nerve abnormalities (e.g., ptosis, difficulty swallowing) followed by peripheral motor weakness. Severe envenomation may result in muscle paralysis, including the muscles of respiration, and lead to death from respiratory failure and aspiration. Sea snake envenomation results in local pain (variable), generalized myalgias, trismus, rhabdomyolysis, and progressive flaccid paralysis; these manifestations can be delayed for several hours.
FIGURE 474-1 Northern Pacific rattlesnake (Crotalus oreganus oreganus) envenomations. A. Moderately severe envenomation. Note edema and early ecchymosis 2 h after a bite to the finger. B. Severe envenomation. Note extensive ecchymosis 5 days after a bite to the ankle.
FIGURE 474-2 Early stages of severe, full-thickness necrosis 5 days after a Russell’s viper (Daboia russelii) bite in southwestern India.
FIGURE 474-3 Severe necrosis 10 days after a pit viper bite in a young child in Colombia. (Courtesy of Jay R. Stanka; with permission.)
MORBIDITY AND MORTALITY
The overall mortality rates for victims of venomous snakebites are low in regions with rapid access to medical care and appropriate antivenoms. In the United States, for example, the mortality rate is <1% for victims who receive antivenom. Eastern and western diamondback rattlesnakes (Crotalus adamanteus and Crotalus atrox, respectively) are responsible for the majority of snakebite deaths in the United States. Snakes responsible for large numbers of deaths in other countries include cobras (Naja spp.), carpet and saw-scaled vipers (Echis spp.), Russell’s vipers (D. russelii), large African vipers (Bitis spp.), lancehead pit vipers (Bothrops spp.), and tropical rattlesnakes (C. durissus).
The incidence of morbidity—defined as permanent functional loss in a bitten extremity—is difficult to estimate but is substantial. Morbidity may be due to muscle, nerve, or vascular injury or to scar contracture. Such morbidity can have devastating consequences for victims in the developing world when they lose the ability to work and provide for their families. In the United States, functional loss tends to be more common and severe after rattlesnake bites than after bites by copperheads (Agkistrodon contortrix) or water moccasins (Agkistrodon piscivorus).
GLOBAL CONSIDERATIONS
![]() In many developing countries where snakebites are common, scarce access to medical care and antivenom resources contributes to high rates of morbidity and mortality. In many countries, the available antivenoms are inappropriate and ineffective against the venoms of medically important indigenous snakes. In those regions, further research is necessary to determine the actual impact of venomous snakebites and the specific antivenom needs in terms of both quantity and spectrum of coverage. Without accurate statistics, it is difficult to persuade antivenom manufacturers to begin and sustain production of appropriate antisera in developing nations. There is evidence that antivenoms can be produced in much more cost-effective ways than those currently being used. Just as important as getting the correct antivenoms into underserved regions is the need to educate populations about snakebite prevention and to train medical care providers in proper management approaches. Local protocols written with significant input from experienced providers in the region of concern should be developed and distributed. Appropriate antivenoms must be available at the likely first point of medical contact for patients (e.g., primary health centers) in order to minimize the common practice of referring victims to more distant, higher levels of care for the initiation of antivenom therapy. Those who care for snakebite victims in these often-remote clinics must have the skills and confidence required to begin antivenom treatment (and to treat possible reactions) as soon as possible when needed.
In many developing countries where snakebites are common, scarce access to medical care and antivenom resources contributes to high rates of morbidity and mortality. In many countries, the available antivenoms are inappropriate and ineffective against the venoms of medically important indigenous snakes. In those regions, further research is necessary to determine the actual impact of venomous snakebites and the specific antivenom needs in terms of both quantity and spectrum of coverage. Without accurate statistics, it is difficult to persuade antivenom manufacturers to begin and sustain production of appropriate antisera in developing nations. There is evidence that antivenoms can be produced in much more cost-effective ways than those currently being used. Just as important as getting the correct antivenoms into underserved regions is the need to educate populations about snakebite prevention and to train medical care providers in proper management approaches. Local protocols written with significant input from experienced providers in the region of concern should be developed and distributed. Appropriate antivenoms must be available at the likely first point of medical contact for patients (e.g., primary health centers) in order to minimize the common practice of referring victims to more distant, higher levels of care for the initiation of antivenom therapy. Those who care for snakebite victims in these often-remote clinics must have the skills and confidence required to begin antivenom treatment (and to treat possible reactions) as soon as possible when needed.
MARINE ENVENOMATIONS
Much of the management of envenomation by marine creatures is supportive in nature. A specific marine antivenom can be used when appropriate.
INVERTEBRATES
Cnidarians The Golgi apparatus of the cnidoblast cells within cnidarians, such as hydroids, fire coral, jellyfish, Portuguese men-of-war, and sea anemones, secretes specialized living stinging organelles called cnidae (also referred to as cnidocysts, a term that encompasses nematocysts, ptychocysts, and spirocysts). Within each organelle resides a stinging mechanism (“thread tube”) and venom. In the stinging process, cnidocysts are released and discharged upon mechanosensory stimulation. The venoms from these organisms contain bioactive substances such as tetramine, 5-hydroxytryptamine, histamine, serotonin, and high-molecular-weight toxins, all of which can, among other effects, change the permeability of cells to ions. Victims usually report immediate prickling or burning, pruritus, paresthesias, and painful throbbing with radiation. The skin becomes reddened, darkened, edematous, and blistered and may show signs of superficial necrosis. A legion of neurologic, cardiovascular, respiratory, rheumatologic, gastrointestinal, renal, and ocular symptoms has been described, especially following stings from anemones, Physalia species, and scyphozoans. Anaphylaxis is possible. Hundreds of deaths have been reported, many of them caused by Chironex fleckeri, Stomolophus nomurai, Physalia physalis, and Chiropsalmus quadrumanus. Irukandji syndrome, associated with the Australian jellyfish Carukia barnesi and other species, is a potentially fatal condition that most commonly is characterized by hypertension; severe back, chest, and abdominal pain; nausea and vomiting; headache; sweating; and, in the most serious cases, myocardial troponin leak, pulmonary edema, and ultimately hypotension. This syndrome is thought to be mediated, at least in part, by the release of endogenous catecholamines followed by cytokines and nitric oxide.
Rescuers should note that envenomations by different cnidarians (typified by jellyfish) may respond differently to similar topical therapies; thus, the recommendations in this chapter must be tailored to local species and clinical practices. During stabilization, the skin should be decontaminated immediately with a generous application of lidocaine (up to 4%), an all-purpose agent that appears to be useful for relieving pain caused by a large number of species. Vinegar (5% acetic acid), rubbing alcohol (40–70% isopropyl alcohol), baking soda (sodium bicarbonate, especially for sea nettle stings), papain (unseasoned meat tenderizer), fresh lemon or lime juice, olive oil, or sugar may be effective, depending on the species of stinging creature. Household ammonia may in and of itself cause skin irritation.
The pressure-immobilization technique is no longer recommended for venom containment in the setting of any jellyfish sting. For the sting of the venomous box jellyfish (C. fleckeri), vinegar should be used. Local application of heat (up to 45°C/113°F), commonly by immersion in hot water, may be as effective. A baking soda slurry (50% baking soda, 50% water) has been recommended for Cyanea and Chrysaora species. Commercial (chemical) cold packs or real ice packs applied over a thin dry cloth or a plastic membrane have been shown to be effective in alleviating mild or moderate Physalia utriculus (bluebottle jellyfish) stings but may be less effective than application of heat. Perfume, aftershave lotion, and high-proof ethanol are not efficacious and may be detrimental; formalin, ether, gasoline, and other organic solvents should not be used. Shaving the skin helps remove remaining nematocysts. Freshwater irrigation and rubbing lead to further stinging by adherent nematocysts and should be avoided.
After decontamination, topical application of an anesthetic ointment (lidocaine, benzocaine), an antihistamine (diphenhydramine), or a glucocorticoid (hydrocortisone) may be helpful. Persistent severe pain after decontamination may be treated with morphine, meperidine, fentanyl, or another narcotic analgesic. Muscle spasms may respond to diazepam (2–5 mg, titrated upward as necessary) or 10% calcium gluconate (5–10 mL) given IV. An ovine-derived IgG antivenom is available from Commonwealth Serum Laboratories (see “Sources of Antivenoms and Other Assistance,” below) for stings from the box jellyfish found in Australian and Indo-Pacific waters. However, despite its reported clinical efficacy, one school of thought holds that perhaps the antivenom is unable to bind the venom rapidly enough to account for its effects. Until further notice, current recommendations for its use apply. Treatment for Irukandji syndrome may require administration of opioid analgesics and MgSO4 as well as aggressive treatment (phentolamine, 5 mg IV) of hypertension. All victims with systemic reactions should be observed for at least 6–8 h for rebound from any therapy, and all elderly adults should be checked for cardiac arrhythmias. Patients may suffer postinflammatory hyperpigmentation and persistent cutaneous hypersensitivity in areas of skin contact.
Safe Sea, a “jellyfish-safe” sunblock (www.nidaria.com) applied to the skin before an individual enters the water, inactivates the recognition and discharge mechanisms of nematocysts, has been tested successfully against a number of marine stingers, and may prevent or diminish the effects of coelenterate stings. Whenever possible, a dive skin or wet suit should be worn when entering ocean waters.
Sea Sponges Many sponges produce crinotoxins that are present on their surface or in their internal secretions. As a result, touching a sea sponge may result in dermatitis or “sponge diver’s disease,” a necrotic skin reaction. Irritant dermatitis may result if small spicules of silica or calcium carbonate penetrate the skin. It is impossible to distinguish between the allergic and spicule reactions, so the treatment is the same for both. Afflicted skin should be gently dried and adhesive tape used to remove embedded spicules. Vinegar should be applied immediately and then for 10–30 min three or four times a day. Rubbing alcohol may be used if vinegar is unavailable. After spicule removal and skin decontamination, glucocorticoid or antihistamine cream may be applied to the skin. Severe vesiculation should be treated with a 2-week tapering course of systemic glucocorticoids. Mild reactions subside in 3–7 days, while involvement of large areas of the skin may result in systemic symptoms of fever, dizziness, nausea, muscle cramps, and formication.
Annelid Worms Annelid worms (bristleworms) possess rows of soft, cactus-like spines capable of inflicting painful stings. Contact results in symptoms similar to those of nematocyst envenomation. Without treatment, pain usually subsides over several hours, but inflammation may persist for up to a week (Fig. 474-4). Victims should resist the urge to scratch because scratching may fracture retrievable spines. Visible bristles should be removed with forceps and adhesive tape or a commercial facial peel; alternatively, a thin layer of rubber cement can be used to entrap and then peel away the spines. Use of vinegar or rubbing alcohol or a brief application of lidocaine or unseasoned meat tenderizer (papain) may provide additional relief. Local inflammation should be treated with topical or systemic glucocorticoids.
FIGURE 474-4 Rash on the hand of a diver from the spines of a bristleworm. (Courtesy of Paul Auerbach, with permission.)
Sea Urchins Venomous sea urchins possess either hollow, venom-filled calcified spines or triple-jawed, globiferous pedicellariae with venom glands. Venom may also be found within the integumentary sheath on the external spine surface of certain species. The venom contains toxic components, including steroid glycosides, hemolysins, proteases, serotonin, and cholinergic substances. Contact with either venom apparatus produces immediate and intensely painful stings. One or more spines entering a joint can cause synovitis that may, over time, progress to arthritis if the spine(s) remain in or near the joint. If multiple spines penetrate the skin, the patient may develop systemic symptoms, including nausea, vomiting, numbness, muscular paralysis, and respiratory distress. A delayed hypersensitivity reaction 7–10 days after resolution of primary symptoms has been described.
The affected part should be immersed immediately in hot water to tolerance (up to 45°C/113°F). Pedicellariae should be removed by shaving so that envenomation cannot continue. Accessible embedded spines should be removed but may break off and remain lodged in the victim. Residual dye from the surface of a spine remaining after the spine’s removal may mimic a retained spine but is otherwise of no consequence. Soft tissue radiography or MRI can confirm the presence of retained spines, which may warrant referral for attempted surgical removal if the spines are near vital structures (e.g., joints, neurovascular bundles). Retained spines may cause the formation of granulomas that are amenable to excision or to intralesional injection with triamcinolone hexacetonide (5 mg/mL). Chronic granulomatous arthritis of the proximal interphalangeal joints has been treated with synovectomy and removal of granulation tissue. Erbium-YAG laser ablation has been deployed to destroy multiple sea urchin spines embedded in the foot and identified visually at the surface level without causing thermal necrosis of the adjacent tissues. Eosinophilic pneumonia and local and diffuse neuropathies have been observed separately after penetration by multiple spines of the black sea urchin (presumed Diadema species). The pathophysiologies of these phenomena have not been determined.
Starfish The crown-of-thorns Acanthaster planci produces venom in glandular tissue underneath the epidermis, which is released via its spiny surfaces (Fig. 474-5). Skin puncture causes pain, bleeding, and local edema, usually with remission over 30–180 min. Multiple punctures may result in reactions such as local muscle paralysis; retained fragments may cause granulomatous lesions and synovitis. There has also been a case report of elevated liver enzymes after A. planci envenomation. Envenomed persons benefit from acute immersion therapy in hot water, local anesthesia, wound cleansing, and possible exploration to remove foreign material.
FIGURE 474-5 Spines on the crown-of-thorns sea star (Acanthaster planci). (Courtesy of Paul Auerbach, with permission.)
Sea Cucumbers Sea cucumbers produce holothurin (a cantharin-like liquid toxin) in their body walls. This toxin is concentrated in the tentacular organs that are projected when the animal is threatened. Underwater, holothurin induces minimal contact dermatitis in the skin but can cause significant corneal and conjunctival irritation. A severe reaction can lead to blindness. Skin should be detoxified with 5% acetic acid (vinegar), papain, or isopropyl alcohol. The eye should be anesthetized with one or two drops of 0.5% proparacaine and irrigated with 100–250 mL of normal saline, with subsequent slit-lamp examination to identify corneal defects.
Cone Snails Cone snails use a detachable dartlike tooth to inject conotoxins into prey, inducing tetanus followed by paralysis. In an unknowing handler, stings result in small, burning punctate wounds followed by local ischemia, cyanosis, and numbness. Dysphagia, syncope, dysarthria, ptosis, blurred vision, and pruritus have also been documented. Some envenomations induce paralysis leading to respiratory failure, coma, and death. There is no antivenom. Pressure-immobilization (see “Octopuses,” below), hot-water soaks, and local anesthetics have been used empirically with success. The wound should be inspected for a foreign body. Edrophonium has been recommended as therapy for paralysis if a Tensilon test is positive.
Octopuses Serious envenomations and deaths have followed bites of Australian blue-ringed octopuses (Octopus maculosus and Octopus lunulata). Although these animals rarely exceed 20 cm in length, their salivary venom contains a potent neurotoxin (maculotoxin) that inhibits peripheral nerve transmission by blocking sodium conductance. Oral numbness and facial numbness develop within several minutes of a serious envenomation and rapidly progress to total flaccid paralysis, including failure of respiratory muscles. Immediately after envenomation, a circumferential pressure-immobilization dressing 15 cm wide should be applied over a gauze pad (~7 × 7 × 2 cm) that has been placed directly over the sting. The dressing should be applied at venous-lymphatic pressure, with the preservation of distal arterial pulses. The limb should then be splinted. Once the victim has been transported to the nearest medical facility, the bandage can be released. Because there is no antidote and passive immunotherapy (rabbit IgG antibody) has been proven effective only against tetrodotoxin in mice, treatment is supportive. Patients with respiratory failure may need to be mechanically ventilated. If respirations are assisted, the victim may remain awake although completely paralyzed. Even with serious envenomations, significant recovery often takes place within 4–10 h, although complete recovery may require 2–4 days. Sequelae are uncommon unless related to hypoxia.
VERTEBRATES
As for all penetrating injuries, first-aid care should be undertaken. In addition, consideration must be given to local wound infection by marine Vibrio species and freshwater Aeromonas hydrophila as well as other “aquatic bacteria,” particularly if spines and needles remain embedded.
Stingrays A stingray injury is both an envenomation and a traumatic wound. Thoracic and cardiac penetration, major vessel laceration, and compartment syndrome have all been observed. The venom, which contains serotonin, 5′-nucleotidase, and phosphodiesterase, causes immediate, intense pain that may last up to 48 h. The wound is very painful (with the pain peaking at 30–60 min and lasting up to 48 h), often becomes ischemic in appearance, and heals poorly, with adjacent soft tissue swelling and prolonged disability. Systemic effects include weakness, diaphoresis, nausea, vomiting, diarrhea, dysrhythmias, syncope, hypotension, muscle cramps, fasciculations, paralysis, and (in rare cases) death. Because of differences in the toxins present on the tissues covering the stingers, freshwater stingrays may cause more severe injuries than marine stingrays.
Scorpionfish The designation scorpionfish encompasses members of the family Scorpaenidae and includes not only scorpionfish but also lionfish and stonefish. A complex venom with neuromuscular toxicity is delivered through 12 or 13 dorsal, 2 pelvic, and 3 anal spines. In general, the sting of a stonefish is regarded as the most serious (severe to life-threatening); that of the scorpionfish is of intermediate seriousness; and that of the lionfish is the least serious. Like that of a stingray, the sting of a scorpionfish is immediately and intensely painful. Pain from a stonefish envenomation may last for days. Systemic manifestations of scorpionfish stings are similar to those of stingray envenomations but may be more pronounced, particularly in the case of a stonefish sting, which may cause severe local tissue necrosis in addition to vital organ failure. The rare deaths that follow stonefish envenomation usually occur within 6–8 h. There is a commercially available stonefish antivenom.
Other Fish Three species of marine catfish—Plotosus lineatus (oriental catfish), Bagre marinus (sail catfish), and Galeichthys felis (common sea catfish)—as well as several species of freshwater catfish are capable of stinging humans. Venom is delivered through a single dorsal spine and two pectoral spines. Clinically, a catfish sting is comparable to that of a stingray, although marine catfish envenomations are generally more severe than those of their freshwater counterparts. Surgeonfish (doctorfish, tang), weeverfish, ratfish, and horned venomous sharks have also envenomed humans.
Platypus The platypus is a venomous mammal. The male has a keratinous spur on each hind limb; the spur is connected to a venom gland within the upper thigh. Skin puncture causes soft tissue edema and pain that may last for days or weeks. Care is supportive, and hot-water therapy does not appear to benefit the victim.
SOURCES OF ANTIVENOMS AND OTHER ASSISTANCE
The best way to locate a specific antivenom in the United States is to call a regional poison control center and ask for assistance. Divers Alert Network, a nonprofit organization designed to assist in the care of injured divers, also may help with the treatment of marine injuries. The network can be reached on the Internet at www.diversalertnetwork.org or by telephone 24 h a day at (919) 684-9111. An antivenom for the box jellyfish (C. fleckeri) and another for stonefish (and severe scorpionfish) envenomation are made in Australia by the Commonwealth Serum Laboratories (CSL; 45 Poplar Road, Parkville, Victoria, Australia 3052; www.csl.com.au; 61-3-9389-1911). When administering the box jellyfish antivenom, time is of the essence. For cardiac or respiratory decompensation, give a minimum of one ampoule and up to six ampoules consecutively IV, preferably in a 1:10 dilution with normal saline. For stonefish (or severe scorpionfish) envenomation, give one ampoule of specific antivenom IM for every one or two punctures, to a maximum of three ampoules.
MARINE POISONINGS
CIGUATERA
Epidemiology and Pathogenesis Ciguatera poisoning is the most common nonbacterial food poisoning associated with fish in the United States; most U.S. cases occur in Florida and Hawaii, although, with transportation of imported fish nationwide, all clinicians need to be aware of ciguatera. The poisoning almost exclusively involves tropical and semitropical marine coral reef fish common in the Indian Ocean, the South Pacific, and the Caribbean Sea. Global estimates predict that 20,000–50,000 people may be affected by this poisoning each year. More than 400 different fish have been associated with ciguatera toxicity, but 75% of poisonings involve the reef-dwelling barracuda, snapper, jack, or grouper. Ciguatera toxin is created by warm-water ocean reef microalgae of the genus Gambierdiscus toxicus, whose consumption by grazing fish allows the toxin to bioaccumulate in the food chain. Three major ciguatoxins are found in the flesh and viscera of ciguateric fish: CTX-1, -2, and -3. Recent research suggests that CTX-1 activates astrocytes and astroglia. In addition, TRPV1, a nonselective cation channel expressed in nociceptive neurons, may play a role in the neurologic disturbances unique to ciguatera poisoning. Most, if not all, ciguatoxins are unaffected by freeze-drying, heat, cold, and gastric acid. None of the toxins affects the odor, color, or taste of fish. Cooking methods may alter the relative concentrations of the various toxins.
Clinical Manifestations The onset of symptoms may come within 15–30 min of ingestion and typically takes place within 2–6 h. Symptoms increase in severity over the ensuing 4–6 h. Most victims develop symptoms within 12 h of ingestion, and virtually all are afflicted within 24 h. The more than 150 signs and symptoms reported include those shown in Table 474-3. Diarrhea, vomiting, and abdominal pain usually develop 3–6 h after ingestion of a ciguatoxic fish. Symptoms may persist for 48 h and then generally resolve (even without treatment). A pathognomonic symptom is the reversal of hot and cold tactile perception, which develops in some persons after 3–5 days and may last for months. More severe reactions tend to occur in persons previously stricken with the disease. Persons who have ingested parrotfish (scaritoxin) may develop classic ciguatera poisoning as well as a “second-phase” syndrome (after 5–10 days’ delay) of disequilibrium with locomotor ataxia, dysmetria, and resting or kinetic tremor. This syndrome may persist for 2–6 weeks.
|
REPRESENTATIVE SIGNS AND SYMPTOMS OF CIGUATERA POISONING |
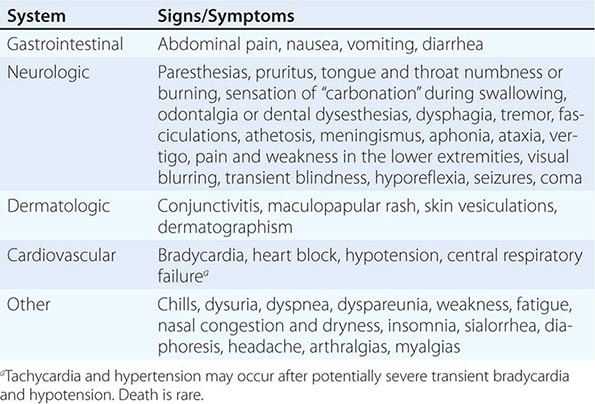
Diagnosis The differential diagnosis of ciguatera includes paralytic shellfish poisoning, eosinophilic meningitis, type E botulism, organophosphate insecticide poisoning, tetrodotoxin poisoning, and psychogenic hyperventilation. At present, the diagnosis of ciguatera poisoning is made on clinical grounds because no routinely used laboratory test detects ciguatoxin in human blood. Liquid chromatography–mass spectrometry is available for ciguatoxins but is of limited clinical value because most health care institutions do not have the equipment needed to perform the test. A ciguatoxin enzyme immunoassay or radioimmunoassay may be used to test small portions of the suspected fish, but even these tests may not detect the very small amount of toxin (0.1 ppb) necessary to render fish flesh toxic. A newer neuroblastoma assay may be sufficiently sensitive to detect small amounts of toxin but is not readily available for clinical use.
DIARRHETIC SHELLFISH POISONING
Diarrhetic shellfish poisoning occurs with consumption of shellfish producing diarrheal illness. The first suspected incident, which occurred in the Netherlands in 1961, was followed by outbreaks in Japan, the United Kingdom, and (most recently) China. The causative agents are the lipophilic compound okadaic acid and the dinophysistoxins, which inhibit serine and threonine protein phosphatases, with consequent protein accumulation and continued secretion of fluid in intestinal cells leading to diarrhea. Shellfish acquire these toxins by feeding on dinoflagellates, particularly of the genera Dinophysis and Prorocentrum.
Symptoms include diarrhea, nausea, vomiting, abdominal pain, and chills. Onset occurs within 30 min to 12 h. The illness is usually self-limited; most patients recover in 3 or 4 days, and only a few require hospitalization. Treatment is supportive and focused on hydration. Toxins can be detected in food samples by a mouse bioassay, an immunoassay, and high-performance liquid chromatography with fluorometric detection (HPLC-FLD).
PARALYTIC SHELLFISH POISONING
Paralytic shellfish poisoning is induced by ingestion of any of a variety of feral or aquacultured filter-feeding organisms, including clams, oysters, scallops, mussels, chitons, limpets, starfish, and sand crabs. The origin of their toxicity is the chemical toxin they accumulate and concentrate by feeding on various planktonic dinoflagellates (e.g., Protogonyaulax, Ptychodiscus, and Gymnodinium) and protozoan organisms. The unicellular phytoplanktonic organisms form the foundation of the food chain, and in warm summer months these organisms “bloom” in nutrient-rich coastal temperate and semitropical waters. In the United States, paralytic shellfish poisoning is acquired primarily from seafood harvested in the Northeast, the Pacific Northwest, and Alaska. These planktonic species can release massive amounts of toxic metabolites into the water and cause mortality in bird and marine populations. The paralytic shellfish toxins are water soluble as well as heat and acid stable; they cannot be destroyed by ordinary cooking or freezing. Contaminated seafood looks, smells, and tastes normal. The best-characterized, most potent, and most frequently identified paralytic shellfish toxin is saxitoxin, which takes its name from the Alaska butter clam Saxidomus giganteus. Saxitoxin appears to block sodium conductance, inhibiting neuromuscular transmission at the axonal and muscle membrane levels. A toxin concentration of >75 µg/100 g of foodstuff is considered hazardous to humans. In the 1972 New England “red tide,” the concentration of saxitoxin in blue mussels exceeded 9000 µg/100 g of foodstuff.
The onset of intraoral and perioral paresthesias (notably of the lips, tongue, and gums) comes within minutes to a few hours after ingestion of contaminated shellfish, and these paresthesias progress rapidly to involve the neck and distal extremities. The tingling or burning sensation later changes to numbness. Other symptoms rapidly develop and include light-headedness, disequilibrium, incoordination, weakness, hyperreflexia, incoherence, dysarthria, sialorrhea, dysphagia, thirst, diarrhea, abdominal pain, nausea, vomiting, nystagmus, dysmetria, headache, diaphoresis, loss of vision, chest pain, and tachycardia. Flaccid paralysis and respiratory insufficiency may follow 2–12 h after ingestion. In the absence of hypoxia, the victim often remains alert but paralyzed. Up to 12% of patients die.
DOMOIC ACID INTOXICATION (AMNESTIC SHELLFISH POISONING)
In late 1987 in eastern Canada, an outbreak of gastrointestinal and neurologic symptoms (amnestic shellfish poisoning) was documented in persons who had consumed mussels found to be contaminated with domoic acid. In this outbreak, the source of the toxin was Nitzschia pungens, a diatom ingested by the mussels. Since the Canadian outbreak, the toxin has been found in shellfish from the United States, the United Kingdom, and Spain. In 1991, an epidemic of domoic acid poisoning in the state of Washington was attributed to the consumption of razor clams. A water-soluble, heat-stable neuroexcitatory amino acid with biochemical analogues of kainic acid and glutamic acid, domoic acid binds to the kainate type of glutamate receptor with three times the affinity of kainic acid and is 20 times as powerful a toxin. Shellfish can be tested for domoic acid by mouse bioassay and HPLC. The regulatory limit for domoic acid in shellfish is 20 parts per million.
The abnormalities noted within 24 h of ingesting contaminated mussels (Mytilus edulis) include arousal, confusion, disorientation, and memory loss. The median time of onset is 5.5 h. Other prominent signs and symptoms include severe headache, nausea, vomiting, diarrhea, abdominal cramps, hiccups, arrhythmias, hypotension, seizures, ophthalmoplegia, pupillary dilation, piloerection, hemiparesis, mutism, grimacing, agitation, emotional lability, coma, copious bronchial secretions, and pulmonary edema. Histologic study of brain tissue taken at autopsy has shown neuronal necrosis or cell loss and astrocytosis, most prominently in the hippocampus and the amygdaloid nucleus—findings similar to those in animals poisoned with kainic acid. Several months after the primary intoxication, victims still display chronic residual memory deficits and motor neuronopathy or axonopathy. Nonneurologic illness does not persist.
SCOMBROID POISONING
Scombroid fish poisoning may be the most common type of seafood poisoning worldwide. It follows consumption of scombroid (mackerel-like) fish, which include albacore, bluefin, and yellowfin tuna; mackerel; saury; needlefish; wahoo; skipjack; and bonito, as well as nonscombroid fish, such as dolphinfish (Hawaiian mahimahi, Coryphaena hippurus), kahawai, sardine, black marlin, pilchard, anchovy, herring, amberjack, and Australian ocean salmon. In the northeastern and mid-Atlantic United States, bluefish (Pomatomus saltatrix) has been linked to scombroid poisoning. Because greater numbers of nonscombroid fish are being recognized as scombrotoxic, the syndrome may more appropriately be called pseudoallergic fish poisoning.
Under conditions of inadequate preservation or refrigeration, the musculature of these dark- or red-fleshed fish undergoes decomposition by Proteus morganii and Klebsiella pneumoniae bacteria, with consequent decarboxylation of the amino acid L-histidine to histamine, histamine phosphate, and histamine hydrochloride. Histamine levels of 20–50 mg/100 g are noted in toxic fish, with levels >400 mg/100 g on occasion. However, it is possible that some other compound may be responsible for this intoxication, because large doses of oral histamine do not reproduce the affliction. It is proposed that this unknown agent works by inhibiting the metabolism of histamine, promoting degranulation of mast cells to release endogenous histamine, or acting as a histamine receptor agonist. Whatever toxin or toxins are involved are heat stable and are not destroyed by domestic or commercial cooking. Affected fish typically have a sharply metallic or peppery taste; however, they may be normal in appearance, color, and flavor. Not all persons who eat a contaminated fish necessarily become ill, perhaps because of uneven distribution of decay within the fish.
Symptoms develop within 15–90 min of ingestion. Most cases are mild, with tingling of lips and mouth, mild abdominal discomfort, and nausea. The more severe and commonly described presentation includes flushing (sharply demarcated; exacerbated by ultraviolet exposure; particularly pronounced on the face, neck, and upper trunk), a sensation of warmth without elevated core temperature, conjunctival hyperemia, pruritus, urticaria, and angioneurotic edema. This syndrome may progress to bronchospasm, nausea, vomiting, diarrhea, epigastric pain, abdominal cramps, dysphagia, headache, thirst, pharyngitis, gingival burning, palpitations, tachycardia, dizziness, and hypotension. Without treatment, the symptoms generally resolve within 8–12 h. Because of blockade of gastrointestinal tract histaminase, the reaction may be more severe in a person who is concurrently ingesting isoniazid.
475 |
Ectoparasite Infestations and Arthropod Injuries |
Ectoparasites include arthropods and creatures from other phyla that infest the skin or hair of animals; the host animals provide them with sustenance and shelter. The ectoparasites may penetrate within or beneath the surface of the host or may attach by mouthparts and specialized claws. These organisms may inflict direct mechanical injury, consume blood or nutrients, induce hypersensitivity reactions, inoculate toxins, transmit pathogens, and incite fear or disgust. Humans are the sole or obligate hosts for many kinds of ectoparasites and serve as facultative or paratenic (accidental) hosts for many others.
Arthropods that are ectoparasitic or otherwise cause injury include insects (such as lice, fleas, bedbugs, wasps, ants, bees, and flies), arachnids (spiders, scorpions, mites, and ticks), millipedes, and centipedes. Certain nematodes (helminths), such as the hookworms (Chap. 256), are ectoparasitic in that they penetrate and migrate through the skin. Infrequently encountered ectoparasites in other phyla include the pentastomes (tongue worms) and leeches.
Arthropods may also cause injury when they attempt to take a blood meal or as they defend themselves by biting, stinging, or exuding venoms. Various arachnids (spiders and scorpions), insects (bees, hornets, wasps, ants, flies, true bugs, caterpillars, and beetles), millipedes, and centipedes produce ill effects during these behaviors. Similarly, certain ectoparasites (e.g., ticks, biting mites, and fleas) that typically infest nonhuman animals can be medically significant. In the United States, lesions caused by arthropod bites and stings are so diverse and variable that it is rarely possible to identify the precise causative organism without a bona fide specimen and taxonomic expertise.
SCABIES
The human itch mite, Sarcoptes scabiei var. hominis, is a common cause of itching dermatosis, infesting ~300 million persons worldwide at any one time. Gravid female mites (~0.3 mm in length) burrow superficially within the stratum corneum, depositing three or fewer eggs per day. Six-legged larvae mature to eight-legged nymphs and then to adults. Gravid adult females emerge to the surface of the skin about 8 days later and then (re)invade the skin of the same or another host. Newly fertilized female mites are transferred from person to person mainly by direct skin-to-skin contact; transfer is facilitated by crowding, poor hygiene, and sex with multiple partners. Generally, these mites die within a day or so in the absence of host contact. Transmission via sharing of contaminated bedding or clothing occurs far less frequently than is often thought. In the United States, scabies may account for up to 5% of visits to dermatologists. Outbreaks occur in preschools, hospitals, nursing homes, and other residential institutions.
The itching and rash associated with scabies derive from a sensitization reaction to the mites and their secretions/excretions. A person’s initial infestation remains asymptomatic for up to 6 weeks before the onset of intense pruritus, but a reinfestation produces a hypersensitivity reaction without delay. Burrows become surrounded by inflammatory infiltrates composed of eosinophils, lymphocytes, and histiocytes, and a generalized hypersensitivity rash later develops in remote sites. Immunity and associated scratching limit most infestations to <15 mites per person. Hyperinfestation with thousands of mites, a condition known as crusted scabies (formerly termed Norwegian scabies), may result from glucocorticoid use, immunodeficiency, and neurologic or psychiatric illnesses that limit the itch and/or the scratch response.
Pruritus typically intensifies at night and after hot showers. Classic burrows are often difficult to find because they are few in number and may be obscured by excoriations. Burrows appear as dark wavy lines in the upper epidermis and are 3–15 mm long. Scabetic lesions are most common on the volar wrists and along the digital web spaces. In males, the penis and scrotum become involved. Small papules and vesicles, often accompanied by eczematous plaques, pustules, or nodules, appear symmetrically at those sites; within intertriginous areas; around the navel and belt line; in the axillae; and on the buttocks and upper thighs. Except in infants, the face, scalp, neck, palms, and soles are usually spared. Crusted scabies often resembles psoriasis: both are characterized by widespread thick keratotic crusts, scaly plaques, and dystrophic nails. Characteristic burrows are not seen in crusted scabies, and patients usually do not itch, although their infestations are highly contagious and have been responsible for outbreaks of classic scabies in hospitals.
Scabies should be considered in patients with pruritus and symmetric superficial, excoriated, papulovesicular skin lesions in characteristic locations, particularly if there is a history of household contact with an infested person. Burrows should be sought and unroofed with a sterile needle or scalpel blade, and the scrapings should be examined microscopically for mites, eggs, and fecal pellets. Examination of skin biopsies (including superficial cyanoacrylate biopsy) or scrapings, dermatoscopic imaging of papulovesicular lesions, and microscopic inspection of clear cellophane tape lifted from lesions also may be diagnostic. In the absence of identifiable mites or eggs, the diagnosis is based on a history of pruritus, a clinical examination, and an epidemiologic link. Diverse kinds of dermatitis from other causes frequently are misdiagnosed as scabies, particularly in presumed “outbreak” situations. Scabies mites of other animals may cause transient irritation, but they do not reside or reproduce in human hosts.
CHIGGERS AND OTHER BITING MITES
Chiggers are the larvae of trombiculid (harvest) mites that normally feed on mice in grassy or brush-covered sites in tropical, subtropical, and (less frequently) temperate areas during warm months. They reside on low vegetation and attach themselves to passing mammalian hosts. While feeding, larvae secrete saliva with proteolytic enzymes to create a tube-like invagination in the host’s skin; this stylostome allows the mite to imbibe tissue fluids. The stylostomal saliva is highly antigenic and causes exceptionally pruritic papular, papulovesicular, or papulourticarial lesions (≤2 cm in diameter). In people previously sensitized to salivary antigens, the papules develop within hours of attachment. While attached, mites appear as tiny red vesicles on the skin. Generally, lesions vesicate and develop a hemorrhagic base. Scratching, however, invariably destroys the body of a mite. Itching and burning often persist for weeks. The rash is common on the ankles and areas where clothing obstructs the further wanderings of the mites. Repellents are useful for preventing chigger bites.
Many kinds of mites that are associated with peridomestic birds and rodents are particularly bothersome when they invade homes and bite people. In North America, the northern fowl mite, chicken mite, tropical rat mite, and house mouse mite normally feed on poultry, various songbirds, and small mammals and are abundant in and around their hosts’ nests. After their natural hosts die or leave the nest, these mites frequently invade human habitations. Although the mites are rarely seen because of their small size, their bites can be painful and pruritic. Once confirmed as the cause of irritation, rodent- and bird-associated mites are best eliminated by excluding their hosts, removing the nests, and cleaning and treating the nesting area with appropriate acaricides. Pyemotes and other mites that infest grain, straw, cheese, hay, or other products occasionally produce similar episodes of rash and discomfort and may produce a unique dermatologic “comet sign” lesion—a paisley-shaped urticarial plaque.
Diagnosis of mite-induced dermatitides (including those caused by chiggers) relies on confirmation of the mite’s identity or elicitation of a history of exposure to the mite’s source. Oral antihistamines or topical steroids may suppress mite-induced pruritus temporarily but do not eliminate the mites.
TICK BITES AND TICK PARALYSIS
Ticks attach superficially to skin and feed painlessly; blood is their only food. Their salivary secretions are biologically active and can produce local reactions, induce fevers, and cause paralysis in addition to transmitting diverse pathogens. The two main families of ticks are the hard (ixodid) and soft (argasid) ticks. Generally, soft ticks attach for <1 h, leaving red macules after they drop off. Some species in Africa, the western United States, and Mexico produce painful hemorrhagic lesions. Hard ticks are much more common and transmit most of the tick-borne infections that are familiar to physicians and patients. Hard ticks attach to the host and feed for several days or sometimes for >1 week. At the site of hard-tick bites, small areas of induration, often purpuric, develop and may be surrounded by an erythematous rim. A necrotic eschar, called a tâche noire, occasionally develops. Chronic nodules (persistent tick-bite granulomas) can be several centimeters in diameter and may linger for months after the feeding tick has been removed. These granulomas can be treated with injected intralesional glucocorticoids or by surgical excision. Tick-induced fever, unassociated with transmission of any pathogen, is often accompanied by headache, nausea, and malaise but usually resolves ≤36 h after the tick is removed.
Tick paralysis, an acute ascending flaccid paralysis that resembles Guillain-Barré syndrome, is believed to be caused by one or more toxins in tick saliva that block neuromuscular transmission and decrease nerve conduction. This rare complication has followed the bites of more than 60 kinds of ticks, although in the United States dog and wood ticks (Dermacentor species) are most commonly involved. Weakness begins symmetrically in the lower extremities ≤6 days after the tick’s attachment, ascends symmetrically during several days, and may culminate in complete paralysis of the extremities and cranial nerves. Deep tendon reflexes are diminished or absent, but sensory examination and findings on lumbar puncture are typically normal. Removal of the tick generally leads to rapid improvement within a few hours and complete recovery after several days, although the patient’s condition may continue to deteriorate for a full day. Failure to remove the tick may lead to dysarthria, dysphagia, and ultimately death from aspiration or respiratory paralysis. Diagnosis depends on finding the tick, which is often hidden beneath scalp hair. An antiserum to the saliva of Ixodes holocyclus, the usual cause of tick paralysis in Australia, effectively reverses paralysis caused by these ticks.
Removal of hard ticks during the first 36 h of attachment nearly always prevents transmission of the agents of Lyme disease, babesiosis, anaplasmosis, and ehrlichiosis, although several tick-borne viruses may be transmitted more quickly. Ticks should be removed by traction with fine-tipped forceps placed firmly around the tick’s mouthparts. Careful handling (to avoid rupture of ticks) and use of gloves may avert accidental contamination with pathogens contained in tick fluids. Use of occlusive dressings, heat, or other substances (in an attempt to induce the tick to detach) merely delay tick removal. Afterward, the site of attachment should be disinfected. Tick mouthparts sometimes remain in the skin but generally are shed spontaneously within days without excision. Although somewhat controversial, current guidelines from the Centers for Disease Control and Prevention suggest that, rather than awaiting the onset of erythema migrans, the results of tick testing, or seroconversion to antigens diagnostic for Lyme disease, administering prophylaxis with a single oral dose of doxycycline (200 mg) within 72 h of tick removal is appropriate in adult patients with bites thought to be associated with deer ticks (Fig. 475-1) in Lyme disease–endemic areas from Maryland to Maine and in Wisconsin and Minnesota.
FIGURE 475-1 Deer ticks (Ixodes scapularis, black-legged ticks) on a U.S. penny: larva (below ear), nymph (right), adult male (above), and adult female (left).
LOUSE INFESTATION (PEDICULIASIS AND PTHIRIASIS)
Nymphs and adults of all three kinds of human lice feed at least once a day, ingesting human blood exclusively. Head lice (Pediculus capitis) infest mainly the hair of the scalp, body lice (Pediculus humanus) the clothing, and crab or pubic lice (Pthirus pubis) mainly the hair of the pubis. The saliva of lice produces a pruritic morbilliform or urticarial rash in some sensitized persons. Female head and pubic lice cement their eggs (nits) firmly to hair, whereas female body lice cement their eggs to clothing, particularly to threads along clothing seams. After ~10 days of development within the egg, a nymph hatches. Empty eggs may remain affixed for months thereafter.
In North America, head lice infest ~1% of elementary school-age children. Head lice are transmitted mainly by direct head-to-head contact rather than by fomites such as shared headgear, bed linens, hairbrushes, and other grooming implements. Chronic infestations by head lice tend to be asymptomatic. Pruritus, due mainly to hypersensitivity to the louse’s saliva, generally is transient and mild. Head lice removed from a person succumb to desiccation and starvation within ~1 day. Head lice are not known to serve as a natural vector for any pathogens.
Body lice remain on clothing except when feeding and generally succumb in ≤2 days if separated from their host. In most Western countries, body lice are generally found on a small proportion of indigent persons but may become increasingly prevalent after upheaval associated with natural or human-caused disasters, when homeless victims are in close contact with infested individuals with whom they share accommodations. Body lice are acquired by direct contact or by sharing of infested clothing and bedding. These lice are vectors for the agents of louse-borne (epidemic) typhus (Chap. 211), louse-borne relapsing fever (Chap. 209), and trench fever (Chap. 197). Pruritic lesions from their bites are particularly common around the neckline. Chronic infestations result in a postinflammatory hyperpigmentation and thickening of skin known as vagabond’s disease.
The crab or pubic louse is transmitted mainly by sexual contact. These lice occur predominantly on pubic hair and less frequently on axillary or facial hair, including the eyelashes. Children and adults may acquire pubic lice by sexual or close nonsexual contact. Intensely pruritic, bluish macules ~3 mm in diameter (maculae ceruleae) develop at the site of bites. Blepharitis commonly accompanies infestations of the eyelashes.
Pediculiasis is often suspected upon the detection of nits firmly cemented to hairs or in clothing. Many bona fide nits, however, are dead or hatched relics of prior infestation, and pseudo-nits are frequently misconstrued to be signs of a louse infestation. Confirmation of a louse infestation, therefore, best relies on the discovery of a live louse.
MYIASIS (FLY INFESTATION)
Myiasis refers to infestations by several kinds of fly larvae (maggots) that invade living or necrotic tissues or body cavities and produce different clinical syndromes, depending on the species of fly.
In forested parts of Central and South America, larvae of the human botfly (Dermatobia hominis) produce furuncular (boil-like) papules or subcutaneous nodules ≤3 cm in diameter. A gravid adult female botfly captures a mosquito or another bloodsucking insect and deposits her eggs on its abdomen. When the carrier insect attacks a human or bovine host several days later, the warmth and moisture of the host’s skin stimulate the eggs to hatch. The emerging larvae promptly penetrate intact skin. After 6–12 weeks of development, mature larvae emerge from the skin and drop to the ground to pupate and then become adults.
The African tumbu fly (Cordylobia anthropophaga) deposits its eggs on damp sand or leaf litter or on drying laundry, particularly that contaminated by urine or sweat. Larvae hatch from eggs upon contact with a host’s body and penetrate the skin, producing boil-like lesions from which mature larvae emerge ~9 days later. Furuncular myiasis is suggested by uncomfortable lesions with a central breathing pore that emits bubbles when submerged in water. A sensation of movement under the patient’s skin may cause severe emotional distress.
Larvae that cause furuncular myiasis may be induced to emerge if the air pore is coated with petrolatum or another occlusive substance. Removal may be facilitated by injection of a local anesthetic into the surrounding tissue, but surgical excision is sometimes necessary because upward-pointing spines of some species hold the larvae firmly in place.
Other fly larvae cause nonfuruncular myiasis. For example, larvae of the horse botfly (Gasterophilus intestinalis) emerge from eggs deposited on the horse’s flanks and may come into contact with and infest human beings. After penetrating human skin, these larvae rarely mature but instead may migrate for weeks in the dermis. The resulting pruritic and serpiginous eruption resembles cutaneous larva migrans caused by canine or feline hookworms (Chap. 256). Larvae of rabbit and rodent botflies (Cuterebra species) occasionally cause dermal or tracheopulmonary myiasis.
Certain flies are attracted to blood and pus, laying their eggs on open or draining sores. Newly hatched larvae enter wounds or diseased skin. Larvae of several types of green bottle flies (Lucilia/Phaenicia species) usually remain superficial and confined to necrotic tissue. Specially raised, sterile “surgical maggots” are sometimes used intentionally for wound debridement. Larvae of screwworm flies, Cochliomyia, and the flesh fly invade viable tissues more deeply and produce large suppurating lesions. Larvae that infest wounds also may enter body cavities such as the mouth, nose, ears, sinuses, anus, vagina, and lower urinary tract, particularly in unconscious or otherwise debilitated patients. The consequences range from harmless colonization to destruction of the nose, meningitis, and deafness. Treatment involves removal of maggots and debridement of tissue.
The maggots responsible for furuncular and wound myiasis also may cause ophthalmomyiasis. Sequelae include nodules in the eyelid, retinal detachment, and destruction of the globe. Most instances in which maggots are found in human feces result from deposition of eggs or larvae by flies on recently passed stools, not from an intestinal maggot infestation.
PENTASTOMIASIS
Pentastomids (tongue worms) inhabit the respiratory passages of reptiles and carnivorous mammals. Human infestation by Linguatula serrata is common in the Middle East and results from the consumption of encysted larval stages in raw liver or lymph nodes of sheep and goats, which are true intermediate hosts for the tongue worms. Larvae migrate to the nasopharynx and produce an acute self-limiting syndrome—known as halzoun or marrara—characterized by pain and itching of the throat and ears, coughing, hoarseness, dysphagia, and dyspnea. Severe edema may cause obstruction that requires tracheostomy. In addition, ocular invasion has been described. Diagnostic larvae measuring ≤10 mm in length appear in copious nasal discharge or vomitus. Individuals become infected with another type of tongue worm, Armillifer armillatus, by consuming its eggs in contaminated food or drink or after handling the definitive host, the African python. Larvae encyst in various organs but rarely cause symptoms. Cysts may require surgical removal as they enlarge during molting, but they usually are encountered as an incidental finding at autopsy. Parasite-induced lesions may be misinterpreted as a malignancy, with the correct diagnosis confirmed histopathologically. Cutaneous larva migrans–type syndromes of other pentastomes have been reported from Southeast Asia and Central America.
LEECH INFESTATIONS
Medically important leeches are annelid worms that attach to their hosts with chitinous cutting jaws and draw blood through muscular suckers. The medicinal leech (Hirudo medicinalis) is still used occasionally for medical purposes to reduce venous congestion in surgical flaps or replanted body parts. This practice has been complicated by intractable bleeding, wound infections, myonecrosis, and sepsis due to Aeromonas hydrophila, which colonizes the gullets of commercially available leeches.
Ubiquitous aquatic leeches that parasitize fish, frogs, and turtles readily attach to the skin of human beings and avidly suck blood. More notorious are arboreal land leeches that live among moist vegetation of tropical rain forests. Attachment is usually painless, and the leeches will detach themselves when satiated with a blood meal. Hirudin, a powerful anticoagulant secreted by the leech, causes continued bleeding after the leech has detached. Healing of a leech-bite wound is slow, and bacterial infections are not uncommon. Several kinds of aquatic leeches in Africa, Asia, and southern Europe can enter the mouth, nose, and genitourinary tract and attach to mucosal surfaces at sites as deep as the esophagus and trachea. Externally attached leeches generally drop off after they have engorged, but removal is hastened by gentle scraping aside of the anterior and posterior suckers the leech uses for attachment and feeding. Some authorities dispute the wisdom of removing leeches with alcohol, salt, vinegar, insect repellent, a flame or heated instrument, or applications of other noxious substances. Internally attached leeches may detach on exposure to gargled saline or may be removed by forceps.
SPIDER BITES
Of the more than 30,000 recognized species of spiders, only ~100 defend themselves aggressively and have fangs sufficiently long to penetrate human skin. The venom that some spiders use to immobilize and digest their prey can cause necrosis of skin and systemic toxicity. Whereas the bites of most spiders are painful but not harmful, envenomations by recluse or fiddleback spiders (Loxosceles species) and widow spiders (Latrodectus species) may be life-threatening. Identification of the offending spider is important because specific treatments exist for bites of widow spiders and because injuries attributed to spiders are frequently due to other causes. Except in cases where the patient actually observes a spider immediately associated with the bite or fleeing from the site, lesions reported as spider-bite reactions are most often due to other injuries or to infections with bacteria such as methicillin-resistant Staphylococcus aureus (MRSA).
Recluse Spider Bites and Necrotic Arachnidism Brown recluse spiders live mainly in the south-central United States and have close relatives in Central and South America, Africa, and the Middle East. Bites by brown recluse spiders usually cause only minor injuries, with edema and erythema. Envenomation, however, occasionally causes severe necrosis of skin and subcutaneous tissue and more rarely causes systemic hemolysis. These spiders are not aggressive toward humans and will bite only if threatened or pressed against the skin. They hide under rocks and logs or in caves and animal burrows. They invade homes and seek dark and undisturbed hiding spots in closets, in folds of clothing, or under furniture and rubbish in storage rooms, garages, and attics. Despite their impressive abundance in some homes, these spiders rarely bite humans. Bites tend to occur while the victim is dressing and are sustained primarily on the hands, arms, neck, and lower abdomen.
Initially, the bite is painless or may produce a stinging sensation. Within the next few hours, the site becomes painful and pruritic, with central induration surrounded by a pale ischemic zone that itself is encircled by a zone of erythema. In most cases, the lesion resolves without treatment in just a few days. In severe cases, the erythema spreads, and the center of the lesion becomes hemorrhagic or necrotic with an overlying bulla. A black eschar forms and sloughs several weeks later, leaving an ulcer that eventually may create a depressed scar. Healing usually takes place in ≤6 months but may take as long as 3 years if adipose tissue is involved. Local complications include injury to nerves and secondary bacterial infection. Fever, chills, weakness, headache, nausea, vomiting, myalgia, arthralgia, maculopapular rash, and leukocytosis may develop ≤72 h after the bite. Reports of deaths attributed to bites of North American brown recluse spiders have not been verified.
Widow Spider Bites The black widow spider, common in the southeastern United States, measures ≤1 cm in body length and 5 cm in leg span and is shiny black with a red hourglass marking on the ventral abdomen. Other dangerous Latrodectus species occur elsewhere in temperate and subtropical parts of the world. The bites of the female widow spiders are notorious for their potent neurotoxins.
Widow spiders spin their webs under stones, logs, plants, or rock piles and in dark spaces in barns, garages, and outhouses. Bites are most common in the summer and early autumn and occur when a web is disturbed or a spider is trapped or provoked. The initial bite is perceived as a sharp pinprick or may go unnoticed. Fang-puncture marks are uncommon. The venom that is injected does not produce local necrosis, and some persons experience no other symptoms. α-Latrotoxin, the most active component of the venom, binds irreversibly to presynaptic nerve terminals and causes release and eventual depletion of acetylcholine, norepinephrine, and other neurotransmitters from those terminals. Painful cramps may spread within 60 min from the bite site to large muscles of the extremities and trunk. Extreme rigidity of the abdominal muscles and excruciating pain may suggest peritonitis, but the abdomen is not tender on palpation and surgery is not warranted. The pain begins to subside during the first 12 h but may recur during several days or weeks before resolving spontaneously. A wide range of other neurologic sequelae may include salivation, diaphoresis, vomiting, hypertension, tachycardia, labored breathing, anxiety, headache, weakness, fasciculations, paresthesia, hyperreflexia, urinary retention, uterine contractions, and premature labor. Rhabdomyolysis and renal failure have been reported, and respiratory arrest, cerebral hemorrhage, or cardiac failure may end fatally, especially in very young, elderly, or debilitated persons.
Tarantulas and Other Spiders Tarantulas are hairy spiders of which 30 species are found in the United States, mainly in the Southwest. The tarantulas that have become popular household pets are usually imported from Central or South America. Tarantulas bite only when threatened and usually cause no more harm than a bee sting, but on occasion the venom causes deep pain and swelling. Several species of tarantulas are covered with urticating hairs that are brushed off in the thousands when a threatened spider rubs its hind legs across its dorsal abdomen. These hairs can penetrate human skin and produce pruritic papules that may persist for weeks. Failure to wear gloves or to wash the hands after handling the Chilean Rose tarantula, a popular pet spider, has resulted in transfer of hairs to the eye with subsequent devastating ocular inflammation. Treatment of bites includes local washing and elevation of the bitten area, tetanus prophylaxis, and analgesic administration. Antihistamines and topical or systemic glucocorticoids are given for exposure to urticating hairs.
Atrax robustus, a funnel-web spider of Australia, and Phoneutria species, the South American banana spiders, are among the most dangerous spiders in the world because of their aggressive behavior and potent neurotoxins. Envenomation by A. robustus causes a rapidly progressive neuromotor syndrome that can be fatal within 2 h. The bite of a banana spider causes severe local pain followed by profound systemic symptoms and respiratory paralysis that can lead to death within 2–6 h. Specific antivenoms for use after bites by each of these spiders are available. Yellow sac spiders (Cheiracanthium species) are common in homes worldwide. Their bites, though painful, generally lead to only minor erythema, edema, and pruritus.
SCORPION STINGS
Scorpions are arachnids that feed on ground-dwelling arthropods and small lizards. They paralyze their prey and defend themselves by injecting venom from a stinger on the tip of the tail. Painful but relatively harmless scorpion stings need to be distinguished from the potentially lethal envenomations that are produced by ~30 of the ~1000 known species and that cause more than 5000 deaths worldwide each year. Scorpions are nocturnal and remain hidden during the day in crevices or burrows or under wood, loose bark, or rocks. They occasionally enter houses and tents and may hide in shoes, clothing, or bedding. Scorpions sting humans only when threatened.
Of the 40 or so scorpion species in the United States, only bark scorpions—e.g., Centruroides sculpturatus (C. exilicauda) in the Southwest—produce venom that is potentially lethal to humans. This venom contains neurotoxins that cause sodium channels to remain open. Such envenomations usually are associated with little swelling, but prominent pain, paresthesia, and hyperesthesia can be accentuated by tapping on the affected area (the tap test). These symptoms soon spread to other locations; dysfunction of cranial nerves and hyperexcitability of skeletal muscles develop within hours. Patients present with restlessness, blurred vision, abnormal eye movements, profuse salivation, lacrimation, rhinorrhea, slurred speech, difficulty in handling secretions, diaphoresis, nausea, and vomiting. Muscle twitching, jerking, and shaking may be mistaken for a seizure. Complications include tachycardia, arrhythmias, hypertension, hyperthermia, rhabdomyolysis, and acidosis. Symptoms progress to maximal severity in ~5 h and subside within a day or two, although pain and paresthesia can last for weeks. Fatal respiratory arrest is most common among young children and the elderly.
Envenomations by Leiurus quinquestriatus in the Middle East and North Africa, by Mesobuthus tamulus in India, by Androctonus species along the Mediterranean littoral and in North Africa and the Middle East, and by Tityus serrulatus in Brazil cause massive release of endogenous catecholamines with hypertensive crises, arrhythmias, pulmonary edema, and myocardial damage. Acute pancreatitis occurs with stings of Tityus trinitatis in Trinidad, and central nervous toxicity complicates stings of Parabuthus and Buthotus scorpions of South Africa. Tissue necrosis and hemolysis may follow stings of the Iranian Hemiscorpius lepturus.
Stings of most other species cause immediate sharp local pain followed by edema, ecchymosis, and a burning sensation. Symptoms typically resolve within a few hours, and skin does not slough. Allergic reactions to the venom sometimes develop.
HYMENOPTERA STINGS
Insects that sting in defense or to subdue their prey belong to the order Hymenoptera, which includes bees, wasps, hornets, yellow jackets, and ants. Their venoms contain a wide array of amines, peptides, and enzymes that cause local and systemic reactions. Although the toxic effect of multiple stings can be fatal to a human, nearly all of the ≥100 deaths due to hymenopteran stings in the United States each year result from allergic reactions.
Bee and Wasp Stings The stinger of the honeybee (Apis mellifera) is unique in being barbed. When a bee stings a foe, its stinging apparatus and attached venom sac tear loose from its body. Muscular contraction of the venom sac continues to inject venom into the skin. Other kinds of bees, ants, and wasps have smooth stinging mechanisms and can sting numerous times in succession. Honeybees, bumblebees, and social wasps generally attack only when a colony is disturbed. Africanized honeybees (now present in South and Central America and the southern and western United States) respond to minimal intrusions more aggressively. Whereas the sting of an Africanized bee contains less venom than that of its non-Africanized relatives, victims tend to sustain far more stings and therefore to receive a far greater overall volume of venom. Most patients who report having sustained a “bee sting,” however, are more likely to have encountered stinging wasps instead.
The venoms of different species of hymenopterans are biochemically and immunologically distinct. Direct toxic effects are mediated by mixtures of low-molecular-weight compounds such as serotonin, histamine, acetylcholine, and several kinins. Polypeptide toxins in honeybee venom include mellitin that damages cell membranes, mast cell–degranulating protein that causes histamine release, the neurotoxin apamin, and the anti-inflammatory compound adolapin. Enzymes in venom include hyaluronidase and phospholipases. There appears to be little cross-sensitization between the venoms of honeybees and wasps.
Uncomplicated hymenopteran stings cause immediate pain, a wheal-and-flare reaction, and local edema, all of which usually subside in a few hours. Multiple stings can lead to vomiting, diarrhea, generalized edema, dyspnea, hypotension, and non-anaphylactic circulatory collapse. Rhabdomyolysis and intravascular hemolysis may cause renal failure. Death from the direct (nonallergic) effects of venom has followed stings of several hundred honeybees. Stings to the tongue or mouth may induce life-threatening edema of the upper airways.
Large local reactions accompanied by erythema, edema, warmth, and tenderness that spread ≥10 cm around the sting site over 1–2 days are not uncommon. These reactions may resemble bacterial cellulitis but are caused by hypersensitivity rather than by secondary infection. Such reactions tend to recur on subsequent exposure but are seldom accompanied by anaphylaxis and are not prevented by venom immunotherapy.
An estimated 0.4–4.0% of the U.S. population exhibits clinical immediate-type hypersensitivity to hymenopteran stings, and 15% may have asymptomatic sensitization manifested by positive skin tests. Persons who experience severe allergic reactions are likely to have similar reactions after subsequent stings by the same or closely related species. Occasionally, persons who have had mild reactions earlier in life will experience more serious reactions to subsequent stings. Mild anaphylactic reactions from insect stings, as from other causes, consist of nausea, abdominal cramping, generalized urticaria, flushing, and angioedema. Serious reactions, including upper airway edema, bronchospasm, hypotension, and shock, may be rapidly fatal. Severe reactions usually begin within 10 min of the sting and only rarely develop after 5 h.
STINGING ANTS
Stinging fire ants are an important medical problem in the United States. Imported fire ants (Solenopsis species) infest southern states from Texas to North Carolina, with colonies now established in California, New Mexico, Arizona, and Virginia. Slight disturbances of their mound nests have provoked massive outpourings of ants and as many as 10,000 stings on a single person. Elderly and immobile persons are at high risk for attacks when fire ants invade dwellings.
Fire ants attach to skin with powerful mandibles and rotate their bodies while repeatedly injecting venom with posteriorly situated stingers. The alkaloid venom consists of cytotoxic and hemolytic piperidines and several proteins with enzymatic activity. The initial wheal-and-flare reaction, burning, and itching resolve in ~30 min, and a sterile pustule develops within 24 h. The pustule ulcerates over the next 48 h and then heals in ≥1 week. Large areas of erythema and edema lasting several days are not uncommon and in extreme cases may compress nerves and blood vessels. Anaphylaxis occurs in fewer than 2% of victims; seizures and mononeuritis have been reported. Stings are treated with ice packs, topical glucocorticoids, and oral antihistamines. Pustules should be cleansed and then covered with bandages and antibiotic ointment to prevent bacterial infection. Epinephrine administration and supportive measures are indicated for anaphylactic reactions. Fire ant whole-body extracts are available for skin testing and immunotherapy, which appears to lower the rate of anaphylactic reactions.
European fire (red) ants (Myrmica rubra) have recently become public health pests in the northeastern United States and southern Canada. The western United States is home to harvester ants (Pogonomyrmex species). The painful local reaction that follows harvester ant stings often extends to lymph nodes and may be accompanied by anaphylaxis. The bullet or conga ant (Paraponera clavata) of South America is known locally as hormiga veinticuatro (“24-hour ant”), a designation that refers to the 24 h of throbbing, excruciating pain following a sting that delivers the potent paralyzing neurotoxin poneratoxin.
DIPTERAN (FLY AND MOSQUITO) BITES
In the process of feeding on vertebrate blood and tissue fluids, adults of certain flies inflict painful bites, produce local allergic reactions, and may transmit pathogenic agents. Bites of mosquitoes, tiny “no-see-um” (ceratopogonid) midges, and phlebotomine sand flies typically produce a wheal and a pruritic papule. Small humpbacked black flies (simuliids) lacerate skin, resulting in a lesion with serosanguineous discharge that is often painful and pruritic. Regional lymphadenopathy, fever, or anaphylaxis occasionally ensues. The widely distributed deerflies and horseflies as well as the tsetse flies of Africa are stout flies measuring ≤25 mm in length that attack during the day and produce large and painful bleeding punctures. Houseflies (Musca domestica) do not consume blood but use rasping mouthparts to scarify skin and feed upon tissue fluids and salt. Beyond direct injury from bites of any kind of fly, risks include transmission of diverse pathogens and secondary infection of the lesion.
FLEA BITES
Common human-biting fleas include the dog and cat fleas (Ctenocephalides species) and the rat flea (Xenopsylla cheopis), which infest their respective hosts and the hosts’ nests and resting sites. Sensitized persons develop erythematous pruritic papules (papular urticaria) and occasionally vesicles and bacterial superinfection at the site of the bite. Symptom-based treatment consists of antihistamines, topical glucocorticoids, and topical antipruritic agents.
Flea infestations are eliminated by removal and treatment of animal nests, frequent cleaning of pet bedding, and application of contact and systemic insecticides to pets and the dwelling. Flea infestations in the home may be abated or prevented if pets are regularly treated with veterinary antiparasitic agents and insect growth regulators.
Tunga penetrans, like other fleas, is a wingless, laterally flattened insect that feeds on blood. Also known as the chigoe flea, sand flea, or jigger (not to be confused with the chigger), it occurs in tropical regions of Africa and the Americas. Adult female chigoes live in sandy soil and burrow under the skin, usually between toes, under nails, or on the soles of bare feet. Gravid chigoes engorge on the host’s blood and grow from pinpoint to pea size during a 2-week interval. They produce lesions that resemble a white pustule with a central black depression and that may be pruritic or painful. Occasional complications include tetanus, bacterial infections, and autoamputation of toes (ainhum). Tungiasis is treated by removal of the intact flea with a sterile needle or scalpel, tetanus vaccination, and topical application of antibiotics.
HEMIPTERAN (TRUE BUG) BITES
Several true bugs of the family Reduviidae inflict bites that produce allergic reactions and are sometimes painful. The cone-nose bugs, so called because of their elongated heads, include the assassin and wheel bugs, which feed on other insects and bite vertebrates only in self-defense, and the kissing bugs, which routinely feed on vertebrate blood. The bites of the night-feeding kissing bugs are painless. Reactions to such bites depend on prior sensitization and include tender and pruritic papules, vesicular or bullous lesions, extensive urticaria, fever, lymphadenopathy, and (rarely) anaphylaxis. Bug bites are treated with topical antipruritics or oral antihistamines. Persons with anaphylactic reactions to reduviid bites should keep an epinephrine kit available. Some reduviids transmit Trypanosoma cruzi, the agent of New World trypanosomiasis (also called Chagas disease) (Chap. 252).
The cosmopolitan bedbugs (Cimex species) hide in crevices of mattresses, bed frames and other furniture, walls, and picture frames and under loose wallpaper. Bedbug populations have resurged, recently attaining levels and spreading to an extent not encountered since the mid-twentieth century. These bugs are now a common pest in homes, dormitories, and hotels; on cruise ships; and even in medical facilities. Generally, the bugs hide during the day and take blood meals at night. Their bite is painless, but minutes to days later, sensitized persons develop erythema, itching, and wheals around a central hemorrhagic punctum. Bedbugs are not known to transmit pathogens.
CENTIPEDE BITES AND MILLIPEDE DERMATITIS
The fangs of centipedes of the genus Scolopendra can penetrate human skin and deliver a venom that produces intense burning pain, swelling, erythema, and sterile lymphangitis. Dizziness, nausea, and anxiety are described occasionally, and rhabdomyolysis and renal failure have been reported. Treatment includes washing of the site, application of cold dressings, oral analgesic administration or local lidocaine infiltration, and tetanus prophylaxis.
Millipedes are docile and do not bite, but some secrete defensive fluids that may burn and discolor human skin. Affected skin turns brown overnight and may blister and exfoliate. Secretions in the eye cause intense pain and inflammation that can result in corneal ulcers and even blindness. Management includes irrigation with copious amounts of water or saline, use of analgesics, and local care of denuded skin.
CATERPILLAR STINGS AND DERMATITIS
Caterpillars of several moth species are covered with hairs or spines that produce mechanical irritation and may contain or be coated with venom. Contact with these caterpillars or their hairs may lead to lepidopterism or caterpillar envenomation. The response typically consists of an immediate burning sensation followed by local swelling and erythema and occasionally by regional lymphadenopathy, nausea, vomiting, and headache. A rare reaction to a South American caterpillar, Lonomia obliqua, can cause disseminated coagulopathy and fatal hemorrhagic shock. In the United States, dermatitis is most often associated with caterpillars of the io, puss, saddleback, and brown-tail moths. Even contact with detached hairs of other caterpillars, such as gypsy moth larvae, can later produce a pruritic urticarial or papular rash called erucism. Spines may be deposited on tree trunks or drying laundry or may be airborne and cause irritation of the eyes and upper airways. Treatment of caterpillar stings consists of repeated application of adhesive or cellophane tape to remove the hairs, which can then be identified microscopically. Local ice packs, topical glucocorticoids, and oral antihistamines relieve symptoms.
BEETLE VESICATION AND DERMITITIS
Several families of beetles have independently developed the ability to produce chemically unrelated vesicating toxins. When disturbed, blister beetles (family Meloidae) extrude cantharidin, a low-molecular-weight toxin that produces thin-walled blisters (≤5 cm in diameter) 2–5 h after contact. The blisters are not painful or pruritic unless broken and resolve without treatment in ≤10 days. Nephritis may follow unusually heavy cantharidin exposure. Contact occurs when individuals sit on the ground, work in the garden, or deliberately handle the beetles. The hemolymph of certain rove beetles (Staphylinidae) contains pederin, a potent vesicant. When these beetles are crushed or brushed against the skin, the released fluid causes painful, red, flaccid bullae. These beetles occur worldwide but are most numerous and problematic in parts of Africa (where they are called “Nairobi fly”) and southwestern Asia. Ocular lesions may develop after impact with flying beetles at night or unintentional transfer of the vesicant on the fingers. Treatment is rarely necessary, although ruptured blisters should be kept clean and bandaged.
Larvae of common carpet beetles are adorned with dense arrays of ornate hairs called hastisetae. Contact with these larvae or their setae results in delayed dermal reactions in sensitized individuals. The lesions are commonly mistaken as bites of bedbugs.
DELUSIONAL INFESTATIONS
The groundless conviction that one is infested with arthropods or other parasites (Ekbom’s syndrome, delusory parasitosis, delusions of parasitosis, and perhaps Morgellons syndrome) is extremely difficult to treat and, unfortunately, is not uncommon. Patients describe uncomfortable sensations of something moving in or on their skin. Excoriations and self-induced ulcerations typically accompany the pruritus, dysesthesias, and imaginary insect bites. Patients often believe that some invisible or as yet undescribed creatures are infesting their skin, clothing, homes, or environment in general. Frequently, patients submit as evidence of infestation specimens that consist of plant-feeding and nonbiting peridomestic arthropods, pieces of skin, vegetable matter, lint, and other inanimate detritus. When evaluating a patient with possible delusional parasitosis, it is imperative to rule out true infestations and bites by arthropods, endocrinopathies, sensory disorders due to neuropathies, opiate and other drug use, environmental irritants (e.g., fiberglass threads), and other causes of tingling or prickling sensations. Frequently, such patients repeatedly seek medical consultations, resist alternative explanations for their symptoms, and exacerbate their discomfort by self-treatment. Long-term pharmacotherapy with pimozide or other psychotropic agents has been more helpful than psychotherapy in treating this disorder. Patients with delusional parasitosis often develop the unshakeable conviction that they are infested by a previously unknown pathogen, while their personal lives, family support, and employment collapse around them.
ACKNOWLEDGMENT
The substantial contributions of Andrew Spielman and James H. Maguire to this chapter in previous editions are gratefully acknowledged.