Heat Illness
Perspective
Humans have been plagued by heat illness throughout recorded history, often as the result of military exercises, athletic events, or recreational activities. When environmental heat stress is maximal, strenuous exercise is not required to produce heat illness. The ancient Greeks named a disease that resembled heatstroke siriasis after the dog star Sirius, which accompanies the summer sun. The U.S. Army reported at least 125 deaths from heatstroke during basic training in the years 1941 to 1944.1 Modern military organizations continue to encounter heat illness because of the requirement to train unacclimatized troops with forced heavy physical exercise. Furthermore, athletes are prone to heat illness; heat stroke is the third leading cause of death among all U.S. athletes. In particular, football has the greatest number of heat stroke fatalities and a 10 times higher rate for heat illness, resulting in time lost from high school athletic activities.2,3
The elderly and poor, often lacking adequate air conditioning and nutrition, and those with preexisting disease are prone to heat illness during environmental extremes. In heat wave years in the United States, approximately 10 times as many deaths are reported as during non–heat wave years. It is also estimated that at least 10 times as many heat-aggravated illnesses occur because of myocardial infarction, cerebrovascular accident, and other causes. More than 700 excess deaths were caused by the heat during the 1995 heat wave in Chicago. The heat wave during the summer of 2003 is estimated to have caused 14,800 deaths in France, and climate models suggest an increase in both frequency and intensity of heat waves in temperate areas in the future.4
Principles of Disease
Heat Production
Heat production can be increased up to 20-fold by strenuous exertion. Rectal temperatures as high as 42° C are recorded without ill effects in trained marathon runners. Metabolic factors, such as hyperthyroidism and sympathomimetic drug ingestion, can dramatically increase heat production. Environmental heat not only adds to the heat load but also interferes with heat dissipation. The physics of heat transfer as it relates to human physiology involves four mechanisms: conduction, convection, radiation, and evaporation.5
Conduction.: Conduction is the transfer of heat energy from warmer to cooler objects by direct physical contact. Air is a good insulator; therefore, only approximately 2% of the body heat loss is by conduction. In contrast, the thermal conductivity of water is at least 25 times that of air.
Convection.: Heat loss to air and water vapor molecules circulating around the body is termed convection. As ambient temperature rises, the amount of heat dissipated by convection becomes minimal, and once air temperature exceeds the mean skin temperature, heat is gained by the body. Convective heat loss varies directly with wind velocity. Loose-fitting clothing maximizes convective (and also evaporative) heat loss.
Radiation.: Radiation is heat transfer by electromagnetic waves. Although radiation accounts for approximately 65% of heat loss in cool environments, it is a major source of heat gain in hot climates. Up to 300 kcal/hr can be gained from radiation when a person is directly exposed to the hot summer sun.6
Evaporation.: Evaporation is the conversion of a liquid to the gaseous phase. Evaporation of 1 mL of sweat from the skin cools the body by 0.58 kcal. As ambient temperature rises, evaporation becomes the dominant mechanism of heat loss. Panting mammals such as dogs have an oropharyngeal countercurrent flow mechanism (carotid rete mirabile) that results in selective cooling of the brain. In humans, respiratory and countercurrent mechanisms are minimal sources of heat loss, and the dominant mode of cooling in hot conditions is evaporation of sweat from the skin.
Heat Regulation
Thermosensors.: Temperature-sensitive structures are located both peripherally in the skin and centrally in the body. Skin temperature changes, however, correlate poorly with changes in the rate of heat loss.7 Thermosensitive neurons are in the preoptic anterior hypothalamus. They are activated when the temperature of the blood circulating through that area exceeds a “set point.”
The skin temperature affects heat loss because a person resting in a warm environment initiates sweating, even though the core temperature remains constant. In contrast, changes in core temperature are more potent than skin temperature changes in producing heat-dissipating responses.8
Central Integrative Area.: The central nervous system (CNS) interprets information received from the thermosensors to properly instruct thermoregulatory effectors. The concept of a central thermostat by which an alteration shifts effector thresholds in the same direction fits a variety of clinical situations. For example, fever, the circadian rhythm of temperature variation, and the 0.5° C difference in rectal temperature after ovulation can be explained by variation of a thermal set point.
Thermoregulatory Effectors.: Sweating and peripheral vasodilation are the major mechanisms by which heat loss can be accelerated. In a warm environment, evaporation of sweat from the skin is the most important mechanism of heat dissipation. Heat loss from the skin by convection and radiation is maximized by increased skin blood flow to facilitate sweating.
The vascular response to heat stress is cutaneous vasodilation and compensatory vasoconstriction of splanchnic and renal beds. These vascular changes are under neurogenic control and allow heat to be dissipated quickly and efficiently, but they place a tremendous burden on the heart.9 For blood pressure to be maintained, cardiac output increases dramatically. For this reason, saunas and hot tubs may be dangerous for patients with cardiac disease. Cardiovascular and baroreceptor reflexes also affect skin blood flow. Reduced forearm sweating and vasodilation are observed in severely dehydrated subjects exercising in a warm environment.10
Acclimatization
Acclimatization is defined as “a constellation of physiologic adaptations that appear in a normal person as the result of repeated exposures to heat stress.” Daily exposure to work and heat for 100 min/day results in near-maximal acclimatization in 7 to 14 days. This is characterized by an earlier onset of sweating (at a lower core temperature), increased sweat volume, and lowered sweat electrolyte concentration. Acclimatization is hastened by modest salt deprivation and delayed by high dietary salt intake. As acclimatization proceeds, the sweat sodium concentration decreases while the volume increases.11
The cardiovascular system plays a major role in both acclimatization and endurance training, largely resulting from an expansion of plasma volume.12 Heart rate is lower and associated with a higher stroke volume. Other physiologic changes include earlier release of aldosterone, although acclimatized individuals generate lower plasma levels of aldosterone during exercise heat stress. Total body potassium depletion of up to 20% (500 mEq) by the second week of acclimatization can occur as a result of sweat and urine losses coupled with inadequate repletion.12
Although many similarities exist between thermoregulatory responses to heat and exercise, the well-conditioned athlete is not necessarily heat acclimatized. For heat and exercise-induced adaptive responses to be maintained, heat exposure needs to continue intermittently at least on 4-day intervals. Plasma volume decreases considerably within 1 week in the absence of heat stress.12
Pathophysiology
Predisposing Factors for Heat Illness
The goal is to maximize voluntary fluid intake and gastric emptying so that fluid can rapidly enter the small intestine, where it is absorbed. Gastric emptying is accelerated to 25 mL/min by large fluid volumes (500-600 mL) and cool temperatures (10-15.8° C). High osmolality inhibits gastric emptying; osmolality of less than 200 mOsm/L is optimal. Most commercially available electrolyte solutions contain excessive sugar. Hydration can be monitored by measurement of body weight before and after training or athletic competition. An athlete with a loss of 2 or 3% body weight (1.5-2 L in a 70-kg man) should drink extra fluid and be permitted to compete only when body weight is within 0.5 to 1 kg (1 or 2 pounds) of the starting weight on the previous day. A weight loss of 5 or 6% represents a moderately severe deficit and usually is associated with intense thirst, scanty urine, tachycardia, and increase in rectal temperature of approximately 2° C. Such athletes should be restricted to light workouts after hydration until they return to normal weight. A loss of 7% or more of body weight represents severe water depletion; participation in sports should not be permitted until the athlete is examined by a physician. Wrestlers frequently fast, restrict food and fluid intake, and exercise vigorously wearing vapor-impermeable clothing to lose weight quickly so that they can compete in a lower weight class.13
Evaporative cooling can be lost when clothing inhibits air convection and evaporation. Loose-fitting clothing or ventilated fishnet jerseys allow efficient evaporation. Light-colored clothing reflects rather than absorbs light. Water evaporated from clothing is much less efficient for body cooling than is water evaporated from the skin.14,15
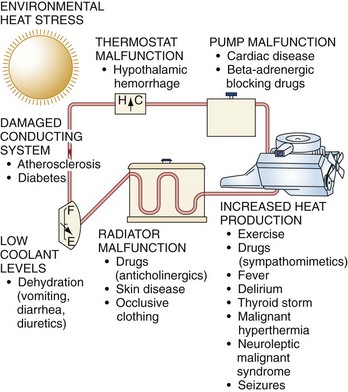
The body’s heat dissipation mechanisms are analogous to the cooling system of an automobile (Fig. 141-1). Coolant (blood) is circulated by a pump (heart) from the hot inner core to a radiator (skin surface cooled by the evaporation of sweat). Temperature is sensed by a thermostat (CNS), which alters coolant flow by a system of pipes, valves, and reservoirs (vasculature). Failure of any of these components can result in overheating.
Radiator function depends on the skin and sweat glands. Occlusive, vapor-impermeable clothing hinders evaporative and convective cooling. Anticholinergic medications and some drugs of abuse interfere with sweating and produce heat illness.11 Various skin diseases, including miliaria (prickly heat rash), extensive burns, scleroderma, ectodermal dysplasia, and cystic fibrosis, are risk factors. Anhidrosis can be secondary to either central or peripheral nervous system disorders as well.
Increased heat production causing heat illness most often accompanies exercise in hot, humid environments. Athletes and military recruits are commonly affected. When heat and humidity are extreme, exertion is not necessary to produce heat-related problems. Several indices help objectify heat strain. The indices can be divided into two categories: heat scales that are based on meteorologic parameters only and heat scales that combine environmental and physiologic parameters.16
The wet bulb globe temperature heat index is an excellent meteorologic measure of environmental heat stress (Box 141-1). It includes the effects of temperature, humidity, and radiant thermal energy from the sun. When climatic conditions exceed 25° C wet bulb, even healthy people are at high risk if they choose to exercise. Above 28° C, exercise and strenuous work should be avoided or limited to extremely short periods.17
The heat strain index is widely accepted as an example of an index that includes environmental and physiologic factors. There are several variations and modified heat strain indices in existence, with varying ease of use and accuracy (Fig. 141-2).16
Before the advent of air conditioning, mortality increased threefold to fivefold in nursing homes and threefold in the general population during heat waves. Mortality in geriatric patients correlates with average weekly peak air temperature. Most deaths in the 2003 European heat wave occurred in elderly patients. Microclimates conducive to heat illness are produced in the interiors of automobiles, tanks, and tents in the sun as well as in engine rooms, hot tubs, and saunas.4 Children are more susceptible to heat stressors because the higher surface area–to–mass ratios allow increased absorption of heat. They also have lower sweat rates per gland.18
Endogenous factors, such as hyperthyroidism and pheochromocytoma, can also drastically increase heat production. An overdose of sympathomimetics or stimulants, such as amphetamines, cocaine, and phencyclidine, can cause fatal hyperpyrexia. High ambient temperature is associated with a significant increase in mortality from cocaine overdose. Many younger patients who die of hyperthermia test positive for cocaine.19 Heatstroke can occur with delirium resulting from ethanol withdrawal.20 There are also reports of heatstroke occurring in well-trained military soldiers or athletes who ingest dietary supplements containing ephedrine or the ergogenic aid creatine.21,22
Certain patients undergoing general anesthesia rapidly experience severe hyperthermia, muscle rigidity, and acidosis. This syndrome, termed malignant hyperthermia, is the result of a genetically determined instability of skeletal muscle sarcoplasmic reticulum that allows inappropriate intracellular calcium release. Dantrolene, which lowers myoplasmic calcium, is effective in the prevention and treatment of this syndrome.23
Malignant hyperthermia is rarely seen in outpatient settings, but a clinically similar entity, neuroleptic malignant syndrome, is often encountered. This syndrome is induced by antipsychotic medications and is characterized by muscle rigidity, severe dyskinesia or akinesia, hyperthermia, tachycardia, dyspnea, dysphagia, and urinary incontinence. Although the lead-pipe rigidity and hyperthermia are reminiscent of malignant hyperthermia, the putative mechanism is different. Dopamine receptor blockade in the corpus striatum caused by butyrophenones and similar agents produces severe muscle spasticity and dystonia, leading to overproduction of heat. Some antipsychotics also cause suppression of thirst recognition. Other miscellaneous risk factors include obesity.24 Individuals with a history of heatstroke, with or without an inherent aberration that predisposed them to the initial episode, are at increased risk for a recurrence.
Fever versus Hyperthermia
It is both diagnostically and therapeutically important to identify patients suffering from a febrile response rather than heat illness. For many years, fever was attributed to pyrogens released by bacteria or viruses (exogenous pyrogens) or to cells undergoing autolysis after phagocytic activity (endogenous pyrogens).25 These circulating pyrogens would directly affect the thermoregulatory control center. It now appears that fever is the result of triggering by pyrogens of pathways involving cytokine receptors or other signals to reset the thermal set point in the preoptic area of the anterior hypothalamus to a new level above 37° C.25
Because fever is the product of a molecular interaction that establishes a new physiologic thermal set point, therapeutic attempts to lower temperature are opposed by body mechanisms that attempt to maintain the new set point. Thus attempts at whole-body cooling produce violent shivering and discomfort.26 The use of agents to block the causative molecular interaction is the most clinically effective approach. Antipyretics block the action of the pyrogen at hypothalamic receptor sites through inhibition of prostaglandin synthesis.27 These antipyretics are not effective against and should not be used to control environmental hyperthermia.
Minor Heat Illness
Physiology
Heat cramps are brief, intermittent, and often severe muscle cramps occurring typically in muscles that are fatigued by heavy work. Heat cramps appear to be related to a salt deficiency. Heat cramps occur most commonly during the first days of work in a hot environment and develop in persons who produce large amounts of thermal sweat and subsequently drink copious amounts of hypotonic fluid.28
Clinical Factors
Athletes, roofers, steel workers, coal miners, field workers, and boiler operators are among the most common victims of heat cramps. Heat cramps tend to occur after exercise when the victim stops working and is relaxing (Box 141-2). In this respect, they differ from the cramps experienced by athletes during exercise, which tend to last for several minutes, are relieved by massage, and resolve spontaneously.
Management
Heat cramps are usually rapidly relieved by salt solutions. Commercially available flavored electrolyte solutions are commonly ingested. Mild cases without concurrent dehydration are treated orally with 0.1 or 0.2% salt solution (two to four 10-grain salt tablets [56-112 mEq] or ¼ to ½ teaspoon of table salt dissolved in a quart of water), which is the general limit of palatability. Severe cases respond rapidly to intravenous isotonic solution (0.9% NaCl). Salt tablets are gastric irritants, delay gastric emptying, and are not recommended.29
Prickly Heat
Physiology and Clinical Features
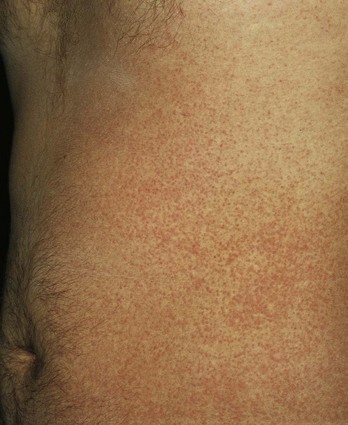
Clinically, this initially produces intensely pruritic vesicles on an erythematous base. The rash is confined to clothed areas, and the affected area is often completely anhidrotic. During approximately the next week, a keratin plug arises and fills these vesicles, causing a deeper obstruction of the sweat gland duct. The obstructed duct then ruptures a second time, producing a deeper vesicle within the dermis. This is known as the profunda stage, and it can persist for weeks. Profunda vesicles are not pruritic and closely resemble the white papules of piloerection; chronic dermatitis is a common complication (Fig. 141-3).
Heat Exhaustion
Clinical Features
The symptoms and signs associated with both types of heat exhaustion are variable and nonspecific and include weakness, fatigue, frontal headache, impaired judgment, vertigo, nausea and vomiting, and occasionally muscle cramps (Box 141-3). Orthostatic dizziness and syncope can occur. Sweating persists and may be profuse. The core temperature is only moderately elevated, usually below 40° C, and signs of severe CNS dysfunction are not present.
Mild heat exhaustion and full-blown heatstroke represent extremes of the spectrum of heat illness, and intermediate cases may prove difficult to differentiate. Nevertheless, heat exhaustion should not be diagnosed in the presence of major CNS dysfunction (seizures and coma) or severe hyperthermia (40.5° C). If uncertainty exists, measurement of hepatic transaminases may prove helpful. Elevations to several thousand units can be seen in patients with heat exhaustion or in healthy runners after a marathon, whereas in patients with heatstroke, such levels are usually in the tens of thousands after 24 hours.30,31
Disposition
Young, otherwise healthy patients who do not have significant laboratory abnormalities and who respond rapidly to hydration do not require hospitalization. These patients can be discharged with instructions to drink plenty of fluids and to avoid heat stress for 24 to 48 hours. Older patients, particularly those with cardiovascular disease or other predisposing factors described in Figure 141-4, require more cautious inpatient fluid and electrolyte replacement and frequent reassessment (Box 141-4).
Heatstroke
Physiology
As heatstroke develops, energy will be insufficient to sustain thermoregulatory mechanisms, resulting in dramatic increases in core temperature and the clinical manifestations of heatstroke.32 An exact temperature at which cellular damage begins to occur in an individual patient varies. Credible reports exist of full recovery despite rectal temperatures of 44.4 to 46.5° C.33 Damage is a function not only of temperature but also of the exposure time.34 The resultant damage to tissues is a function of a complex interaction of body temperature, exposure time, workload, tissue perfusion, and individual factors, which vary markedly.
Neurologic dysfunction is a hallmark of heatstroke, and cerebral edema is common. Other pathologic changes include petechiae in the walls of the third and fourth ventricles and marked cerebellar Purkinje cell damage.35 Interestingly, the hypothalamus, the predominant site of central thermoregulatory control, is usually not damaged.
Prolonged heat stress produces impressive increases in skin blood flow (peripheral vasodilation) and a reduction of the thermal gradient between the core and the skin (Fig. 141-5). Functional hypovolemia is avoided by compensatory vasoconstriction of the splanchnic and renal vasculature. The resulting splanchnic and renal ischemia may explain the nausea, vomiting, and diarrhea observed in runners after a marathon. Hepatic damage is such a consistent feature of heatstroke that its absence should cast doubt on the diagnosis. This is manifested pathologically by centrilobular necrosis with extensive cholestasis.
Clinical Features
Heatstroke versus Heat Exhaustion
The onset of heatstroke is sudden, and the patient’s level of consciousness is altered.35 Prodromal symptoms lasting minutes to hours occur in approximately 20% of cases. These are nonspecific and may include weakness, dizziness, nausea, vomiting, anorexia, frontal headache, confusion, drowsiness, disorientation, muscle twitching, ataxia and signs of cerebellar dysfunction, and psychiatric symptoms ranging from anxiety and irritability to psychosis.36,37
The usual manifestations of heatstroke include hyperpyrexia above 40.5° C, profound CNS dysfunction, and hot skin (Box 141-5). Persistent sweating can be observed in patients with rectal temperatures of 41.5 to 42.4° C with heatstroke. In one large series of exertional heatstroke victims, sweating persisted in 50% of cases.38 Therefore, the cessation of sweating is not the cause of heatstroke, and continued sweating does not preclude the diagnosis.
Classic Heatstroke versus Exertional Heatstroke
The two forms of heatstroke have significantly different presentations and manifestations: classic (epidemic) heatstroke (CHS) and exertional heatstroke (EHS) (Table 141-1).
Table 141-1
Usual Characteristics of Heatstroke
| EXERTIONAL | CLASSIC |
| Healthy | Predisposing factors or medications |
| Younger | Older |
| Exercise | Sedentary |
| Sporadic | Heat wave occurrence |
| Diaphoresis | Anhidrosis |
| Hypoglycemia | Normoglycemia |
| DIC | Mild coagulopathy |
| Rhabdomyolysis | Mild CK elevation |
| Acute renal failure | Oliguria |
| Marked lactic acidosis | Mild acidosis |
| Hypocalcemia | Normocalcemia |
CK, creatine kinase; DIC, disseminated intravascular coagulation.
CHS occurs during periods of sustained high ambient temperatures and humidity, such as during summer heat waves. Victims are often elderly and poor and live in underventilated dwellings without air conditioning. Debilitated patients who have limited access to oral fluids may develop water depletion heat exhaustion, which progresses to heatstroke if it is untreated. Victims of CHS commonly suffer from chronic diseases, alcoholism, or schizophrenia, which predisposes to heat illness. Such patients are often prescribed medications (e.g., diuretics, antihypertensives, neuroleptics, and anticholinergics) that impair the ability to tolerate heat stress. Sweating ceases in the majority of CHS patients. Factors such as advanced age, hypotension, altered coagulation status, and the necessity for endotracheal intubation on arrival at the ED predict a poor outcome despite successful cooling measures.39 In contrast, patients with EHS are usually young and healthy individuals whose heat-dispelling mechanisms are overwhelmed by endogenous heat production. Athletes and military recruits are typical victims.40–42 Rhabdomyolysis and acute renal failure, rarely seen in patients with CHS, are common in patients with EHS.43 Sweating is present in half the cases of EHS. Hypoglycemia may occur as the result of increased glucose metabolism and hepatic damage, resulting in impaired gluconeogenesis. Coagulopathy is common. Hyponatremia with serum sodium levels of less than 130 mmol/L has been detected in summer hikers in the Grand Canyon; many present with neurologic symptoms or seizures.41 Signs of profound CNS dysfunction dominate the early course of heatstroke. Delirium or coma is characteristic, but virtually any neurologic abnormality, including bizarre behavior, opisthotonos, hallucinations, decerebrate rigidity, oculogyric crisis, and cerebellar dysfunction, can be seen.38 Convulsions occur in up to 75% of patients and can be precipitated by therapeutic cooling maneuvers. Profound muscle rigidity with tonic contractions, coarse tremor, and dystonic movements can mimic seizures. Pupils may be fixed and dilated, and the electroencephalogram may be isoelectric. All these changes are potentially reversible, although permanent damage, including cerebellar deficits, hemiplegia, dementia, and personality changes, is common in severe cases. Patients with heatstroke usually have hyperdynamic cardiovascular systems with low peripheral vascular resistance, tachycardia (up to 180 beats/min), and elevated cardiac index.9 Elevation of cardiac troponin I is not uncommon with CHS; however, it is rarer in EHS.44,45 The central venous pressure (CVP) is usually elevated. The combination of elevated CVP with right-sided cardiac dilation suggests right-sided heart failure, which is also seen after shock or sepsis.46 These changes are expected because skin blood vessels dilate to dissipate heat; however, this low peripheral vascular resistance has persisted in patients after reduction of body temperature to nearly normal.
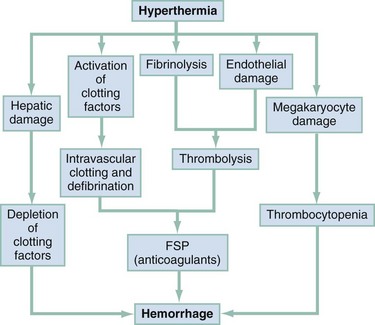
Respiratory alkalosis is a physiologic response to active or passive heating and may be severe enough to produce tetany. Although most patients with CHS have respiratory alkalosis, those with EHS usually have a relatively pure lactic acidosis. Lactic acidosis is associated with a poor prognosis in cases of CHS but not necessarily in cases of EHS. Both CHS and EHS cause the hemoglobin-oxygen dissociation curve to shift to the right. An increase in the temperature denatures the bond between oxygen and hemoglobin, decreasing the concentration of oxyhemoglobin. Aberrations in coagulation are common in patients with severe heatstroke, and their presence is a poor prognostic sign.47 Abnormal hemostasis is manifested clinically by purpura, conjunctival hemorrhage, melena, bloody diarrhea, hemoptysis, hematuria, myocardial bleeding, or hemorrhage into the CNS.48
Hepatic damage is so consistently featured in heatstroke that its absence should cast doubt on the diagnosis. Hepatic injury is evidenced by markedly elevated levels of hepatic aminotransferases (serum aspartate transaminase and alanine transaminase).47 Jaundice typically appears 24 to 72 hours after the onset of severe heatstroke and gradually recedes if the victim survives. Survivors generally have no permanent impairment of liver function. Hypoglycemia with a serum glucose level of less than 65 mg/dL is common in cases of EHS. Renal damage is common. The initial urine specimen, usually obtained by catheterization, is a scanty, brownish, turbid fluid resembling machine oil. Microscopic examination reveals proteinuria with abundant granular casts and red blood cells. Acute oliguric renal failure complicates 25 to 30% of EHS cases and 5% of CHS cases. Glomerular filtration rate, renal plasma flow, urine flow, and sodium excretion diminish markedly during exercise. Heavy physical exertion in hot climates produces acidic and maximally concentrated urine, which can result in acute oliguric renal failure in combination with hypotension and myoglobinuria.28 Cocaine use is also associated with rhabdomyolysis and hyperthermia.49 Diarrhea, probably caused by intense splanchnic vasoconstriction, is commonly seen. Cooling aggravates the diarrhea, creating an unpleasant treatment problem. Pancreatitis is described with elevated serum amylase and lipase levels.
Diagnostic Strategies
Placement of a tympanic temperature sensor directly on the tympanic membrane is difficult and is not used clinically. Commercially available infrared thermometers do not physically contact the tympanic membrane and are unreliable in detection of hyperthermia.50 Only after the initial assessment and cooling have begun is the differential diagnosis relevant. When a history of collapse under conditions of heat stress is present, rapid improvement in mental status and blood pressure with cooling eliminates alternative diagnoses. If, however, the temperature does not respond and the patient does not recover neurologically, other causes of fever and coma should be considered (Box 141-6).
Drug-induced heat illness is an important consideration, particularly anticholinergic poisoning. Differentiation may be difficult because both heatstroke and anticholinergic poisoning produce hyperpyrexia, hot and dry skin, tachycardia, and abnormal mental status. Constricted pupils are present in many heatstroke patients.51 Mydriasis should be present in patients with anticholinergic poisoning, and its absence argues strongly against this diagnosis. Typhoid fever, typhus, delirium tremens, and hypothalamic hemorrhage all produce a symptom complex similar to that of heatstroke.
Management
Cooling
Immediate cooling is the cornerstone of treatment. Mortality correlates with the temperature and the number of dysfunctional organ systems, with an increased risk of death if patients present with anuria, coma, or cardiovascular failure.53 Patients who present to the hospital with heat stroke have high mortality rates ranging from 21 to 63%,4,54 and mortality increases significantly when cooling is delayed.34,55 Cooling efforts take precedence over any time-consuming search for the cause. In the out-of-hospital setting, cooling should be initiated after removal of the patient from the hot environment. When the patient arrives at the hospital, clothes should be removed, a thermistor probe should be inserted, and the temperature should be continuously monitored.
The ideal method of evaporative cooling uses a body cooling unit on which the patient lies suspended on a net surface while being sprayed with atomized 15° C water from above and below.56 Air warmed to 45 to 48° C is blown over the skin surface at 3 m/min. The unit, not widely available, maximizes evaporative cooling by maintaining cutaneous vasodilation and avoiding heat generation caused by shivering.
Less complex equipment can also be used to maximize evaporative cooling, which is the most widely used cooling method. The combination of atomized tepid water at 40° C from a spray bottle and standing fans cools at rates comparable to both body cooling unit and immersion.57 Other evaporative techniques, including the use of downdraft from a helicopter, can be applied successfully.58 Immersion in ice water results in a rapid reduction of core temperature to below 39° C within 10 to 40 minutes but complicates the resuscitation.55 Vigorous skin massage to maintain cutaneous circulation has been advocated, but there is no objective evidence that this is efficacious. When the body temperature reaches 39° C, cooling measures should be discontinued to avoid hypothermic overshoot. Continuous monitoring is necessary to maintain the core temperature at 37 to 38° C.
Military studies of EHS patients treated with ice water immersion have reported no fatalities or permanent sequelae.40 A mortality rate of 14% was reported in a study of 28 patients with CHS treated by immersion.51 Immersion is technically difficult in the ED. Vasoconstriction from ice water immersion may be beneficial to hypotensive patients and may be better than evaporative cooling for victims in shock who have poor peripheral circulation.59
Cooling modalities other than evaporation and immersion should be considered adjunctive treatments (Box 141-7). Application of ice packs to high heat transfer areas (neck, groin, and axillae) is commonly used. Cooling blankets may be a useful adjunct but will not produce rapid cooling if they are used exclusively. Peritoneal dialysis with cold fluids, although successful in a canine model, is invasive. Cold-irrigant gastric or rectal lavage will not provide significant heat exchange if it is used as the primary cooling modality.
Resuscitation
Aspiration and seizures are common in patients with heatstroke, and airway control is essential.60 Hypoxemia may occur because of aspiration, pneumonitis and pulmonary infarction, hemorrhage, or edema. Metabolic demands are high, and normal pulmonary ventilation may be inadequate in this setting.
Circulatory fluid requirements are modest in some cases, averaging 1200 mL of isotonic crystalloid solution in the first 4 hours. Pulmonary edema occurs in patients with heatstroke and can be exacerbated by overzealous fluid administration. The use of a CVP catheter to monitor fluid resuscitation may be deceptive. Most patients have a hyperdynamic circulation with high cardiac index, low peripheral vascular resistance, and elevated CVP as a result of right-sided heart failure. These patients may require only modest intravenous fluids because cooling produces vasoconstriction and increases blood pressure.61 Nevertheless, crystalloid resuscitation is essential.
Hypotension is common in patients with heatstroke and is usually caused by peripheral vasodilation resulting in high-output cardiac failure in addition to dehydration.9 Blood pressure usually rises with cooling. If this does not occur, or if the patient monitored invasively has a low CVP, a fluid challenge of 250 to 500 mL of 0.9% saline should be given rapidly while blood pressure, pulse, and urine output are monitored. If the blood pressure rises, more fluids are given with careful monitoring of the CVP. Aggressive fluid replacement is continued until the blood pressure reaches 90/60 mm Hg or the CVP exceeds 12 mL H2O. On occasion, patients exhibit hypodynamic responses with low cardiac index, elevated CVP, and hypotension. These patients may be cyanotic, whereas patients with hyperdynamic circulation are initially pink. This clinical observation can be helpful in identifying patients who may respond to catecholamines.
Because the pathophysiologic processes of heatstroke and fever differ, antipyretics are not indicated and may be harmful. Salicylates, particularly in large doses, can worsen hyperthermia by uncoupling oxidative phosphorylation and aggravate coagulopathies. Large doses of acetaminophen can result in further hepatic damage. The efficacy of dantrolene is not established.62 If rhabdomyolysis is present, maintenance of urinary output of at least 2 mL/kg/hr is required.63 Urinary alkalinization higher than 6.5 should be considered early in patients with acidemia, dehydration, or underlying renal disease. After volume repletion, administration of mannitol may be considered to increase intravascular volume and to increase glomerular filtration rate. Mannitol should not be used in an oliguric patient.64 Persistent anuria, uremia, or hyperkalemia is an indication for consideration of hemodialysis.
Hematologic evaluation should include arterial blood gas determination; complete blood cell count and platelet counts; electrolyte values and blood urea nitrogen, glucose, creatinine, aspartate transaminase, alanine transaminase, lactate dehydrogenase, creatine kinase, uric and lactic acid, and calcium levels; prothrombin and partial thromboplastin times; international normalized ratio; and fibrin degradation products and hepatic enzymes. Serum cardiac troponin I levels should be obtained. Acidosis is common, especially in patients with EHS. Lactate levels are usually elevated; this may persist or even worsen with improved extremity perfusion.53
Coagulopathies can occur during the first day of illness but are more common on the second and third days. Initial treatment after cooling should include replacement therapy with fresh frozen plasma and platelets.48 The clinician should monitor the laboratory signs of disseminated intravascular coagulation (hypofibrinogenemia, elevated fibrin split products, prolonged prothrombin time, and thrombocytopenia).48 The bleeding diathesis in patients with heatstroke may be the result of fibrinolysis. Although α-aminocaproic acid can impede fibrinolysis, administration of this compound is associated with rhabdomyolysis, and its use is not recommended in patients with heatstroke.
References
1. Dickenson, JG. Heat illness in the services. J R Army Med Corps. 1994;140:7.
2. Centers for Disease Control and Prevention. Heat illness among high school athletes—United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1009.
3. Mueller, FO, Colgate, B. Annual survey of football injury research, 1931-2009. www.unc.edu/depts/nccsi/2009AnnualFootball.pdf.
4. Argaud, L, et al. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med. 2007;167:2177.
5. Bouchama, A, Knochel, JP. Heat stroke. N Engl J Med. 2002;346:1978.
6. Gisolfi, CV, Wenger, CB. Temperature regulation during exercise: Old concepts, new ideas. Exerc Sport Sci Rev. 1984;12:339.
7. Charkoudian, N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clin Proc. 2003;78:603.
8. Rowell, LB. General principles in vascular control. In: Rowell LB, ed. Human Circulation: Regulation during Physical Stress. New York: Oxford University Press, 1986.
9. Wilson, TE, Crandall, CG. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev. 2011;39:12.
10. Nadel, ER, Fortney, SM, Wenger, CB. Effect of hydration state of circulatory and thermal regulations. J Appl Physiol Respir Environ Exerc Physiol. 1980;49:715.
11. Hajat, S, O’Connor, M, Kosatsky, T. Health effects of hot weather: From awareness of risk factors to effective health protection. Lancet. 2010;375:856.
12. Appenzeller, O. Physical training, heat acclimatization and diet. In: Khogali M, Hales JRS, eds. Heat Stroke and Temperature Regulation. New York: Academic Press, 1983.
13. Centers for Disease Control and Prevention. Hyperthermia and dehydration-related deaths associated with intentional rapid weight loss in three collegiate wrestlers—North Carolina, Wisconsin, and Michigan, November-December 1997. MMWR Morb Mortal Wkly Rep. 1998;47:105.
14. Pascoe, DD, Bellingar, TA, McCluskey, BS. Clothing and exercise: II. Influence of clothing during exercise/work in environmental extremes. Sports Med. 1994;18:94.
15. Bernard, T, et al. Critical heat stress evaluation of clothing ensembles with different levels of porosity. Ergonomics. 2010;53:1048.
16. Moran, DS, Shitzer, A, Pandolf, KB. A physiological strain index to evaluate heat stress. Am J Physiol. 1998;275:129.
17. Olshaker, JS, Tek, D. Environmental emergencies. Emerg Med Clin North Am. 1992;10:299.
18. Falk, B. Effect of thermal stress during rest and exercise in the pediatric population. Sports Med. 1998;25:221.
19. Marzuk, PM, et al. Ambient temperature and mortality from unintentional cocaine overdose. JAMA. 1998;279:1795.
20. Martinez, M, Devenport, L, Saussy, J, Martinez, J. Drug-associated heat stroke. South Med J. 2002;95:799.
21. Oh, RC, Henning, JS. Exertional heatstroke in an infantry soldier taking ephedra-containing dietary supplements. Mil Med. 2003;168:129.
22. Bailes, JE, Cantu, RC, Day, AL. The neurosurgeon in sport: Awareness of the risks of heatstroke and dietary supplements. Neurosurgery. 2002;51:283.
23. Ester, EA, Harrison, J, Stasiewicz, ED, Wang, D. Malignant hyperthermia in an adult trauma patient. Am Surg. 2002;68:883.
24. Chung, NK, Pin, CH. Obesity and the occurrence of heat disorders. Mil Med. 1996;161:739.
25. Dinarello, CA. Infection, fever, and exogenous and endogenous pyrogens: Some concepts have changed. J Endotoxin Res. 2004;10:4.
26. Kashmeery, AMS. Physiological studies on heat exhaustion victims among Mecca Pilgrims: Response to relevant hormones, and effect of cold IV infusion on recovery. Acta Med Austriaca. 1995;22:16.
27. Saper, CB, Breder, CD. Endogenous pyrogens in the CNS: Role in the febrile response. Prog Brain Res. 1992;93:419.
28. Noakes, TD. Fluid and electrolyte disturbance in heat illness. Int J Sports Med. 1998;2:5146.
29. Brake, DJ, Bates, GP. Fluid losses and hydration status of industrial workers under thermal stress working extended shifts. J Occup Environ Med. 2003;60:90.
30. Schiff, HB, et al. Myoglobinuria, rhabdomyolysis and marathon running. Q J Med. 1978;47:463.
31. Shibolet, S, et al. Heat stroke: Its clinical picture and mechanism in 36 cases. Q J Med. 1967;36:525.
32. Hubbard, RW, et al. Novel approaches to the pathophysiology of heatstroke: The energy depletion model. Ann Emerg Med. 1987;16:1066.
33. Slovis, CM, Anderson, GF, Casolaro, A. Survival in a heat stroke victim with a core temperature in excess of 46.5° C. Ann Emerg Med. 1982;11:269.
34. Sithinamsuwan, P, et al. Exertional heatstroke: Early recognition and outcome with aggressive combined cooling—a 12-year experience. Mil Med. 2009;174:496.
35. McLaughlin, CT, Kane, AG, Auber, AE. MR imaging of heat stroke, external capsule and thalamic T1 shortening and cerebellar injury. Am J Neuroradiol. 2003;24:1372.
36. Khogali M, Hales JRS, eds. Heat Stroke and Temperature Regulation. New York: Academic Press, 1983.
37. Bark, N. Heat stroke in psychiatric patients: Two cases and a review. J Clin Psychiatry. 1982;43:377.
38. Austin, MG, Berry, JW. Observations on one hundred cases of heat stroke. JAMA. 1956;161:1525.
39. Vicario, SJ, Okabajue, R, Haltom, T. Rapid cooling in classic heatstroke: Effect on mortality rates. Am J Emerg Med. 1986;4:394.
40. Beller, GA, Boyd, AE, III. Heat stroke: A report of 13 consecutive cases without mortality despite severe hyperpyrexia and neurologic dysfunction. Mil Med. 1975;140:464.
41. Backer, HD, et al. Exertional heat illness and hyponatremia in hikers. Am J Emerg Med. 1999;17:532.
42. Porter, AM. Collapse from exertional heat illness: Implications and subsequent decisions. Mil Med. 2003;168:76.
43. Phillips, RA. The relationship between proinflammatory mediators and heat stress induced rhabdomyolysis in exercising marines. Crit Care Nurs Clin North Am. 2003;15:163.
44. Hausfater, P, et al. Elevation of cardiac troponin I during non-exertional heat-related illnesses in the context of a heatwave. Crit Care. 2010;14:R99.
45. Whitcar, R, Laba, D, Smith, S. Exertional heat stroke in a young man with a documented rise in troponin I. Emerg Med J. 2008;25:283.
46. Shahid, MS, et al. Echocardiographic and Doppler study of patients with heatstroke and heat exhaustion. Int J Card Imaging. 1999;15:279.
47. Varghese, GM, et al. Predictors of multi-organ dysfunction in heatstroke. Emerg Med J. 2005;22:185.
48. al-Mashhadani, SA, et al. The coagulopathy of heat stroke: Alterations in coagulation and fibrinolysis in heat stroke patients during the pilgrimage (Haj) to Makkah. Blood Coagul Fibrinol. 1994;5:731.
49. Roth, D. Acute rhabdomyolysis associated with cocaine intoxication. N Engl J Med. 1988;319:673.
50. Hansen, RD, et al. Infrared thermometry in the diagnosis and treatment of heat exhaustion. Int J Sports Med. 1996;17:66.
51. Hart, GR, et al. Epidemic heat stroke: Clinical characteristics and course of 28 patients. Medicine (Baltimore). 1982;61:189.
52. Hamilton, D. The immediate treatment of heatstroke. Anaesthesia. 1976;31:270.
53. Pease, S, et al. Early organ dysfunction course, cooling time and outcome in classic heatstroke. Intensive Care Med. 2009;35:1454.
54. Dematte, JE, et al. Near fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med. 1998;129:173.
55. Costrini, A. Emergency treatment of exertional heatstroke and comparison of whole body cooling techniques. Med Sci Sports Exerc. 1990;22:15.
56. Smith, JE. Cooling methods used in the treatment of exertional heat illness. Br J Sports Med. 2005;39:503.
57. Graham, BS, et al. Nonexertional heat stroke: Physiologic management and cooling in 14 patients. Arch Intern Med. 1986;146:87.
58. Poulton, TJ, Walker, RA. Helicopter cooling of heatstroke victims. Aviat Space Environ Med. 1987;58:358.
59. Bouchama, A, et al. Cooling and hemodynamic management in heatstroke: Practical recommendations. Crit Care. 2007;11:R54.
60. Al-Khawashki, MJ, et al. Clinical presentation of 172 heat stroke cases seen at Mina and Arafat—September 1982. In: Khogali M, Hales JRS, eds. Heat Stroke and Temperature Regulation. New York: Academic Press; 1983:99–108.
61. Atar, S, Royner, E, Rosenfeld, T. Transient cardiac dysfunction and pulmonary edema in exertional heat stroke. Mil Med. 2003;168:671.
62. Krause, T, et al. Dantrolene–a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59:364.
63. Curry, SC, Chang, D, Connor, D. Drug- and toxin-induced rhabdomyolysis. Ann Emerg Med. 1989;18:1068.
64. Malinoski, DJ, Slater, MS, Mullins, RJ. Crush injury and rhabdomyolysis. Crit Care Clin. 2004;20:171.

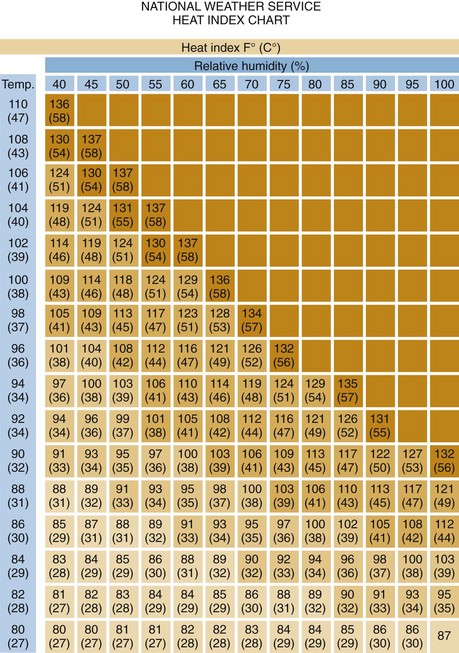
 extreme danger
extreme danger danger
danger