Diseases of the Lung Parenchyma
Chronic obstructive pulmonary disease
Emphysema
Chronic bronchitis
Cystic fibrosis
Idiopathic interstitial pneumonitis
Idiopathic pulmonary fibrosis
Nonspecific interstitial pneumonitis
Sarcoidosis
Bronchiectasis
Pulmonary Langerhans cell histiocytosis
Lymphangioleiomyomatosis
PATHOPHYSIOLOGY AND BASIC MECHANISMS
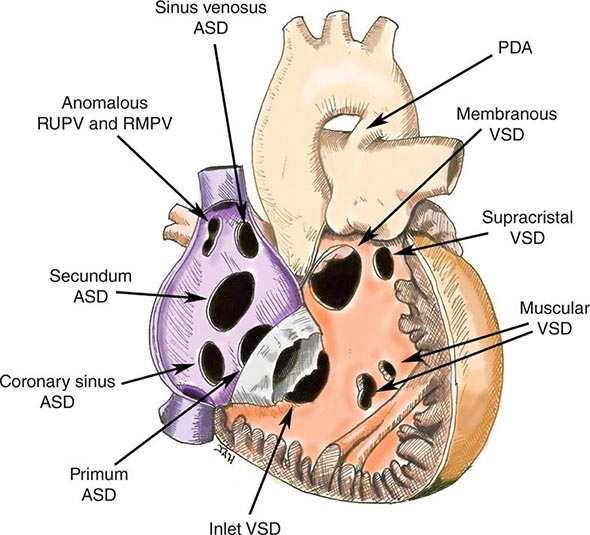
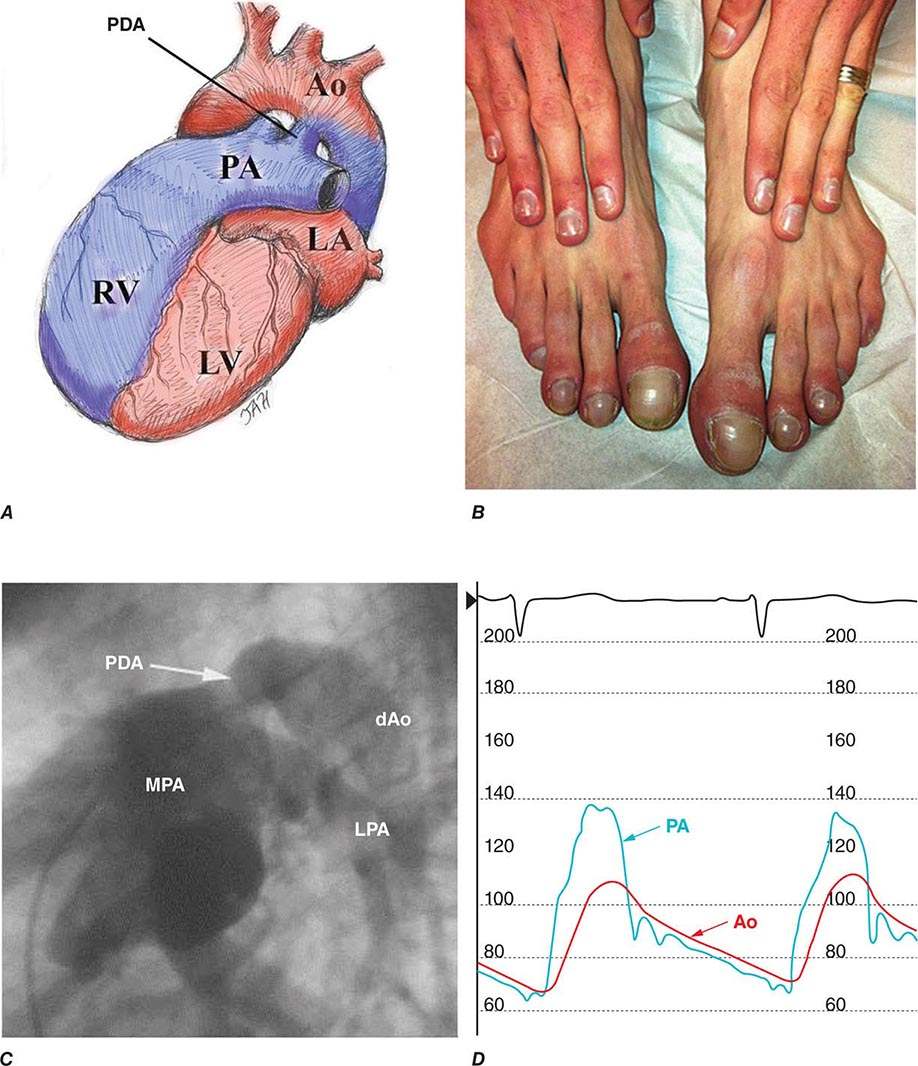
Although many conditions can lead to cor pulmonale, the common pathophysiologic mechanism is pulmonary hypertension that is sufficient to alter RV structure (i.e., dilation with or without hypertrophy) and function. Normally, pulmonary artery pressures are only ~15 mmHg and do not increase even with multiples of resting cardiac output, because of vasodilation and blood vessel recruitment of the pulmonary circulatory bed. But, in the setting of parenchymal lung diseases, primary pulmonary vascular disorders, or chronic (alveolar) hypoxia, the circulatory bed undergoes varying degrees of vascular remodeling, vasoconstriction, and destruction. As a result, pulmonary artery pressures and RV afterload increase, setting the stage for cor pulmonale (Table 279-4). The systemic consequences of cor pulmonale relate to alterations in cardiac output as well as salt and water homeostasis. Anatomically, the RV is a thin-walled, compliant chamber that is better suited to handle volume overload than pressure overload. Thus, the sustained pressure overload imposed by pulmonary hypertension and increased pulmonary vascular resistance eventually causes the RV to fail.
The response of the RV to pulmonary hypertension depends on the acuteness and severity of the pressure overload. Acute cor pulmonale occurs after a sudden and severe stimulus (e.g., massive pulmonary embolus), with RV dilatation and failure but no RV hypertrophy (Chap. 300). Chronic cor pulmonale, however, is associated with a more slowly evolving and progressive pulmonary hypertension that leads to initial modest RV hypertrophy and subsequent RV dilation. Acute decompensation of previously compensated chronic cor pulmonale is a common clinical occurrence. Triggers include worsening hypoxia from any cause (e.g., pneumonia), acidemia (e.g., exacerbation of COPD), acute pulmonary embolus, atrial tachyarrhythmia, hypervolemia, and mechanical ventilation that leads to compressive forces on alveolar blood vessels.
CLINICAL MANIFESTATIONS
Symptoms The symptoms of chronic cor pulmonale generally are related to the underlying pulmonary disorder. Dyspnea, the most common symptom, is usually the result of the increased work of breathing secondary to changes in elastic recoil of the lung (fibrosing lung diseases), altered respiratory mechanics (e.g., overinflation with COPD), or inefficient ventilation (e.g., primary pulmonary vascular disease). Orthopnea and PND are rarely symptoms of isolated right HF and usually point toward concurrent left heart dysfunction. Rarely, these symptoms reflect increased work of breathing in the supine position resulting from compromised diaphragmatic excursion. Abdominal pain and ascites that occur with cor pulmonale are similar to the right HF that ensues in chronic HF. Lower-extremity edema may occur secondary to neurohormonal activation, elevated RV filling pressures, or increased levels of carbon dioxide and hypoxemia, which can lead to peripheral vasodilation and edema formation.
Signs Many of the signs encountered in cor pulmonale are also present in HF patients with a depressed EF, including tachypnea, elevated jugular venous pressures, hepatomegaly, and lower-extremity edema. Patients may have prominent v waves in the jugular venous pulse as a result of tricuspid regurgitation. Other cardiovascular signs include an RV heave palpable along the left sternal border or in the epigastrium. The increase in intensity of the holosystolic murmur of tricuspid regurgitation with inspiration (“Carvallo’s sign”) may be lost eventually as RV failure worsens. Cyanosis is a late finding in cor pulmonale and is secondary to a low cardiac output with systemic vasoconstriction and ventilation-perfusion mismatches in the lung.
DIAGNOSIS
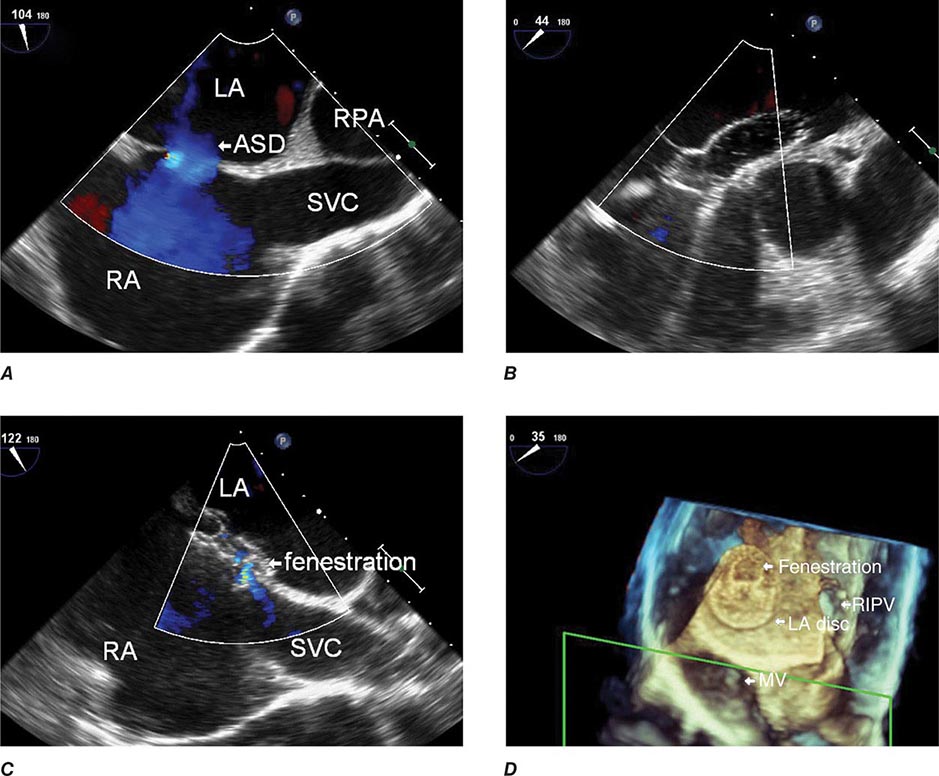
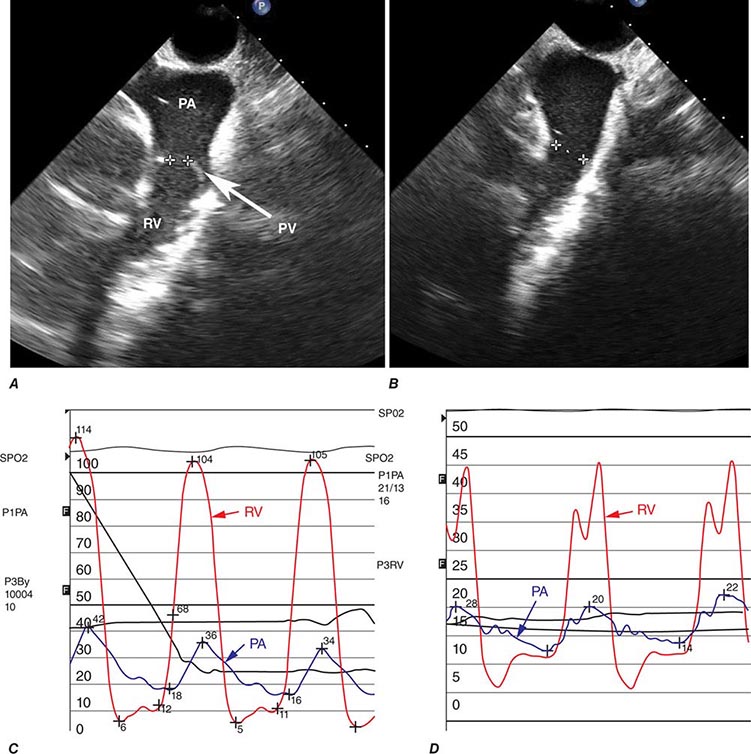
The most common cause of right HF is not pulmonary parenchymal or vascular disease but left HF. Therefore, it is important to evaluate the patient for LV systolic and diastolic dysfunction. The ECG in severe pulmonary hypertension shows P pulmonale, right axis deviation, and RV hypertrophy. Radiographic examination of the chest may show enlargement of the main central pulmonary arteries and hilar vessels. Spirometry and lung volumes can identify obstructive and/or restrictive defects indicative of parenchymal lung diseases; arterial blood gases can demonstrate hypoxemia and/or hypercapnia. Spiral computed tomography (CT) scans of the chest are useful in diagnosing acute thromboembolic disease; however, ventilation-perfusion lung scanning remains best suited for diagnosing chronic thromboembolic disease (Chap. 300). A high-resolution CT scan of the chest can identify interstitial lung disease.
Two-dimensional echocardiography is useful for measuring RV thickness and chamber dimensions. Location of the RV behind the sternum and its crescent shape challenge assessment of RV function by echocardiography, especially when parenchymal lung disease is present. Calculated measures of RV function (e.g., tricuspid annular plane systolic excursion [TAPSE] or the Tei Index) supplement more subjective assessments of RV function. The interventricular septum may move paradoxically during systole in the presence of pulmonary hypertension. As noted, Doppler echocardiography can be used to assess pulmonary artery pressures. MRI is also useful for assessing RV structure and function, particularly in patients who are difficult to image with 2-D echocardiography because of severe lung disease. Right-heart catheterization is useful for confirming the diagnosis of pulmonary hypertension and for excluding elevated left-heart pressures (measured as the pulmonary capillary wedge pressure) as a cause for right HF. BNP and N-terminal BNP levels are elevated in patients with cor pulmonale secondary to RV myocardial stretch and may be dramatically elevated in acute pulmonary embolism.
280 |
Heart Failure: Management |
Distinctive phenotypes of presentation with diverse management targets exemplify the vast syndrome of heart failure. These range from chronic heart failure with reduced ejection fraction (HFrEF) or heart failure with preserved ejection fraction (HFpEF), acute decompensated heart failure (ADHF), and advanced heart failure. Early management evolved from symptom control to disease-modifying therapy in HFrEF with the advent of renin-angiotensin-aldosterone system (RAAS)–directed therapy, beta receptor antagonists, mineralocorticoid receptor antagonists, cardiac resynchronization therapy, and implantable cardio-defibrillators. However, similar advances have been elusive in the syndromes of HFpEF and ADHF, which have remained devoid of convincing therapeutic advances to alter their natural history. In advanced heart failure, a stage of disease typically encountered in HFrEF, the patient remains markedly symptomatic with demonstrated refractoriness or inability to tolerate full-dose neurohormonal antagonism, often requires escalating doses of diuretics, and exhibits persistent hyponatremia and renal insufficiency with frequent episodes of heart failure decompensation requiring recurrent hospitalizations. Such individuals are at the highest risk of sudden or progressive pump failure–related deaths (Chap. 281). In contrast, early-stage asymptomatic left ventricular dysfunction is amenable to preventive care, and its natural history is modifiable by neurohormonal antagonism (not further discussed).
HEART FAILURE WITH PRESERVED EJECTION FRACTION
GENERAL PRINCIPLES
Therapeutic targets in HFpEF include control of congestion, stabilization of heart rate and blood pressure, and efforts at improving exercise tolerance. Addressing surrogate targets, such as regression of ventricular hypertrophy in hypertensive heart disease, and use of lusitropic agents, such as calcium channel blockers and beta receptor antagonists, have been disappointing. Experience has demonstrated that lowering blood pressure alleviates symptoms more effectively than targeted therapy with specific agents.
CLINICAL TRIALS IN HFpEF
The Candesartan in Heart Failure—Assessment of Mortality and Morbidity (CHARM) Preserved study showed a statistically significant reduction in hospitalizations but no difference in all-cause mortality in patients with HFpEF who were treated with the angiotensin receptor blocker (ARB), candesartan. Similarly, the Irbesartan in Heart Failure with Preserved Systolic Function (I-PRESERVE) trial demonstrated no differences in meaningful endpoints in such patients treated with irbesartan. An earlier analysis of a subset of the Digitalis Investigation Group (DIG) trial found no role for digoxin in the treatment of HFpEF. In the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure (SENIORS) trial of nebivolol, a vasodilating beta blocker, the subgroup of elderly patients with prior hospitalization and HFpEF did not appear to benefit in terms of all-cause or cardiovascular mortality. Much smaller mechanistic studies in the elderly with the angiotensin-converting enzyme inhibitor (ACEI) enalapril showed no effect on peak exercise oxygen consumption, 6-minute walk distance, aortic distensibility, left ventricular mass, or peripheral neurohormone expression.
NOVEL TARGETS
A small trial demonstrated that the phosphodiesterase-5 inhibitor sildenafil improved filling pressures and right ventricular function in a cohort of HFpEF patients with pulmonary venous hypertension. This finding led to the phase II trial, Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX), in HFpEF patients (left ventricular ejection fraction [LVEF] >50%) with New York Heart Association (NYHA) functional class II or III symptoms, who received sildenafil at 20 mg three times daily for 3 months, followed by 60 mg three times daily for another 3 months, compared with a placebo. There was no improvement in functional capacity, quality of life, or other clinical and surrogate parameters. Conceptually targeting myocardial fibrosis in HFpEF, the large-scale Aldosterone Antagonist Therapy in Adults with Preserved Ejection Fraction Congestive Heart Failure (TOPCAT) trial has been completed. This trial demonstrated no improvement in the primary composite end-point, but did show a secondary signal of benefit on HF hospitalizations, counterbalanced, however, by an increase in adverse effects, particularly hyperkalemia. However, pessimism has been generated by the negative outcome of the Aldosterone Receptor Blockade in Diastolic Heart Failure (ALDO-DHF) study wherein spironolactone improved echocardiographic indices of diastolic dysfunction but failed to improve exercise capacity, symptoms, or quality-of-life measures. A unique molecule that hybridizes an ARB with an endopeptidase inhibitor, LCZ696, increases the generation of myocardial cyclic guanosine 3′,5′-monophosphate, enhances myocardial relaxation, and reduces ventricular hypertrophy. This dual blocker has been shown to reduce circulating natriuretic peptides and reduce left atrial size to a significantly greater extent than valsartan alone in patients with HFpEF.
CLINICAL PEARLS
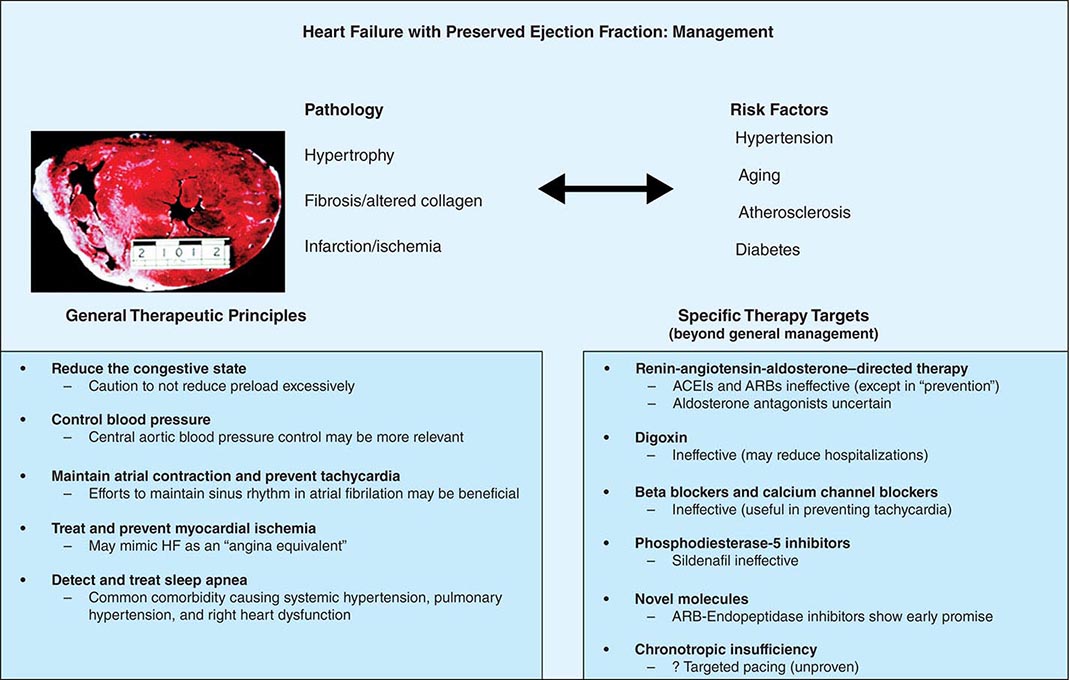
Even as efforts to control hypertension in HFpEF are critical, evaluation for and correction of underlying ischemia may be beneficial. Appropriate identification and treatment of sleep-disordered breathing should be strongly considered. Excessive decrease in preload with vasodilators may lead to underfilling the ventricle and subsequent hypotension and syncope. Some investigators have suggested that the exercise intolerance in HFpEF is a manifestation of chronotropic insufficiency and that such aberrations could be corrected with use of rate responsive pacemakers, but this remains an inadequately investigated contention (Fig. 280-1).
FIGURE 280-1 Pathophysiologic correlations, general therapeutic principles, and results of specific “directed” therapy in heart failure (HF) with preserved ejection fraction. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
ACUTE DECOMPENSATED HEART FAILURE
GENERAL PRINCIPLES
ADHF is a heterogeneous clinical syndrome most often resulting in need for hospitalization due to confluence of interrelated abnormalities of decreased cardiac performance, renal dysfunction, and alterations in vascular compliance. Admission with a diagnosis of ADHF is associated with excessive morbidity and mortality, with nearly half of these patients readmitted for management within 6 months, and a high short-term (5–8% in-hospital) and long-term mortality (20% at 1 year). Importantly, long-term aggregate outcomes remain poor, with a combined incidence of cardiovascular deaths, heart failure hospitalizations, myocardial infarction, strokes, or sudden death reaching 50% at 12 months after hospitalization. The management of these patients has remained difficult and principally revolves around volume control and decrease of vascular impedance while maintaining attention to end-organ perfusion (coronary and renal).
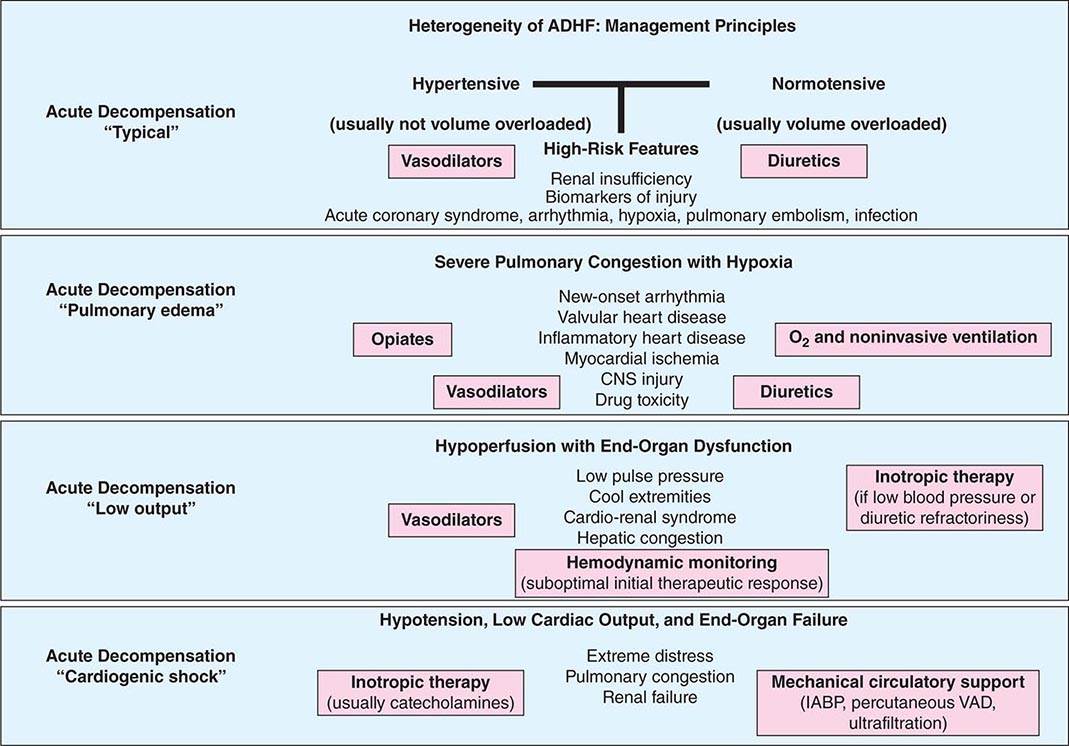
The first principle of management of these patients is to identify and tackle known precipitants of decompensation. Identification and management of medication nonadherence and use of prescribed medicines such as nonsteroidal anti-inflammatory drugs, cold and flu preparations with cardiac stimulants, and herbal preparations, including licorice, ginseng, and ma huang (an herbal form of ephedrine now banned in most places), are required. Active infection and overt or covert pulmonary thromboembolism should be sought, identified, and treated when clinical clues suggest such direction. When possible, arrhythmias should be corrected by controlling heart rate or restoring sinus rhythm in patients with poorly tolerated rapid atrial fibrillation and by correcting ongoing ischemia with coronary revascularization or by correcting offenders such as ongoing bleeding in demand-related ischemia. A parallel step in management involves stabilization of hemodynamics in those with instability. The routine use of a pulmonary artery catheter is not recommended and should be restricted to those who respond poorly to diuresis or experience hypotension or signs and symptoms suggestive of a low cardiac output where therapeutic targets are unclear. Analysis of in-hospital registries has identified several parameters associated with worse outcomes: a blood urea nitrogen level greater than 43 mg/dL (to convert to mmol/L, multiply by 0.357), systolic blood pressure less than 115 mmHg, a serum creatinine level greater than 2.75 mg/dL (to convert to μmol/L, multiply by 88.4), and an elevated troponin I level. A useful clinical schema to identify treatment targets for the various phenotypic presentations and management goals in ADHF is depicted in Fig. 280-2.
FIGURE 280-2 The distinctive phenotypes of acute decompensated heart failure (ADHF), their presentations, and suggested therapeutic routes. (Unique causes of ADHF, such as isolated right heart failure and pericardial disease, and rare causes, such as aortic and coronary dissection or ruptured valve structures or sinuses of Valsalva, are not delineated and are covered elsewhere.) IABP, intraaortic balloon pump; VAD, ventricular assist device.
VOLUME MANAGEMENT
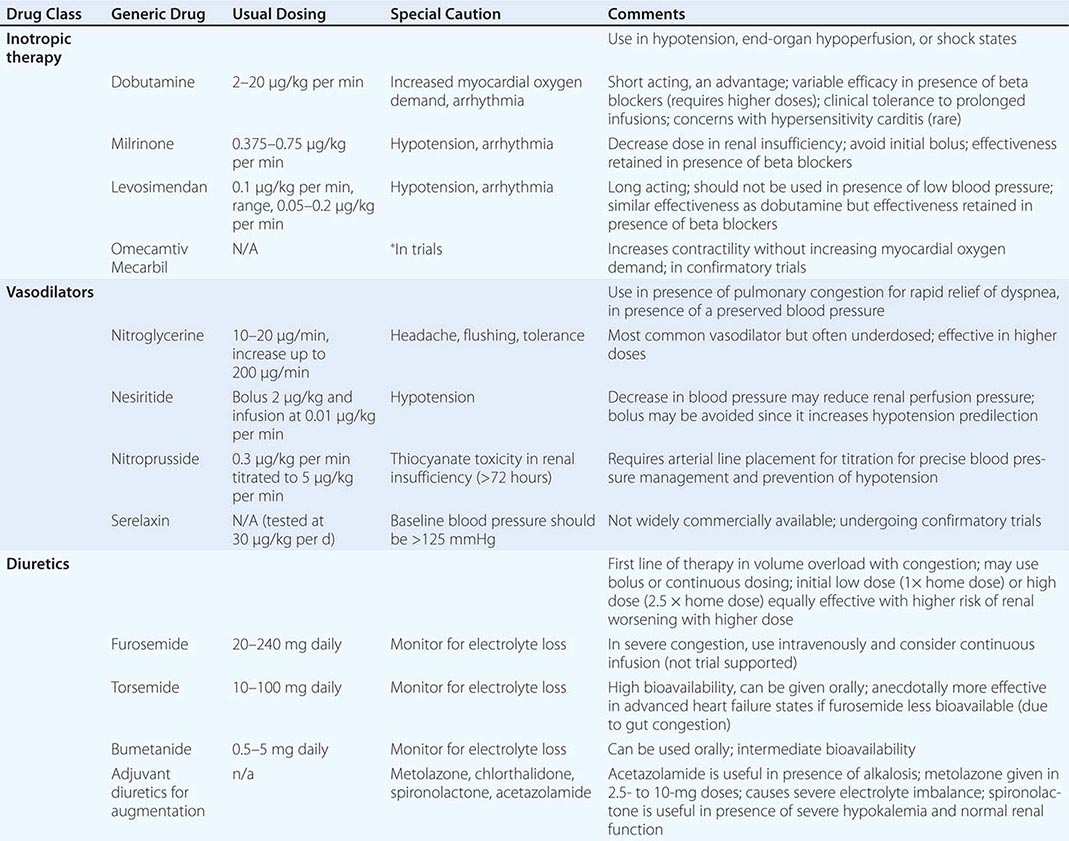
Intravenous Diuretic Agents Intravenous diuretic agents rapidly and effectively relieve symptoms of congestion and are essential when oral drug absorption is impaired. When high doses of diuretic agents are required or when the effect is suboptimal, a continuous infusion may be needed to reduce toxicity and maintain stable serum drug levels. Randomized clinical trials of high-versus low-dose or bolus versus continuous infusion diuresis have not provided clear justification for the best diuretic strategy in ADHF, and as such, the use of diuretic regimens remains an art rather than science. Addition of a thiazide diuretic agent such as metolazone in combination provides a synergistic effect and is often required in patients receiving long-term therapy with loop diuretic agents. Change in weight is often used as a surrogate for adequate diuresis, but this objective measure of volume status may be surprisingly difficult to interpret, and weight loss during hospitalization does not necessarily correlate closely with outcomes. It is generally advisable to continue diuresis until euvolemia has been achieved. Physical examination findings, specifically the jugular venous pressure coupled with biomarker trends, are useful in timing discharge planning.
The Cardiorenal Syndrome The cardiorenal syndrome is being recognized increasingly as a complication of ADHF. Multiple definitions have been proposed for the cardiorenal syndrome, but at its simplest, it can be thought to reflect the interplay between abnormalities of heart and kidney function, with deteriorating function of one organ while therapy is administered to preserve the other. Approximately 30% of patients hospitalized with ADHF exhibit abnormal renal function at baseline, and this is associated with longer hospitalizations and increased mortality. However, mechanistic studies have been largely unable to find correlation between deterioration in renal function, cardiac output, left-sided filling pressures, and reduced renal perfusion; most patients with cardiorenal syndrome demonstrate a preserved cardiac output. It is hypothesized that in patients with established heart failure, this syndrome represents a complex interplay of neurohormonal factors, potentially exacerbated by “backward failure” resulting from increased intra-abdominal pressure and impairment in return of renal venous blood flow. Continued use of diuretic therapy may be associated with a reduction in glomerular filtration rate and a worsening of the cardiorenal syndrome when right-sided filling pressures remain elevated. In patients in the late stages of disease characterized by profound low cardiac output state, inotropic therapy or mechanical circulatory support has been shown to preserve or improve renal function in selected individuals in the short term until more definitive therapy such as assisted circulation or cardiac transplantation is implemented.
Ultrafiltration Ultrafiltration (UF) is an invasive fluid removal technique that may supplement the need for diuretic therapy. Proposed benefits of UF include controlled rates of fluid removal, neutral effects on serum electrolytes, and decreased neurohormonal activity. This technique has also been referred to as aquapheresis in recognition of its electrolyte depletion–sparing effects. Current UF systems function with two large-bore, peripherally inserted venous lines. In a pivotal study evaluating UF versus conventional therapy, fluid removal was improved and subsequent heart failure hospitalizations and urgent clinic visits were reduced with UF; however, no improvement in renal function and no subjective differences in dyspnea scores or adverse outcomes were noted. More recently, in the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial, 188 patients with ADHF and worsening renal failure were randomized to stepped pharmacologic care or UF. The primary endpoint was a change in serum creatinine and change in weight (reflecting fluid removal) at 96 hours. Although similar weight loss occurred in both groups (approximately 5.5 kg), there was worsening in creatinine in the UF group. Deaths and hospitalizations for heart failure were no different between groups, but there were more severe adverse events in the UF group, mainly due to kidney failure, bleeding complications, and intravenous catheter-related complications. This investigation argues against using UF as a primary strategy in patients with ADHF who are nonetheless responsive to diuretics. Whether UF is useful in states of diuretic unresponsiveness remains an open question, and this strategy continues to be employed judiciously in such situations.
VASCULAR THERAPY
Vasodilators including intravenous nitrates, nitroprusside, and nesiritide (a recombinant brain-type natriuretic peptide) have been advocated for upstream therapy in an effort to stabilize ADHF. The latter agent was introduced in a fixed dose for therapy after a comparison with intravenous nitrates suggested more rapid and greater reduction in pulmonary capillary wedge pressure. Enthusiasm for nesiritide waned due to concerns within the pivotal trials for development of renal insufficiency and an increase in mortality. To address these concerns, a large-scale morbidity and mortality trial, the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) study was completed in 2011 and randomly enrolled 7141 patients with ADHF to nesiritide or placebo for 24 to 168 hours in addition to standard care. Nesiritide was not associated with an increase or a decrease in the rates of death and rehospitalization and had a clinically insignificant benefit on dyspnea. Renal function did not worsen, but increased rates of hypotension were noted. Although this trial established the safety for this drug, the routine use cannot be advocated due to lack of significant efficacy. Recombinant human relaxin-2, or serelaxin, is a peptide upregulated in pregnancy and examined in ADHF patients with a normal or elevated blood pressure. In the Relaxin in Acute Heart Failure (RELAX-AHF) trial, serelaxin or placebo was added to a regimen of standard therapy in 1161 patients hospitalized with ADHF, evidence of congestion, and systolic pressure >125 mmHg. Serelaxin improved dyspnea, reduced signs and symptoms of congestion, and was associated with less early worsening of HF. Exploratory endpoints of hard outcomes at 6 months suggested positive signals in favor of mortality reduction. This agent is being tested in a large, more confirmatory trial setting.
INOTROPIC THERAPY
Impairment of myocardial contractility often accompanies ADHF, and pharmacologic agents that increase intracellular concentration of cyclic adenosine monophosphate via direct or indirect pathways, such as sympathomimetic amines (dobutamine) and phosphodiesterase-3 inhibitors (milrinone), respectively, serve as positive inotropic agents. Their activity leads to an increase in cytoplasmic calcium. Inotropic therapy in those with a low-output state augments cardiac output, improves perfusion, and relieves congestion acutely. Although milrinone and dobutamine have similar hemodynamic profiles, milrinone is slower acting and is renally excreted and thus requires dose adjustments in the setting of kidney dysfunction. Since milrinone acts downstream from the β1-adrenergic receptor, it may provide an advantage in patients receiving beta blockers when admitted to the hospital. Studies are in universal agreement that long-term inotropic therapy increases mortality. However, the short-term use of inotropic agents in ADHF is also associated with increased arrhythmia, hypotension, and no beneficial effects on hard outcomes. Inotropic agents are currently indicated as bridge therapy (to either left ventricular assist device support or to transplant) or as selectively applied palliation in end-stage heart failure.
Novel inotropic agents that leverage the concept of myofilament calcium sensitization rather than increasing intracellular calcium levels have been introduced. Levosimendan is a calcium sensitizer that provides inotropic activity, but also possesses phosphodiesterase-3 inhibition properties that are vasodilators in action. This makes the drug unsuitable in states of low output in the setting of hypotension. Two trials, the second Randomized Multicenter Evaluation of Intravenous Levosimendan Efficacy (REVIVE II) and Survival of Patients with Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE), have tested this agent in ADHF. SURVIVE compared levosimendan with dobutamine, and despite an initial reduction in circulating B-type natriuretic peptide levels in the levosimendan group compared with patients in the dobutamine group, this drug did not reduce all-cause mortality at 180 days or affect any secondary clinical outcomes. The second trial compared levosimendan against traditional noninotropic therapy and found a modest improvement in symptoms with worsened short-term mortality and ventricular arrhythmias. Another drug that functions as a selective myosin activator, omecamtiv mecarbil, prolongs the ejection period and increases fractional shortening. Distinctively, the force of contraction is not increased, and as such, this agent does not increase myocardial oxygen demand. In a 600-patient trial called ATOMIC-HF (A Trial of Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure), this agent showed improvement in dyspnea scores in the highest dose cohort, but not across all enrolled patients. How this agent performs in broader populations remains uncertain. Other inotropic agents that increase myocardial calcium sensitivity through mechanisms that reduce cTnI phosphorylation or inhibit protein kinase A are being developed. (Table 280-1 depicts typical inotropic, vasodilator, and diuretic drugs used in ADHF.)
|
INTRAVENOUS THERAPY IN ACUTE DECOMPENSATED HEART FAILURE |
NEUROHORMONAL ANTAGONISTS
Other trials testing unique agents have yielded disappointing results in the situation of ADHF. The Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) trial of selective adenosine antagonism and the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial of an oral selective vasopressin-2 antagonist in ADHF were both negative with respect to hard outcomes.
In patients who fail to respond adequately to medical therapy, mechanical assist devices may be required. This is covered in more detail in Chap. 281.
HEART FAILURE WITH REDUCED EJECTION FRACTION
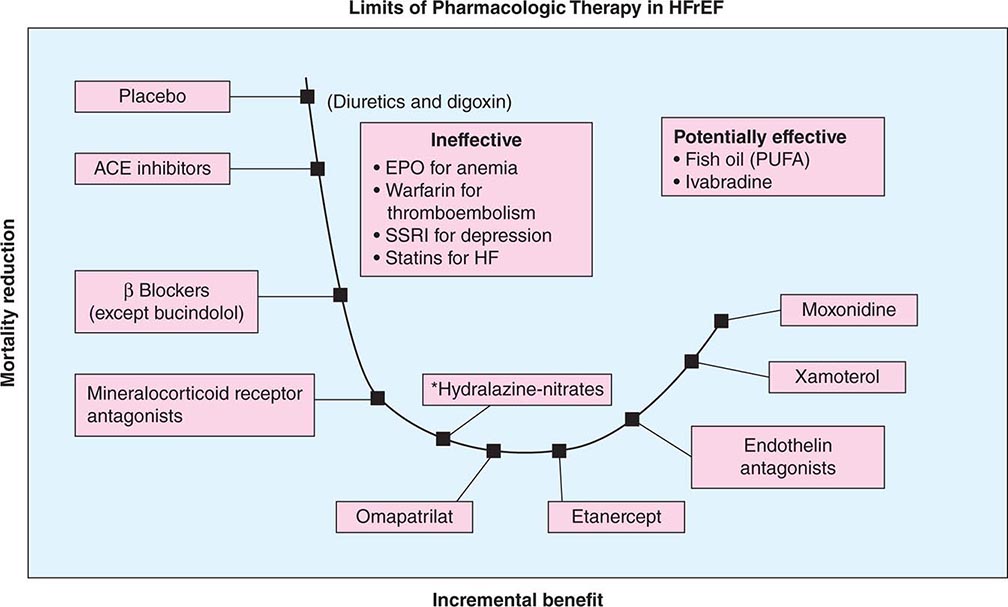
The past 50 years have witnessed great strides in the management of HFrEF. The treatment of symptomatic heart failure that evolved from a renocentric (diuretics) and hemodynamic therapy model (digoxin, inotropic therapy) ushered in the era of disease-modifying therapy with neurohormonal antagonism. In this regard, ACEIs and beta blockers form the cornerstone of pharmacotherapy and lead to attenuation of decline and improvement in cardiac structure and function with consequent reduction in symptoms, improvement in quality of life, decreased burden of hospitalizations, and a decline in mortality from both pump failure and arrhythmic deaths (Fig. 280-3).
FIGURE 280-3 Progressive decline in mortality with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), beta blockers, mineralocorticoid receptor antagonists, and balanced vasodilators (*selected populations such as African Americans); further stack-on neurohormonal therapy is ineffective or results in worse outcome; management of comorbidity is of unclear efficacy. EPO, erythropoietin; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; PUFA, polyunsaturated fatty acid; SSRI, selective serotonin reuptake inhibitor.
NEUROHORMONAL ANTAGONISM
Meta-analyses suggest a 23% reduction in mortality and a 35% reduction in the combination endpoint of mortality and hospitalizations for heart failure in patients treated with ACEIs. Patients treated with beta blockers provide a further 35% reduction in mortality on top of the benefit provided by ACEIs alone. Increased experience with both agents in a broad range of patients with HFrEF has demonstrated the safety of ACEIs in treating patients with mild renal insufficiency and the tolerability of beta blockers in patients with moderately controlled diabetes, asthma, and obstructive lung disease. The benefits of ACEIs and beta blockers extend to advanced symptoms of disease (NYHA class IIIb–IV). However, a substantial number of patients with advanced heart failure may not be able to achieve optimal doses of neurohormonal inhibitors and require cautious reduction in dose exposure to maintain clinical stability. Such individuals with lower exposure to ACEIs and beta blockers represent a high-risk cohort with poor prognosis.
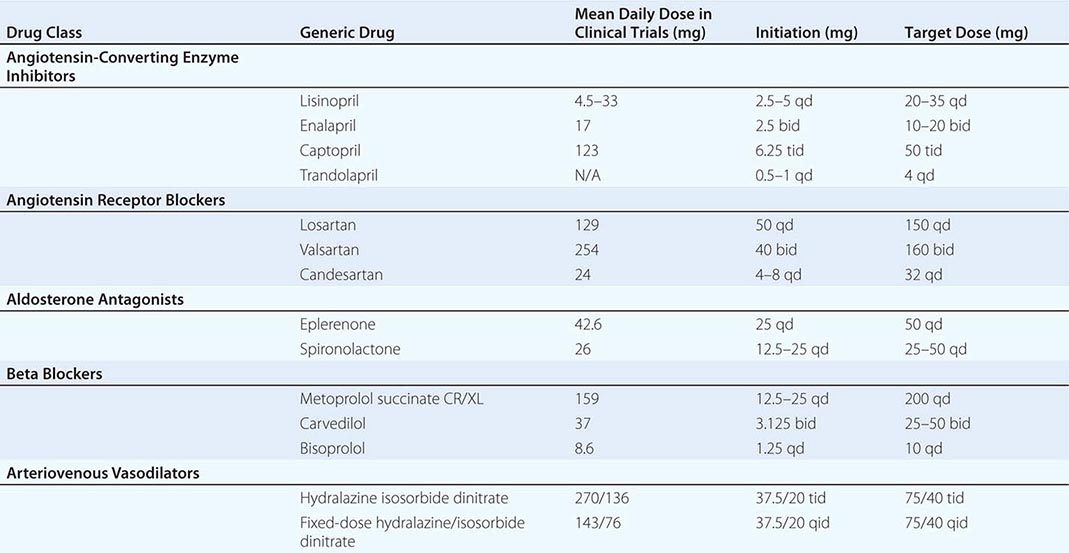
Class Effect and Sequence of Administration ACEIs exert their beneficial effects in HFrEF as a class; however, the beneficial effects of beta blockers are thought to be limited to specific drugs. Beta blockers with intrinsic sympathomimetic activity (xamoterol) and other agents, including bucindolol, have not demonstrated a survival benefit. On the basis of investigations, beta blocker use in HFrEF should be restricted to carvedilol, bisoprolol, and metoprolol succinate—agents tested and proven to improve survival in clinical trials. Whether beta blockers or ACEIs should be started first was answered by the Cardiac Insufficiency Bisoprolol Study (CIBIS) III, in which outcomes did not vary when either agent was initiated first. Thus, it matters little which agent is initiated first; what does matter is that optimally titrated doses of both ACEIs and beta blockers be established in a timely manner.
Dose and Outcome A trial has indicated that higher tolerated doses of ACEIs achieve greater reduction in hospitalizations without materially improving survival. Beta blockers demonstrate a dose-dependent improvement in cardiac function and reductions in mortality and hospitalizations. Clinical experience suggests that, in the absence of symptoms to suggest hypotension (fatigue and dizziness), pharmacotherapy may be up-titrated every 2 weeks in hemodynamically stable and euvolemic ambulatory patients as tolerated.
MINERALOCORTICOID ANTAGONISTS
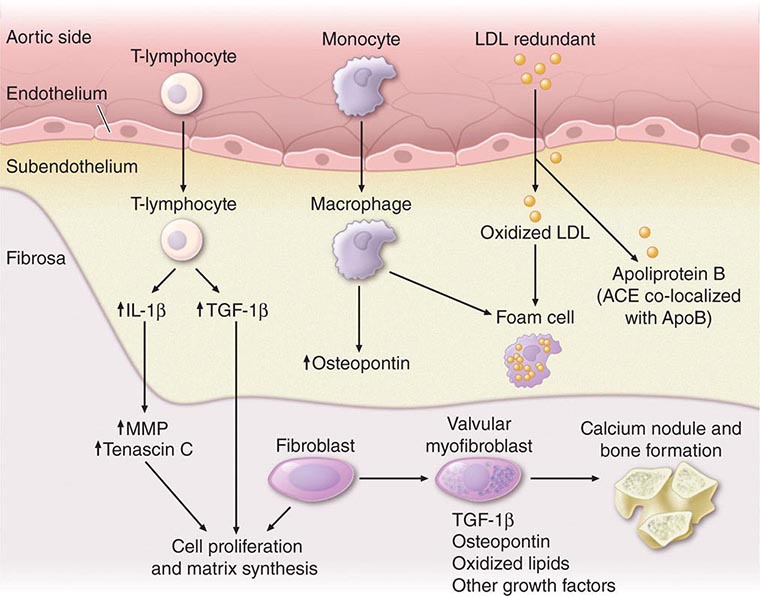
Aldosterone antagonism is associated with a reduction in mortality in all stages of symptomatic NYHA class II to IV HFrEF. Elevated aldosterone levels in HFrEF promote sodium retention, electrolyte imbalance, and endothelial dysfunction and may directly contribute to myocardial fibrosis. The selective agent eplerenone (tested in NYHA class II and post–myocardial infarction heart failure) and the nonselective antagonist spironolactone (tested in NYHA class III and IV heart failure) reduce mortality and hospitalizations, with significant reductions in sudden cardiac death (SCD). Hyperkalemia and worsening renal function are concerns, especially in patients with underlying chronic kidney disease, and renal function and serum potassium levels must be closely monitored.
RAAS THERAPY AND NEUROHORMONAL “ESCAPE”
Neurohormonal “escape” has been witnessed in patients with HFrEF by the finding that circulating levels of angiotensin II return to pretreatment levels with long-term ACEI therapy. ARBs blunt this phenomenon by binding competitively to the AT1 receptor. A large meta-analysis of 24 randomized trials showed the superiority of ARBs to placebo in patients with intolerable adverse effects with ACEIs and their noninferiority in all-cause mortality or hospitalizations when compared with ACEIs. The Valsartan Heart Failure Trial (Val-HeFT) suggested that addition of valsartan in patients already receiving treatment with ACEIs and beta blockers was associated with a trend toward worse outcomes. Similarly, adding valsartan to captopril in patients with heart failure after myocardial infarction who were receiving background beta blocker therapy was associated with an increase in adverse events without any added benefit compared with monotherapy for either group. Thus, the initial clinical strategy should be to use a two-drug combination first (ACEI and beta blocker; if beta blocker intolerant, then ACEI and ARB; if ACEI intolerant, then ARB and beta blocker). In symptomatic patients (NYHA class II–IV), an aldosterone antagonist should be strongly considered, but four-drug therapy should be avoided.
A recent trial called the Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) tested a direct renin inhibitor, aliskiren, in addition to other heart failure medications, within a week after discharge from a hospitalization for decompensated HFrEF. No significant difference in cardiovascular death or hospitalization at 6 or 12 months was noted. Aliskiren was associated with a reduction in circulating natriuretic peptides, but any disease-modifying effect was overcome by excessive adverse events including hyperkalemia, hypotension, and renal dysfunction.
ARTERIOVENOUS VASODILATION
The combination of hydralazine and nitrates has been demonstrated to improve survival in HFrEF. Hydralazine reduces systemic vascular resistance and induces arterial vasodilatation by affecting intracellular calcium kinetics; nitrates are transformed in smooth muscle cells into nitric oxide, which stimulates cyclic guanosine monophosphate production and consequent arterial-venous vasodilation. This combination improves survival, but not to the magnitude evidenced by ACEIs or ARBs. However, in individuals with HFrEF unable to tolerate renin-angiotensin-aldosterone–based therapy for reasons such as renal insufficiency or hyperkalemia, this combination is preferred as a disease-modifying approach. A trial conducted in self-identified African Americans, the African-American Heart Failure Trial (A-Heft), studied a fixed dose of isosorbide dinitrate with hydralazine in patients with advanced symptoms of HFrEF who were receiving standard background therapy. The study demonstrated benefit in survival and hospitalization recidivism in the treatment group. Adherence to this regimen is limited by the thrice-daily dosing schedule. Table 280-2 lists the common neurohormonal and vasodilator regimens for HFrEF.
|
PHARMACOLOGIC THERAPY AND TARGET DOSES IN HEART FAILURE WITH REDUCED EJECTION FRACTION |
HEART RATE MODIFICATION
Ivabradine, an inhibitor of the If current in the sinoatrial node, slows the heart rate without a negative inotropic effect. The Systolic Heart Failure Treatment with Ivabradine Compared with Placebo Trial (SHIFT) was conducted in patients with class II or III HFrEF, a heart rate >70 beats/min, and history of hospitalization for heart failure during the previous year. Ivabradine reduced hospitalizations and the combined endpoint of cardiovascular-related death and heart failure hospitalization. The study population was not necessarily representative of North American patients with HFrEF since, with a few exceptions, most did not receive internal cardioverter-defibrillation or cardiac resynchronization therapy and 40% did not receive a mineralocorticoid receptor antagonist. Although 90% received beta blockers, only a quarter were on full doses. Whether this agent, now available outside the United States, would have been effective in patients receiving robust, guideline-recommended therapy for heart failure remains enigmatic. In the 2012 European Society of Cardiology guidelines for the treatment of heart failure, ivabradine was suggested as second-line therapy before digoxin is considered in patients who remain symptomatic after guideline-based ACEIs, beta blockers, and mineralocorticoid receptor antagonists and with residual heart rate >70 beats/min. Another group in whom potential benefit may be expected includes those unable to tolerate beta blockers.
DIGOXIN
Digitalis glycosides exert a mild inotropic effect, attenuate carotid sinus baroreceptor activity, and are sympathoinhibitory. These effects decrease serum norepinephrine levels, plasma renin levels, and possibly aldosterone levels. The DIG trial demonstrated a reduction in heart failure hospitalizations in the treatment group but no reduction in mortality or improvement in quality of life. Importantly, treatment with digoxin resulted in a higher mortality rate in women than men. Furthermore, the effects of digoxin in reducing hospitalizations were lower in women than in men. It should be noted that low doses of digoxin are sufficient to achieve any potentially beneficial outcomes, and higher doses breach the therapeutic safety index. Although digoxin levels should be checked to minimize toxicity and although dose reductions are indicated for higher levels, no adjustment is made for low levels. Generally, digoxin is now relegated as therapy for patients who remain profoundly symptomatic despite optimal neurohormonal blockade and adequate volume control.
ORAL DIURETICS
Neurohormonal activation results in avid salt and water retention. Loop diuretic agents are often required because of their increased potency, and frequent dose adjustments may be necessary because of variable oral absorption and fluctuations in renal function. Importantly, clinical trial data confirming efficacy are limited, and no data suggest that these agents improve survival. Thus, diuretic agents should ideally be used in tailored dosing schedules to avoid excessive exposure. Indeed, diuretics are essential at the outset to achieve volume control before neurohormonal therapy is likely to be well tolerated or titrated.
CALCIUM CHANNEL ANTAGONISTS
Amlodipine and felodipine, second-generation calcium channel–blocking agents, safely and effectively reduce blood pressure in HFrEF but do not affect morbidity, mortality, or quality of life. The first-generation agents, including verapamil and diltiazem, may exert negative inotropic effects and destabilize previously asymptomatic patients. Their use should be discouraged.
NOVEL NEUROHORMONAL ANTAGONISM
Despite an abundance of animal and clinical data demonstrating deleterious effects of activated neurohormonal pathways beyond the RAAS and sympathetic nervous system, targeting such pathways with incremental blockade has been largely unsuccessful. As an example, the endothelin antagonist bosentan is associated with worsening heart failure in HFrEF despite demonstrating benefits in right-sided heart failure due to pulmonary arterial hypertension. Similarly, the centrally acting sympatholytic agent moxonidine worsens outcomes in left heart failure. The combined drug omapatrilat hybridizes an ACEI with a neutral endopeptidase inhibitor, and this agent was tested in the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) trial. This drug did not favorably influence the primary outcome measure of the combined risk of death or hospitalization for heart failure requiring intravenous treatment. The risk of angioedema was notably higher with omapatrilat than ACEIs alone. LCZ696 and ARB with an endopeptidase inhibitor have shown benefit in a large trial versus ARB alone.
INFLAMMATION
Targeting inflammatory cytokines such as tumor necrosis factor α (TNF-α) by using anticytokine agents such as infliximab and etanercept has been unsuccessful and associated with worsening heart failure. Nonspecific immunomodulation has been tested in the large Advanced Chronic Heart Failure Clinical Assessment of Immune Modulation Therapy (ACCLAIM-HF) trial of 2426 HFrEF patients with NYHA functional class II to IV symptoms. Ex vivo exposure of a blood sample to controlled oxidative stress initiates apoptosis of leukocytes soon after intramuscular gluteal injection of the treated sample. The physiologic response to apoptotic cells results in a reduction in inflammatory cytokine production and upregulation of anti-inflammatory cytokines. This promising hypothesis was not proven, although certain subgroups (those with no history of previous myocardial infarction and those with mild heart failure) showed signals in favor of immunomodulation. Use of intravenous immunoglobulin therapy in nonischemic etiology of heart failure has not been shown to result in beneficial outcomes.
STATINS
Potent lipid-altering and pleiotropic effects of statins reduce major cardiovascular events and improve survival in non–heart failure populations. Once heart failure is well established, this therapy may not be as beneficial and theoretically could even be detrimental by depleting ubiquinone in the electron transport chain. Two trials, Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) and Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiac (GISSI-HF), have tested low-dose rosuvastatin in patients with HFrEF and demonstrated no improvement in aggregate clinical outcomes. If statins are required to treat progressive coronary artery disease in the background setting of heart failure, then they should be employed. However, no rationale appears to exist for routine statin therapy in nonischemic heart failure.
ANTICOAGULATION AND ANTIPLATELET THERAPY
HFrEF is accompanied by a hypercoagulable state and therefore a high risk of thromboembolic events, including stroke, pulmonary embolism, and peripheral arterial embolism. Although long-term oral anticoagulation is established in certain groups, including patients with atrial fibrillation, the data are insufficient to support the use of warfarin in patients in normal sinus rhythm without a history of thromboembolic events or echocardiographic evidence of left ventricular thrombus. In the large Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial, 2305 patients with HFrEF were randomly allocated to either full-dose aspirin or international normalized ratio–controlled warfarin with follow-up for 6 years. Among patients with reduced LVEF who were in sinus rhythm, there was no significant overall difference in the primary outcome between treatment with warfarin and treatment with aspirin. A reduced risk of ischemic stroke with warfarin was offset by an increased risk of major hemorrhage. Aspirin blunts ACEI-mediated prostaglandin synthesis, but the clinical importance of this finding remains unclear. Current guidelines support the use of aspirin in patients with ischemic cardiomyopathy.
FISH OIL
Treatment with long-chain omega-3 polyunsaturated fatty acids (ω-3 PUFAs) has been shown to be associated with modestly improved clinical outcomes in patients with HFrEF. This observation from the GISSI-HF trial was extended to measurements of ω-3 PUFAs in plasma phospholipids at baseline and after 3 months. Three-month treatment with ω-3 PUFAs enriched circulating eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Low EPA levels are inversely related to total mortality in patients with HFrEF.
MICRONUTRIENTS
A growing body of evidence suggests an association between heart failure and micronutrient status. Reversible heart failure has been described as a consequence of severe thiamine and selenium deficiency. Thiamine deficiency has received attention in heart failure due to the fact that malnutrition and diuretics are prime risk factors for thiamine loss. Small exploratory randomized studies have suggested a benefit of supplementation of thiamine in HFrEF with evidence of improved cardiac function. This finding is restricted to chronic heart failure states and does not appear to be beneficial in the ADHF phenotype. Due to the preliminary nature of the evidence, no recommendations for routine supplementation or testing for thiamine deficiency can be made.
ENHANCED EXTERNAL COUNTERPULSATION (EECP)
Peripheral lower extremity therapy using graded external pneumatic compression at high pressure is administered in 1-hour sessions for 35 treatments (7 weeks) and has been proposed to reduce angina symptoms and extend time to exercise-induced ischemia in patients with coronary artery disease. The Prospective Evaluation of Enhanced External Counterpulsation in Congestive Heart Failure (PEECH) study assessed the benefits of enhanced external counterpulsation in the treatment of patients with mild-to-moderate heart failure. This randomized trial improved exercise tolerance, quality of life, and NYHA functional classification but without an accompanying increase in peak oxygen consumption. A placebo effect due to the nature of the intervention simply cannot be excluded.
EXERCISE
The Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study investigated short-term (3-month) and long-term (12-month) effects of a supervised exercise training program in patients with moderate HFrEF. Exercise was safe, improved patients’ sense of well-being, and correlated with a trend toward mortality reduction. Maximal changes in 6-minute walk distance were evident at 3 months with significant improvements in cardiopulmonary exercise time and peak oxygen consumption persisting at 12 months. Therefore, exercise training is recommended as an adjunctive treatment in patients with heart failure.
MANAGEMENT OF SELECTED COMORBIDITY
Sleep-disordered breathing is common in HF and particularly in HFrEF. A range of presentations exemplified by obstructive sleep apnea, central sleep apnea, and its extreme form of Cheyne-Stokes breathing are noted. Frequent periods of hypoxia and repeated micro-and macro-arousals trigger adrenergic surges, which can worsen hypertension and impair systolic and diastolic function. A high index of suspicion is required, especially in patients with difficult-to-control hypertension or with predominant symptoms of fatigue despite reverse remodeling in response to optimal medical therapy. Worsening of right heart function with improvement of left ventricular function noted on medical therapy should immediately trigger a search for underlying sleep-disordered breathing or pulmonary complications such as occult embolism or pulmonary hypertension. Treatment with nocturnal positive airway pressure improves oxygenation, LVEF, and 6-minute walk distance. However, no conclusive data exist to support this therapy as a disease-modifying approach with reduction in mortality.
Anemia is common in heart failure patients, reduces functional status and quality of life, and is associated with increased proclivity for hospital admissions and mortality. Anemia in heart failure is more common in the elderly, in those with advanced stages of HFrEF, in the presence of renal insufficiency, and in women and African Americans. The mechanisms include iron deficiency, dysregulation of iron metabolism, and occult gastrointestinal bleeding. Intravenous iron using either iron sucrose or carboxymaltose (Ferric Carboxymaltose Assessment in Patients with Iron Deficiency and Chronic Heart Failure [FAIR-HF] trial) has been shown to correct anemia and improve functional capacity. Erythropoiesis-regulating agents such as erythropoietin analogues have been studied with disappointing results. The Reduction of Events by Darbepoetin Alfa in Heart Failure (RED-HF) trial evaluated 2278 mild-to-moderate anemia patients with HFrEF and demonstrated that treatment with darbepoetin alfa did not improve clinical outcomes in patients with systolic heart failure.
Depression is common in HFrEF, with a reported prevalence of one in five patients, and is associated with a poor quality of life, limited functional status, and increased risk of morbidity and mortality in this population. Antidepressants may improve depression, promote vascular health, and decrease systemic inflammation in HFrEF. However, the largest randomized study of depression in HFrEF, the Sertraline Against Depression and Heart Disease in Chronic Heart Failure (SADHART-CHF) trial, showed that sertraline was safe, but did not provide greater reduction in depression or improve cardiovascular status among patients with heart failure and depression compared with nurse-driven multidisciplinary management.
Atrial arrhythmias, especially atrial fibrillation, are common and serve as a harbinger of worse prognosis in patients with heart failure. When rate control is inadequate or symptoms persist, pursuing a rhythm control strategy is reasonable. Rhythm control may be achieved via pharmacotherapy or by percutaneous or surgical techniques, and referral to practitioners or centers experienced in these modalities is recommended. Antiarrhythmic drug therapy should be restricted to amiodarone and dofetilide, both of which have been shown to be safe and effective but do not alter the natural history of the underlying disease. The Antiarrhythmic Trial with Dronedarone in Moderate-to-Severe Congestive Heart Failure Evaluating Morbidity Decrease (ANDROMEDA) studied the effects of the novel antiarrhythmic agent dronedarone and found an increased mortality due to worsening heart failure. Catheter ablation and pulmonary vein isolation appear to be safe and effective in this high-risk cohort and compare favorably with the more established practice of atrioventricular node ablation and biventricular pacing.
CARDIAC RESYNCHRONIZATION THERAPY
Nonsynchronous contraction between the walls of the left ventricle (intraventricular) or between the ventricular chambers (interventricular) impairs systolic function, decreases mechanical efficiency of contraction, and adversely affects ventricular filling. Mechanical dyssynchrony results in an increase in wall stress and worsens functional mitral regurgitation. The single most important association of extent of dyssynchrony is a widened QRS interval on the surface electrocardiogram, particularly in the presence of a left bundle branch block pattern. With placement of a pacing lead via the coronary sinus to the lateral wall of the ventricle, cardiac resynchronization therapy (CRT) enables a more synchronous ventricular contraction by aligning the timing of activation of the opposing walls. Early studies showed improved exercise capacity, reduction in symptoms, and evidence of reverse remodeling. The Cardiac Resynchronization in Heart Failure Study (CARE-HF) trial was the first study to demonstrate a reduction in all-cause mortality with CRT placement in patients with HFrEF on optimal therapy with continued moderate-to-severe residual symptoms of NYHA class III or IV heart failure. More recent clinical trials have demonstrated disease-modifying properties of CRT in even minimally symptomatic patients with HFrEF, including the Resynchronization–Defibrillation for Ambulatory Heart Failure Trial (RAFT) and Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT), both of which sought to use CRT in combination with an implantable defibrillator. Most benefit in mildly symptomatic HFrEF patients accrues from applying this therapy in those with a QRS width of >149 ms and a left bundle branch block pattern. Attempts to further optimize risk stratification and expand indications for CRT using modalities other than electrocardiography have proven disappointing. In particular, echocardiographically derived measures of dyssynchrony vary tremendously, and narrow QRS dyssynchrony has not proven to be a good target for treatment. Uncertainty surrounds the benefits of CRT in those with ADHF, a predominant right bundle branch block pattern, atrial fibrillation, and evidence of scar in the lateral wall, which is the precise location where the CRT lead is positioned.
SUDDEN CARDIAC DEATH PREVENTION IN HEART FAILURE
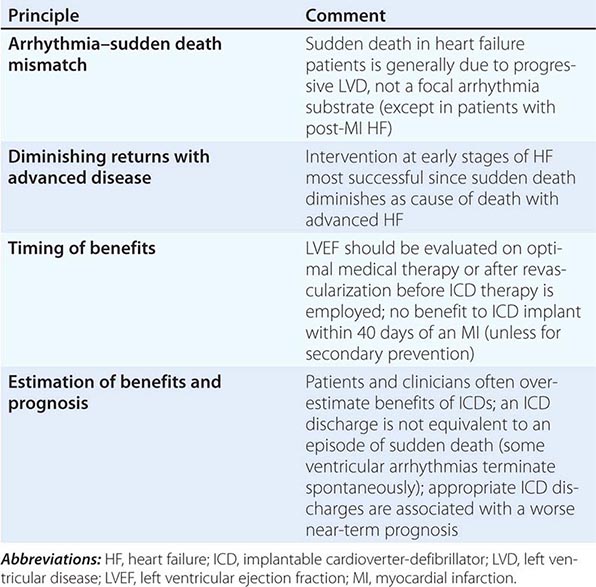
SCD due to ventricular arrhythmias is the mode of death in approximately half of patients with heart failure and is particularly proportionally prevalent in HFrEF patients with early stages of the disease. Patients who survive an episode of SCD are considered to be at very high risk and qualify for placement of an implantable cardioverter-defibrillator (ICD). Although primary prevention is challenging, the degree of residual left ventricular dysfunction despite optimal medical therapy (≤35%) to allow for adequate remodeling and the underlying etiology (post–myocardial infarction or ischemic cardiomyopathy) are the two single most important risk markers for stratification of need and benefit. Currently, patients with NYHA class II or III symptoms of heart failure and an LVEF <35%, irrespective of etiology of heart failure, are appropriate candidates for ICD prophylactic therapy. In patients with a myocardial infarction and optimal medical therapy with residual LVEF ≤30% (even when asymptomatic), placement of an ICD is appropriate. In patients with a terminal illness and a predicted life span of less than 6 months or in those with NYHA class IV symptoms who are refractory to medications and who are not candidates for transplant, the risks of multiple ICD shocks must be carefully weighed against the survival benefits. If a patient meets the QRS criteria for CRT, combined CRT with ICD is often employed (Table 280-3).
|
PRINCIPLES OF ICD IMPLANTATION FOR PRIMARY PREVENTION OF SUDDEN DEATH |
SURGICAL THERAPY IN HEART FAILURE
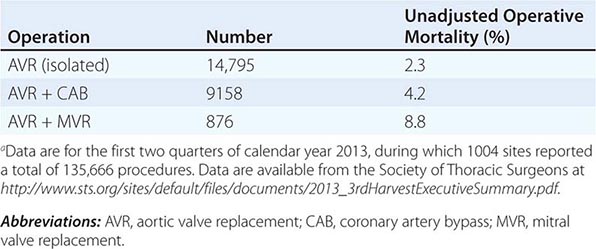
Coronary artery bypass grafting (CABG) is considered in patients with ischemic cardiomyopathy with multivessel coronary artery disease. The recognition that hibernating myocardium, defined as myocardial tissue with abnormal function but maintained cellular function, could recover after revascularization led to the notion that revascularization with CABG would be useful in those with living myocardium. Revascularization is most robustly supported in individuals with ongoing angina and left ventricular failure. Revascularizing those with left ventricular failure in the absence of angina remains controversial. The Surgical Treatment for Ischemic Heart Failure (STICH) trial enrolled 1212 patients with an ejection fraction of 35% or less and coronary artery disease amenable to CABG and randomly assigned them to medical therapy alone or medical therapy plus CABG. There was no significant difference between groups with respect to the primary endpoint of death from any cause. Patients assigned to CABG had lower rates of death from cardiovascular causes and of death from any cause or hospitalization for cardiovascular causes. An ancillary study of this trial also determined that the detection of hibernation pre-revascularization did not materially influence the efficacy of this approach, nor did it help to define a population unlikely to benefit if hibernation was not detected.
Surgical ventricular restoration (SVR), a technique characterized by infarct exclusion to remodel the left ventricle by reshaping it surgically in patients with ischemic cardiomyopathy and dominant anterior left ventricular dysfunction, has been proposed. However, in a 1000-patient trial in patients with HFrEF who underwent CABG alone or CABG plus SVR, the addition of SVR to CABG had no disease-modifying effect. Cardiac symptoms and exercise tolerance improved from baseline to a similar degree in both study groups. SVR resulted in lower left ventricular volumes at 4 months after operation. However, left ventricular aneurysm surgery is still advocated in those with refractory heart failure, ventricular arrhythmias, or thromboembolism arising from an akinetic aneurysmal segment of the ventricle. Other remodeling procedures, such as use of an external mesh-like net attached around the heart to limit further enlargement, have not been shown to provide hard clinical benefits, although favorable cardiac remodeling was noted.
Mitral regurgitation (MR) occurs with varying degrees in patients with HFrEF and dilated ventricles. Annular dilatation and leaflet noncoaptation in the setting of anatomically normal papillary muscles, chordal structures, and valve leaflets characterize functional MR. In patients who are not candidates for surgical coronary revascularization, mitral valve repair remains controversial. Ischemic MR (or infarct-related MR) is typically associated with leaflet tethering and displacement related to abnormal left ventricular wall motion and geometry. No evidence to support the use of surgical or percutaneous valve correction for functional MR exists as disease-modifying therapy even though MR can be corrected.
CELLULAR AND GENE-BASED THERAPY
The cardiomyocyte is no longer considered a terminally differentiated cell and possesses regenerative capacity. Such renewal is accelerated under conditions of stress and injury, such as an ischemic event or heart failure. Investigations that use either bone marrow–derived precursor cells or autologous cardiac-derived cells have gained traction. A number of small- and moderate-scale trials of such therapy have focused on post–myocardial infarction patients and have used autologous bone marrow–derived progenitor or stem cells. These trials have had variable results, with most demonstrating modest improvements in parameters of cardiac structure and remodeling. More promising, however, are cardiac-derived stem cells. Two preliminary pilot trials delivering cells via an intracoronary approach have been reported. In one, autologous c-kit–positive cells isolated from the atria obtained from patients undergoing CABG were cultured and reinfused. In another, cardiosphere-derived cells grown from endomyocardial biopsy specimens were used. These small trials demonstrated improvements in left ventricular function but require far more work to usher in a clinical therapeutic success. The appropriate route of administration, the quantity of cells to achieve a minimal therapeutic threshold, the constitution of these cells (single source or mixed), the mechanism by which benefit accrues, and short- and long-term safety remain to be elucidated.
Targeting molecular aberrations using gene transfer therapy, mostly with an adenoviral vector, is emerging in HFrEF. Several methods of gene delivery have been developed, including direct intramyocardial injection, coronary artery or venous infusion, and injection into the pericardial space. Cellular targets under consideration include β2-adrenergic receptors and calcium cycling proteins such as inhibitors of phospholamban. SERCA2a is deficient in patients with HFrEF and is primarily responsible for reincorporating calcium into the sarcoplasmic reticulum during diastole. A phase II randomized, double-blind, placebo-controlled trial called CUPID (Efficacy and Safety Study of Genetically Targeted Enzyme Replacement Therapy for Advanced Heart Failure) was completed. This study used coronary arterial infusion of adeno-associated virus type 1 carrying the gene for SERCA2a and demonstrated that natriuretic peptides were decreased, reverse remodeling was noted, and symptomatic improvements were forthcoming. Stromal-derived factor 1 enhances myocardial repair and facilitates “homing” of stem cells to the site of tissue injury. Strategies using intramyocardial injections to deploy this gene at sites of injury are being studied.
More advanced therapies for late-stage heart failure such as left ventricular assist devices and cardiac transplantation are covered in detail in Chap. 281.
DISEASE MANAGEMENT AND SUPPORTIVE CARE
Despite stellar outcomes with medical therapy, admission rates following heart failure hospitalization remain high, with nearly half of all patients readmitted to hospital within 6 months of discharge. Recurrent heart failure and related cardiovascular conditions account for only half of readmissions in patients with heart failure, whereas other comorbidity-related conditions account for the rest. The key to achieving enhanced outcomes must begin with the attention to transitional care at the index hospitalization with facilitated discharge through comprehensive discharge planning, patient and caregiver education, appropriate use of visiting nurses, and planned follow-up. Early postdischarge follow-up, whether by telephone or clinic-based, may be critical to ensuring stability because most heart failure–related readmissions tend to occur within the first 2 weeks after discharge. Although routinely advocated, intensive surveillance of weight and vital signs with use of telemonitoring has not decreased hospitalizations. Intrathoracic impedance measurements have been advocated for the identification of early rise in filling pressure and worsened hemodynamics so that preemptive management may be employed. However, this has not been successful and may worsen outcomes in the short term. Implantable pressure monitoring systems do tend to provide signals for early decompensation, and in patients with moderately advanced symptoms, such systems have been shown to provide information that can allow implementation of therapy to avoid hospitalizations by as much as 39% (in the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart failure Patients [CHAMPION] trial). Once heart failure becomes advanced, regularly scheduled review of the disease course and options with the patient and family is recommended including discussions surrounding end-of-life preferences when patients are comfortable in an outpatient setting. As the disease state advances further, integrating care with social workers, pharmacists, and community-based nursing may be critical in improving patient satisfaction with the therapy, enhancing quality of life, and avoiding heart failure hospitalizations. Equally important is attention to seasonal influenza vaccinations and periodic pneumococcal vaccines that may obviate non–heart failure hospitalizations in these ill patients. When nearing end of life, facilitating a shift in priorities to outpatient and hospice palliation is key, as are discussions around advanced therapeutics and continued use of ICD prophylaxis, which may worsen quality of life and prolong death.
GLOBAL CONSIDERATIONS
Substantial differences exist in the practice of heart failure therapeutics and outcomes by geographic location. International guidelines produced by the American College of Cardiology/American Heart Association, European Society of Cardiology, and National Institute for Health and Clinical Excellence (United Kingdom) differ in their approach to evaluation of evidence and prioritization of therapy. The penetrance of CRT and ICD is higher in the United States than in Europe. Conversely, therapy unavailable in the United States, such as ivabradine and levosimendan, is designated as useful in Europe. Although ACEIs appear to be similarly effective across populations, variation in the benefits of beta blockers based on world region remains an area of controversy. In oral pharmacologic therapy trials of HFrEF, patients from southwest Europe have a lower incidence of ischemic cardiomyopathy and those in North America tend to have more diabetes and prior coronary revascularization. There is also regional variation in medication use even after accounting for indication. In trials of ADHF, patients in Eastern Europe tend to be younger, with higher ejection fractions and lower natriuretic peptide levels. Patients from South America tend to have the lowest rates of comorbidities, revascularization, and device use. In contrast, patients from North America have the highest comorbidity burden with high revascularization and device use rates. Given geographic differences in baseline characteristics and clinical outcomes, the generalizability of therapeutic outcomes in patients in the United States and Western Europe may require verification.
281 |
Cardiac Transplantation and Prolonged Assisted Circulation |
Advanced or end-stage heart failure is an increasingly frequent sequela of many types of heart disease, as progressively more effective palliation for the earlier stages of heart disease and prevention of sudden death associated with heart disease become more widely recognized and employed (Chap. 279). When patients with end-stage or refractory heart failure are identified, the physician is faced with the decision of advising compassionate end-of-life care or choosing to recommend extraordinary life-extending measures. For the occasional patient who is relatively young and without serious comorbidities, the latter may represent a reasonable option. Current therapeutic options are limited to cardiac transplantation (with the option of mechanical cardiac assistance as a “bridge” to transplantation) or permanent mechanical assistance of the circulation. In the future, it is possible that genetic modulation of ventricular function or cell-based cardiac repair will be options for such patients. Currently, both of the latter approaches are considered to be experimental.
CARDIAC TRANSPLANTATION
![]() Surgical techniques for orthotopic transplantation of the heart were devised in the 1960s and taken into the clinical arena in 1967. The procedures did not gain widespread clinical acceptance until the introduction of “modern” and more effective immunosuppression in the early 1980s. By the 1990s, the demand for transplantable hearts met, and then exceeded, the available donor supply and leveled off at about 4000 heart transplantations annually worldwide, according to data from the Registry of the International Society for Heart and Lung Transplantation (ISHLT). Subsequently, heart transplantation activity in the United States has remained stable at ~2200 per year, but worldwide activity reported to this registry has decreased somewhat. This apparent decline in numbers may be a result of the fact that reporting is legally mandated in the United States but not elsewhere, and several countries have started their own databases.
Surgical techniques for orthotopic transplantation of the heart were devised in the 1960s and taken into the clinical arena in 1967. The procedures did not gain widespread clinical acceptance until the introduction of “modern” and more effective immunosuppression in the early 1980s. By the 1990s, the demand for transplantable hearts met, and then exceeded, the available donor supply and leveled off at about 4000 heart transplantations annually worldwide, according to data from the Registry of the International Society for Heart and Lung Transplantation (ISHLT). Subsequently, heart transplantation activity in the United States has remained stable at ~2200 per year, but worldwide activity reported to this registry has decreased somewhat. This apparent decline in numbers may be a result of the fact that reporting is legally mandated in the United States but not elsewhere, and several countries have started their own databases.
SURGICAL TECHNIQUE
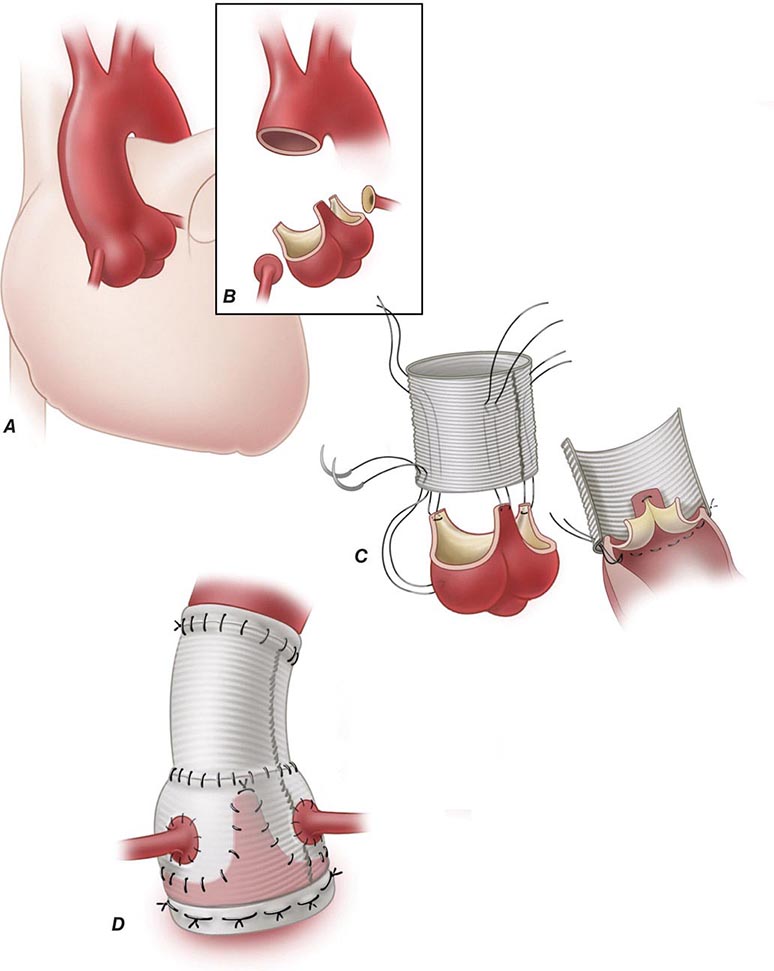
Donor and recipient hearts are excised in virtually identical operations with incisions made across the atria and atrial septum at the mid-atrial level (with the posterior walls of the atria left in place) and across the great vessels just above the semilunar valves. The donor heart is generally “harvested” by a separate surgical team, transported from the donor hospital in a bag of iced saline solution, and reanastomosed into the waiting recipient in the orthotopic or normal anatomic position. The only change in surgical technique since this method was first described has been a movement in recent years to move the right atrial anastomosis back to the level of the superior and inferior venae cavae to better preserve right atrial geometry and prevent atrial arrhythmias. Both methods of implantation leave the recipient with a surgically denervated heart that does not respond to any direct sympathetic or parasympathetic stimuli but does respond to circulating catecholamines. The physiologic responses of the denervated heart to the demands of exercise are atypical but quite adequate for continuation of normal physical activity.
DONOR ALLOCATION SYSTEM
In the United States, the allocation of donor organs is accomplished under the supervision of the United Network for Organ Sharing, a private organization under contract to the federal government. The United States is divided geographically into eleven regions for donor heart allocation. Allocation of donor hearts within a region is decided according to a system of priority that takes into account (1) the severity of illness, (2) the geographic distance from the donor, and (3) the patient’s time on the waiting list. A physiologic limit of ~3 h of “ischemic” (out-of-body) time for hearts precludes a national sharing of hearts. This allocation system design is reissued annually and is responsive to input from a variety of constituencies, including both donor families and transplantation professionals.
At the current time, the highest priority according to severity of illness is assigned to patients requiring hospitalization at the transplantation center for IV inotropic support, with a pulmonary artery catheter in place for hemodynamic monitoring, or to patients requiring mechanical circulatory support—i.e., use of an intra-aortic balloon pump or a right or left ventricular assist device (RVAD, LVAD), extracorporeal membrane oxygenation, or mechanical ventilation. The second highest priority is given to patients requiring ongoing inotropic support, but without a pulmonary artery catheter in place. All other patients are assigned a priority according to time accrued on the waiting list, and matching generally is based only on compatibility in terms of ABO blood group and gross body size.
While HLA matching of donor and recipient would be ideal, the relatively small numbers of patients as well as the time constraints involved make such matching impractical. However, some patients who are “presensitized” and have preexisting antibodies to human leukocyte antigens (HLAs) undergo prospective cross-matching with the donor; these patients are commonly multiparous women or patients who have received multiple transfusions.
INDICATIONS/CONTRAINDICATIONS
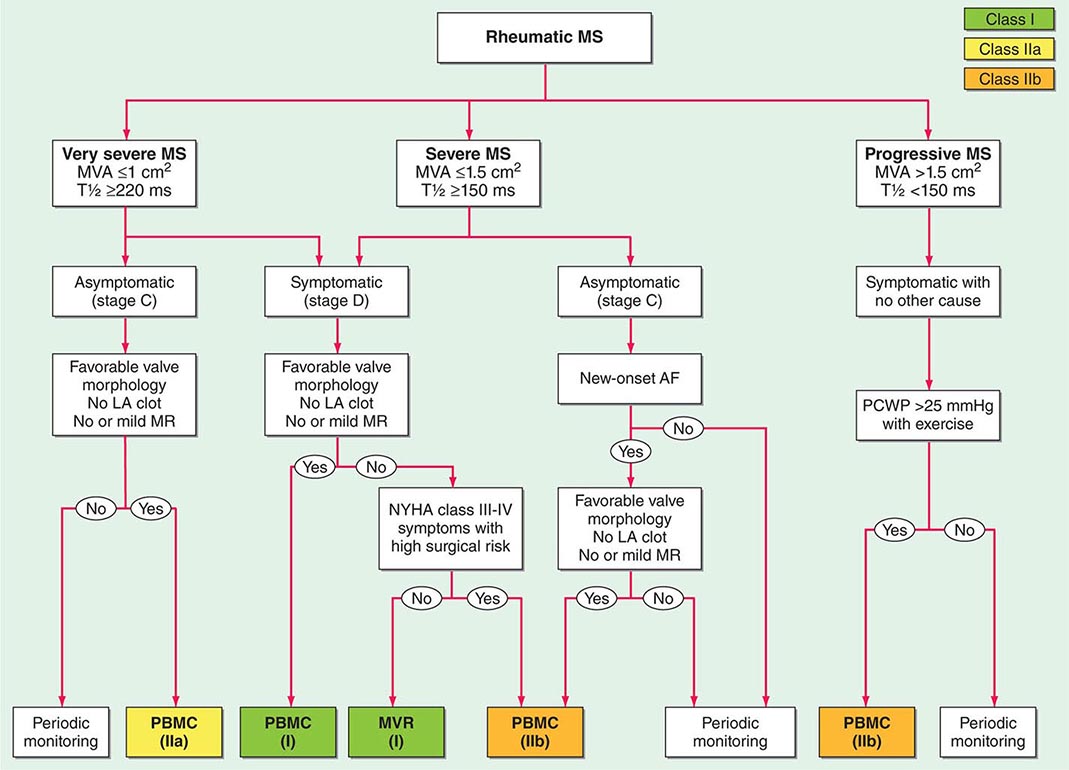
Heart failure is an increasingly common cause of death, particularly in the elderly. Most patients who reach what has recently been categorized as stage D, or refractory end-stage heart failure, are appropriately treated with compassionate end-of-life care. A subset of such patients who are younger and without significant comorbidities can be considered as candidates for heart transplantation. Exact criteria vary in different centers but generally take into consideration the patient’s physiologic age and the existence of comorbidities such as peripheral or cerebrovascular disease, obesity, diabetes, cancer, or chronic infection.
RESULTS
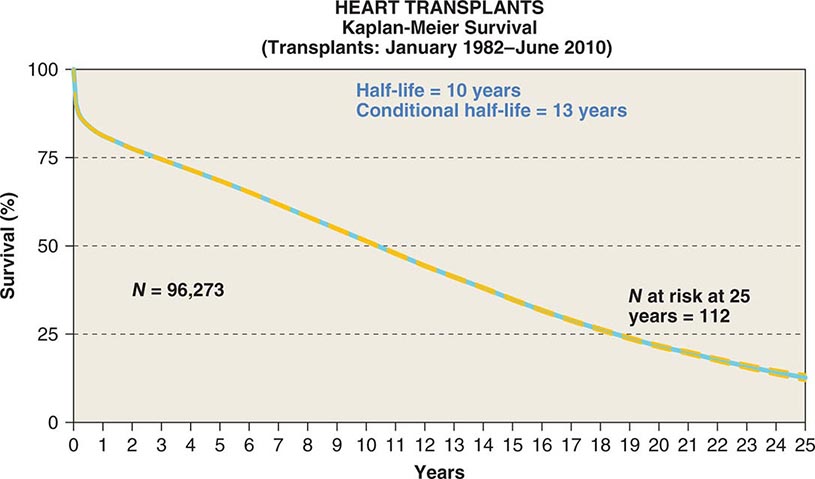
![]() A registry organized by the ISHLT has tracked worldwide and U.S. survival rates after heart transplantation since 1982. The most recent update reveals survival rates of 83% and 76% 1 and 3 years after transplantation, respectively, or a posttransplantation “half-life” of 10.00 years (Fig. 281-1). The quality of life of survivors is generally excellent, with well over 90% of patients in the registry returning to normal and unrestricted function after transplantation.
A registry organized by the ISHLT has tracked worldwide and U.S. survival rates after heart transplantation since 1982. The most recent update reveals survival rates of 83% and 76% 1 and 3 years after transplantation, respectively, or a posttransplantation “half-life” of 10.00 years (Fig. 281-1). The quality of life of survivors is generally excellent, with well over 90% of patients in the registry returning to normal and unrestricted function after transplantation.
FIGURE 281-1 Global survival rates after heart transplantation since 1982. Rates were calculated by the Kaplan-Meier method, which incorporates information from all transplant recipients for whom any follow-up has been provided. Because many patients are still alive and some patients have been lost to follow-up, the survival rates are estimates rather than exact figures because the time of death is not known for all patients. Therefore, 95% confidence limits are provided. (From J Stehlik et al: J Heart Lung Transplant 31:1052, 2012.)
IMMUNOSUPPRESSION
Medical regimens employed to suppress the normal immune response to a solid organ allograft vary from center to center and are in a constant state of evolution, as more effective agents with improved side-effect profiles and less toxicity are introduced. All currently used regimens are nonspecific, providing general hyporeactivity to foreign antigens rather than donor-specific hyporeactivity and also causing the attendant, and unwanted, susceptibility to infections and malignancy. Most cardiac transplantation programs currently use a three-drug regimen that includes a calcineurin inhibitor (cyclosporine or tacrolimus), an inhibitor of T cell proliferation or differentiation (azathioprine, mycophenolate mofetil, or sirolimus), and at least a short initial course of glucocorticoids. Many programs also include an initial “induction” course of polyclonal or monoclonal antibodies to T cells in the perioperative period to decrease the frequency or severity of early posttransplantation rejection. Most recently introduced have been monoclonal antibodies (daclizumab and basiliximab) that block the interleukin 2 receptor and may prevent allograft rejection without additional global immunosuppression.
Cardiac allograft rejection is usually diagnosed by endomyocardial biopsy conducted either on a surveillance basis or in response to clinical deterioration. Biopsy surveillance is performed on a regular basis in most programs for the first year postoperatively (or the first 5 years in many programs). Therapy consists of augmentation of immunosuppression, the intensity and duration of which are dictated by the severity of rejection.
LATE POSTTRANSPLANTATION MANAGEMENT ISSUES
Increasing numbers of heart transplant recipients are surviving for years following transplantation and constitute a population of patients with a number of long-term management issues.
Allograft Coronary Artery Disease Despite usually having young donor hearts, cardiac allograft recipients are prone to develop coronary artery disease (CAD). This CAD is generally a diffuse, concentric, and longitudinal process that is quite different from “ordinary” atherosclerotic CAD, which is more focal and often eccentric. The underlying etiology most likely is primarily immunologic injury of the vascular endothelium, but a variety of risk factors influence the existence and progression of CAD, including nonimmunologic factors such as dyslipidemia, diabetes mellitus, and cytomegalovirus (CMV) infection. It is hoped that newer and improved immunosuppressive modalities will reduce the incidence and impact of these devastating complications, which currently account for the majority of late posttransplantation deaths. Thus far, the immunosuppressive agents mycophenolate mofetil and the mammalian target of the rapamycin (mTOR) inhibitors sirolimus and everolimus have been shown to be associated with short-term lower incidence and extent of coronary intimal thickening; in anecdotal reports, institution of sirolimus was associated with some reversal of CAD. The use of statins also is associated with a reduced incidence of this vasculopathy, and these drugs are now used almost universally in transplant recipients unless contraindicated. Palliation of CAD with percutaneous interventions is probably safe and effective in the short term, although the disease often advances relentlessly. Because of the denervated status of the organ, patients rarely experience angina pectoris, even in advanced stages of disease.
Retransplantation is the only definitive form of therapy for advanced allograft CAD. However, the scarcity of donor hearts makes the decision to pursue retransplantation in an individual patient difficult and ethically complex.
Malignancy An increased incidence of malignancy is a well-recognized sequela of any program of chronic immunosuppression, and organ transplantation is no exception. Lymphoproliferative disorders are among the most frequent posttransplantation complications and, in most cases, seem to be driven by Epstein-Barr virus. Effective therapy includes reduction of immunosuppression (a clear “double-edged sword” in the setting of a life-sustaining organ), administration of antiviral agents, and traditional chemo- and radiotherapy. Most recently, specific antilymphocyte (CD20) therapy has shown great promise. Cutaneous malignancies (both basal cell and squamous cell carcinomas) also occur with increased frequency among transplant recipients and can follow aggressive courses. The role of decreasing immunosuppression in the treatment of these cancers is far less clear.
Infections The use of currently available nonspecific immunosuppressive modalities to prevent allograft rejection naturally results in increased susceptibility to infectious complications in transplant recipients. Although the incidence has decreased since the introduction of cyclosporine, infections with unusual and opportunistic organisms are still the major cause of death during the first postoperative year and remain a threat to the chronically immunosuppressed patient throughout life. Effective therapy depends on careful surveillance for early signs and symptoms of opportunistic infection, an extremely aggressive approach to obtaining a specific diagnosis, and expertise in recognizing the more common clinical presentations of infections caused by CMV, Aspergillus, and other opportunistic agents.
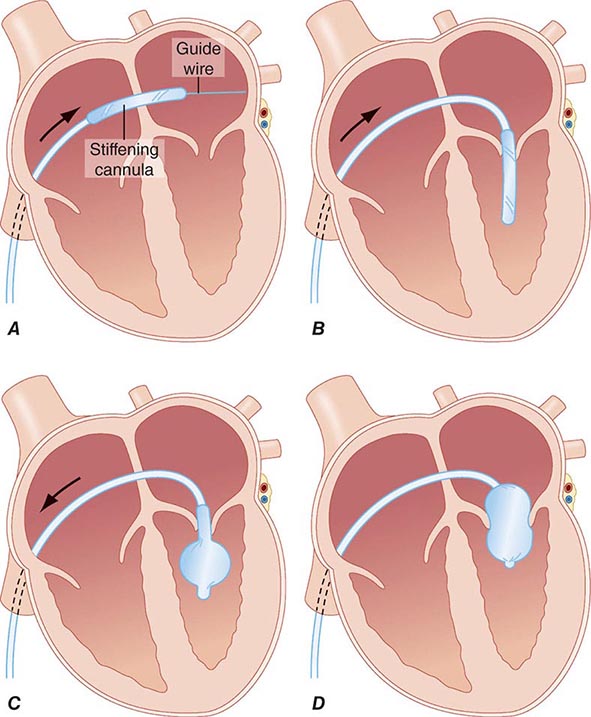
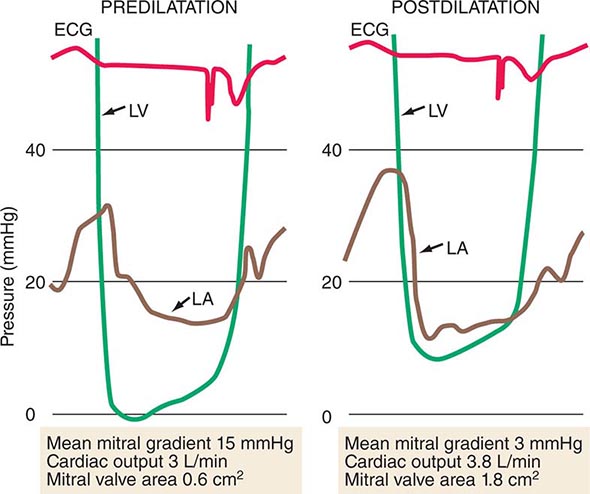
PROLONGED ASSISTED CIRCULATION
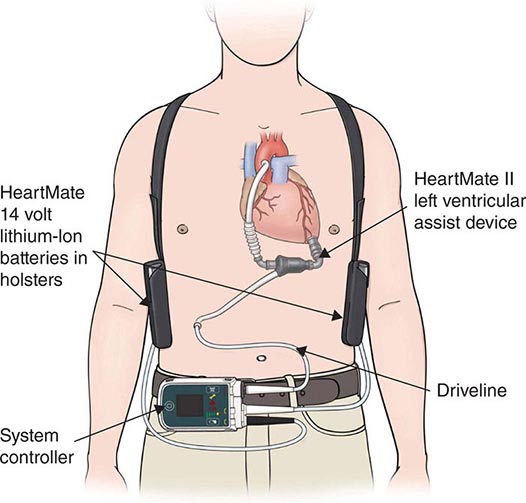
The modern era of mechanical circulatory support can be traced back to 1953, when cardiopulmonary bypass was first used in a clinical setting and ushered in the possibility of brief periods of circulatory support to permit open-heart surgery. Subsequently, a variety of extracorporeal pumps to provide circulatory support for brief periods have been developed. The use of a mechanical device to support the circulation for more than a few hours initially progressed slowly, with the implantation of a total artificial heart in 1969 in Texas by Cooley. This patient survived for 60 h until a donor organ became available, at which point he underwent transplantation. Unfortunately, the patient died of pulmonary complications after transplantation. The entire field of mechanical replacement of the heart then took a decade-long hiatus until the 1980s, when total artificial hearts were reintroduced with much publicity; however, they failed to produce the hoped-for treatment of end-stage heart disease. Starting in the 1970s, in parallel with the development of the total artificial heart, intense research had addressed the development of ventricular assist devices, which provide mechanical assistance for (rather than replacing) the failing ventricle.
Although conceived of initially as alternatives to biologic replacement of the heart, LVADs were introduced—and are still employed primarily—as temporary “bridges” to heart transplantation in candidates in whom medical therapy begins to fail before a donor heart becomes available. Several devices are approved by the U.S. Food and Drug Administration (FDA) and are in widespread use (see later). Those that are implantable within the body are compatible with hospital discharge and offer the patient a chance for life at home during a wait for a donor heart. However successful such “bridging” is for the individual patient, it does nothing to alleviate the scarcity of donor hearts; the ultimate goal in the field remains that of providing a reasonable alternative to biologic replacement of the heart—one that is widely and easily available and cost-effective.
CURRENT INDICATIONS AND APPLICATIONS OF VENTRICULAR ASSIST DEVICES
Currently, there are two major indications for ventricular assistance. First, patients at risk of imminent death from cardiogenic shock are eligible for mechanical support. These patients are generally managed with temporary cardiac assist devices. Second, if patients have a left ventricular ejection fraction <25% or a peak VO2 <14 mL/kg per min or are dependent on inotropic therapy or support with intra-aortic balloon counterpulsation, they may be eligible for mechanical support. If they are eligible for heart transplantation, the mechanical circulatory assistance is termed the “bridge to transplantation.” By contrast, if the patient has a contraindication to heart transplantation, the use of the device is deemed to constitute “destination” left ventricular assistance therapy.
BASIC CONCEPTS
Pulsatile vs. Nonpulsatile Devices Pulsatile devices are ventricular assist devices whose mechanism of action mandates the alternating filling and emptying of a volume chamber within the device that mimics the mechanism of action of the natural heart. Nonpulsatile devices have a mechanism of action that results in continuous blood flow through the device, eliminating the need for pulsatility. The pulsatile devices are larger, bulkier, and associated with greater energy requirements and higher rates of complications than the nonpulsatile devices. However, pulsatile devices provide greater degrees of support and may even be capable of replacing the function of the heart entirely in the form of a total artificial heart. Because of the bulkiness of these devices, many patients are too small to be supported with intracorporeal pulsatile pumps. However, paracorporeal versions are available. These devices are versatile and can be used for right, left, or biventricular assistance/replacement.
Continuous-flow (nonpulsatile) devices are further categorized on the basis of impeller design and mechanism. The older designs have tended to be axial-flow pumps, which operate on the Archimedes screw principle. These devices have an impeller that is in line with the direction of blood flow, and the inlet direction of blood is the same as the outlet direction. Continuous-flow devices have been dependent on the presence of blood-washed bearings within the pump housing and may be associated with an increased risk of blood and platelet activation. The newer devices are centrifugal in design; the blood flow takes a 90° turn between the inlet section of the pump and the outlet section. Another major difference in the newer devices is the absence of blood-washed bearings (with most devices having magnetically levitated impellers). This design allows the construction of smaller pumps with less blood-element activation than the axial-flow designs.
Available Devices In the United States, there are currently four FDA-approved devices that are used as bridges to transplantation in adults. Of these four devices, one is also approved for use as destination therapy or as long-term mechanical support of the heart. A number of other devices are approved only for short-term support in post–cardiac surgery shock or in cardiogenic shock secondary to acute myocardial infarction or fulminant myocarditis; these will not be considered here. So far, no long-term device is totally implantable, and, because of the need for transcutaneous connections, all share a common problem with infectious complications. Likewise, all share some tendency to thromboembolic complications and are subject to the possibility of mechanical failure common to any machine.
The total artificial heart (TAH) (Syncardia, Tucson, AZ) is a pneumatic, biventricular, orthotopically implanted ventricular assist device with an externalized driveline connecting it to its console. The TAH is currently the only FDA-approved device for use in patients who have severe biventricular failure.
The Thoratec LVAD (Thoratec Corp., Pleasanton, CA) is an extracorporeal pump that takes blood from a large cannula placed in the left ventricular apex and propels it forward through an outflow cannula inserted into the ascending aorta. The extracorporeal nature of this pump allows its use in small adults for whom intracorporeal pumps would be too large. This device provides not only left but also right ventricular assistance and can be utilized for biventricular support within the same patient (BiVentricular Assist Device).
The HeartMate II LVAD (Thoratec) similarly uses a drainage cannula in the left ventricular apex to drain blood into a small chamber, where the blood is driven by an electrically powered motor that spins a rotor, accelerating blood outflow into the ascending aorta (Fig. 281-2). This device is currently the only FDA-approved axial-flow pump that can be used both as a bridge to transplantation and as destination therapy.
FIGURE 281-2 Diagram of HeartMate II left ventricular assist device (LVAD). (Reprinted with permission from Thoratec Corp., Pleasanton, CA.)
The HeartWare Ventricular Assist System with the HVAD pump (HeartWare Inc., Framingham, MA) is the first third-generation device to be granted FDA approval for use in patients as a bridge to transplantation. The device is a centrifugal pump that is housed completely within the patient’s pericardial cavity and provides adequate support for many patients.
RESULTS
The use of these devices in the United States is limited mainly to patients with post–cardiac surgery shock and to those who are bridged to transplantation. The results of bridging to transplantation with the available devices are quite good, with nearly 75% of younger patients receiving a transplant by 1 year and having excellent posttransplantation survival rates.
Publication of the REMATCH (Randomized Evaluation of Mechanical Assistance in the Treatment of Heart Failure) trial in 2001 documented a somewhat improved survival rate in patients who had end-stage heart disease, were not candidates for transplantation, and were randomized to a pulsatile LVAD (albeit with a high rate of complications, especially neurologic issues) as opposed to continued medical therapy. This result led to renewed interest in use of the devices for nonbiologic permanent replacement of heart function as well. Subsequently, this device was supplanted by the HeartMate II axial-flow device, which has dramatically improved the survival of patients with severe end-stage heart disease in whom medical therapy has failed. The patients who had this device implanted had a 2-year survival rate of 58%, whereas the survival rate for patients in the medically treated arm of the original REMATCH trial was only 8%. More recent experience has shown that the mean survival period of patients with a continuous-flow LVAD for destination therapy is approaching 5 years.
Several studies have evaluated the benefit of LVAD therapy as a bridge to transplantation. The most recent data come from a series of 140 patients who underwent implantation of a HeartWare HVAD. Of these patients, 94% achieved the principal outcome (defined as survival to transplantation, recovery of heart function, or ongoing device support) at 180 days. With increased experience and improved outcomes using LVADs as a bridge to transplantation, the ability to maintain end-organ function and limit the progression of pulmonary hypertension—or even to decrease pulmonary vascular resistance—makes mechanical unloading a more attractive option than continued inotropic support. The early bridge-to-transplantation experience demonstrated reduced posttransplantation survival compared with medical management; however, more recent experience has shown equivalent outcomes following transplantation. This result is likely secondary to a trend toward earlier device implantation—i.e., prior to the onset of irreversible end-organ damage.
282 |
Congenital Heart Disease in the Adult |
Over a hundred years ago, Sir William Osler, in his classic textbook The Principles and Practice of Medicine (New York, Appleton & Co, 1892, pp 659–663), devoted only five pages to “Congenital Affections of the Heart,” with the first sentence declaring that “[t]hese [disorders] have only limited clinical interest, as in a large proportion of cases the anomaly is not compatible with life, and in others nothing can be done to remedy the defect or even to relieve symptoms.” Fortunately, in the intervening century, considerable progress has been made in understanding the basis for these disorders and their effective treatment.
The most common birth defects are cardiovascular in origin. These malformations are due to complex multifactorial genetic and environmental causes. Recognized chromosomal aberrations and mutations of single genes account for <10% of all cardiac malformations. Congenital heart disease (CHD) complicates ~1% of all live births in the general population—about 40,000 births/year—but occurs more frequently in the offspring (about 4–10%, depending on maternal CHD type) of women with CHD. Owing to the remarkable surgical advances over the last 60 years, >90% of afflicted neonates and children now reach adulthood; women with CHD may now frequently successfully bear children after competent repairs. As such, the population with CHD is steadily increasing. Women with CHD are at increased risk for peri- and postpartum complications, but maternal CHD is generally not considered an absolute contraindication to pregnancy unless the mother has certain high-risk features (e.g., cyanosis, pulmonary hypertension, decompensated heart failure, arrhythmias, aortic aneurysm, among others). Consultation with an adult CHD expert is warranted for all females with CHD who desire to become pregnant.
Nearly one and a half million adults with operated or unoperated CHD live in the United States today; there are now more adults than children with CHD in the United States. Because true surgical cures are rare, and all repairs—be they palliative or corrective—may leave residua, sequelae, or complications, most require some degree of lifetime expert surveillance. The anatomic and physiologic changes in the heart and circulation due to any specific CHD lesion are not static but, rather, progress from prenatal life to adulthood. Malformations that are benign or escape detection in childhood may become clinically significant in the adult. Unfortunately, the growing number of adults with CHD has not been paralleled by an adequate increase in the number of specialists and specialty centers that are trained and equipped to manage this challenging population. Ongoing efforts to increase awareness, resources, and advocacy are essential for the necessary growth of this specialty.
CARDIAC DEVELOPMENT
(See also Chap. 265e) CHD is generally the result of aberrant embryonic development of a normal structure or failure of such a structure to progress beyond an early stage of embryonic or fetal development. This brief section serves to introduce the reader to normal development so that defects may be better understood; by necessity, it is not exhaustive. Cardiogenesis is a finely tuned process with transcriptional control of a complex group of regulatory proteins that activate or inhibit their gene targets in a location- and time-dependent manner. At about 3 weeks of embryonic development, two cardiac cords form and become canalized; at that point, the primordial cardiac tube develops from two sources (cardiac crescent or the first heart field, pharyngeal mesoderm or the second heart field); by 21 days, these fuse into a single cardiac tube beginning at the cranial end. The cardiac tube then elongates and develops discrete constrictions with the following segments from caudal to cranial location: sinus venosus receives the umbilical, vitelline, and common cardinal veins: atrium, ventricle, bulbus cordis, truncus arteriosus, aortic sac, and the aortic arches. The cardiac tube is fixed at the sinus venosus and arterial ends.
Subsequently, in the next few weeks, differential growth of cells causes the tube to elongate and loop as an “S” with the bulboventricular portion moving rightward and the atrium and sinus venosus moving posterior to the ventricle. The primitive atrium and ventricle communicate via the atrioventricular canal from which the endocardial cushion develops into two parts (ventrally and dorsally). The cushions fuse and divide the atrioventricular canal into two atrioventricular inlets and also migrate to help form the ventricular septum. The primitive atrium is divided first by a septum primum membrane, which grows down from the superior wall to the cushions; as this fusion occurs, the mid-portion resorbs in the center forming the ostium secundum. Rightward of the septum primum, a second septum secundum membrane grows down from the ventral-cranial wall toward—but not reaching—the cushions, and covering most, but not all, of the ostium secundum, resulting in a flap of the foramen ovale. The primitive ventricle is partitioned by a finely tuned set of events. The interventricular septum grows up toward the cushions, and the cushions form an upper inlet septum; between the two portions is a hole called the interventricular foramen. The left and right ventricles begin to develop side by side, and the atria and their respective inlet valves align over their ventricles. Finally, these two parts of the septum fuse with the bulboventricular ridges, which, once having septated the truncus arteriosus, extend into the ventricle. The bulbocordis divides into a subaortic portion as the muscular conus resorbs, whereas the subpulmonary section has elongation of its muscular conus. Spiral division of the common truncus arteriosus rotates and aligns the pulmonary artery and aortic portions over their respective outflow tracts, the aortic valve moving posterior over the left ventricle (LV) outflow tract and the pulmonary valve moving anterior over the right ventricle (RV) outflow tract, with a wraparound relationship of the two great arteries.
Early on, the venous systems are bilateral and symmetric and enter two horns of the sinus venosus. Ultimately, except for the coronary sinus, most of the left-sided portions and the left sinus–venosus horn regress, and the systemic venous system empties into the right horn via the inferior and superior vena cavae. The pulmonary venous system, initially connecting to the systemic venous system, develops as buds from the developing lungs, which fuse together in the pulmonary venous confluence, at which point the connection to the systemic system regresses. Simultaneously, a projection from the back wall of the left atrium (the common pulmonary vein) grows posteriorly to merge with the confluence, which then becomes a part of the posterior left atrial wall.
The truncus arteriosus and aortic sac initially develop six paired symmetric arches, which curve posteriorly and become the paired dorsal aortae. The detailed description of the selective regression of some of the arches is not presented in this chapter. In brief summary, this process results in the development of arch 3 as the internal carotid arteries, left arch 4 as the aortic arch and right subclavian artery, and part of arch 6 as the patent ductus arteriosus. The two dorsal thoracic aortae fuse in the abdomen with persistence of the left dorsal aorta.
SPECIFIC CARDIAC DEFECTS
Tables 282-1, 282-2, and 282-3 list CHD malformations as simple, intermediate, or complex. Simple defects generally are single lesions with a shunt or a valvular malformation. Intermediate defects may have two or more simple defects. Complex defects generally have components of an intermediate defect plus more complex cardiac and vascular anatomy, often with cyanosis, and frequently with transposition complexes. The goal of these tables is to suggest when cardiology consultation or advanced CHD specialty care is needed. Patients with complex CHD (which includes most “named” surgeries that usually involve complex CHD) should virtually always be managed in conjunction with an experienced specialty adult CHD center. Patients with intermediate lesions should have an initial consultation and subsequent occasional intermittent follow-up with an adult CHD specialist. Patients with simple lesions often may be managed by a well-informed internist or general cardiologist, although consultation with a specifically trained adult congenital cardiologist is occasionally advisable.
|
SIMPLE ADULT CONGENITAL HEART DISEASE |
|
INTERMEDIATE COMPLEXITY CONGENITAL HEART DISEASE |
|
COMPLEX ADULT CONGENITAL HEART DISEASE |
ATRIAL SEPTAL DEFECT
Atrial septal defect (ASD) is a common cardiac anomaly that may be first encountered in the adult and occurs more frequently in females. Sinus venosus ASD occurs high in the atrial septum near the entry of the superior vena cava into the right atrium and is associated frequently with anomalous pulmonary venous connection from the right lung to the superior vena cava or right atrium (Fig. 282-1). Ostium primum ASDs lie adjacent to the atrioventricular valves, either of which may be deformed and regurgitant. Ostium primum ASDs are common in Down’s syndrome, often as part of complex atrioventricular septal defects with a common atrioventricular valve and a posterior defect of the basal portion of the interventricular septum. The most common ostium secundum ASD involves the fossa ovalis and is midseptal in location; this should not be confused with a patent foramen ovale. Anatomic obliteration of the foramen ovale ordinarily follows its functional closure soon after birth, but residual “probe patency” is a common normal variant; ASD denotes a true deficiency of the atrial septum and implies functional and anatomic patency. The magnitude of the left-to-right shunt depends on the ASD size, ventricular diastolic properties, and the relative impedance in the pulmonary and systemic circulations. The left-to-right shunt causes diastolic overloading of the RV and increased pulmonary blood flow. Patients with ASD are usually asymptomatic in early life, although there may be some physical underdevelopment and an increased tendency for respiratory infections; cardiorespiratory symptoms occur in many older patients. Beyond the fourth decade, a significant number of patients develop atrial arrhythmias, pulmonary arterial hypertension, and right heart failure. Patients exposed to the chronic environmental hypoxemia of high altitude tend to develop pulmonary hypertension at younger ages. In older patients, left-to-right shunting across the ASD increases as progressive systemic hypertension and/or coronary artery disease (CAD) result in reduced compliance of the LV.
FIGURE 282-1 Types and locations of congenital cardiac defects. ASD, atrial septal defect; PDA, patent ductus arteriosus; RMPV, right middle pulmonary vein; RUPV, right upper pulmonary vein; VSD, ventricular septal defect.
Physical Examination Examination usually reveals a prominent RV impulse and palpable pulmonary artery pulsation. The first heart sound is normal or split, with accentuation of the tricuspid valve closure sound. Increased flow across the pulmonic valve is responsible for a midsystolic pulmonary outflow murmur. The second heart sound is widely split and is fixed in relation to respiration. A mid-diastolic rumbling murmur, loudest at the fourth intercostal space and along the left sternal border, reflects increased flow across the tricuspid valve. In ostium primum ASD, an apical holosystolic murmur indicates associated mitral or tricuspid regurgitation or a ventricular septal defect (VSD).
These findings are altered when increased pulmonary vascular resistance causes diminution of the left-to-right shunt. Both the pulmonary outflow and tricuspid inflow murmurs decrease in intensity, the pulmonic component of the second heart sound and a systolic ejection sound are accentuated, the two components of the second heart sound may fuse, and a diastolic murmur of pulmonic regurgitation appears. Cyanosis and clubbing accompany the development of a right-to-left shunt (see “Ventricular Septal Defect” later in this chapter). In adults with an ASD and atrial fibrillation, the physical findings may be confused with mitral stenosis with pulmonary hypertension because the tricuspid diastolic flow murmur and widely split second heart sound may be mistakenly thought to represent the diastolic murmur of mitral stenosis and the mitral “opening snap,” respectively.
Electrocardiogram In ostium secundum ASD, electrocardiogram (ECG) usually shows right-axis deviation and an rSr´ pattern in the right precordial leads representing enlargement of the RV outflow tract. An ectopic atrial pacemaker or first-degree heart block may occur with the sinus venous ASD. In ostium primum ASD, the RV conduction defect is accompanied by left superior axis deviation and counterclockwise rotation of the frontal plane QRS loop. Varying degrees of RV and right atrial (RA) enlargement or hypertrophy may occur with each type of defect, depending on the presence and degree of pulmonary hypertension. Chest x-ray shows an enlarged RA and RV, and pulmonary artery and its branches; increased pulmonary vascular markings of left-to-right shunt vascularity will diminish if pulmonary vascular disease develops.
Echocardiogram Echocardiography reveals pulmonary arterial and RV and RA dilatation with abnormal (paradoxical) ventricular septal motion in the presence of a significant right heart volume overload. The ASD may be visualized directly by two-dimensional imaging, color-flow imaging, or echocontrast. Echocardiography and Doppler examination have supplanted cardiac catheterization. Transesophageal echocardiography is indicated if the transthoracic echocardiogram is ambiguous, which is often the case with sinus venosus defects, or for guiding catheter device closure (Fig. 282-2). Cardiac catheterization is performed if inconsistencies exist in the clinical data, if significant pulmonary hypertension or associated malformations are suspected, if CAD is a possibility, or when attempting transcatheter closure of the ASD.
FIGURE 282-2 A. Transesophageal echocardiogram demonstrating a secundum-type atrial septal defect (ASD) with shunting from the left atrium (LA) to the right atrium (RA). The right pulmonary artery (RPA) and superior vena cava (SVC) are labeled. B. Transcatheter balloon sizing of the ASD. C. Atrial septal occluder placement with a small manually created “fenestration” within the device that continues to allow a small amount of flow from the LA to the RA; this is used as a means of preventing left atrial hypertension after ASD closure. Left atrial hypertension may occur in older patients with decreased left ventricular compliance. D. Three-dimensional image of the septal occluder en-face; note the fenestration in the LA disc. The mitral valve (MV) and right inferior pulmonary vein (RIPV) are labeled.
VENTRICULAR SEPTAL DEFECT
VSD is one of the most common of all cardiac birth defects, either as an isolated defect or as a component of a combination of anomalies (Fig. 282-1). The VSD is usually single and situated in the membranous or midmuscular portion of the septum. The functional disturbance depends on its size and on the status of the pulmonary vascular bed. Only small- or moderate-size VSDs are seen initially in adulthood, as most patients with an isolated large VSD come to medical or surgical attention early in life.
A wide spectrum exists in the natural history of VSD, ranging from spontaneous closure to congestive cardiac failure and death in infancy. Included within this spectrum is the possible development of pulmonary vascular obstruction, RV outflow tract obstruction, aortic regurgitation, or infective endocarditis. Spontaneous closure is more common in patients born with a small VSD, which occurs in early childhood in most. The pulmonary vascular bed is often a principal determinant of the clinical manifestations and course of a given VSD and feasibility of surgical repair. Increased pulmonary arterial pressure results from increased pulmonary blood flow and/or resistance, the latter usually the result of obstructive, obliterative structural changes within the pulmonary vascular bed. It is important to quantitate and compare pulmonary-to-systemic flows and resistances in patients with severe pulmonary hypertension. The term Eisenmenger’s syndrome is applied to patients with a large communication between the two circulations at the aortopulmonary, ventricular, or atrial levels and bidirectional or predominantly right-to-left shunts because of high resistance and obstructive pulmonary hypertension.
Patients with large VSDs and pulmonary hypertension are at greatest risk for developing pulmonary vascular disease. Large VSDs should be corrected early in life when pulmonary vascular disease is not severely elevated. In patients with Eisenmenger’s syndrome, symptoms in adult life consist of exertional dyspnea, chest pain, syncope, and hemoptysis. The right-to-left shunt leads to cyanosis, clubbing, and erythrocytosis (see below). The degree to which pulmonary vascular resistance is elevated before operation is a critical factor determining prognosis. If the pulmonary vascular resistance is one-third or less of the systemic value, progression of pulmonary vascular disease after operation is unusual; however, if a moderate to severe increase in pulmonary vascular resistance exists preoperatively, either no change or a progression of pulmonary vascular disease is common postoperatively. Pregnancy is contraindicated in Eisenmenger’s syndrome. The mother’s health is most at risk if she has a cardiovascular lesion associated with pulmonary vascular disease and pulmonary hypertension (e.g., Eisenmenger’s physiology or mitral stenosis) or severe LV outflow tract obstruction (e.g., aortic stenosis), but she is also at risk of death with any malformation that may cause heart failure or a hemodynamically important arrhythmia. The fetus is most at risk with maternal cyanosis, heart failure, or pulmonary hypertension.
RV outflow tract obstruction develops in ~5–10% of patients who present in infancy with a moderate to large left-to-right shunt. With time, as subvalvular RV outflow tract obstruction progresses, the findings in these patients whose VSD remains sizable begin to resemble more closely those of the cyanotic tetralogy of Fallot. In ~5% of patients, aortic valve regurgitation results from insufficient cusp tissue or prolapse of the cusp through the interventricular defect; the aortic regurgitation then complicates and dominates the clinical course. Echocardiography with spectral and color Doppler examination defines the number and location of defects in the ventricular septum and associated anomalies and the hemodynamic physiology of the defect(s). Hemodynamic and angiographic study may be occasionally required to assess the status of the pulmonary vascular bed and clarify details of the altered anatomy. Cross-sectional imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI) are useful in delineating complex anatomy and assessing extracardiac structures.
In patients with Eisenmenger’s VSD, pulmonary arterial vasodilators and both single- or double-lung transplantation with intracardiac defect repair or heart/lung transplantation show promise for improvement in symptoms (Chaps. 281 and 320e). Chronic hypoxemia in cyanotic CHD results in secondary erythrocytosis due to increased erythropoietin production (Chap. 49). The term polycythemia is a misnomer; white cell counts are normal, and platelet counts are normal to decreased. Compensated erythrocytosis with iron-replete equilibrium hematocrits rarely results in symptoms of hyperviscosity at hematocrits <65% and occasionally not even with hematocrits ≥70%. For this reason, therapeutic phlebotomy is rarely required in compensated erythrocytosis. In contrast, patients with decompensated erythrocytosis fail to establish equilibrium with unstable, rising hematocrits and recurrent hyperviscosity symptoms. Therapeutic phlebotomy, a two-edged sword, allows temporary relief of symptoms but limits oxygen delivery, begets instability of the hematocrit, and compounds the problem by iron depletion. Iron-deficiency symptoms are usually indistinguishable from those of hyperviscosity; progressive symptoms after recurrent phlebotomy are usually due to iron depletion with hypochromic microcytosis. Iron depletion results in a larger number of smaller (microcytic) hypochromic red cells that are less capable of carrying oxygen and less deformable in the microcirculation; with more of them relative to plasma volume, viscosity is greater than for an equivalent hematocrit with fewer, larger, iron-replete, deformable cells. As such, iron-depleted erythrocytosis results in increasing symptoms due to decreased oxygen delivery to the tissues.
Hemostasis is abnormal in cyanotic CHD, due, in part, to the increased blood volume and engorged capillaries, abnormalities in platelet function, and sensitivity to aspirin or nonsteroidal anti-inflammatory agents, as well as abnormalities of the extrinsic and intrinsic coagulation system. Oral contraceptives are often contraindicated for cyanotic women because of the enhanced risk of vascular thrombosis. Symptoms of hyperviscosity can be produced in any cyanotic patient with erythrocytosis if dehydration reduces plasma volume. Phlebotomy for symptoms of hyperviscosity not due to dehydration or iron deficiency is a simple outpatient removal of 500 mL of blood over 45 min with isovolumetric replacement with isotonic saline. Acute phlebotomy without volume replacement is contraindicated. Iron repletion in decompensated iron-depleted erythrocytosis reduces iron-deficiency symptoms, but must be done gradually to avoid an excessive rise in hematocrit and resulting hyperviscosity.
PATENT DUCTUS ARTERIOSUS
The ductus arteriosus is a vessel leading from the bifurcation of the pulmonary artery to the aorta just distal to the left subclavian artery (Fig. 282-1). Normally, the vascular channel is open in the fetus but closes immediately after birth. The flow across the ductus is determined by the pressure and resistance relationships between the systemic and pulmonary circulations and by the cross-sectional area and length of the ductus. In most adults with this anomaly, pulmonary pressures are normal, and a gradient and shunt from aorta to pulmonary artery persist throughout the cardiac cycle, resulting in a characteristic thrill and a continuous “machinery” murmur with late systolic accentuation at the upper left sternal edge. In adults who were born with a large left-to-right shunt through the ductus arteriosus, pulmonary vascular obstruction (Eisenmenger’s syndrome) with pulmonary hypertension, right-to-left shunting, and cyanosis have usually developed. Severe pulmonary vascular disease results in reversal of flow through the ductus; unoxygenated blood is shunted to the descending aorta; and the toes—but not the fingers—become cyanotic and clubbed, a finding termed differential cyanosis (Fig. 282-3). The leading causes of death in adults with patent ductus arteriosus are cardiac failure and infective endocarditis; occasionally, severe pulmonary vascular obstruction may cause aneurysmal dilatation, calcification, and rupture of the ductus.
FIGURE 282-3 A. Patent ductus arteriosus (PDA) in a patient with severe pulmonary hypertension (Eisenmenger’s syndrome). Due to the suprasystemic pulmonary arterial resistance, deoxygenated (cyanotic) blood from the right ventricle (RV) and pulmonary artery (PA) is shunted across the PDA to the aorta (Ao). The left atrium (LA) and left ventricle (LV) are labeled. B. Differential clubbing and cyanosis of the toes due to lower extremity perfusion by the deoxygenated blood crossing the PDA. C. Angiogram in a dilated main pulmonary artery (MPA) with shunting noted across the PDA to the descending aorta (dAo). The left pulmonary artery (LPA) is labeled. D. Direct pressure recordings in the Ao and PA demonstrating suprasystemic PA systolic pressure.
AORTIC ROOT–TO–RIGHT-HEART SHUNTS
The three most common causes of aortic root–to–right-heart shunts are congenital aneurysm of an aortic sinus of Valsalva with fistula, coronary arteriovenous fistula, and anomalous origin of the left coronary artery from the pulmonary trunk. Aneurysm of an aortic sinus of Valsalva consists of a separation or lack of fusion between the media of the aorta and the annulus of the aortic valve. Rupture usually occurs in the third or fourth decade of life; most often, the aorticocardiac fistula is between the right coronary cusp and the RV; but occasionally, when the noncoronary cusp is involved, the fistula drains into the RA. Abrupt rupture causes chest pain, bounding pulses, a continuous murmur accentuated in diastole, and volume overload of the heart. Diagnosis is confirmed by two-dimensional and Doppler echocardiographic studies; cardiac catheterization quantitates the left-to-right shunt, and thoracic aortography visualizes the fistula. Medical management is directed at cardiac failure, arrhythmias, or endocarditis. At operation, the aneurysm is closed and amputated, and the aortic wall is reunited with the heart, either by direct suture or with a patch or prosthesis. Transcatheter device closure is a less invasive and effective alternative to surgery.
Coronary arteriovenous fistula, an unusual anomaly, consists of a communication between a coronary artery and another cardiac chamber, usually the coronary sinus, RA, or RV. The shunt is usually of small magnitude, and myocardial blood flow is not usually compromised; if the shunt is large, there may be a coronary “steal” syndrome with myocardial ischemia and possible angina or ventricular arrhythmias. Potential complications include infective endocarditis; thrombus formation with occlusion or distal embolization with myocardial infarction; rupture of an aneurysmal fistula; and, rarely, pulmonary hypertension and congestive failure. A loud, superficial, continuous murmur at the lower or midsternal border usually prompts a further evaluation of asymptomatic patients. Doppler echocardiography demonstrates the site of drainage; if the site of origin is proximal, it may be detectable by two-dimensional echocardiography. Angiography (classic catheterization, CT, or magnetic resonance angiography) permits identification of the size and anatomic features of the fistulous tract, which may be closed by suture or transcatheter obliteration.
The third anomaly causing a shunt from the aortic root to the right heart is anomalous origin of the left coronary artery from the pulmonary artery. In this condition, oxygenated blood from the aortic root flows via a dilated right coronary artery and collaterals to the left coronary artery and retrograde to the lower pressure pulmonary artery circulation via the anomalous left main coronary artery (which emerges from the pulmonary artery). Myocardial infarction and fibrosis commonly lead to death within the first year, although up to 20% of patients survive to adolescence and beyond without surgical correction. The diagnosis is supported by the ECG findings of an anterolateral myocardial infarction and left ventricular hypertrophy (LVH). Operative management of adults consists of coronary artery reimplantation, coronary artery bypass with an internal mammary artery graft, or saphenous vein–coronary artery graft.
CONGENITAL AORTIC STENOSIS
Malformations that cause obstruction to LV outflow include congenital valvular aortic stenosis, discrete subaortic stenosis, or supravalvular aortic stenosis. Bicuspid aortic valves are more common in males than in females. The congenital bicuspid aortic valve, which may initially be functionally normal, is one of the most common congenital malformations of the heart and may go undetected in early life. Because bicuspid valves may develop stenosis or regurgitation with time or be the site of infective endocarditis, the lesion may be difficult to distinguish in older adults from acquired rheumatic or degenerative calcific aortic valve disease. The dynamics of blood flow associated with a congenitally deformed, rigid aortic valve commonly lead to thickening of the cusps and, in later life, to calcification. Hemodynamically significant obstruction causes concentric hypertrophy of the LV wall. The ascending aorta is often dilated, misnamed “poststenotic” dilatation; this is due to histologic abnormalities of the aortic media and may result in aortic dissection. Diagnosis is made by echocardiography, which reveals the morphology of the aortic valve and aortic root and quantitates severity of stenosis or regurgitation. The clinical manifestations and hemodynamic abnormalities are discussed in Chap. 283.
SUBAORTIC STENOSIS The discrete form of subaortic stenosis consists of a membranous diaphragm or fibromuscular ring encircling the LV outflow tract just beneath the base of the aortic valve. The jet impact from the subaortic stenotic jet on the underside of the aortic valve often begets progressive aortic valve fibrosis and valvular regurgitation. Echocardiography demonstrates the anatomy of the subaortic obstruction; Doppler studies show turbulence proximal to the aortic valve and can quantitate the pressure gradient and severity of aortic regurgitation. Treatment consists of complete excision of the membrane or fibromuscular ring.
SUPRAVALVULAR AORTIC STENOSIS This is a localized or diffuse narrowing of the ascending aorta originating just above the level of the coronary arteries at the superior margin of the sinuses of Valsalva. In contrast to other forms of aortic stenosis, the coronary arteries are subjected to elevated systolic pressures from the LV, are often dilated and tortuous, and are susceptible to premature atherosclerosis. The coronary ostia may also become obstructed by the aortic valve leaflets. In most patients, a genetic defect for the anomaly is located in the same chromosomal region as elastin on chromosome 7. Supravalvular aortic stenosis is the most commonly associated cardiac defect in Williams-Beuren syndrome, typically comprising the following: “elfin” facies, low nasal bridge, cheerful demeanor, mental retardation with retained language skills and love of music, supravalvular aortic stenosis, and transient hypercalcemia.
COARCTATION OF THE AORTA
Narrowing or constriction of the lumen of the aorta may occur anywhere along its length but is most common distal to the origin of the left subclavian artery near the insertion of the ligamentum arteriosum. Coarctation occurs in ~7% of patients with CHD, is more common in males than females, and is particularly frequent in patients with gonadal dysgenesis (e.g., Turner’s syndrome). Clinical manifestations depend on the site and extent of obstruction and the presence of associated cardiac anomalies, most commonly a bicuspid aortic valve. Circle of Willis aneurysms may occur in up to 10%.
Most children and young adults with isolated, discrete coarctation are asymptomatic. Headache, epistaxis, chest pressure, and claudication with exercise may occur, and attention is usually directed to the cardiovascular system when a heart murmur or hypertension in the upper extremities and absence, marked diminution, or delayed pulsations in the femoral arteries are detected on physical examination. Enlarged and pulsatile collateral vessels may be palpated in the intercostal spaces anteriorly, in the axillae, or posteriorly in the interscapular area. The upper extremities and thorax may be more developed than the lower extremities. A midsystolic murmur over the left interscapular space may become continuous if the lumen is narrowed sufficiently to result in a high-velocity jet across the lesion throughout the cardiac cycle. Additional systolic and continuous murmurs over the lateral thoracic wall may reflect increased flow through dilated and tortuous collateral vessels. The ECG usually reveals LV hypertrophy. Chest x-ray may show a dilated left subclavian artery high on the left mediastinal border and a dilated ascending aorta. Indentation of the aorta at the site of coarctation and pre- and poststenotic dilatation (the “3” sign) along the left paramediastinal shadow are essentially pathognomonic. Notching of the third to ninth ribs, an important radiographic sign, is due to inferior rib erosion by dilated collateral vessels. Two-dimensional echocardiography from suprasternal windows identifies the site of coarctation; Doppler quantitates the pressure gradient. Transesophageal echocardiography and MRI or CT allow visualization of the length and severity of the obstruction and associated collateral arteries. In adults, cardiac catheterization is indicated primarily to evaluate the coronary arteries or to perform catheter-based intervention (angioplasty and stent of the coarctation).
The chief hazards of proximal aortic severe hypertension include cerebral aneurysms and hemorrhage, aortic dissection and rupture, premature coronary arteriosclerosis, aortic valve failure, and LV failure; infective endarteritis may occur on the coarctation site or endocarditis may settle on an associated bicuspid aortic valve, which is estimated to be present in 50% of patients.
PULMONARY STENOSIS WITH INTACT VENTRICULAR SEPTUM
Obstruction to RV outflow may be localized to the supravalvular, valvular, or subvalvular levels or occur at a combination of these sites. Multiple sites of narrowing of the peripheral pulmonary arteries are a feature of rubella embryopathy and may occur with both the familial and sporadic forms of supravalvular aortic stenosis. Valvular pulmonic stenosis (PS) is the most common form of isolated RV obstruction.
The severity of the obstructing lesion, rather than the site of narrowing, is the most important determinant of the clinical course. In the presence of a normal cardiac output, a peak systolic pressure gradient <30 mmHg indicates mild PS and >50 mmHg indicates severe PS; pressures between these limits are considered to indicate moderate stenosis. Patients with mild PS are generally asymptomatic and demonstrate little or no progression in the severity of obstruction with age. In patients with more significant stenosis, the severity may increase with time. Symptoms vary with the degree of obstruction. Fatigue, dyspnea, RV failure, and syncope may limit the activity of older patients, in whom moderate or severe obstruction may prevent an augmentation of cardiac output with exercise. In patients with severe obstruction, the systolic pressure in the RV may exceed that in the LV, because the ventricular septum is intact. RV ejection is prolonged with moderate or severe stenosis, and the sound of pulmonary valve closure is delayed and soft. RV hypertrophy reduces the compliance of that chamber, and a forceful RA contraction is necessary to augment RV filling. A fourth heart sound; prominent a waves in the jugular venous pulse; and, occasionally, presystolic pulsations of the liver reflect vigorous atrial contraction. The clinical diagnosis is supported by a left parasternal lift and harsh systolic crescendo-decrescendo murmur and thrill at the upper left sternal border, typically preceded by a systolic ejection sound if the obstruction is due to a mobile nondysplastic pulmonary valve. The holosystolic murmur of tricuspid regurgitation may accompany severe PS, especially in the presence of congestive heart failure. Cyanosis usually reflects right-to-left shunting through a patent foramen ovale or ASD. In patients with supravalvular or peripheral pulmonary arterial stenosis, the murmur is systolic or continuous and is best heard over the area of narrowing, with radiation to the peripheral lung fields.
In mild cases, the ECG is normal, whereas moderate and severe stenoses are associated with RV hypertrophy. The chest x-ray with mild or moderate PS shows a heart of normal size with normal lung vascularity. In pulmonary valvular stenosis, dilatation of the main and left pulmonary arteries occurs in part due to the direction of the PS jet and in part due to intrinsic tissue weakness. With severe obstruction, RV hypertrophy is generally evident. The pulmonary vascularity may be reduced with severe stenosis, RV failure, and/or a right-to-left shunt at the atrial level. Two- and three-dimensional echocardiography visualizes pulmonary valve morphology; the outflow tract pressure gradient is quantitated by Doppler echocardiography (Fig. 282-4).
FIGURE 282-4 A. Transesophageal echocardiogram of a patient with severe pulmonary stenosis due to a mobile and doming pulmonary valve (PV). The pulmonary artery (PA) and the right ventricle (RV) are labeled. B. Following balloon valvuloplasty, the pulmonary valve orifice is larger. C. Simultaneous RV and PA pressure tracings before balloon valvuloplasty; the peak-to-peak gradient across the pulmonary valve is ~70 mmHg. D. After balloon valvuloplasty, the peak-to-peak gradient is reduced to ~25 mmHg.
TETRALOGY OF FALLOT
The four components of the tetralogy of Fallot are malaligned VSD, obstruction to RV outflow, aortic override of the VSD, and RV hypertrophy due to the RV’s response to aortic pressure via the large VSD.
The severity of RV outflow obstruction determines the clinical presentation. The severity of hypoplasia of the RV outflow tract varies from mild to complete (pulmonary atresia). Pulmonary valve stenosis and supravalvular and peripheral pulmonary arterial obstruction may coexist; rarely, there is unilateral absence of a pulmonary artery (usually the left). A right-sided aortic arch and descending thoracic aorta occur in ~25%.
The relationship between the resistance of blood flow from the ventricles into the aorta and into the pulmonary artery plays a major role in determining the hemodynamic and clinical picture. When the RV outflow obstruction is severe, pulmonary blood flow is reduced markedly, and a large volume of desaturated systemic venous blood shunts right-to-left across the VSD. Severe cyanosis and erythrocytosis occur, and symptoms of systemic hypoxemia are prominent. In many infants and children, the obstruction is mild but progressive.
The ECG shows RV hypertrophy. Chest x-ray shows a normal-sized, boot-shaped heart (coeur en sabot) with a prominent RV and a concavity in the region of the pulmonary conus. Pulmonary vascular markings are typically diminished, and the aortic arch and knob may be on the right side. Echocardiography demonstrates the malaligned VSD with the overriding aorta and the site and severity of PS, which may be subpulmonic (fixed or dynamic), at the pulmonary valve or in the main or branch pulmonary arteries. Classic contrast angiography may provide details regarding the RV outflow tract, pulmonary valve and annulus, and caliber of the main branches of the pulmonary artery, as well as about possible associated aortopulmonary collaterals. Coronary arteriography identifies the anatomy and course of the coronary arteries, which may be anomalous. Cardiac MRI and CT complement echocardiography and provide much of the information gathered by angiography as well as additional functional information. MRI is considered the clinical gold standard for quantification of RV volume and function as well as quantification of the pulmonary regurgitation severity.
COMPLETE TRANSPOSITION OF THE GREAT ARTERIES
This condition is commonly called dextro– or D-transposition of the great arteries. The aorta arises rightward anteriorly from the RV, and the pulmonary artery emerges leftward and posteriorly from the LV, which results in two separate parallel circulations; some communication between them must exist after birth to sustain life. Most patients have an interatrial communication, two-thirds have a patent ductus arteriosus, and about one-third have an associated VSD. Transposition is more common in males and accounts for ~10% of cyanotic heart disease. The course is determined by the degree of tissue hypoxemia, the ability of each ventricle to sustain an increased workload in the presence of reduced coronary arterial oxygenation, the nature of the associated cardiovascular anomalies, and the status of the pulmonary vascular bed. Patients who do not undergo surgical palliation generally do not survive to reach adulthood. The long-term outcomes in those that have undergone surgery are in large part determined by the type of surgery performed. By the third decade of life, ~30% of patients with “atrial switch” operations will have developed decreased RV function and progressive tricuspid regurgitation, which may lead to congestive heart failure. Pulmonary vascular obstruction develops by 1–2 years of age in patients with an associated large VSD or large patent ductus arteriosus in the absence of obstruction to LV outflow.
SINGLE VENTRICLE
This is a family of complex lesions with both atrioventricular valves or a common atrioventricular valve opening to a single ventricular chamber. Associated anomalies include abnormal great artery positional relationships, pulmonic valvular or subvalvular stenosis, and subaortic stenosis. Survival to adulthood depends on a relatively normal pulmonary blood flow, yet normal pulmonary resistance and good ventricular function. Modifications of the Fontan approach are generally applied to carefully selected patients with creation of a pathway(s) from the systemic veins to the pulmonary arteries.
TRICUSPID ATRESIA
This malformation is characterized by atresia of the tricuspid valve; an interatrial communication; and, frequently, hypoplasia of the RV and pulmonary artery. The clinical picture is usually dominated by severe cyanosis due to obligatory admixture of systemic and pulmonary venous blood in the LV. The ECG characteristically shows RA enlargement, left-axis deviation, and LV hypertrophy.
Atrial septostomy and palliative operations to increase pulmonary blood flow, often by anastomosis of a systemic artery or vein to a pulmonary artery, may allow survival to the second or third decade. A Fontan atriopulmonary or total cavopulmonary connection may then allow functional correction in patients with normal or low pulmonary arterial resistance pressure and good LV function. There are a number of important long-term considerations with the Fontan circulation, including the development of arrhythmias, progressive liver dysfunction, thromboembolic complications, and potential long-term need for heart or heart and liver transplantation.
EBSTEIN’S ANOMALY
Characterized by a downward displacement of the tricuspid valve into the RV, due to anomalous attachment of the tricuspid leaflets, the Ebstein tricuspid valve tissue is dysplastic and results in tricuspid regurgitation. The abnormally situated tricuspid orifice produces an “atrialized” portion of the RV lying between the atrioventricular ring and the origin of the valve, which is continuous with the RA chamber. Often, the RV is hypoplastic. Although the clinical manifestations are variable, some patients come to initial attention because of either (1) progressive cyanosis from right-to-left atrial shunting, (2) symptoms due to tricuspid regurgitation and RV dysfunction, or (3) paroxysmal atrial tachyarrhythmias with or without atrioventricular bypass tracts (Wolff-Parkinson-White [WPW] syndrome). Diagnostic findings by two-dimensional echocardiography include the abnormal positional relation between the tricuspid and mitral valves with abnormally increased apical displacement of the septal tricuspid leaflet. Tricuspid regurgitation is quantitated by Doppler examination. Surgical approaches include prosthetic replacement of the tricuspid valve when the leaflets are tethered or repair of the native valve.
CONGENITALLY CORRECTED TRANSPOSITION
The two fundamental anatomic abnormalities in this malformation are transposition of the ascending aorta and pulmonary trunk and inversion of the ventricles. This arrangement results in desaturated systemic venous blood passing from the RA through the mitral valve to the LV and into the pulmonary trunk, whereas oxygenated pulmonary venous blood flows from the left atrium through the tricuspid valve to the RV and into the aorta. Thus, the circulation is corrected functionally. The clinical presentation, course, and prognosis of patients with congenitally corrected transposition vary depending on the nature and severity of any complicating intracardiac anomalies and of development of dysfunction of the systemic subaortic RV. Progressive RV dysfunction and tricuspid regurgitation may also develop in one-third of patients by age 30; Ebstein-type anomalies of the left-side tricuspid atrioventricular valve are common. VSD or PS due to obstruction to outflow from the right-sided subpulmonary (anatomic left) ventricle may coexist. Complete heart block occurs at a rate of 2–10% per decade. The diagnosis of the malformation and associated lesions can be established by comprehensive two-dimensional echocardiography and Doppler examination.
MALPOSITIONS OF THE HEART
Positional anomalies refer to conditions in which the cardiac apex is in the right side of the chest (dextrocardia) or at the midline (mesocardia), or in which there is a normal location of the heart in the left side of the chest but abnormal position of the viscera (isolated levocardia). Knowledge of the position of the abdominal organs and of the branching pattern of the main stem bronchi is important in categorizing these malpositions. When dextrocardia occurs without situs inversus, when the visceral situs is indeterminate, or if isolated levocardia is present, associated, often complex, multiple cardiac anomalies are usually present. In contrast, mirror-image dextrocardia is usually observed with complete situs inversus, which occurs most frequently in individuals whose hearts are otherwise normal.
SURGICALLY MODIFIED CONGENITAL HEART DISEASE
Owing to the enormous strides in cardiovascular surgical techniques that have occurred in the past 70 years, a large number of long-term survivors of palliative or corrective operations in infancy and childhood have reached adulthood. These patients are often challenging because of the diversity of anatomic, hemodynamic, and electrophysiologic residua and sequelae of cardiac operations.
The proper care of the survivor of an operation for CHD requires that the clinician understand the details of the malformation before operation; pay meticulous attention to the details of the operative procedure; and recognize the postoperative residua (conditions left totally or partially uncorrected), the sequelae (conditions caused by surgery), and the complications that may have resulted from the operation. Except for ligation of an uncomplicated patent ductus arteriosus, almost every other surgical repair leaves behind or causes some abnormality of the heart and circulation that may range from trivial to serious. Thus, even with results that are considered clinically to be good to excellent, continued long-term postoperative follow-up is advisable.
Cardiac operations involving the atria, such as closure of ASD, repair of total or partial anomalous pulmonary venous return, or venous switch corrections of complete transposition of the great arteries (the Mustard or Senning operations), may be followed years later by sinus node or atrioventricular node dysfunction and/or by atrial arrhythmias (especially atrial flutter). Intraventricular surgery may also result in electrophysiologic consequences, including complete heart block necessitating pacemaker insertion to avoid sudden death. Valvular problems may arise late after initial cardiac operation. An example is the progressive stenosis of an initially nonobstructive bicuspid aortic valve in the patient who underwent aortic coarctation repair. Such aortic valves may also be the site of infective endocarditis. After repair of the ostium primum ASD, the cleft mitral valve may become progressively regurgitant. Tricuspid regurgitation may also be progressive in the postoperative patient with tetralogy of Fallot if RV outflow tract obstruction was not relieved adequately at initial surgery. In many patients with surgically modified CHD, inadequate relief of an obstructive lesion, a residual regurgitant lesion, or a residual shunt will cause or hasten the onset of clinical signs and symptoms of myocardial dysfunction. Despite a good hemodynamic repair, many patients with a subaortic RV develop RV decompensation and signs of left heart failure. In many patients, particularly those who were cyanotic for many years before operation, a preexisting compromise in ventricular performance is due to the original underlying malformation.
A final category of postoperative problems involves the use of prosthetic valves, patches, or conduits in the operative repair. The special risks include infective endocarditis, thrombus formation, and premature degeneration and calcification of the prosthetic materials. There are many patients in whom extracardiac conduits are required to correct the circulation functionally and often to carry blood to the lungs from the RA or RV. These conduits may develop intraluminal obstruction, and if they include a prosthetic valve, it may show progressive calcification and thickening. Many such patients face reintervention (interventional cardiac catheterization or surgical reoperation) one or more times in their lives. Such care should be directed to centers specializing in adults with complex congenital cardiovascular malformations. The effect of pregnancy in postoperative patients depends on the outcome of the repair, including the presence and severity of residua, sequelae, or complications. Contraception is an important topic with such patients. Tubal ligation should be considered in those in whom pregnancy is strictly contraindicated.
Endocarditis Prophylaxis Two major predisposing causes of infective endocarditis are a susceptible cardiovascular substrate and a source of bacteremia. The clinical and bacteriologic profile of infective endocarditis in patients with CHD has changed with the advent of intracardiac surgery and of prosthetic devices. Prophylaxis includes both antimicrobial and hygienic measures. Meticulous dental and skin care are required. Routine antimicrobial prophylaxis is recommended for bacteremic dental procedures or instrumentation through an infected site in most patients with operated CHD, particularly if foreign material, such as a prosthetic valve, conduit, or surgically constructed shunt, is in place. In the case of patches, in the absence of a high-pressure patch leak, or transcatheter devices, prophylaxis is usually recommended for 6 months until there is endothelialization. Individuals with unrepaired cyanotic heart disease are also generally recommended to receive prophylaxis (Chap. 155).
283 |
Aortic Valve Disease |
GLOBAL BURDEN OF VALVULAR HEART DISEASE
![]() Primary valvular heart disease ranks well below coronary heart disease, stroke, hypertension, obesity, and diabetes as a major threat to the public health. Nevertheless, it is the source of significant morbidity and mortality rates. Rheumatic fever (Chap. 381) is the dominant cause of valvular heart disease in developing and low-income countries. Its prevalence has been estimated to range from as low as 1 per 100,000 school-age children in Costa Rica to as high as 150 per 100,000 in China. Rheumatic heart disease accounts for 12–65% of hospital admissions related to cardiovascular disease and 2–10% of hospital discharges in some developing countries. Prevalence and mortality rates vary among communities even within the same country as a function of overcrowding and the availability of medical resources and population-wide programs for detection and treatment of group A streptococcal pharyngitis. In economically deprived areas, tropical and subtropical climates (particularly on the Indian subcontinent), Central America, and the Middle East, rheumatic valvular disease progresses more rapidly than in more-developed nations and frequently causes serious symptoms in patients younger than 20 years of age. This accelerated natural history may be due to repeated infections with more virulent strains of rheumatogenic streptococci. Approximately 15 million to 20 million people live with rheumatic heart disease worldwide, an estimated prevalence characterized by 300,000 new cases and 233,000 case fatalities per year, with the highest mortality rates reported from Southeast Asia (~7.6 per 100,000).
Primary valvular heart disease ranks well below coronary heart disease, stroke, hypertension, obesity, and diabetes as a major threat to the public health. Nevertheless, it is the source of significant morbidity and mortality rates. Rheumatic fever (Chap. 381) is the dominant cause of valvular heart disease in developing and low-income countries. Its prevalence has been estimated to range from as low as 1 per 100,000 school-age children in Costa Rica to as high as 150 per 100,000 in China. Rheumatic heart disease accounts for 12–65% of hospital admissions related to cardiovascular disease and 2–10% of hospital discharges in some developing countries. Prevalence and mortality rates vary among communities even within the same country as a function of overcrowding and the availability of medical resources and population-wide programs for detection and treatment of group A streptococcal pharyngitis. In economically deprived areas, tropical and subtropical climates (particularly on the Indian subcontinent), Central America, and the Middle East, rheumatic valvular disease progresses more rapidly than in more-developed nations and frequently causes serious symptoms in patients younger than 20 years of age. This accelerated natural history may be due to repeated infections with more virulent strains of rheumatogenic streptococci. Approximately 15 million to 20 million people live with rheumatic heart disease worldwide, an estimated prevalence characterized by 300,000 new cases and 233,000 case fatalities per year, with the highest mortality rates reported from Southeast Asia (~7.6 per 100,000).
Although there have been recent reports of isolated outbreaks of streptococcal infection in North America, valve disease in high-income countries is dominated by degenerative or inflammatory processes that lead to valve thickening, calcification, and dysfunction. The prevalence of valvular heart disease increases with age for both men and women. Important left-sided valve disease may affect as many as 12–13% of adults older than the age of 75. In the United States, there were 85,000 hospital discharges with valvular heart disease in 2010, and the vast majority of these were related to surgical procedures for heart valve disease (mostly involving the aortic and mitral valves).
The incidence of infective endocarditis (Chap. 155) has increased with the aging of the population, the more widespread prevalence of vascular grafts and intracardiac devices, the emergence of more virulent multidrug-resistant microorganisms, and the growing epidemic of diabetes. The more restricted use of antibiotic prophylaxis since 2007 has thus far not been associated with an increase in incidence rates. Infective endocarditis has become a relatively more frequent cause of acute valvular regurgitation.
Bicuspid aortic valve disease affects as many as 0.5–1.4% of the general population, with an associated incidence of aortopathy involving root or ascending aortic aneurysm disease or coarctation. An increasing number of childhood survivors of congenital heart disease present later in life with valvular dysfunction. The global burden of valvular heart disease is expected to progress.
As is true for many other chronic health conditions, disparities in access to and quality of care for patients with valvular heart disease have been well documented. Management decisions and outcome differences based on age, gender, race, and geography require educational efforts across all levels of providers.
The role of the physical examination in the evaluation of patients with valvular heart disease is also considered in Chaps. 51e and 267; of electrocardiography (ECG) in Chap. 268; of echocardiography and other noninvasive imaging techniques in Chap. 270e; and of cardiac catheterization and angiography in Chap. 272.
AORTIC STENOSIS
Aortic stenosis (AS) occurs in about one-fourth of all patients with chronic valvular heart disease; approximately 80% of adult patients with symptomatic, valvular AS are male.
ETIOLOGY AND PATHOGENESIS
(Table 283-1) AS in adults is due to degenerative calcification of the aortic cusps and occurs most commonly on a substrate of congenital disease (bicuspid aortic valve), chronic (trileaflet) deterioration, or previous rheumatic inflammation. A pathologic study of specimens removed at the time of aortic valve replacement for AS showed that 53% were bicuspid and 4% unicuspid. The process of aortic valve deterioration and calcification is not a passive one, but rather one that shares many features with vascular atherosclerosis, including endothelial dysfunction, lipid accumulation, inflammatory cell activation, cytokine release, and upregulation of several signaling pathways (Fig. 283-1). Eventually, valvular myofibroblasts differentiate phenotypically into osteoblasts and actively produce bone matrix proteins that allow for the deposition of calcium hydroxyapatite crystals. Genetic polymorphisms involving the vitamin D receptor, the estrogen receptor in postmenopausal women, interleukin 10, and apolipoprotein E4 have been linked to the development of calcific AS, and a strong familial clustering of cases has been reported from western France. Several traditional atherosclerotic risk factors have also been associated with the development and progression of calcific AS, including low-density lipoprotein (LDL) cholesterol, lipoprotein a (Lp[a]), diabetes mellitus, smoking, chronic kidney disease, and the metabolic syndrome. The presence of aortic valve sclerosis (focal thickening and calcification of the leaflets not severe enough to cause obstruction) is associated with an excess risk of cardiovascular death and myocardial infarction (MI) among persons older than age 65. Approximately 30% of persons older than 65 years exhibit aortic valve sclerosis, whereas 2% exhibit frank stenosis.
|
MAJOR CAUSES OF AORTIC VALVE DISEASE |
FIGURE 283-1 Pathogenesis of calcific aortic stenosis. Inflammatory cells infiltrate across the endothelial barrier and release cytokines that act on fibroblasts to promote cellular proliferation and matrix remodeling. LDL is oxidatively modified and taken up by macrophage scavengers to become foam cells. Angiotensin-converting enzyme colocalizes with ApoB. A subset of myofibroblasts differentiates into an osteoblast phenotype capable of promoting bone formation. ACE, angiotensin-converting enzyme; ApoB, apolipoprotein B; LDL, low-density lipoprotein; IL, interleukin; MMP, matrix metalloproteinase; TGF, transforming growth factor. (From RV Freeman, CM Otto: Circulation 111:3316, 2005; with permission.)
Rheumatic disease of the aortic leaflets produces commissural fusion, sometimes resulting in a bicuspid-appearing valve. This condition, in turn, makes the leaflets more susceptible to trauma and ultimately leads to fibrosis, calcification, and further narrowing. By the time the obstruction to left ventricular (LV) outflow causes serious clinical disability, the valve is usually a rigid calcified mass, and careful examination may make it difficult or even impossible to determine the etiology of the underlying process. Rheumatic AS is almost always associated with involvement of the mitral valve and with aortic regurgitation. Mediastinal radiation can also result in late scarring, fibrosis, and calcification of the leaflets with AS.
BICUSPID AORTIC VALVE DISEASE
A bicuspid aortic valve (BAV) is the most common congenital heart valve defect and occurs in 0.5–1.4% of the population with a 2–4:1 male-to-female predominance. The inheritance pattern appears to be autosomal dominant with incomplete penetrance, although some have questioned an X-linked component as suggested by the prevalence of BAV disease among patients with Turner’s syndrome. The prevalence of BAV disease among first-degree relatives of an affected individual is approximately 10%. A single gene defect to explain the majority of cases has not been identified, although a mutation in the NOTCH1 gene has been described in some families. Abnormalities in endothelial nitric oxide synthase and NKX2.5 have been implicated as well. Medial degeneration with ascending aortic aneurysm formation occurs commonly among patients with BAV disease; aortic coarctation is less frequently encountered. Patients with BAV disease have larger aortas than patients with comparable tricuspid aortic valve disease. The aortopathy develops independent of the hemodynamic severity of the valve lesion and is a risk factor for aneurysm formation and/or dissection. A BAV can be a component of more complex congenital heart disease with or without other left heart obstructing lesions, as seen in Shone’s complex.
OTHER FORMS OF OBSTRUCTION TO LEFT VENTRICULAR OUTFLOW
In addition to valvular AS, three other lesions may be responsible for obstruction to LV outflow: hypertrophic obstructive cardiomyopathy (Chap. 287), discrete fibromuscular/membranous subaortic stenosis, and supravalvular AS (Chap. 282). The causes of LV outflow obstruction can be differentiated on the basis of the cardiac examination and Doppler echocardiographic findings.
PATHOPHYSIOLOGY
The obstruction to LV outflow produces a systolic pressure gradient between the LV and aorta. When severe obstruction is suddenly produced experimentally, the LV responds by dilation and reduction of stroke volume. However, in some patients, the obstruction may be present at birth and/or increase gradually over the course of many years, and LV contractile performance is maintained by the presence of concentric LV hypertrophy. Initially, this serves as an adaptive mechanism because it reduces toward normal the systolic stress developed by the myocardium, as predicted by the Laplace relation (S = Pr/h, where S = systolic wall stress, P = pressure, r = radius, and h = wall thickness). A large transaortic valve pressure gradient may exist for many years without a reduction in cardiac output (CO) or LV dilation; ultimately, however, excessive hypertrophy becomes maladaptive, LV systolic function declines because of afterload mismatch, abnormalities of diastolic function progress, and irreversible myocardial fibrosis develops.
A mean systolic pressure gradient >40 mmHg with a normal CO or an effective aortic orifice area of approximately <1 cm2 (or approximately <0.6 cm2/m2 body surface area in a normal-sized adult)—i.e., less than approximately one-third of the normal orifice area—is generally considered to represent severe obstruction to LV outflow. The elevated LV end-diastolic pressure observed in many patients with severe AS and preserved ejection fraction (EF) signifies the presence of diminished compliance of the hypertrophied LV. Although the CO at rest is within normal limits in most patients with severe AS, it usually fails to rise normally during exercise. Loss of an appropriately timed, vigorous atrial contraction, as occurs in atrial fibrillation (AF) or atrioventricular dissociation, may cause rapid progression of symptoms. Late in the course, contractile function deteriorates because of afterload excess, the CO and LV–aortic pressure gradient decline, and the mean left atrial (LA), pulmonary artery (PA), and right ventricular (RV) pressures rise. LV performance can be further compromised by superimposed coronary artery disease (CAD). Stroke volume (and thus CO) can also be reduced in patients with significant hypertrophy and a small LV cavity despite a normal EF. Low-flow, low-gradient AS (with either reduced or normal LV systolic function) is both a diagnostic and therapeutic challenge.
The hypertrophied LV causes an increase in myocardial oxygen requirements. In addition, even in the absence of obstructive CAD, coronary blood flow is impaired to the extent that ischemia can be precipitated under conditions of excess demand. Capillary density is reduced relative to wall thickness, compressive forces are increased, and the elevated LV end-diastolic pressure reduces the coronary driving pressure. The subendocardium is especially vulnerable to ischemia by this mechanism.
SYMPTOMS
AS is rarely of clinical importance until the valve orifice has narrowed to approximately 1 cm2. Even severe AS may exist for many years without producing any symptoms because of the ability of the hypertrophied LV to generate the elevated intraventricular pressures required to maintain a normal stroke volume. Once symptoms occur, valve replacement is indicated.
Most patients with pure or predominant AS have gradually increasing obstruction over years but do not become symptomatic until the sixth to eighth decades. Adult patients with BAV disease, however, develop significant valve dysfunction and symptoms one to two decades sooner. Exertional dyspnea, angina pectoris, and syncope are the three cardinal symptoms. Often, there is a history of insidious progression of fatigue and dyspnea associated with gradual curtailment of activities and reduced effort tolerance. Dyspnea results primarily from elevation of the pulmonary capillary pressure caused by elevations of LV diastolic pressures secondary to impaired relaxation and reduced LV compliance. Angina pectoris usually develops somewhat later and reflects an imbalance between the augmented myocardial oxygen requirements and reduced oxygen availability. CAD may or may not be present, although its coexistence is common among AS patients older than age 65. Exertional syncope may result from a decline in arterial pressure caused by vasodilation in the exercising muscles and inadequate vasoconstriction in nonexercising muscles in the face of a fixed CO, or from a sudden fall in CO produced by an arrhythmia.
Because the CO at rest is usually well maintained until late in the course, marked fatigability, weakness, peripheral cyanosis, cachexia, and other clinical manifestations of a low CO are usually not prominent until this stage is reached. Orthopnea, paroxysmal nocturnal dyspnea, and pulmonary edema, i.e., symptoms of LV failure, also occur only in the advanced stages of the disease. Severe pulmonary hypertension leading to RV failure and systemic venous hypertension, hepatomegaly, AF, and tricuspid regurgitation (TR) are usually late findings in patients with isolated severe AS.
When AS and mitral stenosis (MS) coexist, the reduction in flow (CO) induced by MS lowers the pressure gradient across the aortic valve and, thereby, masks many of the clinical findings produced by AS. The transaortic pressure gradient can be increased in patients with concomitant aortic regurgitation (AR) due to higher aortic valve flow rates.
PHYSICAL FINDINGS
The rhythm is generally regular until late in the course; at other times, AF should suggest the possibility of associated mitral valve disease. The systemic arterial pressure is usually within normal limits. In the late stages, however, when stroke volume declines, the systolic pressure may fall and the pulse pressure narrow. The carotid arterial pulse rises slowly to a delayed peak (pulsus parvus et tardus). A thrill or anacrotic “shudder” may be palpable over the carotid arteries, more commonly the left. In the elderly, the stiffening of the arterial wall may mask this important physical sign. In many patients, the a wave in the jugular venous pulse is accentuated. This results from the diminished distensibility of the RV cavity caused by the bulging, hypertrophied interventricular septum.
The LV impulse is sometimes displaced laterally in the later stages of the disease. A double apical impulse (with a palpable S4) may be recognized, particularly with the patient in the left lateral recumbent position. A systolic thrill may be present at the base of the heart to the right of the sternum when leaning forward or in the suprasternal notch.
Auscultation An early systolic ejection sound is frequently audible in children, adolescents, and young adults with congenital BAV disease. This sound usually disappears when the valve becomes calcified and rigid. As AS increases in severity, LV systole may become prolonged so that the aortic valve closure sound no longer precedes the pulmonic valve closure sound, and the two components may become synchronous, or aortic valve closure may even follow pulmonic valve closure, causing paradoxical splitting of S2 (Chap. 267). The sound of aortic valve closure can be heard most frequently in patients with AS who have pliable valves, and calcification diminishes the intensity of this sound. Frequently, an S4 is audible at the apex and reflects the presence of LV hypertrophy and an elevated LV end-diastolic pressure; an S3 generally occurs late in the course, when the LV dilates and its systolic function becomes severely compromised.
The murmur of AS is characteristically an ejection (mid) systolic murmur that commences shortly after the S1, increases in intensity to reach a peak toward the middle of ejection, and ends just before aortic valve closure. It is characteristically low-pitched, rough and rasping in character, and loudest at the base of the heart, most commonly in the second right intercostal space. It is transmitted upward along the carotid arteries. Occasionally it is transmitted downward and to the apex, where it may be confused with the systolic murmur of mitral regurgitation (MR) (Gallavardin effect). In almost all patients with severe obstruction and preserved CO, the murmur is at least grade III/VI. In patients with mild degrees of obstruction or in those with severe stenosis with heart failure and low CO in whom the stroke volume and, therefore, the transvalvular flow rate are reduced, the murmur may be relatively soft and brief.
LABORATORY EXAMINATION
ECG In most patients with severe AS, there is LV hypertrophy. In advanced cases, ST-segment depression and T-wave inversion (LV “strain”) in standard leads I and aVL and in the left precordial leads are evident. However, there is no close correlation between the ECG and the hemodynamic severity of obstruction, and the absence of ECG signs of LV hypertrophy does not exclude severe obstruction. Many patients with AS have systemic hypertension, which can also contribute to the development of hypertrophy.
Echocardiogram The key findings on TTE are thickening, calcification, and reduced systolic opening of the valve leaflets and LV hypertrophy. Eccentric closure of the aortic valve cusps is characteristic of congenitally bicuspid valves. TEE imaging can display the obstructed orifice extremely well, but it is not routinely required for accurate characterization of AS. The valve gradient and aortic valve area can be estimated by Doppler measurement of the transaortic velocity. Severe AS is defined by a valve area <1 cm2, whereas moderate AS is defined by a valve area of 1–1.5 cm2 and mild AS by a valve area of 1.5–2 cm2. Aortic valve sclerosis, conversely, is accompanied by a jet velocity of less than 2.5 meters/s (peak gradient <25 mmHg). LV dilation and reduced systolic shortening reflect impairment of LV function. There is increasing experience with the use of longitudinal strain and strain rate to characterize earlier changes in LV systolic function, well before a decline in EF can be appreciated. Doppler indices of impaired diastolic function are frequently seen.
Echocardiography is useful for identifying coexisting valvular abnormalities; for differentiating valvular AS from other forms of LV outflow obstruction; and for measurement of the aortic root and proximal ascending aortic dimensions. These aortic measurements are particularly important for patients with BAV disease. Dobutamine stress echocardiography is useful for the evaluation of patients with AS and severe LV systolic dysfunction (low-flow, low-gradient, severe AS with reduced EF), in whom the severity of the AS can often be difficult to judge. Patients with severe AS (i.e., valve area <1 cm2) with a relatively low mean gradient (<40 mmHg) despite a normal EF (low-flow, low-gradient, severe AS with normal EF) are often hypertensive, and efforts to control their systemic blood pressure should be optimized before Doppler echocardiography is repeated. The use of dobutamine stress echocardiography in this setting is under investigation. When there is continued uncertainty regarding the severity of AS in patients with reduced CO, quantitative analysis of the amount of aortic valve calcium with chest computed tomography (CT) may be helpful.
Chest X-Ray The chest x-ray may show no or little overall cardiac enlargement for many years. Hypertrophy without dilation may produce some rounding of the cardiac apex in the frontal projection and slight backward displacement in the lateral view. A dilated proximal ascending aorta may be seen along the upper right heart border in the frontal view. Aortic valve calcification may be discernible in the lateral view, but is usually readily apparent on fluoroscopic examination or by echocardiography; the absence of valvular calcification on fluoroscopy in an adult suggests that severe valvular AS is not present. In later stages of the disease, as the LV dilates, there is increasing roentgenographic evidence of LV enlargement, pulmonary congestion, and enlargement of the LA, PA, and right heart chambers.
Catheterization Right and left heart catheterization for invasive assessment of AS is performed infrequently but can be useful when there is a discrepancy between the clinical and noninvasive findings. Concern has been raised that attempts to cross the aortic valve for measurement of LV pressures are associated with a risk of cerebral embolization. Catheterization is also useful in three distinct categories of patients: (1) patients with multivalvular disease, in whom the role played by each valvular deformity should be defined to aid in the planning of operative treatment; (2) young, asymptomatic patients with noncalcific congenital AS, to define the severity of obstruction to LV outflow, because operation or percutaneous aortic balloon valvuloplasty (PABV) may be indicated in these patients if severe AS is present, even in the absence of symptoms; and (3) patients in whom it is suspected that the obstruction to LV outflow may not be at the level of the aortic valve but rather at the sub- or supravalvular level.
Coronary angiography is indicated to screen for CAD in appropriate patients with severe AS who are being considered for surgery. The incidence of significant CAD for which bypass grafting is indicated at the time of aortic valve replacement (AVR) exceeds 50% among adult patients.
NATURAL HISTORY
Death in patients with severe AS occurs most commonly in the seventh and eighth decades. Based on data obtained at postmortem examination in patients before surgical treatment became widely available, the average time to death after the onset of various symptoms was as follows: angina pectoris, 3 years; syncope, 3 years; dyspnea, 2 years; congestive heart failure, 1.5–2 years. Moreover, in >80% of patients who died with AS, symptoms had existed for <4 years. Among adults dying with valvular AS, sudden death, which presumably resulted from an arrhythmia, occurred in 10–20%; however, most sudden deaths occurred in patients who had previously been symptomatic. Sudden death as the first manifestation of severe AS is very uncommon (<1% per year) in asymptomatic adult patients. Calcific AS is a progressive disease, with an annual reduction in valve area averaging 0.1 cm2 and annual increases in the peak jet velocity and mean valve gradient averaging 0.3 meters/s and 7 mmHg, respectively (Table 283-2).
AORTIC REGURGITATION
ETIOLOGY
(Table 283-1) AR may be caused by primary valve disease or by primary aortic root disease.
Primary Valve Disease Rheumatic disease results in thickening, deformity, and shortening of the individual aortic valve cusps, changes that prevent their proper opening during systole and closure during diastole. A rheumatic origin is much less common in patients with isolated AR who do not have associated rheumatic mitral valve disease. Patients with congenital BAV disease may develop predominant AR, and approximately 20% of patients will require aortic valve surgery between 10 and 40 years of age. Congenital fenestrations of the aortic valve occasionally produce mild AR. Membranous subaortic stenosis often leads to thickening and scarring of the aortic valve leaflets with secondary AR. Prolapse of an aortic cusp, resulting in progressive chronic AR, occurs in approximately 15% of patients with ventricular septal defect (Chap. 282) but may also occur as an isolated phenomenon or as a consequence of myxomatous degeneration sometimes associated with mitral and/or tricuspid valve involvement.
AR may result from infective endocarditis, which can develop on a valve previously affected by rheumatic disease, a congenitally deformed valve, or on a normal aortic valve, and may lead to perforation or erosion of one or more leaflets. The aortic valve leaflets may become scarred and retracted during the course of syphilis or ankylosing spondylitis and contribute further to the AR that derives primarily from the associated root disease. Although traumatic rupture or avulsion of an aortic cusp is an uncommon cause of acute AR, it represents the most frequent serious lesion in patients surviving nonpenetrating cardiac injuries. The coexistence of hemodynamically significant AS with AR usually excludes all the rarer forms of AR because it occurs almost exclusively in patients with rheumatic or congenital AR. In patients with AR due to primary valvular disease, dilation of the aortic annulus may occur secondarily and lead to worsening regurgitation.
Primary Aortic Root Disease AR also may be due entirely to marked aortic annular dilation, i.e., aortic root disease, without primary involvement of the valve leaflets; widening of the aortic annulus and separation of the aortic leaflets are responsible for the AR (Chap. 301). Medial degeneration of the ascending aorta, which may or may not be associated with other manifestations of Marfan’s syndrome; idiopathic dilation of the aorta; annuloaortic ectasia; osteogenesis imperfecta; and severe, chronic hypertension may all widen the aortic annulus and lead to progressive AR. Occasionally AR is caused by retrograde dissection of the aorta involving the aortic annulus. Syphilis and ankylosing spondylitis, both of which may affect the aortic leaflets, may also be associated with cellular infiltration and scarring of the media of the thoracic aorta, leading to aortic dilation, aneurysm formation, and severe regurgitation. In syphilis of the aorta (Chap. 206), now a very rare condition, the involvement of the intima may narrow the coronary ostia, which in turn may be responsible for myocardial ischemia.
PATHOPHYSIOLOGY
The total stroke volume ejected by the LV (i.e., the sum of the effective forward stroke volume and the volume of blood that regurgitates back into the LV) is increased in patients with AR. In patients with severe AR, the volume of regurgitant flow may equal the effective forward stroke volume. In contrast to MR, in which a portion of the LV stroke volume is delivered into the low-pressure LA, in AR the entire LV stroke volume is ejected into a high-pressure zone, the aorta. An increase in the LV end-diastolic volume (increased preload) constitutes the major hemodynamic compensation for AR. The dilation and eccentric hypertrophy of the LV allow this chamber to eject a larger stroke volume without requiring any increase in the relative shortening of each myofibril. Therefore, severe AR may occur with a normal effective forward stroke volume and a normal LVEF (total [forward plus regurgitant] stroke volume/end-diastolic volume), together with an elevated LV end-diastolic pressure and volume. However, through the operation of Laplace’s law, LV dilation increases the LV systolic tension required to develop any given level of systolic pressure. Chronic AR is, thus, a state in which LV preload and afterload are both increased. Ultimately, these adaptive measures fail. As LV function deteriorates, the end-diastolic volume rises further and the forward stroke volume and EF decline. Deterioration of LV function often precedes the development of symptoms. Considerable thickening of the LV wall also occurs with chronic AR, and at autopsy, the hearts of these patients may be among the largest encountered, sometimes weighing >1000 g.
The reverse pressure gradient from aorta to LV, which drives the AR flow, falls progressively during diastole, accounting for the decrescendo nature of the diastolic murmur. Equilibration between aortic and LV pressures may occur toward the end of diastole in patients with chronic severe AR, particularly when the heart rate is slow. In patients with acute severe AR, the LV is unprepared for the regurgitant volume load. LV compliance is normal or reduced, and LV diastolic pressures rise rapidly, occasionally to levels >40 mmHg. The LV pressure may exceed the LA pressure toward the end of diastole, and this reversed pressure gradient closes the mitral valve prematurely.
In patients with chronic severe AR, the effective forward CO usually is normal or only slightly reduced at rest, but often it fails to rise normally during exertion. An early sign of LV dysfunction is a reduction in the EF. In advanced stages, there may be considerable elevation of the LA, PA wedge, PA, and RV pressures and lowering of the forward CO at rest.
Myocardial ischemia may occur in patients with AR because myocardial oxygen requirements are elevated by LV dilation, hypertrophy, and elevated LV systolic tension, and coronary blood flow may be compromised. A large fraction of coronary blood flow occurs during diastole, when arterial pressure is low, thereby reducing coronary perfusion or driving pressure. This combination of increased oxygen demand and reduced supply may cause myocardial ischemia, particularly of the subendocardium, even in the absence of epicardial CAD.
HISTORY
Approximately three-fourths of patients with pure or predominant valvular AR are men; women predominate among patients with primary valvular AR who have associated rheumatic mitral valve disease. A history compatible with infective endocarditis may sometimes be elicited from patients with rheumatic or congenital involvement of the aortic valve, and the infection often precipitates or seriously aggravates preexisting symptoms.
In patients with acute severe AR, as may occur in infective endocarditis, aortic dissection, or trauma, the LV cannot dilate sufficiently to maintain stroke volume, and LV diastolic pressure rises rapidly with associated marked elevations of LA and PA wedge pressures. Pulmonary edema and/or cardiogenic shock may develop rapidly.
Chronic severe AR may have a long latent period, and patients may remain relatively asymptomatic for as long as 10–15 years. However, uncomfortable awareness of the heartbeat, especially on lying down, may be an early complaint. Sinus tachycardia, during exertion or with emotion, or premature ventricular contractions may produce particularly uncomfortable palpitations as well as head pounding. These complaints may persist for many years before the development of exertional dyspnea, usually the first symptom of diminished cardiac reserve. The dyspnea is followed by orthopnea, paroxysmal nocturnal dyspnea, and excessive diaphoresis. Anginal chest pain even in the absence of CAD may occur in patients with severe AR, even in younger patients. Anginal pain may develop at rest as well as during exertion. Nocturnal angina may be a particularly troublesome symptom, and it may be accompanied by marked diaphoresis. The anginal episodes can be prolonged and often do not respond satisfactorily to sublingual nitroglycerin. Systemic fluid accumulation, including congestive hepatomegaly and ankle edema, may develop late in the course of the disease.
PHYSICAL FINDINGS
In chronic severe AR, the jarring of the entire body and the bobbing motion of the head with each systole can be appreciated, and the abrupt distention and collapse of the larger arteries are easily visible. The examination should be directed toward the detection of conditions predisposing to AR, such as bicuspid valve, endocarditis, Marfan’s syndrome, and ankylosing spondylitis.
Arterial Pulse A rapidly rising “water-hammer” pulse, which collapses suddenly as arterial pressure falls rapidly during late systole and diastole (Corrigan’s pulse), and capillary pulsations, an alternate flushing and paling of the skin at the root of the nail while pressure is applied to the tip of the nail (Quincke’s pulse), are characteristic of chronic severe AR. A booming “pistol-shot” sound can be heard over the femoral arteries (Traube’s sign), and a to-and-fro murmur (Duroziez’s sign) is audible if the femoral artery is lightly compressed with a stethoscope.
The arterial pulse pressure is widened as a result of both systolic hypertension and a lowering of the diastolic pressure. The measurement of arterial diastolic pressure with a sphygmomanometer may be complicated by the fact that systolic sounds are frequently heard with the cuff completely deflated. However, the level of cuff pressure at the time of muffling of the Korotkoff sounds (phase IV) generally corresponds fairly closely to the true intraarterial diastolic pressure. As the disease progresses and the LV end-diastolic pressure rises, the arterial diastolic pressure may actually rise as well, because the aortic diastolic pressure cannot fall below the LV end-diastolic pressure. For the same reason, acute severe AR may also be accompanied by only a slight widening of the pulse pressure. Such patients are invariably tachycardic as the heart rate increases in an attempt to preserve the CO.
Palpation In patients with chronic severe AR, the LV impulse is heaving and displaced laterally and inferiorly. The systolic expansion and diastolic retraction of the apex are prominent. A diastolic thrill may be palpable along the left sternal border in thin-chested individuals, and a prominent systolic thrill may be palpable in the suprasternal notch and transmitted upward along the carotid arteries. This systolic thrill and the accompanying murmur do not necessarily signify the coexistence of AS. In some patients with AR or with combined AS and AR, the carotid arterial pulse may be bisferiens, i.e., with two systolic waves separated by a trough (see Fig. 267-2D).
Auscultation In patients with severe AR, the aortic valve closure sound (A2) is usually absent. A systolic ejection sound is audible in patients with BAV disease, and occasionally an S4 also may be heard. The murmur of chronic AR is typically a high-pitched, blowing, decrescendo diastolic murmur, heard best in the third intercostal space along the left sternal border (see Fig. 267-5B). In patients with mild AR, this murmur is brief, but as the severity increases, it generally becomes louder and longer, indeed holodiastolic. When the murmur is soft, it can be heard best with the diaphragm of the stethoscope and with the patient sitting up, leaning forward, and with the breath held in forced expiration. In patients in whom the AR is caused by primary valvular disease, the diastolic murmur is usually louder along the left than the right sternal border. However, when the murmur is heard best along the right sternal border, it suggests that the AR is caused by aneurysmal dilation of the aortic root. “Cooing” or musical diastolic murmurs suggest eversion of an aortic cusp vibrating in the regurgitant stream.
A mid-systolic ejection murmur is frequently audible in isolated AR. It is generally heard best at the base of the heart and is transmitted along the carotid arteries. This murmur may be quite loud without signifying aortic obstruction. A third murmur sometimes heard in patients with severe AR is the Austin Flint murmur, a soft, low-pitched, rumbling mid-to-late diastolic murmur. It is probably produced by the diastolic displacement of the anterior leaflet of the mitral valve by the AR stream and is not associated with hemodynamically significant mitral obstruction. The auscultatory features of AR are intensified by strenuous and sustained handgrip, which augments systemic vascular resistance.
In acute severe AR, the elevation of LV end-diastolic pressure may lead to early closure of the mitral valve, a soft S1, a pulse pressure that is not particularly wide, and a soft, short, early diastolic murmur of AR.
LABORATORY EXAMINATION
ECG In patients with chronic severe AR, the ECG signs of LV hypertrophy become manifest (Chap. 268). In addition, these patients frequently exhibit ST-segment depression and T-wave inversion in leads I, aVL, V5, and V6 (“LV strain”). Left-axis deviation and/or QRS prolongation denote diffuse myocardial disease, generally associated with patchy fibrosis, and usually signify a poor prognosis.
Echocardiogram LV size is increased in chronic AR and systolic function is normal or even supernormal until myocardial contractility declines, as signaled by a decrease in EF or increase in the end-systolic dimension. A rapid, high-frequency diastolic fluttering of the anterior mitral leaflet produced by the impact of the regurgitant jet is a characteristic finding. The echocardiogram is also useful in determining the cause of AR, by detecting dilation of the aortic annulus and root, aortic dissection (see Fig. 270e-5), or primary leaflet pathology. With severe AR, the central jet width assessed by color flow Doppler imaging exceeds 65% of the LV outflow tract, the regurgitant volume is ≥60 mL/beat, the regurgitant fraction is ≥50%, and there is diastolic flow reversal in the proximal descending thoracic aorta. The continuous-wave Doppler profile of the AR jet shows a rapid deceleration time in patients with acute severe AR, due to the rapid increase in LV diastolic pressure. Surveillance transthoracic echocardiography forms the cornerstone of longitudinal follow-up and allows for the early detection of changes in LV size and/or function. For patients in whom transthoracic echocardiography (TTE) is limited by poor acoustical windows or inadequate semiquantitative assessment of LV function or the severity of the regurgitation, cardiac magnetic resonance imaging (MRI) can be performed. This modality also allows for accurate assessment of aortic size and contour. Transesophageal echocardiography (TEE) can also provide detailed anatomic assessment of the valve, root, and portions of the aorta.
Chest X-Ray In chronic severe AR, the apex is displaced downward and to the left in the frontal projection. In the left anterior oblique and lateral projections, the LV is displaced posteriorly and encroaches on the spine. When AR is caused by primary disease of the aortic root, aneurysmal dilation of the aorta may be noted, and the aorta may fill the retrosternal space in the lateral view. Echocardiography, cardiac MRI, and chest CT angiography are more sensitive than the chest x-ray for the detection of root and ascending aortic enlargement.
Cardiac Catheterization and Angiography When needed, right and left heart catheterization with contrast aortography can provide confirmation of the magnitude of regurgitation and the status of LV function. Coronary angiography is performed routinely in appropriate patients prior to surgery.
284 |
Mitral Valve Disease |
The role of the physical examination in the evaluation of patients with valvular heart disease is also considered in Chaps. 51e and 267; of electrocardiography (ECG) in Chap. 268; of echocardiography and other noninvasive imaging techniques in Chap. 270e; and of cardiac catheterization and angiography in Chap. 272.
MITRAL STENOSIS
ETIOLOGY AND PATHOLOGY
Rheumatic fever is the leading cause of mitral stenosis (MS) (Table 284-1). Other less common etiologies of obstruction to left ventricular inflow include congenital mitral valve stenosis, cor triatriatum, mitral annular calcification with extension onto the leaflets, systemic lupus erythematosus, rheumatoid arthritis, left atrial myxoma, and infective endocarditis with large vegetations. Pure or predominant MS occurs in approximately 40% of all patients with rheumatic heart disease and a history of rheumatic fever (Chap. 381). In other patients with rheumatic heart disease, lesser degrees of MS may accompany mitral regurgitation (MR) and aortic valve disease. With reductions in the incidence of acute rheumatic fever, particularly in temperate climates and developed countries, the incidence of MS has declined considerably over the past several decades. However, it remains a major problem in developing nations, especially in tropical and semitropical climates.
|
MAJOR CAUSES OF MITRAL VALVE DISEASE |
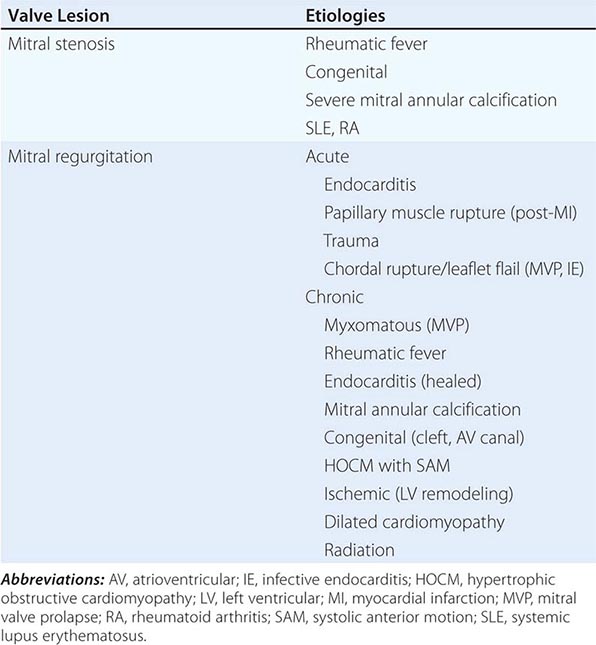
In rheumatic MS, chronic inflammation leads to diffuse thickening of the valve leaflets with formation of fibrous tissue and/or calcific deposits. The mitral commissures fuse, the chordae tendineae fuse and shorten, the valvular cusps become rigid, and these changes, in turn, lead to narrowing at the apex of the funnel-shaped (“fish-mouth”) valve. Although the initial insult to the mitral valve is rheumatic, later changes may be exacerbated by a nonspecific process resulting from trauma to the valve due to altered flow patterns. Calcification of the stenotic mitral valve immobilizes the leaflets and narrows the orifice further. Thrombus formation and arterial embolization may arise from the calcific valve itself, but in patients with atrial fibrillation (AF), thrombi arise more frequently from the dilated left atrium (LA), particularly from within the LA appendage.
PATHOPHYSIOLOGY
In normal adults, the area of the mitral valve orifice is 4–6 cm2. In the presence of significant obstruction, i.e., when the orifice area is reduced to < ~2 cm2, blood can flow from the LA to the left ventricle (LV) only if propelled by an abnormally elevated left atrioventricular pressure gradient, the hemodynamic hallmark of MS. When the mitral valve opening is reduced to <1.5 cm2, referred to as “severe” MS, an LA pressure of ~25 mmHg is required to maintain a normal cardiac output (CO). The elevated pulmonary venous and pulmonary arterial (PA) wedge pressures reduce pulmonary compliance, contributing to exertional dyspnea. The first bouts of dyspnea are usually precipitated by clinical events that increase the rate of blood flow across the mitral orifice, resulting in further elevation of the LA pressure (see below).
To assess the severity of obstruction hemodynamically, both the transvalvular pressure gradient and the flow rate must be measured (Chap. 272). The latter depends not only on the CO but on the heart rate, as well. An increase in heart rate shortens diastole proportionately more than systole and diminishes the time available for flow across the mitral valve. Therefore, at any given level of CO, tachycardia, including that associated with rapid AF, augments the transvalvular pressure gradient and elevates further the LA pressure. Similar considerations apply to the pathophysiology of tricuspid stenosis.
The LV diastolic pressure and ejection fraction (EF) are normal in isolated MS. In MS and sinus rhythm, the elevated LA and PA wedge pressures exhibit a prominent atrial contraction pattern (a wave) and a gradual pressure decline after the v wave and mitral valve opening (y descent). In severe MS and whenever pulmonary vascular resistance is significantly increased, the PA pressure (PAP) is elevated at rest and rises further during exercise, often causing secondary elevations of right ventricular (RV) end-diastolic pressure and volume.
Cardiac Output In patients with severe MS (mitral valve orifice 1–1.5 cm2), the CO is normal or almost so at rest, but rises subnormally during exertion. In patients with very severe MS (valve area <1 cm2), particularly those in whom pulmonary vascular resistance is markedly elevated, the CO is subnormal at rest and may fail to rise or may even decline during activity.
Pulmonary Hypertension The clinical and hemodynamic features of MS are influenced importantly by the level of the PAP. Pulmonary hypertension results from: (1) passive backward transmission of the elevated LA pressure; (2) pulmonary arteriolar constriction (the so-called “second stenosis”), which presumably is triggered by LA and pulmonary venous hypertension (reactive pulmonary hypertension); (3) interstitial edema in the walls of the small pulmonary vessels; and (4) at end stage, organic obliterative changes in the pulmonary vascular bed. Severe pulmonary hypertension results in RV enlargement, secondary tricuspid regurgitation (TR), and pulmonic regurgitation (PR), as well as right-sided heart failure.
SYMPTOMS
In temperate climates, the latent period between the initial attack of rheumatic carditis (in the increasingly rare circumstances in which a history of one can be elicited) and the development of symptoms due to MS is generally about two decades; most patients begin to experience disability in the fourth decade of life. Studies carried out before the development of mitral valvotomy revealed that once a patient with MS became seriously symptomatic, the disease progressed inexorably to death within 2–5 years.
In patients whose mitral orifices are large enough to accommodate a normal blood flow with only mild elevations of LA pressure, marked elevations of this pressure leading to dyspnea and cough may be precipitated by sudden changes in the heart rate, volume status, or CO, as, for example, with severe exertion, excitement, fever, severe anemia, paroxysmal AF and other tachycardias, sexual intercourse, pregnancy, and thyrotoxicosis. As MS progresses, lesser degrees of stress precipitate dyspnea, the patient becomes limited in daily activities, and orthopnea and paroxysmal nocturnal dyspnea develop. The development of persistent AF often marks a turning point in the patient’s course and is generally associated with acceleration of the rate at which symptoms progress. Hemoptysis (Chap. 48) results from rupture of pulmonary-bronchial venous connections secondary to pulmonary venous hypertension. It occurs most frequently in patients who have elevated LA pressures without markedly elevated pulmonary vascular resistances and is rarely fatal. Recurrent pulmonary emboli (Chap. 300), sometimes with infarction, are an important cause of morbidity and mortality late in the course of MS. Pulmonary infections, i.e., bronchitis, bronchopneumonia, and lobar pneumonia, commonly complicate untreated MS, especially during the winter months.
Pulmonary Changes In addition to the aforementioned changes in the pulmonary vascular bed, fibrous thickening of the walls of the alveoli and pulmonary capillaries occurs commonly in MS. The vital capacity, total lung capacity, maximal breathing capacity, and oxygen uptake per unit of ventilation are reduced (Chap. 306e). Pulmonary compliance falls further as pulmonary capillary pressure rises during exercise.
Thrombi and Emboli Thrombi may form in the left atria, particularly within the enlarged atrial appendages of patients with MS. Systemic embolization, the incidence of which is 10–20%, occurs more frequently in patients with AF, in patients >65 years of age, and in those with a reduced CO. However, systemic embolization may be the presenting feature in otherwise asymptomatic patients with only mild MS.
PHYSICAL FINDINGS
(See also Chaps. 51e and 267)
Inspection and Palpation In patients with severe MS, there may be a malar flush with pinched and blue facies. In patients with sinus rhythm and severe pulmonary hypertension or associated tricuspid stenosis (TS), the jugular venous pulse reveals prominent a waves due to vigorous right atrial systole. The systemic arterial pressure is usually normal or slightly low. An RV tap along the left sternal border signifies an enlarged RV. A diastolic thrill may rarely be present at the cardiac apex, with the patient in the left lateral recumbent position.
Auscultation The first heart sound (S1) is usually accentuated in the early stages of the disease and slightly delayed. The pulmonic component of the second heart sound (P2) also is often accentuated with elevated PA pressures, and the two components of the second heart sound (S2) are closely split. The opening snap (OS) of the mitral valve is most readily audible in expiration at, or just medial to, the cardiac apex. This sound generally follows the sound of aortic valve closure (A2) by 0.05–0.12 s. The time interval between A2 and OS varies inversely with the severity of the MS. The OS is followed by a low-pitched, rumbling, diastolic murmur, heard best at the apex with the patient in the left lateral recumbent position (see Fig. 267-5); it is accentuated by mild exercise (e.g., a few rapid sit-ups) carried out just before auscultation. In general, the duration of this murmur correlates with the severity of the stenosis in patients with preserved CO. In patients with sinus rhythm, the murmur often reappears or becomes louder during atrial systole (presystolic accentuation). Soft, grade I or II/VI systolic murmurs are commonly heard at the apex or along the left sternal border in patients with pure MS and do not necessarily signify the presence of MR. Hepatomegaly, ankle edema, ascites, and pleural effusion, particularly in the right pleural cavity, may occur in patients with MS and RV failure.
Associated Lesions With severe pulmonary hypertension, a pansystolic murmur produced by functional TR may be audible along the left sternal border. This murmur is usually louder during inspiration and diminishes during forced expiration (Carvallo’s sign). When the CO is markedly reduced in MS, the typical auscultatory findings, including the diastolic rumbling murmur, may not be detectable (silent MS), but they may reappear as compensation is restored. The Graham Steell murmur of PR, a high-pitched, diastolic, decrescendo blowing murmur along the left sternal border, results from dilation of the pulmonary valve ring and occurs in patients with mitral valve disease and severe pulmonary hypertension. This murmur may be indistinguishable from the more common murmur produced by aortic regurgitation (AR), although it may increase in intensity with inspiration and is accompanied by a loud and often palpable P2.
LABORATORY EXAMINATION
ECG In MS and sinus rhythm, the P wave usually suggests LA enlargement (see Fig. 268-8). It may become tall and peaked in lead II and upright in lead V1 when severe pulmonary hypertension or TS complicates MS and right atrial (RA) enlargement occurs. The QRS complex is usually normal. However, with severe pulmonary hypertension, right axis deviation and RV hypertrophy are often present.
Echocardiogram (See also Chap. 270e) Transthoracic echocardiography (TTE) with color flow and spectral Doppler imaging provides critical information, including measurements of mitral inflow velocity during early (E wave) and late (A wave in patients in sinus rhythm) diastolic filling, estimates of the transvalvular peak and mean gradients and of the mitral orifice area, the presence and severity of any associated MR, the extent of leaflet calcification and restriction, the degree of distortion of the subvalvular apparatus, and the anatomic suitability for percutaneous mitral balloon valvotomy (percutaneous mitral balloon valvuloplasty [PMBV]; see below). In addition, TTE provides an assessment of LV and RV function, chamber sizes, an estimation of the PAP based on the tricuspid regurgitant jet velocity, and an indication of the presence and severity of any associated valvular lesions, such as aortic stenosis and/or regurgitation. Transesophageal echocardiography (TEE) provides superior images and should be used when TTE is inadequate for guiding management decisions. TEE is especially indicated to exclude the presence of LA thrombus prior to PMBV. The performance of TTE with exercise to evaluate the mean mitral diastolic gradient and PA pressures can be very helpful in the evaluation of patients with MS when there is a discrepancy between the clinical findings and the resting hemodynamics.
Chest X-Ray The earliest changes are straightening of the upper left border of the cardiac silhouette, prominence of the main PAs, dilation of the upper lobe pulmonary veins, and posterior displacement of the esophagus by an enlarged LA. Kerley B lines are fine, dense, opaque, horizontal lines that are most prominent in the lower and midlung fields and that result from distention of interlobular septae and lymphatics with edema when the resting mean LA pressure exceeds approximately 20 mmHg.
DIFFERENTIAL DIAGNOSIS
Like MS, significant MR may also be associated with a prominent diastolic murmur at the apex due to increased antegrade transmitral flow, but in patients with isolated MR, this diastolic murmur commences slightly later than in patients with MS, and there is often clear-cut evidence of LV enlargement. An OS and increased P2 are absent, and S1 is soft or absent. An apical pansystolic murmur of at least grade III/VI intensity as well as an S3 suggest significant MR. Similarly, the apical mid-diastolic murmur associated with severe AR (Austin Flint murmur) may be mistaken for MS but can be differentiated from it because it is not intensified in presystole and becomes softer with administration of amyl nitrite or other arterial vasodilators. TS, which occurs rarely in the absence of MS, may mask many of the clinical features of MS or be clinically silent; when present, the diastolic murmur of TS increases with inspiration and the y descent in the jugular venous pulse is delayed.
Atrial septal defect (Chap. 282) may be mistaken for MS; in both conditions, there is often clinical, ECG, and chest x-ray evidence of RV enlargement and accentuation of pulmonary vascularity. However, the absence of LA enlargement and of Kerley B lines and the demonstration of fixed splitting of S2 with a grade II or III mid-systolic murmur at the mid to upper left sternal border all favor atrial septal defect over MS. Atrial septal defects with large left-to-right shunts may result in functional TS because of the enhanced diastolic flow.
Left atrial myxoma (Chap. 289e) may obstruct LA emptying, causing dyspnea, a diastolic murmur, and hemodynamic changes resembling those of MS. However, patients with an LA myxoma often have features suggestive of a systemic disease, such as weight loss, fever, anemia, systemic emboli, and elevated serum IgG and interleukin 6 (IL-6) concentrations. The auscultatory findings may change markedly with body position. The diagnosis can be established by the demonstration of a characteristic echo-producing mass in the LA with TTE.
CARDIAC CATHETERIZATION
Left and right heart catheterization can be useful when there is a discrepancy between the clinical and noninvasive findings, including those from TEE and exercise echocardiographic testing as appropriate. Catheterization is helpful in assessing associated lesions, such as aortic stenosis (AS) and AR. Catheterization and coronary angiography are not usually necessary to aid in decision-making about surgery in patients younger than 65 years of age with typical findings of severe mitral obstruction on physical examination and TTE. In men older than 40 years of age, women older than 45 years of age, and younger patients with coronary risk factors, especially those with positive noninvasive stress tests for myocardial ischemia, coronary angiography is advisable preoperatively to identify patients with critical coronary obstructions that should be bypassed at the time of operation. Computed tomographic coronary angiography (CTCA) (Chap. 270e) is now often used to screen preoperatively for the presence of coronary artery disease (CAD) in patients with valvular heart disease and low pretest likelihood of CAD. Catheterization and left ventriculography may be useful in patients who have undergone PMBV or previous mitral valve surgery for MS, and who have redeveloped limiting symptoms, especially if questions regarding the severity of the valve lesion(s) remain after noninvasive study.
|
TREATMENT |
MITRAL STENOSIS |
(Fig. 284-1) Penicillin prophylaxis of group A β-hemolytic streptococcal infections (Chap. 381) for secondary prevention of rheumatic fever is important for at-risk patients with rheumatic MS. Recommendations for infective endocarditis prophylaxis are similar to those for other valve lesions and are restricted to patients at high risk for complications from infection, including patients with a history of endocarditis. In symptomatic patients, some improvement usually occurs with restriction of sodium intake and small doses of oral diuretics. Beta blockers, nondihydropyridine calcium channel blockers (e.g., verapamil or diltiazem), and digitalis glycosides are useful in slowing the ventricular rate of patients with AF. Warfarin therapy targeted to an international normalized ratio (INR) of 2–3 should be administered indefinitely to patients with MS who have AF or a history of thromboembolism. The routine use of warfarin in patients in sinus rhythm with LA enlargement (maximal dimension >5.5 cm) with or without spontaneous echo contrast is more controversial. The novel oral anticoagulants are not approved for use in patients with significant valvular heart disease.
FIGURE 284-1 Management of rheumatic mitral stenosis. See legend for Fig. 283-2 for explanation of treatment recommendations (class I, IIa, IIb) and disease stages (C, D). Preoperative coronary angiography should be performed routinely as determined by age, symptoms, and coronary risk factors. Cardiac catheterization and angiography may also be helpful when there is a discrepancy between clinical and noninvasive findings. AF, atrial fibrillation; LA, left atrial; MR, mitral regurgitation; MS, mitral stenosis; MVA, mitral valve area; MVR, mitral valve surgery (repair or replacement); NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; PMBC, percutaneous mitral balloon commissurotomy; and T ½, pressure half-time. (Adapted from RA Nishimura et al: 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease. J Am Coll Cardiol doi: 10.1016/j.jacc.2014.02.536, 2014, with permission.)
If AF is of relatively recent onset in a patient whose MS is not severe enough to warrant PMBV or surgical commissurotomy, reversion to sinus rhythm pharmacologically or by means of electrical countershock is indicated. Usually, cardioversion should be undertaken after the patient has had at least 3 consecutive weeks of anticoagulant treatment to a therapeutic INR. If cardioversion is indicated more urgently, then intravenous heparin should be provided and TEE performed to exclude the presence of LA thrombus before the procedure. Conversion to sinus rhythm is rarely successful or sustained in patients with severe MS, particularly those in whom the LA is especially enlarged or in whom AF has been present for more than 1 year.
MITRAL VALVOTOMY
Unless there is a contraindication, mitral valvotomy is indicated in symptomatic (New York Heart Association [NYHA] Functional Class II–IV) patients with isolated severe MS, whose effective orifice (valve area) is < ~1 cm2/m2 body surface area, or <1.5 cm2 in normal-sized adults. Mitral valvotomy can be carried out by two techniques: PMBV and surgical valvotomy. In PMBV (Figs. 284-2 and 284-3), a catheter is directed into the LA after transseptal puncture, and a single balloon is directed across the valve and inflated in the valvular orifice. Ideal patients have relatively pliable leaflets with little or no commissural calcium. In addition, the subvalvular structures should not be significantly scarred or thickened, and there should be no LA thrombus. The short- and long-term results of this procedure in appropriate patients are similar to those of surgical valvotomy, but with less morbidity and a lower periprocedural mortality rate. Event-free survival in younger (<45 years) patients with pliable valves is excellent, with rates as high as 80–90% over 3–7 years. Therefore, PMBV has become the procedure of choice for such patients when it can be performed by a skilled operator in a high-volume center.
FIGURE 284-2 Inoue balloon technique for percutaneous mitral balloon valvotomy. A. After transseptal puncture, the deflated balloon catheter is advanced across the interatrial septum, then across the mitral valve and into the left ventricle. B–D. The balloon is inflated stepwise within the mitral orifice.
FIGURE 284-3 Simultaneous left atrial (LA) and left ventricular (LV) pressure before and after percutaneous mitral balloon valvuloplasty (PMBV) in a patient with severe mitral stenosis. ECG, electrocardiogram. (Courtesy of Raymond G. McKay, MD; with permission.)