11 Heart Failure Caused by Congenital Heart Disease
Basic Principles
Key Points
TABLE 11-1 ECHOCARDIOGRAPHIC INDICES OF VENTRICULAR PERFORMANCE
| Shortening fraction (SF) |  |
| Ejection fraction (EF) |  |
| Heart rate corrected mean velocity of circumferential fiber shortening (Vcfc) |  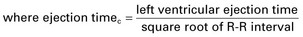 |
| End-systolic wall stress (ESWS) |  |
| Myocardial performance index (MPI) |  |
The Causes of Heart Failure in CHD
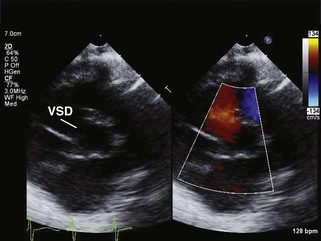
Left-to-Right Shunts
Key Points
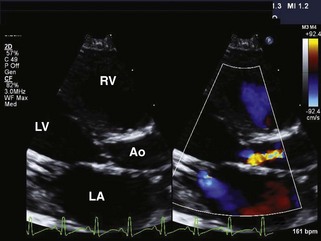
Left Heart Obstructive Lesions
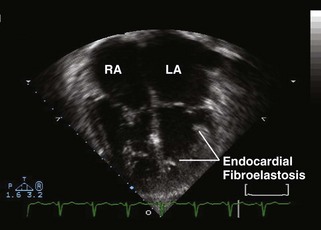
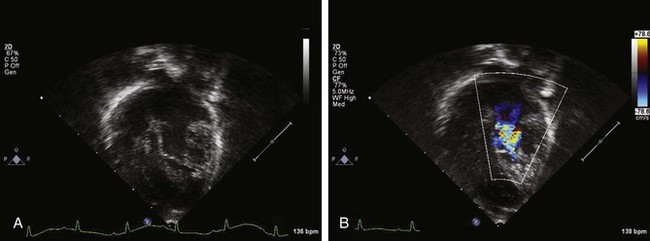
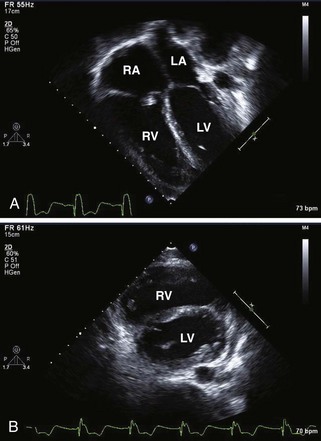
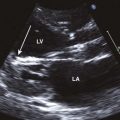
Figure 11-3 Apical 4-chamber view in the same patient as Figure 11-2 with critical aortic stenosis demonstrates a dilated but hypoplastic left ventricle (non–apex forming), with echo-bright papillary muscles consistent with endocardial fibroelastosis. Color Doppler demonstrates mitral regurgitation.
Key Points
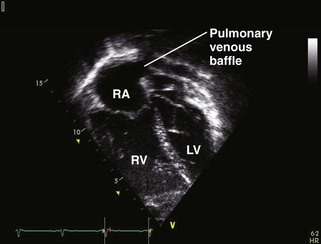
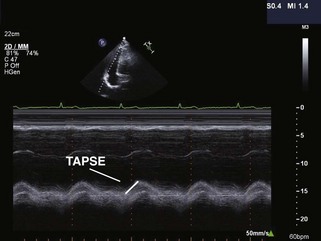
The Systemic Right Ventricle
Key Points
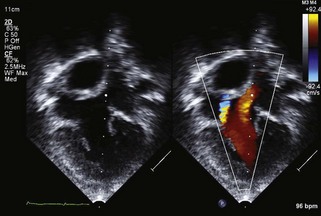
The Single Ventricle
Key Points
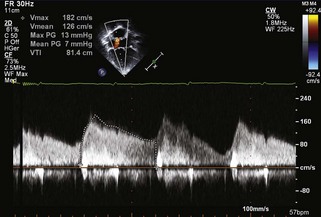
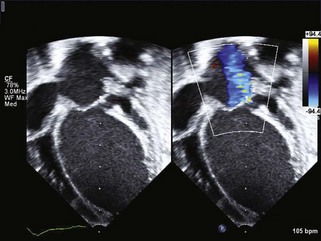
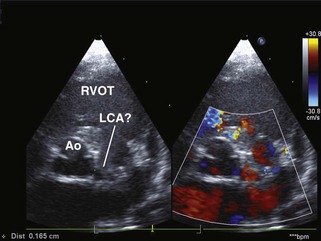
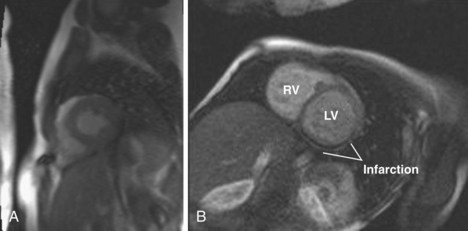
Coronary Insufficiency
Key Points
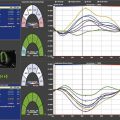
Left Ventricular Dysfunction after Biventricular Repair
Key Points
When Are Other Diagnostic Tests Needed?
1 McDaniel NL, Gutgesell HP. Ventricular septal defects. In: Allen HD, Driscoll DJ, Shaddy RE, Feltes TF, editors. Moss and Adams’ Heart Disease in Infants, Children and Adolescents including the Fetus and Young Adult. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008:667-682.
2 McElhinney DB, Lock JE, Keane JF, et al. Left heart growth, function, and reintervention after balloon aortic valvuloplasty for neonatal aortic stenosis. Circulation. 2005;111:451-458.
3 Lofland GK, McCrindle BW, Williams WG, et al. Critical aortic stenosis in the neonate: A multi-institutional study of management, outcomes, and risk factors. Congenital Heart Surgeons Society. J Thorac Cardiovasc Surg. 2001;121:10-27.
4 Hornung TS, Calder L. Congenitally corrected transposition of the great arteries. Heart. 2010;96:1154-1161.
5 Graham TPJr, Bernard YD, Mellen BG, et al. Long-term outcome in congenitally corrected transposition of the great arteries: A multi-institutional study. J Am Coll Cardiol. 2000;36:255-261.
6 Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838-847.
7 Eidem BW, O’Leary PW, Tei C, et al. Usefulness of the myocardial performance index for assessing right ventricular function in congenital heart disease. Am J Cardiol. 2000;86:654-658.
8 Gewillig M. The Fontan circulation. Heart. 2005;91:839-846.
9 Mahle WT, Coon PD, Wernovsky G, et al. Quantitative echocardiographic assessment of the performance of the functionally single right ventricle after the Fontan operation. Cardiol Young. 2001;11:399-406.
10 Rathod RH, Prakash A, Powell AJ, Geva T. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after Fontan operation. J Am Coll Cardiol. 2010;55:1721-1728.
11 Cohen MS, Herlong R, Silverman ND. Echocardiographic imaging of anomalous origin of the coronary arteries. Cardiol Young. 2010;20(suppl 3):26-34.
12 Pasquali SK, Hasselblad V, Li JS, et al. Coronary artery pattern and outcome of arterial switch operation for transposition of the great arteries: A meta-analysis. Circulation. 2002;106:2575-2580.
13 Cheung EWY, Liang X, Lam WWM, et al. Impact of right ventricular dilation on left ventricular myocardial deformation in patients after surgical repair of tetralogy of Fallot. Am J Cardiol. 2009;104:1264-1270.
14 Tzemos N, Harris L, Carasso S, et al. Adverse left ventricular mechanics in adults with repaired tetralogy of Fallot. Am J Cardiol. 2009;103:420-425.
15 Gillespie MJ, Marino BS, Cohen MS, et al. Systemic atrioventricular valve surgery in 116 pediatric patients: Risk factors for adverse outcomes. Cardiol Young. 2006;16(suppl 3):35-42.
16 Murakami T, Nakazawa M, Nakanishi T, et al. Prediction of postoperative left ventricular pump function in congenital mitral regurgitation. Pediatr Cardiol. 1999;20:418-421.