CHAPTER 56 Heart and great vessels
PERICARDIUM
FIBROUS AND SEROSAL PERICARDIUM
Serosal pericardium
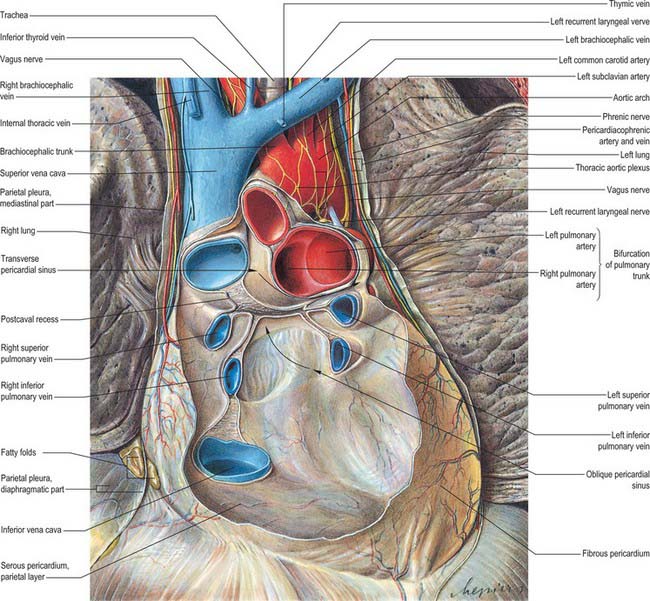
The serosal pericardium is a closed sac within the fibrous pericardium, and has a visceral and a parietal layer. The visceral layer, or epicardium, covers the heart and great vessels and is reflected into the parietal layer, which lines the internal surface of the fibrous pericardium. The reflections of the serosal layer are arranged as two complex ‘tubes’: the aorta and pulmonary trunk are enclosed in one, and the superior and inferior venae cavae and the four pulmonary veins in the other. The tube surrounding the veins has the shape of an inverted J. The cul-de-sac within its curve is behind the left atrium and is termed the oblique sinus. The transverse sinus is a passage between the two pericardial ‘tubes’ (Fig. 56.1). It has the aorta and pulmonary trunk in front and the atria and great veins behind (see Fig. 56.2B,D). The arrangement of the oblique and transverse sinuses, along with that of the main ‘principal’ cavity, is further affected by the development of complex three-dimensional pericardial recesses between adjacent structures. These recesses can be grouped according to the siting of their orifices or ‘mouths’. From the principal pericardial cavity, the postcaval recess projects towards the left behind the atrial termination of the superior vena cava. It is limited above by the right pulmonary artery and below by the upper right pulmonary vein. Its mouth opens superolaterally to the right. The right and left pulmonary venous recesses each project medially and upwards on the back of the left atrium between the superior and inferior pulmonary veins on each side, indenting the side walls of the oblique sinus. The superior aortic recess extends from the transverse sinus. From its mouth, located inferiorly, it ascends posterior to, then to the right of, the ascending aorta and ends at the level of the sternal angle. The inferior aortic recess, also extending from the transverse sinus, is a diverticulum descending from a superiorly located mouth to run between the lower ascending part of the aorta and the right atrium. The left pulmonary recess, with its mouth under the fold of the left vena cava, passes to the left between the inferior aspect of the left pulmonary artery and the upper border of the superior left pulmonary vein. The right pulmonary recess lies between the lower surface of the proximal part of the right pulmonary artery and the upper border of the left atrium.
INNERVATION
The pericardium is innervated by the vagus, together with phrenic nerves and the sympathetic trunks (see Fig. 56.20; see Fig. 58.3). Pericardial pain is typically a sharp severe substernal pain. It may be exacerbated by lying back or on the left side and relieved by leaning forward. It occasionally radiates to the upper border of trapezius.
HEART
The microstructure of cardiac muscle is described in detail in Chapter 6.
GENERAL ORGANIZATION
The heart is a pair of valved muscular pumps combined in a single organ (Fig. 56.2A–D). Although the fibromuscular framework and conduction tissues of these pumps are structurally interwoven, each pump (the so-called ‘right’ and ‘left’ hearts) is physiologically separate, and is interposed in series at different points in the double circulation. Despite this functional disposition in series, the two pumps are usually described topographically in parallel.
The left heart starts at the left atrium, which receives all the pulmonary inflow of oxygenated blood and some coronary venous inflow. It contracts to fill the left ventricle through the left atrioventricular orifice guarded by its mitral valve. The valve is the entry to the inlet of the left ventricle. Ventricular contraction rapidly increases the pressure in the apical trabecular component, closing the mitral valve and opening the aortic valve, enabling the ventricle to eject via the left ventricular outflow tract into the aortic sinuses and the ascending aorta, and thence to the entire systemic arterial tree, including the coronary arteries. This vast vascular bed presents a high peripheral resistance that, with large metabolic demands (especially the sustained requirements of the cerebral tissues), explains the more massive structural organization of the ‘left heart’. The ejection phase of the left ventricle is shorter than that of the right, but its fluctuations in pressure are very much greater. Because of its contrasting functional demands, the heart is far from a simple pair of (structurally combined) parallel pumps, even though the right and left ventricles must deliver more or less the same volume with each contraction. The heart has a complicated, spiral, three-dimensional organization which is markedly skewed when compared with the planes of the body. Terms such as ‘left’ and ‘right’, ‘anterior’ and ‘posterior’, ‘superior’ and ‘inferior’, therefore, do not always assist the descriptions of cardiac anatomy. Another potential source of confusion is the usual study of isolated whole or dissected hearts, with the subsequent difficulty in relating details to the heart as it is positioned within the body. The following preliminary description emphasizes such difficulties in order to circumvent certain misconceptions, before proceeding to an account of more detailed structure.
Cardiac size, shape and external features
The heart is a hollow, fibromuscular organ of a somewhat conical or pyramidal form, with a base, apex and a series of surfaces and ‘borders’. Enclosed in the pericardium, it occupies the middle mediastinum between the lungs and their pleural coverings (Fig. 56.1). It is placed obliquely behind the body of the sternum and the adjoining costal cartilages and ribs. Approximately one-third of the mass lies to the right of the midline.
An average adult heart is 12 cm from base to apex, 8–9 cm at its broadest transverse diameter and 6 cm anteroposteriorly. Its weight varies from 280 to 340 g (average 300 g) in males and from 230 to 280 g (average 250 g) in females. Cardiac weight is 0.45% of body weight in males and 0.40% in females. Adult weight is achieved between the ages of 17 and 20 years. The oblique position of the heart may be emphasized by comparing it to a rather deformed pyramid, with the base facing posteriorly and to the right, and the apex anteriorly and to the left. A line from the apex to the approximate centre of the base, projected posterolaterally, emerges near the right midscapular line. Some surfaces of the cardiac ‘pyramid’ are flat, others more or less convex, these aspects merging along rather ill-defined ‘borders’. Precise definition of surfaces and intervening ‘borders’ is, therefore, difficult. In the account that follows, official nomenclature (Terminologia Anatomica 1998) and more generally used terms from clinical practice are given as alternatives. The heart is described as having a base and apex, its surfaces being designated as sternocostal (anterior), diaphragmatic (inferior) and right and left (pulmonary). Its borders are termed upper, inferior (‘acute’ margin or border) and left (‘obtuse’ margin or border). Some name the right surface a ‘border’, despite its extent. One avoidable source of confusion is the use of ‘posterior’, which can be replaced with the unambiguous term ‘diaphragmatic’. If posterior is to be used for a cardiac surface, it should be reserved for the base. (However, compounding this difficulty, there are a number of different usages of the term ‘cardiac base’.)
The heart is placed obliquely in the thorax. The atrial and ventricular septal structures are virtually in line, but inclined forwards and to the left at 45° to a sagittal plane. The planes of the mitral and tricuspid valves, although vertical and not precisely co-planar, are broadly at right angles to the septal plane. The right atrium, therefore, is not only to the right, but also anterior and inferior to the left atrium. It is also partly anterior to the left ventricle, an important atrioventricular septum intervening. The right ventricle forms most of the anterior aspect of the ventricular mass (Fig. 56.3), only its inferior end is to the right of the left ventricle, its upper left extremity (pulmonary orifice) is to the left and superior relative to the aortic valve. The left atrium forms most of the posterior aspect of the heart, whereas the left ventricle is only prominent inferiorly, running along the left margin to reach the apex. The atria are essentially right of and posterior to their respective ventricles. These general dispositions are of the greatest importance in planning or interpreting radiographs, scans, angiocardiograms and echocardiograms.
Grooves on the cardiac surface
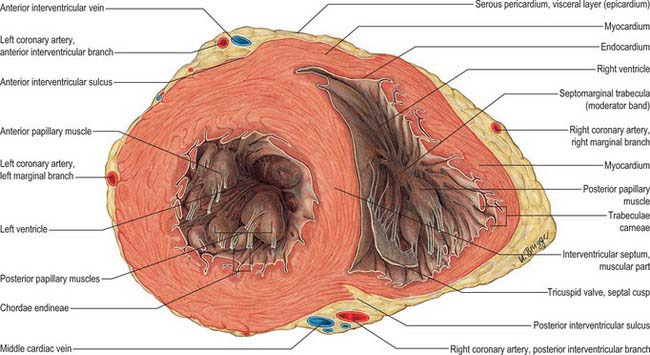
The division of the heart into four chambers produces boundaries that are visible externally as grooves (sulci). Some are deep and obvious and contain prominent structures. Others are less distinct, even barely perceptible, and are sometimes obscured, in part, by the major structures that cross them. The interatrial groove is a shallow groove separating the two atria. The lateral limits are defined by the borders of the atria. The atrioventricular (coronary) groove (or sulcus) separates the atria from the ventricles. This groove, containing the main trunks of the coronary arteries, is oblique. It descends to the right on the sternocostal surface, separating the right atrium (and its auricle or appendage) from the oblique right margin of the right ventricle and its infundibulum. Its upper left part is obliterated where it is crossed by the pulmonary trunk and, behind this, the aorta, from which the coronary arteries originate. Continuing to the left, the groove curves around the ‘obtuse’ margin and descends to the right, separating the atrial base from the diaphragmatic surface of the ventricles (Fig. 56.2A,D). This diaphragmatic part of the atrioventricular groove then curves around the ‘acute’ margin at its lower right end to become confluent with the sternocostal part. Thus the groove passes from high on the left to low on the right, with the diaphragmatic part being a little to the left of the sternocostal. A section that includes the atrioventricular groove is at 45° to the sagittal plane and at a greater but variable angle to the transverse and coronal planes. It approximately traverses the lines of attachment of the atrioventricular valves and (even less precisely) those of the aortic and pulmonary valves. A line at right angles to the centre of this plane will descend forwards and leftwards to the cardiac apex.
Cardiac base, apex, surfaces and borders
The true cardiac base is somewhat quadrilateral, with curved lateral extensions. It faces back and to the right, separated from the thoracic vertebrae (fifth to eighth in the recumbent, sixth to ninth in the erect posture) by the pericardium, right pulmonary veins, oesophagus and aorta. It is formed mainly by the left atrium, and only partly by the posterior part of the right atrium (Fig. 56.2B,D). It extends superiorly to the bifurcation of the pulmonary trunk and inferiorly to the posterior part of the atrioventricular groove, which contains the coronary sinus and branches of the coronary arteries. It is limited to the right and left by the rounded surfaces of the corresponding atria. These are separated by the shallow interatrial groove. The point of junction of the atrioventricular, interatrial and posterior interventricular grooves is termed the crux of the heart. Two pulmonary veins on each side open into the left atrial part of the base, whereas the superior and the inferior vena cava open into the upper and lower parts of the right atrial basal region. The area of the left atrium between the openings of right and left pulmonary veins forms the anterior wall of the oblique pericardial sinus (Fig. 56.1). This description of the anatomical base reflects the usual position of the heart in the thorax. Some confusion is produced by other current usages of the term ‘base’. It is often applied to the segment of the atrioventricular and ventriculoarterial junctions seen after dissections through the atrioventricular groove. This area is better termed the base of the ventricles. In clinical practice, auscultation in or near the parasternal parts of the second intercostal spaces is often described as occurring at the clinical ‘base’, to make the contrast with the clinical ‘apex’. Such descriptions, while less than perfect anatomically, will almost certainly persist.
Anterior, sternocostal surface of the heart
Facing forwards and upwards, the anterior surface has an acute right and a more gradual left convexity (Fig. 56.3). It consists of an atrial area above and to the right, and a ventricular part below and to the left of the atrioventricular groove. The atrial area is occupied almost entirely by the right atrium. The left atrium is largely hidden by the ascending aorta and pulmonary trunk. Only a small part of the left appendage projects forwards to the left of the pulmonary trunk. Of the ventricular region, about one-third is made up by the left and two-thirds by the right ventricle. The site of the septum between them is indicated by the anterior interventricular groove. The sternocostal surface is separated by the pericardium from the body of the sternum, the sternocostal muscles and the third to the sixth costal cartilages. Because of the bulge of the heart to the left, more of this surface is behind the left costal cartilages than behind the right ones. It is also covered by the pleural membranes and by the thin anterior edges of the lungs, except for a triangular area at the cardiac incisure of the left lung. The lungs and their pleural coverings are variable in their degree of overlap of the heart.
Inferior, diaphragmatic surface of the heart
Largely horizontal, the inferior surface of the heart slopes down and forwards a little towards the apex (Fig. 56.2B,D). It is formed by the ventricles (chiefly the left) and rests mainly upon the central tendon but also, apically, on a small area of the left muscular part of the diaphragm. It is separated from the anatomical base by the atrioventricular groove and is traversed obliquely by the posterior interventricular groove.
This is atrial (mainly the left atrium). Anterior to it are the ascending aorta and the pulmonary trunk (Fig. 56.1). At its extremity, the superior vena cava enters the right atrium.
Right atrium
General and external features
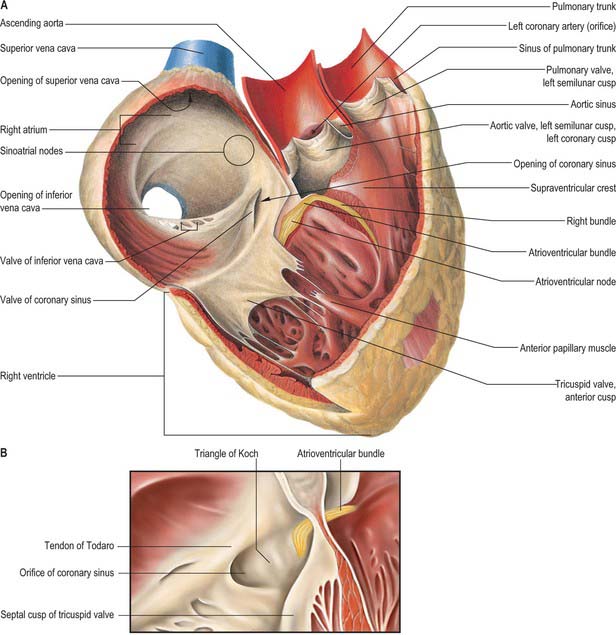
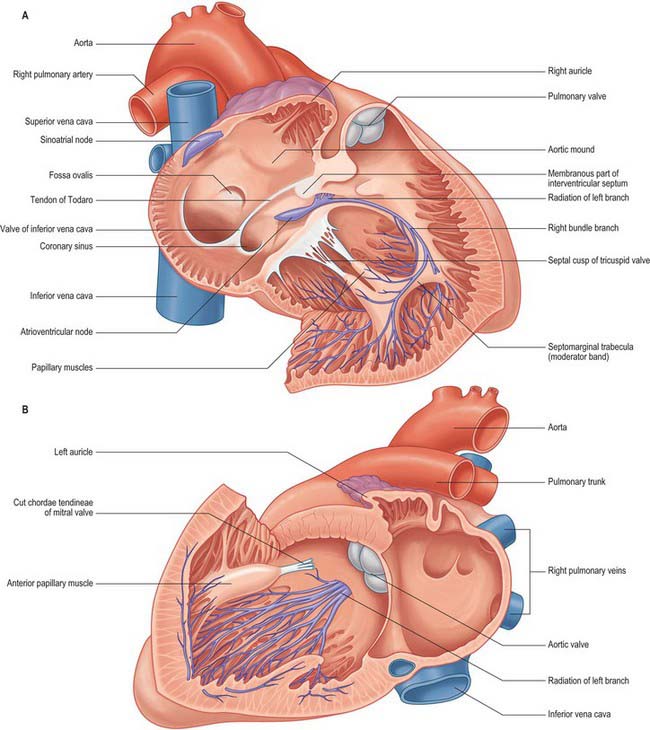
The interatrial septum (or atrial septum) is oblique, so the right atrium is both anterior and to the right of the left atrium (Fig. 56.2A–D), also extending inferior to it. Its walls form the right upper sternocostal surface, the convex right (pulmonary surface) and a little of the right side of the anatomical base. The superior vena cava opens into its dome and the inferior vena cava into its lower posterior part (Fig. 56.2B,D). An extensive muscular pouch, the auricle, projects anteriorly to overlap the right side of the ascending aorta. The auricle is a broad, triangular structure and has a wide junction with the true atrial component of the atrium (Fig. 56.2A,C). The junction between the venous part (sinus venosus) and the atrium proper is marked externally by a shallow groove, the sulcus terminalis, extending between the right sides of the openings of the two venae cavae. The sulcus terminalis corresponds, internally, to the terminal crest (crista terminalis) which is the site of origin of the extensive pectinate muscles that arise serially at right angles from the crest. Posteriorly, the vertical interatrial groove descends to the crux.
Interior surface
The interior surface of the right atrium can be divided into three regions: a smooth-walled venous component posteriorly that leads, anteriorly, to the vestibule of the tricuspid valve and the auricle (Fig. 56.4A,B). The wall of the vestibule is smooth, but its junction with the auricle is ridged all around the atrioventricular junction. The smooth-walled part receives the opening of the venae cavae and the coronary sinus. It represents the venous component (sinus venosus) of the developing heart. The wall of the vestibule has a ridged surface and that of the auricle is trabeculated. Both are derived from the embryonic atrium proper.
The superior and inferior venae cavae open into the venous component. The superior vena cava returns blood from head, neck and upper limb through an orifice that faces inferoanteriorly and has no valve, and also receives blood from the chest wall and the oesophagus via the azygos system. The inferior vena cava is larger than its superior counterpart: it drains blood from all structures below and including the diaphragm into the lowest part of the atrium near the septum. Anterior to its orifice is a flap-like valve, the Eustachian valve or valve of the inferior vena cava (Fig. 56.4A,B). Of varying size, this valve is found along the lateral, or right, margin of the vein. When traced inferiorly, it runs into the sinus septum (see below), where it is contiguous with the valve of the coronary sinus. The lateral part of the Eustachian valve becomes continuous with the lower end of the terminal crest. The valve is a fold of endocardium enclosing a few muscular fibres. It is large during fetal life, when it serves to direct richly oxygenated blood from the placenta through the foramen ovale of the atrial septum into the left atrium. The valve varies markedly in size in postnatal life; it is sometimes cribriform or filamentous but often is absent. A particularly prominent recess, behind the Eustachian valve, is seen posteroinferiorly relative to the ostium of the coronary sinus.
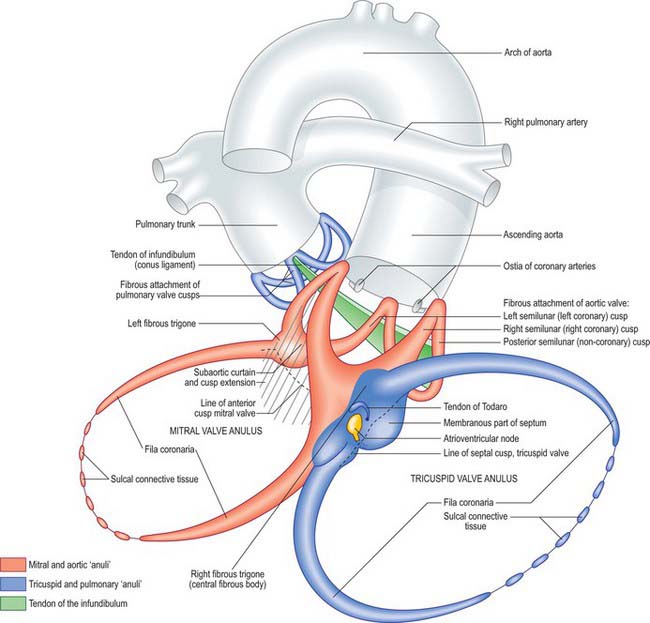
The coronary sinus opens into the venous atrial component between the orifice of the inferior vena cava, the fossa ovale and the vestibule of the atrioventricular opening (Fig. 56.4A,B). The coronary sinus is often guarded by a thin, semicircular valve that covers the lower part of the orifice (Thebesius’ valve, also known as the Thebesian valve). The upper limb of this valve joins the Eustachian valve; a tendinous structure, the tendon of Todaro, runs from this commissure into the sinus septum, which is the septum between the coronary sinus and the fossa ovale. The tendon of Todaro runs forwards to insert into the central fibrous body and is one of the landmarks of the triangle of Koch (see below). The ostium of the coronary sinus forms a prominent landmark in the right atrium. The sinus itself lies within the left atrioventricular groove (Fig. 56.2B,D), and is the conduit for return of most of the venous blood from the heart, although some atrial veins drain directly to the right or left atrial chambers. The coronary sinus begins at the point where the oblique vein of the left atrium joins the great cardiac vein. The sinus receives the middle and small cardiac veins close to its junction with the right atrium.
The atrium proper and the auricle are separated from the venous sinus by the crista terminalis. This smooth, muscular ridge begins on the upper part of the septal surface and, passing anterior to the orifice of the superior vena cava, skirts its right margin to reach the right side of the orifice of the inferior vena cava. It marks the site of the right venous valve of the embryonic heart, and corresponds externally to the terminal groove. The sinu-atrial node is located within the superior part of the groove, lateral to and extending below the orifice of the superior vena cava (Fig. 56.4A,B).
Anteroinferior in the right atrium is the large, oval vestibule leading to the orifice of the tricuspid valve. A triangular zone, the triangle of Koch, is defined between the attachment of the septal cusp of the tricuspid valve, the anteromedial margin of the ostium of the coronary sinus, and the round, collagenous, palpable, subendocardial tendon of Todaro (Fig. 56.4A,B). The triangle is a landmark of particular surgical importance, indicating the site of the atrioventricular node and its atrial connections. Anterosuperior to the insertion of the tendon of Todaro, the septal wall is formed by the atrioventricular component of the membranous septum, intervening between the right atrium and subaortic outlet of the left ventricle (Fig. 56.5). The atrial wall bulges anterosuperiorly above the membranous septum. This area is the aortic mound (torus aorticus) and marks the location of the non-coronary sinus of the aorta with its enclosed valvular cusp.
Right ventricle
External features
The convex anterosuperior surface of the right ventricle makes up a large part of the sternocostal aspect of the heart, and is separated from the thoracic wall only by the pericardium (Fig. 56.2A–D). The left pleura and, to a lesser extent, the anterior margin of the left lung are interposed above and to the left. The inferior surface is flat and is related mainly, with the interposition of the pericardium, to the central tendon and a small adjoining muscular part of the diaphragm. The left and posterior wall is the ventricular septum. This is slightly curved and bulges into the right ventricle so that, in sections across the cardiac axis, the outline of the right ventricle is crescentic. A delicate collagenous band, the tendon of the infundibulum (conus ligament), is believed by some to connect the pulmonary muscular infundibulum posteriorly to the root of the aorta. The wall of the right ventricle is significantly thinner (3–5 mm on average) than that of the left, the ratio of the thickness of the two walls usually being 1 : 3.
Internal features
The inlet and outlet components of the ventricle, supporting and surrounding the cusps of the tricuspid and pulmonary valves respectively, are separated in the roof of the ventricle by the prominent supraventricular crest (crista supraventricularis) (Fig. 56.4A). The crest is a thick, muscular, highly arched structure, extending obliquely forwards and to the right from a septal limb high on the interventricular septal wall to a mural or parietal limb on the anterolateral right ventricular wall. The posterolateral aspect of the crest provides a principal attachment for the anterosuperior cusp of the tricuspid valve. The septal limb of the crest may be continuous with, or embraced by, the septal limbs of the septomarginal trabecula. The inlet and outlet regions extend apically into and from the prominent coarsely trabeculated component of the ventricle. The inlet component is itself also trabeculated, whereas the outlet component (or infundibulum) has predominantly smooth walls. The trabeculated appearance is caused by a myriad of irregular muscular ridges and protrusions, which are known collectively as trabeculae carneae, and are lined by endocardium. These protrusions and intervening grooves impart great variation in wall thickness; the protrusions vary in extent from mere ridges to trabeculae, which are fixed at both ends but otherwise free. Other conspicuous protrusions are the papillary muscles, which are inserted at one end onto the ventricular wall and are continuous at the other end with collagenous cords, the chordae tendineae, inserted on the free edge and elsewhere on the free aspect of the atrioventricular valves. One protrusion in the right ventricle, the septomarginal trabecula or septal band, is particularly prominent. It reinforces the septal surface where, at the base, it divides into limbs that embrace the supraventricular crest. Towards the apex, it supports the anterior papillary muscle of the tricuspid valve and, from this point, crosses to the parietal wall of the ventricle as the ‘moderator band’ (this alternative name records an old idea that the septomarginal trabecula prevents overdistension of the ventricle). A further series of prominent trabeculae, the septoparietal trabeculations, extend from its anterior surface and run onto the parietal ventricular wall. The smooth-walled outflow tract, or infundibulum (conus arteriosus), ascends to the left above the septoparietal trabeculations and below the arch of the supraventricular crest to the pulmonary orifice.
Tricuspid valve
Tricuspid valvular orifice
The tricuspid valve orifice is best seen from the atrial aspect and measures on average 11.4 cm in circumference in males and 10.8 cm in females. It has a clear line of transition from the atrial wall or septum to the lines of attachment of the valvular cusps. Its margins are not precisely in a single plane. It is almost vertical, but at 45° to the sagittal plane and slightly inclined to the vertical, such that it ‘faces’ (on its ventricular aspect) anterolaterally to the left and somewhat inferiorly (Fig. 56.6). Roughly triangular, its margins are described as anterosuperior, inferior and septal, corresponding to the lines of attachment of the valvular cusps.
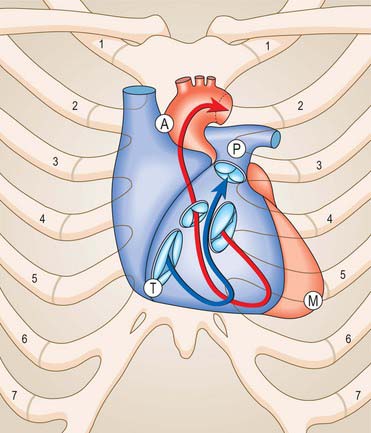
Fig. 56.6 Relation of the sternocostal surface and valves of the heart to the thoracic cage. The right heart is blue, the arrow denotes the inflow and outflow channels of the right ventricle; the left heart is treated similarly in red. The positions, planes and relative sizes of the cardiac valves are shown. The position of the letters A, P, T and M indicate respectively the aortic, pulmonary, tricuspid and mitral auscultation areas of clinical practice. Note that, for the purpose of illustration, the orifices of the aortic, mitral and tricuspid valves are shown with some separation between them. In reality, the cusps of the three valves are in fibrous continuity (see Fig. 56.10).
Tricuspid valve cusps
The anterosuperior cusp is the largest component of the tricuspid valve. It is attached chiefly to the atrioventricular junction on the posterolateral aspect of the supraventricular crest, but extends along its septal limb to the membranous septum, ending at the anteroseptal commissure. One or more notches often indent its free margin. The attachment of the septal cusp passes from the inferoseptal commissure on the posterior ventricular wall across the muscular septum and then angles across the membranous septum to the anteroseptal commissure. The septal cusp defines one of the borders of the triangle of Koch, thereby aiding location of the atrioventricular node, which lies at the apex of the triangle, and ensuring that this area can be avoided when operating on the tricuspid valve (Figs 56.4A,B; 56.5).
Chordae tendineae (tendinous cords)
The chordae tendineae are fibrous collagenous structures supporting the cusps of the atrioventricular valves. False chordae connect papillary muscles to each other or to the ventricular wall including the septum, or pass directly between points on the wall (or septum, or both); they are irregular in numbers and dimensions in the right ventricle. The true chordae usually arise from small projections on the tips or margins of the apical one-thirds of papillary muscles, but sometimes arise from the bases of papillary muscles or directly from the ventricular walls and the septum. They are attached to various parts of the ventricular aspects or the free margins of the cusps. They have been classified into first-, second- and third-order chordae according to the distance of the attachment from the margins of the cusps; this scheme has little functional or morphological merit.
Opening of the tricuspid valve
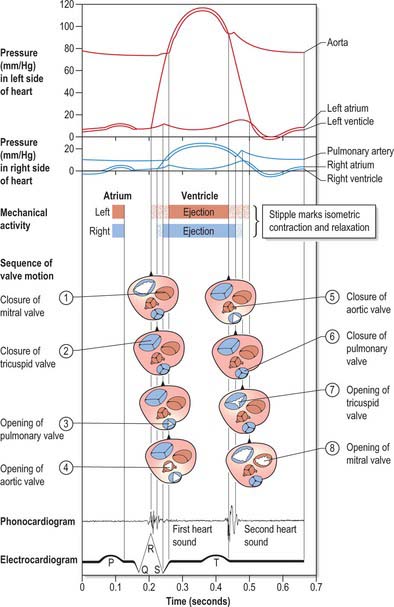
Despite its name, the tricuspid valve acts more like a bicuspid valve, because the septal cusp, the smallest of the three cusps, is fixed between the right and left fibrous trigones and the atrial and ventricular septa. The remainder of the tricuspid anulus is muscular. During diastole the right ventricle relaxes, the anulus dilates and the large anterior and posterior cusps move away from the plane of the anulus into the right ventricle. During systole the anulus constricts as the right ventricle contracts, and the two major cusps move like sails to abut a relatively immobile septal cusp and the septum itself (Fig. 56.7).
Pulmonary valve
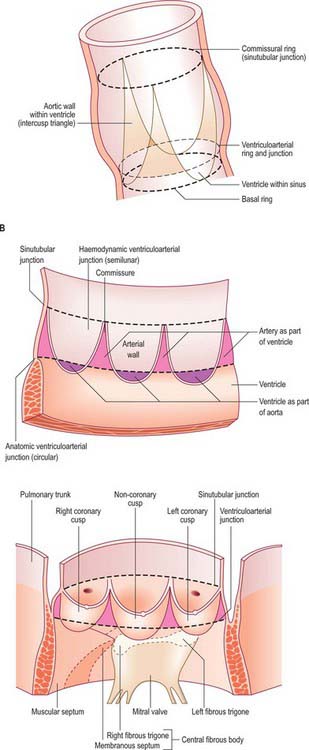
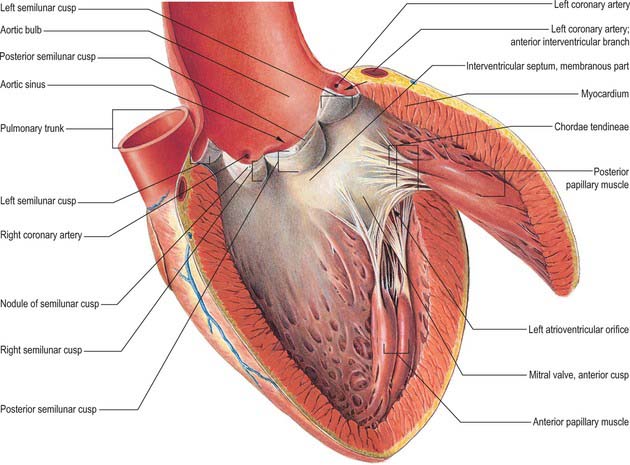
The pulmonary valve, guarding the outflow from the right ventricle, surmounts the infundibulum and is situated at some distance from the other three cardiac valves (Figs 56.8, 56.9, 56.10). Its general plane faces superiorly to the left and slightly posteriorly. It has three semilunar cusps attached by convex edges partly to the infundibular wall of the right ventricle and partly to the origin of the pulmonary trunk. The line of attachments is curved, rising at the periphery of each cusp near their zones of apposition (the commissures) and reaching the sinutubular ridge of the pulmonary trunk (Fig. 56.11A–C). Removal of the cusps shows that the fibrous semilunar attachments enclose three crescents of infundibular musculature within the pulmonary sinuses, whereas three roughly triangular segments of arterial wall are incorporated within the ventricular outflow tract beneath the apex of each commissural attachment. There is, thus, no proper circular ‘anulus’ supporting the cusps of the valve, and the fibrous semilunar attachment is an essential requisite for snug closure of the nodules and lunules of the cusps (see below) during ventricular diastole. It is difficult to name the cusps and corresponding sinuses of the pulmonary valve and trunk precisely according to the coordinates of the body, because the valvular orifice is obliquely positioned. The official nomenclature (Terminologia Anatomica 1998) refers to an anterior, a posterior and a septal cusp, based on their position in the fetus. The position changes with development and in the adult there is one anterior semilunar cusp, and right and left semilunar cusps.
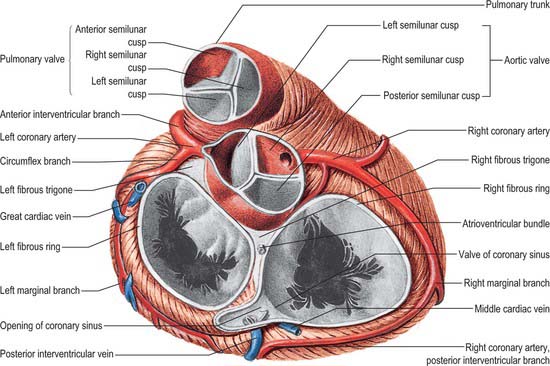
Fig. 56.9 The base of the ventricles, after removal of the atria and the pericardium. Coronary arteries and cardiac veins can be seen. Contrast the planes and positions of the aortic and pulmonary valves. Contrast with Fig. 56.10.
(From Sobotta 2006.)
Opening of the pulmonary valve
During diastole, the pulmonary valve is closed and all three cusps of the valve are tightly apposed. The pulmonary valve is difficult to visualize at echocardiography and usually only the posterior cusp is visible when the valve is closed; atrial systole may cause a slight posterior movement of the valve cusps. The pulmonary valve opens passively during ventricular systole and then closes rapidly at the end of systole (Fig. 56.7).
Left atrium
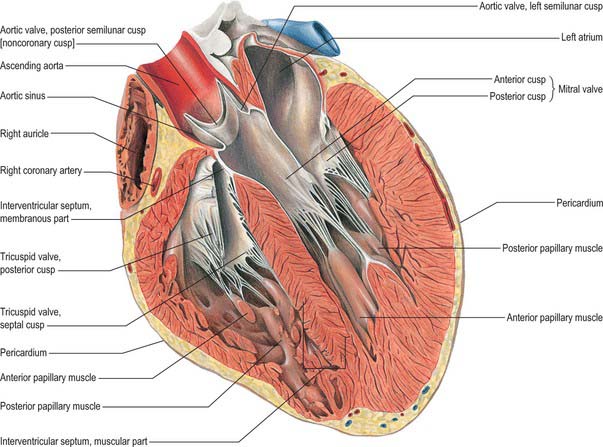
Although smaller in volume than the right, the left atrium has thicker walls (3 mm on average). Its cavity and walls are formed largely by the proximal parts of the pulmonary veins, which are incorporated into the atrium during development. The only clear derivative of the left part of the embryonic atrium is the auricle, together with the vestibule of the mitral valve. The left atrium is roughly cuboidal and extends behind the right atrium, separated from it by the obliquely positioned septum, thus the right atrium is in front and anterolateral to the right part of the left atrium. The left part is concealed anteriorly by the initial segments of the pulmonary trunk and aorta: part of the transverse pericardial sinus lies between it and these arterial trunks. Anteroinferiorly, and to the left, it adjoins the base of the left ventricle at the orifice of the mitral valve. Its posterior aspect forms most of the anatomical base of the heart and is approximately quadrangular, receiving the terminations of (usually) two pulmonary veins from each lung. It forms the anterior wall of the oblique pericardial sinus (Fig. 56.1). This surface ends at the shallow vertical interatrial groove, which descends to the cardiac crux. The left atrial auricle is constricted at its atrial junction and all the pectinate muscles of the left atrium are contained within it. It is characteristically longer, narrower and more hooked than the right auricle, its margins being more deeply indented. It turns forwards to the left of the pulmonary trunk, overlapping its origin (Fig. 56.8). Interiorly, the four pulmonary veins open into the upper posterolateral surfaces of the left atrium, two on each side. Their orifices are smooth and oval, the left pair frequently opening via a common channel. Some minimal cardiac veins return blood directly from the myocardium to the cavity of the left atrium. The left atrial aspect of the septum has a characteristically rough appearance, bounded by a crescentic ridge, concave upwards, which marks the site of the foramen ovale.
Left ventricle
General and external features
The left ventricle is constructed in accordance with its role as a powerful pump that sustains pulsatile flow in the high-pressured systemic arteries. Variously described as half-ellipsoid or cone-shaped, it is longer and narrower than the right ventricle, extending from its base in the plane of the atrioventricular groove to the cardiac apex. Its long axis descends forwards and to the left. In transverse section, at right angles to the axis, its cavity is oval or nearly circular, with walls about three times thicker (8–12 mm) than those of the right ventricle (Fig. 56.12). It forms part of the sternocostal, left and inferior (diaphragmatic) cardiac surfaces. Except where obscured by the aorta and pulmonary trunk, the base of the ventricular cone is superficially separated from the left atrium and atrial auricle by part of the atrioventricular groove; the coronary sinus runs in the posterior aspect of the groove to reach the right atrium (Fig. 56.2B,D). The anterior and posterior interventricular grooves indicate the lines of mural attachment of the ventricular septum and the limits of the left and right ventricular territories. The sternocostal surface of the ventricle curves bluntly into its left surface at the obtuse margin.
Internal features
The left ventricle has an inlet region, guarded by the mitral valve (ostium venosum), an outlet region, guarded by the aortic valve (ostium arteriosum), and an apical trabecular component. The left atrioventricular orifice admits atrial blood during diastole, flow being towards the cardiac apex. After closure of the mitral cusps, and throughout the ejection phase of systole, blood is expelled from the apex through the aortic orifice. In contrast to the orifices within the right ventricle, those of the left ventricle are in close contact, with fibrous continuity between the cusps of the aortic and mitral valves (the subaortic curtain; Fig. 56.13). The inlet and outlet turn sharply round this fibrous curtain (Fig. 56.9).
The anterolateral wall is the concavo-convex ventricular septum, a muscular wall the convexity of which is the posteromedial profile of the right ventricle as seen in section. It thus completes the circular outline of the left ventricle (Fig. 56.12). Towards the aortic orifice, the septum becomes the thin, collagenous interventricular component of the membranous septum, an oval or round area below and confluent with the fibrous triangle separating the right and the non-coronary cusps of the aortic valve.
Between the lower limits of the free margins of the cusps of the mitral valve and the apex of the ventricle, the muscular walls are deeply trabeculated. These trabeculae carneae are finer and more intricate than those of the right ventricle, but similar in structure. Trabeculation is characteristically well developed near the apex, whereas the upper reaches of the septal surface are smooth (Fig. 56.13).
Hypertrophy of heart muscle
In hypertrophic cardiomyopathy, there is an increase in the thickening of the myocardial walls, particularly the interventricular septum, which is disproportionately thickened in comparison with the posterior wall. Echocardiography allows accurate assessment of the thickening and of systolic function. Other features in hypertrophic cardiomyopathy are dynamic left ventricular outflow obstruction, systolic anterior motion of the anterior mitral valve cusp, and midsystolic closure of the aortic valve. A degree of diastolic dysfunction is also present in some cases of hypertrophic cardiomyopathy. Serial short-axis magnetic resonance imaging (MRI) allows accurate measurement of wall thickness and is particularly useful in assessing hypertrophy confined to the apex. Gradient-echo MRI also allows some functional assessment of the hypertrophy. A number of histological changes are observed, including disarray of the cardiac myocytes with replacement fibrosis, and expansion of the collagen component. Treatment is usually medical, except for refractory cases and those in whom the left ventricular outflow tract obstruction has a gradient of greater than 50 mmHg. Ventricular septal myotomy and myectomy are performed in such cases. More recently, catheter alcohol septal ablation has been introduced as a non-surgical alternative. A number of patients may also require implantation of cardiac defibrillators to prevent sudden cardiac death.
Mitral valve
Mitral valvular orifice
The anulus is strongest at the internal aspects of the left and right fibrous trigones. Extending from these structures, the anterior and posterior coronary prongs (which are tapering, fibrous, subendocardial tendons) partly encircle the orifice at the atrioventricular junction (Figs 56.9, 56.10). Between the tips of the prongs, the atrial and ventricular myocardial masses are separated by a more tenuous sheet of deformable fibroelastic connective tissue. Spanning anteriorly between the trigones, the fibrous core of the central part of the anterior aortic cusp of the mitral valve is a continuation of the fibrous subaortic curtain that descends from the adjacent halves of the left and non-coronary cusps of the aortic valve (Fig. 56.13).
Mitral valve cusps
When the valve is laid open, the anterior cusp (aortic, septal, ‘greater’ or anteromedial) is seen to guard one-third of the circumference of the orifice and to be semicircular or triangular, with few or no marginal indentations. Its fibrous core (lamina fibrosa) is continuous, on the outflow aspect, beyond the margins of the fibrous subaortic curtain, with the right and left fibrous trigones (Figs 56.5, 56.9, 56.11C). Between these, it is continuous with the fibrous curtain itself and, beyond the trigones, with the roots of the anular fibrous prongs (Fig. 56.10). The cusp has a deep crescentic rough zone, which receives various chordae tendineae. The ridge limiting the outer margin of the rough zone indicates the maximal extent of surface contact with the mural cusp in full closure. A clear zone is seen between the rough zone and the valvular anulus, which is devoid of attachments of chordae, although its fibrous core carries extensions from chordae attached in the rough zone. The anterior cusp has no basal zone, continuing instead into the valvular curtain. Hinging on its anular attachment, and continuous with the subaortic curtain, it is critically placed between the inlet and the outlet of the ventricle. During passive ventricular filling and atrial systole, its smooth atrial surface is important in directing a smooth flow of blood towards the body and apex of the ventricle. After the onset of ventricular systole and closure of the mitral valve, the ventricular aspect of its clear zone merges into the smooth surface of the subaortic curtain which, with the remaining fibrous walls of the subvalvular aortic vestibule, forms the smooth boundaries of the ventricular outlet.
The posterior cusp (mural, ventricular, ‘smaller’ or posterolateral) usually has two or more minor indentations. Lack of definition of major intervalvular commissures has previously led to disagreement and confusion concerning the territorial extent of this cusp and the possible existence of accessory ‘scallops’. Examination of the valve in the closed position, however, shows that the posterior cusp can conveniently be regarded as all the valvular tissue posterior to the anterolateral and posteromedial ends of the major zone of apposition with the aortic cusp. Thus defined, it has a wider attachment to the anulus than does the anterior cusp, guarding two-thirds of the circumferential attachments. Further indentations usually divide the mural cusp into a relatively large middle scallop and smaller anterolateral and posteromedial commissural scallops. Each scallop has a crescentic, opaque rough zone, receiving on its ventricular aspect the attachments of the chordae that define the area of valvular apposition in full closure. From the rough zone to within 2–3 mm of its anular attachment, there is a membranous clear zone devoid of chordae. The basal 2–3 mm is thick and vascular, and receives basal chordae. The ratio of rough to clear zone in the anterior cusp is 0.6; in the middle scallop of the posterior cusp it is 1.4. Much more of the mural cusp is in apposition with the aortic cusp during closure of the mitral valve.
Mitral chordae tendineae (tendinous cords)
The chordae tendineae resemble those supporting the tricuspid valve. False chordae (trabeculae carneae; Fig. 56.13) are also irregularly distributed as in the right ventricle. They occur in about 50% of all human left ventricles, and often cross the subaortic outflow. Many contain extensions from the ventricular conducting tissues. These left ventricular bands can often be identified by cross-sectional echocardiography. Their role, if any, has still to be determined. True chordae of the mitral valve may be divided into intercusp (or commissural) chordae, rough zone chordae, including the special strut chordae, so-called ‘cleft’ chordae and basal chordae. Most true chordae divide into branches from a single stem soon after their origin from the apical one-third of a papillary muscle, or proceed as single chordae that divide into several branches near their attachment. Basal chordae, in contrast, are solitary structures passing from the ventricular wall to the mural cusp.
Papillary muscles
The two muscles supporting the cusps of the mitral valve also vary in length and breadth and may be bifid. The anterolateral muscle arises from the sternocostal mural myocardium, the posteromedial from the diaphragmatic region (Fig. 56.12). Chordae tendineae arise mostly from the tip and apical one-third of each muscle, but sometimes take origin near their base. The chordae from each papillary muscle diverge and are attached to corresponding areas of closure on both valvular cusps.
Opening of the mitral valve
At the onset of diastole, opening is passive but rapid, the cusps parting and projecting into the ventricle as left atrial pressure exceeds left ventricular diastolic pressure. Passive ventricular filling proceeds as atrial blood pours to the apex, directed by the pendant aortic cusp of the valve. The cusps begin to float passively together, hinging on their anular attachments, partially occluding the ventricular inlet. Atrial systole now occurs, jetting blood apically and causing re-opening of the cusps. As maximal filling is achieved, the cusps again float rapidly together. Closure is followed by ventricular systole, which starts in the papillary muscles and continues rapidly as a general contraction of the walls and septum. Coordinated contraction of the papillary muscles increases the tension in the chordae and promotes joining of the corresponding points on opposing cusps, preventing their eversion. With general mural and septal excitation and contraction, left ventricular pressure increases rapidly (Fig. 56.7). The cusps ‘balloon’ towards the atrial cavity and the atrial aspects of the rough zones come into maximal contact. Precise papillary contraction, and increasing tension in the chordae, continue to prevent valvular eversion and maintain valvular competence.
Aortic valve
The smooth left ventricular outflow tract, or aortic vestibule, ends at the cusps of the aortic valve. Although stronger in construction, the aortic valve resembles the pulmonary (Figs 56.9, 56.11, 56.13) in possessing three semilunar cusps, supported within the three aortic sinuses of Valsalva. Although the aortic valve, like the pulmonary valve, is often described as possessing an anulus in continuity with the fibrous skeleton, there is no complete collagenous ring supporting the attachments of the cusps. As with the pulmonary valve, the anatomy of the aortic valve is dominated by the fibrous semilunar attachment of the cusps (Fig. 56.11C).
Aortic valve cusps
The cusps are attached in part to the aortic wall and in part to the supporting ventricular structures. The situation is more complicated than in the pulmonary valve, because parts of the cusps also take origin from the fibrous subaortic curtain, and are continuous with the aortic cusp of the mitral valve (Fig. 56.13). This area of continuity is thickened at its two ends to form the right and left fibrous trigones (Fig. 56.9). However, as with the pulmonary valve, the semilunar attachments incorporate segments of ventricular tissue within the base of each aortic sinus. These sinuses and cusps are conveniently named as right, left and non-coronary, according to the origins of the coronary arteries (Fig. 56.11C). The semilunar attachments also incorporate three triangular areas (trigones) of aortic wall within the apex of the left ventricular outflow tract. As these triangular areas are part of the wall of the aorta rather than of the left ventricle, and are interposed between the bulbous aortic sinuses, they separate the cavity of the left ventricle from the pericardial space. Removal of the trigones in an otherwise intact heart is instructive in demonstrating the relationships of the aortic valve, which can justly be considered as the keystone of the heart. The base of the triangle between the non-coronary and the left coronary cusps is continuous inferiorly with the fibrous aortic-mitral curtain. The apex of this triangle ‘points’ into the transverse pericardial space. The triangle between right and non-coronary cusps has, as its base, the membranous components of the interventricular septum and thus ‘faces’ the right ventricle, whereas its apex ‘points’ towards the transverse pericardial space behind the origin of the right coronary artery. The third triangle, between the two coronary cusps, has its base on the muscular ventricular septum. Its apex ‘points’ to the plane of space found between the aortic wall and the free-standing sleeve of right ventricular infundibular musculature that supports the cusps of the pulmonary valve. Although the basal attachments of each cusp are thickened and collagenous at their ventricular origins, there is no continuous collagenous skeleton supporting, in circular fashion, all the attachments of the cusps of the aortic valve. Valvular function depends primarily upon the semilunar attachments of the cusps.
Currently, three sets of names are used to describe the aortic cusps. Posterior, right and left refer to presumed fetal positions before full cardiac rotation has occurred (Ch. 59). Corresponding terms based on the approximate positions in maturity are anterior, left posterior and right posterior. However, as already indicated, widespread clinical terminology links both cusps and sinuses to the origins of the coronary arteries. Thus, the anterior is termed the right coronary cusp, the left posterior is the left coronary, and the right posterior is the noncoronary: these clinical terms are preferable, in the normal heart, because they are simple and unambiguous.
Aortic sinuses (of Valsalva)
The aortic sinuses are more prominent than those in the pulmonary trunk. The upper limit of each sinus reaches considerably beyond the level of the free border of the cusp and forms a well-defined complete circumferential sinotubular ridge when viewed from the aortic aspect (Fig. 56.11C). Coronary arteries usually open near this ridge within the upper part of the sinus, but are markedly variable in their origin. The walls of the sinuses are largely collagenous near the attachment of the cusps, but the amount of lamellated elastic tissue increases with distance from the zone of attachment. Strands of myocardium may enter this fibroelastic wall. At the mid level of each sinus, its wall is about half the thickness of the supravalvular aortic wall and less than one-quarter of the thickness of the sinutubular ridge. At this level, the mean luminal diameter of the beginning of the aortic root is almost double that of the ascending aorta. These details are functionally significant in the mechanism of valvular motion.
Opening of the aortic valve
During diastole, the closed aortic valve supports an aortic column of blood at high but slowly diminishing pressure (Fig. 56.7). Each sinus and its cusp form a hemispherical chamber. The three nodules are apposed and the margins and lunular parts of adjacent cusps are tightly apposed on their ventricular aspects. From the aortic aspect, the closed valve is triradiate, three pairs of closely compressed lunules radiating from their nodules to their peripheral commissural attachments at the sinutubular junction (Fig. 56.9). As ventricular systolic pressure increases, it exceeds aortic pressure and the valve is passively opened. The fibrous wall of the sinuses nearest the aortic vestibule is almost inextensible but, in the upper parts of sinuses, the wall is fibroelastic. Under left ventricular ejection pressure, the radius here increases 16% in systole. Hence the commissures move apart, making the orifice triangular when fully open. The free margins of the cusps then become almost straight lines between peripheral attachments. However, they do not flatten against the sinus walls, even at maximal systolic pressure, which is probably an important factor in subsequent closure. During ejection, most blood enters the ascending aorta, but some enters the sinuses, forming vortices that help to maintain the triangular ‘mid position’ of the cusp during ventricular systole and probably initiate their approximation with the end of systole. Tight and full closure ensues, with the rapid decrease in ventricular pressure in diastole. Commissures narrow, nodules aggregate and the valve reassumes its triradiate form. Experiments indicate that 4% of ejected blood regurgitates through a valve with normal sinuses, whereas 23% regurgitates through a valve without them. The normal structure of the aortic sinuses also promotes non-turbulent flow into the coronary arteries.
Echocardiography
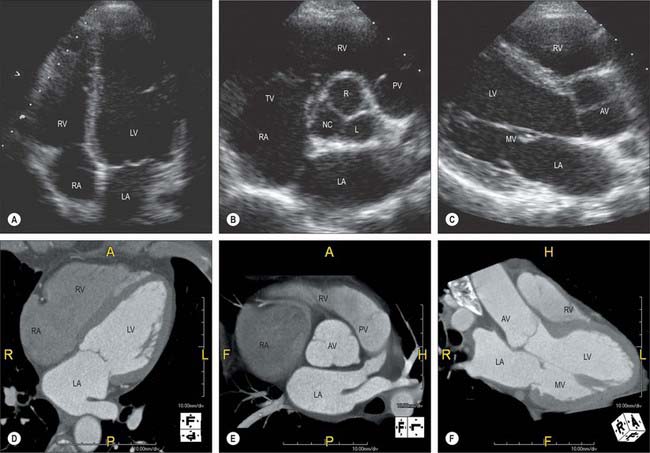
The gross anatomy of the heart can be evaluated by two-dimensional echocardiography in the para-sternal, apical, suprasternal and subcostal positions (Fig. 56.14). The standardized planes used are long axis, short axis and four-chamber. Echocardiography allows a detailed assessment of the functional anatomy of the heart. The long-axis view is obtained by placing the ultrasound transducer in the left apicosternal position and provides detailed images of the left ventricle, aorta, left atrium, and mitral and aortic valves (Fig. 56.14C). Angling the beam towards the right also allows assessment of the right atrium, right ventricle and tricuspid valves. Rotating the transducer by 90° in the clockwise direction produces the short-axis view, which allows assessment of the left ventricle, papillary muscles, chordae tendineae and mitral valves (Fig. 56.14B). The four-chamber view demonstrates the ventricles, atria, and mitral and tricuspid valves (Fig. 56.14A). Rotation of the transducer allows two-chamber views of the heart and more detailed assessment of the aorta and aortic valves. Cardiac magnetic resonance and computer tomography provide similar information on cardiac structure and function (Fig. 56.14D–F), together with complementary information on great vessels and other extracardiac intrathoracic stuctures.
CONNECTIVE TISSUE AND FIBROUS SKELETON OF THE HEART
From epicardium to endocardium, and from the orifices of the great veins to the roots of the arterial trunks, the intercellular spaces between contractile and conduction elements are everywhere permeated by connective tissue. The amount varies greatly in arrangement and texture in different locations.
Although it is often stated that all four valves are contained within this skeleton, this is not the case. The cusps of the pulmonary valve are supported on a free-standing sleeve of right ventricular infundibulum which can easily be removed from the heart without disturbing either the fibrous skeleton or the left ventricle. The fibrous skeleton is strongest at the junction of the aortic, mitral and tricuspid valves, the so-called central fibrous body (see below) (Figs 56.9, 56.10). Two pairs of curved, tapering, collagenous prongs, fila coronaria, extend from the central fibrous body. They are stronger on the left, passing partially around the mitral and tricuspid orifices, which are almost co-planar and incline to face the cardiac apex. The aortic valve, in contrast, faces up, right and slightly forwards. It is anterosuperior and to the right of the mitral orifice. Two of the cusps of the aortic valve are in fibrous continuity with the aortic cusp of the mitral valve: this aortic-mitral or subaortic curtain is also an integral part of the fibrous skeleton (Figs 56.9, 56.11C). The two ends of the curtain are strengthened as the right and left fibrous trigones, which are the strongest part of the skeleton. The right trigone, together with the membranous septum, constitutes the central fibrous body, which is penetrated by the bundle of His, or atrioventricular bundle, i.e. by the mechanism for atrioventricular conduction. The membranous septum is crossed on its right aspect by the attachment of the tricuspid valve, which divides the septum into atrioventricular and interventricular components.
CONGENITAL CARDIAC MALFORMATIONS
Acyanotic cardiac defects
Acyanotic heart defects are the result of either left to right cardiac shunting through heart defects (intra- or extra-cardiac), or of obstruction. Left to right shunting leads to an increased workload and stress on the heart and the lungs as a consequence of increased pulmonary blood flow and, in turn, increased pulmonary venous return. Depending on the location of the shunt and the magnitude of left to right shunting, patients are at risk of developing pulmonary arterial hypertension, unless timely heart surgery is undertaken. Examples of defects leading to left to right shunting are simple septal defects such as atrial or ventricular septal defects or patent ductus arteriosus, or more complex cardiac defects, including atrioventricular septal defects, and/or a combination of any of these defects with abnormal atrioventricular or ventriculoarterial connections (e.g. a double outlet right ventricle, where more than 50% of both the aorta and the pulmonary artery originate from the right ventricle) (Ch. 59). Obstructive lesions may involve the atrioventricular or semilunar valves (mitral, aortic or pulmonary valve stenosis), or narrow vessels (e.g. coarctation of the aorta or interrupted aortic arch). Depending on the level and severity of the obstruction, patients may have a range of clinical presentations from an asymptomatic heart murmur (common) to cardiovascular collapse (uncommon). Treatment in all cases is directed towards normalizing heart workload, systemic and pulmonary blood flow, and cardiac output, and may be surgical and/or catheter-based.
Cyanotic cardiac defects
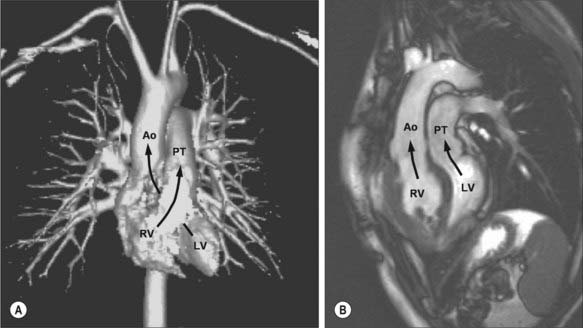
Cyanotic cardiac defects may be attributable to a right to left intra- or extra- cardiac shunt or to a severe reduction in pulmonary blood flow. They may be caused by simple lesions such as severe pulmonary stenosis with an atrial septal defect and right to left shunting; moderate lesions including Fallot’s tetralogy, in which there is a ventricular septal defect, right ventricular outflow obstruction, right ventricular hypertrophy and an over-riding aorta; or more complex lesions including transposition of the great arteries (Fig. 56.15A,B) and tricuspid and pulmonary atresia (often in the setting of hypoplastic right ventricle and ‘single’ ventricular physiology). Neonates with severe cyanotic congenital heart defects may be dependent on the patency of the ductus arteriosus: early diagnosis, treatment with intravenous prostaglandin infusion and timely transfer to a tertiary centre for more definitive therapy, is key to survival and good long-term outcomes.
CONDUCTION TISSUE
Overview of the conduction system
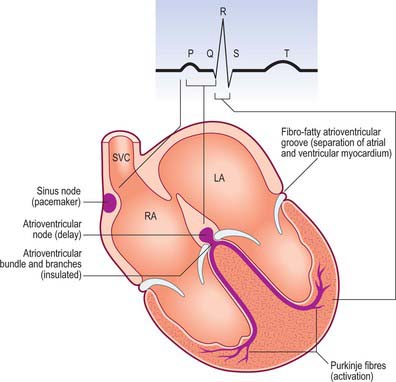
The human heart beats ceaselessly at 70 cycles every minute for many decades, maintaining perfusion of pulmonary and systemic tissues. The rate and stroke volume fluctuate in response to prevailing physiological demands. Figure 56.7 summarizes the principal events in a cardiac cycle, including: the electrical events recorded in the electrocardiogram; the mechanical sequences of diastole, atrial systole, isovolumetric contraction, ejection and isovolumetric relaxation in ventricular systole; the acoustic phenomena recorded in the phonocardiogram; the pressure profiles of right and left hearts and arterial trunks: the sequences of valvular events. Cardiac efficiency depends on precise timing of the operation in interdependent structures. Passive diastolic filling of the atria and ventricles is followed by atrial systole, stimulated by discharge from the sinu-atrial node, which completes ventricular filling. Excitation and contraction of the atria must be synchronous and finish before ventricular contraction. This is effected by a delay in the conduction of excitation from atria to ventricles. Thereafter, ventricular contraction proceeds in a precise manner. A specialized ventricular conduction system ensures that closure of atrioventricular valves is followed rapidly by a wave of excitation and contraction, which spreads from the ventricular apices towards the outflow tracts and orifices, rapidly accelerating the blood during ejection.
Cardiac contraction originates unequivocally in specialized myocytes, but neural influences are important in adapting the intrinsic cardiac rhythm to functional demands from the entire body. All cardiac myocytes are excitable, and display autonomous rhythmic depolarization and repolarization of the cell membrane, conduction of waves of excitation via gap junctions to adjacent myocytes, and excitationcontraction coupling to their actomyosin complexes. These properties are developed to different degrees in different sites and in different types of myocyte (see Ch. 6). The rate of depolarization and repolarization is slowest in the ventricular myocardium, intermediate in the atrial muscle and fastest in the myocytes of the sinoatrial node. These last over-ride those generating slower rhythms and, in the normal heart, are the locus for the rhythmic initiation of cardiac cycles. Conversely, conduction velocity is slow in nodal myocytes, intermediate in general ‘working’ cardiac myocytes and fastest in the myocytes of the ventricular conduction system.
The nodes and networks of the so-called specialized myocardial cells constitute the cardiac conduction system (Figs 56.4A, 56.16A,B and 56.17). The components of this system are the sinu-atrial and atrioventricular nodes, the atrioventricular bundle with its left and right bundle branches and the subendocardial plexus of ventricular conduction cells (Purkinje fibres). Within the system, the main pacemaker rhythm of the heart is generated (sinus), is influenced by nerves (sinus and its innervation) and is transmitted specifically from atria to ventricles (atrioventricular node and bundle) and, within the ventricles, to all their musculature. The spread of excitation is very rapid, but not instantaneous. Different parts of the ventricles are excited at slightly different times, with important functional consequences. Failure of the conduction system will not block cardiac contraction, but the system will become poorly coordinated or uncoordinated. The rhythm will be slower because it originates from a spontaneous (myogenic) activity in the working cardiac myocytes or in a subsidiary pacemaker in a more distal part of the diseased or disrupted conduction system.
There are no specialized internodal and interatrial conduction pathways. The excitation emanating from the sinoatrial node spreads to the atrial musculature and to the atrioventricular node through ordinary atrial working myocardium. The geometric arrangement of fibres along well-organized atrial muscle bundles, e.g. the terminal crest and the rims of the fossa ovale, ensure that conduction is marginally more rapid than elsewhere within the atrium.
Sinoatrial node
The sinoatrial node is an elliptical structure, 10–20 mm long. It is located at the junction between parts of the right atrium derived from the embryonic venous sinus and the atrium proper (Figs 56.4A, 56.17). The node is often covered by a plaque of subepicardial fat, making it visible in some instances to the naked eye. It extends between 1 and 2 cm on the right from the crest of the right auricle and runs posteroinferiorly into the upper part of the terminal groove. In a small proportion of individuals, about 1 in 10, it extends in horseshoe fashion across the crest of the auricle. Nodal tissue does not occupy the full thickness of the right atrial wall from epicardium to endocardium in humans, but rather sits as a wedge of specialized tissue subepicardially within the terminal groove. Its location is marked consistently by a large central artery, which is a branch of either the left circumflex or posterior descending branch of the right coronary artery. Nodal cells are grouped circumferentially around this artery and interwoven into its dense collagenous adventitia. These cells are now considered the ‘pacemakers’, although the functional implications of this relationship are not fully understood. Many nerve fibres are present, although none appears to terminate on cells. There are no autonomic ganglion cells within the node, although many border it anteriorly and posteriorly. P cells are most abundant in the central region. They are small, empty-looking cells, 5–10 μm in greatest diameter, with a large central nucleus. Their pale appearance is attributable to the sparsity of organelles: myofibrils are few and irregularly arranged, and there is no proper sarcotubular system and little glycogen. P cells are less abundant in the periphery of the node, where they mix with slender fusiform transitional cells which are part of a heterogeneous group that is intermediate in appearance between P cells and normal working cardiac cells, and which link P cells to other cells.
Atrioventricular node
The atrioventricular node is an atrial structure that lies at the root of an extensive tree of conduction tissue reaching the apex of the ventricles, the papillary muscles and other regions of the ventricles (Fig. 56.17). The node, with its transitional zones, is located within the atrial component of the muscular atrioventricular septum. Its anatomical landmarks are the boundaries of the triangle of Koch (the attachment of the septal cusp of the tricuspid valve inferiorly, the ostium of the coronary sinus basally, and the tendon of Todaro superiorly) (Fig. 56.4A,B). The compact node is a half oval set against the central fibrous body towards the apex of this triangle. Its atrial aspect is convex, and is overlain by atrial myocardium. Its left margin is concave and abuts on the superior aspect of the central fibrous body. Its basal end projects into the atrial muscle and its anteroinferior end enters the central fibrous body to become the penetrating atrioventricular bundle. The node is pervaded by an irregular collagenous reticulum enmeshing the myocytes, but this is less dense than in the sinu-atrial node. Its arterial supply is from a characteristic vessel that originates from the dominant coronary artery at the crux of the heart. The node has a well-formed compact zone made up of interlocking nodal cells, which frequently show stratification. The transitional cell zones are found superficially and posteriorly. The larger component of atrioventricular delay is probably produced in these transitional zones of the node.
Atrioventricular bundle
The atrioventricular bundle is the direct continuation of the atrioventricular node, becoming oval, quadrangular or triangular in transverse sectional profile as it enters the central fibrous body (Fig. 56.4A). Traversing the fibrous body, it branches on the crest of the muscular interventricular septum, the branching tract being sandwiched between the muscular and the membranous components of the septum. The right branch of the bundle (crus dextrum) is a narrow, discrete round group of fascicles that courses at first within the myocardium and then subendocardially towards the apex of the ventricle, entering the septomarginal trabecula to reach the anterior papillary muscle. It gives few branches to the ventricular walls in its septal course. At the origin of the anterior papillary muscle, it divides profusely into fine subendocardial fascicles that diverge and, first, embrace the papillary muscle, and then recurve sub-endocardially to be distributed to the remaining ventricular walls. The left branch (crus sinistrum) arises as numerous fine intermingling fascicles that leave the left margin of the branching bundle through much of its course along the crest of the muscular ventricular septum (Fig. 56.16A,B). These fascicles form a flattened sheet down the smooth left ventricular septal surface. The sheet diverges apically and subendocardially across the left aspect of the ventricular septum, separating into anterior, septal and posterior divisions. Fine branches leave the sheets, forming subendocardial networks, which first surround the papillary muscles and then curve back subendocardially to be distributed to all parts of the ventricle.
The principal branches of the bundle are insulated from the surrounding myocardium by sheaths of connective tissue. Functional contacts between ventricular conduction and working myocytes become numerous only in the subendocardial terminal ramifications. Hence, papillary muscles contract first, followed by a wave of excitation and ensuing contraction that travels from the apex of the ventricle to the arterial outflow tract. Because the Purkinje network is subendocardial, muscular excitation proceeds from the endocardial to the epicardial aspect. In the developing heart, it can be shown that the bundle responsible for atrioventricular conduction is a much more extensive structure. Immunohistochemical analysis has revealed that the precursor of the system is a ring of cells that surrounds the inlet and outlet components of the developing ventricular loop (Ch. 59). This ring becomes modified after septation of the ventricles, so that it encircles the right atrioventricular orifice and the aortic outlet from the left ventricle. With subsequent growth, only the septal components of this ‘figure of eight’ persist as the atrioventricular conduction tract. However, remnants of the aortic ring can persist as a ‘dead-end tract’.
The Wolff–Parkinson–White syndrome is caused by abnormal small strands of otherwise unremarkable ventricular myocardium, which connect the atrial and ventricular myocardial masses at some point around the atrioventricular junctions. Histologically, they are strands of working myocardium running through the fibroareolar tissue of the atrioventricular groove.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
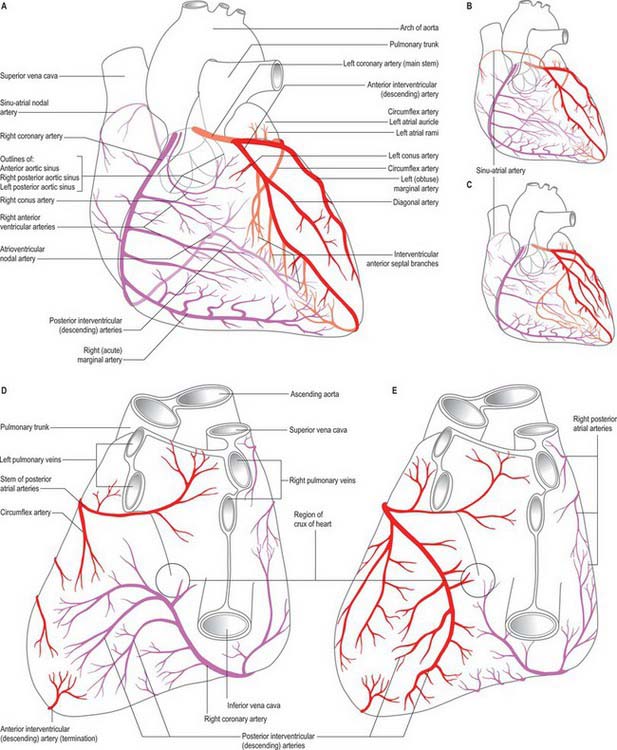
Coronary arterial supply
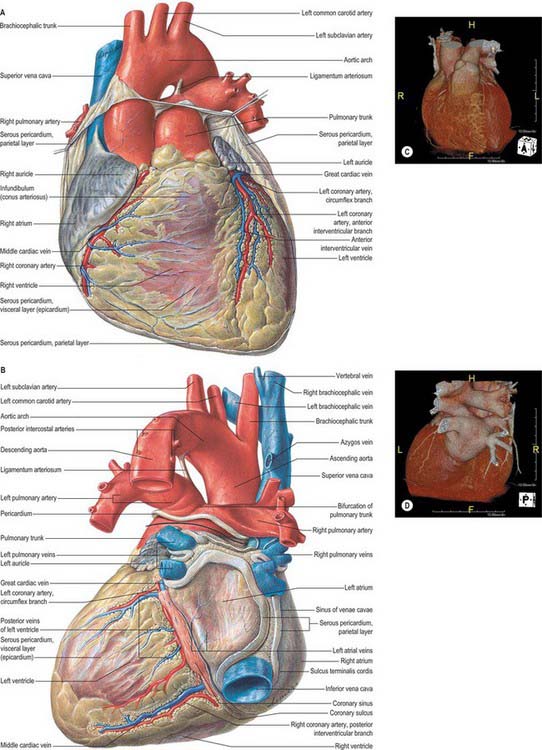
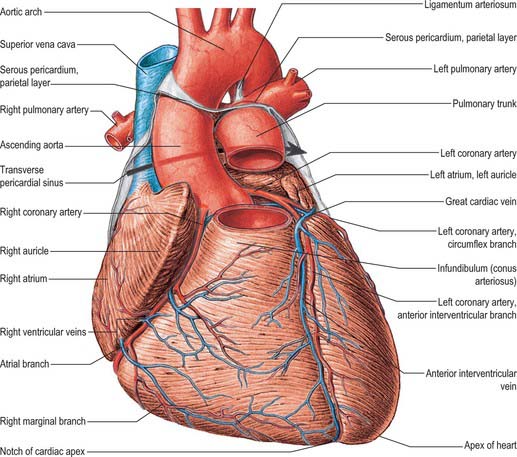
The right and left coronary arteries arise from the ascending aorta in its anterior and left posterior sinuses (Figs 56.8, 56.9, 56.18A-E). The levels of the coronary ostia are variable. The two arteries, as indicated by their name, form an oblique inverted crown, in which an anastomotic circle in the atrioventricular groove is connected by marginal and interventricular (descending) loops intersecting at the cardiac apex (Fig. 56.18A–E). This is, of course, only an approximation. The degree of anastomosis varies and is usually insignificant. The main arteries and major branches are usually subepicardial, but those in the atrioventricular and interventricular grooves are often deeply sited, and occasionally hidden by overlapping myocardium or embedded in it.
Right coronary artery
Although the atrial branches of the right coronary artery are sometimes described as anterior, lateral (right or marginal) and posterior groups, they are usually small single vessels 1mm in diameter. The right anterior and lateral branches are occasionally double, very rarely triple, and mainly supply the right atrium. The posterior branch is usually single and supplies the right and left atria. The artery of the sinoatrial node is an atrial branch, distributed largely to the myocardium of both atria, mainly the right. Its origin is variable: it comes from the circumflex branch of the left coronary in 35%. However, more commonly it arises from the anterior atrial branch of the right coronary artery, less often from its right lateral part, least often from its posterior atrioventricular part. This ‘nodal’ artery thus usually passes back in the groove between the right auricular appendage and aorta. Whatever its origin, it usually branches around the base of the superior vena cava, typically as an arterial loop from which small branches supply the right atrium. A large ‘ramus cristae terminalis’ traverses the sinu-atrial node (Fig. 56.18A–C); it would seem more appropriate to name this branch the ‘nodal artery’, as most of the currently named vessel actually supplies the atria and serves as the ‘main atrial branch’.
Left coronary artery
The left coronary artery is larger in calibre than the right, and supplies a greater volume of myocardium, including almost all the left ventricle and atrium, except in so-called ‘right dominance’, when the right coronary artery partly supplies a posterior region of the left ventricle (Fig. 56.18A-C). The left coronary artery usually supplies most of the interventricular septum. It arises from the left posterior (left ‘coronary’) aortic sinus; the ostium is below the margin of the cusps in 15%, and may be double, leading into major initial branches, usually the circumflex and anterior interventricular (descending) arteries. Its initial portion, between its ostium and its first branches, varies in length from a few millimetres to a few centimetres. The artery lies between the pulmonary trunk and the left atrial auricle, emerging into the atrioventricular groove, in which it turns left. This part is loosely embedded in subepicardial fat and usually has no branches, but may give off a small atrial ramus and, rarely, the sinu-atrial nodal artery.
Coronary distribution
Details of coronary distribution require integration into a concept of total cardiac supply. Most commonly, the right coronary artery supplies all of the right ventricle (except a small region to the right of the anterior interventricular groove); a variable part of the diaphragmatic aspect of the left ventricle; the posteroinferior one-third of the intraventricular septum; the right atrium and part of the left atrium; the conduction system as far as the proximal parts of the right and left crura. Left coronary distribution is reciprocal, and includes most of the left ventricle; a narrow strip of right ventricle; the anterior two-thirds of the interventricular septum; most of the left atrium. As noted previously (Fig. 56.18A–E), variations in the coronary arterial system mainly affect the diaphragmatic aspect of the ventricles and reflect the relative ‘dominance’ of supply by the left or the right coronary artery. The term is misleading, because the left artery almost always supplies a greater volume of tissue than the right. In ‘right dominance’, the posterior interventricular artery is derived from the right coronary artery; in ‘left dominance’ it is derived from the left coronary artery. In the so-called ‘balanced’ pattern, branches of both arteries run in or near the posterior interventricular groove.
Coronary anastomosis
Coronary arteriovenous anastomoses and numerous connections between the coronary circulation and cardiac cavities, producing so-called ‘myocardial sinusoids’ and ‘arterioluminal’ vessels, have been reported; their importance in coronary disease is uncertain.
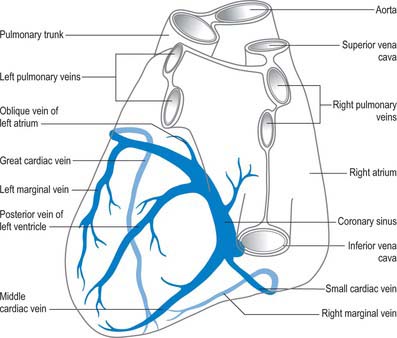
Cardiac veins
Coronary sinus
The large majority of cardiac veins drain into the wide coronary sinus, 2 or 3 cm long, lying in the posterior atrioventricular groove between the left atrium and ventricle (Fig. 56.2B,D; Fig. 56.19). The sinus opens into the right atrium between the opening of the inferior vena cava and the right atrioventricular orifice; the opening is guarded by an endocardial fold (semilunar valve of the coronary sinus; Fig. 56.4A). Its tributaries are the great, small and middle cardiac veins, the posterior vein of the left ventricle and the oblique vein of the left atrium; all except the last have valves at their orifices.
The great cardiac vein begins at the cardiac apex, ascends in the anterior interventricular groove to the atrioventricular groove and follows this, passing to the left and posteriorly to enter the coronary sinus at its origin (Fig. 56.19). It receives tributaries from the left atrium and both ventricles, including the large left marginal vein that ascends the left aspect (obtuse border) of the heart.
The small cardiac vein lies in the posterior atrioventricular groove between the right atrium and ventricle and opens into the coronary sinus near its atrial end (Fig. 56.19). It receives blood from the posterior part of the right atrium and ventricle. The right marginal vein passes right, along the inferior cardiac margin (acute border). It may join the small cardiac vein in the atrioventricular groove, but more often opens directly into the right atrium.
The middle cardiac vein (Fig. 56.19) begins at the cardiac apex, and runs back in the posterior interventricular groove to end in the coronary sinus near its atrial end.
Posterior vein of the left ventricle
The posterior vein of the left ventricle (Fig. 56.19) is found on the diaphragmatic surface of the left ventricle a little to the left of the middle cardiac vein. It usually opens into the centre of the coronary sinus, but sometimes opens into the great cardiac vein.
Oblique vein of the left atrium
The small vessel that is the oblique vein of the left atrium (Fig. 56.19) descends obliquely on the back of the left atrium to join the coronary sinus near its end. It is continuous above with the ligament of the left vena cava. The two structures are remnants of the left common cardinal vein.
Anterior cardiac veins
The anterior cardiac veins drain the anterior part of the right ventricle (Figs 56.2A,C; 56.8). Usually two or three, sometimes even five, they ascend in subepicardial tissue to cross the right part of the atrioventricular groove, passing deep or superficial to the right coronary artery. They end in the right atrium, near the groove, separately or in variable combinations. A subendocardial collecting channel, into which all may open, has been described. The right marginal vein courses along the inferior (acute) cardiac margin, draining adjacent parts of the right ventricle, and usually opens separately into the right atrium. It may join the anterior cardiac veins or, less often, the coronary sinus. Because it is commonly independent, it is often grouped with the small cardiac veins, but it is larger in calibre, being comparable to the anterior cardiac veins or even wider.
INNERVATION
Cardiac plexus
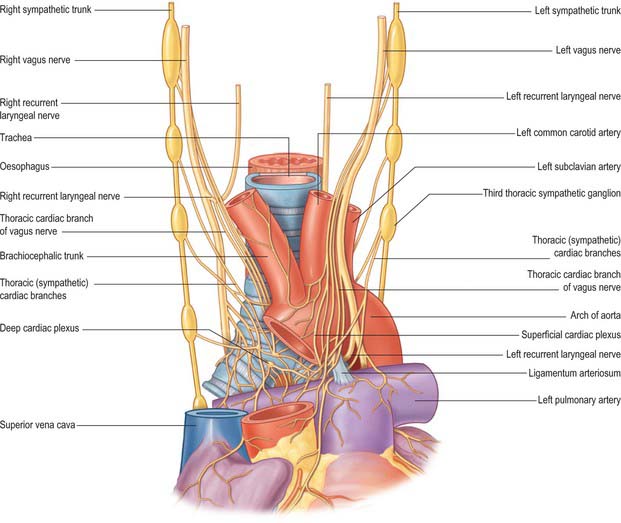
Nearing the heart, the autonomic nerves form a mixed cardiac plexus, usually described in terms of a superficial component inferior to the aortic arch lying between it and the pulmonary trunk, and a deep part between the aortic arch and tracheal bifurcation. The cardiac plexus is also described by regional names for its coronary, pulmonary, atrial and aortic extensions (Fig. 56.20). These plexuses contain ganglion cells. Ganglion cells, confined to the atrial tissues, and with a preponderance adjacent to the sinu-atrial node, are also found in the heart along the distribution of branches of the plexus. Their axons are considered to be largely, if not exclusively, postganglionic parasympathetic. Cholinergic and adrenergic fibres, arising in or passing through the cardiac plexus, are distributed most profusely to the sinu-atrial and atrioventricular nodes; the supply to the atrial and ventricular myocardium is much less dense. Adrenergic fibres supply the coronary arteries and cardiac veins. Rich plexuses of nerves containing cholinesterase, adrenergic transmitters and other peptides, e.g. neuropeptide Y, are found in the subendocardial regions of all chambers and in the cusps of the valves.
Deep (dorsal) part of the cardiac plexus
Branches from the right half of the deep part of the cardiac plexus pass in front of and behind the right pulmonary artery. Those anterior to it, the more numerous, supply a few filaments to the right anterior pulmonary plexus and continue on to form part of the right coronary plexus. Those behind the pulmonary artery supply a few filaments to the right atrium and then continue into the left coronary plexus. The left half of the deep part of the cardiac plexus is connected with the superficial, and supplies filaments to the left atrium and left anterior pulmonary plexus. It forms much of the left coronary plexus.
MAJOR BLOOD VESSELS
ARTERIES
Pulmonary trunk
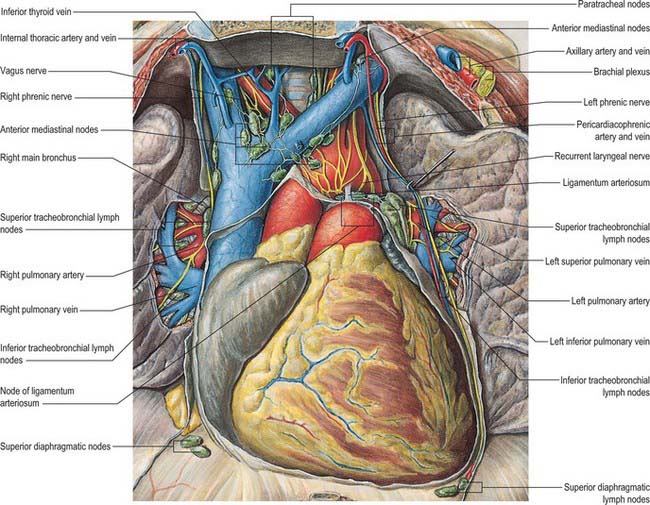
The pulmonary trunk, or pulmonary artery, conveys deoxygenated blood from the right ventricle to the lungs (Fig. 56.2A,C; see Fig. 57.8). About 5 cm in length and 3 cm in diameter, it is the most anterior of the cardiac vessels and arises from the base of the right ventricle (from the pulmonary anulus surmounting the conus arteriosus) above and to the left of the supraventricular crest. It slopes up and back, at first in front of the ascending aorta, then to its left. Below the aortic arch it divides, level with the fifth thoracic vertebra and to the left of the midline, into right and left pulmonary arteries of almost equal size. The pulmonary trunk bifurcation lies below, in front and to the left of the tracheal bifurcation (which is also associated with the inferior tracheobronchial lymph nodes and the deep cardiac nerve plexus). In the fetus, at the level of the bifurcation the pulmonary artery is connected to the aortic arch by the ductus arteriosus, which lies in the same direction as the pulmonary artery.
Thoracic aorta
Ascending aorta
The ascending aorta is typically 5 cm long and begins at the base of the left ventricle, level with the lower border of the third left costal cartilage; it ascends obliquely, curving forwards and to the right, behind the left half of the sternum to the level of the upper border of the second left costal cartilage (Figs 56.2A, B, 56.8, 56.18A-C; see Figs 53.2, 57.8). At its origin, proximal to the aortic anulus, the sectional profile is larger and not circular because of three almost hemispherical outward bulges (sinuses of Valsalva), one posterior (non-coronary), one left and one right, which correspond to the three cusps of the aortic valve (see above). Distal to the aortic anulus there are three aortic sinuses, beyond which the calibre of the vessel is slightly increased by a bulging of its right wall. This aortic bulb gives the vessel an oval section.
The ascending aorta is within the fibrous pericardium, enclosed in a tube of serosal pericardium with the pulmonary trunk (Figs 56.1, 56.2A,C). Anterior to its lower part are the infundibulum, the initial segment of the pulmonary trunk and the right auricle. Superiorly, it is separated from the sternum by the pericardium, right pleura, anterior margin of the right lung, loose areolar tissue and the remains of the thymus gland. Posterior are the left atrium, right pulmonary artery and principal bronchus. Right lateral are the superior vena cava and right atrium, the former partly posterior, and left lateral are the left atrium and, at a higher level, the pulmonary trunk. At least two structures, aorticopulmonary bodies (reminiscent of the carotid arterial chemoreceptors and baroreceptors), lie between the ascending aorta and the pulmonary trunk. The inferior aorticopulmonary body is near the heart and anterior to the aorta, and the middle aorticopulmonary body is near the right side of the ascending aorta.
Aortic arch
The aortic arch continues from the ascending aorta (Figs 56.1, 56.2A,C). Its origin, slightly to the right, is level with the upper border of the second right sternocostal joint. The arch first ascends diagonally back and to the left over the anterior surface of the trachea, then back across its left side and finally descends to the left of the fourth thoracic vertebral body, continuing as the descending thoracic aorta. It ends level with the sternal end of the second, left costal cartilage. Thus the aortic arch lies wholly in the superior mediastinum. It curves around the hilum of the left lung, and extends upwards to the mid level of the manubrium of the sternum. The shadow of the arch is easily identified in anteroposterior radiographs and its left profile is sometimes called the ‘aortic knuckle’ (see Fig. 55.16). The arch may also be visible in left anterior oblique views enclosing a pale space, ‘the aortic window’, in which shadows of the pulmonary trunk and its left branch may be discerned. Its diameter at the origin is the same as in the ascending aorta, 28 mm, but it is reduced to 20 mm at the end, after the issue of its large collateral branches. At the border with the thoracic aorta, a small stricture (aortic isthmus), followed by a dilatation, can be recognized. In fetal life the isthmus lies between the origin of the left subclavian artery and the opening of the ductus arteriosus.
Anteriorly and to the left of the aortic arch is the left mediastinal pleura. Deep to the pleura it is crossed, in anteroposterior order by: the left phrenic nerve, left lower cervical cardiac branch of the vagus, left superior cervical cardiac branch of the sympathetic and left vagus (Figs 56.1, 56.20). As the left vagus crosses the arch, its recurrent laryngeal branch hooks below the vessel left and behind (developmentally caudal to) the ligamentum arteriosum and then ascends on the right of the arch. The left superior intercostal vein ascends obliquely forwards on the arch, superficial to the left vagus, deep to the left phrenic nerve. The left lung and pleura separate all these from the thoracic wall. Posterior and to the right are the trachea and deep cardiac plexus, the left recurrent laryngeal nerve, oesophagus, thoracic duct and vertebral column. Above, the brachiocephalic, left common carotid and left subclavian arteries arise from its convexity, and are crossed anteriorly near their origins by the left brachiocephalic vein. Below are the pulmonary bifurcation, left principal bronchus, ligamentum arteriosum, superficial cardiac plexus and left recurrent laryngeal nerve. Best viewed from the left, the concavity of the aortic arch is the upper curved limit through which structures gain access to or leave the hilum of the left lung.
Three branches arise from the convex aspect of the arch: the brachiocephalic trunk, left common carotid and left subclavian arteries (Figs 56.1, 56.2A-D). They may branch from the beginning of the arch or the upper part of the ascending aorta. The distance between these origins varies, the most frequent being approximation of the left common carotid artery to the brachiocephalic trunk.
Coarctation of the aorta
The postductal type of coarctation has been attributed to abnormal extension of the ductal tissue into the aortic wall, stenosing both vessels as the duct contracts after birth. This form can permit years of normal life, allowing the development of an extensive collateral circulation to the aorta distal to the stenosis (Fig. 56.21). High vascularity of the thoracic wall is important and clinically characteristic; many arteries arising indirectly from the aorta, proximal to the coarctation segment, anastomose with vessels connected with it distal to the block, and all of these vessels become greatly enlarged. Thus, in the anterior thoracic wall, the thoraco-acromial, lateral thoracic and subscapular arteries (from the axillary artery), the suprascapular artery (from the subclavian artery), and the first and second posterior intercostal arteries (from the costocervical trunk), anastomose with other posterior intercostal arteries, and the internal thoracic artery and its terminal branches anastomose with the lower posterior intercostal and inferior epigastric arteries. Posterior intercostal arteries are always involved, and enlargement of their dorsal branches may eventually groove (‘notch’) the inferior margins of the ribs. The radiographic shadow of the enlarged left subclavian artery is also increased. Enlargement of the scapular vessels and anastomoses may lead to widespread interscapular pulsation (easily appreciated with the palm of the hand, and sometimes heard on auscultation).
Brachiocephalic artery
The brachiocephalic (innominate) artery, the largest branch of the aortic arch, is 4–5 cm in length (Fig. 56.2A,C; see Fig. 28.14). It arises from the convexity of the arch posterior to the centre of the manubrium of sternum, and ascends posterolaterally to the right, at first anterior to the trachea, then on its right. Level with the upper border of the right sternoclavicular joint, it divides into the right common carotid and right subclavian arteries.
Descending thoracic aorta
The thoracic aorta is the segment of descending aorta confined to the posterior mediastinum (see Fig. 55.4B). It begins level with the lower border of the fourth thoracic vertebra, continuous with the aortic arch, and ends anterior to the lower border of the 12th thoracic vertebra in the diaphragmatic aortic aperture. At its origin it is left of the vertebral column, as it descends it approaches the midline, and at its termination is directly anterior to it.
Aortic bodies
Aortic bodies develop progressively during fetal life. They attain maximum size in the first three postnatal years, when the largest are two brownish bodies, 1 cm long, which flank the abdominal aorta and are usually united anterior to it by a horizontal mass immediately above the inferior mesenteric artery, thereby forming an inverted crescentic or H-shaped arrangement which is intimately related to the intermesenteric and superior hypogastric plexuses (see Fig. 15.16). Their constituent cells disperse and atrophy and by 14 years they may have disintegrated completely. When well developed, they consist of masses of polygonal chromaffin cells embedded in wide-meshed capillary plexuses and secreting noradrenaline (norepinephrine). Other small chromaffin bodies are also widespread in the fetus in the abdominal and pelvic prevertebral sympathetic plexuses. They reach a maximum size between the fifth and eighth fetal months, and survive in adults mainly near the coeliac and superior mesenteric arteries and as microscopic collections of cells that persist in the lower parts of the intermesenteric plexus.
Common carotid arteries
Right common carotid artery
The right common carotid artery and its relations are described in Chapter 28.
Left common carotid artery
The left common carotid artery (see Fig. 28.14) ascends until level with the left sternoclavicular joint, where it enters the neck. It is 20–25 mm long and it lies at first in front of the trachea, then it inclines to the left. The further course of the artery is described in Chapter 28.
VEINS
Superior vena cava
The superior vena cava returns blood to the heart from the tissues above the diaphragm. It is approximately 7 cm in length, and is formed by the junction of the brachiocephalic veins behind the lower border of the first right costal cartilage near the sternum. It descends vertically behind the first and second intercostal spaces, and ends in the upper right atrium behind the third right costal cartilage. Its inferior half is within the fibrous pericardium, which it pierces level with the second costal cartilage. Covered anterolaterally by serous pericardium (from which a retrocaval recess projects), it is slightly convex to the right (Figs 56.1, 56.3; see Fig. 28.14). The superior vena cava has no valves.
The anterior margins of the right lung and pleura are anterior and the pericardium intervenes below: these structures separate the superior vena cava from the internal thoracic artery, first and second intercostal spaces, and second and third costal cartilages. The trachea and right vagus are posteromedial, the right lung and pleura are posterolateral, and the right pulmonary hilum is posterior. The right phrenic nerve and pleura are right lateral and the brachiocephalic artery and ascending aorta are left lateral, the aorta overlapping the superior vena cava (see Fig. 58.3).
Inferior vena cava
The inferior vena cava returns blood to the heart from the tissues below the diaphragm. It passes through the diaphragm between the right leaf and central area of the central tendon of the diaphragm at the level of the eight and ninth thoracic vertebrae, and drains into the inferoposterior part of the right atrium (see Fig. 58.1). The thoracic part is very short, and is partly inside and partly outside the pericardial sac. The extrapericardial part is separated from the right pleura and lung by the right phrenic nerve, and the intrapericardial part is covered, except posteriorly, by inflected serous pericardium. The abdominal course of the inferior vena cava is described in Chapter 62.
Brachiocephalic veins
The right and left brachiocephalic veins join to form the superior vena cava.
Right brachiocephalic vein
The right brachiocephalic vein is about 2.5 cm long, and begins posterior to the sternal end of the right clavicle. It descends almost vertically to join the left brachiocephalic vein, forming the superior vena cava posterior to the lower border of the first right costal cartilage, near the right sternal border. It is anterolateral to the brachiocephalic artery and right vagus nerve; the right pleura, phrenic nerve and internal thoracic artery are posterior to it above, and become lateral below (see Fig. 28.14). Its tributaries are the right vertebral, internal thoracic, inferior thyroid and sometimes the first right posterior intercostal veins.
Anderson RH, Ho SY, Becker AE. The surgical anatomy of the conduction tissues. Thorax. 1983;38:408-420.
Carvalho JS, Moscoso G, Ville Y. First-trimester transabdominal fetal echocardiography. Lancet. 1998;351:1023-1027.
Federative Committee on Anatomical Terminology. Terminologia Anatomica. Stuttgart: Thieme, 1998.
Ho SY, McCarthy KP, Josen M, Rigby ML. Anatomic-echocardiographic correlates: an introduction to normal and congenitally malformed hearts. Heart. 2001;86:3-11.
James TN. Congenital disorders of cardiac rhythm and conduction. J Cardiovasc Electrophysiol. 1993;4:702-718.
Mizeres NJ. The cardiac plexus in man. Am J Anat. 1963;112:141-151.