Headache Disorders*
Headache is a common complaint, accounting for approximately 3 million visits to the emergency department (ED) per year in the United States.1 In addition, many more patients present with headache as part of a constitutional illness, making the symptom of headache one of the most frequent complaints in the ED.1
Headache commonly is divided into primary and secondary disorders.2 The primary headache disorders include migraine, cluster, and tension-type headaches, which represent the majority of headaches seen in clinical emergency practice.3–5 Secondary headache disorders include a variety of organic illnesses in which head pain is a symptom of an identifiable, distinct pathologic process. To facilitate a standardized approach to headache management, the International Headache Society published a classification system and diagnostic criteria for headache disorders, cranial neuralgias, and facial pain.2 This comprehensive and widely accepted system includes 14 major categories of headache disorders and uses specific operational diagnostic criteria to define each headache type (Box 103-1). Most patients presenting to an ED with headache have a benign primary headache disorder requiring only symptomatic treatment and referral. The challenge for the emergency physician is to identify the very small subset of patients who have headache as a symptom of a serious or potentially life-threatening disease.
Primary Headache Disorders
Principles of Disease
Migraine is a common, chronic, sometimes incapacitating neurovascular disease characterized by recurrent attacks of severe headache, autonomic nervous system dysfunction, and, in some patients, an aura causing visual, sensory, motor, or other neurologic symptoms.6 It is a primary headache disorder with a genetic basis.7
Migraine headaches account for approximately 1 million visits to the ED per year.8 Migraine attacks typically begin in the second decade of life and peak in prevalence in the fourth decade of life, with a 1-year period prevalence of 7% of men and 24% of women.9 Overall, migraine is more prevalent among women (18%) than among men (6%).9 During childhood, however, there is no gender difference in the prevalence of migraine. After menarche, a correlation between migraine and menstruation is noted in half of female migraine sufferers. After menopause, the prevalence of migraine among women decreases.10
Historically, migraine headaches have been considered to be vascular in origin. However, this hypothesis is no longer tenable as alterations in cerebral blood flow do not correlate with the various phases of the headache attack or vascular territories and do not explain features of an acute migraine such as premonitory mood disturbances, nausea, and osmophobia. Rather, vascular changes are now thought to be an epiphenomenon to what is a primary neurologic event.11 Abnormal trigeminal nerve activation, possibly triggered by cortical spreading depression or, less likely, a sterile neuropeptide-induced inflammatory process, leads to pain and sensitization of higher order neurons in the brainstem and thalamus. Descending modulation is likely to be compromised as well. It is not yet known what initiates the pathophysiologic process that leads to a migraine attack. Migraine is commonly thought of in two major categories: migraine without aura, or common migraine, which is the most frequent form of migraine and accounts for approximately 80% of all cases (Box 103-2); and migraine with aura, or classic migraine, which has specific reversible neurologic symptoms that precede the actual headache (Box 103-3) and is seen less frequently.6
Clinical Features
The aura of classic migraine consists of focal neurologic symptoms that precede and herald the migraine attack. By definition, the aura is fully reversible and typically lasts 10 to 20 minutes, although it may continue for as long as 1 hour. The most common aura is visual; features may include scintillating scotomas (bright rim around an area of visual loss), teichopsias (subjective visual image perceived with eyes open or closed), fortification spectrums (zigzagged wall of fortress slowly drifting across visual field), photopsias (poorly formed brief flashes or sparks of light), and blurred vision. Less common auras include somatosensory phenomena such as tingling or numbness, motor disturbances, and cognitive or language disorders.6
Ophthalmoplegic migraine is a rare syndrome associated with paresis of one or more ocular nerves, most commonly the third cranial nerve. Patients typically present with ipsilateral headache associated with extraocular muscle paresis and occasionally pupillary changes. The ophthalmoplegia or pupillary changes may last for days to weeks and rarely may become permanent. Because of the neurologic abnormalities, secondary causes including intracranial aneurysm and mass lesion should be ruled out in the absence of a clear history of recurrent identical episodes.12
Hemiplegic migraine is characterized by a motor aura consisting of hemiparesis or hemiplegia. The progression of the motor deficit is gradual and in most cases is accompanied by a visual, sensory, or speech disturbance. The neurologic symptoms last up to 60 minutes, followed by headache. Rarely, the motor deficit is persistent, resulting from a true migrainous stroke.2 A familial version of hemiplegic migraine is associated with genetic mutations of ion transporters.7
Basilar-type migraines arise with an aura referable to the brainstem and are associated with multiple neurologic findings, including visual symptoms (often total blindness), dysarthria, tinnitus, vertigo, bilateral paresthesias, paresis, and altered level of consciousness. The symptoms are stereotypic and resolve spontaneously.2
Many factors can trigger migraine headaches in predisposed persons. Common precipitants include sleep deprivation, stress, hunger, hormonal changes including menstruation, and use of certain drugs including oral contraceptives and nitroglycerin.13 In addition, some patients report specific food sensitivities to chocolate, caffeine, and foods rich in tyramine, monosodium glutamate, and nitrates. Alcohol, specifically red or port wine, has also been implicated. In others, certain sensory stimuli, such as a strong glare or strong odors, loud noises, and weather changes, can trigger an attack.6
Diagnostic Evaluation
Routine neuroimaging is not necessary for patients with typical recurrent migraine headaches.14 An increasing appreciation of medical imaging–induced radiation toxicity warrants prudent use of computed tomography (CT) scanning in younger patients. Neuroimaging should be considered for older or immunocompromised patients with new-onset headaches, headaches with a progressive course, headaches worsened by Valsalva maneuver, headache causing awakening from sleep, or headaches associated with unexplained neurologic abnormalities.14 Such patients have a higher likelihood of having a secondary cause of headache, such as tumor, arteriovenous malformation, or structural lesion. In addition, patients who present with a thunderclap or abrupt-onset headache require a lumbar puncture to rule out subarachnoid hemorrhage if findings on a CT scan are normal.15 The description “worst headache of life” may be associated with subarachnoid hemorrhage but suffers from interobserver variability.16
Treatment
The pharmacologic treatment of migraine is divided into abortive therapies, which attempt to limit the intensity and duration of a given episode, and prophylactic therapies, which are intended to decrease the frequency and intensity of attacks. The goals of acute migraine therapy include rapid pain relief, minimization of headache recurrence and medication side effects, restoration of the patient’s ability to function, and minimization of the use of backup and rescue medications.17
There are several approaches to treatment of the acute headache episode, depending on the severity of the attack (Table 103-1). The choice of agents depends on the patient’s previous response to specific therapies, the existence of comorbid conditions, and the presence or absence of nausea or vomiting. Gastric stasis is common during acute migraine attacks and may limit the effectiveness of oral agents.18
For mild to moderate attacks, simple analgesics such as acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs) are often effective.19,20 In the presence of nausea or vomiting, the addition of an agent such as metoclopramide enhances the absorption and effectiveness of these medications.21 Appropriate doses and possible side effects are listed in Table 103-1.
Dopamine antagonists, such as the neuroleptics prochlorperazine and chlorpromazine, and the antiemetics metoclopramide and droperidol have been shown to be highly effective as monotherapy for acute migraine attacks.22–24 Because of their efficacy, safety, tolerability, and few contraindications, they are used by many emergency physicians as first-line therapy for many migraine patients. For this class of medication, clinical research has outpaced preclinical work, and thus a compelling mechanism of action is lacking. However, migraine pathogenesis is likely to involve dopamine pathways.25 The most common side effects after parenteral administration include sedation, postural hypotension, and extrapyramidal symptoms, most notably akathisia, which can be treated with diphenhydramine or midazolam.26
DHE is administered intravenously (IV) in a dose of 1.0 mg during 2 minutes; this can be repeated in 1 hour if pain control has not been achieved. Because DHE can cause nausea and vomiting, patients should be pretreated with an antiemetic such as metoclopramide 10 mg IV or prochlorperazine 10 mg IV. Repeated administration of the intravenous form of DHE is effective in patients with status migrainosus.27 Contraindications to use of DHE include pregnancy, breast-feeding, poorly controlled hypertension, coronary artery disease, and peripheral vascular disease. DHE should not be used if the patient has already taken any drug in the triptan class or if the patient is using macrolides or protease inhibitors.
Sumatriptan, the first-approved medication of the triptan class, a class of selective 5-HT (1B/1D) receptor agonists, is available for subcutaneous administration, and it is the most common triptan preparation used in the ED setting.8 Other triptans available in the United States include eletriptan, almotriptan, zolmitriptan, naratriptan, frovatriptan, and rizatriptan. The initial dose of sumatriptan is 6 mg given subcutaneously, which may be repeated once in 1 hour if the patient has a partial response to the first dose. Common side effects include tingling, flushing, warm or hot sensations, heaviness in the chest, and initial worsening of the underlying headache.28 Sumatriptan has contraindications similar to those for DHE and should not be used within hours of administration of an ergotamine-containing medication or DHE. A smaller dose of subcutaneous sumatriptan may limit side effects.29
Opioid analgesics such as morphine should be reserved for patients who do not respond to any of the medications listed or have contraindications to all standard migraine therapies. Although opioids are frequently used, they have been shown to be less efficacious than other agents and are associated with frequent ED visits.30,31
Recurrence of migraine within 24 hours of ED discharge is common, regardless of medication administered or pain intensity at discharge.32 Corticosteroids are effective at decreasing the rate of migraine recurrence after ED discharge, although with a number needed to treat (NNT) of 9, these medications are not necessarily indicated for all patients.33 Oral naproxen 500 mg and sumatriptan 100 mg are reasonable treatment options for the recurrent headache.34
Prophylactic Therapy
Prophylactic therapy is indicated for patients who have frequent attacks (more than two or three functionally disabling episodes per month), prolonged attacks lasting more than 48 hours, or attacks that are severe and debilitating. Several classes of medications are used for the prophylaxis of migraine, including beta-blockers, tricyclic antidepressants, and antiepileptic drugs.35,36 Prophylactic therapy is best initiated in consultation with the patient’s primary care provider or neurologist.
Cluster Headache
Cluster headache is the only headache syndrome that is more common in men than in women. It typically occurs in young to middle-aged adults who smoke, with a peak incidence in the late 20s.37 The headaches tend to occur repeatedly during a defined time interval, hence the term cluster. Several attacks can occur in 1 day, and a typical cluster period may last weeks to months. Several precipitating factors have been implicated, most notably the ingestion of alcohol. Stress and climatic changes may also play a role in susceptible persons.
Clinical Features
Cluster headaches occur suddenly with little warning, and multiple episodes can occur within a 24-hour period. Each headache lasts from 15 minutes up to 3 hours. The headache typically begins with a unilateral sharp, stabbing pain in the eye, which may awaken the patient from sleep. The attacks occur exclusively in the territory of the trigeminal nerve.38 Unlike the migraineur, the patient with cluster headache predictably presents agitated and anxious, rocking, rubbing the head, and pacing. The attack subsides rapidly, often leaving the patient exhausted.
Accompanying the headache are ipsilateral autonomic symptoms such as ptosis, miosis, and forehead or facial sweating. The eye often is injected and tearing, and many patients have unilateral nasal congestion.2
Differential Diagnosis
Other headache disorders that mimic cluster headache include carotid artery dissection, trigeminal neuralgia, and the rare trigeminal autonomic cephalalgia paroxysmal hemicrania. Carotid dissection should be excluded as the diagnosis in patients who present with unilateral face or neck pain and Horner’s syndrome. With trigeminal neuralgia, the pain peaks within seconds, lasts only a couple of minutes, and can be provoked by specific trigger points on the face or oral mucosa. The trigeminal autonomic cephalalgias are manifested by a brief unilateral headache that recurs dozens of times per day, often accompanied by the same unilateral eye and nasal symptoms as cluster.2
Treatment
Cluster headache is brief in duration and may resolve before a patient presents to medical attention. High-flow oxygen should be considered first-line therapy. Delivered through a non-rebreather mask at a rate of 12 L/min, it has been shown to abort the headache within 15 minutes in 78% of patients, with an NNT of 2 compared with patients who breathe room air.39 Subcutaneous sumatriptan is also an effective therapy for acute cluster headache and should be dosed at 6 mg.40 Larger doses do not confer much benefit, whereas smaller doses may decrease some of the medication side effects. Alternatively, octreotide or intravenous dopamine antagonists may be effective.41,42
Once the acute attack has been relieved, the emergency physician’s work is not done. The acute attack is part of an ongoing “cluster” of headaches that likely will recur the following day. Corticosteroids have long been theorized to help break the cluster, although high-quality evidence is not available. A recommended regimen is 60 mg of prednisone daily for 10 days, followed by a 1-week taper. Verapamil, dosed at 120 mg three times a day, will decrease the frequency of attacks by the end of the first week of therapy.43
Tension-type Headache
Tension-type headache is the most common recurrent headache disorder, affecting more than 50% of the population, but it is an infrequent cause of ED visits.4,44 Women are affected slightly more frequently than men are, with peak prevalence in the fourth decade of life. These headaches typically do not cause substantial functional disability, and patients are able to continue with their normal daily activities. The median frequency of headaches is six per month, and stress and lack of sleep are implicated as triggering factors.2,45 By definition, episodic tension-type headache lasts as little as 30 minutes and as long as 7 days.
Little is known about the pathophysiology of tension-type headache. There is no clear evidence that increased muscle activity is present, and the physical examination will reveal tender areas of the scalp and neck with both tension and migraine headaches. Despite different epidemiologic profiles, similar response to many therapeutics suggests that tension and migraine headaches may be part of a continuum with similar pathophysiologic mechanisms.46,47
Clinical Features
Patients typically complain of a tight, bandlike discomfort around the head that is nonpulsating and dull. They also may experience tightening of the neck muscles. A majority do not seek medical assistance because the headache usually is mild in intensity and not functionally disabling. On occasion, the discomfort can build up slowly and fluctuate in severity for several days. Unlike in migraine, the headache does not worsen with physical activity, and accompanying symptoms, such as nausea, vomiting, phonophobia, and photophobia, are unusual. Anxiety and depression may coexist with chronic tension headache, which by definition occurs more than 15 days a month and can be daily and unremitting.2
Differential Diagnosis
Tension headache is the least distinct of all of the primary headache disorders, and its diagnosis is based mainly on the absence of features that would suggest another headache diagnosis. Because of this lack of specificity, the clinician often may hesitate to make this benign diagnosis without other investigations to exclude organic disease. The most common disorders mimicking tension headache are migraine, idiopathic intracranial hypertension, oromandibular dysfunction, cervical spondylosis, sinus or eye disease, and intracranial masses.48
Treatment
For a majority of patients with tension headaches, simple analgesics, such as acetaminophen or an NSAID, are adequate for pain control. Because tension-type headache is more common in sedentary persons, a regular exercise program may help.37 Antiemetic dopamine antagonists may be useful therapy.49 Patients with chronic symptoms may exhibit signs of depression or anxiety, and these patients often respond to medications and nonpharmacologic regimens that treat these conditions. Some nonpharmacologic regimens are meditation, massage, and biofeedback.
Secondary Headache Disorders
Principles of Disease
Subarachnoid hemorrhage (SAH) refers to extravasated blood in the subarachnoid space. Presence of the blood activates meningeal nociceptors, leading to diffuse occipital pain along with signs of meningismus. SAH accounts for up to 10% of all strokes and is the most common cause of sudden death from a stroke.50
Approximately 80% of patients with nontraumatic SAH have ruptured saccular aneurysms.15 Other causes include arteriovenous malformations, cavernous angiomas, mycotic aneurysms, neoplasms, and blood dyscrasias. SAH may be caused secondarily by an intraparenchymal hematoma that dissects its way into the subarachnoid space.
The risk for aneurysmal SAH increases with age; most cases occur between the ages of 40 and 60 years.51 In children and adolescents, aneurysms are uncommon, and when SAH occurs, it usually is secondary to an arteriovenous malformation. It is estimated that 2% of the general population harbor a berry aneurysm, and the risk of rupture may increase with aneurysmal size.52 Other risk factors associated with SAH include hypertension, smoking, excessive alcohol consumption, and sympathomimetic drugs.53 Increased systolic blood pressure values and long-term hypertension before aneurysm rupture seem to predict fatal SAH independently of aneurysm size or the patient’s age at the time of rupture; patient gender also does not influence mortality.54 A familial association of cerebral aneurysms with several diseases, including autosomal dominant polycystic kidney disease, coarctation of the aorta, Marfan syndrome, and Ehlers-Danlos syndrome type IV, has been described.
Of all patients presenting to the ED with a primary complaint of headache, less than 1% have SAH. Many patients with SAH die before reaching the hospital; preadmission mortality rates range from 3 to 26%.15 Because of the significant morbidity and mortality associated with this condition and the high likelihood of clinical deterioration in patients who initially are misdiagnosed, SAH should be a primary consideration in the initial ED evaluation of all patients with nontraumatic headache. Accordingly, familiarity with its presentation is essential.
Clinical Features
A majority of patients with SAH present with a sudden, cataclysmic thunderclap headache, which often is described as “the worst headache of [their] life.” The onset of headache may be associated with exertion, the Valsalva maneuver, or sexual intercourse in up to 20% of patients.15 The headache of SAH classically peaks in intensity within seconds to minutes. Headaches that take longer to peak in intensity are less likely to be SAH.55 Associated signs and symptoms include nausea and vomiting in approximately 75% of patients, neck stiffness in 25%, and seizures in 17%.51 Physical findings depend on the extent of the SAH. Meningismus is present in more than 50% of patients, and up to 20% have focal neurologic abnormalities.56 Funduscopic examination may reveal retinal or subhyaloid hemorrhages, and patients also may have an isolated third or sixth nerve palsy. Oculomotor (third) nerve compression secondary to an expanding aneurysm leads to pupillary dilation. Approximately 50% of patients with a ruptured aneurysm are restless or have an altered level of consciousness. Although a majority do not have focal neurologic signs, such signs when present may indicate the site of the aneurysm.57
The patient’s prognosis is related to neurologic status at hospital admission. The Hunt and Hess scale stratifies patients according to their clinical signs and symptoms at the time of presentation and is predictive of outcome (Table 103-2).58 Patients who present with a grade 1 or grade 2 hemorrhage tend to have a good prognosis. Patients with grade 4 or grade 5 hemorrhage tend to do poorly, and these patients have an altered mental status, ranging from stupor to deep coma, together with focal neurologic signs and symptoms. Patients with grade 3 hemorrhage present with drowsiness or confusion and are at risk for rapid clinical deterioration.
Table 103-2
Hunt and Hess Clinical Grading Scale for Cerebral Aneurysms and Subarachnoid Hemorrhage
| GRADE | CONDITION |
| 0 | Unruptured aneurysm |
| 1 | Asymptomatic or minimal headache and slight nuchal rigidity |
| 2 | Moderate or severe headache, nuchal rigidity, no neurologic deficit other than cranial nerve palsy |
| 3 | Drowsiness, confusion, or mild focal deficit |
| 4 | Stupor, moderate to severe hemiparesis |
| 5 | Deep coma, decerebrate posturing, moribund appearance |
Diagnostic Studies
A non–contrast-enhanced head CT scan is the first-line test for the diagnosis of SAH and should be ordered emergently when this diagnosis is suspected (Fig. 103-1). For acute hemorrhage less than 24 hours old, the sensitivity of CT in identifying hemorrhage is greater than 90%; however, sensitivity decreases to approximately 50% by the end of the first week.59 Because the sensitivity of head CT is limited, particularly as the duration of headache increases, the cerebrospinal fluid (CSF) should be examined for blood or xanthochromia, the yellowish tinting of CSF by hemoglobin breakdown products, for SAH to be ruled out definitively. A common pitfall in ED diagnostic workups of headache is to overestimate the sensitivity of the CT scan for subarachnoid blood. Alternatively, an appropriate strategy may be to perform lumbar puncture without first performing a CT scan to decrease throughput time in carefully selected young patients who have completely normal physical examination findings, who are not immunocompromised, and in whom SAH is not strongly suspected.60 This strategy may be particularly appropriate for patients in whom the differential diagnosis includes other conditions requiring a CSF analysis, such as meningitis. An alternative strategy is to perform a non–contrast-enhanced head CT scan followed by CT angiography rather than the lumbar puncture; this is reasonably sensitive and may be appropriate for patients at lower risk of disease.59 Concerns of additional radiation exposure and contrast agent toxicity need to be weighed against the pain caused by a lumbar puncture. Finally, several published clinical decision rules can help risk stratify patients.16,61
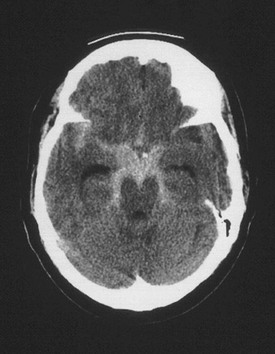
Figure 103-1 Cerebral aneurysm. Shown is a computed tomography scan of an aneurysmal subarachnoid hemorrhage in a 55-year-old woman. Subarachnoid blood can be seen within the interpeduncular and ambient cisterns and the right sylvian fissure from a ruptured aneurysm at the junction of the right carotid artery and the posterior communicating artery. (From Brisman JL: Neurosurgery for cerebral aneurysm. Emedicine, updated Sep 23, 2010. Available at http://emedicine.medscape.com/article/252142-overview#a1.)
After lumbar puncture is performed, interpretation of the results can be challenging because up to one third of spinal fluid analyses may be falsely positive.62 To differentiate a traumatic lumbar puncture from SAH, the patient’s CSF should be spun and the supernatant observed for xanthochromia. The yellowish pigmentation is secondary to the metabolism of hemoglobin to pigmented molecules of oxyhemoglobin and bilirubin, a process that can take up to12 hours to occur.63 The method of comparing the red blood cell count in the first and the last tubes of CSF is less accurate, although SAH cannot be ruled out if a substantial numbers of red blood cells persist in tube 4.15 CSF xanthochromia in association with normal findings on the CT scan is suggestive of SAH. After the diagnosis is established, angiography should be performed to study the vascular anatomy and to identify the source of hemorrhage.
A normal non–contrast-enhanced head CT scan followed by a normal spinal fluid analysis definitively rules out SAH and does not need to be followed with angiography, even in patients at high risk of disease.64 However, this strategy does not rule out other causes of thunderclap headache that may be in the differential diagnosis, such as carotid artery dissection, cerebral venous sinus thrombosis, and reversible cranial vasoconstrictor syndrome.
Up to 90% of patients with SAH have cardiac arrhythmias or electrocardiographic abnormalities suggestive of acute cardiac ischemia, which may lead to an erroneous primary cardiac diagnosis. Typical electrocardiographic findings include ST-T wave changes, U waves, and QT prolongation.65
Treatment
The management of SAH is complex and includes initial resuscitation, stabilization, and emergent neurosurgical consultation. The goals of management are to treat the acute medical and neurologic complications, to prevent recurrent hemorrhage, and to forestall the ischemic complications of vasospasm. Because of an altered level of consciousness, patients with SAH of grade 3 or higher are at risk for respiratory depression and hypercapnia, which can lead to further increases in intracranial pressure (ICP); therefore, these patients may require early endotracheal intubation. Blood pressure also should be closely monitored because of the risk of continued bleeding or recurrent hemorrhage. Nimodipine, a calcium channel blocker, should be started soon after a diagnosis of aneurysmal SAH is made to lessen the likelihood of poor outcome.66 Because nimodipine may cause transient hypotension in some patients, hemodynamic monitoring is required during its administration. The recommended dose is 60 mg by mouth or nasogastric tube every 4 hours. Antifibrinolytics (e.g., aminocaproic acid) do not improve overall outcome because the reduction in the rate of rebleeding is offset by an increase in poor outcome caused by cerebral ischemia.67 Similarly, corticosteroids have not been demonstrated to be of benefit.68
Blood pressure management should be determined by the patient’s clinical status with involvement of the treating neurosurgeon. The typical treatment goal is a systolic blood pressure below 160 mm Hg or a mean arterial pressure below 130 mm Hg unless vasospasm is present.69
Analgesics, including opioids, should be used for persistent headache. In patients who are nauseated or at risk for vomiting, antiemetics should be administered. Agitated patients require sedation, and all patients should be placed at bed rest in a quiet and dark environment. Clinically evident seizures should be treated with anticonvulsants, but the prophylactic use of these drugs is controversial.70 A majority of patients require hemodynamic and ICP monitoring in an intensive care setting. For definitive management, endovascular coil embolization is preferable to neurosurgical clipping, but this decision is based on size, location, and morphologic features of the aneurysm as well as local expertise.71
Intracranial Neoplasm
Headache, the most common presenting complaint among patients with brain tumor, is reported by approximately 50% of the patients.72 Headache is less common in older patients with brain tumors, presumably because of age-related atrophy. Headache can be caused by benign or malignant primary neoplasms of the central nervous system as well as by metastatic lesions. The most common causes of metastasis are lung and breast carcinoma, followed by malignant melanoma and carcinomas of the gastrointestinal tract.73
The headache of intracranial neoplasms can be caused by several mechanisms, including direct involvement of and traction on pain-sensitive structures such as meninges or larger cerebral vessels, or it may be a symptom of increased ICP. The pain patterns produced are highly variable, depending on the location and size of the mass and the structures involved. Rapidly growing tumors are more likely to be associated with headache. Headaches often but not always are on the same side as the tumor.72,74
Clinical Presentation
The typical patient presents with complaints of a worsening headache that has been present for weeks to months. The headache may have been present initially only on awakening, gradually becoming continuous. The classic triad of brain tumor headache—sleep disturbances, severe pain, and nausea and vomiting—is seen in a minority of patients.73 Vomiting, when it is present, may be projectile and not preceded by nausea and often can be attributed to increased ICP. If increased ICP is present, the headache often is bilateral and worsened by coughing, sneezing, bending, defecation, and sexual intercourse. Other presentations of intracranial neoplasms include seizures, personality changes, and cognitive difficulties.73
Diagnostic Evaluation
The diagnosis of brain tumor may be suspected from the history and neurologic examination. Early in the course, patients present with just a headache and an intact neurologic examination, although the majority of intracranial neoplasms eventually will cause focal neurologic deficits. Neuroimaging with CT or magnetic resonance imaging (MRI) is the most efficient way to confirm the diagnosis. Contrast enhancement on CT often improves the identification of the underlying mass lesion and helps differentiate it from other causes, including abscess, hematoma, and vascular malformation.75
Treatment
Management consists of urgent referral to neurosurgery and treatment of any acute complications, including increased ICP and seizures. For patients who present with symptoms suggestive of increased ICP (e.g., headache, nausea, vomiting, confusion, weakness), treatment with steroids has been shown to be beneficial. Dexamethasone is the high-potency steroid used most often to treat edema associated with brain tumors. It has several advantages over other glucocorticoids, including a longer half-life, reduced mineralocorticoid effect, and lower associated incidence of cognitive and behavioral complications.75 The exact dose of steroids necessary for each patient varies in accordance with histologic features, size, and location of the tumor and the amount of edema present. In general, most patients require between 8 and 16 mg of dexamethasone per day. An appropriate starting dose in the ED is 10 mg IV, followed by 4 mg every 6 hours.
Patients who have a seizure should receive anticonvulsant therapy. A multitude of antiepileptic drugs are available, and none has demonstrated superiority in this population of patients. Newer generation antiepileptics may be more tolerable. The choice of antiepileptic should be made in consultation with the specialist. Empirical or prophylactic treatment does not appear to delay or to prevent the onset of seizure activity and may expose the patient to unnecessary complications and toxicity.76
Giant Cell Arteritis
Giant cell arteritis, or temporal arteritis, is a systemic inflammatory process of the small and medium-sized arteries. Extracranial branches of the aortic arch and the ophthalmic vessels most commonly are involved, but the process may affect any artery in the body. The mean age at onset is 71 years, and it is rare before the age of 50 years. Women are affected more commonly than men are.77
Clinical Presentation
Headache is the most common initial manifestation of giant cell arteritis and occurs in more than 70% of patients with this disorder.78 The headache often is of 2 to 3 months’ duration and can be continuous or intermittent and often worsens at night or on exposure to cold. The pain may be described as sharp, throbbing, boring, or aching and usually is localized to the temporal region but may occur anywhere in the head. The physical examination may reveal tenderness over the scalp in the area of the temporal artery, with exacerbation of the pain by wearing a hat or resting the head on a pillow. Patients also may experience jaw claudication secondary to vascular insufficiency of the masseter and temporalis muscles. Systemic signs and symptoms, including fever, anorexia, and weight loss, often are present. Approximately 40% of patients develop symptoms of polymyalgia rheumatica, pain in their large proximal joints, with symptoms referable to the neck, torso, and lower back. Typically, pain and stiffness are worse in the morning and lessen as the day goes on.78
The most serious complication of giant cell arteritis is permanent visual loss, which eventually occurs in one third of untreated cases. Amaurosis fugax also can occur before permanent visual loss. Other complications include peripheral neuropathies, transient ischemic attacks, and stroke.77
Diagnostic Evaluation
The physical examination may reveal abnormalities of the temporal arteries, including tenderness, reduced or absent pulsations, erythema, and nodularity or swelling, best detected by light palpation just anterior and slightly superior to the tragus of the ear. Visual acuity, visual field testing, and thorough funduscopic examination should be performed.79
A majority of patients have a significant elevation of the erythrocyte sedimentation rate (ESR), usually to more than 50 mm/hr and often more than 100 mm/hr, although an elevated ESR is not specific for the disorder and a normal value does not rule out the diagnosis. Other abnormalities on laboratory studies include mild to moderate anemia, elevated C-reactive protein level, liver function abnormalities, and thrombocytosis. Temporal arteritis is substantially less likely in patients with an ESR below 50 mm/hr and a C-reactive protein level below 2.45 mg/dL, although patients in whom there is high suspicion of disease should be referred for biopsy even if both of these biomarkers are normal.80,81 The diagnosis is confirmed by temporal artery biopsy. Because this is a patchy disease with skip lesions, multiple biopsy specimens of a long segment of the artery may need to be examined.
Carotid and Vertebral Artery Dissection
Carotid and vertebral dissections are the most frequent cause of stroke in persons younger than 45 years, accounting for approximately 20% of all cases in this age group.82 Although dissections may occur spontaneously, careful history taking frequently identifies an association with sudden neck movement or trauma preceding the event.83 Reported mechanisms include neck torsion, chiropractic manipulation, coughing, minor falls, and motor vehicle collisions. Early symptoms and signs may be subtle, and delays in diagnosis are common in the absence of neurologic findings. The median delay from symptom onset to diagnosis can be several days.84,85
The pathologic lesion is intramural hemorrhage within the media of the arterial wall. The hematoma can be localized or extend circumferentially along the length of the vessel, resulting in partial or complete occlusion. Platelet aggregation and thrombus formation also occur, further compromising vessel patency or causing distal embolization. The timing of these events is variable, and a patient may experience symptoms of cerebral ischemia days to years after dissection.83
Clinical Presentation
The typical presentation of the patient with carotid or vertebral dissection is the abrupt onset of pain in the neck or face. Neurologic findings usually occur within the first few hours, but autopsy studies have shown that strokes may occur months later.82
Carotid Dissection.: The classic triad of symptoms for carotid dissection includes unilateral headache, ipsilateral Horner’s syndrome, and contralateral hemispheric findings that may include aphasia, neglect, visual disturbances, or hemiparesis. The headache is often severe and throbbing but may be subacute and similar to previous headaches. Acute severe retro-orbital pain in a previously healthy person with no history of cluster headaches is particularly suggestive of carotid dissection.86 Most patients eventually develop signs of cerebral ischemia. Warning symptoms include transient ischemic attacks, amaurosis fugax, episodic lightheadedness, and syncope. Spontaneous dissection of the carotid artery has a favorable prognosis and recurrence is uncommon. Factors associated with a worse prognosis include old age, occlusive disease on angiography, and stroke as the initial presenting symptom.83
Vertebral Dissection.: Vertebral artery dissections are less common than carotid dissections. The classic presentation is that of a relatively young person with severe, unilateral posterior headache and neurologic findings. The majority of patients develop a rapidly progressive neurologic deficit with symptoms of brainstem and cerebellar ischemia. Common findings include vertigo, severe vomiting, ataxia, diplopia, hemiparesis, unilateral facial weakness, and tinnitus. Spontaneous vertebral artery dissection appears to be relatively rare. Approximately 10% of patients who develop a vertebral dissection die during the acute phase, secondary to massive stroke. For patients who survive, the prognosis is usually good.87
Diagnosis and Treatment
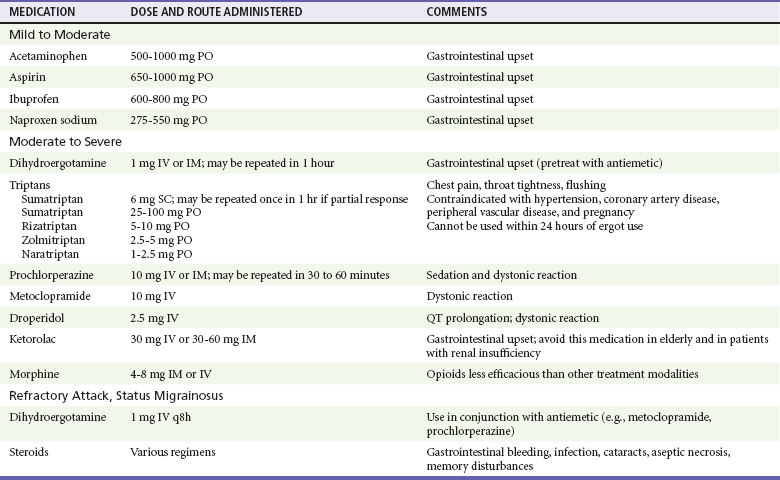
Identification of patients with dissection is challenging. The emergency physician should consider the diagnosis in any young patient who presents with head or neck pain with focal neurologic findings. A non–contrast-enhanced head CT scan is often normal in uncomplicated dissection. Further imaging studies, such as CT angiography, magnetic resonance angiography, and catheter angiography, are required to confirm the diagnosis.88 Figure 103-2 shows an example of carotid artery dissection on MRI. Duplex imaging is not sufficiently sensitive to rule out disease.89 Treatment is aimed at prevention of stroke. It is not yet clear if early anticoagulation offers an outcome benefit over antiplatelet therapy.90
Cerebral Venous Sinus Thrombosis
Thrombosis of the intracranial veins and sinuses is a rare cause of stroke but tends to affect younger patients without traditional cerebrovascular risk factors.91 Because of the significant associated morbidity, however, cerebral venous sinus thrombosis (CVST) is an important consideration in the differential diagnosis for headache in patients with suggestive signs and symptoms.
Thrombophilia is usually identified in patients with CVST, including genetic and hypercoagulable disorders, pregnancy and the puerperium, inflammatory systemic disorders including vasculitis, connective tissue disorders, and medications such as oral contraceptives. Other risk factors include head trauma, head and orofacial infections, and neurosurgical procedures.91
Clinical Presentation
The clinical presentation can be variable, depending on the location of the thrombosis. Common symptoms and signs include headache, seizures, decreased level of consciousness that may progress to coma, and focal neurologic deficits. Papilledema is less common with acute presentations. With cavernous sinus thrombosis, the clinical picture is dominated by ocular findings including orbital pain, proptosis, and paralysis of extraocular movements.91
Diagnostic Evaluation
An elevated D-dimer level is present in approximately 90% of cases of CVST and along with the clinical findings can be used to exclude the diagnosis in individual patients at low risk of disease.92,93 The definitive diagnosis of CVST is based on neuroimaging of the area of thrombosis. CT by itself is an insensitive test, but it may reveal nonspecific late lesions, such as an infarct, hemorrhage, or edema. The key to diagnosis is to image the venous system itself. This is best accomplished by a combination of MRI to visualize the thrombosed vessel and magnetic resonance venography to detect nonvisualization of the same vessel.94 CT angiography and venography may also be used to visualize the cerebral venous system.
Treatment
Specific treatment of CVST includes anticoagulation to prevent propagation of the thrombosis as well as complications (e.g., pulmonary embolism). In patients whose clinical condition worsens despite anticoagulation, thrombolysis or thrombectomy may be considered in centers with expertise in interventional procedures. Seizures should be treated with anticonvulsants.95
The prognosis with CVST is based on the underlying etiology, condition at time of diagnosis, and development of complications. Although overall mortality is low compared with other strokes, victims of this disease tend to be younger and healthier patients.94
Idiopathic Intracranial Hypertension
Although this disease is sometimes called pseudotumor cerebri or benign intracranial hypertension, the term idiopathic intracranial hypertension (IIH) reflects the current incomplete understanding of the pathophysiology and the fact that this disorder is not benign—a substantial minority of patients develop permanent visual loss. Compared with other headache disorders, IIH is a relatively uncommon neurologic disease seen primarily in young obese women of childbearing age. Several predisposing factors have been identified, including the use of oral contraceptives, anabolic steroids, tetracyclines, and vitamin A.96
Pathophysiology and Clinical Features
Visual complaints are common, and patients may experience transient visual obscuration several times a day secondary to ischemia of the visual pathways. These episodes can be followed by prolonged periods of visual loss, which can become permanent in up to 10% of patients. Patients also may complain of nausea, vomiting, dizziness, and pulsatile tinnitus. The physical examination will reveal papilledema and visual field defects, including an enlarged blind spot initially, followed by loss of peripheral vision. On occasion, a sixth nerve palsy is noted.96
Diagnosis
The diagnosis of IIH should not be made without neuroimaging and measurement of ICP. Diagnostic criteria are listed in Box 103-4. Venous sinus disease should be excluded in all patients with this diagnosis.2
Treatment
Predisposing factors (e.g., discontinuation of implicated medications) should be corrected. Symptomatic treatment often includes lowering of ICP and management of the headache. Evidence-based therapies are lacking. Acetazolamide (a carbonic anhydrase inhibitor) can be used to decrease CSF production alone or with a loop diuretic such as furosemide.97 Steroids also have been used, although their mechanism of action is unclear. Prolonged therapy is a problem, and rebound headache often occurs when doses are tapered. In patients with impending visual loss or incapacitating symptoms, placement of a ventricular shunt or optic nerve sheath fenestration may be indicated.96
Post-traumatic Headache
Headache is the most common symptom after minor head injury. It often is part of a complex syndrome that can include dizziness, fatigue, insomnia, irritability, memory loss, and difficulty with concentration. There are approximately 2 million closed head injuries per year, and post-traumatic headache (PTHA) occurs in an estimated 30 to 50% of patients with these injuries.98 Acute PTHA develops hours to days after the injury and resolves within 3 to 6 months.99 Chronic PTHA may last several months to years and may mimic other forms of headache, including tension and migraine headaches. The presence of headache, dizziness, or nausea on initial presentation is strongly associated with the development of chronic PTHA.100
Acute Glaucoma
Treatment includes topical miotics, topical beta-blockers, oral carbonic anhydrase inhibitors (e.g., acetazolamide, 250 mg four times daily), intravenous osmotic agents (e.g., mannitol), and prompt referral to an ophthalmologist. The potential for diagnostic confusion between acute glaucoma, iritis, and cluster headache should be recognized. Although cluster headache may arise with pain, nausea, and a red eye, vision is not affected and the pupil generally is small and the eyelid is ptotic (from an oculosympathetic paresis). Acute iritis also arises with a painful red eye, but only acute angle closure glaucoma is associated with markedly elevated intraocular pressure.101,102
Post–Dural Puncture Headache
Principles of Disease and Pathophysiology
Headache is the most common complication of lumbar puncture, occurring in up to 40% of patients.103 The incidence is highest in the 18- to 30-year age group, but this complication is uncommon in young children and in adults older than 60 years. Although the onset often is immediate, patients may not report symptoms for several days. In a majority of affected persons, the duration of headache is less than 5 days.103
The cause of post–dural puncture headache (PDPH) is not entirely clear. The most likely explanation is a persistent CSF leak that exceeds CSF production, resulting in CSF hypotension. If sufficient CSF is lost, the brain descends in the cranial vault when the patient assumes the upright position, leading to increased traction on the pain fibers. Thus the headache is characteristically positional and increases with the upright position and decreases with recumbency. The amount of time a patient remains recumbent after lumbar puncture does not appear to affect the incidence of headache.104
Certain factors have been implicated as causes of PDPH, including the size or diameter of the spinal needle, the orientation of the bevel during the procedure, and the amount of fluid withdrawn. Smaller-diameter needles cause less leakage, and it is postulated that insertion of the needle with the bevel up (i.e., bevel pointing up when the patient is in the lateral position) minimizes damage to the dural fibers. Use of atraumatic needles or pencil-point needles (e.g., Whitaker or Sprotte) also has been shown to reduce the incidence of PDPH significantly.105,106
Clinical Features
PDPH typically is bilateral, throbbing, and exacerbated by the upright position. Associated signs and symptoms include neck stiffness, nausea, vomiting, auditory disturbances including tinnitus and hearing loss (hypoacusis), and ocular symptoms including blurred vision and diplopia.103
Treatment
Most PDPHs resolve spontaneously within a few days with bed rest, adequate hydration, and mild analgesics. For persistent headaches, methylxanthine agents may be useful. Caffeine sodium benzoate (500 mg in 1 L of fluid) may be effective.107 For severe headaches lasting longer than 24 hours, an epidural blood patch (autologous blood clot) relieves the headache in the majority of patients.108
Intracranial Infection
With acute bacterial meningitis, the patient often has a severe bursting headache that rapidly increases in severity during a short period. These patients typically have significant meningismus, with both Kernig’s and Brudzinski’s signs. With viral meningitis, patients also may complain of severe headache and nuchal rigidity, but the course is more indolent than with bacterial meningitis.109
The severity of headache associated with encephalitis depends on the type of virus involved. For example, the headache is usually mild with mumps encephalitis. With herpes simplex infection, however, the headache is abrupt and severe and frequently is associated with confusion, fever, altered level of consciousness, seizures, and focal neurologic signs. Patients with brain abscess often have headache as their presenting complaint. As the infection progresses, vomiting, focal neurologic signs, and depressed level of consciousness typically develop.110
Headache is a frequent complaint in patients with human immunodeficiency virus infection and can be caused by a number of conditions, including aseptic meningitis, toxoplasmosis, cryptococcal or tuberculous meningitis, and cytomegalovirus encephalitis. Cryptococcal meningitis in particular can be indolent but eventually lethal. Because spinal fluid analysis may be relatively bland, clinicians need to maintain a high index of suspicion for this particular pathogen as T-cell counts dwindle.111
In a majority of cerebral infections, the mechanism of head pain includes meningeal irritation and increased ICP. In addition, headache may be a general reaction to fever or the toxic products of the infecting agent.110
Hypertensive Headache
Contrary to common belief, hypertension is not an important cause of headache, and the occurrence of headache and hypertension in the same patient is often coincidental. Whether some patients with mild to moderate hypertension suffer from headache caused by elevated blood pressure is uncertain. The rate of blood pressure increase is more important as a cause of headache than the absolute blood pressure value is. Diastolic pressures lower than 130 mm Hg are rarely the cause of headache.2
Nonetheless, the association of headache with severe hypertension is well documented. Acute, severe headache is a prominent symptom of hypertensive encephalopathy, and most patients have blood pressure readings in the range of 250/150 mm Hg. Other conditions include headache secondary to toxic agents (e.g., drug-induced hypertension), pheochromocytoma, and eclampsia.2
Cervicogenic Headache
Cervicogenic headache refers to headache originating from disorders of the neck. Diagnosis is based on the presence of one of the following three distinct sets of symptoms112:
Medication-Overuse Headache
Excessive analgesic or antimigraine medication use and withdrawal from these medications can worsen an existing headache disorder in susceptible patients. Medication-overuse headache is underdiagnosed and often difficult to manage. It occurs in patients with a primary headache disorder (e.g., migraine, tension type) who use immediate-relief medications on a near-daily basis, often in excessive quantities. Medications that have been implicated include NSAIDs, aspirin or acetylsalicylic acid, acetaminophen, barbiturate-analgesic combinations, opioids, caffeine, ergotamine, and triptans. Often, patients with this disorder describe preemptive use of drugs, in anticipation of—rather than for—headache. Women are affected more commonly than men are, and the most frequently affected age group is that of persons between 30 and 40 years. The headache itself is variable and may be accompanied by asthenia, nausea, anxiety, depression, and difficulty with concentration. Typically, it is worse on awakening in the morning and after physical exertion.113
Treatment requires complete withdrawal of the medication being overused while preventive therapy is initiated and a new acute headache therapeutic. In addition, these patients require a comprehensive education and follow-up program with pharmacologic, dietary, and behavioral components.113
High-Altitude Headache
Headache is one of the cardinal manifestations of acute mountain sickness and can occur at altitudes higher than 5000 feet above sea level in unacclimatized persons. The headache is throbbing in nature, located in the temporal or occipital areas, and probably caused by a mild increase in ICP secondary to brain swelling.114 It is worse at night or in the early morning and exacerbated by the Valsalva maneuver or bending forward.115 Other findings associated with high-altitude illness include fatigue, nausea, vomiting, dizziness, insomnia, and altered mental status. Pulmonary edema and cerebral edema develop in severe cases. The treatment of these conditions includes supplemental oxygen and descent to a lower altitude.
Reversible Cranial Vasoconstriction Syndrome
Reversible cranial vasoconstriction syndrome (RCVS) is a disorder of cerebral arteries characterized by segmental areas of vasoconstriction. It causes recurrent thunderclap headache in susceptible patients and is a cause of ischemic or hemorrhagic stroke. The headache is often described as severe and throbbing and is associated with nausea, vomiting, and photophobia. The headache may be provoked by use of vasoactive medications or substances. RCVS has been called by the eponym Call-Fleming syndrome and is the same disease as postpartum angiopathy or migrainous vasospasm. Typically, patients present with a thunderclap headache with neurologic deficits or seizures, and during cerebral vascular imaging, abnormal areas of vasoconstriction, described as sausage on a string, are noted. Repeated vascular imaging within 3 months shows resolution of the areas of vasoconstriction. Treatment with calcium channel blockers has been described, although when to initiate treatment is not clear. The prevalence of this presumably rare disorder is not known. However, many patients who present to an ED with a thunderclap headache cannot be assigned a specific diagnosis. It is possible that the prevalence of this disorder is greater than currently understood.116,117
Other Headache Disorders and Facial Pain
Relatively recently described, nummular headache is a highly localized headache, rarely more than moderately painful, that is felt exclusively in a rounded, 2- to 6-cm area of the head. Epidemiology and pathogenesis are not yet well understood. Nummular headache is usually responsive to nonsteroidals, although a high frequency of headaches may be prevented with tricyclic antidepressants. In patients with new onset of headache, underlying disease of the scalp or dura should be excluded.118
Trigeminal Neuralgia
Trigeminal neuralgia is a painful unilateral affliction of the face characterized by brief electric shock–like (lancinating) pains limited to the distribution of one or more divisions of the trigeminal nerve. Pain is commonly evoked by trivial stimuli (e.g., washing, shaving, smoking, talking, or brushing teeth) but also may occur spontaneously. Individual attacks are brief, lasting a few seconds to less than 2 minutes, and are stereotypic in the individual patient. The lightning-like pains and unilateral grimaces characteristic of trigeminal neuralgia led to the designation tic douloureux. The diagnosis is straightforward in most patients on the basis of clinical criteria. However, because these symptoms also can be caused by an underlying mass lesion or multiple sclerosis, MRI is indicated in previously undiagnosed patients and when sensory loss or motor dysfunction is present.119
Several oral drugs have been effective in treatment of trigeminal neuralgia, including carbamazepine and oxcarbazepine. Phenytoin and baclofen may also be useful. However, approximately 30% of patients fail to respond to medical therapy. In these patients, surgical management, by alcohol or glycerol injection or microvascular decompression, may be indicated. For patients with acute severe pain, parenteral treatment with an antiepileptic drug such as phosphenytoin may be useful.120
Coital Headache
Coital cephalgia is a recurrent, benign headache associated with sexual activity and is more common in men than in women. Different types have been described, including headaches that occur before, during, or immediately after orgasm. They typically are occipital in location and may increase in severity with mounting sexual excitement. Their duration can be minutes to hours. On occasion, some patients experience a sudden, explosive headache that occurs during orgasm. In these patients, SAH should be ruled out.121,122
Cough and Exertional Headache
In some patients, severe headache can be provoked by rapid increase in intra-abdominal pressure, such as coughing, sneezing, laughing, heavy lifting or exertion, and Valsalva maneuver. The pain starts within a few seconds of the precipitant and typically is brief when it is associated with cough but can last as long as 24 hours when it is associated with exertion. The headache is bilateral and throbbing in nature and in a majority of patients resolves spontaneously without persistent neurologic symptoms (e.g., neck stiffness or photophobia). In some patients, the headache may be secondary to structural lesions, especially in the posterior fossa113; therefore, all previously undiagnosed patients require CT, or preferably MRI, followed by lumbar puncture to rule out intracranial disease, including SAH. For patients with recurrent benign exertional headache, treatment includes avoidance of the underlying triggering mechanism and use of analgesics as necessary. For patients with exertional headache, NSAIDs, including indomethacin, have been effective.121,122
References
1. McCaig, LF, Nawar, EW. National Hospital Ambulatory Medical Care Survey: 2004 emergency department summary. Adv Data. 2006;372:1–29.
2. The Classification Subcommittee of the International Headache Society. International Classification of Headache Disorders, 2nd ed. Cephalalgia. 2004;24(Suppl 1):1–151.
3. Bigal, M, Bordini, CA, Speciali, JG. Headache in an emergency room in Brazil. Sao Paulo Med J. 2000;118:58–62.
4. Friedman, BW, et al. Applying the International Classification of Headache Disorders to the emergency department: An assessment of reproducibility and the frequency with which a unique diagnosis can be assigned to every acute headache presentation. Ann Emerg Med. 2007;49:409–419.
5. Luda, E, Comitangelo, R, Sicuro, L. The symptom of headache in emergency departments. The experience of a neurology emergency department. Ital J Neurol Sci. 1995;16:295–301.
6. Goadsby, PJ, Lipton, RB, Ferrari, MD. Migraine—current understanding and treatment. N Engl J Med. 2002;346:257–270.
7. de Vries, B, Frants, RR, Ferrari, MD, van den Maagdenberg, AM. Molecular genetics of migraine. Hum Genet. 2009;126:115–132.
8. Vinson, DR. Treatment patterns of isolated benign headache in US emergency departments. Ann Emerg Med. 2002;39:215–222.
9. Lipton, RB, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349.
10. Brandes, JL. The influence of estrogen on migraine: A systematic review. JAMA. 2006;295:1824–1830.
11. Sprenger, T, Goadsby, PJ. Migraine pathogenesis and state of pharmacological treatment options. BMC Med. 2009;7:71.
12. Troost, BT. Ophthalmoplegic migraine. Biomed Pharmacother. 1996;50:49–51.
13. Diamond, S, Diamond, ML. Emergency treatment of migraine. Insights into current options. Postgrad Med. 1997;101:169–172.
14. Frishberg, BM. The utility of neuroimaging in the evaluation of headache in patients with normal neurologic examinations. Neurology. 1994;44:1191–1197.
15. Edlow, JA, Caplan, LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29–36.
16. Perry, JJ, et al. High risk clinical characteristics for subarachnoid haemorrhage in patients with acute headache: Prospective cohort study. BMJ. 2010;341:c5204.
17. Lipton, RB, Hamelsky, SW, Dayno, JM. What do patients with migraine want from acute migraine treatment? Headache. 2002;42(Suppl 1):3–9.
18. Aurora, S, Kori, S, Barrodale, P, Nelsen, A, McDonald, S. Gastric stasis occurs in spontaneous, visually induced, and interictal migraine. Headache. 2007;47:1443–1446.
19. Rabbie, R, Derry, S, Moore, RA, McQuay, HJ. Ibuprofen with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. (10):2010.
20. Derry, S, Moore, RA, McQuay, HJ. Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. (11):2010.
21. Tfelt-Hansen, P. The effectiveness of combined oral lysine acetylsalicylate and metoclopramide (Migpriv) in the treatment of migraine attacks. Comparison with placebo and oral sumatriptan. Funct Neurol. 2000;15(Suppl 3):196–201.
22. Colman, I, et al. Parenteral metoclopramide for acute migraine: Meta-analysis of randomised controlled trials. BMJ. 2004;329:1369–1373.
23. Kelly, AM, Walcynski, T, Gunn, B. The relative efficacy of phenothiazines for the treatment of acute migraine: A meta-analysis. Headache. 2009;49:1324–1332.
24. Silberstein, SD, Young, WB, Mendizabal, JE, Rothrock, JF, Alam, AS. Acute migraine treatment with droperidol: A randomized, double-blind, placebo-controlled trial. Neurology. 2003;60:315–321.
25. Akerman, S, Goadsby, PJ. Dopamine and migraine: Biology and clinical implications. Cephalalgia. 2007;27:1308–1314.
26. Parlak, I, et al. Midazolam vs. diphenhydramine for the treatment of metoclopramide-induced akathisia: A randomized controlled trial. Acad Emerg Med. 2007;14:715–721.
27. Raskin, NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36:995–997.
28. Akpunonu, BE, et al. Subcutaneous sumatriptan for treatment of acute migraine in patients admitted to the emergency department: A multicenter study. Ann Emerg Med. 1995;25:464–469.
29. Landy, SH, McGinnis, JE, McDonald, SA. Pilot study evaluating preference for 3-mg versus 6-mg subcutaneous sumatriptan. Headache. 2005;45:346–349.
30. Friedman, BW, Kapoor, A, Friedman, MS, Hochberg, ML, Rowe, BH. The relative efficacy of meperidine for the treatment of acute migraine: A meta-analysis of randomized controlled trials. Ann Emerg Med. 2008;52:705–713.
31. Colman, I, Rothney, A, Wright, SC, Zilkalns, B, Rowe, BH. Use of narcotic analgesics in the emergency department treatment of migraine headache. Neurology. 2004;62:1695–1700.
32. Ducharme, J, Beveridge, RC, Lee, JS, Beaulieu, S. Emergency management of migraine: Is the headache really over? Acad Emerg Med. 1998;5:899–905.
33. Colman, I, et al. Parenteral dexamethasone for acute severe migraine headache: Meta-analysis of randomised controlled trials for preventing recurrence. BMJ. 2008;336:1359–1361.
34. Friedman, BW, et al. Treating headache recurrence after emergency department discharge: A randomized controlled trial of naproxen versus sumatriptan. Ann Emerg Med. 2010;56:7–17.
35. Linde, K, Rossnagel, K. Propranolol for migraine prophylaxis. Cochrane Database Syst Rev. (2):2004.
36. Mulleners, WM, Chronicle, EP. Anticonvulsants in migraine prophylaxis: A Cochrane review. Cephalalgia. 2008;28:585–597.
37. Steiner, TJ, Fontebasso, M. Headache. BMJ. 2002;325:881–886.
38. Mathew, NT. Cluster headache. Semin Neurol. 1997;17:313–323.
39. Cohen, AS, Burns, B, Goadsby, PJ. High-flow oxygen for treatment of cluster headache: A randomized trial. JAMA. 2009;302:2451–2457.
40. Ekbom, K, et al. Subcutaneous sumatriptan in the acute treatment of cluster headache: A dose comparison study. The Sumatriptan Cluster Headache Study Group. Acta Neurol Scand. 1993;88:63–69.
41. Matharu, MS, Levy, MJ, Meeran, K, Goadsby, PJ. Subcutaneous octreotide in cluster headache: Randomized placebo-controlled double-blind crossover study. Ann Neurol. 2004;56:488–494.
42. Rozen, TD. Olanzapine as an abortive agent for cluster headache. Headache. 2001;41:813–816.
43. Leone, M, et al. Verapamil in the prophylaxis of episodic cluster headache: A double-blind study versus placebo. Neurology. 2000;54:1382–1385.
44. Schwartz, BS, Stewart, WF, Simon, D, Lipton, RB. Epidemiology of tension-type headache. JAMA. 1998;279:381–383.
45. Iversen, HK, Langemark, M, Andersson, PG, Hansen, PE, Olesen, J. Clinical characteristics of migraine and episodic tension-type headache in relation to old and new diagnostic criteria. Headache. 1990;30:514–519.
46. Cady, RK. The convergence hypothesis. Headache. 2007;47(Suppl 1):S44–S51.
47. Lipton, RB, Cady, RK, Stewart, WF, Wilks, K, Hall, C. Diagnostic lessons from the spectrum study. Neurology. 2002;58(9 Suppl 6):S27–S31.
48. Crystal, SC, Robbins, MS. Epidemiology of tension-type headache. Curr Pain Headache Rep. 2010;14:449–454.
49. Cicek, M, et al. Prospective, randomised, double blind, controlled comparison of metoclopramide and pethidine in the emergency treatment of acute primary vascular and tension type headache episodes. Emerg Med J. 2004;21:323–326.
50. Becker, KJ. Epidemiology and clinical presentation of aneurysmal subarachnoid hemorrhage. Neurosurg Clin North Am. 1998;9:435–444.
51. Locksley, HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg. 1966;25:219–239.
52. Rinkel, GJ, Djibuti, M, Algra, A, van Gijn, J. Prevalence and risk of rupture of intracranial aneurysms: A systematic review. Stroke. 1998;29:251–256.
53. Broderick, JP, et al. Major risk factors for aneurysmal subarachnoid hemorrhage in the young are modifiable. Stroke. 2003;34:1375–1381.
54. Juvela, S. Prehemorrhage risk factors for fatal intracranial aneurysm rupture. Stroke. 2003;34:1852–1857.
55. Linn, FH, Rinkel, GJ, Algra, A, van Gijn, J. Headache characteristics in subarachnoid haemorrhage and benign thunderclap headache. J Neurol Neurosurg Psychiatry. 1998;65:791–793.
56. Linn, FH, et al. Prospective study of sentinel headache in aneurysmal subarachnoid haemorrhage. Lancet. 1994;344:590–593.
57. Morgenstern, LB. Worst headache and subarachnoid hemorrhage. Ann Emerg Med. 1999;33:478.
58. Hunt, WE, Hess, RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20.
59. McCormack, RF, Hutson, A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med. 2010;17:444–451.
60. Schull, MJ. Lumbar puncture first: An alternative model for the investigation of lone acute sudden headache. Acad Emerg Med. 1999;6:131–136.
61. Locker, TE, Thompson, C, Rylance, J, Mason, SM. The utility of clinical features in patients presenting with nontraumatic headache: An investigation of adult patients attending an emergency department. Headache. 2006;46:954–961.
62. Perry, JJ, et al. Should spectrophotometry be used to identify xanthochromia in the cerebrospinal fluid of alert patients suspected of having subarachnoid hemorrhage? Stroke. 2006;37:2467–2472.
63. Roost, KT, Pimstone, NR, Diamond, I, Schmid, R. The formation of cerebrospinal fluid xanthochromia after subarachnoid hemorrhage. Enzymatic conversion of hemoglobin to bilirubin by the arachnoid and choroid plexus. Neurology. 1972;22:973–977.
64. Savitz, SI, Levitan, EB, Wears, R, Edlow, JA. Pooled analysis of patients with thunderclap headache evaluated by CT and LP: Is angiography necessary in patients with negative evaluations? J Neurol Sci. 2009;276:123–125.
65. Lanzino, G, Kongable, GL, Kassell, NF. Electrocardiographic abnormalities after nontraumatic subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1994;6:156–162.
66. Dorhout Mees, SM, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. (3):2007.
67. Roos, Y, Rinkel, G, Vermeulen, M, Algra, A, van Gijn, J. Antifibrinolytic therapy for aneurysmal subarachnoid hemorrhage: A major update of a Cochrane review. Stroke. 2003;34:2308–2309.
68. Feigin, VL, et al. Corticosteroids for aneurysmal subarachnoid haemorrhage and primary intracerebral haemorrhage. Cochrane Database Syst Rev. (3):2005.
69. Bederson, JB, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025.
70. Riordan, KC, et al. Anticonvulsant drug therapy after aneurysmal subarachnoid hemorrhage: A critically appraised topic. Neurologist. 2010;16:397–399.
71. van der Schaaf, I, et al. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. (4):2005.
72. Valentinis, L, et al. Headache attributed to intracranial tumours: A prospective cohort study. Cephalalgia. 2010;30:389–398.
73. Kirby, S, Purdy, RA. Headache and brain tumors. Curr Neurol Neurosci Rep. 2007;7:110–116.
74. Pfund, Z, Szapáry, L, Jászberényi, O, Nagy, F, Czopf, J. Headache in intracranial tumors. Cephalalgia. 1999;19:787–790.
75. Newton, HB, Turowski, RC, Stroup, TJ, McCoy, LK. Clinical presentation, diagnosis, and pharmacotherapy of patients with primary brain tumors. Ann Pharmacother. 1999;33:816–832.
76. Teall, J, et al. Rizatriptan (MAXALT) for the acute treatment of migraine and migraine recurrence. A placebo-controlled, outpatient study. Rizatriptan 022 Study Group. Headache. 1998;38:281–287.
77. Nordborg, E, Nordborg, C. Giant cell arteritis: Epidemiological clues to its pathogenesis and an update on its treatment. Rheumatology (Oxford). 2003;42:413–421.
78. Hellmann, DB. Temporal arteritis: A cough, toothache, and tongue infarction. JAMA. 2002;287:2996–3000.
79. Smetana, GW, Shmerling, RH. Does this patient have temporal arteritis? JAMA. 2002;287:92–101.
80. Parikh, M, et al. Prevalence of a normal C-reactive protein with an elevated erythrocyte sedimentation rate in biopsy-proven giant cell arteritis. Ophthalmology. 2006;113:1842–1845.
81. Walvick, MD, Walvick, MP. Giant cell arteritis: Laboratory predictors of a positive temporal artery biopsy. Ophthalmology. 2011;118:1201–1204.
82. Norris, JW, Beletsky, V. Cervical arterial dissection. Adv Neurol. 2003;92:119–125.
83. Schievink, WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898–906.
84. Ahmad, HA, Gerraty, RP, Davis, SM, Cameron, PA. Cervicocerebral artery dissections. J Accid Emerg Med. 1999;16:422–424.
85. Silbert, PL, Mokri, B, Schievink, WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517–1522.
86. Dodick, DW, Rozen, TD, Goadsby, PJ, Silberstein, SD. Cluster headache. Cephalalgia. 2000;20:787–803.
87. Arnold, M, et al. Vertebral artery dissection: Presenting findings and predictors of outcome. Stroke. 2006;37:2499–2503.
88. Vertinsky, AT, et al. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29:1753–1760.
89. Nebelsieck, J, et al. Sensitivity of neurovascular ultrasound for the detection of spontaneous cervical artery dissection. J Clin Neurosci. 2009;16:79–82.
90. Lyrer, P, Engelter, S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev. (10):2010.
91. Ferro, JM, et al. Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664–670.
92. Crassard, I, et al. A negative D-dimer assay does not rule out cerebral venous thrombosis: A series of seventy-three patients. Stroke. 2005;36:1716–1719.
93. Kosinski, CM, et al. Do normal D-dimer levels reliably exclude cerebral sinus thrombosis? Stroke. 2004;35:2820–2825.
94. Bousser, MG, Ferro, JM. Cerebral venous thrombosis: An update. Lancet Neurol. 2007;6:162–170.
95. Einhäupl, K, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229–1235.
96. Friedman, DI. Idiopathic intracranial hypertension. Curr Pain Headache Rep. 2007;11:62–68.
97. Kesler, A, Hadayer, A, Goldhammer, Y, Almog, Y, Korczyn, AD. Idiopathic intracranial hypertension: Risk of recurrences. Neurology. 2004;63:1737–1739.
98. Packard, RC. Epidemiology and pathogenesis of posttraumatic headache. J Head Trauma Rehabil. 1999;14:9–21.
99. Linder, SL. Post-traumatic headache. Curr Pain Headache Rep. 2007;11:396–400.
100. De Kruijk, JR, et al. Prediction of post-traumatic complaints after mild traumatic brain injury: Early symptoms and biochemical markers. J Neurol Neurosurg Psychiatry. 2002;73:727–732.
101. Amerasinghe, N, Aung, T. Angle-closure: Risk factors, diagnosis and treatment. Prog Brain Res. 2008;173:31–45.
102. Hedges, TR. An ophthalmologist’s view of headache. Headache. 1979;19:151–155.
103. Evans, RW. Complications of lumbar puncture. Neurol Clin. 1998. [1683–1105].
104. Dougados, M, et al. Efficacy of celecoxib, a cyclooxygenase 2–specific inhibitor, in the treatment of ankylosing spondylitis: A six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum. 2001;44:180–185.
105. Richman, JM, et al. Bevel direction and postdural puncture headache: A meta-analysis. Neurologist. 2006;12:224–228.
106. Thomas, SR, Jamieson, DR, Muir, KW. Randomised controlled trial of atraumatic versus standard needles for diagnostic lumbar puncture. BMJ. 2000;321:986–990.
107. Halker, RB, et al. Caffeine for the prevention and treatment of postdural puncture headache: Debunking the myth. Neurologist. 2007;13:323–327.
108. Duffy, PJ, Crosby, ET. The epidural blood patch. Resolving the controversies. Can J Anaesth. 1999;46:878–886.
109. Attia, J, Hatala, R, Cook, DJ, Wong, JG. The rational clinical examination. Does this adult patient have acute meningitis? JAMA. 1999;282:175–181.
110. Gladstone, J, Bigal, ME. Headaches attributable to infectious diseases. Curr Pain Headache Rep. 2010;14:299–308.
111. Price, RW. Neurological complications of HIV infection. Lancet. 1996;348:445–452.
112. Sjaastad, O, Fredriksen, TA, Pfaffenrath, V. Cervicogenic headache: Diagnostic criteria. Headache. 1990;30:725–726.
113. Dodick, D, Freitag, F. Evidence-based understanding of medication-overuse headache: Clinical implications. Headache. 2006;46(Suppl 4):S202–S211.
114. Hackett, PH, Roach, RC. High-altitude illness. N Engl J Med. 2001;345:107–114.
115. Harris, MD, Terrio, J, Miser, WF, Yetter, JF, 3rd. High-altitude medicine. Am Fam Physician. 1998;57:1907–1914.
116. Calabrese, LH, Dodick, DW, Schwedt, TJ, Singhal, AB. Narrative review: Reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34–44.
117. Chen, SP, Fuh, JL, Wang, SJ. Reversible cerebral vasoconstriction syndrome: An under-recognized clinical emergency. Ther Adv Neurol Disord. 2010;3:161–171.
118. Grosberg, BM, Solomon, S, Lipton, RB. Nummular headache. Curr Pain Headache Rep. 2007;11:310–312.
119. Bagheri, SC, Farhidvash, F, Perciaccante, VJ. Diagnosis and treatment of patients with trigeminal neuralgia. J Am Dent Assoc. 2004;135:1713–1717.
120. Cruccu, G, et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008:151013–151028.
121. Cutrer, FM, Boes, CJ. Cough, exertional, and sex headaches. Neurol Clin. 2004;22:133–149.
122. Pascual, J, Iglesias, F, Oterino, A, Vázquez-Barquero, A, Berciano, J. Cough, exertional, and sexual headaches: An analysis of 72 benign and symptomatic cases. Neurology. 1996;46:1520–1524.