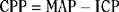CHAPTER 103 HEAD TRAUMA
Traumatic brain injury (TBI) is a major cause of death and disability in the United States and many other parts of the world. An estimated 1.5 million people sustain a TBI each year in the United States. There are 50,000 deaths from TBI, which accounts for one third of all injury-related deaths. Among TBI survivors, more than 200,000 require hospitalization, and 80,000 to 90,000 experience the onset of a long-term or lifelong disability associated with a TBI. The social and economic costs related to TBI are enormous. Direct and indirect costs of TBI were estimated to be $56.3 billion in 1995. These statistics indicate that TBI is a major public health problem with significant socioeconomic implications.
TRAUMA CARE SYSTEMS
The development of organized trauma care systems has also played a role in improving the outcomes of patients with TBI and other injuries. These systems, which have been introduced in the United States since the late 1970s, have been shown to decrease mortality after major trauma.1 The most advanced of these is the level 1 trauma center, which maintains trauma surgeons and anesthesiologists in the hospital 24 hours per day and ready access to trauma surgical suites. Neurosurgeons and other specialists are immediately available as needed. The next echelon is the level 2 trauma center, which has immediate access to surgeons and anesthesiologists but does not maintain in-house physician staffing. A level 2 center is able to initiate definitive care for all injured patients but may have to refer some tertiary trauma care needs to a level 1 center. Some cities also have specialized pediatric trauma centers.
TRAUMATIC BRAIN INJURY CLASSIFICATION
Glasgow Coma Scale and Glasgow Outcome Scale
A simple and reliable clinical assessment tool is needed for evaluating acute TBI. An accurate baseline assessment is crucial, and serial assessments are also needed, because neurological status may change with time, sometimes rapidly. TBI care is provided by a wide range of physicians, nurses, and allied health personnel. Many classification schemes have been developed; however, the Glasgow Coma Scale (GCS) (Table 103-1), introduced by Teasdale and Jennett in 1974, is the most widely used.2 The GCS has proved to be accurate, capable of detecting clinically important changes in neurological status, and easy to use by a variety of health care professionals.
| Activity | Score | Performance |
|---|---|---|
| Eye opening | ||
| 4 | Spontaneously | |
| 3 | In response to voice | |
| 2 | In response to pain | |
| 1 | None | |
| Best verbal response | ||
| 5 | Oriented | |
| 4 | Confused | |
| 3 | Inappropriate words | |
| 2 | Incomprehensible sounds | |
| 1 | None | |
| Best motor response | ||
| 6 | Follows commands | |
| 5 | Localizes to pain | |
| 4 | Withdraws in response to pain | |
| 3 | Abnormal flexion in response to pain | |
| 2 | Abnormal extension in response to pain | |
| 1 | None | |
Total score ranges from 3 to 15.
Scores on the GCS range from 3 to 15. A GCS score of 3 to 8 is indicative of severe head injury, with a mortality rate of 35% to 40%. A GCS score of 9 to 12 is indicative of moderate head injury, with a mortality rate of 5% to 10%, and a score of 13 to 15 is indicative of mild head injury, with a mortality rate of less than 2%. Children older than 2 years and teenagers have a better prognosis than do adults. The postresuscitation GCS is one of the strongest predictors of ultimate outcome after TBI.
A reliable scale is also needed to assess the long-term outcomes of patients as they recover from TBI. The Glasgow Outcome Scale (Table 103-2) is a commonly used system that has been developed for this purpose. Serial testing indicates that more than two thirds of patients reach their final outcome category on the Glasgow Outcome Scale within 3 months of injury.
TABLE 103-2 The Glasgow Outcome Scale
| Outcome Category | Definition |
|---|---|
| Good recovery | Patient able to return to former occupation, although not necessarily at the same level; may have minor neurological or psychological impairments |
| Moderate disability | Patient unable to return to work but otherwise able to perform the activities of daily living independently |
| Severe disability | Patient requires assistance to perform daily activities and cannot live independently |
| Persistent vegetative | Absence of speech or no evidence of mental function in a patient who appears awake with spontaneous eye opening |
| Death |
IMAGING OF TRAUMATIC BRAIN INJURY
The clinical severity of TBI is not always correlated with the magnitude of findings seen on CT. For example, in a patient with clinically mild TBI, an intracranial hematoma may be apparent on the admitting computed tomographic scan. With modern high-resolution scanning techniques, head CT displays abnormal findings in up to 50% of cases of mild TBI.3 Conversely, a patient, who is deeply unconscious with a severe TBI, may undergo initial head CT that yields completely normal findings. This underscores the fact that the clinician cannot rely solely on clinical findings or on results of CT when evaluating patients with TBI. The clinical and imaging examinations are complementary, and both are needed for the optimal evaluation of patients with TBI.
SPECIFIC INJURIES
Concussion
Another serious complication of seemingly mild head injury is the second-impact syndrome, a rare condition that has been described in athletes who sustain a second head injury while still symptomatic from an earlier injury. Although the individual appears to have only minimal impairment from the first injury, malignant cerebral edema, which is refractory to all interventions, occurs soon after the second injury. The mortality rate with second-impact syndrome is 50% to 100%. Recognition and increasing understanding of this syndrome have led to the development of guidelines that allow for a safe return to athletic competition while avoiding the devastating complications of second-impact syndrome. In addition to neurological examination and imaging studies, neuropsychological testing is now an important part of the decision-making process for allowing athletes to return to competition after TBI (Bailes, Day, 2001).4
Contusion
Cerebral contusion is the classic example of focal TBI. In the pre-CT era, cerebral contusion could be diagnosed only in the operating room during craniotomy or at the autopsy table. Thus, contusion was considered only in cases of severe TBI and was therefore thought to be pathognomonic for severe injury. Since the advent of CT, especially current high-resolution techniques, contusions have commonly been observed in patients with clinically mild and moderate TBI. It is now recognized that there is a wide range of severity associated with cerebral contusion. Tiny punctate contusions in patients with mild TBI have little or no clinical significance and carry the same prognosis as normal findings on CT.5 At the other end of the spectrum, large contusions with significant mass effect in patients with severe TBI can be life-threatening.
Contusions can be classified as coup or contrecoup injuries. Coup contusions occur at the location of impact, whereas contrecoup contusions occur on the opposite side or at a point distant from the impact. Contusions may be present in any part of the brain but are most common in the frontal and temporal lobes. Figure 103-1 shows a typical hemorrhagic contusion in the left inferior frontal region, just above the roof of the orbit.
Intracerebral Hematoma
Intracerebral hemorrhage is bleeding within the brain parenchyma and generally occurs after severe head injury. As with contusion, the natural history of intracerebral hemorrhage is one of progressive enlargement during the first few days after injury. Intracerebral hemorrhage may develop on a delayed basis in areas of contusion. Individuals with coagulopathy from liver disease or anticoagulant medications are at increased risk for intracerebral hemorrhage. In these individuals, intracerebral hemorrhage may occur after low-impact injury. Small intracerebral hemorrhages can be observed, whereas larger intracerebral hemorrhages that cause significant mass effect should be resected.
Subdural Hematoma
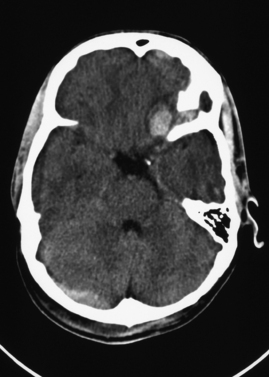
Chronic subdural hematoma represents the gradual accumulation of liquefied hematoma in the subdural space, occurring over 2 or more weeks. Chronic subdural hematoma is usually present in elderly persons, who have more prominent subdural spaces as a result of cerebral atrophy. Chronic subdural hematoma, occurs most commonly after minor head injury. Sometimes the patient and family cannot even recall when the injury occurred. Over time, the hematoma gradually enlarges as a result of repeated episodes of minor bleeding and/or the drawing of fluid into the hematoma as a result of an osmotic effect. As in other forms of intracranial hemorrhage, risks of chronic subdural hematoma are elevated in individuals with coagulopathy caused by liver disease or anticoagulant medications. Surgery is often required and may involve either burr hole drainage or a craniotomy. At surgery, a chronic subdural hematoma is liquefied and is described as having a “crank case oil” appearance. The prognosis is guarded, and there is substantial risk of recurrent subdural hematoma that necessitates further surgery. Figure 103-2 shows bilateral chronic subdural hematomas.
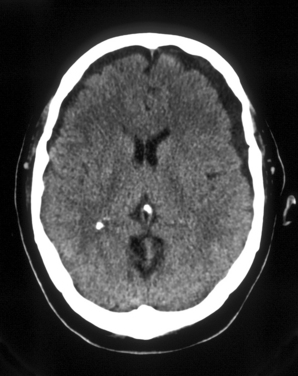
Subdural hematoma may have both acute and chronic components if there is ongoing bleeding in the subdural space. The presence of a mixed acute and chronic subdural hematoma is readily identified on CT (Figure 103-3).
Epidural Hematoma
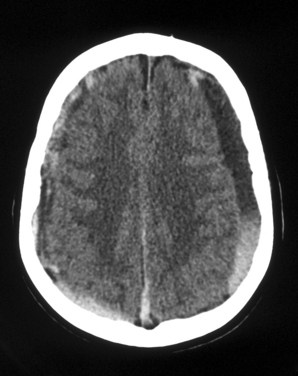
Epidural hematoma represents acute bleeding into the epidural space. This bleeding may be either arterial or venous. The classic epidural hematoma is observed with a linear skull fracture of the temporal bone, which tears the middle meningeal artery, allowing blood to accumulate under pressure in the epidural space. However, sizable epidural hematoma of venous origin may also occur. Epidural hematoma is usually observed in children and young adults but is rare in elderly persons, because of the firm adherence of the dura to the inner table of the skull. A convexity epidural hematoma is usually readily identified on routine head CT; however, an epidural hematoma low in the middle cranial fossa may be missed on axial images as a result of artifact from the adjacent skull base. In such cases, coronal imaging may be helpful in demonstrating the hematoma. A right frontal epidural hematoma is shown in Figure 103-4.
Figure 103-4 Computed tomographic head scan shows a right frontal epidural hematoma.
(Courtesy of Daniel F. Broderick, MD.)
The typical clinical scenario of a patient developing an epidural hematoma is one in which the patient, often a child, sustains a blow to the head and is initially stunned but then rapidly regains awareness, only to deteriorate again over the next few minutes to hours. Typical clinical findings include dilation of a pupil, caused by a cranial nerve III palsy, ipsilateral to the injury, and progressive obtundation. Emergency craniotomy is usually required for an epidural hematoma. The lesion must be identified and evacuated as soon as possible in order to prevent brain herniation and to obtain a good outcome.
Skull Fracture
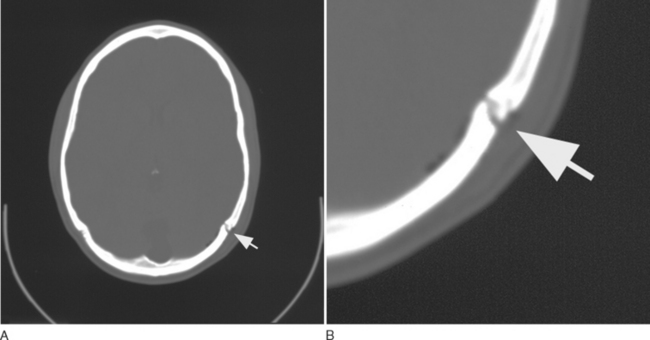
Most skull fractures are readily identified on CT. Fractures of the skull base may be harder to visualize with standard head CT protocols. In these cases, specialized CT techniques may be required, including coronal imaging, thin slices, and bone windows. Figure 103-5 depicts a linear skull fracture through the left lambdoid suture with a small amount of adjacent pneumocephalus.
A simple, linear, nondepressed skull fracture requires no specific treatment but does have some prognostic significance. In one large study of minor TBI, 3% of patients with a skull fracture deteriorated to the point of needing a neurosurgical procedure, whereas the risk of significant deterioration in patients without a skull fracture was less than 1%.6 This indicates that in cases of clinically mild TBI, the prognosis is worse in patients with a skull fracture than in those whose head CT does not reveal a fracture. These data have led some investigators to recommend that a skull fracture be an indication for overnight admission to the hospital for patients with clinically mild TBI.
CLINICAL ASSESSMENT
Adverse Effects of Anticoagulant Medications
There is a seemingly small risk of thrombotic complications related to the use of recombinant factor VIIa. The major drawback to this medication is its cost. A 1.2-mg vial costs about $1000. However, in cases of life-threatening intracranial hemorrhage, the benefits of this product clearly outweigh the expense and risks of its use.
“Talk and Die” Injuries
It is now well known that patients with seemingly mild TBI can have a brief period of stability, or even improvement, followed by dramatic neurological deterioration. This ominous clinical scenario was first reported by Reilly and associates in 1975.7 This initial report focused on patients who talked at some point after the injury and then died. These injuries came to be known as “talk and die” injuries and, in their milder form, as “talk and deteriorate” injuries.
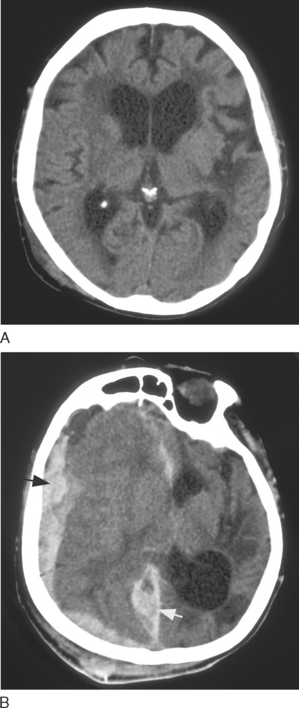
Figure 103-6 illustrates the dramatic worsening that may occur with TBI. The case involved a 72-year-old woman who presented to the emergency department with a clinically mild TBI (GCS score, 15) after a fall at home. Figure 103-6A shows the initial computed tomographic head scan, which was unremarkable, aside from extracranial soft tissue swelling in the right parietal region. The patient was dismissed from the emergency department in the care of her family. Later that day, the patient exhibited marked deterioration and was brought back to the hospital by her family. She was deeply comatose, and the GCS score had decreased from 15 to 6. Figure 103-6B shows the follow-up computed tomographic head scan, obtained 10 hours after the initial scan, which reveals interval development of a large acute right subdural hematoma. Emergency craniotomy was required. This was an example of a “talk and deteriorate” injury.
TREATMENT
Hospital Admission
In cases of clinically mild TBI, one of the first decisions to be made is whether to admit the patient to the hospital for observation or to have the patient observed at home. There is no evidence that hospital admission improves the outcomes of patients with mild TBI. Two large clinical series in which investigators compared outcomes of observation of inpatients and outpatients with mild TBI failed to show a benefit of inpatient care.8,9 Nevertheless, large numbers of patients with mild TBI are admitted to the hospital for overnight observation, generally for medicolegal reasons.
The most important consideration is for the patient to be in an environment where he or she can be observed carefully for any evidence of neurological deterioration. The decision to admit or observe on an outpatient basis depends on clinical judgment, results of CT in selected patients, and consideration of a patient’s social situation. As a general rule, the patient with a GCS score of 15, no evidence of skull fracture on CT, and a responsible parent or other adult available to monitor the patient may be observed at home. In pediatric patients, the possibility of child abuse is an additional consideration. A child should not be discharged home if the clinical and radiographic findings are at all suggestive of nonaccidental injury.
Intracranial Pressure Monitoring
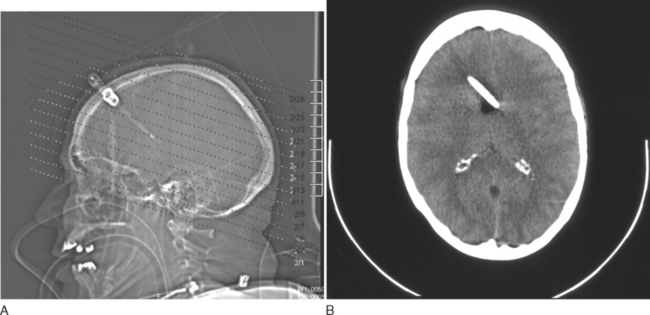
ICP monitoring should be considered for clinically severe TBI (GCS score, 3 to 8). There are various types of ICP monitors, including intraventricular, intraparenchymal, subdural, and epidural devices. Intraventricular monitors are more accurate and have the added advantage of allowing withdrawal of CSF to lower ICP. The main drawback to intraventricular monitoring is difficulty with catheter placement if the ventricles are severely compressed as a result of cerebral edema caused by severe TBI. In these cases, it may not be technically possible to cannulate the ventricles. Other concerns with the intraventricular technique include the risk of intraparenchymal hemorrhage associated with passing a catheter through brain tissue and the risk of infection. An intraventricular ICP monitor is visible in Figure 103-7.
Management of Raised Intracranial Pressure
After placement of the ICP monitor, efforts are made to keep ICP lower than 20 mm Hg and CPP higher than 50 mm Hg. General measures include elevating the head of the bed 30 to 45 degrees, avoidance of hypoxia, maintenance of normocapnia (carbon dioxide tension, 35 to 40 mm Hg), and light sedation. If raised ICP persists, the follow measures may be needed: heavy sedation with fentanyl and paralysis with vecuronium; drainage of CSF in 3- to 5-mL increments; mannitol, 0.25 to 1 g/kg, followed by 0.25 g/kg every 6 hours; and mild hyperventilation to a carbon dioxide tension of 30 to 35 mm Hg. If mannitol is used, serum electrolytes and osmolality must be monitored closely. Mannitol must be discontinued if serum osmolality exceeds 320 mOsm/L. Head CT should be repeated if there is a persistent problem with raised ICP. Finally, high-dose barbiturate therapy (barbiturate coma) should be considered as a last resort for raised ICP that is refractory to all other measures.
Anticonvulsant Therapy
Total prevention of seizures would obviously be of great benefit to the patient. Administration of an anticonvulsant medication to prevent a first seizure (prophylactic anticonvulsants) has been the subject of considerable investigation. Data indicate that in adults, prophylactic anticonvulsants lower the risk of early seizures but do not improve outcomes. Prophylactic anticonvulsants also do not lower the risk of late seizures.10 In Guidelines for the Management of Severe Head Injury, the American Association of Neurological Surgeons has rated various treatments as standards, guidelines, or options. Prophylactic anticonvulsants are considered by this organization to be an option of unclear benefit to the patient.11 This means that the clinician may choose to prescribe or withhold this treatment, on the basis of clinical judgment. If prophylactic anticonvulsants are being considered, the decision to prescribe must be made in view of the known potential for serious complications associated with these medications. Prophylaxis, when prescribed, is usually phenytoin, and the usual duration of therapy is 1 week.
Steroids
The use of glucocorticoids in severe TBI dates back to the 1960s. Early laboratory and clinical work suggested that glucocorticoids improved cerebral edema, lowered ICP, and had other beneficial effects. As a result, these agents became a standard part of the management of severe TBI. However, more recent investigation indicates that glucocorticoids do not lower ICP or improve outcome in patients with severe TBI. Therefore, the routine use of glucocorticoids is no longer recommended for any type of TBI.11
OUTCOMES
Prognosis in TBI is well correlated with the admission GCS score, measured after resuscitation. As mentioned previously, severe TBI (GCS score, 3 to 8) carries a mortality rate of 35% to 40%; moderate TBI (GCS score, 9 to 12) carries a mortality rate of 5% to 10%; and mild TBI (GCS score, 13 to 15) carries a mortality rate of less than 2%. Children older than 2 years and teenagers have a better prognosis than do adults in all categories.
CONCLUSIONS AND RECOMMENDATIONS
Bailes JE, Day AL, editors. Neurological Sports Medicine: A Guide for Physicians and Athletic Trainers. Rolling Meadows, IL: American Association of Neurological Surgeons, 2001.
Brain Trauma Foundation. Guidelines for the Management of Severe Head Injury, 2nd ed. Park Ridge, IL: American Association of Neurological Surgeons, 2000.
Greenberg MS. Handbook of Neurosurgery, 5th ed., New York: Thieme; 2001:626-685.
Popp AJ, editor. A Guide to the Primary Care of Neurological Disorders. Park Ridge, IL: American Association of Neurological Surgeons, 1998.
1 Mendeloff JM. Trauma systems and public policy. Annu Rev Publ Health. 1991;12:401-424.
2 Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81-84.
3 Fay GC, Jaffe KM, Polissar NL, et al. Mild pediatric traumatic brain injury: a cohort study. Arch Phys Med Rehabil. 1993;74:895-901.
4 Broshek DK, Barth JT. Neuropsychological assessment of the amateur athlete. In: Bailes JE, Day AL, editors. Neurological Sports Medicine: A Guide for Physicians and Athletic Trainers. Rolling Meadows, IL: American Association of Neurological Surgeons; 2001:155-168.
5 Hahn YS, McLone DG. Risk factors in the outcome of children with minor head injury. Pediatr Neurosurg. 1993;19:135-142.
6 Dacey RG, Alves WM, Rimel RW, et al. Neurosurgical complications after apparently minor head injury: assessment of risk in a series of 610 patients. J Neurosurg. 1986;65:203-210.
7 Reilly PL, Graham DI, Adams JH, et al. Patients with head injury who talk and die. Lancet. 1975;2:375-377.
8 Lowdon IMR, Briggs M, Cockin J. Post-concussional symptoms following minor head injury. Injury. 1989;20:193-194.
9 Miller JD, Murray LS, Teasdale GM. Development of a traumatic intracranial hematoma after a “minor” head injury. Neurosurgery. 1990;27:669-673.
10 Temkin NR, Dikmen SS, Wilensky AJ. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497-502.
11 Brain Trauma Foundation. Guidelines for the Management of Severe Head Injury, 2nd ed. Park Ridge, IL: American Association of Neurological Surgeons, 2000.