Head and Neck Sinuses and Masses
Lesions of the head and neck in children can be subdivided by etiology as those resulting from infection, trauma, neoplasm, or those of congenital origin. The more common benign neoplasms including hemangiomas, lymphangiomas, and cystic hygromas are discussed in Chapter 72. Malignant neoplasms of childhood (e.g., neuroblastoma, lymphoma, and rhabdomyosarcoma), which occur as primary or metastatic masses in the head and neck, lesions of the thyroid and parathyroid, as well as traumatic injuries of the head and neck, also are discussed in other chapters. In this chapter, common congenital head and neck malformations are discussed, and inflammatory lesions are reviewed.
Lesions of Embryonic Origin
Congenital cysts and sinuses in the neck result from embryonic structures that have failed to mature or have persisted in an aberrant fashion.1,2 Both diagnosis and therapy depend on a working knowledge of the embryologic origin and differentiation of the head and neck structures.3,4 This knowledge is particularly important because complete resection of cartilaginous remnants, remnants of the branchial arch and cleft structures, and midline fusion abnormalities is needed to avoid recurrence. Congenital lesions of the head and neck, in descending order of frequency, are thyroglossal duct cysts, preauricular pits and sinuses, branchial cleft anomalies, dermoid cysts, and median cervical clefts.
Thyroglossal Duct Cyst
One of the most common lesions in the midline of the neck is the thyroglossal duct cyst. Thyroglossal duct remnants are found in 7% of the population and most are asymptomatic.4,5 Although embryonic in origin, it is rare for these lesions to manifest in the newborn period.1 More commonly, they are noted in preschool-age children.1 Thyroglossal duct cysts also are common in young adults and, with the exception of thyroid goiter, are the most common midline neck mass.6
The embryogenesis of the thyroglossal duct is intimately involved with that of the thyroid gland, the hyoid bone, and the tongue.7 The foramen cecum is the site of the development of the thyroid diverticulum. In the embryo, this structure develops caudal to the central tuberculum impar, which is one of the pharyngeal buds that leads to the formation of the tongue. As the tongue develops, the thyroid diverticulum descends into the neck, maintaining its connection to the foramen cecum. The hyoid bone is developing from the second branchial arch at this time. The thyroid gland develops between weeks 4 and 7 of gestation and descends into its pretracheal position in the neck.8 As a result of these multiple events occurring simultaneously, the thyroglossal duct may pass in front of or behind the hyoid bone, but most commonly, it passes through it. Normally, the duct disappears by the time the thyroid reaches its appropriate position by 5 to 8 weeks gestation.5,9 Thyroglossal duct cysts never have a primary external opening because the embryologic thyroglossal tract never reaches the surface of the neck.8 A cyst can be located anywhere along the migratory course of the thyroglossal tract if it fails to become obliterated (Fig. 73-1). Occasionally, the cysts attach to the pyramidal lobe or may be intrathyroidal.10 Complete failure of migration of the thyroid results in a lingual thyroid, which develops beneath the foramen cecum at the base of the tongue.11 In this instance, no thyroid tissue is found in the neck.
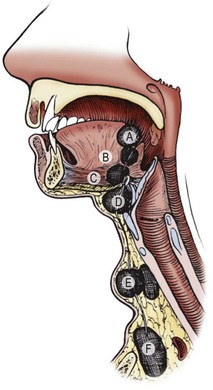
FIGURE 73-1 Thyroglossal duct cysts can be located anywhere from the base of the tongue to behind the sternum. A and B, Lingual (rare); C and D, adjacent to hyoid bone (common); E and F, suprasternal fossa (rare). (From Welch KJ, Randolph JG, Ravitch MM, et al. editors. Pediatric Surgery. 4th ed. Chicago: Year Book Medical; 1986. p. 549.)
Two-thirds of thyroglossal duct anomalies are discovered within the first three decades of life.5 Classically, the thyroglossal cysts are located in the midline at or just below the hyoid bone (Fig. 73-2). Suprahyoid thyroglossal cysts must be distinguished from submental dermoid cysts and from submental lymph nodes.12 Rarely, the cysts are suprasternal in location. The initial sign is usually a painless mass in the midline of the neck, with 66% found adjacent to the hyoid bone.9 On physical examination, the thyroglossal duct cyst is smooth, soft, and nontender. To distinguish this lesion from the more superficial dermoid cyst, one should palpate the lesion while the child sticks out his or her tongue. Owing to its attachment to the foramen cecum, the thyroglossal duct cyst usually moves cephalad when the tongue protrudes. This maneuver is more reliable than asking the child to swallow and determining whether the mass moves with swallowing. Owing to the communication to the mouth via the foramen cecum, thyroglossal cysts can become infected with oral flora. One-third of patients will present with a concurrent or prior infection, and one-quarter will present with a draining sinus from spontaneous or incisional drainage of an abscess.9 Some patients may present with a foul taste in the mouth from spontaneous drainage of the cyst via the foramen cecum.
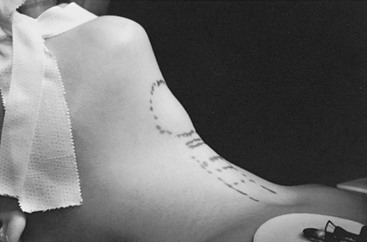
FIGURE 73-2 A classic thyroglossal duct cyst located in the midline just below the hyoid bone. Markings on the neck represent the thyroid, cricoid and tracheal cartilages.
The preoperative evaluation for a patient with a suspected thyroglossal duct cyst includes a complete history and physical examination. Patients with findings concerning for hypothyroidism should undergo thyroid function testing and additional imaging to exclude median ectopic thyroid. The incidence of ectopic thyroid tissue in or near the duct is reported to be from 10–45%, and some clinicians have advocated preoperative thyroid scanning or ultrasound (US) to eliminate the possibility of an ectopic thyroid gland masquerading as a thyroglossal duct cyst.13–17 Ultrasound appears to be very accurate and avoids the need for radiation and possible sedation in younger children.16 Although ultrasound has been noted to have significant limitations in differentiating thyroglossal duct cysts from other midline neck masses, it can be useful in differentiating cysts and ectopic thyroid.18 Ninety per cent of ectopic thyroid tissue lies at the base of the tongue, and thyroglossal duct cysts are rarely found there. Abnormal thyroid function tests, a suggestive history, or a solid mass on ultrasound should prompt a preoperative thyroid scan to ensure the lesion is not the only thyroid gland present, which occurs in less than 1–2% of thyroglossal duct cysts.9,15,19 If ectopic thyroid tissue is found, the management is controversial, but some clinicians suggest a trial of medical suppression to decrease the size of the mass.19
Elective surgical excision a of thyroglossal duct cyst is advised to avoid the complications of infection and the small risk (<1%) of cancer (papillary thyroid carcinoma) developing in the cyst.15 The operation includes complete excision of the cyst and its tract upward to the base of the tongue (Fig. 73-3), and resection of the central portion of the hyoid bone as described by Sistrunk in 1920.20–22 Several other studies have shown that multiple smaller tracts can connect through the hyoid bone to the floor of the mouth, requiring wide resection of tracts above the hyoid.20–22 If these suprahyoid tracts remain, the incidence of recurrence increases.23 The best chance for successful excision is adequate wide resection at the initial procedure.24
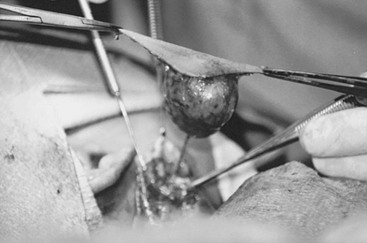
FIGURE 73-3 Complete excision of a previously infected thyroglossal duct cyst. Surrounding skin was removed because of changes related to a previous infection. Note the well-defined tract leading toward the hyoid bone and the floor of the mouth. The operation was completed by excising the central portion of the hyoid bone and suture ligating the tract.
As with all neck surgery, the patient should be supine with the neck slightly hyperextended (Fig. 73-4). The thyroglossal cyst is exposed through a transverse incision. The cyst has a characteristic appearance, distinctly different from that of thyroid tissue. The dissection should continue cephalad to the hyoid, resecting a block of tissue along the tract. Transecting the hyoid is simplified by using angled scissors, similar to Potts scissors, or by using a side-cutting bone cutter. Alternatively, some advocate use of an ultrasonic osteotomy device to minimize trauma to surrounding soft tissues.25 The base of the tract at the floor of the mouth is ligated with absorbable suture. The wings of the hyoid do not need to be approximated. The incision is closed in layers. If the floor of the mouth is entered inadvertently, this can be repaired with absorbable suture. The incision is copiously irrigated. Occasionally, the dissection may be made simpler by having the anesthesiologist place his or her finger at the base of the child’s tongue to identify the cephalad extent of the dissection. With complete excision, including the central portion of the hyoid bone, the risk of recurrence is low, 2–5%.5,9,23,26 Some authors advocate more extensive excision of the infrahyoid tissue as well as exposure of the posterior hyoid space, noting that extensive arborization of thyroglossal ducts exists at all levels, and residual ducts can result in recurrence. Recurrence rates in series employing these extended excisions range from 0–1.67%.27,28 Risk factors for recurrence include simple cyst excision alone (recurrence rates of 38–70%), intraoperative cyst rupture, presence of a cutaneous component secondary to infection, and postoperative wound infections.5,9,29 The cyst is usually connected to the foramen cecum by single or multiple tracts, which pass through the hyoid. Under histologic exam, the duct lining is stratified squamous epithelium or ciliated pseudostratified columnar epithelium, with associated mucus-secreting glands.9 The cyst contains a characteristic glairy mucus.
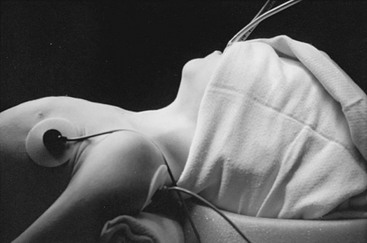
FIGURE 73-4 Positioning a child for a cervical operation. Hyperextension of the head with support under the shoulders and stabilization with a bean bag keeps the child in a stable position and facilitates exposure. The head of the bed should be elevated 30° to decrease venous pressure in the neck.
Infected cysts or sinuses should be initially managed by treating the infection. The usual route of infection is via the mouth; thus the common organisms are Haemophilus influenzae, Staphylococus aureus, and Staphylococcus epidermidis.9 Directed antibiotic therapy should be initiated. Needle aspiration may be required to decompress the cyst and allow for identification of the organism, but formal incision and drainage should be avoided. This may seed ductal cells outside of the cyst and increase recurrence rates.9 If incision and drainage is required, the incision should be placed so that it can be completely excised during a formal Sistrunk procedure once the infection clears. Excision of acutely infected cysts should be avoided, as recurrence can occur in 25% of patients.30 Three months should be allowed for inflammation to resolve prior to definitive operative treatment.
If a solid mass is found, it should be sent for frozen section to rule out median ectopic thyroid. If it is normal thyroid tissue and there is additional functional thyroid tissue in the normal location, a Sistrunk procedure with excision of the mass should be performed.9 If there is no other thyroid tissue present, management is controversial. One option is to leave the tissue in situ or reposition it into the strap muscles. This is done to prevent the patient from becoming permanently hypothyroid; however, most patients still require thyroid hormone therapy for hypothyroidism or to control the size of the ectopic thyroid. Due to this likely need for long-term therapy and possible malignant degeneration, some surgeons recommend completely excising the ectopic thyroid tissue regardless of the presence or absence of additional thyroid tissue.9 If the mass is found to represent carcinoma, then management is dependent on the extent of disease. The vast majority of cases are papillary carcinoma. If confined to the cyst specimen, it can be adequately treated with a Sistrunk procedure alone.31 More extensive cancers should undergo risk stratification and generally warrant total thyroidectomy and consideration of adjuvant therapy, including radioactive iodine in some cases.32
Remnants of Embryonic Branchial Apparatus
Branchial anomalies represent approximately 30% of congenital neck masses and can present as cysts, sinuses, or fistulae.5,33 They are equally common in males and females and present in childhood or early adulthood.
During weeks 4 to 8 after fertilization, four pairs of well-developed ridges (branchial arches) dominate the lateral cervicofacial area of the human embryo.34 These four pairs are accompanied by two rudimentary pairs, which are analogous to the gill apparatus of fish.2,34 No true gill mechanisms are found in any stage of the human embryo. These pharyngeal arches and clefts are formed without a true connection between the outer ectodermal clefts and the inner endodermal pharyngeal pouches (Fig. 73-5). The mature structures of the head and neck are derivatives of several branchial arches and their intervening clefts.34,35 The clefts and pouches are gradually obliterated by mesenchyme to form those structures. Branchial cleft anomalies result if that process is incomplete.33
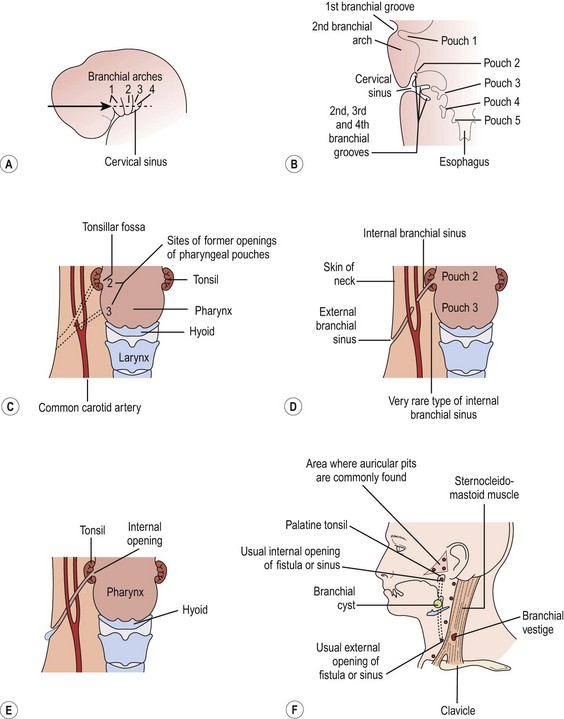
FIGURE 73-5 (A) The head and neck region of a 5-week-old embryo. (B) Horizontal section through the embryo illustrating the relationship of the cervical sinus to the branchial arches and pharyngeal pouches. (C) The child’s neck region, indicating the former sites of openings of the cervical sinus and the pharyngeal pouches. The dotted lines indicate possible courses of branchial fistulas. (D) The embryologic basis of various types of branchial sinuses. (E) A branchial fistula resulting from persistence of parts of the second branchial cleft and the second pharyngeal pouch. (F) Possible sites of branchial cysts and openings of branchial sinuses and fistulas. A branchial vestige also is illustrated. (From Moore KL. The Developing Human: Clinically Oriented Embryology. Philadelphia: WB Saunders; 1977.)
Each arch transforms during gestation into a defined anatomic pattern. Understanding this pattern and its relationship to normal neck structures is key in the diagnosis and treatment of these anomalies. Each anomaly is classified by the cleft or pouch of origin which can be determined by the internal opening of the sinus as well as its relationship to nerves, arteries, and muscles. Careful attention to these relationships is necessary to prevent injury to surrounding tissues and ensure complete resection.33 The embryology and anatomy for each cleft will be discussed later.
Branchial anomalies are lined with either respiratory or squamous epithelium; sinuses and fistulas usually the former and cysts the later.33 One can also see lymphoid tissue, sebaceous glands, salivary tissue or cholesterol crystals. Squamous cell cancer can be seen in adults, but it is rare and it can be difficult to distinguish a primary branchogenic anomaly from a metastatic lesion or an occult primary.5
Complete fistulas are more common than external sinuses. Both are more common than branchial cysts, at least during childhood.36,37 In adults, cysts predominate.36 By definition, all branchial remnants are truly congenital and are present at birth.37,38 Cysts are remnants of sinuses without an external opening and usually appear later in childhood than do sinuses, fistulas, and cartilaginous remnants, which appear in infancy.5,38 Sinuses have the persistence of the external opening only, while fistulas involve the persistence of the branchial groove with breakdown of the branchial membrane.5 Commonly, the tiny external opening of the fistula and the external sinuses remain unnoticed for some time. Spontaneous mucoid drainage from the ostium along the border of the sternocleidomastoid muscle (SCM) usually heralds its presence and initiates the parent’s concern and the reason for the child’s referral. The first clinical presentation may be an infected mass as a result of the inability of the thick mucoid material to drain spontaneously. Infection is, however, less common in fistulas and external sinuses than in cysts.1 The cutaneous openings are occasionally marked by skin tags or cartilaginous remnants. The tract itself may be palpable. A cordlike structure can sometimes be felt ascending in the neck by hyperextending the child’s neck and making the skin taut. Compression along the tract may produce mucoid material exiting from the ostium.
The evaluation of these lesions starts with a thorough history and physical examination. Palpating the tract and observing the mucoid discharge can be confirmatory. Although colored dye or radiopaque material may be injected to delineate the tract, these manipulations generally are unnecessary. Upper endoscopy may be helpful to locate the pharyngeal opening. Both the pyriform sinus and tonsillar fossas should be examined. Cysts may be more difficult to diagnose. They lie deep to the skin along the anterior border of the SCM.1 They can usually be distinguished from cystic hygromas, which are subcutaneous and can be transilluminated. Ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) can help define the lesion and may be helpful in narrowing the differential diagnosis, but CT is most often used and can demonstrate a fistula in two-thirds of cases.39 Barium esophagram has 50–80% sensitivity for third and fourth branchial fistulas.40 While fine needle aspiration is necessary in adults to exclude metastatic carcinoma, it is not necessary in children and incisional biopsy should be avoided.33
The goal of treating all congenital neck sinuses, cysts, and fistulas is usually complete excision, when no inflammation is present.41 Timing of resection is controversial with some advocating for early resection to prevent infection while others wait until age 2 or 3 years.33,42,43 As with thyroglossal duct cysts, if the lesion is infected at clinical presentation, antibiotic therapy and warm soaks to encourage spontaneous drainage of mucoid plugs should precede definitive excision. Approximately 20% of lesions will have been infected at least once prior to surgery.42 Attempts at complete excision in an inflamed, infected field increase the risk of nerve injury and incomplete resection. Aspiration or limited incision and drainage (I&D) is sometimes necessary to resolve the infection. Complete surgical excision is delayed until the inflammation subsides and the surrounding skin is supple. Endoscopic cauterization of fourth branchial cleft sinuses has been described either at the time of initial abscess drainage or four to six weeks later. Recurrence with this technique seems unusual.44
Surgical resection is performed under general anesthesia with the positioning as shown in Figure 73-4. A small transverse elliptical incision is made around the external opening and deepened beneath the cervical fascia. The initial dissection is along the inferior border of the incision, so that the ascending tract is identified from below and not injured. Placement of a 2-0 or 3-0 monofilament suture or probe within the tract can facilitate dissection. Dissection proceeds cephalad, staying on the tract until visualization of the most superior portion of the tract becomes difficult. At this level, a second, more cephalad, parallel ‘stair-step’ incision or extension of the first incision may be necessary for adequate exposure. The tract is pulled through the second incision, and the dissection is continued cephalad between the bifurcation of the carotid artery to the point where the tract dives into the pharynx. The fistula is suture ligated with absorbable suture. The incision is closed in layers with absorbable sutures. No drains are needed if excision is complete. Recurrences are rare and imply that a portion of the epithelium-lined tract was overlooked. The incidence of recurrence is higher in patients with previously infected lesions. The specific embryology, anatomy, and treatment for each type will now be discussed.
First Cleft Anomalies
The first branchial arch forms the mandible and contributes to the maxillary process of the upper jaw.35,38,45 Abnormal development of the first branchial arch results in a host of facial deformities, including cleft lip and palate, abnormal shape or contour of the external ear, and malformed internal ossicles.35,38 The first branchial cleft contributes to the tympanic cavity, eustachian tube, middle ear cavity, and mastoid air cells. Microtia and aural atresia occur with failure of the first branchial cleft to develop.34,35
First branchial anomalies are rare and account for less than 1% of branchial cleft malformations.33 Cysts are seen as swellings posterior or anterior to the ear or inferior to the earlobe in the submandibular region. External openings, if found, are located inferior to the mandible in a suprahyoid position. One-third open into the external auditory canal.6 The tract may be intimately associated with, or course through, the parotid gland. This association and the proximity of cranial nerve VII make resection difficult, particularly in the younger patient who is likely to have a tract deep to the facial nerve.39 First cleft anomalies are classified as type I or type II (Figs 73-6 and 73-7).5,33 Type I remnants contain only ectoderm, course lateral to the facial nerve, and present as swellings near the ear. Type II lesions consist of both mesoderm and ectoderm, can contain cartilage, pass medial to the facial nerve, and present as swellings inferior to the angle of the mandible or anterior to the SCM in a preauricular, infra-auricular, or postauricular position. First branchial anomalies are more common in females than males and are often misdiagnosed leading to delay in excision.46 Presentation can include cervical, parotid, or auricular signs. Cervical signs include drainage from a pit-like depression at the angle of the mandible. Parotid signs result from rapid enlargement due to inflammation. Auricular signs can consist of swelling or otorrhea.
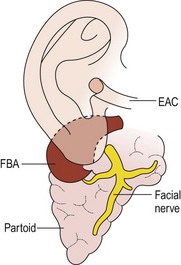
FIGURE 73-6 Type I first branchial cleft anomaly (FBA). Note that the anomaly, located in the parotid gland, has no connection to the external auditory canal (EAC). (From Mukherji SK, Fatterpekar G, Castillo M, et al. Imaging of congenital anomalies of the branchial apparatus. Neuroimaging Clin North Am 2000;10:75–93.)
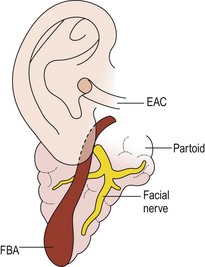
FIGURE 73-7 Type II first branchial cleft anomaly (FBA). The anomaly connects with the external auditory canal (EAC) and extends deep into the parotid gland. (From Mukherji SK, Fatterpekar G, Castillo M, et al. Imaging of congenital anomalies of the branchial apparatus. Neuroimaging Clin North Am 2000;10:75–93.)
Resection of first arch anomalies often requires at least partial facial nerve dissection and superficial parotidectomy. It is important to resect any involved skin or cartilage of the external auditory canal. If it extends medial to the tympanic membrane, a second procedure may be necessary to remove the medial component. Tracts that go to the middle ear are more likely to travel deep or split around the facial nerve.46 A superficial parotidectomy incision allows good exposure and facial nerve monitoring may decrease the risk of nerve injury.47 Another option is to open the fistula tract longitudinally and facilitate the dissection by visualizing it from the inside, but this approach requires microsurgical techniques.48 In any case, identification of the nerve is essential, as the risk of temporary and permanent facial paralysis is significantly higher when the nerve is not identified.49 Recurrence is common, and 2.4 procedures are required on average for complete resection.50 Each repeat operation places the facial nerve at greater risk due to prior scarring, emphasizing the importance of complete resection at the first attempt.33
Second Cleft Anomalies
The second arch forms the hyoid bone and the cleft of the tonsillar fossa.1,2 The second pouch gives rise to the tonsillar and supratonsillar fossa.33 The external ostium of the second branchial cleft is along the anterior border of the SCM, generally at the junction of the lower and middle thirds.36 Owing to its embryonic origin, the second cleft tract penetrates the platysma and cervical fascia to ascend along the carotid sheath to the level of the hyoid bone. Remnants may be found anywhere along this course. The residual tract turns medially between the branches of the carotid artery, behind the posterior belly of the digastric and stylohyoid muscles, and in front of the hypoglossal nerve to end in the tonsillar fossa36 (Fig. 73-8). Although the internal opening can be anywhere in the nasopharynx or oropharynx, it is most commonly found in the tonsillar fossa. Figure 73-9 demonstrates the four types of second arch anomalies. Type I lies anterior to the SCM and does not come into contact with the carotid sheath. Type II is the most common, passing deep to the SCM and anterior or posterior to the carotid sheath. Type III passes between the internal and external carotid arteries, ending adjacent to the pharynx. Type IV is medial to the carotid sheath adjacent to the tonsillar fossa.
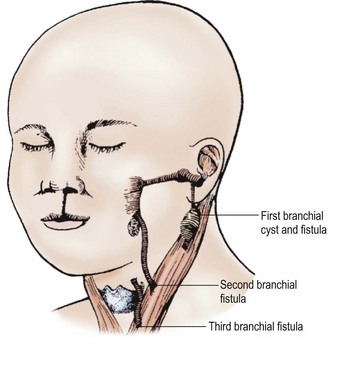
FIGURE 73-8 A child with a cleft lip and remnants of the first three branchial systems. Note the important relation to the sternocleidomastoid muscle and the fistula’s origin. (From Welch KJ, Randolph JG, Ravitch MM, et al. editors. Pediatric Surgery. 4th ed. Chicago: Year Book Medical; 1986. p. 543.)
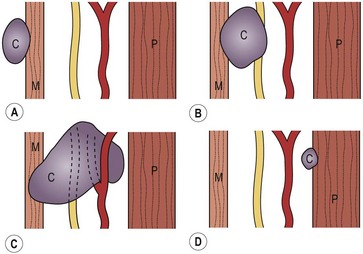
FIGURE 73-9 Types I to IV second branchial cleft anomalies. (A) Type I: the cyst (C) is superficial to the sternocleidomastoid muscle (M). (B) Type II: the cyst is adjacent to the carotid sheath. (C) Type III: the cyst passes between the internal and external carotid arteries to the lateral wall of the pharynx (P). (D) Type IV: the cyst is deep to the carotid sheath abutting the pharynx. (From Mukherji SK, Fatterpekar G, Castillo M, et al. Imaging of congenital anomalies of the branchial apparatus. Neuroimaging Clin North Am 2000;10:75–93.)
Second branchial cleft anomalies represent 95% of all branchial cleft anomalies. About 10% of second branchial remnants are bilateral.36 These anomalies commonly present as a fistula or cyst in the lower, anterolateral neck. Fistulas are commonly diagnosed in infancy or childhood after presenting with chronic drainage from an opening anterior to the SCM in the lower third of the neck. Cysts usually present during the third to fifth decades of life with an acute increase in size following an upper respiratory infection.5,33 When operating on a fistula, dissection of the tract follows the course as described above with care taken during the resection to protect the spinal accessory, hypoglossal, and vagus nerves. A finger or bougie in the oropharynx can help identify the opening in the tonsillar fossa and the tract must be carefully ligated at this entry point. An alternative to open resection of the fistula is a stripping technique utilizing a guidewire passed between the opening in the neck and the internal opening in the tonsillar fossa. There is, however, only limited experience with this technique, and the long-term recurrence risk is not known.51 Second cleft cysts can be approached via a conventional cervical incision or a retroauricular hairline incision for improved cosmesis.52 Endoscopic cyst resections have also been described and noted to have low recurrence rates, but the learning curve for such procedures may limit their widespread adoption.53
Third and Fourth Cleft Anomalies
Third and fourth branchial anomalies are rare. The third and fourth pouches form the pharynx below the hyoid bone. Thus these fistulas enter into the pyriform sinus. The third cleft migrates lower in the neck to form the inferior parathyroid glands and the thymus.7,36 The descent of the fourth cleft stops higher in the neck to form the superior parathyroid glands. The fourth pouch has added significance in that its ventral portion develops into the ultimobranchial body, which contributes thyrocalcitonin-producing parafollicular cells to the thyroid gland.7 It is unusual to find cysts and sinuses from the third branchial cleft.2,35 When found, they are in the same area as those of the second cleft but ascend posterior to the carotid artery rather than through the bifurcation (Fig. 73-10).35 The fistula pierces the thyrohyoid membrane and enters the pyriform sinus. Fourth branchial fistulae are difficult to differentiate from other associated anomalies. Fourth pouch cysts also are highly unusual and must be differentiated from laryngoceles. These tracts originate at the apex of the pyriform sinus, descend beneath the aortic arch, and then ascend anterior to the carotid artery to end in the vestigial cervical sinus of His (Fig. 73-11).54 Other anomalies arising from the third and fourth branchial pouches may appear as cystic structures in the neck. Thymic cysts can occur as a result of incomplete degeneration of the thymal pharyngeal duct or of progressive cystic degeneration of epithelial remnants of Hassall corpuscles.54 Most are found on the left side of the neck. Parathyroid cysts may be located anywhere around the thyroid gland or in the mediastinum. These are usually not associated with biochemical abnormalities, although reports of hyperparathyroidism secondary to functioning cysts have been seen. The etiology of these cysts is not clear, but they may be embryologic remnants of third and fourth branchial pouches, or may represent cystic degeneration of adenomas or gradual enlargement of microcysts.54
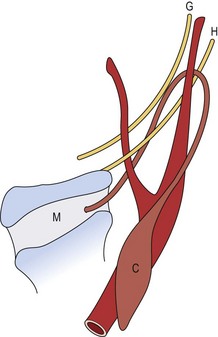
FIGURE 73-10 Third branchial cleft anomaly. The cyst (C) is posterior to the sternocleidomastoid muscle, and the tract ascends posterior to the internal carotid artery. It then passes medially between the hypoglossal (H) and glossopharyngeal (G) nerves. It pierces the thyroid membrane (M) to enter the pyriform sinus. (From Mukherji SK, Fatterpekar G, Castillo M, et al. Imaging of congenital anomalies of the branchial apparatus. Neuroimaging Clin North Am 2000;10:75–93.)
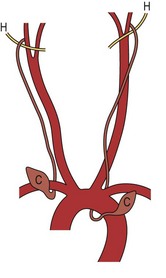
FIGURE 73-11 Fourth branchial cleft anomaly. The cysts (C) are located anterior to the aortic arch on either side. The tract hooks either the subclavian artery or the aortic arch, depending on the side, and ascends to loop over the hypoglossal nerve (H). (From Mukherji SK, Fatterpekar G, Castillo M, et al. Imaging of congenital anomalies of the branchial apparatus. Neuroimaging Clin North Am 2000;10:75–93.)
Both third and fourth cleft lesions can present at any age. In the neonate, both can present with tracheal compression or airway compromise due to rapid enlargement. Infectious complications are the most common presentation outside the neonatal period. A recent review found that 39% of third arch anomalies presented as neck abscesses and 33% as acute suppurative thyroiditis.55 For fourth arch anomalies, 42% presented initially as abscesses and 45% as thyroiditis in one series.56
The operative approach for third and fourth arch anomalies is similar to the second arch, with a few notable exceptions. Endoscopy should be used to find the pyriform sinus entry point to allow cannulation of the tract to aid with dissection. Fourth arch anomaly resections require ipsilateral hemithyroidectomy for complete excision and partial resection of the thyroid cartilage may be necessary to expose the pyriform sinus.57 In cases presenting with infection, antibiotics should be administered initially and operative excision delayed until the inflammation has resolved, which helps to decrease the risk of recurrent nerve injury.56 For both third and fourth arch anomalies, children older than 8 years generally have fewer postoperative complications than younger patients. As such, it has been suggested that initial management in children older than 8 years should be excision of the fistula tract while management in younger children should begin with endoscopic cauterization of the internal fistula opening. While ipsilateral thyroidectomy appears to decrease recurrence rates for fourth arch fistulas, there is no role for this procedure in the management of third arch anomalies.55,56
Preauricular Pits, Sinuses, and Cysts
Preauricular pits, cysts, and sinuses are not of true branchial cleft origin.58 They represent ectodermal inclusions, which are related to embryonic ectodermal mounds (auditory hillocks) that essentially form the auricles of the ear.35,59 The sinuses are often short and end blindly. They never connect internally to the external auditory canal or eustachian tube, and they characteristically end in thin strands that blend with the periosteum of the external auditory canal.58 Some authors propose that they are a marker of teratogenic exposure.59 Preauricular cysts are located in the subcutaneous layer superficial to the parotid fascia, but may seem deeper if they become infected. The lining cells of these cysts and sinuses are stratified squamous epithelium. They do not contain hair-bearing follicles owing to their origin from the ectoderm associated with external ear formation.41
The estimated incidence of preauricular sinuses is 0.1–0.9% in the USA and as high as 4–10% in Africa.59 Preauricular cysts and sinuses are commonly noted at birth, more commonly on the right side.59 The parent may remark about the familial and bilateral nature of these lesions.36 Although preauricular pits are associated with renal abnormalities in certain syndromes, renal ultrasound is generally indicated only in cases of multiple anomalies or a family history of such syndromes.60 Excision of these preauricular sinuses is not needed unless there is a history of drainage. Sinuses that drain are often connected to subcutaneous cysts that have an increased likelihood of staphylococcal infection. Ideally these lesions should be completely excised before becoming infected (Fig. 73-12). Prior infection increases the difficulty of complete surgical excision, which increases the risk of recurrence.
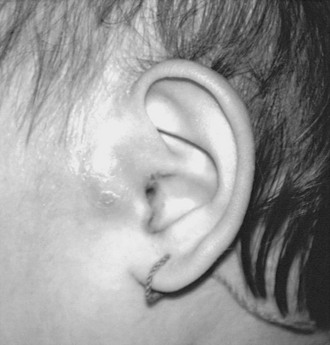
FIGURE 73-12 An infected preauricular cyst. The pit anterior to the helix is difficult to see. Note the swelling and skin changes anterior to the tragus. Preauricular sinuses that drain sebaceous material should be excised electively. Warm compresses and antibiotics allowed the inflammation to diminish. The cyst and sinus were then completely excised.
Complete surgical excision of the sinus tract and subcutaneous cyst to the level of the temporalis fascia is the treatment of choice in the uninfected draining sinus. It is important to avoid rupture of the sinus and to completely excise the sinus/cyst to decrease the risk of recurrence.61 If infection supervenes, the lesion is treated with antibiotics and warm soaks to encourage drainage and control of the surrounding inflammation. Occasionally, as with infected branchial cysts, I&D or needle aspiration may be required to control the infection. Excision is often accomplished through an elliptic incision with a small, chevron skin flap surrounding the sinus. The cyst is then dissected from the subcutaneous tissue and removed in its entirety.58 The cyst or sinus may have multiple branches, making complete resection difficult. Removal of a small bit of adjacent cartilage reduces the risk of missing one of these branching tracts.59 The incidence of recurrence is as high as 42% owing to these multiple branches, and some clinicians have advocated an extended preauricular incision to enhance exposure.61 Alternatively, extension of the elliptical excision superiorly allows removal of the subcutaneous tissue between the temporalis fascia and the helix perichondrium, which is the plane in which the sinus tracts exist. This facilitates complete excision without necessitating visualization of all the tracts, and has been noted to decrease recurrence rates.62
Dermoid and Epidermoid Cysts
Dermoid cysts embryologically represent ectodermal elements that either were trapped beneath the skin along median or paramedian embryonic lines of fusion or failed to separate from the neural tube.63–65 Dermoid cysts are differentiated from epidermoid cysts histologically by the accessory glandular structures found in dermoids.63 Dermoid cysts contain sebaceous glands, hair follicles, connective tissues, and papillae.63 Both contain sebaceous material within the cyst cavity.
Most dermoid or epidermoid cysts are diagnosed before the patient is 3 years of age.5 The most common location for dermoid cysts in children is along the supraorbital palpebral ridge. This lesion commonly appears as a characteristic swelling in the corner of the eyebrow and is most commonly first noticed at birth or within the first 1 to 3 months of life. Although usually attached to the underlying bony fascia, this lesion is movable and nontender. Occasionally, the mass may be dumbbell shaped and penetrate through the orbital bone. Dermoid cysts may occur along the midline or in atypical locations such as the medial orbital wall, nose, floor of the mouth, or submental and submaxillary areas.66 Midline dermoid cysts can be confused with midline thyroglossal duct cysts. Dermoid cysts, however, are more superficial and are usually mobile in the soft tissues, but do not move cephalad when the tongue protrudes as they lack a connecting tract to the hyoid bone. Nasal dermoid cysts may present as a cyst or sinus anywhere from the glabella to the base of the columella.66 Any midline scalp lesion suspected of being a dermoid cyst should undergo preoperative radiographic evaluation to exclude intracranial extension. This is especially important for nasal dermoid cysts which have been reported to extend to the cribiform plate in 12–45% of cases.66 Imaging is also recommended for orbital dermoids with palpably indistinct margins, as such lesions may require deep orbital dissection.67 The best way to evaluate for deep extension of a midline lesion is controversial. Both CT and MRI may be used in a complementary fashion, with CT better evaluating bony abnormalities and MRI better defining soft tissue structures. Some clinicians argue that CT alone is adequate; however, false negatives have been reported.66 Dermoids and epidermoid cysts gradually increase in size due to the accumulation of sebum.65 Infection is rare, but the cysts can rupture resulting in granulomatous inflammation.5,65 Fine needle aspirate may also be helpful in distinguishing an infected thyroglossal duct from a ruptured dermoid.65
Excision is the treatment of choice for all dermoid and epidermoid cysts, especially those which are symptomatic, enlarging, or have ruptured. This has been most commonly performed as an open operation, although increasing experience with removing these lesions is accruing by using endoscopic minimally invasive techniques.68 Proponents of the open technique report good cosmetic results with minimal complications, while early reports using the endoscopic approach have reported a few partial facial nerve injuries especially early in the surgeon’s learning curve.69 Excellent cosmetic results have also been reported using an eyelid crease incision to excise angular dermoids.70 It is important to completely remove the capsule and avoid intraoperative rupture to decrease the risk of recurrence. Infection is possible secondary to repeated local trauma. Malignant degeneration of dermoids also is possible but rare.24 Complete surgical removal is curative.
Torticollis
Torticollis in childhood may be congenital or acquired. Congenital torticollis resulting from fibrosis and shortening in the SCM is the most common type.71–73 The shortening of the SCM characteristically pulls the head and neck to the side of the lesion. The resulting ‘mass’ represents the fibrous tissue palpable within the muscle. The etiology of this ‘fibrous tumor’ is debatable.72 The significant incidence of breech presentations and other abnormal obstetric positions has been used to support both the injury and tumor etiology. Those who favor tumor see the abnormal presentation as the result of the fixed abnormal head position, whereas those who favor trauma see the difficult extraction as the cause of injury.72,74 No one theory completely explains this condition. The etiology of acquired torticollis includes cervical hemivertebrae and imbalance of the ocular muscles. In children in whom no identifiable muscular etiology is found, a high likelihood exists of Klippel–Feil anomalies or a neurologic disorder as the cause.75 Acquired torticollis also should raise the suspicion of otolaryngologic infection, gastroesophageal reflux (Sandifer syndrome), or the possibility of a neoplastic condition as the underlying cause.74,75
Histologically, the basic abnormality in congenital torticollis is endomysial fibrosis—the deposition of collagen and fibroblasts around individual muscle fibers that undergo atrophy.72 The sarcoplasmic nuclei are compacted to form ‘muscle giant cells,’ which appear to be multinucleated.72 The severity and distribution of fibrosis differ widely from patient to patient. Some cases of fibrosis occur bilaterally. The fact that mature fibrous tissue is present even in the neonate suggests that the disease begins well before birth and is probably not due to a difficult delivery.
In a series of 100 infants with torticollis, 66% had a ‘tumor’ in the muscle, and the other 34% had fibrosis but no tumor.26,76 A series of 1,086 cases from China noted only 42.7% with a tumor.77 In the typical case, the mass is not found in the newborn period but is noted at the first ‘well-baby’ check-up, some 6 weeks after birth. The infant has the characteristic posture, with the face and chin rotated away from the affected side and the head tilted toward the ipsilateral shoulder (Fig. 73-13).73 Acquired torticollis may develop at any age, and it is important to keep in mind the various causes of the acquired lesion. Its appearance depends on the severity of the lesion, the distribution of the fibrosis, and the child’s growth pattern. With time, facial and cranial asymmetry develop, and a notable flattening of the facial structures on the side of the lesion occurs. This may become irreversible by age 12 years, although reports have described good results when the operative correction is performed after age 10.78,79

FIGURE 73-13 This young child has left torticollis. He is sitting on his mother’s lap. The face and chin are tilted away from the involved side and the head is tilted toward the ipsilateral shoulder.
Experience with this condition has shown that more than 90% of patients respond to nonoperative management.77 The key to successful treatment is early recognition and prompt physical therapy.71,80 The longer the shortening of the muscle persists, the more facial and cranial asymmetry develops, and the deeper the cervical tissues become involved in the process. The need for potential surgical intervention is associated with the severity of the rotational deficit, an older age at presentation, and the presence of ‘tumor’ rather than fibrosis without tumor.77 Ultrasound may be used not only as a diagnostic tool but also to help determine which children may be more likely to need operative therapy. In another study from China, the cross-sectional as well as longitudinal extent of fibrosis in the SCM correlated well with the need for operation.81
In most instances, complete correction can be achieved by early range-of-motion and stretching exercises, and positional changes with the baby in the crib. The parents should be taught to perform these exercises with the baby several times each day. One parent holds the child’s shoulder down against a firm surface, and the other rotates the head toward the opposite shoulder. When the child’s head is rotated toward the opposite shoulder, the muscle is gently kneaded along its entire course. Often one parent can accomplish the stretching exercises by placing the baby on his or her lap, turning the baby’s head, and gently extending the head and neck over the parent’s knees. An additional maneuver is rearranging the baby’s room, changing objects in the crib, and encouraging the baby to look toward the side opposite the involved muscle. One study showed a mean duration of 4.7 months for successful nonoperative resolution.82 Some clinicians have used botulinum toxin injections in select patients who have failed to improve after six months of aggressive physical therapy, avoiding operation in 74–95% of patients.83,84
Some clinicians have suggested that the criterion for operation, regardless of age, is the development of facial hemihypoplasia.80 In children with significant torticollis, facial hemihypoplasia is invariably present, not always with a linear relation between the two conditions.80 The muscle can be divided anywhere, but transection in the middle third, through a lateral collar incision, is the simplest and provides the most aesthetically acceptable scar.80 Through this incision, one can divide the fascia colli of the neck, which is often tight and may need to be divided anteriorly as far as the midline and posteriorly to the anterior border of the trapezius. Intensive physiotherapy, including full rotation of the neck in both directions and full extension of the cervical spine, is instituted as soon as possible. For optimal results, application of a soft cervical collar for at least 3 months following fibrous band release is recommended.85 Some authors advise delaying operative intervention until the patient is at least five years of age as studies have shown better long-term outcomes due to improved compliance with physical therapy and collar therapy in older children.86
Inflammatory Lesions
Enlarged cervical lymph nodes are by far the most common neck masses in childhood. In most instances, they are the result of nonspecific reactive hyperplasia.1 The etiology is often viral or related to an upper respiratory tract or skin infection. In such cases, the adenitis resolves spontaneously. Most cases are first seen with enlarged nodes bilaterally. Because the anterior cervical nodes drain the mouth and pharynx, almost all upper respiratory and pharyngeal infections have some effect on the anterior cervical nodes. Enlarged cervical lymph nodes are frequently palpable in children between ages 2 and 10 years. Palpable nodes are uncommon in infants. A mass in a child younger than 2 years is more likely to be a cystic hygroma, thyroglossal duct cyst, dermoid cyst, or branchial cyst.87
As the head contains so many structures through which bacteria or viruses can enter the body, the cervical lymph nodes frequently become involved in many infections and inflammatory diseases. Cervical nodes also may be the first clinical manifestations of various tumors, particularly lymphomas.1 The most frequent inflammatory lesion of the cervical lymph nodes is suppurative lymphadenitis (Fig. 73-14). Others of importance are cat-scratch disease, atypical mycobacterial lymphadenitis, and tuberculous lymphadenitis. Less common but important considerations in the differential diagnosis of cervical adenitis include Kawasaki disease and acquired immunodeficiency syndrome (AIDS).

FIGURE 73-14 Acute suppurative cervical adenitis. Skin is shiny and taut over the centralized abscess cavity.
Acute Suppurative Cervical Lymphadenitis
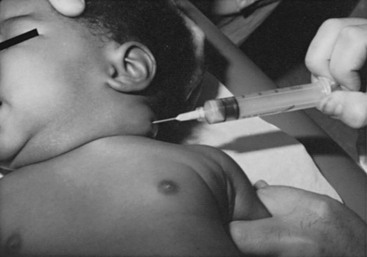
The most common cause of acute cervical lymphadenopathy is a bacterial infection arising in the oropharynx or elsewhere in the drainage area.88 The most common organisms are penicillin-resistant Staphylococcus aureus and Streptococcus pyogenes, although cultures of the pus often yield a mixture of both or prove to be sterile.88 Staphylococcus may be more prevalent in infants.89 Anaerobes, although common in the oropharynx, are not common pathogens in cervical adenitis. Fever is variable and usually mild. Initial treatment with antibiotics is often followed by resolution without suppuration. Without treatment, the node often enlarges and becomes fluctuant, eventually leading to thinning of the overlying skin and abscess formation. Needle aspiration can be both diagnostic and therapeutic. Aspiration of purulent material confirms the diagnosis. The material obtained can be cultured (Fig. 73-15). Frequently, needle aspiration and drainage of the purulent material coupled with judicious antibiotic therapy may alleviate the necessity of formal I&D. Occasionally, repeated aspirations may be necessary.
Mycobacterial Lymphadenitis
The prevalence and the relative incidence of infections caused by different mycobacteria vary with the success of preventive health measures in particular populations.90 Seventy to 95% of mycobacterial lymphadenitis in the United States is caused by nontuberculous strains, usually the atypical mycobacteria of the Mycobacterium avium-intracellulare-scrofulaceum (MAIS) complex.90 Internationally adopted terminology (MAIS) defines this group of 10 to 15 mycobacteria, which produce a specific and localized form of lymphadenitis.90 The portal of entry is primarily through the mucous membranes of the pharynx. Lymphadenitis resulting from infection with M. tuberculosis is thought to be an extension of a primary pulmonary infection and usually involves the supraclavicular nodes.91 Infection with the atypical strains usually involves higher cervical nodes, most commonly the submandibular or submaxillary.91 This finding is consistent with the etiology being a primary infection and not pulmonary disease. Infection is most commonly seen between ages 1 and 5 years; occurrence before age 1 year is rare. The disease involves unilateral nodes, and dissemination is rare. Atypical mycobacteria enter from the environment and are not contagious, although the reservoir may be the mouth and oropharynx of apparently healthy children.89 Person-to-person spread of disease has not been documented, and isolation is not necessary.
Infection with atypical mycobacteria is generally limited to the lymph nodes, but fluctuance and spontaneous drainage occur in up to 50% of cases, with sinus tracts forming in up to 10%. The nodes are usually nontender. Spontaneous regression of atypical nodal infection may occur but is likely to lead to breakdown of the nodes and sinus or fistula formation. Children with tuberculous scrofula usually are symptomatic. Most have pulmonary tuberculosis when the diagnosis is made.92 It is rare for the infection to progress from cervical adenopathy to pulmonary disease if the initial chest roentgenograms are clear. Degeneration of nodes with abscess and fistula formation is unusual.
In children, tuberculous or atypical mycobacterial lymphadenitis presents with a clinical picture of chronic lymph node hypertrophy.93 Pulmonary tuberculosis on chest radiograph helps to identify the cause of the cervical swelling. Patients with MAIS usually have a normal chest roentgenogram. Skin testing helps differentiate these diseases. All children with tuberculosis should show positive test results to second-strength purified protein derivative (PPD). Children with atypical mycobacterial infection have either a negative or a doubtful skin test. If the initial PPD is inconclusive, second-strength PPD may help confirm the mycobacterial etiology. A history of familial exposure to tuberculosis should suggest tuberculosis as more likely than atypical mycobacteria. Final diagnosis may depend on culture results or histopathology after excision of the involved nodes.94
It is important to distinguish tuberculous from MAIS lymphadenitis because the treatment is significantly different. In tuberculous infections, antituberculous chemotherapy is required, usually resulting in marked resolution of the lymphadenopathy within a few months. Chemotherapy is continued for 2 years.92 Surgical intervention in a human tuberculosis infection is confined to an excisional biopsy of a node, if the diagnosis cannot be made on other grounds. Most children with tuberculous lymphadenitis respond well to chemotherapy with standard drugs. Treatment of MAIS infections has been primarily surgical. Clarithromycin, ethambutol, and rifampicin may be used in patients whose lesions are too close to the facial nerve or other key structures, or for involvement of nodes deep to the SCM without overlying skin involvement.95,96 Patients requiring operation need careful, thorough excision of all clinically involved nodes, sinus tracts, and overlying skin. This procedure should ideally be performed before extensive ulceration of the overlying skin occurs. Children with atypical mycobacterial infection respond well to complete surgical excision without additional drug therapy.
Cat-Scratch Disease
The incidence of cat-scratch disease varies greatly in different parts of the world. In developed countries, cat-scratch disease is the most common cause of nonbacterial chronic lymphadenopathy.97 Bartonella henselae, a Gram-negative, rickettsial organism, is responsible for most cases of cat-scratch disease.98 The disease is usually transmitted via a superficial wound caused by a cat, dog, or monkey. The healthy kitten is the most frequent vector. The disease begins as a superficial infection or pustule forming in 3 to 5 days and is followed by regional adenopathy in 1 to 2 weeks. Generally, only one node is involved. Nodal involvement corresponds to the inoculation site and the nodes that drain it. The axilla is the most commonly involved area.1 The diagnosis can be made by a commercially available indirect fluorescent antibody test for detection of antibody or by polymerase chain reaction studies for B. henselae on a fine needle aspirate.98 Complete excision of the involved node is recommended to confirm the diagnosis, if necessary. Patients usually have tender lymphadenopathy and few systemic symptoms. On rare occasion, complications include encephalitis, retinitis, and osteomyelitis. Treatment for cat-scratch disease is most often symptomatic as the disease is usually self-limited. Lymphadenopathy resolves spontaneously over a period of weeks to months with only occasional suppuration. Specific antimicrobial therapy against B. henselae has not proven efficacious, although it is susceptible to many common antibiotics. Azithromycin, rifampin, ciprofloxacin, and trimethorprim-sulfamethoxazole may be efficacious if antibiotics are needed.99,100
References
1. Filston, HC. Head and neck-sinuses and masses. In: Holder TM, Ashcraft KW, eds. Pediatric Surgery. Philadelphia: WB Saunders; 1980:1062–1079.
2. Gray, SW, Skandalakis, JE. The pharynx and its derivatives. In: Skandalakis JE, Gray SW, eds. Embryology for Surgeons: The Embryological Basis for the Treatment of Congenital Defects. Philadelphia: WB Saunders; 1972:15–62.
3. Guarisco, JL. Congenital head and neck masses in infants and children, Part II. Ear Nose Throat J. 1991; 70:75–82.
4. Telander, RL, Deane, SA. Thyroglossal and branchial cleft cysts and sinuses. Surg Clin North Am. 1977; 57:779–796.
5. Enepekides, DJ. Management of congenital anomalies of the neck. Facial Plast Surg Clin North Am. 2001; 9:131–145.
6. Roback, SA, Telander, RL. Thyroglossal duct cysts and branchial cleft anomalies. Semin Pediatr Surg. 1994; 3:142–146.
7. Gray, SW, Skandalakis, JE, Akin, JT, Jr. Embryological considerations of thyroid surgery: Developmental anatomy of the thyroid, parathyroids, and the recurrent laryngeal nerve. Am Surg. 1976; 42:621–628.
8. Ward, PA, Straham, RW, Acquerelle, M, et al. The many faces of cysts of the thyroglossal tract. Trans Am Acad Ophthalmol Otolaryngol. 1970; 74:310–316.
9. Folley, DS, Fallat, ME. Thyroglossal duct and other congenital midline cervical anomalies. Semin Pediatr Surg. 2006; 15:70–75.
10. Sonnino, RE, Spigland, N, Laberge, JM, et al. Unusual patterns of congenital neck masses in children. J Pediatr Surg. 1989; 24:966–969.
11. Katz, AD, Zager, WT. The lingual thyroid. Arch Surg. 1971; 102:582–585.
12. Welch, KJ, Tapper, D, Vawter, GP. Surgical treatment of thymic cysts and neoplasms in children. J Pediatr Surg. 1979; 14:691–698.
13. Strickland, AL, Macfee, JA, VanWyk, JJ, et al. Ectopic thyroid glands simulating thyroglossal duct cysts. JAMA. 1969; 208:307–310.
14. Nanson, EM. Salivary gland drainage into the thyroglossal duct. Surg Gynecol Obstet. 1979; 149:203–205.
15. Radkowski, D, Arnold, J, Healy, GB, et al. Thyroglossal duct remnants: Preoperative evaluation and management. Arch Otolaryngol Head Neck Surg. 1991; 117:1378–1381.
16. Gupta, P, Maddalozzo, J. Preoperative sonography in presumed thyroglossal duct cysts. Arch Otolaryngol. 2001; 127:200–202.
17. Kessler, A, Eviatar, E, Lapinsky, J, et al. Thyroglossal duct cyst: Is thyroid scanning necessary in the preoperative evaluation? Israel Med Assoc J. 2001; 3:409–410.
18. Sidell, DR, Shapiro, NL. Diagnostic accuracy of ultrasonography for midline neck masses in children. Otolaryngol Head Neck Surg. 2011; 144:431–434.
19. Pinczower, E, Crockett, DM, Atkinson, JB, et al. Preoperative thyroid scanning in presumed thyroglossal duct cysts. Arch Otolaryngol Head Neck Surg. 1992; 118:985–988.
20. Sistrunk, WE. Technique of removal of cyst and sinuses of the thyroglossal duct. Surg Gynecol Obstet. 1928; 46:109–112.
21. Bennett, KG, Organ, CH, Jr., Williams, GR. Is the treatment for thyroglossal duct cysts too extensive? Am J Surg. 1986; 152:602–605.
22. Obiako, MN. The Sistrunk operation for the treatment of thyroglossal cysts and sinuses. Ear Nose Throat J. 1985; 64:196–201.
23. Ein, SH, Shandling, B, Stephens, CA, et al. Management of recurrent thyroglossal duct remnants. J Pediatr Surg. 1984; 19:437–439.
24. Hoffman, MA, Schuster, SR. Thyroglossal duct remnants in infants and children: Reevaluation of histopathology and methods for resection. Ann Otol Rhinol Laryngol. 1988; 97:483–486.
25. Salgarelli, AC, Robiony, M, Consolo, U, et al. Piezosurgery to perform hyoid bone osteotomies in thyroglossal duct cyst surgery. J Craniofac Surg. 2011; 22:2272–2274.
26. Mukel, RA, Calcaterra, TC. Management of recurrent thyroglossal duct cyst. Arch Otolaryngol. 1983; 109:34–36.
27. Ahmed, J, Leong, A, Jonas, N, et al. The extended Sistrunk procedure for the management of thyroglossal duct cysts in children: How we do it. Clin Otolaryngol. 2011; 36:271–275.
28. Maddalozzo, J, Alderfer, J, Modi, V. Posterior hyoid space as related to excision of the thyroglossal duct cyst. Laryngoscope. 2010; 120:1773–1778.
29. Ostlie, DJ, Burjonrappa, SC, Snyder, CL, et al. Thyroglossal duct infections and surgical outcomes. J Pediatr Surg. 2004; 39:396–399.
30. Kaselas, C, Tsikopoulos, G, Chortis, C, et al. Thyroglossal duct cyst’s inflammation. When do we operate? Pediatr Surg Int. 2005; 21:991–993.
31. Motamed, M, McGlashan, JA. Thyroglossal duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2004; 12:106–109.
32. Forest, VI, Murali, R, Clark, JR. Thyroglossal duct cyst carcinoma: Case series. J Otolaryngol Head Neck Surg. 2011; 40:151–156.
33. Waldhausen, JHT. Branchial cleft and arch anomalies in children. Semin Pediatr Surg. 2006; 15:64–69.
34. Moore, GW, Hutchins, GM, O’Rahilly, R. The estimated age of staged human embryos and early fetuses. Obstetrics. 1982; 139:500–506.
35. Burge, D, Middleton, A. Persistent pharyngeal pouch derivatives in the neonate. J Pediatr Surg. 1983; 18:230–234.
36. Soper, RT, Pringle, KC. Cysts and sinuses of the neck. In: Welch K, et al, eds. Pediatric Surgery. 4th ed. Chicago: Year Book Medical; 1986:539–551.
37. Frazer, JE, Bertwistle, AP. The nomenclature of disease states caused by certain vestigial structures in the neck. Br J Surg. 1923; 2:131–134.
38. Randall, P, Royster, HP. First branchial cleft anomalies. Plast Reconstr Surg. 1963; 31:497–501.
39. D’Souza, AR, Uppal, HS, De, R, et al. Updating concepts of first branchial cleft defects: A literature review. Int J Pediatr Otolaryngol. 2002; 62:103–109.
40. Shrime, M, Kacker, A, Bent, J, et al. Fourth branchial complex anomalies: A case series. Int J Pediatr Otolaryngol. 2003; 67:1227–1233.
41. Lee, K, Klein, TR. Surgery of cysts and tumors of the neck. In: Paparella MM, Shumrick DA, eds. Otolaryngology. Philadelphia: WB Saunders, 1973.
42. Roback, SA, Telander, RL. Thyroglossal duct cysts and branchial cleft anomalies. Semin Pediatr Surg. 1994; 3:142–146.
43. O’Mara, W, Amedee, R. Anomalies of the branchial apparatus. J La State Med Soc. 1998; 150:570–573.
44. Jordan, JA, Graves, JE, Manning, SC, et al. Endoscopic cauterization for treatment of fourth branchial cleft sinuses. Arch Otolaryngol. 1998; 124:1021–1024.
45. Gaisford, JC, Anderson, VS. First branchial cleft cysts and sinuses. Plast Reconstr Surg. 1975; 55:299–304.
46. Triglia, JM, Nicollas, R, Ducroz, V, et al. First branchial cleft anomalies. Arch Otolaryngol Head Neck Surg. 1998; 124:291–295.
47. Bajaj, Y, Tweedie, D, Ifeacho, S, et al. Surgical technique for excision of first branchial cleft anomalies: How we do it. Clin Otolaryngol. 2011; 36:371–374.
48. Chen, Z, Wang, Z, Dai, C. An effective surgical technique for the excision of first branchial cleft fistula: Make-inside-exposed method by tract incision. Eur Arch Otorhinolaryngol. 2010; 267:267–271.
49. D’Souza, AR, Uppal, HS, De, R, et al. Updating concepts of first branchial cleft defects: A literature review. Int J Pediatr Otorhinolaryngol. 2002; 62:103–109.
50. Ford, GR, Balakrishman, A, Evans, JN, et al. Branchial cleft and pouch anomalies. J Laryngol Otol. 1992; 106:137–143.
51. Van Zele, T, Katrien, B, Philippe, D, et al. Stripping of a fistula for complete second branchial cleft. J Plast Reconstr Aesthet Surg. 2010; 63:1052–1054.
52. Roh, JL, Yoon, YH. Removal of pediatric branchial cleft cyst using a retroauricular hairline incision (RAHI) approach. Int J Pediatr Otorhinolaryngol. 2008; 72:1503–1507.
53. Chen, LS, Sun, W, Wu, PN, et al. Endoscope-assisted versus conventional second branchial cleft cyst resection. Surg Endosc. 2012; 26:1397–1402.
54. Benson, MT, Dalen, K, Mancuso, AA, et al. Congenital anomalies of the branchial apparatus: Embryology and pathologic anatomy. Radiographics. 1992; 12:943–960.
55. Nicoucar, K, Giger, R, Jaecklin, T, et al. Management of congenital third branchial arch anomalies: A systematic review. Otolaryngol Head Neck Surg. 2010; 142:21–28.e2.
56. Nicoucar, K, Giger, R, Pope, HG, et al. Management of congenital fourth branchial arch anomalies: A review and analysis of published cases. J Pediatr Surg. 2009; 44:1432–1439.
57. Nicollas, R, Ducroz, V, Garabedian, EN, et al. Fourth branchial pouch anomalies: A study of six cases and a review of the literature. Int J Pediatr Otorhinolaryngol. 1998; 44:5–10.
58. Singer, R. A new technique for extirpation of preauricular cysts. Am J Surg. 1966; 111:291–295.
59. Scheinfeld, NS, Silverberg, NB, Weinberg, JM, et al. The preauricular sinus: A review of its clinical presentation, treatment, and associated conditions. Ped Derm. 2004; 21:191–196.
60. Tan, T, Constantinides, H, Mitchell, TE. The preauricular sinus: A review of its aetiology, clinical presentation and management. Int J Pediatr Otorhinolaryngol. 2005; 69:1469–1474.
61. Currie, AR, King, WW, Vlantis, AC, et al. Pitfalls in the management of preauricular sinuses. Br J Surg. 1996; 83:1722–1724.
62. Leopardi, G, Chiarella, G, Conti, S, et al. Surgical treatment of recurring preauricular sinus: supra-auricular approach. Acta Otorhinolaryngol Ital. 2008; 28:302–305.
63. Gold, BC, Skeinkopf, DE, Levy, B. Dermoid, epidermoid and teratomatous cysts of the tongue and the floor of the mouth. J Oral Surg. 1974; 32:107–111.
64. Smirniotopoulos, JG, Chiechi, MV. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics. 1995; 15:1437–1455.
65. Acierno, SP, Waldhausen, JHT. Congenital cervical cysts, sinuses, and fistulae. Otolaryngol Clin N Am. 2007; 40:161–176.
66. Sreetharan, V, Kangesu, L, Sommerlad, BC. Atypical congenital dermoids of the face: A 25-year experience. J Plast Reconstr Aesthet Surg. 2006; 60:1025–1029.
67. Cavazza, S, Laffi, GL, Lodi, L, et al. Orbital dermoid cyst of childhood: Clinical pathologic findings, classification and management. Int Ophthalmol. 2011; 31:93–97.
68. Huang, MG, Cohen, SR, Burstein, FD, et al. Endoscopic pediatric plastic surgery. Ann Plast Surg. 1997; 38:1–8.
69. Gur, E, Drielsma, R, Thomson, HG. Angular dermoid cysts in the endoscopic era: Retrospective analysis of aesthetic results using the direct, classic method. Plast Reconstr Surg. 2004; 113(5):1324–1329.
70. Cozzi, DA, Mele, E, d’Ambrosio, G, et al. The eyelid crease approach to angular dermoid cysts in pediatric general surgery. J Pediatr Surg. 2008; 43:1502–1506.
71. Jones, PG. Torticollis. In: Welch KJ, ed. Pediatric Surgery. 4th ed. Chicago: Year Book Medical; 1986:552–556.
72. Mickelson, MR, Cooper, RR, Ponseti, IV. Ultrastructure of the sternocleidomastoid muscle in muscular torticollis. Clin Orthop. 1975; 110:11–18.
73. Dunn, PM. Congenital postural deformities: Perinatal associations. Proc R Soc Med. 1972; 65:735–739.
74. Kahn, ML, Davidson, R, Drummond, DS. Acquired torticollis in children. Orthop Rev. 1991; 20:667–674.
75. Bredenkamp, JK, Maceri, DR. Inflammatory torticollis in children. Arch Otolaryngol Head Neck Surg. 1990; 116:310–313.
76. Ling, CM, Low, YS. Sternomastoid tumor and muscular torticollis. J Bone Joint Surg Br. 1969; 41:432–437.
77. Cheng, JC, Tang, SP, Chen, TM, et al. The clinical presentation and outcome of treatment of congenital muscular torticollis in infants–a study of 1,086 cases. J Pediatr Surg. 2000; 35:1091–1096.
78. Yu, SW, Wand, NH, Chin, LS, et al. Surgical correction of muscular torticollis in older children. Chung Hua I Hsueh Tsa Chih Taipei. 1995; 5:168–171.
79. Cheng, JCY, Tang, SP. Outcome of surgical treatment of congenital muscular torticollis. Clin Orthop. 1999; 362:190–200.
80. Soeur, R. Treatment of congenital torticollis. J Bone Joint Surg. 1940; 38:35–40.
81. Lin, JN, Chou, ML. Ultrasonographic study of the sternocleidomastoid muscle in the management of congenital muscular torticollis. J Pediatr Surg. 1997; 32:1648–1651.
82. Emery, C. The determinants of treatment duration for congenital muscular torticollis. Phys Ther. 1994; 74:921–929.
83. Oleszek, JL, Chang, N, et al. Botulinum toxin type A in the treatment of children with congenital muscular torticollis. Am J Phys Med Rehabil. 2005; 84(10):813–816.
84. Joyce, MB, de Chalain, TMB. Treatment of recalcitrant idiopathic muscular torticollis in infants with botulinum toxin type A. J Craniofacial Surg. 2005; 16(2):321–327.
85. Lee, IJ, Lim, SY, Song, HS, et al. Complete tight fibrous band release and resection in congenital muscular torticollis. J Plast Reconstr Aesthet Surg. 2010; 63:947–953.
86. Shim, JS, Jang, HP. Operative treatment of congenital torticollis. J Bone Joint Surg Br. 2008; 90:934–939.
87. Jones, PG. Glands of the neck. In: Welch K, ed. Pediatric Surgery. 4th ed. Chicago: Year Book Medical; 1986:517–520.
88. Hieber, JP, Davis, AT. Staphylococcal cervical adenitis in young infants. Pediatrics. 1976; 57:424–426.
89. Bodenstein, L, Altman, RP. Cervical lymphadenitis in infants and children. Semin Pediatr Surg. 1994; 3:134–141.
90. Altman, RP, Margeleth, AM. Cervical lymphadenopathy from atypical mycobacteria: Diagnosis and surgical treatment. J Pediatr Surg. 1975; 10:419–422.
91. Belin, RP, Richardson, JD, Richardson, DL, et al. Diagnosis and management of scrofula in children. J Pediatr Surg. 1974; 9:103–107.
92. Kent, PC. Tuberculous lymphadenitis: Not a localized disease process. Am J Med Sci. 1967; 254:866–874.
93. Lincoln, EM, Gilbert, LA. Disease in children due to mycobacteria other than Mycobacterium tuberculosis. Am Rev Respir Dis. 1972; 105:683–690.
94. Pinder, SE, Colville, A. Mycobacterial cervical lymphadenitis in children: Can histological assessment help differentiate infections caused by non-tuberculous mycobacteria from Mycobacterium tuberculosis? Histopathology. 1993; 22:59–64.
95. Fraser, L, Moore, P, et al. Atypical mycobacterial infection of the head and neck in children: A five year retrospective review. Otolaryngol. 2008; 138:311–314.
96. Harris, RL, Modayil, P, Adam, J, et al. Cervicofacial nontuberculous mycobacterium lymphadenitis in children: Is surgery always necessary? Int J Pediatr Otorhinolaryngol. 2009; 73:1297–1301.
97. Carithers, HA. Cat scratch skin test antigen: Purification by heating. Pediatrics. 1977; 60:928–929.
98. Scott, MA, McCurley, TL, Vnencak-Jones, CL, et al. Cat scratch disease: Detection of Bartonella henselae DNA in archival biopsies from patients with clinically, serologically, and histologically defined disease. Am J Pathol. 1996; 149:2161–2167.
99. Chia, JKS, Nakate, MM, Lami, JLM, et al. Azithromycin for the treatment of cat-scratch disease. Clin Infectious Dis. 1998; 26:193–194.
100. Margileth, AM. Antibiotic therapy for cat-scratch disease: Clinical study of therapeutic outcome in 268 patients and a review of the literature. Pediatr Infect Dis J. 1992; 11:474–478.