CHAPTER 15 Head and Neck Manifestations in the Immunocompromised Host
Spectrum of Immunosuppression
Immunosuppression occurs in the setting of genetic, infectious, and other acquired disorders. The cells of the immune system derive from hematopoietic stem cells in the bone marrow, circulate in the blood and lymph, and are present in nearly every tissue.1 Protection of the host occurs by two mechanisms: innate and adaptive. The innate immune system is widely conserved among many vertebrate species and is the first line of defense. The primary cells involved are neutrophils, eosinophils, basophils, macrophages/monocytes, dendritic cells, and natural killer cells. Adaptive immunity protects the host against pathogens that escape innate immune responses and is a characteristic of higher vertebrates. The cellular components involved in adaptive immunity are T cells and B lymphocytes. Immunodeficiencies can affect any of the components of the innate and adaptive immune systems.
Congenital immunodeficiencies, which are less common than acquired, rarely affect the innate immune system. Severe combined immunodeficiency (SCID) affects both the T and B cells of the adaptive immune system, and children born with this disorder often die of infections in early childhood. Other genetic immunodeficiencies that affect T and B cells include DiGeorge’s syndrome, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, common variable immunodeficiency, and selective immunoglobulin deficiencies in which serum concentrations of one or more immunoglobulin subclasses are reduced. Clinical characteristics of T-cell dysfunction include onset of symptoms in early infancy (4 to 5 months) with recurrent fungal, viral, and mycobacterial organisms and infections with opportunistic infections such as Pneumocystis.2 B-cell deficiencies are marked by recurrent infections with encapsulated organisms such as sinopulmonary infections, otitis media and sepsis, and increased incidence of atopy, but no increased susceptibility to fungal or viral infections.2
Acquired immunodeficiencies are much more common than genetic immunodeficiencies. Acquired immunodeficiency may result from infection with the human immunodeficiency virus (HIV); hematologic malignancies and myeloproliferative disorders such as multiple myeloma or leukemia; diabetes mellitus; or iatrogenic drug-induced immunosuppression from chemotherapeutic agents, corticosteroids, and other immunosuppressive agents after solid organ and bone marrow transplantation (Table 15-1). Patients with immunosuppression are at higher risk than their immunocompetent counterparts for developing fungal, bacterial, and viral infections, and have higher rates of certain malignancies. Much of this pathology may manifest in the head and neck and thus otolaryngologists should be familiar with the spectrum of disease affecting immunocompromised patients.
| Genetic |
Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome
Biology and Immunology
HIV infects and debilitates lymphocytes and macrophages leading to progressive immune compromise. Progressive decline in immune function in later stages of infection culminates in the acquired immunodeficiency syndrome (AIDS). Anecdotal reports3 of unusual infections and idiopathic immune deficiency, primarily in homosexual men, began in the early 1980s and rapidly grew into the HIV/AIDS epidemic that has affected the world population. Every day, more than 6800 people become infected with HIV and 5700 people die of AIDS.4 Worldwide, approximately 33.2 million people are living with HIV infection, with sub-Saharan Africa being the most severely affected region, where AIDS is the leading cause of death. After many years of increase, the global prevalence of HIV has stabilized and in some countries has even decreased.4 With wider availability of effective treatment, rates of death have also recently begun to decline.
Transmission of HIV occurs through body fluids and tissues. Virus from an infected patient may inoculate the bloodstream of another individual through a breach in skin or mucosa, or via intravenous infusion. Modes of transmission include sexual intercourse, the sharing of needles by intravenous drug users, vertical transmission from mother to child, and, rarely, transfusion of contaminated blood products or accidental exposure in health care workers. More than 50% of new HIV infections in the United States occur in men who have sex with men; infection after heterosexual intercourse and injection drug use lead to 32% and 18% of new infections, respectively.4
HIV is a retrovirus in the Lentivirus subfamily, named for the slow progression of disease in affected individuals. These viruses establish chronic infections with a long incubation time and slow progression of disease. Viruses in the Lentivirus family typically infect cells involved in immune modulation; in the case of HIV, that is primarily CD4 T cells and macrophages, resulting in defects in both humoral and cell-mediated immunity. The virus life cycle begins when the virus binds to the CD4 receptor, a surface protein on the helper T subset of T lymphocytes, which is also expressed on macrophages. Fusion of the viral and cell membranes allows entry of the viral core into the cell. The reverse transcriptase enzyme, a protein carried by the virus, allows transcription of RNA into DNA (a reversal of normal transcription), then mediates transcription of the viral RNA genome into viral DNA. Viral integrase, another viral protein, then facilitates incorporation of the viral DNA into the host genome. The viral DNA is transcribed into multiple RNA copies by the host cell. This newly created RNA may be spliced and translated into viral proteins or it may remain intact as a future viral genome. Translation of some viral RNA sequences results in protein precursors or multiple proteins bound together. These precursors undergo proteolytic processing by a viral protease that liberates the functional viral proteins. These proteases are required for viral infectivity. Following replication of the viral genome and proteins, the new viruses bud from the infected cell and proceed to infect new cells. The viral DNA polymerase is error-prone, incorporating one mismatched nucleotide per genome per round of transcription. This, combined with the amount of replication that occurs, establishes a vast pool of genetic diversity.5,6 The development of vast genetic diversity also gives the virus an advantage in the development of drug resistance and provides a critical barrier to vaccine development.
Diagnosis and Classification
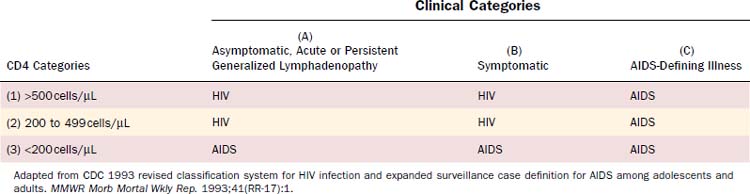
HIV infection is diagnosed when anti-HIV antibodies are detected in the serum by screening enzyme-linked immunosorbent assay (ELISA) and is confirmed with specific antibody detection with Western blot. Persistent antibodies against HIV typically occur within 3 months of infection. The CDC classification system for HIV, last updated in 1993, is based on the clinical manifestations of disease and the patient’s CD4 count (Table 15-2).7 These classifications assist in assessing the level of immune compromise and the corresponding risk for development of opportunistic infection or neoplasm. Three clinical categories have been identified: (A) asymptomatic HIV infection, persistent generalized lymphadenopathy, or acute HIV infection (a mononucleosis-type syndrome); (B) symptomatic conditions such as oral thrush, oral hairy leukoplakia, and fungal sinusitis that are attributed to HIV infection but do not fall into category A or C; (C) AIDS-defining conditions (Table 15-3). Patients may be further stratified according to their CD4 counts: (1) >500 cells/µL; (2) 200 to 499 cells/µL; (3) <200 cells/µL. The lowest accurate CD4 count, not the most recent, is used for classification purposes. AIDS is diagnosed for any patient who develops an AIDS-defining illness (category C) or CD4 less than 200 cells/µL (category 3). The World Health Organization (WHO) classification for HIV infection includes four clinical stages8:
Adapted from CDC 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1993;41(RR-17):1.
Highly Active Antiretroviral Therapy
When left untreated, HIV infection causes gradual debilitation of the immune system over a period of years, resulting in profound immunocompromise and AIDS. Advances in understanding of HIV biology have allowed for the development of medications targeting the reverse transcriptase and protease enzymes critical to the viral life cycle. Current drugs do not eradicate the virus, thus the major goals of treatment are the complete inhibition of HIV replication, reduction in HIV-related morbidity, and prevention of vertical transmission.9–11 Antiretroviral agents from five classes of drugs are currently available to treat HIV infection. These include nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), entry inhibitors (EIs), and integrase inhibitors.11 Combinations of three or more of these agents allow for such effective suppression of viral replication that the emergence of drug-resistant variants is delayed, forming the basis of highly active antiretroviral therapy (HAART).10 The use of HAART is credited for a 45% decline in mortality rates during the late 1990s, although in more recent years, the decline in mortality has been more modest, only 8% between 2000 and 2004.12
The U.S. Department of Health and Human Services recommends initiation of HAART for patients with history of an AIDS-defining illness or with CD4 less than 350 cells/µL.11 The timing of HAART initiation in asymptomatic patients with CD4 greater than 350 cells/µL is not well defined and the decision should be guided by the potential benefits and risks associated with therapy, patient comorbidities, and willingness to adhere to long-term treatment. Drug resistance testing is recommended for all patients with diagnosed HIV, regardless of whether treatment will be initiated. Two types of combination regimens are generally used: NNRTI-based (one NNRTI with two NRTI) and PI-based (one or two PI with two NRTI). The choice of regimen is determined by patient comorbidities (e.g., tuberculosis infection, liver disease, and cardiovascular disease), dosing convenience, potential drug interactions, and results of genotypic drug resistance testing.
Immune Restoration Disease
Recently, infections with opportunistic organisms occurring in HIV-positive patients who initiate antiretroviral therapy have been recognized and are known as immune reconstitution syndrome or immune restoration disease (IRD).13,14 Diagnostic criteria for IRD include a previous diagnosis of AIDS, concurrent antiretroviral therapy with increasing CD4 cell count, and an exacerbation or atypical presentation of opportunistic infections.15 The most common pathogens associated with this condition are Mycobacterium (tuberculous and nontuberculous), Cryptococcus, herpesviruses, and hepatitis B and C viruses.13 Symptoms may occur as soon as a few days following HAART initiation, although most patients develop symptoms 2 to 8 weeks after beginning HAART.15 IRD may occur in 10% to 50% of patients initiating HAART, but opportunistic infections are rare, occurring in 5% of patients.16 It is also seen in 5% of transplant recipients after dose reduction in immunosuppressive medications.17 IRD is thought to be caused by overly exuberant pathogen-specific immune responses as patients recover immune function.13–1517 IRD-associated mycobacterial infections generally present with fever, lymphadenopathy, and worsening respiratory function.18 HIV-positive patients not already receiving HAART who present with mycobacterial infections should not be started on HAART until after the infection is controlled.15 Trials examining the role of steroidal and nonsteroidal anti-inflammatory agents in the treatment of IRD are ongoing.18
Occupational Exposure to Human Immunodeficiency Virus Infection
With more than 1.3 million HIV-positive children and adults in the United States4 and with the high prevalence of otolaryngologic complaints among these patients, most otolaryngologists will treat HIV-positive patients. An understanding of the risks of occupational transmission as well as precautions that may minimize such transmission is essential. There have been 57 documented cases of HIV seroconversion among U.S. health care personnel following occupational exposure, the most recent occurring in 2000.19 Because of the voluntary nature of the reporting system, there may be underreporting of cases. Exposures that carry a risk for HIV transmission are percutaneous injuries (e.g., needlestick or cut with a sharp object) or contact of mucous membrane or nonintact skin with blood, tissue, or body fluids that are potentially infectious. The risk of transmission from fluid other than blood or transmission through nonintact skin is too low to be estimated in prospective studies. Even though cerebrospinal fluid carries a risk of transmission, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are not considered infectious unless they are visibly bloody.20 Pooled prospective data suggest an average risk of HIV transmission of 0.3% for needlesticks and 0.09% following mucous membrane exposure.21 Whereas injury from suture needles has been suggested as a possible source of occupational exposure to HIV, such an injury has not been implicated as a source of transmission in prospective studies. Although a lower viral load (<1500 RNA copies/mL) or one that is below the limits of detection probably indicates a lower titer exposure, it does not rule out the possibility of transmission.
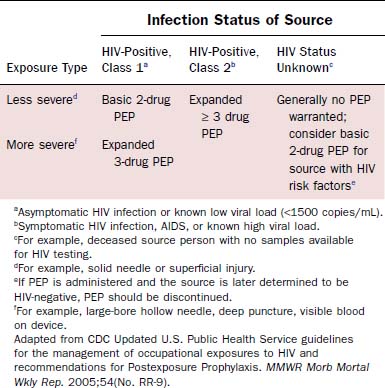
Prevention of blood exposure through the use of safe practices, barrier precautions, safe needle devices, and other innovations is the best way to prevent infection with HIV and other blood-borne pathogens.22 Simple strategies such as using double gloves, eye protection, impermeable gowns, and special techniques for passing sharp objects have all been suggested to minimize exposure risk. Despite such precautions, needlesticks and mucosal exposures will continue. After exposure to blood or other infectious fluids, the following actions should be taken immediately22: Wash needlestick areas and cuts with soap and water; flush nose, mouth, or skin with water; irrigate eyes with clean water, saline, or sterile irrigants. To minimize the risk of disease transmission, postexposure prophylaxis (PEP) should be initiated within hours of exposure and should continue for 4 weeks.20 The number of agents used depends on the type of exposure and risk profile of the source patient (Table 15-4). Treatment may be discontinued if the source patient subsequently tests negative for HIV infection and does not demonstrate any evidence of acute HIV infection. Exposed individuals should be tested for HIV at the time of exposure with follow-up testing at 6 weeks, 12 weeks, and 6 months after exposure.20 Prevention of HIV transmission following PEP is not complete. There have been at least 20 cases of seroconversion despite the use of PEP,21 with some of the patients having received multiple drug regimens. Thus careful adherence to universal precautions with avoidance of exposure remains the most prudent way to prevent infection.
Immunosuppression and Malignancy
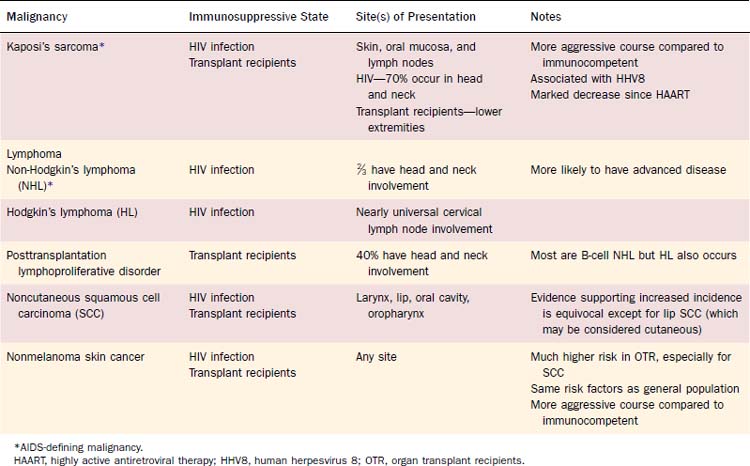
Patients with immune deficiency are at increased risk for developing malignancy (Table 15-5). The mechanism of oncogenesis is unknown, but the striking similarity between increased malignancies in immunosuppressed transplant recipients and patients with HIV suggests that immune function plays a vital role. In HIV, three types of cancer occur at markedly increased rates during later stages of the disease and are thus considered AIDS-defining malignancies. These include KS, non-Hodgkin’s lymphoma (NHL), and invasive cervical cancer. A meta-analysis comparing the incidence of cancer in patients with HIV/AIDS and immunosuppressed transplant recipients demonstrated a striking similarity in the pattern of increased rates of most types of cancer. Both groups had increased rates of AIDS-defining malignancies, Hodgkin’s lymphoma, squamous cell carcinoma of the lip, and nonmelanoma skin cancer.23 Non–AIDS-defining malignancies in patients with HIV also occur later in the disease course, often after the diagnosis of AIDS.24,25 These findings suggest that immune deficiency plays a role in the pathogenesis of cancer, although the mechanism of oncogenesis is unknown. There is no clear evidence linking immune depression with other types of head and neck cancer.
Kaposi’s Sarcoma
Epidemiology and Pathogenesis
KS is an angioproliferative disorder that causes lesions marked by spindle cell proliferation, neoangiogenesis, inflammation, and edema.26,27 There are four clinical variants with identical histologic features but with distinct epidemiologic patterns: classic KS, endemic KS, immunosuppression—or transplant-related KS, and HIV-associated KS (AIDS-KS).28–30 The classic form of KS was first described in older men of Eastern European or Mediterranean descent, with lesions typically occurring on the upper and lower extremities.29 An endemic variety of KS was also recognized in black adults and children in Africa, where it was and continues to be very common.31 In 1981, Friedman-Kien published a report of KS occurring in otherwise healthy young homosexual men32 and eventually the association between HIV infection and the development of KS became well established. By 1989, HIV-KS was reported in 15% of all US patients with AIDS.33 The overall risk of KS in AIDS patients was estimated to be 20,000 times that of the general population and more than 300 times that of other immunosuppressed patients. Since the introduction of effective antiretroviral therapy, there has been a marked decrease in the incidence of AIDS-KS,34,35 with estimated incidence now being approximately 5%.36 As solid organ and hematopoietic transplantation became more common, transplant recipients were also found to be at increased risk for developing KS.37–39 Prevalence reports vary depending on geographic location, ranging from 0.5% in the United States to 5.3% in Saudi Arabia.40 The risk for KS in organ transplant recipients is highest within the first 2 years after transplant and is increased with HLA mismatches.41
During the early AIDS-KS epidemic, a striking relationship was seen between the mode of HIV infection and the risk of developing KS. The risk for acquiring AIDS-KS was reported to be as low as 1% for patients who acquired HIV via blood transfusion, whereas in the population of men who have sex with men (MSM), the risk was 21%.33 KS was seen in only 2% of AIDS cases in women, with most of them reporting sexual contact with MSM.31 These epidemiologic disparities led to speculation that an infectious component, possibly sexually transmitted, played a role in AIDS-KS.33 In 1994, Chang and colleagues identified Kaposi’s sarcoma–associated herpesvirus (KSHV), a new herpesvirus, within KS lesions.42 KSHV is formally designated as human herpesvirus 8 (HHV8) and, along with Epstein-Barr virus (EBV), is a member of the gammaherpesvirus subfamily of herpesviruses.43,44 HHV8 has been identified in more than 95% of all KS lesions regardless of the epidemiologic subtype.29,30 The varying prevalence of HHV8 infection worldwide may explain the wide range of KS prevalence in different countries.45 The mechanism of oncogenesis has not been fully elucidated, in that KSHV is present in both immunosuppressed and immunocompetent individuals.46 It is believed that HHV8 infection coupled with the host’s state of immunosuppression plays a causal role.29,47,48
HHV8 infection itself appears to be a worldwide epidemic whose onset may have predated the HIV epidemic.47,49–51 Despite its widespread prevalence, the modes of transmission of HHV8 are incompletely understood. Some studies suggest that sex between men is an important route of transmission.49,52,53 In populations with high rates of endemic KS, familial clustering of HHV8 seropositivity was seen.54 Data suggesting vertical transmission from mother to child have also been reported,55,56 but do not explain the increased prevalence of HHV8 infection in prepubescent children. Some researchers have concluded there may be a role for child-to-child nonsexual transmission, perhaps via saliva.56–58
Presentation and Diagnosis
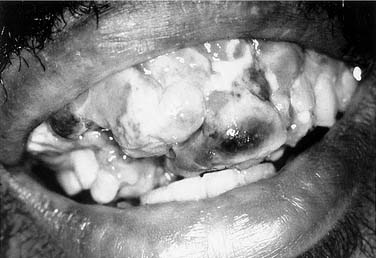
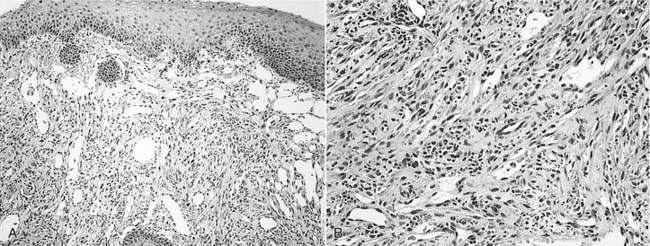
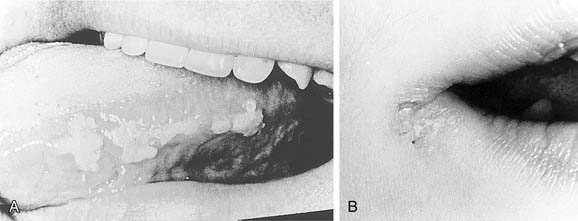
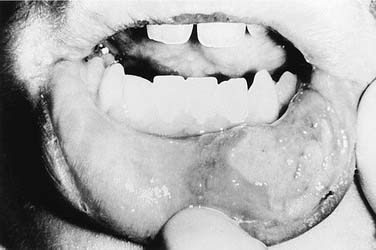
Compared with the classic and endemic forms of KS, immunosuppression-related KS and AIDS-KS are more aggressive. The clinical course of AIDS-KS ranges from an indolent, slowly progressive disease to a rapidly progressive and fatal course.28 AIDS-KS is associated with a shortened life expectancy, although most patients die of opportunistic infection or lymphoma and not KS per se.59 Multiple lesions develop in many patients, who more commonly have lymph node or visceral organ involvement. Frequently involved sites include the skin, oral mucosa, and lymph nodes. AIDS-KS occurs in the head and neck in as many as 70% of cases,60,61 whereas immunosuppression-related KS tends to occur in the lower extremities.30 Oral KS occurs in roughly one third of AIDS-KS,62 whereas in immunosuppression-related KS cutaneous lesions are most common.63 Cutaneous disease presents as multicentric macular and papular lesions that are nontender and nonblanching. These frequently coalesce and progress to violaceous, nodular lesions (Fig. 15-1). They are usually asymptomatic but may become pruritic and aesthetically displeasing. Mucosal KS commonly occurs in the oral cavity, and oral KS, which is associated with lower CD4 counts than cutaneous disease,66 may be a first sign of HIV infection.64,65 Oral KS may resemble cyclosporine-associated gingival hyperplasia in organ transplant recipients but cyclosporine usually causes generalized fibrotic gingival hyperplasia whereas oral KS produces a more localized red-purple enlargement.67 The most frequently affected sites in oral KS are the hard palate, gingiva, and tongue.64 Mucosal KS is more likely to be symptomatic than cutaneous disease. Lesions may cause loose teeth and are associated with pain, ulceration, and bleeding.
Depending on its location, visceral KS may be asymptomatic or rapidly fatal. Postmortem studies suggest that more than 25% of AIDS-KS patients have visceral lesions.30 These most commonly involve the gastrointestinal (GI) tract, liver, spleen, and lungs. GI disease is often asymptomatic, whereas median survival in pulmonary KS without treatment is only a few months.68 The most common presenting symptoms of pulmonary KS are dyspnea and cough, usually without fever unless concomitant infection is present. It may be difficult to distinguish pulmonary KS from other neoplastic and infectious diseases clinically, thus radiologic studies play an important role in its diagnosis. Computed tomography (CT) of the chest is often sufficient to diagnose pulmonary KS69 and may identify lymphatic or extrapulmonary involvement.68
Otolaryngologists may encounter patients with KS of the larynx associated with symptoms ranging from chronic cough or hoarseness to acute upper airway obstruction.70–73 If diagnosis and treatment proceed appropriately, acute airway obstruction may be avoided. Local treatment in isolated disease or systemic treatment in multicentric disease is recommended to avoid progression to airway compromise.71
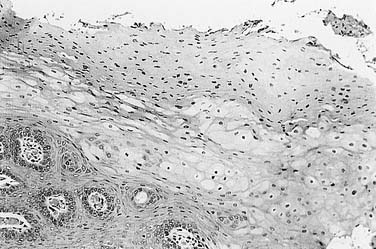
Once the diagnosis of KS is suspected, biopsy may be performed for pathologic confirmation. The histopathology of KS is characterized by abnormal proliferation of lymphatics. Lesions may occur in three forms: patch, plaque, and nodule.74 Nodular lesions are more common in immunosuppression-related and AIDS-KS, and are marked by proliferation of slitlike vascular channels, extravasated erythrocytes, and spindle-cell proliferation (Fig. 15-2). KS lesions may be mimicked by bacillary angiomatosis, which also causes vascular proliferative lesions. The presence of pleomorphic bacilli on Warthin-Starry silver stain helps distinguish bacillary angiomatosis.75,76 The identification of HHV8 DNA may help distinguish KS from other vascular lesions.77 If KS is identified in a patient without a known history of HIV infection, an HIV test is warranted.
Treatment
Despite multiple therapeutic options, treatment is palliative and there is no cure. The disease course is variable; many patients achieve remission of KS but succumb to other causes of death. The course is complicated by increased susceptibility for developing opportunistic infections related to immunosuppression. Treatment of KS is determined by the extent and location of disease, as well as the severity of symptoms. Specific indications for treatment of KS include cosmetically disfiguring lesions, symptomatic oral lesions, symptomatic visceral lesions, or pain or edema associated with lymphadenopathy or extensive cutaneous disease. Local therapies may be useful in localized lesions or for cosmesis, but they do not prevent new lesions from developing in untreated areas78 and recurrence rates are high.30 Local treatments include alitretinoin topical gel (the only FDA-approved topical treatment for KS), local radiation, intralesional chemotherapy injection, cryotherapy, laser therapy, and surgical excision.
Restoration of immune function when possible should be a primary goal, because lesions often regress with the reversal of immunosuppression. Withdrawal of immunosuppressive agents in transplant recipients may, however, lead to transplant failure in up to half of patients.29 Some data suggest that using sirolimus instead of cyclosporine-based immunosuppression regimens may result in regression of immunosuppression-related KS lesions, but further investigation is needed to substantiate these results.63,79 HAART is now recognized to be a cornerstone of AIDS-KS treatment of all stages.34,78,80 Antiretrovirals have been shown to decrease KS tumor growth, and active investigation in the use of protease inhibitors for non-AIDS patients with KS is ongoing.81,82 The AIDS Clinical Trial Group (ACTG) staging system for KS originally took into account tumor burden, immune status as reflected by CD4 count, and the presence of systemic illness to predict likelihood of survival.83 More recent data have demonstrated that only high tumor burden and systemic illness portend an unfavorable prognosis.84
Four agents are currently FDA-approved for systemic treatment of KS. These include liposomal anthracyclines (doxorubicin and daunorubicin), paclitaxel, and interferon-alpha. Other commonly used agents include vinca alkaloids (vincristine and vinblastine) and bleomycin.34,85 Caution must be exercised when using paclitaxel because serious drug interactions with various components of HAART may occur. Multidrug chemotherapy is associated with increased toxicity, including myelosuppression, and results have been disappointing in widespread disease.85 HIV-positive patients on chemotherapy are also at increased risk of developing opportunistic infections.86 As the molecular basis of KS pathogenesis becomes more clearly defined, therapies targeting specific pathways are being developed. These experimental treatments have mostly focused on angiogenesis, HHV8 replication and life cycle, and cytokine regulation.87 There are ongoing clinical trials investigating antiangiogenic compounds such as imatinib mesylate,88 matrix metalloproteinase inhibitors,89 and interleukin-12.90
Cutaneous Neoplasms
Immunosuppressed organ transplant recipients and HIV-positive patients have a higher risk of developing skin cancer compared to immunocompetent individuals. Risk factors for the development of skin cancer in immunosuppressed patients are the same as those in the general population, with fair skin, sun exposure, and family history of skin cancer being the principal factors.91–94 Even though the rate of nonmelanoma skin cancer (NMSC) is increased in both groups, organ transplant recipients have a markedly higher risk than people with HIV/AIDS.23 The incidence of NMSC in OTR can be as high as 34%.95,96 In the general population, basal cell carcinoma (BCC) is more common than cutaneous squamous cell carcinoma (SCC), whereas organ transplant recipients develop SCC twice as frequently as BCC.97 The risk of developing NMSC increases dramatically with longer posttransplant times.97,98 At 10 years after transplantation, the prevalence of NMSC is 32%.99 Within the HIV-positive population, BCC is second behind KS as the most common cutaneous malignancy, with a prevalence of 1.8%.93 CD4 count does not correlate with the incidence or severity of NMSC.100,101 Both groups have increased rates of lip SCC.23,24 The evidence linking immunosuppression to increased risk of melanoma is less clear than for NMSC, in that increased rates of detection may reflect greater surveillance in immunosuppressed patients.92,101
Compared to NMSC in the general population, NMSC in these patients, particularly SCC, is more often invasive and aggressive, with higher rates of metastasis and recurrence.92,100–102 Melanoma also has more aggressive features in the setting of immune depression, and shorter disease-free intervals and overall survival have been demonstrated in HIV-positive patients.103
Treatment outcomes vary with the type of skin cancer. Although more aggressive types of BCC in HIV-positive patients have been reported,104–106 standard excisional techniques result in cure rates similar to those in the general population.100,101 In contrast, cutaneous SCC is associated with higher mortality in immunosuppressed patients, with mortality around 3% in organ transplant recipients107 and up to 50% in HIV patients who have metastatic SCC.108 As in the management of Kaposi’s sarcoma, reduction of immunosuppression in organ transplant recipients is associated with reduced incidence and improved outcomes for skin cancer.102 There are no clear guidelines for the management of cutaneous SCC or melanoma in HIV patients, but local excision with early consideration for systemic therapy is generally recommended.101 Because of the aggressive nature of skin cancers in immunosuppressed patients, early detection with a low threshold for biopsy of suspicious lesions is recommended.
Squamous Cell Carcinoma
Data regarding the incidence of noncutaneous SCC in immunosuppressed patients are controversial. Several studies have reported increased incidence of noncutaneous SCC in organ transplant recipients, with one large series reporting a 0.5% incidence, which was a 20-fold increase over the control population.109,110 In particular, patients undergoing liver transplantation had an even greater risk.111,112 There have also been reports of increased head and neck SCC in HIV-positive patients.24,113 Confounding factors of pretransplantation tobacco and alcohol use, however, may contribute to the increased incidence seen in both groups.109,112–114 High levels of immunosuppression in organ transplant recipients109 and lower CD4 count in HIV-positive patients115 may confer an increased risk. Immunosuppressed patients, however, do appear to experience a more virulent clinical course than the general population. Organ transplant recipients and HIV-positive patients tend to present at an earlier age and with more advanced disease than their immunocompetent counterparts.109,110,115 As in immunocompetent individuals, the larynx is the most common site of involvement, followed by the oral cavity and oropharynx.114,116 Tobacco cessation and alcohol abuse counseling, as well as early detection through biopsies of suspicious lesions, are warranted.
Higher susceptibility to infection with HPV in organ transplant recipients and HIV-positive patients may play an important role in the development of noncutaneous head and neck SCC.24,116 HPV infection has been associated with head and neck SCC, particularly of the tonsil.117 Organ transplant recipients and patients with HIV/AIDS also have increased incidence of other HPV-related cancers, such as cervical and anogenital cancer.23 It is thought that immune compromise could lead to increased infection rates and failure to resolve the infections.118 As more insight is gained into the pathophysiology and clinical behavior of HPV-related cancers, management strategies and the role of prophylactic HPV vaccination in immunosuppressed patients may be better defined.
Treatment of organ transplant recipients and HIV-positive patients with head and neck SCC should follow established guidelines. Even though surgical intervention remains an important option, patients often require combined therapy for advanced disease. The use of chemotherapy in these patients may put them at significant risk for opportunistic infection. Little data exist regarding the tolerance and efficacy of radiation therapy; however, patients with HIV have been shown to tolerate radiation therapy to the head and neck.119
Lymphoma
HIV-positive patients and organ transplant recipients are at higher risk for developing non-Hodgkin’s lymphoma (NHL) and Hodgkin’s lymphoma (HL) than immunocompetent individuals. EBV-associated NHL is the most common type of lymphoma to develop in both groups. NHL comprises a heterogeneous group of tumors, but the overwhelming majority (>95%) are B-cell derived.78 Lymphomas occurring in immunosuppressed organ transplant recipients are categorized within a disease entity known as posttransplantation lymphoproliferative disorder (PTLD). The WHO classification system divides PTLD into four categories120,121:
The WHO classification for AIDS-related NHL includes three groups122:
Most AIDS-related NHL are EBV-associated B-cell lymphomas such as Burkitt’s lymphoma, diffuse large B-cell lymphoma, and plasmablastic lymphoma (PBL).78,85,123,124
EBV, along with HHV8, is a member of the gammaherpesvirus family. It is estimated that more than 90% of the world adult population is infected.125 It is found in up to 50% of AIDS-related NHL126 and nearly 90% of PTLD.127 EBV alters tumor suppressor p53 gene expression and protein regulation, which is thought to play a role in oncogenesis,44,85 but given the nearly ubiquitous presence of EBV infection, it may be that the intensity of immune suppression determines the risk of developing NHL.126 However, immunosuppressed patients have not been found to have increased rates of EBV-associated nasopharyngeal cancer,128 and a large proportion of AIDS-related NHL have not demonstrated EBV. Thus the role of EBV in NHL oncogenesis remains unclear and is an area of ongoing investigation.
AIDS-Related Non-Hodgkin’s Lymphoma
Epidemiology and Presentation
AIDS-related NHL occurs in up to 19% of HIV-positive patients85 and is the second most common malignancy to develop in HIV-positive patients.129 Patients are much more likely than their immunocompetent counterparts to present with advanced disease, B symptoms (fever, chills, night sweats, and weight loss), and extranodal disease, including bone marrow involvement.78,85,129 Seventy percent to 80% of AIDS-related NHL is diagnosed with initial stage III or IV disease, in stark contrast to only 10% to 15% of high-grade NHL in the HIV-negative population.130–133 Whereas most NHLs develop in the setting of advanced immune suppression, NHL may occur in the setting of relative immune competence and thus cannot be ruled out based on a high CD4 count or low viral load. Burkitt’s lymphoma, in particular, has been noted to occur in patients with a relatively high CD4 count.134,135 Even though the incidence of Kaposi’s sarcoma has clearly declined since the advent of HAART, the effect of HAART on AIDS-related NHL has been less significant. Although some studies suggest a trend toward declining incidence, others have found an increased incidence of NHL in the HAART era.78,136
As with immunocompetent patients with NHL, AIDS-related NHL most commonly presents in the head and neck, with nearly two thirds of patients with head and neck manifestations.129 Extranodal disease is twice as common in AIDS-related NHL compared to non-HIV associated NHL.129,137,138 Extranodal head and neck sites include the oral cavity, sinonasal region, pharynx, nasopharynx, orbit, parotid gland, larynx, mandible, and CNS.139–145 Other sites of extranodal disease include the GI tract, bone marrow, and liver. Nodal disease predominates in the neck with frequent involvement of the submandibular, jugulodigastric, and supraclavicular regions.146
NHL of the head and neck often presents as a growing mass. Constitutional symptoms are frequently present with fever, night sweats, and unintentional weight loss (greater than 10% of body mass) present in 82% of patients.147 Initial symptoms are dependent upon the location of disease. Sinonasal lymphoma usually presents with nasal obstruction or other nonspecific symptoms consistent with chronic rhinosinusitis.148 NHL of the oral cavity most commonly affects the gingiva and palate and may manifest as a persistent sore, an enlarging mass, or loose teeth. Hoarseness, respiratory symptoms, and dysphagia may indicate laryngeal or pharyngeal disease. Nasopharyngeal lymphoma may present with nasal obstruction and serous otitis media. Because of the close association with HIV, patients newly diagnosed with NHL should be screened for HIV infection.
PBL is a unique type of diffuse large B-cell lymphoma that typically involves the oral cavity and mandible but has been reported in other regions including the paranasal sinuses, skin, and lymph nodes.78,149 Although first described as an AIDS-related NHL, there are reports of PBL occurring in HIV-negative patients and OTR.150–152 The gingiva and hard palate mucosa are most commonly involved and tumors have a propensity for adjacent bone invasion.153 These lesions must be distinguished from benign gingival enlargements like pyogenic granuloma and peripheral giant cell granuloma.149 Unlike other NHLs, PBLs are generally HHV8 and EBV-negative,150 although some series report positivity.152,154
Diagnosis
The diagnosis of lymphoma in HIV-positive patients begins with a high index of suspicion. Multiple common manifestations of HIV disease such as peripheral generalized lymphadenopathy and benign oral ulcerations may mimic the findings for patients with lymphoma. The combination of a rapidly enlarging lesion and constitutional symptoms is particularly concerning for NHL. The diagnosis of lymphoma may be made with fine needle aspiration (FNA) biopsy. Even though histologic type may be determined from a cell block following aspiration, this test may not be performed and some pathologists prefer larger tissue specimens for diagnosis and determination of histologic type. Thus open biopsies may be considered. The prognosis and management depend upon the presence of extranodal disease; therefore a thorough investigation of the CNS, mediastinum, and abdomen should be performed with magnetic resonance imaging (MRI), or CT if MRI is not available. Leptomeningeal disease is the most common manifestation of CNS involvement, occurring in up to 10% of patients,155 and is best evaluated with MRI.156 A bone scan and bone marrow biopsy may also be useful in the staging process.
Prognosis and Treatment
The International Prognostic Index (IPI), developed in 1993, is a clinical tool used to predict survival in NHL.157 Factors associated with poor prognosis include age older than 60 years, advanced tumor stage, elevated serum lactate dehydrogenase, poor performance status and more than one extranodal site of disease. It has been validated in patients with AIDS-related lymphoma, in that high-risk IPI scores have been shown to predict poor survival.130,158,159 One large study demonstrated 3-year survival rates for low, low-intermediate, high-intermediate, and high-risk groups to be 66%, 42%, 35%, and 8% respectively in the HAART era.160 Recent data suggest that, in the HAART era, the relationship between CD4 count and survival in NHL may be less significant than was previously thought.158,161 However, other reports show that CD4 count continues to be a predictor of survival.136,162,163 Before advent of HAART, overall survival in NHL was only 10%.78 Numerous studies have demonstrated improved survival when combining HAART with standard combination chemotherapy. Up to 92% of patients achieve complete remission136 and nearly 50% achieve 3-year survival even with high-intermediate risk IPI scores.130,164
AIDS-related NHL is typically treated with multiagent chemotherapy in combination with HAART.165 The therapy must balance the need to eradicate the neoplasm with the risk of further immune suppression. Systemic treatment of NHL may be complicated by marrow suppression, immunosuppression, mucositis, and opportunistic infections, resulting in a high rate of morbidity and mortality.85 Rituximab, an anti-CD20 antibody, has shown promising results in the treatment of NHL in HIV-negative patients, and clinical trials are ongoing to investigate its use in AIDS-related NHL. Radiotherapy has a role for patients with localized disease or for palliation of symptomatic lesions. NHL confined to the cervical nodes has an improved response to therapy with longer associated survival than extranodal disease involving the paranasal sinuses, mandible, and other extranodal sites.145 Primary CNS lymphoma has perhaps the poorest prognosis with its tendency to recur and association with profound immune suppression.133,166 Outcomes for relapsed, aggressive NHL are extremely poor and no effective treatments are available. Whereas the treatment of choice for relapsed NHL in immunocompetent patients is autologous stem cell transplantation (ASCT), opportunistic infections and toxicity have limited its use in AIDS-related NHL.165
Posttransplantation Lymphoproliferative Disorder
PTLD is a complication of solid organ and hematopoietic transplantation marked by EBV-driven abnormal lymphoproliferation. The overall incidence in adults is approximately 2% to 3%,127 while in children the incidence is close to 8%.167 It is the second most common malignancy to develop in organ transplant recipients, after cutaneous malignancy.114 Nearly 40% of patients with PTLD present with findings in the head and neck, especially in Waldeyer’s ring and cervical lymph nodes.168 PTLD involving the head and neck usually presents with mononucleosis-like symptoms and adenotonsillar hypertrophy, and may be associated with upper airway obstruction.114,168,169 There are also reports of PTLD involving the nasal cavity and paranasal sinuses,170,171 as well as facial cutaneous manifestations.140
Most PTLDs are NHLs of the B-cell type.114 Incidence varies significantly with the type of organ transplanted, with the highest rates in multiorgan or intestinal transplants and lowest rates in renal transplants.127 PTLD develops in the setting of severe immunosuppression, which impairs the formation of a cytotoxic T lymphocyte immune response to EBV. The risk of developing PTLD is highest in EBV-naive patients, which may account for the higher incidence in children. It occurs in a bimodal temporal distribution, with most cases occurring within the first 2 years after transplantation and a smaller peak occurring later than 2 years after transplantation.127
Treatment should be directed first toward decreasing immunosuppression regimens to allow an adequate T-cell response. Up to 50% of patients respond to immunosuppression reduction.172,173 Patients who fail to respond to immunosuppression reduction may require systemic therapy such as combination chemotherapy, cytokine therapy, or anti-CD20 therapy (rituximab).114,127 Surgery is reserved for management of local symptoms, and acute upper airway obstruction caused by tonsillar hypertrophy may require tonsillectomy.174
Hodgkin’s Lymphoma
Hodgkin’s lymphoma can be seen in transplant recipients and patients with HIV. In transplant patients, it is a much less common manifestation of PTLD than NHL, comprising only 1.8% to 3.5% of all PTLDs,175 but its incidence is still 15-fold greater than that in the general population.176 Risk factors for the development of HL in transplant recipients include bone marrow transplantation and a history of graft-versus-host disease (GVHD).175,177 HL-like PTLD is clinically similar to classic HL occurring in the posttransplant setting but is distinguished by its pathologic features such as cell markers and background proliferation.175,177 HL occurring in the setting of PTLD has a better prognosis than other PTLD lymphomas176 and should be managed like other PTLD lymphomas with a focus on reduction of immunosuppression and appropriate chemotherapy.175
Hodgkin’s Lymphoma in Human Immunodeficiency Virus
Although not considered an AIDS-defining malignancy, HL is the most common non–AIDS-defining tumor occurring in patients with HIV.123,178 The incidence of HL is increased nearly 5- to 15-fold in patients with HIV (HIV-HL) compared to that in HIV-negative patients.175,179 Data suggest that HIV-HL is more common in homosexual and intravenous drug–using men than in other HIV risk groups.85,180 Several features distinguish HIV-HL from HL occurring in HIV-negative patients. HIV-HL is more likely to present with advanced disease, extranodal involvement including bone marrow in up to 50%, and B symptoms in 40% (compared to 27% in HIV-negative patients).178,180,181 In both groups, cervical lymphatic involvement is common; one series demonstrated cervical node involvement in all cases of HIV-HL.180 Notably, EBV is present in more than 90% of HIV-HL patients compared to less than 50% of non-HIV patients.123,181 The histology of HIV-HL is also distinct from that of the general population. HIV-positive patients more frequently develop the aggressive mixed cellularity and lymphocyte-depleted subtypes in comparison to the predominance of nodular lymphocytic subtypes in the general population.179 The most surprising finding in HIV-HL is that its incidence has unexpectedly increased in the HAART era. Patients with moderate immunosuppression (CD4 between 225 and 249 cells/µL) are more than twice as likely to develop HIV-HL than patients with severe immunosuppression (CD4 < 25 cells/µL).179 It is believed that increased CD4 counts allow for improved survival of Reed Sternberg cells, which are the characteristic pathologic cells seen in HL.182 Upon diagnosis of HIV-HL, staging evaluation should include CT imaging of the brain, chest, and abdomen, as well as a bone marrow biopsy.123 Evaluation of the patient’s immune status should also be performed with CD4 count and viral load testing.
There have been no randomized controlled trials for treatment of HIV-HL,183 but the accepted standard of treatment consists of combination chemotherapy and antiretroviral therapy. Patients treated with HAART in combination with chemotherapy have shown improvement in response to therapy, longer disease-free survival, and longer overall survival when compared with patients treated with combination chemotherapy alone.181,184 Estimated 2-year survival improved from 45% in patients treated with chemotherapy alone to 62% in those treated with combined HAART and chemotherapy.185 Complete response to therapy and overall survival are much worse than in HIV-negative patients, however. Overall survival rates in HIV-HL patients compared with HIV-negative patients were 68% vs. 92% at 1 year and 41% vs. 77% at 5 years.181
Salivary Gland Disease
Parotid Lesions in Human Immunodeficiency Virus
Parotid lesions are common in HIV-positive patients, especially children in whom up to 18% may present with parotid masses.186–188 These lesions may result from AIDS-related malignancies such as NHL143,144 or KS189,190 or from diffuse infiltrative lymphocytosis syndrome (DILS), but the majority of parotid enlargement in HIV-positive patients is the result of a benign process known as benign lymphoepithelial cyst (BLEC). BLEC of the parotid produces persistent, nontender parotid enlargement associated with cervical lymphadenopathy in up to 90% of patients.191 Inspissated secretions can lead to duct obstruction with resulting sialadenitis and pain.192 These lesions have varying proportions of cystic and solid components,193 and even though only unilateral clinical disease may be evident, radiologic evaluation nearly always reveals bilateral changes.194,195 Histologically, BLEC has cyst walls lined by hyperplastic and metaplastic squamous epithelium, and contains aggregates of lymphoid proliferation.191 The differential diagnosis of cystic parotid lesions includes Sjögren’s syndrome, cystic Warthin’s tumor, and branchial cleft cysts. Bilateral cystic Warthin’s tumors may be differentiated radiologically from BLEC based on the presence of focal nodularity in Warthin’s tumors and the associated lymphadenopathy of BLEC.195
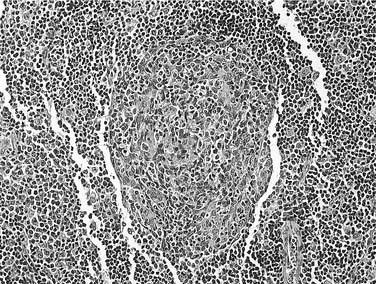
FNA can be useful in the diagnosis of parotid masses. Unilateral masses, or masses suspicious for malignancy, should undergo FNA. A study of 99 parotid FNAs in HIV-positive patients found 75% to be consistent with BLEC, 14% were infectious/inflammatory, and 6% were neoplastic. Of the neoplastic lesions, all were malignant, with three NHLs, one multiple myeloma, one metastatic adenocarcinoma from the lung, and one direct extension from a cutaneous BCC. In 6% of patients, the FNA was nondiagnostic.196 FNA of BLEC reveals a heterogeneous lymphoid population, scattered foamy macrophages, and anucleated squamous cells in a proteinaceous background.191 Germinal centers, myoepithelial islands representing metaplasia of ductal epithelium, and cystic ductal dilatation (Fig. 15-3) differentiate BLEC from lymphoma.197 Aspiration may also be useful for the relief of symptoms in larger cysts, although the lesions may recur. Initial aspirates should be sent for cytologic and microbiologic evaluation.
A variety of treatment options exist for patients with BLEC. Patients not already receiving HAART should be referred for treatment initiation because these lesions may regress with antiviral therapy.198,199 For minimally symptomatic patients without significant cosmetic deformity, observation alone represents the best option. Low-dose radiation treatment results in a greater than 50% reduction in the size of the lesion. This improvement, however, typically lasts less than 10 months.200 Some patients are treated with repeated needle aspirations although the repetitive nature of this treatment is suboptimal. Needle aspiration combined with doxycycline or tetracycline sclerotherapy may result in significant size decrease.201–203 A 1 mg/mL doxycycline solution has been used, injecting 1 to 2 mL through an intravenous catheter following cyst aspiration. Most patients are left with smaller residual fibrotic masses but long-term results of this treatment are unknown.201,202
HIV-associated BLEC typically does not require parotidectomy. Parotidectomy may be considered in those rare cases of BLEC that undergo rapid size change, are disfiguring, or have significant pressure symptoms. Other indications for parotidectomy include FNA cytology suggestive of neoplasm or unilateral masses with a significant solid component or features worrisome for malignancy. There have been no reports of malignant transformation of BLEC.191
Diffuse Infiltrative Lymphocytosis Syndrome
Parotid gland enlargement associated with sicca symptoms was recognized in HIV-positive patients in the 1980s, and Itescu and colleagues first categorized DILS as a discrete entity in 1990.204 DILS occurs in the setting of HIV and is marked by salivary gland enlargement with circulating and visceral CD8 lymphocytosis. It is often associated with cervical lymphadenopathy, and up to 60% of patients also report sicca symptoms such as xerostomia and xerophthalmia.192,205,206 In the pre-HAART era, DILS occurred in approximately 3% to 4% of HIV-positive patients, but in the HAART era its incidence has dropped significantly to less than 1%.205 It occurs twice as frequently in black patients as in whites.207,208 Diagnosis is confirmed with minor salivary gland biopsy demonstrating lymphocytic infiltration or with positive gallium Ga67 scintigraphy when biopsy is not possible.192
DILS is phenotypically similar to Sjögren’s syndrome in terms of salivary gland enlargement, sicca symptoms, and salivary gland histology. However, whereas parotid swelling is universal in DILS, it occurs in less than one third of patients with Sjögren’s syndrome.192 In addition, DILS is characterized by more frequent occurrence of extraglandular lymphocytic infiltration, predominance of CD8 cells in lymphoid aggregates (as opposed to CD4 in Sjögren’s syndrome), and the infrequent presence of serum autoantibodies.192,205 Extraglandular disease in DILS may include lymphocytic interstitial pneumonitis in up to half of patients,209 but this complication is becoming less common in the HAART era.205
Xerostomia in Human Immunodeficiency Virus and Transplant Recipients
HIV-positive patients as well as patients who suffer from chronic graft-versus-host disease (GVHD) are significantly affected by xerostomia. The prevalence of xerostomia is reported to be 2% to 10% in HIV-positive patients.210 Chronic GVHD may develop in up to 40% to 70% of patients receiving transplants from matched, unrelated donors,211 and 80% of patients with extensive GVHD complain of xerostomia.212 In the setting of HIV, some cases are iatrogenically induced by the use of HAART, antidepressants, and other drugs, whereas other cases are caused by chronic mouth breathing secondary to sinonasal disease or adenoidal hypertrophy. Still others are related to DILS as noted earlier. Salivary flow rates are diminished in the setting of HIV infection or GVHD,210,213,214 which leads to increased incidence of dental caries and impaired deglutition. Salivary substitutes, frequent saline rinses, and sialogogues help alleviate these problems. Dental caries can be prevented with fluoride.
Cervical Lymphadenopathy in Immunosuppressed Patients
Although constitutional symptoms by themselves are not a specific indicator of infection or malignancy, the presence of such symptoms without a known cause warrants further investigation. Cervical lymphadenopathy caused by lymphoma in immunosuppressed patients is associated with B symptoms in nearly 50% of patients.78,178,180,215 The distribution, size, and mobility of neck nodes may suggest infectious or malignant etiology. Cervical lymphadenopathy that is greater than 2 cm, unilateral, painful, deep, or asymmetric is suspicious for a pathologic cause, specifically granulomatous disease or lymphoma.216 Tender adenopathy is more likely to be secondary to bacterial infections, including tuberculosis, whereas nontender, enlarging neck nodes may result from malignancy.217 A thorough head and neck examination should search for potential primary sites of infection or malignancy.
Organ transplant recipients are at higher risk for development of cervical involvement, which is seen in 6% of patients, and extrapulmonary tuberculosis, which is seen in 10% to 20%.218 Head and neck sites of tuberculosis infection in organ transplant recipients include the cervical lymph nodes,219 larynx,220,221 and middle ear.222 Nontuberculous mycobacterial infections also occur in immunocompromised patients and frequently cause pulmonary infections, cervical lymphadenitis, and ulcerative skin lesions, but has also been reported to involve the temporal bone.223
Cervical Adenopathy in Human Immunodeficiency Virus
Data regarding the incidence of cervical adenopathy are sparse, but one series demonstrated that of all HIV-positive patients presenting for FNA of an enlarged lymph node, 54% had cervical involvement.224 Approximately 40% of HIV-positive patients with adenopathy have benign reactive lymphadenopathy.225–227 and 20% to 30% have tubercular etiology.226 In this background of hyperplastic adenopathy, however, cases of infectious and neoplastic etiology exist, including Mycobacterium tuberculosis, fungal infection, Pneumocystis jiroveci (formerly Pneumocystis carinii), lymphoma, KS, and other processes that also occur in the general population (Table 15-6).224,225,227 Malignancy may be present in up to 10% of cases. Differentiating among these processes remains challenging, yet critical to the appropriate management of lymphadenopathy in the setting of immunosuppression. The tendencies for multiple pathologic processes to coexist in this population and the poor sensitivity of many clinical findings and tests often make it necessary to perform microbiologic and histologic evaluations of lymph node tissue.
Table 15-6 Differential Diagnosis of Lymphadenopathy in Immunosuppressed Patients
Extrapulmonary tuberculosis is the second most common cause of cervical lymphadenopathy in HIV-positive patients.224,225,227 Tuberculosis is the leading cause of death in HIV-positive patients and effective treatments are available, thus prompt diagnosis and initiation of therapy are essential. The most common site of extrapulmonary tuberculosis involvement in HIV-positive patients is the lymph nodes,224 but other head and neck sites that may be involved include the larynx,220,228,229 oral cavity and lip,230,231 or the parotid gland.232
The patient’s immune status, as indicated by the history of opportunistic infection, CD4 count, and viral load, may help narrow the differential diagnosis of cervical adenopathy. Whereas NHL and mycobacterial infection are more likely to be present in the setting of advanced immunosuppression, the CD4 count alone is not sufficient to exclude malignancy or infection.134,135,179 The purified protein derivative (PPD) or tuberculin skin test may facilitate the diagnosis of mycobacterial lymphadenitis. In the setting of advanced HIV infection and immune compromise, however, the patient may become anergic, resulting in a low sensitivity of the PPD test. The criterion for a positive result in an HIV-infected patient is a skin reaction greater than 5 mm in diameter rather than 10 mm as in the general population.
FNA biopsy should be the initial method of tissue sampling in most cases of suspicious cervical lymphadenopathy in the HIV-infected patient. If possible, a cell block should be created from the sample and the sample should be sent for cytology, flow cytometry, and culture and stains for aerobic and anaerobic bacteria, mycobacteria, and fungi. In the largest series among HIV-positive patients, FNA provided a definitive diagnosis in more than half of patients and directed further clinical investigation in another 30%, while 20% of the initial samples were inadequate for pathologic evaluation.227 A subsequent study in children corroborated these results, with only 8% of samples being inadequate for diagnosis.225
An FNA diagnosis of follicular hyperplasia should be correlated with the clinical picture and should not, by itself, absolve the clinician’s suspicion of lymphoma. The decision to perform a diagnostic open biopsy should be driven by a suspicion of malignancy or infection in the setting of a negative or inconclusive FNA.217,233 Conditions that would favor proceeding with an open biopsy in the setting of an inconclusive finding include nodes greater than 2 cm and growing; onset associated with a low CD4 count; asymmetric, unilateral, or localized lymphadenopathy; constitutional symptoms of unknown origin; mediastinal adenopathy; or hepatosplenomegaly. Open biopsy of suspected metastatic carcinoma should be avoided and, if possible, the diagnosis should made by FNA. When metastatic carcinoma is diagnosed, a thorough examination of the upper aerodigestive tract should be performed under general anesthesia in search of a primary tumor.
Sinonasal Infection
Presentation and Pathogenesis
As in the general population, sinonasal complaints are common among immunosuppressed patients. Reports of prevalence among HIV-positive patients range from 10% to 68%, depending on the criteria used.234–237 Nearly 50% of patients undergoing allogeneic stem cell transplantation (STC) complain of rhinosinusitis, and 12% of solid organ transplant recipients develop acute sinusitis.238,239 Chronic graft-versus-host disease is a risk factor for sinusitis in hematopoietic transplant recipients.238,240 The maxillary and ethmoid sinuses are most frequently involved,234,241,242 and symptoms of acute or chronic sinusitis are similar to those of the general population. These include fever, facial pain or pressure, nasal congestion, and mucopurulent nasal discharge and postnasal drainage. Patients with chronic sinusitis are often relatively asymptomatic, reporting only congestion and nasal discharge. Some patients present initially with pulmonary complaints such as bronchospasm or infection, resulting from postnasal drainage.
Several pathogenic mechanisms have been proposed to account for the high incidence of sinusitis in immunocompromised patients. First, impaired systemic and local immunity due to HIV infection or immunosuppressive therapy leaves the host susceptible to infection.237 Second, decreased mucociliary clearance times in patients with HIV infection243,244 and ciliary abnormalities in the nasal mucosa of bone marrow transplant recipients245 have been noted. Impaired mucociliary function may result in stasis of secretions and increased susceptibility to sinonasal infection, or may reflect damage to the nasal microenvironment from repeated infection resulting in reduced mucociliary clearance. Finally, some data suggest that polyclonal B-cell activation with increased production of immunoglobulin E leads to increased atopy in HIV-positive patients, manifesting as new or increased allergic symptoms (allergic rhinitis, drug allergies, and asthma).246 However, attempts to directly correlate sinusitis with atopic disease in HIV-positive patients have been unsuccessful,235 and a recent study in HIV-positive children did not find increased prevalence of atopy.247
The spectrum of bacteria implicated in immunosuppression-associated sinusitis is similar to that seen in the general population. The most common pathogens include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in acute sinusitis and Staphylococcus, Pseudomonas, and anaerobes in chronic sinusitis.248,249 Other unusual nonfungal pathogens cultured from immunocompromised patients include Legionella pneumophila,250 Acanthamoeba,251–253 Mycobacterium kansasii, Pasteurella multocida,254 and cytomegalovirus.255
The clinical and radiologic characteristics of sinusitis are closely linked to the degree of immunosuppression. Patients with relatively normal immune function (CD4 ≥ 200 cells/µL) should be treated with standard regimens for acute and chronic sinusitis.248,256 As immune function deteriorates, patients are more susceptible to extensive disease and chronic sinusitis is more likely to develop,257 thus antibiotic coverage should include Staphylococcus, Pseudomonas, and anaerobes.248 One report found that patients with a diagnosis of AIDS had significantly worse CT findings by the Lund Mackay classification system258 than did patients with HIV.234 Patients in more advanced stages of immunosuppression as reflected by CD4 less than 200 cells/µL or absolute neutrophil count (ANC) less than 600 cells/µL are at risk for infection with more aggressive disease such as pseudomonal or invasive fungal sinusitis.259,260
The incidence of invasive fungal sinusitis (IFS) is 1.7% after hematopoietic transplantation,238 with the highest risk occurring in the immediate posttransplant period.261,262 It is much more common in hematologic malignancy such as acute myelogenous leukemia (14% incidence) or acute lymphoblastic leukemia (4% incidence) than in HIV/AIDS or solid organ transplantation.263,264 This difference in susceptibility may be explained by the fact that neutrophils are essential in the immune response to fungal infections.265,266 Solid organ transplant recipients and patients with HIV/AIDS generally retain neutrophil function, but patients in the later stages of HIV can become neutropenic and typically are at risk for IFS when CD4 counts fall below 50 cells/µL.267 Regardless of the cause of immunosuppression, the major determinant of risk is the presence and duration of neutropenia (ANC < 600 cells/µL).263,265 Prophylactic antifungal therapy in patients with hematologic malignancies and hematopoietic and solid organ transplant recipients has not been shown to be effective for the prevention of IFS.262 Aspergillus fumigatus is the most common fungal pathogen in invasive and noninvasive sinusitis for both immunosuppressed and immunocompromised patients.260 Other fungi that have been isolated include Candida albicans,268 Rhizopus arrhizus,269 Cryptococcus neoformans,270 Exserohilum,271 and Valsa sordida,272 among others.263
Diagnosis
The initial evaluation of an immunosuppressed patient with symptoms of sinusitis should include a thorough head and neck examination as well as nasal endoscopy in order to identify any mucosal or structural abnormalities. Endoscopy-guided cultures should be obtained if discharge from the middle meatus or sphenoethmoid recess is identified. The diagnosis of IFS can pose a challenge, because physical examination findings may be very subtle. The endoscopic findings range from pale, ischemic mucosa to well-circumscribed necrotic plaques. Perforations of the nasal septum and hard palate may be present. Fungal infection may be accompanied by suppuration, causing confusion with bacterial sinusitis.260
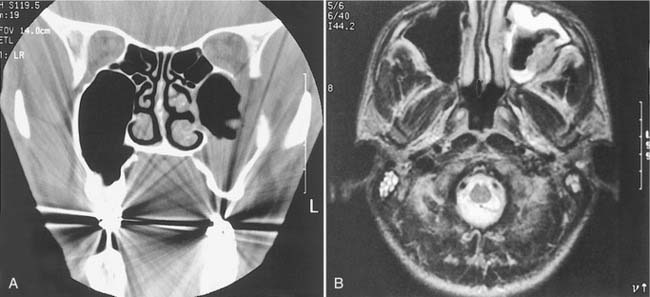
Radiologic studies often play a key role in diagnosis. Unilateral edema of the sinonasal mucosa with or without bony erosion on seen CT scan (Fig. 15-4) should lead to suspicion of fungal sinusitis or a neoplasm. But even though CT imaging is the study of choice to identify bony erosion, MRI is superior in delineating the intracranial or orbital extent of disease.261 Changes seen on T1 images are isointense in bacterial and fungal infections, but the T2 images demonstrate low signal intensity for fungal disease and high intensity for bacterial disease. A low threshold for imaging patients with low neutrophil or CD4 counts is advised.
Invasive fungal sinusitis represents a diagnostic and therapeutic emergency and any suspicion should be addressed as soon as possible. Consideration may be made for inferior and/or middle turbinate biopsy263,273 or diagnostic antral lavage226 with biopsy and histologic examination. Frozen section of biopsy specimen is often used to accelerate initiation of treatment with antifungal agents. Drainage from the middle meatus and biopsy tissue should be collected and sent for bacterial and fungal culture. There are as yet no uniform guidelines for the diagnosis of IFS, although any suspicious lesions should undergo biopsy with silver staining, histopathology, and culture.
Fungal sinusitis can extend via thrombophlebitic or hematologic spread and thus may enter into the orbit or intracranially without histologic evidence of mucosal invasion.274 The angiocentric invasion pattern of Aspergillus may allow extension of disease into the orbit or intracranially without evidence of bony destruction on imaging study. Thus, in the clinical scenario consistent with fungal sinusitis and fungal elements identified on silver stain or culture, treatment of fungal sinusitis should begin regardless of the histologic confirmation of invasion.
Treatment
The goals of management of sinusitis in immunocompromised patients include swift treatment of bacterial sinusitis and early identification of fungal sinusitis or neoplasm. Initial medical management of acute bacterial sinusitis consists of broad-spectrum antibiotics, decongestants, and saline irrigation. Antibiotics should have good coverage of Streptococcus, Staphylococcus, and H. influenzae; amoxicillin/clavulanate or cefuroxime represent good first-line choices. Antibiotic coverage should also include Pseudomonas and anaerobes in patients with moderate immunosuppression or those who do not respond to initial antibiotic treatment.248 Topical decongestants may enhance sinus drainage and provide symptomatic improvement.275 Patients should be followed closely, and if fever or local symptoms persist after 10 days of therapy, surgical drainage should be considered. Patients with a rapidly progressive course or toxic presentation should undergo early imaging and aggressive treatment, including parenteral antibiotics and possible surgical intervention. As culture and biopsy results become available, the antibiotic coverage may be narrowed. A total of 4 to 6 weeks of antibiotics should be administered.256
Invasive fungal sinusitis requires immediate, aggressive treatment. Without treatment, mortality is reported to be 50% to 80% due to intracranial and orbital involvement.261 Ideal treatment involves three components: systemic antifungal therapy, surgical debridement of infected tissue, and restoration of immune function. In particular, immunosuppression should be reversed because restoration of neutrophil count is required to prolong survival.263,264 Granulocyte colony-stimulating factor has not been substantiated to be helpful in the treatment of invasive fungal sinusitis. Initiation of HAART should be considered in an attempt to improve the underlying immune status of HIV-positive patients. High-dose, broad-spectrum antifungal medication such as amphotericin B should be administered intravenously in all cases with a clinical picture compatible with IFS, regardless of whether invasion has been demonstrated histologically. Liposomal amphotericin B,261,276 echinocandins such as caspofungin277,278 or micafungin,279 and broad-spectrum triazoles such as posaconazole280 are alternative medical therapies for patients with poor renal function.
Surgical debridement of involved tissue is important to minimize fungal load, in that medical therapy alone is typically insufficient. The severity of infection and level of immune impairment should be considered when planning surgical intervention. Sinusotomy alone is insufficient; as much as possible, involved tissue should be debrided to bleeding margins.261 Intraoperative frozen sections may assist in defining the extent of resection by determining the boundaries of invasion.281 Transfacial approaches may be required in the setting of extensive disease, which may require maxillectomy, orbital exenteration, or craniofacial resection for optimal disease control; however, the chances of survival with intracranial extension are dismal.282 Aggressive surgery must be considered in the context of the patient’s overall condition and prognosis. Postoperative management should continue intravenous antifungals with consideration for antifungal-containing saline irrigation.280 Careful follow-up and a low threshold for repeated surgical exploration is required. Patients in whom fungal disease appears clinically to have resolved may develop persistent chronic bacterial sinusitis, thus continued follow-up is necessary.283
Although more commonly involving the paranasal sinuses, invasive fungal infections of the pharynx and larynx have been described in HIV-positive284–286 and other immunosuppressed patients.287–290 Diagnosis and treatment of these invasive infections requires a high degree of suspicion and prompt initiation of medical therapy. In one case, a total laryngectomy was required for disease control.291
Immunosuppressed patients with chronic sinusitis are also candidates for surgical intervention. Friedman and colleagues found that endoscopic sinus surgery in HIV-positive patients with chronic sinusitis resulted in symptomatic improvement in 75% of patients, comparable to their results in the general population. This effect was found to be independent of CD4 count and has led the authors to suggest the same treatment algorithm for chronic sinusitis in patients regardless of HIV status.292 These findings were bolstered by Murphy and colleagues who showed a significant decrease in the symptoms of sinusitis and increased quality of life in HIV-positive patients with chronic sinusitis following endoscopic sinus surgery.293 Follow-up time in these studies was limited. Because of the devastating nature of invasive fungal sinusitis and the susceptibility of posttransplant patients to opportunistic infections, some authors advocate pretransplant endoscopic sinus surgery in patients who demonstrate evidence of sinusitis.239,294
Otologic and Neurotologic Manifestations of Immunosuppression
External and Middle Ear
Malignant Otitis Externa
Although most commonly occurring in older diabetic patients, malignant otitis externa (MOE) also occurs in immunocompromised patients,295 often in younger and nondiabetic patients with hematologic malignancy or HIV.296 The susceptibility of the immunocompromised patient to MOE is attributed to a combination of impaired chemotaxis and neutrophil function, and depressed humoral immunity.297 The pathogenesis of MOE is further facilitated by the breakdown of the local cutaneous barrier in the external auditory canal (EAC) secondary to dermatologic lesions (e.g., eczema, seborrhea) and self-induced trauma caused by pruritus. Otitis externa (OE) may progress to malignant OE if not managed aggressively and in a timely fashion. Spread to the skull base with resulting temporal bone osteomyelitis occurs via the fissures of Santorini and the tympanomastoid suture.297 Temporal bone osteomyelitis should be suspected when otalgia, swelling, and otorrhea persist despite therapy or when there is onset of facial nerve paralysis or other cranial nerve dysfunction. Unlike in diabetic patients, fungal MOE accounts for a significant portion of infections in patients with immunocompromise secondary to HIV or hematologic malignancy.298,299 Aspergillus species are most common in fungal infections. As in the general population, Pseudomonas aeruginosa is the primary etiologic agent in bacterial MOE.
Early diagnosis and management of MOE is essential in that this is a life-threatening condition, especially in patients with low CD4 counts and neutropenia.297 Otoscopy may reveal granulation tissue at the bony cartilaginous junction of the EAC, although fungal MOE has a propensity to involve the middle ear and mastoid without EAC involvement early in the disease course.299 EAC debris should be cultured and stained for bacteria, fungi, acid-fast bacilli (AFB), and Pneumocystis, although culture results may be negative due to previously administered topical or oral antibiotics. CT may show erosion of the EAC bone or suggest skull base involvement, whereas MRI is more useful for detecting soft tissue changes. Technetium-99 scintigraphy is a very sensitive but relatively nonspecific test for detecting increased osteoblastic activity. In contrast, gallium-67 citrate scintigraphy is more specific for osteomyelitis and some suggest its use to monitor response to therapy.295,297 Others, however, have noted persistently positive scans in the setting of recurrent disease.296
Oral fluoroquinolones have become the treatment of choice in bacterial MOE with cure rates near 90%.296 However, recent data demonstrate nearly 35% resistance of P. aeruginosa to ciprofloxacin, thus ciprofloxacin in combination with the third-generation cephalosporin ceftazidime has been recommended.295 Management of fungal MOE consists of long-term intravenous antifungals with adequate coverage against Aspergillus. Serial gallium-67 bone scans may help monitor the response to therapy.
Middle Ear
Eustachian tube obstruction caused by adenoidal hypertrophy or sinonasal disease is common in HIV-infected children and adults. It is therefore not surprising that otitis media (OM) commonly occurs in the HIV-infected population. Although there is no clear link between immunosuppression and increased incidence of OM,226 limited data suggest that HIV-positive patients may be more prone to systemic complications related to acute otitis media (AOM).300,301 Serous otitis media (SOM) and conductive hearing loss (CHL) are more prevalent in adults and older children, whereas AOM more frequently occurs in young children.300–302 The bacteriology of AOM parallels that in HIV-negative patients, with S. pneumoniae, H. influenzae, group A Streptococcus, and M. catarrhalis predominating.301,303 Therefore initial therapy for acute OM is the same as that for the HIV-negative patient. In the severely immunocompromised child, antibiotic coverage should be expanded to include S. aureus, because there may be an increased incidence of this pathogenic organism in HIV-positive children.304 If OM persists, tympanocentesis should be considered to guide therapy by culture and sensitivity results.
HIV-positive patients who progress to develop chronic otitis media, cholesteatoma, or persistent tympanic membrane perforations should be evaluated for surgical intervention. Advances in the treatment of HIV infection have resulted in HIV-positive patients living longer with relatively less immune compromise. The improved overall prognosis of HIV-positive patients may lead to an increase in chronic ear disease, and HIV patients undergoing elective otologic surgery have not been shown to have adverse events related to their immunosuppression.305
Systemic complications of AOM are uncommon in immunocompetent children, and some data suggest that these complications are more likely to occur in the setting of advanced immunocompromise.301,306 Patients with suppurative OM refractory to antibiotics should undergo early tympanocentesis for culture and special stains, and granulation tissue, polyps, and other masses should be carefully biopsied to rule out neoplasms and atypical infections.
Special Considerations
P. jiroveci (formerly P. carinii) and A. fumigatus represent organisms that may cause otomastoiditis in immunocompromised patients. Extrapulmonary manifestations of P. jiroveci have become more common with the routine use of pentamidine for P. jiroveci pneumonia,307 and otomastoiditis and caused by P. jiroveci often occurs in the absence of pulmonary disease.307–311 The route of transmission of P. jiroveci to the temporal bone is not clear, but retrograde spread from the nasopharynx through the eustachian tube and hematogenous spread have been proposed. Patients with Pneumocystis otomastoiditis commonly present with unilateral otalgia, otorrhea, hearing loss, and a polypoid mass on otoscopy. CT of the temporal bones reveals bony sclerosis without erosions and opacification of the middle ear and mastoid air spaces. A persistent aural polyp accompanied by signs and symptoms of OM despite otic drops and systemic antibiotics should undergo biopsy for histopathology and special stains. Silver stains should be requested and may reveal P. jiroveci organisms surrounded by a foamy granular exudate. P. jiroveci otitis externa is resolved with recovery of hearing using oral trimethoprim-sulfamethoxazole.307,308
A. fumigatus infection of the ear may present as a chronic OE in relatively immunocompetent patients, but can also cause a rapidly fatal invasive otomastoiditis or malignant OE in patients with poor immune function. Risk factors for invasive aspergillosis of the temporal bone include a low CD4 count, AIDS diagnosis, neutropenia, corticosteroid therapy, antineoplastic therapy, and prolonged antibiotic therapy. Otomastoiditis caused by A. fumigatus may start in the middle ear space or extend from the EAC.299 Invasive disease results in destruction of ossicles, erosion of the facial canal with nerve invasion, and destruction of dural plates with possible extension to the dural sinuses and other intracranial structures.312 Vertigo indicates labyrinthine invasion by Aspergillus, whereas meningismus and mental status changes indicate intracranial extension. Facial nerve weakness indicates bone invasion, which can often be confirmed by the presence of bony erosion on temporal bone CT.
OE and OM caused by invasive A. fumigatus require prompt surgical and drug therapy.313 High-dose amphotericin B should be started immediately. As much Aspergillus-infected tissue as possible should be removed to slow down extension of disease to vital structures and to facilitate the ameliorating effects of drug therapy and host immunity. In the case of OE caused by invasive Aspergillus, infected soft tissue and bone of the EAC should be debrided. Limited disease may be addressed by a transcanal approach, but a transmastoid approach with removal of the canal wall will provide more complete debridement and less risk to the facial nerve. Otomastoiditis should be managed with a radical mastoidectomy if middle ear contents and mastoid air cells are to be removed. The management of the facial nerve in these cases is controversial because invasion of the nerve by Aspergillus is not easily proven without biopsy. When the facial nerve is paralyzed and gross evidence of invasion and necrosis is present, the nerve should be resected and grafted when appropriate.
Neuropathies in Human Immunodeficiency Virus
Neurologic disorders are a common manifestation of HIV infection resulting from the neurotropic and immunosuppressive properties of the virus and susceptibility to CNS infections such as CMV, toxoplasmosis, or cryptococcal meningitis. In the pre-HAART era, 30% to 50% of patients in later stages of immunodeficiency developed the AIDS dementia complex, also known as HIV-associated dementia (HAD).314,315 The prevalence of neurologic complications of AIDS has decreased by nearly half in the HAART era, but 10% to 15% of patients still develop HAD.316 HAD is a subcortical dementia marked by memory loss, apathy, and difficulty with reading and comprehension in the early stages. Patients gradually progress over months to develop global dementia and often have concomitant motor and sensory neuropathies.316 It is thought to be caused either by repeated exposure to infected monocytes that cross the blood-brain barrier or by autonomous viral production in the brain, which serves as a reservoir for the virus.314
The incidence of peripheral neuropathy, now the most common neurologic manifestation of AIDS, has also decreased dramatically in the HAART era. Symptomatic peripheral neuropathy is present in approximately 30% of patients.317,318 Cranial nerve involvement is rare, with 2% to 3% incidence cited in older studies wherein the most commonly affected nerves were the facial, trigeminal, optic, and cochleovestibular nerves.319,320 Peripheral neuropathy associated with HIV infection may affect motor nerves, causing spastic paraparesis, but more commonly affects sensory nerves in a phenomenon known as distal sensory polyneuropathy (DSP).316 DSP is characterized by symmetric polyneuropathy and axonal degeneration. A similar pattern of neuropathy can selectively affect cranial nerves in the form of a mononeuritis multiplex, resulting in a transient mononeuropathy or progressive sensorimotor polyneuropathy.321 Although previously noted to occur in patients with lower CD4 counts, in the HAART era, CD4 count does not seem to correlate with rates or severity of symptomatic DSP.317,318 The pathogenic mechanisms of DSP are not fully understood, but direct neural invasion by HIV is not likely to play an important role.317 Rather, its pathogenesis is likely multifactorial. One important factor may be the HIV envelope glycoprotein gp120, which is thought to bind to chemokine receptors and induce neuronal injury in the dorsal root ganglion.316,317 Direct mitochondrial toxicity from antiretroviral NRTI is also thought to contribute to neuropathy in HIV patients.316–318322 Finally, necrotizing vasculitis associated with CMV or varicella zoster virus infection may also play a role in axonal degeneration.322
HAD must be differentiated from other causes of CNS dysfunction such as CMV encephalitis, progressive multifocal leukoencephalopathy (PML), meningitis, primary CNS lymphoma, and depression.316 PML is a demyelinating disease of the CNS associated with papovavirus infection. In PML, there is progressive deterioration of mentation and other neurologic functions, including cranial nerve weakness. Toxoplasma gondii infection of the brain produces large areas of coagulation necrosis. The resulting mass lesion causes altered levels of consciousness and focal deficits, including cranial nerve dysfunction. Toxoplasmosis is definitively diagnosed by brain biopsy but is usually diagnosed presumptively by the presence of an enhancing lesion on MRI or CT, and a clinical and radiographic response to empiric management with pyrimethamine and either sulfadiazine or clindamycin.
Cranial nerve deficits may result from primary or secondary CNS lymphoma, with the trigeminal and facial nerves most commonly being involved.323–325 Aseptic meningitis is also associated with cranial neuropathies secondary to increased intracranial pressure and perineural inflammation.326 Idiopathic facial nerve paralysis has previously been reported to occur at much higher frequencies in HIV-positive patients.327 However, given the extremely rare occurrence of idiopathic facial nerve paralysis even among HIV-positive patients, these findings have not been substantiated over time. The diagnosis of cranial neuropathies may be complicated by the presence of multiple concurrent pathologic processes of the CNS and peripheral nervous system.
Hearing Loss
Early in the HIV epidemic, several small studies suggested higher levels of idiopathic sensorineural hearing loss (SNHL), primarily affecting the high frequencies in patients with HIV. However, more recent data have not demonstrated an increased incidence of idiopathic hearing loss in the HIV-positive population.226,328 Antiretroviral treatment does not appear to cause increased rates of hearing loss.329 Although patients with HIV may not have clinical evidence of auditory dysfunction, many studies have demonstrated delayed wave latencies on auditory brainstem evoked response (ABR) testing.330–333 These ABR abnormalities occur in patients with symptomatic or asymptomatic HIV infection. The central auditory pathway in the upper brainstem is dysfunctional in earlier stages of HIV infection as indicated by prolonged I-V and III-V intervals, whereas the lower brainstem is affected in advanced HIV infection as indicated by a prolonged I-III interval.334 These findings were attributed to the central nervous effects of HIV infection.
The labyrinth is not as common a site of HIV-related pathology as is the central auditory system. Few abnormal findings are present on temporal bone histopathology, even on specimens from which viruses have been cultured.335 However, a recent study demonstrated that patients with HIV had abnormal posturography results compared to normal individuals and that vestibular dysfunction worsens with advanced immunosuppresssion.336 These findings may reflect the CNS effects of HIV infection.
Opportunistic diseases including CNS toxoplasmosis, tuberculosis meningitis, cryptococcal meningitis, and NHL may also cause SNHL in the AIDS patient. Sudden deafness and vestibular hypofunction in association with acute HIV infection and aseptic meningitis has been demonstrated, possibly as a result of a viral labyrinthitis or cochleovestibular neuropathy.337
Cryptococcal Meningitis
Cryptococcal meningitis is an opportunistic infection by the fungus C. neoformans and is the AIDS-defining illness in 40% to 60% of patients who are diagnosed with AIDS.338,339 Since the introduction of HAART, the incidence of opportunistic infections with C. neoformans in patients with HIV has decreased dramatically from up to 66 cases per 1000 persons in 1992 to 2 to 7 cases per 1000 persons in 2000. Meningitis is the most common manifestation, occurring in nearly 75% of cases.338,340 Cryptococcal meningitis is associated with severe immunocompromise and rarely occurs when the CD4 count is greater than 100 cells/µL. Bilateral SNHL occurs in 18% to 30% of patients with cryptococcal meningitis.341,342 Hearing loss demonstrates sudden onset with slow progression, and is usually reversible with treatment of the underlying disorder.342 Cryptococcal meningitis in patients with AIDS differs from that occurring in immunocompetent individuals in that AIDS patients develop fewer mass lesions, have higher fungal burdens, and have a poor cerebrospinal fluid (CSF) inflammatory response.326,343 Cryptococcal meningitis may present with cranial nerve palsies affecting the facial or vestibular nerves due to elevated intracranial pressure.343,344 Temporal bone histopathology in patients with cryptococcal meningitis demonstrates extensive invasion and destruction of the fibers and spiral ganglion of the cochlear nerve. Relative sparing of cochlear structures and vestibular nerves occurs in most cases, except in fulminant infections.345–348
The diagnosis of cryptococcal meningitis may be delayed because of its sometimes indolent course. The most common presentation is headache, fever, malaise and altered mental status over several weeks,343 but patients with cryptococcal meningitis may only complain of sudden hearing loss.348 Hearing loss is the most common cranial nerve manifestation followed by abnormal papillary reflexes, extraocular muscle paralysis, facial nerve paralysis, and blurred vision. Intracranial hypertension is thought to be the primary cause of visual and extraocular abnormalities associated with cryptococcal meningitis.343
If SNHL resulting from cryptococcal meningitis is misdiagnosed as idiopathic sudden hearing loss or autoimmune inner ear disease, management with corticosteroids places the patient at risk of developing fulminant life-threatening cryptococcal infection. Sudden and rapidly progressive hearing loss in an immunocompromised HIV-infected patient should therefore include serum cryptococcal antigen and a lumbar puncture before corticosteroid therapy is considered. Elevated CSF cell count is seen in immunocompetent patients, but is often low in patients with HIV.343 The diagnosis is made by the detection of cryptococcal antigen and by the identification of C. neoformans on culture. Visualization of the encapsulated budding yeast using India ink is a quick and easy but less sensitive test. The current standard therapy is at least 2 weeks of amphotericin B (0.7 to 1 mg/kg/day), or liposomal amphotericin B for patients with renal dysfunction.343 The addition of 5-fluorocytosine has been shown to have additive benefit.349,350 Patients are treated with fluconazole (200 mg/day) for 6 to 12 months, with consideration for discontinuation after CD4 count is above 100 to 200 cells/µL for at least 6 to 12 months.343 Persistent intracranial hypertension may require serial lumbar punctures, acetazolamide, or ventricular-peritoneal shunt placement.351
As with mycobacteria, Cryptococcus infections in HIV-positive patients may be associated with IRD. Cryptococcal infections in IRD may present with lymphadenitis, CNS lesions, or skin or soft tissue lesions, but aseptic meningitis is most commonly reported.17,18 These infections usually represent reactivation from previously treated infections.18 Differentiating IRD-related cryptococcal infections from true opportunistic infections is difficult but important, because management differs in these patients. There are no universally accepted guidelines, but tapered doses of prednisone have been proposed.17 Moreover, HAART-naive patients who present with opportunistic infections may benefit from deferral of HAART initiation until after the initial treatment phase has succeeded.15,18
Otosyphilis
Otosyphilis should be suspected in any HIV-infected patient who presents with cochleovestibular complaints. In turn, HIV infection should be suspected in all patients diagnosed with syphilis. An alarming trend of increased rates of syphilis has emerged with the HIV epidemic. After steady declines in the incidence of infection until 2000, the incidence in the United States by 2005 was up to 3 cases per 100,000 people.352 The primary demographic group affected has been men who have sex with men who account for 65% of cases.352,353 The reasons for the change include a decrease in safer sex practices and increased illicit drug use.352 Patients with HIV differ from HIV-negative patients with syphilis in that HIV infection is associated with a higher risk of developing neurosyphilis.352 The interval between primary infection and otosyphilis appears to be shortened to 2 to 5 years in HIV infection compared to 15 to 30 years in the general population.354 Even in patients clinically cured of syphilis, viable treponemes have been isolated from perilymph and temporal bone tissue.355,356 Thus it has been suggested that residual treponemes, which remain dormant in the temporal bone, are reactivated as cell-mediated immunity deteriorates in the setting of HIV. Neurosyphilis can occur at any stage of syphilis infection, thus otosyphilis should be suspected in an HIV-infected patient with cochleovestibular abnormalities and a previous history of treated syphilis because labyrinthine disease may result from reactivation, reinfection, or persistent treponemal infection.
The incidence of hearing loss has been reported to be nearly 20% in patients with latent syphilis and 54% of patients with neurosyphilis.357 Hearing loss resulting from syphilis is usually bilateral, may be progressive or fluctuate, or may have a sudden onset.357,358 Tinnitus, aural fullness, and dysequilibrium occasionally occur. The audiometric curve often shows a low-frequency hearing loss in association with diminished speech discrimination scores. Fortunately, the majority of patients either recover hearing or have stable hearing after appropriate treatment.357,359
The diagnosis of otosyphilis is based upon history and serologic testing in patients with no other apparent cause for cochleovestibular dysfunction. Traditionally, nontreponemal tests such as Venereal Disease Research Laboratory (VDRL) and rapid plasma reagin (RPR) tests have been used for initial screening. In recent years, however, nontreponemal testing using an enzyme immunosorbent assay (EIA) has become the screening test of choice.360 If positive, confirmatory testing with treponemal-specific tests such as fluorescent treponemal antibody absorption (FTA-ABS) or microhemagglutinin-treponema pallidum (MHA-TP) should be performed. CSF of any patient suspected of neurosyphilis should be examined.361 Treponemal-specific tests remain positive for life, even after successful eradication of infection. Nontreponemal EIA testing is not helpful in cases of reinfection, and VDRL may be negative in tertiary syphilis. Patients with reactive treponemal tests but nonreactive nontreponemal tests who have clinical findings consistent with otosyphilis should be presumptively treated. Temporal bone CT findings in otosyphilis may be normal, but irregular lucencies in the otic capsule or ossicular chain with a characteristic “moth-eaten” appearance can help confirm the diagnosis.362
Treatment of syphilis is the same in HIV-positive and HIV-negative patients. The current CDC recommendation for treatment for otosyphilis is aqueous crystalline penicillin G (18 to 24 million U per day) for 10 to 14 days, followed by benzathine penicillin G (2.4 million U intramuscularly weekly) for up to 3 weeks.361 Corticosteroids are also often used in otosyphilis, but in HIV-infected patients may be associated with a risk of further immunocompromise, leading to infectious complications. Corticosteroid therapy should be of short duration and be given in close consultation with the patient’s primary care provider.
Approach to Sensorineural Hearing Loss in Human Immunodeficiency Virus–Positive Patients
Management of hearing loss in the HIV-infected population should focus on the underlying cause (Table 15-7). As mentioned earlier, indiscriminate administration of corticosteroids in the setting of HIV infection is risky. There may be multiple causes of hearing loss during the course of HIV infection. As a result, the clinician should be prepared to repeat the evaluation in search of new causes of SNHL in cases of sudden and rapid deterioration of residual hearing. Finally, auditory rehabilitation with hearing aids has an important role in maintaining the quality of life in an otherwise disabling and isolating disease. Initial experience with cochlear implantation in patients with HIV has demonstrated successful results with no increase in postoperative complications.363,364
Table 15-7 Differential Diagnosis of Sensorineural Hearing Loss in HIV Infection
* Associated with late HIV infection, including acquired immunodeficiency syndrome.
Hearing Loss in Transplant Recipients
Patients with chronic renal failure are known to have higher rates of sensorineural hearing loss, as demonstrated by both pure tone audiometry and ABR testing.365,366 Whereas treatment with dialysis has not been shown to improve hearing function, one small study demonstrated improvement in ABR findings after renal transplantation.366 In contrast, patients who undergo liver transplantation have increased rates of SNHL. Nearly 15% of children undergoing liver transplantation were found to have SNHL by audiologic testing.367,368 Studies in adults suggest that immunosuppression with tacrolimus may be an important factor in the development of posttransplantation hearing loss.369,370
Oral Cavity
Oral lesions occur in many patients during the course of HIV infection. The most common oral manifestation is candidiasis, followed by oral hairy leukoplakia.371 Other common findings include KS, periodontal and gingival infections, aphthous ulcers, herpes simplex stomatitis, and xerostomia (Table 15-8). The risk for developing an HIV-related oral lesion correlates with lower CD4 counts, although some data suggest that this relationship weakens with longer durations of HIV infection (>10 years).371 The use of HAART has caused a 10% to 50% decrease in HIV-associated oral disease, particularly for oral candidiasis,372 although in children this beneficial effect is not as significant.373 Overall, oral complications develop in approximately 20% of HIV-positive patients on HAART.374 KS and NHL are the most prevalent malignancies occurring in the oral cavity and pharynx. However, as the longevity of AIDS patients increases, so does their risk of developing other malignancies. The clinician should be prepared to handle this increasing challenge (1) by being familiar with the oral lesions that commonly occur in this patient population, (2) by performing biopsies of all lesions that are suspicious or that do not respond to a short course of empiric therapy, and (3) by not assuming that multiple concurrent lesions have the same pathogenesis.
Table 15-8 Differential Diagnosis of Oral Lesions in HIV Infection
* May be associated with bone erosion.
Oral Candidiasis
Candidal infection is the most common oral manifestation of HIV infection. In the era of HAART, the incidence of oral candidiasis has decreased by nearly 50%.375 Reports of prevalence of oral candidiasis are 10% to 24% for patients not on HAART, and 2% to 10% for those on HAART.376,377 With CD4 counts less than 200 cells/µL, the prevalence increases to 39% for patients not receiving HAART.376 It also occurs in up to 20% of organ transplant recipients.378,379 The most common organism responsible is Candida albicans, and up to 80% of all HIV-positive patients have oral colonization with C. albicans.380 Oral candidiasis is strongly related to lower CD4 counts and higher viral loads, and possibly with smoking.381,382 The development of OC in patients on HAART is predictive for immune and virologic failure.374,383
Candidal infection of the oral mucosa can take four forms384:
The differential diagnosis of this lesion includes leukoplakia, carcinoma in situ, and oral hairy leukoplakia. Candidal infection can be diagnosed based on the resolution of these lesions with empiric anticandidal therapy. More definitive diagnosis can be made by a potassium hydroxide preparation or Gram stain of a scraping or by periodic acid–Schiff stain of a biopsy specimen. Candida species have also been reported to cause necrotizing lesions of the epiglottis in immunocompromised patients.385,386
Oral candidiasis in patients with CD4 greater than 200 cells/µL is successfully managed with topical antifungals—nystatin suspension 5 mL four times per day, or clotrimazole troches 10 mg five times per day.387 For patients with CD4 counts less than 200 cells/µL, oral fluconazole or itraconazole is recommended, with ketoconazole as an alternative.388 Posaconazole has been demonstrated to be effective for oral candidiasis refractory to azoles.389,390 The use of fluconazole prophylaxis for the prevention of oral candidiasis is not recommended due to the risk of side effects and lack of survival benefit.391
Oral Hairy Leukoplakia
Oral hairy leukoplakia is a white lesion with a corrugated and shaggy surface; it most frequently occurs on the lateral surface of the tongue. Oral hairy leukoplakia affects 3% to 10% of HIV-positive patients,392,393 and may also occur in immunosuppressed transplant recipients.378,394 It is rare in children, occurring in only 3% to 4% of HIV-positive children.186 Oral hairy leukoplakia is caused by EBV,387 and its presence in an otherwise asymptomatic patient is a strong indicator of a diagnosis of HIV infection with moderate to severe immunosuppression. Risk factors for the development of oral hairy leukoplakia include low CD4 count, high viral load, and the presence of oral candidiasis.393,395,396 The presence of oral hairy leukoplakia is not as strongly predictive for immune and virologic failure as oral candidiasis.383
When an oral lesion persists despite a short course of anticandidal therapy, a biopsy is indicated to rule out a malignant or premalignant lesion. The differential diagnosis of oral hairy leukoplakia includes leukoplakia, carcinoma in situ, hypertrophic candidiasis, and lichen planus. Oral hairy leukoplakia is diagnosed on biopsy by the presence of hyperkeratosis, acanthosis, clear or “balloon” cells in the upper spinous cell layer with minimal inflammation, and the presence of EBV in the basal epithelial cells (Fig. 15-6).384 Once the diagnosis is made, further management is rarely needed because OHL is often asymptomatic and does not undergo malignant transformation. There are insufficient data to support evidence-based recommendations for treatment. Topical treatments such as podophyllin or retinoin have been investigated, but recurrence is common. One small study found that the addition of acyclovir with podophyllin cream resulted in complete remission at 12 months.397 The use of systemic antivirals (desciclovir, valacyclovir, acyclovir, ganciclovir, foscarnet, and famciclovir) has been reported, but the body of evidence is too small to determine their efficacy.387
Herpes Simplex Virus
Infections of the oral cavity by herpes simplex virus increase in all stages of HIV infection. Seropositivity for HSV approaches 95% in the HIV-positive community, compared to 80% in the general population.398 The prevalence of HSV lesions has decreased in the HAART era, and recent prevalence is reported at 2% to 3%.186,399 Herpetic lesions are particularly persistent when the CD4 count is less than 100 cells/µL. Herpes labialis is the most common manifestation of herpes simplex infection of the oral cavity. When this manifestation occurs in the HIV-infected patient, these “fever blisters” are generally larger, more numerous, persist longer, and recur more frequently than in the general population. Intraoral infection by herpes simplex usually affects the keratinized and attached mucosa of the hard palate and gingiva and the dorsum of the tongue. The characteristic appearance is that of small round ulcers without an erythematous halo. Multiple ulcers can produce significant discomfort with mastication and swallowing, threatening nutrition. Herpes simplex virus (HSV) infection in the HIV-infected patient is likely to be prolonged and less localized than in the general population.
Herpes simplex infection is usually diagnosed empirically by the characteristic appearance of lesions and is treated with oral famciclovir, valacyclovir, or acyclovir for 7 days, although severe lesions may require intravenous acyclovir.398 For lesions that persist after adequate treatment, cell scrapings from the base of the lesion should be cultured for sensitivity testing. The treatment of choice for acyclovir-resistant HSV is intravenous foscarnet. Daily suppressive therapy with oral acyclovir, famciclovir, or valacyclovir should be considered for patients who have frequent or severe recurrences.
Oral Histoplasmosis
Histoplasmosis is a granulomatous fungal disease caused by Histoplasma capsulatum and is an AIDS-defining illness.400 H. capsulatum is endemic in areas of North, Central, and South America, Asia, and Africa, but it can be found throughout the world.401 Nearly 50% to 80% of adults in endemic areas may be infected, with most being asymptomatic.402 Although immunocompetent patients can develop oral lesions, oral histoplasmosis is strongly associated with HIV infection. In endemic areas, the prevalence of oral histoplasmosis is 3% in HIV-infected patients and less than 0.1% in non–HIV-infected patients,400,403 thus any patient who presents with oral histoplasmosis should undergo HIV testing. Symptomatic histoplasmosis infection presents as acute pulmonary, chronic pulmonary, or disseminated disease.403 Mucocutaneous involvement most often occurs in the setting of disseminated histoplasmosis but can occur in isolation.400 Histoplasmosis affecting the nasal cavity404 and parotid gland405 of HIV-positive patients has been reported, and oral histoplasmosis may also occur in immunosuppressed organ transplant recipients.406 The presence of oral histoplasmosis is associated with CD4 counts less than 50 cells/µL and higher mortality at 6 months than for patients with disseminated histoplasmosis without oral involvement.407,408
Patients with oral histoplasmosis present with painful, erythematous patches that progress to raised, granulomatous lesions that may be covered with an adherent pseudomembrane.400 Associated cervical adenopathy may be present.409 Diagnosis is established by antigen testing in body fluids and by biopsy of lesions with histology and culture for H. capsulatum.402 For oral histoplasmosis occurring in the setting of disseminated disease, treatment depends upon the severity of disease.401 For moderately severe or severe disease, systemic amphotericin B (3 mg/kg/day) for 1 to 2 weeks followed by oral itraconazole (200 mg three times daily for 3 days then 200 mg twice daily for 12 months) is recommended. For mild to moderate disease, oral itraconazole alone may be sufficient. Patients with CD4 counts less than 150 cells/µL or other immunosuppressed patients with recurrent disease should be placed on daily itraconazole prophylaxis.
Gingivitis and Periodontal Disease
Periodontal disease associated with HIV infection is classified into three categories410:
With the use of HAART, the prevalence of periodontal disease has dropped from less than 10% to as low as less than 1% in one study.410 The presence of necrotizing periodontal disease in a patient not known to have HIV infection should prompt serologic testing, because this disease is strongly associated with HIV. Patients with CD4 less than 200 cells/µL are much more likely to develop necrotizing periodontal disease than those with CD4 greater than 200 cells/µL.
The differential diagnosis of a destructive process that involves the soft tissue and bone of the alveolar crests includes lymphoma, SCC, KS, bacillary angiomatosis, fungal infection, and mycobacterial infection. The presence of bone destruction out of proportion with the soft tissue changes is more likely to be caused by bacillary angiomatosis than necrotizing ulcerative periodontitis.76 Tissue should be sent for histopathologic and microbiologic testing. Periodontal consultation should be requested for appropriate and expeditious management of periodontal disease.
The mainstay of therapy for HIV-related gingivitis and periodontitis includes dental plaque removal and oral rinses with 10% povidone-iodine, with 0.1% to 0.2% chlorhexidine gluconate.384 Because periodontal disease in HIV-positive patients is closely associated with candidiasis, topical or systemic antifungal treatment should also be employed.410 If present, necrotic tissue should be debrided and the teeth scaled. If patients fail to respond to initial management, parenteral antibiotic therapy with clindamycin or metronidazole should be instituted.
Aphthous Ulcers
Aphthous ulcers are of three types, all of which affect unattached oral mucosa. Herpetiform ulcers are smaller than 0.2 mm in diameter and are self-limited. Minor aphthous ulcers are well-circumscribed, painful ulcers less than 6 mm in diameter with an erythematous halo. In the HIV-infected patient, ulcers frequently coalesce to form larger lesions lasting about 2 weeks. Major aphthous ulcers (Sutton’s disease) are larger than 6 mm in diameter (Fig. 15-7). They are painful, persist for weeks, and threaten nutritional intake. They are difficult to grossly differentiate from malignancy. In the HAART era, the incidence of aphthous ulcers ranges from 1% to 8% in HIV-positive patients.375,399,411
Management should focus on ruling out malignancy, providing symptomatic relief, and monitoring nutritional status. The edge of the ulcer should be biopsied and submitted for histopathologic study to rule out lymphoma and SCC. The size, chronicity, and exquisite tenderness of major aphthous ulcers mandate close monitoring of body weight and the provision of liquid nutritional supplements. Initial treatment for minor ulcers with over-the-counter topical protective and analgesic medications is recommended.412 For major or nonhealing minor lesions, thalidomide (200 mg/day) has been shown to be effective for treatment, but ineffective for prevention of recurrence.387 Intralesional or systemic steroids have also been found to be effective.412
Human Papillomavirus
Human papillomavirus (HPV) may cause oral or genital warts, which, like other HIV-associated oral lesions, occur with lower CD4 counts.413 The incidence of oral warts in HIV-positive patients is 1.6%, and interestingly, the incidence of oral warts increases with lower viral loads.414 Whether this phenomenon is explained by a mechanism similar to immune reconstitution syndrome is unclear. Oral HPV infection rates have not decreased in the HAART era, and in fact may have increased in white men infected with HIV.387 Few studies have investigated optimal management of these lesions in the HIV population, but surgery is the most common therapy for symptomatic lesions. Alternative therapies include cidofovir, bleomycin, cimetidine, podophyllin, or intralesional interferon.
Miscellaneous
Although more commonly involving the paranasal sinuses, invasive fungal infections of the pharynx and larynx have been described in HIV-positive284–286 and other immunosuppressed patients.287–290 Diagnosis and treatment of these invasive infections requires a high degree of suspicion and prompt initiation of medical therapy. In one case, a total laryngectomy was required for disease control.291
Human Immunodeficiency Virus–Associated Facial Lipoatrophy
First described in 1998,415 lipodystrophy is a term used to refer collectively to the changes in fat distribution that occur in HIV-positive patients. These changes include peripheral lipoatrophy affecting the face and extremities; lipohypertrophy, which may manifest as increased fat deposition in dorsocervical fat pads (“buffalo hump”), increased breast size and abdominal girth, and visceral adipose tissue; and metabolic disturbances such as hypertriglyceridemia, hypercholesterolemia, insulin resistance, type 2 diabetes mellitus, and elevated hepatic transaminases.416–418 There is not yet consensus regarding the definition and classification of the manifestations of lipodystrophy, which hinders interpretation of the literature.418 Reports of prevalence vary widely due this lack of standardized case definitions and differences in methodology,419 but a review of recent literature found that the prevalence of peripheral lipoatrophy in HIV-positive men and women was 22% to 38%.420 The cause and mechanism of lipoatrophy remain unknown. Many reports have demonstrated that the use of antiretroviral therapy including NRTIs and protease inhibitors, but not NNRTIs,418,419,421 is associated with lipoatrophy. These findings have led some to conclude that inhibition of lipid and adipocyte regulatory proteins by antiretrovirals or mitochondrial toxicity from NRTIs is responsible.418 However, other studies have shown a strong correlation with AIDS diagnosis, CD4 count, and viral load, and not with HAART, suggesting a role for HIV infection itself.418,422 This discrepancy may be due to different methodologies used in the studies, and it is possible that these various factors may have an impact on some but not others of the different manifestations of lipodystrophy.416
Facial lipoatrophy is characterized by fat atrophy in the malar, buccal, melolabial, and temporal regions (Fig. 15-8).423,424 Patients affected by peripheral lipoatrophy may feel embarrassed or stigmatized by their aesthetic appearance, and studies have shown that these negative effects may lead to decreased adherence to HAART.418 Objective assessment of the severity of lipoatrophy is difficult, because CT and MRI are both costly and not practical for routine use. Ultrasound has been shown in some studies to be useful,425,426 but other data suggest that changes detected with ultrasound do not correlate with changes seen on CT.427 This controversy may reflect difference in methodology used.
Figure 15-8. Moderately severe facial lipoatrophy, before (A) and after (B) treatment.
(Courtesy of Dr. Michael Echavez.)
Many different treatment strategies have been proposed for facial lipoatrophy, but unfortunately no medical therapies have been shown to be effective. Surgical options include autologous fat grafting,424,428 hyaluronic acid injection,429,430 poly-L-lactic acid injection,431–433 calcium hydroxylapatite injection,434,435 malar or submalar synthetic implant placement,436 and other injectable fillers.437–439 Current FDA-approved treatments for facial lipoatrophy include calcium hydroxylapatite and poly-L-lactic acid. Few comparative studies have been performed and have found no difference in successful outcomes between implant placement and injection with fat or synthetic substances.440–442 The decision to treat facial lipoatrophy and the method used to correct the cosmetic deformity should be guided by patient and surgeon preference.
Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027.
Biggar RJ, Jaffe ES, Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786.
Chhieng DC, Argosino R, McKenna BJ, et al. Utility of fine-needle aspiration in the diagnosis of salivary gland lesions in patients infected with human immunodeficiency virus. Diagn Cytopathol. 1999;21:260.
Cooley TP. Non-AIDS-defining cancer in HIV-infected people. Hematol Oncol Clin North Am. 2003;17:889.
Dave SP, Pernas FG, Roy S. The benign lymphoepithelial cyst and a classification system for lymphocytic parotid gland enlargement in the pediatric HIV population. Laryngoscope. 2007;117:106.
Ellison E, Lapuerta P, Martin SE. Fine needle aspiration (FNA) in HIV+ patients: results from a series of 655 aspirates. Cytopathology. 1998;9:222.
Ferrari S, Vento S, Monaco S, et al. Human immunodeficiency virus-associated peripheral neuropathies. Mayo Clin Proc. 2006;81:213.
Friedman GD, Jeffrey Fessel W, Udaltsova NV, et al. Cryptococcosis: the 1981-2000 epidemic. Mycoses. 2005;48:122.
Funk E, Brissett AE, Friedman CD, et al. HIV-associated facial lipoatrophy: establishment of a validated grading scale. Laryngoscope. 2007;117:1349.
Gerlini G, Romagnoli P, Pimpinelli N. Skin cancer and immunosuppression. Crit Rev Oncol Hematol. 2005;56:127.
Gourin CG, Terris DJ. Head and neck cancer in transplant recipients. Curr Opin Otolaryngol Head Neck Surg. 2004;12:122.
Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59.
Gurney TA, Lee KC, Murr AH. Contemporary issues in rhinosinusitis and HIV infection. Curr Opin Otolaryngol Head Neck Surg. 2003;11:45.
Harris NL, Swerdlow SH, Frizzera G, et al. Post-transplant lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, et al, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001:264-269.
Hengge UR, Ruzicka T, Tyring SK, et al. Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis. 2002;2:281.
Hunt SM, Miyamoto RC, Cornelius RS, et al. Invasive fungal sinusitis in the acquired immunodeficiency syndrome. Otolaryngol Clin North Am. 2000;33:335.
Itescu S, Brancato LJ, Buxbaum J, et al. A diffuse infiltrative CD8 lymphocytosis syndrome in human immunodeficiency virus (HIV) infection: a host immune response associated with HLA-DR5. Ann Intern Med. 1990;112:3.
Mirza N, Lanza DC. Diagnosis and management of rhinosinusitis before scheduled immunosuppression: a schematic approach to the prevention of acute fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33:313.
Noguchi K, Fukazawa H, Murakami Y, et al. Gamma-herpesviruses and cellular signaling in AIDS-associated malignancies. Cancer Sci. 2007;98:1288.
Preciado DA, Matas A, Adams GL. Squamous cell carcinoma of the head and neck in solid organ transplant recipients. Head Neck. 2002;24:319.
Ramirez-Amador V, Ponce-de-Leon S, Anaya-Saavedra G, et al. Oral lesions as clinical markers of highly active antiretroviral therapy failure: a nested case-control study in Mexico City. Clin Infect Dis. 2007;45:925.
Raphael M, Borisch B, Jaffe ES. Lymphomas associated with infection by the human immunodeficiency virus (HIV). In: Jaffe ES, Harris NL, Stein H, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2001.
Rubin Grandis J, Branstetter BFt, Yu VL. The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis. 2004;4:34.
Singh B, Poluri A, Shaha AR, et al. Head and neck manifestations of non-Hodgkin’s lymphoma in human immunodeficiency virus-infected patients. Am J Otolaryngol. 2000;21:10.
Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis. 2007;44:1222.
1. Goronzy JJ, Cornelia MW. The innate and adaptive immune systems. In Goldman L, editor: Cecil Medicine, 23rd ed, Philadelphia: Elsevier, 2007.
2. Ballow M. Primary immunodeficiency diseases. In Goldman L, editor: Cecil Medicine, 23rd ed, Philadelphia: Elsevier, 2007.
3. Centers for Disease Control and Prevention. Pneumocystis pneumonia—Los Angeles. MMWR Morb Mortal Wkly Rep. 1981;30:250.
4. Joint United Nations Programme on HIV/AIDS. In AIDS Epidemic Update. Geneva, Switzerland: World Health Organization; 2007.
5. Mansky LM, Temin HM. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J Virol. 1995;69:5087.
6. Pathak VK, Temin HM. Broad spectrum of in vivo forward mutations, hypermutations, and mutational hotspots in a retroviral shuttle vector after a single replication cycle: substitutions, frameshifts, and hypermutations. Proc Natl Acad Sci U S A. 1990;87:6019.
7. Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1993;41(RR-17):1.
8. WHO Case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva, Switzerland: World Health Organization, 2006.
9. Masur H, Healey L, Hadigan C. Treatment of human immunodeficiency virus infection and acquired immunodeficiency syndrome. In Goldman L, editor: Cecil Medicine, 23rd ed, Philadelphia: Elsevier, 2007.
10. Simon V, Ho DD, Abdool Karim Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet. 2006;368:489.
11. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Bethesda, MD: US Department of Health and Human Services, 2007.
12. Hariri S, McKenna MT. Epidemiology of human immunodeficiency virus in the United States. Clin Microbiol Rev. 2007;20:478.
13. French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615.
14. Shelburne SA3rd, Darcourt J, White ACJr, et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:1049.
15. Battegay M, Nuesch R, Hirschel B, et al. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6:280.
16. French MA. Disorders of immune reconstitution in patients with HIV infection responding to antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:16.
17. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395.
18. Murdoch DM, Venter WD, Van Rie A, et al. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther. 2007;4:9.
19. Centers for Disease Control and Prevention. Fact Sheet: Surveillance of Occupationally Acquired HIV/AIDS in Healthcare Personnel, as of December 2006, in 2007.
20. CDC. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Morb Mortal Wkly Rep. 54(No. RR-9), 2005.
21. CDC. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Morb Mortal Wkly Rep. 2001;50(No. RR-11):1.
22. Centers for Disease Control and Prevention. Exposure to Blood. What Health Care Personnel Need to Know. National Institute for Occupational Safety and Health; 2003.
23. Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59.
24. Frisch M, Biggar RJ, Engels EA, et al. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736.
25. Grulich AE, Li Y, McDonald A, et al. Rates of non-AIDS-defining cancers in people with HIV infection before and after AIDS diagnosis. AIDS. 2002;16:1155.
26. Delabesse E, Oksenhendler E, Lebbe C, et al. Molecular analysis of clonality in Kaposi’s sarcoma. J Clin Pathol. 1997;50:664.
27. Tappero JW, Conant MA, Wolfe SF, et al. Kaposi’s sarcoma. Epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371.
28. Feller L, Wood NH, Lemmer J. HIV-associated Kaposi sarcoma: pathogenic mechanisms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:521.
29. Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027.
30. Hengge UR, Ruzicka T, Tyring SK, et al. Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis. 2002;2:281.
31. Volberding PA. Hematology and oncology in patients with human immunodeficiency virus infection. In Goldman L, editor: Cecil Medicine, 23rd ed, Philadelphia: Elsevier, 2007.
32. Friedman-Kien AE. Disseminated Kaposi’s sarcoma syndrome in young homosexual men. J Am Acad Dermatol. 1981;5:468.
33. Beral V, Peterman TA, Berkelman RL, et al. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123.
34. Pantanowitz L, Dezube BJ. Advances in the pathobiology and treatment of Kaposi sarcoma. Curr Opin Oncol. 2004;16:443.
35. Portsmouth S, Stebbing J, Gill J, et al. A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi’s sarcoma. AIDS. 2003;17:F17.
36. International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92:1823.
37. Shepherd FA, Maher E, Cardella C, et al. Treatment of Kaposi’s sarcoma after solid organ transplantation. J Clin Oncol. 1997;15:2371.
38. Farge D. Kaposi’s sarcoma in organ transplant recipients. The Collaborative Transplantation Research Group of Ile de France. Eur J Med. 1993;2:339.
39. Qunibi W, Akhtar M, Sheth K, et al. Kaposi’s sarcoma: the most common tumor after renal transplantation in Saudi Arabia. Am J Med. 1988;84:225.
40. Campistol JM, Schena FP. Kaposi’s sarcoma in renal transplant recipients—the impact of proliferation signal inhibitors. Nephrol Dial Transplant. 2007;22(Suppl 1):i17.
41. Mbulaiteye SM, Engels EA. Kaposi’s sarcoma risk among transplant recipients in the United States (1993-2003). Int J Cancer. 2006;119:2685.
42. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865.
43. Moore PS, Gao SJ, Dominguez G, et al. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcoma. J Virol. 1996;70:549.
44. Noguchi K, Fukazawa H, Murakami Y, et al. Gamma-herpesviruses and cellular signaling in AIDS-associated malignancies. Cancer Sci. 2007;98:1288.
45. Boeckle E, Boesmueller C, Wiesmayr S, et al. Kaposi sarcoma in solid organ transplant recipients: a single center report. Transplant Proc. 2005;37:1905.
46. Webster-Cyriaque J, Duus K, Cooper C, et al. Oral EBV and KSHV infection in HIV. Adv Dent Res. 2006;19:91.
47. Dukers NH, Rezza G. Human herpesvirus 8 epidemiology: what we do and do not know. AIDS. 2003;17:1717.
48. Hengge UR, Ruzicka T, Tyring SK, et al. Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 2: pathogenesis, Castleman’s disease, and pleural effusion lymphoma. Lancet Infect Dis. 2002;2:344.
49. Melbye M, Cook PM, Hjalgrim H, et al. Risk factors for Kaposi’s-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981-1996. Int J Cancer. 1998;77:543.
50. O’Brien TR, Kedes D, Ganem D, et al. Evidence for concurrent epidemics of human herpesvirus 8 and human immunodeficiency virus type 1 in US homosexual men: rates, risk factors, and relationship to Kaposi’s sarcoma. J Infect Dis. 1999;180:1010.
51. Osmond DH, Buchbinder S, Cheng A, et al. Prevalence of Kaposi sarcoma-associated herpesvirus infection in homosexual men at beginning of and during the HIV epidemic. JAMA. 2002;287:221.
52. Martin JN, Ganem DE, Osmond DH, et al. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948.
53. Kedes DH, Operskalski E, Busch M, et al. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918.
54. Angeloni A, Heston L, Uccini S, et al. High prevalence of antibodies to human herpesvirus 8 in relatives of patients with classic Kaposi’s sarcoma from Sardinia. J Infect Dis. 1998;177:1715.
55. Bourboulia D, Whitby D, Boshoff C, et al. Serologic evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus infection. JAMA. 1998;280:31.
56. Plancoulaine S, Abel L, van Beveren M, et al. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet. 2000;356:1062.
57. Mayama S, Cuevas LE, Sheldon J, et al. Prevalence and transmission of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77:817.
58. Andreoni M, Sarmati L, Nicastri E, et al. Primary human herpesvirus 8 infection in immunocompetent children. JAMA. 2002;287:1295.
59. Chachoua A, Krigel R, Lafleur F, et al. Prognostic factors and staging classification of patients with epidemic Kaposi’s sarcoma. J Clin Oncol. 1989;7:774.
60. Lager I, Altini M, Coleman H, et al. Oral Kaposi’s sarcoma: a clinicopathologic study from South Africa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:701.
61. Singh B, Har-el G, Lucente FE. Kaposi’s sarcoma of the head and neck in patients with acquired immunodeficiency syndrome. Otolaryngol Head Neck Surg. 1994;111:618.
62. Di Lorenzo G, Konstantinopoulos PA, Pantanowitz L, et al. Management of AIDS-related Kaposi’s sarcoma. Lancet Oncol. 2007;8:167.
63. Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317.
64. Ficarra G, Berson AM, Silverman SJr, et al. Kaposi’s sarcoma of the oral cavity: a study of 134 patients with a review of the pathogenesis, epidemiology, clinical aspects, and treatment. Oral Surg Oral Med Oral Pathol. 1988;66:543.
65. Goldberg AN. Kaposi’s sarcoma of the head and neck in acquired immunodeficiency syndrome. Am J Otolaryngol. 1993;14:5.
66. Glick M, Muzyka BC, Lurie D, et al. Oral manifestations associated with HIV-related disease as markers for immune suppression and AIDS. Oral Surg Oral Med Oral Pathol. 1994;77:344.
67. Darling M, Thompson I, Meer M. Oral Kaposi’s sarcoma in a renal transplant patient: case report and literature review. J Can Dent Assoc. 2004;70:617.
68. Aboulafia DM. The epidemiologic, pathologic, and clinical features of AIDS-associated pulmonary Kaposi’s sarcoma. Chest. 2000;117:1128.
69. Hartman TE, Primack SL, Muller NL, et al. Diagnosis of thoracic complications in AIDS: accuracy of CT. AJR Am J Roentgenol. 1994;162:547.
70. Angouridakis N, Constantinidis J, Karkavelas G, et al. Classic (Mediterranean) Kaposi’s sarcoma of the true vocal cord: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2006;263:537.
71. Goldberg AN, Crans CA, Trulock EP. Management of Kaposi’s sarcoma of the larynx in acquired immunodeficiency syndrome. Trans Am Bronchoesophagological Assoc. 1994:27-32.
72. Schiff NF, Annino DJ, Woo P, et al. Kaposi’s sarcoma of the larynx. Ann Otol Rhinol Laryngol. 1997;106:563.
73. Beitler AJ, Ptaszynski K, Karpel JP. Upper airway obstruction in a woman with AIDS-related laryngeal Kaposi’s sarcoma. Chest. 1996;109:836.
74. Schoen FJ. Blood vessels. In Kumar V, editor: Robbins and Cotran: Pathologic Basis of Disease, 7th ed, Philadelphia: Saunders, 2005.
75. Lopez de Blanc S, Sambuelli R, Femopase F, et al. Bacillary angiomatosis affecting the oral cavity. Report of two cases and review. J Oral Pathol Med. 2000;29:91.
76. Glick M, Cleveland DB. Oral mucosal bacillary epithelioid angiomatosis in a patient with AIDS associated with rapid alveolar bone loss: case report. J Oral Pathol Med. 1993;22:235.
77. Jin YT, Tsai ST, Yan JJ, et al. Detection of Kaposi’s sarcoma-associated herpesvirus-like DNA sequence in vascular lesions. A reliable diagnostic marker for Kaposi’s sarcoma. Am J Clin Pathol. 1996;105:360.
78. Cheung MC, Pantanowitz L, Dezube BJ. AIDS-related malignancies: emerging challenges in the era of highly active antiretroviral therapy. Oncologist. 2005;10:412.
79. Lebbe C, Euvrard S, Barrou B, et al. Sirolimus conversion for patients with posttransplant Kaposi’s sarcoma. Am J Transplant. 2006;6:2164.
80. Vanni T, Sprinz E, Machado MW, et al. Systemic treatment of AIDS-related Kaposi sarcoma: current status and perspectives. Cancer Treat Rev. 2006;32:445.
81. Sgadari C, Barillari G, Toschi E, et al. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002;8:225.
82. Sgadari C, Monini P, Barillari G, et al. Use of HIV protease inhibitors to block Kaposi’s sarcoma and tumour growth. Lancet Oncol. 2003;4:537.
83. Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1989;7:1201.
84. Nasti G, Talamini R, Antinori A, et al. AIDS-related Kaposi’s sarcoma: evaluation of potential new prognostic factors and assessment of the AIDS Clinical Trial Group Staging System in the HAART era—the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naive from Antiretrovirals. J Clin Oncol. 2003;21:2876.
85. Epstein JB, Cabay RJ, Glick M. Oral malignancies in HIV disease: changes in disease presentation, increasing understanding of molecular pathogenesis, and current management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:571.
86. Levine AM, Tulpule A. Clinical aspects and management of AIDS-related Kaposi’s sarcoma. Eur J Cancer. 2001;37:1288.
87. Dittmer DP, Krown SE. Targeted therapy for Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus. Curr Opin Oncol. 2007;19:452.
88. Koon HB, Bubley GJ, Pantanowitz L, et al. Imatinib-induced regression of AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2005;23:982.
89. Dezube BJ, Krown SE, Lee JY, et al. Randomized phase II trial of matrix metalloproteinase inhibitor COL-3 in AIDS-related Kaposi’s sarcoma: an AIDS Malignancy Consortium Study. J Clin Oncol. 2006;24:1389.
90. Little RF, Aleman K, Kumar P, et al. Phase 2 study of pegylated liposomal doxorubicin in combination with interleukin-12 for AIDS-related Kaposi sarcoma. Blood. 2007;110:4165.
91. Bouwes Bavinck JN, Euvrard S, Naldi L, et al. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol. 2007;127:1647.
92. Gerlini G, Romagnoli P, Pimpinelli N. Skin cancer and immunosuppression. Crit Rev Oncol Hematol. 2005;56:127.
93. Smith KJ, Skelton HG, Yeager J, et al. Cutaneous neoplasms in a military population of HIV-1-positive patients. Military Medical Consortium for the Advancement of Retroviral Research. J Am Acad Dermatol. 1993;29:400.
94. Lobo DV, Chu P, Grekin RC, et al. Nonmelanoma skin cancers and infection with the human immunodeficiency virus. Arch Dermatol. 1992;128:623.
95. Ulrich C, Schmook T, Sachse MM, et al. Comparative epidemiology and pathogenic factors for nonmelanoma skin cancer in organ transplant patients. Dermatol Surg. 2004;30:622.
96. Bordea C, Wojnarowska F, Millard PR, et al. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation. 2004;77:574.
97. Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol. 2006;154:498.
98. Ramsay HM, Reece SM, Fryer AA, et al. Seven-year prospective study of nonmelanoma skin cancer incidence in U.K. renal transplant recipients. Transplantation. 2007;84:437.
99. Ramsay HM, Fryer AA, Reece S, et al. Clinical risk factors associated with nonmelanoma skin cancer in renal transplant recipients. Am J Kidney Dis. 2000;36:167.
100. Cooley TP. Non-AIDS-defining cancer in HIV-infected people. Hematol Oncol Clin North Am. 2003;17:889.
101. Wilkins K, Turner R, Dolev JC, et al. Cutaneous malignancy and human immunodeficiency virus disease. J Am Acad Dermatol. 2006;54:189.
102. Lewis KG, Jellinek N, Robinson-Bostom L. Skin cancer after transplantation: a guide for the general surgeon. Surg Clin North Am. 2006;86:1257.
103. Rodrigues LK, Klencke BJ, Vin-Christian K, et al. Altered clinical course of malignant melanoma in HIV-positive patients. Arch Dermatol. 2002;138:765.
104. Kagen MH, Hirsch RJ, Chu P, et al. Multiple infundibulocystic basal cell carcinomas in association with human immunodeficiency virus. J Cutan Pathol. 2000;27:316.
105. Rahimizadeh A, Shelton R, Weinberg H, et al. The development of a Marjolin’s cancer in a human immunodeficiency virus-positive hemophilic man and review of the literature. Dermatol Surg. 1997;23:560.
106. Sitz KV, Keppen M, Johnson DF. Metastatic basal cell carcinoma in acquired immunodeficiency syndrome-related complex. JAMA. 1987;257:340.
107. Penn I. Tumors after renal and cardiac transplantation. Hematol Oncol Clin North Am. 1993;7:431.
108. Nguyen P, Vin-Christian K, Ming ME, et al. Aggressive squamous cell carcinomas in persons infected with the human immunodeficiency virus. Arch Dermatol. 2002;138:758.
109. Preciado DA, Matas A, Adams GL. Squamous cell carcinoma of the head and neck in solid organ transplant recipients. Head Neck. 2002;24:319.
110. Pollard JD, Hanasono MM, Mikulec AA, et al. Head and neck cancer in cardiothoracic transplant recipients. Laryngoscope. 2000;110:1257.
111. Saigal S, Norris S, Muiesan P, et al. Evidence of differential risk for posttransplantation malignancy based on pretransplantation cause in patients undergoing liver transplantation. Liver Transpl. 2002;8:482.
112. Duvoux C, Delacroix I, Richardet JP, et al. Increased incidence of oropharyngeal squamous cell carcinomas after liver transplantation for alcoholic cirrhosis. Transplantation. 1999;67:418.
113. Singh B, Balwally AN, Shaha AR, et al. Upper aerodigestive tract squamous cell carcinoma. The human immunodeficiency virus connection. Arch Otolaryngol Head Neck Surg. 1996;122:639.
114. Gourin CG, Terris DJ. Head and neck cancer in transplant recipients. Curr Opin Otolaryngol Head Neck Surg. 2004;12:122.
115. Singh B, Sabin S, Rofim O, et al. Alterations in head and neck cancer occurring in HIV-infected patients—results of a pilot, longitudinal, prospective study. Acta Oncol. 1999;38:1047.
116. Haigentz MJr. Aerodigestive cancers in HIV infection. Curr Opin Oncol. 2005;17:474.
117. Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32(Suppl 1):59.
118. Steben M, Duarte-Franco E. Human papillomavirus infection: epidemiology and pathophysiology. Gynecol Oncol. 2007;107:S2.
119. Kao GD, Devine P, Mirza N. Oral cavity and oropharyngeal tumors in human immunodeficiency virus-positive patients: acute response to radiation therapy. Arch Otolaryngol Head Neck Surg. 1999;125:873.
120. Harris NL, Swerdlow SH, Frizzera G, et al. Post-transplant lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, et al, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001:264-269.
121. Harris NL, Ferry JA, Swerdlow SH. Posttransplant lymphoproliferative disorders: summary of Society for Hematopathology Workshop. Semin Diagn Pathol. 1997;14:8.
122. Raphael M, Borisch B, Jaffe ES. Lymphomas associated with infection by the human immunodeficiency virus (HIV). In: Jaffe ES, Harris NL, Stein H, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2001.
123. Berretta M, Cinelli R, Martellotta F, et al. Therapeutic approaches to AIDS-related malignancies. Oncogene. 2003;22:6646.
124. Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413.
125. Rickinson A. Epstein-Barr virus. Virus Res. 2002;82:109.
126. Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16:405.
127. Lim WH, Russ GR, Coates PT. Review of Epstein-Barr virus and post-transplant lymphoproliferative disorder post-solid organ transplantation. Nephrology (Carlton). 2006;11:355.
128. Melbye M, Cote TR, West D, et al. Nasopharyngeal carcinoma: an EBV-associated tumour not significantly influenced by HIV-induced immunosuppression. The AIDS/Cancer Working Group. Br J Cancer. 1996;73:995.
129. Singh B, Poluri A, Shaha AR, et al. Head and neck manifestations of non-Hodgkin’s lymphoma in human immunodeficiency virus-infected patients. Am J Otolaryngol. 2000;21:10.
130. Lim ST, Karim R, Tulpule A, et al. Prognostic factors in HIV-related diffuse large-cell lymphoma: before versus after highly active antiretroviral therapy. J Clin Oncol. 2005;23:8477.
131. Matthews GV, Bower M, Mandalia S, et al. Changes in acquired immunodeficiency syndrome-related lymphoma since the introduction of highly active antiretroviral therapy. Blood. 2000;96:2730.
132. Rabkin CS, Biggar RJ, Horm JW. Increasing incidence of cancers associated with the human immunodeficiency virus epidemic. Int J Cancer. 1991;47:692.
133. Levine AM. Acquired immunodeficiency syndrome-related lymphoma. Blood. 1992;80:8.
134. Roithmann S, Tourani JM, Andrieu JM. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991;338:884.
135. Beral V, Peterman T, Berkelman R, et al. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991;337:805.
136. Palmieri C, Treibel T, Large O, et al. AIDS-related non-Hodgkin’s lymphoma in the first decade of highly active antiretroviral therapy. Q J Med. 2006;99:811.
137. Carbone A, Tirelli U, Vaccher E, et al. A clinicopathologic study of lymphoid neoplasias associated with human immunodeficiency virus infection in Italy. Cancer. 1991;68:842.
138. Kaplan LD, Abrams DI, Feigal E, et al. AIDS-associated non-Hodgkin’s lymphoma in San Francisco. JAMA. 1989;261:719.
139. Knowles DM. Etiology and pathogenesis of AIDS-related non-Hodgkin’s lymphoma. Hematol Oncol Clin North Am. 2003;17:785.
140. Steinberg MJ, Herrera AF, Barakat RG. Posttransplant lymphoproliferative disorder resembling a chronic orocutaneous infection in an immunosuppressed patient. J Oral Maxillofac Surg. 2004;62:1033.
141. Jordan RC, Chong L, DiPierdomenico S, et al. Oral lymphoma in HIV infection. Oral Dis. 1997;3(Suppl 1):S135.
142. Vazquez-Pineiro T, Viana de Frias L, Cristobal E, et al. HIV-associated oral pleomorphic B-cell malignant lymphoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:142.
143. Rosenstiel DB, Carroll WR, Listinsky CM. MALT lymphoma presenting as a cystic salivary gland mass. Head Neck. 2001;23:254.
144. Corr P, Vaithilingum M, Thejpal R, et al. Parotid MALT lymphoma in HIV infected children. J Ultrasound Med. 1997;16:615.
145. Finn DG. Lymphoma of the head and neck and acquired immunodeficiency syndrome: clinical investigation and immunohistological study. Laryngoscope. 1995;105:1.
146. Shapiro AL, Shechtman FG, Guida RA, et al. Head and neck lymphoma in patients with the acquired immune deficiency syndrome. Otolaryngol Head Neck Surg. 1992;106:258.
147. Levine AM. Epidemiology, clinical characteristics, and management of AIDS-related lymphoma. Hematol Oncol Clin North Am. 1991;5:331.
148. Shohat I, Berkowicz M, Dori S, et al. Primary non-Hodgkin’s lymphoma of the sinonasal tract. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:328.
149. Desai RS, Vanaki SS, Puranik RS, et al. Plasmablastic lymphoma presenting as a gingival growth in a previously undiagnosed HIV-positive patient: a case report. J Oral Maxillofac Surg. 2007;65:1358.
150. Carbone A, Gloghini A. KSHV/HHV8-associated lymphomas. Br J Haematol. 2008;140:13.
151. Borenstein J, Pezzella F, Gatter KC. Plasmablastic lymphomas may occur as post-transplant lymphoproliferative disorders. Histopathology. 2007;51:774.
152. Verma S, Nuovo GJ, Porcu P, et al. Epstein-Barr virus—and human herpesvirus 8-associated primary cutaneous plasmablastic lymphoma in the setting of renal transplantation. J Cutan Pathol. 2005;32:35.
153. Flaitz CM, Nichols CM, Walling DM, et al. Plasmablastic lymphoma: an HIV-associated entity with primary oral manifestations. Oral Oncol. 2002;38:96.
154. Dong HY, Scadden DT, de Leval L, et al. Plasmablastic lymphoma in HIV-positive patients: an aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasm. Am J Surg Pathol. 2005;29:1633.
155. Sarker D, Thirlwell C, Nelson M, et al. Leptomeningeal disease in AIDS-related non-Hodgkin’s lymphoma. AIDS. 2003;17:861.
156. Davies CL, Chinn R, Nelson M, et al. Outcome in AIDS-related systemic non-Hodgkin lymphoma and leptomeningeal disease is not predicted by a CT brain scan. AJNR Am J Neuroradiol. 2007;28:1988.
157. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987.
158. Navarro JT, Ribera JM, Oriol A, et al. Advanced stage is the most important prognostic factor for survival in patients with systemic acquired immunodeficiency syndrome-related non-hodgkin’s lymphoma treated with CHOP and highly active antiretroviral therapy. Int J Hematol. 2007;86:337.
159. Rossi G, Donisi A, Casari S, et al. The International Prognostic Index can be used as a guide to treatment decisions regarding patients with human immunodeficiency virus-related systemic non-Hodgkin lymphoma. Cancer. 1999;86:2391.
160. Mounier N, Spina M, Gabarre J, et al. AIDS-related non-Hodgkin lymphoma: final analysis of 485 patients treated with risk-adapted intensive chemotherapy. Blood. 2006;107:3832.
161. Miralles P, Berenguer J, Ribera JM, et al. Prognosis of AIDS-related systemic non-Hodgkin lymphoma treated with chemotherapy and highly active antiretroviral therapy depends exclusively on tumor-related factors. J Acquir Immune Defic Syndr. 2007;44:167.
162. Bower M, Gazzard B, Mandalia S, et al. A prognostic index for systemic AIDS-related non-Hodgkin lymphoma treated in the era of highly active antiretroviral therapy. Ann Intern Med. 2005;143:265.
163. Simcock M, Blasko M, Karrer U, et al. Treatment and prognosis of AIDS-related lymphoma in the era of highly active antiretroviral therapy: findings from the Swiss HIV Cohort Study. Antivir Ther. 2007;12:931.
164. Ratner L, Lee J, Tang S, et al. Chemotherapy for human immunodeficiency virus-associated non-Hodgkin’s lymphoma in combination with highly active antiretroviral therapy. J Clin Oncol. 2001;19:2171.
165. Mounier N, Spina M, Gisselbrecht C. Modern management of non-Hodgkin lymphoma in HIV-infected patients. Br J Haematol. 2007;136:685.
166. Robotin MC, Law MG, Milliken S, et al. Clinical features and predictors of survival of AIDS-related non-Hodgkin’s lymphoma in a population-based case series in Sydney, Australia. HIV Med. 2004;5:377.
167. Pickhardt PJ, Siegel MJ, Hayashi RJ, et al. Posttransplantation lymphoproliferative disorder in children: clinical, histopathologic, and imaging features. Radiology. 2000;217:16.
168. Herrmann BW, Sweet SC, Hayashi RJ, et al. Otolaryngological manifestations of posttransplant lymphoproliferative disorder in pediatric thoracic transplant patients. Int J Pediatr Otorhinolaryngol. 2006;70:303.
169. Fairley JW, Hunt BJ, Glover GW, et al. Unusual lymphoproliferative oropharyngeal lesions in heart and heart-lung transplant recipients. J Laryngol Otol. 1990;104:720.
170. Gordon AR, Loevner LA, Sonners AI, et al. Posttransplantation lymphoproliferative disorder of the paranasal sinuses mimicking invasive fungal sinusitis: case report. AJNR Am J Neuroradiol. 2002;23:855.
171. Herrmann BW, Sweet SC, Molter DW. Sinonasal posttransplant lymphoproliferative disorder in pediatric lung transplant patients. Otolaryngol Head Neck Surg. 2005;133:38.
172. Paya CV, Fung JJ, Nalesnik MA, et al. Epstein-Barr virus-induced posttransplant lymphoproliferative disorders. ASTS/ASTP EBV-PTLD Task Force and the Mayo Clinic Organized International Consensus Development Meeting. Transplantation. 1999;68:1517.
173. Starzl TE, Nalesnik MA, Porter KA, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583.
174. Myer CM3rd, Reilly JS. Airway obstruction in an immunosuppressed child. Arch Otolaryngol. 1985;111:409.
175. Said JW. Immunodeficiency-related Hodgkin lymphoma and its mimics. Adv Anat Pathol. 2007;14:189.
176. Caillard S, Agodoa LY, Bohen EM, et al. Myeloma, Hodgkin disease, and lymphoid leukemia after renal transplantation: characteristics, risk factors and prognosis. Transplantation. 2006;81:888.
177. Semakula B, Rittenbach JV, Wang J. Hodgkin lymphoma-like posttransplantation lymphoproliferative disorder. Arch Pathol Lab Med. 2006;130:558.
178. Vaccher E, Spina M, Tirelli U. Clinical aspects and management of Hodgkin’s disease and other tumours in HIV-infected individuals. Eur J Cancer. 2001;37:1306.
179. Biggar RJ, Jaffe ES, Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786.
180. Poluri A, Shah KG, Carew JF, et al. Hodgkin’s disease of the head and neck in human immunodeficiency virus-infected patients. Am J Otolaryngol. 2002;23:12.
181. Glaser SL, Clarke CA, Gulley ML, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988-1998. Cancer. 2003;98:300.
182. Gloghini A, Carbone A. Why would the incidence of HIV-associated Hodgkin lymphoma increase in the setting of improved immunity? Int J Cancer. 2007;120:2753.
183. Marti-Carvajal AJ, Cardona AF, Rodriguez ML. Interventions for treating AIDS-associated Hodgkin s lymphoma in treatment-naive adults. Cochrane Database Syst Rev. 2007;CD006149.
184. Spina M, Berretta M, Tirelli U. Hodgkin’s disease in HIV. Hematol Oncol Clin North Am. 2003;17:843.
185. Gerard L, Galicier L, Boulanger E, et al. Improved survival in HIV-related Hodgkin’s lymphoma since the introduction of highly active antiretroviral therapy. AIDS. 2003;17:81.
186. Vaseliu N, Carter AB, Kline NE, et al. Longitudinal study of the prevalence and prognostic implications of oral manifestations in Romanian children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 2005;24:1067.
187. Santos LC, Castro GF, de Souza IP, et al. Oral manifestations related to immunosuppression degree in HIV-positive children. Braz Dent J. 2001;12:135.
188. Magalhaes MG, Bueno DF, Serra E, et al. Oral manifestations of HIV positive children. J Clin Pediatr Dent. 2001;25:103.
189. Steele NP, Sampogna D, Sessions RB. Kaposi’s sarcoma of an intraparotid lymph node leading to a diagnosis of HIV. Laryngoscope. 2005;115:861.
190. Rizos E, Drosos AA, Ioannidis JP. Isolated intraparotid Kaposi sarcoma in human immunodeficiency virus type 1 infection. Mayo Clin Proc. 2003;78:1561.
191. Dave SP, Pernas FG, Roy S. The benign lymphoepithelial cyst and a classification system for lymphocytic parotid gland enlargement in the pediatric HIV population. Laryngoscope. 2007;117:106.
192. Reveille JD. Rheumatic manifestations of human immunodeficiency virus infection. In Harris ED, Budd RC, Genovese MC, et al, editors: Kelley’s Textbook of Rheumatology, 7th ed, Philadelphia: Elsevier, 2005.
193. Sperling NM, Lin PT. Parotid disease associated with human immunodeficiency virus infection. Ear Nose Throat J. 1990;69:475.
194. Shugar JM, Som PM, Jacobson AL, et al. Multicentric parotid cysts and cervical adenopathy in AIDS patients. A newly recognized entity: CT and MR manifestations. Laryngoscope. 1988;98:772.
195. Som PM, Brandwein MS, Silvers A. Nodal inclusion cysts of the parotid gland and parapharyngeal space: a discussion of lymphoepithelial, AIDS-related parotid, and branchial cysts, cystic Warthin’s tumors, and cysts in Sjogren’s syndrome. Laryngoscope. 1995;105:1122.
196. Chhieng DC, Argosino R, McKenna BJ, et al. Utility of fine-needle aspiration in the diagnosis of salivary gland lesions in patients infected with human immunodeficiency virus. Diagn Cytopathol. 1999;21:260.
197. Smith FB, Rajdeo H, Panesar N, et al. Benign lymphoepithelial lesion of the parotid gland in intravenous drug users. Arch Pathol Lab Med. 1988;112:742.
198. Mandel L, Surattanont F. Regression of HIV parotid swellings after antiviral therapy: case reports with computed tomographic scan evidence. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:454.
199. Morales-Aguirre JJ, Patino-Nino JA, Mendoza-Azpiri M, et al. Parotid cysts in children infected with human immunodeficiency virus: report of 4 cases. Arch Otolaryngol Head Neck Surg. 2005;131:353.
200. Beitler JJ, Vikram B, Silver CE, et al. Low-dose radiotherapy for multicystic benign lymphoepithelial lesions of the parotid gland in HIV-positive patients: long-term results. Head Neck. 1995;17:31.
201. Lustig LR, Lee KC, Murr A, et al. Doxycycline sclerosis of benign lymphoepithelial cysts in patients infected with HIV. Laryngoscope. 1998;108:1199.
202. Suskind DL, Tavill MA, Handler SD. Doxycycline sclerotherapy of benign lymphoepithelial cysts of the parotid: a minimally invasive treatment. Int J Pediatr Otorhinolaryngol. 2000;52:157.
203. Echavez MI, Lee KC, Sooy CD. Tetracycline sclerosis for treatment of benign lymphoepithelial cysts of the parotid gland in patients infected with human immunodeficiency virus. Laryngoscope. 1994;104:1499.
204. Itescu S, Brancato LJ, Buxbaum J, et al. A diffuse infiltrative CD8 lymphocytosis syndrome in human immunodeficiency virus (HIV) infection: a host immune response associated with HLA-DR5. Ann Intern Med. 1990;112:3.
205. Basu D, Williams FM, Ahn CW, et al. Changing spectrum of the diffuse infiltrative lymphocytosis syndrome. Arthritis Rheum. 2006;55:466.
206. Mandel L, Kim D, Uy C. Parotid gland swelling in HIV diffuse infiltrative CD8 lymphocytosis syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:565.
207. Mbopi-Keou FX, Belec L, Teo CG, et al. Synergism between HIV and other viruses in the mouth. Lancet Infect Dis. 2002;2:416.
208. Kazi S, Cohen PR, Williams F, et al. The diffuse infiltrative lymphocytosis syndrome. Clinical and immunogenetic features in 35 patients. AIDS. 1996;10:385.
209. Itescu S, Winchester R. Diffuse infiltrative lymphocytosis syndrome: a disorder occurring in human immunodeficiency virus-1 infection that may present as a sicca syndrome. Rheum Dis Clin North Am. 1992;18:683.
210. Navazesh M, Mulligan R, Barron Y, et al. A 4-year longitudinal evaluation of xerostomia and salivary gland hypofunction in the Women’s Interagency HIV Study participants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:693.
211. Storek J, Gooley T, Siadak M, et al. Allogeneic peripheral blood stem cell transplantation may be associated with a high risk of chronic graft-versus-host disease. Blood. 1997;90:4705.
212. Alborghetti MR, Correa ME, Adam RL, et al. Late effects of chronic graft-vs.-host disease in minor salivary glands. J Oral Pathol Med. 2005;34:486.
213. Nagler RM, Nagler A. The molecular basis of salivary gland involvement in graft-vs-host disease. J Dent Res. 2004;83:98.
214. Lin AL, Johnson DA, Stephan KT, et al. Alteration in salivary function in early HIV infection. J Dent Res. 2003;82:719.
215. LaCasce AS. Post-transplant lymphoproliferative disorders. Oncologist. 2006;11:674.
216. Shapiro AL, Pincus RL. Fine-needle aspiration of diffuse cervical lymphadenopathy in patients with acquired immunodeficiency syndrome. Otolaryngol Head Neck Surg. 1991;105:419.
217. Burton F, Patete ML, Goodwin WJJr. Indications for open cervical node biopsy in HIV-positive patients. Otolaryngol Head Neck Surg. 1992;107:367.
218. Chen CH, Lian JD, Cheng CH, et al. Mycobacterium tuberculosis infection following renal transplantation in Taiwan. Transpl Infect Dis. 2006;8:148.
219. Ishikawa K, Hoshinaga K, Maruyama T, et al. Mycobacterium tuberculosis infection of bilateral cervical lymph nodes after renal transplantation. Int J Urol. 2001;8:640.
220. Jha V, Kohli HS, Sud K, et al. Laryngeal tuberculosis in renal transplant recipients. Transplantation. 1999;68:153.
221. Tato AM, Pascual J, Orofino L, et al. Laryngeal tuberculosis in renal allograft patients. Am J Kidney Dis. 1998;31:701.
222. Ergun I, Keven K, Sengul S, et al. Tuberculous otitis media in a renal transplant recipient. Am J Kidney Dis. 2004;43:e1.
223. Redaelli de Zinis LO, Tironi A, Nassif N, et al. Temporal bone infection caused by atypical mycobacterium: case report and review of the literature. Otol Neurotol. 2003;24:843.
224. Reid AJ, Miller RF, Kocjan GI. Diagnostic utility of fine needle aspiration (FNA) cytology in HIV-infected patients with lymphadenopathy. Cytopathology. 1998;9:230.
225. Michelow P, Meyers T, Dubb M, et al. The utility of fine needle aspiration in HIV positive children. Cytopathology. 2007.
226. Prasad HK, Bhojwani KM, Shenoy V, et al. HIV manifestations in otolaryngology. Am J Otolaryngol. 2006;27:179.
227. Ellison E, Lapuerta P, Martin SE. Fine needle aspiration (FNA) in HIV+ patients: results from a series of 655 aspirates. Cytopathology. 1998;9:222.
228. Lui SL, Tang S, Li FK, et al. Tuberculous infection in southern Chinese renal transplant recipients. Clin Transplant. 2004;18:666.
229. Nasti G, Tavio M, Rizzardini G, et al. Primary tuberculosis of the larynx in a patient infected with human immunodeficiency virus. Clin Infect Dis. 1996;23:183.
230. Miziara ID. Tuberculosis affecting the oral cavity in Brazilian HIV-infected patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:179.
231. Ilyas SE, Chen FF, Hodgson TA, et al. Labial tuberculosis: a unique cause of lip swelling complicating HIV infection. HIV Med. 2002;3:283.
232. Rangel AL, Coletta RD, Almeida OP, et al. Parotid mycobacteriosis is frequently caused by Mycobacterium tuberculosis in advanced AIDS. J Oral Pathol Med. 2005;34:407.
233. Davidson BJ, Morris MS, Kornblut AD, et al. Lymphadenopathy in the HIV-seropositive patient. Ear Nose Throat J. 1990;69:478.
234. Miziara ID, Araujo Filho BC, La Cortina RC, et al. Chronic rhinosinusitis in HIV-infected patients: radiological and clinical evaluation. Rev Bras Otorrinolaringol (Engl Ed). 2005;71:604.
235. Garcia-Rodriguez JF, Corominas M, Fernandez-Viladrich P, et al. Rhinosinusitis and atopy in patients infected with HIV. Laryngoscope. 1999;109:939.
236. Porter JP, Patel AA, Dewey CM, et al. Prevalence of sinonasal symptoms in patients with HIV infection. Am J Rhinol. 1999;13:203.
237. Tami TA. The management of sinusitis in patients infected with the human immunodeficiency virus (HIV). Ear Nose Throat J. 1995;74:360.
238. Ortiz E, Sakano E, De Souza CA, et al. Chronic GVHD: predictive factor for rhinosinusitis in bone marrow transplantation. Rev Bras Otorrinolaringol (Engl Ed). 2006;72:328.
239. Dhong HJ, Lee JC, Ryu JS, et al. Rhinosinusitis in transplant patients. Clin Otolaryngol Allied Sci. 2001;26:329.
240. Savage DG, Taylor P, Blackwell J, et al. Paranasal sinusitis following allogeneic bone marrow transplant. Bone Marrow Transplant. 1997;19:55.
241. Mofenson LM, Korelitz J, Pelton S, et al. Sinusitis in children infected with human immunodeficiency virus: clinical characteristics, risk factors, and prophylaxis. National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. Clin Infect Dis. 1995;21:1175.
242. Zurlo JJ, Feuerstein IM, Lebovics R, et al. Sinusitis in HIV-1 infection. Am J Med. 1992;93:157.
243. Rosen EJ, Calhoun KH. Alterations of nasal mucociliary clearance in association with HIV infection and the effect of guaifenesin therapy. Laryngoscope. 2005;115:27.
244. Milgrim LM, Rubin JS, Small CB. Mucociliary clearance abnormalities in the HIV-infected patient: a precursor to acute sinusitis. Laryngoscope. 1995;105:1202.
245. Cordonnier C, Gilain L, Ricolfi F, et al. Acquired ciliary abnormalities of nasal mucosa in marrow recipients. Bone Marrow Transplant. 1996;17:611.
246. Sample S, Chernoff DN, Lenahan GA, et al. Elevated serum concentrations of IgE antibodies to environmental antigens in HIV-seropositive male homosexuals. J Allergy Clin Immunol. 1990;86:876.
247. Bowser CS, Kaye J, Joks RO, et al. IgE and atopy in perinatally HIV-infected children. Pediatr Allergy Immunol. 2007;18:298.
248. Truitt TO, Tami TA. Otolaryngologic manifestations of human immunodeficiency virus infection. Med Clin North Am. 1999;83:303.
249. Milgrim LM, Rubin JS, Rosenstreich DL, et al. Sinusitis in human immunodeficiency virus infection: typical and atypical organisms. J Otolaryngol. 1994;23:450.
250. Schlanger G, Lutwick LI, Kurzman M, et al. Sinusitis caused by Legionella pneumophila in a patient with the acquired immune deficiency syndrome. Am J Med. 1984;77:957.
251. Nachega JB, Rombaux P, Weynand B, et al. Successful treatment of Acanthamoeba rhinosinusitis in a patient with AIDS. AIDS Patient Care STDS. 2005;19:621.
252. Rivera MA, Padhya TA. Acanthamoeba: a rare primary cause of rhinosinusitis. Laryngoscope. 2002;112:1201.
253. Teknos TN, Poulin MD, Laruentano AM, et al. Acanthamoeba rhinosinusitis: characterization, diagnosis, and treatment. Am J Rhinol. 2000;14:387.
254. Schmulewitz L, Chandesris MO, Mainardi JL, et al. Invasive Pasteurella multocida sinusitis in a renal transplant patient. Transpl Infect Dis. 2007.
255. Upadhyay S, Marks SC, Arden RL, et al. Bacteriology of sinusitis in human immunodeficiency virus-positive patients: implications for management. Laryngoscope. 1995;105:1058.
256. Gurney TA, Lee KC, Murr AH. Contemporary issues in rhinosinusitis and HIV infection. Curr Opin Otolaryngol Head Neck Surg. 2003;11:45.
257. Godofsky EW, Zinreich J, Armstrong M, et al. Sinusitis in HIV-infected patients: a clinical and radiographic review. Am J Med. 1992;93:163.
258. Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117:S35.
259. Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004;10(Suppl 1):31.
260. Hunt SM, Miyamoto RC, Cornelius RS, et al. Invasive fungal sinusitis in the acquired immunodeficiency syndrome. Otolaryngol Clin North Am. 2000;33:335.
261. Gillespie MB, O’Malley BW. An algorithmic approach to the diagnosis and management of invasive fungal rhinosinusitis in the immunocompromised patient. Otolaryngol Clin North Am. 2000;33:323.
262. Malani PN, Kauffman CA. Prevention and prophylaxis of invasive fungal sinusitis in the immunocompromised patient. Otolaryngol Clin North Am. 2000;33:301.
263. Park AH, Muntz HR, Smith ME, et al. Pediatric invasive fungal rhinosinusitis in immunocompromised children with cancer. Otolaryngol Head Neck Surg. 2005;133:411.
264. Parikh SL, Venkatraman G, DelGaudio JM. Invasive fungal sinusitis: a 15-year review from a single institution. Am J Rhinol. 2004;18:75.
265. Hejny C, Kerrison JB, Newman NJ, et al. Rhino-orbital mucormycosis in a patient with acquired immunodeficiency syndrome (AIDS) and neutropenia. Am J Ophthalmol. 2001;132:111.
266. Rodriguez TE, Falkowski NR, Harkema JR, et al. Role of neutrophils in preventing and resolving acute fungal sinusitis. Infect Immun. 2007;75:5663.
267. Robinson MR, Fine HF, Ross ML, et al. Sino-orbital-cerebral aspergillosis in immunocompromised pediatric patients. Pediatr Infect Dis J. 2000;19:1197.
268. Colmenero C, Monux A, Valencia E, et al. Successfully treated candida sinusitis in an AIDS patient. J Craniomaxillofac Surg. 1990;18:175.
269. Blatt SP, Lucey DR, DeHoff D, et al. Rhinocerebral zygomycosis in a patient with AIDS. J Infect Dis. 1991;164:215.
270. Choi SS, Lawson W, Bottone EJ, et al. Cryptococcal sinusitis: a case report and review of literature. Otolaryngol Head Neck Surg. 1988;99:414.
271. Lasala PR, Smith MB, McGinnis MR, et al. Invasive Exserohilum sinusitis in a patient with aplastic anemia. Pediatr Infect Dis J. 2005;24:939.
272. Kalkanci A, Kustimur S, Sucak GT, et al. Fulminating fungal sinusitis caused by Valsa sordida, a plant pathogen, in a patient immunocompromised by acute myeloid leukemia. Med Mycol. 2006;44:531.
273. Gillespie MB, Huchton DM, O’Malley BW. Role of middle turbinate biopsy in the diagnosis of fulminant invasive fungal rhinosinusitis. Laryngoscope. 2000;110:1832.
274. Lortholary O, Meyohas MC, Dupont B, et al. Invasive aspergillosis in patients with acquired immunodeficiency syndrome: report of 33 cases. French Cooperative Study Group on Aspergillosis in AIDS. Am J Med. 1993;95:177.
275. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1.
276. Handzel O, Landau Z, Halperin D. Liposomal amphotericin B treatment for rhinocerebral mucormycosis: how much is enough? Rhinology. 2003;41:184.
277. Said T, Nampoory MR, Nair MP, et al. Safety of caspofungin for treating invasive nasal sinus aspergillosis in a kidney transplant recipient. Transplant Proc. 2005;37:3038.
278. Groll AH, Walsh TJ. Caspofungin: pharmacology, safety and therapeutic potential in superficial and invasive fungal infections. Expert Opin Investig Drugs. 2001;10:1545.
279. Jacobs P, Wood L, Du Toit A, et al. Eradication of invasive mucormycosis—effectiveness of the echinocandin FK463. Hematology. 2003;8:119.
280. Vener C, Carrabba M, Fracchiolla NS, et al. Invasive fungal sinusitis: an effective combined treatment in five haematological patients. Leuk Lymphoma. 2007;48:1577.
281. Ghadiali MT, Deckard NA, Farooq U, et al. Frozen-section biopsy analysis for acute invasive fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2007;136:714.
282. Kennedy CA, Adams GL, Neglia JP, et al. Impact of surgical treatment on paranasal fungal infections in bone marrow transplant patients. Otolaryngol Head Neck Surg. 1997;116:610.
283. Otto KJ, Delgaudio JM. Invasive fungal rhinosinusitis: what is the appropriate follow-up? Am J Rhinol. 2006;20:582.
284. Athanassiadou F, Kourti M, Papageorgiou T, et al. Invasive aspergillosis of the larynx in a child with acute lymphoblastic leukemia. Pediatr Infect Dis J. 2005;24:190.
285. Kingdom TT, Lee KC. Invasive aspergillosis of the larynx in AIDS. Otolaryngol Head Neck Surg. 1996;115:135.
286. Sriskandabalan P, Roy RB. Aspergillus infection of the epiglottis in a HIV positive patient. Genitourin Med. 1996;72:431.
287. Nagasawa M, Itoh S, Tomizawa D, et al. Invasive subglottal aspergillosis in a patient with severe aplastic anemia: a case report. J Infect. 2002;44:198.
288. Nakahira M, Matsumoto S, Mukushita N, et al. Primary aspergillosis of the larynx associated with CD4+ T lymphocytopenia. J Laryngol Otol. 2002;116:304.
289. Barnes C, Berkowitz R, Curtis N, et al. Aspergillus laryngotracheobronchial infection in a 6-year-old girl following bone marrow transplantation. Int J Pediatr Otorhinolaryngol. 2001;59:59.
290. Morelli S, Sgreccia A, Bernardo ML, et al. Primary aspergillosis of the larynx in a patient with Felty’s syndrome. Clin Exp Rheumatol. 2000;18:523.
291. Lapointe A, Parke RB, Kearney DL, et al. Laryngectomy for fungal abscesses of the larynx. Otolaryngol Head Neck Surg. 2004;131:1007.
292. Friedman M, Landsberg R, Tanyeri H, et al. Endoscopic sinus surgery in patients infected with HIV. Laryngoscope. 2000;110:1613.
293. Murphy C, Davidson TM, Jellison W, et al. Sinonasal disease and olfactory impairment in HIV disease: endoscopic sinus surgery and outcome measures. Laryngoscope. 2000;110:1707.
294. DelGaudio JM, Martinez EJ. Endoscopic sinus surgery in patients with chronic hepatic failure awaiting liver transplant. Am J Rhinol. 2004;18:253.
295. Franco-Vidal V, Blanchet H, Bebear C, et al. Necrotizing external otitis: a report of 46 cases. Otol Neurotol. 2007;28:771.
296. Rubin Grandis J, Branstetter BFt, Yu VL. The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis. 2004;4:34.
297. Sreepada GS, Kwartler JA. Skull base osteomyelitis secondary to malignant otitis externa. Curr Opin Otolaryngol Head Neck Surg. 2003;11:316.
298. Bellini C, Antonini P, Ermanni S, et al. Malignant otitis externa due to Aspergillus niger. Scand J Infect Dis. 2003;35:284.
299. Yao M, Messner AH. Fungal malignant otitis externa due to Scedosporium apiospermum. Ann Otol Rhinol Laryngol. 2001;110:377.
300. Weber R, Pinheiro Neto CD, Miziara ID, et al. HAART impact on prevalence of chronic otitis media in Brazilian HIV-infected children. Rev Bras Otorrinolaringol (Engl Ed). 2006;72:509.
301. Shapiro NL, Novelli V. Otitis media in children with vertically-acquired HIV infection: the Great Ormond Street Hospital experience. Int J Pediatr Otorhinolaryngol. 1998;45:69.
302. Miziara ID, Weber R, Araujo Filho BC, et al. Otitis media in Brazilian human immunodeficiency virus infected children undergoing antiretroviral therapy. J Laryngol Otol. 2007;121:1048.
303. Marchisio P, Principi N, Sorella S, et al. Etiology of acute otitis media in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1996;15:58.
304. Ugochukwu EF, Ezechukwu CC, Undie N, et al. Pattern of pathogens in ear discharge of HIV-infected children in Nnewi, Southeast Nigeria. Niger J Clin Pract. 2007;10:130.
305. Kohan D, Giacchi RJ. Otologic surgery in patients with HIV-1 and AIDS. Otolaryngol Head Neck Surg. 1999;121:355.
306. Linstrom CJ, Pincus RL, Leavitt EB, et al. Otologic neurotologic manifestations of HIV-related disease. Otolaryngol Head Neck Surg. 1993;108:680.
307. Praveen CV, Terry RM, Elmahallawy M, et al. Pneumocystis carinii infection in bilateral aural polyps in a human immunodeficiency virus-positive patient. J Laryngol Otol. 2002;116:288.
308. Breda SD, Hammerschlag PE, Gigliotti F, et al. Pneumocystis carinii in the temporal bone as a primary manifestation of the acquired immunodeficiency syndrome. Ann Otol Rhinol Laryngol. 1988;97:427.
309. Gherman CR, Ward RR, Bassis ML. Pneumocystis carinii otitis media and mastoiditis as the initial manifestation of the acquired immunodeficiency syndrome. Am J Med. 1988;85:250.
310. Kohan D, Rothstein SG, Cohen NL. Otologic disease in patients with acquired immunodeficiency syndrome. Ann Otol Rhinol Laryngol. 1988;97:636.
311. Wasserman L, Haghighi P. Otic and ophthalmic pneumocystosis in acquired immunodeficiency syndrome. Report of a case and review of the literature. Arch Pathol Lab Med. 1992;116:500.
312. Strauss M, Fine E. Aspergillus otomastoiditis in acquired immunodeficiency syndrome. Am J Otol. 1991;12:49.
313. Connolly JL, Carron JD. Invasive Aspergillus of the temporal bone. Am J Otolaryngol. 2007;28:134.
314. Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86.
315. Ghafouri M, Amini S, Khalili K, et al. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28.
316. McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543.
317. Cornblath DR, Hoke A. Recent advances in HIV neuropathy. Curr Opin Neurol. 2006;19:446.
318. Ferrari S, Vento S, Monaco S, et al. Human immunodeficiency virus-associated peripheral neuropathies. Mayo Clin Proc. 2006;81:213.
319. Elder GA, Sever JL. AIDS and neurological disorders: an overview. Ann Neurol. 1988;23(Suppl):S4.
320. Uldry PA, Regli F. [Isolated and recurrent peripheral facial paralysis in human infection with human immunodeficiency virus (HIV)]. Schweiz Med Wochenschr. 1988;118:1029.
321. Dalakas MC, Pezeshkpour GH. Neuromuscular diseases associated with human immunodeficiency virus infection. Ann Neurol. 1988;23(Suppl):38.
322. Luciano CA, Pardo CA, McArthur JC. Recent developments in the HIV neuropathies. Curr Opin Neurol. 2003;16:403.
323. Danchaivijitr N, Hesselink JR, Aryan HE, et al. Cerebello-Pontine angle (CPA) lymphoma with perineural extension into the middle fossa: case report. Surg Neurol. 2004;62:80.
324. Iplikcioglu AC, Dinc C, Bikmaz K, et al. Primary lymphoma of the trigeminal nerve. Br J Neurosurg. 2006;20:103.
325. Sandlund JT, Murphy SB, Santana VM, et al. CNS involvement in children with newly diagnosed non-Hodgkin’s lymphoma. J Clin Oncol. 2000;18:3018.
326. Mohan S, Ahmed SI, Alao OA, et al. A case of AIDS associated cryptococcal meningitis with multiple cranial nerve neuropathies. Clin Neurol Neurosurg. 2006;108:610.
327. Belec L, Georges AJ, Bouree P, et al. Peripheral facial nerve palsy related to HIV infection: relationship with the immunological status and the HIV staging in Central Africa. Cent Afr J Med. 1991;37:88.
328. McNaghten AD, Wan PC, Dworkin MS. Prevalence of hearing loss in a cohort of HIV-infected patients. Arch Otolaryngol Head Neck Surg. 2001;127:1516.
329. Schouten JT, Lockhart DW, Rees TS, et al. A prospective study of hearing changes after beginning zidovudine or didanosine in HIV-1 treatment-naive people. BMC Infect Dis. 2006;6:28.
330. Matas CG, Leite RA, Magliaro FC, et al. Audiological and electrophysiological evaluation of children with acquired immunodeficiency syndrome (AIDS). Braz J Infect Dis. 2006;10:264.
331. Smith T, Jakobsen J, Gaub J, et al. Clinical and electrophysiological studies of human immunodeficiency virus-seropositive men without AIDS. Ann Neurol. 1988;23:295.
332. Ollo C, Johnson RJr, Grafman J. Signs of cognitive change in HIV disease: an event-related brain potential study. Neurology. 1991;41:209.
333. Reyes-Contreras L, Silva-Rojas A, Ysunza-Rivera A, et al. Brainstem auditory evoked response in HIV-infected patients with and without AIDS. Arch Med Res. 2002;33:25.
334. Pagano MA, Cahn PE, Garau ML, et al. Brain-stem auditory evoked potentials in human immunodeficiency virus-seropositive patients with and without acquired immunodeficiency syndrome. Arch Neurol. 1992;49:166.
335. Chandrasekhar SS, Siverls V, Sekhar HK. Histopathologic and ultrastructural changes in the temporal bones of HIV-infected human adults. Am J Otol. 1992;13:207.
336. Dellepiane M, Medicina MC, Mora R, et al. Static and dynamic posturography in patients with asymptomatic HIV-1 infection and AIDS. Acta Otorhinolaryngol Ital. 2005;25:353.
337. Grimaldi LM, Luzi L, Martino GV, et al. Bilateral eighth cranial nerve neuropathy in human immunodeficiency virus infection. J Neurol. 1993;240:363.
338. Dromer F, Mathoulin-Pelissier S, Fontanet A, et al. Epidemiology of HIV-associated cryptococcosis in France (1985-2001): comparison of the pre- and post-HAART eras. AIDS. 2004;18:555.
339. Mirza SA, Phelan M, Rimland D, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992-2000. Clin Infect Dis. 2003;36:789.
340. Friedman GD, Jeffrey Fessel W, Udaltsova NV, et al. Cryptococcosis: the 1981-2000 epidemic. Mycoses. 2005;48:122.
341. Linthicum FHJr, Krutikova YV. Temporal bone histopathology case of the month: acute hearing loss due to cryptococcal meningitis in a patient with acquired immunodeficiency syndrome. Otol Neurotol. 2007;28:723.
342. Wang HC, Chang WN, Lui CC, et al. The prognosis of hearing impairment complicating HIV-negative cryptococcal meningitis. Neurology. 2005;65:320.
343. Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2004;72:99.
344. Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. 2000;30:47.
345. Harada T, Sando I, Myers EN. Temporal bone histopathology in deafness due to cryptococcal meningitis. Ann Otol Rhinol Laryngol. 1979;88:630.
346. Igarashi M, Weber SC, Alford BR, et al. Temporal bone findings in cryptococcal meningitis. Arch Otolaryngol. 1975;101:577.
347. Kwartler JA, Linthicum FH, Jahn AF, et al. Sudden hearing loss due to AIDS-related cryptococcal meningitis—a temporal bone study. Otolaryngol Head Neck Surg. 1991;104:265.
348. McGill TJ. Mycotic infection of the temporal bone. Arch Otolaryngol. 1978;104:140.
349. Brouwer AE, Rajanuwong A, Chierakul W, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363:1764.
350. van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med. 1997;337:15.
351. Johnston SR, Corbett EL, Foster O, et al. Raised intracranial pressure and visual complications in AIDS patients with cryptococcal meningitis. J Infect. 1992;24:185.
352. Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis. 2007;44:1222.
353. Stevenson J, Heath M. Syphilis and HIV infection: an update. Dermatol Clin. 2006;24:497.
354. Smith ME, Canalis RF. Otologic manifestations of AIDS: the otosyphilis connection. Laryngoscope. 1989;99:365.
355. Wiet RJ, Milko DA. Isolation of the spirochetes in the perilymph despite prior antisyphilitic therapy: a case report. Arch Otolaryngol. 1975;101:104.
356. Mack LWJr, Smith JL, Walter EK, et al. Temporal bone treponemes. Arch Otolaryngol. 1969;90:11.
357. Yimtae K, Srirompotong S, Lertsukprasert K. Otosyphilis: a review of 85 cases. Otolaryngol Head Neck Surg. 2007;136:67.
358. Darmstadt GL, Harris JP. Luetic hearing loss: clinical presentation, diagnosis, and treatment. Am J Otolaryngol. 1989;10:410.
359. Klemm E, Wollina U. Otosyphilis: report on six cases. J Eur Acad Dermatol Venereol. 2004;18:429.
360. French P. Syphilis. BMJ. 2007;334:143.
361. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55:1.
362. Sonne JE, Zeifer B, Linstrom C. Manifestations of otosyphilis as visualized with computed tomography. Otol Neurotol. 2002;23:806.
363. Roland JTJr, Alexiades G, Jackman AH, et al. Cochlear implantation in human immunodeficiency virus-infected patients. Otol Neurotol. 2003;24:892.
364. Vincenti V, Pasanisi E, Bacciu A, et al. Cochlear implantation in a human immunodeficiency virus-infected patient. Laryngoscope. 2005;115:1079.
365. Thodi C, Thodis E, Danielides V, et al. Hearing in renal failure. Nephrol Dial Transplant. 2006;21:3023.
366. Bains KS, Chopra H, Sandhu JS, et al. Cochlear function in chronic kidney disease and renal transplantation: a longitudinal study. Transplant Proc. 2007;39:1465.
367. Bucuvalas JC, O’Connor A, Buschle K, et al. Risk of hearing impairment in pediatric liver transplant recipients: a single center study. Pediatr Transplant. 2003;7:265.
368. Deutsch ES, Bartling V, Lawenda B, et al. Sensorineural hearing loss in children after liver transplantation. Arch Otolaryngol Head Neck Surg. 1998;124:529.
369. Rifai K, Kirchner GI, Bahr MJ, et al. A new side effect of immunosuppression: high incidence of hearing impairment after liver transplantation. Liver Transpl. 2006;12:411.
370. Norman K, Bonatti H, Dickson RC, et al. Sudden hearing loss associated with tacrolimus in a liver transplant recipient. Transpl Int. 2006;19:601.
371. Sroussi HY, Villines D, Epstein J, et al. The correlation between prevalence of oral manifestations of HIV and CD4+ lymphocyte counts weakens with time. J Acquir Immune Defic Syndr. 2006;42:516.
372. Hodgson TA, Greenspan D, Greenspan JS. Oral lesions of HIV disease and HAART in industrialized countries. Adv Dent Res. 2006;19:57.
373. Parveen Z, Acheampong E, Pomerantz RJ, et al. Effects of highly active antiretroviral therapy on HIV-1-associated oral complications. Curr HIV Res. 2007;5:281.
374. Ramirez-Amador V, Ponce-de-Leon S, Anaya-Saavedra G, et al. Oral lesions as clinical markers of highly active antiretroviral therapy failure: a nested case-control study in Mexico City. Clin Infect Dis. 2007;45:925.
375. Ramirez-Amador V, Esquivel-Pedraza L, Sierra-Madero J, et al. The changing clinical spectrum of human immunodeficiency virus (HIV)-related oral lesions in 1000 consecutive patients: a 12-year study in a referral center in Mexico. Medicine (Baltimore). 2003;82:39.
376. Umadevi KM, Ranganathan K, Pavithra S, et al. Oral lesions among persons with HIV disease with and without highly active antiretroviral therapy in southern India. J Oral Pathol Med. 2007;36:136.
377. Yang YL, Lo HJ, Hung CC, et al. Effect of prolonged HAART on oral colonization with Candida and candidiasis. BMC Infect Dis. 2006;6:8.
378. de la Rosa-Garcia E, Mondragon-Padilla A, Irigoyen-Camacho ME, et al. Oral lesions in a group of kidney transplant patients. Med Oral Patol Oral Cir Bucal. 2005;10:196.
379. Gulec AT, Demirbilek M, Seckin D, et al. Superficial fungal infections in 102 renal transplant recipients: a case-control study. J Am Acad Dermatol. 2003;49:187.
380. Erkose G, Erturan Z. Oral Candida colonization of human immunodeficiency virus infected subjects in Turkey and its relation with viral load and CD4+ T-lymphocyte count. Mycoses. 2007;50:485.
381. Chattopadhyay A, Caplan DJ, Slade GD, et al. Risk indicators for oral candidiasis and oral hairy leukoplakia in HIV-infected adults. Community Dent Oral Epidemiol. 2005;33:35.
382. Mercante DE, Leigh JE, Lilly EA, et al. Assessment of the association between HIV viral load and CD4 cell count on the occurrence of oropharyngeal candidiasis in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;42:578.
383. Miziara ID, Weber R. Oral candidosis and oral hairy leukoplakia as predictors of HAART failure in Brazilian HIV-infected patients. Oral Dis. 2006;12:402.
384. Greenspan D, Greenspan JS, Schiodt M, et al. AIDS and the mouth. Copenhagen: Munksgaard; 1990.
385. Sharma N, Berman DM, Scott GB, et al. Candida epiglottitis in an adolescent with acquired immunodeficiency syndrome. Pediatr Infect Dis J. 2005;24:91.
386. Sengor A, Willke A, Aydin O, et al. Isolated necrotizing epiglottitis: report of a case in a neutropenic patient and review of the literature. Ann Otol Rhinol Laryngol. 2004;113:225.
387. Baccaglini L, Atkinson JC, Patton LL, et al. Management of oral lesions in HIV-positive patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(Suppl):50 e1.
388. Pienaar ED, Young T, Holmes H. Interventions for the prevention and management of oropharyngeal candidiasis associated with HIV infection in adults and children. Cochrane Database Syst Rev. 2006;3:CD003940.
389. Vazquez JA, Skiest DJ, Tissot-Dupont H, et al. Safety and efficacy of posaconazole in the long-term treatment of azole-refractory oropharyngeal and esophageal candidiasis in patients with HIV infection. HIV Clin Trials. 2007;8:86.
390. Winslow DL. Posaconazole for azole-refractory candidiasis in patients with HIV infection. AIDS Alert. 2007;22:59.
391. Goldman M, Cloud GA, Wade KD, et al. A randomized study of the use of fluconazole in continuous versus episodic therapy in patients with advanced HIV infection and a history of oropharyngeal candidiasis: AIDS Clinical Trials Group Study 323/Mycoses Study Group Study 40. Clin Infect Dis. 2005;41:1473.
392. Greenspan D, Gange SJ, Phelan JA, et al. Incidence of oral lesions in HIV-1-infected women: reduction with HAART. J Dent Res. 2004;83:145.
393. Eyeson JD, Tenant-Flowers M, Cooper DJ, et al. Oral manifestations of an HIV positive cohort in the era of highly active anti-retroviral therapy (HAART) in South London. J Oral Pathol Med. 2002;31:169.
394. Casiglia J, Woo SB. Oral hairy leukoplakia as an early indicator of Epstein-Barr virus-associated post-transplant lymphoproliferative disorder. J Oral Maxillofac Surg. 2002;60:948.
395. Moura MD, Grossmann Sde M, Fonseca LM, et al. Risk factors for oral hairy leukoplakia in HIV-infected adults of Brazil. J Oral Pathol Med. 2006;35:321.
396. Margiotta V, Campisi G, Mancuso S, et al. HIV infection: oral lesions, CD4+ cell count and viral load in an Italian study population. J Oral Pathol Med. 1999;28:173.
397. Moura MD, Guimaraes TR, Fonseca LM, et al. A random clinical trial study to assess the efficiency of topical applications of podophyllin resin (25%) versus podophyllin resin (25%) together with acyclovir cream (5%) in the treatment of oral hairy leukoplakia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:64.
398. Benson CA, Kaplan JE, Masur H, et al. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm Rep. 2004;53:1.
399. Patton LL, Phelan JA, Ramos-Gomez FJ, et al. Prevalence and classification of HIV-associated oral lesions. Oral Dis. 2002;8(Suppl 2):98.
400. Hernandez SL, Lopez De Blanc SA, Sambuelli RH, et al. Oral histoplasmosis associated with HIV infection: a comparative study. J Oral Pathol Med. 2004;33:445.
401. Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807.
402. Wheat LJ. Endemic mycoses. In Cohen J, Powderly WG, editors: Infectious Diseases, 2nd ed, Edinburgh: Mosby, 2004.
403. Mitchell TG. Systemic fungi. In Cohen J, Powderly WG, editors: Infectious Diseases, 2nd ed, Edinburgh: Mosby, 2004.
404. Felix F, Gomes GA, Pinto PC, et al. Nasal histoplasmosis in the acquired immunodeficiency syndrome. J Laryngol Otol. 2006;120:67.
405. Vargas PA, Mauad T, Bohm GM, et al. Parotid gland involvement in advanced AIDS. Oral Dis. 2003;9:55.
406. Motta AC, Galo R, Lourenco AG, et al. Unusual orofacial manifestations of histoplasmosis in renal transplanted patient. Mycopathologia. 2006;161:161.
407. Couppie P, Clyti E, Nacher M, et al. Acquired immunodeficiency syndrome-related oral and/or cutaneous histoplasmosis: a descriptive and comparative study of 21 cases in French Guiana. Int J Dermatol. 2002;41:571.
408. Cunha VS, Zampese MS, Aquino VR, et al. Mucocutaneous manifestations of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome: particular aspects in a Latin-American population. Clin Exp Dermatol. 2007;32:250.
409. Ferreira OG, Cardoso SV, Borges AS, et al. Oral histoplasmosis in Brazil. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:654.
410. Yin MT, Dobkin JF, Grbic JT. Epidemiology, pathogenesis, and management of human immunodeficiency virus infection in patients with periodontal disease. Periodontol 2000. 2007;44:55.
411. Hamza OJ, Matee MI, Simon EN, et al. Oral manifestations of HIV infection in children and adults receiving highly active anti-retroviral therapy [HAART] in Dar es Salaam, Tanzania. BMC Oral Health. 2006;6:12.
412. Kerr AR, Ship JA. Management strategies for HIV-associated aphthous stomatitis. Am J Clin Dermatol. 2003;4:669.
413. Lilly EA, Cameron JE, Shetty KV, et al. Lack of evidence for local immune activity in oral hairy leukoplakia and oral wart lesions. Oral Microbiol Immunol. 2005;20:154.
414. King MD, Reznik DA, O’Daniels CM, et al. Human papillomavirus-associated oral warts among human immunodeficiency virus-seropositive patients in the era of highly active antiretroviral therapy: an emerging infection. Clin Infect Dis. 2002;34:641.
415. Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51.
416. Waters L, Nelson M. Long-term complications of antiretroviral therapy: lipoatrophy. Int J Clin Pract. 2007;61:999.
417. Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121.
418. Carr A. HIV lipodystrophy: risk factors, pathogenesis, diagnosis and management. AIDS. 2003;17(Suppl 1):141.
419. Sattler F. Body habitus changes related to lipodystrophy. Clin Infect Dis. 2003;36:S84.
420. Tien PC, Grunfeld C. What is HIV-associated lipodystrophy? Defining fat distribution changes in HIV infection. Curr Opin Infect Dis. 2004;17:27.
421. Tien PC, Barron Y, Justman JE, et al. Antiretroviral therapies associated with lipoatrophy in HIV-infected women. AIDS Patient Care STDS. 2007;21:297.
422. Kotler DP, Rosenbaum K, Wang J, et al. Studies of body composition and fat distribution in HIV-infected and control subjects. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:228.
423. Funk E, Brissett AE, Friedman CD, et al. HIV-associated facial lipoatrophy: establishment of a validated grading scale. Laryngoscope. 2007;117:1349.
424. Davison SP, Timpone JJr, Hannan CM. Surgical algorithm for management of HIV lipodystrophy. Plast Reconstr Surg. 2007;120:1843.
425. Ascher B, Katz P. Facial lipoatrophy and the place of ultrasound. Dermatol Surg. 2006;32:698.
426. Gulizia R, Vercelli A, Gervasoni C, et al. Controversy concerning role of ultrasonographic lipoatrophy assessments in HIV patients. AIDS. 2006;20:789.
427. Carey D, Wand H, Martin A, et al. Evaluation of ultrasound for assessing facial lipoatrophy in a randomized, placebo-controlled trial. AIDS. 2005;19:1325.
428. Domergue S, Psomas C, Yachouh J, et al. Fat microinfiltration autografting for facial restructuring in HIV patients. J Craniomaxillofac Surg. 2006;34:484.
429. Denton AB, Tsaparas Y. Injectable hyaluronic acid for the correction of HIV-associated facial lipoatrophy. Otolaryngol Head Neck Surg. 2007;136:563.
430. Gooderham M, Solish N. Use of hyaluronic acid for soft tissue augmentation of HIV-associated facial lipodystrophy. Dermatol Surg. 2005;31:104.
431. Lam SM, Azizzadeh B, Graivier M. Injectable poly-L-lactic acid (Sculptra): technical considerations in soft-tissue contouring. Plast Reconstr Surg. 2006;118:55S.
432. Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336.
433. Moyle GJ, Brown S, Lysakova L, et al. Long-term safety and efficacy of poly-L-lactic acid in the treatment of HIV-related facial lipoatrophy. HIV Med. 2006;7:181.
434. Comite SL, Liu JF, Balasubramanian S, et al. Treatment of HIV-associated facial lipoatrophy with Radiance FN (Radiesse). Dermatol Online J. 2004;10:2.
435. Silvers SL, Eviatar JA, Echavez MI, et al. Prospective, open-label, 18-month trial of calcium hydroxylapatite (Radiesse) for facial soft-tissue augmentation in patients with human immunodeficiency virus-associated lipoatrophy: one-year durability. Plast Reconstr Surg. 2006;118:34S.
436. Funk E, Bressler FJ, Brissett AE. Contemporary surgical management of HIV-associated facial lipoatrophy. Otolaryngol Head Neck Surg. 2006;134:1015.
437. Loutfy MR, Raboud JM, Antoniou T, et al. Immediate versus delayed polyalkylimide gel injections to correct facial lipoatrophy in HIV-positive patients. AIDS. 2007;21:1147.
438. Orentreich D, Leone AS. A case of HIV-associated facial lipoatrophy treated with 1000-cs liquid injectable silicone. Dermatol Surg. 2004;30:548.
439. Ramon Y, Fodor L, Ullmann Y. Preliminary experiences with Bio-Alcamid in HIV facial lipoatrophy. Dermatology. 2007;214:151.
440. Mori A, Lo Russo G, Agostini T, et al. Treatment of human immunodeficiency virus-associated facial lipoatrophy with lipofilling and submalar silicone implants. J Plast Reconstr Aesthet Surg. 2006;59:1209.
441. Negredo E, Higueras C, Adell X, et al. Reconstructive treatment for antiretroviral-associated facial lipoatrophy: a prospective study comparing autologous fat and synthetic substances. AIDS Patient Care STDS. 2006;20:829.
442. Nelson L, Stewart KJ. Experience in the treatment of HIV-associated lipodystrophy. J Plast Reconstr Aesthet Surg. 2007.