8 Head and neck cancer
Aetiology
The important aetiological factors in squamous cell carcinomas of the head and neck include:
Pathogenesis and pathology
SCC is thought to develop in a stepwise progression from squamous metaplasia to dysplasia, through carcinoma-in-situ to invasive squamous cell carcinoma. These histological changes are accompanied by molecular alterations which disrupt the regulation of cell proliferation, by inactivating tumour suppressor genes and activating oncogenes.
The majority (90%) of malignant tumours of the head and neck are squamous cell carcinomas. The WHO classification of head and neck cancers is outlined in Box 8.1.
Anatomy
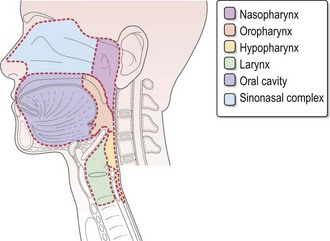
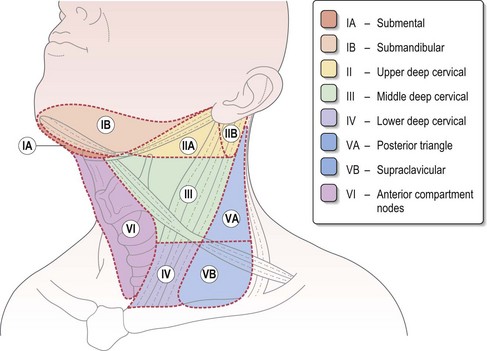
Figure 8.1 shows the anatomical sites in the head and neck. Figure 8.2 demonstrates the anatomical levels of neck nodes and the typical regional lymphatic drainage for head and neck subsites, which are important in planning surgery and radiotherapy. In the unoperated neck, the pattern of lymph node drainage is relatively predictable for different tumour subsites. The risk of occult lymph node metastasis varies according to the primary site and the size of the primary tumour. Clinical assessment of this risk of cervical nodal metastasis dictates subsequent decisions on inclusion of lymph node groups within a neck dissection or radiotherapy target volume during the definitive treatment.
Evaluation of patients with head and neck cancer
Important features in the history for head and neck cancer patients are:
Investigations and staging
The objectives of the clinical assessment of a patient with a suspected head and neck cancer are:
Imaging
Staging
TNM staging is based on the primary tumour size and/or extent, regional lymph node metastasis and distant metastatic spread (Box 8.2).
Box 8.2
A general TNM staging of head and neck tumour
| Stage I | T1N0M0 | tumour of ≤2 cm |
| Stage II | T2N0M0 | tumour of >2–4 cm |
| Stage III | T3N0M0 | tumour of >4 cm |
| T1–3N1M0 | ipsilateral single node ≤3 cm | |
| Stage IV | T4N0–1M0 | involving adjacent structures |
| Any T N2M0 | ipsilateral single node >3–6 cm (N2a) | |
| ipsilateral multiple nodes <6 cm (N2b) | ||
| contralateral or bilateral nodes <6 cm (N2c) | ||
| Any T N3M0 | nodes >6 cm | |
| Any T, any N, M1 | Distant metastasis |
Management of head and neck cancers
Pre-treatment assessment
Management of head and neck cancers is complex, and needs a multidisciplinary approach. All patients require assessment of performance status, dentition, swallowing and nutrition. Dental assessment is important in minimizing late side effects of radical radiotherapy such as dental caries and osteoradionecrosis. Maintaining adequate nutrition is a challenge in patients with head and neck cancer. Patients who are malnourished or at risk of becoming malnourished need dietetic assessment and often intervention is needed. All patients with locally advanced head and neck cancer need a swallowing and language therapy (SALT) assessment prior to radical treatment. Patients are encouraged to stop smoking and minimize alcohol intake during radiotherapy.
Principles of treatment
Early stage disease (stages I–II/T1–2N0M0)
Early stage disease is usually managed with either surgery or radiotherapy. The choice of treatment is based on location of tumour and anticipated morbidity. Radiotherapy results in a local control rate of 85–95% for T1 and 70–85% for T2 lesions. Treatment of the neck should be considered in addition to the treatment to the primary site. Node negative head and neck cancers with a >15–20% risk of occult cervical node metastasis (all cancers except <2 cm lesions in oral cavity and T1 glottic cancers) need elective management of neck nodes – either by a neck dissection or neck irradiation. The level(s) of nodes to be treated depends on the primary site of tumour and T stage, and the choice of treatment modality depends on the treatment of the primary site (Box 8.3).
Box 8.3
Recommended elective node treatment in stage I–II (T1–2N0) head and neck cancer
| Primary site | Nodal irradiation in N0 disease | Selective node dissection in N0 disease |
|---|---|---|
| Oral cavity: | ||
| T2 well lateralized | Ipsilateral level I–II | I–III |
| T2 reaching midline | Bilateral level I–III | |
| Oropharynx: | ||
| T1 Tonsil | Ipsilateral Ib–II | II–IV |
| T2 lateralized tonsil | Ipsilateral Ib–IV | |
| All other N0 | Bilateral Ib–V | |
| Larynx: | ||
| T1–2 Glottic | No nodal radiotherapy | II–IV and if extends below glottis, II–V |
| T1/2 Supraglottic | Bilateral level Ib–III | |
| All other N0 | Bilateral Ib–V | |
| Hypopharynx: | ||
| All N0 | Bilateral I–V | II–V |
| Nasopharynx: | ||
| T1N0 squamous carcinoma | No neck irradiation | – |
| All N0 | Bilateral I–V |
Radiotherapy in head and neck cancer (Box 8.4)
Box 8.4
Radical radiotherapy in head and neck cancer
Radical radiotherapy
The indications for postoperative radiotherapy are based on risk factors (Table 8.1). The treatment target is generally the tumour bed and involved neck to a dose of 60–66 Gy in 30–33 fractions (Box 8.4). Consideration may be given to prophylactic nodal irradiation to a lower dose depending on risk.
Table 8.1 Risk factors for predicting locoregional recurrence in the postoperative setting
| Primary site | Neck nodes | |
|---|---|---|
| Major risk factors | Positive resection margins | Extracapsular spread |
| Minor risk factors | Close resection margins (<5 mm) | 2 or more involved nodes |
| Invasion of soft tissues | More than one lymph node level involved | |
| Multifocal primary | Involved node >3 cm in diameter | |
| Perineural invasion | ||
| Vascular invasion | ||
| Poorly differentiated | ||
| T3 or T4 disease |
| High risk of recurrence is associated with the presence of one major risk factor or two minor risk factors. |
| Intermediate risk of recurrence is associated with the presence of one minor risk factor. |
| Factors of less importance in predicting risk of recurrence include: oral cavity primary site; presence of carcinoma-in-situ or dysplasia at the resection margin; and uncertain surgical or pathological findings. |
Alternate fractionation schedule studied in head and neck cancer include hyperfractionation (80.5 Gy in 70 fractions over 7 weeks using two 1.15 Gy fractions per day), accelerated fractionation (68 Gy in 34 fractions giving six fractions per week). The CHART trial failed to improve local control in locally advanced head and neck cancer. Though generally hyperfractionation and acceleration improve local control by a modest amount compared with conventional fractionation, these are not universally adopted in clinical practice because of practicality of delivery and the advent of chemoradiotherapy.
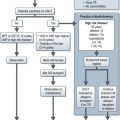
Role of primary chemoradiation (Box 8.5)
Box 8.5
Systemic treatment in head and neck cancer
Cisplatin with concurrent radiotherapy
New treatment approaches
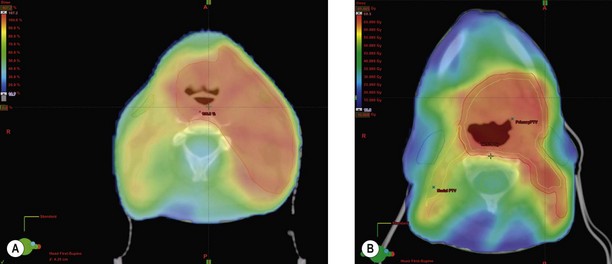
Intensity modulated radiotherapy (IMRT) (Figure 8.5) – the role of IMRT in head and neck cancer is evolving, whereby radiation dose can be reduced to critical structures e.g. spinal cord and side effects can be modified, e.g. minimizing salivary gland toxicity with additional scope for increasing the dose to gross disease.
Role of chemotherapy
The use of neoadjuvant (induction) and adjuvant chemotherapy in the treatment of locally advanced head and neck cancer has been controversial. A large meta-analysis of trials studying the role of chemotherapy in locally advanced head and neck SCC showed no significant benefit for induction or adjuvant chemotherapy. More recent studies have supported a role for induction chemotherapy in improving laryngeal preservation in locally advanced SCC of the larynx. A study comparing cisplatin-5-FU with docetaxel, cisplatin and 5-FU showed a better survival with three drugs (Box 8.5).
Care during and after treatment
Radiotherapy toxicity is discussed on p. 348. Rehabilitation of the patient after completion of treatment involves input from a multidisciplinary team including clinical nurse specialists, speech and language therapists, dietitians, physiotherapists, occupational therapists, dental surgeons, dental hygienists and prosthetic specialists.
Treatment of recurrent disease
The management options for advanced, recurrent or metastatic head and neck cancer depend on factors such as co-morbid conditions, previous treatment, performance status, patient preferences and the nature of their symptoms. Advanced head and neck cancer can have a devastating impact on personal appearance and vital functions such as speech and swallowing. Quality of life is of paramount importance when deciding management in recurrent disease.
Management of specific cancers
Tumours of the eye
Malignant tumours of the eyelids include basal cell carcinoma and squamous cell carcinoma, which are managed similarly to skin cancers (p. 237).
Malignant melanoma is the most common primary intraocular tumour in adults, which behaves similarly to melanoma elsewhere. In localized disease, treatment is by surgical excision and management of advanced disease is the same as melanoma arising from skin (p. 231).
A variety of malignant tumours, such as sarcomas and lacrimal gland cancers, can arise in the orbit. Sarcomas are managed with exenteration of the eye, with adjuvant radiotherapy with or without chemotherapy (p. 254). Lacrimal gland tumours are treated with complete surgical excision if possible and radiotherapy is indicated for inoperable tumours and for close/positive margins postoperatively.
Cancers of pinna
Management of basal cell carcinomas and squamous cell carcinomas is dealt within Chapter 14 (p. 231).
Hypopharynx
Presentation and evaluation
Evaluation includes endoscopy and biopsy. Local staging is with CT and MRI.
Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet.. 2008;371:1695-1709.
Grégoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiother Oncol.. 2003;69:227-236.
Machtay M, Moughan J. Trotti A t al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol.. 2008;26:3582-3589.
Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys.. 2006;64:47-56.
Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys.. 1991;21:109-122.
Rivera F, García-Castaño A, Vega N, Vega-Villegas ME, Gutiérrez-Sanz L. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther.. 2009;9:1421-1428.
Corry J, Peters LJ, Rischin D. Optimising the therapeutic ratio in head and neck cancer. Lancet Oncol.. 2010;11:287-291.
Dirix P, Nuyts S. Evidence-based organ-sparing radiotherapy in head and neck cancer. Lancet Oncol.. 2010;11:85-91.
Bernier J, Bentzen SM, Vermorken JB. Molecular therapy in head and neck oncology. Nat Rev Clin Oncol.. 2009;6:266-277.