22 Hands and feet
Summary and Key Features
• Extrinsic and intrinsic factors cause skin laxity, dyspigmentation and fat loss in the hands.
• Soft tissue augmentation with a variety of fillers has been well studied and shown to be safe and efficacious to camoflouge exposed tendons and vessels and improve lipoatrophy of the hands.
• The most common fillers for the hands are calcium hydroxyapatite, hyaluronic acid and autologous fat.
• Poly-L-lactic acid has fallen out of favor for hand augmentation because of the risk of visible nodules under lax skin.
• Use of disposable blunt tipped cannulas has improved the ease and reduced the side effect profile for hand filling.
• In addition to standard post-filler instructions like ice and elevation, patient should be told to massage the hand after treatment.
• Potential complications of hand augmentation include visible product, Tyndall effect, persistent edema and pain.
• Dermal fillers have been used to improve symptoms and speed healing of diabetic foot ulcers. The use to reduce metatarsalgia symptoms from chronic wearing of high heels is being used but has not been well studied.
Soft tissue augmentation of the hands
Calcium hydroxylapatite
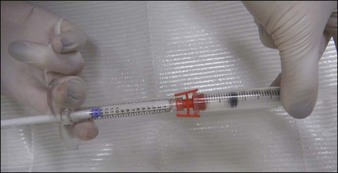
As an opaque, white substance, CaHA has the advantage of acting as both a filler and camouflage for underlying anatomical architecture. However, its use for hand rejuvenation was initially limited by the product’s high viscosity and pain upon injection. In 2007, Busso & Applebaum reported a novel approach that overcame both issues. Utilizing a Luer-to-Luer lock connector (Fig. 22.1A), the authors mixed 0.1 mL 2% lidocaine without epinephrine with 1.3 mL of CaHA (the standard syringe volume at that time). After tenting the skin of the central dorsal hand to separate it from underlying large vessels and tendons, they injected a single bolus of 0.5–1.4 mL of the lidocaine–CaHA mixture into the space created between the subcutaneous layer and the superficial fascia. The bolus was then massaged and molded into a cosmetically smooth appearance. The addition of lidocaine reduced both the viscosity and pain.
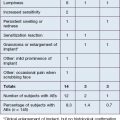
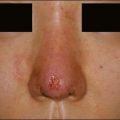
Several larger studies by Busso et al, Bank, and Marmur et al have recently been published supporting the safety and efficacy of CaHA as an anti-aging hand filler. Busso et al published a multicenter, randomized, controlled trial of 101 patients followed over 6 months and established a new hand volume severity scale 3 to assess results (Table 22.1). Following administration of a lidocaine bolus preoperatively, CaHA was administered as a bolus into the dorsal hand using a 27-gauge 0.75-inch (20 mm) needle. Aside from reporting statistically significant improvement in the treatment group, the study also found that adverse events, including bruising, itching, pain, redness, and swelling (Fig. 22.2), were frequent yet short in duration and did not affect overall patient function significantly. Similarly, Sadick noted only brief and minor side effects in 10 patients following CaHA treatment with a 25-gauge 0.5-inch (12 mm) needle to the dorsal hands and residual cosmetic correction lasting up to 1 year postoperatively.
| Severity | Characteristics of hand |
|---|---|
| 4 | All three central tendons are fully exposed when hand is at rest |
| 3 | All three central tendons are partially exposed, with one or two tendons fully exposed when hand is at rest |
| 2 | All three central tendons are partially exposed when hand is at rest |
| 1 | One or two central tendons are slightly exposed when hand is at rest |
| 0 | No tendon is exposed when hand is at rest |
Adapted from Busso M, Moers-Carpi M, Storck R, et al 2010 Multicenter, randomized trial assessing the effectiveness and safety of calcium hydroxylapatite for hand rejuvenation. Dermatologic Surgery 36(s1):790-797.
Most recently, the availability of blunt-tipped small-gauge cannulas has revolutionized the procedure by reducing the number of puncture sites required, patient discomfort, and postoperative bruising. The authors recommend delivering boluses or linear threads with one entry point at the dorsal wrist (Fig. 22.3). The product is massaged until smooth. Patients are instructed to massage several times daily and use topical ice packs for the first 48 hours. A small latex-free examination glove can be applied immediately with an ice pack (Fig. 22.4). Despite these interventions, some patients complain of severe pain, which limits range of motion. The authors’ clinical experience validates the mean volume of 3.1 mL for optimal improvement of both hands reported by Busso et al. We have found that dividing the treatment into two planned sessions at 2–4-week intervals has reduced the incidence of significant pain and made the treatment viable for patients who work with their hands.
Balkin SW, Kaplan L. Silicone injection management of diabetic foot ulcers: a possible model for prevention of pressure ulcers. Decubitus. 1991;4(4):38–40.
Bank DE. A novel approach to treatment of the aging hand with Radiesse. Journal of Drugs in Dermatology. 2009;8(12):1122–1126.
Beck M. Toe the line: doctors fight cosmetic foot surgery. Health Journal. 2010. 27 July. Online. Available http://online.wsj.com/article/.
Busso M, Applebaum D. Hand augmentation with Radiesse (calcium hydroxylapatite). Dermatologic Therapy. 2007;20(6):385–387.
Busso M, Moers-Carpi M, Storck R, et al. Multicenter, randomized trial assessing the effectiveness and safety of calcium hydroxylapatite for hand rejuvenation. Dermatologic Surgery. 2010;36(s1):790–797.
Butterwick KJ. Rejuvenation of the aging hand. Dermatologic Clinics. 2005;23:515–527. vii
Butterwick KJ, Bevin AA, Iyer S. Fat transplantation using fresh versus frozen fat: a side-by-side two-hand comparison pilot study. Dermatologic Surgery. 2006;32(5):640–644.
Coleman SR. Hand rejuvenation with structural fat grafting. Plastic and Reconstructive Surgery. 2002;110(7):1731–1744. discussion 1745-1747
Edelson KL. Hand recontouring with calcium hydroxylapatite (Radiesse). Journal of Cosmetic Dermatology. 2009;8(1):44–51.
Giunta RE, Eder M, Machens HG, et al. Structural fat grafting for rejuvenation of the dorsum of the hand. Handchirurgie, Mikrochirurgie, plastische Chirurgie. 2010;42(2):143–147.
Gutowski KA. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plastic and Reconstructive Surgery. 2009;124(1):272–280.
Haq S, Storck R, Martine B, et al. Multinational, multipatient study of calcium hydroxylapatite for treatment of the aging hand: European cosmetic physician group on hand augmentation. Dermatologic Surgery. 2010;36(s1):782–789.
Hartmann V, Bachmann F, Plaschke M, et al. Hand augmentation with stabilized hyaluronic acid (Macrolane VRF20 and Restylane Vital, Restylane Vital Light). Journal der Deutschen Dermatologischen Gesellschaft. 2010;8(1):41–44.
Man J, Rao J, Goldman M. A double-blind, comparative study of nonanimal-stabilized hyaluronic acid versus human collagen for tissue augmentation of the dorsal hands. Dermatologic Surgery. 2008;34(8):1026–1031.
Marmur ES, Al Quran H, De Sa Earp AP, et al. A five-patient satisfaction pilot study of calcium hydroxylapatite injection for treatment of aging hands. Dermatologic Surgery. 2009;35(12):1978–1984.
Pu LL, Coleman SR, Cui X, et al. Autologous fat grafts harvested and refined by the Coleman technique: a comparative study. Plastic and Reconstructive Surgery. 2008;122(3):932–937.
Redaelli A. Cosmetic use of polylactic acid for hand rejuvenation: report on 27 patients. Journal of Cosmetic Dermatology. 2006;5(3):233–238.
Rendon MI, Cardona LM, Pinzon-Plazas M. Treatment of the aged hand with injectable poly-l-lactic acid. Journal of Cosmetic and Laser Therapy. 2010;12(6):284–287.
Sadick NS. A 52-week study of safety and efficacy of calcium hydroxylapatite for rejuvenation of the aging hand. Journal of Drugs in Dermatology. 2011;10(1):47–51.
Sadick NS, Anderson D, Werschler WP. Addressing volume loss in hand rejuvenation: a report of clinical experience. Journal of Cosmetic and Laser Therapy. 2008;10(4):237–241.
Vleggaar D. Soft-tissue augmentation and the role of poly-L-lactic acid. Plastic and Reconstructive Surgery. 2006;3 suppl:S46–S54.
Werschler PBM, Busso M. Prepackaged injectable soft-tissue rejuvenation of the hand and other nonfacial area. Body Rejuvenation. 2010;7:221–225.