HAEMORRHAGE AND CIRCULATORY SHOCK
Introduction
Blood volume in the textbook subject is of the order of 5 L (see Chapter 1) and at any one time about 65% of this volume is in the venous compartment of the circulation. A reduction in blood volume may occur in many ways. Loss of whole blood through blood vessel trauma either directly out of the subject or into other tissues such as the lumen of the stomach or a thigh muscle is referred to as haemorrhage. However, excessive vasodilatation of peripheral blood vessels will induce similar physiological responses to loss of blood volume. These processes increase the ‘volume’ of the circulatory system whilst the volume of blood it contains remains unchanged leading to a relative hypovolaemia.
Normal physiological compensatory responses will usually cope with low levels of blood loss (up to 10% blood volume) without problem. This happens frequently on a voluntary basis with people who lose 500 mL blood while attending a blood donor session. The fundamentals of these physiological responses are firstly short-term maintenance of arterial blood pressure so that tissue perfusion is sustained and, secondly, over a longer time course, the lost blood volume is replaced. These mechanisms, which link together many of the individual topics covered in greater detail earlier in this book, will now be reviewed. Severe insults to the circulatory system lead to the syndrome of circulatory shock. This topic is discussed at the end of this chapter. A case history of a patient who has suffered a haemorrhage is shown in Case 14.1:1.
Arterial blood pressure changes in response to haemorrhage
Rapid (time course in minutes) blood volume losses of the order of 5–10% in someone with normally functioning circulatory reflexes produce little if any change in mean arterial pressure. A rapid 15–20% haemorrhage will cause a modest reduction in mean arterial pressure from a normal textbook value of about 93 mm Hg (see Chapter 9) to about 80–90 mm Hg. Recovery from such a haemorrhage using normal physiological mechanisms should be uneventful. A 20–30% blood volume loss might typically result in a drop in mean arterial blood pressure to 60–80 mm Hg and generate some indications of shock responses but such a haemorrhage would not normally be fatal. A blood volume loss of 30–40% however would lead to a substantial reduction of mean arterial blood pressure to 50–70 mm Hg with serious shock responses which may become irreversible. Blood volume loss beyond 50% would normally be fatal.
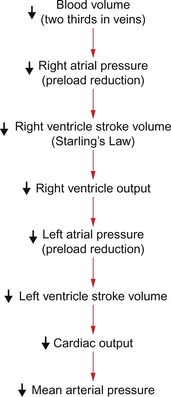
The fundamental links between blood loss and hypotension are described in Chapter 4 and are summarized in Figure 14.1. Decreased blood volume leads to decreased right atrial pressure and consequently decreased filling of the right ventricle. This leads to a decrease in stroke volume (Starling’s law) and therefore to a reduction in cardiac output. A 20% reduction in blood volume would typically result in an initial reduction in resting cardiac output from about 5 L/min to about 3 L/min. As mean arterial pressure is the product of cardiac output and peripheral resistance there is a fall in blood pressure as identified above. An important first aid point is that it is inadvisable to prop up someone who has suffered a haemorrhage to a sitting position. This would only further reduce the preload effects on the heart.
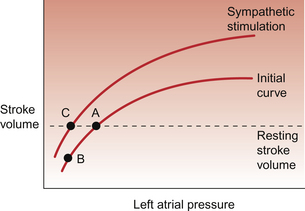
The compensatory responses which would help to restore blood pressure are triggered by the cardiovascular reflexes discussed in Chapter 10. Of prime importance is the carotid sinus baroreceptor reflex. The modified nerve endings which constitute the baroreceptor become less stretched as blood pressure falls. Fewer action potentials will therefore pass up the glossopharyngeal nerve and enter the brain at the level of the medulla. Following central processing of the baroreceptor input there will be activation of sympathetic outflow and inhibition of the parasympathetic nerve supply to the heart. The consequences are an increased heart rate (tachycardia) and an increase in cardiac contractility, both of which contribute to a raised cardiac output despite decreased preload effects on the heart (see Chapter 4). Re-establishment of a normal resting stroke volume as a result of a sympathetically induced increase in cardiac contractility is illustrated in Figure 14.2. In otherwise normal subjects, a significant tachycardia does not occur until the blood loss has exceeded 700–800 mL (10–15%).
Sympathetic nervous system activation also leads toα-receptor-mediated vasoconstriction (see Chapter 9). The regions of the circulation which are particularly affected by sympathetic vasoconstriction responses include the skin, gut, kidney and skeletal muscle. The brain and coronary circulations are relatively preserved especially during the sequel to a haemorrhage amounting to about 20% or less blood volume loss. Concurrent sympathetically mediated venoconstriction will help to maintain central venous pressure and hence limit the fall in preload on the right side of the heart. An unfortunate practical aspect of this venoconstriction is that it may hinder attempts to gain access to the circulation with a venous cannula so that infusion of blood or other replacement body fluids can commence.
The patient who has suffered a haemorrhage and has therefore activated these compensatory responses appears pallid due to cutaneous vasoconstriction, with cold clammy skin, possibly with peripheral cyanosis, and a weak rapid pulse. The responses to haemorrhage will vary considerably between individuals depending on factors such as age and gender, nutrition and fluid balance, season of the year and environmental temperature as well as less easily defined individual characteristics. The extent and speed of the haemorrhage will be important as well. When haemorrhage reaches levels of the order of 25–35% blood loss the sympathetic activation outlined above is succeeded by inhibition of sympathetic outflow and progression to circulatory shock as described later in this chapter. Peripheral cyanosis (see Chapter 1) is associated with a reduced local blood flow and hence greater than normal extraction of oxygen from the remaining blood. Sweating is regulated by the sympathetic nervous system and so is another manifestation of the baroreceptor reflex. Some of these autonomic nervous system mediated changes feature in the case history of Linda Hamilton outlined in Case 14.1:1. A simple but quite effective test of peripheral circulatory function is the nail capillary refill test. Pressure is applied to a finger nail bed until it has blanched. This indicates that blood has been forced away from the area. When pressure is removed the time taken for blood to return to the nail bed (indicated by a change in colour) is measured. Normally capillary refill time will be less than 2 seconds. A longer time indicates reduced tissue perfusion. This test is particularly useful in children as other signs of imminent circulatory collapse appear relatively late.
Short-term responses which help to restore lost blood volume
In Chapter 11 the ‘Starling forces’ which determine the movement of water across capillary walls are described. Fundamentally, the capillary blood pressure (hydrostatic pressure) tends to move water out of the capillary bed into the interstitial fluid. This is opposed by a gradient of colloid osmotic pressure which will tend to draw water from the interstitial compartment back into the capillary.
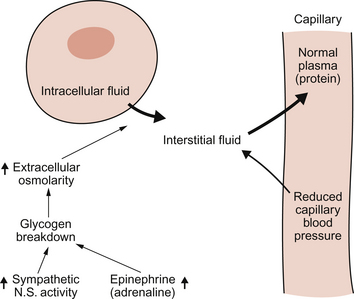
Following a haemorrhage, there may be a fall in mean arterial pressure but, in addition, a compensatory peripheral vasoconstrictor response occurs. As the main resistance vessels (see Chapter 9) are the arterioles, vessels which come immediately before the capillaries, there will be a fall in capillary blood pressure following a haemorrhage. This will alter the balance of the Starling forces and, as the colloid osmotic pressure of plasma is initially unchanged, this will result in a net movement of water from the interstitial space into the blood. This is illustrated in Figure 14.3. The rate and extent of this internal replacement of lost blood volume will depend on the rate and degree of blood loss. A rapid loss of 20% of blood volume (about a 1000 mL haemorrhage in the ‘textbook person’) will lead to perhaps 600 mL of interstitial fluid entering the blood over a period of around 10 minutes. This is sometimes referred to as an ‘internal transfusion’. As skeletal muscle constitutes about 50% of body weight this tissue is a major source of the interstitial fluid for the internal transfusion.
A further factor which helps to maintain the internal transfusion is hormonally driven glycogen breakdown in the liver (Fig. 14.3). Sympathetic activation and an associated increase in adrenaline (epinephrine) secretion from the adrenal medulla lead to release of glucose from glycogen stores and, as a consequence, an increase in plasma and interstitial fluid osmolarity. More than half of total body water is held inside cells (see Chapter 11) and the rise in osmolarity draws fluid into the interstitial space from the intracellular compartment. It has been estimated that this fluid movement from the intracellular compartment contributes about half of the volume of the internal transfusion.
A consequence of the internal transfusion is haemodilution, a fall in haematocrit. Many patients who have suffered a haemorrhage will already have a low haematocrit by the time they reach hospital. While on the one hand this reduced haematocrit will lower blood viscosity (see Chapter 8) and make it easier for blood to flow round the body, the reduced viscosity also lowers the resistance to blood flow and potentially contributes to a further fall in blood pressure. The reduced oxygen carrying capacity of blood as a result of haemodilution will restrict oxygen delivery to already poorly perfused tissues.
Longer term responses which help to restore lost blood volume and electrolytes
Shifting water between the various fluid compartments in the body as a short-term measure still leaves the problem of replacing the overall water loss following a haemorrhage. This is achieved by a combination of changes in glomerular filtration rate in the kidneys and hormonally mediated changes in kidney tubular function (Fig. 14.4). Assuming an adequate oral salt and water intake, in the case of a 20% blood volume loss the body fluid volumes would be returned to normal over a period of about 3 days.
The main hormones involved in salt and water retention are described below.
Renin-angiotensin aldosterone system (see Chapter 9)
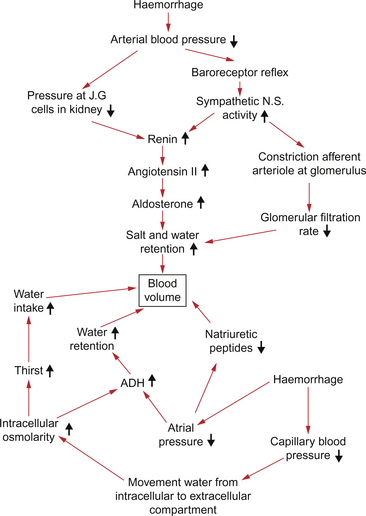
The main triggers for increased renin release following haemorrhage are firstly an increase in sympathetic nerve activity which, via a β-adrenoceptor mechanism on the juxtaglomerular (JG) cells, will increase renin secretion (Fig. 14.4). This increase in renin secretion is therefore linked to the fall in blood pressure at the carotid sinus baroreceptor. Secondly, the JG cells respond directly to a fall in renal artery blood pressure to increase renin secretion. An increase in renin secretion will increase the generation of angiotensin II (Ang II). This has two beneficial roles in the acute response to haemorrhage. The immediate vasoconstrictor effect of Ang II helps to maintain arterial blood pressure. Ang II is also the major stimulus to increased aldosterone synthesis in the zona glomerulosa of the adrenal cortex.
Aldosterone has a sodium retaining and potassium excreting action on the distal segments of the nephron. Water is absorbed osmotically along with the sodium retained. Ang II is also involved, by its action on the subfornical organ of the brain, in promoting thirst responses. A patient who had suffered an acute 20% haemorrhage and has had no fluid replacement therapy would experience an intense thirst within an hour or so. This is mainly due to changes in the environment of the thirst centre in the hypothalamus associated with the fluid shifts which generate the internal transfusion (see p. 164).
Natriuretic peptide hormones (ANP and BNP)
Release of these hormones, particularly from the atria of the heart, is an important aspect of normal blood volume regulation (Fig. 14.4). Expansion of blood volume with the consequent increase in central venous pressure and increased stretch of the right atrial wall is the normal trigger for release of natriuretic peptides. Actions of natriuretic peptides include inhibition of the action of aldosterone and a vasodilator action, especially within the kidney, which increases GFR. Following a haemorrhage, natriuretic peptide hormone levels will be suppressed as a consequence of the reduction in central venous pressure. The sodium and water losing effects of the peptides will be reduced thus contributing to maintaining the extracellular fluid volume.
Antidiuretic hormone (ADH)
This peptide hormone is synthesized in the hypothalamus and released into the circulation from the posterior pituitary (Fig. 14.4). The major physiological triggers for secretion are a fall in blood volume, detected by stretch receptors in the atria and great veins (see Chapter 10), or a rise in the osmolarity of blood passing through the hypothalamus. ADH secretion will therefore be increased following haemorrhage.
Decompensated or irreversible shock following haemorrhage
The decline in blood pressure has an adverse effect on cardiac function with reduced coronary perfusion. This results in a dangerous positive feedback loop as the fall in cardiac output will lead to further reduction in blood pressure and therefore further impaired coronary blood flow and hence further reduced cardiac performance. The poor tissue perfusion also has an adverse effect in peripheral tissues as it leads to local metabolic acidosis. Accumulation of tissue metabolites, such as H+ (see Chapter 9), leads to vasodilatation and a further reduction in arterial blood pressure.
Causes of shock
1. hypovolaemic shock—reduction of blood volume
2. cardiogenic shock—pump failure
3. distributive shock—a fall in peripheral resistance and, consequently, decreased arterial blood pressure
In each case tissue perfusion is reduced and there is an inadequate supply of oxygen to the tissues.
In cardiogenic shock following events such as myocardial infarction (see Chapter 5) or serious arrhythmias there will be a fall in systemic arterial blood pressure as a result of the fall in cardiac output. In contrast to hypovolaemic shock, central venous pressure and pulmonary vein pressure are both likely to be increased. Poor left ventricular performance will lead to a rise in filling pressure on the left side of the heart and the possibility of pulmonary oedema. Fluid replacement therapy must therefore be very carefully managed. The changes in kidney function, which are primarily triggered by the fall in arterial blood pressure, will be broadly similar in haemorrhagic and cardiogenic shock.
Anaphylactic shock is another form of distributive shock. It represents an immediate hypersensitivity reaction which usually occurs within 30 minutes of exposure to an antigen such as a drug (e.g. penicillin), a specific protein (e.g. in a peanut) or an insect venom (e.g. a bee sting). It is mediated by immunoglobulin E (IgE) and leads to the release of mediators such as histamine which are stored in granules within mast cells. Vasodilatation and an increase in capillary permeability follow the release of histamine (see Chapter 11).
Fluid replacement therapy
The primary reason for fluid replacement therapy is to maintain tissue perfusion by ensuring an adequate cardiac output and a sufficiently high arterial blood pressure. Optimizing the preload effects on the heart in order to maintain cardiac output is an important aspect of fluid management. However care must be taken to avoid overload as this may lead to pulmonary oedema, especially if the patient is already in cardiac failure (see Chapter 6).
• estimate the amount of fluid already lost
• allow for future anticipated abnormal loss as for example in cases of excessive sweating or diarrhoea
• maintain normal daily fluid intake requirements (typically about 2500 mL/day).
Use of blood as a replacement fluid
• Cross-matching of blood does take some time which may be critical in an emergency situation. Even then adverse immunological reactions may take place.
• Transfusion which increases the haematocrit above an optimal level of 30–35% may increase the viscosity of blood (see Chapter 8) and hence the flow characteristics may change. Old stored red cells lose part of the flexibility which is essential for them to pass through capillaries (see Chapter 11). This may contribute to the obstruction of small vessels and cause local hypoxia.
• Stored blood loses platelets and is deficient in clotting factors. This may leave the patient with reduced ability to coagulate blood, a dangerous situation particularly during surgery. An effective clotting mechanism can be restored by administering clotting factors and extra platelets.
• Citrate is used as an anticoagulant for stored blood and, as this is metabolized, a metabolic alkalosis (see Chapter 1) will develop over the next 1–2 days.
• 2,3,DPG (see Chapter 1) which is generated inside red cells disappears during storage. This means that the affinity of haemoglobin to bind oxygen increases. The red cells pick up oxygen at the lungs without problem but fail to deliver it adequately in the tissues.
• Other potential sources of problems include a low [Ca++] and high [K+] in stored blood.
Use of crystalloid solutions as replacement fluids
Hypertonic saline solutions (1.8% up to 10% saline) are available for use in special circumstances.
The characteristic of crystalloid solutions is that as the solutes are small they cross capillary walls. The volume of distribution is therefore at least all of the extracellular fluid compartment. The distribution of Na+ and Cl− in body fluids is predominantly extracellular and so saline infusion will mainly expand the extracellular space. Dextrose solutions are handled differently. Once infused into the circulation the glucose will be taken up into cells and either metabolized or stored as glycogen. Giving isotonic dextrose solution is therefore ultimately equivalent to an infusion of distilled water. The difference is that there is a sufficient time delay for the solution to become distributed around the body and mix with other body fluids before the glucose is removed. There is therefore dilution of body fluids but no osmotic lysis of cells. Some of the water will enter the intracellular compartment by moving down the osmotic gradient created by the dilution of the extracellular fluid. The volume of distribution of the water is therefore the same as for total body water, i.e. two thirds intracellular and one third extracellular (see Chapter 1).
Use of colloid solutions as replacement fluids
Human albumin (rmm = 69 000) is quantitatively the most important contributor to the colloid osmotic pressure of blood. It is normally held within the vascular compartment unless capillary permeability is increased (see Chapter 11). Infusion of an isotonic solution containing human albumin can be used to increase blood volume. However the half-life of albumin in plasma is relatively short, 10% of administered albumin leaves the circulation within 2 hours and 95% within 2 days. Human albumin is used for volume replacement in shock and in burned patients but is not recommended for routine volume replacement because supplies are limited and effective cheaper alternatives are available.
Selection of suitable volume replacement therapy is a complex decision for which the volume and composition of the fluid lost is a prime consideration. It is also an area of considerable controversy. The relative merits of crystalloid or colloid solutions for fluid replacement during surgery and in resuscitation is a subject for heated debate.
Chien, S. Role of the sympathetic nervous system in haemorrhage. Phys. Rev.. 1967; 47:214–288.
Länne, T., Lundvall, J. Mechanisms in man for rapid refill of the circulatory system in hypovolaemia. Acta Phys. Scand.. 1992; 146:299–306.
Schadt, J. C., Ludbrook, J. Haemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am. J. Phys.. 1991; 260:H305–H318.