16.5 Haemolytic uraemic syndrome
Introduction
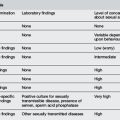
Epidemiology
Non-diarrhoeal-associated HUS (D– HUS) cases account for 10% of cases. These are secondary to:
Investigations
Renal biopsy is rarely indicated in children who have the characteristic features of HUS.
Treatment
No added potassium is required unless serum levels are below normal values. Hyperkalaemia must be anticipated and treated in a timely fashion (refer to management of hyperkalaemia in Chapter 10.5 on electrolytes).
Hypertension responds well to treatment with short-acting calcium channel blockers, e.g. nifedipine. Intravenous nitroglycerine can be used if oral medication is not tolerated. Nitroprusside is not favoured because of the danger of cyanide poisoning in renal failure. Labetalol by intravenous bolus or continuous infusion can also be used to manage hypertension (see Chapter 16.3). Treatment of hypertension can prevent development of encephalopathy and congestive heart failure.
Complications
The frequency of complications is higher in D– HUS patients.
Pancreatitis can develop, sometimes with diabetes, and hepatic involvement can occur.
Prevention
Controversies and future directions
 In one study, a strategy was developed in which a recombinant E. coli was generated that had on its surface a lipopolysaccharide (LPS), which mimicked the Shiga toxin receptor. These recombinant E. coli bind to the Shiga toxin thus preventing the binding of Shiga toxin to target cells.
In one study, a strategy was developed in which a recombinant E. coli was generated that had on its surface a lipopolysaccharide (LPS), which mimicked the Shiga toxin receptor. These recombinant E. coli bind to the Shiga toxin thus preventing the binding of Shiga toxin to target cells. Strategies for immunisation have been explored. The first steps in evaluation of development of a vaccine against Shiga-toxin-producing strains of E. coli have been undertaken in a preclinical study.
Strategies for immunisation have been explored. The first steps in evaluation of development of a vaccine against Shiga-toxin-producing strains of E. coli have been undertaken in a preclinical study. An oligovalent water-soluble carbohydrate pentamer with five pairs of trisaccharide ligands linked to a central dendrimer, and called ‘Starfish’, neutralises all five B subunits of Shiga toxin and protects human cells from Shiga toxin in culture.
An oligovalent water-soluble carbohydrate pentamer with five pairs of trisaccharide ligands linked to a central dendrimer, and called ‘Starfish’, neutralises all five B subunits of Shiga toxin and protects human cells from Shiga toxin in culture. Administration of intravenous γ-globulin IgG and gabexate mesilate, a synthetic serine protease inhibitor used as an anticoagulant resulted in amelioration of disease in five children with D+HUS. The IgG itself has no proven benefit on the course of D + HUS.
Administration of intravenous γ-globulin IgG and gabexate mesilate, a synthetic serine protease inhibitor used as an anticoagulant resulted in amelioration of disease in five children with D+HUS. The IgG itself has no proven benefit on the course of D + HUS.Corrigan J.J.Jr, Boineau F. Hemolytic uremic syndrome. Pediatr Rev. 2001;22:365-368.
Cronan K., Norman M. Renal and electrolyte emergencies. In: Fleisher G.R., Ludwig S., editors. Textbook of pediatric emergency medicine. 4th ed. Philadelphia: Lippincott, Williams & Wilkins; 2000:847-848. Chapter 86
Elliott E.J., Robins-Browne R.M., O’ Loughlin E.V., et al. Nationwide study of haemolytic uraemic syndrome: Clinical, microbiological, and epidemiological features. Arch Dis Child. 2001;85:125-131.
Garg A.X., Suri R.S., Barrowman N., et al. Long-term renal prognosis of diarrhoea-associated hemolytic uremic syndrome: a systematic review, meta-analysis and meta-regression. JAMA. 2003;290(10):1360-1370.
Kaplan B., Meyers K. The pathogenesis of hemolytic uremic syndrome. J Am Soc Nephrol. 1998;9:1126-1133.
Ray P., Liu X. Pathogenesis of Shiga toxin-induced hemolytic uremic syndrome. Pediatr Nephrol. 2001;16:823-839.
Ring G.H., Lakkis F.G., Badr K.F. Microvascular diseases of the kidney, 6th edn. Brenner B., editor. The kidney, vol. 2. Philadelphia: WB Saunders. 2000:1597-1603. Chapter 35
Siegler R.L. The hemolytic uremic syndrome. Pediatr Clin N Am. 1995;42(6):1505-1522.
Stewart C.L., Tina L.U. Hemolytic uremic syndrome. Pediatr Rev. 1993;14(6):218-224.
Tarr P.I., Gordon C.A., Chandler W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073-1086.
Trachtman H., Christen E. Pathogenesis, treatment and therapeutic trials in hemolytic uremic syndrome. Curr Opin Pediatr. 1999;11:162-168.