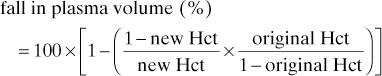H
H2 receptor antagonists. Competitive antagonists of H2 histamine receptors. Used to reduce histamine-mediated gastric acid secretion in: peptic ulcer disease; gastro-oesophageal reflux; those at risk of aspiration of gastric contents; and to reduce gastric/duodenal bleeding in patients in ICU. Their use in critically ill patients receiving enteral feeding is declining because they increase the risk of nosocomial chest infections. Have also been used with antihistamine drugs to reduce the severity of adverse drug reactions and other allergic responses involving histamine.
 cimetidine: introduced first. Cheapest, but with more side effects. Inhibits hepatic microsomal enzymes.
cimetidine: introduced first. Cheapest, but with more side effects. Inhibits hepatic microsomal enzymes.
 ranitidine: fewer side effects than cimetidine and does not cause hepatic enzyme inhibition. Longer duration of action.
ranitidine: fewer side effects than cimetidine and does not cause hepatic enzyme inhibition. Longer duration of action.
 nizatidine: similar to ranitidine.
nizatidine: similar to ranitidine.
Marik PE, Vasu T, Hirani A, Pachinburavan M (2010). Crit Care Med; 38: 2222–8
Haemaccel, see Gelatin solutions
Haematocrit (Hct). Total red cell volume as a proportion of blood volume. Slightly higher in venous than arterial blood because of entry of chloride ions (chloride shift) into red cells with accompanying water entry. An easily measured index of O2-carrying capacity of the blood, assuming normal red cell haemoglobin concentration and function. Normal values: 0.4–0.54 (male); 0.37–0.47 (female). Haemodilution to a Hct of 0.3–0.35 may be beneficial for tissue O2 delivery (e.g. in critically ill patients) because of reduced blood viscosity and increased flow; a value below this level is thought to compromise O2 delivery.
Haemoconcentration. Increase in haematocrit and haemoglobin concentration following dehydration or plasma loss. Degree of haemoconcentration may indicate the extent of fluid deficiency. Does not occur immediately after haemorrhage, since red cells and plasma are lost together; compensatory mechanisms restoring blood volume cause subsequent haemodilution. In prolonged severe ‘irreversible’ shock, however, fluid shifts into the interstitium with resulting haemoconcentration.
Haemodiafiltration. Modification of continuous arteriovenous or venovenous haemofiltration (CAVHD or CVVHD respectively) in order to improve efficiency and solute clearance rate. Circuitry and other aspects are identical to those for haemofiltration except that 1–2 l/h of dialysate fluid is allowed to run counter-current to the blood flow on the filtrate side of the haemofilter (Fig. 79). Solute is cleared by a combination of diffusion and convection.
Haemodialysis. Dialytic technique for removal of solutes and water from blood by their passage across a semipermeable membrane into dialysis fluid (dialysate). Indications include renal failure, fluid overload and pulmonary oedema, electrolyte disturbances, severe acidosis and some cases of drug poisoning and overdoses.
 vascular access: usually via a: ‘single needle’ single-lumen catheter (through which blood is withdrawn into the dialyser and then returned to the patient in an alternating cycle); double-lumen central venous catheter (or two single ones); Silastic arteriovenous shunt connecting adjacent vessels, e.g. radial artery/cephalic vein (Scribner shunt); or permanent arteriovenous fistula (see Shunt procedures).
vascular access: usually via a: ‘single needle’ single-lumen catheter (through which blood is withdrawn into the dialyser and then returned to the patient in an alternating cycle); double-lumen central venous catheter (or two single ones); Silastic arteriovenous shunt connecting adjacent vessels, e.g. radial artery/cephalic vein (Scribner shunt); or permanent arteriovenous fistula (see Shunt procedures).
 passage of blood via an extracorporeal circuit to a semipermeable cellophane membrane or hollow fibre system. Traditional cellulose-based membranes may be associated with complement activation and subsequent inflammatory cascade; thus newer synthetic membranes (e.g. polyacrylonitrile, polysulphone) are increasingly used, although more expensive. Dialysate may be passed on the other side of the membrane, usually in a counter-current fashion. Blood flow is usually 150–300 ml/min. The following are exchanged:
passage of blood via an extracorporeal circuit to a semipermeable cellophane membrane or hollow fibre system. Traditional cellulose-based membranes may be associated with complement activation and subsequent inflammatory cascade; thus newer synthetic membranes (e.g. polyacrylonitrile, polysulphone) are increasingly used, although more expensive. Dialysate may be passed on the other side of the membrane, usually in a counter-current fashion. Blood flow is usually 150–300 ml/min. The following are exchanged:
– solutes: pass by diffusion from blood to dialysate, depending on the concentration gradient, size (mw) of solute, membrane porosity and duration of dialysis. Thus fluids of different composition may be used to remove different amounts of solute as required. Most dialysis fluids contain sodium, chloride, calcium, magnesium, acetate or bicarbonate (as an alkali source; bicarbonate itself cannot be added directly since it may precipitate calcium and magnesium) and variable amounts of glucose and potassium. Solutes may also pass across the membrane by applying a hydrostatic pressure across the membrane, thereby removing water (ultrafiltration); solutes that can pass through the membrane pores are swept along with the water (solvent drag).
 anticoagulation of the extracorporeal circuit is required, e.g. with heparin or prostacyclin infused into the line upstream to the dialysis machine. Control of coagulation with infusion of protamine to the downstream line has been used but may be difficult.
anticoagulation of the extracorporeal circuit is required, e.g. with heparin or prostacyclin infused into the line upstream to the dialysis machine. Control of coagulation with infusion of protamine to the downstream line has been used but may be difficult.
Performed intermittently, e.g. for 4–6 h daily/weekly as required.
 technical, e.g. related to vascular access and bleeding, air embolism, clotting within the circuit. Modern machines usually incorporate alarms and monitors for air bubbles.
technical, e.g. related to vascular access and bleeding, air embolism, clotting within the circuit. Modern machines usually incorporate alarms and monitors for air bubbles.
 hypotension: may be related to hypovolaemia, disequilibrium syndrome or acetate in the dialysate (thought to cause vasodilatation and cardiac depression; replacement with bicarbonate has been suggested, although it is more complex to achieve).
hypotension: may be related to hypovolaemia, disequilibrium syndrome or acetate in the dialysate (thought to cause vasodilatation and cardiac depression; replacement with bicarbonate has been suggested, although it is more complex to achieve).
 hypoxaemia: mechanism is unclear.
hypoxaemia: mechanism is unclear.
[Belding H Scribner (1921–2003), Seattle nephrologist]
Haemodilution. Lowering of haematocrit and haemoglobin concentration due to fluid shift, retention or administration. May follow compensatory restoration of blood volume after haemorrhage, or iv fluid therapy with only partial replacement of red cell losses. Also occurs as a physiological process in pregnancy. Reduction of haematocrit lowers blood viscosity and increases blood flow, although O2 content falls. Optimal haematocrit following acute blood loss is thought to be about 0.3; in addition to improved tissue blood flow, hazards of blood transfusion and risk of DVT are reduced. Animal studies of cerebral ischaemia have suggested reduced infarct size if early haemodilution is achieved, although human evidence is lacking.
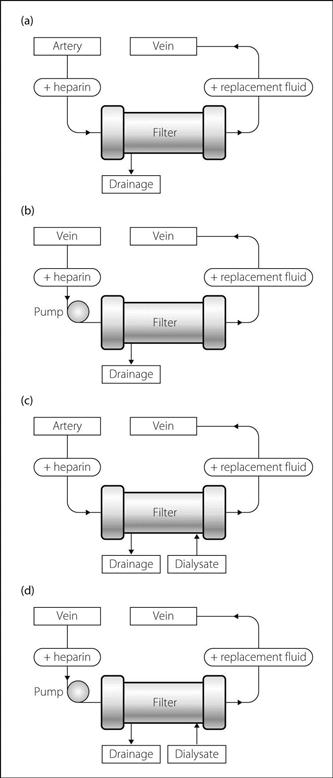
Haemofiltration. Common form of renal replacement therapy used in the ICU. First described in 1977; its main benefit over haemodialysis is cardiovascular stability. Initially described as continuous arteriovenous haemofiltration (CAVH; Fig. 79a) using an extracorporeal circuit via a surgically performed arteriovenous shunt (see Shunt procedures) or using large-bore arterial and venous cannulae. Blood flow through the circuit relies upon the arterial–venous pressure difference.
Now more frequently employs a continuous veno-venous circuit (CVVH; Fig. 79b) using a large-bore double-lumen venous cannula and a peristaltic roller pump that incorporates monitors to detect air embolism and extremes of circuit pressure. Blood is pumped at 100–200 ml/min. Anticoagulation of the extracorporeal circuit, but not the patient, is achieved using heparin (200–1000 U/h) or prostacyclin (2–10 ng/kg/min). Anticoagulation is not usually necessary if the patient has a coagulopathy.
Both CAVH and CVVH rely on the passage of blood through a filter containing a highly permeable membrane (polysulphone, polyamide or polyacrylonitrile; surface area 0.6–1.0 m2) that acts as an artificial glomerulus. Ultrafiltration occurs by virtue of the hydrostatic pressure gradient between the blood and ultrafiltrate sides of filter; solute removal occurs because of convection. Water and solutes lost from the plasma are replaced by haemofiltration fluid containing water and electrolytes. Ultrafiltration can be slow and titrated to the patient response. Replacement fluid is infused into the ‘arterial’ limb of the circuit (pre-dilution) or, more usually, into the ‘venous’ limb after the filter (post-dilution). Pre-dilution may be useful when there is a high filtrate removal rate (> 10 l/day) or a high haematocrit (> 35%) as it decreases viscosity and subsequent clotting in the circuit. However, pre-dilution decreases the efficiency of the system as the blood being filtered contains a lower concentration of waste products.
Both CAVH and CVVH require an exchange of 12–20 l/day to achieve adequate solute clearance. Filtration can be increased by applying a negative pressure to the filtrate side of the filter or by increasing the distance between the filter and filtrate collecting chamber. In haemodiafiltration, dialysate fluid is passed through the filter to improve efficiency and solute clearance rate (Fig. 79c and 79d).
Complications are related to the extracorporeal circuit and vascular access (air embolism, clotting, haemorrhage, complement activation, infection), ultrafiltration (hypovolaemia), electrolyte loss (hyponatraemia, hypocalcaemia), hypothermia, metabolic alkalosis (use of large volumes of lactate-rich replacement fluid) and removal of therapeutic drugs, including parenteral nutrition.
It has been suggested that CAVH and CVVH have a beneficial effect in sepsis by removing pro-inflammatory cytokines, although this is controversial.
Haemoglobin (Hb). Red-coloured pigment in erythrocytes, composed of:
– Hb A (adult): two α chains, two β chains.
– Hb A2 (usually 2–3%): two α chains, two δ chains.
– Hb F (fetal): two α chains, two γ chains.
There are 141 amino acid residues in α chains; 146 in β, δ and γ chains.
Fetal Hb is normally replaced by Hb A within 6 months of birth, unless polypeptide chain production is abnormal, e.g.:
– thalassaemia: reduced synthesis of normal chains.
– haemoglobinopathies, e.g. sickle cell anaemia: abnormal β chains are synthesised.
 haem: porphyrin derivative containing iron in the ferrous (Fe2+) state. One haem moiety, containing one iron atom, is conjugated to each polypeptide. Oxidation of the iron to the ferric (Fe3+) state forms methaemoglobin (high levels of which cause methaemoglobinaemia).
haem: porphyrin derivative containing iron in the ferrous (Fe2+) state. One haem moiety, containing one iron atom, is conjugated to each polypeptide. Oxidation of the iron to the ferric (Fe3+) state forms methaemoglobin (high levels of which cause methaemoglobinaemia).
 the iron atom in each haem moiety, remaining in the ferrous state but sharing one of its electrons, can reversibly bind one O2 molecule, forming oxyhaemoglobin. Thus each Hb molecule can bind four O2 molecules. In its deoxygenated form, the Hb molecule exists in a ‘taut’ configuration. Binding of one O2 molecule breaks salt linkages between α- and β-globin chains and produces a more ‘relaxed’ configuration. This results in increased affinity for further binding (cooperativity), resulting in the sigmoid-shaped oxyhaemoglobin dissociation curve. Affinity is reduced by increasing PCO2 (Bohr effect), acidity, temperature and amount of 2,3-DPG present. Fetal Hb has greater affinity for O2 than has adult Hb.
the iron atom in each haem moiety, remaining in the ferrous state but sharing one of its electrons, can reversibly bind one O2 molecule, forming oxyhaemoglobin. Thus each Hb molecule can bind four O2 molecules. In its deoxygenated form, the Hb molecule exists in a ‘taut’ configuration. Binding of one O2 molecule breaks salt linkages between α- and β-globin chains and produces a more ‘relaxed’ configuration. This results in increased affinity for further binding (cooperativity), resulting in the sigmoid-shaped oxyhaemoglobin dissociation curve. Affinity is reduced by increasing PCO2 (Bohr effect), acidity, temperature and amount of 2,3-DPG present. Fetal Hb has greater affinity for O2 than has adult Hb.
 CO2 may bind reversibly to amino groups of the polypeptide chains, forming carbamino compounds (RNH2 + CO2 → RNHCO2H). Deoxygenated Hb has a greater affinity for CO2 than oxygenated (Haldane effect).
CO2 may bind reversibly to amino groups of the polypeptide chains, forming carbamino compounds (RNH2 + CO2 → RNHCO2H). Deoxygenated Hb has a greater affinity for CO2 than oxygenated (Haldane effect).
 imidazole groups of histidine residues act as buffers in the blood and provide a large buffering capacity owing to their abundance. Deoxygenated Hb is a weaker acid and better buffer than oxygenated.
imidazole groups of histidine residues act as buffers in the blood and provide a large buffering capacity owing to their abundance. Deoxygenated Hb is a weaker acid and better buffer than oxygenated.
– with carbon monoxide, forming carboxyhaemoglobin.
– formation of methaemoglobin.
– causing sulphaemoglobinaemia.
– prolonged exposure to raised glucose levels in diabetes mellitus, forming glycosylated Hb.
Normal blood Hb concentration is 13–17 g/dl (men), 12–16 g/dl (women).
Hsia CCW (1998). N Engl J Med; 338: 239–47 – old but still the best
See also, Anaemia; Carbon dioxide transport; Carbon monoxide poisoning; Methaemoglobinaemia; Myoglobin; Oxygen transport; Polycythaemia
Haemoglobinopathies. Diseases of abnormal haemoglobin production (cf. thalassaemias: impaired production of normal haemoglobin). Over 300 variants have been described, mostly due to single amino acid substitutions. Originally named after letters of the alphabet, then after the place of origin of the first patient described. Most are clinically insignificant, but some may lead to acute or chronic haemolysis, and some are associated with impaired O2 binding and secondary polycythaemia. Sickle cell anaemia is the most important; it may be combined with other abnormalities, e.g. haemoglobin C. The latter on its own may cause mild haemolytic anaemia and splenomegaly.
Haemolysis. Abnormal destruction of erythrocytes. Normal red cell survival is about 120 days; bone marrow compensation may restore red cell volume if the lifespan is shortened. Anaemia may result if haemolysis is excessive, bone marrow abnormal, or haematinics (e.g. iron) are deficient. Haemolysis may result in jaundice, decreased haptoglobin concentration (see below) and reticulocytosis.
 genetic red cell abnormalities:
genetic red cell abnormalities:
– membrane abnormalities, e.g. hereditary spherocytosis, elliptocytosis.
– haemoglobinopathies, thalassaemia.
– enzyme deficiencies, e.g. glucose 6-phosphate dehydrogenase deficiency, pyruvate kinase deficiency.
– immune:
– primary.
– malignancy.
– infection, e.g. viral, mycoplasma.
– drugs, e.g. penicillins, methyldopa, rifampicin, sulphonamides.
– incompatible blood transfusion (including rhesus blood group incompatibility).
– infection, e.g. malaria, generalised sepsis.
– drugs, e.g. sulphonamides, phenacetin.
– trauma, e.g. prosthetic heart valves, extracorporeal circuits. Also associated with red cell damage following contact with vasculitic endothelium (e.g. haemolytic–uraemic syndrome).
– burns.
– paroxysmal nocturnal haemoglobinuria.
 extravascular: most common type; involves sequestration of red cells from the circulation.
extravascular: most common type; involves sequestration of red cells from the circulation.
Haemolytic–uraemic syndrome. Acquired condition involving thrombocytopenia, a microangiopathic haemolytic anaemia and endothelial injury to the renal vasculature, leading to acute kidney injury. Usually occurs in children, especially following diarrhoea (usually due to Shiga toxin-producing Escherichia coli) or upper respiratory infection, but may occur in adults. May occur in cancer, infections and during chemotherapy administration. Closely related to thrombotic thrombocytopenic purpura, but neurological features such as CVA that characterise the latter are uncommon. A similar condition may occur postpartum or in women taking the contraceptive pill.
Treatment is mainly supportive. Although heparin and prostacyclin have been used, their benefit is unproven. Plasmapheresis and immunosuppressive drugs have also been used.
Haemoperfusion. Removal of toxic substances from plasma by adsorption on to special filters, e.g. amberlite resin, activated charcoal granules coated in acrylic gel or cellulose. Performed in poisoning and overdoses, and hepatic failure. Modern devices are extremely efficient; complete removal of toxin from the body is limited by tissue binding. Thus haemoperfusion is most effective for poisons with small volumes of distribution, e.g. barbiturates, disopyramide, theophylline, meprobamate and methaqualone; these are rarely taken in overdose. Tricyclic antidepressants are not removed. Requires vascular cannulation (e.g. femoral vein), extracorporeal circuit and heparinisation. Blood flow of 100–200 ml/min is employed, continued for several hours according to the clinical condition or plasma toxin levels.
Complications: as for dialysis. Thrombocytopenia was common with earlier adsorption columns.
Haemophilia. X-linked recessive coagulation disorder with an incidence (type A) of 1 : 5000–10 000. One-third of cases are new mutations. Predominantly affects males, although female carriers may exhibit mild disease. Female homozygotes almost always die in utero. Results in deficiency of factor VIII (haemophilia A) or IX (haemophilia B; Christmas disease; one-tenth as common), leading to increased bleeding into muscles, joints and internal organs. The intrinsic coagulation pathway is slowed, with activated partial thromboplastin time prolonged; prothrombin and bleeding times are normal. Specific factor VIII/IX assay reveals reduced activity, and von Willebrand factor assay is normal.
• Intensive care/anaesthetic considerations:
– factor VIII is given as a concentrate (preferred), as cryoprecipitate, or as fresh frozen plasma, with haematological advice and monitoring of blood levels. Half-life is 8–12 h; adequate levels are required for at least a week postoperatively. About 15% of patients have circulating antibodies to factor VIII or IX, making control more difficult; eptacog alfa may be useful in such cases.
Desmopressin 0.4 µg/kg iv may transiently increase levels of factor VIII by 3–6 times in mild cases, and tranexamic acid 1 g orally may also be given.
– care should be taken with any invasive procedure, including venesection or arterial blood sampling.
– NSAIDs and antiplatelet drugs should be avoided.
 high risk of HIV infection in haemophiliacs given pooled factor VIII before the availability of recombinant factor VIII in the mid/late 1980s and of recombinant factor IX in 1997.
high risk of HIV infection in haemophiliacs given pooled factor VIII before the availability of recombinant factor VIII in the mid/late 1980s and of recombinant factor IX in 1997.
[Stephen Christmas (1947–1993); name of British patient in whom the disease was first described]
Finjvandraat K, Cnossen MH, Leebeek FWG, Peters M (2012). Br Med J; 344: 36–40
Haemorrhage. Physiological effects of acute haemorrhage:
 blood volume is reduced, leading to reduced venous return and cardiac output.
blood volume is reduced, leading to reduced venous return and cardiac output.
 arterial BP falls, with activation of the baroreceptor reflex, reduced parasympathetic activity and increased sympathetic activity. Tachycardia, peripheral arterial vasoconstriction (to skin, viscera and kidneys) and venous constriction restore BP, initially. Classified in ATLS guidelines as follows:
arterial BP falls, with activation of the baroreceptor reflex, reduced parasympathetic activity and increased sympathetic activity. Tachycardia, peripheral arterial vasoconstriction (to skin, viscera and kidneys) and venous constriction restore BP, initially. Classified in ATLS guidelines as follows:
– I: up to 15% of blood volume lost (usually little physiological change).
– II: 15–30% lost (tachycardia, peripheral vasoconstriction, postural hypotension).
– III: 30–40% lost (hypotension, mental confusion, maximum tachycardia).
– IV: > 40% lost (cardiovascular collapse and shock). Bradycardia and hypotension may occur with over 20–30% of loss; thought to be vagally mediated, due to cardiac afferent C-fibre discharge caused by ventricular distortion and underfilling.
 increased vasopressin secretion and renin/angiotensin system activity causes vasoconstriction, sodium and water retention and thirst.
increased vasopressin secretion and renin/angiotensin system activity causes vasoconstriction, sodium and water retention and thirst.
 catecholamine and corticosteroid secretion increase as part of the stress response.
catecholamine and corticosteroid secretion increase as part of the stress response.
 increased movement of interstitial fluid to the intravascular compartment and third space.
increased movement of interstitial fluid to the intravascular compartment and third space.
 increased 2,3-DPG production, increasing tissue O2 delivery.
increased 2,3-DPG production, increasing tissue O2 delivery.
 increased plasma protein synthesis.
increased plasma protein synthesis.
 increased erythropoietin secretion and erythropoiesis.
increased erythropoietin secretion and erythropoiesis.
Volume restoration takes 1–3 days after moderate haemorrhage, with reduction of haematocrit and plasma protein concentration.
• Features: as for hypovolaemia.
 large-bore intravenous cannulae and iv fluid administration:
large-bore intravenous cannulae and iv fluid administration:
– cross-matched blood is best (but some benefit in cardiac output and tissue flow is derived from haemodilution).
– colloid maintains intravascular expansion for longer than crystalloid.
– crystalloid: saline is more effective than dextrose.
– CVP and urine output measurement are useful for monitoring volume replacement.
See also, Blood loss, perioperative; Blood transfusion; Colloid/crystalloid controversy; Damage control resuscitation
Haemostasis, see Coagulation
Hagen–Poiseuille equation. For laminar flow of a fluid of viscosity η through a tube of length L and radius r, with pressure gradient P across the length of the tube:
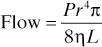
Originally derived by observing flow of liquid through rigid cylinders of different dimensions, with different driving pressures. Applied to blood flow through blood vessels, and gas flow through breathing systems and airways, although these tubes are neither rigid nor perfect cylinders.
[Jean Poiseuille (1797–1869), French physiologist;
Gotthilf HL Hagen (1797–1884), German engineer]
Haldane effect. Increased capacity of deoxygenated blood for CO2 transport compared with oxygenated blood.
 increased binding of reduced haemoglobin to CO2, forming carbamino groups (accounts for 70% of the effect).
increased binding of reduced haemoglobin to CO2, forming carbamino groups (accounts for 70% of the effect).
Half-life (t1/2). The time taken for a substance undergoing decay to decrease by half. Commonly used to describe an exponential process (in which the half-life is constant), but may also refer to a non-exponential process (in which the half-life varies).
 radioactive half-life (time taken for half the number of atoms to disintegrate).
radioactive half-life (time taken for half the number of atoms to disintegrate).
 drug half-life (time taken for drug concentration to fall by half, whether resulting from redistribution or clearance).
drug half-life (time taken for drug concentration to fall by half, whether resulting from redistribution or clearance).
In pharmacokinetics, context-sensitive half-life refers to the time for plasma concentration of a drug to decrease by 50% after terminating an iv infusion that has maintained steady-state plasma concentration. For example, for propofol it is approximately 20 min after 2 h infusion, 30 min after 6 h infusion and 50 min after 9 h infusion. Corresponding figures for midazolam and alfentanil are in the order of 40 min, 70 min and 80 min; for fentanyl: 40 min, 4 h and 5 h. Ultra-short-acting drugs are less affected by ‘context’ (i.e. duration of infusion); for example, for remifentanil it is approximately 3 min, irrespective of the duration of the infusion.
Hall, Richard, see Halsted, William Stewart
Haloperidol. Butyrophenone used as a sedative and antipsychotic drug. Acts by blocking central dopamine receptors. Also acts on cholinergic, serotonergic, histaminergic and α-adrenergic receptors. Has tranquillising effects without impairing consciousness; used to sedate psychotic patients in ICU. Half-life is approximately 20 h.
• Dosage:
 1.5–5.0 mg orally bd/tds; up to 100 mg may be needed in severe schizophrenia.
1.5–5.0 mg orally bd/tds; up to 100 mg may be needed in severe schizophrenia.
• Side effects include prolonged Q–T syndromes, extrapyramidal symptoms (parkinsonism, dystonia, restlessness and tardive dyskinesia); can trigger the neuroleptic malignant syndrome. Other side effects are similar to those of chlorpromazine, but with less sedation and fewer antimuscarinic effects.
Halothane. 2-Bromo-2-chloro-1,1,1-trifluoroethane (Fig. 80). Inhalational anaesthetic agent, introduced in 1956. Its use rapidly spread because of its greater potency, ease of use, non-irritability and non-inflammability compared with diethyl ether and cyclopropane. Risks of arrhythmias and liver damage on repeated administration (halothane hepatitis) and introduction of newer agents (e.g. sevoflurane, which has replaced halothane as the agent of choice for inhalational induction) have led to a decline in its use. Discontinued for human use in the UK in 2007.
 colourless liquid; vapour has characteristic pleasant smell and is 6.8 times denser than air.
colourless liquid; vapour has characteristic pleasant smell and is 6.8 times denser than air.
 SVP at 20°C 32 kPa (243 mmHg).
SVP at 20°C 32 kPa (243 mmHg).
 MAC 0.76%.
MAC 0.76%.
 supplied in liquid form with thymol 0.01%; decomposes slightly in light.
supplied in liquid form with thymol 0.01%; decomposes slightly in light.
• Effects:
– smooth rapid induction, with rapid recovery.
– increases cerebral blood flow but reduces intraocular pressure.
– respiratory depressant, with increased respiratory rate and reduced tidal volume.
– bronchodilatation and inhibition of secretions.
– myocardial O2 demand decreases.
– arrhythmias are common, e.g. bradycardia, nodal rhythm, ventricular ectopics/bigemini.
– sensitises the myocardium to catecholamines, e.g. endogenous or injected adrenaline.
– dose-dependent uterine relaxation.
Up to 20% is metabolised in the liver. Metabolites include bromine, chlorine and trifluoroacetic acid; negligible amounts of fluoride ions are produced. Repeat administration after recent use may result in hepatitis.
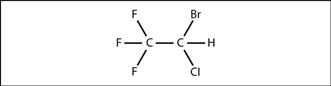
Fig. 80 Structure of halothane
‘Halothane shakes’, see Shivering, postoperative
Halsted, William Stewart (1852–1922). US surgeon, pioneer of local anaesthetic nerve blocks with Hall and others. He and Hall described blocks of most of the nerves of the face, head and limbs, experimenting on each other and becoming cocaine addicts in the process. Chief of surgery and professor at Johns Hopkins University, where he started the first formal surgical training programme in the USA. Pioneer of aseptic technique, introducing use of rubber gloves during surgery.
Hamburger shift, see Chloride shift
Hanging drop technique. Method of identifying the epidural space, e.g. during epidural or spinal anaesthesia. A drop of saline is placed at the hub of a needle that is advanced towards the epidural space; when the space has been entered the drop is drawn into the needle by the negative pressure within the space. Not always reliable, since negative pressure is not always present.
Haptoglobin. Alpha-globulin synthesised by the liver, that binds free haemoglobin in the blood. Normal serum level is 30–190 mg/dl; this usually binds 100–140 mg free haemoglobin per 100 ml plasma. Haptoglobin–haemoglobin complex is rapidly removed from the circulation by the reticuloendothelial system; if the liver is unable to produce new haptoglobin quickly enough, the plasma haptoglobin level falls. Although the reduction in haptoglobin is used mainly as a sensitive indicator of intravascular haemolysis, it may also occur if haemolysis is extravascular.
Harmonics. Related sine waveforms; the frequency of each is a multiple of the fundamental frequency of the first harmonic, the slowest component of the series. Complex waveforms may be produced by adding higher harmonics to the first (fundamental) harmonic (Fourier analysis). Monitoring equipment must be able to reproduce harmonics of high enough frequency for the signal recorded; e.g. up to the 10th harmonic for many recorders. More harmonics are required for more complex waveforms with higher frequencies, increasing the required frequency response of the monitor concerned, e.g. ECG 0.5–80 Hz, EEG 1–60 Hz, EMG 2–1200 Hz.
Harnesses. Used to secure breathing attachments or facemasks to the patient. May damage soft tissues around the face, and airway obstruction may still occur.
Formerly widely used during ‘mask’ anaesthesia, their use has declined with increasing use of the LMA, though many modern facemasks are still supplied with plastic hooks for attachment. Their disposable silicone/rubber equivalents are used for non-invasive positive pressure ventilation or CPAP.
Hartmann’s solution (Ringer’s lactate; Compound sodium lactate). IV fluid containing sodium 131 mmol/l, potassium 5 mmol/l, calcium 2 mmol/l, chloride 111 mmol/l and sodium lactate 29 mmol/l. pH is 5–7. Originally formulated from Ringer’s solution for fluid replacement and treatment of metabolic acidosis in children, using an isotonic solution containing more sodium than chloride. Lactate is metabolised to glucose and bicarbonate within a few hours, and the hazards of bicarbonate administration avoided. Now widely used as the crystalloid of choice for ECF replacement, since it is more ‘physiological’ in make-up; however, its advantage over saline solutions for routine use has been questioned.
Often avoided in patients with renal failure because of the risk of hyperkalaemia, in sick patients or those with hepatic failure because of the risk of hyperlactataemia (although the clinical relevance of this is unclear), and in diabetics because of the risk of hyperglycaemia (although the actual increase in blood glucose concentration is likely to be small). Due to its calcium content, may cause clotting of stored blood when transfused through a line that has not first been flushed with saline.
Hayek oscillator, see Intermittent negative pressure ventilation
Hb, see Haemoglobin
Hct, see Haematocrit
HDU, see High dependency unit
Head injury. Common cause of morbidity and mortality in trauma, especially in young males; it should be suspected in all trauma cases, especially those involving the chest and neck. Divided into closed or penetrating (the latter having worse outcomes), whilst brain injury can be divided into:
 that occurring after the initial injury (secondary) resulting from potentially preventable or treatable causes (e.g. hypoxaemia, hypotension, hypercapnia). Compression due to cerebral oedema or intracerebral, intraventricular, epidural or subdural haemorrhage can be included here, although the first two are usually associated with direct brain injury.
that occurring after the initial injury (secondary) resulting from potentially preventable or treatable causes (e.g. hypoxaemia, hypotension, hypercapnia). Compression due to cerebral oedema or intracerebral, intraventricular, epidural or subdural haemorrhage can be included here, although the first two are usually associated with direct brain injury.
 external signs of head injury, e.g. bruising, lacerations. Cervical spine fractures or serious ligamentous injuries should be assumed until proven otherwise. Chest trauma and other injuries may be present.
external signs of head injury, e.g. bruising, lacerations. Cervical spine fractures or serious ligamentous injuries should be assumed until proven otherwise. Chest trauma and other injuries may be present.
 impaired consciousness and amnesia. Often associated with alcohol or other cause of coma. Brainstem death may occur.
impaired consciousness and amnesia. Often associated with alcohol or other cause of coma. Brainstem death may occur.
 pupils: the third cranial nerve on the side of an expanding cerebral lesion is stretched over the edge of the tentorium cerebelli, causing ipsilateral dilatation and absence of the direct light reflex with the consensual light reflex preserved. Eventually, the contralateral pupil is also affected.
pupils: the third cranial nerve on the side of an expanding cerebral lesion is stretched over the edge of the tentorium cerebelli, causing ipsilateral dilatation and absence of the direct light reflex with the consensual light reflex preserved. Eventually, the contralateral pupil is also affected.
 bradycardia and hypertension may occur (Cushing’s reflex), and irregular respiration as compression continues.
bradycardia and hypertension may occur (Cushing’s reflex), and irregular respiration as compression continues.
– disturbance of CSF dynamics, causing CSF leak (rhinorrhoea or otorrhoea) or hydrocephalus.
– respiratory, e.g. aspiration pneumonitis, infection, PE, pulmonary oedema, ARDS.
– diabetes insipidus or syndrome of inappropriate antidiuretic hormone secretion, cerebral salt-wasting syndrome.
– DIC.
 as for coma and trauma, i.e. CPR: O2 administration, maintenance of airway, breathing, circulation. The Glasgow coma scale and the simpler AVPU scale are widely used for assessment. Frequent reassessment is important.
as for coma and trauma, i.e. CPR: O2 administration, maintenance of airway, breathing, circulation. The Glasgow coma scale and the simpler AVPU scale are widely used for assessment. Frequent reassessment is important.
 opioid analgesic drugs should be used with caution in spontaneously breathing patients, because of the risk of central and respiratory depression and their effects on the pupils.
opioid analgesic drugs should be used with caution in spontaneously breathing patients, because of the risk of central and respiratory depression and their effects on the pupils.
 cervical spine and skull X-rays to identify fractures, and CT scanning to identify haemorrhage, oedema and evidence of raised intracranial pressure. Ultrasound has been used to identify midline shift, before subsequent CT scanning.
cervical spine and skull X-rays to identify fractures, and CT scanning to identify haemorrhage, oedema and evidence of raised intracranial pressure. Ultrasound has been used to identify midline shift, before subsequent CT scanning.
– unconsciousness or amnesia at any time.
– difficulty in assessment, e.g. confusion, alcohol.
– deterioration in conscious level, pupillary signs or other observations.
– fractured skull and confusion.
 criteria for consulting a neurosurgeon:
criteria for consulting a neurosurgeon:
– coma persisting after resuscitation (i.e. Glasgow coma score [GCS] < 8).
– deterioration in conscious level or the development of other neurological signs.
– persistent confusion, even without a skull fracture.
– compound depressed skull fracture.
– suspected base of skull fracture.
Transfer for CT scan requires tracheal intubation if conscious level is depressed, i.e. GCS ≤ 8.
 if injury is severe, tracheal intubation and IPPV are performed, traditionally maintaining arterial PCO2 at about 4–4.5 kPa to reduce ICP and cerebral oedema. During intubation, steps should be taken to prevent aspiration of gastric contents or worsening of any associated cervical spine injury. Continued hyperventilation is less often employed now as it is recognised that in head injury cerebral blood flow may already be reduced and aggressive hyperventilation may result in excessive cerebral vasoconstriction and cerebral ischaemia; PCO2 is therefore acutely lowered by hyperventilation only to offset any acute increases in ICP. Other indications for IPPV include:
if injury is severe, tracheal intubation and IPPV are performed, traditionally maintaining arterial PCO2 at about 4–4.5 kPa to reduce ICP and cerebral oedema. During intubation, steps should be taken to prevent aspiration of gastric contents or worsening of any associated cervical spine injury. Continued hyperventilation is less often employed now as it is recognised that in head injury cerebral blood flow may already be reduced and aggressive hyperventilation may result in excessive cerebral vasoconstriction and cerebral ischaemia; PCO2 is therefore acutely lowered by hyperventilation only to offset any acute increases in ICP. Other indications for IPPV include:
– respiratory irregularity, hypo- or hyperventilation.
– uncontrolled convulsions, raised ICP or hyperthermia.
– extensor or flexor posturing.
Other measures to prevent a high ICP and reduce cerebral O2 requirements include:
– heavy sedation + neuromuscular blockade (the latter has been implicated in increased mortality but the evidence is weak). Thiopental coma or other forms of cerebral protection/resuscitation are sometimes employed, although controversial.
– use of diuretics to reduce cerebral oedema (e.g. mannitol). Recent evidence (CRASH trial: corticosteroid randomisation after significant head injury) suggests that corticosteroids may increase mortality.
– hyperglycaemia may worsen outcome and glycaemic control should be instituted.
– prophylactic use of anticonvulsant drugs is controversial but is common if craniotomy is required or if the cerebral perfusion pressure falls.
 in some ICUs, ICP monitoring is undertaken and cerebral perfusion pressure calculated. EEG and related monitoring, evoked potentials, transcranial Doppler ultrasound and jugular bulb catheterisation have been used to follow progress.
in some ICUs, ICP monitoring is undertaken and cerebral perfusion pressure calculated. EEG and related monitoring, evoked potentials, transcranial Doppler ultrasound and jugular bulb catheterisation have been used to follow progress.
 other drug therapy includes antibiotic cover for CSF leaks and prophylaxis for stress ulcers (e.g. pantoprazole sodium).
other drug therapy includes antibiotic cover for CSF leaks and prophylaxis for stress ulcers (e.g. pantoprazole sodium).
• Anaesthesia for patients with head injuries: as for neurosurgery and emergency surgery. In particular:
Moppett IK (2007). Br J Anaesth; 99: 18–31
Helmy A, Vizcaychipi M, Gupta AK (2007). Br J Anaesth; 99: 32–42
See also, Cerebral metabolic rate for oxygen; Coning; Maxillofacial surgery
Health and safety issues, see COSHH regulations; Environmental safety of anaesthetists; Scavenging
Healthcare Commission. Non-governmental organisation, formed in 2003–4 from the Commission for Health Improvement (CHI), the Audit Commission (audits NHS organisations and evaluates their cost-effectiveness) and the National Care Standards Commission (regulates independent hospitals and care homes). Its remit was the improvement of patient care by assessing NHS organisations, investigating serious failures, ensuring compliance with national guidelines and advising on best practice in clinical governance.
Abolished in 2009, with its responsibilities assumed by the Care Quality Commission.
Healthcare Quality Improvement Partnership (HQIP). Body established in 2008 and contracted by the UK Department of Health to oversee national audits, registers and confidential enquiries. Led by a consortium including the Academy of Medical Royal Colleges, the Royal College of Nursing and patient representatives. Programmes of relevance to anaesthesia include audits/registers of various cancers, paediatric intensive care, cardiac surgery/cardiology, pain, carotid endartectomy, hip fracture, and the Confidential Enquiries into Maternal Deaths.
Heart. Develops from a single tube that doubles up forming primitive atrium and ventricle; divided by septa into left and right sides (see Atrial septal defect; Ventricular septal defect). The primitive arterial outlet (truncus arteriosus) splits to form the aorta and pulmonary trunk; the venous inlet (truncus venosus) absorbs into the smooth-walled part of the right atrium.
 right border: from third to sixth right costal cartilages, 1–1.5 cm from the left sternal edge.
right border: from third to sixth right costal cartilages, 1–1.5 cm from the left sternal edge.
The left border is composed mainly of left ventricle, the right border mainly of right atrium, and the base mainly of right ventricle, as on the CXR. Weighs about 300 g. Enclosed within pericardium.
– bears the right auricular appendage (remnant of the original atrium).
– receives superior and inferior venae cavae, and coronary sinus.
– crescent-shaped in cross-section, due to bulging of the left ventricle.
– pulmonary valve: composed of three cusps.
 left atrium: receives four valveless pulmonary veins.
left atrium: receives four valveless pulmonary veins.
– aortic valve: composed of three semilunar cusps, one anterior and two posterior.
• Blood supply: see Coronary circulation
• Nerve supply: from vagus and sympathetic nervous system from upper thoracic and cervical ganglia; mainly T1–4. The cardiac plexus receives branches from both components of the autonomic nervous system.
[Antoine Louis (1723–1792), French surgeon]
See also, Action potential; Atrial …; Cardiac …; Coronary …; Heart …; Left ventricular …; Myocardial …; Ventricular …
Heart, artificial. Device used to replace or assist the heart in end-stage heart failure when heart transplantation is unavailable, or following cardiac surgery.
Shah KB, Tang DG, Cooke RH, et al. (2011). Clin Cardiol; 34: 147–52
Heart block. Usually refers to atrioventricular (AV) block, i.e. interruption of impulse propagation between atria and ventricles. Bundle branch block refers to interruption distal to the atrioventricular node.
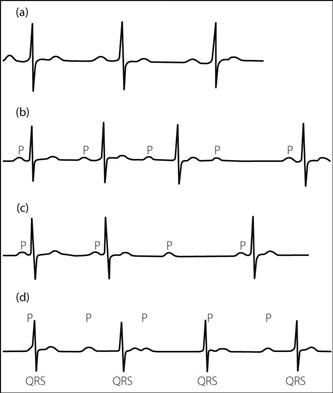
 first-degree (Fig. 81a): delay at the AV node:
first-degree (Fig. 81a): delay at the AV node:
– P–R interval > 0.2 s at normal heart rate.
– usually clinically insignificant.
– ageing.
– drugs, e.g. halothane, digoxin.
– cardiomyopathy, myocarditis.
 second-degree: occasional complete block. May be:
second-degree: occasional complete block. May be:
– Mobitz type I (Wenckebach phenomenon) (Fig. 81b):
– AV delay; P–R interval lengthens with successive P waves until complete AV block occurs, i.e. no QRS complex follows the P wave. The cycle then repeats.
– usually due to AV conduction delay.
– rarely proceeds to complete heart block.
– at risk of developing complete heart block, particularly if it occurs regularly.
 third-degree (Fig. 81d): complete heart block, at the AV node or below:
third-degree (Fig. 81d): complete heart block, at the AV node or below:
– ECG demonstrates independent atrial and ventricular activity, the latter arising from an ectopic site. The ventricular rate is usually slow, especially if the ectopic site is far from the AV node (wide QRS complexes; rate < 45/min).
– wide pulse pressure (large stroke volume).
– escape ventricular arrhythmias may occur.
– old age.
For patients undergoing surgery who have pre-existing complete heart block or predisposing conditions, a pacing wire should be inserted preoperatively. Drugs decreasing AV conduction (e.g. β-receptor antagonists and halothane) should be avoided. Isoprenaline 0.02–0.2 µg/kg/min may be used to increase ventricular rate in complete heart block, and should be available, as should a back-up pacing box. Cardiac output should be maintained where possible. Antiarrhythmic drugs suppressing ventricular activity should be avoided.
[Karl Wenckebach (1848–1904), Dutch physician;
Woldemar Mobitz (1889–1951), German cardiologist]
Heart, conducting system. Composed of:
 atrioventricular node (AV node): lies in the atrial septum, above the coronary sinus opening. Normally the only means of conduction between atria and ventricles; the bundle of Kent is an abnormal accessory conduction pathway that bypasses the AV node, and is present in Wolff–Parkinson–White syndrome.
atrioventricular node (AV node): lies in the atrial septum, above the coronary sinus opening. Normally the only means of conduction between atria and ventricles; the bundle of Kent is an abnormal accessory conduction pathway that bypasses the AV node, and is present in Wolff–Parkinson–White syndrome.
– the left bundle branch divides into anterior and posterior fascicles.
– Purkinje fibres spread from the ends of bundle branches/fascicles to the rest of the ventricles.
Interruption of impulse conduction may result in bundle branch block and heart block.
Heart failure, see Cardiac failure
Heart–lung transplantation. First performed in 1968 but with poor results; outcomes have steadily improved since the 1980s. In the UK ~50 are performed each year, with 1-year survival ~85%. Isolated lung transplantation initially produced poor results but interest has increased more recently.
• Indications include: pulmonary hypertension, Eisenmenger’s syndrome, pulmonary fibrosis, cystic fibrosis and emphysema.
• Donor:
 as for heart transplantation, with normal CXR, minimal sputum and no history of aspiration or pulmonary oedema. Arterial PO2 should exceed 13.3 kPa (100 mmHg) with FIO2 0.4.
as for heart transplantation, with normal CXR, minimal sputum and no history of aspiration or pulmonary oedema. Arterial PO2 should exceed 13.3 kPa (100 mmHg) with FIO2 0.4.
 immunosuppression and general management as for heart transplantation.
immunosuppression and general management as for heart transplantation.
 PEEP and inotropes are usually required; isoprenaline is particularly useful because of its ability to reduce pulmonary vascular resistance. Pulmonary oedema and sputum retention are common. The cough reflex is destroyed and ciliary activity impaired.
PEEP and inotropes are usually required; isoprenaline is particularly useful because of its ability to reduce pulmonary vascular resistance. Pulmonary oedema and sputum retention are common. The cough reflex is destroyed and ciliary activity impaired.
 weaning from IPPV is as for cardiac surgery.
weaning from IPPV is as for cardiac surgery.
 lung rejection may occur without heart rejection, and may be difficult to detect. Graft-versus-host disease may also occur.
lung rejection may occur without heart rejection, and may be difficult to detect. Graft-versus-host disease may also occur.
Heart murmurs. General principles:
 systolic murmurs may be physiological; diastolic murmurs are always pathological.
systolic murmurs may be physiological; diastolic murmurs are always pathological.
 low murmurs are heard best with the stethoscope bell, high-pitched ones with the diaphragm.
low murmurs are heard best with the stethoscope bell, high-pitched ones with the diaphragm.
 left-sided murmurs are heard best at end-expiration; right-sided ones best at end-inspiration.
left-sided murmurs are heard best at end-expiration; right-sided ones best at end-inspiration.
 murmurs radiate usually in the direction of turbulent flow.
murmurs radiate usually in the direction of turbulent flow.
 when auscultating the heart, murmurs are best described by:
when auscultating the heart, murmurs are best described by:
– VSD.
– aortic stenosis or sclerosis (although severe disease can produce a pansystolic murmur).
– ASD.
See also, Cardiac cycle; Preoperative assessment; Pulmonary valve lesions; Tricuspid valve lesions
Heart rate. Normal range in adults is usually defined as 60–100 beats/min, but it may be less than 50 beats/min in fit young subjects, and increase up to 200 beats/min on exercise. Rate is about 100 beats/min in the denervated heart. In children, heart rate decreases with age from 110–160 beats/min during infancy, reaching adult values at about 12 years.
 sympathetic activity, e.g. secondary to hypotension, hypoxaemia, hypercapnia, pain, fear, anger, exercise.
sympathetic activity, e.g. secondary to hypotension, hypoxaemia, hypercapnia, pain, fear, anger, exercise.
 hormones, e.g. catecholamines, thyroxine.
hormones, e.g. catecholamines, thyroxine.
 Bainbridge reflex and inspiration.
Bainbridge reflex and inspiration.
 parasympathetic activity via the vagus nerve, e.g. secondary to the baroreceptor reflex, fear, pain, raised ICP, expiration.
parasympathetic activity via the vagus nerve, e.g. secondary to the baroreceptor reflex, fear, pain, raised ICP, expiration.
See also, Arrhythmias; Pacemaker cells; Sinus arrhythmia; Sinus bradycardia; Sinus rhythm; Sinus tachycardia
 quiet in obese or well-built subjects. Also in pericarditis.
quiet in obese or well-built subjects. Also in pericarditis.
 second sound is quiet in aortic stenosis, since valve mobility is reduced.
second sound is quiet in aortic stenosis, since valve mobility is reduced.
 first sound is quiet in mitral regurgitation, since valve closure is incomplete.
first sound is quiet in mitral regurgitation, since valve closure is incomplete.
 first sound is increased in mitral stenosis, since the valve is kept open right up to systole by increased left atrial pressure, instead of gradual closure at end of diastole.
first sound is increased in mitral stenosis, since the valve is kept open right up to systole by increased left atrial pressure, instead of gradual closure at end of diastole.
• Splitting of the second sound:
 heard at the left sternal edge.
heard at the left sternal edge.
 increased in inspiration, when right ventricular contraction is delayed by increased preload. Fixed in ASD; reversed in left bundle branch block and severe aortic stenosis, when left ventricular contraction is markedly delayed.
increased in inspiration, when right ventricular contraction is delayed by increased preload. Fixed in ASD; reversed in left bundle branch block and severe aortic stenosis, when left ventricular contraction is markedly delayed.
 third heart sound (thought to be due to ventricular filling). Occurs shortly after the second sound. May occur in normal subjects, also in reduced ventricular compliance/increased volume, e.g. in cardiac failure, mitral regurgitation, VSD.
third heart sound (thought to be due to ventricular filling). Occurs shortly after the second sound. May occur in normal subjects, also in reduced ventricular compliance/increased volume, e.g. in cardiac failure, mitral regurgitation, VSD.
 fourth heart sound (thought to be due to atrial contraction under pressure with forceful ventricular distension). Occurs shortly before the first sound. Always pathological, reflecting poor ventricular compliance; may occur in aortic stenosis, pulmonary stenosis, hypertension.
fourth heart sound (thought to be due to atrial contraction under pressure with forceful ventricular distension). Occurs shortly before the first sound. Always pathological, reflecting poor ventricular compliance; may occur in aortic stenosis, pulmonary stenosis, hypertension.
 gallop rhythm: all four sounds, e.g. cardiac failure.
gallop rhythm: all four sounds, e.g. cardiac failure.
 others, e.g. the late ejection click of aortic stenosis, the late systolic click of mitral valve prolapse, and the early diastolic opening snap of mitral stenosis.
others, e.g. the late ejection click of aortic stenosis, the late systolic click of mitral valve prolapse, and the early diastolic opening snap of mitral stenosis.
Heart transplantation. First performed in humans in 1967 by Barnard. Initial problems were infection and rejection. Interest resurged in the late 1970s with the introduction of ciclosporin, leading to establishment of centres worldwide.
• Indications: mostly end-stage ischaemic heart disease and cardiomyopathy; also congenital heart disease, valvular disease and others.
• Contraindications include age over 50 years, insulin-dependent diabetes mellitus, active infection, and recent PE/MI; these contraindications have been relaxed as expertise increases and chances of survival improve.
• Donor:
 initial management including heparin and cardioplegia is as for cardiac surgery. The heart is placed in cold crystalloid solution and both the atria opened; it is transported in ice.
initial management including heparin and cardioplegia is as for cardiac surgery. The heart is placed in cold crystalloid solution and both the atria opened; it is transported in ice.
 immunosuppressive drugs include ciclosporin, corticosteroids, azathioprine and antithymocyte immunoglobulin.
immunosuppressive drugs include ciclosporin, corticosteroids, azathioprine and antithymocyte immunoglobulin.
 general management is as for cardiac surgery. After cardiopulmonary bypass and aortic cross-clamping, the ventricles are removed, leaving most of the atria. The atria and aortas are anastomosed. The donor heart may also be piggy-backed next to the original heart, anastomosing left atria, aortas, pulmonary arteries and venae cavae.
general management is as for cardiac surgery. After cardiopulmonary bypass and aortic cross-clamping, the ventricles are removed, leaving most of the atria. The atria and aortas are anastomosed. The donor heart may also be piggy-backed next to the original heart, anastomosing left atria, aortas, pulmonary arteries and venae cavae.
 inotropes are usually required for over 24 h. The denervated heart rate is usually faster than that of the innervated heart, with no response to indirectly acting drugs, e.g. atropine. Drugs acting directly on the myocardium (e.g. isoprenaline) are used. The rate responds slowly to circulating catecholamines.
inotropes are usually required for over 24 h. The denervated heart rate is usually faster than that of the innervated heart, with no response to indirectly acting drugs, e.g. atropine. Drugs acting directly on the myocardium (e.g. isoprenaline) are used. The rate responds slowly to circulating catecholamines.
Postoperative care is as routine, but barrier nursing is required.
Ramakrishna H, Jaroszewski DE, Arabia FA (2009). Ann Card Anaesth; 12: 71–8
Heat. Form of energy associated with movement of particles (e.g. molecules, atoms) within a substance or system; temperature describes the propensity for transfer of heat energy between systems.
 radiation: especially from bright shiny bodies to dark matt bodies.
radiation: especially from bright shiny bodies to dark matt bodies.
 conduction: especially via good conductors of heat, e.g. metals.
conduction: especially via good conductors of heat, e.g. metals.
All these routes may be important in heat loss during anaesthesia.
Heat capacity. Quantity of heat required to raise the temperature of a mass by 1 K (J/K). Specific heat capacity (C) is the quantity of heat required to raise the temperature of unit mass of substance by 1 K (J/kg/K). The total heat capacity of an object may be calculated from knowledge of the mass and values for C of its constituent parts.
For a gas, Cp is specific heat capacity at constant pressure, and Cv is specific heat capacity at constant volume. Cp−Cv equals the work done in expansion of the gas. For an ideal gas, Cp−Cv = R (universal gas constant).
Heat loss, during anaesthesia. Prevention is important because of the adverse effects of hypothermia and the increased postoperative O2 consumption (up to 10 times) caused by shivering. In addition, the duration of action of neuromuscular blocking drugs is prolonged and drug clearance delayed. Particularly important in neonates and children, and in the elderly, because of reduced reserves.
• Temperature may fall by several °C during prolonged surgery, via:
– convection (15%): increased if the patient is uncovered.
– conduction (3%): usually a less important route; increased by use of cold irrigating solutions.
 reduced heat production and impaired temperature regulation. The latter may be peripheral (e.g. vasodilatation, shivering and impaired piloerection) or central (central effects of drugs).
reduced heat production and impaired temperature regulation. The latter may be peripheral (e.g. vasodilatation, shivering and impaired piloerection) or central (central effects of drugs).
 identification of high-risk patients:
identification of high-risk patients:
– prolonged surgery/open body cavity.
 temperature measurement during anaesthesia.
temperature measurement during anaesthesia.
 covering during transfer to the operating suite.
covering during transfer to the operating suite.
 maintenance of ambient temperature at 22–24°C and humidity about 50% (compromise between patient temperature and staff comfort).
maintenance of ambient temperature at 22–24°C and humidity about 50% (compromise between patient temperature and staff comfort).
 covering with drapes, reflective garments and head coverings (especially children).
covering with drapes, reflective garments and head coverings (especially children).
 warming of all skin cleansing solutions and iv fluids.
warming of all skin cleansing solutions and iv fluids.
 humidification of inspired gases.
humidification of inspired gases.
Heat of vaporisation, see Latent heat
Heat–moisture exchanger (HME; Swedish nose; hygroscopic condenser). Passive humidifier device. Positioned between the breathing tubing and the facemask, tracheal/tracheostomy tube or other airway device. Contains material (e.g. sponges, paper or metal gauze) that acts as a screen that can absorb large amounts of water. As expired gas passes through, water vapour condenses on the filter screen that also gains heat. The water and heat are given up when dry, cool inspired gas passes through in the next phase of breathing. May be disposable or refillable.
Heatstroke, see Hyperthermia
Heavy metal poisoning. Poisoning by exposure to any toxic metal (regardless of its molecular weight), including lead, mercury, arsenic, cadmium, bismuth, aluminium, antimony, chromium, cobalt, copper, gold, manganese, nickel, thallium, vanadium and zinc. Renal, hepatic, neurological and gastrointestinal damage are common following ingestion; acute lung injury often follows inhalation of fumes. Antidotes (chelating agents) include dimercaprol (for antimony, arsenic, bismuth, gold, mercury, thallium and lead), penicillamine (copper and lead), ascorbic acid (chromium), succimer (arsenic, mercury), unithiol (arsenic, mercury, nickel) and sodium calcium edetate (lead, manganese, zinc).
Hedonal. Obsolete anaesthetic agent, used iv (1905), rectally and orally. Very slow in onset and offset.
Heidbrink valve, see Adjustable pressure-limiting valve
Heimlich manoeuvre. Method of relieving choking caused by a foreign body using forceful compression of the upper abdomen. The resultant rise in intrathoracic pressure expels the object from the upper airway. The operator stands behind the subject with hands clenched over the subject’s epigastrium, the operator’s arms passing under the subject’s. A sharp thrust is delivered inwards and upwards. A similar manoeuvre may be performed with the subject lying. Compression of the lower chest has been found to be as effective. Clearance of one’s own airway by falling forwards on to the back of a chair has been reported.
Heimlich valve. Disposable device used in the treatment of pneumothorax. Consists of a flattened rubber tube within a clear plastic tube; attached to a thoracostomy tube, it allows the venting of air from within the chest and prevents the ingress of air. May block/malfunction if blood passes through valve. Useful during the prehospital phase of resuscitation or during interhospital transfer. Now infrequently used since the introduction of plastic underwater seal bottles and portable, disposable bag/valve assemblies.
Helium. Inert gas, present in natural gas and to a lesser extent in air. Less dense than nitrogen. Thus, if flow is turbulent, greater flow of a helium/O2 mixture will occur than of a nitrogen/O2 mixture, as turbulent flow depends on fluid density (and density of 21% O2 in helium is 34% of that of 21% O2 in nitrogen). Used therefore to increase alveolar O2 supply in upper airway obstruction. Of less use in lower airway obstruction (e.g. asthma) because most peripheral flow is laminar and therefore depends on viscosity, which is greater for helium/O2 mixtures than for nitrogen/O2. However, some benefit may occur where flow is turbulent.
Supplied in cylinders with brown shoulders and body, at 137 bar. Also available with 21% O2, in brown-bodied cylinders with brown and white quartered shoulders, at the same pressure.
Has also been used to investigate small airway resistance to flow, by comparing flow–volume loops breathing air and helium/O2. The two curves are more similar in small airway obstruction than in normal lungs.
Because of its very low solubility, helium is also used in measurement of lung volumes.
HELLP syndrome (Syndrome of haemolysis elevated liver enzymes and low platelets). Condition first recognised in 1982; thought to represent part of the spectrum of pre-eclampsia characterised primarily by abnormal blood tests rather than hypertension, proteinuria and oedema (although they may occur). HELLP syndrome is associated with significant maternal morbidity, including DIC, placental abruption, acute kidney injury, pulmonary oedema and hepatic rupture. General principles of management are as for pre-eclampsia; in addition plasma exchange, administration of fresh frozen plasma and prostacyclin have been used.
Henderson–Hasselbalch equation. Equation describing the relationship between the concentrations of dissociated and undissociated acid or base, acid or base dissociation constants (Ka and Kb respectively) and pH. In its generic form for weak acids (e.g. carbonic acid, thiopental sodium):
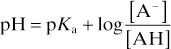
and for weak bases (e.g. local anaesthetic agents):
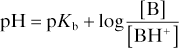
Applicable to any buffer system; commonly illustrated using the bicarbonate buffer system. For the reaction of CO2 with water to form carbonic acid, which dissociates to form bicarbonate and hydrogen ions:
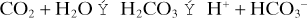
The acid dissociation constant Ka for the dissociation of H2CO3 then equals
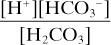
Taking logarithms of both sides:
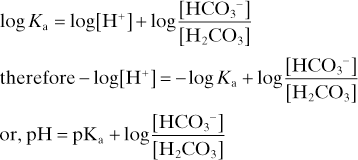
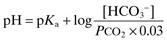
with PCO2 measured in mmHg
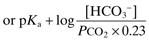
with PCO2 measured in kPa.
Thus used to explain changes in pH, PCO2 and [HCO3–] in disturbances of acid–base balance.
When applied to pharmacokinetics, the equation can be used to predict the effect of plasma/tissue pH on the fraction of unionised drug; e.g. acidosis reduces the unionised fraction of lidocaine hydrochloride, rendering it less effective in infected tissues.
[Lawrence Henderson (1878–1942), US biochemist; Karl Hasselbalch (1874–1962), Danish physiologist]
Henry’s law. Amount of gas dissolved in a solvent is proportional to the partial pressure of the gas when in equilibrium with the solvent, at constant temperature.
Heparin sodium/calcium. Anticoagulant drug, used for secondary prevention in acute coronary syndromes and for the prophylaxis and treatment of thromboembolism. Has also been used in DIC. Discovered in 1916. A mucopolysaccharide, derived from animal lung and intestine. Strongly acidic and electronegative, binding strongly to proteins and amines.
• Actions:
 potentiates the action of antithrombin III, a naturally occurring inhibitor of activated coagulation factors XII, XI, IX, X and thrombin.
potentiates the action of antithrombin III, a naturally occurring inhibitor of activated coagulation factors XII, XI, IX, X and thrombin.
 inhibits platelet aggregation by fibrin.
inhibits platelet aggregation by fibrin.
 activates lipoprotein lipase, involved in fat transport.
activates lipoprotein lipase, involved in fat transport.
 thought to be involved in immunological/inflammatory reactions, possibly via binding to histamine and 5-HT. Contained within mast cells.
thought to be involved in immunological/inflammatory reactions, possibly via binding to histamine and 5-HT. Contained within mast cells.
Fast onset but short-acting, with half-life of about 90 min; therefore most effectively given by iv infusion (heparin sodium). Effects persist for 4–6 h and are monitored by measuring the activated partial thromboplastin time (APTT), although thrombin and clotting times are also prolonged. In low dosage, acts by preventing spontaneous activation of factor X in hypercoagulable states (e.g. postoperatively), without affecting the ability to form clots when required. Thus given sc to prevent DVT, although there may be individual variation in effect.
• Dosage:
– prophylaxis of DVT: 5000 units sc 2 h preoperatively, then bd/tds until the patient is walking.
– during arterial surgery, 100 units/kg iv; for cardiac surgery, 300 units/kg. Also used to anticoagulate extracorporeal circuits, e.g. cardiopulmonary bypass, haemofiltration.
– enoxaparin 150 units/kg (1.5 mg/kg) od.
– bemiparin: 115 units/kg od for 5–9 days.
– treatment of acute coronary syndromes:
– dalteparin: 120 units/kg bd (maximum 18 000 units bd).
– enoxaparin 100 units/kg (1 mg/kg) bd.
Higher doses are required in pregnancy.
 increased bleeding: cessation of infusion is usually adequate; protamine may be given but only partially reverses low-mw heparin.
increased bleeding: cessation of infusion is usually adequate; protamine may be given but only partially reverses low-mw heparin.
 heparin-induced thrombocytopenia (HIT): antibody-mediated condition occurring in up to 3% of cases, typically 5–10 days after starting therapy. May be associated with increased tendency to thrombosis. Heparinoids and hirudins are alternatives if this occurs.
heparin-induced thrombocytopenia (HIT): antibody-mediated condition occurring in up to 3% of cases, typically 5–10 days after starting therapy. May be associated with increased tendency to thrombosis. Heparinoids and hirudins are alternatives if this occurs.
 osteoporosis following prolonged use.
osteoporosis following prolonged use.
 inhibition of aldosterone secretion has been described and regular monitoring of plasma potassium concentration is recommended if the duration of therapy exceeds 7 days.
inhibition of aldosterone secretion has been described and regular monitoring of plasma potassium concentration is recommended if the duration of therapy exceeds 7 days.
Side effects are less frequent with low-mw heparins than with unfractionated heparin.
Heparinoids. Heteropolysaccharide derivatives of heparin; used for the prevention and treatment of thromboembolism in patients with a history of heparin-induced thrombocytopenia. Danaparoid is the only preparation licensed for use in the UK.
Hepatic failure. May follow:
 chronic disease and cirrhosis:
chronic disease and cirrhosis:
– chronic autoimmune hepatitis.
– drugs, e.g. methyldopa, alcohols.
– non-alcoholic fatty liver disease.
– metabolic disease, e.g. haemochromatosis, Wilson’s disease, α1-antitrypsin deficiency, other inborn errors of metabolism.
– biliary disease, e.g. primary biliary cirrhosis.
– vascular lesions, e.g. venous occlusion, chronic cardiac failure.
 acute disease (fulminant hepatic failure):
acute disease (fulminant hepatic failure):
– drugs, e.g. paracetamol, halothane, chloroform, chlorpromazine, monoamine oxidase inhibitors, phenytoin, isoniazid.
– others: less common, including:
– poisons, e.g. carbon tetrachloride.
– acute fatty liver of pregnancy.
– shock.
– jaundice.
– gynaecomastia and testicular atrophy, caused by decreased metabolism of circulating oestrogens.
– encephalopathy: thought to be caused by reduced clearance of toxic waste products, e.g. ammonia, methionine and fatty acids. May be provoked by stress, including surgery, trauma and infection. Classified thus:
– stage 1: altered personality or cognition. EEG is usually normal.
– stage 4: unresponsive; comatose with depressed reflexes.
Treatment includes reduction of nitrogen intake, and oral administration of lactulose (20–50 ml/day) and/or neomycin (1 g 4–6-hourly) to reduce ammonia-producing GIT bacteria and encourage nitrogen-utilising bacteria.
– portal hypertension is caused by vascular occlusion, possibly due to fibrotic changes in cirrhosis. It may cause splenomegaly and enlargement of portal–systemic vascular anastomoses, e.g. oesophagogastric junction, retroperitoneal and umbilical vessels. Oesophageal varices may cause severe haemorrhage; treatment may include:
– medical treatment: iv vasopressin analogues (e.g. terlipressin), somatostatin, proton pump inhibitors (e.g. pantoprazole sodium).
– band ligation or injection of varices with sclerosant/tissue adhesive.
– use of a Sengstaken–Blakemore tube.
– cirrhotic cardiomyopathy may be present, with features of heart failure, arrhythmias and reduced response to inotropic drugs.
– GIT haemorrhage: apart from oesophageal varices, may also be caused by gastric erosions or peptic ulcer disease. Coagulation factors and platelets may be reduced. Anaemia is common.
– hypoproteinaemia, with reduced plasma oncotic pressure, drug binding, immunoglobulins, cholinesterase and coagulation factors.
– fluid retention: may cause ascites, pleural effusion, peripheral oedema and hyperdynamic circulation. Treatment includes spironolactone, sodium restriction and drainage of ascites/pleural effusion.
– hypoxaemia is common, caused by 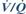 mismatch, atelectasis, diaphragmatic splinting or pleural effusion. Portopulmonary hypertension may be present in end-stage disease due to accumulation of vasoactive substances normally cleared by the liver.
mismatch, atelectasis, diaphragmatic splinting or pleural effusion. Portopulmonary hypertension may be present in end-stage disease due to accumulation of vasoactive substances normally cleared by the liver.
– infection is common, especially bacterial.
– metabolic and respiratory alkalosis may occur.
 acute fulminant hepatic failure: defined as hepatic failure occurring within 8 weeks of illness, in a previously normal liver. It presents with rapidly progressing encephalopathy, coma and cerebral oedema, hypoglycaemia, hyponatraemia, hypokalaemia, alkalosis, hypothermia, acute lung injury, haemorrhage, coagulopathy and renal failure. Renal failure may be due to hepatorenal syndrome or acute tubular necrosis. Jaundice is uncommon initially. DIC and infection may occur.
acute fulminant hepatic failure: defined as hepatic failure occurring within 8 weeks of illness, in a previously normal liver. It presents with rapidly progressing encephalopathy, coma and cerebral oedema, hypoglycaemia, hyponatraemia, hypokalaemia, alkalosis, hypothermia, acute lung injury, haemorrhage, coagulopathy and renal failure. Renal failure may be due to hepatorenal syndrome or acute tubular necrosis. Jaundice is uncommon initially. DIC and infection may occur.
Treatment is supportive and includes O2 therapy, IPPV, vitamin K, blood products, neomycin/lactulose, H2 receptor antagonists/omeprazole, prophylactic antibiotics and nutritional support with dextrose. ICP monitoring may be useful and measures (e.g. head-up tilt, mannitol) employed to reduce ICP if raised. Liver dialysis techniques have been used and liver transplantation may be required. Experimental therapies include auxiliary liver transplantation, temporising hepatectomy, artificial liver systems (live liver cells within an extracorporeal circuit), intraperitoneal hepatocyte transplantation, use of liver growth factors and xenotransplantation.
• Anaesthetic management of patients with hepatic failure or chronic liver disease:
 directed towards the above complications, particularly preoperative assessment for, and improvement of:
directed towards the above complications, particularly preoperative assessment for, and improvement of:
– encephalopathy, and haematological and coagulation abnormalities.
– pulmonary and renal function.
– fluid, acid–base and electrolyte disturbance.
Vitamin K may be given if coagulation is abnormal, fresh frozen plasma if surgery is urgent.
 anaesthetic technique: increased doses of iv agents and neuromuscular blocking drugs may be required in cirrhosis, due to increased volume of distribution, but elimination may be prolonged; all sedative drugs require careful titration if encephalopathy is present. Opioids should be used cautiously. Drug metabolism is reduced; isoflurane/desflurane and atracurium are often preferred as reliance on metabolism is less than with other agents. Hypocapnia exacerbates the reduction in hepatic blood flow during general anaesthesia.
anaesthetic technique: increased doses of iv agents and neuromuscular blocking drugs may be required in cirrhosis, due to increased volume of distribution, but elimination may be prolonged; all sedative drugs require careful titration if encephalopathy is present. Opioids should be used cautiously. Drug metabolism is reduced; isoflurane/desflurane and atracurium are often preferred as reliance on metabolism is less than with other agents. Hypocapnia exacerbates the reduction in hepatic blood flow during general anaesthesia.
 screening for infectious hepatitis should be performed.
screening for infectious hepatitis should be performed.
 maintenance of good peri- and postoperative renal function is important (see Jaundice).
maintenance of good peri- and postoperative renal function is important (see Jaundice).
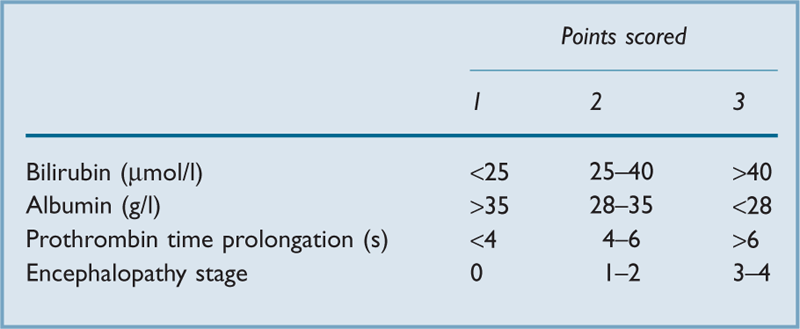
A scoring system has been devised for assessment of risk, depending on preoperative blood tests and clinical assessment (Table 23). Good operative risk is suggested by < 6 points, moderate by 7–9 points, and poor risk by > 10 points.
Al-Khafaji A, Huang DT (2011). Crit Care Med; 39: 1157–66
Hepatitis. Acute hepatitis may be:
– RNA enterovirus, spread via the orofaecal route. Incubation period is 3–5 weeks.
– causes fever, headache, GIT symptoms, impaired liver function tests, jaundice and hepatomegaly.
– recovery is usually within 6 weeks, although malaise may persist longer.
– passive immunisation with immunoglobulin is available.
– features are as for hepatitis A but more severe. May lead to recovery, acute fulminant hepatic failure (rarely) or a chronic infective state. The last includes asymptomatic carriage or chronic hepatitis that may lead to hepatocellular carcinoma or hepatic failure.
– HBsAg: increases 1 month after exposure, peaks at 2–3 months, and falls at 4–5 months.
– HBeAg: increases at 1 month, peaks at 2 months and falls at 3 months.
– anti-HBc: increases at 2 months, peaks at 4 months and falls slowly thereafter.
– anti-HBe: increases at 2–3 months, remaining elevated.
– anti-HBs: increases at 1–2 months, remaining elevated.
– screening of blood for transfusion, use of disposable needles, etc.; operating theatre precautions as for HIV infection. Pregnant women should be screened for HbsAg to reduce fetal transmission.
– hepatitis C (causes most cases of what was previously called non-A non-B hepatitis):
– RNA virus, identified with a serological marker.
– acute infection is usually asymptomatic; however, about 85% become carriers, with 20–40% developing chronic liver disease and cirrhosis. About 5% develop hepatocellular carcinoma. Patients with chronic infection may receive interferon-α and ribavirin.
– screening of blood products began in the UK in 1991.
– hepatitis D (delta): RNA virus, dependent on coexistent hepatitis B infection.
– other viral infections include cytomegalovirus, herpes simplex, varicella zoster and glandular fever.
 due to other infections, e.g. toxoplasmosis, leptospirosis.
due to other infections, e.g. toxoplasmosis, leptospirosis.
– idiosyncratic, e.g. phenothiazines, monoamine oxidase inhibitors, tricyclic antidepressants, chloroform, methyldopa, indometacin, erythromycin, rifampicin, chlorpropamide. ‘Halothane hepatitis’ was first described in 1958, with an estimated incidence of 1 : 6000 to 1 : 30 000, typically following repeated exposure within a short period. The cause is uncertain but a direct toxic effect, an immune reaction to halothane or hepatocytes altered by halothane, or halothane-induced hepatic hypoxia have all been implicated. Hepatitis has also followed exposure to more modern volatile anaesthetic agents, but a causative role is unclear, the incidence is thought to be extremely low, and repeated exposure is generally considered safe.
– dose-related, e.g. paracetamol, carbon tetrachloride, alcohol.
 metabolic, e.g. Wilson’s disease, or associated with pregnancy.
metabolic, e.g. Wilson’s disease, or associated with pregnancy.
 associated with circulatory abnormalities, e.g. right ventricular failure, severe hypotension.
associated with circulatory abnormalities, e.g. right ventricular failure, severe hypotension.
Hepatorenal syndrome (HRS). Renal impairment secondary to severe hepatic dysfunction, usually cirrhosis. May coexist with or mimic other causes of renal failure (e.g. hypovolaemia, glomerulonephritis, urinary tract obstruction); therefore considered a diagnosis of exclusion.
 serum creatinine > 130 µmol/l and no improvement after 2 days of volume expansion with albumin and withdrawal of diuretics.
serum creatinine > 130 µmol/l and no improvement after 2 days of volume expansion with albumin and withdrawal of diuretics.
 no recent/current treatment with nephrotoxic drugs.
no recent/current treatment with nephrotoxic drugs.
 normal renal ultrasound and no evidence of renal parenchymal disease.
normal renal ultrasound and no evidence of renal parenchymal disease.
Classified according to speed of onset and progression; type 1 is rapidly progressive (often due to a precipitating cause, such as infection) and associated with MODS, while type 2 is associated with slowly worsening ascites and haemodynamic function. Median survival is 1–3 weeks and 6 months respectively.
Prognosis is poor, even with renal replacement therapy, unless hepatic function improves or transplant is performed. Administration of albumin and inotropic drugs (e.g. terlipressin, noradrenaline) improves renal function in up to 50% of patients; early improvement in MAP predicts response to treatment.
 Echinacea: activates the immune system with reports of allergy (including anaphylaxis), decreased effectiveness of immunosuppressive drugs with short-term use and immunosuppression with long-term use.
Echinacea: activates the immune system with reports of allergy (including anaphylaxis), decreased effectiveness of immunosuppressive drugs with short-term use and immunosuppression with long-term use.
 Ephedra (ma huang): contains ephedrine and other sympathomimetic alkaloids. Causes dose-dependent tachycardia and hypertension with subsequent risk of myocardial ischaemia and CVA. Increases risk of arrhythmias when inhalational anaesthetic agents such as halothane are used. Haemodynamic instability may occur with concomitant use of monoamine oxidase inhibitors. Should be discontinued 24 h preoperatively.
Ephedra (ma huang): contains ephedrine and other sympathomimetic alkaloids. Causes dose-dependent tachycardia and hypertension with subsequent risk of myocardial ischaemia and CVA. Increases risk of arrhythmias when inhalational anaesthetic agents such as halothane are used. Haemodynamic instability may occur with concomitant use of monoamine oxidase inhibitors. Should be discontinued 24 h preoperatively.
 garlic (ajo): inhibits platelet aggregation (sometimes irreversibly), increasing the risk of perioperative bleeding. Should be discontinued 7 days preoperatively.
garlic (ajo): inhibits platelet aggregation (sometimes irreversibly), increasing the risk of perioperative bleeding. Should be discontinued 7 days preoperatively.
 ginkgo: inhibits platelet-activating factor with potential for increased bleeding. Should be discontinued 36 h preoperatively.
ginkgo: inhibits platelet-activating factor with potential for increased bleeding. Should be discontinued 36 h preoperatively.
 ginseng: may cause hypoglycaemia and inhibition of platelet aggregation. Should be discontinued 7 days preoperatively.
ginseng: may cause hypoglycaemia and inhibition of platelet aggregation. Should be discontinued 7 days preoperatively.
 St John’s wort (Hypericum): causes enzyme induction of the cytochrome oxidase system (especially the cytochrome P450 family). Should be discontinued at least 5 days preoperatively.
St John’s wort (Hypericum): causes enzyme induction of the cytochrome oxidase system (especially the cytochrome P450 family). Should be discontinued at least 5 days preoperatively.
Hereditary angio-oedema. Congenital deficiency of C1 esterase inhibitor, leading to complement activation with swelling of the face, mouth, skin and intestine. Of autosomal dominant inheritance, but new mutations account for 25% of cases; prevalence is 1 in 50 000. May occur spontaneously or after a trigger (e.g. trauma, infection, surgery, stress), possibly via activation of kinins, plasmins or other proteases. An acquired form may occur in lymphomas.
Attacks may mimic an acute surgical abdomen, with pain, diarrhoea and vomiting. May also cause upper airway obstruction, carrying a mortality of 25–40%. Management of an acute episode is as for airway obstruction; iv adrenaline, corticosteroids, antihistamine drugs, aprotinin, antifibrinolytic drugs and danazol have been tried, with varying results. Synthetic, partially purified C1 esterase inhibitor is the treatment of choice for the termination of acute attacks, and is effective in 30–45 min. 2–3 units of fresh frozen plasma (which contains C1 esterase inhibitor) may also be given.
Levy JH, Freiberger DJ, Roback J (2010). Anesth Analg; 110: 1271–80
Hereditary angioneurotic oedema, see Hereditary angio-oedema
Hering–Breuer reflex (Inflation reflex). Inhibition of respiratory muscles following lung inflation, leading to termination of inspiration. Afferent pathway is thought to be from pulmonary stretch receptors via the vagus. Of minor importance in humans, but active in many other mammals; bilateral vagotomy produces slow deep breathing in the latter but not the former.
[Karl Hering (1834–1918), German physiologist; Josef Breuer (1852–1925), Austrian psychiatrist]
Hetastarch, see Hydroxyethyl starch
Hewer, Christopher Langton (1896–1986). English anaesthetist, of major importance in the establishment and evolution of anaesthesia in the UK. Popularised the use of trichloroethylene in 1941. Edited Anaesthesia for its first 20 years, also Recent Advances in Anaesthesia and Analgesia for 50 years. Received many honours and medals.
Hewitt, Frederick (1857–1916). English anaesthetist, practised at St George’s Hospital. Renowned for many contributions to anaesthesia, including the first fixed proportion N2O/O2 machine, inhalers, airways and other equipment. A strong advocate of teaching and high standards in anaesthesia. Knighted in 1911.
Hexafluorenium. Obsolete drug used to prolong the action of suxamethonium by inhibiting plasma cholinesterase. May cause arrhythmias and bronchospasm.
Hexamethonium. Obsolete ganglion blocking drug used in hypotensive anaesthesia.
Hexastarch, see Hydroxyethyl starch
Hexobarbital. Obsolete iv anaesthetic agent, introduced in 1932. Replaced by the safer and more predictable thiopental.
HFJV, HFPPV, HFO, HFV, see High-frequency ventilation
More common in the elderly and in obesity.
 epigastric pain, belching, indigestion.
epigastric pain, belching, indigestion.
 regurgitation; may lead to stricture formation.
regurgitation; may lead to stricture formation.
 aspiration of gastric contents:
aspiration of gastric contents:
– chronic pulmonary damage due to repeated aspiration.
– risk of acute aspiration perioperatively.
[Rudolph Nissen (1896–1981), Swiss surgeon]
See also, Diaphragmatic herniae; Gastro-oesophageal reflux; Lower oesphageal sphincter
Hiccups. Intense synchronous contraction of the diaphragm and inspiratory intercostal muscles lasting about 500 ms, followed approximately 30 ms after its onset by glottic closure. May involve phrenic or vagal efferents. Results in a characteristic inspiratory sound associated with discomfort. On average, occur at a rate of less than 30/min. Frequent in the newborn. Frequency is decreased by breath-holding or a raised arterial PCO2 and increased by a lowered arterial PCO2. Episodes may be terminated by a sudden shock. May occur recurrently with neurological disease (e.g. brainstem tumours, encephalitis, meningitis), metabolic disease (e.g. uraemia) and many thoracic, abdominal or cardiac conditions. During anaesthesia, hiccups may be provoked by surgical stimulation, especially around the diaphragm, and particularly in the presence of inadequate paralysis or anaesthesia.
Hickman, Henry Hill (1800–1830). English surgeon, practising in Ludlow and Shifnall, Shropshire. First described the production of insensibility in animals by exposure to a gas (CO2) and suggested its use for painless surgery, in 1824. His efforts to publicise his experiments were unsuccessful, both in the UK and abroad.
Hickman line, see Central venous cannulation, long-term
High dependency unit (HDU). Area providing a level of care between that of a general ward and an ICU (i.e. level 1 and 2 care). Provides care for patients with a single failing organ system, those stepping down from ICU and high-risk patients requiring postoperative care. Costs and nursing requirements are less than for an ICU; they usually have a nurse : patient ratio of 1 : 2.
See also, Care of the critically ill surgical patient; Medical emergency team; Postoperative care team; Safe transport and retrieval team; Transportation of critically ill patients
High-frequency ventilation (HFV). Mechanism of respiratory support developed in the 1970s. Small breaths are delivered at high frequencies, maintaining gas exchange without barotrauma or other deleterious effects of IPPV. May be superimposed on spontaneous ventilation.
 high-frequency positive pressure ventilation (HFPPV): 60–150 cycles/min, delivered via an intratracheal insufflation catheter, bronchoscope or tracheal tube. Tidal volumes of 100–400 ml are used. Fluidic valves are often used, without moving parts. Possible using some conventional ventilators, especially paediatric ones.
high-frequency positive pressure ventilation (HFPPV): 60–150 cycles/min, delivered via an intratracheal insufflation catheter, bronchoscope or tracheal tube. Tidal volumes of 100–400 ml are used. Fluidic valves are often used, without moving parts. Possible using some conventional ventilators, especially paediatric ones.
 high-frequency jet ventilation (HFJV): 60–600 cycles/min, delivered via a cannula inserted through the cricothyroid membrane, placed within a bronchoscope, or incorporated near the tip of a tracheal tube. Expiration is continuous through the open system. Principles of gas entrainment are as for injector techniques. Tidal volume is up to 150 ml. Produces positive airway pressure of about 5 cmH2O. Most ventilators employ electrical solenoid valves.
high-frequency jet ventilation (HFJV): 60–600 cycles/min, delivered via a cannula inserted through the cricothyroid membrane, placed within a bronchoscope, or incorporated near the tip of a tracheal tube. Expiration is continuous through the open system. Principles of gas entrainment are as for injector techniques. Tidal volume is up to 150 ml. Produces positive airway pressure of about 5 cmH2O. Most ventilators employ electrical solenoid valves.
The mechanism of gas exchange is unclear but is thought to involve continuous mixing of gases. HFJV is most commonly used, particularly for ENT and thoracic procedures such as sleeve resection of the trachea/bronchi and tracheobronchial fistula, in which increased airway pressures and excessive movement may be especially detrimental. HFO has been used in acute lung injury in all ages. It reduces airway pressures and splints the lungs above closing capacity, unlike conventional IPPV, in which pressure and volume changes exhibit large swings. HFJV via cricothyrotomy is an alternative to tracheal intubation and IPPV in respiratory failure.
Hirudin. Peptide (65 amino acid) originally derived from leech saliva, now manufactured using recombinant techniques. Specifically inhibits the actions of thrombin in the coagulation pathway; unlike heparin, it does not require antithrombin III as a cofactor and is not inhibited by anti-heparin proteins. It also does not affect platelets directly and may also inhibit thrombin bound to a fibrin clot. Has been studied as a means of preventing primary and recurrent MI and DVT; initial studies have been encouraging, although bleeding may be a problem, as with heparin. Lepirudin and bivalirudin are recombinant forms available in the UK.
His bundle electrography. Technique for investigating cardiac conduction defects and tachycardias, using transvenous intracardiac bipolar electrodes at various sites. Concurrent recording of a formal ECG is usually undertaken. Assesses conduction through different parts of the heart conducting system, identifying conduction defects and accessory pathways. May also be used to distinguish supraventricular from ventricular arrhythmias; ventricular complexes in the former are preceded by His bundle activity. The effects of pacing stimuli at different sites may also be observed, e.g. in assessment of refractory tachycardias.
Histamine and histamine receptors. Histamine, an amine, is present in mast cells, basophils, gastric mucosa and the CNS. It is involved in the inflammatory response and gastric acid secretion, and is thought to be a neurotransmitter, although its role as the latter is unclear. Involved in many other inflammatory mediator pathways (e.g. cytokines, complement, leukotrienes) and with coagulation and other processes. Synthesised by decarboxylation of L-histidine, and broken down by deamination and/or methylation with renal excretion.
• Histamine receptor subtypes have been identified:
– cause vascular smooth muscle relaxation and dilatation, with increased vascular permeability.
– cause stimulation and irritation of cutaneous nerve endings.
– cause some vasodilatation (but less than H1 receptors).
– increase acid, pepsin and intrinsic factor secretion from gastric mucosa.
 H4: present in GIT and basophils and involved in chemotaxis.
H4: present in GIT and basophils and involved in chemotaxis.
The histamine receptors are all G protein-coupled receptors; H2 actions are thought to be mediated via cAMP, and H1 via cyclic guanosine monophosphate.
Specific receptor antagonists have been developed; they are called antihistamine drugs (H1) and H2 receptor antagonists largely for historical reasons (the latter were designed many years after the former).
Histamine is released from mast cells following iv injection of certain drugs, e.g. tubocurarine and morphine. The amount released depends partly on the rate of injection. Skin wheals, hypotension and bronchospasm may occur.
Histamine receptor antagonists, see Antihistamine drugs; H2 receptor antagonists