Guillain-Barré Syndrome
After reading this chapter, you will be able to:
• List the anatomic alterations of the lungs associated with Guillain-Barré syndrome.
• Describe the causes of Guillain-Barré syndrome.
• List the cardiopulmonary clinical manifestations associated with Guillain-Barré syndrome.
• Describe the general management of Guillain-Barré syndrome.
• Describe the clinical strategies and rationales of the SOAPs presented in the case study.
• Define key terms and complete self-assessment questions at the end of the chapter and on Evolve.
Anatomic Alterations of the Lungs Associated with Guillain-Barré Syndrome
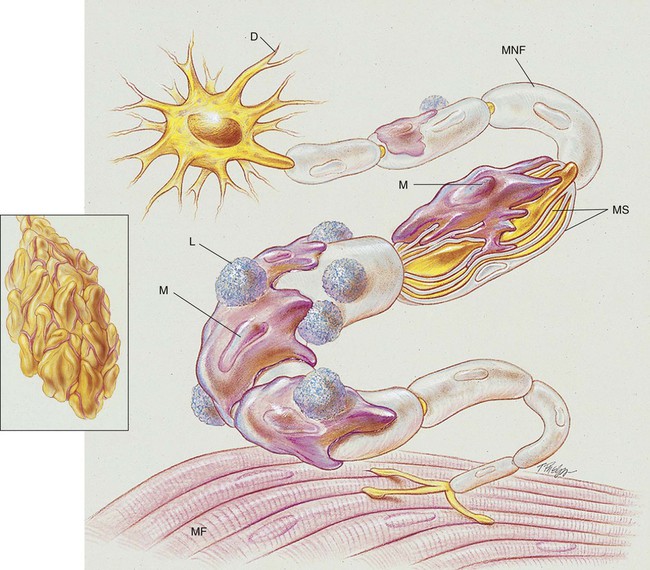
Paralysis of the skeletal muscles develops in response to various pathologic changes in the peripheral nerves. Microscopically, the nerves show demyelination, inflammation, and edema. As the anatomic alterations of the peripheral nerves intensify, the ability of the neurons to transmit impulses to the muscles decreases, and eventually paralysis ensues (see Figure 28-1). Box 28-1 lists other names in the literature for Guillain-Barré syndrome.
Etiology and Epidemiology
The precise cause of Guillain-Barré syndrome is not known. It is probably an immune disorder that causes inflammation and deterioration of the patient’s peripheral nervous system. Elevated levels of immunoglobulin M (IgM) antibodies against myelin glycolipid have been found in the serum of patients with Guillain-Barré syndrome. Antibodies that are cell-mediated are thought to be responsible for peripheral nerve demyelination and inflammation. Lymphocytes and macrophages appear to attack and strip off the myelin sheath of the peripheral nerves and leave swelling and fragmentation of the neural axon (see Figure 28-1). It is believed that the myelin sheath covering the peripheral nerves (or the myelin-producing Schwann cell) is the actual target of the immune attack.
General Management of Guillain-Barré Syndrome
Good clinical indicators of acute ventilatory failure include the following:
• NIF <−25 cm H2O—In other words, the patient is unable to generate a negative inspiratory pressure of 25 cm H2O or more. For example, an NIF of only −15 would confirm severe muscle weakness and, important, that acute ventilatory failure was likely.
Respiratory Care Treatment Protocols
Oxygen Therapy Protocol
Oxygen therapy is used to treat hypoxemia, decrease the work of breathing, and decrease myocardial work. Because of the hypoxemia that may develop in Guillain-Barré syndrome, supplemental oxygen may be required. However, because of the alveolar consolidation and atelectasis associated with Guillain-Barré syndrome, capillary shunting may be present. Hypoxemia caused by capillary shunting or alveolar hypoventilation is refractory to oxygen therapy (see Oxygen Therapy Protocol, Protocol 9-1).
Bronchopulmonary Hygiene Therapy Protocol
Because of the excessive mucous production and accumulation associated with Guillain-Barré syndrome, a number of bronchopulmonary hygiene modalities may be used to enhance the mobilization of bronchial secretions (see Bronchopulmonary Hygiene Therapy Protocol, Protocol 9-2).
Mechanical Ventilation Protocol
Mechanical ventilation may be necessary to provide and support alveolar gas exchange and eventually return the patient to spontaneous breathing. Because acute ventilatory failure is seen in patients with severe Guillain-Barré syndrome, continuous mechanical ventilation is often required. Continuous mechanical ventilation is justified because the acute ventilatory failure is thought to be reversible (see Mechanical Ventilation Protocols, Protocol 9-5, Protocol 9-6, and Protocol 9-7).
CASE STUDY
Guillain-Barré Syndrome
Admitting History and Physical Examination
Respiratory Assessment and Plan
S N/A (intubated on ventilator)
O Vital signs: BP 126/82, HR 68, RR 12 (IMV); afebrile; no spontaneous breaths; CXR: normal; normal breath sounds; ABGs (on Fio2 = 0.40): pH 7.51, Paco2 29, 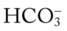 22, Pao2 204; Spo2 98%
22, Pao2 204; Spo2 98%
• Acute alveolar hyperventilation with excessive oxygenation (ABGs)
• Excessive alveolar ventilation (increased pH and decreased Paco2)
P Adjust mechanical ventilator settings (decrease tidal volume and Fio2) according to Mechanical Ventilation Protocol and Oxygen Therapy Protocol. Monitor closely and reevaluate.
Respiratory Assessment and Plan
O Skin color good; crackles and rhonchi over both lung fields; moderate amount of whitish, clear secretions being suctioned regularly; vital signs: BP 124/83, HR 74, T 37.7° C (99.8° F); CXR: unremarkable; ABGs (Fio2 = 0.4): pH 7.44, Paco2 35, 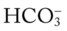 24, and Pao2 98; Spo2 97%
24, and Pao2 98; Spo2 97%
• Normal acid-base and oxygenation status on present ventilator settings (ABGs)
• Excessive sputum accumulation; possible progression to mucous plugging and atelectasis (crackles, rhonchi, whitish and clear secretions)
P Begin Bronchopulmonary Hygiene Therapy Protocol (vigorous tracheal suctioning and obtain sputum stain and culture). Begin Lung Expansion Therapy Protocol (10 cm H2O positive end-expiratory pressure [PEEP] to offset any early development of atelectasis). Monitor and reevaluate (4 × per shift).
Respiratory Assessment and Plan
O Skin color good; crackles and rhonchi over both lung fields improving; small amount of clear secretions suctioned; vital signs: BP 118/79, HR 68, T normal; no spontaneous respirations; CXR: normal; ABGs (Fio2 = 0.4): pH 7.42, Paco2 37, 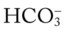 24, Pao2 97; Spo2 97%
24, Pao2 97; Spo2 97%
• Normal acid-base and oxygenation status on present ventilator settings (ABGs)
• Respiratory insufficiency (no spontaneous respirations)
• Secretion control improving (crackles, rhonchi, clear secretions)
P Continue Ventilator Management Protocol. Continue Bronchopulmonary Hygiene Therapy Protocol. Continue Lung Expansion Therapy Protocol. Monitor and reevaluate (forced expiratory volume in 1 second [FEV1], negative inspiratory force [NIF] 2 × per shift).
Discussion
As shown during the first assessment, when acute ventilatory failure developed, the patient was transferred to the ICU, intubated, and placed on a mechanical ventilator. Shortly after the patient was placed on the ventilator, his arterial blood gas values showed hyperoxia and acute alveolar hyperventilation—both of which were caused by the ventilator settings. The appropriate response was to immediately adjust the ventilator settings by reducing the tidal volume or frequency (or both) and the Fio2. At the time of the assessment, the patient exhibited no evidence of airway obstruction or secretions. Therefore the Bronchial Hygiene Therapy Protocol (Protocol 9-2), was not indicated. Indeed, all that needed to be done at that time was to ensure adequate ventilation and oxygenation on the ventilator.
However, 3 days later, at the time of the second assessment, crackles and rhonchi were heard over all lung fields. Clearly the time had come to initiate the Bronchial Hygiene Therapy Protocol with suctioning and possibly even therapy with mucolytic agents. Because of the risk of atelectasis, the Lung Expansion Therapy Protocol (Protocol 9-3), in the form of PEEP on the ventilator, was indicated. In such a case the sputum should be cultured to see whether any infectious organisms are present.



 values will be lower than expected for a particular Pa
values will be lower than expected for a particular Pa
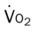
 )
)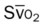

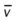 )O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;
)O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio; 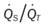 , pulmonary shunt fraction;
, pulmonary shunt fraction; 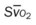 , mixed venous oxygen saturation;
, mixed venous oxygen saturation; 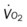 , oxygen consumption.
, oxygen consumption.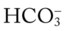 23, and Pa
23, and Pa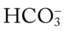 22, and Pa
22, and Pa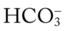 24, and Pa
24, and Pa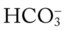 24, and Pa
24, and Pa