Chapter 14 Guidance of Catheter-Based Cardiac Interventions
Over the past 2 decades, percutaneous interventions for structural heart disease have become increasingly common. Interventional cardiologists are now treating a variety of lesions that previously required surgery, and these interventions have become more technically complex over time. Although fluoroscopy, two-dimensional transesophageal echocardiography (2DTEE), and intracardiac echocardiography (ICE) are most typically used for procedural guidance, real-time three-dimensional TEE (RT3DTEE) offers several important advantages over these modalities (Figure 14-1).1
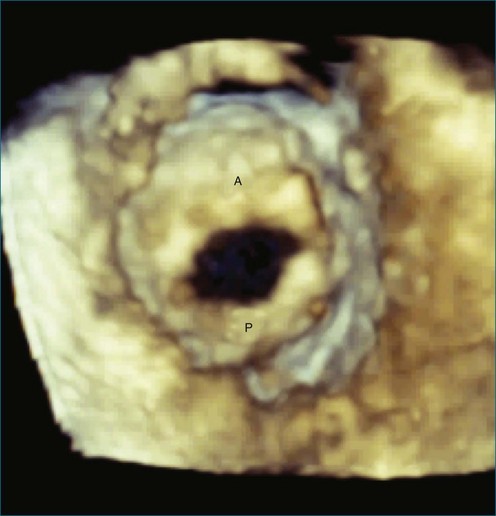
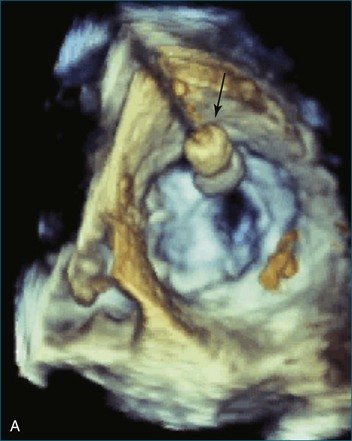
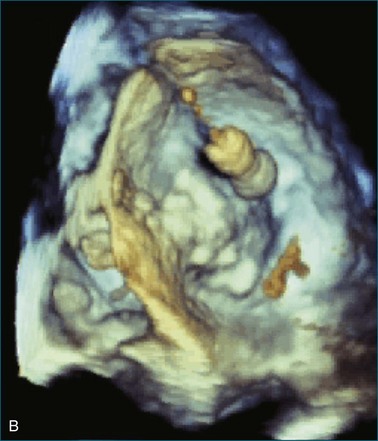
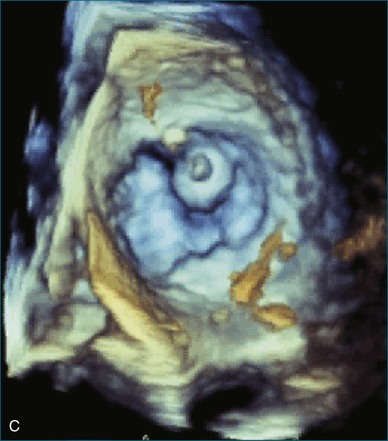
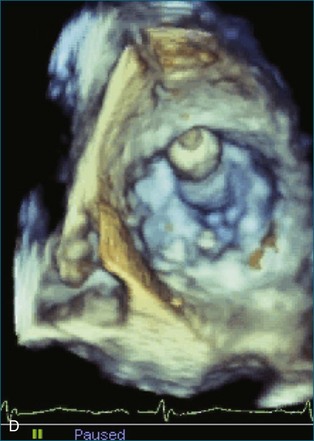
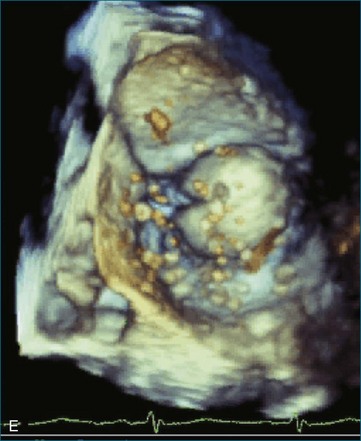
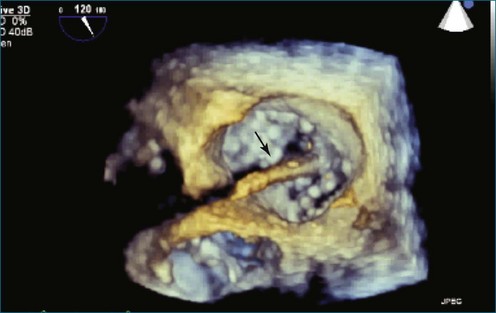
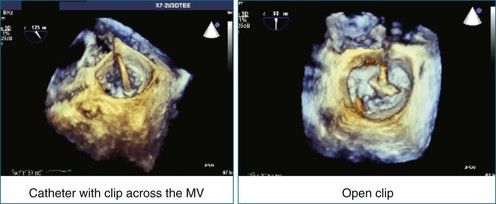
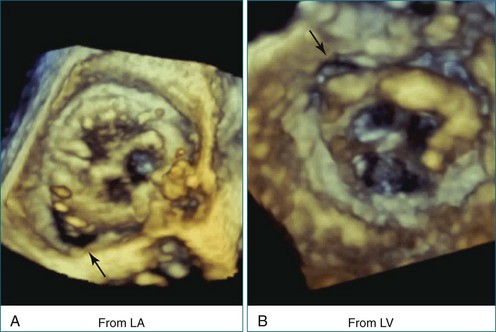
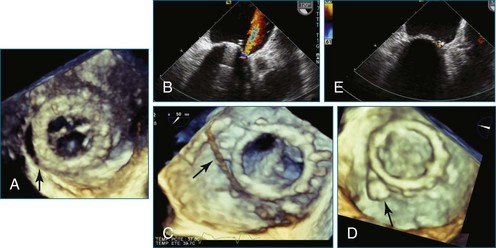
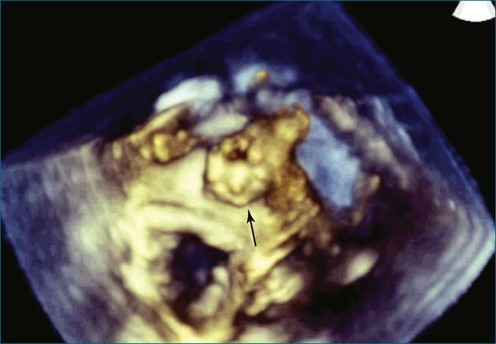
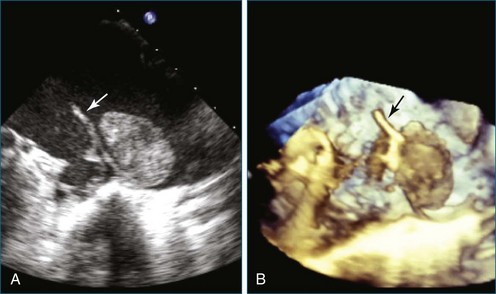
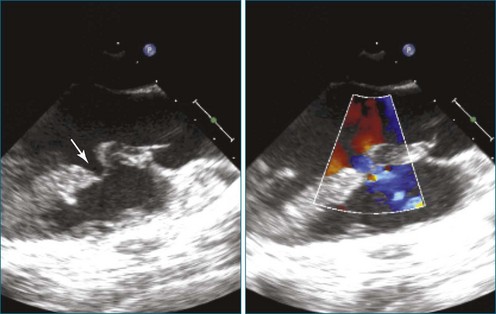
First and foremost, RT3DTEE represents the anatomy and pathology of interest in an intuitive manner. Although the echocardiographer’s expertise is required for image acquisition, those with relatively little training in cardiac imaging can understand 3DTEE images with little difficulty. For example, simultaneous visualization of all six mitral valve scallops is possible in one view in the vast majority of patients, making RT3DTEE ideal for transcutaneous mitral valve repair using the Mitra Clip device (Abbott Vascular, Abbott Park, IL).2–4 In this procedure, the “clip” is delivered to the left atrium via transseptal puncture. RT3DTEE can facilitate this portion of the procedure by demonstrating tenting of the interatrial septum, allowing optimal positioning of the Brockenbrough needle for the septal puncture. For this particular procedure, it is optimal to be slightly superior and posterior to the center of the fossa ovalis. Once the mitral valve clip has been passed into the left atrium, it must then be positioned perpendicular to the line of leaflet coaptation, in the center of the valvular orifice. The clip is deployed to grasp the A2 and P2 scallops. Because spatial relationships on RT3DTEE closely mirror the actual anatomy, an interventionalist can use RT3DTEE to guide catheter movements, verify device placement, and confirm that the mitral valve orifice has been appropriately cleaved in two (Figures 14-2 to 14-8; Videos 14-1 and 14-2).
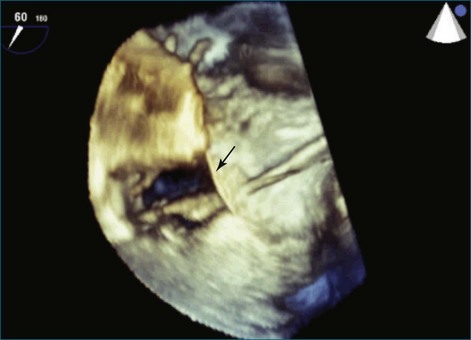
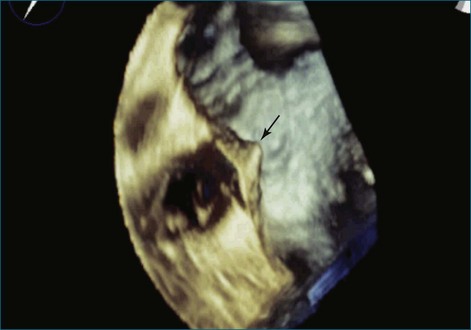
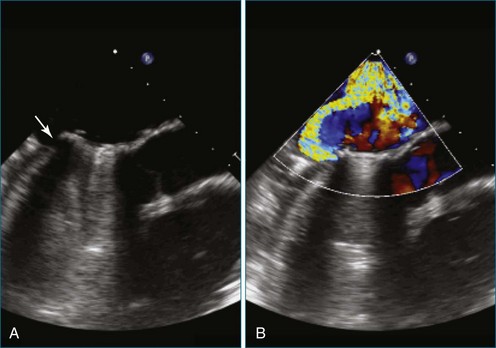
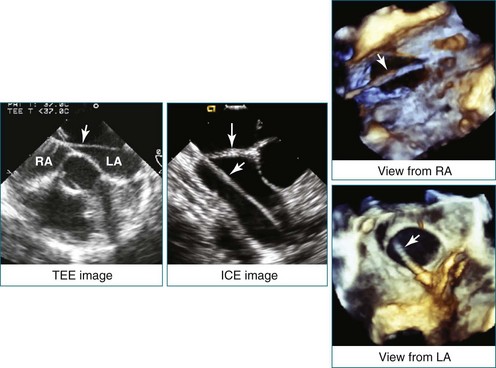
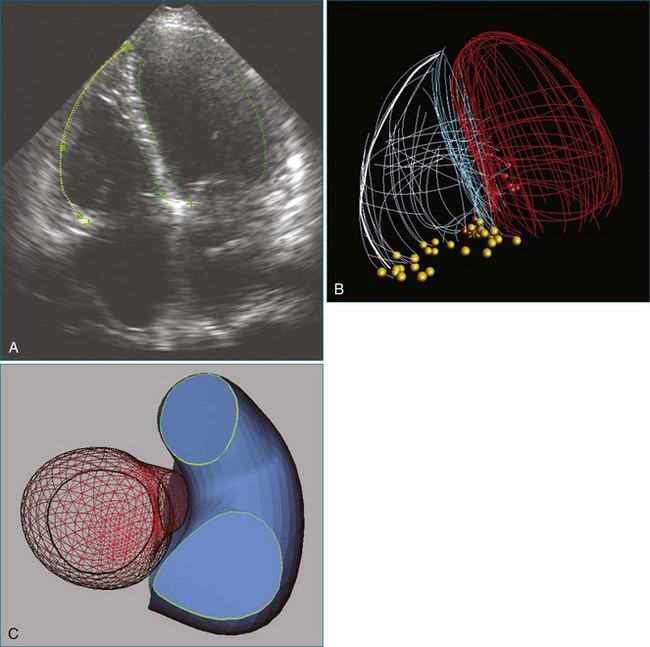
Figure 14-3 Shown is a continuation of the procedure in Figure 14-2. The catheter can then safely traverse the interatrial septum (arrow) to gain access to the left atrium, the mitral valve, or both. In Video 14-2, it is clear that two catheters are present.
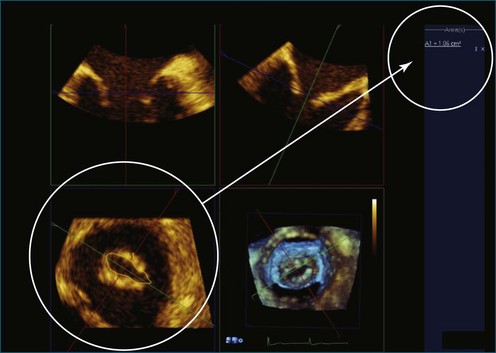
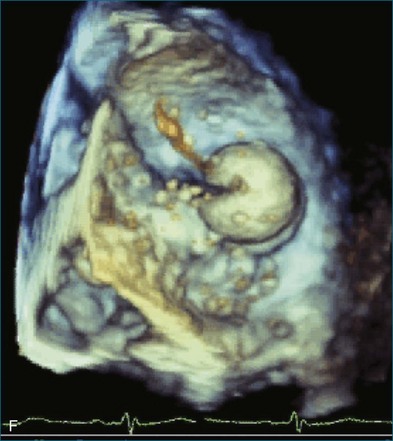
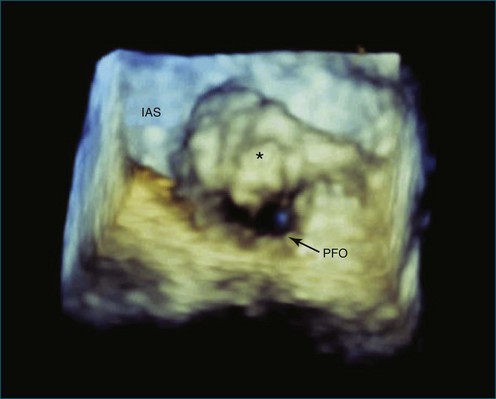
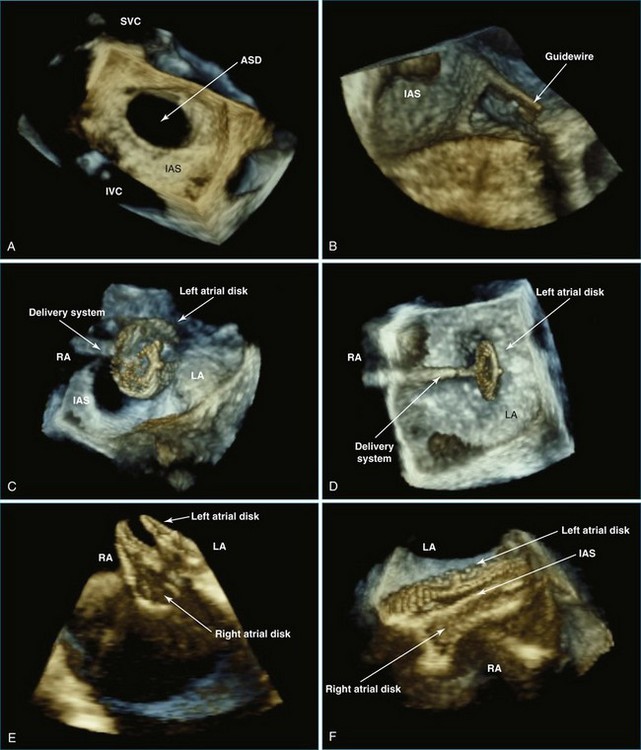
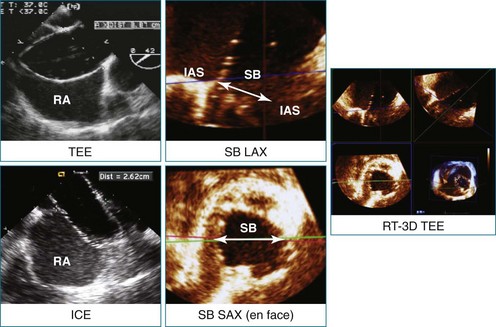
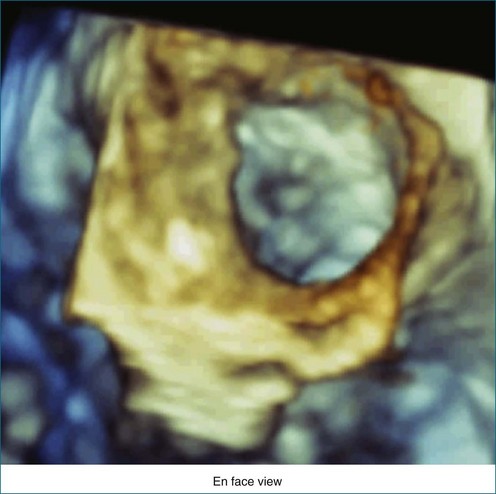
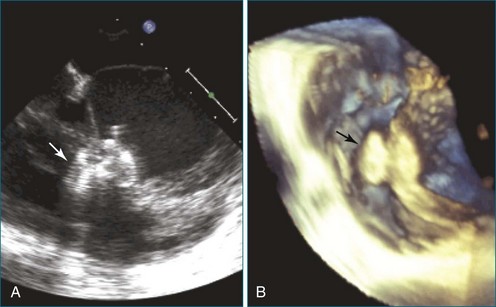
RT3DTEE is invaluable in preprocedural planning, particularly when device sizing is a concern. For instance, when a device must be selected for atrial septal defect (ASD), ventricular septal defect (VSD), or patent foramen ovale closure, 3DTEE can provide a true en face view of the defect so that its dimensions can be accurately measured. Such a defect often is elliptical or fenestrated such that 2D imaging cannot fully capture its complex shape.5 Furthermore, the relationship of a defect to vital structures such as the aortic root (in ASD closure) or mitral valve (in VSD closure) is easier to appreciate on RT3DTEE. This may help the interventionalist avoid complications during device deployment (Figure 14-9).
Because RT3DTEE can accurately characterize orifice areas and shapes, it also is well suited for catheter-based left atrial appendage (LAA) closure. RT3DTEE allows optimal visualization of the LAA in the vast majority of patients. 2DTEE tends to underestimate orifice area values, but RT3DTEE findings were shown to correlate well with computed tomography in one small study.6 Accurate device sizing and positioning are critical in this procedure to avoid complications such as device embolization, stroke, and hemopericardium from appendage laceration. RT3DTEE is used to guide transseptal puncture, catheter manipulation in the left atrium, and device deployment. Prior to the conclusion of the procedure, RT3DTEE confirms stability of the device.
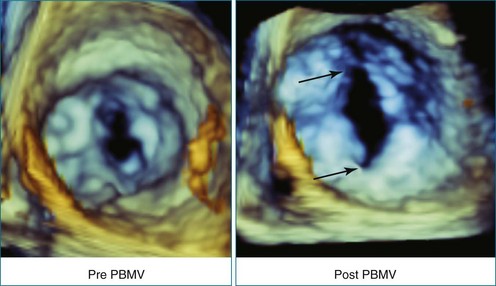
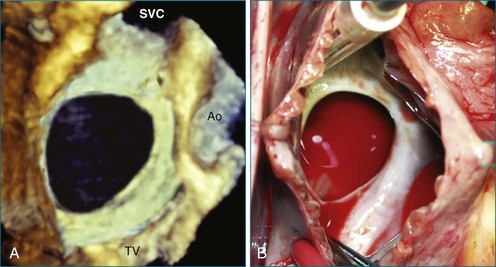
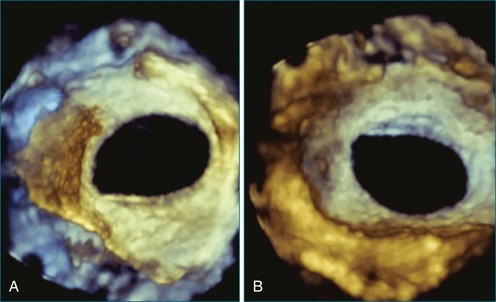
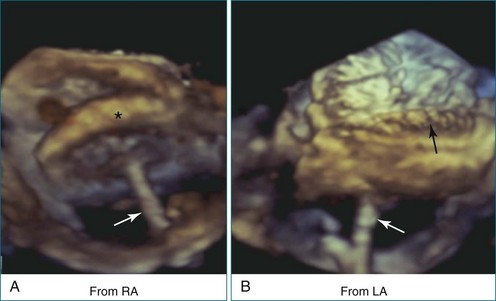
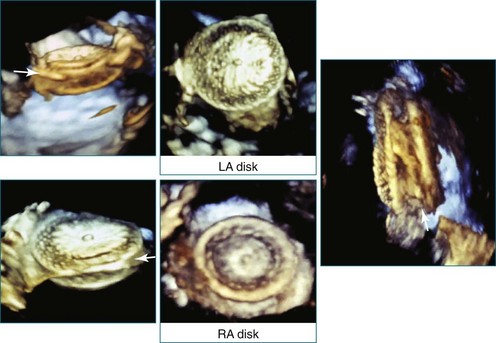
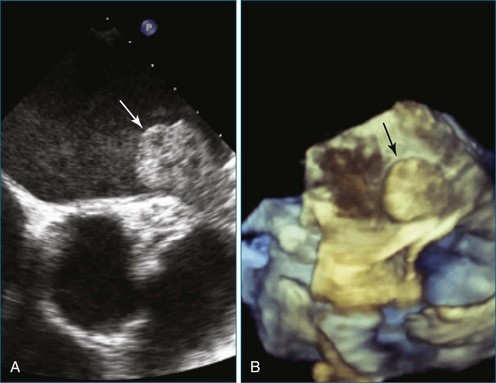
Perhaps the most spatially complex lesions addressed in the catheterization laboratory are periprosthetic valvular leaks. Dehiscence of a mitral valve annuloplasty ring or prosthesis, for instance, often results in a single elliptical defect, but multiple defects also may be present. Before starting such an intervention, a thorough evaluation of the relationship between the mitral annulus and the implant is essential to identify the site of the largest defect and the most significant periprosthetic regurgitation. In this setting, RT3DTEE provides significant additional detail that 2DTEE cannot.7,8 During the intervention, RT3DTEE facilitates positioning of wires, catheters, and the closure device. In a patient with a mechanical valve, this modality can help ensure that device deployment does not restrict prosthetic disk motion. Finally, RT3DTEE can be used to reevaluate mitral regurgitation at the conclusion of the intervention. In summary, transcatheter repair of periprosthetic regurgitation is very difficult, if not impossible, to perform effectively without the use of RT3DTEE guidance (Figures 14-10 to 14-16; Video 14-3).
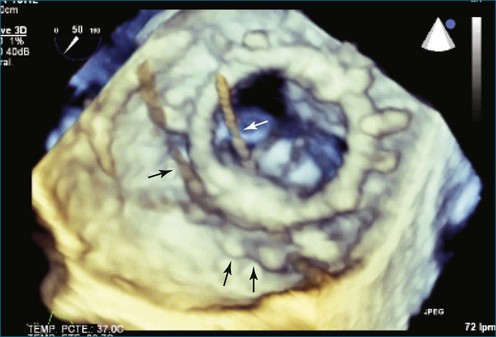
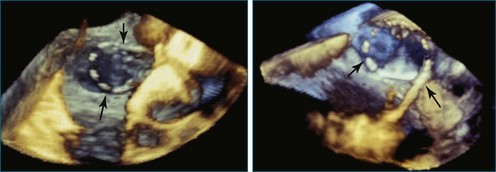
In addition to interventions for structural heart disease, RT3DTEE also shows promise for guidance of catheter-based arrhythmia ablation. In pulmonary vein isolation for atrial fibrillation, RT3DTEE can identify the orifices of the veins and confirm appropriate catheter tip placement. Unlike 2DTEE or ICE, it clearly demonstrates the relationship of the catheters to the endocardial surface. With RT3DTEE, it often is possible to see the entire length of a catheter within a region of interest, facilitating precise catheter manipulation (Figures 14-17 to 14-32).9 The utility of RT3DTEE in electrophysiologic procedures is an area that deserves further study.
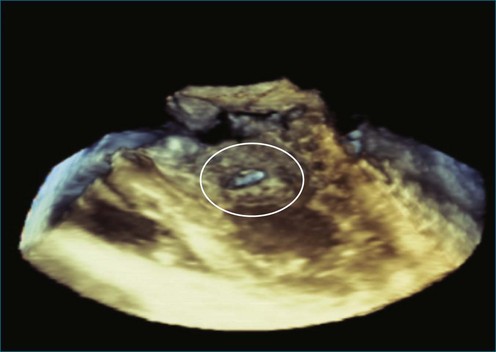
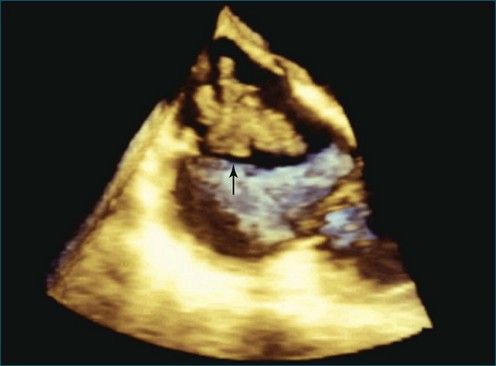
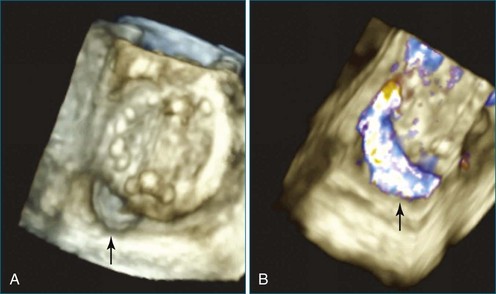
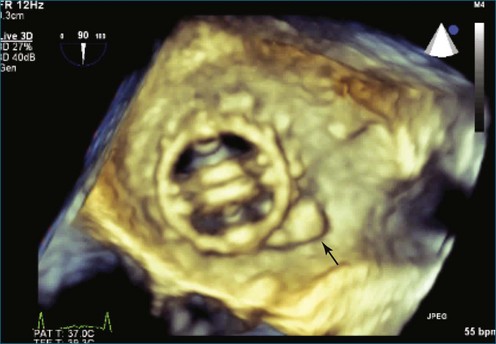
Figure 14-31 This real-time three-dimensional transesophageal echocardiography zoom mode image, obtained from the mid-esophageal window, shows the ventricular septal defect (VSD) in Figure 14-30 en face (oval) from the left ventricle. It is possible to measure the VSD’s dimensions precisely to facilitate selection of a repair device. Color Doppler could be used to illustrate flow across the defect.
Because image acquisition is relatively rapid with current technology, RT3DTEE does not appear to lengthen procedure times. One small, retrospective study of interatrial communication closures found that RT3DTEE, when added to 2DTEE, was associated with a reduction in fluoroscopy times.10 In a catheterization laboratory where many complex interventions are performed, such a finding could translate into a meaningful reduction in radiation exposure for staff and patients.
Current limitations of RT3DTEE include motion artifacts, which are worsened by respiratory motion; tissue dropout, which can result in blurred images; and visual delay with moving structures. Anterior cardiac structures, such as the tricuspid and aortic valves, may be less well visualized than more posterior structures, such as the mitral valve and left atrial appendage.4 Larger, controlled trials are needed to determine definitively whether RT3DTEE can improve procedural outcomes.
1 Perk G, Lang RM, Garcia-Fernandez MA, et al. Use of real-time three-dimensional transesophageal echocardiography in intracardiac catheter based interventions. J Am Soc Echocardiogr. 2009;22:865–882.
2 Sugeng L, Shernan SK, Salgo IS, et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J Am Coll Cardiol. 2008;52:446–449.
3 Lee PW, Lam YY, Yip GWK, et al. Role of real-time three-dimensional transesophageal echocardiography in guidance of interventional procedures in cardiology. Heart. 2010;96(18):1485–1493.
4 Altiok E, Becker M, Hamada S, et al. Optimized guidance of percutaneous edge-to-edge repair of the mitral valve using real-time 3-D transesophageal echocardiography. Clin Res Cardiol. 2011;100(8):675–681.
5 Lodato JA, Cao QL, Weinert L, et al. Feasibility of real-time three-dimensional transoesophageal echocardiography for guidance of percutaneous atrial septal defect closure. Eur J Echocardiogr. 2009;10:543–548.
6 Shah SJ, Bardo DM, Sugeng L, et al. Real time three-dimensional transesophageal echocardiography of the left atrial appendage: Initial experience in the clinical setting. J Am Soc Echocardiogr. 2008;21:1362–1368.
7 Kronzon K, Sugeng L, Perk G, et al. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J Am Coll Cardiol. 2009;53:1543–1547.
8 Kim MS, Casserly IP, Garcia JA, et al. Percutaneous transcatheter closure of prosthetic mitral paravalvular leaks: Are we there yet? J Am Coll Cardiol Interven. 2009;2:81–90.
9 Yang HS, Srivathsan K, Wissner E, Chandrasekaran K. Images in cardiovascular medicine: Real-time 3-dimensional transesophageal echocardiography: Novel utility in atrial fibrillation ablation with a prosthetic mitral valve. Circulation. 2008;117:e304–e305.
10 Balzer J, van Hall S, Rassaf T, et al. Feasibility, safety, and efficacy of real-time three-dimensional echocardiography for guiding device closure of interatrial communications: Initial clinical experience and impact on radiation exposure. Eur J Echocardiogr. 2010;11:1–8.