Gram-Negative, Oxidase-Positive Motile Rods
• P. aeruginosa is the most common clinically significant pseudomonad.
1. Gram-negative, oxidase-positive, aerobic rods with one to three flagella
3. Positive nitrate reduction test
6. Blue-green pigment (pyocyanin) or other diffusible pigments produced by many strains
1. Pili promote adherence to respiratory epithelium.
2. Capsule is antiphagocytic and promotes adherence to tracheal epithelium.
3. Exotoxin A inhibits protein synthesis by adenosine diphosphate (ADP)-ribosylation of elongation factor-2 (EF-2), similar to diphtheria toxin (see Chapter 10, Fig. 10-1C).
4. Biofilm production occurs when sufficient bacteria trigger quorum sensing.
5. Other virulence factors include the endotoxin exoenzyme S, which prevents phagocytic killing; degradative enzymes that damage host tissues; and antibiotic resistance.
• P. aeruginosa causes a wide variety of opportunistic infections, most commonly in hospitalized, immunocompromised, and debilitated individuals.
1. Urinary tract infection (UTI)
• P. aeruginosa is a common cause of UTI, especially in patients with indwelling catheters who are on antimicrobial therapy, which selects for drug-resistant strains.
• Vesicular and pustular lesions; cellulitis, abscesses, and subcutaneous infections
• In cystic fibrosis patients, P. aeruginosa and Staphylococcus aureus are the most common causes of chronic pulmonary infection, which exacerbates the underlying disease and is difficult to eradicate.
• In neutropenic and other immunocompromised individuals, P. aeruginosa acquired from contaminated respiratory therapy equipment can cause diffuse, bilateral bronchopneumonia.
6. Keratitis (fulminating ulceration of the cornea)
• Initial trauma to the eye followed by exposure to P. aeruginosa can lead to eye-threatening panophthalmitis without prompt treatment.
7. Disseminated infections of the immunocompromised host (e.g., transplant patient).
8. P. aeruginosa osteomyelitis
• P. aeruginosa is ubiquitous in the environment and in moist reservoirs (e.g., respiratory and dialysis equipment, cut flowers, sinks, and bars of soap) throughout hospitals and other institutions.
• Aminoglycoside (or fluoroquinolone) + antipseudomonal β-lactam antibiotic, depending on the susceptibility of the isolate
III Vibrio Species and Related Bacteria
• Vibrio, Aeromonas, and Plesiomonas species are all gram-negative, oxidase-positive motile rods that are salt tolerant or salt requiring.
• Comma-shaped rods grow in freshwater ponds and brackish water (e.g., where rivers empty into ocean).
• They can be cultured on most media used for stool culture (e.g., blood agar and MacConkey agar).
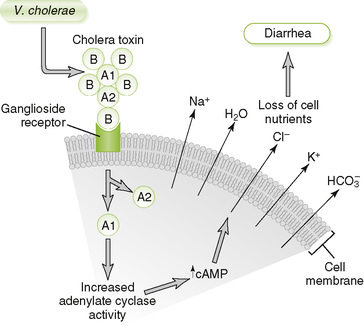
• Pili and other adhesins permit V. cholerae to adhere tightly to the mucosal epithelium.
• A-B type exotoxin ADP-ribosylates stimulatory G protein, leading to increased cyclic adenosine monophosphate (cAMP) level within mucosal cells and secretion of ions and fluid (Fig. 13-1).
• Rapid onset occurs 2 or 3 days after inoculation.
• Initial clinical manifestations are vomiting and severe watery diarrhea with mucous flecks (rice-water stools), leading to dehydration (volume depletion) and electrolyte imbalance.
• Complications in untreated patients include shock, acidosis, cardiac arrhythmia, and renal failure; there is a high mortality rate.
• Fecal-oral route spreads V. cholerae via water, fish, and shellfish.
a. Large inocula are required to establish infection; thus, direct person-to-person spread is unlikely.
• Carriers who have recovered from cholera may shed organisms and are an important reservoir of V. cholerae in endemic regions.
• Good public sanitation measures are important in reducing contamination of water sources with cholera-containing feces.
• Vaccination is largely ineffective, providing only short-term protection against the least serious strain of V. cholerae.
• Supportive care with fluid and electrolyte replacement is required.
B Other Vibrio species, Aeromonas species, and Plesiomonas species (Table 13-1)
TABLE 13-1
Vibrio cholerae and Related Organisms
| Organism | Source of infection | Disease manifestations |
| V. cholerae | Ingestion of contaminated water, raw fish, or shellfish | Severe, watery diarrhea with rice-water stools |
| V. parahaemolyticus | Ingestion of contaminated shellfish | Explosive, watery diarrhea, cramps, nausea; self-limited |
| V. vulnificus | Exposure to contaminated seawater or raw oysters | Wound infection with swelling, erythema, pain; eventual tissue necrosis and septicemia |
| Aeromonas hydrophila and Plesiomonas species | Ingestion of or exposure to contaminated water or food | Watery or bloody diarrhea, cramps, vomiting; wound infection; systemic disease in immunocompromised patients |
IV Campylobacter and Helicobacter Species
• Organisms in these genera are gram-negative, oxidase-positive motile rods that are microaerophilic and have complex growth requirements.
• Seagull-shaped rod with a single flagellum
• Selective medium (Campy plate) used for isolation from stool specimens
• C. jejuni invades and destroys mucosal surfaces of the jejunum, ileum, and colon.
• Endotoxin, enterotoxins, and cytotoxins are produced, but their role in pathogenesis is ill defined.
• Organisms are inactivated by gastric juices and sensitive to complement- and antibody-mediated killing.
3. Acute enteritis caused by C. jejuni
• Profuse watery or bloody diarrhea, malaise, fever, abdominal pain, and cramps are common symptoms.
• Disease is usually self-limited, but symptoms usually last for at least 1 week.
4. Association with Guillain-Barré syndrome
• Infection is commonly acquired by ingestion of contaminated food (especially poultry), milk, or water.
• Asymptomatic human carriers and animal reservoirs (puppies) promote the spread of C. jejuni.
• The incidence of C. jejuni enteritis peaks in young adults.
• Lack of gastric acids and hypogammaglobulinemia increase risk for C. jejuni infection and severity of disease.
• Curved rod with multiple flagella
• Neutralization of local stomach acid by urease-produced ammonia (NH3) promotes initial colonization.
• Rapid penetration of gastric mucus in mucus barrier is facilitated by multiple flagella.
• Localized tissue damage by mucinase and cytotoxin stimulates infiltration of inflammatory cells.
• Increased secretion of gastrin and gastric acid stimulated by infection promotes gastric metaplasia, which increases the risk for developing gastric cancer.
2. Diseases caused by H. pylori
• Most common cause of stomach and duodenal ulcers
• Chronic atrophic gastritis of pylorus and antrum with possible progression to gastric adenocarcinoma
3. Tests to identify H. pylori
• Campylobacter-like organism (CLO) test