Grafts Frequently Used During Orthognathic Surgery and for Adjunctive Procedures
Bone Grafting: Definitions and Options
During maxillofacial surgery, grafting is a procedure that replaces missing structures with tissues from the patient’s own body or with artificial, synthetic, or natural substitutes.24,33,40,50,56,60,62,69–71,78,106,111,112 It is estimated that more than 500,000 bone-grafting procedures are performed annually in the United States, and these numbers easily double globally. Approximately half of the procedures that involve grafts relate to spinal fusion. The exact number of grafts used in head and neck reconstruction is not known, but it is significant.
Bone grafting is possible because bone—unlike most other biologic tissues—has the ability to regenerate completely if it is provided with space into which it can grow.3–5,7,13,29,34,49,64,65,83,125–128 As native bone grows, it will generally replace any resorbable graft material in its path, which results in a fully integrated region of new bone. The biologic mechanisms that provide a rationale for bone grafting are osteoconduction, osteoinduction, and osteogenesis.
Osteoconduction occurs when the graft material serves as a scaffold that facilitates new growth from the native bone (Fig. 18-1, A). Osteoblasts from the margin of the defect use the graft material as a framework upon which to spread and generate new bone. The selected graft material for the management of a bone defect should at the very least be osteoconductive.
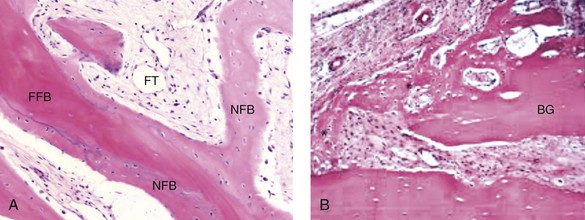
Figure 18-1 A, Osteoconduction. A photomicrograph of a bone biopsy, where NFB surrounds the pre-existing bone and the residual of FFB. NFB presents features of mature bone, with well-organized lamellae and numerous small osteocytic lacunae. B, Osteoinduction. Peripheral region of the graft at 10 days. New bone formation, with primary bone tissue from the bone graft (BG) fragment and the parietal bone. A from Acocella A, Bertolai R, Nissan J, Sacco R: Clinical, histological and histomorphometrical study of maxillary sinus augmentation using cortico-cancellous fresh frozen chips. J Craniomaxillofac Surg 39:192–199, 2011. B from Esteves JC, Borrasca AG, Aranega AM, et al: Histomorphometric analysis of the repair process of autogenous bone grafts fixed at rat calvaria with cyanoacrylate. J Appl Oral Sci 19:529–534, 2011.
Osteoinduction involves the stimulation of osteoprogenitor cells to differentiate into osteoblasts that then begin new bone formation (Fig. 18-1, B). The most widely studied types of osteoinductive cell mediators are bone morphogenic proteins (BMPs). A bone graft material that is both osteoconductive and osteoinductive will serve as a scaffold for existing osteoblasts, and it will also stimulate the formation of new osteoblasts, thereby theoretically promoting the faster integration of the graft.
Types of Bone Grafts
Bone Autografts
Fresh autogenous cancellous and corticocancellous bone are benchmark graft materials that allograft and bone substitutes attempt to match in their in vivo performance.2,25,117–124 They incorporate all of the aforementioned properties, and they have no associated risk of viral transmission. They are incorporated into surrounding bone through creeping substitution. Unfortunately, the availability of an autograft for a specific need may be limited, and the harvest may be associated with donor site morbidity.10
Bone Graft Substitutes
The use of premarket-approved rh-BMP-2 (Infuse Bone Graft; Medtronic, Minneapolis, Minn) as an autograft replacement for spinal fusion and the treatment of open tibia fractures. This substance has also been approved for maxillary sinus augmentation and localized alveolar ridge augmentation.
The use of rh-BMP-7 (OP-1 Implant; Olympus Biotech, Hopkington, Mass) as a humanitarian device. This substance has been approved as an autograft substitute for the non-union of long bones.
The use of rh-BMP-7 (Op-1 Putty; Olympus Biotech, Hopkington, Mass) as a humanitarian device alternative to autograft in compromised patients. This substance is approved for patients who require revision posterolateral lumbar spinal fusion or for those in whom autologous bone and bone marrow recovery (i.e., harvesting) are not feasible or are not expected to promote fusion.
Fresh autogenous cancellous bone and, to a lesser extent, cortical bone are the benchmark graft materials. Their shortcomings include limited availability, donor site morbidity, and the potential for resorption.
The advantages of allogenic bone include its availability in various sizes and shapes as well as the avoidance of host donor harvesting. The transmission of infection (particularly of human immunodeficiency virus) has been virtually eliminated as a concern when the grafts are properly treated.
The ideal bone graft substitute is biocompatible, bioresorbable, osteoconductive, osteoinductive, structurally similar to bone, easy to use, and cost-effective. Bone substitute products are currently available on the market. They vary with regard to their composition and their claimed mechanism of action.
U.S. Food and Drug Administration approvals of specific uses of recombinant human growth factors (i.e., rh-BMP-2 and rh-BMP-7) for the spine and the long bones have been made on the basis of demonstrated bone repair in human trials. Approved head and neck applications are limited but are likely to increase.
The orthognathic surgeon has choices in the realm of bone grafting. Selection should be based on reasonable burdens of proof. Examining the products’ claims and whether such claims are supported by preclinical and clinical studies for the specific sites to be used during clinical practice should be considered.
Grafting Interpositional Defects and Gaps of the Mandible and the Maxilla
Autogenous Iliac (Particulate Cancellous) Graft Donor Site ( Video 8)
Video 8)
Iliac (hip) particulate cancellous bone remains the preferred graft material for the management of congenital cleft defects of the alveolus, the palate, and the floor of the nose.1,9,12,15,26,28,46–48,52,63,66,77,82,90,115 This is especially true when the canine tooth is expected to erupt through the graft (i.e., cleft defect); when a tooth will be orthodontically moved into the grafted site; or when a dental implant will later be placed (Fig. 18-2). The cleft defect that requires a graft may be on one side (i.e., with unilateral cleft lip and palate) or on both sides (i.e., with bilateral cleft lip and palate). Previous attempts at grafting a bone deficiency may have failed as a result of an unsuitable graft material being selected; a soft-tissue deficiency or poor flap management; or inadequate postoperative management or patient cooperation (see Chapters 32 and 33).
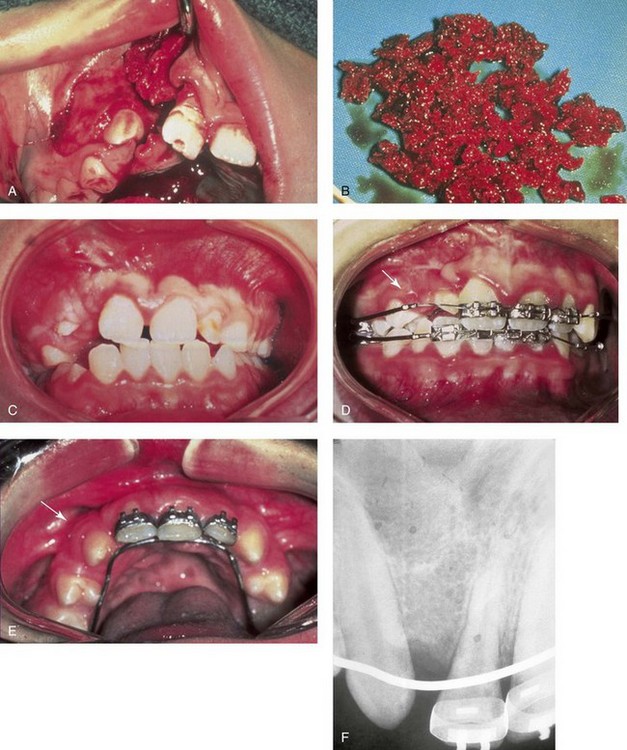
Figure 18-2 A child was born with unilateral cleft lip and palate and underwent arch expansion orthodontic treatment. This was followed by autogenous iliac bone grafting and fistula closure during the mixed dentition. A, Intraoperative view after the elevation of the mucogingival flap indicating the extent of the cleft defect. B, Harvested autogenous cancellous iliac graft used to fill the cleft defect. C, Occlusal view early after surgery. D, Later occlusal view with the early eruption of the canine. E, Occlusal and palate view after the eruption of the permanent canine through the bone graft. The canine and the other posterior teeth are orthodontically guided anteriorly to close the cleft and dental gap in the region of the congenitally missing lateral incisor. F, Periapical radiograph indicating normal alveolar ridge formation after the eruption of the canine through the bone-grafted cleft site.
Donor site discomfort in the anterior iliac region is generally caused by the injury or contusion of the surrounding muscles on the medial (i.e., external oblique, internal oblique, and rectus abdominis) and lateral (i.e., medial gluteus) sides of the bone.* In a thin individual with a minimal fat layer and without baseline ankle, knee, or lumbar region arthritis or myalgia who is relatively young (i.e., <50 years), only minimum discomfort should occur. However, there are reports of complications associated with anterior iliac crest graft harvesting, including gait disturbance, paresthesia, superficial infections, hematoma, poor cosmesis, and chronic donor site pain.6,20,41,42,53,57,81,92,103–105,110,113,114,131 The experienced surgeon can harvest the graft rapidly and efficiently. There is no need for the placement of a drain, minimal blood loss (i.e., <50 cc) is expected, and the head and neck region will have been simultaneously prepped and draped.
Step-by-Step Approach (Fig. 18-3)
Preparation and Draping
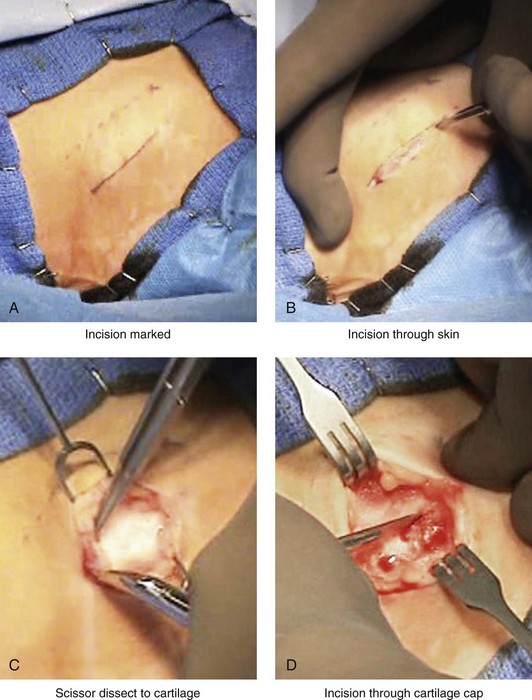
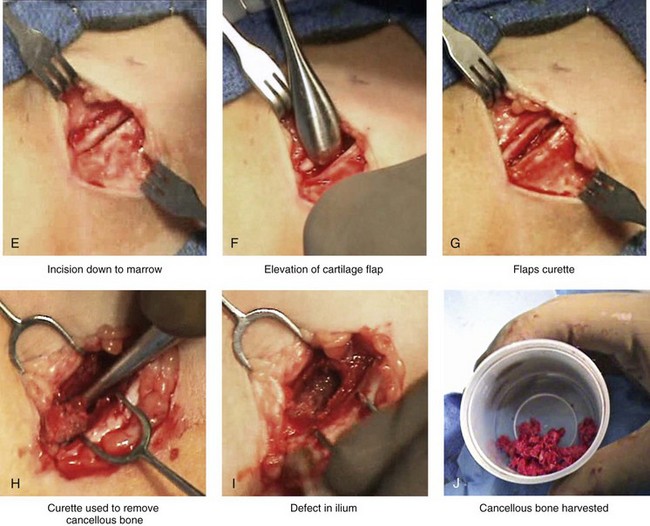
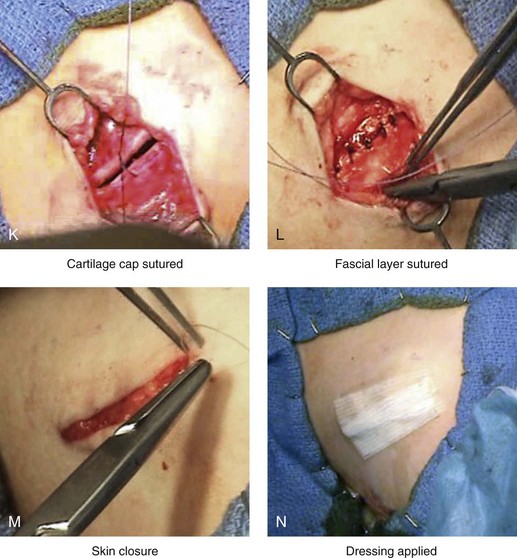
Figure 18-3 Autogenous iliac (particularly cancellous) graft donor site harvesting. A, The planned incision is parallel and 1 cm lateral to the iliac crest. The incision starts 1 cm posterior to the anterior superior iliac spine. B, After the injection of local anesthesia, the incision is carried out through the skin. C, Scissors dissection continues through the subcutaneous tissue and the superficial and deep fascia while avoiding the cutting and contusion of the gluteus muscle laterally or the abdominal oblique and transverse abdominis muscles medially. D, With a knife, the cartilaginous cap is split down the center directly over the crest and down to the marrow cavity. E, With a knife, perpendicular relaxing incisions are completed through the cartilage at either end of the main incision. F, With a periosteal elevator, each half of the cartilaginous cap (i.e., medial and lateral) is elevated off of the underlying marrow space. G, The cancellous marrow bone is now exposed. H, Exposed cancellous bone is harvested with curettes. I, With the necessary cancellous bone harvested, a defect in the marrow space can be seen. J, Harvested cancellous marrow bone to be used for reconstruction. K, The cartilaginous cap is repositioned back in place with interrupted ties (3-0 Vicryl). L, The deep and superficial fascia layers are closed with interrupted suture ties (3-0 Vicryl). M, Subdermal closure is performed with interrupted suture ties (4-0 Vicryl). The skin is closed with subcuticular running suture (5-0 Monocryl). N, Steri-Strips and then an occlusive dressing (Tegaderm) are placed over the skin.
• An endotracheal tube is placed and secured in accordance with the patient’s head and neck reconstruction needs.
• Ophthalmic ointment and corneal shields are placed for eye protection.
• The patient is supine on the operating room table.
• The patient’s arm is tightly tucked to his or her side.
• Betadine solution is used for both hip and head and neck preparation.
• At the hip, the patient is prepped halfway down the thigh, to the groin, across the abdominal midline, up to the rib cage, and posterior down the side of the buttock.
• Draping is completed, with limited exposed skin left around the planned incision site.
Skin Incision and Flap Elevation
• The incision is carried through the skin, the subcutaneous tissue, and the superficial and deep fascia directly over the iliac crest where the graft is to be harvested.
• The cutting or contusion of the gluteus muscle laterally or of the abdominal oblique and transverse abdominis muscles medially is to be avoided. Injury to these muscles when approaching the iliac crest should be avoided, because this is the primary cause of buttock and abdominal wall discomfort after surgery.
Graft Harvesting
Cartilage Cap Over Crest (Patient Typically <12 Years Old)
• If a cartilaginous cap remains over the crest, it is split down the center directly over the crest and down to the marrow cavity. Perpendicular relaxing incisions are completed through the cartilage at either end of the main incision. This allows for the lifting of each half of the cartilage cap (i.e., medial and lateral) off of the underlying marrow space. The lifting of the vascularized cartilage flaps is accomplished with a periosteal elevator.
• The exposed cancellous bone is then harvested with curettes.
No Cartilage Cap Over Crest (Patient Typically >12 Years Old)
• When the iliac crest is mature, no cartilaginous cap remains. In this case, a window of cortical bone is removed from the medial aspect of the crest and down the medial plate for approximately 2 cm. This is an efficient method for the exposure of the medullary cavity.
• The subperiosteal dissection of the medial crest and down the medial plate is followed by the placement of a toed-out retractor down the medial plate.
• The removal of the cortex is facilitated with the following: 1) an oscillating saw with a short fan blade on a long shaft to cut through the cortex of the medial plate at the inferior aspect; 2) an oscillating saw with a wide fan blade on a short shaft to cut through the cortex of the medial crest; and 3) a reciprocating saw with a short, straight blade to cut through the cortices of the medial plate to connect the other two cuts.
• The removal of the window of cortical bone is then easily accomplished with the use of a chisel and a mallet.
• With the window of cortical bone removed, cancellous marrow is harvested with curettes.
• The removed cortical bone may also be chopped or used as an intact cortical graft. There is no advantage to replacing the small cortical bone segment, because the strength of the crest has not been compromised. The bone defect will not be visible or palpable through the closed skin wound. The removed marrow and cortical bone regenerate themselves.
Wound Closure
• After adequate graft is harvested, bone wax is used for hemostasis. The bone wax is not used to fill the cavity; rather, a thin layer is placed directly over the bleeding medullary surfaces. Interestingly, the wax does not prevent the regeneration of bone that will fill the cavity.
• If present, the cartilaginous cap is repositioned and sutured back into place with interrupted ties (3-0 Vicryl). If a cap is not present, then the overlying periosteum is closed with interrupted ties (3-0 Vicryl).
• The deep and superficial fascia layers are closed with interrupted suture ties (3-0 Vicryl).
• Subdermal closure is performed with interrupted suture ties (4-0 Vicryl).
• The skin is closed with subcuticular running suture (5-0 Monocryl).
Autogenous Iliac (Corticocancellous Bloc) Graft Donor Site ( Video 9)
Video 9)
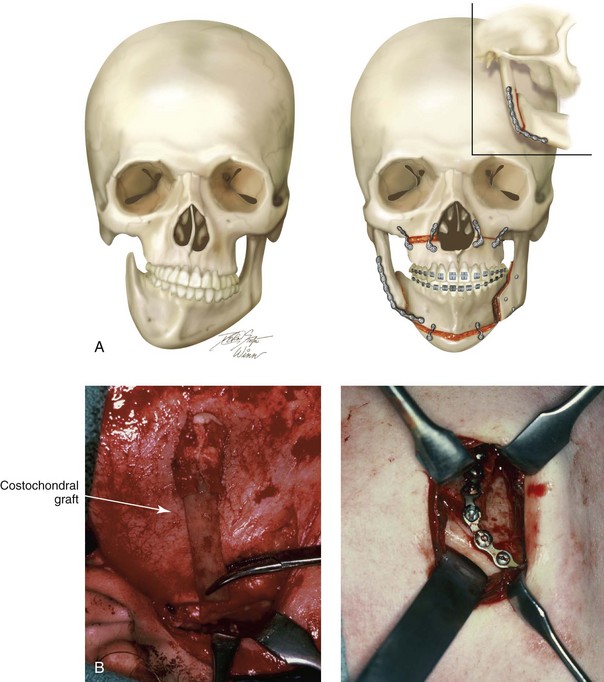
When a Le Fort I osteotomy with significant horizontal advancement and vertical lengthening is carried out, an interpositional defect (i.e., dead space) is created. Despite rigid plate and screw fixation of the repositioned maxilla, inadequate bone contact may jeopardize successful healing (e.g., fibrous union; see Chapter 16) or leave the upper jaw prone to skeletal relapse (see Chapter 17). The advantage of placing an interpositional graft is sometimes obvious and at other times borderline. When an interpositional graft is deemed necessary, I prefer to use either autogenous or allogenic iliac bone.107 A crafted corticocancellous anterior iliac (hip) graft is interposed between the pyriform and the zygomatic titanium plates that are used to secure the upper jaw in its new location (Fig. 18-4). The graft is tightly wedged between the advanced and lengthened anterior maxillary wall and the more posterior baseline maxillary wall. Any sharp edges of the inset graft are smoothed using a rotary drill with a watermelon bur. The graft is then fixed in place with an additional titanium plate and screws.
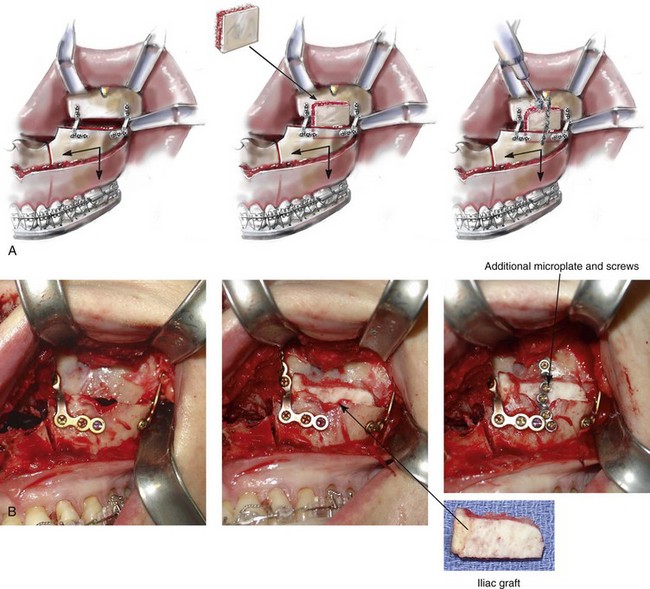
Figure 18-4 When the extent of horizontal advancement and vertical lengthening of the maxilla after Le Fort I osteotomy are judged to be significant, then an interpositional bone graft may be required and beneficial for the achievement of precise healing. In these circumstances, I prefer to use either autogenous or allogenic iliac bone graft. A bloc of corticocancellous bone is crafted and tightly wedged into the gap on each side. Each graft is inset in between the pyriform rim and zygomatic buttress titanium plates. An additional plate is then contoured to extend from the anterior maxilla above, across the graft, and then onto the alveolar process of the anterior maxilla. Titanium screws (1.2 mm in diameter) are then used to secure the plate to the bone. A, Illustrations and B, intraoperative views of interpositional corticancellous iliac graft in a Le Fort I osteotomy site.
Another indication for a corticocancellous bloc graft is when a ramus osteotomy (e.g., an inverted L or straight horizontal osteotomy) is completed. After the proximal segment is seated with the condyle in the glenoid fossa (i.e., the terminal hinge position) and the distal mandible is secured to the maxillary teeth via intermaxillary fixation, a significant interpositional gap may remain. In these circumstances, a crafted corticocancellous iliac graft can be tightly interposed between the proximal and distal segments with additional cancellous bone packed into the remaining dead space. Rigid plate and screw fixation of the osteotomy segments and the graft is always required (see Chapter 28).
When an anterior maxillary or mandibular segmental alveolar defect requires reconstruction (e.g., after trauma or tumor resection), a crafted corticocancellous anterior iliac graft is generally preferable to other options. After successful graft healing (i.e., 4 to 6 months), dental rehabilitation including implant and crown placement may follow (see Chapter 35).2
Donor site discomfort in the anterior iliac region is generally caused by the injury or contusion of the surrounding muscles on the medial (i.e., external oblique, internal oblique, and rectus abdominis) and lateral (i.e., medial gluteus) sides of the bone.* In a thin individual with a minimal fat layer and without baseline ankle, knee, or lumbar region arthritis or myalgia who is relatively young (i.e., <50 years old), only minimum muscle contusion and discomfort should occur. However, there are reports of complications associated with anterior iliac crest graft harvesting, including gait disturbance, paresthesia, superficial infections, hematoma, poor cosmesis, and chronic donor site pain.6,20,41,42,53,57,81,92,103,104,105,110,113,114,131 The experienced surgeon can harvest the graft rapidly and effectively. In general, there is no need for the placement of a drain, minimal blood loss (i.e., <50 cc) is expected, and the head and neck region will have been simultaneously prepped and draped.
Step-by-Step Approach (Fig. 18-5)
Preparation and Draping
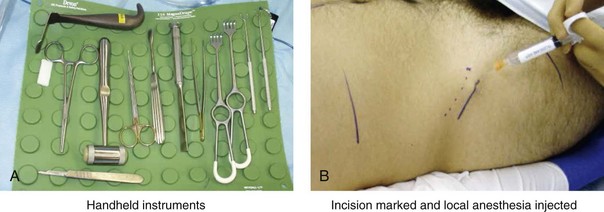
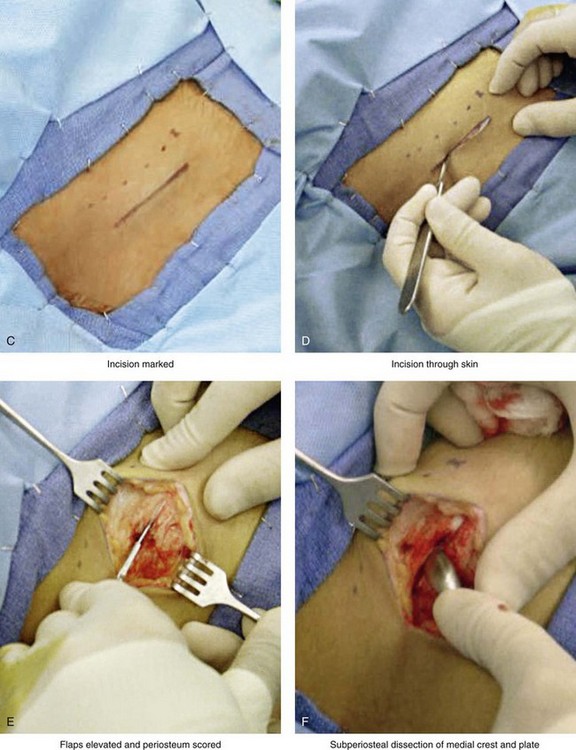
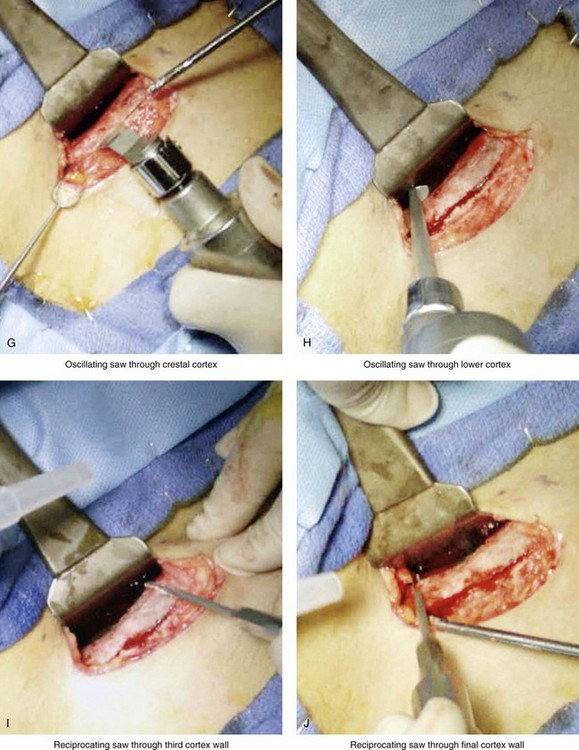
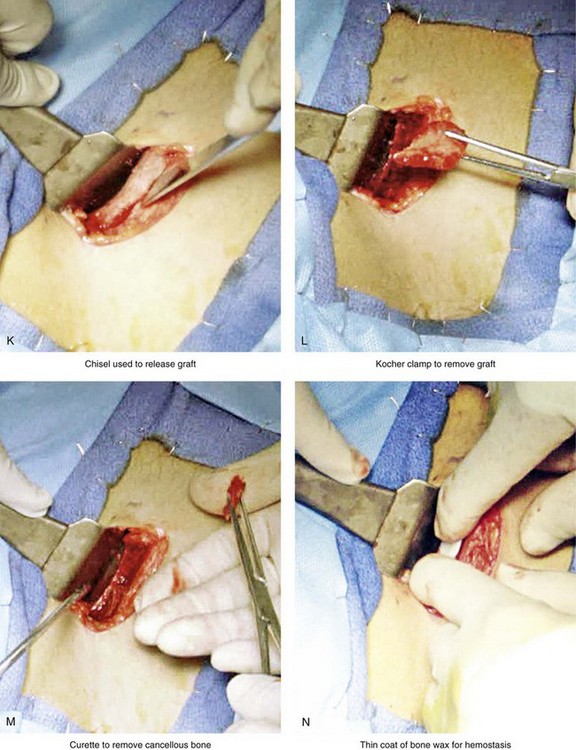
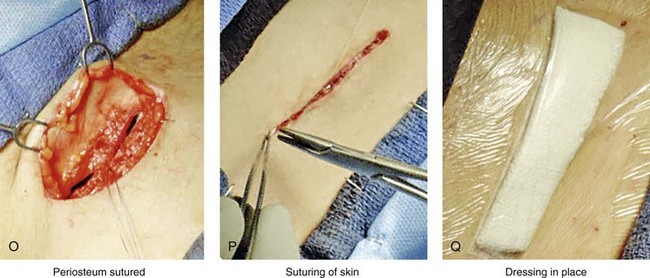
Figure 18-5 Intraoperative views of the harvesting of autogenous iliac corticocancellous bone. A, The basic handheld instruments that are used to harvest the graft. B, The planned incision is parallel and lateral to the iliac crest. It starts just posterior to the anterior superior iliac spine. Local anesthesia is injected below the skin directly over the surgical marking and down to the periosteum over the iliac crest. C, The planned incision is shown. D, With a knife, the incision is carried through the skin and the subcutaneous tissue. The scissors dissection continues through superficial and deep fascia directly over the crest, where the graft is to be harvested. The contusion of the gluteus muscle laterally or the abdominal oblique and transverse abdominis muscles medially is avoided. E, With the use of a knife, the periosteum is scored directly over the midline of the crest from just posterior to the anterior superior iliac spine. The incision continues posteriorly for a distance that depends on the extent of exposure required for the planned graft. F, Subperiosteal dissection of the medial crest and down the medial plate continues. G, A towed-out wide retractor is placed down the medial plate. The efficient removal of a bloc of corticocancellous bone graft is performed by first cutting through all four cortical wall borders. An oscillating saw with a wide blade on a short shaft is used to cut through the wall directly over the medial aspect of the crest. H, An oscillating saw with a short fan blade on a long shaft is next used to cut through the inferior cortical wall. I, A reciprocating saw with a short straight blade is used to cut through the lateral wall. J, A reciprocating saw with a short straight blade is used to cut through the last cortical wall. K, The separation and removal of the corticocancellous block is accomplished with the use of a chisel that is 10 mm in width. After the chisel is in place, a twisting motion is carried out to fully separate the graft. L, The bloc graft is removed with a Kocher clamp. M, Additional cancellous marrow is harvested with curettes as needed. N, A thin coat of bone wax is placed onto the marrow walls for hemostasis. O, The periosteum is closed with interrupted suture ties (3-0 Vicryl). P, After closing the deep and superficial fascia layers with interrupted sutures (3-0 Vicryl), subdermal and then subcuticular closure are accomplished (5-0 Monocryl). Q, Steri-Strips and then an occlusive dressing (Tegaderm) are placed over the skin.
• An endotracheal tube is placed and secured in accordance with the patient’s head and neck reconstruction needs.
• Ophthalmic ointment and corneal shields are placed for eye protection.
• The patient is supine on the operating room table.
• The patient’s arm is tightly tucked to his or her side.
• Betadine solution is used for both hip and head and neck preparation.
• At the hip, the patient is prepped halfway down the thigh, to the groin, across the abdominal midline, up to the rib cage, and posterior down the side of the buttock.
• Draping is completed, with limited exposed skin left around the planned incision.
Skin Incision and Flap Elevation
• The incision is carried through the skin, the subcutaneous tissue, and the superficial and deep fascia directly over the iliac crest where the graft is to be harvested.
• The cutting or contusion of the gluteus muscle laterally or of the abdominal oblique and transverse abdominis muscles medially is to be avoided. Injury to these muscles when approaching the iliac crest is the primary cause of buttock and abdominal wall discomfort after surgery.
Graft Harvesting
• With the use of a knife (no. 15 blade), the periosteum is scored directly over the midline of the crest from just posterior of the anterior superior iliac spine. The incision continues posteriorly for a distance that is dependent on the extent of exposure required for the planned graft.
• Subperiosteal dissection of the medial crest and down the medial plate continues. A toed-out retractor is placed down the medial plate.
• The efficient removal of a bloc of corticocancellous bone graft is accomplished using an oscillating saw (with a short fan blade on a long shaft and a wide fan blade on a short shaft) and a reciprocating saw (with a short, straight blade) to cut through all four bordering cortical walls.
• The corticocancellous bloc graft is then removed from the medial aspect of the crest and down the medial plate. The length of the graft along the crest and its depth into the medullary cavity are variable, depending on the patient’s reconstructive needs.
• The actual removal of the corticocancellous bloc is accomplished with the use of a 10-mm chisel and a twisting motion to fully separate the graft.
• With the bloc graft removed, additional cancellous marrow is harvested with curettes, as needed.
• The strength of the crest and of the ilium as a whole should not be compromised by the bloc graft removal. In general, the bone defect will not be detectable through the closed skin wound, because the lateral aspect of crest is preserved. The removed marrow and the cortical bone will regenerate, often with greater than normal thickness.
Wound Closure
• After adequate graft is harvested, bone wax is used for hemostasis. The bone wax is not used to fill the cavity; rather, a thin layer is placed directly over the bleeding medullary surfaces. The wax should not prevent bone regeneration.
• The periosteum is closed with interrupted suture ties (3-0 Vicryl).
• The deep and superficial fascia layers are closed with interrupted suture ties (3-0 Vicryl).
• Subdermal closure is performed with interrupted suture ties (4-0 Vicryl).
• The skin is closed with subcuticular running suture (5-0 Monocryl).
Alloplastic Graft (Porous Hydroxyapatite Bloc Implant)
When either a chin osteotomy with significant vertical lengthening or a Le Fort I osteotomy with significant horizontal advancement or vertical lengthening is carried out, an interpositional defect (i.e., dead space) is created. Despite rigid plate and screw fixation, inadequate bone contact across the osteotomy site may jeopardize successful healing (e.g., fibrous union) or leave the upper jaw or chin prone to skeletal relapse. When an interpositional graft is deemed necessary, I prefer to use either autogenous or allogenic anterior iliac crest. The disadvantage of an autogenous graft for this purpose relates to the potential for donor site morbidity. The use of porous bloc hydroxyapatite as an interpositional bone substitute in specific circumstances has been advocated by some.11,18,27,30,87,93,94,98,95,97,129,134,139
Coralline-derived porous bloc hydroxyapatite has been used successfully as an option when an interpositional graft is needed for chin-lengthening procedures (Fig. 18-6). Rosen and colleagues as well as other authors have published clinical reports of the use of this substance in the above-mentioned manner with low rates of complications and rapid healing.93–98 When used as an interpositional graft after Le Fort I osteotomy (as compared with at a chin osteotomy site), higher incidences of infection, sinus displacement, and extrusion have been reported and remain of concern.139
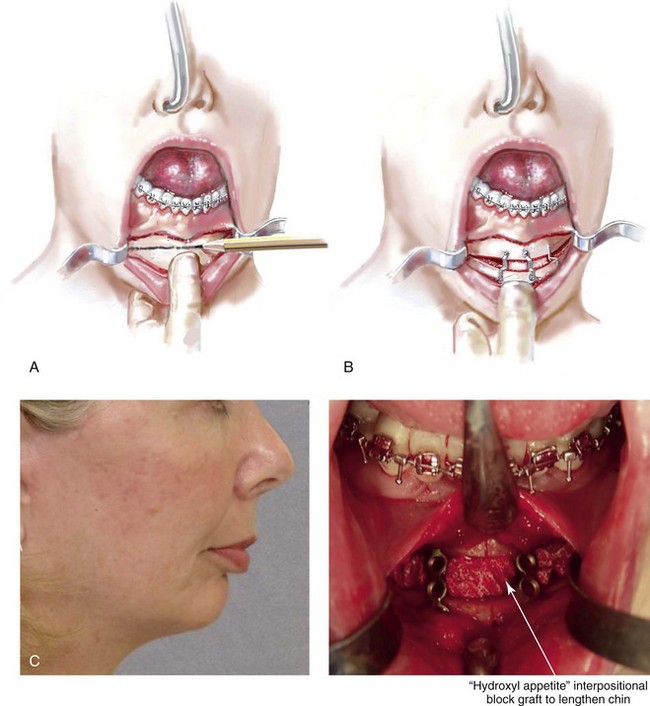
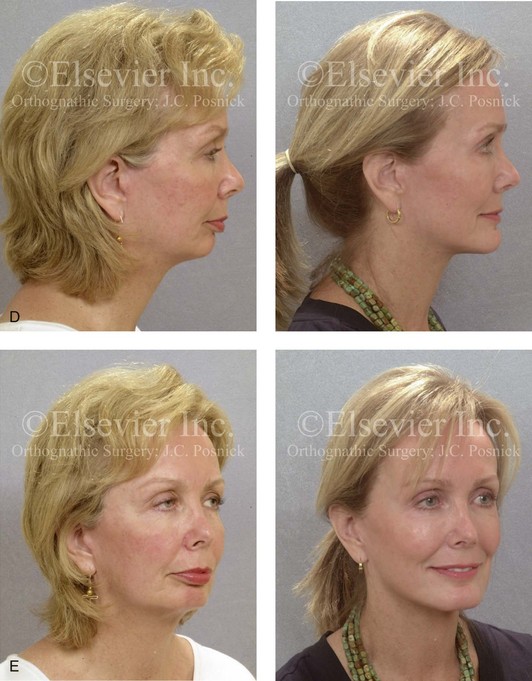
Figure 18-6 In conjunction with an osseous genioplasty, if significant vertical lengthening is carried out, an interpositional graft (e.g., autograft, allograft, bloc hydroxyapatite) is crafted and placed in the gap. The graft fills the central gap in between the fixation plates. I do not find it necessary to place a graft in all of the lateral aspects of the gaps. An additional plate with screws is generally placed vertically in the midline across the osteotomy site directly over the graft. A, Illustration of a chin with vestibular incision exposure. The marking pencil indicates the proposed osteotomy. B, Illustration of osseous genioplasty with an interpositional graft. C, Profile view of a woman with a retrognathic mandible and a vertically short chin. Intraoperative view of the same patient indicating the completed osseous genioplasty with an interpositional hydroxyapatite graft. D, Profile views of the same patient shown before and after sagittal ramus osteotomies (horizontal advancement) and osseous genioplasty (vertical lengthening with interpositional hydroxyapatite graft). E, Oblique facial views before and after reconstruction (see Fig. 25-2).
Grafting Condyle and Ascending Ramus Defects
Autogenous Rib (Costochondral) Donor Site
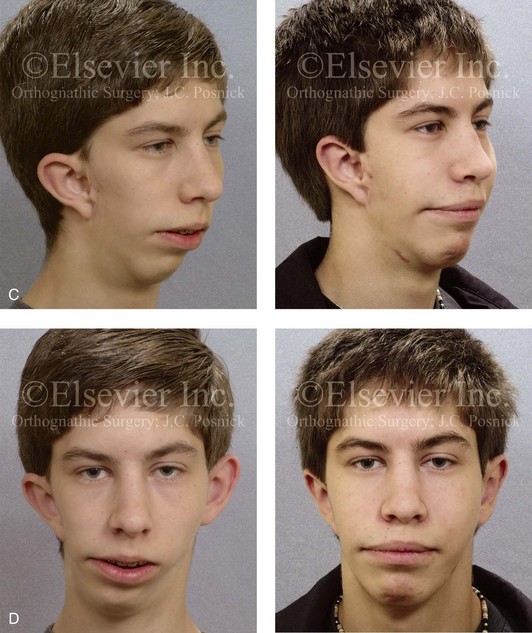
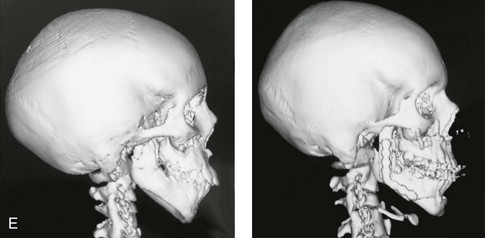
When the condyle and portions of the ascending ramus have been removed (e.g., after tumor or trauma) or are congenitally absent (e.g., in a patient with hemifacial microsomia with Kaban type IIB malformation), there will be a limited number of reconstructive options to choose from, including an autogenous costochondral graft; an artificial total joint replacement; an autogenous vascularized fibular composite flap; or a sagittal split ramus osteotomy with proximal segment repositioning (see Chapters 27, 28, 35, and 36). For a patient who requires condyle and ascending ramus reconstruction, these options are not likely to give equivalent results. Clinical judgment and experience are required to assess the defect and the patient-specific factors (i.e., age, medical condition, quality of the recipient bed, and dental rehabilitation needs) before recommending the option that is best suited to the individual’s reconstructive needs.
Step-by-Step Approach
Preparation and Draping
• Nasotracheal intubation (i.e., RAE tube) is completed and secured in accordance with the patient’s head and neck reconstruction needs.
• Ophthalmic ointment and corneal shields are placed for eye protection.
• The rib to be harvested is palpated and identified.
• Betadine solution is used for both chest wall and head and neck preparation.
• The patient is prepped below the rib cage, past the chest midline, superior to the nipple, and laterally to the midaxillary line.
• Draping is completed, with limited skin exposure left parallel to and on all sides of the planned incision.
Skin Incision and Flap Elevation
• With the use of a knife (no. 15 blade), the incision is carried through the skin, the subcutaneous tissue, the fascia, and the muscle directly over the rib to be harvested.
• Superior and inferior flaps are elevated to provide deep exposure of approximately 6 cm of bone and 2 cm of adjoining cartilage.
Graft Harvesting
• The periosteum is scored directly over the mid portion of the long axis of the rib to be harvested but not through bone or into the cartilaginous cap.
• Curved and straight elevators are used to dissect in the subperiosteal plane circumferentially around the rib to be harvested.
• A curved subperiosteal dissector (e.g., Doyen) is then inserted underneath and around the rib. This instrument is used to further strip and gain exposure in the subperiosteal plane, including underneath the cartilaginous cap.
• For protection, the retractor (e.g., Doyen) is placed deep and directly underneath the location for incising the cartilaginous cap.
• A knife (no. 15 blade) is then used to incise the full thickness of the cartilaginous cap, including not more than 1 cm of cartilage attached to the bone.
• The retractor (e.g., Doyen) is removed, and the rib cutter is inserted to cut and completely separate the rib according to the length of bone required for the purposes of reconstruction. The graft is removed.
Wound Closure
• The wound is checked for hemostasis and any exposure into the lung cavity; this is done by filling the wound with saline. The anesthesiologist introduces maximum end tidal volume and holds the area under pressure with the Valsalva maneuver. With the confirmation of no pneumothorax, wound closure is accomplished.
• The periosteal sleeve is approximated with interrupted suture ties (3-0 Vicryl).
• The deep and superficial fascia layers are closed with interrupted suture ties (3-0 Vicryl).
• Subdermal closure is performed with interrupted suture ties (4-0 Vicryl).
• The skin is closed with subcuticular running suture (5-0 resorbable).
Postoperative Care
• A postoperative chest radiograph is used to confirm that there is no injury to the lungs.
• The patient may shower without concern about moisture on the occlusive dressing that is covering the wound.
• The dressing remains intact for approximately 7 to 10 days.
• When the dressing is removed, no additional bandages are placed.
Cartilage Grafting for Nasal Reconstruction
Over the last several decades of cosmetic rhinoplasty, the paradigm has shifted from aggressive reductive procedures to augmentation procedures. More attention is now focused on the use of grafts to augment deficiencies and the appropriateness of the graft material selected for each indication. When surgically altering the shape of the nose, two frequent reconstructive concerns are inadequate structural integrity and volume. These problems are generally best addressed with the use of cartilage grafts taken from a limited number of donor sources, such as the septum, the conchal bowl of the ear, or the rib cartilage. The choice of grafts and of the techniques used to place them remain both an art and science (see Chapter 38).14,31
Autogenous Nose (Septal) Cartilage Donor Site
Septal cartilage is generally reasonably straight and resilient; it provides structural support similar to that of the native nasocartilaginous framework. The septal cartilage can be contoured, carved, and sutured into an exact location. Significant resorption, infection, or warping is uncommon unless the cartilage is subjected to high-tension forces or crushing during preparation. Preferred uses for septal cartilage in nasal reconstruction often include caudal struts, lower lateral cartilage (LLC) crural grafts, spreader grafts, and tip grafts (e.g., shield grafts) to increase projection or definition (Fig. 18-8).38,39
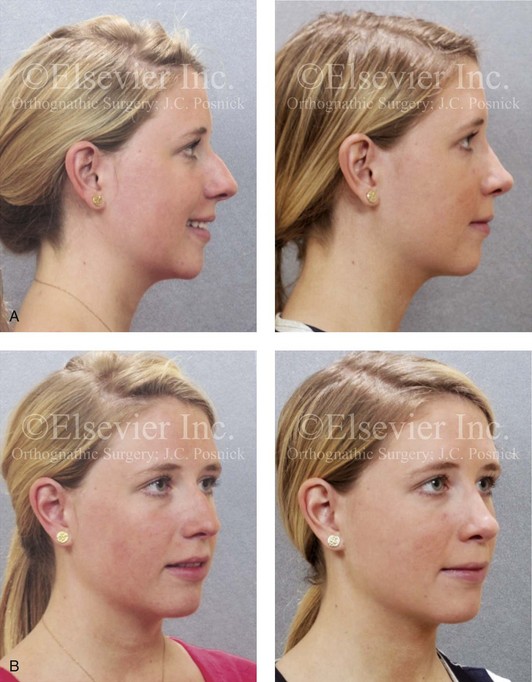
Figure 18-8 A, Profile views of a teenaged girl before and after rhinoplasty with the use of a septal (caudal strut) cartilage graft to improve tip position and projection as described in Fig. 18-9. B, Oblique facial views before and after reconstruction.
A main disadvantage of the use of septal cartilage in nasal reconstruction is the limited amount that is often available. Anterior to the vomer and perpendicular plate of the ethmoid, septal cartilage can be harvested without concern, but always with the maintenance of the anterior structural support of the cartilaginous septum (i.e., adequate dorsal and caudal struts) to prevent dorsal or caudal collapse. In most individuals, unless the septal cartilage has been previously removed through submucous resection or is absent for other reasons (e.g., infection, congenital condition, ischemic necrosis), adequate cartilage can generally be harvested to serve as a caudal strut graft (i.e., to provide tip support); spreader grafts (i.e., to widen the nasal valves or to straighten the cartilaginous dorsum); LLC support (i.e., for the cleft or “pinched” nose); or as additional tip augmentation (see Chapters 29, 32, 33, 35, and 38).
Step-by-Step Approach (Fig. 18-9)
Preparation and Draping for Rhinoplasty
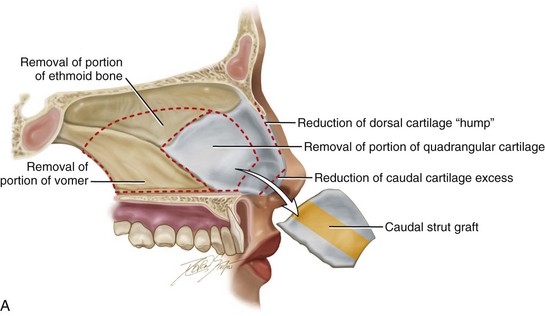
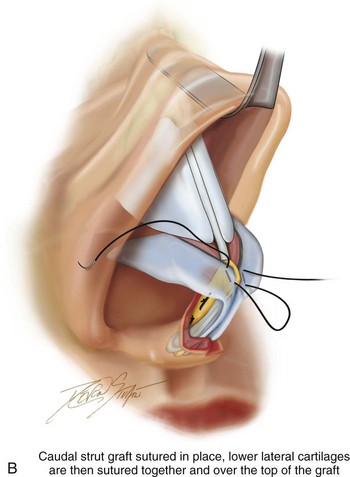
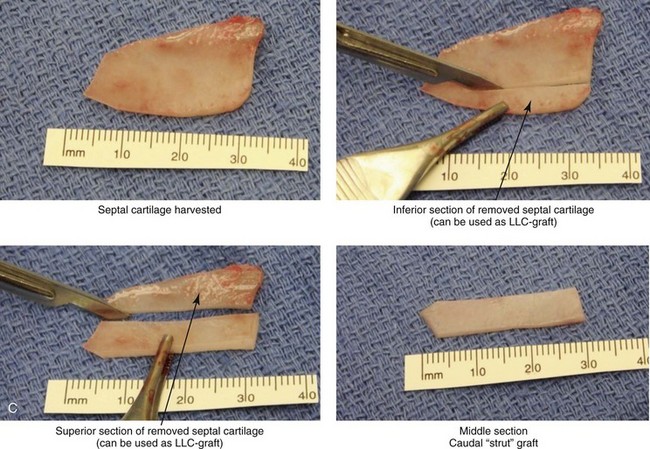
Figure 18-9 A, Illustrations that demonstrate the location of the harvesting of a septal cartilage graft and B, of the graft’s placement as a caudal strut. C, Intraoperative views of removed septal cartilage that can be used for grafts to reconstruct the nose. The cartilage can be crafted as a caudal strut, spreader grafts, a lower lateral cartilage augmentation graft, and as tip onlay grafts.
• Orotracheal intubation (i.e., RAE tube) is placed as required for the rhinoplasty procedure. The tube is taped to the chin to prevent dislodgement.
• Ophthalmic ointment and corneal shields are placed for eye protection.
• If the patient’s hair is long, it is tied in a ponytail.
• Betadine solution is used for the preparation of the scalp, forehead, external ears, face, and neck. (Do not use soap, alcohol, Hibiclens, and so on.)
Local Anesthesia
• Xylocaine (1% with 1 : 100,000 epinephrine) is injected deep into the skin within the soft-tissue envelope of the nose as is routine for rhinoplasty.
• Xylocaine (1% with 1 : 100,000 epinephrine) is injected on each side of the septum, deep to the mucosa and superficial to the cartilage.
• Cocaine-soaked pledgets (5 cc of 4% solution) are placed in each nasal cavity.
Graft Harvesting
• Separate the lower lateral cartilages with Stevens scissors. Dissect down to and identify the anterior aspect of the caudal septal cartilage. Use a sharp periosteal elevator (e.g., Cottle) to initiate the submucosal dissection on each side of the caudal edge of the septal cartilage. Extend the submucosal dissection inferior to the maxilla and posterior to the vomer. Continue the subperiosteal dissection as required for the resection of the deviated bony septum.
• Use a knife (no. 15 blade) to initiate a caudal and dorsal septal incision through the quadrangular cartilage for the insertion of a swivel knife. The incision is located to maintain approximately 10 mm of caudal and 10 mm of dorsal septal cartilage.
• Insert the swivel knife, and incise the septal cartilage for graft harvesting and for submucous resection as indicated.
Wound Closure
• After the completion of the septorhinoplasty procedure, the closure of wounds is performed per routine rhinoplasty.
• To approximate the columella skin (i.e., the stair-step incision), a single dermal suture is placed (5-0 Vicryl).
• Columella skin closure is performed with interrupted suture ties (6-0 nylon).
• Intranasal wound closure is performed with interrupted ties (5-0 chromic).
Placement of Dressing
• The placement of dressings is routine as per the septorhinoplasty procedure.
• Flexible splints (e.g., Reuter Type-Medtronic USA INC, Jacksonville, FL.) are generally placed on each side of the septum and secured together with a single suture tie (3-0 nylon).
• Antibiotic ointment (e.g., Bactroban) is generally injected into each nostril cavity.
• Packing (e.g., folded Telfa) is generally inserted equally into each nostril cavity.
• Tape (e.g., a Steri-Strip) is placed over dorsum of nose as per rhinoplasty procedure.
• A custom splint (e.g., Aquaplast) is molded over the dorsum of nose as per rhinoplasty.
Autogenous Ear (Conchal) Cartilage Donor Site
Conchal cartilage may be required for augmentation during secondary rhinoplasty when prior harvest, trauma, infection, or a genetic deficiency has rendered the nasal septal framework deficient and unavailable as a graft source.76,79 In these cases, conchal cartilage may be the default option for non-structural nasal reconstruction applications. The conchal cartilage is pliable, resilient, flat, and thin. The conchal bowl may also have the natural contour to fit a specific nasal reconstructive need. Histologically, auricular cartilage is elastic hyaline. The flat base of the conchal bowl serves as the donor site and is harvested through a postauricular skin incision without leaving a noticeable aesthetic change in the external ear. The conchal bowl is composed of two components: the superior cymba and the inferior cavum. These are divided by the conchal extension of the helical root. The conchal bowl is bordered by the helical root and the external auditory meatus. Human anatomic dissection confirms the dimensions of the conchal bowl: it has a thickness of 1.9 mm to 4.4 mm; a maximum width of 1.9 cm to 2.9 cm; and a maximum height of 1.9 cm to 3.1 cm that can be harvested. During the harvesting of a conchal graft, the sidewalls are preserved to maintain the structural stability of the ear. A conchal graft can be useful for the reconstruction of the LLC (e.g., cleft nasal deformity, overresected iatrogenic deformity); spreader grafts (i.e., when minimal structural support is required); or nasal tip augmentation (i.e., when the underlying support is adequate). It is almost always a second or third donor site choice when nasal structural support is required, because it lacks sufficient strength to be used routinely as either a dorsum or caudal strut framework. In most cases, septal cartilage is preferred for these indications. For situations in which significant stretching of the overlying soft-tissue envelope is required to achieve tip projection (e.g., cocaine saddle deformity, bilateral cleft nasal deformity, Binder nasal deformity), a rib cartilage graft will best provide the needed framework support (see Chapters 29, 32, 33, 35, and 38).
Step-by-Step Approach
Preparation and Draping
• Orotracheal intubation (i.e., RAE tube) is placed as required for the rhinoplasty procedure. The RAE tube is taped to the chin to prevent dislodgement.
• Ophthalmic ointment and corneal shields are placed for eye protection.
• If the patient’s hair is long, it is tied in a ponytail.
• Betadine solution is used for the preparation of the scalp, forehead, external ears, face, and neck. (Do not use soap, alcohol, Hibiclens, and so on.)
• Draping is completed, with exposure of the forehead, the external ears, the face, and the neck down to the clavicles left in the operative field as per the routine for rhinoplasty.
Graft Harvesting
• The place at which the flat and curved portions of the conchal bowl meet is identified.
• The planned incision is marked to follow the natural curve of the conchal cartilage at the location at which the flat bowl begins.
• With a fresh knife (no. 15 blade), the full thickness of the conchal cartilage is incised at the surgical marking, without perforation through the anterior skin.
• With the use of a sharp periosteal elevator (e.g., Cottle), the incised cartilage is freed from the tightly attached overlying anterior skin along the flat portion of the conchal bowl.
• A decision about the volume of the conchal cartilage graft required is made.
• The conchal bowl is incised at the desired location to achieve graft requirements.
• Hemostasis and the integrity of the anterior auricular skin are confirmed.
Placement of Dressing
• A thick coat of antibiotic ointment is placed in the postauricular fold and over the anterior ear skin surface.
• A sheet of gauze (e.g., Xeroform) is placed in the postauricular groove and also on the anterior skin surface.
• Two or three pieces of dry “fluff” gauze are placed over the anterior surface of the ear.
• Gauze rolls (e.g., Kerlix) are used as a wrap to hold the fluff gauze and to apply gentle pressure. The gauze dressing is looped around the anterior neck and the forehead to secure it.
• An Ace wrap is loosely placed as an outer layer of the head and ear dressing.
• The whole dressing is removed without replacement 5 to 7 days later.
Postoperative Care
• After the ear dressing is removed, the patient is encouraged to shower and shampoo the hair, the external ears, the face, and the neck once or twice daily as per the rhinoplasty routine.
• The donor ear is dabbed dry (i.e., not rubbed) after washing.
• No vigorous sports activities are allowed during the early phase of healing (i.e., approximately 4 to 6 weeks) as per rhinoplasty.
Autogenous Rib (Cartilage Only) Donor Site (Video 10 )
)
When significant structural support is required for either the caudal septum or dorsum of the nose within a tight soft-tissue envelope, neither conchal nor septal cartilage will generally be adequate. In these cases, autogenous rib cartilage will frequently be the graft material of choice (Fig. 18-10) (see Chapters 29, 32, 33, 35, and 38).37,59,68,84–86,108,109 Costal cartilage is available in abundance, it undergoes minimal postoperative resorption, and it is relatively easy to carve into a strut graft. Its perceived donor site harvesting difficulties often dissuade surgeons from choosing it; these include the need for a small scar on the anterior lower chest wall as well as concerns about pneumothorax and postoperative pain. With meticulous surgical technique, these potential disadvantages are generally overcome. However, somewhat unpredictable occasional graft warping that may jeopardize the long-term aesthetic results when used as either a dorsal or caudal strut remains a concern. Gibson’s experimental studies provide the principles for understanding and managing cartilage warping.31 He suggested the use of “balancing cross sectional carving along the long axis of the cartilage as a way to limit/prevent warping.” The general approach when carving costal cartilage involves symmetrical removal from both sides and then using the central core of the cartilage for reconstruction and augmentation whenever feasible. In vitro studies suggest that the full distortion of the graft may be demonstrated within 30 minutes of carving. At a minimum, using Gibson’s balanced carving principles and waiting 15 minutes before graft placement are likely to uncover warping tendencies in a majority of cases. We often use internal stabilization of the dorsal strut (and, less often, of the caudal strut) via the placement of a Kirschner wire (K-wire; no. 35 threaded) through the spine of the graft (see Chapters 29, 35, and 38).
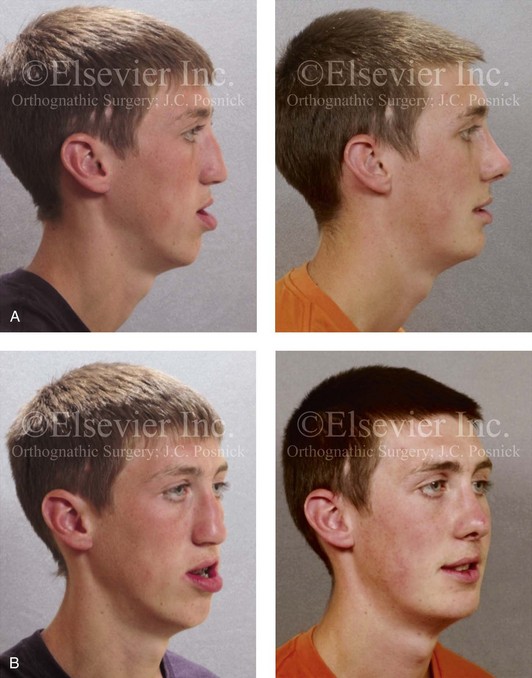
Figure 18-10 Facial views of a teenager who was born with unilateral cleft lip and palate before and after cleft jaw reconstruction followed by cleft rhinoplasty. The nasal reconstruction involved the use of a rib cartilage (caudal strut) graft as described in Figure 18-11. A, Profile facial views before and after reconstruction. B, Oblique facial views before and after reconstruction.
Definitive rhinoplasty will be required for the reconstruction of the residual bilateral cleft lip nasal deformity at some point after the early teenage years. A “short” columella generally defines the presenting nasal deformity in the individual with bilateral cleft lip and palate (see Chapter 33). A crafted autogenous rib cartilage caudal strut provides the structural support to adequately stretch the columella skin and the soft-tissue envelope for favorable tip projection (see Fig. 18-10). The graft is stabilized to the base of the maxilla with a short, buried K-wire (no. 35 threaded). The LLCs are then sutured together over the top of the rib cartilage caudal strut to form the most superficial aspect of the tip (see Chapter 38).
For the reconstruction of the severe posttraumatic or congenital saddle nose deformity, rib cartilage often has advantages as compared with bone in that it restores at least a degree of springiness to the cartilaginous vault and tip; it is considered easier and less morbid to harvest; and it is not prone to resorption (see Chapter 35). A rib cartilage dorsal strut may extend from the radix to the nasal tip or just from the inferior aspect of the nasal bones to the tip. A K-wire may be inserted through the spine of the dorsal strut to prevent warping; this is done before graft inset. The dorsal strut is combined with a separate rib cartilage caudal strut, which extends from the base of the maxilla to the tip. A short buried K-wire secures the caudal strut to the base of the maxilla. The two grafts are then sutured together to form the new tip. The LLCs are sutured together and then over the top of the grafts to establish the most superficial aspect of the tip (see Chapter 38). The soft-tissue envelope is redraped over the reconstructed cartilaginous vault and tip.
For the correction of the cocaine-induced or iatrogenic saddle deformity of the nose, a rib cartilage graft is typically crafted into two struts (see Chapter 38). The first is a caudal strut that extends from the base of the maxilla to the new nasal tip. Assuming adequate height of the nasal bones (i.e., the osseous vault), the second graft (i.e., the dorsal strut) is inset deep and then flush with the bony dorsum. The dorsal strut extends to the new nasal tip. Each cartilage graft is secured in place at its point of insertion, and the grafts are then sutured to each other where they join at the tip. The LLCs are sutured together and then over the top of the grafts to form the most superficial aspect of the tip (see Fig. 18-9). The soft-tissue envelope is redraped over the reconstructed cartilaginous vault and tip (see Chapter 38).
Step-by-Step Approach (Fig. 18-11)
Preparation and Draping
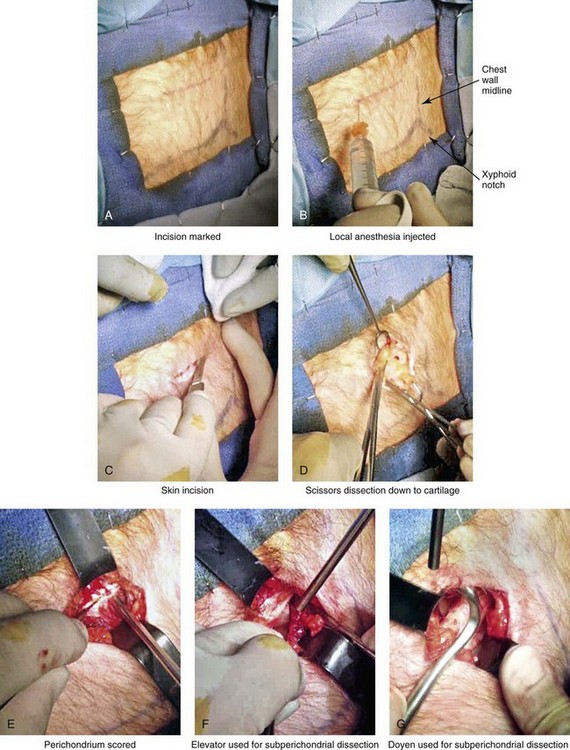
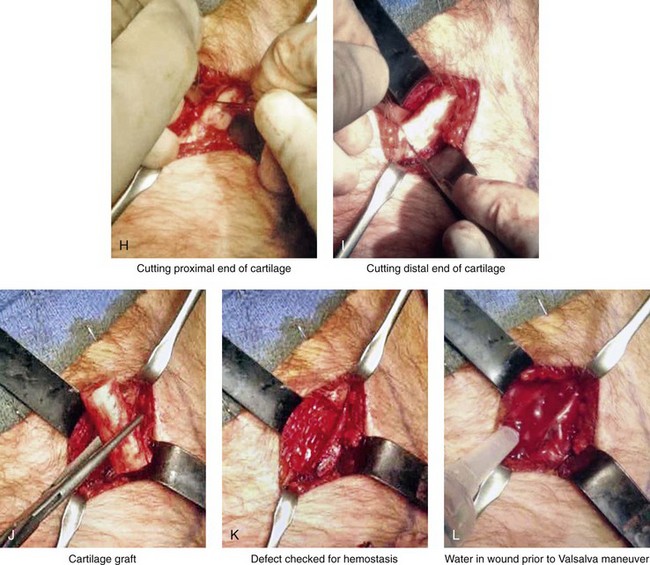
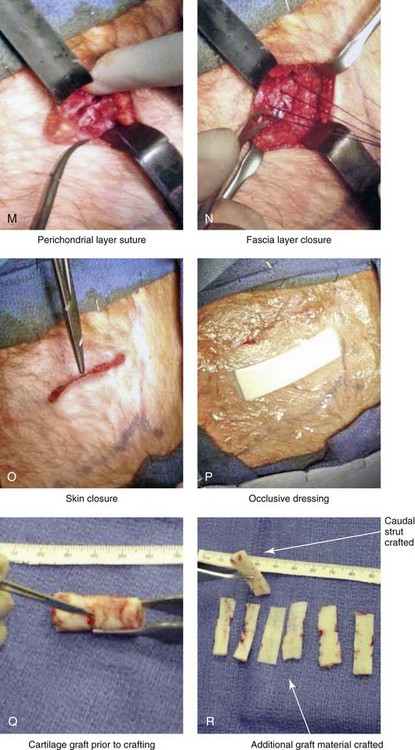
Figure 18-11 Intraoperative views of autogenous rib (cartilage only) donor site harvesting. A, The planned incision is marked directly over the rib cartilage to be harvested (≈4 cm incision length) before prepping and draping. B, Local anesthesia (Xylocaine 1% with epinephrine) is injected in the soft tissues below the skin and to the depth of the rib cartilage to be harvested. C, With a knife, the incision is carried through the skin and the subcutaneous tissue. D, Scissors dissection continues through the fascia directly over the rib cartilage. E, The perichondrium is scored with a knife directly over the mid portion of the long axis of the rib cartilage to be harvested. F, Curved and straight elevators are used to dissect in the subperichondrial plane circumferentially around the cartilage. G, A curved dissector (Doyen) is inserted underneath and around the rib cartilage. The Doyen is used to gain further exposure in the subperichondrial plane. H, A retractor is placed directly underneath the location for the full-thickness incision of the cartilage. A knife is then used to incise the full thickness of the cartilage on one end. I, A retractor is placed on the other end of the cartilage, and the knife is used to incise the cartilage. J, The cartilage graft is removed. K, After the removal of the cartilage graft, the defect is inspected for hemostasis. L, The wound is filled with saline, and the anesthesiologist introduces maximum end tidal volume and holds the area under pressure with the Valsalva maneuver. This confirms that no pneumothorax is present. M, The perichondrial sleeve is approximated with interrupted suture ties (3-0 Vicryl). N, The deep and superficial fascia are closed with interrupted ties (3-0 Vicryl). O, Subdermal closure and then subcuticular closure are accomplished (5-0 Monocryl). P, Steri-Strips and an occlusive dressing (Tegaderm) are placed over the skin. Q, The harvested rib cartilage is crafted to suit the purposes of the recipient site. R, A crafted caudal strut graft can be seen. Additional grafts have also been crafted that can be used to reconstruct specific deficits (i.e., lower lateral cartilages, additional tip grafts, dorsal grafts).
• Orotracheal intubation (i.e., RAE tube) is placed as required for the rhinoplasty procedure. The RAE tube is taped to the chin to prevent dislodgement.
• The specific rib to be harvested is palpated and identified.
• Betadine solution is used for the preparation of both the hip and the head and neck. (Do not use soap, alcohol, Hibiclens, and so on.)
• The patient is prepped below the rib cage, past the chest midline, superior to the nipple, and laterally to the midaxillary line.
• Draping is completed, with limited skin exposure left parallel to and on all sides of the planned incision.
Skin Incision and Flap Elevation
• With the use of a knife (no. 15 blade), the incision is carried through the skin, the subcutaneous tissue, and the fascia directly over the rib cartilage to be harvested.
• Superior and inferior flaps are elevated to provide access to the rib cartilage.
• This should provide exposure of approximately 4 cm of rib cartilage.
Graft Harvesting
• The perichondrium is scored directly over the mid portion of the long axis of the rib cartilage to be harvested.
• Curved and straight elevators are used to dissect in the subperichondrial plane circumferentially around the cartilage to be harvested.
• A curved subperichondrial dissector (e.g., Doyen) is inserted underneath and around the rib cartilage. This instrument is used to gain further exposure in the subperichondrial plane.
• A retractor (e.g., Doyen) is placed deep and directly underneath the location for the incising of the cartilage graft. A knife (no. 15 blade) is then used to incise the full thickness of the cartilage.
• The retractor (e.g., Doyen) is then repositioned just below the other end of the cartilage at the location for incision. The knife (no. 15 blade) is used to incise the cartilage.
Wound Closure
• The wound is checked for hemostasis and any exposure into the lung cavity; this is done by filling the wound with saline. The anesthesiologist introduces maximum end tidal volume and holds the area under pressure with the Valsalva maneuver. With the confirmation of no pneumothorax, wound closure is accomplished.
• The perichondrial sleeve is approximated with interrupted suture ties (3-0 Vicryl).
• The deep and superficial fascia layers are closed with interrupted suture ties (3-0 Vicryl).
• Subdermal closure is with interrupted suture ties (4-0 Vicryl).
• The skin is closed with subcuticular running suture (5-0 resorbable).
Alloplastic Grafts and Implants for the Augmentation of the Facial Skeleton
Porous Polyethylene Augmentation Implants
Specific patterns of skeletal dysmorphology may lead the individual to request and the surgeon to agree to augmentation and camouflage procedures for the sole purpose of enhancing facial aesthetics.17,33,67,98,116,132,133,135,137 To avoid the need for graft harvesting and to limit concerns about the resorption of the graft over time, consideration of the use of alloplastic synthetic materials remains attractive. Alloplastic materials have been used to enhance and augment the facial skeleton over the years have included silicon, Biocoral, Biofax, Proplast, Teflon, Plasti-Pore, Seramic, polyamide-mesh, hydroxyapatite, Gore-Tex, methyl methacrylate, and porous polyethylene. These materials have been used with varying levels of success.18,19,30,32,33,43–45,89,91,99,101,102,136 Their intrinsic biomedical properties generally fall short of ideal, but they may be adequate in specific clinical settings.
Of the currently available alloplastic synthetic implants that are commercially available, the materials that are seemingly most favorable for the augmentation of the facial skeleton are those made of porous polyethylene. Histologically, fibrous ingrowth into the polyethylene pores without capsule formation represents the key attribute of this particular material. The implants are placed in the subperiosteal plane and then preferably fixed in their new location to the underlying skeleton with titanium screws. Systemic antibiotics are administered perioperatively to limit the risk of infection. Technique-sensitive decisions regarding favorable sites for implant placement, preferred incision locations, extent of soft-tissue dissection, the size and shape of the implant selected, the crafting of the implant, the method of fixation, and postoperative wound management are all important factors for the limiting of morbidity and the achievement of desired results. Alloplastic implant extrusion, migration, infection, underlying bone resorption, and unsatisfactory aesthetic results all remain of concern.142,140,141
Augmenting the skeleton to camouflage facial dysmorphology with the use of porous polyethylene implants has been championed by Yaremchuk.140–142 That author and others have demonstrated the effectiveness of this alloplastic graft approach for craniofacial reconstructive procedures and for purely facial aesthetic augmentation in specific clinical settings. The use of these implants in load-bearing locations as interpositional grafts or for purposes of nasal augmentation have consistently fallen short of the mark.
References
1. Abramowicz, S, Katsnelson, A, Forbes, PW, Padwa, BL. Anterior versus posterior approach to iliac crest for alveolar cleft bone grafting. J Oral Maxillofac Surg. 2012; 70:211–215.
2. Acocella, A, Bertolai, R, Nissan, J, Sacco, R. Clinical, histological and histomorphometrical study of maxillary sinus augmentation using cortico-cancellous fresh frozen chips. J Craniomaxillofac Surg. 2011; 39:192–199.
3. Albee, F. Bone graft surgery. Philadelphia: Saunders; 1915.
4. Albee, F. The inlay bone graft versus Lane plates in the treatment of fractures. Am J Surg. 1915; 29:77.
5. Albee, F. A statistical study of 539 cases of Pott’s disease treated by bone graft. Am J Orthop Surg. 1916; 14:134.
6. Arrington, ED, Smith, WJ, Chambers, HG, et al. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996; 329:300.
7. Axhausen, G. Die histologischen und klinischen gesetze der frien osteoplastik auf grund von teierversuchen. Arch Klin Chir. 1909; 88:23.
8. Banwart, JC, Asher, MA, Hassanein, RS. Iliac crest bone graft harvest donor site morbidity: A statistical evaluation. Spine. 1995; 20:1055–1060.
9. Baqain, Z, Anabtawi, M, Karaky, A, Malkawi, Z. Morbidity from anterior iliac crest bone harvesting for secondary alveolar bone grafting: An outcome assessment study. J Oral Maxillofac Surg. 2009; 67:570–575.
10. Barone, A, Ricci, M, Mangano, F, Covani, U. Morbidity associated with iliac crest harvesting in the treatment of maxillary mandibular atrophies: A 10-year analysis. J Oral Maxillofac Surg. 2011; 69:2298–2304.
11. Bojescul, JA, Polly, DW, Jr., Kuklo, TR, et al. Backfill for iliac-crest donor sites: A prospective randomized study of coralline hydroxyapatite. Am J Orthop. 2005; 34:377–382.
12. Boyne, PJ, James, RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Maxillofac Surg. 1980; 38:613–616.
13. Brunski, JB, Puleo, DA, Nanci, A. Biomaterials and biomechanics of oral and maxillofacial implants: Current status and future developments. Int J Oral Maxillofac Implants. 2000; 15:15.
14. Clark, JM, Cook, TA. Immediate reconstruction of extruded alloplastic nasal implants with irradiated homograft costal cartilage. Laryngoscope. 2002; 112(6):968–974.
15. Cohen, M, Figueroa, AA, Haviv, Y, et al. Iliac versus cranial bone for secondary grafting of residual alveolar clefts. Plast Reconstr Surg. 1991; 87:423–428.
16. Consolo, U, Politi, M, Niconi, PF, Salgarelli, A. Prelievi d’osso da cresta iliaca. Revisione della casistica e valutazioni clinico-statistiche. Minerva Stomatol. 1990; 39:133–137.
17. Converse, JM. Restoration of facial contour by bone grafts introduced through the oral cavity. Plast Reconstr Surg. 1950; 6:295.
18. Cottrell, DA, Wolford, LM. Long-term evaluation of the use of coralline hydroxyapatite in orthognathic surgery. J Oral Maxillofac Surg. 1998; 56:935.
19. Davis, PKB, Jones, SM. The complications of silastic implants: Experience with 137 consecutive cases. Br J Plast Surg. 1971; 24:405.
20. Dawson, KH, Egbert, MA, Myall, RW. Pain following iliac crest bone grafting of alveolar clefts. J Craniomaxillofac Surg. 1996; 24:151.
21. De La Torre, JI, Tenenhaus, M, Gallagher, PM, Sachs, SA. Harvesting iliac bone graft: Decreasing the morbidity. Cleft Palate Craniofac J. 1999; 36:388–390.
22. De Riu, G, Meloni, SM, Raho, MT, et al. Delayed iliac abscess as complication of an iliac bone graft in an orthognathic case. Int J Oral Maxillofac Surg. 2008; 37:1156–1158.
23. Dingman, RO. The use of iliac bone on the repair of facial and cranial defects. Plast Reconstr Surg. 1950; 3:24.
24. Doyen, E. Technique chirurgicale. Paris: Masson et Cie; 1897.
25. Esteves, JC, Borrasca, AG, Aranega, AM, et al. Histomorphometric analysis of the repair process of autogenous bone grafts fixed at rat calvaria with cyanoacrylate. J Appl Oral Sci. 2011; 19:529–534.
26. Eufinger, H, Leppänen, H. Iliac crest donor site morbidity following open and closed methods of bone harvest for alveolar cleft alveoplasty. J Craniomaxillofac Surg. 2000; 28:31–38.
27. Finn, RA, Bell, WH, Brammer, JA. Interpositional “grafting” with autogenous bone and coralline hydroxyapatite. J Maxillofac Surg. 1980; 8:217–227.
28. Freihofer, HP, Borstlap, WA, Kuijpers-Jagtman, AM. Timing and transplant materials for closure of alveolar clefts: A clinical comparison of 296 cases. J Craniomaxillofac Surg. 1993; 21:143.
29. Gallie, WE, Robertson, DE. The transplantation of bone. JAMA. 1918; 70:1134.
30. Ganguli, A, Steward, C, Butler, SL, et al. Bacterial adhesion to bisphosphonate coated hydroxyapatite. J Mater Sci Mater Med. 2005; 16:283–287.
31. Gibson, T, Davis, WB. The distortion of autogenous cartilage grafts: Its causes and prevention. Br J Plast Surg. 1958; 10:257.
32. Godin, MS, Waldman, R, Johnson, CM. The use of expanded polytetrafluoroethylene (Gore-Tex) in rhinoplasty. Arch Otolaryngol Head Neck Surg. 1995; 121:1131.
33. Gosain, AK, Persing, JA. Report on the Symposium “Biomaterials in the Face: Benefits and Risks. ”. J Craniofac Surg. 1999; 10:1.
34. Gottsauner, A, Hardt, N. Technik and erfahrungen mit der Sinuslift-OP und enos-salen implantalen. Z Zahnärztl Implantol. 1993; 9:184–187.
35. Goulet, JA, Senunas, LE, DeSilva, GL, Greenfield, ML. Autogenous iliac crest bone graft: Complications and functional assessment. Clin Orthop Relat Res. 1997; 339:76–81.
36. Grossman, MG, Ducey, SA, Nadler, SS, Levy, AS. Meralgia paresthetica: Diagnosis and treatment. J Am Acad Orthop Surg. 2001; 9:336–344.
37. Gunter, JP, Clark, CP, Friedman, RM. Internal stabilization of autogenous rib cartilage grafts in rhinoplasty: A barrier to cartilage warping. Plast Reconstr Surg. 1997; 100:162.
38. Gunter, JP, Friedman, RM. Lateral crural strut graft: Technique and clinical applications in rhinoplasty. Plast Reconstr Surg. 1997; 99:943–952.
39. Gunter, JP, Rohrich, RJ. Augmentation rhinoplasty: Onlay grafting using shaped autogenous septal cartilage. Plast Reconstr Surg. 1990; 86:39.
40. Hacker, Von. Ersatz von Schaedeldefekten durch unter der kopfschwartz verscholbener oder un gelappte periostknochen. Bietr Klin Chir. 1903; 37:499.
41. Hall, HD, Posnick, JC. Early results of secondary bone grafts in 106 alveolar clefts. J Oral Maxillofac Surg. 1983; 41:289.
42. Hill, NM, Horne, JG, Devane, PA. Donor site morbidity in the iliac crest bone graft. Aust N Z J Surg. 1999; 69:726.
43. Hiraga, Y. Complications of augmentation rhinoplasty in the Japanese. Ann Plast Surg. 1980; 4:495.
44. Holmes, RE. Bone regeneration within a coralline hydroxyapatite implant. Plast Reconstr Surg. 1979; 63:626–633.
45. Holmes, RE, Wardrop, RW, Wolford, LM. Hydroxylapatite as a bone graft substitute in orthognathic surgery: Histologic and histometric findings. J Oral Maxillofac Surg. 1988; 46:661.
46. Hughes, CW, Revington, PJ. The proximal tibia donor site in cleft alveolar bone grafting: Experience of 75 consecutive cases. J Craniomaxillofac Surg. 2002; 30:12.
47. Ilankovan, V, Stronczek, M, Telfer, M, et al. A prospective study of trephined bone grafts of the tibial shaft and iliac crest. Br J Oral Maxillofac Surg. 1998; 36:434–439.
48. Isaksson, S. Evaluation of three bone grafting techniques for severely resorbed maxillae in conjunction with immediate endosseous implants. Int J Oral Maxillofac Implants. 1994; 9:679–688.
49. Ivy, R. Bone grafting for restoration of defects of the mandible. Plast Reconstr Surg (1946). 1951; 7:333.
50. Jackson, IT, Adham, H, Bite, U, Marx, R. Update on cranial bone grafts in craniofacial surgery. Ann Plast Surg. 1987; 18:37–40.
51. James, JD, Geist, ET, Gross, BD. Adynamic ileus as a complication of iliac bone removal. J Oral Surg. 1981; 39:289.
52. Johannson, B, Ohlsson, A. Bone grafting and dental orthopaedics in primary and secondary cases of cleft lip and palate. Acta Chir Scand. 1961; 112:112.
53. Joshi, A, Kostakis, GC. An investigation of post-operative morbidity following iliac crest graft harvesting. Br Dent J. 2004; 196:167.
54. Kager, AN, Marks, M, Bastrom, T, Newton, PO. Morbidity of iliac crest bone graft harvesting in adolescent deformity surgery. J Pediatr Orthop. 2006; 26:132–134.
55. Kalk, W, Raghoebar, G, Jansma, J, Boering, G. Morbidity from iliac crest bone harvesting. J Oral Maxillofac Surg. 1996; 54:1424.
56. Kappis, A. Zur deckung von schaedeldefekten. Centralblat Chir. 1917; 42:409.
57. Keller, EE, Triplett, WW. Iliac bone grating: Review of 160 consecutive cases. J Oral Maxillofac Surg. 1987; 45:11.
58. Kessler, P, Thorwarth, M, Bloch-Birkholz, A, et al. Harvesting if bone from the iliac crest—a comparison of the anterior and the posterior sites. Br J Oral Maxillofac Surg. 2005; 43:51–56.
59. Kim, DW, Shah, AR, Toriumi, DM. Concentric and eccentric carved costal cartilage: A comparison of warping. Arch Facial Plast Surg. 2006; 8(1):42–46.
60. Klapp, R, Schroeder, H. Die unterkieferschussbruche. Berlin: Hermann Meusser; 1917.
61. Kline Jr, RM, Wolfe, SA. Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg. 1995; 95:5.
62. Koenig, F. Der knoecherne ersatz schaedeldefekten. Centralblat Chir. 1890; 17:497.
63. Koole, R, Bosker, H, Norman van der Dussen, F. Late secondary autogenous bone grafting in cleft patients comparing mandibular and iliac crest grafts. J Craniomaxillofac Surg. 1989; 17:28–30.
64. Kübler, N, Reuther, J, Kirschner, T, et al. Osteoinductive, morphologic and biomechanical properties of autolyzed, antigen-extracted, allogenic bone. J Oral Maxillofac Surg. 1993; 51:1346–1357.
65. Kübler, NR. Osteoinduction und reparation. Mund Kiefer Gesichts Chir. 1997; 1:2–25.
66. LaRossa, D, Buchman, S, Rothkopf, DM, et al. A comparison of iliac and cranial bone in secondary grafting of alveolar clefts. Plast Reconstr Surg. 1995; 96:789–797.
67. LaTrenta, GS. Atlas of aesthetic face and neck surgery. Philadelphia: Saunders; 2004.
68. Laurie, JW, Kaban, LB, Mulliken, JB, Murray, JE. Donor site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984; 73:933–938.
69. Lebedinsky, J, Virenque, M. Prothese. Chirurgie Cranio-Maxillo-Faciale. Paris: Librarie J B Bailliere et Fils; 1918.
70. Lindermann, A. Bruhn’s ergebnisse aus dem Düsseldorfer Lazareff. Wiesbaden: Kieferschussverletzungen; 1916.
71. Lindemann, A. Quoted by Dolomore WH. Br Dent J. 1917; 38:16.
72. Marx, RE, Morales, MJ. Morbidity from bone harvest in major jaw reconstruction: A randomized trial comparing the lateral anterior and posterior approaches to the ilium. J Oral Maxillofac Surg. 1988; 48:196–203.
73. Massey, EW. Meralgia paraesthetica secondary to trauma of bone graft. J Trauma. 1980; 20:342–343.
74. Mazock, JB, Schow, SR, Triplett, RG. Posterior iliac crest bone harvest: Review of technique, complications and use of an epidural catheter for postoperative pain control. J Oral Maxillofac Surg. 2003; 61:1497–1503.
75. Mischkowski, RA, Selbach, I, Neugebauer, J, et al. Lateral femoral cutaneous nerve and iliac crest bone grafts—anatomical and clinical considerations. Int J Oral Maxillofac Surg. 2006; 35:366–372.
76. Mowlavi, A, Pham, S, Wilhelmi, B, et al. Anatomical characteristics of the conchal cartilage with suggested clinical applications in rhinoplasty surgery. Aesthet Surg J. 2010; 30:523–526.
77. Mowlem, R. Cancellous chips bone grafts: Report on 72 cases. Lancet. 1944; 2:746.
78. Mueller, W. Zur frage der temporaren schaedelresektion an stelle der trepanation. Centralblat Chir. 1890; 17:65.
79. Murrell, GL. Auricular cartilage grafts and nasal surgery. Laryngoscope. 2004; 114(12):2092–2102.
80. Nkenke, E, Weisbach, V, Winckler, E, et al. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: A prospective study. Int J Oral Maxillofac Surg. 2004; 33:157–163.
81. Nocini, PF, Bedogni, A, Valsecchi, S, et al. Fractures of the iliac crest following anterior and posterior bone graft harvesting. Review of the literature and case presentation. Minerva Stomatol. 2003; 52:441.
82. Nwoku, AL, Al Atel, A, Al Shlash, S, et al. Retrospective analysis of secondary alveolar cleft grafts using iliac or chin bone. J Craniofac Surg. 2005; 16:864–868.
83. Ollier, L. Reserches experimentales sur les greffes osseuses. J Physiol D Homme et des Animaux. 1860; 3:88.
84. Ortiz-Monasterio, F, Olmedo, A, Oscoy, LO. The use of cartilage grafts in primary aesthetic rhinoplasty. Plast Reconstr Surg. 1981; 67:597.
85. Peer, L. Transplantation of tissues, vol 1. Baltimore: Williams & Wilkins; 1955.
86. Peer, LA. Cartilage grafting. Br J Plast Surg. 1955; 7:250.
87. Piecuch, JF, Topazian, RG, Skoly, S, et al. Experimental ridge augmentation with porous hydroxyapatite implants. J Dent Res. 1983; 62:148–154.
88. Porchet, F, Jacques, B. Unusual complications at iliac crest bone graft donor site: Experience with two cases. Neurosurgery. 1996; 39:856–859.
89. Raghavan, U, Jones, NS, Romo, R, III. Immediate autogenous cartilage grafts in rhinoplasty after alloplastic implant rejection. Arch Facial Plast Surg. 2004; 6:192–196.
90. Rawashdeh, MA. Morbidity of iliac crest donor site following open bone harvesting in cleft lip and palate patients. Int J Oral Maxillofac Surg. 2008; 37:223–227.
91. Rees, TD, Krupp, S, Wood-Smith, D. Secondary rhinoplasty. Plast Reconstr Surg. 1970; 46:332.
92. Robertson, PA, Wray, AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: A prospective analysis of morbidity. Spine. 2001; 26:1473.
93. Rosen, HM. Surgical correction of the vertically deficient chin. Plast Reconstr Surg. 1988; 82:247–255.
94. Rosen, HM. Porous block hydroxyapatite as an interpositional bone-graft substitute in orthognathic surgery. Plast Reconstr Surg. 1989; 83:985.
95. Rosen, HM. Definitive surgical correction of vertical maxillary deficiency. Plast Reconstr Surg. 1990; 85:215–221.
96. Rosen, HM. Response of porous hydroxyapatite to contiguous tissue infection. Plast Reconstr Surg. 1991; 88:1076.
97. Rosen, HM, Ackerman, JL. Porous block hydroxyapatite in orthognathic surgery. Angle Orthod. 1991; 61:185.
98. Rosen, HM, McFarland, MM. The biologic behavior of hydroxyapatite implanted into the maxillofacial skeleton. Plast Reconstr Surg. 1990; 85:718–723.
99. Rubin, JP, Yaremchuk, MJ. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: A comprehensive review of the literature. Plast Reconstr Surg. 1997; 100:1336.
100. Sadove, AM, Nelson, CL, Eppley, BL, Nguyen, B. An evaluation of calvarial and iliac donor sites in alveolar cleft grafting. Cleft Palate J. 1990; 27:225–228.
101. Sailer, HF. Experiences with the use of lyophilized bank cartilage for facial contour correction. J Maxillofac Surg. 1976; 4:149.
102. Salyer, KE, Ubinas, EE, Snively, SL. Porous, hydroxyapatite as an onlay graft in maxillofacial surgery. Plastic Surgical Forum. 1986; 8:61.
103. Sasso, RC, LeHuec, JC, Shaffrey, C. Spine Interbody Research Group: Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005; 18:S77–S81.
104. Schnee, CL, Freese, A, Weil, RJ, Marcotte, PJ. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine (Phila Pa 1976). 1997; 22:2222–2227.
105. Seiler, JG, III., Johnson, J. Iliac crest autogenous bone grafting: Donor site complications. J South Orthop Assoc. 2000; 9:91.
106. Seydel, H. Eine neue methode, grosse knochendefekte des schaedels zu decken. Centralblat Chir. 1889; 12:209.
107. Sheehan, JH. Use of iliac bone in the facial and cranial repair. Am J Surg. 1941; 52:55.
108. Sheen, JH. Spreader graft: A method of reconstructing the roof of the middle nasal vault following rhinoplasty. Plast Reconstr Surg. 1984; 73(2):230–239.
109. Sheen, JH. The ideal dorsal graft: A continuing quest. Plast Reconstr Surg. 1998; 102:2490.
110. Silber, JS, Anderson, DG, Daffner, SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single level anterior cervical discectomy and fusion. Spine. 2003; 28:134.
111. Skyoff, W. Zur frage der knochenplastik am unterkeifer. Centralblatt f. Chirurgie. 1900; 35:881.
112. Soehr, O. Zur frage des schaedelplastik. Beitr Klin Chir. 1907; 55:465.
113. Stevenson, S. Biology of bone grafts. Orthop Clin North Am. 1999; 30:543.
114. Summers, BN, Eisenstein, SM. Donor site pain from the ilium. J Bone Joint Surg Br. 1989; 71A:677.
115. Swan, MC, Goodacre, TE. Morbidity at the iliac crest donor site following bone grafting of the cleft alveolus. Br J Oral Maxillofac Surg. 2006; 44:129–133.
116. Terino, EO. Alloplastic facial contouring by zonal principles of skeletal anatomy. Clin Plast Surg. 1992; 19:487–510.
117. Tessier, P. Autogenous bone grafts taken from the calvarium for facial and cranial applications. Clin Plast Surg. 1982; 9:531.
118. Tessier, P, Kawamoto, H, Matthews, D, et al. Autogenous bone grafts and bone substitutes—tools and techniques: I. A 20,000-case experience in maxillofacial and craniofacial surgery. Plast Reconstr Surg. 2005; 116(5 Suppl):6S–24S.
119. Tessier, P, Kawamoto, H, Matthews, D, et al. Complications of harvesting autogenous bone grafts: A group experience of 20,000 cases. Plast Reconstr Surg. 2005; 116(5 Suppl):72S–73S.
120. Tessier, P, Kawamoto, H, Matthews, D, et al. Taking bone grafts from the anterior and posterior ilium—tools and techniques: II. A 6800-case experience in maxillofacial and craniofacial surgery plastic and reconstructive surgery. Plast Reconstr Surg. 2005; 116(5 Suppl):25S–37S.
121. Tessier, P, Kawamoto, H, Matthews, D, et al. Taking long rib grafts for facial reconstruction—tools and techniques: III. A 2900-case experience in maxillofacial and craniofacial surgery plastic and reconstructive surgery. Plast Reconstr Surg. 2005; 116(5 Suppl):38S–46S.
122. Tessier, P, Kawamoto, H, Matthews, D, et al. Taking tibial grafts in the diaphysis and upper epiphysis—tools and techniques: IV. A 650-case experience in maxillofacial and craniofacial surgery plastic and reconstructive surgery. Plast Reconstr Surg. 2005; 116(5 Suppl):47S–53S.
123. Tessier, P, Kawamoto, H, Matthews, D, et al. Taking calvarial grafts, either split in situ or splitting of the parietal bone flap ex vivo—tools and techniques: V. A 9650-case experience in craniofacial and maxillofacial surgery. Plast Reconstr Surg. 2005; 116(5 Suppl):54S–71S.
124. Tessier, P, Kawamoto, H, Matthews, D, et al. Taking calvarial grafts—tools and techniques: VI. The splitting of a parietal bone “flap. ”. Plast Reconstr Surg. 2005; 116(5 Suppl):74S–88S.
125. Urist, MR. Bone: Formation by autoinduction. Science. 1965; 150:893–899.
126. Virenque, M. Chirurgie reparatrice maxillo-faciale. Paris: Librarie Maloine; 1940.
127. Virenque, M. Quelques notions nouvelles en chirurgie reparatrice maxillo-faciale. Rev Stomatol. 1942; 43:149.
128. Von Eiselsberg, F. Zur behandlung von ernobenen schaedelknochen defekten. Bielage Zentralblat Chir. 1895; 22:44.
129. Waite, PD, Matukas, VJ. Zygomatic augmentation with hydroxyapatite: A preliminary report. J Oral Maxillofac Surg. 1986; 44:349.
130. Weikel, A, Habal, M. Meralgia paresthetica: A complication of iliac bone procurement. Plast Reconstr Surg. 1977; 4:572.
131. Westrich, GH, Geller, DS, O’Malley, MJ, et al. Anterior iliac crest bone graft harvesting using the corticocancellous reamer system. J Orthop Trauma. 2001; 15:500.
132. Whitaker, LA. Aesthetic augmentation of the malar-midface structures. Plast Reconstr Surg. 1987; 90:337–341.
133. Whitaker, LA. Temporal and malar-zygomatic reduction and augmentation. Clin Plast Surg. 1991; 18:55.
134. White, E, Shors, EC. Biomaterial aspects of Interpore-200 porous hydroxyapatite. Dent Clin North Am. 1986; 30:49–67.
135. Wilkinson, TS. Complications in aesthetic malar augmentation. Plast Reconstr Surg. 1983; 71:643.
136. Williams, JD, Romo, T, III., Sclafani, AP, Cho, H. Porous high-density polyethylene implants in auricular reconstruction. Arch Otolaryngol Head Neck Surg. 1997; 123:578.
137. Wolfe, SA. Malar augmentation using autogenous materials. Clin Plast Surg. 1991; 18:39.
138. Wolfe, SA, Kawamoto, HK. Taking the iliac-bone grafts. J Bone Joint Surg Am. 1978; 60:411.
139. Wolford, LH, Wardrop, RW, Hartog, J. Coralline porous hydroxyapatite as a bone graft substitute in orthognathic surgery. J Oral Maxillofac Surg. 1987; 45:1034–1042.
140. Yaremchuk, MJ. Infraorbital rim augmentation. Plast Reconstr Surg. 2001; 107:1585–1590.
141. Yaremchuk, MJ. Improving periorbital appearance in the “morphologically prone. ”. Plast Reconstr Surg. 2004; 114:980–987.
142. Yaremchuk, MJ, Israeli, D. Paranasal implants for correction of midface concavity. Plast Reconstr Surg. 1998; 102:1676–1684.
143. Zijderveld, SA, ten Bruggenkate, CM, van Den Bergh, JP, Schulten, EA. Fractures of the iliac crest after split-thickness bone grafting for preprosthetic surgery: Report of 3 cases and review of the literature. J Oral Maxillofac Surg. 2004; 62:781–786.