Chapter 169 Gout
 Diagnostic Summary
Diagnostic Summary
• Acute onset, frequently nocturnal, with typically monoarticular joint pain, involving the metatarsophalangeal joint of the big toe in about 50% of cases
• Elevated serum uric acid level
• Asymptomatic periods between acute attacks
• Identification of urate crystals in joint fluid
• Aggregated deposits of monosodium urate monohydrate (tophi) chiefly in and around the joints of the extremities but also in subcutaneous tissue, bone, cartilage, and other tissues
 General Considerations
General Considerations
Gout is a common type of arthritis caused by an increased concentration of uric acid (the final breakdown product of purine metabolism) in biological fluids. In gout, uric acid crystals (monosodium urate) are deposited in joints, tendons, kidneys, and other tissues, where they cause considerable inflammation and damage.1 Gout is a condition characterized biochemically by increased serum uric acid levels, leukotriene levels, and neutrophil accumulation. Gout may lead to debilitation owing to the tophaceous deposits around the joints and tendons; renal involvement may result in kidney failure due to either parenchymal disease or urinary tract obstruction.
Gout is associated with affluence and is often called the “rich man’s disease.” Throughout history, the sufferer of gout has been depicted as a portly, middle-aged man sitting in a comfortable chair with one foot resting painfully on a soft cushion as he consumes great quantities of meat and wine. In fact, the traditional picture does have some basis in reality, as meats, particularly organ meats, are high-purine foods, whereas alcohol inhibits uric acid secretion by the kidneys. Furthermore, even today, gout is primarily a disease of adult men; more than 95% of sufferers of gout are men older than age 30. The incidence of diagnosed gout cases has been estimated at 2.13% of the 2009 U.S. population, although 10% to 20% of the adult population has hyperuricemia. Gout is a strong predictor of the metabolic syndrome and an increased risk for type 2 diabetes.1–4
Causes of Gout
Gout is classified into two major categories: primary and secondary. Primary gout accounts for about 90% of all cases, whereas secondary gout accounts for only 10%. Primary gout is usually idiopathic (i.e., the underlying metabolic defect is unknown). However, there are several genetic defects in which the exact cause of the elevated uric acid is known.1 The synthesis and degradation of purines are summarized in Figure 169-1.
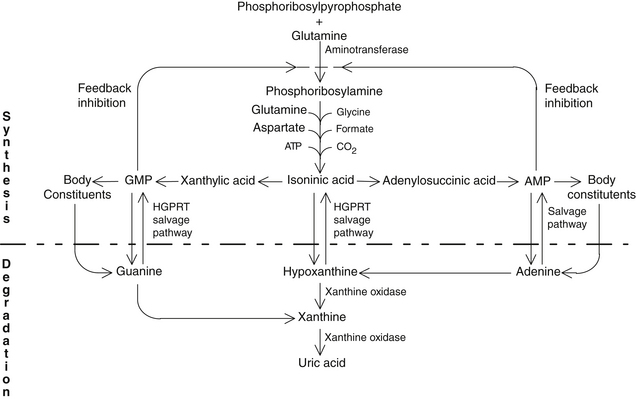
FIGURE 169-1 Purine synthesis and degradation.
(From Nutrition Foundation. Nutrition reviews’ present knowledge in nutrition, ed 5. Washington, DC: Nutrition Foundation, 1984:740-756.)
The term secondary gout refers to those cases in which the elevated uric acid level is secondary to some other disorder such as excessive breakdown of cells or some form of renal disease. Diuretic therapy for hypertension and low-dose aspirin therapy are also important causes of secondary gout because they cause decreased uric acid excretion.
• Increased synthesis of uric acid, found in a majority of individuals
• Reduced ability to excrete uric acid, typical of a smaller group (about 30%)
• Overproduction of uric acid, as well as underexcretion of uric acid—a small minority
Although the exact metabolic defect in gout is unknown in the majority of cases, gout is one of the most controllable metabolic diseases. Box 169-1 summarizes the causes of gout.
BOX 169-1 Causes of Hyperuricemia
Metabolic
• Increased production of purine (primary)
• Specific enzyme defects (e.g., Lesch-Nyhan syndrome, glycogen storage disease)
• Decreased enzyme activity (e.g., hypoxanthine-guanine phosphoribosyltransferase is decreased in 1% to 2% of adults with gout)
• Increased enzyme activity (e.g., phosphoribosylpyrophosphate synthetase)
• Increased production of purine (secondary)
• Increased turnover of purines
• Myeloproliferative disorders
• Lymphoproliferative disorders
• Carcinoma and sarcoma (disseminated)
• Increased de novo synthesis (e.g., glucose-6-phosphatase deficiency)
• Increased catabolism of purines
Renal
• Decreased renal clearance of uric acid (primary)
• Decreased renal clearance of uric acid (secondary)
• Functional impairment of tubular secretion
• Drug-induced (e.g., thiazides, probenecid, salicylates, ethambutol, pyrazinamide)
• Hyperlacticemia (e.g., lactic acidosis, alcoholism, toxemia of pregnancy, chronic beryllium disease)
• Hyperketoacidemia (e.g., diabetic ketoacidosis, fasting, starvation)
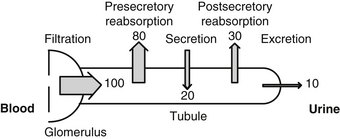
Almost all of the plasma urate is filtered at the glomerulus: only the small amount bound to protein is not filtered. Renal excretion is peculiar in that about 80% of the filtered uric acid is reabsorbed in the proximal tubule of the nephron. Actually, the distal tubule secretes most of the uric acid found in the urine. Distal to this site, some postsecondary reabsorption occurs. These events are summarized in Figure 169-2.
Uric acid is a highly insoluble molecule; at pH 7.4 and body temperature, the serum is saturated at 6.4 to 7 mg/100 mL. Although higher concentrations do not necessarily result in urate deposition (some unknown factor in serum appears to inhibit urate precipitation), the chance of an acute attack is greater than 90% when the level is above 9 mg/100 mL (Table 169-1). Lower temperatures decrease the saturation point of uric acid, which may explain why urate deposits tend to form in areas such as the pinna of the ear, where the temperature is lower than the mean body temperature (Table 169-2). Uric acid is insoluble below pH 6 and can precipitate as the urine is concentrated in the collecting ducts and passed to the renal pelvis.
TABLE 169-1 Prevalence of Gouty Arthritis by Maximum Urate Level
| SERUM URATE (mg/100 mL) | MEN (%) | WOMEN (%) |
|---|---|---|
| <6 | 0.6 | 0.08 |
| 6-6.9 | 1.9 | 3.3 |
| 7-7.9 | 16.7 | 17.4 |
| 8-8.9 | 25 | 0 |
| 9+ | 90 | 0 |
Data from Faller J, Fox IH. Ethanol-induced hyperuricemia: evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med 1982;307:1598-1602.
TABLE 169-2 Solubility of Urate Ion as a Function of Temperature in 140 mM Na+
| TEMPERATURE (°C) | MAXIMUM SOLUBILITY (mg/100 mL) |
|---|---|
| 37 | 6.8 |
| 35 | 6 |
| 30 | 4.5 |
| 25 | 3.3 |
| 20 | 2.5 |
| 15 | 1.8 |
| 10 | 1.2 |
Data from Faller J, Fox IH. Ethanol-induced hyperuricemia: evidence for increased urate production by activation of adenine nucleotide turnover. N Engl J Med 1982;307:1598-1602.
Signs and Symptoms
Subsequent attacks are common, with the majority of such individuals having another attack within 1 year. However, nearly 7% never have a second attack. Chronic gout rarely is an issue owing to the advent of dietary therapy and drugs that lower uric acid levels. When it does occur, chronic gout is due to poor compliance or inadequate response to treatment, or it may arise in patients with high flare frequency, tophi, and the inability to maintain serum urate levels below 6 mg/dL.2 Some degree of kidney dysfunction occurs in nearly 90% of subjects with gout, and there is a higher risk of kidney stones.
 Therapeutic Considerations
Therapeutic Considerations
• Drugs like allopurinol or febuxostat to keep uric acid levels within a normal range
• Controlled weight loss in obese individuals
• Avoidance of known precipitating factors such as heavy alcohol consumption or a diet rich in purines or refined carbohydrates
Several dietary factors are known to cause gout: consumption of alcohol, especially beer and hard liquor; foods high in purine content (e.g., organ meats, meat, yeast, poultry); fats; refined carbohydrates, especially high-fructose corn syrup; and overconsumption of calories.5 Individuals with gout are typically obese; they are prone to hypertension, the metabolic syndrome, and diabetes and are at a greater risk for cardiovascular disease. Obesity is probably the most important dietary factor. Thiazide and loop diuretics are also associated with a higher risk of incident gout and higher rate of gout flares.5
In concept, the naturopathic approach for chronic gout does not differ substantially from the standard medical approach. However, naturopaths focus on dietary and herbal measures to keep uric acid levels within the normal range rather than on the use of drugs. In the conventional medical treatment of gout, drugs that inhibit xanthine oxidase are often too much relied upon. Allopurinol, a structural isomer of hypoxanthine (a naturally occurring purine in the body), has been the mainstay treatment for decades. However, in February 2009, the U.S. Food and Drug Administration approved febuxostat (Uloric), another xanthine oxidase inhibitor; it is a more effective treatment for lowering and maintaining serum urate levels.6 Febuxostat is beginning to supplant allopurinol in the conventional management of gout. Uricosuric agents (probenecid, sulfinpyrazone, and benzbromarone) are used as second-line therapy for patients with underexcretion of uric acid.
Lead Toxicity
An additional item of concern relates to lead toxicity. A secondary type of gout, sometimes called saturnine gout, can result from lead toxicity. Historically, saturnine gout was due to the consumption of alcoholic beverages stored in containers with lead in them. An unexpected and fairly common source of lead appears to be leaded crystal, because port wine elutes lead when stored in a crystal decanter.6 Lead concentration increases with storage time, reaching toxic levels after several months. Even a few minutes in a crystal glass results in a measurable increase in the level of lead in the wine. Lead promotes hyperuricemia as a result of decreasing renal urate excretion.
Dietary Considerations
The dietary treatment of gout involves the following guidelines:
• Elimination of alcohol intake
• Achievement of ideal body weight
Low-Purine Alkaline-Ash Diet
An alkaline-ash diet is recommended in the dietary treatment of gout, because a more alkaline pH increases uric acid solubility. An alkaline-ash diet was shown to increase uric acid excretion from 302 mg/day at pH 5.9 to 413 mg/day at pH 6.5.8
Weight Reduction
Excess weight is associated with an increased rate of gout. Weight reduction in obese individuals significantly reduces levels of serum uric acid levels.9 Weight reduction should involve the use of a low glycemic diet designed to improve insulin sensitivity. Such a diet also helps to manage the elevated cholesterol and triglycerides that are common in obesity.
Carbohydrates, Fats, and Protein
Refined carbohydrates and saturated fats should be kept to a minimum, as the former increase uric acid production, whereas the latter increase uric acid retention. In addition, one of the key dietary goals in the treatment of gout appears to be the enhancement of insulin sensitivity.9
Protein intake should not be excessive (i.e., >0.8 g/kg per day), because it has been shown that uric acid synthesis may be accelerated in both normal and gouty patients by a high protein intake.5 Adequate protein is necessary (0.8 g/kg daily); however, amino acids decrease the resorption of uric acid in the renal tubules, thus increasing uric acid excretion and reducing serum uric acid concentrations.7
Nutritional Supplements
Folic Acid
Folic acid has been shown to inhibit xanthine oxidase, the enzyme responsible for producing uric acid.10 Research has demonstrated that a derivative of folic acid is an even greater inhibitor of xanthine oxidase than allopurinol, suggesting that folic acid at pharmacologic doses may be an effective treatment for gout.11 Positive results in the treatment of gout have been reported, but the data are incomplete and uncontrolled.12
Quercetin
The bioflavonoid quercetin has demonstrated several effects in experimental studies, indicating its possible benefit to individuals with gout.13–15 Quercetin may offer significant protection by inhibiting the following:
For more information on the pharmacology of quercetin, see Chapter 92.
Botanical Medicines
Cherries
Consuming one-half pound of fresh or canned cherries per day has been shown to be effective in lowering uric acid levels and preventing attacks of gout.18 To assess the physiologic effects of cherry consumption, one study measured plasma urate, antioxidant, and inflammatory markers in 10 healthy women who consumed two servings (280 g) of cherries after an overnight fast.19 Blood and urine samples were taken before the cherry dose and at 1.5, 3, and 5 hours afterward. Plasma urate decreased 5 hours after the cherry consumption by an average of 30 mmol/L. This reduction correlated with an increase in urine urate excretion. Plasma C-reactive protein and nitric oxide concentrations decreased slightly after the 3-hour mark.
• They have the unique ability to actually cross-link collagen fibers, resulting in reinforcement of the natural cross-linking of collagen that forms the collagen matrix of connective tissue (e.g., ground substance, cartilage, tendon).
• They prevent free radical damage through their potent antioxidant and free radical scavenging action.
• They inhibit enzymatic cleavage of collagen by enzymes secreted by leukocytes during inflammation.
• They prevent the release and synthesis of compounds that promote inflammation, such as histamine, serine proteases, prostaglandins, and leukotrienes.
 Therapeutic Approach
Therapeutic Approach
• Dietary and herbal measures that maintain uric acid levels within the normal range
• Controlled weight loss in obese individuals
• Avoidance of known precipitating factors (such as heavy alcohol consumption and a high-purine diet)
• The use of nutritional substances to prevent further acute attacks
• The use of herbal and nutritional substances to inhibit the inflammatory process
Botanical Medicines
• Choose one of the following:
• Cherry fruit extract (10:1): 500 to 1000 mg three times a day
• Grape seed extract (>95% procyanidolic oligomers): 100 to 300 mg/day
• Pine bark extract (>90% procyanidolic oligomers): 100 to 300 mg/day
• Anthocyanoside extracts (e.g., Vaccinium myrtillus): equivalent to 80 mg anthocyanoside content a day
• Quercetin: 200 to 400 mg three times a day between meals (Note: Consider EMIQ at a dosage of 100 to 200 mg/day, see Chapter 92 for more information).
1. Richette P., Bardin T. Gout. Lancet. 2010 Jan 23;375(9711):318–328.
2. Brook R.A., Forsythe A., Smeeding J.E., et al. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin. 2010 Dec;26(12):2813–2821.
3. Hernández-Cuevas C.B., Roque L.H., et al. First acute gout attacks commonly precede features of the metabolic syndrome. J Clin Rheumatol. 2009 Mar;15(2):65–67.
4. Choi H.K., De Vera M.A., Krishnan E. Gout and the risk of type 2 diabetes among men with a high cardiovascular risk profile. Rheumatology (Oxford). 2008 Oct;47(10):1567–1570.
5. Singh J.A., Reddy S.G., Kundukulam J. Risk factors for gout and prevention: a systematic review of the literature. Curr Opin Rheumatol. 2011 Mar;23(2):192–202.
6. Schumacher H.R., Jr., Becker M.A., Wortmann R.L., et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008 Nov 15;59(11):1540–1548.
7. Graziano J.H., Blum C. Lead exposure from lead crystal. Lancet. 1991;337:141–142.
8. Kanbara A., Hakoda M., Seyama I. Urine alkalization facilitates uric acid excretion. Nutr J. 2010 Oct 19;9:45. Published online 2010 October 19 http://dx.doi.org/10.1186/1475-2891-9-45
9. Dessein P.H., Shipton E.A., Stanwix A.E., et al. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis. 2000;59:539–543.
10. Lewis A.S., Murphy L., McCalla C., et al. Inhibition of mammalian xanthine oxidase by folate compounds and amethopterin. J Biol Chem. 1984;259:12–15.
11. Oster K.A. Folic acid and xanthine oxidase. Ann Intern Med. 1977;86:367.
12. Flouvier B., Devulder B. Folic acid, xanthine oxidase, and uric acid. Ann Intern Med. 1978 Feb;88(2):269.
13. Bindoli A., Valente M., Cavallini L. Inhibitory action of quercetin on xanthine oxidase and xanthine dehydrogenase activity. Pharmacol Res Commun. 1985;17:831–839.
14. Busse W.W., Kopp D.E., Middleton E., Jr. Flavonoid modulation of human neutrophil function. J Allergy Clin Immunol. 1984;73:801–809.
15. Yoshimoto T., Furukawa M., Yamamoto S., et al. Flavonoids: potent inhibitors of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun. 1983;116:612–618.
16. Stein H.B., Hasan A., Fox I.H. Ascorbic acid-induced uricosuria: a consequence of megavitamin therapy. Ann Intern Med. 1976;84:385–388.
17. Gershon S.L., Fox I.H. Pharmacologic effects of nicotinic acid on human purine metabolism. J Lab Clin Med. 1974;84:179–186.
18. Blau L.W. Cherry diet control for gout and arthritis. Texas Rep Biol Med. 1950;8:309–311.
19. Jacob R.A., Spinozzi G.M., Simon V.A., et al. Consumption of cherries lowers plasma urate in healthy women. J Nutr. 2003;133:1826–1829.