Chapter 24 Glycosides
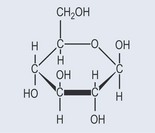
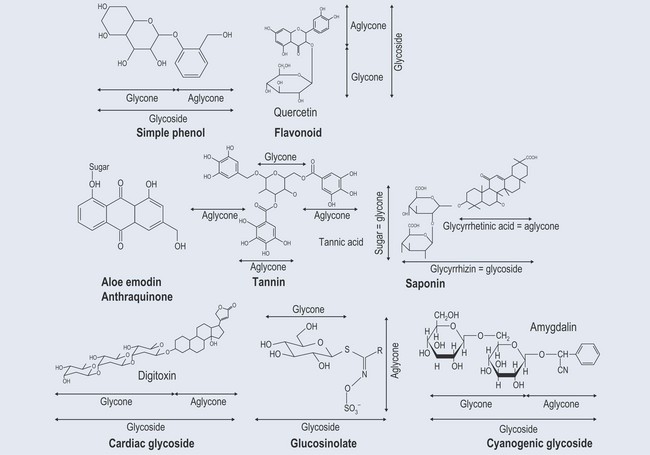
Many of the plant secondary metabolites are found naturally attached to sugars (glycosides). Mostly, the sugars are monosaccharides (Figure 24.1), such as glucose, but they can be more complicated. The sugars link to a non-sugar part called an aglycone and can be attached by separate bonds or, more commonly, as di-, tri- or tetrasaccharides. This is done by one sugar attaching to the aglycone and the others linking onto that sugar.
The linkage between the two groups can vary. It can be:
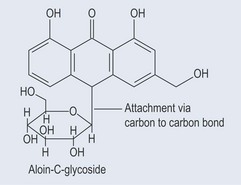
Figure 24.2 The C-glycoside linkage. The sugars attached vary in composition and can consist of one or more sugars.
The well-established naming of glycosides using the termination ‘in’ (e.g. salicin, aloin) has persisted and results in some confusion, as some substances (e.g. pectin) are not glycosides. Similar examples of this, involving isoflavones, are discussed in Chapter 21 ‘Phenols’ (p. 161). The more modern termination ‘-oside’ can be used (e.g. sennoside) to prevent confusion.
Glycosides are generally inactive and are activated by a process of hydrolysis into:
Anthraquinones
It has been shown experimentally that the sugar moiety certainly of sennosides A and B (see Figure 21.9, p. 155) remains unaltered in both the stomach and gut, but is cleaved off in the caecum by the activity of microorganisms, which convert them to dianthrones. The dianthrones that remain in the gut are cleaved again to form an anthrone and anthraquinone (see Figure 21.8, p. 154), which produce the laxative effects.
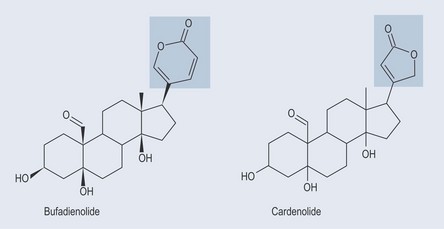
Cardiac Glycosides
The Heart
Cardiac glycosides exert a positive inotropic effect on the heart in cardiac failure (see Chapter 26 ‘Cardiovascular disorders’, p. 191). Cardiac failure occurs when the heart is unable to pump blood effectively at a rate that meets the needs of the body. The heart muscle can perform only weakly, particularly on ventricular contraction. This reduced pumping capacity results in a reduced heart output. However, as new blood continues to enter the heart, the volume of blood in the heart increases. As the heart is unable to pump this blood out, it becomes congested, hence ‘congestive heart failure’. Cardiac glycosides (or the aglycones) increase the capacity of the heart muscle to pump.
The mechanism of action of the cardiac glycosides is still not clear, but the most widely accepted idea is that the cardiac glycosides inhibit the membrane-bound sodium and potassium pumps responsible for the sodium and potassium exchange (see Figure 31.1, p. 235).
Cyanogenic Glycosides
Cyanogenic glycosides (Figure 24.4) yield hydrocyanic acid (HCN) as one of the products of hydrolysis, hence the name ‘cyanogenic’. They are capable of producing cyanide when broken down.