Chapter 95 Glutamine
 Introduction
Introduction
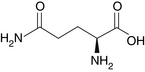
Glutamine (Figure 95-1) is the most abundant amino acid in blood and muscle tissue. It comprises approximately 6% of mixed whole body protein and is unique among amino acids in that it is a preferred fuel of rapidly dividing cells, such as intestinal and immune cells, and is important in maintaining pancreatic function.1–3 Glutamine is involved in the transport of circulating amino nitrogen and is an important intermediary that allows for accelerated gluconeogenesis from amino acids that are released by the skeletal muscle during stress states.4 In addition, glutamine is used as a precursor for DNA and glutathione synthesis.5 As one of the principal fuels used by the cells of the intestinal lining, it accounts for 35% of enterocyte energy production.
Although readily available in the diet and synthesized in the body from glutamate and ammonia, supplementation is known to enhance the energy metabolism of the gastrointestinal mucosa, thus stimulating regeneration.6 Although glutamine is not considered essential in healthy people, there is evidence that the increased need for glutamine in stressed states such as burns, septicemia, endotoxemia, intestinal failure, and critical illness may result in it being “conditionally essential.”3,7,8
Forms
The nomenclature of L-glutamine and glutamine are used interchangeably. D-glutamine is the stereoisomer of L-glutamine and does not have any known biological activity. L-glutamine is not soluble in water, and aqueous solutions are unstable at temperatures of 22° C to 24° C. As a result, the more soluble and more stable dipeptides such as alanyl-glutamine are used as delivery forms of L-glutamine in total parenteral nutrition solutions.9,10
Physiologic Effects
Intestinal Repair and Protection
Animal and human studies suggest that glutamine stimulates intestinal mucosal growth11 and protects from mucosal atrophy. Glutamine prevents intestinal mucosal damage and was shown to decrease bacterial leakage across the intestines after they are damaged, presumably by stimulating repair.12 Glutamine is thought to accomplish this by strengthening epithelial tight junctions and also by preventing paracellular permeabilities through an epidermal growth factor receptor–dependent mechanism.
In one tissue culture experiment, intestinal epithelium cells were treated with acetaldehyde to compromise barrier function. These cells were treated with L-glutamine, D-glutamine, L-asparagine, L-arginine, L-lysine, or L-alanine. Only the L-glutamine demonstrated a benefit by decreasing aldehyde effects on transepithelial resistance. Furthermore, L-glutamine–treated cells decreased permeability that was dose dependent. L-glutamine reduced the acetaldehyde-induced disturbance of transmembrane structures, such as occludin, zonula occludens-1, E-cadherin, and β-catenin from the intercellular junctions. Lastly, L-glutamine induced a rapid increase in the tyrosine phosphorylation of the epidermal growth factor receptor. No other amino acids demonstrated this effect.13
Acid Base Balance
Glutamine plays an important role in acid-base homeostasis.14 Glutamine is synthesized from glutamate and the toxic alkaline waste product ammonia by the enzyme glutamine synthetase, which requires magnesium and adenosine triphosphate. When ammonia levels are elevated, the body effectively removes ammonia from the blood by synthesizing glutamine. Conversely, if the blood is too acidic, the glutamine can be broken down into glutamate and ammonia, which increases blood pH. Ammonia can bind hydrogen ions to produce ammonium cations, which are excreted in the urine along with chloride anions. Bicarbonate ions are simultaneously released into the bloodstream. Clinical studies showed that relatively small oral doses of glutamine can elevate plasma bicarbonate concentrations in healthy adults.
In one study, 2 g of glutamine were dissolved in a cola drink and ingested over a 20-minute period 45 minutes after a light breakfast. Control subjects were given soda only. Blood samples were taken 1 week before, at baseline, and subsequently at three separate 30-minute intervals after ingestion of the glutamine drink or placebo. Eight of nine subjects responded to the oral glutamine load with a significant increase in plasma glutamine at 30 and 60 minutes before returning to the baseline value at 90 minutes. Ninety minutes after the glutamine was administered, plasma bicarbonate concentration was found to be increased. Circulating plasma growth hormone concentration was elevated as well. Concomitant with enhanced renal acid secretion, glutamine ingestion also caused an increase in the glomerular filtration rate.15
The authors of this study explained that their results showed that it was unlikely L-glutamine was a direct precursor of bicarbonate. Instead, L-glutamine appeared to play an indirect role in accelerating acid secretion through mechanistic changes in the kidneys. Human studies showed that urinary ammonium excretion is altered by changes in glutamine intake.16
Chronic metabolic acidosis is a common clinical problem encountered in catabolic states such as sepsis, shock, and diabetes, and is a major factor in many biological derangements.17 Because glutamine becomes an essential amino acid in catabolic states when the increased demand exceeds the body’s capability to synthesize it,18 glutamine supplementation may be quite useful to maintain pH homeostasis in patients with acidotic conditions.
Glutathione Repletion
Glutathione is a tripeptide consisting of glutamate, cysteine, and glycine. As a reservoir source for glutamate in the body, the availability of glutamine appears crucial for the regeneration of glutathione stores in the liver during hepatic injury; in skeletal muscle after major trauma, sepsis, or surgery; and in chemotherapy-injured heart muscle.19–21 Glutamine can enhance intracellular repletion of glutathione, an important scavenger of reactive oxygen species.22 Rat studies demonstrated that during 5-fluorouracil–induced free radical–mediated hepatic injury, glutamine increased glutathione biosynthesis and preserved the glutathione stores in hepatic tissue.19 Seventeen patients who underwent a standardized surgical procedure were prospectively given 0.56 g/kg body weight/day of glutamine or a placebo. Using percutaneous muscle biopsies and blood samples, there were no significant decreases in total or reduced glutathione in the glutamine-supplemented group 24 and 72 hours after the operation. In contrast, the placebo group experienced total muscle glutathione losses of 47 ± 8% and 37 ± 11%, as well as reduced glutathione decreases of 53 ± 10% and 45 ± 16% at 24 and 72 hours, respectively.
Protein Sparing
Glutamine is a regulator of muscle proteolysis,23 and supplementation can attenuate loss of protein in the muscle. Experiments using animal cancer models demonstrated decreased protein loss and simultaneous protection of immune and gut-barrier function during radiation therapy in patients with advanced cancer.5 In children with severe muscle wasting, 5 hours of oral glutamine was shown to have protein-sparing effect (see later discussion on “Cachexia”).24
Immune Support
Although poorly understood, it appears that glutamine has an immune-modulating effect by enhancing interleukin (IL)-6 levels25 and lymphocyte function.26 IL-6 plays an essential role in the final differentiation of β-cells into immunoglobulin-secreting cells, nerve cell differentiation, and acute phase reactants in hepatocytes. Exercise by itself is known to induce an eleven-fold increase in plasma IL-6. Glutamine supplementation further enhances IL-6 levels.25 The ability of lymphocytes to proliferate and generate lymphokine-activated killer cell activity in vitro was found to be glutamine dependent.27 Additionally, glutamine-enriched parental nutrition demonstrated enhanced lymphocyte activity in patients who received high doses of chemotherapy after stem cell transplantation for hematologous malignancy.
 Clinical Applications
Clinical Applications
Intestinal Permeability–Related Conditions
A number of conditions are linked to intestinal permeabilities, including chronic urticaria,28 inflammatory bowel disease (Crohn’s disease),29–31 celiac disease,32 liver and biliary cirrhosis and cases of portal hypertension,33,34 systemic sclerosis,35 diabetes,36 rheumatologic disorders,37,38 cystic fibrosis,39 alcohol overuse,40 adult and child asthma,41 human immunodeficiency virus (HIV)/acquired immune deficiency syndrome,42 nonsteroidal antiinflammatory drug–treated arthritis patients,43 moderate to major burn injuries,44 corticosteroid use,45 cardiopulmonary bypass patients,46,47 and acute metal toxicities.48 To evaluate these permeabilities, sucrose serves as a marker for gastroduodenal permeability and the urinary lactulose/mannitol ratio for intestinal permeability, after administration of these sugars.28 From a naturopathic perspective, the underlying cause of many of these conditions may stem from food allergies that contribute first to chronic inflammation in the intestinal tract28 and then to systemic endotoxemia. Certain conditions such as cardiopulmonary bypass can cause intestinal ischemia,49 which is then the primary insult that causes permeabilities in these patients. The use of glutamine can help heal these permeabilities, thus removing a mode of pathogenesis in these variegated conditions.
Infectious Diarrhea
Animal models showed the usefulness of glutamine in diarrhea to augment sodium and water absorption and to enhance blood glucose and body weight.50 A rat model of cholera toxin–induced diarrhea showed that glutamine was able to improve water and electrolyte intestinal absorption even better than traditional glucose solutions.10 One placebo-controlled, double-blind, randomized trial human study evaluated glutamine to treat acute diarrhea in 128 otherwise healthy children. Of these 6- to 24-month-olds, 63 received 0.3 g/kg per day of glutamine and 65 controls received a placebo for 7 days. The average duration of diarrhea in the glutamine-treated group was significantly shorter than that of the placebo group (3.40 ± 1.96 vs 4.57 ± 2.48 days, respectively). However, no differences in serum IL-8 and secretory immunoglobulin-A were found between groups at the beginning of treatment or 1 week later.51
Clearly, glutamine holds promise for enhancing repair of mucosal injury caused by a wide range of infections or toxic agents and thus has great potential as a nutritional therapeutic for patients with enteric infection.52
Postsurgical Complications of the Gastrointestinal Tract
Patients undergoing abdominal surgeries such as gastrectomies, sigmoidectomies, cholecystectomies, colectomies, and rectal resections are at risk for the development of intestinal failure or short bowel syndrome (SBS). In SBS, a serious malabsorption of fluid, electrolytes, and other nutrients can occur, placing the patient at higher morbidity and mortality risk.53 Trauma from abdominal surgery may also compromise the intestinal mucosa to the point where bacteria and endotoxins can easily transfer through the intestinal wall and invade tissue and blood in an event called bacterial translocation. Through inflammatory mechanisms, bacteria, and endotoxic septic conditions, the intestinal mucosal barrier can be adversely affected and cause further damage, thus forming a vicious circle. Severe cases result in systemic inflammatory response syndrome and multiple organ dysfunction syndrome.54
In a regimen that includes growth hormone and diet changes, glutamine can help difficult cases to enhance bowel adaptation. In one study of 10 patients with SBS who previously failed to adapt to enteral nutrients, 8 subjects received exogenous growth hormone, supplemental glutamine, and a modified high-carbohydrate, high-fiber diet. Two patients were treated with the modified diet alone. Three weeks of treatment with growth hormone, glutamine, and a modified diet significantly increased total caloric absorption from approximately 60% to 75%, protein absorption from 49% to 63%, and carbohydrate absorption from 60% to 82%. Water absorption increased from 46% to 65%, and sodium from 49% to 69%. Fat absorption did not change. Diet alone did not influence nutrient absorption or stool output. After 28 days of therapy, the patients were discharged and instructed to continue the diet and glutamine treatment.55 It is unknown whether glutamine and diet changes alone, without concomitant growth hormone administration, would have the same positive effect.
In a second study, 20 patients who underwent abdominal surgery were randomized into two groups receiving oral administration of 30 g of glutamine or a placebo in divided doses for 7 days. Serum glutamine concentration was significantly decreased in the placebo group and increased in the glutamine group after 7 days. Markers of intestinal permeability were significantly increased in the placebo group and decreased in the glutamine group. Additionally, the serum markers of endotoxin, diamine oxidase, and malondialdehyde concentrations were significantly decreased in the glutamine group compared with those in the placebo group. Temperatures, heart rates, and white blood cell counts were also significantly lower in the glutamine group.54
Ischemia reperfusion of the gut is also a common event in various clinical conditions, such as trauma, burn, septic shock, cardiac or aortic surgery, and liver or small bowel transplantation, and is associated with a high death rate. Intestinal ischemia reperfusion can cause edema and disruption of the structural integrity and function of the intestinal mucosa and associated vascular tissue. It may set the stage for endotoxemic translocation of a number of bacterium, including Escherichia coli, Enterococcus, Pseudomonas, Proteus, and Staphylococcus. Studies of animal models demonstrated that glutamine, when supplemented as total parenteral nutrition, protected the intestines from morphologic and functional mucosal injury after intestinal ischemia reperfusion. Furthermore, intestinal permeabilities and the incidence of bacterial translocation in intestinal ischemia reperfusion animals were also prevented in a dose-dependent manner by glutamine supplementation.56,57
Chemotherapy and Radiation Side Effects
Standard cancer therapies often include the use of chemotherapy and radiation, which can injure rapidly dividing intestinal cells. It was shown that during chemotherapeutic and radiotherapy insult, glutamine reduced degeneration of intestinal mucosa in rats, prevented intestinal mucosal injury,56 protected liver function through enhanced glutathione biosynthesis and storage in hepatic tissue, increased immune function, and reduced permeability of the gut.19,26
In one investigation, 70 patients with colorectal cancer were randomly assigned to oral glutamine at 18 g/day or placebo before the first regimen of 5-fluorouracil and folinic acid administered intravenously for 5 days. Glutamine was given 5 days before, during, and after chemotherapy. Using D-xylose urinary excretion and cellobiose/mannitol evaluation, damage to the intestines was assessed at baseline, as well as 4 and 5 days after the end of the first cycle of chemotherapy. After one cycle of chemotherapy, the reduction in D-xylose absorption and reduction of mannitol was significantly greater in the placebo group (7.1% vs 3.8% and 9.2% vs 4.5%, respectively). Urinary recovery of cellobiose was not different between the study arms. Accordingly, the cellobiose/mannitol ratio increased more in the placebo treatment group. Furthermore, diarrhea parameters, as well as the average number of antidiarrheal opiate loperamide tablets needed, were reduced in the glutamine arm, thus supporting the positive clinical effect of this low-cost supplement.11
Oropharyngeal mucositis, or mouth sores, and accompanying swallowing difficulty are other untoward results of radiotherapy and can be a major source of suffering in patients with head and neck cancer. Glutamine during and after chemotherapy appears to be an excellent way to safely decrease the incidence of mouth sores. One investigation of 17 patients with head and neck cancer who received primary or adjuvant mouth irradiation for 5 days a week were randomized to either adjunctive glutamine suspension of 16 g in 240 mL normal saline or a saline placebo. Patients were instructed to swish the test solutions (30 mL) four times daily. The duration of objective oral mucositis was significantly shorter in the glutamine arm.58 A second randomized, double-blind crossover trial observed 24 patients who were given a glutamine or placebo suspension to swish and swallow on days of chemotherapy administration and for at least 14 days after therapy. Significant improvement was observed in the glutamine group. Additionally, the duration of mouth pain was 4.5 days less in chemotherapy courses with concomitant glutamine supplementation. The severity of oral pain was reduced so significantly when glutamine was used that a patient could venture past soft foods 4 days sooner compared with placebo.59
Glutamine studies validating its use are also beginning to emerge in other areas of oncology. In a study of esophageal cancer patients, 13 patients were randomized into two groups, controls and a group that received oral glutamine supplemented at a dosage of 30 g/day for 4 weeks. It was observed that supplementation of glutamine enhanced lymphocyte mitogenic function and reduced permeability of the gut during radiochemotherapy.5 Patients who underwent bone marrow transplant and myelosuppressive chemotherapy for acute myeloid leukemia also found that parenteral glutamine therapy could improve neutrophil recovery, although no change in neutropenic fever was shown.60 Given that glutamine improves the structure and function of the gut, it is understandable that multiple parameters and markers of healthy physiologic function will improve with its use.
It should be noted that glutamine’s efficacy may depend on a number of other factors, including the specific chemotherapeutic prescribed and dosage. A study of 65 patients with advanced breast cancer receiving doxifluridine were prescribed 30 g/day of glutamine in three divided doses of 10 g each or a placebo for 8 consecutive days during each interval between chemotherapy, which was administered from days 1 to 4. In this case, there was no statistical difference with regard to diarrhea morbidity, nor did glutamine affect the severity and duration of tumor growth.61 Interestingly, a study of bone marrow transplantation patients found that allogeneic transplantation patients (those receiving bone marrow from another individual) did not have the same beneficial mouth pain reduction that autologous transplantation patients (those who donated their own marrow) experienced when receiving glutamine support. However, in the work mentioned previously, the amounts of glutamine were less than those used in other studies.
It was also theorized that methotrexate use in the allogeneic patients might have been responsible for the decreased protection. Nevertheless, in the allogeneic patients, the 28-day survival was still increased.62 A third multicenter study of 129 patients found no protection against diarrhea when used adjunctively with pelvic radiation therapy. These patients received 4 g of glutamine or a placebo by mouth, which was also a significantly lower dose than the more successful studies employed.63
Although intestinal function is greatly compromised with chemotherapy and radiation treatment, cardiac function is commonly affected as well. The use of doxorubicin therapy for breast cancer is often limited by cardiomyopathic heart changes that often result in congestive heart failure. One rat study simulated doxorubicin treatment with and without glutamine support and found that oxidative damage to the heart was diminished in the glutamine-treated group, probably as a result of glutamine’s ability to maintain cardiac tissue glutathione levels (see later discussion on “Cardiac Disease”).21
Cachexia
Cancer-related cachexia is caused by a diverse combination of accelerated protein breakdown and slowed protein synthesis.64 There has been considerable interest in giving supplemental glutamine to cancer patients because glutamine is taken up by the growing tumor, and any subsequent deficiency of glutamine in the host may cause cancer cachexia.5
One animal study found that glutamine levels in plasma and skeletal muscle were decreased in tumor-bearing rats, whereas glutamine production and the conversion of arginine to glutamine were increased. In rats supplemented with glutamine, total parenteral nutrition demonstrated a reduced whole body protein breakdown rate during chemotherapy.5 A clinical study of patients with Stage IV solid malignancies who had weight loss of at least 5% were randomly assigned in a double-blind fashion to either a control mixture of nonessential amino acids or treatment of 14 g/day of glutamine, along with the leucine metabolite β-hydroxy-β-methylbutyrate (3 g/day) and L-arginine (14 g/day). Within 4 weeks, the patients supplemented with the glutamine mixture gained 0.95 ± 0.66 kg of body mass, whereas control subjects lost 0.26 ± 0.78 kg during the same period. This effect continued over the 24 weeks, with no negative effect of treatment on the incidence of adverse effects or quality of life measures.64
Because the glutamine derived from skeletal muscle is trapped by the tumor, there is a theoretic concern that glutamine supplementation in cancer patients could potentially encourage tumor growth. One research group, however, showed that glutamine supplementation does not appear to enhance DNA content in tumor cells.5,65 Additionally, some tissue culture studies provided evidence that glutamine might even inhibit cancer promotion.66 Although research is necessary to clarify this point, given the immediate risk of mortality due to protein loss in cancer patients, it still would seem prudent to administer glutamine to those patients at greater immediate risk of cachexia-related death.
Human Immunodeficiency Virus
Loss of body cell mass and drug-associated gastrointestinal problems often occur in patients with HIV infection. In these cases, the patient’s ability to survive can be affected in the long term. Given the role glutamine plays in cachexic body mass loss (see previous section on “Cachexia”), reversal of malabsorption,67 and protection of the small intestine,68 glutamine deficiency is a probable causal factor in HIV-associated wasting.69
Preliminary clinical studies suggested improvements in HIV-positive patients dosed at 8 g/day with regard to intestinal permeability and intestinal absorption. The authors of this study correctly suggested that at least 20 g/day might be necessary for more significant improvements.70 A double-blind, placebo-controlled trial of 26 patients with greater than 5% weight loss since their disease onset used a glutamine and antioxidant regimen, including 40 g/day of glutamine in divided doses or 40 g of a glycine placebo for 12 weeks. Over 3 months, the glutamine/antioxidant group gained 2.2 kg in body weight (3.2%), whereas the control group gained only 0.3 kg (0.4%).
The glutamine-antioxidant group gained 1.8 kg in body cell mass, whereas the control group gained 0.4 kg. Of note, the intracellular water increased in the glutamine-antioxidant group but not in the control group.71 Glutamine can help HIV patients decrease the severity of iatrogenic diarrhea. Twenty-five patients suffering for more than a month from nelfinavir-associated diarrhea were randomized in a double-blind, placebo-controlled, crossover trial to receive L-glutamine at 30 g/day or a placebo for 10 days. In this study, the L-glutamine significantly reduced the severity of nelfinavir-associated diarrhea and produced improved quality of life compared with placebo.72
Peptic Ulcers
Cabbage juice, a key source of glutamine, has been well documented as having remarkable success in treating peptic ulcers. One liter per day of the fresh juice, taken in divided doses, resulted in total ulcer healing in an average of only 10 days. Further research showed that the high glutamine content of the juice is probably responsible for the efficacy of cabbage in treating these ulcers. In a double-blind clinical study of 57 patients, 24 using 1.6 g/day of glutamine and the rest using conventional therapy (antacids, antispasmodics, milk, and bland diet), glutamine proved to be the more effective treatment. Half of the glutamine patients showed complete healing (according to radiographic analysis) within 2 weeks, and 22 of the 24 showed complete relief and healing within 4 weeks.22 Although the mechanism for these results is unknown, it was postulated by the authors to be due to the role of glutamine in the biosynthesis of the hexosamine moiety in certain mucoproteins. These moieties may stimulate mucin synthesis, which would benefit peptic ulcer patients.
Severe Burns
Plasma glutamine levels were demonstrated to be profoundly decreased after severe burns in adults. This may at least partially explain the impaired cellular immunity that is seen in burn patients. Burn victims given glutamine showed better intestinal repair, a higher quality of wound healing, and reduced hospitalization. In one study, 48 severe burn patients were randomly divided into two groups: a control group that took a placebo and a glutamine-treated group that received 0.5 g of glutamine/kg body weight/day, both for 14 days. After taking glutamine for 14 days, plasma glutamine concentration was significantly increased in the glutamine group compared to the control group. In addition, a greater quality of wound healing as well as shorter hospital stays were experienced in the glutamine-treated burn patients.73
In another study, dosage of L-glutamine at 0.6 g/kg per day did not result in an immediate whole body protein gain (an important factor in a burn patient’s convalescence) and resulted in an insignificant increase in plasma glutamine.4 However, this study measured only the first 48 hours, which might not have been enough time to show a long-term benefit.
Another study of 45 severely burned adults found that those randomized to receive enteral glutamine experienced a reduction in blood infection by a factor of three, and mortality risk was lowered.74 Another investigation of burned patients whose total body surface burns ranged from 50% to 80% and third-degree burns ranged from 20% to 40%, but who did not have respiratory injuries found improved gut permeability, initially decreased plasma endotoxin levels, and reduced hospitalization.75
Low-Birth-Weight Infants
Infants with a birth weight of less than 1000 to 1500 g may be especially susceptible to glutamine depletion, as nutritional supply of glutamine is limited in the first weeks after birth. This may increase morbidity by contributing to problems with gut integrity, as well as immune suppression.3 One study of 35 ill preterm neonates of less than 1000 g were randomized to receive either glutamine-supplemented parenteral nutrition or standard parenteral nutrition. Although there were no significant differences between the groups in white cell count, differential white cell count, blood urea nitrogen, plasma ammonia, lactate, pyruvate, plasma glutamine, or glutamate, the median time to achieving full enteral nutrition was shorter in the glutamine group (13 versus 21 days). Parenteral glutamine was well tolerated and considered safe for these preterm neonates.76
However, other studies found that formula supplemented with glutamine in growing preterm infants was entirely metabolized in the gut and did not have a discernable effect on whole-body protein and nitrogen kinetics.77 A large multicenter, double-blind, randomized trial of infants with a birth weight of 401 to 1000 g were given either a control or 20% isonitrogenous solution by parenteral nutrition for up to either 120 days of age, death, or discharge from the hospital. Of the 721 infants who were assigned to glutamine supplementation, 370 (51%) died or developed late-onset sepsis, compared with 343 of the 712 infants (48%) assigned to control. Although no adverse effects were noted as a result of being given glutamine, this study demonstrated that glutamine did not decrease mortality. This study and others also found no reduction in the incidence of sepsis in these young patients.78,79
Exercise and Weight Lifting
Glutamine is considered to have an anabolic effect on skeletal muscle. Given the benefits on glutathione reserves, protein catabolism, and intestinal integrity, some glutamine enthusiasts believe that glutamine supplementation may be useful for exercise and strength training as well. One small study suggested that oral glutamine increases growth hormone release.15 Even so, clinical trials studying glutamine as an exercise performance enhancer are not encouraging.
A double-blind, placebo-controlled, crossover study had six resistance-trained men lift weights after the ingestion of glutamine or glycine at 0.3 g/kg body weight or a placebo. One hour after ingestion, subjects performed four total sets of exercise to momentary muscular failure, including two sets of leg presses at 200% of body weight and two sets of bench presses at 100% of body weight. Despite glutamine’s possible role in exercise, there were no actual differences in the average number of maximal repetitions performed in the leg press or bench press exercises among these three groups.80 Other studies using 0.3 to 0.9 g/kg of body weight also demonstrated no changes in exercise performance, body composition, or muscle protein degradation in young healthy adults.81,82 It is possible that beneficial effects of glutamine are best detected only in patients with chronic illness and those with compromised physiology instead of normal, healthy individuals.
Cardiac Disease
Cardiac disease is a recognized stress on the physiology of the gastrointestinal and immune system. Animal studies supported the notion that glutamine can help recovery from cardiac ischemia and help the heart recover after reperfusion injury.83,84 As noted earlier, glutamine can protect the heart from damage due to chemotherapy regimens by its glutathione-replenishing effect (see earlier discussion on “Chemotherapy and Radiation Side Effects”).19 The benefits to the gastrointestinal system in those heart patients experiencing ischemic gut episodes after cardiac bypass were also previously noted.46,47 Animal research showed that greater levels of plasma glutamine also prevented decreases in the ratio between adenosine triphosphate to adenosine diphosphate in myocardial tissue.84
Unfortunately, little research has been done to evaluate the clinical use of glutamine in various cardiac situations. One investigation of patients with chronic stable angina received a single 80 mg/kg oral dose of glutamine or a placebo in a double-blind, random fashion 40 minutes before a standard exercise test. This single dose of glutamine significantly increased plasma glutamine concentration from 419 to 649 µmol/L. Moreover, the glutamine appeared to encourage positive changes in ST depression on the echocardiogram.84 Clearly, more research is required, but given that heart disease is the leading reason for mortality, natural and safe treatments like glutamine should be further explored.
Other Conditions
In alcoholics, glutamine supplementation (1 g/day) was shown to reduce voluntary alcohol consumption in uncontrolled human studies and experimental animal studies.85–88 Despite the fact that this research is about 50 years old, there has never been any follow-up to these preliminary studies. This is unfortunate, because the results were quite promising, finding glutamine to be safe and relatively inexpensive. Related to alcoholism in many patients, individuals with acute pancreatitis also benefited from parenteral glutamine treatment with improvement in immune function, decreased systemic inflammation, and a trend toward shorter hospital stays.1
In an interesting study of 20 brain injury patients, 10 subjects were randomized to receive either an early enteral diet or the same formula with glutamine and probiotic added for a range of 5 to 14 days. The infection rate was found to be 100% in the control group, but only 50% in the glutamine group. The median number of infections per patient was significantly greater in the control group compared with the study group. Critical care stay and ventilation requirements were more than halved in the treatment group (10 vs 22 days, and 14 vs 7 days, respectively). Interestingly, probiotics were also used in the treatment group. This synergistic enhancement in gut flora may be useful to augment the already known benefits of glutamine.89
 Dosage
Dosage
• Severe burns: enteral dosage for individuals with severe burns was approximately 0.5 g/kg per day in most studies.
• Preventing and treating the side effects of chemotherapy involves the following:
• Patients treated with 5-fluorouracil received up to 18 g/day 5 days before, then during, and 5 days after treatment.11
• High-dose chemotherapy after stem cell transplantation: total parenteral nutrition enriched with glutamine 20 g.26
• Oral mucositis: 16 g mixed with 240 mL normal saline, and 30 mL is swished four times a day.58
• Esophageal cancer radiotherapy was given with a successful adjunctive oral glutamine supplement of 30 g/day for 4 weeks.5
• Cachexia patients were tested with dosages of 14 g/day combined with other amino acids.64
• Pediatric oncology patients: 0.65 g/kg was found to be a safe dose of glutamine to use in a clinical study in pediatric oncology patients.90
• Children with acute diarrhea: 0.3 g/kg per day was used successfully.51
• Peptic ulcers: drinking 1 L a day in divided doses was sufficient or 1.6 g/day for 1 month.23
• HIV patients: 30 to 40 g/day of glutamine to prevent medication-associated diarrhea and to improve intestinal permeability71,72; coadministration of antioxidants might also be helpful.
• Postabdominal surgeries: glutamine can be dissolved in warm water and taken orally or by gastric tube after the operation at 30 g/day for 7 days.54
 Toxicity
Toxicity
Glutamine, even at high doses, is without apparent side effects and is well tolerated.4,52 Glutamine is synthesized from glutamate and the toxic alkaline waste product ammonia. If the blood is too acidic, the glutamine can be broken down into glutamate and ammonia, which will increase blood pH. Glutamate levels in the blood can increase slightly with high doses of supplemental glutamine administration (around 15 g in a single dose), but not with moderate doses (of about 5 g in a single dose). The higher doses may contribute to glutamate levels, and should be used with caution in patients with neurodegenerative diseases such as amyotrophic lateral sclerosis and multiple sclerosis.91 In one pediatric oncology patient, a single dose of 0.75/kg was found to raise the blood ammonia level to an unacceptably high limit. Related to this, it was difficult to disperse the glutamine adequately at this dose, resulting in the suspension being found unpalatable.
 Drug Interactions
Drug Interactions
In cancer treatment, glutamine does not appear to change the efficacy of cancer drugs, rate of relapse, or progression of malignancy.62 Some animal studies suggested that glutamine supplementation might even preferentially increase tumor retention of methotrexate, thus increasing the therapeutic window of this drug.92 Many antiseizure medications, including phenobarbital, phenytoin, carbamazepine, primidone, and valproic acid, work to block glutamate activity in the brain. Because glutamine can convert to glutamate, clinicians should be cautious when using glutamine in patients using these medications.
1. Ockenga J., Borchert K., Rifai K., et al. Effect of glutamine-enriched total parenteral nutrition in patients with acute pancreatitis. Clin Nutr. 2002;21:409–416.
2. Souba W.W. Cytokine control of nutrition and metabolism in critical illness. Curr Probl Surg. 1994;31:577–643.
3. van den Berg A., van Elburg R.M., Twisk J.W., et al. Glutamine-enriched enteral nutrition in very low birth weight infants. Design of a double-blind randomised controlled trial. BMC Pediatr. 2004;4:17.
4. Sheridan R.L., Prelack K., Yu Y.M., et al. Short-term enteral glutamine does not enhance protein accretion in burned children: a stable isotope study. Surgery. 2004;135:671–678.
5. Yoshida S., Kaibara A., Ishibashi N., et al. Glutamine supplementation in cancer patients. Nutrition. 2001;17:766–768.
6. Souba W.W. Glutamine: a key substrate for the splanchnic bed. Annu Rev Nutr. 1991;11:285–308.
7. Souba W.W., Klimberg V.S., Plumley D.A., et al. The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J Surg Res. 1990;48:383–391.
8. Qin H.L., Cui H.G., Zhang C.H., et al. Effects of glutamine on structure and function of gut in endotoxemic rats. China Natl J New Gastroenterol. 1996;2:69–72.
9. PDR health. L-Glutamine. Available online at http://www.pdrhealth.com/drug_info/nmdrugprofiles/nutsupdrugs/lgl_0125.shtml Accessed 9/23/2004
10. Carneiro-Filho B.A., Bushen O.Y., Brito G.A., et al. Glutamine analogues as adjunctive therapy for infectious diarrhea. Curr Infect Dis Rep. 2003;5:114–119.
11. Daniele B., Perrone F., Gallo C., et al. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: a double blind, placebo controlled, randomised trial. Gut. 2001;48:28–33.
12. Klimberg V.S., Salloum R.M., Kasper M., et al. Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch Surg. 1990;125:1040–1045.
13. Seth A., Basuroy S., Sheth P., et al. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G510–G517.
14. Welbourne T., Claville W., Langford M. An oral glutamine load enhances renal acid secretion and function. Am J Clin Nutr. 1998;67:660–663.
15. Welbourne T.C. Increased plasma bicarbonate and growth hormone after an oral glutamine load. Am J Clin Nutr. 1995;61:1058–1061.
16. Welbourne T., Weber M., Bank N. The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease. J Clin Invest. 1972;51:1852–1860.
17. Pan M., Meng Q., Choudry H.A., et al. Stimulation of intestinal glutamine absorption in chronic metabolic acidosis. Surgery. 2004;136:127–134.
18. Souba W.W. Glutamine: Physiology, Biochemistry, and Nutrition in Critical Illness. Austin, TX: RG Landes Publishing; 1991.
19. Yu J.C., Jiang Z.M., Li D.M. Glutamine: a precursor of glutathione and its effect on liver. World J Gastroenterol. 1999;5:143–146.
20. Flaring U.B., Rooyackers O.E., Wernerman J., et al. Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin Sci (Lond). 2003;104:275–282.
21. Cao Y., Kennedy R., Klimberg V.S. Glutamine protects against doxorubicin-induced cardiotoxicity. J Surg Res. 1999;85:178–182.
22. Harward T.R., Coe D., Souba W.W., et al. Glutamine preserves gut glutathione levels during intestinal ischemia/reperfusion. J Surg Res. 1994;56:351–355.
23. May P.E., Barber A., D’Olimpio J.T., et al. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–479.
24. Jensen G.L., Miller R.H., Talabiska D.G., et al. A double-blind, prospective, randomized study of glutamine-enriched compared with standard peptide-based feeding in critically ill patients. Am J Clin Nutr. 1996;64:615–621.
25. Hiscock N., Petersen E.W., Krzywkowski K., et al. Glutamine supplementation further enhances exercise-induced plasma IL-6. J Appl Physiol. 2003;95:145–148.
26. Piccirillo N., De Matteis S., Laurenti L., et al. Glutamine-enriched parenteral nutrition after autologous peripheral blood stem cell transplantation: effects on immune reconstitution and mucositis. Haematologica. 2003;88:192–200.
27. Noyer C.M., Simon D., Borczuk A. A double-blind placebo-controlled pilot study of glutamine therapy for abnormal intestinal permeability in patients with AIDS. Am J Gastroenterol. 1998;93:972–975.
28. Buhner S., Reese I., Kuehl F., et al. Pseudoallergic reactions in chronic urticaria are associated with altered gastroduodenal permeability. Allergy. 2004;59:1118–1123.
29. Danese S., Sans M., Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev. 2004;3:394–400.
30. Peeters M., Geypens B., Claus D., et al. Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterol. 1997;113:802–807.
31. Puspok A., Oberhuber G., Wyatt J., et al. Gastroduodenal permeability in Crohn’s disease. Eur J Clin Invest. 1998;28:67–71.
32. Smecuol E., Bai J.C., Vazquez H., et al. Gastrointestinal permeability in celiac disease. Gastroenterol. 1997;112:1129–1136.
33. Di Leo V., Venturi C., Baragiotta A., et al. Gastroduodenal and intestinal permeability in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2003;15:967–973.
34. Campillo B., Pernet P., Bories P.N., et al. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur J Gastroenterol Hepatol. 1999;11:755–759.
35. Catanoso M., Lo Gullo R., Giofre M.R., et al. Gastro-intestinal permeability is increased in patients with limited systemic sclerosis. Scand J Rheumatol. 2001;30:77–81.
36. Vaarala O. The gut immune system and type 1 diabetes. Ann N Y Acad Sci. 2002;958:39–46.
37. Katz J.P., Lichtenstein G.R. Rheumatologic manifestations of gastrointestinal diseases. Gastroenterol Clin North Am. 1998;27:533–562.
38. Parke A.L. Gastrointestinal disorders and rheumatic diseases. Curr Opin Rheumatol. 1991;3:160–165.
39. Van Elburg R.M., Uil J.J., van Aalderen W.M., et al. Intestinal permeability in exocrine pancreatic insufficiency due to cystic fibrosis or chronic pancreatitis. Pediatr Res. 1996;39:985–991.
40. Keshavarzian A., Holmes E.W., Patel M., et al. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207.
41. Hijazi Z., Molla A.M., Al-Habashi H., et al. Intestinal permeability is increased in bronchial asthma. Arch Dis Child. 2004;89:227–229.
42. Uil J.J., van Elburg R.M., van Overbeek F.M., et al. Clinical implications of the sugar absorption test: intestinal permeability test to assess mucosal barrier function. Scand J Gastroenterol Suppl. 1997;223:70–78.
43. Weber P., Brune T., Ganser G., et al. Gastrointestinal symptoms and permeability in patients with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2003;21:657–662.
44. Deitch E.A. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107:411–416.
45. Kiziltas S., Imeryuz N., Gurcan T., et al. Corticosteroid therapy augments gastroduodenal permeability to sucrose. Am J Gastroenterol. 1998;93:2420–2425.
46. Riddington D.W., Venkatesh B., Boivin C.M., et al. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA. 1996;275:1007–1012.
47. Sinclair D.G., Haslam P.L., Quinlan G.J., et al. The effect of cardiopulmonary bypass on intestinal and pulmonary endothelial permeability. Chest. 1995;108:718–724.
48. Gotteland M., Araya M., Pizarro F., et al. Effect of acute copper exposure on gastrointestinal permeability in healthy volunteers. Dig Dis Sci. 2001;46:1909–1914.
49. Videm V., Svennevig J.L., Fosse E., et al. Plasma endotoxin concentrations during cardiac surgery may be related to atherosclerosis. Perfusion. 2000;15:421–426.
50. Brooks H.W., White D.G., Wagstaff A.J., et al. Evaluation of a glutamine-containing oral rehydration solution for the treatment of calf diarrhea using an Escherichia coli model. Vet J. 1997;153:163–169.
51. Yalcin S.S., Yurdakok K., Tezcan I., et al. Effect of glutamine supplementation on diarrhea, interleukin-8 and secretory immunoglobulin A in children with acute diarrhea. J Pediatr Gastroenterol Nutr. 2004;38:494–501.
52. Majamaa H., Isolauri E., Saxelin M., et al. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20:333–338.
53. Matarese L.E., Seidner D.L., Steiger E. Growth hormone, glutamine, and modified diet for intestinal adaptation. J Am Diet Assoc. 2004;104:1265–1272.
54. Quan Z.F., Yang C., Li N., et al. Effect of glutamine on change in early postoperative intestinal permeability and its relation to systemic inflammatory response. World J Gastroenterol. 2004;10:1992–1994.
55. Byrne T.A., Morrissey T.B., Nattakom T.V., et al. Growth hormone, glutamine, and a modified diet enhance nutrient absorption in patients with severe short bowel syndrome. J Parenter Enteral Nutr. 1995;19:296–302.
56. Wu G.H., Wang H., Zhang Y.W., et al. Glutamine supplemented parenteral nutrition prevents intestinal ischemia-reperfusion injury in rats. World J Gastroenterol. 2004;10:2592–2594.
57. Zhang W.X., Zhou L.F., Zhang L., et al. Protective effects of glutamine preconditioning on ischemia-reperfusion injury in rats. Hepatobiliary Pancreat Dis Int. 2011;10:78–82.
58. Huang E.Y., Leung S.W., Wang C.J., et al. Oral glutamine to alleviate radiation-induced oral mucositis: a pilot randomized trial. Int J Radiat Oncol Biol Phys. 2000;46:535–539.
59. Anderson P.M., Schroeder G., Skubitz K.M. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer. 1998;83:1433–1439.
60. Scheid C., Hermann K., Kremer G., et al. Randomized, double-blind, controlled study of glycyl-glutamine-dipeptide in the parenteral nutrition of patients with acute leukemia undergoing intensive chemotherapy. Nutrition. 2004;20:249–254.
61. Bozzetti F., Biganzoli L., Gavazzi C., et al. Glutamine supplementation in cancer patients receiving chemotherapy: a double-blind randomized study. Nutrition. 1997;13:748–751.
62. Anderson P.M., Ramsay N.K., Shu X.O., et al. Effect of low-dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transplant. 1998;22:339–344.
63. Kozelsky T.F., Meyers G.E., Sloan J.A. North Central Cancer Treatment Group. Phase III double-blind study of glutamine versus placebo for the prevention of acute diarrhea in patients receiving pelvic radiation therapy. J Clin Oncol. 2003;21:1669–1674.
64. May P.E., Barber A., D’Olimpio J.T., et al. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–479.
65. Klimberg V.S., Souba W.W., Salloum R.M., et al. Glutamine-enriched diets support muscle glutamine metabolism without stimulating tumor growth. J Surg Res. 1990;48:319–323.
66. Kaufmann Y., Kornbluth J., Feng Z., et al. Effect of glutamine on the initiation and promotion phases of DMBA-induced mammary tumor development. J Parenter Enteral Nutr. 2003;27:411–418.
67. Dwyer J.T. Nutrition support of HIV+ patients. Henry Ford Hosp Med J. 1991;39:60–65.
68. Klimberg V.S., Souba W.W., Dolson D.J., et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990;66:62–68.
69. Shabert J.K., Wilmore D.W. Glutamine deficiency as a cause of human immunodeficiency virus wasting. Med Hypotheses. 1996;46:252–256.
70. Noyer C.M., Simon D., Borczuk A., et al. A double-blind placebo-controlled pilot study of glutamine therapy for abnormal intestinal permeability in patients with AIDS. Am J Gastroenterol. 1998;93:972–975.
71. Shabert J.K., Winslow C., Lacey J.M., et al. Glutamine-antioxidant supplementation increases body cell mass in AIDS patients with weight loss: a randomized, double-blind controlled trial. Nutrition. 1999;15:860–864.
72. Huffman F.G., Walgren M.E. L-glutamine supplementation improves nelfinavir-associated diarrhea in HIV-infected individuals. HIV Clin Trials. 2003;4:324–329.
73. Peng X., Yan H., You Z., et al. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns. 2004;30:135–139.
74. Garrel D., Patenaude J., Nedelec B., et al. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial. Crit Care Med. 2003;31:2444–2449.
75. Zhu M., Tang D., Zhao X., et al. Impact of glutamine of gut permeability and clinical prognosis on the aging patients undergoing gastric-intestinal operation. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2000;22:425–427. :[Chinese]
76. Thompson S.W., McClure B.G., Tubman T.R. A randomized, controlled trial of parenteral glutamine in ill, very low birth-weight neonates. J Pediatr Gastroenterol Nutr. 2003;37:550–553.
77. Parimi P.S., Devapatla S., Gruca L.L., et al. Effect of enteral glutamine or glycine on whole-body nitrogen kinetics in very-low-birth-weight infants. Am J Clin Nutr. 2004;79:402–409.
78. Poindexter B.B., Ehrenkranz R.A., Stoll B.J., et al. National Institute of Child Health and Human Development Neonatal Research Network. Parenteral glutamine supplementation does not reduce the risk of mortality or late-onset sepsis in extremely low birth weight infants. Pediatrics. 2004;113:1209–1215.
79. Vaughn P., Thomas P., Clark R., et al. Enteral glutamine supplementation and morbidity in low birth weight infants. J Pediatr. 2003;142:662–668.
80. Antonio J., Sanders M.S., Kalman D., et al. The effects of high-dose glutamine ingestion on weightlifting performance. J Strength Cond Res. 2002;16:157–160.
81. Candow D.G., Chilibeck P.D., Burke D.G., et al. Effect of glutamine supplementation combined with resistance training in young adults. Eur J Appl Physiol. 2001;86:142–149.
82. Haub M.D., Potteiger J.A., Nau K.L., et al. Acute L-glutamine ingestion does not improve maximal effort exercise. J Sports Med Phys Fitness. 1998;38:240–244.
83. Khogali S.E., Harper A.A., Lyall J.A., et al. Effects of L-glutamine on post-ischaemic cardiac function: protection and rescue. J Mol Cell Cardiol. 1998;30:819–827.
84. Khogali S.E., Pringle S.D., Weryk B.V., et al. Is glutamine beneficial in ischemic heart disease? Nutrition. 2002;18:123–126.
85. Rogers L.L., Pelton R.B., Williams R.J. Voluntary alcohol consumption by rats following administration of glutamine. J Biol Chem. 1955;214:503–506.
86. Rogers L.L., Pelton R.B. Glutamine in the treatment of alcoholism; a preliminary report. Q J Stud Alcohol. 1957;18:581–587.
87. Ravel J.M., Felsing B., Lansford E.M., et al. Reversal of alcohol toxicity by glutamine. J Biol Chem. 1955;214:497–501.
88. Rogers L.L., Pelton R.B., Williams R.J. Voluntary alcohol consumption by rats following administration of glutamine. J Biol Chem. 1955;214:503–506.
89. Falcao de Arruda I.S., de Aguilar-Nascimento J.E. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci (Lond). 2004;106:287–292.
90. Ward E., Picton S., Reid U., et al. Oral glutamine in pediatric oncology patients: a dose finding study. Eur J Clin Nutr. 2003;57:31–36.
91. Dharmananda S. Amino acid supplements I: glutamine with Reference to the Related Compound Glutamate. Director, Institute for Traditional Medicine, Portland, Oregon. http://www.itmonline.org/arts/glutamine.htm. Accessed 4/7/2009.
92. Rubio I.T., Cao Y., Hutchins L.F., et al. Effect of glutamine on methotrexate efficacy and toxicity. Ann Surg. 1998;227:772–778.