Chapter 168 Glaucoma
Acute (Angle Closure) and Chronic (Open Angle)
 General Considerations
General Considerations
In the United States, approximately 3 million people have glaucoma, which is undetected in 25% of them.1 The chronic open-angle type, for which there appears to be no consistent anatomic basis, accounts for 70% to 75% of these cases. Histologically, however, there is a strong correlation between the content and composition of collagen and the glaucomatous eye.2
Collagen is the most abundant protein in the body, including the eye. In the eye it provides tensile strength and integrity to the tissues (e.g., cornea, sclera, lamina cribrosa, trabecular meshwork, vitreous). Inborn errors of collagen metabolism (e.g., osteogenesis imperfecta, Ehlers-Danlos syndrome, Marfan syndrome) are often associated with ocular complications: glaucoma, myopia, retinal detachment, ectopia lentis, and blue sclera.3 Morphologic changes in the lamina cribrosa (the scleral area that is pierced by the optic nerve fibers and blood vessels), trabecular meshwork (the connective tissue network through which aqueous humor must pass to reach the canal of Schlemm), and papillary blood vessels in the eye have all been observed in glaucomatous eyes.2,4–6 These changes may result in elevated IOP readings or, perhaps more significantly, lead to the progression of peripheral visual loss. Changes in collagen structure would explain the following2,4–6:
• Similar peripheral vision loss in patients with normal and elevated IOP
 Diagnosis
Diagnosis
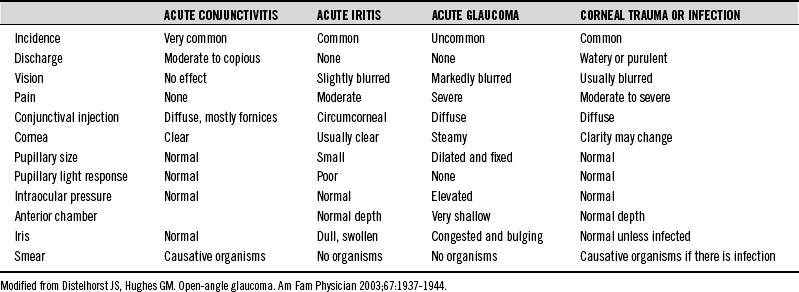
The primary challenge with acute glaucoma is early recognition, because delay in referral for surgical intervention increases the risk of blindness. Table 168-1 provides an overview of the differential diagnosis of the inflamed eye.
 Therapeutic Considerations
Therapeutic Considerations
Corticosteroids
The importance of collagen destruction in the etiology of glaucoma is apparent in corticosteroid-induced glaucoma.2 Corticosteroid use should be discouraged in the glaucoma patient, as it is known to inhibit the biosynthesis of collagen and glycosaminoglycans, thereby worsening the patient’s glaucoma.2
Supplements and Diet
Vitamin C
Of foremost importance in achieving collagen integrity are optimal tissue concentrations of ascorbic acid (AA). Furthermore, AA has been demonstrated to lower IOP levels in many clinical studies.7–11 A daily dose of 0.5 g/kg, whether in single or divided doses, reduces the IOP by an average of 16 mm Hg.11 Near normal tension levels were achieved in some patients unresponsive to acetazolamide (a carbonic anhydrase inhibitor) and 2% pilocarpine (a miotic agent).11
The hypotonic action of AA on the eye is long-lasting if supplementation is continued, and intravenous administration results in an even greater initial reduction in IOP.7,9–11 The patient must be monitored to determine the appropriate individual dose, because some patients respond to as little as 2 g/day, whereas others will respond only to extremely high doses (e.g., 35 g/day).7–11 Abdominal discomfort as a side effect of high doses is common but usually resolves after 3 to 4 days.11
The proposed mechanisms by which AA lowers IOP include the following:
Flavonoids
To further aid normal collagen metabolism, flavonoids should be supplemented, particularly the anthocyanosides (the blue-red pigments found in berries) and proanthocyanosides. These compounds elicit an AA-sparing effect, improve capillary integrity, and stabilize the collagen matrix by preventing free radical damage, inhibiting enzymatic cleavage of the collagen matrix, and cross-linking with collagen fibers directly to form a more stable collagen matrix.12–14 Vaccinium myrtillus (European bilberry) extract is particularly rich in these flavonoid and anthocyanidin compounds and has been used in Europe with good results in reducing myopia, improving nocturnal vision, and reversing diabetic retinopathy.15 Mirtogenol, a combination of bilberry anthocyanoside extract (160 mg Mirtoselect) and pine bark extract (80 mg Pycnogenol) was studied in 38 asymptomatic subjects with intraocular hypertension. Patients were given either Mirtogenol (20 subjects) or were not treated (18 subjects). After 2 months of supplementation with Mirtogenol, the mean IOP decreased from a baseline of 25.2 to 22.2 mm Hg. After 3 months of treatment with Mirtogenol, the IOP was significantly lowered compared with that of untreated controls (P <0.05) to 22 mm Hg. No further improvement was found after 6 months. Of the 20 patients taking Mirtogenol, 19 had a decreased IOP after 3 months. Only marginal effects on the IOP were found in the 18 control subjects. No side effects were observed. Ocular blood flow (central retinal, ophthalmic, and posterior ciliary arteries) improved both in the systolic and diastolic components as measured by ultrasound. After 3 months of treatment, the improvement in ocular blood flow was significant as compared with both baseline and the control group.16 Rutin has also been demonstrated to lower IOP when used as an adjunct in patients unresponsive to miotics alone.17
In patients with normotensive glaucoma (NTG), Ginkgo biloba extract (GBE) may be helpful, based on the results of two double blind studies. In the first study, which comprised healthy human volunteers, GBE (120 mg daily) significantly increased end-diastolic velocity in the ophthalmic artery (23% change), with no change in the placebo group. GBE did not alter arterial blood pressure, heart rate, or IOP.18 In the second study, patients with NTG received either 40 mg GBE or placebo three times daily for 4 weeks, followed by a washout period of 8 weeks, then 4 weeks of the opposite treatment. After GBE treatment, a significant improvement in visual field indices was recorded, showing that GBE improves preexisting visual field damage in some patients with NTG.19
Allergy
The successful treatment of chronic glaucoma by antiallergy measures has been reported in the literature.20 In one study, many of the 113 patients demonstrated an immediate rise in intraocular pressure of up to 20 mm Hg (in addition to other typical allergic symptoms) when challenged with the appropriate allergen, whether food borne or environmental. The author speculated that the known allergic responses of altered vascular permeability and vasospasm could result in the congestion and edema characteristic of glaucoma.
Magnesium
Because channel-blocking drugs benefit some glaucoma patients, a group of researchers at the University Eye Clinic in Basel, Switzerland, decided to evaluate the effect of supplemental magnesium, which has been referred to as “nature’s physiologic calcium-channel blocker.” The trial included 10 glaucoma patients (6 with primary open-angle glaucoma and 4 with normal-tension glaucoma). All patients had a digital cold-induced vasospasm. Magnesium was given at a dose of 121.5 mg twice daily for 1 month. After 4 weeks of treatment, the visual fields improved, as noted by a decrease in the mean defect and square root of loss variance scores. All three nail fold–capillaroscopic parameters improved, as well as digital temperature. These results demonstrate that magnesium supplementation improves the peripheral circulation and seems to have a beneficial effect on the visual field in patients with glaucoma.21
To evaluate the effect of oral magnesium therapy on ocular blood flow and visual field perimetry indices in patients with normotensive glaucoma (NTG), 15 patients with NTG (the study group) received 300 mg of oral magnesium (citrate) for 1 month, while 15 patients with NTG (the control group) received no treatment. In the study group, significant improvements were noted in visual field measurements (e.g., mean deviation improved from –3.7 at baseline to -2.5 and pattern standard deviation improved from 3.6 baseline to 2.8). There was no change in ocular blood flow, so the exact mechanism of magnesium’s effect is not known.22
Chromium
Ascorbic acid and chromium potentiate insulin receptors, which help to sustain strong focusing activity in the ciliary muscle. Deficiencies of either ascorbic acid or chromium were associated with elevated intraocular pressure, which tends to stretch the normal eye, thereby reducing focusing power.23
Fish Oil
In an interesting speculative study, rabbits were fed food soaked with cod liver oil, resulting in a drop in IOP from 25 to 11 mm Hg. Intramuscular injections of cod liver oil produced a dose-dependent reduction in IOP. When the animals were taken off cod liver oil, their IOP returned to baseline. Control animals given liquid lard or safflower oil experienced no change in IOP.24 Preliminary studies in humans with docosahexaenoic acid have had encouraging results.25
Caffeine
Many physicians instruct patients with glaucoma to avoid coffee, although clinical data supporting this practice are insufficient. To estimate the effect of coffee drinking on IOP, the results of the consumption of regular coffee (180 mg caffeine in 200 mL of coffee) and decaffeinated coffee (3.6 mg caffeine in 200 mL of coffee) were compared in patients with normotensive glaucoma or ocular hypertension in a double-blind crossover study. IOP was monitored in both groups at 30, 60, and 90 minutes after coffee ingestion. In patients with normotensive glaucoma who drank regular coffee, the mean changes in IOP at 30, 60, and 90 minutes were 0.9, 3.6, and 2.3 mm Hg, respectively; in those who drank decaffeinated coffee, they were 0.75, 0.70, and 0.4 mm Hg, respectively. The corresponding values in patients with ocular hypertension were as follows: after regular coffee, 1.1, 3.4, and 3 mm Hg; and after decaffeinated coffee, 0.6, 0.9, and 0.5 mm Hg, respectively. This study showed clearly that subjects who drank regular coffee demonstrated a greater elevation in IOP, which may be clinically significant.26
Other Recommendations
Exercise
Exercise can lead to an immediate and prolonged reduction in IOP. Within 5 minutes of starting exercise, IOP initially increases and then gradually decreases to its lowest level 60 minutes following exercise. The drop in IOP is approximately 23% in normal individuals, while people with glaucoma usually experience a greater drop and longer duration of postexercise recovery than do those with normal eyes.27 Specifically, the drop in IOP for glaucoma patients following walking/jogging and running was 7.2% and 12.7%, respectively, more than the decrease in IOP experienced by persons normal eyes. Similarly, the mean duration of the IOP-lowering effect following running was approximately 84 minutes in glaucomatous eyes and 63 minutes in normal eyes. The mechanism of acute lowering of IOP is independent of systemic blood pressure and level of sympathetic stimulation but may be partly due to increased serum osmolarity.
Exercise appears to be effective in lowering IOP in sedentary subjects engaging in moderate to heavy exercise but is somewhat less effective in physically fit subjects.28 However, individuals who are more physically fit tend to have lower IOP. If one stops exercising, the effect wears off in 3 weeks. Although exercise may not be effective in lowering IOP in everyone, it can lead to significant improvements in many. One study found that IOP was lowered by at least 2 mm Hg by exercise in 34% of subjects; however, 57% had no change in IOP, while 9% had an IOP elevation.29
Helicobacter pylori
Several studies have found an association between Helicobacter pylori infection and open-angle glaucoma.30 A particularly interesting 2-year study found that successful eradication of H. pylori resulted in decreased IOP.31 See Chapter 198 for a discussion of the treatment of H. pylori.
 Therapeutic Approach
Therapeutic Approach
Acute closed-angle glaucoma is an ocular emergency; such patients should be referred immediately to an ophthalmologist. Unless adequately treated within 12 to 48 hours, the patient will become permanently blind within 2 to 5 days. An asymptomatic eye with a narrow anterior chamber angle may convert spontaneously to angle-closure glaucoma. The process can be precipitated by anything that dilates the pupil, such as atropine and epinephrine-like drugs. Typical signs and symptoms include extreme pain, blurring of vision, conjunctivitis, and a fixed, dilated pupil.1
Agents that dilate the pupils must be strictly avoided in any patient suspected of having glaucoma.
1. Distelhorst J.S., Hughes G.M. Open-angle glaucoma. Am Fam Physician. 2003;67:1937–1944.
2. Tengroth B., Ammitzboll T. Changes in the content and composition of collagen in the glaucomatous eye: basis for a new hypothesis for the genesis of chronic open-angle glaucoma. Acta Ophthalmol (Copenh). 1984;62:999–1008.
3. Weiss J., Jayson M. Collagen in health and disease. Edinburgh; New York: Churchill Livingstone; 1982. 388-403
4. Quigley H., Addicks E. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99:137–143.
5. Krakau T., Bengtsson B., Holmin C. The glaucoma theory updated. Acta Ophthalmol (Copenh). 1983;61:737–741.
6. Rohen J.W. Why is intraocular pressure elevated in chronic simple glaucoma? Anatomical considerations. Ophthalmology. 1983;90:758–765.
7. Bietti G. Further contributions on the value of osmotic substances as means to reduce intra-ocular pressure. Trans Ophthalmol Soc Aust. 1967;26:61–71.
8. Fishbein S., Goodstein S. The pressure lowering effect of ascorbic acid. Ann Ophthalmol. 1972;4:487–491.
9. Linner E. The pressure lowering effect of ascorbic acid in ocular hypertension. Acta Ophthalmol (Copenh). 1969;47:685–689.
10. Shen T.M., Yu M.C. Clinical evaluation of glycerin-sodium ascorbate solution in lowering intraocular pressure. Chinese Med J (Engl). 1975;1:64–68.
11. Virno M., Bucci M., Pecori-Giraldi J., et al. Oral treatment of glaucoma with vitamin C. Eye Ear Nose Throat Monthly. 1967;46:1502–1508.
12. Gabor M. Pharmacologic effects of flavonoids on blood vessels. Angiologica. 1972;9:355–374.
13. Monboisse J., Braquet P., Borel J. Oxygen-free radicals as mediators of collagen breakage. Agents Actions. 1984;15:49–50.
14. Hagerman A., Butler L. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256:4494–4497.
15. Bever B., Zahnd G. Plants with oral hypoglycemic action. Q J Crude Drug Res. 1979;17:139–196.
16. Steigerwalt R.D., Gianni B., Paolo M., et al. Effects of Mirtogenol on ocular blood flow and intraocular hypertension in asymptomatic subjects. Mol Vis. 2008 Jul 10;14:1288–1292.
17. Stocker F. New ways of influencing the intraocular pressure. N Y St J Med. 1949;49:58–63.
18. Chung H.S., Harris A., Kristinsson J.K., et al. Ginkgo biloba extract increases ocular blood flow velocity. J Ocul Pharmacol Ther. 1999 Jun;15(3):233–240.
19. Quaranta L., Bettelli S., Uva M.G., et al. Effect of Ginkgo biloba extract on preexisting visual field damage in normal tension glaucoma. Ophthalmology. 2003 Feb;110(2):359–362.
20. Raymond L.F. Allergy and chronic simple glaucoma. Ann Allergy. 1964;22:146–150.
21. Gaspar A.Z., Gasser P., Flammer J. The influence of magnesium on visual field and peripheral vasospasm in glaucoma. Ophthalmologica. 1995;209:11–13.
22. Aydin B., Onol M., Hondur A., et al. The effect of oral magnesium therapy on visual field and ocular blood flow in normotensive glaucoma. Eur J Ophthalmol. 2010 Jan-Feb;20(1):131–135.
23. Lane B.C. Diet and glaucomas. J Am Coll Nutr. 1991;10:536.
24. McGuire R. Fish oil cuts lower ocular pressure. Med Tribune. 1991;19:25.
25. Cellini M., Caramazza N., Mangiafico P., et al. Fatty acid use in glaucomatous optic neuropathy treatment. Acta Ophthalmol Scand Suppl. 1998;227:41–42.
26. Avisar R., Avisar E., Weinberger D. Effect of coffee consumption on intraocular pressure. Ann Pharmacother. 2002;36:992–995.
27. Qureshi I.A. The effects of mild, moderate, and severe exercise on intraocular pressure in glaucoma patients. Jpn J Physiol. 1995;45:561–569.
28. Qureshi I.A. Effects of exercise on intraocular pressure in physically fit subjects. Clin Exp Pharmacol Physiol. 1996;23:648–652.
29. Era P., Parssinen O., Kallinen M., et al. Effect of bicycle ergometer test on intraocular pressure in elderly athletes and controls. Acta Ophthalmol (Copenh). 1993;71:301–307.
30. Kountouras J., Zavos C., Chatzopoulos D. Induction of apoptosis as a proposed pathophysiological link between glaucoma and Helicobacter pylori infection. Med Hypotheses. 2004;62:378–381.
31. Kountouras J., Mylopoulos N., Chatzopoulos D., et al. Eradication of Helicobacter pylori may be beneficial in the management of chronic open-angle glaucoma. Arch Intern Med. 2002;162:1237–1244.