Chapter 9 Glaucoma
Introduction
The diagnosis of open-angle glaucoma is most commonly made with a combination of intraocular pressure (IOP) measurement, central corneal thickness (CCT) measurement by pachymetry, automated visual field testing (Humphrey Visual Field Analyzer), and optic disc evaluation. Slit lamp examination, while usually normal in primary open-angle glaucoma and normal tension glaucoma, can provide evidence of other open-angle glaucoma mechanisms including pigmentary dispersion, pseudoexfoliation, uveitis, and remote ocular trauma. While slit lamp examination of the anterior segment may provide clues to angle closure,1 gonioscopy is essential for the proper diagnosis and management of the angle-closure glaucoma mechanisms.2 Indentation gonioscopy can help distinguish between appositional angle closure and permanent synechial angle closure.3
A-scan ultrasonography in the glaucoma patient may provide axial length measurements that are longer than normal, indicating myopia. Myopia is more commonly seen in patients with primary open-angle glaucoma and pigmentary glaucoma. Hyperopic patients, with shorter than average axial length, are more likely to have narrow anatomical angles and frank acute, sub-acute or chronic angle-closure glaucoma.4
Preoperative A-scan ultrasonography is essential for intraocular lens implant determination for cataract surgery (Chapter 7). In the presurgical glaucoma patient, A-scan ultrasonography can alert the clinician to longer ocular axial length, a risk factor for inadvertent ocular penetration during retrobulbar anesthetic administration (Chapter 18). Nanophthalmos, typically diagnosed in patients with an ocular axial length less than 21.0 mm, is a risk factor for uveal effusion after routine cataract surgery and for flat anterior chamber and aqueous misdirection after glaucoma filtering surgery.
Anterior chamber angle evaluation
Ultrasound biomicroscopy (UBM) provides excellent, high-resolution images of the ocular anterior segment.5 It can assess conjunctival lesions, corneal disorders and measure the narrowness of the angle and assess the ciliary body. Its high frequency makes it excellent for anterior segment evaluation, but not for posterior segment evaluation because of poor posterior penetration of the high-frequency ultrasound (Chapter 4). Lower frequency B-scan ultrasonography is preferred for posterior segment evaluation in glaucoma patients. UBM requires a highly skilled technician, a supine patient and a water-bath coupling probe on the patient’s eye.
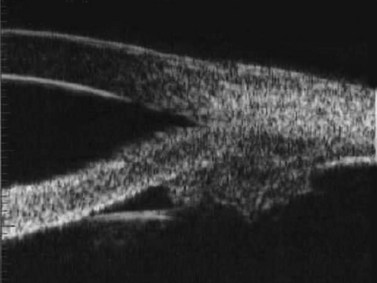
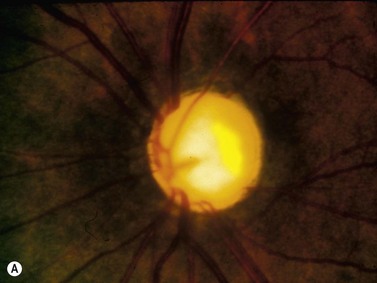
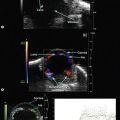
In the normal eye, the UBM will show the cornea and a clear, deep anterior chamber. Just behind that is the flat, undilated iris. Details of the iris stroma and the posterior iris pigment layer are discernable. The central anterior lens capsule surface can be seen just behind the pupil and central iris. More peripherally behind the iris, the ciliary processes, and sometimes zonules, can be seen (Figure 9.1).
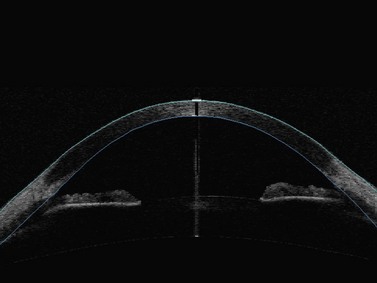
Anterior segment optical coherence tomography (OCT) provides images of the angle structures similar to anterior segment UBM (Figure 9.2).6 It has the advantage of being a non-contact procedure and requires less technical skill than UBM.7,8 Very high frequency digital UBM offers some of the advantages of the anterior segment OCT (Chapter 6).
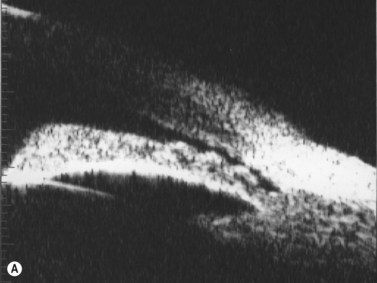
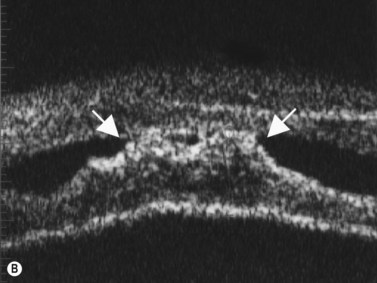
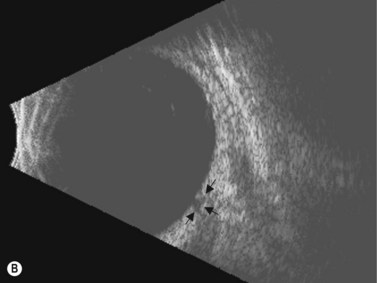
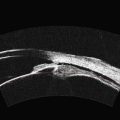
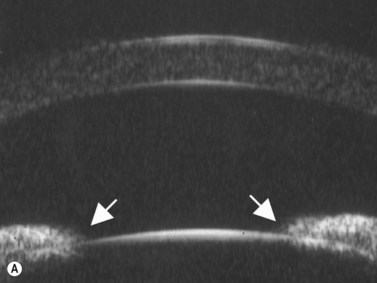
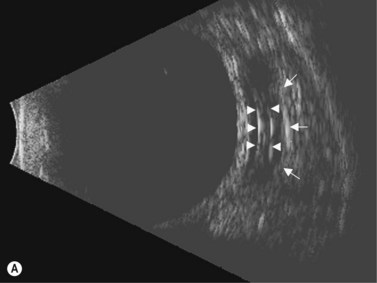
In eyes with a normal and open angle, the iris has a flat approach from the pupil peripherally to the scleral spur. In eyes with a narrow angle, the iris has an anterior convexity and the UBM will show both if the angle is narrowed and by how much (Figure 9.3). In eyes with greater angle narrowing, the UBM will show the peripheral iris touching the scleral spur and the trabecular meshwork region of the angle. This is a dynamic process with the angle opening tending to increase in bright illumination and decrease with angle narrowing in dim illumination.
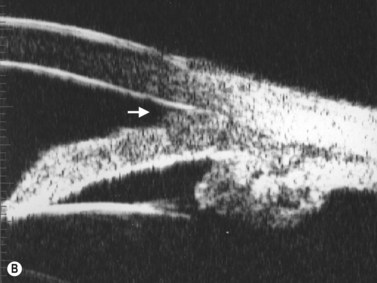
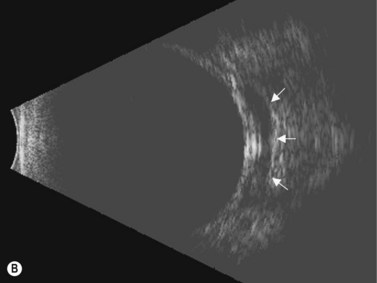
Primary angle-closure glaucoma may present as an acute attack, as intermittent episodes of subacute angle closure, or most commonly, as chronic progressive angle closure, which early on may be asymptomatic. The first two present often with dramatic symptoms of blurred vision, haloes, ocular pain, headache and nausea and vomiting. Shallow anterior chamber, corneal edema, ocular injection, glaucomflecken, and iris atrophy may be seen on slit lamp examination. While glaucoma medical management can reduce intraocular pressure and reduce pain, early laser peripheral iridotomy (LPI) is the treatment of choice for the primary and many secondary angle-closure glaucoma mechanisms. After a successful laser PI, the peripheral iris moves more posteriorly and the anterior chamber deepens. A post-procedural UBM should show a more open angle with less anterior iris convexity. However, peripheral anterior synechiae, adhesions of the iris to the trabecular meshwork and cornea, will persist. The UBM may also show the patency of the LPI (Figure 9.4).
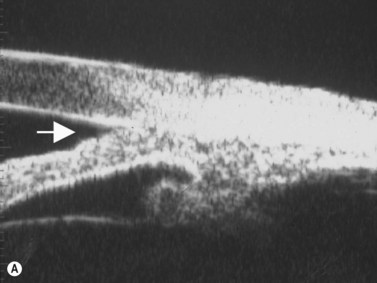
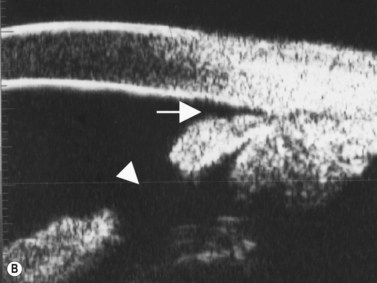
Reproduced with permission from: Rockwood EJ, Sharma S, Hayden BC, Singh AD. Glaucoma. Ultrasound Clin 2008; 3(2):207–215.4
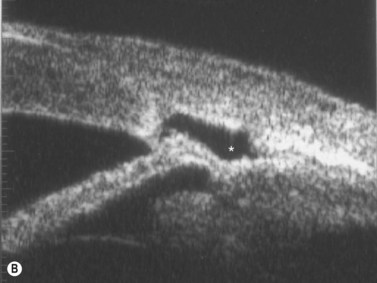
Plateau iris configuration may be seen during gonioscopy. The approach of the iris from the peripupillary region to the peripheral iris may appear relatively flat. However, as the iris approaches the scleral spur, it drops more posteriorly, creating the iris plateau configuration. Indentation gonioscopy will push the mid peripheral iris more posteriorly; however the ciliary body in these eyes is more anteriorly placed and resists posterior movement of the peripheral iris during indentation gonioscopy. In some cases, pharmacologic dilation of eyes with plateau iris may precipitate angle closure despite the presence of a patent laser PI. An additional laser procedure, a laser peripheral iridoplasty, may be corrective. UBM confirms the relatively flat iris approach toward the angle with a steeper peripheral descent of the iris toward the scleral spur (Figure 9.5).9 Iridociliary body cysts may cause a plateau iris-like configuration (Figure 9.6).10
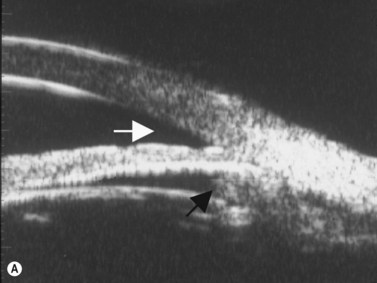
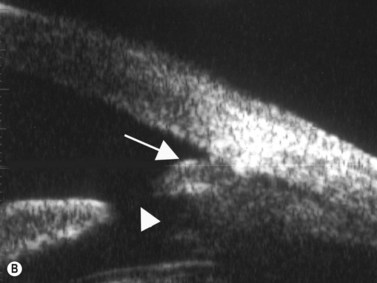
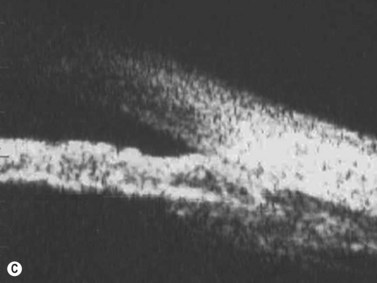
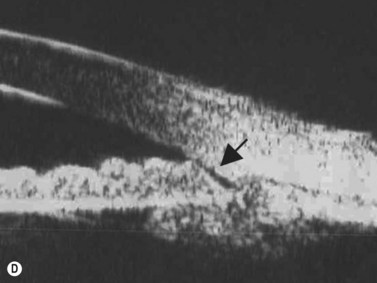
Reproduced with permission from: Rockwood EJ, Sharma S, Hayden BC, Singh AD. Glaucoma. Ultrasound Clin 2008; 3(2):207–215.4 Angle appearance before (C) and after (D) laser iridoplasty. Note separation of peripheral iris from the trabecular meshwork (D, arrow). C and D courtesy of M. Willet, MD and J Eisengart, MD, Cleveland, Ohio.
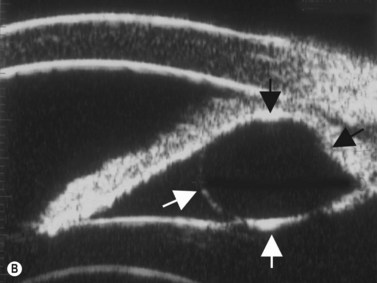
Reproduced with permission from: Rockwood EJ, Sharma S, Hayden BC, Singh AD. Glaucoma. Ultrasound Clin 2008; 3(2):207–215.4
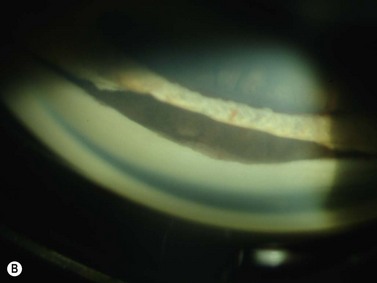
In pigment dispersion syndrome, typically seen in young myopes and in males more often than females, the posterior peripheral and mid-peripheral iris rubs against the anterior lens zonules and releases pigment granules into the anterior segment with resulting deposition on the corneal endothelium (Kruckenberg spindle), iris and in the trabecular meshwork. Anterior segment OCT and UBM often show a posterior bowing of the mid-peripheral iris in pigment dispersion syndrome and pigmentary glaucoma (Figure 9.7). Laser PI sometimes reduces this “reverse” pupillary block, resulting in a flatter iris configuration and sometimes a reduction of IOP.
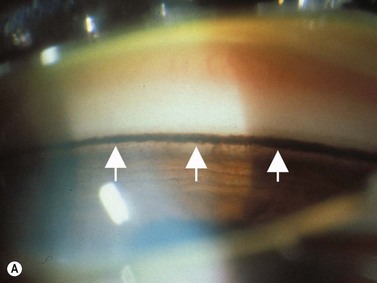
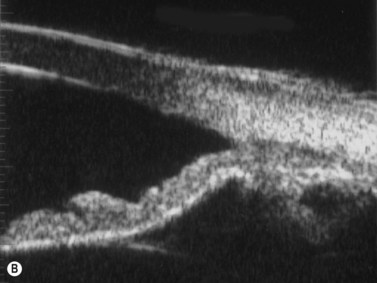
Reproduced with permission from: Rockwood EJ, Sharma S, Hayden BC, Singh AD. Glaucoma. Ultrasound Clin 2008; 3(2):207–215.4
Peripheral anterior synechiae may be clearly shown on UBM (Figure 9.8). These may occur in both the primary and the secondary angle-closure glaucomas. After laser PI, different areas of the angle may appear open and other areas closed with peripheral anterior synechiae after laser PI.
Secondary glaucoma
Intraocular inflammatory debris can directly cause open-angle glaucoma and the topical corticosteroids used to treat the uveitis may cause a corticosteroid-induced IOP elevation. Intraocular inflammation from any cause, uveitis, trauma, or post-surgery, can cause the formation of posterior and/or peripheral anterior synechiae. Extensive posterior synechiae may lead to pupillary block (iris bombé) and angle closure (Figure 9.9). A laser PI is necessary. Progressive formation of peripheral anterior synechiae from recurrent episodes of uveitis will cause angle-closure glaucoma, which is not responsive to laser PI.
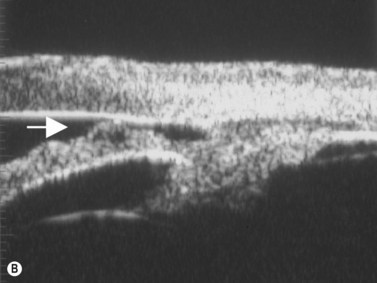
Reproduced with permission from: Rockwood EJ, Sharma S, Hayden BC, Singh AD. Glaucoma. Ultrasound Clin 2008; 3(2):207–215.4
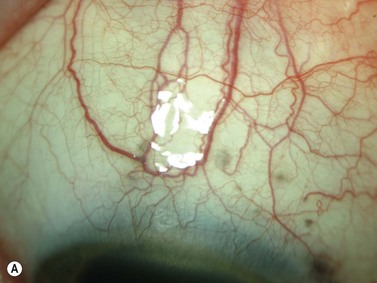
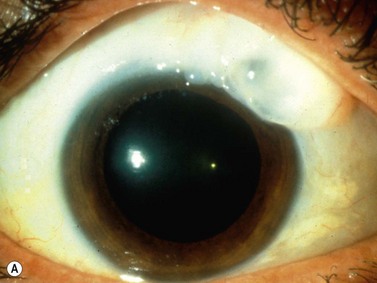
A secondary pigmentary glaucoma may develop after ciliary sulcus placement of an intraocular lens (IOL) during cataract surgery. Plate-haptic IOLs in the sulcus are more likely to cause pigment dispersion. Instead of the typical radial iris transillumination defects seen in primary pigmentary glaucoma, large blotchy iris transillumination defects are seen over the IOL haptic. Occasionally erosion of the plate into the iris can also cause bleeding and chronic inflammation, also known as the uveitis, glaucoma, hyphema or UGH syndrome. UBM can identify the location of the offending haptic(s) (Chapter 8).
The group of anterior segment entities collectively referred to as the iridocorneal endothelial syndromes (Chandlers’ syndrome, Cogan–Reese syndrome and essential iris atrophy) often result in the production of high peripheral anterior synechiae (Figure 9.10). Progressive synechiae and corneal endothelial disease lead both to corneal decompensation and severe secondary angle-closure glaucoma, neither requiring nor responsive to laser PI.
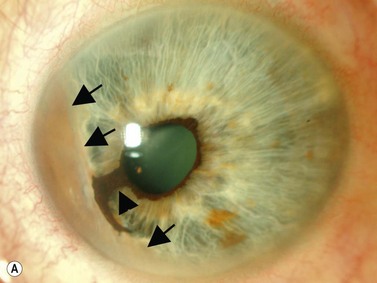
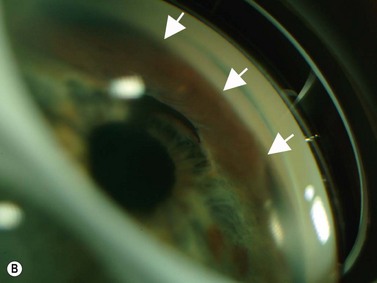
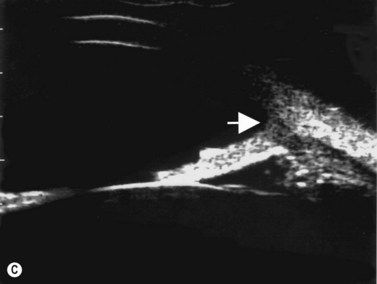
Reproduced with permission from: Rockwood EJ, Sharma S, Hayden BC, Singh AD. Glaucoma. Ultrasound Clin 2008; 3(2):207–215.4
Uveal effusion can push the lens forward, narrowing or closing the anterior chamber angle.11 Uveitis, hypotony and nanophthalmos are a few of the potential causes of uveal effusion. UBM and anterior segment OCT can be used to evaluate for angle closure and B-scan ultrasonography can be used to assess and monitor the uveal effusion (Chapter 12). Angles may also narrow or close in posterior scleritis or after a heavy panretinal laser photocoagulation. A laser PI is usually not indicated in these situations (except nanophthalmos with primary angle closure). For treatment, cycloplegia with atropine, homatropine, or scopolamine and topical corticosteroids may be sufficient. A laser peripheral iridoplasty is sometimes beneficial. Post laser, the peripheral angle will be more open on UBM or anterior segment OCT scan.
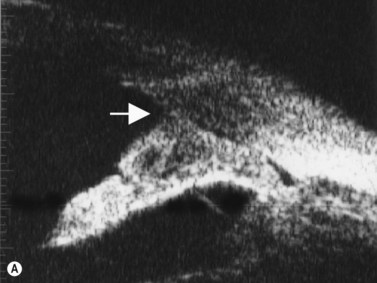
Intraocular tumors are an uncommon cause of glaucoma. Anterior melanomas of the iris or ciliary body can release tumor cells and pigment, which may cause reduction of trabecular meshwork outflow and increased IOP. Direct invasion of the anterior chamber angle is a second mechanism for glaucoma. Larger posterior segment melanomas can push the iris and lens forward and narrow the anterior chamber angle (Figure 9.11). Melanomas, retinoblastoma and other intraocular malignant tumors may cause a neovascular glaucoma (Chapter 11). UBM and anterior segment OCT can be used to measure the extent of angle involvement of the tumor.
Congenital glaucoma
For the child presenting with new glaucoma and unilateral or bilateral corneal haziness from edema and if the retina is not visible, it is important to determine if there is other intraocular pathology associated with glaucoma. B-scan ultrasonography can be used to evaluate for intraocular tumor, retinal detachment, or persistent hyperplastic primary vitreous (Chapters 10, 11, 15).
Evaluation after glaucoma laser and surgery
UBM performed after laser PI typically shows some residual shallowing of the central anterior chamber due to the presence of the enlarged lens in the hyperopic eye. The iris may appear “draped” on the anterior lens surface. The mid-peripheral iris moves more posteriorly after laser PI. Because of the thin profile of most intraocular lens implants, a UBM scan performed after cataract surgery in the eye with primary angle-closure glaucoma will show deepening of both the central and peripheral anterior chamber and a flattening of the entire iris (Figure 9.4).
Different trabeculectomy postoperative glaucoma filtering blebs may have greatly varying IOP control and varying thickness and morphology, depending on whether the conjunctival filtering bleb was constructed intraoperatively as a limbus or fornix-based conjunctival flap and whether a non-US FDA approved anti-fibrosing intraoperative or postoperative agent such as 5-fluorouracil or mitomycin C was used. Fornix-based conjunctival trabeculectomy flaps tend to be lower and more diffuse and limbus-based conjunctival trabeculectomy flaps tend to be higher, especially just posterior to the limbus. Some patients, more commonly males, develop thicker, encapsulated glaucoma filtering blebs, often giving higher postoperative intraocular pressures. Both 5-fluorouracil and mitomycin C tend to produce higher and thinner glaucoma filtering blebs and increase both the amount of reduction of postoperative IOP and an increased risk of thinner glaucoma filtering blebs. Both the UBM and anterior segment OCT have been used to map out and categorize the superficial and internal bleb morphology (Figure 9.12).
Reproduced with permission from: Rockwood EJ, Sharma S, Hayden BC, Singh AD. Glaucoma. Ultrasound Clin 2008; 3(2):207–215.4
Glaucoma implants (tubes, tube shunts, valves) tend to produce thicker, more encapsulated filtering blebs over the glaucoma implant plate whether a valved or non-valved implant is used. The quality of trabeculectomy blebs can usually be readily evaluated at the slit lamp. The bleb formed after glaucoma implants, especially if the plate is sutured into place at or posterior to the ocular equator, may be difficult to evaluate at the slit lamp. Ultrasonography may be used to identify the presence or absence of a glaucoma filtering bleb over the glaucoma implant plate especially if the IOP is not controlled after a previous glaucoma implant surgery (Figure 9.13). If a bleb is present, but IOP control is inadequate, some clinicians have advocated a glaucoma implant bleb revision and others, the placement of a second glaucoma implant in another ocular quadrant for improved IOP control. If the tube is blocked, it can usually be recognized at the slit lamp. Iris or loose vitreous are the most likely ocular tissues to cause glaucoma implant tube obstruction. Either of these can be corrected with laser or surgical clearing of the tube blockage.
Reproduced with permission from: Rockwood EJ, Sharma S, Hayden BC, Singh AD. Glaucoma. Ultrasound Clin 2008; 3(2):207–215.4
Aqueous misdirection, formerly known as malignant glaucoma, can present after glaucoma filtering surgery with shallow anterior chamber and elevated IOP despite a patent laser or surgical iridectomy. Choroidal hemorrhage can present similarly; however moderate to large choroidal elevation is typically seen (Chapter 10). If the posterior ocular segment cannot be adequately visualized, B-scan ultrasonography can be used to help differentiate between aqueous misdirection and choroidal hemorrhage. If the anterior chamber is shallow and the IOP is low after glaucoma filtering surgery, a wound leak or over filtration should be suspected. Non-hemorrhagic choroidal effusions may be visible or detected with B-scan ultrasonography (Chapter 10). UBM scanning performed on eyes with either choroidal effusion or choroidal hemorrhage will show a shallowing of both the central and peripheral anterior chamber. The natural lens or intraocular lens implant will be pushed forward and may be close to the corneal endothelial surface.
Optic disc evaluation
Ultrasonography typically does not play a role in the diagnosis and management of most open-angle glaucoma, ocular hypertensive, and glaucoma suspect patients. However, the patient with glaucoma but an inadequate view of the posterior segment, may benefit because of the added information gained for the planning of glaucoma medical, laser and surgical management. For the patient presenting with very high IOP, iris neovascularization, and corneal edema, the two most likely diagnoses are proliferative diabetic retinopathy and central retinal venous occlusive disease. B-scan ultrasonography can be performed to evaluate for vitreous hemorrhage, macular edema and tractional or rhegmatogenous retinal detachment (Chapter 10). After initiation of appropriate medical therapy, plans can be made for the administration of intraocular anti-vascular endothelial growth factor injection.
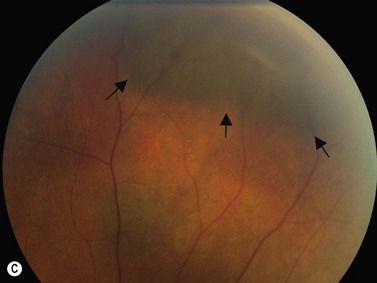
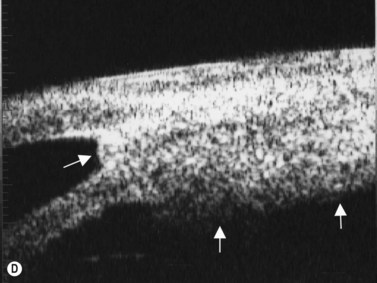
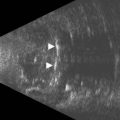
If vitreoretinal surgery is indicated, information obtained from the B-scan ultrasonography can assist the retinal surgeon in preoperative and intraoperative surgical management. Involvement of the glaucoma surgeon for intraoperative surgical management (e.g., glaucoma implant) of the IOP may be helpful. A crude assessment of the amount of preoperative optic disc cupping can be determined with B-scan ultrasonography preoperatively in these patients with no view of the optic disc (Figure 9.14). This can add to the clinicians’ armamentarium in preoperative discussions with the patient and family members.
1 Friedman DS, He M. Anterior chamber angle assessment techniques. Surv Ophthalmol. 2008;53(3):250-273.
2 Fisch B. Gonioscopy and the glaucomas. Boston: Butterworth-Heinemann; 1993.
3 Forbes M. Gonioscopy with corneal indentation. A method for distinguishing between appositional closure and synechial closure. Arch Ophthalmol. 1966;76(4):488-492.
4 Rockwood EJ, Sharma S, Hayden BC, et al. Glaucoma. Ultrasound Clin. 2008;3(2):207-215.
5 Pavlin CJ, Harasiewicz K, Sherar MD, et al. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991;98(3):287-295.
6 Nolan WP, See JL, Chew PT, et al. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology. 2007;114(1):33-39.
7 Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123(8):1053-1059.
8 Radhakrishnan S, See J, Smith SD, et al. Reproducibility of anterior chamber angle measurements obtained with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48(8):3683-3688.
9 Pavlin CJ, Ritch R, Foster FS. Ultrasound biomicroscopy in plateau iris syndrome. Am J Ophthalmol. 1992;113(4):390-395.
10 Azuara-Blanco A, Spaeth GL, Araujo SV, et al. Plateau iris syndrome associated with multiple ciliary body cysts. Report of three cases. Arch Ophthalmol. 1996;114(6):666-668.
11 Quigley HA. What’s the choroid got to do with angle closure? Arch Ophthalmol. 2009;127(5):693-694.
12 Bollinger K, Singh A, Singh AD. Glaucoma and intraocular tumors. In: Shaarawy TM, Sherwod MB, Hitchings R, et al, editors. Glaucoma: Medical Diagnosis and Therapy, Vol 1. Philadelphia: Saunders-Elsevier; 2009.