12 Glabella
Summary and Key Features
• Botulinum toxin type A for treatment of glabellar rhytides was FDA approved for cosmetic use in 2002, but has been used for this purpose for over 20 years
• Injection of botulinum toxin into the glabellar complex (corrugator supercilii and procerus muscles) and the lateral eyebrow may achieve reduction of glabellar rhytides and lower forehead rhytides, and brow lift
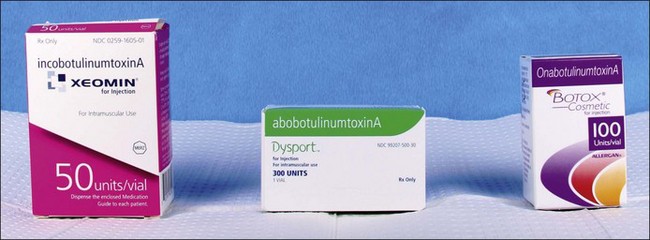
• There are currently three types of botulinum type A toxin injectables specifically FDA approved for the treatment of glabellar furrows, including Botox®, Dysport®, and Xeomin®, at doses of 20 units (U), 50 U, and 20 U respectively
• Dose variation may be required to obtain optimal correction and higher doses may be required in men
Introduction
The use of botulinum toxin type A over the last two decades for treatment of glabellar rhytides has revolutionized the field of cosmetic dermatology and plastic surgery. Several multicenter, double-blind, randomized, placebo-controlled studies have proven its efficacy (e.g. that by Carruthers et al in 2003). It is the first quick, less invasive and effective, non-surgical technique for both brow lift and rhytid treatment. The same group demonstrated in 2010 that botulinum toxin type A injection to the glabella has been shown to improve both dynamic glabellar rhytides and those in repose. Subsequent uses of botulinum toxin type A for cosmetic improvement of forehead, perioral area, chin, crow’s feet, and other rhytides have been successful, but its use in the glabella is the first and still the most widely used.
Anatomy
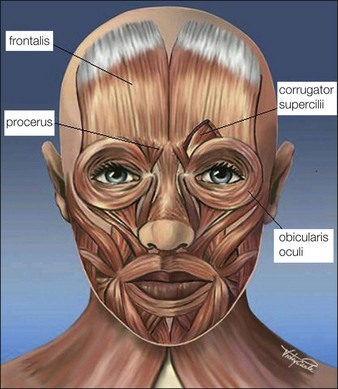
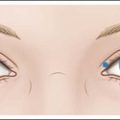
The glabellar complex consists of the two corrugator supercilii muscles and the procerus muscle that collectively serve upon contraction to pull the brow medially and downward (Fig. 12.1). The corrugator supercilii are two sets of horizontally oriented muscle fibers that lie beneath the medial eyebrow to about the mid-pupillary line. In some patients, the corrugators extend beyond the mid-pupillary line (these muscles can be visualized at maximum contraction when ‘frowning’). The procerus is a vertically oriented muscle that lies in between the eyebrows. The frontalis muscle of the forehead is vertically oriented and the medial belly interpolates with the glabellar complex, and its lateral portion interpolates with the lateral orbicularis oculi. Its main function is to elevate the brow. The orbicularis oculi is a thin circular muscle around the eyes that lies on top of the lateral portion of the corrugator supercilli. The lateral portion of the orbicularis oculi under the tail of the brow is a powerful brow depressor upon contraction. The levator palpebrae muscle lies beneath the orbicularis oculi, underneath the bony orbital rim, and its function is eyelid opening. Rhytides are typically perpendicular to the orientation of muscle fibers, thus contraction of the glabellar muscles typically produces vertically oriented lines between the brows.
 Injection technique (see Video ‘Botulinum Toxin Glabella’)
Injection technique (see Video ‘Botulinum Toxin Glabella’)
The proper preparation, storage, and handling of botulinum toxin is discussed in Chapters 9 and 11. Insulin syringes or 1 mL syringes with 30–32 gauge needles are typically used for injection.
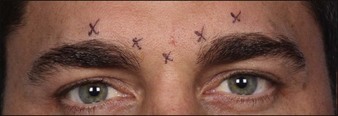
In the glabella, there are typically five injection sites: one at each medial corrugator, one at each lateral corrugator (1 cm above orbital rim at the mid-pupillary line), and a single injection into the procerus (Fig. 12.2). For some patients with particularly long or stronger corrugator muscles, an additional injection site may be given midway between the medial and lateral corrugator injection sites (a total of seven injections).
Dosing
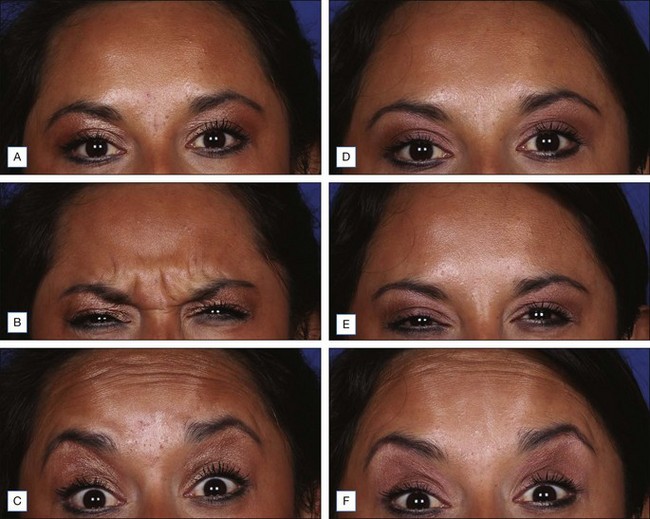
Botox® (onabotulinumtoxinA) (Fig. 12.3)
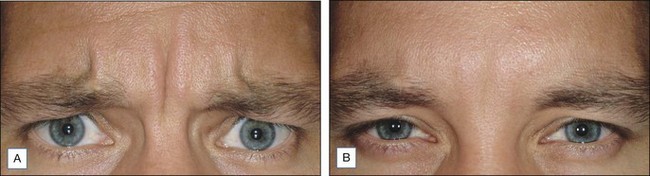
Onset of response is typically 1–14 days and results last for 3–4 months. In a 2011 study by Beer et al of 45 patients who received a 20 U injection into the glabella, nearly half of patients may experience onset of response by day 1, with 100% by day 14. A meta-analysis by Kane and colleagues of the duration of efficacy pooled results from four global Phase III pivotal trials of onabotulinumtoxinA treatment of glabellar lines and demonstrated that, in 523 subjects of diverse ethnic backgrounds, treatment with a 20 U dose resulted in more than half of the responders sustaining clinical benefit for 4 months. In fact, Dailey and co-workers found that injection of 20 U of onabotulinumtoxinA to the glabellar complex every 4 months for 20 months significantly reduces or progressively eliminates glabellar rhytides for up to 6 months after the last treatment.
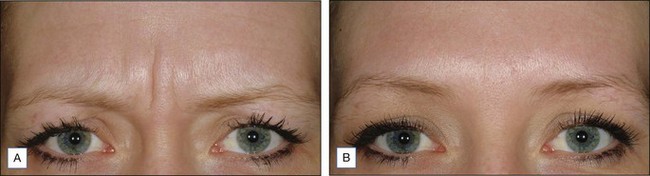
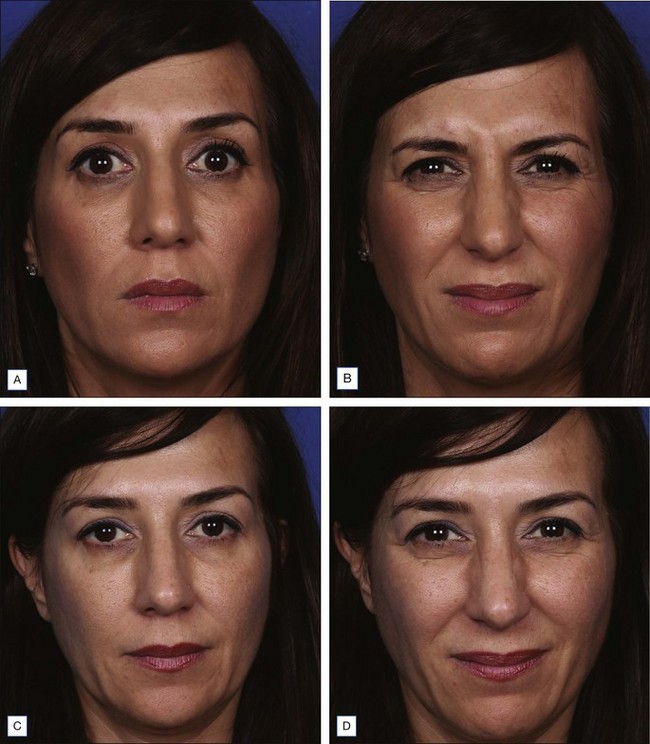
Dysport® (abobotulinumtoxinA) (Figs 12.4 and 12.5)
Xeomin® (incobotulinumtoxinA) (Fig. 12.6)
Myobloc® (rimabotulinumtoxinB)
Unlike Botox®, Dysport®, and Xeomin®, which are derived from type A strains of botulinum toxin, Myobloc® (Soltice Neurosciences, South San Francisco, CA) is derived from botulinum toxin type B. This type cleaves synaptobrevin (or VAMP, vesicle-associated membrane complex) rather than SNAP-25 of the SNARE complex to prevent acetylcholine release and thus muscle contraction. Myobloc® at a dose of 2500 U was shown by Alster & Lupton to be effective in the treatment of glabellar rhytides, particularly in those who showed decreased or negligible clinical effect to botulinum toxin type A. However, the duration of effect was shorter (2–3 months). Myobloc® is currently FDA approved only for cervical dystonia. (Table 12.1 and Fig. 12.7)
Table 12.1 Optimal dosing for glabellar injection of botulinum toxin in women, by brand, in the United States
| Type | Units* |
|---|---|
| Botox® (Allergan)† | 20 |
| Dysport (Ipsen Biopharm)† | 50 |
| Xeomin® (Merz Pharmaceuticals)† | 20 |
| Myobloc® (Soltice Neurosciences) | 2500 |
* Doses are based on published studies with standard dilution of the product recommended by the manufacturer.
† Botox®, Dysport®, and Xeomin® are the only forms of botulinum toxin that are currently FDA-approved for glabellar injection (see Fig. 13.6).
Special considerations
Men
A 2005 study by Carruthers & Carruthers concluded that men often require a higher dose of botulinum toxin in the glabellar complex. Men often prefer to have a straighter brow appearance as opposed to an arched eyebrow. Brow-lift technique is useful in men with mild eyelid ptosis at baseline; however, in the younger man, brow lift can result in a more feminized brow appearance. In men who do not require a brow lift, in order to retain a straighter and less arched eyebrow appearance, we often inject 3 U at the junction of each temporalis, frontalis, and orbicularis oculi muscles, approximately 1–1.5 cm above the lateral brow.
Potential adverse events
In the first multicenter, double-blind, randomized, placebo-controlled trials of the safety of botulinum toxin type A in the treatment of glabellar lines, performed by Carruthers and colleagues in 2002, out of the 264 patients studied (BoNT-A 203, placebo 61), 5.4% (11/203) had mild blepharoptosis in the BoNT-A group, which had resolved by day 120. The group’s second multicenter randomized trial for safety and efficacy, published 1 year later, showed that in 273 patients (BoNT-A 202, placebo 71) the most common adverse event was headache (BoNT-A 11%, placebo 20%). In this study the incidence of blepharoptosis was 1% (2/202) for the botulinum toxin group. The incidence of blepharoptosis decreased with subsequent treatments in a 1-year follow-up study of the two trials where botulinum toxin was administered to glabellar rhytides at day one and then at two subsequent treatments 4 months apart.
Alster TS, Lupton JR. Botulinum toxin type B for dynamic glabellar rhytides refractory to botulinum toxin type A. Dermatologic Surgery. 2003;29(5):516–518.
Beer KR, Boyd C, Patel RK, et al. Rapid onset of response and patient-reported outcomes after onabotulinumtoxina treatment of moderate-to-severe glabellar lines. Journal of Drugs in Dermatology. 2011;10(1):39–44.
Borodic GE. Botulinum A toxin for (expressionistic) ptosis overcorrection after frontalis sling. Ophthalmic Plastic and Reconstructive Surgery. 1992;8(2):137–142.
Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. Journal of the American Academy of Dermatology. 2009;61(6):961–970. e1–11
Carli L, Montecucco C, Rossetto O. Assay of diffusion of different botulinum neurotoxin type a formulations injected in the mouse leg. Muscle and Nerve. 2009;40(3):374–380.
Carruthers A, Carruthers J. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin. Dermatologic Surgery. 1998;24(11):1189–1194.
Carruthers A, Carruthers J. Prospective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type A in men with glabellar rhytids. Dermatologic Surgery. 2005;31(10):1297–1303.
Carruthers A, Carruthers J. Eyebrow height after botulinum toxin type A to the glabella. Dermatologic Surgery. 2007;33(1 spec. no.):S26–S31.
Carruthers A, Carruthers J, Lei X, et al. OnabotulinumtoxinA treatment of mild glabellar lines in repose. Dermatologic Surgery. 2010;36(suppl 4):2168–2171.
Carruthers A, Carruthers J, Lowe NJ, et al. One-year, randomized, multicenter, two-period study of the safety and efficacy of repeated treatments with botulinum toxin type A in patients with glabellar lines. Journal of Clinical Research. 2004;7:1–20.
Carruthers A, Carruthers J, Said S. Dose-ranging study of botulinum toxin type A in the treatment of glabellar rhytids in females. Dermatologic Surgery. 2005;31(4):414–422. discussion 422
Carruthers JA, Lowe NJ, Menter MA, et al. Glabellar Lines I Study Group. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. Journal of the American Academy of Dermatology. 2002;46(6):840–849.
Carruthers JD, Carruthers JA. Treatment of glabellar frown lines with C. botulinum-A exotoxin. Journal of Dermatologic Surgery and Oncology. 1992;18(1):17–21.
Carruthers JD, Lowe NJ, Menter MA, et al. Botox Glabellar Lines II Study Group. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plastic and Reconstructive Surgery. 2003;112(4):1089–1098.
Dailey RA, Philip A, Tardie G. Long-term treatment of glabellar rhytides using onabotulinumtoxina. Dermatologic Surgery. 2011;37(7):918–928.
Dressler D, Mander GJ, Fink K. Equivalent potency of Xeomin and Botox. Movement Disorders. 2008;23(suppl 1):S20–S21.
Dressler D, Mander G, Fink K. Measuring the potency labelling of onabotulinumtoxinA (Botox(r)) and incobotulinumtoxinA (Xeomin(r)) in an LD50 assay. Journal of Neural Transmission. 2012;119(1):13–15.
Fagien S, Carruthers JD. A comprehensive review of patient-reported satisfaction with botulinum toxin type a for aesthetic procedures. Plastic and Reconstructive Surgery. 2008;122(6):1915–1925.
Flynn TC. Botulinum toxin: examining duration of effect in facial aesthetic applications. American Journal of Clinical Dermatology. 2010;11(3):183–199.
Huang W, Rogachefsky AS, Foster JA. Browlift with botulinum toxin. Dermatologic Surgery. 2000;26(1):55–60.
Huilgol SC, Carruthers A, Carruthers JD. Raising eyebrows with botulinum toxin. Dermatologic Surgery. 1999;25(5):373–375. discussion 376
Hunt T, Clarke K. Potency evaluation of a formulated drug product containing 150-kd botulinum neurotoxin type A. Clinical Neuropharmacology. 2009;32(1):28–31.
Kane M, Glogau R, Caulkins C, et al n.d. Pooled analysis of duration of efficacy of onabotulinumtoxinA in glabellar lines (poster presented first at the Winter Clinical Dermatology meeting, Hawaii, Jan 14-19 Jan 2011)
Kranz G, Haubenberger D, Voller B, et al. Respective potencies of Botox and Dysport in a human skin model: a randomized, double-blind study. Movement Disorders. 2009;24(2):231–236.
Lewis T, Jacobsen G, Ozog D. Intrafollicular orifice injection technique for botulinum toxin type A. Archives of Dermatology. 2008;144(12):1657–1658.
Lowe P, Patnaik R, Lowe N. Comparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: a double-blind, randomized study. Journal of the American Academy of Dermatology. 2006;55(6):975–980.
Majlesi G. GaAs laser treatment of bilateral eyelid ptosis due to complication of botulinum toxin type A injection. Photomedicine and Laser Surgery. 2008;26(5):507–509.
Moers-Carpi M, Tan K, Fulford-Smith A 2011 A multicentre, randomized, double-blind study to evaluate the efficacy of onabotulinumtoxinA (20 units) in the treatment of glabellar lines, when compared to incobotulinumtoxinA (30 units) (poster presented at the 7th European masters in aesthetic and anti-aging medicine, September 30-October 1, Paris, France)
Monheit G, Carruthers A, Brandt F, et al. A randomized, double-blind, placebo-controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal dose. Dermatologic Surgery. 2007;33(1 spec. no.):S51–S59.
Nestor MS, Ablon GR. Comparing the clinical attributes of abobotulinumtoxina and onabotulinumtoxina utilizing a novel contralateral frontalis model and the frontalis activity measurement standard. Journal of Drugs in Dermatology. 2011;10(10):1148–1157.
Roche N, Schnitzler A, Genêt FF, et al. Undesirable distant effects following botulinum toxin type a injection. Clinical Neuropharmacology. 2008;31(5):272–280.
Sattler G, Callander MJ, Grablowitz D, et al. Noninferiority of incobotulinumtoxinA, free from complexing proteins, compared with another botulinum toxin type A in the treatment of glabellar frown lines. Dermatologic Surgery. 2010;36(suppl 4):2146–2154.
Trindade de Almeida AR, Marques E, de Almeida J, et al. Pilot study comparing the diffusion of two formulations of botulinum toxin type A in patients with forehead hyperhidrosis. Dermatologic Surgery. 2007;33(1 spec. no.):S37–S43.
Vartanian AJ, Dayan SH. Complications of botulinum toxin A use in facial rejuvenation. Facial Plastic Surgery Clinics of North America. 2005;13(1):1–10.
Wu Y, Zhao G, Li H, et al. Botulinum toxin type A for the treatment of glabellar lines in Chinese: a double-blind, randomized, placebo-controlled study. Dermatologic Surgery. 2010;36(1):102–108.