Giant Papillary Conjunctivitis
Introduction
Giant papillary conjunctivitis is an inflammatory condition seen in the upper tarsal conjunctiva, initially reported by Spring in 1974.1 The author noted a papillary reaction, similar to that seen in patients with allergic conjunctivitis, though his findings were reported in soft contact lens wearers. Allansmith and colleagues further detailed the syndrome, suggesting that it may be immunologic in origin, with the proteinaceous deposits on the contact lenses serving as the antigen.2 As the constellation of findings is predominantly associated with the use of contact lenses, the disease is also referred to as contact lens-induced papillary conjunctivitis (CLPC); however, the condition has also been reported in patients with ocular dermoids, ocular prostheses, exposed sutures following ocular procedures, extruded scleral buckles, filtering blebs, exposed corneal deposits, as well as tissue adhesives (Fig. 16.1).3–10
Epidemiology
Giant papillary conjunctivitis (GPC) is most often associated with wearing soft contact lenses, though it can occur with any type of contact lens. In a study of 221 patients, 85% were wearing soft contact lenses, with the remaining 15% of participants wearing rigid lenses.11 One report noted an average interval of 10 months for the development of GPC for soft lens wearers, compared to 8.5 years for rigid lens wearers.2 The type and material of the lens also contributes to the severity of the disease, with soft lens wearers suffering from more severe manifestations of disease than their counterparts in rigid gas-permeable lenses.11 Silicone hydrogel lenses also appear to have similar effects with prolonged use, though the findings are seen more commonly in earlier designs.12
Researchers have also studied the association of other atopic conditions in patients with GPC, due to the similarities between GPC and other immunologically derived ocular disorders (e.g. vernal keratoconjunctivitis). Allergic rhinitis and hay fever are often estimated to range 10–20% in the general population, and in the subset of GPC patients, the incidence has been reported to be as low as 12% to over 26%.11,13 One particular cohort reports that patients who suffer from allergies seem to have more severe signs and symptoms of GPC, compared to those who did not; however, the presence or absence of other allergic conditions did not have any effect on the ultimate treatment of those individuals or their long-term use of contact lenses.11
Pathophysiology
Neutrophils and lymphocytes are present in the epithelium and substantia propria of normal conjunctival tissue. Mast cells and plasma cells are also present, though they are sequestered to the substantia propria. In patients with GPC, these cells increase in number and are often found throughout the epithelium and the substantia propria, and are found in conjunction with other inflammatory cells, such as basophils and eosinophils.14 These findings, along with elevation of cytokines and chemokines in the tear film of GPC patients, suggest a possible allergic mechanism for the development of disease. Interleukin-6 (IL-6) along with IL-6 soluble receptor (IL-6sr) have been noted to be elevated four- to eightfold compared to controls, and IL-6sr has been postulated to be an important mediator in the formation of the papillae.15 Locally produced tear immunoglobulins (e.g. IgE, IgG, and even IgM in severe cases) are also found to be elevated in the tears of GPC patients, with the degree of elevation correlated to the severity of symptoms. It is interesting to note, that with discontinuation of lens wear and resolution of the signs and symptoms, these tear immunoglobulin levels return to normal.12
The proteinaceous deposits on the lens surface have classically been cited as the possible nidus for the development of inflammation and thus, the papillae associated with GPC. These various substances can cover 50% of the contact lens surface within 30 minutes of lens insertion, and 90% after 8 hours of wear time.16,17 Even with the best cleaning regimens involving enzymatic treatment, a residual coating still exists, and as new coating material is constantly built on the surface of the lens, the overall lens coating increases.18 The lens type and material also affect the rate and amount of accumulation of protein coating, as well as the total percentages of lipid, calcium and protein that deposit on the lens.12 Differences in the polymer content, structure and charge determine the extent to which protein is deposited on the anterior lens surface.19
The nature of these deposits is similar in patients with and without GPC. In addition, there are no morphologic or biochemical findings that differentiate the coating on the contact lenses in these two groups; however, those affected with GPC generally have more coating on their contact lenses, and lenses of GPC patients can promote a clinical picture of injection, thickening and a papillary reaction on the upper tarsal conjunctiva in monkeys in a laboratory setting.20 Cellular infiltrates seen in the biopsy samples from the monkeys are also similar to those seen in GPC; thus, the animal model strongly suggests that an antigen exists in the contact lens coating that may produce the same inflammatory reaction seen clinically as GPC.20 These findings support the allergic hypothesis for GPC, as does the fact that immunoglobulins G, A and M (IgG, IgA, and IgM), were also found in the protein deposits on GPC-associated contact lenses; however, eosinophil major basic protein, a material elaborated from eosinophils found in allergic reactions, was not found to a significant degree on lenses of GPC patients.21–24
In contrast, the mechanical hypothesis is supported by the association between GPC and inert objects, such as cyanoacrylate adhesives, exposed sutures or scleral buckle elements, dermoids and orbital prostheses. Researchers have postulated that the irritation and friction from the lens damages conjunctival epithelial cells and causes release of chemotactic factors (e.g. neutrophil chemotactic factor).24 Injection of these factors into the upper tarsal conjunctiva of rabbits produced a GPC-like reaction, suggesting that a combination of direct trauma and the resulting inflammatory cascade can stimulate a hypersensitivity reaction to lens-bound antigens.
Clinical Findings
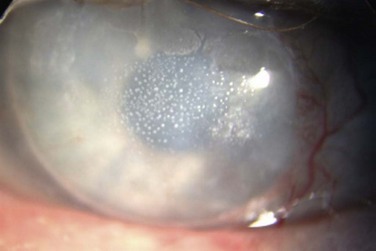
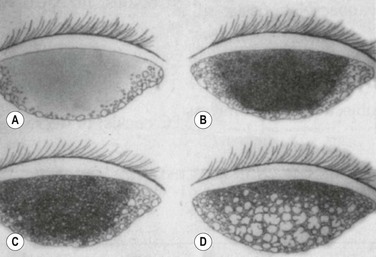
The biomicroscopic findings of normal tarsal conjunctiva, include a vascular arcade of fine, radiating vessels running perpendicular to the lid margin and a smooth, moist and pink surface. This has been termed a ‘satin’ appearance.2 Generally, the surface is devoid of papillae, or there may be a fine, fairly uniform papillary appearance detectable after the instillation of fluorescein dye and examination with a cobalt blue filter. These papillae, if present, are often smaller than 0.3 mm. Non-specific signs of inflammation, such as thickening of the tarsal conjunctiva with mild hyperemia, may be noted in early cases. In addition, bulbar conjunctival injection, superior corneal pannus, and corneal opacities may also be found on examination. As the disease progresses, non-uniform papillary changes develop, and finally giant papillae are seen, defined as a papillary reaction greater than 0.3 mm (Fig. 16.2).
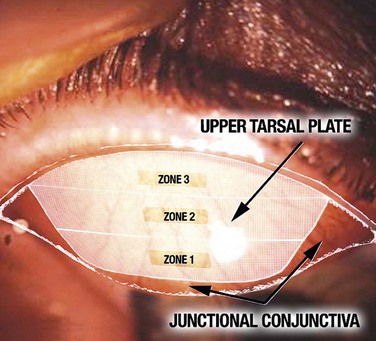
Further characterization of the size and location of the papillae is also helpful in correlating clinical findings with patient symptoms. Delineation of the upper tarsal plate into three zones, as well as two areas medially and laterally (Fig. 16.3), can be useful to the clinician in determining whether findings are within the normal variance or whether it represents disease. For example, large papillae in the junctional or transitional zone are not considered pathologic and should be disregarded in the assessment of the upper tarsal conjunctiva.12
Diagnosis
Giant papillary conjunctivitis shares morphologic and histologic similarities with vernal conjunctivitis, but is best differentiated from this and other immunologically based conjunctival inflammation by the associated clinical history (e.g. contact lens wear, surgical intervention). Biomicroscopic findings may also be helpful, as the location and appearance of the papillae are associated with the type and material of the lens. Soft contact lens wearers often exhibit a generalized papillary response, whereas those wearing rigid gas-permeable lenses or silicone hydrogels manifest papillae in a more localized fashion. Korb and colleagues25,26 have reported that papillae in soft and hard lens wearers differ in the location on the tarsal conjunctiva. Soft lens wearers usually form papillae nearest the upper margin of the tarsal plate (zones 1 and 2) and progress to diffuse involvement of all 3 zones, whereas rigid lens wearers and those using silicone hydrogel lenses develop papillae near the lid margin (zones 2 and 3) and persist in a more localized pattern.12
In addition, other associated signs and symptoms can aid in the diagnosis of GPC. These signs and symptoms are often classified into four stages:2
 Stage 1, or preclinical disease: minimal mucus discharge is usually noted upon awakening, and patients may report occasional itching after lens removal. Examination of the lenses reveals a mild protein coating. The tarsal conjunctiva may appear normal, or exhibit mild hyperemia with normal vascular structures.
Stage 1, or preclinical disease: minimal mucus discharge is usually noted upon awakening, and patients may report occasional itching after lens removal. Examination of the lenses reveals a mild protein coating. The tarsal conjunctiva may appear normal, or exhibit mild hyperemia with normal vascular structures.
 Stage 2, or mild disease: increased mucus production is noted, along with itching, increased lens awareness and marked coating of the contact lenses. Blurring of vision may occur. Mild to moderate injection of the tarsal conjunctiva is noted with some loss of the normal vascular pattern (i.e. superficial vessels are typically obscured, with deeper vessels still visible). The papillary reaction noted on examination may show variability in terms of the size of the papillae, which may be attributed to thickening of the underlying tissue. Some of the papillae measure 0.3 mm or greater at this stage, and can be best detected upon examination after instillation of fluorescein dye.
Stage 2, or mild disease: increased mucus production is noted, along with itching, increased lens awareness and marked coating of the contact lenses. Blurring of vision may occur. Mild to moderate injection of the tarsal conjunctiva is noted with some loss of the normal vascular pattern (i.e. superficial vessels are typically obscured, with deeper vessels still visible). The papillary reaction noted on examination may show variability in terms of the size of the papillae, which may be attributed to thickening of the underlying tissue. Some of the papillae measure 0.3 mm or greater at this stage, and can be best detected upon examination after instillation of fluorescein dye.
 Stage 3, or moderate disease: itching and mucus formation, along with lens coating, are more prominent at this point, and patients often report having difficulty with lens tolerability and keeping the lenses clean. Increased lens awareness and excessive lens movement with blinking often result in fluctuating quality of vision and reduction in lens wear time. The tarsal conjunctiva now shows marked thickening and injection, with obscuration of normal vascular pattern. The papillae have increased in both size and number, and the papillae appear more elevated secondary to changes in the underlying tissue (e.g. subconjunctival fibrosis and thickening).
Stage 3, or moderate disease: itching and mucus formation, along with lens coating, are more prominent at this point, and patients often report having difficulty with lens tolerability and keeping the lenses clean. Increased lens awareness and excessive lens movement with blinking often result in fluctuating quality of vision and reduction in lens wear time. The tarsal conjunctiva now shows marked thickening and injection, with obscuration of normal vascular pattern. The papillae have increased in both size and number, and the papillae appear more elevated secondary to changes in the underlying tissue (e.g. subconjunctival fibrosis and thickening).
 Stage four, or severe disease: patients are often unable to wear their lenses at all at this stage, with intense discomfort and cloudy vision upon initial insertion of the lenses. There is excessive lens movement, as well as poor centration of the contact lens. Increased mucus secretion is also reported, often to the point where patients note that their eyelids are stuck together in the mornings. The normal vascular pattern is completely obscured, and the papillae have enlarged to sizes of 1 mm or greater. Subconjunctival scarring and fluorescein staining of the apices of the papillae may be present (Fig. 16. 4).
Stage four, or severe disease: patients are often unable to wear their lenses at all at this stage, with intense discomfort and cloudy vision upon initial insertion of the lenses. There is excessive lens movement, as well as poor centration of the contact lens. Increased mucus secretion is also reported, often to the point where patients note that their eyelids are stuck together in the mornings. The normal vascular pattern is completely obscured, and the papillae have enlarged to sizes of 1 mm or greater. Subconjunctival scarring and fluorescein staining of the apices of the papillae may be present (Fig. 16. 4).
It is important to note that, although these stages can aid in the characterization of the disease, patient presentation is often variable. Some patients may have minimal complaints but exhibit marked inflammatory changes on the tarsal conjunctiva; in contrast, patients with severe symptoms may present with only mild or early tarsal changes. Also, despite the fact that GPC is often noted bilaterally, the clinical findings may be grossly asymmetric. In some cases, the disparity between eyes is easily explained (e.g. poor lens fit), but in other cases, no specific etiology can be determined.27
Treatment
Depending on the severity of disease, several or all of the strategies for reducing lens coating or mechanical stimulation may need to be employed. One study noted that if only the cleaning regimen was changed, only 50% of affected patients were able to continue wearing the contact lenses.11 A decrease in the wear time only resulted in 20% of patients who could continue to wear their lenses. Refitting the patients with new lenses resulted in a range of success rates: 68% of patients could tolerate wearing their lenses if they were refitted with the same type of lens; a change to a gas-permeable lens allowed for 82% of patients to return to contact lens wear; and frequent replacement of a daily wear lens resulted in a 91% success rate.11
The literature on immune response modulation for GPC has mainly focused on topical therapy, most commonly mast cell stabilizers and loteprednol etabonate (Lotemax®). Mast cell stabilizers have been shown to be effective, with one study finding a 70% success rate in patients with moderate to severe GPC that suffered a recurrence of symptoms despite changes in the contact lens polymer or design.28,29 The use of a steroid, such as loteprednol, has been found to reduce the presence of papillae, itching, and lens intolerance.30,31 However, chronic treatment with a steroid is generally not recommended in these cases.
A combination of the two therapeutic options, therefore, seems most appropriate for initiating treatment for the patient suffering from GPC. For stage 1, or preclinical cases, more frequent observation (e.g. 4–6 month follow-up visits) may be all that is required, as these individuals are generally asymptomatic but will be predisposed to developing GPC. Treatment for stage 2 to stage 3 should begin with discontinuation of contact lenses anywhere from 2–4 weeks, with re-evaluation of the conjunctiva and refit with frequent replacement contact lenses. These can range from daily disposables to lenses that are replaced every 2 weeks. For those patients changing their lenses every 1–2 weeks, a lens cleaning regimen utilizing a hydrogen peroxide-based cleaner is preferred.12 If there is a return of symptoms despite a change in the lens type and polymer, discontinue the lenses for another 2–4 weeks, refit with a daily disposable lens (or rigid gas-permeable lens) and add a mast cell stabilizer. These patients should generally be re-evaluated 3–4 times per year.
For severe, or stage 4 disease, discontinuation of contact lens wear may be required for at least 4 weeks, along with refitting the contact lens with either a daily disposable or rigid gas-permeable lens. The key for therapy at this stage is to determine if resolution of the associated findings of corneal and apical papillary staining has occurred; it is often noted that the appearance and size of the papillae may not change during the course of treatment for severe GPC. If the associated inflammatory signs have resolved, then attempts to refit the patient with a new lens may have a greater success rate. Specifically, if the patient is refitted with a daily wear contact lens and replaces the lens in an interval of 4 weeks or less, the rate of developing GPC drops to 4.5%. Individuals utilizing daily disposable lenses have not been reported to develop GPC.32
References
1. Spring, TF. Reaction to hydrophilic lenses. Med J Aust. 1974;1:449–450.
2. Allansmith, MR, Korb, DR, Greiner, JV, et al. Giant papillary conjunctivitis in contact lens wearers. Am J Ophthalmol. 1977;83:697–708.
3. Jolson, AS, Jolson, SC. Suture barb giant papillary conjunctivitis. Ophthalmic Surg. 1984;15:139–140.
4. Vengayil, S, Vanathi, M, Dada, T, et al. Filtering bleb-induced giant papillary conjunctivitis. Cont Lens Anterior Eye. 2008;31:41–43.
5. Srinivasan, BD, Jakobiec, FA, Iwamoto, T, et al. Giant papillary conjunctivitis with ocular prostheses. Arch Ophthalmol. 1979;97:892–895.
6. Skrypuch, OW, Willis, NR. Giant papillary conjunctivitis from an exposed prolene suture. Can J Ophthalmol. 1986;21:189–192.
7. Manners, RM, Vardy, SJ, Rose, GE. Localised giant papillary conjunctivitis secondary to a dermolipoma. Eye (Lond). 1995;9(Pt 3):376–378.
8. Dunn, Jr, JP., Weissman, BA, Mondino, BJ, et al. Giant papillary conjunctivitis associated with elevated corneal deposits. Cornea. 1990;9:357–358.
9. Carlson, AN, Wilhelmus, KR. Giant papillary conjunctivitis associated with cyanoacrylate glue. Am J Ophthalmol. 1987;104:437–438.
10. Robin, JB, Regis-Pacheco, LF, May, WN, et al. Giant papillary conjunctivitis associated with an extruded scleral buckle. Case report. Arch Ophthalmol. 1987;105:619.
11. Donshik, PC. Giant papillary conjunctivitis. Trans Am Ophthalmol Soc. 1994;92:687–744.
12. Donshik, PC, Ehlers, WH, Ballow, M. Giant papillary conjunctivitis. Immunol Allergy Clin North Am. 2008;28:83–103. [vi].
13. Friedlaender, MH. Some unusual nonallergic causes of giant papillary conjunctivitis. Trans Am Ophthalmol Soc. 1990;88:343–349. [discussion 349–51].
14. Allansmith, MR, Korb, DR, Greiner, JV. Giant papillary conjunctivitis induced by hard or soft contact lens wear: quantitative histology. Ophthalmology. 1978;85:766–778.
15. Shoji, J, Inada, N, Sawa, M. Antibody array-generated cytokine profiles of tears of patients with vernal keratoconjunctivitis or giant papillary conjunctivitis. Jpn J Ophthalmol. 2006;50:195–204.
16. Fowler, SA, Allansmith, MR. The surface of the continuously worn contact lens. Arch Ophthalmol. 1980;98:1233–1236.
17. Fowler, SA, Allansmith, MR. Evolution of soft contact lens coatings. Arch Ophthalmol. 1980;98:95–99.
18. Fowler, SA, Allansmith, MR. The effect of cleaning soft contact lenses. A scanning electron microscopic study. Arch Ophthalmol. 1981;99:1382–1386.
19. Minarik, L, Rapp, J. Protein deposits on individual hydrophilic contact lenses: effects of water and ionicity. Clao J. 1989;15:185–188.
20. Ballow, M, Donshik, PC, Rapacz, P, et al. Immune responses in monkeys to lenses from patients with contact lens induced giant papillary conjunctivitis. Clao J. 1989;15:64–70.
21. Trocme, SD, Kephart, GM, Bourne, WM, et al. Eosinophil granule major basic protein in contact lenses of patients with giant papillary conjunctivitis. Clao J. 1990;16:219–222.
22. Richard, NR, Anderson, JA, Tasevska, ZG, et al. Evaluation of tear protein deposits on contact lenses from patients with and without giant papillary conjunctivitis. Clao J. 1992;18:143–147.
23. Jones, B, Sack, R. Immunoglobulin deposition on soft contact lenses: relationship to hydrogel structure and mode of use and giant papillary conjunctivitis. Clao J. 1990;16:43–48.
24. Elgebaly, SA, Donshik, PC, Rahhal, F, et al. Neutrophil chemotactic factors in the tears of giant papillary conjunctivitis patients. Invest Ophthalmol Vis Sci. 1991;32:208–213.
25. Korb, DR, Allansmith, MR, Greiner, JV, et al. Biomicroscopy of papillae associated with hard contact lens wearing. Ophthalmology. 1981;88:1132–1166.
26. Korb, DR, Greiner, JV, Finnemore, VM, et al. Biomicroscopy of papillae associated with wearing of soft contact lenses. Br J Ophthalmol. 1983;67:733–736.
27. Palmisano, PC, Ehlers, WH, Donshik, PC. Causative factors in unilateral giant papillary conjunctivitis. Clao J. 1993;19:103–107.
28. Meisler, DM, Berzins, UJ, Krachmer, JH, et al. Cromolyn treatment of giant papillary conjunctivitis. Arch Ophthalmol. 1982;100:1608–1610.
29. Kruger, CJ, Ehlers, WH, Luistro, AE, et al. Treatment of giant papillary conjunctivitis with cromolyn sodium. Clao J. 1992;18:46–48.
30. Asbell, P, Howes, J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. Clao J. 1997;23:31–36.
31. Bartlett, JD, Howes, JF, Ghormley, NR, et al. Safety and efficacy of loteprednol etabonate for treatment of papillae in contact lens-associated giant papillary conjunctivitis. Curr Eye Res. 1993;12:313–321.
32. Donshik, PC, Porazinski, AD. Giant papillary conjunctivitis in frequent-replacement contact lens wearers: a retrospective study. Trans Am Ophthalmol Soc. 1999;97:205–216. [discussion 216–20].