Chapter 42 Gestational Trophoblastic Neoplasia
 Genetics of Gestational Trophoblastic Disease
Genetics of Gestational Trophoblastic Disease
The cytogenetic analysis of tissue obtained from molar pregnancies offers some clue to the genesis of these lesions. Figure 42-1 illustrates the genetic composition of molar pregnancies.
 Classification
Classification
The term gestational trophoblastic neoplasia is of clinical value because often the diagnosis is made and therapy instituted without definitive knowledge of the precise histologic pattern. GTN may be benign or malignant and nonmetastatic or metastatic (Box 42-1).
Metastatic GTN can be subdivided into good prognosis and poor prognosis groups, depending on the sites of metastases and other clinical variables (Box 42-2).
 Pathologic Features
Pathologic Features
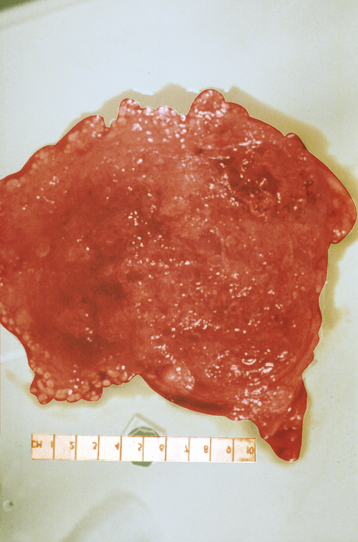
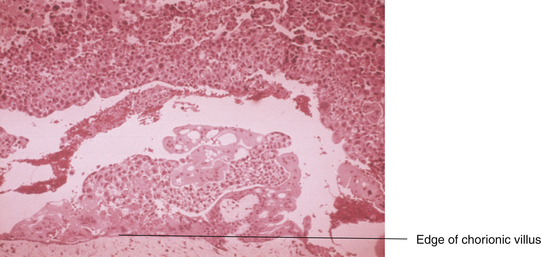
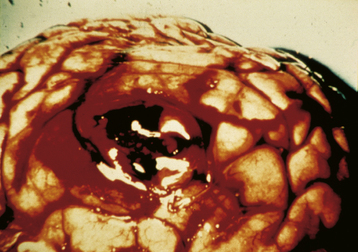
Grossly, a hydatidiform mole appears as multiple vesicles that have been classically described as a “bunch of grapes” (Figure 42-2). The characteristic histopathologic findings associated with a complete molar pregnancy are (1) hydropic villi, (2) absence of fetal blood vessels, and (3) hyperplasia of trophoblastic tissue (Figure 42-3). Invasive mole differs from hydatidiform mole only in its propensity to invade locally and to metastasize.
 Hydatidiform Mole
Hydatidiform Mole
SYMPTOMS
Most patients with hydatidiform mole present with irregular or heavy vaginal bleeding during the first or early second trimester of pregnancy (Box 42-3). The bleeding is usually painless, although it can be associated with uterine contractions. In addition, the patient may expel molar “vesicles” from the vagina and occasionally may have excessive nausea, even hyperemesis gravidarum. Irritability, dizziness, and photophobia may occur because some patients experience preeclampsia. Patients may occasionally exhibit symptoms relating to hyperthyroidism, such as nervousness, anorexia, and tremors.
STAGING
The International Federation of Gynecology and Obstetrics (FIGO) staging system for gestational trophoblastic tumors is shown in Table 42-1.
TABLE 42-1 INTERNATIONAL FEDERATION OF GYNECOLOGY AND OBSTETRICS (FIGO) STAGING OF GESTATIONAL TROPHOBLASTIC NEOPLASIA
| Stage I | Disease confined to uterus |
| Stage Ia | Disease confined to uterus with no risk factors |
| Stage Ib | Disease confined to uterus with one risk factor |
| Stage Ic | Disease confined to uterus with two risk factors |
| Stage II | Gestational trophoblastic tumor extending outside uterus but limited to genital structures (adnexa, vagina, broad ligament) |
| Stage IIa | Gestational trophoblastic tumor involving genital structures without risk factors |
| Stage IIb | Gestational trophoblastic tumor extending outside uterus but limited to genital structures with one risk factor |
| Stage IIc | Gestational trophoblastic tumor extending outside uterus but limited to genital structures with two risk factors |
| Stage III | Gestational trophoblastic disease extending to lungs with or without known genital tract involvement |
| Stage IIIa | Gestational trophoblastic tumor extending to lungs with or without genital tract involvement and with no risk factors |
| Stage IIIb | Gestational trophoblastic tumor extending to lungs with or without genital tract involvement and with one risk factor |
| Stage IIIc | Gestational trophoblastic tumor extending to lungs with or without genital tract involvement and with two risk factors |
| Stage IV | All other metastatic sites |
| Stage IVa | All other metastatic sites without risk factors |
| Stage IVb | All other metastatic sites with one risk factor |
| Stage IVc | All other metastatic sites with two risk factors |
Risk factors affecting staging include the following: (1) human chorionic gonadotropin greater than 100,000 IU/mL and (2) duration of disease longer than 6 months from termination of antecedent pregnancy. The following factors should be considered and noted in reporting: (1) prior chemotherapy has been given for known gestational trophoblastic tumor, (2) placental-site tumors should be reported separately, (3) histologic verification of disease is not required.
TREATMENT
Monitoring Levels of the β Subunit of Human Chorionic Gonadotropin
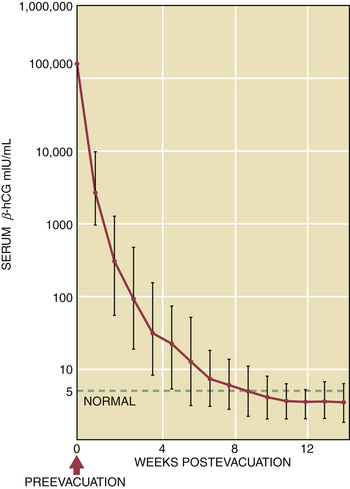
After the evacuation of a hydatidiform mole, the patient must be monitored with weekly serum assays of β-hCG. Because the titers drop to a low level, a nonspecific pregnancy test cannot be used because of the possibility of cross-reactivity with luteinizing hormone. The radioimmunoassay, sensitive to levels of 1 to 5 mIU/mL, should be used. There are currently several reference standards used to measure hCG in serum, each with its own scale. It is very important, therefore, either to use the same standard each time for measurement or to accurately adjust for any differences between reference standards before making any comparisons between test results. Following the evacuation, the β-hCG levels should steadily decline to undetectable levels, usually within 12 to 16 weeks. A normal regression curve for β-hCG levels following evacuation of a molar pregnancy is shown in Figure 42-5.
 Choriocarcinoma
Choriocarcinoma
 Treatment of Gestational Trophoblastic Neoplasia
Treatment of Gestational Trophoblastic Neoplasia
NONMETASTATIC AND METASTATIC GESTATIONAL TROPHOBLASTIC NEOPLASIA WITH A GOOD PROGNOSIS
The chemotherapy most often employed is either methotrexate or actinomycin D (Box 42-4). Methotrexate is usually given as a daily dose for 5 consecutive days or every other day for 8 days, alternating with folinic acid (leucovorin). This folinic acid “rescue” regimen is associated with significantly less bone marrow, gastrointestinal, and liver toxicity. Actinomycin D is given for 5 consecutive days intravenously or every other week as a single dose.
El-Helw L.M., Hancock B.W. Treatment of gestational trophoblastic neoplasia (Review). Lancet Oncol. 2007;8:715-724.
Garner E.I., Goldstein D.P., Feltmate C.M., Berkowitz R.S. Gestational trophoblastic disease. Clin Obstet Gynecol. 2007;50:112-122.
Lurian J.R., Singh D.K., Schink J.C. Role of surgery in the management of high-risk gestational trophoblastic neoplasia. J Reprod Med. 2006;51:773-776.
Ngan S., Seckl M.J. Gestational trophoblastic neoplasia management: An update (Review). Curr Opin Oncol. 2007;19:486-491.
Soper J.T., Spillman M., Sampson J.H., et al. High-risk gestational neoplasia with brain metastases: Individualized multidisciplinary therapy in the management of four patients. Gynecol Oncol. 2007;104:691-694.

 Epidemiology and Etiology
Epidemiology and Etiology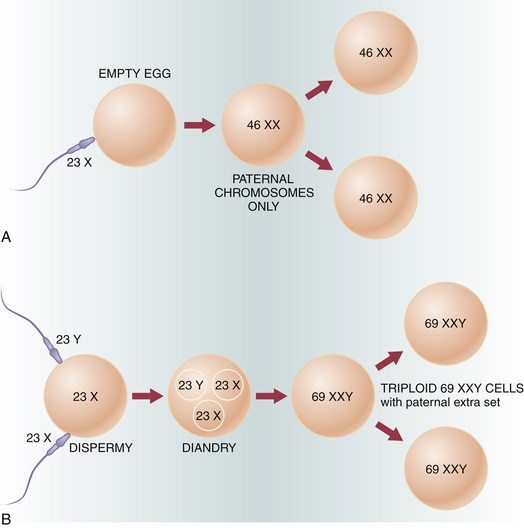
 Partial Mole
Partial Mole Invasive Mole
Invasive Mole