Genitourinary System
Perspective
Genitourinary trauma is frequently a covert entity and is associated with a wide array of injury. Approximately 10% of all multiply injured patients have some manifestation of genitourinary involvement.1 Because of its relatively infrequent occurrence and often subtle presentation, it may be overlooked in the initial evaluation of the multiply injured patient. Prompt identification and appropriate management of genitourinary injuries can minimize potential long-term complications, including renal insufficiency, chronic hypertension, incontinence, and sexual dysfunction. The astute emergency physician can accomplish these goals by a stepwise evaluation that considers the mechanism of injury, pertinent physical examination findings, urinalysis, and adjunctive diagnostic imaging.
Historical Perspective
The basic tenets of lower urinary tract injury have not changed appreciably in recent decades. A thoroughly performed physical examination, pelvic radiography, and the recognition of gross hematuria or blood at the urethral meatus identify virtually all significant lower urinary tract injuries. Major advances in the identification of significant upper tract genitourinary injuries, their clinical markers, and ultimate staging procedures have come to the forefront over the last few decades. Before 1985, all trauma patients with any amount of microhematuria were labeled “at risk” for genitourinary injury and underwent intravenous pyelography. This was neither diagnostically definitive nor cost-effective and simply perpetuated the existing confusion and controversy. In 1985, Nicolaisen and colleagues published the first of a series of articles that established guidelines for identifying significant upper tract genitourinary injuries, their markers, and the diagnostic studies that define the exact extent of these injuries and aid in subsequent patient management.2 In addition, the advent of ultrasonography has greatly simplified the diagnosis and management of external genitalia trauma.
Clinical Features
Physical Examination
Examination of the genitalia can be informative. The emergency physician should examine for evidence of hematoma or ecchymosis of the penile shaft, scrotal skin, or perineum. Gross blood at the urethral meatus is suggestive of a urethral injury and suggests the need for retrograde urethrography. In circumstances in which emergency surgical exploration for life-threatening injuries is indicated, retrograde urethrography can be performed in the operating room or after the operative procedure. For the male patient, classic teaching dictates that a Foley catheter should never be introduced when urethral injury is possible without prior evaluation of urethral integrity by retrograde urethrography. The concern is that trauma from the catheter placement may convert a partial urethral tear into a complete disruption. Although the literature on this topic is sorely lacking, one small retrospective review of 13 cases of urethral injury demonstrated no evidence that a careful blind attempt to insert a urinary catheter worsened the initial injury.3
Careful inspection for blood at the vaginal introitus is particularly important in the female patient known to have a pelvic fracture. A vaginal examination can discern vaginal lacerations or urethral disruption caused by displaced bony pelvic fracture fragments. This examination should be conducted carefully so as to protect the hands of the examiner from injury as well as to prevent worsening of the injuries themselves. Urethral injuries are uncommon in female patients owing to the short length and increased mobility of the female urethra and the relative protection from injury afforded by the symphysis pubis. Unlike with male urethral injuries, urethrography is technically difficult and not routinely recommended in females with suspected urethral injury. The inability to pass a Foley catheter in a premenopausal female patient with a pelvic fracture signifies the potential for urethral injury and possible need for suprapubic urinary drainage. Successful passage of a Foley catheter in a patient with blood at the vaginal introitus does not exclude urethral injury, however, and these worrisome physical examination findings are conveyed to the urologist, who can plan subsequent endoscopic or radiographic urethral evaluation accordingly.4–6 In an older postmenopausal female trauma patient, urethral injury is distinguished from a superiorly retracted urethral meatus and accompanying meatal stenosis. These preexisting conditions are common in an atrophic vaginal setting, and a 12- or 14-Fr coudé or Foley catheter usually is required to achieve successful bladder access.
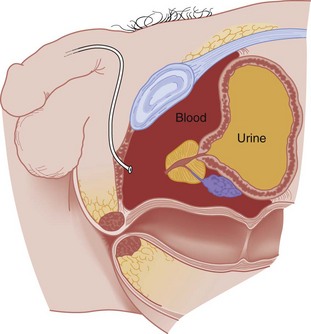
The digital rectal examination evaluates sphincter tone, bowel wall integrity, the presence or absence of gross blood, and the position of the prostate. Normally the posterior lobe of the prostate is palpable and well defined (Fig. 47-1). A pelvic fracture may disrupt the puboprostatic ligaments and the prostatomembranous urethra, resulting in significant retropubic venous bleeding. This may produce a large pelvic hematoma that can displace the prostate superiorly, resulting in a boggy, ill-defined mass on rectal examination (Fig. 47-2). Although it has long been recommended as part of the routine evaluation of all trauma patients, multiple studies have questioned the utility of the rectal examination in this setting and have specifically demonstrated that a palpably abnormal prostate is an insensitive indicator of urethral injury.7–10 Thus the decision to evaluate for urethral injury should consider additional clinical features, especially the presence or absence of gross hematuria or blood at the urethral meatus, and not rely on the findings of the rectal examination.
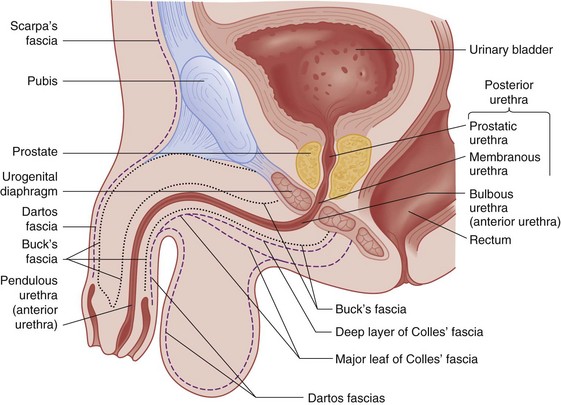
Figure 47-1 Anatomy of male genitalia.
Lower Urinary Tract
Anatomy
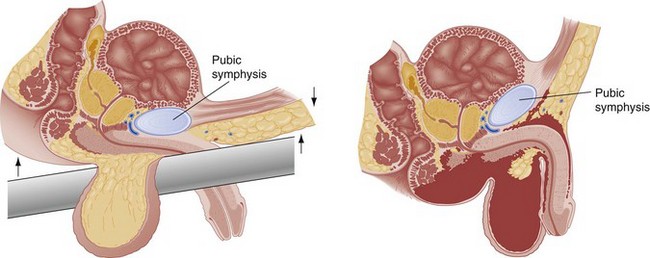
The urogenital diaphragm divides the anterior (bulbous and pendulous) urethra from the posterior (membranous and prostatic) urethra. Contrary to early descriptions, the urogenital diaphragm does not completely encircle the membranous urethra, but rather forms an incomplete sling that offers posterior and lateral support.5 The prostatic urethra is attached to the posterior symphysis pubis by the puboprostatic ligaments. A fracture of the pelvis with displacement of the symphysis may result in a laceration or avulsion of the prostatic urethra because of the shearing force on the fixed prostatic and membranous urethra. Injuries to the anterior and posterior urethra are caused by different mechanisms, involve different symptoms, and are treated differently.
Pathophysiology
Urethral disruption is the most significant injury to be identified. Failure to do so may lead to significant morbidity. Overly aggressive urethral manipulation can convert a partial urethral tear into a complete tear, thus precluding accurate assessment of urinary output and subsequently potentiating the long-term complications of urethral trauma (e.g., urethral stricture formation and urinary incontinence). Anterior urethral injuries are most often caused by straddle injuries, falls, gunshot wounds, and self-instrumentation (Fig. 47-3).
Pelvic fractures account for most posterior urethral injuries (see Fig. 47-2). The risk of urethral injury varies with the specific type of pelvic fracture. High-risk fractures include the straddle fracture, in which all four pubic rami are involved, and the Malgaigne fracture, which involves fracture and displacement of the hemipelvis—that is, both ischiopubic rami anteriorly and the ipsilateral sacrum, sacroiliac joint, or ilium posteriorly. Urethral injuries are rarely associated with fractures that do not involve the ischiopubic rami.5,11 In a prospective, single-center study of 203 consecutive male patients with pelvic fractures, 51 sustained urethral injuries. Malgaigne and straddle fractures were significantly associated with urethral injuries, with odds ratios of 3.4 and 3.85, respectively. In the same series, the highest risk for urethral injury was seen in patients sustaining a straddle fracture with concurrent sacroiliac diastasis (odds ratio 24). Among those sustaining fractures not involving the anterior pelvic arch, none had urinary tract injury.11
Diagnostic Strategies
Catheter Placement: The following technique for catheter placement assumes a normal urethra and includes the use of sterile technique, proper control of the foreskin, the use of copious amounts of lubricating jelly, and the gentle passage of a 14- or 16-Fr Foley or coudé catheter into the bladder. In all uncircumcised patients, continuous foreskin retraction with a folded 4 × 4-inch gauze pad is necessary to control the foreskin during catheter placement (Fig. 47-4). Without this maneuver, the foreskin tends to repeatedly reduce itself over the glans penis, which contaminates the field and complicates the catheterization attempt. Slight resistance to the advancing catheter should be expected secondary to voluntary contraction of the external sphincter. This is more apt to occur in a combative, anxious trauma patient than in a cooperative or unconscious patient. When this occurs, the patient should be reassured and asked to relax the perineum and rectal area while gentle advancing pressure is applied to the catheter. This combined approach allows the catheter to navigate the external sphincter successfully and pass easily into the bladder. If reassurance and relaxation do not allow easy passage of the catheter in a male patient with clinical features suggestive of urethral injury, it should be removed and retrograde urethrography performed. The catheter is passed to its fullest extent to ensure that the balloon is completely in the bladder when inflated, The catheter is then withdrawn to the point of balloon approximation with the bladder neck, secured to the patient’s leg to prevent accidental dislodgement, and left to drain. Inflation of the catheter balloon under any other circumstances may result in iatrogenic urethral trauma.
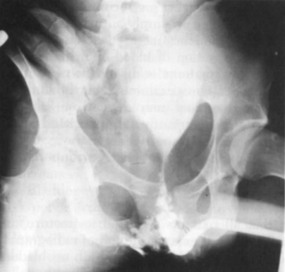
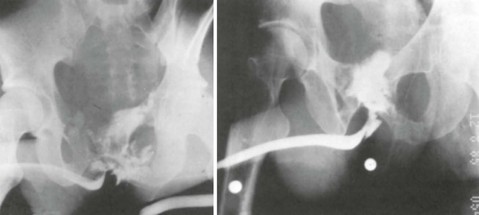
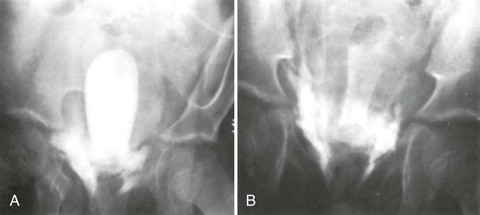
Successful passage of a Foley catheter precludes a complete urethral disruption. Nonetheless, the possibility of a partial urethral injury not manifested by history, mechanism of injury, or physical examination does exist. If a possible urethral injury is suggested by physical examination findings before the placement of a Foley catheter, a retrograde urethrogram should be obtained. The presence of urethral extravasation together with contrast material filling the bladder is diagnostic of a partial urethral injury (Fig. 47-5). Identification of a partial urethral injury enables one careful attempt at urethral placement of a 12- or 14-Fr Foley or coudé catheter, depending on the size of the patient.6 If any difficulty is encountered, the catheter should be removed and a urologist consulted. If a partial urethral tear is suggested subsequent to successful passage of a Foley catheter, a small feeding tube can be placed alongside the urethral catheter and a modified retrograde urethrogram obtained. In this circumstance the urethrogram is for documentation and subsequent management purposes only because appropriate therapy (Foley catheter drainage) has already been instituted.
Radiology: In male patients with possible urethral injury, retrograde urethrography is the diagnostic procedure of choice. Retrograde urethrography is not an emergency procedure and should follow more critical diagnostic and resuscitative measures.
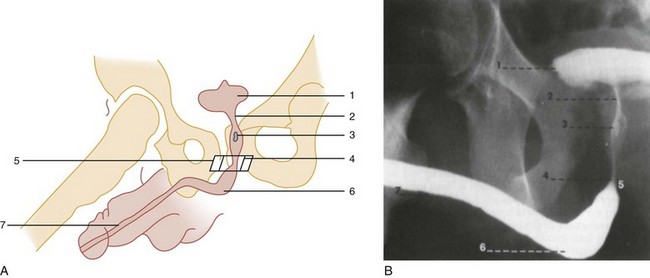
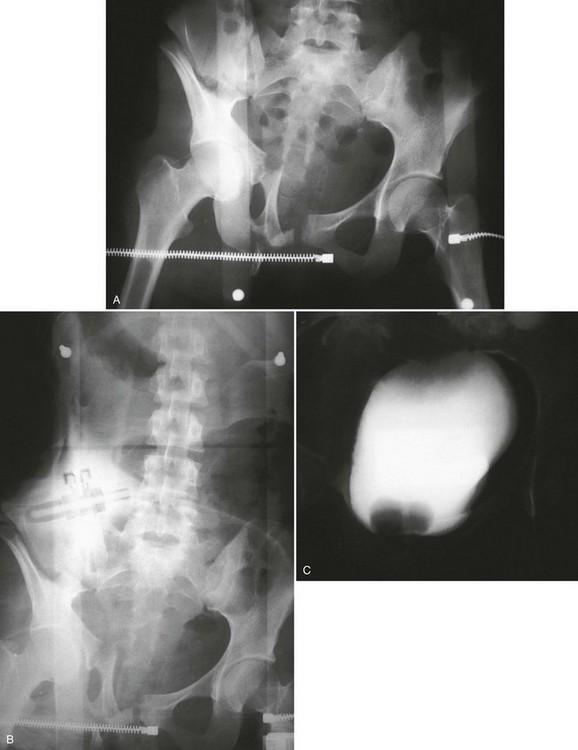
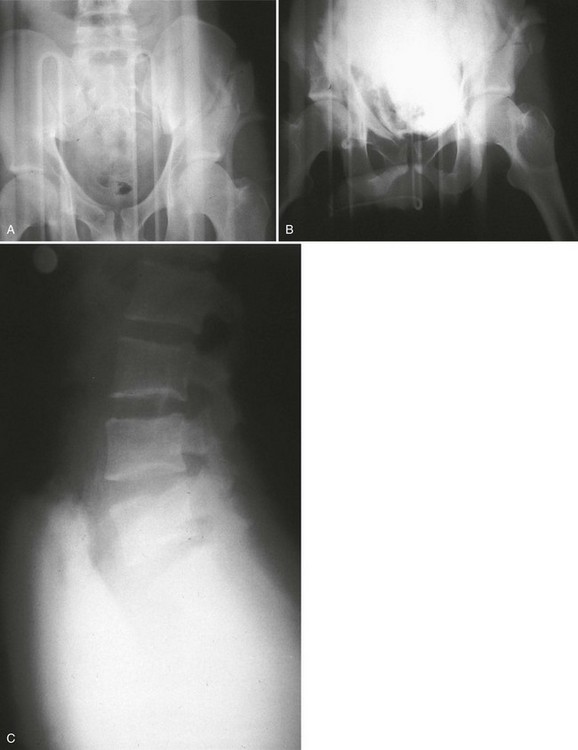
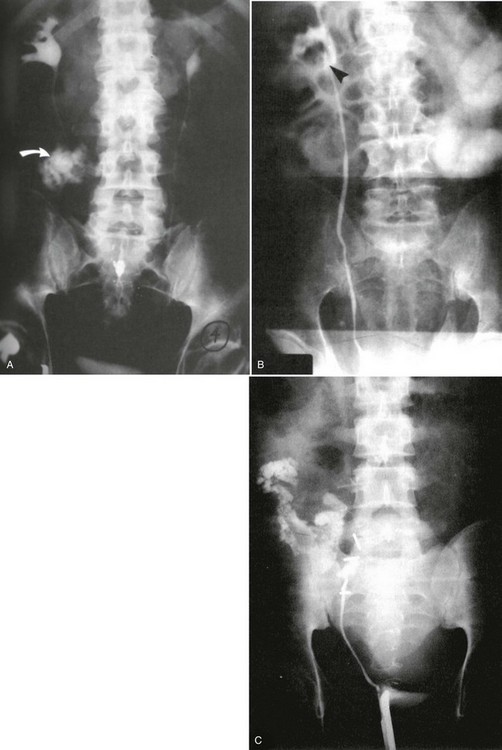
A preinjection kidney, ureter, and bladder (KUB) film is first obtained. In uncircumcised patients, the penile foreskin is retracted and secured with a folded 4 × 4-inch gauze pad (see Fig. 47-4). The penis should be held between the long and ring fingers of the nondominant hand. A simple Christmas tree adapter, a Cooke adapter placed on the end of a 60-mL Toomey syringe, or a Toomey syringe alone is gently passed into the urethral meatus until a snug fit inside the meatus is confirmed. The distal end of the syringe is secured with the thumb and index fingers of the nondominant hand (Fig. 47-6). Some authors have recommended inflation of a Foley catheter balloon just proximal to the fossa navicularis or the use of other cumbersome adjuncts to facilitate injection of contrast media. These techniques should be avoided, however, because they promote leakage of contrast material around the penis, which can simulate extravasation on the urethrogram and promote a spurious examination. Next, 60 mL (or 0.6 mL/kg in children) of full-strength or half-strength water-soluble contrast medium is injected slowly over 30 to 60 seconds. Overly forceful injection may result in intravasation of contrast material into the urethral venous plexus. A radiograph is taken during the injection of the last 10 mL of contrast material. Retrograde flow through the urethra and into the bladder without extravasation ensures continuity of the urethra and absence of urethral injury (Fig. 47-7). Extravasation of contrast material outside the urethra with concomitant evidence of bladder filling distinguishes a partial urethral injury (see Fig. 47-5) from a complete urethral disruption, in which contrast material will be absent from inside the bladder (Fig. 47-8). The latter situation requires urologic consultation for appropriate management, the timing and specifics of which remain controversial and depend on the location and mechanism of injury.6,12,13 In the interim, if measurement of urinary output is essential, the bladder should be accessed by the suprapubic placement of a peel-away sheath and Foley catheter through use of the Seldinger technique (Fig. 47-9). When venous intravasation of contrast is suggested, a postvoid film demonstrates clearing of any intravasated material while extravasated contrast from a urethral injury remains. Retrograde urethrography should be deferred if pelvic angiography is indicated, as extravasated contrast material from a urethral injury may obscure computed tomography (CT) scans and angiographic images and complicate attempts to control significant pelvic hemorrhage by vascular embolization.14
Management
The optimal definitive management of urethral injuries remains a subject of controversy in the urologic literature.6,12,13 Pertinent variables include the location of the injury (anterior vs. posterior), extent of the disruption (partial vs. complete), mechanism of injury, hemodynamic stability of the patient, and presence of associated injuries. Treatment options vary from allowing a partial disruption to heal over a stenting urethral catheter to early or delayed, open or endoscopic repair. Adjunctive suprapubic urinary drainage is frequently indicated. Regardless of the specific management plan, the ultimate goals are the preservation of urinary continence and sexual function and avoidance of the disruption of any pelvic hematoma.
In female patients, proximal urethral injuries are managed by immediate surgical exploration and repair, as conservative management with proximal urinary diversion alone is associated with an increased risk of urethrovaginal fistulae or obliterative urethral strictures. Distal injuries may be managed by urethral catheterization. Regardless of the location of the urethral injury, associated vaginal lacerations require transvaginal repair to reduce the incidence of fistula formation.6
Bladder Trauma
Pathophysiology
More than two thirds of bladder injuries result from blunt trauma. Approximately 90% result from motor vehicle collisions (MVCs) as a result of trauma sustained during ejection from the vehicle or the compressive force of the seat belt on a distended bladder. Approximately 80% of blunt bladder injuries are associated with fractures of the bony pelvis.15 Additional life-threatening, nonurologic injuries are common and confer a significant mortality risk.15,16 Penetrating injuries may be inflicted by gunshot wounds, stab wounds, or impalement injuries. The diagnostic evaluation of the bladder, like that of the urethra, can be accomplished quickly without elaborate radiographic equipment or can be part of the CT evaluation performed for the evaluation of nonurologic injuries.
Extraperitoneal rupture occurs almost exclusively with pelvic fractures when the associated shearing forces result in tearing of the anterolateral bladder wall at its fascial attachments. Occasionally, extraperitoneal rupture results from bladder laceration by a bone spicule from the fractured pelvis.15 Extravasated urine may be confined to the perivesical space or may dissect along tissue planes and extend to the penis, scrotum, thigh, anterior abdominal wall, obturator foramen, or retroperitoneum.15,17,18
Diagnostic Strategies
Laboratory: Gross hematuria is the cardinal sign of bladder injury and is present in more than 95% of cases.15,19,20 In the setting of pelvic fracture, gross hematuria indicates the need to investigate for bladder injury. Relative indications for bladder imaging include gross hematuria without pelvic fracture and microhematuria in the setting of pelvic fracture.15 Grossly clear urine in a blunt trauma patient without a pelvic fracture virtually eliminates the possibility of bladder rupture.
Radiology: Conventional retrograde cystography and retrograde CT cystography are the diagnostic procedures of choice for suggested bladder injury.21–23 It is key that these studies not be done in an antegrade fashion, as such studies (e.g., injecting intravenous contrast material, clamping the Foley catheter, and allowing the examination to depend on antegrade filling of the bladder from renal excretion of progressively dilute contrast material) may produce incomplete and spurious findings owing to inadequate distention of the bladder.
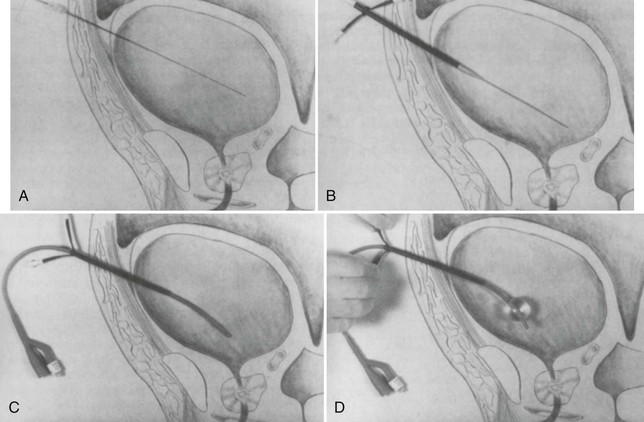
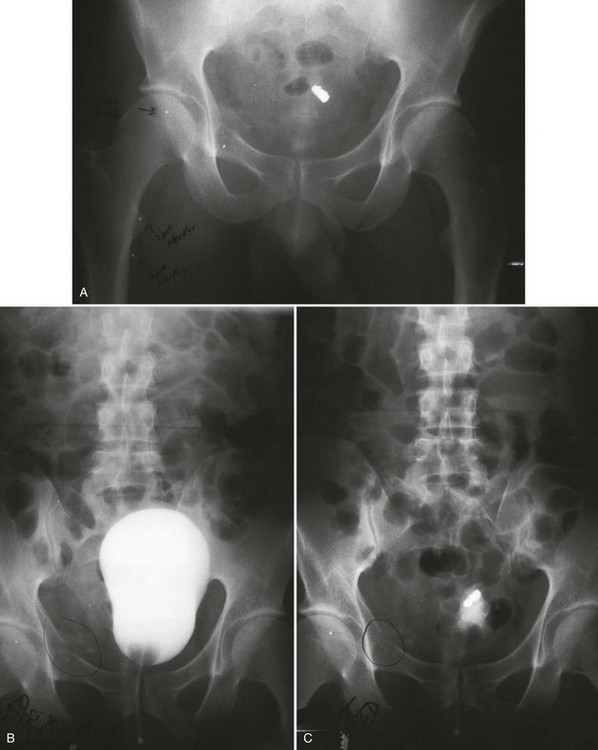
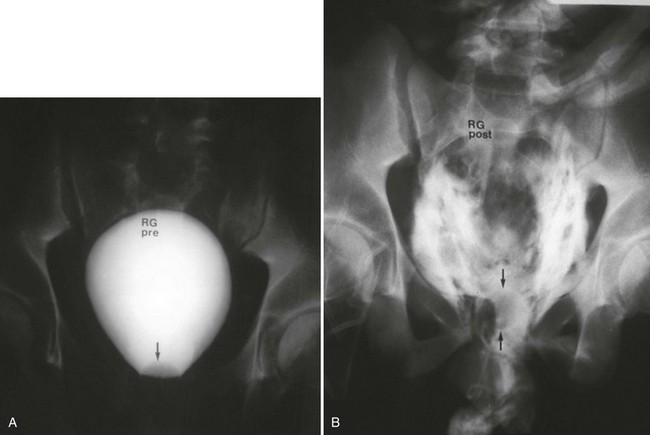
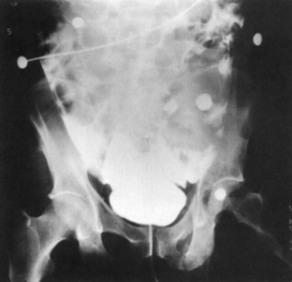
Conventional Retrograde Cystogram.: Performance of either conventional plain film retrograde cystography or CT retrograde cystography assumes prior evaluation of urethral integrity and the presence of an indwelling Foley catheter in the bladder. A Toomey syringe alone without its central piston is used for gravity instillation of contrast material (Fig. 47-10). Allowing the contrast material to freely infuse from a hanging bottle connected to an indwelling Foley catheter runs the risk of the tubing becoming disconnected, with contrast material subsequently leaking onto the examination table (Fig. 47-11A and B). This promotes an inaccurate examination that may result in an unnecessary operative procedure (Fig. 47-11C). In the setting of pelvic fracture, it is imperative that the patient remain supine throughout the examination. This lessens the potential for rebleeding from an organized retropubic hematoma.
A preliminary KUB or scout film is obtained to provide a baseline evaluation of the pelvis, abdomen, and surrounding bony structures. It will become the film of reference for the postevacuation radiograph obtained after completion of the cystogram. Potential areas of extravasation on the postevacuation film will be confirmed when compared with the preliminary KUB film (Fig. 47-12). Contrast material should not be instilled into the bladder until the quality and anatomic information on the preliminary KUB film have been confirmed.
1. Instillation of 100 mL with immediate fluoroscopic evidence of gross extravasation.
2. A total instillation of 300 to 400 mL in any patient 11 years of age or older; in patients younger than 11 years, the correct amount of contrast medium is determined by the following formula: (age in years divided by 2) × 30.
3. The instillation of a lesser amount than 100 mL that initiates a bladder contraction. This becomes evident by the retrograde filling of the Toomey syringe with bladder contents.
After a few minutes, the original contrast material can again be instilled to the point of stimulating a bladder contraction, at which time an additional 50 mL of full-strength contrast material should be injected slowly but forcefully into the bladder. The Foley catheter is clamped, and an anteroposterior radiograph is taken of the filled bladder (Fig. 47-13A and B). A lateral film may help clarify any areas in question (Fig. 47-13C). After the film of the filled bladder meets standards for quality and detail, the bladder should be completely evacuated into a large basin or, preferably, into an available bedside drainage bag. Any spillage of contrast material onto the pelvic genitalia or examination table may lead to false-positive findings on the postevacuation radiograph. The postevacuation film may disclose evidence of posterior bladder wall or extraperitoneal extravasation not seen on the anteroposterior radiograph of the filled bladder (Figs. 47-12 and 47-14).
In cases of extraperitoneal bladder perforation, contrast material is evident in the area of the pubic symphysis and pelvic outlet (Fig. 47-15). With intraperitoneal perforation, contrast material outlines intraperitoneal structures (e.g., loops of bowel, the liver, and spleen) (Fig. 47-16).
Several studies have documented that false-negative results are associated with the use of less than 300 to 400 mL, or an age-inappropriate amount, of contrast material for cystography.17 This has been seen primarily in penetrating bladder injuries in which the perforation from a small-caliber gunshot wound or a thin-blade stab wound can be missed. The anatomically interlacing bladder wall muscle fibers are arranged such that these wounds lend themselves to immediate muscle fiber reapproximation and tenuous sealing of the wound by covering peritoneum and intra-abdominal mesentery. Unless an adequate amount of full-strength contrast material is used to fully distend or even overdistend the bladder, extravasation will not be evident, the injury will be missed, and there will be potential for significant morbidity.
Computed Tomography Retrograde Cystogram.: The same anatomic information regarding bladder injury may be obtained with retrograde CT cystography rather than routine plain film radiography.21–23 CT cystography is best performed in trauma patients who are undergoing CT evaluation for other possible injuries.
Following standard CT imaging of the abdomen and pelvis to evaluate for injury, the bladder is filled with water-soluble contrast medium in the same manner as for the retrograde cytogram, and additional CT images are obtained. Postvoid images are not necessary given the additional detail evident on CT imaging. Intraperitoneal rupture is diagnosed on helical CT scan by the presence of extravasated contrast material throughout the abdominal cavity (i.e., contrast ascites). Extraperitoneal extravasation is more difficult to visualize but can be appreciated on images taken through the area of the bladder neck and lower pelvis (Fig. 47-17A and B).
Management
In cases of bladder contusions, there is no evidence of extravasation on retrograde cystography. For these injuries, expectant management with or without Foley catheter drainage is standard practice.15,24 Most uncomplicated extraperitoneal bladder ruptures heal spontaneously with urinary catheter drainage alone. Indications for operative repair of extraperitoneal ruptures include the presence of concomitant injury to the rectum or vagina, injury involving the bladder neck, or situations in which laparotomy is required for other injuries. Intraperitoneal bladder rupture requires surgical repair. Without operative intervention, lower urinary tract contamination will infect initially sterile urine and promote the development of subsequent bacterial peritonitis. Bladder repairs are never emergencies and normally follow operative repair of life-threatening injuries. Prompt urology consultation is indicated to allow for coordination between surgeons and determination of the optimal timing of the repair.
Upper Tract
Perspective
Renal trauma rarely occurs in isolation. Concomitant nonurologic injuries may result in hemodynamic instability, necessitating prompt intervention and relegating the search for renal injury to a position of secondary importance. Significant renal injuries define a small subset of the trauma population at large, and mortality from renal trauma accounts for less than 0.1% of trauma deaths.25 This fact may lead to complacency and causes some of these injuries to be overlooked initially. Renal injuries are graded 1 through 5 according to the American Association for the Surgery of Trauma Organ Injury Scaling Committee guidelines, which identify most injuries requiring operative intervention.26,27
Complications
Urinary extravasation is the most common complication of renal trauma, occurring in 10 to 30% of penetrating trauma and 2 to 18% of blunt injuries.25 There is considerable controversy in the urologic literature regarding the incidence of hypertension after renal trauma, with reported rates varying greatly from as little as 0.2% to as much as 55%.25,28 Many experts currently believe the overall risk of hypertension after most renal injuries to be quite low.25,28,29 Factors affecting the risk of hypertension after renal trauma include the specific injury type and severity. Renal artery occlusion, although rare, imparts a risk of hypertension of up to 50%.25,29
Anatomy and Physiology
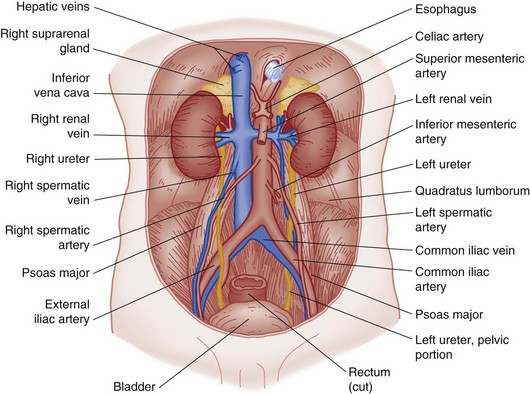
The kidneys are located in the retroperitoneal space, are surrounded by adipose tissue and loose areolar connective tissue, and lie along the lower two thoracic vertebrae and the first four lumbar vertebrae (Fig. 47-18). The kidneys are not fixed. They move with the diaphragm and are supported by their renal arteries, veins, and adipose tissue, which is connected to a layer of fibrous tissue called the renal fascia or Gerota’s fascia.
The indented medial border of the kidney is called the hilum. The major renal vessels and ureter make up the renal pedicle and enter and exit at the hilum. The longitudinal section of the kidney (Fig. 47-19) shows an outer renal cortex and an inner renal medulla with its columns of Bertin. Each column of Bertin forms a papilla that empties into the renal pelvis. The renal pelvis is a funnel-shaped sac with cup-shaped extensions called calyces, which receive urine from each papilla and are the important decompression areas for rises in intrapelvic pressure.
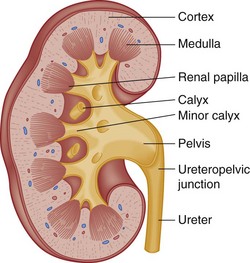
Figure 47-19 Longitudinal section of kidney.
The kidneys are perfused by 1200 mL of blood per minute, or 20 to 25% of cardiac output. Of this, 90% goes to the cortex and 10% to the medulla. Reduced blood flow to the kidney, whether from blunt or penetrating injury, causes renin to be released from the juxtaglomerular cells. Renin enters the bloodstream and combines with a plasma protein to form angiotensin. Angiotensin raises blood pressure by causing arteriolar vasoconstriction and acting on the adrenal cortex to augment aldosterone secretion. Aldosterone acts on the renal tubules to promote sodium reabsorption. Water follows passively with subsequent increase in blood volume. These changes increase blood flow to the kidney and other organs. The body requires only one third of normal renal function to sustain life. It is unusual in cases of genitourinary trauma to lose total renal function unless the patient had only one kidney prior to injury, which carries a 1 in 1000 to 1 in 5000 incidence.30
Epidemiology
Renal injury occurs in 1 to 5% of hospitalized trauma patients and represents the most common of all genitourinary injuries.13,29 Associated organ injuries occur in 61 to 100% of penetrating trauma and 35 to 55% of blunt injuries.25 Blunt trauma accounts for approximately 90% of injuries and occurs most often after MVCs, falls from heights, or direct blows to the flank. The pathophysiologic mechanisms include rapid deceleration, displacement, and, rarely, an explosion-type injury of the ureteropelvic junction. Penetrating injuries tend to be more severe and are associated with a higher nephrectomy rate.13
Diagnostic Strategies
Hematuria.: The presence, absence, or degree of hematuria correlates poorly with the severity of renal injury. This is especially true in the setting of penetrating trauma. In select cases of blunt trauma, the distinction between gross and microscopic hematuria can be used to guide clinical decision-making when considered along with the patient’s hemodynamic status and the mechanism and severity of injury.13,29,31,32 Formerly, a trauma patient who exhibited gross or microscopic hematuria was labeled “at risk” for urologic injury and underwent intravenous pyelography. Experience has shown that most of the identifiable injuries were renal contusions that could be managed expectantly. Moreover, in a significant number of severely traumatized patients, vigorous initial fluid resuscitation precluded satisfactory contrast concentration in the kidney, yielding an incomplete, nondiagnostic initial radiographic examination.
In 1989, Mee and associates published the hallmark article that established guidelines for the evaluation and treatment of renal trauma. Their 10-year prospective study of 1146 consecutive patients with blunt (88%) and penetrating renal trauma established that clinically significant blunt renal injuries are associated with gross hematuria, microscopic hematuria with shock (any systolic blood pressure less than 90 mm Hg), or, rarely but importantly, a history of sudden deceleration injury without hematuria or shock. In the same series, there was no correlation between the presence or absence of hematuria and the extent of renal injury among 139 cases of penetrating trauma.32
Pediatrics.: In children the kidney is the most commonly injured genitourinary organ.33 Controversy exists in the urologic literature as to whether the adult criteria for renal imaging after blunt trauma can be safely applied to the pediatric population.29,34,35 Owing to significant differences in physiology, hypotension is a late and unreliable indicator of shock in children.13,29 In addition, unlike in adults, major blunt renal injuries can occur in the presence of microhematuria. The literature has defined 50 RBCs per high-power field (hpf) as the threshold below which imaging could be confidently deferred without missing significant injuries in children.35,36 Current guidelines support imaging for pediatric (16 years or younger) patients with blunt renal trauma in the presence of gross hematuria, with microhematuria greater than 50 RBCs/hpf, or with significant deceleration injuries.29,37 As with adults, intravenous contrast-enhanced helical CT scanning is the diagnostic imaging technique of choice.
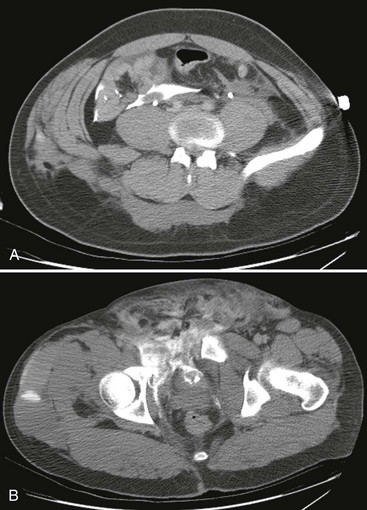
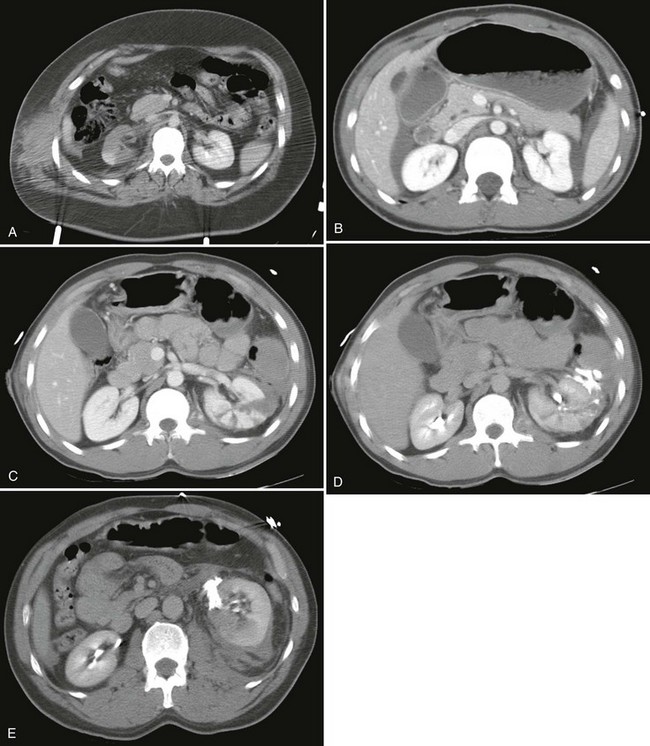
Computed Tomography.: Intravenous contrast-enhanced helical CT is the diagnostic radiographic procedure of choice in evaluating significant upper tract renal trauma.13,29,31,38 CT detects renal contusions, lacerations, renal pedicle injuries, devitalized segments, and urinary extravasation (Fig. 47-20). It allows for grading of renal injuries and provides important information about concomitant nonurologic injuries to abdominal and pelvic structures. Additional images obtained 10 minutes after contrast injection enable the detection of delayed extravasation and increase diagnostic accuracy.
Angiography.: Angiography may be a useful adjunct to CT imaging in the stable trauma patient with suggested renovascular injury, such as renal artery thrombosis, laceration, or pseudoaneurysm, and may be therapeutic in cases in which stenting or embolization is indicated.21,31,38
Intravenous Pyelography.: Formerly, intravenous pyelography was the most common imaging modality used in the evaluation of renal trauma. It has been shown to be less accurate than CT, is time-consuming and labor-intensive, and images only the urinary tract.31 Despite these significant limitations, it may play a limited role in the initial evaluation of suspected renal trauma when CT is not readily available. In patients requiring immediate surgical intervention for other indications, a “one-shot intravenous pyelogram” (IVP) obtained in the emergency department or, more commonly, on the operating table may provide limited information to help the surgeon stage upper tract injuries and confirm bilateral renal function. This test is performed by obtaining a single KUB film 10 minutes after the injection of 2 mL of intravenous contrast per kilogram (maximum 150 mL).30
Ultrasound.: Ultrasound is not sufficiently sensitive to rule out possible renal trauma, as it has been shown to miss up to 78% of known renal injuries.31,38 In the multiply injured trauma patient, ultrasound is useful in the evaluation for free fluid in the abdomen and pelvis, which may herald other significant nonrenal injuries; however, this modality cannot reliably distinguish blood from other types of fluid such as ascites or extravasated urine.39 Moreover, free intraperitoneal fluid may be absent in up to 65% of isolated renal injuries.21,38
Management
Blunt Injury: In the absence of a significant deceleration mechanism, adult blunt trauma patients without gross hematuria or shock (any systolic blood pressure <90 mm Hg) and pediatric blunt trauma patients with microhematuria of 50 RBCs or fewer per high-power field can be confidently discharged from the emergency department if hospital admission is not otherwise indicated. Outpatient urology follow-up until microhematuria has cleared is advisable to be certain it does not represent another more serious underlying condition.
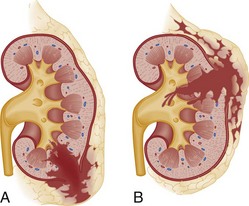
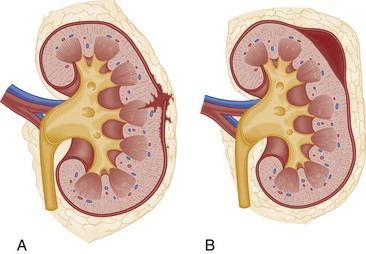
The optimal management of blunt renal trauma depends on the type and severity of injury (Figs. 47-21 and 47-22), the patient’s hemodynamic status, and the management plan for any associated nonurologic injuries. The American Association for the Surgery of Trauma organ injury severity scale for the kidney stratifies injuries and has been shown to correlate with the need for surgical intervention and the rate of nephrectomy.26 Grade I injuries include simple contusions and subcapsular, nonexpanding hematomas without parenchymal laceration. Grade II injuries are parenchymal lacerations less than 1 cm in depth without urinary extravasation and a stable, nonexpanding hematoma. Grade III injuries are parenchymal lacerations greater than 1 cm in depth without urinary extravasation. Grade IV injuries involve lacerations extending through the cortex, medulla, and collecting system or main renal artery or vein injuries with contained hemorrhage. Grade V injuries involve completely shattered kidneys or avulsion of the renal hilum and devascularization of the kidney.26 In one large retrospective review of 2467 patients with renal injury, the need for surgery increased from 0% of grade I injuries to 93% of grade V injuries. Likewise, the nephrectomy rate increased from 0% of grade I and II injuries to 86% of grade V injuries. Given that 80 to 90% are grade I or II, most blunt renal injuries can be managed nonoperatively with bed rest until any gross hematuria clears and with periodic follow-up imaging to assess for injury resolution and to document renal function.
Immediate operative intervention is indicated for persistent life-threatening hemorrhage of possible renal origin. Restoration of normal renal function is unlikely after main renal artery injury, and nephrectomy may be required, especially in the presence of longer than 2 to 3 hours of complete or 6 hours of partial renal ischemia.29,40 In select patients, arteriography with hemorrhage control by embolization is a reasonable alternative to laparotomy.13 Of note, arterial embolization results in infarction of the affected renal segment, imparting a risk of infection and post-traumatic hypertension. These risks can be minimized by superselective catheterization and embolization of peripheral branches of the renal artery when possible, based in the specific injury and expertise of the interventionalist. When laparotomy is required for other injuries before adequate radiographic evaluation, blunt renal trauma may be surgically staged. In this setting, renal exploration is indicated in the presence of an expanding, pulsatile, or uncontained retroperitoneal hematoma that is thought to indicate renal pedicle avulsion or when the injured kidney is not visualized on the one-shot IVP.31
Penetrating Injury: In cases of penetrating renal trauma, the presence or absence of hematuria is of no consequence in predicting upper urinary tract injury. The location of the penetrating injury in relation to the urinary tract is the most important determining factor in deciding the need for radiographic investigation. Therefore the absence of hematuria in a patient with a gunshot or stab wound in proximity to the urinary tract does not eliminate the need for intravenous contrast-enhanced CT as the initial diagnostic examination. Significant injuries to the kidney and ureter may occur in penetrating trauma without hematuria.13,29,32 Additional images obtained at 10 minutes after contrast injection are indicated to evaluate for delayed contrast extravasation and maximize the sensitivity of the study. The majority of penetrating renal injuries require surgical intervention.
Ureteral Trauma
Approximately 80% of ureteral injuries are iatrogenic, resulting from complications of abdominal or pelvic surgery, with the remainder caused by external trauma. The ureters are relatively protected from injury by the bony pelvis, vertebral column, and psoas muscle. Thus ureteral trauma is rare, present in approximately 3 per 10,000 trauma admissions.41 Gunshot wounds are the most frequently reported mechanism, with ureteral injuries complicating 2 to 3% of abdominal gunshot wounds.42,43
Blunt force trauma causes ureteral injury when a significant decelerating force results in avulsion of the ureter from its fixed points at the ureteropelvic junction or, less commonly, the ureterovesicular junction. Owing to the significant force required for ureteral injury, concomitant major organ injury occurs in approximately 90% of cases, and hypotension is present in more than 50%.44
Clinical Features
There are no reliable signs and symptoms of ureteral injury in the acutely injured patient. Hematuria (gross or microscopic) is frequently present but may be absent in more than 25% of patients, and thus its absence alone cannot be relied on to exclude the diagnosis.44 After blunt trauma, ureteral injury should be considered in patients with a significant decelerating mechanism, gross hematuria, or microscopic hematuria with hypotension. With penetrating mechanisms, ureteral injury should be considered when the injuring force occurs in proximity to the anatomic course of the ureter. Missed ureteral injury may manifest in a delayed fashion with a variety of signs and symptoms, including fever, nausea and vomiting, hematuria, flank pain, and a palpable flank mass.
Diagnostic Strategies
Intravenous contrast-enhanced CT of the abdomen and pelvis is highly sensitive for visualization of upper tract injuries and is often indicated for the investigation of associated nonurologic injuries. Additional images obtained 10 minutes after contrast injection enable the detection of delayed extravasation and increase diagnostic accuracy.21
Retrograde pyelography (Fig. 47-23) is slightly more sensitive than intravenous contrast-enhanced CT for the evaluation of ureteral injury, but its performance is frequently impractical in the multiply injured patient and it is rarely undertaken in lieu of CT. Likewise, a formal IVP, although sensitive, is of limited usefulness owing to the significant time required for study completion.44 In patients requiring immediate surgical intervention for other indications, a one-shot IVP performed in the emergency department or, more commonly, on the operating table may provide limited information to help the surgeon stage upper tract injuries and confirm bilateral renal function. This test is performed by obtaining a single KUB film 10 minutes after the injection of 2 mL/kg of intravenous contrast (maximum 150 mL). The presence or absence of ureteral injury is subsequently confirmed during operative exploration.
Management
Operative repair is indicated for all ureteral injuries. With early diagnosis and appropriate treatment, the kidney and ureter can be saved in most cases. The complications of missed ureteral injuries are significant and include extended hospital stays, persistent urinoma, sepsis, loss of renal function, and death.45,46
External Genitalia
Anatomy
The penis contains three masses of erectile tissue (Fig. 47-24). The two corpora cavernosa constitute the main bulk and lie in the center of the penis. The smaller corpus spongiosum lies on the ventral surface of the penis, encases the urethra, and expands at the penile tip to form the glans penis. Blood is supplied by arteries lying in each of the three erectile masses and by the two dorsal penile arteries. A single dorsal vein drains most of the penis. The tunica albuginea is a dense fibrous envelope that surrounds the corpus spongiosum and each corpus cavernosum. Buck’s fascia overlies the tunica albuginea, enclosing all three of the erectile masses, the dorsal arteries, dorsal nerve, and deep dorsal vein within a common compartment.
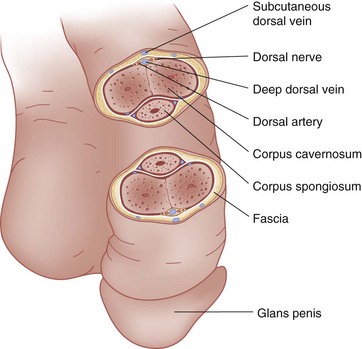
Figure 47-24 Cross-sectional view of the penis.
Clinical Features
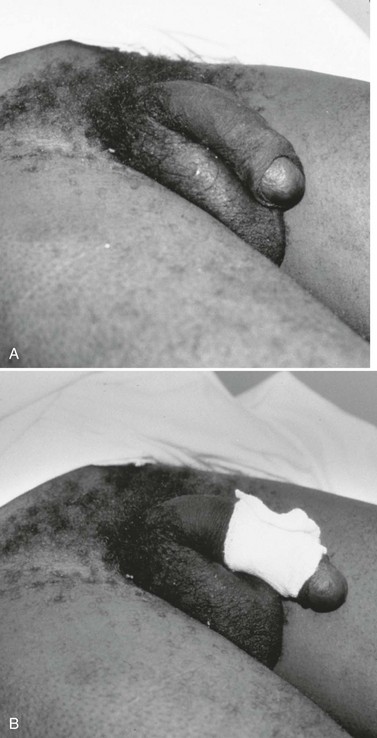
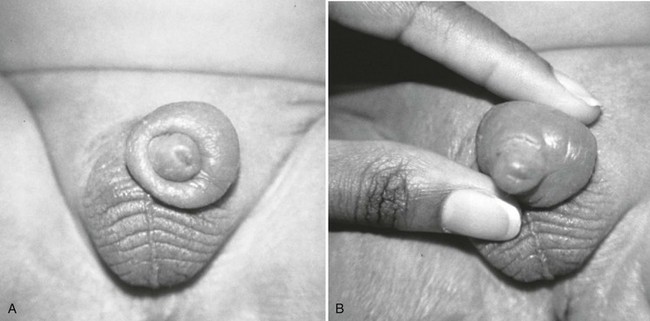
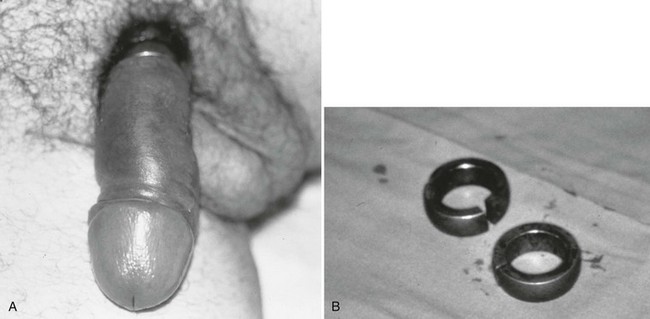
Important historical factors to ascertain include the mechanism of injury and the state of the penis, flaccid or erect, at the time the injuring force was inflicted, as well as tetanus immunization status. Penile injuries range from small lacerations or contusions to skin degloving or amputation. Strangulation injuries of the penile shaft inflicted by an encircling piece of string or hair may be seen in children (Fig. 47-25). Adolescents and adults may have various objects of penile incarceration such as bottles, washers, and metal rings (Fig. 47-26). These objects are often used to facilitate masturbation, prevent detumescence, and heighten sexual pleasure. Prolonged application of these devices may result in loss of superficial skin, deep urethral necrosis, or eventual need for penile amputation.47
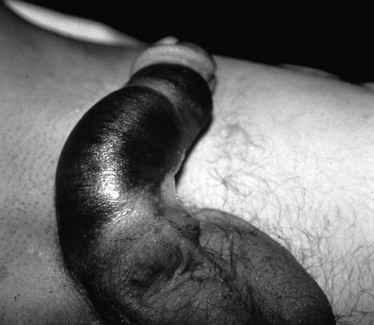
The pendulous nature of the flaccid penis provides a measure of protection from blunt injury. By contrast, forceful bending of the erect penis may result in traumatic rupture of the corpus cavernosum, or penile fracture, owing to tearing of the tunica albuginea. This injury may occur during vigorous sexual intercourse or masturbation.48 The patient often reports hearing a snapping sound followed by immediate pain, detumescence, and a slowly progressive penile hematoma (Fig. 47-27). In the majority of cases, the swelling and ecchymosis are localized to the penile shaft and contained by Buck’s fascia. The resultant appearance has been described as an “eggplant deformity.” If Buck’s fascia is torn, blood may track along fascial planes to the scrotum, perineum, and pubis. The corporal defect may be palpable on physical examination. Urethral injury occurs in 10 to 38% of penile fractures.47 The diagnosis is suggested by the presence of gross hematuria, blood at the urethral meatus, or the inability to void, but may be possible even in the absence of these clinical findings. Urethral injury is present in up to 50% of all penetrating penile injuries.21
Penetrating trauma to the external genitalia may be inflicted by stab wounds or gunshot wounds. These injuries are frequently associated with concomitant injuries to the bladder, urethra, rectum, testis, and iliac and femoral vasculature.47 Penile amputation may result from interpersonal violence or be self-induced as a result of severe psychiatric disease.
The testicles are vulnerable to injury because of their location but are relatively protected by the mobility of the scrotum, the tough tunica albuginea that surrounds the testes, and the cremasteric reflex. Eighty-five percent of testicular injuries are inflicted by blunt trauma, most commonly during sporting endeavors.49 Mechanisms include falls, kicks, and direct strikes by thrown objects. Testicular rupture occurs in more than 40% of blunt scrotal trauma in patients who come to the hospital.13,50 Additional injuries include scrotal hematoma, hematocele, intratesticular hematoma, traumatic testicular torsion, testicular avulsion, testicular displacement, and epididymal injury. Symptoms include severe pain, faintness, nausea, vomiting, and occasionally urinary retention secondary to pain. Physical examination is often inadequate owing to significant pain and swelling.50
Genital bite wounds may be inflicted by humans during sexual activity or by animals. The predominant human oral bacterial organism in genital bite wounds is Eikenella corrodens; however, transmission of viral infections, including hepatitis and human immunodeficiency virus (HIV), is possible.47 Dog and cat bites may lead to pathogenic infections with Pasteurella multocida and anaerobic organisms. With certain animal bites, the possibility of rabies transmission is considered.
Diagnostic Strategies
A careful physical examination is usually sufficient to diagnose most blunt penile injuries. Retrograde urethrography is indicated in cases of suggested urethral injury, which should be considered in the presence of concomitant penile or scrotal hematoma, gross hematuria, or blood at the urethral meatus. Rarely, atypical presentations of penile fracture may require adjunctive imaging. Corpus cavernosography, ultrasound, and magnetic resonance imaging have all been advocated for this indication.47,51,52 The acquisition of penile imaging should occur in consultation with the treating urologist.
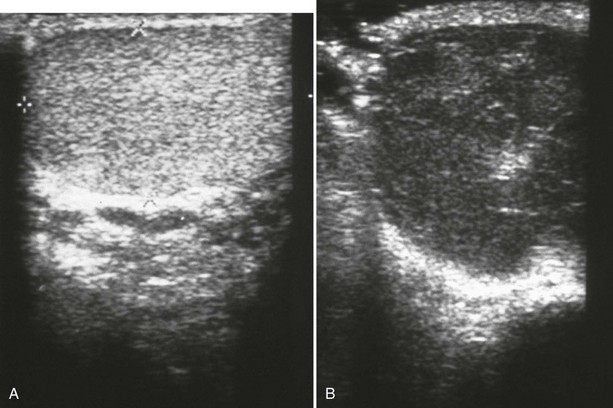
Unlike with penile injuries, physical examination is frequently inadequate for the assessment of blunt scrotal trauma. Ultrasound is the test of choice owing to its higher than 95% sensitivity for testicular rupture when the diagnosis is based on the finding of a heterogeneous echo pattern of the testicular parenchyma with a loss of contour definition (Fig. 47-28).50,53 Scrotal imaging is rarely indicated after penetrating trauma because surgical exploration is usually warranted.
Management
Constricting devices are promptly identified and removed, a procedure that can test the ingenuity of even the most experienced emergency physician (see Fig. 47-26). Various creative techniques with saws, metal cutters, or emery wheels may be necessary to remove some metal objects. Reconstructive surgery of the penis may eventually be needed but is usually delayed until penile tissue viability has been determined. Fortunately, each corpora body has a separate blood supply and may be preserved even though the penile skin may slough, necessitating skin grafting.
Superficial penile hematomas without rupture of the tunica albuginea and with no immediate detumescence of the erect penis may be treated conservatively with the local application of ice and administration of nonsteroidal anti-inflammatory drugs (NSAIDs).13 Superficial lacerations of the penile and scrotal skin may be primarily approximated with 4-0 chromic or Vicryl absorbable suture. Patients with degloving penile injuries and scrotal skin loss should be treated by a urologist and plastic surgeon in the operating room with irrigation, débridement, and skin flaps or skin grafts.
With penile fracture, early surgical repair of the tunica albuginea defect (within 24-36 hours after injury) is indicated to maximize functional outcomes.13,49,51 Early surgical exploration and repair are recommended for most penetrating injuries to the penis.54 Penile amputation may be managed by reimplantation or local reshaping. Successful reimplantation may be achieved even up to 24 hours after amputation.47,49 The recovered amputated penis should be carefully wrapped in sterile, saline-moistened gauze and placed in a plastic bag. That bag is then placed in a second plastic bag containing ice. Hemostasis of a bleeding penile stump can usually be achieved with direct pressure.
After blunt scrotal trauma, surgical exploration is indicated for treatment of testicular rupture, large hematocele, traumatic torsion, and testicular dislocation. Testicular salvage rates are 80 to 90% when surgical exploration occurs within 72 hours and less than 50% when exploration is delayed beyond 72 hours.49 In patients with a sonographically normal, homogenous-appearing testicle, exploration is not necessary.50 Testicular contusions are treated with bed rest, ice packs, NSAIDs, and urologic follow-up. Surgical exploration is indicated in nearly all cases of penetrating scrotal trauma.50,54
Patients with simple bite wounds within 6 to 12 hours after injury without gross contamination can be treated with irrigation and primary closure.47,49 Broad-spectrum antibiotic coverage with an agent such as amoxicillin-clavulanate is indicated. Grossly contaminated or infected wounds should be irrigated, covered with a sterile dressing, and managed in conjunction with the consulting urologist.
References
1. McAninch, JW. Injuries to the genitourinary tract. In Tanagho EA, McAninch JW, eds.: Smith’s General Urology, 18th ed, New York: McGraw-Hill, 2008.
2. Nicolaisen, GS. Re-evaluation of the indications for radiological assessment. J Urol. 1985;133:183.
3. Shlamovitz, GZ, McCullough, L. Blind urethral catheterization in trauma patients suffering from lower urinary tract injuries. J Trauma. 2007;62:330.
4. Perry, MO, Husmann, DA. Urethral injuries in female subjects following pelvic fractures. J Urol. 1992;147:139.
5. Rosenstein, DI, Alsikafi, NF. Diagnosis and classification of urethral injuries. Urol Clin North Am. 2006;33:73.
6. Brandes, S. Initial management of anterior and posterior urethral injuries. Urol Clin North Am. 2006;33:87.
7. Shlamovitz, GZ, et al. Poor test characteristics for the digital rectal examination in trauma patients. Ann Emerg Med. 2007;50:25.
8. Ziran, BH, Chamberlin, E, Shuler, FD, Shah, M. Delays and difficulties in the diagnosis of lower urologic injuries in the context of pelvic fractures. J Trauma. 2005;58:533.
9. Esposito, TJ, et al. Reasons to omit digital rectal exam in trauma patients: No fingers, no rectum, no useful additional information. J Trauma. 2005;59:1314.
10. Ball, CG, et al. Traumatic urethral injuries: Does the digital rectal examination really help us? Injury. 2009;40:984.
11. Koraitim, MM, Marzouk, ME, Atta, MA, Orabi, SS. Risk factors and mechanism of urethral injury in pelvic fractures. Br J Urol. 1996;77:876.
12. Chapple, C, et al. Consensus statement on urethral trauma. BJU Int. 2004;93:1195.
13. Lynch, TH, et al. EAU guidelines on urological trauma. Eur Urol. 2005;47:1.
14. Spencer Netto, FA, et al. Retrograde urethrocystography impairs computed tomography diagnosis of pelvic arterial hemorrhage in the presence of a lower urologic tract injury. J Am Coll Surg. 2008;206:322.
15. Gomez, RG, et al. Consensus statement on bladder injuries. BJU Int. 2004;94:27.
16. Cass, AS, Luxenberg, M. Features of 164 bladder ruptures. J Urol. 1987;138:743.
17. Corriere, JN, Sandler, CM. Mechanisms of injury, patterns of extravasations and management of extraperitoneal bladder rupture due to blunt trauma. J Urol. 1988;139:43.
18. Andrich, DE, Mundy, AR. The nature of urethral injury in cases of pelvic fracture urethral trauma. J Urol. 2001;165:1492.
19. Brewer, ME, Wilmoth, RJ, Enderson, BL, Daley, BJ. Prospective comparison of microscopic and gross hematuria as predictors of bladder injury in blunt trauma. Urology. 2007;69:1086.
20. Morey, AF, et al. Bladder rupture after blunt trauma: Guidelines for diagnostic imaging. J Trauma. 2001;51:683.
21. Jankowski, JT, Spirnak, JP. Current recommendations for imaging in the management of urologic traumas. Urol Clin North Am. 2006;33:365.
22. Deck, AJ, Shaves, S, Talner, L, Porter, JR. Computerized tomography cystography for the diagnosis of traumatic bladder rupture. J Urol. 2000;164:43.
23. Vaccaro, JP, Brody, JM. CT cystography in the evaluation of major bladder trauma. Radiographics. 2000;20:1373.
24. Corriere, JN, Jr., Sandler, CM. Diagnosis and management of bladder injuries. Urol Clin North Am. 2006;33:67.
25. Al-Qudah, HS, Santucci, RA. Complications of renal trauma. Urol Clin North Am. 2006;33:41.
26. Santucci, RA, et al. Validation of the American Association for the Surgery of Trauma organ injury severity scale for the kidney. J Trauma. 2001;50:195.
27. Moore, EE, et al. Organ injury scaling: Spleen, liver, and kidney. J Trauma. 1989;29:1664.
28. Broghammer, JA, Fisher, MB, Santucci, RA. Conservative management of renal trauma: A review. Urology. 2007;70:623.
29. Santucci, RA, et al. Evaluation and management of renal injuries: Consensus statement of the renal trauma subcommittee. BJU Int. 2004;93:937.
30. Morey, AF, McAninch, JW, Tiller, BK, Duckett, CP, Carroll, PR. Single shot intraoperative excretory urography for the immediate evaluation of renal trauma. J Urol. 1999;161:1088.
31. Alsikafi, NF, Rosenstein, DI. Staging, evaluation, and nonoperative management of renal injuries. Urol Clin North Am. 2006;33:13.
32. Mee, SL, McAninch, JW, Robinson, AL, Auerbach, PS, Carroll, PR. Radiographic assessment of renal trauma: A 10-year prospective study of patient selection. J Urol. 1989;141:1095.
33. Brown, SL, Elder, JS, Spirnak, JP. Are pediatric patients more susceptible to major renal injury from blunt trauma? A comparative study. J Urol. 1998;160:138.
34. Santucci, RA, Langenburg, SE, Zachareas, MJ. Traumatic hematuria in children can be evaluated as in adults. J Urol. 2004;171:822.
35. Morey, AF, Bruce, JE, McAninch, JW. Efficacy of radiographic imaging in pediatric blunt renal trauma. J Urol. 1996;156:2014.
36. Buckley, JC, McAninch, JW. Pediatric renal injuries: Management guidelines from a 25-year experience. J Urol. 2004;172:687.
37. Buckley, JC, McAninch, JW. The diagnosis, management, and outcomes of pediatric renal injuries. Urol Clin North Am. 2006;33:33.
38. Smith, JK, Kenney, PJ. Imaging of renal trauma. Radiol Clin North Am. 2003;41:1019.
39. Jones, AE, Mason, PE, Tayal, VS, Gibbs, MA. Sonographic intraperitoneal fluid in patients with pelvic fracture: Two cases of traumatic intraperitoneal bladder rupture. J Emerg Med. 2003;25:373.
40. Master, VA, McAninch, JW. Operative management of renal injuries: Parenchymal and vascular. Urol Clin North Am. 2006;33:21.
41. Siram, SM, et al. Ureteral trauma: Patterns and mechanisms of injury in an uncommon condition. Am J Surg. 2010;199:566.
42. Elliott, SP, McAninch, JW. Ureteral injuries from external violence: The 25-year experience at San Francisco General Hospital. J Urol. 2003;170:1213.
43. Perez-Brayfield, MR, et al. Gunshot wounds to the ureter: A 40-year experience at Grady Memorial Hospital. J Urol. 2001;166:119.
44. Elliott, SP, McAninch, JW. Ureteral injuries: External and iatrogenic. Urol Clin North Am. 2006;33:55.
45. Kunkle, DA, Kansas, BT, Pathak, A, Goldberg, AJ, Mydlo, JH. Delayed diagnosis of traumatic ureteral injuries. J Urol. 2006;176:2503.
46. Carver, BS, Bozeman, CB, Venable, DD. Ureteral injury due to penetrating trauma. South Med J. 2004;97:462.
47. Wessells, H, Long, L. Penile and genital injuries. Urol Clin North Am. 2006;33:117.
48. Koifman, L, Barros, R, Júnior, RA, Cavalcanti, AG, Favorito, LA. Penile fracture: Diagnosis, treatment and outcomes of 150 patients. Urology. 2010;76:1488.
49. Morey, AF, Metro, MJ, Carney, KJ, Miller, KS, McAninch, JW. Consensus on genitourinary trauma: External genitalia. BJU Int. 2004;94:507.
50. Buckley, JC, McAninch, JW. Diagnosis and management of testicular ruptures. Urol Clin North Am. 2006;33:111.
51. Lee, SH, et al. Trauma to male genital organs: A 10-year review of 156 patients, including 118 treated by surgery. BJU Int. 2008;101:211.
52. Bhatt, S, Kocakoc, E, Rubens, DJ, Seftel, AD, Dogra, VS. Sonographic evaluation of penile trauma. J Ultrasound Med. 2005;24:993.
53. Guichard, G, et al. Accuracy of ultrasonography in diagnosis of testicular rupture after blunt scrotal trauma. Urology. 2008;71:52.
54. Cline, KJ, Mata, JA, Venable, DD, Eastham, JA. Penetrating trauma to the male external genitalia. J Trauma. 1998;44:492.