CHAPTER 2 GENITOURINARY PATHOLOGY
BLADDER AND PROSTATE PATHOLOGY
INTRODUCTION
TESTICULAR AND PARATESTICULAR PATHOLOGY
TESTICULAR GERM CELL TUMORS
SEMINOMA
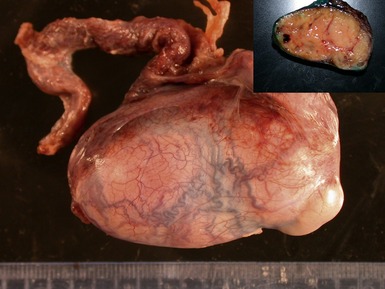
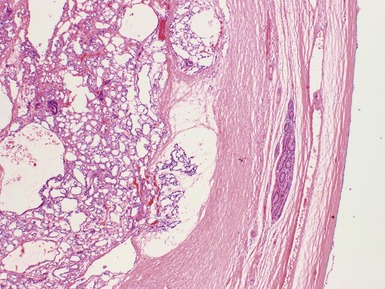
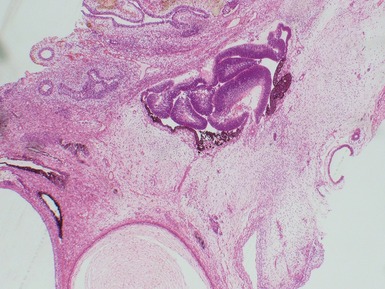
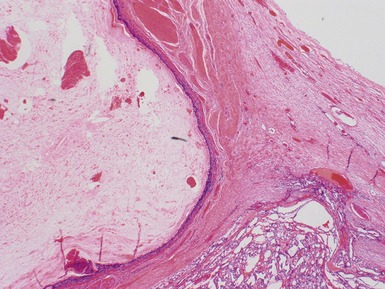
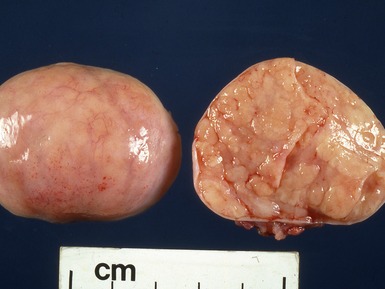
Fig 2.3 Photograph of a testis with a malignant yolk sac tumor. The capsule is stretched and the cut surface (inset) is solid with areas of hemorrhage.
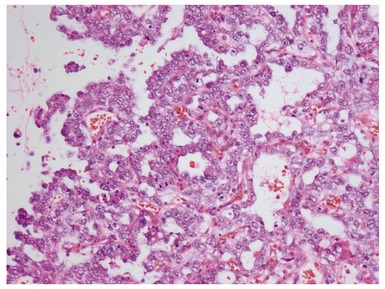
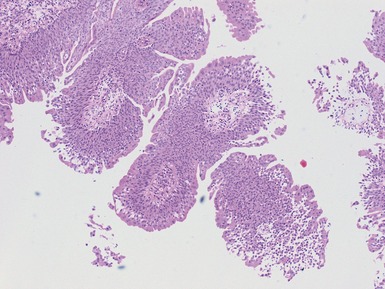
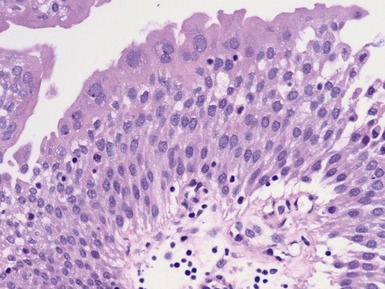
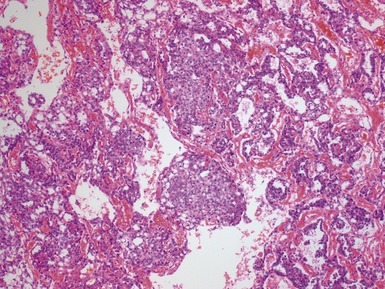
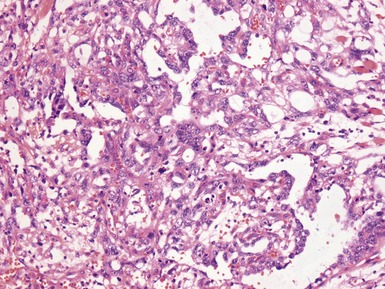
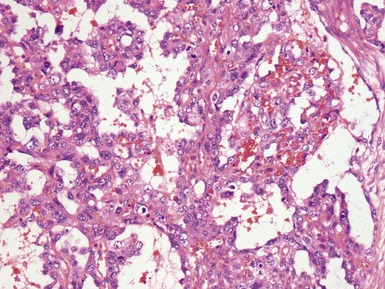
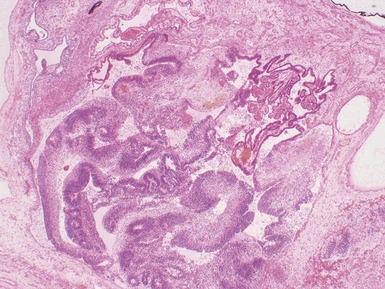
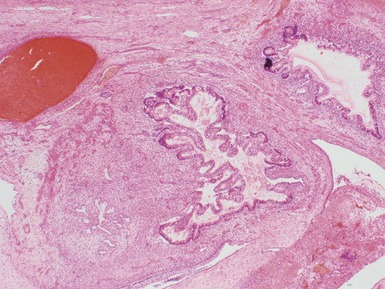
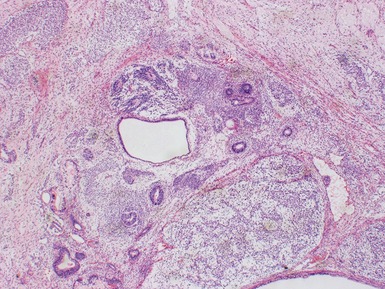
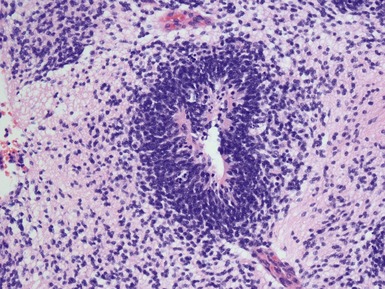
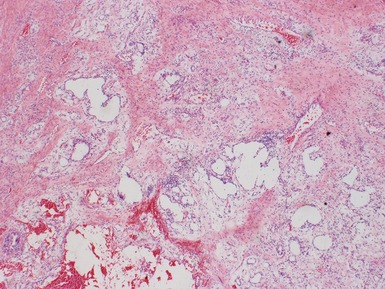
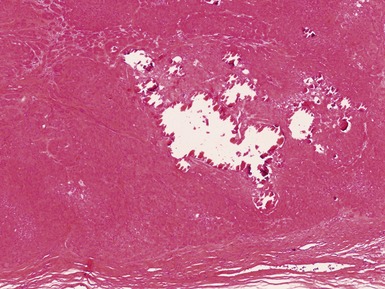
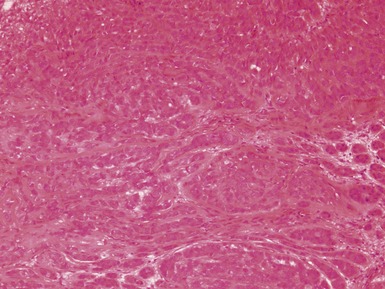
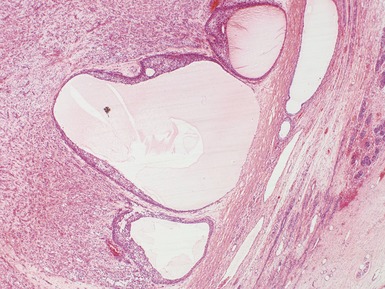
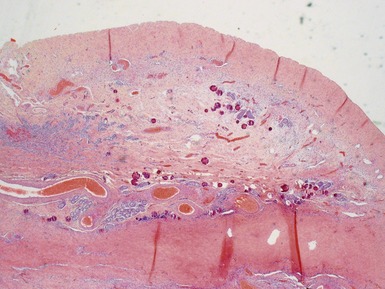
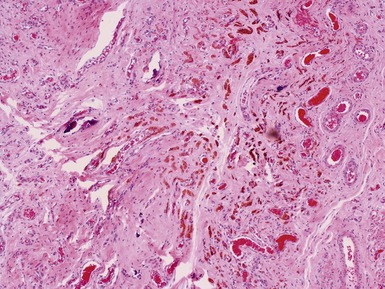
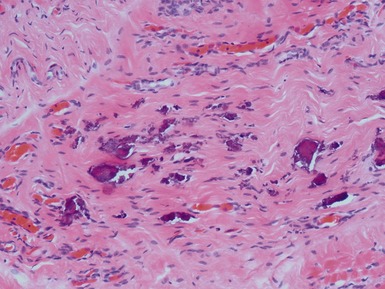
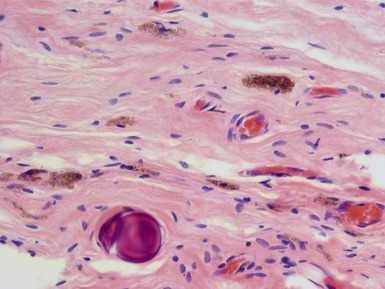
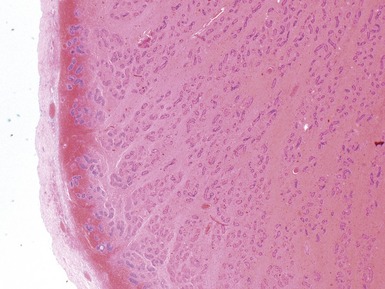
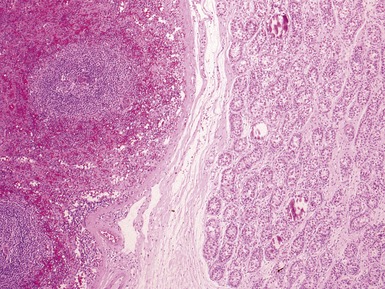
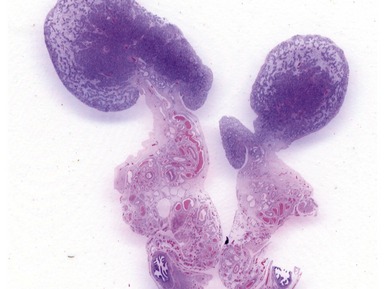
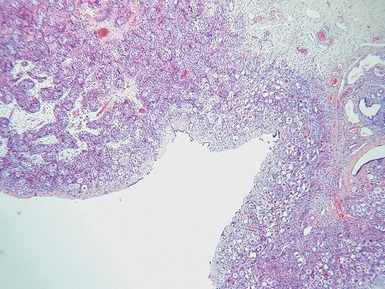
Figs 2.4–2.8 Photomicrographs of testicular malignant yolk sac tumors, demonstrating the range of morphological appearances including microcystic, reticular, solid and papillary patterns.
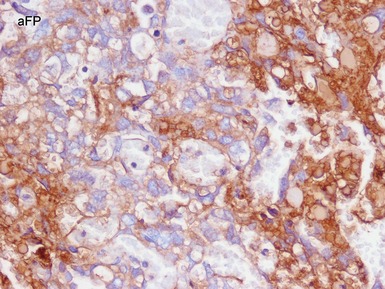
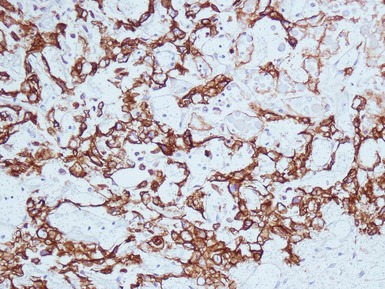
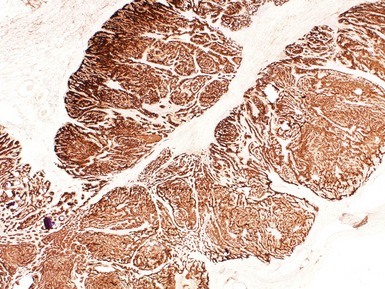
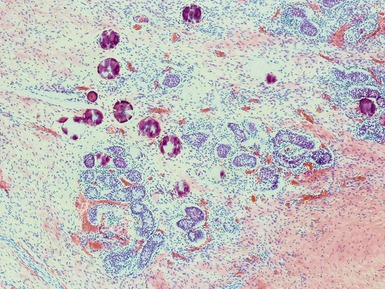
Figs 2.9–2.10 Photomicrographs of testicular malignant yolk sac tumors, demonstrating the tumor cells highlighted by cytokeratin (Fig 2.9) and alphafetoprotein (Fig 2.10) immunostaining.
TERATOMA
MIXED GERM CELL TUMOR
Differential diagnoses and pitfalls
TESTICULAR SEX-CORD STROMAL TUMORS
LEYDIG CELL TUMORS
Histopathological features
SERTOLI CELL TUMOR
GONADOBLASTOMA
SECONDARY TESTICULAR INVOLVEMENT
VANISHED TESTIS / TESTICULAR REGRESSION SYNDROME (TRS)
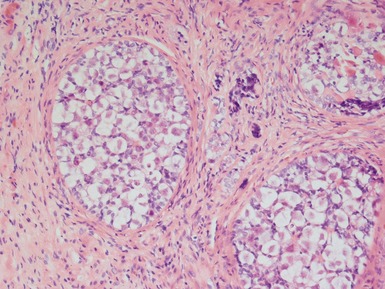
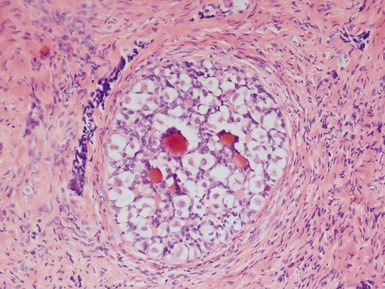
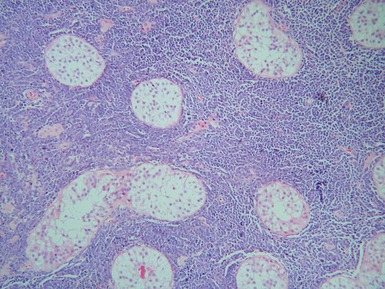
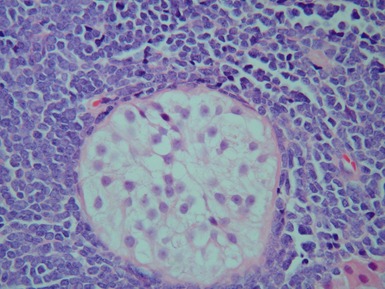
CRYPTORCHIDISM
Clinical features
 optimal timing of orchidopexy still uncertain, <1 year of age may be best to also preserve fertility (Leung & Robson 2004)
optimal timing of orchidopexy still uncertain, <1 year of age may be best to also preserve fertility (Leung & Robson 2004)Histopathological features
Immunohistochemical stains
 caution in using these markers in prepubertal children since normal immature germ cells also express CD117 and PLAP (Law et al 2006)
caution in using these markers in prepubertal children since normal immature germ cells also express CD117 and PLAP (Law et al 2006)TESTICULAR AND TESTICULAR APPENDAGE TORSION
Epidemiology
PARATESTICULAR / ADNEXAL TUMORS
Adenomatoid tumor
NONTUMOROUS PARATESTICULAR MASSES
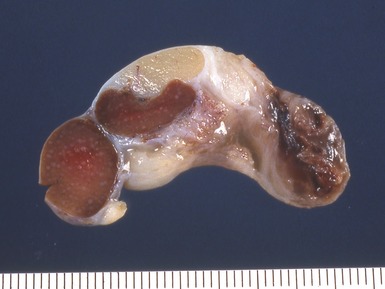
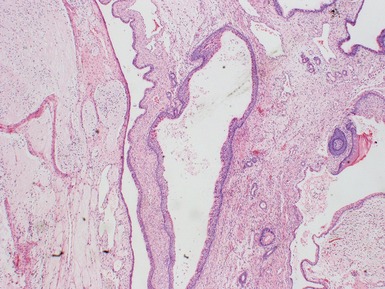
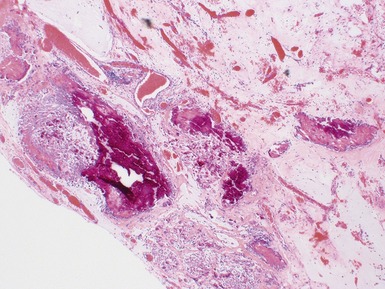
Fig 2.34 Photograph of a case of splenogonadal fusion, demonstrating juxtaposed but not admixed, splenic and gonadal elements.
CYSTIC DYSPLASIA OF THE RETE TESTIS (CDRT)
Clinical features
INTERSEX / DISORDERS OF SEXUAL DIFFERENTIATION (DSD)
 associated modification of risk for germ cell tumors in specific diagnostic groups (Cools et al 2006)
associated modification of risk for germ cell tumors in specific diagnostic groups (Cools et al 2006)46XY DSD
Other
Bouron-Dal Soglio D, Harvey I, Jovanovic M, Oligny LL, Fournet JC. Bilateral cystic dysplasia of the rete testis with renal adysplasia. Pediatr Dev Pathol. 2006;9:157-160.
Cools M, Drop SL, Wolffenbuttel KP, Oosterhuis JW, Looijenga LH. Germ cell tumors in the intersex gonad: old paths, new directions, moving frontiers. Endocr Rev. 2006;27:468-484.
Law H, Mushtaq I, Wingrove K, Malone M, Sebire NJ. Histopathological features of testicular regression syndrome: relation to patient age and implications for management. Fetal Pediatr Pathol. 2006;25:119-129.
Lee PA, Houk CP, Ahmed SF, Hughes IA. International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Pediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics.. 2006;118:e488-e500.
Leung AK, Robson WL. Current status of cryptorchidism. Adv Pediatr. 2004;51:351-377.
Murphy FL, Law H, Mushtaq I, Sebire NJ. Testicular and paratesticular pathology in infants and children: the histopathological experience of a tertiary pediatric unit over a 17-year period. Pediatr Surg Int. 2007;23:867-872.
Park E, Morrison S. Cystic dysplasia of the rete testis in an adolescent with VATER association. Pediatr Radiol. 2008;38:123.
Walsh TJ, Dall’Era MA, Croughan MS, Carroll PR, Turek PJ. Prepubertal orchiopexy for cryptorchidism may be associated with lower risk of testicular cancer. J Urol. 2007;178:1440-1446.