chapter 31 Genetic conditions
INTRODUCTION AND OVERVIEW
Until recently, the focus has been on genetic conditions, usually rare, that are directly due to a genetic variation. There are many thousands of these conditions, and they include changes to chromosome number and structure, pathogenic variants in single genes. For example, around 2300 comparatively rare conditions are now known to be primarily due to an inherited mutation in a single gene.1 As they follow a pattern of inheritance in families first defined by Mendel over 100 years ago, these conditions are often referred to as Mendelian. Still, the genes and mutations causing around 1600 or so Mendelian conditions remain to be identified.1 Also, more than one gene can be involved in the causation of some Mendelian conditions.
For all these genetic conditions, treatment is limited and symptomatic. Gene therapy—treatment to correct the underlying genetic change—is still in its infancy and research is continuing, to ensure its safety and efficacy.2 Currently the only preventive strategy is detection through genetic testing prenatally and termination of an affected pregnancy or pre-implantation. These strategies can only be used where pregnancies are identified as at-risk. This determination results from genetic counselling and genetic testing where appropriate, and may be available based on a positive family history or ethnicity.
An emerging area is the use of DNA genetic testing to guide prescribing and drug response (pharmacogenomics).3 This field epitomises the move to tailored medicine.
EPIGENETICS
Epigenetic mechanisms, and their effects in gene activation and inactivation, are increasingly being found to play a critical role in phenotype transmission and development. The study of epigenetics is therefore telling us much about the mechanisms whereby lifestyle, environmental and psychosocial factors can influence the activation and progression of chronic illnesses, including cancer,4 dementia5 and ageing.6 But, more optimistically, it also tells us much about the potential for lifestyle and environmental modification to alter disease progression.
LIFESTYLE FACTORS AND GENETICS
Our genes are programmed not just by the DNA but also by the ‘epigenome’, which is the chemicals and proteins laid down over the genes and which alter their expression. Epigenetic patterns are sculpted during fetal development, and are affected by maternal nutrition and exposure to environmental toxins as well as psychological stress.7 It is now also known that extensive lifestyle changes, such as those undertaken in the Ornish program, are associated with alterations of cancer gene expression8 and improved telomerase-based genetic repair.9 Thus, alterations in genetic function and repair probably explain a significant amount of the benefit of lifestyle-based interventions.
Some further examples and implications are explored below.
Mind–body and DNA modulation
Psychological stress increases DNA damage10 in animal studies11,12 and studies on humans. There is an increase in DNA damage in humans under stress—in students taking examinations, for example—and stress increases the number of genetic mutations and probably impairs the body’s ability to repair them13 (due to the effect of the mediators of the stress response14), increases oxidation and affects DNA repair enzymes.
DNA damage stimulates the body’s DNA repair mechanisms in order to compensate for the damage.15 A study of healthy medical students showed that during the exam period, compared with low-stress periods after vacations, there was an increase in DNA repair capacity (DRC) in nearly all the study participants.16 Students who had higher and more consistent levels of stress and mood disturbance during exam periods had a reduction in DRC, or no change when they needed it most, suggesting that the response had been impaired in some way due to emotional stress. The implications of DRC are important. A study comparing DRC in women with breast cancer and in cancer-free women found significant differences.17 Women with breast cancer have a lower DRC (5.6% DRC) than women without breast cancer (8.7% DRC). Younger breast cancer patients had a more significant reduction in DRC. A low DRC increased susceptibility to breast cancer, with a 1% decrease in DRC corresponding to a 22% increase in breast cancer risk.
Not only is DNA function, damage and repair affected by psychological states, but the mind also affects genetic ageing. Telomeres are segments of DNA at the ends of the DNA strands that help the DNA to not ‘unravel’, which would cause the cell to die. A study of healthy premenopausal women who were carers for someone with a major disability found that psychological stress, in terms of both perception and duration, was significantly associated with markers of cell death and longevity. The women who were coping least well with the demands showed higher oxidative stress, lower telomerase activity (the enzyme that repairs telomeres) and shorter telomere length. Women with the highest levels of perceived stress, compared with those with the lowest, had telomeres shorter on average by the equivalent of 9–17 years of additional ageing.18 A similar finding has been demonstrated for people with depression.19 Low telomerase activity is associated with high allostatic load and also with other risk factors for cardiovascular disease, such as smoking, lipids, high blood pressure, high fasting glucose, and metabolic syndrome.20
Much work is now going into investigating whether practices such as mindfulness training, which have been found to reduce stress and improve mental health, can also slow the ageing process, measured by markers such as telomere length and telomerase activity. Interestingly, the research is suggesting that ageing may be able to be slowed, or even reversed to an extent, through the use of these practices. ‘We review data linking telomere length to cognitive stress and stress arousal and present new data linking cognitive appraisal to telomere length. Given the pattern of associations revealed so far, we propose that some forms of meditation may have salutary effects on telomere length by reducing cognitive stress and stress arousal and increasing positive states of mind and hormonal factors that may promote telomere maintenance. Aspects of this model are currently being tested in ongoing trials of mindfulness meditation.’21
Psychological states also affect genetic expression in conditions as varied as addictive behaviours,22 cardiovascular disease,23 mental health problems such as depression24 and schizophrenia,25,26 and asthma,27 which goes some way to explaining why stress is such a common trigger for many diseases. For example, chronic pain is associated with depression, but it is the emotional reactivity to chronic pain that can sensitise the brain to pain messages, increase the likelihood of a genetic disposition to depression activating itself, and impair the ability of the brain to make new neurons (neurogenesis).28
Spirituality and religion may have important effects on lifestyle, including assisting in lowering rates of substance abuse in those genetically at risk.29 For example, this may be mediated by the reduced risk of expressing a genetic disposition to smoking and other addictive behaviours in those with a high level of self-rated religiosity.30
Exercise
Physical activity levels have been linked with genetic function, expression and repair.31 How exercise does this is coming to be understood, but it is not necessary to understand how it works in order to enjoy the benefits. Regular moderate exercise helps to reduce oxidative damage and is associated with improved DNA repair, which may be one of the reasons that exercise is associated with slower ageing and cell death.32 Extreme training, on the other hand, is associated with more oxidative damage,33 although it does stimulate DNA repair.34 These effects can be seen for a week after extreme exertion, such as after a marathon.35 Part of the protective effect of exercise may relate to it producing a similar effect to calorie restriction—that is, it helps to change the balance of calories and exceeding calories out.36 The anti-inflammatory effect of exercise may also be a factor. Exercise also has beneficial effects on mental health which, bearing in mind the preceding section on stress management and genetics, may be an important factor in protecting our genes. All in all, healthy genes are just another reason to exercise regularly.
Nutrition
Nutrition has an important role in the function, repair, expression and ageing of DNA.37–39 Various micronutrients, vitamins and minerals are required for DNA synthesis, prevention of oxidative damage and DNA maintenance. For example, low levels of folate are associated with increased risk for a baby with a neural tube defect, whereas increasing folate is associated with better DNA repair.40 Also, the minerals selenium, manganese and zinc minerals are cofactors for DNA repair enzymes.41 The Mediterranean diet, rich in olive oil and vegetables, is associated with a reduced incidence of cancer, which is postulated to be partly because of effects on DNA damage and repair.42
GENETIC COUNSELLING AND GENETICS SERVICES
Genetic counselling is provided as part of a comprehensive genetics service43,44 whose elements include clinical, laboratory and education. Genetic counselling provides information, supportive counselling regarding the diagnosis and risk for a genetic condition in the family, genetic testing where appropriate and management of rare conditions in some cases. The healthcare professional team providing genetic counselling may consist of clinical geneticists or other medical specialists, genetic counsellors and social workers. Internationally, genetic counselling is increasingly provided by genetic counsellors working as part of mainstream medicine.
Taking and verifying the family history is at the core of genetic counselling and the provision of appropriate genetic testing. The history is, optimally, collected by the general practitioner at the first visit and updated regularly as it can provide information for preventive strategies. The family history changes with time, and ideally the patient should maintain their own copy of their family health history, update it opportunistically and be able to answer the question: ‘Do you have a family history of XX condition?’. For example, the family health record can be downloaded from the NSW Health (New South Wales government) Centre for Genetics Education website.45
While it may not always be possible, the record of the family history will optimally be three-generational, including primary relatives (children, siblings, parents) and secondary relatives (aunts, uncles and grandparents) on both sides of the family, and noting in particular: ethnic background (ancestry and culture); adoption; age at diagnosis; age and cause of death; birth defects, stillbirths and miscarriages. It is not always possible to assume ethnicity from country of birth or surname. More information can be obtained by asking patients where their parents, grandparents or great-grandparents were born.46 There are no privacy implications in taking a family history.
GENETIC TESTING
Genetic testing for changes in chromosome number and structure traditionally used cytogenetic karyotyping, but increasingly will rely on molecular cytogenetic testing, which can reveal very small changes not identifiable under the light microscope.47 DNA genetic testing also is changing rapidly, with increased use of sequencing of parts, or the whole, of a patient’s genome.
Genetic screening is done for a particular condition in individuals, groups or populations without family history of the condition (e.g. genetic carrier screening for genetic conditions common in the Ashkenazi Jewish population48). Usually, a panel of mutations that are common to the population group will be tested for, so rarer mutations will not be detected.
Genetic testing is done for a particular condition where an individual is suspected of being at increased risk due to their family history or as the result of a genetic screening test. Direct DNA gene testing looks at the presence or absence of a known mutation in the gene. The mutation may be family-specific. The test is very accurate and is used for diagnosis and screening, including prenatal, genetic carrier testing, cascade testing, and presymptomatic and predictive testing (Table 31.1). Limitations of direct DNA genetic testing include the following:
| Type of test | Use |
|---|---|
| Genetic carrier testing | Testing an individual to determine whether they are a carrier of a faulty, or mutated, gene for a particular condition |
| Cascade testing | Testing of blood relatives when a mutation has been identified in a family member |
| Pre-implantation testing | Testing an at-risk embryo for a genetic condition, prior to implantation, thereby obviating the need to terminate an affected pregnancy |
| Prenatal testing | Testing to detect fetal abnormalities |
| Diagnostic testing | Specific testing in an individual who has symptoms suggestive of a genetic condition |
| Presymptomatic testing | Testing to determine, prior to any sign of the condition, whether an individual will, without appropriate intervention, develop the condition in the future |
| Predictive testing | Testing prior to any sign of the presence of the condition, to determine whether an individual is at risk for developing the condition in the future |
ORDERING GENETIC TESTS
Costs to the patient of genetic testing vary according to how pathology testing is funded in each country—that is, whether there is a national health system or private health insurance. With developments in technology, such as sequencing the patient’s whole DNA, rather than just looking at specific genes using direct gene testing, costs are expected to decrease in the next few years.49
Before obtaining consent, the patient should be informed about the purpose and personal/family implications of a genetic test. Patients who have had a predictive or presymptomatic genetic test have a duty to inform life insurers of the test result when applying for a new, or altering an existing, policy.50
The patient should be encouraged to share the information with their relatives, and supported in doing so.46 Changes to the Australian National Privacy Act in 2006 allow, in appropriate exceptional circumstances, for a healthcare professional working in the private sector to release information to genetic relatives without the consent of the person being tested.51 However, this should only be seen as the last resort after counselling patients to inform their relatives where there is a serious risk to their life, health or safety. When such action is considered, guidelines are available.52 A general practitioner has no duty to inform a patient’s relatives about a positive genetic test result.53
A number of ethical issues arise from genetic testing that are relevant not only for individuals and families but for society in general as well.54 These issues have been addressed internationally55–57 and include:
Increasingly, genetic tests are being marketed directly to consumers through the internet (direct to consumer (DTC) genetic testing). Laboratories conducting DTC genetic testing are largely based in the United States58,59 as regulation in countries such as Australia allows laboratories to conduct tests for health conditions only when referred from a medical practitioner.60 DNA samples are collected at home using kits provided (a cheek swab or saliva sample) and sent to the laboratory through the mail. Payment is made via the internet. Support may or may not be available for explanation of the results, again via the internet. Concern has been raised about the marketing and scientific credibility of these DTC genetic tests internationally.61,62 Further, for genetic tests that purport to provide evidence for lifestyle and dietary modification, caution should be exercised in interpreting the results and recommendations provided, on the basis that analysis of the person’s DNA is in a non-holistic context.63
CHROMOSOMAL CONDITIONS
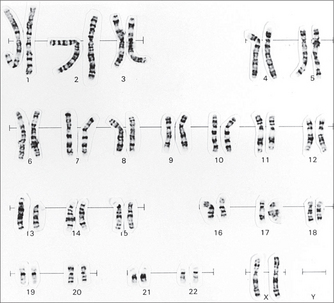
TRISOMY 21, DOWN SYNDROME64–66
Aetiology
History
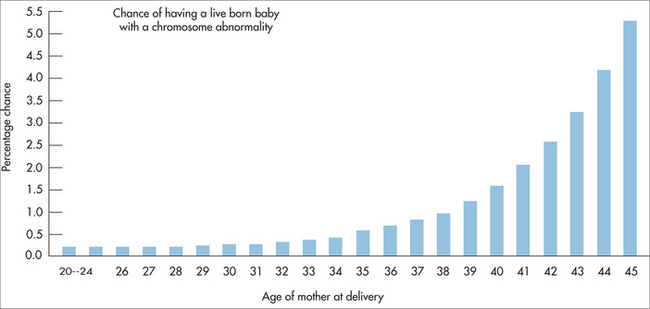
Associated with increased maternal age (Fig 31.2). A family history may indicate the translocation type of trisomy 21.
Examination
Management
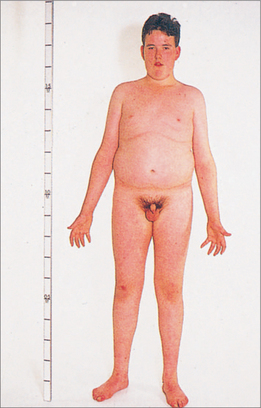
47,XXY KLINEFELTER SYNDROME69
Incidence: 1 in 500 total births (1 in 1000 male births).
Aetiology
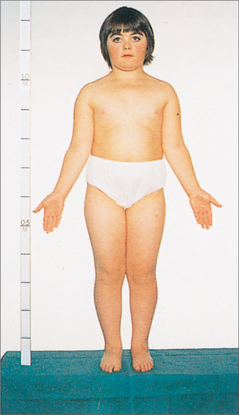
45,X TURNER SYNDROME70
Incidence: 1 in 2000 total births.
Aetiology
Examination
Poor growth or pubertal delay is highly characteristic. Other features include:
SINGLE GENE CONDITIONS
Of the 20,000 or so human genes identified, pathogenic variants in about 12,000 of these result in a genetic condition that follows a clear pattern of inheritance in families.1 Again, each one is individually rare but several are more commonly found in particular population groups—these include cystic fibrosis and haemoglobinopathies such as alpha and beta forms of thalassaemia and sickle cell anaemia.
CYSTIC FIBROSIS71–74
Aetiology
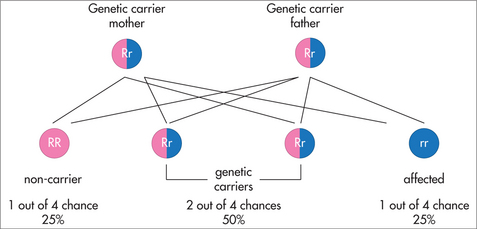
Cystic fibrosis (CF) is caused by mutations in the CFTR (cystic fibrosis transmembrane regulator) gene. It follows an autosomal recessive pattern of inheritance (Fig 31.6), so both parents must carry the mutated gene. There is often no family history of the condition, because the mutation has previously only been present in healthy carriers on each side of the family. CFTR regulates the transport of chloride and sodium ions in the epithelial surfaces of the airway, pancreatic and biliary ducts, gastrointestinal tract, sweat ducts and vas deferens. Pathogenic mutations either remove or reduce the function of the CFTR gene. There are more than 1500 known CFTR mutations, but not all are associated with classic CF (see below).75 A genetic carrier for CF—that is, a person carrying only one mutated copy of the CFTR gene—will still produce sufficient amounts of the salt-transport protein for normal body function.
Management
Prevention
Pre-conceptional cascade genetic carrier testing and screening in appropriate population groups to identify mutation carriers enables prenatal testing and reproductive choice. The most common mutation in the CFTR gene is known as p.Phe508del (traditionally known as ΔF508 or dF508), which accounts for approximately 70% of all CF gene mutations in those of northern European ancestry. Laboratories test for varying numbers of the common mutations. Testing for 12 or more mutations covers at least 80% of possible mutations in Australians of northern European ancestry.77 The rest of the mutations are extremely rare. Mutations that are common in populations other than those whose ancestry is northern European may not be included in the panel of mutations that are routinely tested for by laboratories.
Important pitfalls
In the absence of a family history of CF, a negative screen for CFTR gene mutations is reassuring, but cannot absolutely rule out the possibility of being a carrier, as not all mutations can be tested. Therefore if a couple choose screening for CF there is still a small risk of having a baby with CF if their carrier test is negative. The major selection criterion for lung transplantation is a life expectancy predicted to be 50% or less at 2 years. This can be indicated by increasing decline in respiratory function, quality of life and weight, and more frequent need for IV therapy.
HAEMOGLOBINOPATHIES (including alpha- and beta-thalassaemia and sickle cell disease)78–80
Aetiology
Haemoglobinopathies are caused by mutations in the genes of the ‘alpha-globin chain’ and/or the ‘beta-globin chain’ of haemoglobin. They follow an autosomal-recessive pattern of inheritance (Fig 31.6), so both parents must carry the mutated gene.
Examination
Investigations
Management
Prevention
Medical
Important pitfalls
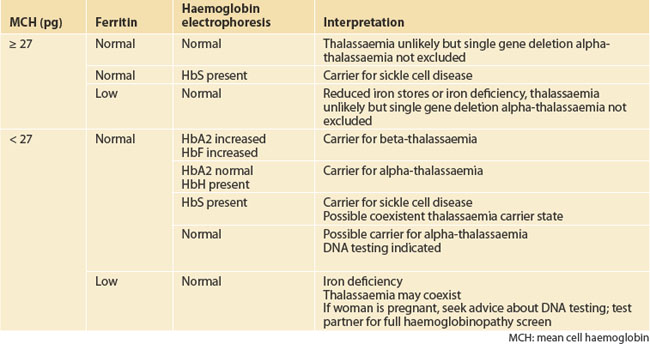
A haematologist or thalassaemia service should be consulted for assistance in interpreting haemoglobinopathy testing results, as interpretation is influenced by the clinical picture (Table 31.2). Prenatal diagnosis is available to couples where there is a risk of having a child affected by a haemoglobinopathy and the causative globin mutations carried by the parents are known. Because of the time-consuming nature of DNA testing to identify causative gene mutations, it is important that, wherever possible, DNA studies are carried out pre-pregnancy.
INHERITED GENETIC SUSCEPTIBILITY
Many of the more common adult-onset conditions seen in general practice may be indirectly due to an inherited mutation. These conditions, such as some forms of cancer, cardiac and haematological conditions, involve inherited predisposition and are usually associated with a positive family history. Where the family history is suggestive of an inherited predisposition, genetic counselling and, where appropriate and available, genetic testing may identify the causative predisposing inherited mutation. National guidelines have been produced for healthcare professionals to triage on the basis of the family history into those who are at potentially high risk for the condition running in the family due to an inherited predisposition. For example, those whose family history suggests they are at potentially high risk for breast, ovarian or colorectal cancer could be referred to a family cancer service.43 If appropriate, genetic testing may then be used to identify the family-specific mutation and enable predictive genetic testing for other blood relatives.
BREAST AND OVARIAN CANCER INHERITED SUSCEPTIBILITY81
Incidence: About 5% of all breast and ovarian cancers.
Aetiology
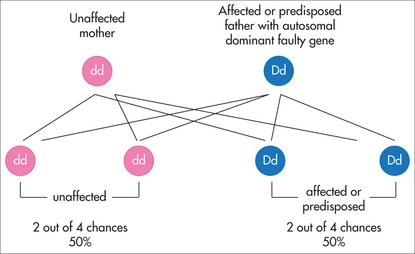
A mutation in either the BRCA1 or the BRCA2 gene is identified in a blood relative who has or had breast and/or ovarian cancer and who was identified at potentially high risk on the basis of her family history. Inheritance of the mutated gene follows a pattern of autosomal-dominant inheritance (Fig 31.7). The BRCA1 or BRCA2 genes are tumour suppressor genes that normally function in both breast and ovarian tissues as ‘cancer protection’ genes in the cell, contributing to the maintenance of controlled cell growth. The woman’s breast and/or ovarian cancer is due to her having inherited a mutation in one copy of her BRCA1 or BRCA2 gene in her cells. For breast and/or ovarian cancer to develop, other mutations must build up over time in other ‘cancer protection’ genes in the cells of the breast and/or ovary. The mutation is family-specific, and now predictive genetic testing for the mutation can be offered to unaffected blood relatives.
History
The BRCA1 or BRCA2 mutations can be inherited from either the mother or the father. The inheritance pattern is described as autosomal-dominant (Fig 31.7). A child of a parent who carries the mutated gene has a 1 in 2, or 50%, chance of inheriting the mutation and being predisposed to develop breast and/or ovarian cancer.
Management
Prevention
Early detection using screening strategies. For breast cancer, regular breast examinations, mammography (with or without breast ultrasound) and breast magnetic resonance imaging (MRI) may be beneficial for women at high risk of breast cancer, as they can help to detect breast cancer at an early stage. Prophylactic mastectomy is an option for some women.82 Ovarian cancer surveillance is not recommended for women at high or potentially high risk. Evidence shows that ultrasound or CA125, singly or in combination, is not effective at detecting early ovarian cancer. The most effective risk-reducing strategy for ovarian cancer is bilateral salpingo-oophorectomy.83,84
Complementary therapies
There are currently no recommendations for the prevention of breast or ovarian cancer.
LYNCH SYNDROME INHERITED SUSCEPTIBILITY85,86 (formerly hereditary non-polyposis colorectal cancer, HNPCC)
Incidence: Between 1% and 4% of all colorectal cancers.
Aetiology
In Lynch syndrome, a small number of polyps develop in the bowel and can develop into bowel cancer. Cancers in both men and women can also occur in the kidney, ureter and parts of the gut such as the small bowel, stomach or pancreas. Women also have an increased risk of cancer of the inner lining of the uterus and the ovaries, although the risk of bowel cancer is higher. Age of onset is very variable (from 20s to 80s). The condition is due to an inherited mutation in one of the copies of one of their mismatch repair (MMR) genes. Inheritance of the mutated gene follows a pattern of autosomal-dominant inheritance (Fig 31.3). The MMR genes normally function as ‘cancer protection’ genes in the cell, contributing to the maintenance of controlled cell growth by repairing any changes in the DNA that occur as the cells are copied. Genetic testing to identify the mutated MMR gene involved is undertaken in a family member who has or had colorectal cancer and who is identified as potentially high risk on the basis of their family history. For the colorectal cancer to have developed, other mutations must build up over time in other ‘cancer protection’ genes in the cells of the colon. The mutation is family-specific, and now predictive genetic testing for the mutation can be offered to unaffected blood relatives.
Management
Prevention
Lifestyle
Association of Genetic Support of Australasia Inc. http://www.agsa-geneticsupport.org.au.
Australasian Genetic Alliance. http://www.australasiangeneticalliance.org.au.
Cancer Council Australia. http://www.cancer.org.au.
Centre for Genetics Education, NSW Health (Australia). http://www.genetics.edu.au.
Cystic Fibrosis Australia Inc. http://www.cysticfibrosisaustralia.org.au.
Down Syndrome NSW, Australia. http://www.dsansw.org.au.
House of Lords Science and Technology Committee, Subcommittee Report, Genomic Medicine (2009). http://www.publications.parliament.uk/pa/ld200809/ldselect/ldsctech/107/10702.htm.
March of Dimes, Genetics and your practice. http://www.marchofdimes.com/gyponline/index.bm2.
National Human Genome Research Institute, Frequently asked questions about genetic testing. http://www.genome.gov/19516567.
NHMRC, eGenetics. http://www.nhmrc.gov.au/your_health/egenetics/.
Thalassaemia Society of New South Wales. http://www.thalnsw.org.au.
Turner Syndrome Association of Australia Ltd. http://www.turnersyndrome.org.au.
1 John Hopkins University. Online Mendelian inheritance in man. Online. Available: http://www.ncbi.nlm.nih.gov/sites/entrez?db=OMIM&itool=toolbar.
2 Barlow-Stewart K. Gene therapy. Barlow-Stewart K, editor. The Australasian genetics resource book. Royal North Shore Hospital. Sydney: Centre for Genetics Education; 2007. Online. Available: http://www.genetics.edu.au/factsheet/fs27.html, Feb 2008.
3 Stanford University, Pharmacogenomics knowledge base. Online. Available: http://www.pharmgkb.org/index.jsp.
4 Nise MS, Falaturi P, Erren TC. Epigenetics: origins and implications for cancer epidemiology. Med Hypotheses. 2010;74(2):377-382.
5 Johnson W, Deary IJ, McGue M, et al. Genetic and environmental transactions linking cognitive ability, physical fitness, and education in late life. Psychol Aging. 2009;24(1):48-62.
6 Ostojić S, Pereza N, Kapović M. A current genetic and epigenetic view on human aging mechanisms. Coll Anthropol. 2009;33(2):687-699.
7 Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reprod Toxicol. 2007;24(1):9-19.
8 Ornish D, Magbanua MJ, Weidner G, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci USA. 2008;105(24):8369-8374.
9 Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057. Erratum in: Lancet Oncol. 2008;9(12):1124.
10 Gidron Y, Russ K, Tissarchondou H, et al. The relation between psychological factors and DNA damage: a critical review. Biol Psychol. 2006;72(3):291-304.
11 Adachi S, Kawamura K, Takemoto K. Oxidative damage of nuclear DNA in liver of rats exposed to psychological stress. Cancer Research. 1993;53(18):4153-4155.
12 Fischman H, Pero R, Kelly D. Psychogenic stress induces chromosomal and DNA damage. Int J Neurosci. 1996;84(1–4):219-227.
13 Kiecolt-Glaser J, Glaser R. Psychoneuroimmunology and immunotoxicology: implications for carcinogenesis. Psychosom Med. 1999;61(3):271-272.
14 Flint MS, Baum A, Chambers WH, et al. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology. 2007;32(5):470-479.
15 Oldham KM, Wise SR, Chen L, et al. A longitudinal evaluation of oxidative stress in trauma patients. J Parenteral Nutr. 2002;26(3):189-197.
16 Cohen L, Marshall G, Cheng L, et al. DNA repair capacity in healthy medical students during and after exam stress. J Behav Med. 2000;23(6):531-545.
17 Ramos JM, Ruiz A, Colen R, et al. DNA repair and breast carcinoma susceptibility in women. Cancer. 2004;100(7):1352-1357.
18 Epel ES, Blackburn EH, Lin J, et al. Proc Natl Acad Sci USA. 2004;101(49):17312-17315.
19 Simon NM, Smoller JW, McNamara KL, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60(5):432-435.
20 Epel ES, Lin J, Wilhelm FH, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277-287.
21 Epel E, Daubenmier J, Moskowitz JT, et al. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann NY Acad Sci. 2009;1172:34-53.
22 Self D, Nestler E. Relapse to drug seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51(1–2):49-60.
23 Cui Y, Gutstein W, Jabr S, et al. Control of human vascular smooth muscle cell proliferation by sera derived from ‘experimentally stressed’ individuals. Oncol Rep. 1998;5(6):1471-1474.
24 Lopez J, Chalmers D, Little K, et al. Regulation of serotonin 1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43(8):547-573.
25 Benes F. The role of stress and dopamine-GABA interactions in the vulnerability for schizophrenia. J Psychiatr Res. 1997;31(2):257-275.
26 Kato T. Epigenomics in psychiatry. Neuropsychobiology. 2009;60(1):2-4.
27 Mrazek DA, Klinnert M, Mrazek PJ, et al. Prediction of early-onset asthma in genetically at-risk children. Pediatr Pulmonol. 1999;27(2):85-94.
28 Duric V, McCarson KE. Persistent pain produces stress-like alterations in hippocampal neurogenesis and gene expression. J Pain. 2006;7(8):544-555.
29 Kendler KS, Liu XQ, Gardner CO, et al. Dimensions of religiosity and their relationship to lifetime psychiatric and substance use disorders. Am J Psychiatry. 2003;160(3):496-503.
30 Timberlake DS, Rhee SH, Haberstick BC, et al. The moderating effects of religiosity on the genetic and environmental determinants of smoking initiation. Nicotine Tob Res. 2006;8(1):123-133.
31 Teran-Garcia M, Rankinen T, Bouchard C. Genes, exercise, growth, and the sedentary, obese child. J Appl Physiol. 2008;105(3):988-1001.
32 Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6(1):71-75.
33 Pittaluga M, Parisi P, Sabatini S, et al. Cellular and biochemical parameters of exercise-induced oxidative stress: relationship with training levels. Free Radic Res. 2006;40(6):607-614.
34 Radák Z, Apor P, Pucsok J, et al. Marathon running alters the DNA base excision repair in human skeletal muscle. Life Sci. 2003;72(14):1627-1633.
35 Tsai K, Hsu TG, Hsu KM, et al. Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise. Free Radic Biol Med. 2001;31(11):1465-1472.
36 Kritchevsky D. Caloric restriction and experimental carcinogenesis. Hybrid Hybridomics. 2002;21(2):147-151.
37 Tyson J, Mathers JC. Dietary and genetic modulation of DNA repair in healthy human adults. Proc Nutr Soc. 2007;66(1):42-51.
38 Mathers JC, Coxhead JM, Tyson J. Nutrition and DNA repair—potential molecular mechanisms of action. Curr Cancer Drug Targets. 2007;7(5):425-431.
39 Mathers JC. Nutritional modulation of ageing: genomic and epigenetic approaches. Mech Ageing Dev. 2006;127(6):584-589.
40 Wei Q, Shen H, Wang LE, et al. Association between low dietary folate intake and suboptimal cellular DNA repair capacity. Cancer Epidemiol Biomarkers Prev. 2003;12(10):963-969.
41 Sheng Y, Pero RW, Olsson AR, et al. DNA repair enhancement by a combined supplement of carotenoids, nicotinamide, and zinc. Cancer Detect Prev. 1998;22:284-292.
42 Machowetz A, Poulsen HE, Gruendel S, et al. Effect of olive oils on biomarkers of oxidative DNA stress in Northern and Southern Europeans. FASEB J. 2007;21(1):45-52.
43 Centre for Genetics Education. Find a genetics service. Online. Available: http://www.genetics.edu.au/services/index.html, 2008. Feb 2008.
44 Human Genetics Society of Australasia. Certification in genetic counselling. Online. Available: http://www.hgsa.com.au/index.cfm?pid=111440, May 2010.
45 Centre for Genetics Education, NSW Health. Family history. Online. Available: www.genetics.com.au/publications/family_history.asp, 2007. May 2010.
46 Barlow-Stewart K, Emery J, Metcalfe S. Genetics in practice (Ch 17). Genetics in family medicine: the Australian handbook for general practitioners. Biotechnology Australia, Commonwealth Department of Industry, Tourism and Resources; 2007. Online. Available: http://www.nhmrc.gov.au/your_health/egenetics/practitioners/gems.htm, May 2010.
47 Saam J, Gudgeon J, Aston E, et al. How physicians use array comparative genomic hybridisation results to guide patient management in children with developmental delay. Genet Med. 2008;10(3):181-186.
48 Pacific Laboratory Medicine Services. Fact sheets on genetic conditions in the Ashkenazi Jewish community. Online. Available: http://www.palms.com.au/Education/Genetics/genlist.shtml, 2007. May 2010.
49 Tucker T, Marra M, Friedman JM. Massively parallel sequencing: the next big thing in genetic medicine. Am J Hum Gen. 2009;85(2):142-154.
50 Centre for Genetics Education, NSW Health. Life insurance products and genetic testing in Australia. Online. Available: http://www.genetics.com.au/factsheet/fs23a.asp, 2007. May 2010.
51 Privacy Legislation Amendment Act 2006. Online. Available: http://www.comlaw.gov.au/ComLaw/Legislation/Act1.nsf/all/search/592A1E3B62096FD6CA2571ED0012E038, May 2010.
52 National Health & Medical Research Council. Guidelines approved under Section 95AA of the Privacy Act 1988 (Cth). Online. Available: http://www.nhmrc.gov.au/publications/synopses/e96syn.htm, 2009. May 2010.
53 Otlowski MFA. Disclosure of genetic information to at-risk relatives: recent amendments to the Privacy Act 1988 (Cth). Med J Aust. 2007;187(7):398-399.
54 Barlow-Stewart K, Christadoulou J. It’s all in our genes. In: Oates K, Currow K, Hu W, et al, editors. Child health: a manual for general practice. Sydney: MacLennan & Petty, 2001.
55 Australian Law Reform Commission. Essentially yours: the protection of human genetic information in Australia. Online. Available: http://www.austlii.edu.au/au/other/alrc/publications/reports/96/index.htm, 2003. May 2010.
56 National Institute for Health and Clinical Excellence. NHS evidence: genetic conditions. Ethical, legal and social implications (ELSI) of genetic information. Online. Available: http://www.library.nhs.uk/GENETICCONDITIONS/ViewResource.aspx?resID=59911, May 2010.
57 US Department of Energy Office of Science, Office of Biological and Environmental Research, Human Genome Program. Online. Available: www.ornl.gov/sci/techresources/Human_Genome/elsi/elsi.shtml, May 2010.
58 23andMe. Choose the DNA test that’s right for you. Online. Available: http://www.23andme.com/, May 2010.
59 Genetics and Public Policy Center, Washington DC, USA. Direct-to-consumer genetic testing companies. Online. Available: http://www.dnapolicy.org/resources/DTCcompanieslist.pdf, May 2010.
60 National Pathology Accreditation Advisory Council (NPAAC). Laboratory accreditation standards and guidelines for nucleic acid detection and analysis; 2006. Online. Available: http://www.health.gov.au/internet/main/publishing.nsf/Content/npaac-nucleic-acid-toc~npaac-nucleic-acid-diag~npaac-nucleic-acid-gen.
61 National Health & Medical Research Council. Direct to consumer (DTC) DNA tests. Online. Available: http://www.nhmrc.gov.au/your_health/issues/genetics/testing/dtc.htm, May 2010.
62 Hunter D, Khoury M, Drazen J. Letting the genome out of the bottle—will we get our wish? N Engl J Med. 2008;358(2):105-107.
63 Saukko P. Pitching products, pitching ethics: selling nutrigenetic tests as lifestyle or medicine. In: Castle D, Ries N, editors. Nutrition and genomics: issues of ethics, law, regulation and communication. Sydney: Elsevier, 2009.
64 Gardner RJM, Sutherland G. Chromosome abnormalities and genetic counselling. 3rd edn. New York: Oxford University Press, 2004.
65 Morris JK, Mutton DE, Alberman E. Recurrences of free trisomy 21: analysis of data from the national Down syndrome cytogenetic register. Prenat Diagn. 2005;25:1120-1128.
66 Australian Institute of Health and Welfare. Australia’s babies, their health and wellbeing. Bulletin Issue 21. Online. Available: http://www.aihw.gov.au/publications/aus/bulletin21, 2004. Jan 2008
67 Morris JK, Mutton DE, Alberman E. Revised estimates of maternal age specific live birth prevalence of Down syndrome. J Med Screen. 2002;9:2-6.
68 Barlow-Stewart K. Trisomy 21—Down syndrome. Barlow-Stewart K, editor. The Australasian genetics resource book. Sydney: Centre for Genetics Education, Royal North Shore Hospital; 2007. Online. Available: http://www.genetics.edu.au/factsheet/fs28.html, Feb 2008.
69 Barlow-Stewart K, Emery J, Metcalfe S. Chromosomal conditions (Ch 7). Genetics in family medicine: the Australian handbook for general practitioners. Biotechnology Australia, Commonwealth Department of Industry, Tourism and Resources; 2007. Online. Available: http://www.nhmrc.gov.au/your_health/egenetics/practitioners/gems.htm, May 2010.
70 Neilsen J, Naeraa R. Turner’s syndrome and Turner contact groups: an orientation. 3rd edn, rev. Denmark. Online. Available: http://www.aaa.dk/TURNER/ENGELSK/TURN_ORI.HTM, 1991. May 2010
71 Barlow-Stewart K, Emery J, Metcalfe S. Cystic fibrosis (Ch 9). Genetics in family medicine: the Australian handbook for general practitioners. Biotechnology Australia, Commonwealth Department of Industry, Tourism and Resources; 2007. Online. Available: http://www.nhmrc.gov.au/your_health/egenetics/practitioners/gems.htm, May 2010.
72 Cystic Fibrosis Mutation Database. Online. Available: http://www.genet.sickkids.on.ca/cftr/, May 2010.
73 Massie J, Clements B. Diagnosis of cystic fibrosis after newborn screening: the Australasian experience—twenty years and five million babies later: a consensus statement from the Australasian Pediatric Respiratory Group. Pediatr Pulmonol. 2005;39:440-446.
74 Wilcken B, Wiley V. Newborn screening. Pathology. 2008;40:104-115.
75 Human Genetics Society of Australasia. Cystic fibrosis population screening position paper. Online. Available: http://www.hgsa.com.au/index.cfm?pid=111468, 2009. May 2010.
76 Barlow-Stewart K. Autosomal recessive inheritance—traditional patterns of inheritance (Ch 1). Barlow-Stewart K, editor. The Australasian genetics resource book. Sydney: Centre for Genetics Education, Royal North Shore Hospital; 2007. Online. Available: http://www.genetics.edu.au/factsheet/fs8.html, Feb 2008.
77 Massie Rj, Delayticki MB, Bankier A. Screening couples for cystic fibrosis status: why are we waiting? Med J Aust. 2005;183:501-502.
78 Barlow-Stewart K, Emery J, Metcalfe S. Haemoglobinopathies (Ch 12). Genetics in family medicine: the Australian handbook for general practitioners. Biotechnology Australia, Commonwealth Department of Industry, Tourism and Resources; 2007. Online. Available: http://www.nhmrc.gov.au/your_health/egenetics/practitioners/gems.htm, May 2010.
79 Bowden DK. Screening for thalassaemia. Australian Prescriber. 2001;24:120-123.
80 Trent R, Webster B, Bowden D, et al. Complex phenotypes in the haemoglobinopathies: recommendations on screening and DNA testing. Pathology. 2006;38:507-519.
81 National Breast and Ovarian Cancer Centre. Advice about familial aspects of breast cancer and epithelial ovarian cancer. Online. Available: http://www.nbocc.org.au/view-document-details/bog-advice-about-familial-aspects-of-breast-cancer-and-ovarian-cancer, 2006. May 2010
82 Centre for Genetics Education, NSW Health. Information for women considering preventive mastectomy because of a strong family history of breast cancer. Online. Available: http://www.genetics.com.au/publications/cancer.asp.
83 National Breast and Ovarian Cancer Centre Position Statement. Surveillance of women at high or potentially high risk of ovarian cancer. Online. Available: http://www.nbocc.org.au/our-organisation/position-statements/surveillance-of-women-at-high-or-potentially-high-risk-of-ovarian-cancer, 2009. May 2010.
84 Centre for Genetics Education, NSW Health. Risk management options for women at increased risk of developing ovarian cancer. Online. Available: http://www.genetics.com.au/publications/cancer.asp, May 2010.
85 Cancer Institute NSW. Hereditary non-polyposis colon cancer (HNPCC). Online. Available: http://www.cancerinstitute.org.au/cancer_inst/programs/hnpcc.html, May 2010.
86 Australian Cancer Network and NHMRC. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer. Online. Available: http://www.cancer.org.au/Healthprofessionals/clinicalguidelines/cancergenetics.htm, 2006. May 2010.