CHAPTER 9 Genetic Applications
An Overview
Basics
Definition
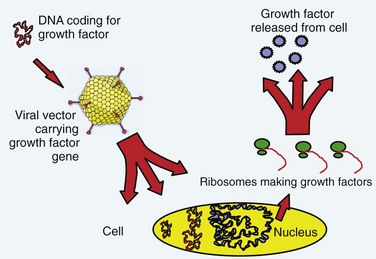
The term gene therapy was previously used to describe replacement of a defective gene with a functional copy by means of gene transfer. The diseases originally targeted for gene therapy were classic, heritable genetic disorders. The term now broadly defines therapy involving the transfer of exogenous genes (complementary DNA [cDNA]) encoding therapeutic proteins into cells to treat disease.1 The genetically altered cells are made into protein-producing “factories” churning out disease-altering gene products. Specifically, the host cell transcribes the exogenous gene (or transgene) into messenger RNA (mRNA); cytoplasmic ribosomes translate mRNA into the protein product. These products can affect not only the metabolism of cells from which they were made but also the metabolism of adjacent non–genetically altered cells via paracrine mechanisms (Fig. 9–1).
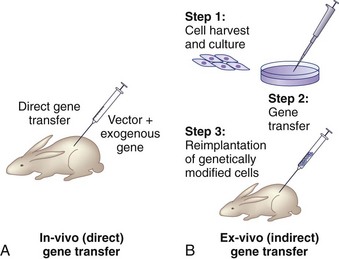
There are two basic strategies for delivering exogenous genes to target cells. The first is the in vivo method, in which a gene-carrying vector is directly transferred to an intended population of cells within the host. The second approach, known as ex vivo gene therapy, involves removing target cells from the body, genetically altering them in vitro, and reimplanting them in the body (Fig. 9–2). Ex vivo methods are more complex and involve multiple, time-intensive steps. This approach is relatively safer, however, because the genetically altered cells may be observed for abnormal behavior before implantation. The ex vivo strategy allows the opportunity for in vitro selection of cells that express the gene of interest at high levels. Gene transfer via the in vivo method is technically simpler. There are relative advantages and disadvantages to both approaches that depend on the anatomy and physiology of the target organs, the pathophysiology of the disease being treated, the vector of choice, and safety considerations.2
Nonviral Vectors
Nonviral vectors include liposomes, DNA-ligand complexes, gene guns, and microbubble-enhanced ultrasound. Liposomes are phospholipid vesicles that deliver genetic material into a cell by fusing with the cell membrane. Liposome vectors are simple, inexpensive, and safe, but drawbacks include transient expression of the transgene, cytotoxicity at higher concentrations, and low efficiency of transfection. DNA-ligand complexes and gene gun are nonpathogenic and relatively inexpensive to construct, but there is concern with lower transfer efficiencies and limited persistence of gene expression. Nishida and colleagues3 showed that ultrasound transfection with microbubbles significantly enhanced the transfection efficiency of plasmid DNA into the nucleus pulposus cells of rats in vivo, observing transgene expression up to 24 weeks. The overall transfection efficiency and level of gene expression of these nonviral vectors are generally inferior, however, to that of viral-mediated gene transfer. Consequently, most current studies involving gene therapy employ viral vectors.4
Viral Vectors
Retroviruses are small RNA viruses that replicate their genomic RNA into double-stranded DNA (dsDNA) via the action of reverse transcriptase. The dsDNA is integrated into the host genome at a random location where it is able to express transgene for the life of the cell. Exogenous dsDNA is replicated by the transduced cell and passed on to all progeny cells during cell division. Gene delivery with retroviral vectors results in stable, long-term expression because the gene is integrated into the cell’s genome. Because the integration is at a random site, however, the risk of potential mutagenesis of oncogenes exists. Until more recently, this risk was considered only a theoretical possibility, but preliminary reports from a gene therapy trial involving retroviral vectors suggest one of the enrolled subjects developed leukemia as a result of oncogene mutation.5 Another disadvantage of the most commonly used retroviral vector, the murine leukemia virus (MLV), is that it infects and transduces only actively dividing cells. For these reasons, MLV is most suited for ex vivo applications. Lentiviruses, another class of retroviruses, are capable of infecting nondividing cells. Their drawbacks lie in their wild-type pathogenicity and complex genomic configuration, which make molecular processing for gene transfer much more intricate.
Spinal Application: Fusion
Clinical Problem
Disorders of the spine often necessitate intervertebral fusion. Although internal fixation devices can successfully achieve temporary stabilization at practically all levels, long-term stability requires osseous consolidation. In contrast to fracture healing, spinal arthrodesis involves deposition of new bone in intersegmental locations that are not biologically structured for bone formation. Consequently, the nonunion rate is 40%6,7 with single-level fusions and higher when multiple levels are attempted. Although instrumentation has improved the rate of bony union, pseudarthrosis remains a considerable clinical problem. Autogenous bone graft may be scarce in volume in cases such as pediatric fusions and revision surgery. It is associated with substantial donor site morbidity. Owing to these significant obstacles to clinical success, extensive research has been directed at developing molecular therapies to facilitate intersegmental fusion.
Augmentation of Spinal Fusion with Bone Morphogenetic Proteins
Bone morphogenetic proteins (BMPs) are a group of osteoinductive cytokines that play an essential role in the formation and maturation of osseous tissues. Urist and colleagues8,9 originally recognized these proteins for their ability to form ectopic bone by inducing mesenchymal stem cell differentiation into chondrocytes and osteoblasts. Numerous subsequent animal studies have shown the ability of BMPs to enhance bone deposition at fusion sites.10–19 This was followed by several clinical trials that validated these preclinical findings. In a study by Patel and colleagues,20 patients undergoing posterolateral lumbar fusion augmented with iliac crest autograft and recombinant human BMP-7 (rhBMP-7) had better outcomes as measured by the Oswestry score and radiographic analysis than patients who received iliac crest autograft alone. In another human trial using rhBMP-2 in interbody fusion cages for single-level lumbar degenerative disc disease, patients who received cages filled with collagen sponge–delivered rhBMP-2 had superior clinical and radiographic results compared with control patients who received cages filled with autogenous bone graft.21 Various other clinical trials have similarly shown the efficacy of BMPs to enhance spinal arthrodesis.22,23
Gene Therapy for Spinal Fusion
Gene therapy represents a potential next step in the evolution of therapies directed at promoting a spinal fusion. Gene therapy techniques to deliver various BMP genes could overcome the barriers of high dosing and complex carrier systems and achieve long-term, controllable BMP expression. The transduced cells would secrete the BMP extracellularly, delivering it to the environment at physiologically appropriate doses for a sustained period, maximizing the osteoinductive potential of these growth factors. In addition, BMP expression could be regulated in a temporal fashion by using vectors with inducible promoters, which would allow the ability to control the activity of the protein tightly to the clinical setting. Another potential advantage is the capacity to deliver gene therapy for spine arthrodesis in a minimally invasive procedure with percutaneous injections to the spine. Lieberman and colleagues24 found an increase in the total volume of new bone with improved histologic quality when the gene for BMP-2 was delivered compared with rhBMP-2 protein alone.
Several studies have shown the feasibility of using gene therapy to enhance spinal fusion. Alden and colleagues25 showed new enchondral bone formation in paraspinal muscles injected with adenoviral BMP-2 constructs (Ad-BMP-2). Important observations made by this study included the absence of bone deposition distant from the injection site and the absence of neural compromise, suggesting that this approach may be safe for the clinical setting. In addition, Helm and colleagues26 documented that the direct injection of Ad-BMP-9 resulted in fusion in a rodent model without the development of nerve root compression or systemic side effects. In a rabbit model, Riew and associates27 showed that mesenchymal cells transduced with BMP-2 can promote spinal arthrodesis.
Many of these studies used first-generation adenoviral vectors for gene delivery to immunocompromised animals, allowing for sustained expression. Gene therapy experiments with immunocompetent animals have led to a relative paucity of bone formation,27 however, owing to the immune response elicited by adenoviral vectors. Further studies using second-generation adenoviral vectors or other vectors such as lentivirus could minimize these responses and maximize gene expression.
Another molecular avenue for bypassing the limitation of adenoviral immunogenicity is to deliver a gene for a factor that is “upstream” from the actions of BMP cytokines. In this way, neither efficient transduction nor sustained duration would be necessary because this factor would start a cascade of BMP activity after only a short period of expression. This intriguing strategy has been developed by Boden and colleagues,28,29 who showed that a novel intracellular transcription factor, LIM mineralization protein-1 (LMP-1), could be used to upregulate the expression of BMPs and their receptors. LMP-1 initiates a cascade of events intracellularly, which stimulates the secretion of osteoinductive factors, which increases BMP activity. All of the study animals that were implanted with peripheral blood buffy coat cells genetically modified with Ad-LMP-1 showed successful lumbar fusion.28 None of the 10 controlled rabbits had evidence of any bone formation, and the investigators concluded that local gene therapy could reliably induce spinal fusion in an immunocompetent animal.
Spinal Application: Intervertebral Disc Degeneration
Clinical Problem
Degenerative disc disease is a chronic process that can clinically manifest in multiple disorders, such as idiopathic low back pain, disc herniation, radiculopathy, myelopathy, and spinal stenosis. It is a significant source of patient pain and morbidity, using a large portion of health care resources.30 Available treatment options include conservative measures such as bed rest, anti-inflammatory agents, analgesic medications, and physical therapy. When conservative measures fail, invasive surgical procedures such as discectomy, instrumentation, or fusion, with their inherent risks and expenses, are often required. Conservative and surgical treatment modalities focus on the clinical symptoms of intervertebral disc degeneration without addressing the underlying pathologic processes occurring throughout the course of degeneration.
Although the precise pathophysiology of degenerative disc disease remains to be delineated, it is known to involve a complex interaction of biologic, genetic, and biomechanical factors. The progressive decline in aggrecan, the primary proteoglycan of the nucleus pulposus, is known to be a significant and characteristic factor.31–33 At the biochemical level, aggrecan homeostasis is altered by various combinations of decreased synthesis and increased breakdown. With reductions in proteoglycan content, the nucleus pulposus dehydrates, decreasing disc height and its load-bearing capacity.34–36 This situation may directly affect biomechanical function by altering the loads experienced by the facet joints, leading to degenerative changes. Although disc degeneration most probably evolves in response to a complex interplay of multiple biochemical and biomechanical factors,37 the ability to restore proteoglycan content may have therapeutic benefit by increasing disc hydration and potentially improving biomechanics.
Growth Factors
The ability to increase proteoglycan synthesis in the intervertebral disc was shown by Thompson and colleagues,38 who showed that the exogenous application of human transforming growth factor (TGF)-β1 to canine disc tissue in culture stimulated in vitro proteoglycan synthesis. The authors suggested that growth factors may be useful for the treatment of disc degeneration. Subsequent studies with other growth factors, such as insulinlike growth factor (IGF)-I, BMP-2, and osteogenic protein (OP)-1 (OP-1) also exhibited the ability to upregulate proteoglycan content in intervertebral disc cells.39,40 Owing to the relatively brief half-life of these factors, however, practical application of growth factor therapy to chronic conditions such as degenerative disc disease would necessitate repeated administrations. Consequently, efforts were directed at developing approaches to induce endogenous synthesis of growth factors via gene therapy such that genetically modified disc cells manufacture the desired growth factors on a continuous basis, enabling long-term regulation of matrix synthesis with the potential to prevent or delay degenerative disc disease.
Previous Studies of Intradiscal Gene Therapy
The notion of using gene transfer for intervertebral disc applications was initially introduced by Wehling and colleagues.41 In an in vitro study, these investigators reported on a retroviral mediated transfer of two different genes to cultured chondrocytic cells from bovine intervertebral endplates: (1) the bacterial β-galactosidase marker gene (LacZ) and (2) the cDNA of the human interleukin-1 receptor antagonist (IL-1Ra). Transfer of LacZ resulted in transduction of approximately 1% of the cell population. Transfer of the IL-1Ra cDNA resulted in significant levels of IL-1Ra protein by 48 hours. The authors concluded that this ex vivo approach, involving harvesting of endplate tissue from a degenerating disc, transducing these cultured cells with therapeutic genes, and reimplanting the genetically modified cells into the disc, could provide a novel strategy for treating degenerative diseases of the spine.
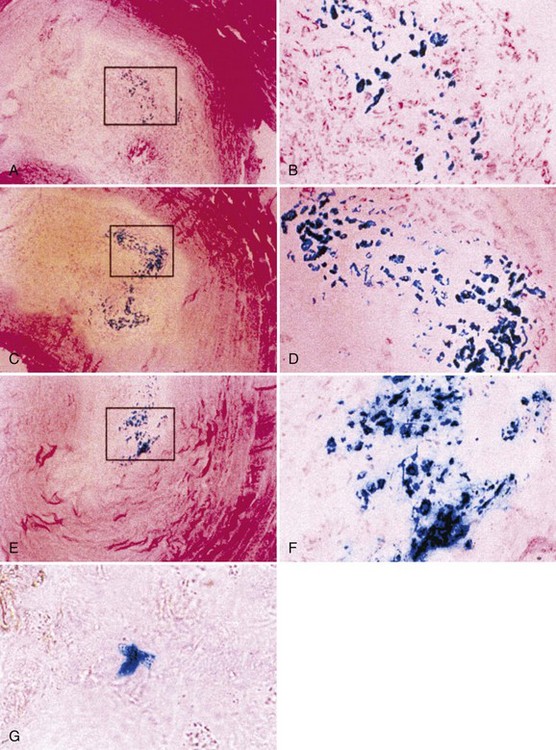
Nishida and colleagues42 reported the first successful in vivo gene transfer to the intervertebral disc using an adenoviral vector to deliver the LacZ marker gene to the rabbit lumbar disc. The authors were able to show sustained transgene production with no significant reduction in expression 3 months after transduction (Fig. 9–3). The rabbits used in these studies showed no signs of systemic illness in response to the adenoviral vector and its transgene synthesis. In addition, no histologic changes suggesting a cellular immune response were observed.
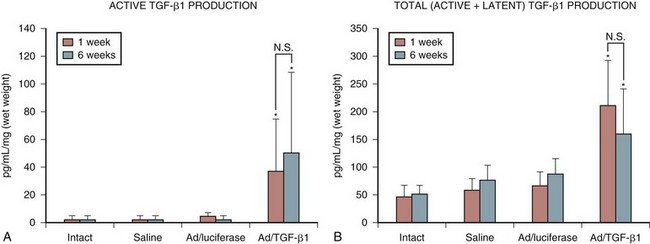
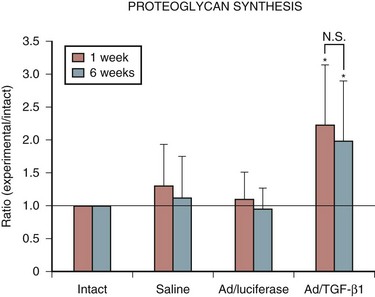
Encouraged by these results with a marker gene, the successful in vivo transduction of the intervertebral disc with a therapeutic gene, human TGF-β1, was soon accomplished.43 This study showed a 30-fold increase in active TGF-β1 synthesis and a 5-fold increase in total TGF-β1 production in discs injected with the adenoviral–growth factor construct (Fig. 9–4). Biologic modulation was also documented by a 100% increase in proteoglycan synthesis (Fig. 9–5). As in the previous studies, no signs of local or systemic immune response were noted.
Additional in vitro studies with cultured human nucleus pulposus cells yielded similar promising results. Successful transduction of the LacZ marker gene delivered with adenoviral vectors was achieved in human cells from degenerated discs.44 The response of human cells from degenerated discs to adenoviral-mediated delivery of TGF-β1 was subsequently assessed.45 Increased production of TGF-β1, proteoglycan, and collagen was shown in cells receiving gene therapy compared with controls. Cells receiving the adenovirus–TGF-β1 construct showed increased proteoglycan and collagen synthesis compared with cells receiving exogenous TGF-β1 protein, presumably in response to the sustained expression of this growth factor with gene transfer.
The viral load required to increase proteoglycan synthesis was significantly less than the load necessary for transduction of the entire cell population, perhaps highlighting the ability of a transduced cell to influence the biologic activity of non–genetically altered neighboring cells. The concept that successfully transduced cells exert a paracrinelike effect on their nontransduced neighboring cells implies that significant alteration in protein synthesis can be achieved with only a few transduced cells.2 A better understanding of this paracrine effect may enable the use of decreased viral loads to achieve a therapeutic effect, minimizing potential viral toxicity.
Additional in vitro studies with other promising growth factors such as BMP-2 and IGF-I documented the potential of adenoviral delivery of these factors to increase proteoglycan synthesis in a viral dose–dependent manner.46,47 Adenoviral delivery of tissue inhibitor of metalloproteinase (TIMP)-1 showed the same ability.47 TIMP-1 is an endogenous inhibitor of matrix metalloproteinases, enzymes capable of degrading the extracellular matrix of the intervertebral disc. This finding established a second gene therapy strategy to modify the disrupted balance of synthesis and catabolism occurring in the degenerated intervertebral disc: inhibition of matrix degradation with ensuing net increase in proteoglycan content.
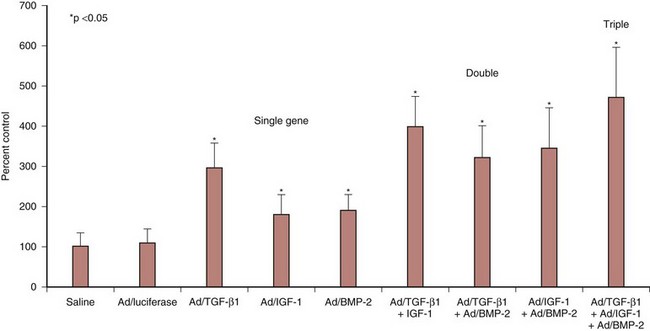
Considering the potential adverse effects of viral vectors, studies have been undertaken to develop strategies to minimize viral loads while maintaining the same biologic effects. Experiments with combination gene therapy involving TGF-β1, IGF-I, and BMP-2 suggested that these growth factors are synergistic in amplifying matrix synthesis.46 Adenoviral delivery of a single growth factor increased proteoglycan synthesis by a range of 180% to 295%, whereas combination gene therapy with two agents resulted in increases of 322% to 398%. When all three growth factors were combined, proteoglycan synthesis was increased by 471% (Fig. 9–6). It remains to be determined if combination gene therapy with an anabolic growth factor and a catabolic inhibitor such as TIMP-1 would have a similar synergistic effect.
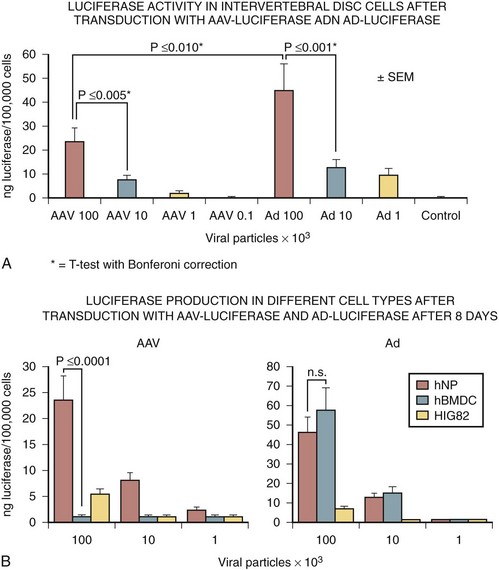
Lattermann and colleagues48 investigated the transduction efficiency of the AAV vector on nucleus pulposus cells compared with an adenoviral vector in vitro and in vivo. This study showed that the transduction efficacy of the AAV vector on human nucleus pulposus cells in vitro was high, but 48% lower than adenovirus (Fig. 9–7). Next, in vivo gene expression in rabbits after transduction with an AAV vector carrying the luciferase marker gene was achieved at all time points up to 6 weeks (Fig. 9–8). Similar to the in vitro results, the maximum transgene expression using the AAV-luciferase was approximately 50% of the maximum obtained after transduction with the adenoviral vector. Although transgene expression in vivo was decreased compared with levels achieved after adenoviral vector transduction, the overall amount of luciferase was high, likely exceeding any potential therapeutic dose. The authors concluded these experiments showed the feasibility of the AAV vector, as these levels of gene expression may be sufficient for the sustained delivery of a growth factor gene to the nucleus pulposus.
Other studies have focused on the role of LIM mineralization protein (LMP)-1 and Sox9 in the intervertebral disc. LMP-1 is an intracellular regulatory protein that upregulates expression and enhances anabolic activity of the BMP family of proteins. Yoon and colleagues49 showed an increase in total proteoglycan and aggrecan synthesis in vitro and in vivo after transduction of rat intervertebral disc cells with an adenoviral vector construct containing LMP-1. They found significant increases in expression of BMP-2 and BMP-7 mRNA after in vitro transduction with LMP-1. Sox9 is a transcription factor responsible for chondrogenesis and type II collagen expression. Paul and colleagues50 treated human intervertebral disc cells with an adenoviral vector construct containing Sox9 and found an increase in type II collagen production compared with controls. These studies make LMP-1 and Sox9 attractive candidates for further investigation as effective tools for intervertebral disc degeneration gene therapy.
Gene Expression Time Frame
Adenoviral vectors have been frequently used in gene therapy studies for the intervertebral disc owing to their ability to transduce efficiently highly differentiated, nondividing cells such as the cells of the nucleus pulposus. Successful gene therapy for the degenerated disc depends not only on efficient gene transfer but also on the expression of transgene for sufficiently long periods. The duration of gene expression after adenoviral transfer to an immunocompetent animal is limited in most organs and tissues by immune reactions to viral proteins and to foreign proteins encoded by the transgene. Specifically, expression for longer than 12 weeks has been difficult to achieve in most musculoskeletal tissues after adenoviral delivery, owing to brisk immune responses. Gene expression can occur for longer periods, however, in an “immune privileged” site. In studies by Nishida and colleagues,42,43 intradiscal marker gene expression was achieved in the rabbit lumbar disc for 1 year after transduction by adenovirus. Because the intervertebral disc is well encapsulated and avascular, it seems to act as an “immune privileged” organ, permitting longer durations of transgene expression compared with other musculoskeletal tissues.
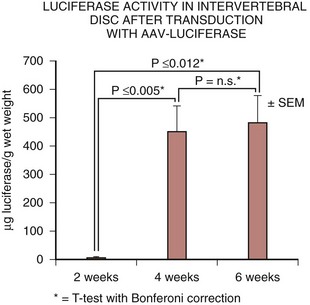
Compared with the adenoviral vector, the AAV vector has a relatively slower initial gene expression after transduction, likely attributable to an increased latency period. This delay in gene expression was also observed in the previously described in vivo experiments involving the intervertebral disc.48 At 2 weeks, transgene expression was present but low compared with the adenovirus. At 4 weeks, transgene expression increased 60-fold, however, and that level was maintained at 6 weeks. This characteristic delay in gene expression could potentially preclude the use of AAV vectors in acute processes such as fracture healing, but there is no theoretical disadvantage when treating more chronic diseases such as degenerative disc disease.
Future Directions
The aforementioned studies documented the feasibility of in vivo gene transfer to the intact intervertebral disc. There are reasonable concerns, however, regarding the feasibility of efficient gene transfer to a degenerated disc in which the cell population and extracellular environment are distorted. More recent studies have identified possible genetic causes for disc degeneration.47–49 Gene transfer to replace the defective gene with a normal copy may have great therapeutic benefit in susceptible populations. Although the intervertebral disc seems to be an “immune privileged” organ, the issue of viral vector immunogenicity is still crucial in light of a reported death of a patient undergoing a clinical trial with the adenovirus. Consequently, other vectors, such as the AAV vector, have been rigorously investigated because of their decreased side-effect profile.
To address the issue of transgene product toxicity, regulatory control systems may be necessary to control transgene expression, including the ability to turn on or off expression in the event of an errant injection or leakage of the injection contents into surrounding tissues.51 The ability to turn the transgene on or off based on the application of an externally applied stimulus (i.e., oral pharmaceutical) is an advantageous safety feature and a method to modify treatment by titrating transgene expression. Numerous inducible gene expression systems have been investigated, including systems using heat shock proteins, metallothionein, steroid regulatory promoters, tetracycline, and, most recently, the EcR insect receptor. Regulation systems with tissue-specific promoters would further optimize efficacy and minimize treatment side effects.
Another strategy for gene modulation that has emerged is RNA interference (RNAi). RNAi is mediated by small interfering RNA (siRNA) molecules that bind in a sequence-specific manner to target mRNAs, marking them for degradation and preventing translation of the target gene. The efficacy of RNAi mediated by siRNAs has been reported in nucleus pulposus cells in vitro using an exogenous reporter gene and an endogenous gene.52 To overcome the short half-life of the siRNA, Nishida and colleagues53 used DNA vector–based RNAi and showed a stable downregulation of specific gene expression. RNAi has potential use as a novel strategy of gene therapy for treatment of degenerative disc disease by silencing expression of catabolic genes.
1 Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg Am. 1995;77:1103-1114.
2 Thompson JP, Oegema TRJr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253-260.
3 Wehling P, Schulitz KP, Robbins PD, et al. Transfer of genes to chondrocytic cells of the lumbar spine: Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine. 1997;22:1092-1097.
4 Boden SD, Titus L, Hair G, et al. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1). Spine. 1998;23:2486-2492.
5 Nishida K, Kang JD, Suh JK, et al. Adenovirus-mediated gene transfer to nucleus pulposus cells: Implications for the treatment of intervertebral disc degeneration. Spine. 1998;23:2437-2442. discussion 2443
1 Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35-47.
2 Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg Am. 1995;77:1103-1114.
3 Nishida K, Doita M, Takada T, et al. Sustained transgene expression in intervertebral disc cells in vivo mediated by microbubble-enhanced ultrasound gene therapy. Spine. 2006;31:1415-1419.
4 Sobajima S, Kim JS, Gilbertson LG, et al. Gene therapy for degenerative disc disease. Gene Ther. 2004;11:390-401.
5 Hacein-Bey-Abina S, von Kalle C, Schmidt M, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255-256.
6 DePalma AF, Rothman RH. The nature of pseudarthrosis. Clin Orthop Relat Res. 1968;59:113-118.
7 Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992;284:80-90.
8 Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893-899.
9 Urist MR, Sato K, Brownell AG, et al. Human bone morphogenetic protein (hBMP). Proc Soc Exp Biol Med. 1983;173:194-199.
10 Boden SD, Martin GJJr, Morone MA, et al. Posterolateral lumbar intertransverse process spine arthrodesis with recombinant human bone morphogenetic protein 2/hydroxyapatite-tricalcium phosphate after laminectomy in the nonhuman primate. Spine. 1999;24:1179-1185.
11 Cheung KMC, Leong JCY, Liu SL. Augmentation of intertransverse spinal fusion in primates using OP-1. Annual Meeting of the International Society for the Study of the Lumbar Spine, Kona, HI, 1999.
12 Cook SD, Dalton JE, Tan EH, et al. In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implants as a bone graft substitute for spinal fusions. Spine. 1994;19:1655-1663.
13 Frenkel SR, Moskovich R, Spivak J, et al. Demineralized bone matrix: Enhancement of spinal fusion. Spine. 1993;18:1634-1639.
14 Holliger EH, Trawick RH, Boden SD, et al. Morphology of the lumbar intertransverse process fusion mass in the rabbit model: A comparison between two bone graft materials—rhBMP-2 and autograft. J Spinal Disord. 1996;9:125-128.
15 Lovell TP, Dawson EG, Nilsson OS, et al. Augmentation of spinal fusion with bone morphogenetic protein in dogs. Clin Orthop Relat Res. 1989;243:266-274.
16 Magin M: BMP-7 (rhOP-1) is able to enhance lumbar vertebral interbody fusion: Results of a sheep trial and clinical impact. Annual Meeting of the International Society for the Study of the Lumbar Spine, Kona, HI, 1999.
17 Oikarinen J. Experimental spinal fusion with decalcified bone matrix and deep-frozen allogeneic bone in rabbits. Clin Orthop Relat Res. 1982;162:210-218.
18 Schimandle JH, Boden SD, Hutton WC. Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine. 1995;20:1326-1337.
19 Sheehan JP, Kallmes DF, Sheehan JM, et al. Molecular methods of enhancing lumbar spine fusion. Neurosurgery. 1996;39:548-554.
20 Patel TC, Vaccaro AR, Truumees E: A safety and efficacy study of OP-1 (rhBMP-7) as an adjunct to posterolateral lumbar fusion. Annual Meeting of the North American Spine Society, New Orleans, 2000.
21 Boden SD, et al. The use of rhBMP-2 in interbody fusion cages: Definitive evidence of osteoinduction in humans: A preliminary report. Spine. 2000;25:376-381.
22 Burkus KJ, Transfeldt EE, Kitchel SH: A prospective and randomized study assessing the clinical and radiographic outcomes of patients treated with RhBMP-2 and threaded cortical bone dowels in the lumbar spine. Annual Meeting of the North American Spine Society, New Orleans, 2000.
23 Kleeman TJ, Talbot-Kleeman A: Laparoscopic ALIF: rhBMP-2 with titanium cages vs autograft with bone dowels. Annual Meeting of the North American Spine Society, New Orleans, 2000.
24 Lieberman JR, Le LQ, Wu L, et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998;16:330-339.
25 Alden TD, Pittman DD, Beres EJ, et al. Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J Neurosurg. 1999;90(1 Suppl):109-114.
26 Helm GA, Alden TD, Beres EJ, et al. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J Neurosurg. 2000;92(2 Suppl):191-196.
27 Riew KD, Wright NM, Cheng S, et al. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif Tissue Int. 1998;63:357-360.
28 Boden SD, Titus L, Hair G, et al. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1). Spine. 1998;23:2486-2492.
29 Viggeswarapu M, Boden SD, Liu Y, et al. Adenoviral delivery of LIM mineralization protein-1 induces new-bone formation in vitro and in vivo. J Bone Joint Surg Am. 2001;83:364-376.
30 Conrad DA. Cost of low back pain problems: An economic analysis. In: Weinstein JN, Gordon SL, editors. Low Back Pain: A Clinical and Scientific Overview. Rosemont, IL: American Association of Orthopedic Surgeons, 1996.
31 Adler JH, Schoenbaum M, Silberberg R. Early onset of disk degeneration and spondylosis in sand rats (Psammomys obesus). Vet Pathol. 1983;20:13-22.
32 Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307-1314.
33 Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987;5:198-205.
34 Butler D, Trafimow JH, Andersson GB, et al. Discs degenerate before facets. Spine. 1990;15:111-113.
35 Urban JP, McMullin JF. Swelling pressure of the intervertebral disc: Influence of proteoglycan and collagen contents. Biorheology. 1985;22:145-157.
36 Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: Influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179-187.
37 Garfin SR. The intervertebral disc disease—does it exist? In: Weinstein WJN, editor. The Lumbar Spine. Philadelphia: WB Saunders; 1990:369-380.
38 Thompson JP, Oegema TRJr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253-260.
39 Osada R, Ohshima H, Ishihara H, et al. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690-699.
40 Takegami K, Thonar EJ, An HS, et al. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27:1318-1325.
41 Wehling P, Schulitz KP, Robbins PD, et al. Transfer of genes to chondrocytic cells of the lumbar spine: Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine. 1997;22:1092-1097.
42 Nishida K, Kang JD, Suh JK, et al. Adenovirus-mediated gene transfer to nucleus pulposus cells: Implications for the treatment of intervertebral disc degeneration. Spine. 1998;23:2437-2442. discussion 2443
43 Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: An in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419-2425.
44 Moon SH, Gilbertson LG, Nishida K, et al. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer: Implications for the clinical management of intervertebral disc disorders. Spine. 2000;25:2573-2579.
45 Moon SH: Proteoglycan synthesis in human intervertebral disc cells cultured in alginate beads; exogenous TGF-beta1 vs. adenovirus-mediated gene transfer of TGF beta1 cDNA. Orthopaedic Research Society, Orlando, FL, 2000.
46 Moon SH, Nishida K, Gilbertson LG, et al. Biologic response of human intervertebral disc cells to gene therapy cocktail. Spine. 2008;33:1850-1855.
47 Wallach CJ, Sobajima S, Watanabe Y, et al. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine. 2003;28:2331-2337.
48 Lattermann C, Oxner WM, Xiao X, et al. The adeno associated viral vector as a strategy for intradiscal gene transfer in immune competent and pre-exposed rabbits. Spine. 2005;30:497-504.
49 Yoon ST, Park JS, Kim KS, et al. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29:2603-2611.
50 Paul R, Haydon RC, Cheng H, et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003;28:755-763.
51 Vadala G, Sowa GA, Smith L, et al. Regulation of transgene expression using an inducible system for improved safety of intervertebral disc gene therapy. Spine. 2007;32:1381-1387.
52 Nishida K, Kakutani K, Doita M. Down regulation of Fas ligand expression in nucleus pulposus cells in vitro mediated by RNA interference: successful gene silencing of endogenous gene. International Society of the Study of the Lumbar Spine. New York. 2005.
53 Nishida K, Kakutani K, Doita M. DNA vector based RNA interference in nucleus pulposus cells in vitro. New York: International Society of the Study of the Lumbar Spine; 2005.