General Reaction Patterns
1. All morphologic changes represent a dose-dependent effect in a “time-space window.” That is, first, below a lower-dose threshold of functional alterations, no morphologic lesions occur despite the patient’s apparent illness, and, second, there is a time delay between the occurrence of a functional disturbance and the development of morphologic changes (called morphogenesis). Space refers to the fact that morphologic lesions are most extensive at the site of “toxic impact” and become less severe (and possibly less typical) with increasing distance. This should be kept in mind when taking biopsies for pathologic evaluation.
2. Whatever the quality of injury, the living organism reacts with a limited number of patterns. There are variations to these patterns, which may provide us with clues to the etiology of the injury, but no entirely new reactions can be expected, even when a new pathologic agent (such as human immunodeficiency virus) arises.
General Reaction Patterns
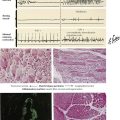
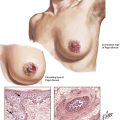
The following figures provide examples of the 5 reaction patterns in different tissues and organs.
TABLE 1-1
BASIC TYPES OF B-CELL AND T-CELL IMMUNOREACTIONS*
| Gell and Coombs Type | Alias | Mechanism |
| B-cell reactions | ||
| Type I IR | Allergic IR Atopic IR Anaphylactic IR |
Cytophilic antibodies (e.g., IgE) bind to mast cells; antigen binding to these cell-bound antibodies causes mast cell degranulation with release of vasoactive mediators (e.g., histamine), which initiate the microvascular inflammatory response (thrombocytes and eosinophils cooperate). |
| Type II IR | Toxic or cytotoxic IR | Complement-binding antibodies (on antigen binding) activate complement cascade, members of which initiate inflammatory response by activating cell chemotaxis and phagocytosis, ultimately causing toxic cell and tissue damage. |
| Type III IR | Immunocomplex IR | Persistence of antigen-antibody complexes are recognized by the immune system as foreign and induce the production of secondary anticomplex antibodies (i.e., anti-antibodies, such as rheumatoid factor); these bind and activate complement and cause tissue lesion through complement components (see above). |
| T-cell reactions | ||
| Type IV IR | Cell-mediated IR T-cell cytotoxic IR CTL response |
a. Direct destruction of target antigenic cells by binding of CTL, Fas-related induction of apoptosis, and/or release of perforin and granzymes b. T-cell cytokine response activation of macrophages: granulomatous (e.g., IFN-γ, TNF) reaction c. T-cell cytokine response activation of mast cells: basophil reaction (e.g., IL-3, IL-5) d. T-cell cytokine response: activation of vasoproliferative factors (e.g., IL-3, IL-8) |
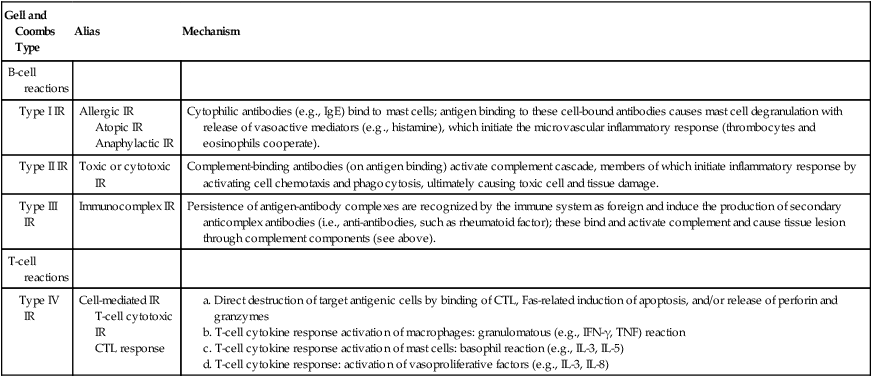
*CTL indicates cytotoxic T lymphocytes; Fas, cellular apoptosis receptor; Ig, immunoglobulin; IL, interleukin; IFN, interferon; IR, immunoreaction; TNF, tumor necrosis factor.
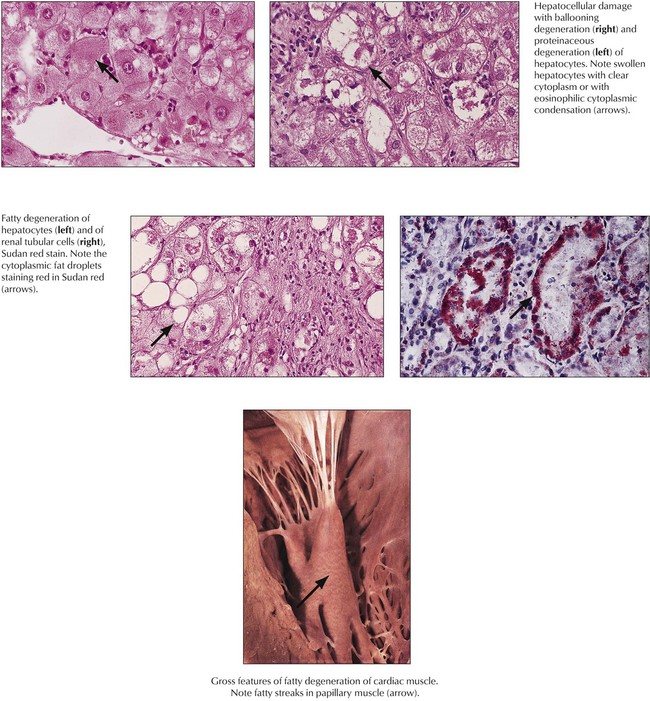
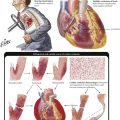
Degeneration, the reversible cell response to injury, has 2 major forms: cellular swelling (proteinaceous, hydropic or ballooning degeneration) and fatty degeneration (fatty change or steatosis). Ultrastructurally, cells show bleb formation, loss of microvilli, loss of intracellular attachments, and swelling of mitochondria and endoplasmic reticulum with granular and fibrillar disaggregation of nuclear chromatin. Fat vacuoles result from disintegration of lipid membranes (fat phanerosis) or accumulation of metabolic lipids (fat thesaurosis). Causes include trauma, chemical injury, metabolic and nutritional factors (hypoxidosis, toxic metabolites, malnutrition), and infectious or immunologic injuries. Enhanced influx of calcium ions into the cell and inactivation of the sodium pump (increase of intracellular sodium and loss of potassium) result in increased intracellular water (swelling). Under certain conditions (e.g., sustained acidosis, hypercalcemia), degenerating cells may accumulate precipitated calcium salts, leading to dystrophic calcification.
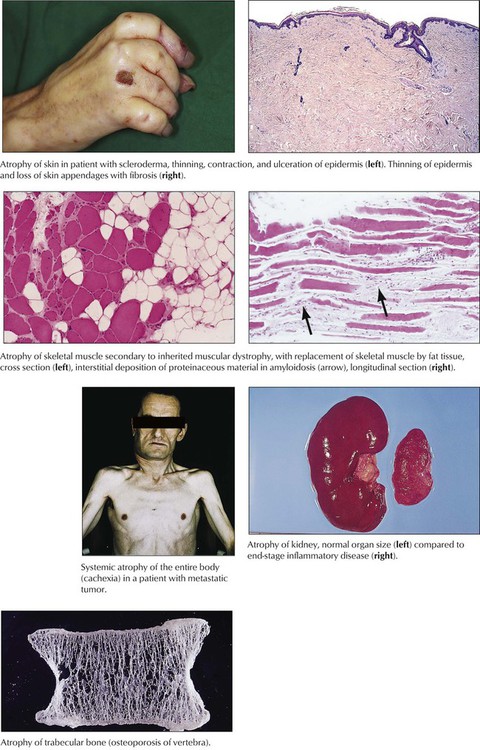
Atrophy of cells or tissues indicates a catabolic metabolism that is not immediately lethal. Cells and organs shrink with or without accumulation of metabolic products (e.g., lipofuscin, brown atrophy). Tissue atrophy may be symmetric, with reduction of all tissue components, or asymmetric, with reduction of only some components. Symmetric atrophy is commonly caused by reduced blood supply or old age, whereas asymmetric atrophy suggests a variety of causes, such as decreased workload, nutritional deficiencies, decreased neural or endocrine stimulation, and chronic low-level injury (radiation, chemical toxins). Cellular atrophy (associated with reduction of functional activity) can be reversible on restoration of normal environmental conditions. Cachexia or wasting syndrome refers to systemic catabolic changes and symmetric atrophy of the entire body such as that accompanying advanced tumors or chronic consumptive infections (e.g., tuberculosis, acquired immunodeficiency syndrome [AIDS]).
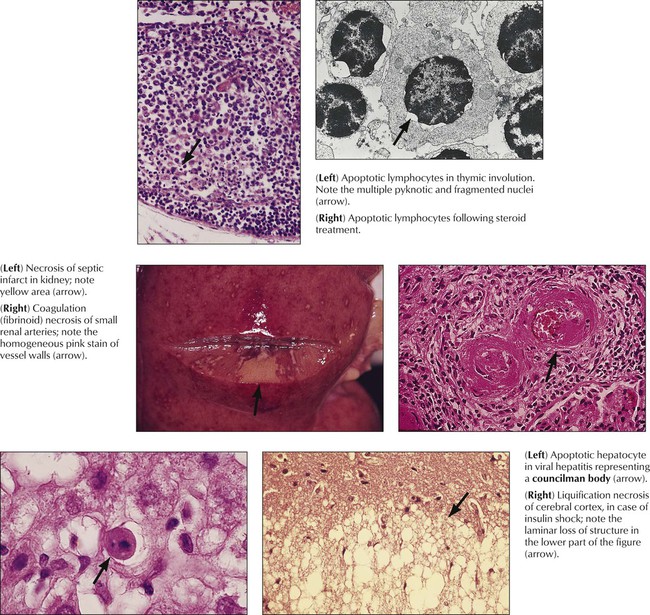
Apoptosis (programmed cell death) serves the process of physiologic cell turnover in development and aging and the disposal of damaged or functionally incapable cells. It follows the specific stimulation of cell membrane receptors (Fas receptor) or genomic damage and is initiated by activation of endonucleases and caspases, DNA fragmentation, and mitochondrial disruption. In light microscopy, the key morphologic change is nuclear condensation and fragmentation followed by cell shrinkage, engulfment, and further disposal by macrophages. Electron microscopy reveals compartmentalization and dissolution of cytoplasmic organelles. Apoptosis is observed in the lymphocyte turnover in antigenically stimulated germinal centers (apoptotic cells in germinal center macrophages, i.e., tingible body macrophages), developing tissues during ontogenesis, other fast-growing tissues including cancer, virus infection, ionizing radiation, and hormonal or toxic conditions.
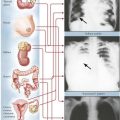
Necrosis, which follows irreversible cell and tissue injury, starts with cell membrane damage, swelling, denaturation, and coagulation of intracellular proteins with breakdown of organelles. Later stages are accompanied by nuclear pyknosis (shrinkage with condensation), loss of the nuclear membrane, and dissolution of nuclei. Coagulative necrosis occurs in tissues with normal protein content, and liquefaction necrosis occurs in tissues poor in protein (brain, fat tissue). Necrosis arises from enzymatic autodigestion (autolysis = self digestion; heterolysis = digestion of adjacent cells and tissues by enzymes released from dying cells). Breakdown products induce chemotaxis and cause a neutrophilic inflammation serving the disposal of necrotic debris. Common causes of necrosis are ischemia, physical trauma, chemical toxins, complex biologic injuries (toxins from infections, arthropods, snakes, plants), and immunologic factors.
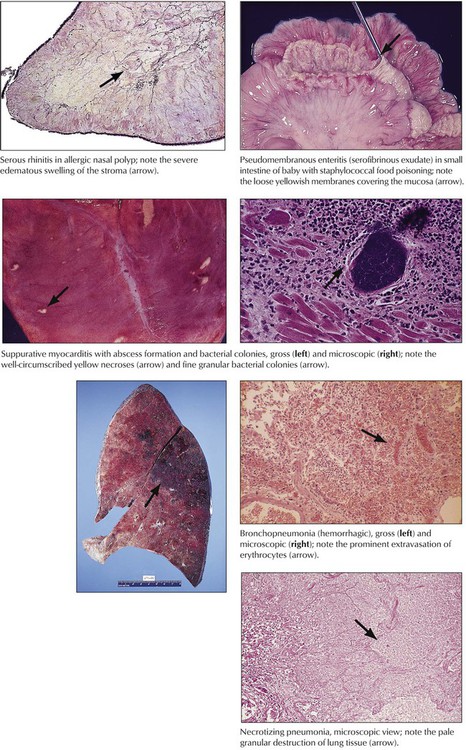
Acute inflammation describes alterations in microvascular circulation (hyperemia, peristasis, and stasis) with increased vascular permeability and exudation of fluids (edema, fibrinous exudates). After additional toxic effects, local thrombosis or necrosis may complicate the reaction. The type of inflammatory response is determined by the nature of the etiologic agent and its distribution in the body and by the composition of the reacting tissue. Acute neutrophilic inflammation (suppurative inflammation) is commonly caused by bacterial infection. Acute viral infection causes lymphocytic infiltrations (stimulation of the immune system by viruses, virus-infected cells, or both). Bacterial (or fungal) toxins may induce necrosis or abscesses by exotoxins or hemorrhage by endotoxins. Endotoxemia and a systemic inflammatory response can lead to circulatory shock.
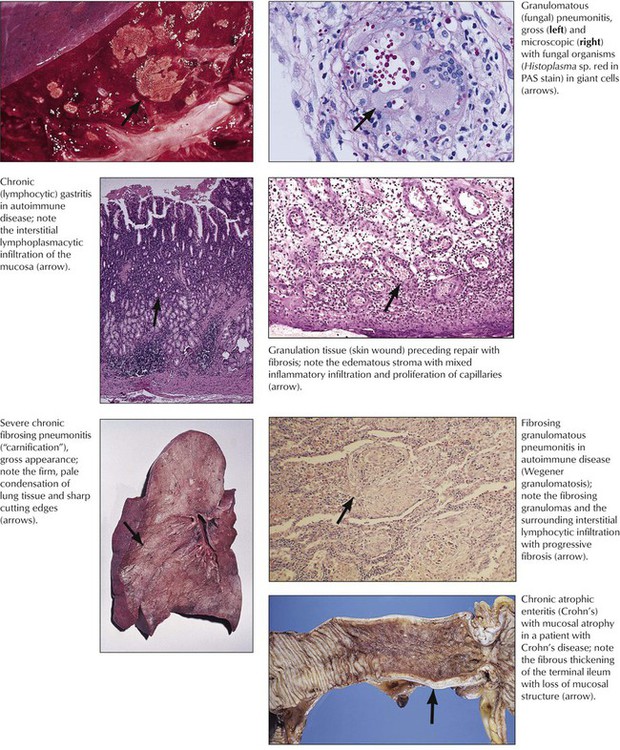
Chronic inflammation follows the initiation of repair (“organization”) of acute inflammation and is characterized by activation of the immune system and of phagocytosis with subsequent proliferation of new capillaries and fibroblasts, production of collagen, and scarring. Lymphohistiocytic infiltration accompanied by capillaries in an edematous stroma and increasing numbers of fibroblasts is called granulation tissue. When inflammation involves a significant T-cell immune response, as in tuberculosis, salmonellosis, or yersiniosis, granuloma formation may result. The form and course of noninfectious inflammation depends on the toxic dose and duration of the pathologic stimulus. For example, acute low-dose radiation (sun exposure) causes hyperemia, a higher dose (sunburn) causes hyperemia and edema, and a very high dose (sunburn grade III) causes necrosis and secondary inflammation. Chronic low-dose exposure (sun or other radiation) causes mild persistent edema followed by atrophy and fibrosis.
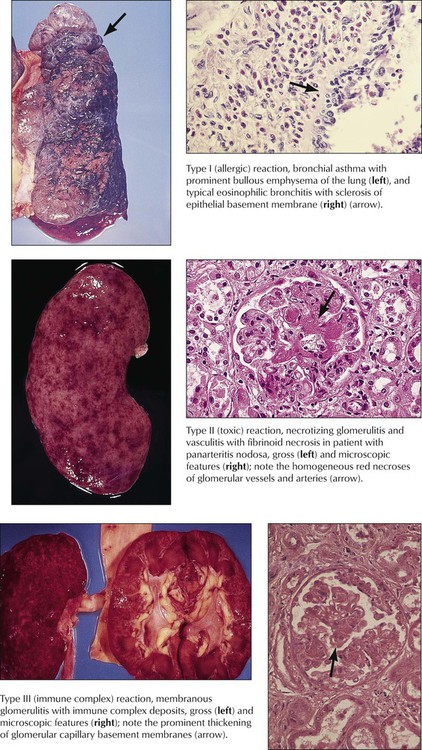
The morphology of immunologically induced inflammation depends on the initiating antigen and the reacting component of the immune system (Table 1-1). Type I B-cell immune reaction (allergy type) is characterized by increased vascular permeability with edema, platelet aggregation, and infiltration by eosinophils (e.g., allergic rhinitis, asthma bronchiale). Type II B-cell reaction causes lysis of the antigenic target cell or necrosis of tissue components (e.g., autoimmune hemolytic anemia, nephrotoxic glomerulonephritis). Type III B-cell immune reactions or immune complex reactions are characterized by accumulations of antigen-antibody complexes and in situ complement activation with subsequent serofibrinous exudates; thickening of basement membranes; and slow, secondary development of granulation tissue at the site of immune complex deposition (e.g., membranoproliferative glomerulonephritis, certain lesions in lupus erythematosus, and rheumatoid arthritis). More acute reactions cause acute vasculitis with or without microhemorrhage (Arthus-type reaction).
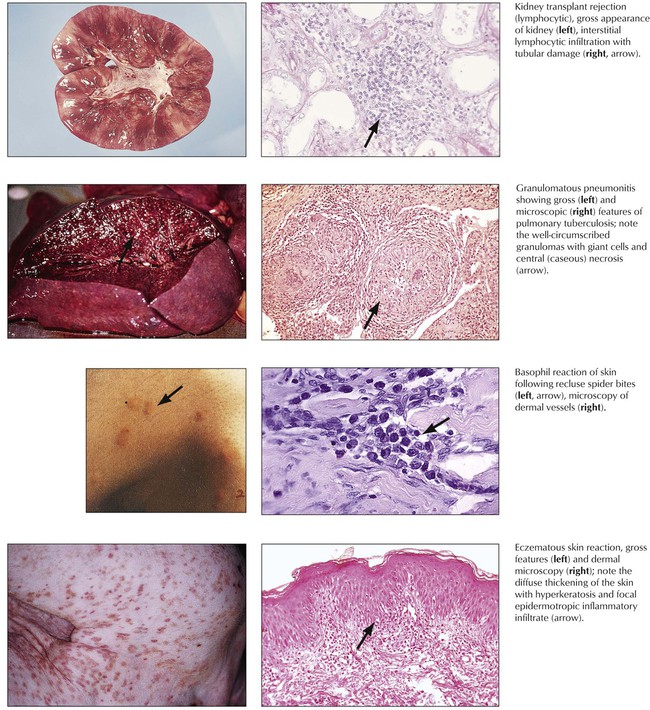
T-cell immune reactions are divided into the lymphocytotoxic reaction (classic type IV reaction or tuberculin-type cellular immune reaction), the granulomatous reaction, the basophil reaction (Jones-Mote–type reaction), and the contact allergy–type reaction (Table 1-1). The lymphocytotoxic reaction is brought about by direct action of cytotoxic T lymphocytes on the cellular antigen, as in acute transplant rejection. Granulomatous reactions are initiated by T-cell–induced accumulation and activation of phagocytes with typical tissue reactions in certain infectious diseases such as tuberculosis. Basophil reactions are caused by secretion of specific T-cell cytokines with attraction of basophils to the site of the antigen deposit. This can be seen in certain arthropod reactions, such as spider bites. The contact allergy–type reaction with production of vasoproliferative factors and other cytokines is caused by antigens such as heavy metals. Eczema is characteristic of contact allergy–type reaction.
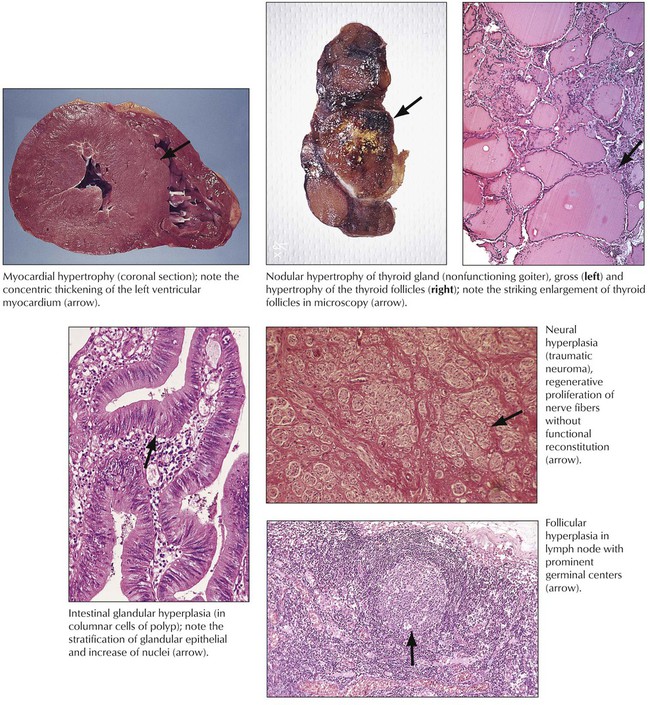
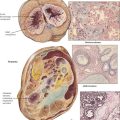
Regeneration, hypertrophy, and hyperplasia are forms of functional or structural repair or both of damaged cells and tissues. Regeneration may be complete with restitution of normal structure and function or incomplete. Hypertrophy is an increase in cell mass without cell division (i.e., increase in functional units such as organelles, nuclear ploidy). There are at least 2 identified stimuli for hypertrophy: mechanical triggers (i.e., stretching of cardiac or skeletal muscles) and trophical triggers (i.e., neuroendocrine activation). Compensation for structural or functional deficiency or both by hypertrophy remains limited, and degenerative changes occur when hypertrophic cells can no longer compensate for the increased burden.
Hyperplasia results from an increase in cell division and may follow or coincide with hypertrophy in nonpostmitotic tissues. It is initiated by growth factors produced by cells adjacent to the functionally or structurally damaged area. Hyperplasia compensates for a decrease or loss of cellular function or is a response to increased functional requirement. Examples are hyperplastic intestinal crypts in chronic inflammation, follicular hyperplasia of a lymph node in antigenic stimulation, and axonal proliferation after trauma (traumatic neuroma). Positive effects of hyperplasia are limited by the extent of the blood supply to the newly formed tissue. When hyperplasia becomes out of balance with vascularization, focal degeneration, hypoxidotic necroses, or both occur.
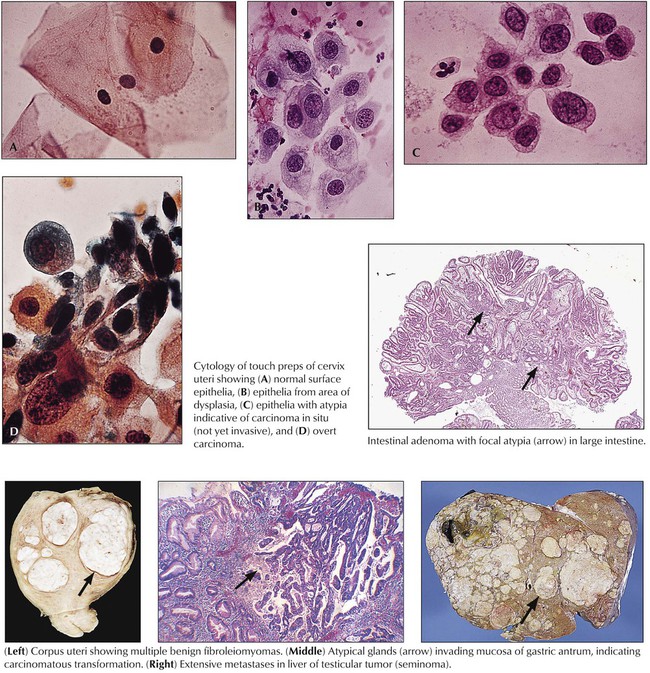
Dysplasia refers to restitution or tissue growth with altered features. Atypia refers to cellular changes. Dysplasia describes an abnormal structural regeneration that may become malignant, such as the adenomatous polyp of the colon. Typical dysplastic changes can be observed in proliferating mucosa of intestinal polyps (adenomas) or in the cervix uteri with chronic inflammation and mucosal regeneration. They are characterized by irregular glandular patterns, occasionally with some loss of cellular polarity. Cellular atypia indicating malignant potential is characterized by nuclear enlargement with hyperchromasia (polyploidy and aneuploidy) with increase in the nuclear/cytoplasmic ratio and irregular nucleoli, loss of cell polarity and contact inhibition, and increased and atypical mitotic figures.
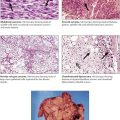
Neoplasia (“new growth”) results from a disturbance of physiologic growth regulation with persistent activity of growth-promoting factors or loss of proliferation inhibition functions (or of physiologic apoptosis). It leads to tumorous growth patterns independent of or at the expense of surrounding cells and tissues. Benign neoplasias (tumors), such as the uterine myoma in the figure, exhibit expansive growth with compression and atrophy of surrounding tissues but no true invasion or metastasis. Benign tumors are often designated by their tissue of origin with the affix –oma, such as myoma, hemangioma, and neurinoma. Although “benign,” such tumors can cause severe disturbances and death when they interfere with the function of other organs, such as by compression (as a meningioma compresses the brain) or obstruction of canalicular structures.
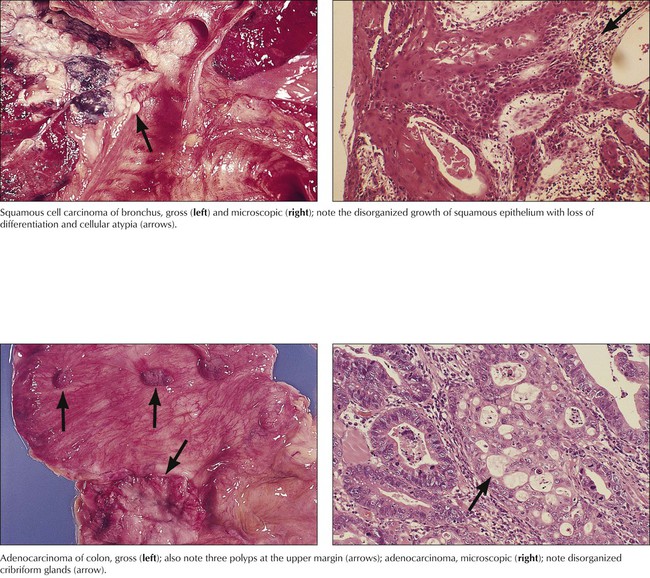
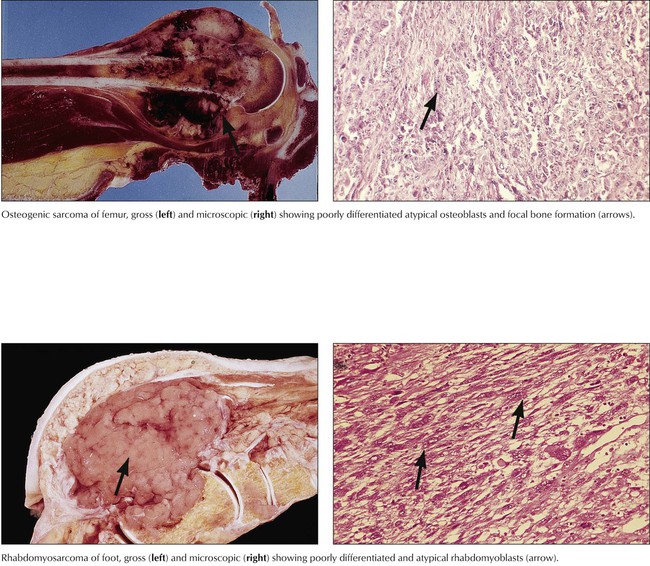
Malignant tumors grow progressively at the expense of other tissues and cause death by damaging vital organs or by causing cachexia and infections. Malignancy is morphologically defined by cellular atypia, invasive and destructive growth, and metastatic spread via lymphatic vessels, hematogenously, or within other canalicular systems and body cavities. Malignant tumors of epithelial origin are carcinomas. Tumors of mesenchymal origin are sarcomas (e.g., squamous cell carcinoma, adenocarcinoma, fibrosarcoma, osteosarcoma). There are exceptions to this nomenclature, such as malignant lymphomas and leukemias for hemolymphatic malignancies and astrocytoma, ependymoma, glioblastoma, and others for malignant brain tumors. The degree of malignancy of a given tumor is assessed by tumor classification and staging, the histologic tumor type, its degree of differentiation, and its local invasion and metastatic spread. Classification and staging determine the choice of treatment and the patient’s prognosis.