Chapter 20 General Principles of Infection
Etiology
Surgeon-Dependent Factors
Skin Preparation
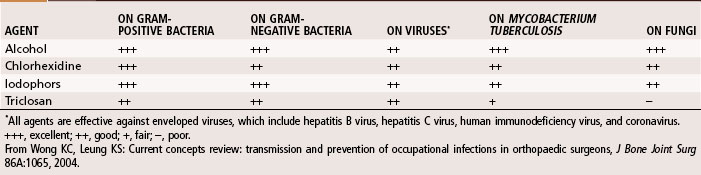
Hand washing is the most important procedure for prevention of nosocomial infections. Studies suggest that hand scrubbing for 2 minutes is as effective as traditional hand scrubbing for 5 minutes. The optimal duration of hand scrubbing has yet to be determined. Hand rubbing with an aqueous alcohol solution that is preceded by a 1-minute nonantiseptic hand washing for the first case of the day was found by Parienti et al. to be just as effective in prevention of surgical site infections as traditional hand scrubbing with antiseptic soap. The effectiveness of common antiseptics is summarized in Table 20-1.
Prophylactic Antibiotic Therapy
 Methicillin-Resistant Staphylococcus Aureus
Methicillin-Resistant Staphylococcus Aureus
The evolution of S. aureus into a multiple-drug–resistant pathogen (methicillin-resistant S. aureus [MRSA]) has become a major health concern worldwide. Approximately 57% of S. aureus bacteria are methicillin resistant, and now vancomycin-resistant strains are being reported. This is probably one of the most worrisome problems in the fight against bacterial infections. Initially, MRSA was seen only in hospital settings and long-term care facilities; however, it is now becoming increasingly prevalent in young, healthy individuals in the community (Table 20-2; at-risk groups), and it is particularly virulent. The mortality rate associated with invasive MRSA infections is 20%.
TABLE 20-2 At-Risk Groups and Risk Factors for Community-Acquired Methicillin-Resistant Staphylococcus aureus
| AT-RISK GROUPS | RISK FACTORS |
|---|---|
From Marcotte AL, Trzeciak MA: Community-acquired methicillin-resistant Staphylococcus aureus: an emerging pathogen in orthopaedics, J Am Acad Orthop Surg 16:98, 2008.
Because of the prevalence of community acquired (CA)-MRSA, it is necessary to rapidly identify the organism, determine antibiotic sensitivity, and begin antibiotic therapy (for empirical coverage see Table 22-2). For invasive infections, intravenous vancomycin is recommended or, alternatively, daptomycin, gentamicin, and linezolid can be used. In cases of necrotizing fasciitis, clindamycin, gentamicin, rifampin, trimethoprim-sulfamethoxazole, and vancomycin are effective. Until a sensitivity determination can be made, antimicrobial coverage specifically of CA-MRSA is recommended. For deep subperiosteal abscess or superficial abscess, irrigation and débridement are necessary to reduce bacterial counts. Obtaining an infectious disease consult is highly recommended.
Diagnosis
Laboratory Studies
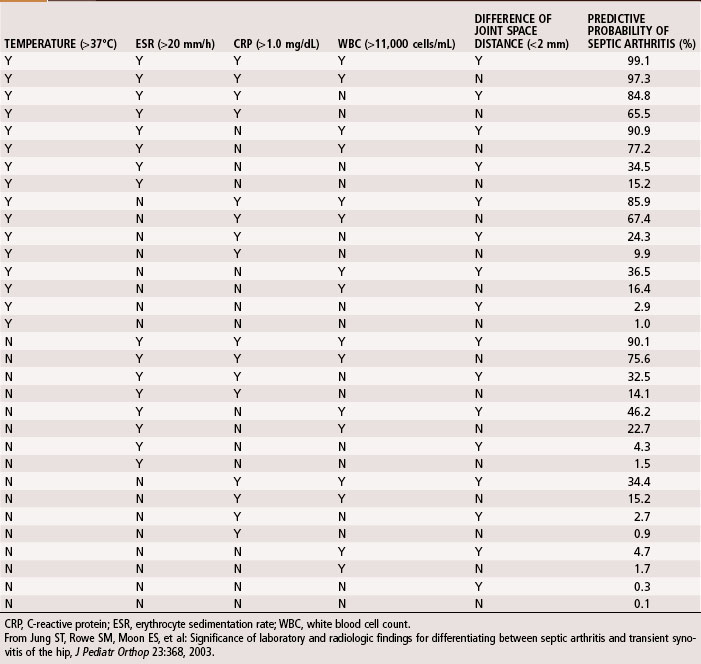
A complete blood cell count, including differential and erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), should be obtained during initial evaluation of bone and joint infections. The white blood cell count is an unreliable indicator of infection and often is normal even when infection is present. The differential shows increases in neutrophils during acute infections. The ESR becomes elevated when infection is present, but this does not occur exclusively in the presence of infection. Fractures or other underlying diseases can cause elevation of the ESR. The ESR also is unreliable in neonates, patients with sickle cell disease, patients taking corticosteroids, and patients whose symptoms have been present for less than 48 hours. Peak elevation of the ESR occurs at 3 to 5 days after infection and returns to normal approximately 3 weeks after treatment is begun. CRP, synthesized by the liver in response to infection, is a better way to follow the response of infection to treatment. CRP increases within 6 hours of infection, reaches a peak elevation 2 days after infection, and returns to normal within 1 week after adequate treatment has begun. Other tests, such as the S. aureus surface antigen or antibody test and counterimmunofluorescence studies of the urine, are promising, but their usefulness in clinical situations has not been proved. Material obtained from aspiration of joint fluid can be sent to the laboratory for a cell count and differential to distinguish acute septic arthritis from other causes of arthritis. In septic arthritis, the cell count usually is greater than 80,000/mm3, with more than 75% of the cells being neutrophils (Table 20-3). Jung et al. devised an algorithm to predict the probability of septic arthritis in children (Table 20-4). A Gram stain also should be obtained. Gram stains identify the types of organisms (gram-positive or gram-negative) in about a third of bone and joint aspirates. Intraoperative frozen section also should be obtained in cases in which infection is suspected. A white blood cell count greater than 10 per high-power field is considered indicative of infection, whereas a count less than 5 per high-power field all but excludes infection.
| LEUKOCYTES | NEUTROPHILS (%) | |
|---|---|---|
| Normal | <200 | <25 |
| Traumatic | <5,000 | <25 |
| Toxic synovitis | 5,000-15,000 | <25 |
| Acute rheumatic fever | 10,000-15,000 | 50 |
| Juvenile rheumatoid arthritis | 15,000-80,000 | 75 |
| Septic arthritis | >80,000 | >75 |
From Morrissy RT: Septic arthritis. In Gustilo RB, Genninger RP, Tsukayama DT, editors: Orthopaedic infection: diagnosis and treatment, Philadelphia, 1989, WB Saunders.
Imaging Studies
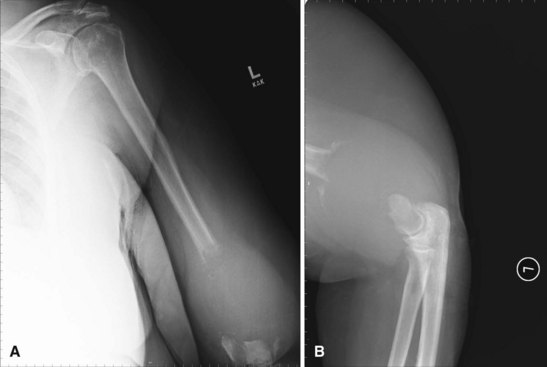
Radiographic studies are helpful but are not as useful in the diagnosis of acute bone and joint infections as they are in following responses to treatment. Plain radiographs show soft tissue swelling, joint space narrowing or widening, and bone destruction (Fig. 20-1). Bone destruction is not apparent on radiographs, however, until an infection has been present for 10 to 21 days. In addition, 30% to 50% of the bone matrix must be lost to show a lytic lesion on radiographs (Fig. 20-2). Wheat found that fewer than 5% of plain radiographs were initially abnormal in bone and joint infections, and fewer than 30% were abnormal at 1 week; however, 90% were abnormal at 3 to 4 weeks. If initial radiographs are normal in the evaluation of bone and joint infections, other imaging methods that show soft tissue swelling and loss of normal fat planes around the involved bone or joint should be used.
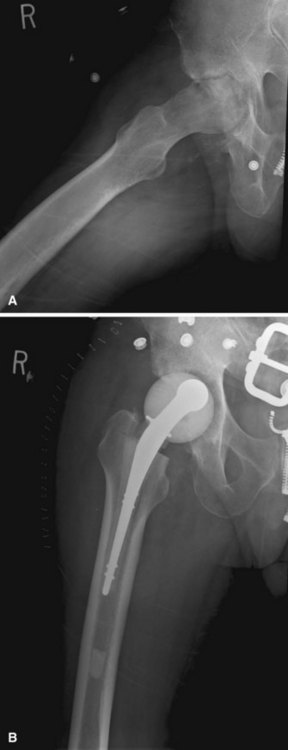
FIGURE 20-1 A, Radiographic evidence of acute bone and joint infection. B, Treatment with antibiotic cement spacer.
(Courtesy of Andrew Crenshaw.)
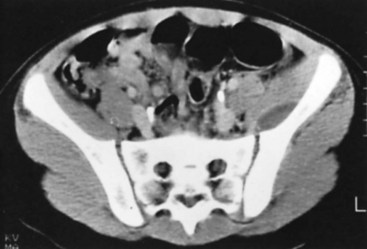
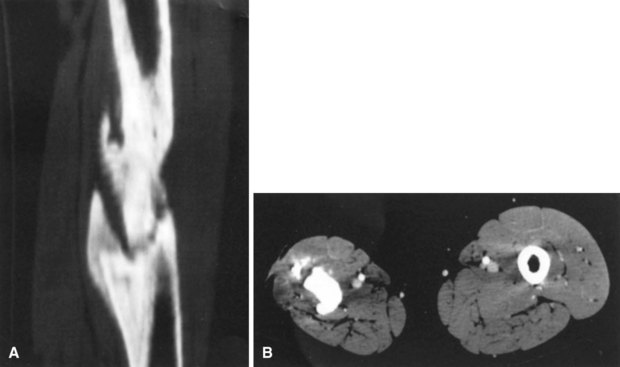
Conventional tomography can be useful in identifying a sequestrum or subchondral bony plate destruction, although it has largely been replaced with more conventional radiographic methods. Arthrography helps document proper aspiration of a suspected septic joint. Dye should be injected only after fluid is obtained from the joint because the bactericidal effect of iodinated contrast material can cause a false-negative culture result. CT can help determine the extent of medullary involvement. Pus within the medullary cavity replaces the marrow fat, causing an increased density on the CT scan. Adjacent soft tissue abscesses also are seen easily (Fig. 20-3). CT diagnosis of acute osteomyelitis is based on detection of intraosseous gas, osteolysis, soft tissue masses, abscesses, or foreign bodies. Additionally, increased vascularity after administration of a contrast agent also can aid in the diagnosis. Narrowing of the medullary cavity by granulation tissue and new bone is readily shown during the healing phase of osteomyelitis. CT identifies sequestra in chronic osteomyelitis (Fig. 20-4). It also is helpful in identifying alterations in areas poorly seen on plain films, such as the sternoclavicular joint, sacroiliac joint, and spine. Contrast material can be used to delineate abscesses in necrotic tissue that does not enhance from surrounding hyperemic tissue.
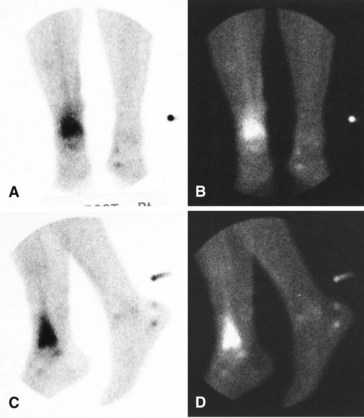
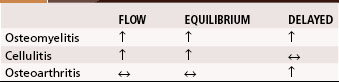
A major disadvantage of three-phase 99mTc phosphate bone scintigraphy is that the increased uptake caused by osteomyelitis is difficult to distinguish from that caused by degenerative joint disease or posttraumatic or postsurgical changes. The relative activity in each of the three phases may be helpful in differentiating other causes of increased uptake. Cellulitis causes increased activity during the flow and equilibrium phases and a decreased or normal uptake in the delayed phase. Osteomyelitis causes increased uptake in all three phases (Fig. 20-5). Increased uptake in the delayed phase but not in the flow or equilibrium phase suggests degenerative joint disease (Table 20-5). 99mTc phosphate bone scans are unreliable in neonates (<6 weeks old) and usually are negative in 60% of these patients with bone or joint infections.
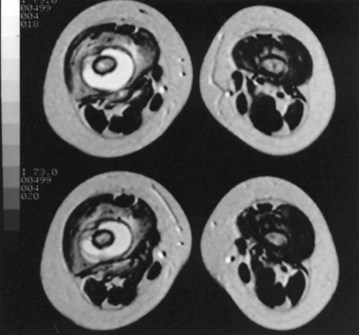
MRI has been used for evaluating bone and joint infections. MRI is a complex imaging method that aligns the body’s protons along the axis of a powerful external magnetic field and records the motion of the protons as they return to the magnetic field alignment after absorbing energy from a radiofrequency-generating coil. Each type of tissue has its own unique signal characteristics. Two parameters are evaluated. The first is the echo time (TE), which is the time that elapses between the initial radiofrequency pulse and its return back to the radio antenna (akin to a sonar ping). The second is repetition time (TR), which is the time between the applied consecutive radiofrequency pulses to the patient (the frequency of pings). When the TR and TE are short, a T1 image is produced that shows fat as a high, bright signal. When the TR and TE are long, a T2 image is obtained that shows water as a bright signal. An additional signal is obtained by suppressing the fat signal; this is called short tau inversion recovery (STIR). STIR signals have a high negative predictive value for osteomyelitis of almost 100%; however, STIR cannot be used to differentiate fluid collections (e.g., abscesses) from circumscribed soft tissue edema. The reported abnormal images reflect an increase in water content, resulting from edema in the marrow cavity. Marrow fat is replaced by edema and cellular infiltrates that are lower in signal than fat on T1 images and higher in signal than fat on T2 and STIR images. The classic findings of osteomyelitis on MRI are a decrease in the normally high marrow signal on T1 images and a normal or increased signal on T2 images (Fig. 20-6). According to Boutin et al., MRI is the most appropriate tool to rule out cartilaginous epiphyseal infection. Mazur et al. showed that MRI was superior in sensitivity (97%) and specificity (92%) to 99mTc phosphate bone scintigraphy for detection of osteomyelitis. MRI detects changes (e.g., lytic areas) much earlier in the course of disease than radiographs because it shows the condition of the intramedullary cavity. The signal changes seen on MRI are nonspecific, and anything that causes edema or hyperemia (e.g., fractures, tumors, and inflammatory processes) produces signal changes similar to that of osteomyelitis. Although MRI is good for detailing marrow involvement and discitis, it does little to detect early cortical bone involvement.
1. Patients suspected of musculoskeletal infection should have plain radiographs of the area in question.
2. CT is useful in detecting bone abnormalities such as sequestra.
3. Ultrasonography helps to establish if a joint effusion is present and to localize needle aspiration to diagnose septic arthritis.
4. A three-phase bone scan accurately detects osteomyelitis in nonviolated bone. If hardware is in place or if there has been previous trauma to the bone or a Charcot joint for instance is present, a three-phase bone scan is only useful as a screening test. A white blood cell–labeled bone scan helps with detection of complicated osteomyelitis, and combining this with a colloid scan maximizes accuracy. This is especially useful in total joint infections and diabetic feet but less so in neuropathic joints. It is not helpful with spinal osteomyelitis.
5. MRI shows surrounding tissue and is excellent in detecting osteomyelitis. Adding gallium improves detection of spinal osteomyelitis.
6. FDG-PET also is helpful in the diagnosis of spinal infection and chronic osteomyelitis but is not readily available in all health care institutions.
Treatment
Several routes of antibiotic treatment exist. Oral antibiotics are still the most commonly used. Intravenous application may be required for more serious infections that do not respond to oral antibiotics. Local delivery of antibiotics also can be beneficial. Polymethyl methacrylate (PMMA) beads impregnated with heat-stable antibiotics (tobramycin, vancomycin, and gentamicin) have been used since the early 1970s. A 2- to 3-cm area around each bead has a high concentration of antibiotic. With tobramycin and vancomycin, the peak concentration of antibiotic delivered to local tissue occurs on the first day and lasts for only approximately 1 week. This local delivery system avoids systemic toxicity; however, it requires removal (usually surgical) within 4 weeks. A more attractive biodegradable system is the collagen-gentamicin sponge, which obviates the need for surgical removal and delivers higher concentrations of antibiotics than PMMA beads. It has been suggested that antibiotic release by this method may be complete within 4 days. Lactic acid polymerase may be the next step in local biodegradable antibiotic delivery systems. This system delivers a high concentration of quinolines (bactericidals for probable pathogens of chronic osteomyelitis) for 60 days, with a peak release of antibiotics at day 15. An additional method of local antibiotic delivery is that of mixing autogenous iliac crest bone graft with piperacillin or vancomycin. Antibiotics must be chosen carefully. For example, heat-stable antibiotics are required for PMMA applications; quinolones have shown detrimental effects on chondrocytes and fracture healing; and tobramycin at intermediate levels of concentration (400 µg/mL) can decrease cell replication. In general, vancomycin is less toxic to osteoblasts at high local concentrations than other aminoglycosides and rifampin and the quinolones should not be administered when bone regeneration is an issue. An infectious disease consult can help guide the appropriate antibiotic in each patient and can be especially useful with the ever-changing microbial picture. Even though many surgical techniques have been described for the treatment of osteomyelitis (see Chapter 21), prevention is still the best course, and adherence to the basic principles of treatment of infections helps achieve success.
Human Immunodeficiency Virus
Four stages of HIV infection have been identified, although not all individuals infected with HIV go through all four stages. The stages are (1) acute primary HIV infection, (2) chronic asymptomatic HIV infection, (3) symptomatic HIV infection, and (4) advanced HIV-associated opportunistic disease or AIDS. Acute primary HIV infection appears clinically similar to infectious mononucleosis and occurs 2 to 6 weeks after viral transmission. Clinical features include pharyngitis, dysphagia, lymphadenopathy, rash, fever, fatigue, hepatosplenomegaly, and leukopenia. This stage is self-limiting, and most patients do not seek medical attention. Within 3 months after viral transmission, most patients develop positive serology, and virtually all patients seroconvert by 6 months, although delayed seroconversion 1 year after infection has been reported. After acute infection, a prolonged period ranging from 5 to more than 15 years of symptomless, chronic infection ensues. In the third stage (AIDS-related complex) the HIV-infected patient is no longer symptom free but has not yet developed AIDS-defining opportunistic infection as defined by the CDC or an absolute CD4 cell count of less than 200/mm3. In the final stage, a potentially life-threatening opportunistic disease develops as a result of the severe cell-mediated immunodeficiency. The epidemiological data on HIV transmission overwhelmingly indicate that the virus is transmitted through sexual, parenteral, and maternal-infant routes. HIV has been isolated from many organs and tissues, including bone. Blood, semen, vaginal secretions, bone, breast milk, and possibly saliva have been implicated in HIV transmission. With current screening of donors and HIV testing techniques, the risk of HIV infection per unit of blood transfused is 1 in 2 million. Although the risk from any single transfusion is low, each transfusion has the potential to be fatal. This potential has increased physician awareness and decreased the elective use of allogeneic blood. When an individual does become infected from a transfusion, the development of AIDS seems to be more rapid than with other forms of transmission. In the United States, two donors were responsible for the transmission of HIV in four musculoskeletal grafts in 1985 and 1988. No further transmissions of HIV through allografts have been reported since 1988. However, the risk of transmission of HIV through allografts is estimated to be 1 in 1.6 million (one to two cases every 2 years) related to the fact that a window period still exists between testing methods and the patient having detectable viral antibodies. Nucleic antibody testing has a window period for HIV and hepatitis C of 7 days and 8 days for hepatitis B. Additionally, there has been one reported case of hepatitis B and two of hepatitis C, with the most recent occurring in 2002. With better screening techniques including patient history and serological and nucleic acid testing, rates remain low. Additionally, chemical sterilization techniques have also decreased the opportunity for disease transmission through allografts. The current risk of acquiring an infection from the allograft remains well below the overall perioperative nosocomial risk. Intraoperative culturing of the allograft has a low sensitivity and is generally not recommended. However, it is important that each surgeon knows the specifications of the tissue bank that he or she uses and to ensure that it is American Association of Tissue Banks (AATB) accredited (Table 20-6).
Adapted from Azar FM: Tissue processing: role of secondary sterilization techniques, Clin Sports Med 28:191, 2009.
Musculoskeletal Syndromes in Human Immunodeficiency Virus–Infected Patients
The most common musculoskeletal syndromes in HIV-infected patients are manifestations of drug toxicity, reactive arthritis, infectious arthritis, myositis, tendinitis, and bursitis. General principles to be kept in mind when evaluating an HIV-infected patient with musculoskeletal problems include the following: (1) Any musculoskeletal syndrome that occurs in non–HIV-infected patients can occur in HIV-infected patients; (2) HIV infection can alter the clinical presentation, severity, and course of musculoskeletal problems; and (3) early diagnosis of infections is especially important to prevent their spread in an immunocompromised patient (Table 20-7).
TABLE 20-7 Musculoskeletal Syndromes in Human Immunodeficiency Virus–Infected Patients
| CONDITION | COMMENTS |
|---|---|
| Arthralgias | Causes include systemic bacterial infection, inflammation, drug toxicity |
| Reactive arthritis (Reiter syndrome) | Possibly more severe in HIV disease |
| Psoriatic arthritis | Most commonly Staphylococcus aureus or Streptococcus pneumoniae |
| Osteomyelitis | Reported in HIV disease as a result of extension of infection from septic joint |
| Myositis | |
| Pyomyositis | Focal pain, tenderness |
| Idiopathic | Focal pain, tenderness |
| From zidovudine | Usually resolves when zidovudine is discontinued |
From Lane N: HIV disease and arthritis: diagnostic and therapeutic dilemmas. In Cohen PT, Sande MA, Volberding PA, editors: The AIDS knowledge base, Boston, 1994, Little, Brown.
Risks and Prevention
During orthopaedic surgical procedures, contact with blood and other body fluids containing blood in gross or microscopic amounts is frequent (3.7%). Lacerations from bone fragments and edges and cuts and needle sticks must be avoided. The estimated risk after a mucocutaneous exposure was reported to be 0.09% based on one seroconversion in six studies. The American Academy of Orthopaedic Surgeons (AAOS) has developed several basic recommendations for procedures in the operating room (Box 20-1). These precautions involve wearing surgical gowns that offer protection against contact with blood, using nontouch techniques for surgery and suturing, not passing sharp instruments from hand to hand (establishing a “hands-free” zone), and proper removal of contaminated gowns and postoperative scrub. Specific recommendations by the AAOS can be found in their information statement Preventing the Transmission of Bloodborne Pathogens (2008).
Box 20-1
American Academy of Orthopaedic Surgeons Recommendations for Operating Room Procedures
1. Avoid the use of sharp instruments if possible.
2. Avoid direct passing of sharp instruments between team members.
4. Use a scalpel for skin incisions only, then scissors and electrocautery.
5. Avoid simultaneous suture of the same layer by two members of a team.
6. Preferably use blunt suture needles.
8. Comply with regulations for elimination of disposable material.
9. Always wear gloves when handling material covered with blood.
Adapted from Lemaire R, Masson JB: Risk of transmission of blood-borne viral infection in orthopaedic and trauma surgery, J Bone Joint Surg 82B:313, 2000.
After exposure of a health care worker to blood, a rapid HIV test should be performed on the source. If it is negative, no chemoprophylaxis should be offered. However, if it is positive, chemoprophylaxis should be offered. The rapid HIV test does have a low false-positive rate; therefore, all positive results should be followed with standard enzyme immunoassay and a Western blot assay. The test also will not identify HIV-positive patients if they have been infected less than 3 months. A decrease in seroconversion rates of 79% has been shown with the use of chemoprophylaxis after exposure using zidovudine and lamivudine, chain terminators for reverse transcriptase. Adding a protease inhibitor, indinavir, further decreases antiretroviral activity. These drugs should be started within 2 hours of exposure and generally are recommended for at least a 4-week course. In 2005, the CDC updated U.S. Public Health Service Guidelines for the management of occupational exposures to hepatitis B virus, hepatitis C virus, and HIV; recommendations for chemoprophylaxis can be found in Table 20-8. The most current postexposure prophylaxis (PEP) drug regimen can be found at the National HIV/AIDS Clinicians Consultation Center (http://www.ucsf.edu/hivcntr). Also questions about PEP can be answered at National Clinicians postexposure prophylaxis hot line (PEPline) at (888) HIV-4911. Most exposures to HIV-infected blood do not cause seroconversion, and toxicity of a chemoprophylactic regimen must be considered before the initiation of treatment. If available, consultation with infectious disease is recommended.
Barie PS. Surgical site infections: epidemiology and prevention. Surg Infect. 3, 2002. S–9
Bhars CH, Marschal M, Weise K, et al. Acute musculoskeletal infection: comparison of different methods for intraoperative bacterial identification. Acta Chir Orthop. 2006;73:237.
Centers for Disease Control and Prevention, Update: allograft-associated bacterial infections–United States, 2002 Available at www.cdc.gov/mmwr/preview,mmwrhtmlmm5110a2.htm Accessed November 18, 2010
Edlich RF, Wind TC, Hill LG, et al. Creating another barrier to the transmission of bloodborne operative infections with a new glove gauntlet. J Long Term Eff Med Implants. 2003;13:97.
Fluit AC, Verhoef J, Schmitz FJ, European SENTRY Participants. Frequency of isolation and antimicrobial resistance of gram-negative and gram-positive bacteria from patients in intensive care units of 25 European university hospitals participating in the European arm of the SENTRY Antimicrobial Surveillance Program 1997-1998. Eur J Clin Microbiol Infect Dis. 2001;20:617.
French GL. What’s new and not so new on the antimicrobial horizon? Clin Microbiol Infect. 2008;14:19.
Kak V, Chandrasekar PH. Bone and joint infections in injection drug users. Infect Dis Clin North Am. 2002;16:681.
Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopaedic surgery. J Bone Joint Surg. 2010;92A:1820.
Klevens RM. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763.
Luedicke C, Slickers P, Ehricht R, Monecke S. Molecular fingerprinting of Staphylococcus aureus from bone and joint infections. Eur J Clin Microbiol Infect Dis. 2010;29:457.
Marcotte AL, Trzeciak MA. Community-acquired methicillin resistant Staphylococcus aureus: an emerging pathogen in orthopaedics. J Am Acad Orthop Surg. 2008;16:98.
Parienti JJ, Thibon P, Heller R, et al. Hand-rubbing with an aqueous alcoholic vs. traditional surgical hand-scrubbing and 30-day surgical site infection rates: a randomized equivalence study. JAMA. 2002;288:722.
Schmidt AH, Swiontkowski MF. Pathophysiology of infections after internal fixation of fractures. J Am Acad Orthop Surg. 2000;8:285.
Schwarzkopf R, Takemoto RC, Immerman I, et al. Prevalence of Staphylococcus aureus colonization in orthopaedic surgeons and their patients. J Bone Joint Surg. 2010;92A:1815.
Stefani S, Goglio A. Methicillin-resistant Staphylococcus aureus: related infections and antibiotic resistance. Int J Infect Dis. 2010;14S4:S19.
Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 3, 2002. CD003087
Wilson ML, Winn W. Laboratory diagnosis of bone, joint, soft-tissue, and skin infections. Clin Infect Dis. 2008;46:453.
Wong KC, Leung KS. Transmission and prevention of occupational infections in orthopaedic surgeons. J Bone Joint Surg. 2004;86A:1065.
DeWinter F, Van de Wiele C, Vogelaers D, et al. Fluorine-18 fluorodeoxyglucose-positron emission tomography: a highly accurate imaging modality for the diagnosis of chronic musculoskeletal infections. J Bone Joint Surg. 2001;83A:651.
Jung ST, Rowe SM, Moon ES, et al. Significance of laboratory and radiologic findings for differentiating between septic arthritis and transient synovitis of the hip. J Pediatr Orthop. 2003;23:368.
Kattapuram TM, Treat ME, Kattapuram SV. Magnetic resonance imaging of bone and soft tissue infections. Curr Clin Top Infect Dis. 2001;21:190.
Levine BR, Evans BG. Use of blood culture vial specimens in intraoperative detection of infection. Clin Orthop Relat Res. 2001;382:222.
Moojen DJF, Spijkers SNM, Schot CS, et al. Identification of orthopaedic infections using broad-range polymerase chain reaction and reverse line blot hybridization. J Bone Joint Surg. 2007;89:1298.
Morrissy RT, Bone and joint sepsis. Morrissy, RT, Weinstein, SL. Lovell and Winter’s pediatric orthopaedics, ed 5, Philadelphia: Lippincott-Raven, 2001.
Palestro CJ, Lov C, Miller TT. Imaging of musculoskeletal infections. Best Pract Res Clin Rheumatol. 2006;20:1197.
Prandini N, Lazzeri E, Rossi B, et al. Nuclear medicine imaging of bone infections. Nucl Med Comm. 2006;27:633.
Pruthi S, Thapa MM. Infectious and inflammatory disorders. Magn Reson Imaging Clin North Am. 2009;17:423.
Quigley AM, Gananasegaran G, Buscombe JR, Hilson AJ. Technetium-99m-labelled sulesomab (LeukoScan) in the evaluation of soft-tissue infections. Med Princ Pract. 2008;17:447.
Sonmezoglu K, Sonmezoglu M, Halac M, et al. Usefulness of 99mTc-ciprofloxacin (infection) scan in diagnosis of chronic orthopedic infections: comparative study with 99mTc-HMPAO leukocyte scintigraphy. J Nucl Med. 2001;42:567.
Teller RE, Christie MJ, Martin W, et al. Sequential indium-labeled leukocyte and bone scans to diagnose prosthetic joint infection. Clin Orthop Relat Res. 2000;373:241.
Turecki MB, Taljanovic MS, Stubbs AY, et al. Imaging of musculoskeletal soft-tissue infections. Skeletal Radiol. 2010;39:957.
Vicente AG, Almoguera M, Alonso JC, et al. Diagnosis of orthopedic infection in clinical practice using Tc-99m sulesomab (antigranulocyte monoclonal antibody fragment fab 2). Clin Nucl Med. 2004;29:781.
Wolf G, Aigner RM, Schwarz T. Diagnosis of bone infection using 99mTc-HMPAO labeled leukocytes. Nucl Med Commun. 2001;22:1201.
Hanssen AD. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res. 2005;437:91.
Holtom PD, Patzakis MJ. Newer methods of antimicrobial delivery for bone and joint infections. Instr Course Lect. 2003;52:745.
Kanellakopoulou K, Giamarellos-Bourboulis EJ. Carrier systems for local delivery of antibiotics in bone infections. Drugs. 2000;59:1223.
Nathwani D. Place of parenteral cephalosporins in the ambulatory setting: clinical evidence. Drugs. 2000;3:47.
O’Donnell JA, Gelone SP. Fluoroquinolones. Infect Dis Clin North Am. 2000;14:489.
Tarkin IS, Dunman PM, Garvin KL. Improving the treatment of musculoskeletal infections with molecular diagnostics. Clin Orthop Relat Res. 2005;437:83.
American Academy of Orthopaedic Surgeons Advisory Statement, Preventing the transmission of bloodborne pathogens February 2001, revised 2008. Available at www.aaos.org/wordhtml/papers/advistmt/1018.htm
American College of Surgeons Committee on Perioperative Care. Statement on sharps safety. Bull Am Coll Surg. 2007;92:34.
Azar FM. Tissue processing: role of secondary sterilization techniques. Clin Sports Med. 2009;28:191.
Bassett IV, Freedberg KA, Walensky RP. Two drugs or three? Balancing efficacy, toxicity, and resistance in postexposure prophylaxis for occupational exposure to HIV. Clin Infect Dis. 2004;39:395.
Berguer R, Heller PJ. Strategies for preventing sharps injuries in the operating room. Surg Clin North Am. 2005;855:1299.
Biviji AA, Paiement GD, Steinbach LS. Musculoskeletal manifestations of human immunodeficiency virus infection. J Am Acad Orthop Surg. 2002;10:312.
Bouvet E, Pellissier G, Abiteboul D, L’Hériteau F, Group for the Prevention of Occupational Infections in Healthcare Workers. Is double gloving an effective barrier to protect surgeons against blood exposure due to needlestick injury? Infect Control Hosp Epidemiol. 2009;30:928.
Centers for Disease Control and Prevention. Updated U.S. public health service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Morb Mortal Wkly Rep. 50(RR-11), 2001.
Centers for Disease Control and Prevention. Division of HIV/AIDS prevention: basic statistics. www.cdc.gov/hiv/stats.htm#hivest, 2003. Available at
Centers for Disease Control and Prevention. Public Health Service guidelines for the management occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Morbid Mortal Wkly Rep. 2005;54:1.
Centers for Disease Control and Prevention, Surveillance of occupationally acquired HIV/AIDS in healthcare personnel as of December 2006 Available at www.cdc.gov/ncidod/dhqp/bp_hcp_w_hiv.html Accessed October 5, 2010
Centers for Disease Control and Prevention, HIV in the United States Available at www.cdc.gov/hiv Accessed November 18, 2010
Corey KE, Servoss JC, Casson D, et al. Pilot study of postexposure prophylaxis for hepatitis C virus in health care workers. Infect Control Hosp Epidemiol. 2009;30:1000.
Friedlaender GE. Appropriate screening for prevention of infection transmission by musculoskeletal allografts. Instr Course Lect. 2000;49:615.
Judd WR, Romanelli F, Smith KM, Murphy BS, Postexposure prophylaxis: a guide for prevention of human immunodeficiency virus transmission in orthopaedic surgery Available at www.orthosupersite.com/print.aspx?rid=27829 Accessed August 26, 2010
Laine T, Aarnio P. Glove perforation in orthopaedic and trauma surgery: a comparison between single, double indicator gloving and double gloving with two regular gloves. J Bone Joint Surg. 2004;86B:898.
Lemaire R, Masson JB. Risk of transmission of blood-borne viral infection in orthopaedic and trauma surgery. J Bone Joint Surg. 2000;82B:313.
McAllister DR, Joyce MJ, Mann BJ, Vangsness T. Allograft update: The current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35:2148.
Mikhael MM, Huddleston PM, Zobitz ME, et al. Mechanical strength of bone allografts subjected to chemical sterilization and other terminal processing methods. J Biomech. 2008;41:2816.
Mroz TE, Joyce MJ, Steinmetz MP, et al. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16:559.
Panlilio A. Current issues and update on human immunodeficiency virus infection in the orthopaedic setting. Instr Course Lect. 2000;49:621.
Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated US Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1.
Robert LM, Chamberland ME, Cleveland JL, et al. Investigation of patients of health care workers infected with HIV. Centers for Disease Control and Prevention database. Ann Intern Med. 1995;122:653.
Stramer SL. Current risks of transfusion-transmitted agents: a review. Arch Pathol Lab Med. 2007;131:702.
UNAIDS/WHO (Joint United Nations Program on HIV/AIDS—World Health Organization). Global summary of HIV and AIDS epidemic. www.unaids.org/html/pub/topics/epidemiology/slides02/12-04/epicoredec04slide001_en_ppt.htm, December 2004. Available at
Vangsness CT, Dellamaggiora RD. Current safety sterilization and tissue banking issues for soft tissue allografts. Clin Sports Med. 2009;28:183.
Wittmann A, Kralj N, Köver J, et al. Study of blood contact in simulated surgical needlestick injuries with single or double latex gloving. Infect Control Hosp Epidemiol. 2009;30:53.
World Health Organization Regional Office for the Western Pacific, Fact sheets: 3 × 5—responding to a global crisis Available at www.wpro.who.int/media_centre/fact_sheets/fs_200312_3X5AIDS.htm
Worldwide HIV & AIDS statistics, 2009 AIDS epidemic update Available at www.avert.org/worldstats.htm Accessed October 5, 2010
Ayliffe GA. Role of the environment of the operating suite in surgical wound infection. Rev Infect Dis. 1991;13:S800.
Bowerman SG, Green NE, Mencio GA. Decline of bone and joint infections attributable to Haemophilus influenzae type b. Clin Orthop Relat Res. 1997;341:128.
Boxma H, Broekhuizen T, Patka P, et al. Randomised controlled trial of single-dose antibiotic prophylaxis in surgical treatment of closed fractures: the Dutch trauma trial. Lancet. 1996;27:1133.
Brennan PJ, DeGirolamo MP. Musculoskeletal infections in immunocompromised hosts. Orthop Clin North Am. 1991;22:389.
Christou N. Perioperative nutritional support: immunologic defects. J Parenter Enter Nutr. 1990;14:186.
Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281.
Deshmukh N, Kramer JW, Kjellberg SI. A comparison of 5-minute povidone-iodine scrub and 1-minute povidone-iodine scrub followed by alcohol foam. Mil Med. 1998;163:145.
Donatto KC. Orthopedic management of septic arthritis. Rheumatol Dis Clin North Am. 1998;24:275.
Esterhai, T, Gristina, AG, Poss, R. Musculoskeletal infection. Chicago: American Academy of Orthopaedic Surgeons, 1992.
Evans RP, Nelson CL, Harrison BH. The effect of wound environment on the incidence of acute osteomyelitis. Clin Orthop Relat Res. 1993;286:289.
Foster MR, Heppenstall RB, Friedenberg ZB, et al. A prospective assessment of nutritional status and complications in patients with fractures of the hip. J Orthop Trauma. 1990;4:49.
Garre MA, Boles JM, Youinou PY. Current concepts in immune derangement due to undernutrition. J Parenter Enter Nutr. 1987;11:309.
Gentry LO, Rodriquez GG. Oral ciprofloxacin compared with parenteral antibiotics in the treatment of osteomyelitis. Antimicrob Agents Chemother. 1990;34:40.
Gentry LO, Rodriquez-Gomez G. Ofloxacin versus parenteral therapy for chronic osteomyelitis. Antimicrob Agents Chemother. 1991;35:538.
Gillespie WJ. Epidemiology in bone and joint infection. Infect Dis Clin North Am. 1990;4:361.
Hingst V, Juditzki I, Heeg P, et al. Evaluation of the efficacy of surgical hand disinfection following a reduced application time of 3 instead of 5 min. J Hosp Infect. 1992;20:79.
Janik J. Electric cautery lowers contamination threshold for infection by laparotomies. Am J Surg. 1998;175:263.
Jensen JE, Jensen TG, Smith TK, et al. Nutrition in orthopaedic surgery. J Bone Joint Surg. 1982;64A:1263.
Lee JT. Commentary on the “guideline for prevention of surgical site infection, 1999,”. AJIC. 1999;27:96.
Louis SS, Steinberg EL, Gruen OA, et al. Outer gloves in orthopaedic procedures: a polyester/stainless steel wire weave glove liner compared with latex. J Orthop Trauma. 1998;12:101.
Ludwig KA, Carlson MA, Condon RE. Prophylactic antibiotics in surgery. Annu Rev Med. 1993;44:385.
Mader JT, Mohan D, Calhoun J. A practical guide to the diagnosis and management of bone and joint infections. Drugs. 1997;54:253.
Maranan MC, Moreira B, Boyle-Vavra S, Daum RS. Antimicrobial resistance in staphylococci: epidemiology, molecular mechanisms, and clinical relevance. Infect Dis Clin North Am. 1997;11:813.
Marshall JC, Meakins JL, Immune responses and musculoskeletal disease. Evarts, CM, ed. Surgery of the musculoskeletal system, ed 2, New York: Churchill Livingstone, 1990.
Masterson BJ. Cleansing the surgeon’s hands. Sci Am Surgeon. 1996;2:3.
Moro ML, Carrieri MP, Tozzi AE, et al. Risk factors for surgical wound infections in clean surgery: a multicenter study. Italian PRINOS Study Group, An Ital Chir, 1996;67.
Namias N, Harvill S, Ball S, et al. Cost and morbidity associated with antibiotic prophylaxis in the ICU. J Am Coll Surg. 1999;188:225.
Nelson CL, Prevention of infection. Evarts, CM, ed. Surgery of the musculoskeletal system, ed 2, New York: Churchill Livingstone, 1990.
Norden CW. Antibiotic prophylaxis in orthopaedic surgery. Rev Infect Dis. 1991;13:13.
Oishi CS, Carrion WV, Hoaglund FT. Use of parenteral prophylactic antibiotics in clean orthopaedic surgery. Clin Orthop Relat Res. 1993;296:249.
O’Shaughnessy M, O’Malley VP, Corbett G, et al. Optimum duration of surgical scrub-time. Br J Surg. 1991;78:685.
Puskarich CL, Nelson CL, Nusbickel FR, et al. The use of two nutritional indicators in identifying long bone fracture patients who do and do not develop infections. J Orthop Res. 1990;8:799.
Rainey-McDonald CG, Holliday RL, Wells GA, et al. Validity of a two-variable nutritional index for use in electing candidates for nutritional support. J Parenter Enter Nutr. 1983;7:15.
Salvati EA, Robinson RP, Zeon SM, et al. Infection rates after 3175 total hip and total knee replacements with and without a horizontal unidirectional filtered air-flow system. J Bone Joint Surg. 1982;64A:525.
Wheelock SM, Lookinland S. Effect of surgical hand scrub time on subsequent bacterial growth. AORN J. 1997;65:1087.
Becker W, Palestro CJ, Winship J, et al. Rapid imaging of infections with a monoclonal antibody fragment (LeukoScan). Clin Orthop Relat Res. 1996;329:263.
Boutin RD, Brossmann J, Sartoris DJ, et al. Update on imaging of orthopedic infections. Orthop Clin North Am. 1998;29:41.
Dietz FR, Koontz FP, Found EM, et al. The importance of positive bacterial cultures of specimens obtained during clean orthopaedic operations. J Bone Joint Surg. 1991;73A:1200.
Faden H, Grossi M. Acute osteomyelitis in children: reassessment of etiologic agents and their clinical characteristics. Am J Dis Child. 1991;145:65.
Hakki S, Harwood S, Morissey M, et al. Comparative study of monoclonal antibody scan in diagnosing orthopaedic infection. Clin Orthop Relat Res. 1997;335:275.
Hoeffel DP, Hinrichs SH, Garvin KL. Molecular diagnostics for the detection of musculoskeletal infection. Clin Orthop Relat Res. 1999;360:37.
Howard CB, Einhorn M, Dagan R, et al. Ultrasound in diagnosis and management of acute haematogenous osteomyelitis in children. J Bone Joint Surg. 1993;75B:79.
Lewin JS, Rosenfeld NS, Hoffer PB, et al. Acute osteomyelitis in children: combined Tc-99m and Ga-67 imaging. Radiology. 1986;158:795.
Mazur JM, Ross G, Cummings J, et al. Usefulness of magnetic resonance imaging for the diagnosis of acute musculoskeletal infections in children. J Pediatr Orthop. 1995;15:144.
McAfee JG. What is the best method for imaging focal infections [editorial]? J Nucl Med. 1990;31:413.
Patzakis MJ, Wilkins J, Kumbar J, et al. Comparison of the results of bacterial cultures from multiple sites in chronic osteomyelitis of long bones. J Bone Joint Surg. 1994;76A:664.
Perry CR, Pearson RL, Miller GA. Accuracy of cultures of material from swabbing of the superficial aspect of the wound and needle biopsy in the preoperative assessment of osteomyelitis. J Bone Joint Surg. 1991;73A:745.
Royle SG. Investigation of the irritable hip. J Pediatr Orthop. 1992;12:396.
Scott RJ, Christofersen MR, Robertson WWJ, et al. Acute osteomyelitis in children: a review of 116 cases. J Pediatr Orthop. 1990;10:649.
Shauwecker DS, Braunstein EM, Wheat LJ. Diagnostic imaging of osteomyelitis. Infect Dis Clin North Am. 1990;4:441.
Spangehl MJ, Younger AS, Masri BA, et al. Diagnosis of infection following total hip arthroplasty. Instr Course Lect. 1998;47:285.
Unkila-Kallio L, Kallio MJT, Eskola J, et al. Serum C-reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of children. Pediatrics. 1994;93:59.
Wegener WA, Alavi A. Diagnostic imaging of musculoskeletal infection. Orthop Clin North Am. 1991;22:401.
Wheat J. Diagnostic strategies in osteomyelitis. Am J Med. 1985;78:218.
Zawin JK, Hoffer FA, Rand FF, et al. Joint effusion in children with an irritable hip: US diagnosis and aspiration. Radiology. 1993;187:459.
Chan YS, Ueng SW, Wang CJ, et al. Management of small infected tibial defects with antibiotic-impregnated autogenic cancellous bone grafting. J Trauma. 1998;45:758.
Gentry LO. Newer concepts in antimicrobial therapy. Clin Orthop Relat Res. 1990;261:23.
Lister J. The antiseptic system: on a new method of treating compound fracture, abscess, etc, with observations on the conditions of suppuration. Lancet. 1867;1:326.
Wachol-Drewek Z, Pfeiffer M, Scholl E. Comparative investigation of drug delivery of collagen implants saturated in antibiotic solutions and a sponge containing gentamicin. Biomaterials. 1996;17:1733.
American Academy of Orthopaedic Surgeons Advisory Statement. HIV-infected orthopaedic surgeons. Park Ridge, IL: AAOS; 1991.
American Academy of Orthopaedic Surgeons Task Force on AIDS and Orthopaedic Surgery. Recommendations for the prevention of human immunodeficiency virus (HIV) transmission in the practice of orthopaedic surgery. Park Ridge, IL: AAOS; 1989.
Bell D. Human immunodeficiency virus transmission in health care settings: risk and reduction. Am J Med. 1991;91:294S.
Bell KM, Clement DA. Eye protection for the surgeon. J R Coll Surg Edinb. 1991;36:178.
Boyce T, Edwards J, Scarborough N. Allograft bone: the influence of processing on safety and performance. Orthop Clin North Am. 1999;30:571.
Buck BE, Resnick L, Shah SM, et al. Human immunodeficiency virus cultured from bone: implications for transplantation. Clin Orthop Relat Res. 1990;251:249.
Burcham J, Marmor M, Dubin N, et al. CD4 is the best predictor of the development of AIDS in a cohort of HIV-infected homosexual men. AIDS. 1991;5:365.
Centers for Disease Control. Provisional Public Health Service interagency recommendations for screening donated blood and plasma for antibody to the virus causing acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep. 1985;32:1.
Centers for Disease Control. Recommendations for prevention of human immunodeficiency virus transmission in health care settings. MMWR Morb Mortal Wkly Rep. 1987;36:3.
Centers for Disease Control. Update: acquired immunodeficiency syndrome and human immunodeficiency virus infection among health care workers. MMWR Morb Mortal Wkly Rep. 1988;37:229.
Centers for Disease Control. Estimates of HIV prevalence and projected AIDS cases. MMWR Morb Mortal Wkly Rep. 1990;39:110.
Centers for Disease Control. HIV prevalence estimates and AIDS care projections for the U.S. MMWR Morb Mortal Wkly Rep. 1990;39(RR-16):1.
Centers for Disease Control. Recommendations for preventing transmission of human immunodeficiency virus and hepatitis B virus to patients during exposure-prone invasive procedures. MMWR Morb Mortal Wkly Rep. 1991;40(RR-8):1.
Centers for Disease Control. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41(RR-17):1.
Centers for Disease Control. Guidelines for the performance of CD4 T-cell determinations in persons with HIV. MMWR Morb Mortal Wkly Rep. 1992;41(RR-8):1.
Centers for Disease Control. Projections of the number of persons diagnosed with AIDS and the number of immunosuppressed HIV infected persons—U.S. 1992-1994. MMWR Morb Mortal Wkly Rep. 1992;41(RR-18):1.
Centers for Disease Control and Prevention. National HIV serosurveillance summary: results through 1992. Atlanta: U.S. Department of Health and Human Services, Public Health Service; 1993. publication HIV/NCID/11-93/036
Centers for Disease Control and Prevention. HIV/AIDS surveillance report. MMWR Morb Mortal Wkly Rep. 1995;7:20.
Centers for Disease Control and Prevention. Public health service guidelines for the management of health care workers’ exposure to HIV and recommendations for postexposure prophylaxis. MMWR Morb Mortal Wkly Rep. 47(RR-7), 1998.
Chamberland ME, Bell DM. HIV transmission from health care worker to patient: what is the risk? Ann Intern Med. 1992;116:871.
Chamberland ME, Ciesielski CA, Howard RJ, et al. Occupational risk of infection with human immunodeficiency virus. Surg Clin North Am. 1995;75:1057.
Chamberland ME, Conley LJ, Bush TJ, et al. Health care workers with AIDS: national surveillance update. JAMA. 1991;266:3459.
Chang HJ, Luck JV, Jr., Bell DM, et al. Transmission of human immunodeficiency virus infection in the surgical setting. J Am Acad Orthop Surg. 1996;4:279.
Chou L, Reynolds MR, Esterhai JL, Jr. Hazards to the orthopaedic trauma surgeon: occupational exposure to HIV and viral hepatitis. J Orthop Trauma. 1996;10:289.
Conley LJ, Holmberg SD. Transmission of AIDS from blood screened negative for antibody to the human immunodeficiency virus. N Engl J Med. 1992;326:1499.
Emanuel EJ. Do physicians have an obligation to treat patients with AIDS? N Engl J Med. 1988;318:1686.
Fahey BJ, Henderson DK. Minimizing risk for occupational blood-borne infections. JAMA. 1990;264:1189.
Fahey JL, Taylor JM, Detels R, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type I. N Engl J Med. 1990;322:166.
Fitch KN, Alvarez P, Medina R, et al. Occupational transmission of HIV in health care workers. Eur J Public Health. 1995;5:175.
Gant P, Wong PK, Meyer RD. Pyomyositis in a patient with the acquired immunodeficiency syndrome. Arch Intern Med. 1988;148:1608.
Gerberding JL, Quebbeman EJ, Rhodes RS. Hand protection. Surg Clin North Am. 1995;75:1133.
Hardy WD. Natural history of HIV infection and disease. In: Wilson, SE, Williams, RA. Surgical problems in the AIDS patient. New York: Igaku-Shoin, 1994.
Herbert AM, Walker DM, Kavies KJ, et al. Occupationally acquired hepatitis C virus infection. Lancet. 1992;339:305.
Hirsch MS. Chemotherapy of human immunodeficiency virus infections: current practice and future prospects. J Infect Dis. 1990;161:845.
Howard RJ. Transfusion and transplantation: blood transfusions and HIV; tissue transplantation. In: Wilson, SE, Williams, RA. Surgical problems in the AIDS patient. New York: Igaku-Shoin, 1994.
Ippolito G, Puro V, De Carli G. The risk of occupational human immunodeficiency virus infection in health care workers: Italian multicenter study. The Italian Study Group on Occupational Risk of HIV Infection. Arch Intern Med. 1993;153:1451.
Jochen ABB. Occupationally acquired hepatitis C virus infection. Lancet. 1992;339:305.
Joint United Nations Programme on HIV/AIDS (UNAIDS)-WHO. Revised recommendations for the selection and use of HIV antibody tests. Wkly Epidemiol Rec. 1997;72:81.
Karon JM, Buehler JW, Byers RH, et al. Projections of the number of persons diagnosed with AIDS and the number of immunosuppressed HIV-infected persons—United States, 1992-1994. MMWR Morb Mortal Wkly Rep. 1992;41:1.
Lane N. HIV disease and arthritis: diagnostic and therapeutic dilemmas. In: Cohen, PT, Sande, MA, Volberding, PA. The AIDS knowledge base. Boston: Little, Brown, 1994.
Learmont J, Tindall B, Evans L, et al. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet. 1992;340:863.
Lee CA, Phillips AN, Elford J, et al. Progression of HIV disease in a hemophiliac cohort followed for 11 years and the effect of treatment. BMJ. 1991;303:1093.
Lot F, Sequier JC, Fegueux S, et al. Probable transmission of HIV from an orthopedic surgeon to a patient in France. Ann Intern Med. 1999;5:1.
Luck JV, Jr., Logan LR, Benson DR, et al. Human immunodeficiency virus infection: complications and outcome of orthopaedic surgery. J Am Acad Orthop Surg. 1996;4:297.
Mast S, Gerberding JL. Factors predicting infectivity following needlestick exposure to HIV: an in vitro model. Clin Res. 1991;1:39.
McCarthy ML, Bosse MJ, Preas MA, et al. Orthopedic trauma surgeon’s attitudes and practices towards bloodborne pathogens. J Orthop Trauma. 1996;10:383.
McKinney WP, Young MJ. The cumulative probability of occupationally acquired HIV infection: the risks of repeated exposures during a surgical career. Infect Control Hosp Epidemiol. 1990;11:243.
McLeod GX, Hammer SM. Zidovudine: five years later. Ann Intern Med. 1992;117:487.
Nichols RL. Percutaneous injuries during operation: who is at risk for what? JAMA. 1992;267:2938.
Palca J. The sobering geography of AIDS. Science. 1991;252:373.
Panlilio AL, Foy DR, Edwards JR, et al. Blood contacts during surgical procedures. JAMA. 1991;265:1533.
Panlilio AL, Shapiro CN, Schable CA, et al. Serosurvey of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among hospital-based surgeons. J Am Coll Surg. 1995;180:16.
Pantaleo G, Graziosi C, Fauci A. The immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327.
Polish LP, Tong MT, Co RL, et al. Risk factors for hepatitis C virus infection among health care personnel in a community hospital. Am J Infect Control. 1993;21:196.
Popejoy SL, Fry DE. Blood contact and exposure in the operating room. Surg Gynecol Obstet. 1991;172:480.
Quebbeman EJ, Telford GL, Hubbard S, et al. Risk of blood contamination and injury to the operating room personnel. Ann Surg. 1991;214:614.
Phillips AN, Lee CA, Elford J, et al. Serial CD4 lymphocyte counts and development of AIDS. Lancet. 1991;337:389.
Ranki A, Krohn M, Allain J-P, et al. Long latency precedes overt seroconversion in sexually transmitted human immunodeficiency virus infection. Lancet. 1987;2:589.
Royce RA, Luckmann RS, Fusaro RE, et al. The natural history of HIV-1 infection: staging classification of disease. AIDS. 1991;5:355.
Schiff SJ. A surgeon’s risk of AIDS. J Neurosurg. 1990;73:651.
Shapiro CN. Occupational risk of infection with hepatitis B and hepatitis C virus. Surg Clin North Am. 1995;75:1047.
Stein DS, Korvick JA, Vermund SH. CD4 lymphocyte cell enumeration for prediction of clinical course of human immunodeficiency virus disease: a review. J Infect Dis. 1992;165:352.
Stein, JH, ed. Internal medicine, ed 4, St. Louis: Mosby, 1994.
Tokars JI, Bell DM, Culver DH, et al. Percutaneous injuries during surgical procedures. JAMA. 1992;267:2899.
Tokars JI, Chamberland ME, Schable CA, et al. A survey of occupational blood contact and HIV infection among orthopedic surgeons. JAMA. 1992;268:489.
Tokars JI, Marcus J, Culver DH, et al. Surveillance of HIV infection and zidovudine use among health care workers after occupational exposure to HIV-infected blood. The CDC Cooperative Needlestick Surveillance Group. Ann Intern Med. 1993;118:913.
Volberding PA, Legakos SW, Koch MA, et al. Zidovudine in asymptomatic human immunodeficiency virus infection: a controlled trial in persons with fewer than 500 CD4-positive cells per cubic millimeter. N Engl J Med. 1990;322:941.
Yankauer A. The deadliest plague. Am J Public Health. 1989;79:821. (editorial)
Yarchoan R, Venzon DJ, Pluda JM, et al. CD4 count and the risk for death in patients infected with HIV receiving antiretroviral therapy. Ann Intern Med. 1991;115:184.