CHAPTER 229 General Principles in Evaluating and Treating Peripheral Nerve Pathology, Injuries, and Entrapments and Their Historical Context
During much of the 20th century, peripheral nerve surgery struggled with a single question—how should a damaged nerve be managed? Reliable surgical approaches did not emerge until after World War II. Before this period, surgeons first debated the utility of performing any kind of repair and then watched with disappointment as they realized that many direct repairs failed. Much of this history has been reviewed in detail recently.1
In the mid-19th century there were reports that recovery could take place after a nerve was severed, even without surgical repair,2 and other published reports showed that suture repair could produce good results.3,4 However, over the course of the following 100 years, no reliable success was achieved with any method.
Wars can lead to medical advances, and nerve surgery has not been an exception. One of the lessons from World War II was that gunshot or shell fragment wounds, whether they sever a nerve (<15% of cases) or leave it in continuity, are blunt injuries.5 Such injuries have an element of stretch/contusion as a result of implosion and then explosion affecting a length of nerve, even when a missile does not make a direct hit but passes close by. This leads to injury over the length of the nerve. The extent of this injury is unpredictable acutely, so even if the nerve was severed and the ends distracted, World War II studies showed a large measure of failure when the nerve was repaired acutely or what some would designate primarily.
Another lesson learned from large American and less so from British studies of such nerve injuries secondary to shell fragments was that inspection and palpation of the lesion at the operating table did not always provide an accurate prediction for or against recovery.6 Review of pathologic slides submitted from resected nerves indicated that many lesions in continuity had adequate anatomic regeneration and, most likely, had unnecessarily been resected. The reverse implication was that many other nerves had probably, after operative inspection and perhaps only neurolysis, not undergone needed resection and repair.
These observations led Nulsen and Lewey to propose the use of simple stimulation to evaluate such lesions.7 Such evaluation was valuable because positive distal muscle responses could antedate clinical recovery by weeks. The difficulty with the test was that one had to wait many months with major nerve lesions before the test could show regeneration. If the response was negative after such an elapse of time, repair would be greatly delayed and result in outcomes less than optimal.
These observation led in the late 1960s to the development of nerve action potential (NAP) or nerve-to-nerve recording across initially experimental and eventually actual clinical lesions in continuity.8 This permitted operative evaluation within a few months because the finding of an NAP across such a lesion correlated with the presence of 3000 to 4000 fibers just distal to the lesion. These fibers were usually of moderate size and had some degree of early myelination. Recovery to a grade 3 or better Louisiana State University Health Sciences Center (LSUHSC) level occurred in such patients with positive NAPs about 93% of the time. Absence of such a response leading to resection was confirmed pathologically as a neurotmetic (Seddon classification) or grade IV (Sunderland classification) lesion that had little or no opportunity for spontaneous recovery. Occasionally, the presence of an NAP was due to regrowth in a portion of the cross section of the nerve with poor growth elsewhere. This led to split repair in which a portion of the nerve underwent neurolysis and another portion underwent repair, usually with interfascicular grafts.
Seddon experimented with the use of nerve autografts after World War II but generally had little success.9,10 It was only at the end of the 1960s that Hanno Millesi finally solved this problem with use of carefully constructed grouped interfascicular grafts.11 He discovered that Seddon’s failure with nerve grafts was due to technical surgical factors that could be corrected to achieve reliable success. He also showed that many of the previous attempts at direct repair failed when the repair line was under tension or scar tissue formed in the nerve ends and blocked regrowing axons. The 1970s ushered in a series of confirmatory experiments,12–14 and the 1980s saw the onset of full-scale acceptance of interposition nerve graft repair after stump excision as a paradigm to guide management.15
The all too frequent lesions in continuity with serious distal loss can usually be observed for several months. If no clinical or electromyographic evidence of recovery has taken place by this time, exploration and decisions about complete repair are aided by intraoperative electrophysiologic studies,16 especially NAP recordings across the lesions.
Guided by these principles, David Kline and Alan Hudson gained a large volume of experience from the 1970s through the 1990s that has provided the basis for classification and prognostic assessment of a wide variety of different types of clinical nerve injury situations.17,18 Their work, along with that of Mackinnon and Dellon,15 Birch and colleagues,19 and others, also resulted in the development of a complete set of surgical techniques and exposures that provided approaches and exposure methods for virtually every nerve in the body.15,17,19–21 Outcomes have been graded for sensory and motor recovery with the LSUHSC system17 and, in many other instances, the British Medical Research Council system.22 Some current work has and certainly future work will emphasize the practical usefulness of the limb and indices of patient satisfaction in addition to motor and sensory grades.
Significant progress has also been made in understanding the proper use of a variety of possible types of nerve transfer procedures. This body of knowledge now provides a great deal of guidance for the development of reinnervation/reanimation strategies in the setting of severe brachial plexus injuries.23
Blunt injuries differ from sharp ones by knife or glass that sever the nerve completely or incompletely. When a nerve is transected by these sharp forces, relatively acute (within 72 hours) repair is indicated. When transected, nerves tend to retract because of their elasticity. With more acute repair, retraction is minimal, scar has not had time to become established, and the surgical anatomy is straightforward. Data on both sharply and bluntly transected nerves show that when the timing of repair is matched to the situation, the results are greatly optimized, even with difficult challenges in nerve surgery such as the brachial plexus.17
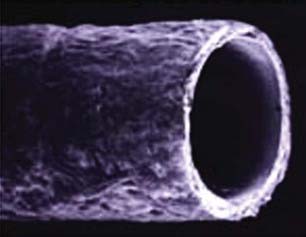
The next major technical advance in nerve repair included the solution to the problem of how to make functional synthetic tubes24–26 for use in place of interposition autografts (Fig. 229-1). A randomized prospective outcome trial demonstrated that tubes can be used reliably for successful repair of short nerve gaps up to 3 cm in length without grafts.27 These synthetics have greatly simplified repair of some nerve injuries.
Entrapment Syndromes
Recognition of distal entrapments such as carpal tunnel syndrome and cubital tunnel syndrome led to useful surgical procedures as early as the 1930s.28 For most patients with these conditions, treatment has changed little since that time. Newer surgical methodologies such as endoscopic carpal tunnel release have resulted in faster recovery times from surgery, but complications of endoscopic release, such as a higher rate of laceration of the recurrent branch of the median nerve and the ulnar nerve than with open surgery, have inhibited the widespread use of endoscopic methods. Advances in endoscopic technology have mostly resolved this differential complications issue,29 but outcomes have been so good with open carpal tunnel surgery that it is still the most common method used by neurosurgeons.30,31
Ulnar nerve surgery has been somewhat of a confused area because of the availability of other operations, such as subcutaneous or submuscular transposition or epicondylectomy. Outcome studies have failed to show any advantage over the older, simpler in situ decompressive surgery in most cases.32 Exceptions may be ulnar entrapment with more severe grades of loss or previous operations, in which case submuscular transposition may have an advantage.17,20
Minimal-Access Surgery for Major Proximal Nerve Entrapments
Among the major advances in peripheral nerve surgery in the early 21st century has been the introduction of minimal-access approaches for release of proximal nerve entrapments.33,34 Until recently, the extensive exposure required for repair of complex and severe nerve injuries has tended to guide the approaches used by nerve surgeons trying to treat small focal entrapments of proximal nerves. In the treatment of sciatic nerve pain syndromes, for instance, large operations that require transection of the gluteal muscle mass can lead to an impaired gait that can be more disruptive to the patient than the original pain syndrome. The introduction of small nondestructive approaches to the brachial plexus and pelvic nerves has led to outpatient management of these conditions with no formal hospital admission for surgery. The application of adhesiolystic agents initially developed for other surgical specialities (e.g., hyaluronate-based Seprafilm) has made it possible that neuroplasty, in which nerves are released, will not in most cases be reversed by recurrent adhesions according to a recent study,33 although it remains to be fully proved that decreases in the occurrence of postoperative adhesions per se result in better outcomes.
Impact of Nerve Imaging and Open Magnetic Resonance–Guided Injections
Just as angiography, computed tomography, and MRI transformed the process of diagnosis and surgical management in cranial and spinal neurosurgery, the late arrival of effective imaging for peripheral nerves is starting to transform and energize the field of peripheral nerve surgery.35–38 The results have, in some cases, provided insight into long-standing disputes in nerve surgery.
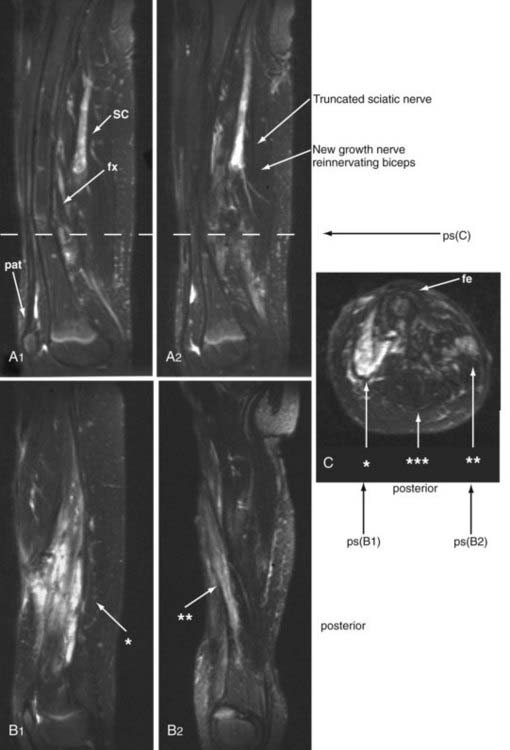
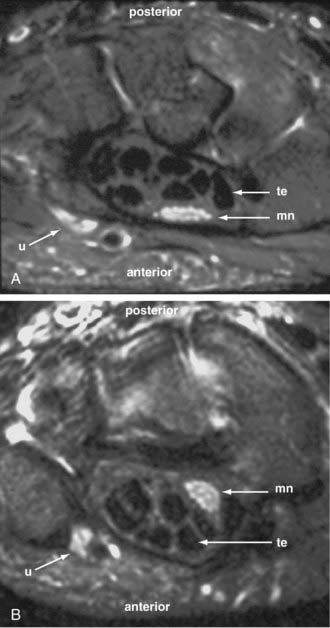
Through much of the 19th century and well into the 20th century, some surgeons believed that severed nerves could recover distal innervation without surgical repair. Subsequently, patients who did not recover underwent surgical exploration and were found to need nerve repair for recovery. However, without imaging, there was no way to know what happened to patients who may have suffered a torn or severed nerve but then recovered without surgery. The presumption has been that these patients had bruised nerves with gross continuity that recovered. However, in 2002, magnetic resonance neurography revealed that as in other mammals, severed human nerves could sprout viable new terminal nerve elements in some situations39 (Fig. 229-2)—thus finally confirming Virchow’s much doubted claim from the 1860s. Nonetheless, it is clear that virtually all severed nerves need surgical repair if recovery is to be expected.
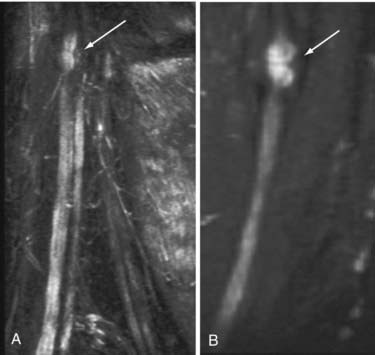
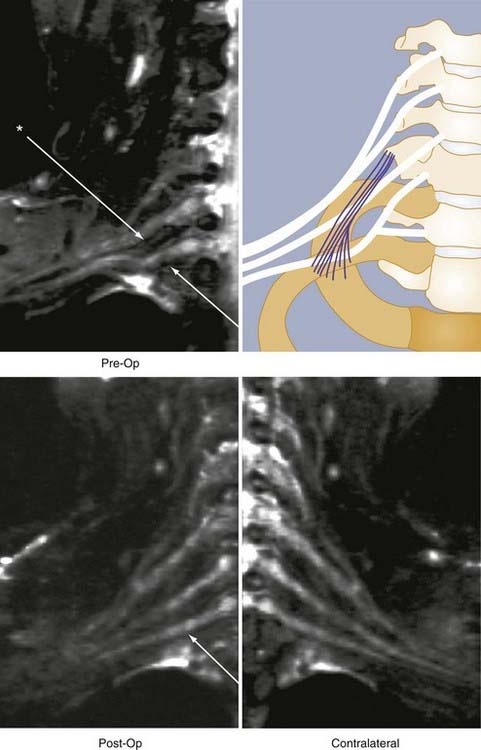
It is no longer necessary to wait for failure of improvement to occur and surgical exploration to learn whether a nerve is severed (Fig. 229-3). Therefore, as soon as the postinjury edema has resolved, a patient may be imaged and early repair of severed or disrupted nerves can be planned. If a patient is failing to improve after nerve repair, it is sometimes possible to discover why a repair has failed before additional months elapse (Fig. 229-4). Distraction of previously repaired stumps or large neuroma formation may be seen and indicate the need for further repair.
In addition to compressive nerve entrapment, magnetic resonance neurography has shown that neuropathic pain and disability can result from alteration of the course of the nerve and failure of normal nerve movement. An image showing the median nerve palmar to the tendons with the wrist in flexion fits expectations from anatomy, but an image showing the nerve dorsal to the tendons with the wrist in extension is a surprise (Fig. 229-5), particularly when we learn that the two images were obtained at the same level of the wrist in the same asymptomatic and normal individual. This finding led to the discovery of a class of carpal tunnel syndrome patients in whom there is no mechanical compression but tethering of the median nerve to the palmar surface of the carpal tunnel leads to the syndrome; that is, in symptomatic imaging subjects, the nerve did not translocate with wrist extension but did move in those without symptoms.
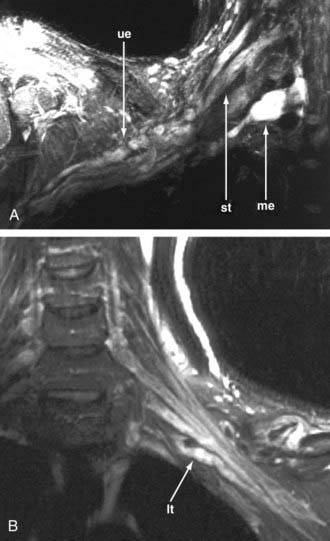
The very existence of thoracic outlet syndrome has been questioned on philosophic grounds and because of the absence of agreement on diagnostic tests—even with more than a hundred thousand operations for the condition being performed annually. With imaging, one can typically see the distorted course of the C8 and or T1 spinal nerves and lower trunk of the brachial plexus in a patient with hand pain and weakness involving both the thenar and hypothenar musculature (true neurogenic thoracic outlet syndrome) (Fig. 229-6). It has also proved feasible to see that surgical release of the entrapment site correlates with postoperative resolution of the symptoms. This promises to resolve a century-old battle of opinion regarding the routine matter of a treatable nerve entrapment—simply because it is now possible to observe abnormalities on a diagnostic image and correlate them with the clinical picture.
Electroneural Prostheses
As myoelectric prostheses have advanced considerably in quality and complexity, the possibility of constructing truly eloquent multiple-joint robotic limbs with dextrous hand and finger movement has now become a reality. To provide detailed control of such devices, new surgical techniques for deployment of the severed nerve in severed limbs have emerged.40,41 It is now possible to split the distal components of the proximal stump of a severed motor nerve to establish a grid of subcutaneous muscle activation zones that can be detected by external sensors and used to drive and control elaborate robotic movements with replacement limbs.
Birch R, Bonney G, Wynn Parry CB. Surgical Disorders of the Peripheral Nerves. New York: Churchill Livingstone; 1998.
Ecklund JM, Ling GS. From the battlefront: peripheral nerve surgery in modern day warfare. Neurosurg Clin N Am. 2009;20:107.
Filler AG. Diagnosis and management of pudendal nerve entrapment syndromes: impact of MR neurography and open MR-guided injections. Neurosurgy Q. 2008;18:1.
Filler AG, Haynes J, Jordan SE, et al. Sciatica of nondisc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J Neurosurg Spine. 2005;2:99-115.
Filler AG, Howe FA, Hayes CE, et al. Magnetic resonance neurography. Lancet. 1993;341:659.
Filler AG, Kliot M, Howe FA, et al. Application of magnetic resonance neurography in the evaluation of patients with peripheral nerve pathology. J Neurosurg. 1996;85:299.
Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin. 2004;22:643.
Kim DH, Midha R, Murovic JA, et al. Kline & Hudson’s Nerve injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors, 2nd ed. Philadelphia: WB Saunders; 2008.
Kline DG, DeJonge BR. Evoked potentials to evaluate peripheral nerve injuries. Surg Gynecol Obstet. 1968;127:1239.
Kline DG, Hudson AR, Kim DH. Atlas of Peripheral Nerve Surgery. Philadelphia: WB Saunders; 2001.
Kuiken TA, Miller LA, Lipschutz RD, et al. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007;369:371.
Little KM, Zomorodi AR, Selznick LA, et al. An eclectic history of peripheral nerve surgery. Neurosurg Clin N Am. 2004;15:109.
Medical Research Council. Aids to the Examination of the Peripheral Nervous System, vol 45. London: Her Majesty’s Stationery Office. 1976.
Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16:E5.
Terzis JK, Smith KL. The Peripheral Nerve: Structure, Function, and Reconstruction. New York: Raven Press; 1990.
Zlowodzki M, Chan S, Bhandari M, et al. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2007;89:2591.
1 Little KM, Zomorodi AR, Selznick LA, et al. An eclectic history of peripheral nerve surgery. Neurosurg Clin N Am. 2004;15:109.
2 Virchow RLK. Die krankhaften Geschwülste; dreissig Vorlesungen, gehalten wärhend des Wintersemester 1862-1863 an den Universität zu Berlin, vol 1. Berlin: Hirschwald. 1863.
3 Nelaton A. Correspondence to the Society de Chirurgie, seance du 22 Juin 1864. Lancette Fr. 1864;37:307.
4 Laugier S. Seance de l’Academie des Sciences, Paris. Lancette Fr. 1864;37:297.
5 Lyons WR, Woodhall B. Atlas of Peripheral Nerve Injuries. Philadelphia: WB Saunders; 1949.
6 Woodhall B, Nulsen FE, White J, et al. Neurosurgical implications. In: Woodhall B, editor. Peripheral Nerve Regeneration. Washington, DC: Veterans Administration, U.S. Government Printing Office; 1957:569.
7 Nulsen FE, Lewey FH. Intraneural bipolar stimulation: a new aid in the assessment of nerve injuries. Science. 1947;106:301.
8 Kline DG, DeJonge BR. Evoked potentials to evaluate peripheral nerve injuries. Surg Gynecol Obstet. 1968;127:1239.
9 Seddon HJ. Nerve grafting. J Bone Joint Surg Br. 1963;45:447.
10 Seddon HJ. The use of autogenous nerve grafts for the repair of large gaps in peripheral nerves. Br J Surg. 1947;35:151.
11 Millesi H. [On the problem of overbridging defects of the peripheral nerves.]. Wien Med Wochenschr. 1968;118:182.
12 Millesi H, Meissl G, Berger A. The interfascicular nerve-grafting of the median and ulnar nerves. J Bone Joint Surg Am. 1972;54:727.
13 Millesi H, Meissl G, Berger A. Further experience with interfascicular grafting of the median, ulnar, and radial nerves. J Bone Joint Surg Am. 1976;58:209.
14 Terzis J, Faibisoff B, Williams B. The nerve gap: suture under tension vs. graft. Plast Reconstr Surg. 1975;56:166.
15 Mackinnon SE, Dellon AL. Surgery of the Peripheral Nerve. New York: Thieme; 1988.
16 Kline DG. Early evaluation of peripheral nerve lesions in continuity with a note on nerve recording. Am Surg. 1968;34:77.
17 Kim DH, Midha R, Murovic JA, et al. Kline & Hudson’s Nerve injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors, 2nd ed. Philadelphia: WB Saunders; 2008.
18 Kline DG, Hudson AR. Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. Philadelphia: WB Saunders; 1995.
19 Birch R, Bonney G, Wynn Parry CB. Surgical Disorders of the Peripheral Nerves. New York: Churchill Livingstone; 1998.
20 Kline DG, Hudson AR, Kim DH. Atlas of Peripheral Nerve Surgery. Philadelphia: WB Saunders; 2001.
21 Terzis JK, Smith KL. The Peripheral Nerve: Structure, Function, and Reconstruction. New York: Raven Press; 1990.
22 Medical Research Council. Aids to the Examination of the Peripheral Nervous System, vol 45. London: Her Majesty’s Stationery Office. 1976.
23 Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16:E5.
24 Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990;85:419.
25 Archibald SJ, Krarup C, Shefner J, et al. A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J Comp Neurol. 1991;306:685.
26 Archibald SJ, Shefner J, Krarup C, et al. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J Neurosci. 1995;15:4109.
27 Weber RA, Breidenbach WC, Brown RE, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036.
28 Learmonth JR. The principle of decompression in the treatment of certain diseases of the peripheral nerves. Surg Clin North Am. 1933;13:905.
29 Hankins CL, Brown MG, Lopez RA, et al. A 12-year experience using the Brown two-portal endoscopic procedure of transverse carpal ligament release in 14,722 patients: defining a new paradigm in the treatment of carpal tunnel syndrome. Plast Reconstr Surg. 2007;120:1911.
30 Huang JH, Zager EL. Mini-open carpal tunnel decompression. Neurosurgery. 2004;54:397.
31 Lo SL, Raskin K, Lester H, et al. Carpal tunnel syndrome: a historical perspective. Hand Clin. 2002;18:211.
32 Zlowodzki M, Chan S, Bhandari M, et al. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2007;89:2591.
33 Filler AG, Haynes J, Jordan SE, et al. Sciatica of nondisc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J Neurosurg Spine. 2005;2:99-115.
34 Filler AG. Diagnosis and management of pudendal nerve entrapment syndromes: impact of MR neurography and open MR-guided injections. Neurosurg Q. 2008;18:1.
35 Filler AG, Howe FA, Hayes CE, et al. Magnetic resonance neurography. Lancet. 1993;341:659.
36 Filler AG, Kliot M, Howe FA, et al. Application of magnetic resonance neurography in the evaluation of patients with peripheral nerve pathology. J Neurosurg. 1996;85:299.
37 Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin. 2004;22:643.
38 Filler AG. MR neurography and diffusion tensor imaging: origins, history & clinical impact of the first 50,000 cases with an assessment of efficacy and utility in a prospective 5,000 patient study group. Neurosurgery. in press
39 Filler AG. Demonstration of reinnervation of muscle by sprouting from a severed nerve in a human subject. Paper presented at the 32nd Annual Meeting of the Society for Neuroscience. Orlando, Florida. 2002.
40 Kuiken TA, Miller LA, Lipschutz RD, et al. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007;369:371.
41 Ecklund JM, Ling GS. From the battlefront: peripheral nerve surgery in modern day warfare. Neurosurg Clin N Am. 2009;20:107.