27 General Abdominal and Urologic Surgery
ABDOMINAL SURGERY AND UROLOGIC interventions after infancy make up a large fraction of anesthetic practice for the pediatric anesthesiologist. The field is rapidly evolving, with increased use of laparoscopic surgery, including robot-assisted procedures.1 This chapter focuses on the specific issues related to abdominal and urologic surgery, particularly in young children. The management of infants for pyloromyotomy and other neonatal abdominal procedures is discussed in Chapter 36.
General Principles of Abdominal Surgery
“The Full Stomach”: the Risk for Pulmonary Aspiration of Gastric Contents
A large number of abdominal surgeries are emergency procedures that require a rapid induction of anesthesia and protection of the airway to prevent regurgitation and aspiration. Children who present with these emergencies should be considered to be at risk for vomiting and/or regurgitation and aspiration during induction of and emergence from anesthesia. Adhering to fasting guidelines for elective surgery2 does not ensure that the stomachs of children with acute abdomens are empty of liquids and solids. The only metric that has been associated with gastric emptying after an acute emergency in children is the time interval between the last food ingested and the occurrence of the pathologic event or trauma.2 However, there is no firm fasting interval after a trauma that predicts a zero risk of regurgitation and aspiration. In a postal survey, 83% of anesthesiologists use a rapid-sequence induction (RSI) for children with a forearm fracture within 2 hours of feeding and after opioid administration, whereas fewer than 20% use an RSI for this fracture if the child had not eaten for 6 hours since the injury.3 The presence or absence of bowel sounds is also not predictive of gastric emptying or of the risk of regurgitation. Preoperatively, some children with acute abdomens are administered oral contrast agent before abdominal ultrasonography and/or computed tomography, in order to visualize the stomach contents and to estimate their volumes. However, these radiologic tools may not provide reliable estimates of the volume of the gastric contents.4,5 Indeed, the absence of gastric contents in these scans does not eliminate the risk of vomiting and regurgitation. Consequently, there is no evidence that delaying the surgical procedure for the express purpose of emptying the stomach will reduce the risk of regurgitation; it cannot predict when the risk of regurgitation will be zero and may actually increase the risk of complications by delaying surgical attention to the acute abdomen.
Rapid-Sequence Induction
RSI is recommended for children with a full stomach, to quickly secure the airway. This approach is intended to minimize the risk of aspiration, although it is not evidence based. The strategy is to predetermine the drug doses and have all the required airway equipment ready to use. Predetermined drug doses are administered in a rapid sequence and when muscle relaxation is present, the trachea is intubated and the cuff (if used) inflated. Many clinicians include cricoid pressure to occlude the esophageal lumen during RSI, although no randomized studies have demonstrated that this combination prevents regurgitation and aspiration compared with an inhalational or slow IV induction. This lack of evidence, combined with both theoretical and actual complications associated with RSI and cricoid pressure in children, has generated skepticism regarding their role in preventing vomiting in children who are at risk.6 Only 74% of anesthesiologists in Northern Ireland perform RSI for children scheduled for appendectomy, 78% in the United States use it for pyloromyotomy and 83% in England use it for forearm fractures within 2 hours of eating and after recent opioid administration.3,7,8 In two surveys, 16% and 28% of anesthesiologists in the United States and UK, respectively, reported that a number of children with full stomachs had experienced gastric regurgitation despite using RSI with cricoid pressure, and several of them had progressed to serious harm and even death.7,9 Despite the lack of evidence that RSI is effective in children with full stomachs, we continue to recommend this approach for the majority of children at risk. Whether cricoid pressure contributes substantively to preventing regurgitation when RSI is performed remains unclear. Complications of RSI, for the most part, relate to improperly performed RSI (e.g., excessive cricoid pressure distorting the anatomy of the airway,10 causing difficulty in securing the airway) or poor selection of patients (those with a known difficult airway, where a more measured approach must take precedence over concerns for possible aspiration).
RSI in infants and children requires more planning than it does in older children and adults for several reasons. First, the induction drugs should be flushed into the child’s veins using a separate flush syringe, to ensure a rapid bolus administration of the drugs. Succinylcholine remains useful for rapid-sequence tracheal intubation for brief procedures. The introduction of intermediate-acting neuromuscular blocking drugs (NMBDs) with rapid onset, coupled with concerns about the risk of hyperkalemia after succinylcholine in children with undiagnosed neuromuscular diseases (especially males less than 8 years of age), have dramatically reduced the use of succinylcholine in elective surgery. With the shift from succinylcholine to nondepolarizing NMBDs for rapid paralysis in young children, inability to intubate and prolonged paralysis may present serious, possible life-threatening problems. Although sugammadex reverses some NMBDs, its effectiveness is limited to rocuronium and vecuronium and is not always available.11–13 Second, preoxygenation is often difficult in infants and children because they commonly resist the tight application of the face mask needed to deliver high concentrations of oxygen. Failure to ventilate the lungs after induction and before tracheal intubation may result in desaturation more rapidly in young infants and children than older children, and in those with upper respiratory tract infections or other causes of a limited oxygen reserve.14,15 During laryngoscopy and intubation, mask ventilation with 100% oxygen should begin when the saturation decreases to 95%, to attenuate the nadir in oxygen saturation that follows.14 Third, the force needed to occlude the esophagus when applying cricoid pressure to infants and children is poorly understood and poorly applied, can distort the view of the larynx during laryngoscopy, may not occlude the lumen of the esophagus, and may actually deform the lumen of the trachea if excessive force is applied.6,10,16 Conversely, effective cricoid pressure that occludes the esophagus in children, may permit bag mask ventilation with up to 40 cm H2O peak inspiratory pressure without gastric insufflation.17 This is known as a modified RSI. Thus, if the first attempt at tracheal intubation fails or the child desaturates during laryngoscopy, properly maintained cricoid pressure allows bag and mask ventilation of the lungs to restore oxygenation, without increasing the risk of regurgitation.
Indications for Preoperative Nasogastric Tube Placement
Although there are no published guidelines on this subject, it is reasonable to insert a nasogastric tube preoperatively to allow drainage of gastrointestinal fluids in cases of documented bowel obstruction (e.g., ileus, strangulated bowel, pyloric obstruction) or in other situations in which aspiration is judged to be a substantial risk. The child may experience discomfort while a nasogastric tube is inserted, but this must be balanced against the need to decompress the stomach and reduce the risk of regurgitation during induction of anesthesia. In the remaining cases, the placement of a nasogastric tube can wait until after tracheal intubation. It should be noted that placement of a nasogastric tube may decrease lower esophageal sphincter tone, increase the risk for reflux, and reduce the ability to clear refluxed gastric contents from the distal esophagus.18,19 Thus the anesthesiologist is faced with the dilemma of whether or not to remove the nasogastric tube before induction. It is reasonable to apply suction to the nasogastric tube, evacuate all of the gastric contents before induction, and to remove the nasogastric tube before induction, because it is unclear if even properly applied cricoid pressure can prevent wicking of gastric contents along the path created by the nasogastric tube. It should be further noted that placing a nasogastric tube, although comforting, does not ensure an empty stomach.
Fluid Balance
To date, published studies indicate that resuscitation with crystalloid fluids yields similar results to that with colloids.20 In most children, initial resuscitation is undertaken with balanced salt solutions. Although colloid therapy may result in less tissue edema and less volume infused, the added expense of colloids may not justify their routine use in children.
Presence of Abdominal Compartment Syndrome
Acute intraabdominal disease processes may lead to a critically increased intraabdominal pressure (IAP).21 If the IAP increases above the capillary perfusion pressure of the intraabdominal organs, an abdominal compartment syndrome can develop. Organ perfusion will become compromised and ischemia and/or necrosis may develop. The most commonly affected organs in this situation are the bowels, kidneys, and liver. Abdominal compartment syndrome occurs less frequently in children than in adults.22 Causes of abdominal compartment syndrome include burns, extracorporeal membrane oxygenation,23 closure of gastroschisis or omphalocoele,24 abdominal trauma,25,26 abdominal surgery,27 and a variety of other intraabdominal pathologies, including necrotizing enterocolitis, Hirschsprung enterocolitis, perforated bowel, diaphragmatic hernia, and Wilms tumor.23,28,29 Insufficient perfusion of the bowel may cause an ileus, translocation of bacteria, lactate accumulation, and production of mediators that cause hemodynamic instability. Increased IAP can reduce liver blood flow that will reduce hepatic function,30 mainly manifested as an inability to metabolize lactate, a delay in drug metabolism,30,31 and, in severe cases, impaired synthesis of coagulation factors. Because the pressure is also transmitted to the retroperitoneal space, renal function may become impaired, resulting in oliguria or anuria and reduced excretion of drugs.28 In addition, cranial displacement of the abdominal contents and splinting of the diaphragm may seriously compromise ventilation.32
If acute intraabdominal compartment syndrome is suspected, then the IAP should be measured to determine if it exceeds the critical threshold of 20 to 25 mm Hg. Some suspect a compartment syndrome when the vesicular (bladder) pressure exceeds 10 to 12 mm Hg.33,34 These pressures can be measured either via a nasogastric tube or bladder catheter.35 The diagnosis of intra-abdominal compartment syndrome should be suspected when the triad of (1) massive abdominal distention, (2) increased bladder pressures and increased peak inspiratory airway pressures, and (3) evidence of renal and/or cardiac dysfunction are present.29,36,37
Children with acute intraabdominal compartment syndrome can become extremely hemodynamically unstable. Although decompression of the abdomen by a laparotomy will immediately normalize the IAP, reperfusion of the ischemic tissues almost always releases a host of biologically active substances that cause serious hypotension. These substances may also precipitate acute renal failure and lead to a disseminated intravascular coagulopathy. As in the case of sepsis, the anesthesiologist must be fully prepared to address these challenges by having blood products in the operating room and vasopressors available before induction of anesthesia. Some children will require a patch abdominoplasty as a temporizing measure to protect abdominal organs with primary closure of the anterior abdominal wall at some time in the future.29,36
Preoperative Laboratory Testing and Investigations
Most minor elective cases (e.g., umbilical or inguinal hernia repair) do not require any further preoperative work-up beyond a basic history and physical examination. Many centers require a preoperative urine (or hemoglobin) screen for pregnancy in females who have reached menarche.38 More complex elective cases may warrant additional laboratory testing, including basic hematology screening and electrolyte profile.
Monitoring Requirements
Routine elective cases rarely require more than standard monitoring equipment. In children undergoing major intraabdominal procedures, invasive arterial and central venous blood pressure monitoring may be indicated. A multiple-lumen central venous line inserted at the beginning of the procedure will facilitate administration of inotropic and/or vasoactive drugs, in addition to measuring central venous pressure; ultrasound-guided insertion is strongly recommended.39 These lines are of great value in the immediate postoperative period for blood sampling, drug administration, ongoing assessment of intravascular volume status, and parenteral nutrition. Transesophageal echocardiographic or transesophageal Doppler evaluation may provide valuable intraoperative and postoperative information regarding the child’s volume status, as well as cardiac contractility.40–50
Monitoring IAP is important during laparoscopic surgery, although it will be of minimal value in omphalocele and gastroschisis surgeries, as long as the abdomen remains open. Once the abdomen is closed however, IAP will provide useful prognostic information regarding intraabdominal organ blood flow, circulatory stability, and respiratory embarrassment (see Chapter 36).32
Choice of Anesthetic
The anesthesiologist may use his or her personal preference of anesthetic technique for the management of both elective and emergency intraabdominal surgery in children. However, airway management associated with intraabdominal surgery requires careful consideration. Even when the child is not at increased risk for regurgitation and aspiration, the risk of regurgitation can be increased if the surgeon manipulates the bowel, when the child is positioned head down, and if gas is insufflated into the peritoneal cavity (as occurs in laparoscopic procedures). A particular concern arises when the surgeon decides to decompress distended bowel. One method is to perform an enterotomy and directly drain the fluid, whereas the other is to massage (strip) the bowel in a retrograde direction until the contents can be vented with a nasogastric tube. The latter method has caused massive intestinal regurgitation that exceeded the capacity of the nasogastric tube, required clearance of the mouth with a Yankauer suction tip, and caused pulmonary aspiration.51 The laryngeal mask airway should not be used during intraabdominal surgery in general, and instead we strongly recommend the use of tracheal intubation with a cuffed tracheal tube as the standard in the majority of these cases.
Regional anesthetic techniques may be useful adjuncts in children undergoing both minor and major abdominal surgery. However, in critically unstable or septic children, the use of epidural anesthesia is not recommended, because sympathetic blockade may further exacerbate the hemodynamic instability, and there is an increased risk of epidural abscess formation. Simple local infiltration of the port insertion sites contributes to adequate postoperative analgesia in most children who have had laparoscopic procedures,52 whereas those who have had an open procedure will require IV opioids (see Chapter 43). Analgesia is commonly supplemented with NSAIDs and acetaminophen.
General Principles of Urologic Surgery
With the exception of acute drainage of urinary obstruction (i.e., ultrasound-guided nephrostomy or cystostomy procedures) and torsion of the testis, most pediatric urologic surgeries are elective. In the vast majority of cases, these children are otherwise healthy or with stable medical conditions that do not require more than a careful history, physical examination, and review of the child’s medical record. Children who undergo urologic procedures may be suffering from emotional disturbance because of repeated interventions and sensitivity of the surgical site. This mandates special psychological attention before and after anesthesia. The occurrence of anaphylaxis resulting from exposure to latex-related products is of special concern in children with chronic urologic disorders.53–56 Children with spina bifida are at greater risk for latex allergy than those without spina bifida,57–60 presumably because of early and repeated exposure to latex urethral catheters, although every child with congenital malformations of the urinary tract and repeat latex exposure to mucous membranes beginning in the neonatal period is at significant risk for developing latex hypersensitivity.61,62 Thus latex-free management is highly recommended in this population.63–65
Reduced Renal Function
Children with chronic renal disease have impaired renal function, which may affect drug dosing and disposition, as well as cause secondary effects on the cardiovascular system. In the most severe cases, the child may require dialysis to balance fluids and electrolytes. In children with renal disease, it is essential to determine the degree of renal impairment by consulting the child’s nephrologist and reviewing the serum creatinine, blood urea nitrogen, sodium, and potassium concentrations (see also Chapter 26). Because renal impairment may also affect clotting, a coagulation profile, including platelet count, should be reviewed preoperatively if substantial blood loss is anticipated. These children are prone to fluid overload, particularly those who are anuric and dialysis dependent. Apart from clinical signs associated with fluid overload, measuring the child’s weight and comparing it with their normal weight is a simple means to assess the child’s current volume status. If cardiac function or volume status remains in doubt, an echocardiogram should be obtained. Children with chronic renal insufficiency often have impaired left ventricular function even before they require dialysis, so a preoperative echocardiogram may be indicated66–70; pericardial effusion is also a concern in these children.67,71,72
For children who are dialyzed, the most recent date of dialysis should be documented. Overhydration and/or hypervolemia should be corrected preoperatively with dialysis. Although dialysis corrects hyperkalemia and overhydration, and may transiently improve platelet function (peritoneal dialysis yielding more consistent improvement than hemodialysis), it is best to not perform dialysis within 12 hours of anesthesia, so as to avoid a relative hypovolemia and to allow sufficient time for body fluids to re-equilibrate (see Chapter 26).73,74 Postdialysis laboratory indices of serum electrolytes (particularly potassium), hemoglobin or hematocrit, renal function (creatinine and blood urea nitrogen), and the child’s weight loss should be recorded. For children who undergo hemodialysis, IV access should not be established in the extremity ipsilateral to the arteriovenous fistula.
Systemic Hypertension
Systemic hypertension is common in renal insufficiency in adults, but far less common in children. Nonetheless, some children with urologic disorders develop significant systemic hypertension associated with disturbances in the renin-angiotensin system.75–78 As in adults, it is important to control systemic hypertension before induction to avoid wide swings in blood pressure. In contrast to adults, however, hypervolemia is an important cause of hypertension in children with renal insufficiency and should be considered and treated preoperatively. Children and adults are often treated with similar antihypertensive medications.77,78 These medications should be continued up to and including the morning of surgery to maintain intraoperative and postoperative hemodynamic stability. However, angiotensin-converting enzyme inhibitors are exceptions to this rule79: consideration should be given to holding the medication at least 1 day before surgery to avoid intraoperative hypotension80,81; conversely, holding the medication may be associated with rebound hypertension postoperatively. If these medications are not withheld, vasopressors may be required during anesthesia to stabilize the blood pressure.82 Because therapy-resistant renal hypertension is an indication for nephrectomy, one should anticipate and be prepared to treat wide fluctuations in blood pressure, including severe hypertension during the first stage of the operation and profound hypotension when the responsible kidney is removed. As a consequence, long-acting antihypertensive agents are best avoided during the early stages of a nephrectomy in a child.
Corticosteroid Medications
Children with renal disease may be chronically treated with corticosteroids as part of their medical management (e.g., children with proteinuria or who have undergone previous renal transplant surgery). In such cases, a stress dose of parenteral corticosteroids during surgery is indicated, with continued supplementation until the child resumes his or her normal corticosteroid medication by the enteral route. In more complex situations, consultation with a pediatric nephrologist or endocrinologist is warranted to optimize corticosteroid supplementation, although in more straightforward cases, a dose of 2.5 mg/kg of IV hydrocortisone two to three times each day is usually adequate (see Chapter 25).
Monitoring Requirements
Invasive monitors (i.e., invasive blood pressure monitoring, central venous access for pressure measurements and administration of vasoactive drugs) are indicated for major surgical interventions, and in cases with concomitant problems (e.g., decreased renal function, significant hypertension, associated cardiac dysfunction, sepsis). If central venous access is deemed to be necessary, the coagulation status of the child should be evaluated, because platelet function may be substantially compromised. Ultrasound guidance improves safety in securing central venous access in cases in which a coagulopathy is present or suspected on clinical grounds (see Chapter 48). In the unstable child, even more complex monitoring (e.g., esophageal Doppler monitoring or transesophageal echocardiography) should be considered.
Laparoscopic Surgery
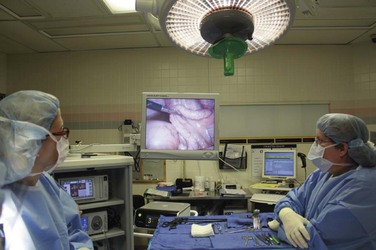
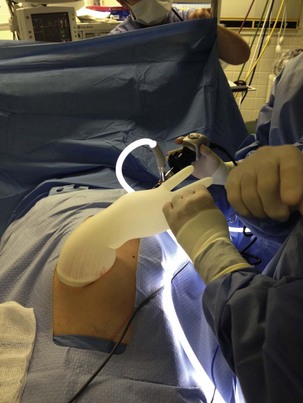
Although laparoscopic surgery was introduced more than 80 years ago, its role in pediatric surgery has only become notable in the past 10 to 15 years. To a large extent, this has been due to improved technology in optics and miniaturization of the instruments. An increasing number of general and urologic surgical procedures (including appendectomy, cholecystectomy, and splenectomy, as well as those to treat inguinal hernia and undescended testicles) are performed either laparoscopically or using robotic techniques in children of all ages, including neonates (Figs. 27-1 to 27-4).83–91 More sophisticated laparoscopic techniques have enabled the performance of complex surgical procedures, including Nissen fundoplication, colectomy, pyeloplasty, bowel pull-through, and removal of large organs, including the kidney and spleen.89,92–95 Technological advances now permit laparoscopic surgery in neonates and small infants, including those with hypoplastic left heart syndrome after stage 1 or 2 repair.91,96–98 It must be noted however that laparoscopic surgery in children with cyanotic heart disease carries with it a substantial risk that exceeds most other populations having this form of surgery. Although several reports suggest that infants and children of all ages, at all stages of palliative repair of their cyanotic heart disease, tolerate laparoscopic surgery,98–100 the risk for those with Fontan physiology undergoing laparoscopic surgery is substantial. It is essential to understand that in Fontan physiology, pulmonary blood flow is passive, and decreases in venous return (whether from increases in intrathoracic pressure or head-up positioning) or increases in pulmonary vascular resistance (because of carbon dioxide absorption or decreased minute ventilation) could severely reduce the cardiac output.101 Creating a pneumoperitoneum, as in the case in laparoscopic surgery, and extreme positions increase IAP and arterial carbon dioxide tensions (increasing pulmonary artery pressure), reduce venous return, and increase the risk of reducing the cardiac output in children with Fontan physiology (see later discussion). Successful management of these children during laparoscopic surgery requires a multidisciplinary team that functions in concert to optimize the child’s outcome (1) by optimizing the child’s condition preoperatively and identifying any cardiac issues, (2) by recruiting surgeons who can complete the surgery quickly and efficiently, (3) by maintaining the children in the supine position throughout the surgery, (4) by maintaining normocapnia, (5) by monitoring the child with a transesophageal echo probe as needed, as well as with an arterial invasive pressure monitor for blood gas analysis, and (6) by insufflating the abdomen to the lowest (less than 8 mm Hg) IAP that is surgically feasible. Larger studies are required before children with cyanotic heart disease, particularly those with Fontan physiology, can routinely undergo laparoscopic surgery in any center except those with specialized teams to manage these children.
Laparoscopic surgery offers a number of advantages over open surgery, including more rapid emergence from anesthesia, faster ambulation, earlier discharge from the hospital, and reduced perioperative complications.102–105 Robot-assisted surgical technology is discussed later.
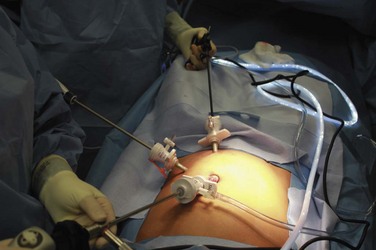
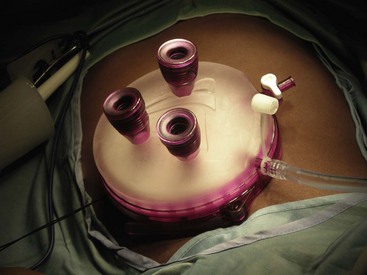
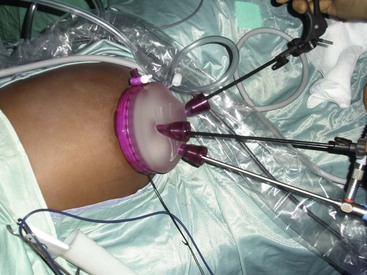
Laparoscopic surgery involves the insufflation of gas into the abdominal cavity in order to visualize the intraabdominal organs. When preparing for laparoscopic surgery, if the surgery involves the upper abdomen, the stomach should be decompressed using a nasogastric or orogastric tube whereas if the surgery involves the lower abdomen, the bladder should be emptied using a urinary catheter. Surgical access to the peritoneal cavity is achieved with trocars introduced through three (or more) small (3 to 10 mm in diameter) incisions. Through the trocars, the laparoscopic instruments and a camera are then passed. Recently a laparo-endoscopic single site (LESS), single port, single incision laparoscopic surgery (SILS), single incision multiport laparoscopy (SIMPL), or “belly button” surgery has been developed, in which all instruments pass through a single incision and a single large trocar (often in the umbilicus).106–109 Through the single trocar, the instruments and a camera are inserted.109a (E-Figs. 27-1 through 27-3). “Clashing” (or colliding of objects) is a common problem because of the close proximity of the cables exiting the single trocar, as well as the proximity of the multiple hands performing the procedure. The clashing of cables has been resolved by fusing them into a single cable as they exit the trocar.
E-FIGURE 27-2 A commercially available SIMPL device for a child in which a single sheath is inserted through a flap lifted in the umbilicus. The ports and gas insufflator are mounted on the outside. For more information see Reference 109a. (Courtesy Dr. K. Vali, Pediatric Surgery, Women and Children’s Hospital of Buffalo, Buffalo, N.Y.)
E-FIGURE 27-3 A commercially available SIMPL device in a child through which the camera and two laparoscopic instruments have access to the abdominal cavity. This is achieved through the umbilicus with one surgical incision. For more information, see Reference 109a. (Courtesy Dr. K. Vali, Pediatric Surgery, Women and Children’s Hospital of Buffalo, Buffalo, N.Y.)
Pneumoperitoneal pressure is a major concern for all laparoscopic approaches. Experimental evidence in adult and newborn pigs demonstrated that the risks of cardiorespiratory consequences and potentially fatal emboli were directly related to the peak pneumoperitoneal pressure.110,111 In infants and children, the optimal pneumoperitoneal pressure is the least pressure that enables adequate surgical access. Carbon dioxide should be insufflated through one of the trocars until the IAP reaches 6 to 15 mm Hg; most surgeons currently limit the IAP in neonates and young infants to 6 to 8 mm Hg and in children to 10 to 12 mm Hg.112 The IAP is maintained throughout the surgery by intermittently insufflating additional CO2. Although carbon dioxide is most commonly used, a number of gases have been investigated (see later discussion).113 Carbon dioxide is the preferred gas because it does not support combustion, is rapidly cleared from the peritoneal cavity at the end of surgery, and does not expand into bubbles or spaces.93,114,115 However, the increased IAP may cause mechanical difficulties, such as circulatory or respiratory depression, hypothermia due to dry gas leakage, pneumothorax or subcutaneous emphysema, endobronchial intubation resulting from the upward shift of tracheal bifurcation, and injury due to paracentesis. The major disadvantage of CO2 is that it is rapidly absorbed from the peritoneum, with the absorption and washout of CO2 being more rapid and the peak end-tidal partial pressure of CO2 (Petco2) less in infants than in older children.116,117 The inverse relationship between age and rate of CO2 absorption from the abdomen has been attributed to the thinner peritoneum and the reduced peritoneal fat deposits in the abdomen of infants compared with older children.117 Evidence indicates that only 10% to 20% of the Petco2 originates from the insufflated CO2 after 10 to 20 minutes of insufflation at an IAP of 10 to 15 mm Hg.118 During CO2 insufflation, both the Petco2 and Paco2 increase up to 20 mm Hg or 20% to 50% above baseline.94,112,114,116,119 Hypercapnia may occur if surgery lasts more than 1 hour, particularly in neonates,120 necessitating an increase in minute ventilation of 50% to 100% to maintain a physiologic pH.93,121 Again, it must be emphasized that all of these adverse physiologic changes that occur with laparoscopic surgery could have catastrophic implications for children with Fontan physiology and that consideration to performing an open procedure may be the safest approach.
The difference between the partial pressures of arterial and end-tidal CO2 (Pa−Petco2) often increases during insufflation of CO2,119,121 although a number of studies reported negative Pa−Petco2 gradients, particularly in infants. The explanation for these negative gradients has been elusive, but may be due to sampling or technical errors or ventilation-perfusion mismatch.119,121 The Pa−Petco2 gradient before and after pneumoperitoneum increases from a mean of 5.7 mm Hg to 13.4 mm Hg.122 In neonates and in children with cyanotic congenital heart disease, the Petco2 may not reliably track the Paco2 during CO2 insufflation, leading some to recommend arterial blood gas monitoring to validate the Petco2 measurements.119,122,123
Increased Paco2 may also trigger spontaneous respiratory efforts that could interfere with surgery. Additionally it may also initiate a sympathetic response, including an increase in the heart rate, blood pressure, and cerebral blood flow, as well as ventricular arrhythmias, although this occurs rarely today because sevoflurane has replaced halothane in many countries. A sudden increase in Petco2 may also suggest a diagnosis of malignant hyperthermia. If this diagnosis proves to be difficult to confirm or reject (see Chapter 40), it may be necessary to desufflate the abdomen and determine if the clinical and laboratory findings suggestive of MH resolve. Insufflated CO2 originating from the abdomen continues to be exhaled for the first 30 minutes after desufflation.118
Gas emboli have been reported in several laparoscopic studies, most of which were interesting curiosities of no clinical consequence, although in several, the result was profound cardiovascular collapse. Most consider the emboli to be intravascular CO2 bubbles. Intravascular embolization of CO2 may occur when the insufflation pressure exceeds the venous pressure, forcing CO2 bubbles into the venous circulation, resulting in sudden cardiovascular collapse.124 Continuous precordial Doppler or CO2 partial pressure are effective in detecting a gas embolus, although the Doppler may be overly sensitive, with numerous false-positives. It has been suggested that many episodes of subclinical gas embolisms are unrecognized because the symptoms are mild and nonspecific. However, children with right-to-left shunts or potential right-to-left shunts, such as a patent foramen ovale, are vulnerable to the systemic effects of these emboli. Although CO2 is soluble in blood and rapidly buffered, CO2 emboli dissolve slowly in blood, taking 2 to 3 minutes to disappear.125 Hence, large emboli may block blood flow in the heart for several minutes or more, before they dissolve. The minimum rate of infusion of CO2 into blood that triggers cardiovascular collapse in pigs is 1.2 mL/kg/min125; comparable data in humans are lacking. Anesthesiologists should be aware of the high-risk conditions that predispose to CO2 emboli (e.g., increased abdominal insufflation pressure, hypovolemia and reduced venous pressure, spontaneous respirations, and resection of vessel-rich parenchymatous organs) and correct the condition or warn the surgeons.
Some investigators suggest that clinically important gas emboli are actually nitrogen, not carbon dioxide gas emboli.124 Whereas transient emboli are thought to be composed of CO2, emboli that persist may be nitrogen gas.124 Nitrogen is insoluble in blood (blood/gas partition coefficient of 0.014), which explains its persistence as an embolus in blood. These emboli may arise from air either present in or entrained by the trocar during insufflation of the peritoneum, that is forced into a transected blood vessel while the carboperitoneum is pressurized.124 This mechanism is a rare source for gas in the circulation, which may explain, in part, why the emboli that occur during laparoscopy very rarely result in cardiovascular instability and arrest.
In contrast to CO2, insufflation with oxygen, air, and nitrous oxide for laparoscopic surgery has been eschewed because they all support combustion. However, repeat desufflations of the pneumoperitoneal gas during laparoscopy in pigs whose lungs were ventilated with 66% nitrous oxide in oxygen, has been shown to prevent concentrations of nitrous oxide from exceeding 10% in the pneumoperitoneal cavity.126 Nitrous oxide is still not recommended for either insufflation or as an adjunctive anesthetic gas during laparoscopy, because it also expands into gas-filled cavities should gas emboli appear in the circulation.114 Its use during laparoscopic surgery may distend the bowel in bowel obstruction and obscure the surgeon’s view, as well as expand any CO2 emboli that develop.
The inert gases argon and helium were also candidate gases for insufflation to create a pneumoperitoneum as they cannot be oxidized (and therefore ignited), although they are much more expensive than CO2. When argon was used to create a pneumoperitoneum in pigs, embolization occurred more frequently than with CO2.127 In theory, both argon and helium can cause serious sequelae if embolized into the vascular system because they are insoluble in blood (blood/gas solubility of helium is 0.007 and argon is 0.029) and are likely to persist.93,114,115,127
Pulmonary Effects
As the pressure within the peritoneal cavity increases, so too do the possible adverse respiratory effects, particularly at IAP greater than 15 cm H2O. The respiratory manifestations of increased IAP include cephalad displacement of the diaphragm, decreased excursion of the diaphragm, and decreases in pulmonary and thoracic compliance, vital capacity, functional residual capacity, and closing volume.128 Cephalad displacement of the diaphragm shifts ventilation to the nondependent parts of the lungs, creating ventilation-perfusion mismatch which may require that minute ventilation is increased from 50% to 100% to offset these effects. With a small functional residual capacity in children, cephalad displacement of the diaphragm further compresses the lungs, causing collapse of the small airways, ventilation-perfusion mismatch, and possibly hypoxemia. Pulmonary mechanics after insufflation of a pneumoperitoneum increases peak inspiratory pressures by 27% and decreases compliance by about 39%.129 These physiologic changes are compounded by the extreme body tilting (i.e., extreme head-up or head-down positions) often requested by surgeons.114,130 Positioning the child head down (i.e., Trendelenburg position) decreases compliance by 17% and pneumoperitoneum decreases compliance by 27%, requiring increases in peak inflation pressures of 19% and 32%, respectively.131 Pulmonary function appears to be restored more readily after laparoscopic than open surgery.93 In some instances, the pneumoperitoneum and the extreme position shift the tracheal tube in a rostral direction, as far as 1.2 to 2.7 cm, possibly impacting on the carina or passing into a bronchus.132 With these changes, inspired oxygen concentrations in excess of 30% may be required, along with positive end-expiratory pressure to restore adequate oxygenation. A persistent 5% decrease in oxygen saturation has been associated with partial or intermittent endobronchial intubation.133
Securing the airway for laparoscopic surgery requires particular attention. Cuffed tracheal tubes are preferred over uncuffed tubes to maintain an adequate minute ventilation.123 If the child has a mature tracheotomy, the air leak around the tracheotomy must be assessed before surgery. If the leak is excessive, then the IAP may prevent adequate ventilation during surgery. In such a situation, the tracheotomy should be replaced with a cuffed tracheotomy or an armored (cuffed) tracheal tube. If the air leak around the tracheotomy is insignificant, then ventilation should be adequate in the presence of increased IAP during laparoscopic surgery.
Excessive IAP may cause gas to track across the diaphragm, causing a pneumomediastinum or pneumothorax.115 This is more common in hiatus hernia surgery and Nissen fundoplication, during which dissection of the esophagus may create passages for gas to traverse the diaphragm. Pneumomediastinum should be suspected if subcutaneous emphysema appears. If surgery creates a transdiaphragmatic passage for CO2 to accumulate in the pleural space, the resulting pneumothorax may produce cardiorespiratory manifestations. A chest radiograph should be obtained if subcutaneous emphysema appears during or after surgery, or if there is a high index of suspicion that a pneumothorax has formed. In laparoscopic surgeries that are brief (less than 30 minutes), both laryngeal mask airways and ProSeal supraglottic airways have been used without complications, although most clinicians prefer cuffed tracheal tubes.115,134 Both pressure- and volume-controlled ventilation have been used during laparoscopic abdominal surgery in infants and children. In a single randomized study, both ventilation strategies with 5 mm Hg positive end-expiratory pressure maintained effective ventilation and gas exchange.135
Although pneumoperitoneum offers the surgeons excellent operating conditions, it is a source of cardiorespiratory compromise. An alternative to insufflating CO2 to create a pneumoperitoneum is the gasless laparoscopic approach. This requires lifting the anterior abdominal wall to create an intra-abdominal tent.115,136 Implementation of the gasless approach in pediatric medicine has been rather slow, presumably because of technical difficulties and the scarcity of instruments for infants and children.
Cardiovascular Effects
A pneumoperitoneum can adversely affect cardiovascular indices.115,136 Three major factors contribute to cardiovascular changes: (1) IAP, (2) position (i.e., steep head-up or reverse Trendelenburg), and (3) release of neurohumoral vasoactive substances.128 Increased IAP exerts a biphasic effect on venous return and cardiac output (CO). In neonatal pigs, the cardiac index (CI) decreased 55% when IAP exceeded 20 mm Hg.115 The magnitude of the increase in IAP determines the degree to which the circulation is depressed.123,137 For example, at IAP less than 15 mm Hg, blood is compressed out of the splanchnic circulation increasing venous return which either increases or results in no effect on CO. In contrast, at IAP greater than 15 mm Hg, the inferior vena cava is compressed, reducing venous return and therefore CO. Studies in children yielded similar results. When the IAP exceeded 12 mm Hg, myocardial contractility138 and venous return114 decreased mildly. In both infants and children, CO2 insufflation to IAP 10 to 13 mm Hg decreased CI approximately 13%.139–141 However, an IAP less than or equal to 5 mm Hg maintained the CI in infants during a CO2 pneumoperitoneum.142 In all of the studies in infants and children in which the CI decreased during increased IAP (10 to 12 mm Hg), the CI returned to preinsufflation values when the abdomen was desufflated.138,139,141 Left ventricular systolic function was diminished and septal wall motion abnormalities have been reported with an IAP of 10 to 12 mm Hg in children with a CO2 pneumoperitoneum.138,139 No significant changes in echocardiographic indices of left ventricular work, preload or afterload, have been noted if the IAP was less than 10 mm Hg during the pneumoperitoneum.112 When standard indices of hemodynamics were measured in infants and young children during laparoscopic Nissen fundoplication, IAP less than or equal to 10 mm Hg yielded no significant changes in heart rate and blood pressure but a slight increase in CI.142,143 If IAP is maintained at less than or equal to 10 mm Hg, then the impact on hemodynamics (and in particular, CO) should be clinically insignificant, because venous return is enhanced as a result of displacement of blood from the splanchnic bed, and afterload is not increased.114,123,142
Body position during laparoscopic surgery may exaggerate cardiovascular changes. For Nissen fundoplication, a steep head-up position (greater than 20-degree incline) has been used, which reduces venous return.130 In adult pigs, laparoscopic surgery for Nissen fundoplication increased pleural and mediastinal pressures that in turn, reduced CO episodically at an IAP of 15 mm Hg.144 These decreases in CO were manifested by episodes of hypotension and hypoxia. When children were positioned in the steep head-up position, they developed transient hypotension and bradycardia that were reversed immediately with fluid loading and atropine.145
It is imperative to continuously monitor IAP to minimize the cardiorespiratory effects of laparoscopic surgery and to avoid excessive insufflation pressures. In adults, induction of anesthesia, IAP to 14 mm Hg, and 10-degree head-up tilt decreased the CI more than 50%.146 Some clinicians recommend a maximum IAP during laparoscopy in children of 6 to 8 mm Hg to limit the cardiorespiratory effects,147 although the consensus appears to be closer to pressures of 10 to 12 mm Hg. In neonates and infants, 6 to 8 mm Hg appears to be the accepted peak IAP. Based on the current literature, the net cardiovascular effects of insufflating the abdomen to pressures less than or equal to 12 mm Hg, combined with the head-up position, are likely to be well-tolerated if adequate hydration is maintained and bradycardia is avoided.145
Central Nervous System Effects
Laparoscopic surgery must be carefully evaluated if planned for a child with increased intracranial pressure, or in the presence of a ventriculoperitoneal (VP) shunt. The combination of increased IAP, increased systemic vascular resistance, increased Paco2 tension, and Trendelenburg position (as in lower abdominal surgery) may dramatically increase intracranial pressure. In adults during extreme head-down position (40 degrees) for prolonged periods, such as in robot-assisted surgery in the pelvis, cerebral tissue oxygen saturation is well-maintained, as evidenced by near-infrared spectroscopy.148 Under similar surgical conditions, intraocular pressure increased 100%, and scleral edema and blurred vision may develop.149
Patency of a VP shunt should be evaluated before the procedure to prevent sudden increases in intracranial pressure during the procedure. Children with reduced brain compliance may sustain dramatic increases in intracranial pressure if the IAP is sufficient to attenuate the drainage of cerebrospinal fluid into the abdominal cavity. In these children, laparoscopic surgery may be relatively contraindicated.130 The risks should be thoroughly explored and discussed with the neurosurgeon, general surgeon, anesthesiologist, and the family. Studies have reported a range of responses to laparoscopy in children with VP shunts, from dramatic increases in intracranial pressure to no change at all.128,150,151 Accordingly, some advocate externalizing the shunt and clamping the distal (intraabdominal) end of the shunt before surgery to prevent carbon dioxide from passing retrograde up the shunt or from the laparoscopic pressure disrupting the shunt valve, although an IAP of 80 mm Hg is required for this to occur.153 Others discourage externalizing the shunt as sequelae have been reported, and instead recommend monitoring the intracranial pressure to prevent and detect increases in intracranial pressure, and further recommend retraction of abdominal tissue during the laparoscopic surgery.153 For the surgeon, using a SILS approach to laparoscopic surgery in these children reduces the risk of both traumatizing and infecting the VP shunt.152 A recent review demonstrated that laparoscopy in children with VP shunts is safe and not associated with an increased risk of shunt infection.153 No single strategy can provide the optimal management for every child with a VP shunt undergoing laparoscopic surgery.
Renal Function and Fluid Requirements
Increased IAP decreases renal blood flow, renal function (creatinine clearance and glomerular filtration rate), and urine output.123,128,154 The effects on renal function and renal filtration are poorly understood. Decreases in urine output during laparoscopic surgery in children vary, in part, with the age of the child: oliguria occurs in older children and anuria in infants less than 1 year of age.128,130,155 The etiology of the renal dysfunction and oliguria is multifactorial, but includes direct and indirect effects of IAP on renal perfusion, antidiuretic hormone (ADH), endothelin, and nitric oxide (NO).128,156 ADH concentrations increase as a result of reduced renal blood flow, resorbing water, and decreasing urine output. IAP increases renal endothelin (endothelin-1), resulting in renal venoconstriction, which reduces renal blood flow and urine output.128,156 Inhibiting endogenous NO exacerbates the renal dysfunction during pneumoperitoneum through several mechanisms, including reduced renal perfusion and increased salt and water resorption (e.g., oliguria).156 Thus pretreating patients with an NO donor (such as l-arginine or nondepressor doses of nitroglycerine) may attenuate the detrimental effects of a pneumoperitoneum on renal dysfunction, a supposition that awaits human studies.156 Renal tubular injury does not contribute to the renal dysfunction associated with increased IAP.157 In adult donor nephrectomy patients, an overnight infusion of fluids followed by a colloid bolus immediately before the pneumoperitoneum attenuated the adverse hemodynamic effects and reduced the magnitude of changes in creatinine clearance associated with increased IAP.158 Comparable data in children have not been forthcoming.
Fluid administration during laparoscopic surgery should be carefully monitored. Open abdominal surgeries may require 10 to 15 mL/kg/hr to account for “third space fluid” losses resulting from extensive bowel manipulation. Although the existence of and manipulation of a real “third space” are debated, the conceptual fluid shift is real. During laparoscopic surgery, however, these fluid requirements are reduced because little fluid is lost and the bowels are minimally manipulated. In fact, care must be taken to avoid fluid overload during these surgeries. Urine output is often used as an index of preload in children undergoing abdominal surgery, but 88% of infants (less than 1 year of age) and 33% of children develop anuria or oliguria during laparoscopic surgery. Because these completely resolve within several hours of desufflation, it is not necessary to fluid challenge these children, as fluid overload is a real possibility.155 Transient oliguria in children after laparoscopic surgery should not be viewed as an early indicator of impending renal dysfunction.
Pain Management
Postoperative pain after open general and urologic surgery is primarily directed at the pain from the skin and muscle incisions. With the small incisions used during laparoscopic surgery, perioperative pain is less than with open surgery.159–161 Pain after laparoscopic surgery arises from several sources, including the incision sites, residual gas in the abdomen, referred pain from the diaphragm, and stretch on nerves from peculiar patient positions. A long-acting local anesthetic should be infiltrated around the incision sites at the end of laparoscopic surgery to prevent postoperative incisional pain. Some children develop pain after laparoscopic surgery, including back and shoulder pain. In these instances, multimodal pain therapy, including acetaminophen, nonsteroidal antiinflammatory agents, and (less commonly) opioids, are effective.94,95,115 Recent evidence suggests that SIMPL for appendectomy causes less pain than surgery with a multiport system.162
Robot-Assisted Surgery
Robot-assisted surgery is relatively new to pediatric surgery and urology, with only limited experience reported, although it has been used extensively in adults since the late 1990s to facilitate minimally invasive endoscopic surgery. Enhanced with three-dimensional magnifying views and feedback-controlled enhanced motions of human hands, robot-assisted surgery enables very fine manipulation of surgical instruments without natural human tremor, in a way that was not possible in the past. It has great potential as the future direction for pediatric surgery and urology.1,148,163,164
Initially introduced as a tool for remote battlefield surgery, robotic surgery is now available to assist pediatric surgeons in performing complex surgery, with less tissue and organ damage on extremely small surgical targets. Most of the information that is currently available is limited to one product (da Vinci Surgical System, Intuitive Surgical, Sunnyvale, Calif.) and to adult urologic procedures. However, rapid innovation and miniaturization of equipment has resulted in several pediatric centers undertaking robot-assisted laparoscopic surgery, with excellent outcomes (Videos 27-1 and 27-2)![]() . Concerns from the anesthesiologist’s perspective regarding this relatively new approach are summarized in Table 27-1. Most of the perceived problems relate to the steep Trendelenburg position used, although numerous surgeries have been performed without this position.148,163
. Concerns from the anesthesiologist’s perspective regarding this relatively new approach are summarized in Table 27-1. Most of the perceived problems relate to the steep Trendelenburg position used, although numerous surgeries have been performed without this position.148,163
TABLE 27-1 Issues Regarding Current Robotic-Assisted Procedures from the Anesthetist’s Perspective
Increased intracranial pressure, ocular pressure, and impairment of cerebral perfusion
Optic nerve or retinal morbidity
Cerebro-facial congestion, airway edema, vocal cord palsy, delayed awakening
Possible overstretching of nerves passing through the axilla and nerve plexus damage
Extended lithotomy leading to compartment syndrome in the lower legs
Tendency to apply greater intraabdominal pressure
Exploration of new surgical approaches and complex tasks at awkward angles
Longer surgical time and longer setting up time, hypothermia, pressure sores
Unpredictable movement of robot and camera arms,
Hitting or compressing the child’s face
Organization of all tubing and cables, including intravenous tubing and breathing hoses.
Specific General Surgical and Urologic Conditions
Nissen Fundoplication
Children who require a Nissen fundoplication often have a cerebral neurologic injury (i.e., cerebral palsy) that causes esophageal dysmotility. This dysmotility heralds esophageal reflux that, if severe, may result in aspiration pneumonia. If medical and gastric tube therapies fail, a Nissen fundoplication may be considered. The anesthetic considerations are few because this surgery is not associated with postoperative pain, large fluid shifts, or large blood loss. Positioning a bougie within the esophagus during surgery allows the surgeons to gauge how tight to tie the muscles around the esophagus; without a bougie, the muscles around the esophagus may be overtightened, causing an esophageal obstruction. Care must be taken to avoid dislodging the tracheal tube in the perioperative period. At the end of the procedure, it is common for the surgeon to request that 50 to 60 mL of air be insufflated into the stomach via the gastric tube to ensure that there are no anastomotic leaks. In general, this surgery is completed in less than 1 hour in experienced hands, with a complication rate of about 10% and an average hospital stay of about 1.6 days. Postoperative pain is easily managed.165,166
Pectus Excavatum
Although this is a deformity of the chest wall, pediatric surgeons most often carry out the corrective procedure. Correction of the pectus excavatum by the classic approach involves an open procedure with fracture of the sternum, removal of multiple costal cartilages, and lifting of the sternum anteriorly with fixation, using one or two stainless steel bars. The Nuss procedure, a less invasive technique,167,168 was initially a technique whereby a U-shaped bar was blindly passed through the thorax hugging the undersurface of the sternum. Once across the chest, the bar was flipped, through which process the sternum was pushed anteriorly without fracturing it, thus avoiding the creation of a flail chest by the removal of the costal cartilages. This procedure has since been modified, whereby the bar is passed through the thorax under direct vision, using thoracoscopy to reduce possible perforation of major structures (e.g., the heart or lungs).169–171 Although the risk of this complication is reduced when the Nuss procedure is performed under direct vision, it may still occur.172–174 Both blind and thoracoscopic approaches cause significant postoperative pain, which may be treated with patient-controlled analgesia, a thoracic epidural catheter, or a lumbar epidural catheter and epidural morphine (see Chapters 41 to 44).175 Because these procedures are generally performed in teenagers, the thoracic epidural is preferably placed with the teenager awake but sedated. Compliance with inserting the epidural catheter while awake may be difficult in less mature teenagers. It is unclear whether the thoracic epidural provides improved analgesia compared with standard patient-controlled analgesia for this procedure.176 It should be noted that these children will return for removal of the pectus bar after several years. Occasionally, the bar has become adherent to the pericardium or lung, resulting in a severe, sudden, and catastrophic rupture of a major vessel or chamber in the heart when the pectus bar is removed.177 It would be prudent to establish ample IV access to provide the means to rapidly transfuse fluids and blood should a catastrophic blood loss occur.
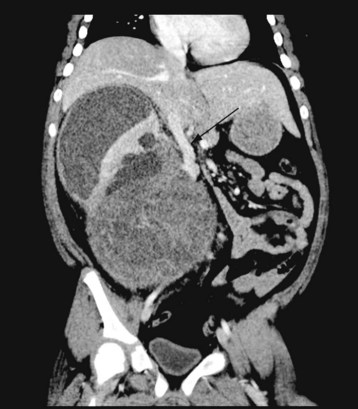
Pheochromocytoma
Pheochromocytoma is a rare but potentially life-threatening neuroendocrine tumor that arises from the chromaffin cells of the adrenal medulla in 80% of cases, or extraadrenal paraganglionic tissue in 20% of cases.178,179 Intraabdominal tumors are most commonly found around renal blood vessels or the corpora paraaortica (organs of Zu), which displays the largest collection of chromaffin tissue. Extraadrenal paragangliomas may occur anywhere in the body from the base of the skull to the pelvis.180 Only cells that stain positive for chromaffin secrete catecholamines. These tumors derive their name from the dark grey-brown immunostaining of chromogranin A by chromium salts.180 The Greek word “pheo,” meaning twilight, refers to the dark grey or twilight-colored immunostaining.
The incidence of pheochromocytoma in the general population is approximately 0.3 to 1 per million per year.180 In children, the incidence of benign pheochromocytoma is 1 per 10 million and of the malignant form, 1 per 50 million. Approximately 10% to 20% of all pheochromocytomas are diagnosed in childhood, with an average age at the time of onset of 11 years.179,180 In childhood, there is a preponderance of males, whereas during the reproductive years, there is a preponderance of females. The net effect throughout childhood is an equal distribution in the sexes.181 In contrast to adults, pheochromocytomas in children are more often benign, bilateral, multiple in number, and extra-adrenal.180 With adequate preparation preoperatively and close monitoring intraoperatively, the mortality associated with extirpation of the tumor is less than 3% in children.178
Most of these tumors are benign, with only 5% to 10% malignant (although some reports suggest a malignancy rate as great as 50%). Malignancies of pheochromocytomas have been found in the bones, lungs, lymph nodes, and liver.178,179 A diagnosis of pheochromocytoma can be confirmed by positive staining for chromaffin cells. The paraganglioma tumors with mutations in succinate dehydrogenase subgroup B (see later discussion) are most likely to metastasize.178 Malignant tumors are more likely to secrete dopamine than benign ones.178 The 5-year survival of metastatic pheochromocytoma is 50%, with no effective treatment.178 In adults, radical surgery and iobenguane I 123 therapy have been effective in 80% of adults, although only 5% go into remission.182
In children, 40% to 60% of pheochromocytomas are inherited179,180 in an autosomal dominant pattern,179,180 with the remainder occurring spontaneously. Four hereditary syndromes associated with pheochromocytomas have been identified in five genes’ mutations178,183–185: von Hippel-Lindau type 2, multi-glandular multiple endocrine neoplasia (MEN) type 2, neurofibromatosis 1, and paraganglionic syndromes (mitochondrial complex II succinate dehydrogenase type B and D). Two additional (nonhereditary) syndromes have also been associated with pheochromocytomas: Tuberous sclerosis and Carney triad. The gene defect responsible for the MEN syndromes is located on chromosome 10q11.2.179 MEN type 2A (Sipple syndrome) includes medullary thyroid cancer, parathyroid adenoma, and pheochromocytoma. MEN type 2B includes medullary thyroid cancer, multiple neuromas, Marfan habitus, and pheochromocytoma. Pheochromocytomas associated with MEN secrete both epinephrine and norepinephrine.178 These tumors are bilateral in 50% to 80% of cases, but rarely metastasize.178 The von Hippel-Lindau syndrome type 2 (type 1 does not involve pheochromocytomas) is the most common cause of familial pheochromocytoma.180 The von Hippel-Lindau gene is located on 3p25-26.178,179 Pheochromocytoma is present in 10% to 20% of children with the gene. Three subtypes of the gene are distinguished by their association with other organs: type 2A involves hemangioblastomas of the central nervous system, endolymphatic sac tumors, and ependymal cystadenomas; type 2B involves all the organs in type 2A, as well as renal cell and pancreatic cysts and tumors. The only tissues associated with type 2C are pheochromocytomas. When associated with the von Hippel-Lindau gene, pheochromocytomas secrete exclusively norepinephrine.178 These tumors occur bilaterally in the adrenal glands, but rarely metastasize.179 Neurofibromatosis type 1 involves neurofibromas and café au lait spots. The genetic mutation is found on chromosome 17q11.2.178 Pheochromocytomas occur in less than 5% of children with neurofibromatosis.179
Paraganglioma syndromes arise from mutations in the gene that codes for succinate dehydrogenase on cytochrome oxidase II in the respiratory chain of mitochondria. Two genetic mutations involve pheochromocytomas: subgroup B, which is coded on chromosome 1p36 and subgroup D, which is coded on chromosome 11q23.178,179 These mutations present with paragangliomas of the adrenal, extra-adrenal, and head and neck regions. Subgroup B occurs more commonly in children (approximately 20% of pheochromocytomas bear this mutation), with a 30% to 50% metastatic rate. Subgroup D is inherited strictly along paternal lines.179
Pheochromocytomas can secrete epinephrine, norepinephrine, and/or dopamine. Most pheochromocytomas secrete norepinephrine as the predominant hormone; only 10% to 20% secrete epinephrine and dopamine as the predominant hormones. Hypertension is the most common presenting sign of this tumor. It is often accompanied by regular, intermittent headaches that are associated with nausea and vomiting. In children, nausea is a common presenting finding. The classic triad of headaches, sweating and palpitations, along with hypertension constitute the presenting signs in greater than 90% of patients with pheochromocytomas. Sustained hypertension is present in 60% to 90% of children with the tumor, but is not a requirement for the diagnosis.186 In fact, there is no relationship between circulating catecholamine concentrations and overt symptoms. Epinephrine-secreting tumors can present as circulatory shock due to decreased intravascular volume, as a result of sustained high concentrations of catecholamines and their effects on vascular resistance. Tumors that secrete predominantly dopamine do not usually present with hypertension.
Other presenting findings include weight loss, nausea and vomiting, polyuria, visual disturbances, and anxiety.186 Presenting signs may include pallor, orthostatic hypotension, tremor, and syncope, as well as abdominal pain, diarrhea and other gastrointestinal manifestations, hyperglycemia, low-grade fever, and behavioral disturbances. On occasion, catecholamine release may be triggered by anesthesia, micturition (as in a urinary bladder pheochromocytoma), foods, and drugs, such as metoclopramide, tricyclic antidepressants, glucagon, and radiology contrast dye. Pheochromocytomas have been described as the great mimic, giving support to a differential diagnosis that includes acute coronary infarction, carcinoid tumor, thyroid storm, and cocaine (or other amphetamine-like drug) overdose. In other emergent circumstances, surgery undertaken for acute appendicitis with an undiagnosed pheochromocytoma has been terminated prematurely once the diagnosis of a pheochromocytoma was strongly suspected. The acute appendicitis resolved with conservative nonoperative management while the pheochromocytoma was investigated, the patient was treated with α-adrenergic blocking agents, and the pheochromocytoma removed electively.187
Surgical resection of pheochromocytoma is optimally managed on an elective basis, after the child’s medical status has been properly investigated, medical conditions are stabilized, the location of the tumor(s) determined and the α-adrenergic receptors completely blocked. Preoperative α-adrenergic blockade is critical, as its routine use has reduced perioperative complications during pheochromocytoma resection from 60% to 3%.188 It must be emphasized that β-adrenergic blockade should NEVER be introduced until α-adrenergic blockade has been well-established, because this could cause unopposed paroxysmal systemic hypertension, acute coronary or stroke signs, and death. Thus preoperative preparation of these children first requires the institution of a noncompetitive α-adrenergic blocker, such as phenoxybenzamine. Phenoxybenzamine irreversibly alkylates α-adrenergic receptors reducing the risk of an α-adrenergic medicated paroxysmal increase in blood pressure during surgery. Phenoxybenzamine may be administered either orally or IV.183 Oral doses between 0.25 and 1.0 mg/kg/day in divided doses are most common, although doses as great as 2 mg/kg/day have been used.180,183 Oral bioavailability of phenoxybenzamine is only 20% to 30% with a 24-hour onset of action.183 Children are usually treated for 3 to 15 days with oral phenoxybenzamine before surgery, although there is no means to reliably assess the completeness of α-adrenergic blockade.183 Some think that α-adrenergic blockade has been established when the systolic blood pressure returns to normal limits for the child’s age.179 Alternatively, IV phenoxybenzamine has been used to more rapidly and reliably block α1 receptors, although aggressive monitoring for peripheral vasodilatation, decreases in blood pressure, and relative hypovolemia must be followed until the hemodynamics have equilibrated. The action of phenoxybenzamine is terminated only by synthesizing new α-adrenergic receptors. The disadvantage of irreversibly blocking α-adrenergic receptors, as conferred by phenoxybenzamine, is that reactive hypotension may follow removal of the tumor, with resistance to interventions that are intended to increase the peripheral vascular resistance. In contrast, phentolamine has also been used because it has a much shorter half-life than does phenoxybenzamine and it is reversible. Competitive α-adrenergic blockade with doxazosin, which also has a brief duration of action when compared with phenoxybenzamine, may be displaced from the α-adrenergic receptors by increased concentrations of circulating catecholamines.178 It is important to reemphasize that β-adrenergic blockade should only be introduced once α-adrenergic receptor blockade has been established.
Perioperative fluid and electrolyte management in children as α-adrenergic blockade develops requires close monitoring. It is tempting to administer generous volumes of balanced salt solutions preoperatively to prevent hypotension, but this should be tempered with the understanding that children are at increased risk for catecholamine-induced pulmonary edema.183 The plasma potassium concentration should be monitored closely as hypokalemia may develop as a result of increased renin concentrations.179 Postoperative hypotension, a direct consequence of α-adrenergic receptor blockade, may be attenuated by augmenting the sodium intake in the diet preoperatively, and by the judicious use of IV balanced salt solutions in the perioperative period.180
Perioperative Evaluation
Preparation should be made for general anesthesia with invasive arterial and central venous catheters, together with transesophageal echocardiogram, if deemed necessary. Infusions to manage hypertensive crises should be available, including sodium nitroprusside, magnesium sulfate, and esmolol. Magnesium causes vasodilation by inhibiting catecholamine receptors and catecholamine release as well as antagonizing endogenous calcium effects.189 Vasopressors, including ephedrine and vasopressin, may be required to restore the blood pressure if hypotension occurs, especially postoperatively.178,189,190
Laboratory Findings
Biochemical testing for pheochromocytoma should be performed in children who present with signs suggestive of pheochromocytoma, whether a primary tumor or a recurrence. The most accurate biochemical tests are those for free plasma metanephrine and normetanephrine concentrations, and 24-hour urine for fractionated metanephrines,191 although even these tests are not without problems.178,183,184 These tests have supplanted 24-hour urinary and plasma epinephrine, norepinephrine, and dopamine concentrations, and the degradation product, urinary vanillylmandelic acid. Reference standards for metanephrine must be adjusted for the child’s age, because the concentrations may be up to 33% less than those in adults. The current tests are much more reliable than older methods (vanillylmandelic acid concentration) for establishing a diagnosis of a pheochromocytoma. Plasma catecholamine concentrations correlate well with the urine concentrations during sustained tumor catecholamine secretion, although they may be exaggerated during episodes of cardiovascular instability. In both sporadic and familial pheochromocytoma, the test with the greatest sensitivity for normetanephrine and metanephrine is the measurement of plasma free concentrations.191 Plasma metanephrines are present in 99% of sporadic pheochromocytomas. However, this test may be difficult to perform in children because they must remain supine and relaxed for 30 minutes before sampling. The presence of increased urine and plasma catecholamine concentrations can be attributed to numerous physiologic and pathologic conditions, as well as to medications (e.g., acetaminophen, tricyclic antidepressants, β-adrenergic blockers, and calcium channel blockers). However, when the upper reference limits are adjusted, sensitivities of 100% and specificities of 80% to 94% can be obtained (sensitivities for familial forms are less than for sporadic forms, whereas specificities for familial forms are greater than for sporadic forms).178,184 Concentrations of metanephrine and normetanephrine (as determined by high pressure liquid chromatography) that exceed the 99th percentile upper limit for normal levels, and thus suggest a “likely” diagnosis of a pheochromocytoma are: blood, free metanephrines greater than 0.42 nmol/L and normetanephrine greater than 1.4 nmol/L; urine, metanephrine greater than 2880 nmol per 24 hours and normetanephrine greater than 6550 nmol per 24 hours.178 In some cases, results of the biochemical tests for diagnosing a pheochromocytoma are borderline. In such cases, oral clonidine may be administered as a suppression test, suppressing the release of norepinephrine. If clonidine suppresses the concentration of plasma normetanephrine greater than 40% below the upper reference limit, then a diagnosis of a pheochromocytoma may be excluded.192 Caution should be exercised when administering clonidine, as α2-adrenoceptor agonists may cause substantial hypotension and bradycardia in children. Suppression tests are infrequently used today because of the sensitivity and specificity of the current catecholamine assays.
Chronic exposure to increased circulating catecholamine concentrations may lead to a cardiomyopathy, congestive heart failure, and arrhythmias. Preoperatively, an electrocardiogram, chest radiograph, and echocardiogram should be performed.183 The electrocardiogram may reveal arrhythmias, evidence of myocardial ischemia, or ventricular hypertrophy. The chest radiograph may show an increased cardiothoracic ratio suggestive of a cardiomyopathy or heart failure. Echocardiography will identify reduced cardiac function, heart failure, cardiomyopathy, or simply ventricular hypertrophy. Cardiac function should be optimized medically before embarking on general anesthesia. Transesophageal echocardiography and central venous pressure and arterial pressure monitoring may be indicated.
Once the biochemical diagnostic tests have been completed and the presence of a pheochromocytoma has been confirmed, the tumor(s) must be located. The most common location in children is in the abdomen, both within and without the adrenal gland; computed axial tomography (CAT) and magnetic resonance imaging (MRI) are used to locate these tumors in children.183 CAT scans are very rapid, but they expose the children to radiation. MRI studies are slower and require general anesthesia, but they avoid radiation exposure. The accuracy of locating a pheochromocytoma with these two techniques is similar,183 96% to 100%, although both may, on occasion, have difficulty distinguishing a pheochromocytoma from other intraabdominal lesions, especially with small tumors (less than 1 cm in diameter). For small tumors or for extraadrenal tumors, a supplementary radiologic investigation known as the iobenguane I-123 test may be used. Studies with iobenguane yield positive responses with both pheochromocytomas and neuroblastomas, necessitating additional studies to distinguish between the two.179 Iobenguane uptake by the tumor may be impaired in the presence of labetalol and tricyclic antidepressants. Although metastatic paragangliomas may lose their ability to take up iobenguane, the combination of a positive MRI and iobenguane uptake usually confirms the diagnosis. In cases where iobenguane cannot be used or yields negative results, or when the tumor remains elusive, positron emission tomography with fluorodeoxyglucose F-18 may be used. The latter test offers greater sensitivity and possibly specificity than does the iobenguane I-123 test, especially during investigation for a metastatic pheochromocytoma.
Anesthetic Management
Anxiolytics (such as midazolam) are useful to maintain circulatory homeostasis before surgical resection of pheochromocytomas. General anesthesia and tracheal intubation is required whether the tumor is resected using an open laparotomy or a laparoscopy. Induction of anesthesia may be undertaken with IV anesthetics, while avoiding sympathomimetics (e.g., ketamine). Steroidal muscle relaxants (e.g., vecuronium), benzodiazepines, opioids, and inhalational anesthetics may be used. Some have suggested succinylcholine may trigger a sympathetic response via stimulation of the sympathetic ganglia or fasciculations, although the evidence for such a response is weak.193 Surges in blood pressure may be managed with propofol, increasing the concentration of the inhalational anesthetic, or administering vasodilators (magnesium or sodium nitroprusside infusions). Most pheochromocytomas are resected using an open laparotomy approach, although more recently, laparoscopic surgery has found favor.194 The latter approach reduces the duration of surgery and speeds recovery. However, insufflation of the abdomen with carbon dioxide may precipitate a sympathetic response and a decrease in venous return. If the child was not euvolemic before insufflating the abdomen, a rapid decrease in venous return and blood pressure may ensue. This should be treated aggressively, using balanced salt solutions while relieving the intraabdominal pressure until the blood pressure stabilizes. If abdominal insufflation triggers a sympathetic response, an IV bolus of propofol should be administered, the concentration of inhaled anesthetic increased, and/or antihypertensives administered as needed. Contraindications to laparoscopic surgery include large tumors (greater than 15 cm), coagulopathy, and invasive metastatic disease.
Potential Perioperative Problems
Hypertension can occur during the surgery, before or during manipulation of the tumor, despite α-adrenergic blockade. Measures that may be used to control the blood pressure include increasing the inspired concentration of inhalational anesthetic, IV propofol (bolus or infusion), sodium nitroprusside infusion (0.5-8 µg/kg/min), IV magnesium sulfate (30 mg/kg loading dose over 30 minutes, followed by an infusion of 10 mg/kg/hr), α-adrenergic blockers (e.g., phentolamine) and calcium channel blockers (e.g., diltiazem or nicardipine).189
Hypotension can occur once the tumor has been extirpated, as a result of the sudden removal of the source of catecholamines and/or irreversible α-adrenergic blockade by phenoxybenzamine. Hypotension may continue for several days postoperatively. Supportive treatment with fluids and vasopressors (including vasopressin) may be effective in restoring normal blood pressure.189
Circumcision
In infants and children, circumcision is performed under general anesthesia. Multimodal pain therapy includes acetaminophen 30 to 40 mg/kg rectally or 10 to 15 mg/kg IV, parenteral opioids (i.e., morphine 0.05-0.1 mg/kg), and/or local anesthetic without epinephrine (dorsal penile block, caudal block, subcutaneous ring block, and topical lidocaine–prilocaine [EMLA]) (see Chapter 41).195 A comparison of a subcutaneous ring block of the penis with a suprapubic penile block, showed the latter provided better analgesia.196 When a caudal block was compared with penile blocks and parenteral analgesics, a Cochrane review concluded that, with a caudal block, both rescue analgesia and nausea and vomiting were reduced compared with parenteral analgesics, although the analysis was limited because of a dearth of studies.197
Hypospadias and Chordee
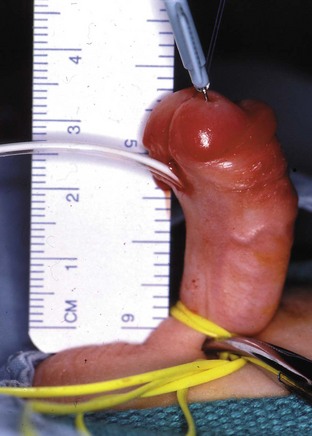
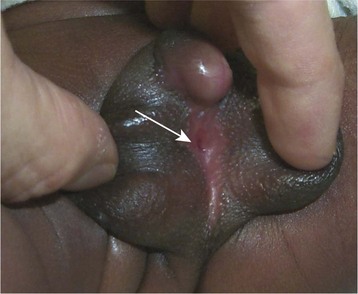
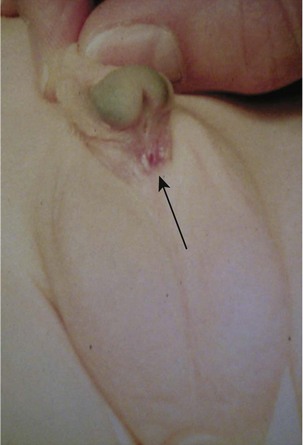
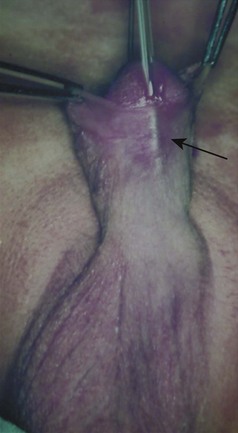
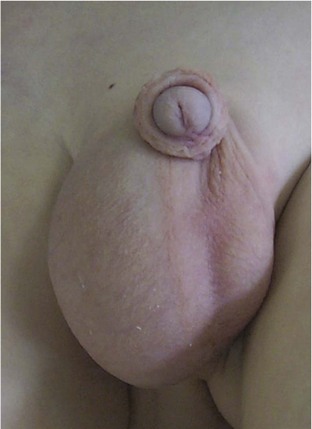
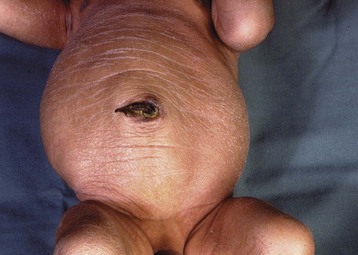
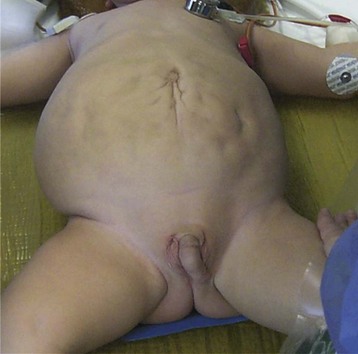
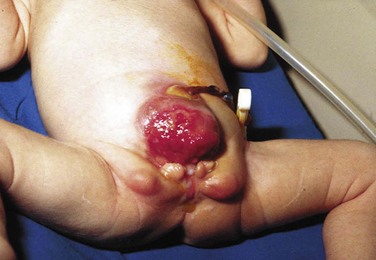
This congenital malformation occurs in 1 of 250 liveborn males. It often occurs in isolation, without other congenital anomalies. Hypospadias refers to a malposition of the meatus of the urethra: rather than opening at the distal tip of the penis, the urethra opens along the undersurface of the penis anywhere from just proximal to the glans to the scrotum (Fig. 27-5). The majority of hypospadias defects are distal, occurring near or at the glans of the penis. Between 15% and 50% of hypospadii have an associated chordee, whereas 8% have an undescended testis. A small number of children with hypospadias have urethral openings remote from the glans of the penis, including the scrotum (Fig. 27-6, E-Figs. 27-4 and 27-5).
Surgery is undertaken with an expected duration of between 1 and 4 hours depending on the severity of the hypospadias. It is important to establish an understanding with the urologist of the type of regional block that will suit the extent of surgery: those requiring a minor hypospadias repair (single-stage procedure, i.e., MAGPI [meatal advancement and glanuloplasty technique] or Mathieu repair) may be managed with a facemask, laryngeal mask airway, or tracheal tube. The anesthetic is at the discretion of the anesthesiologist; the children are outpatients and receive either a penile block or a single-shot caudal block. Those with more extensive hypospadias who require longer surgery will require either a laryngeal mask airway or a tracheal tube. They will be admitted to a hospital for one to two nights and need a strategy for continuous postoperative analgesia. For the latter, either a caudal catheter or a lumbar epidural catheter can be used during surgery to reduce the anesthetic requirements and postoperatively for analgesia. If opioids are avoided, a caudal-epidural block consisting of only local anesthetic does not cause delayed micturition after the urinary catheter has been removed.198
Cryptorchidism and Hernias: Inguinal and Umbilical
These surgeries, together with hydrocele repair, are common outpatient procedures. Orchiopexy refers to mobilizing the undescended testis that is either in the inguinal canal or, less commonly, within the abdominal cavity (Fig. 27-7), and securing it firmly in the scrotum. Approximately 33% of preterm infant males are born with one undescended testis, whereas only 3% of full-term males are similarly afflicted. Although the incidence of undescended testis decreases to 1% by 3 months of age, the incidence remains at 1% thereafter. Cryptorchidism usually occurs in isolation, although it is associated with a number of conditions, including Prader-Willi syndrome, Noonan syndrome, and cloacal exstrophy.
An inguinal hernia in a child is a congenital failure of the processus vaginalis to obliterate. In this case, a loop of bowel protrudes beyond the internal ring, causing a bulge in the inguinal region or scrotum. These protuberances may appear periodically, with complete resolution in the interim. On occasion, a small sac of fluid is present in the scrotum, known as a hydrocele, and is confused for a loop of bowel in the scrotum (E-Fig. 27-6). Hydroceles are removed electively with the same approach as for an inguinal hernia. On occasion, the loop of bowel does not reduce spontaneously from the hernia and remains trapped in the canal, necessitating a visit to the emergency department. A surgeon is often required to manually reduce the trapped bowel. In these cases, the hernia repair is then scheduled as an urgent or elective surgery, depending on whether there is suspicion of persistent or potential recurrent ischemia to the bowel. In some cases, the entrapped bowel cannot be reduced and an incarcerated hernia or obstructed bowel is diagnosed. Incarcerated hernias and bowel obstructions are surgical emergencies that require general anesthesia, and muscle relaxation to reduce the strangulated bowel. The emergency nature of the surgery requires careful questioning regarding the time interval between the last meal and the onset of abdominal pain and strangulated bowel. It is usually assumed that these children have a full stomach. At the time of open reduction, if the bowel does not appear to have adequate perfusion, a segment of the ischemic or necrotic bowel may have to be resected.
Management of cryptorchidism and inguinal hernia requires general anesthesia (facemask, laryngeal mask airway, or tracheal tube) and an adequate pain management strategy. Multimodal pain therapy, as described earlier, may be used together with a regional block. When the surgeon pulls on the foreskin, hernia sac, or testis during surgery, laryngospasm may occur if the depth of anesthesia is inadequate. Anesthesia can be deepened most rapidly by an IV bolus of propofol, although some increase the inspired concentration of inhalational anesthetic. Regional blocks (ilioinguinal, iliohypogastric, scrotal block; caudal-epidural block; or transversus abdominis plane block) are used for both orchiopexy and inguinal hernia surgery, using either a landmark-based or ultrasound-guided method199 (see Chapter 41).
Torsion of the Testis
Presentation of a male with sudden onset of acute scrotal pain in the absence of trauma requires immediate investigation and possible surgery to preserve a potentially viable testis. The differential diagnosis of acute onset of torsion of the testis (Fig. 27-8) includes torsion of the testicular appendix, torsion of the spermatic cord, epididymitis, and incarcerated hernia. The majority of testes can be saved if surgery is performed within 6 hours of the onset of pain if the diagnosis is confirmed by Doppler ultrasonography or suspected on clinical grounds.200 The salvage rate for the testis decreases to 50% if surgery is undertaken 6 to 12 hours after the onset of pain. Children with suspected acute testicular torsion are assumed to have a full stomach and require RSI and tracheal intubation. Although pain at the time of induction of anesthesia may be intense, when the torsion is relieved, the pain abates. Hence, at the conclusion of surgery many of these children no longer have substantive pain that warrants aggressive treatment.
Posterior Urethral Valves
Posterior urethral valves (PUV) is a spectrum of urethral obstruction that varies from mild to severe. The diagnosis is often made antenatally (preferably by 24 weeks gestation) by ultrasound identification of bladder distention, megaureters, and hydronephrosis. When PUV is diagnosed antenatally, the severity of the disease tends to be greater.201 Decompression of the urogenital system may be achieved by a vesicoamniotic shunt in utero. Although some recommend that an intervention should be undertaken as quickly as possible to minimize the impact on renal function, evidence suggests that early intervention does not substantively affect the outcome, because renal damage may have already occurred in utero.202,203
Postnatally, a lack of or decrease in urine output, urinary retention, or a poor urine stream may be the only indications of the presence of these valves.204 Affected children can have associated renal insufficiency resulting from congenital renal dysplasia and urethral valve obstruction. Because the renal concentrating mechanism is often impaired, these infants commonly present with greater than normal urine output. Consequently, careful monitoring of urine output and balanced salt solution infusion rate is necessary. Primary valve ablation is required to decompress the urogenital system.
Several indices have been postulated as predictors of poor long-term renal function in infants with PUV, including a creatinine 0.8 mg/dL or greater at birth, antenatal diagnosis, proteinuria, moderate or severe hydronephrosis, and renal dysplasia.201,205 A recent analysis indicated that a nadir creatinine greater than 1 mg/dL and bladder dysfunction were the only independent predictors of long-term renal dysfunction.206
Prune-Belly Syndrome
Prune-belly syndrome is a disorder that occurs predominantly (97% of the time) in males, with an incidence of 1 in 40,000 births. Affected infants present with a range of findings, from stillborn to a full-term neonate, with a host of possible organ and chromosomal abnormalities.205 Affected organs may involve orthopedic in 50% (congenital hip dislocation and scoliosis), gastrointestinal in 30% (malrotation and volvulus), congenital heart disease in 10% (tetralogy of Fallot and ventricular septal defect), and chromosomal defects (trisomy 18 and trisomy 21).205
In utero, the child’s abdomen often swells with fluid (in the presence of oligohydramnios) that is resorbed by birth, leaving the characteristic wrinkled redundant abdominal wall (Fig. 27-9 and E-Fig. 27-7). The pathophysiology of this syndrome is unclear, but it has been suggested that a urethral obstruction in utero leads to dilatation of the urethra (megaurethra is a common finding), which, combined with bladder distention and ascites, causes distention of the abdomen in utero. This ultimately leads to vesicoureteral reflux and ureteral dilatation in 80% of affected children.
Abdominal overdistention in utero causes weak rectus abdominis muscles that undermine the child’s ability to exhale forcefully, and to generate a strong cough to clear secretions. As a result, chronic aspiration pneumonia may contribute to an early demise. Some have suggested that aggressive intervention to correct the weak rectus muscles by plication and muscle transfer may improve respiratory function,207,208 reduce back strain and pain, decreased bladder volume, and arrest scoliosis.209 However, this view is not shared by others who prefer to observe the child for signs of regurgitation and aspiration before intervening.210 Controlling the type of feeds, preventing gastrointestinal reflux disease, and using antibiotics to treat pneumonia permit the child to grow. Constipation is a frequent problem that may be exacerbated by their inability to increase their IAP during defecation. Stool softeners are often prescribed to prevent constipation and abdominal distention. Pneumonia must be aggressively treated and completely resolved before entertaining surgery. Percutaneous endoscopic gastroscopy tubes are generally eschewed in these children when feeding is a problem, as abdominal wall surgery is difficult. These children often require urologic surgery to correct vesicoureteral reflux and orchiopexy. Urethral obstruction may be due to the angulation of the urethra within the prostate.
Trisomy 18, the second most common autosomal trisomy, occurs in 1 in approximately 7,000 live births, and is associated with prune-belly syndrome. In contrast to the simple prune-belly syndrome, 60% to 80% of these infants are female. Trisomy 18 is characterized by severe neurologic developmental problems (including microcephaly), micrognathia and/or retrognathia, microstomia, auricular abnormalities, and others. In fact, 95% die in utero, with only 5% to 10% surviving 1 year and only 1% reaching 10 years of age. Mortality results from cardiac anomalies (90% of affected children have a ventricular septal defect, valvular heart defect, atrial septal defect, hypoplastic left heart syndrome, tetralogy of Fallot, or other cardiac defect), renal anomalies, failure to thrive, and apnea.211,212 Additional findings include pulmonary hypoplasia and gastrointestinal anomalies (including prune-belly syndrome, omphalocele, ileal atresia, and esophageal atresia).
General anesthesia with tracheal intubation is required for most surgeries in these children. Controlled ventilation is recommended, because of the variability in the strength of the abdominal muscles. It is prudent to suction the lungs once the trachea has been intubated, in order to assess the severity of secretions. As little muscle relaxant as possible should be used during the surgery, with preferably no muscle relaxant administered during the last hour, to ensure the child’s strength has had time to recover to have a successful extubation. Although opioids should be used cautiously, regional anesthesia may be preferred, in view of their difficulty with clearing secretions from the tracheobronchial tree.213
Ureteral Reimplantation
Vesicoureteral reflux, in which urine passes retrograde up the ureter, is a congenital disorder affecting 0.5% to 2% of children. Recurrent episodes of pyelonephritis may occur, leading to renal scarring and reduced renal function, depending on the severity of the reflux. The pathology is thought to be an anatomically abnormal insertion of the ureter into the bladder that fails to close tight when the bladder fills and contracts. A voiding cystourethrogram is the test required to diagnose the reflux and assess its severity. Mild forms of vesicoureteral reflux are managed with daily antibiotics until the child outgrows the reflux. However, more severe forms or those who develop kidney infections despite antibiotic therapy, require surgical correction. The classic surgical approach for vesicoureteral reflux is an open procedure in which the affected ureters are reimplanted into the bladder wall, re-creating a normal muscle flap valve.214 This involves a lower abdominal incision and 3 to 4 hours of surgery. Postoperatively, the pain is often intense, necessitating 2 to 3 days of continuous infusion of local anesthetic via an indwelling caudal or epidural catheter.
More recently, laparoscopic techniques have been developed, with and without robotic control, for reimplantation of the ureters.214 Preliminary evidence suggests an excellent success rate (greater than 90%) for this approach,215 although the time for the surgery is more than twice the open technique and more complications were identified in children with small bladders.
Surgical alternatives in the past 20 years have spawned a number of compounds for injection into the terminal submucosal tract of the ureter, by passing a needle through a specially designed cystoscope. Initially, polytetrafluoroethylene (Teflon) was injected endoscopically, but a safer, more durable polymer has been developed over the past 20 years that is a mixture of dextranomer and hyaluronic acid (Deflux). In this way, surgeons create a swelling just inferior to the opening of the ureter into the bladder that prevents urine from refluxing (Fig. 27-10, before and after injection of polymer). The technique is successful in 80% of cases after one injection and in greater than 90% after two injections. Although this procedure still requires general anesthesia, its advantages far outweigh any disadvantages, in that the duration of the procedure is very brief (usually 15 to 30 minutes), the procedure avoids an abdominal incision, causes no postoperative pain, and its greatest advantage is that the child can be an outpatient. However, the results of this injection technique continue to be monitored because long-term outcomes and sequelae have not been determined.
Pyeloplasty
Pyeloplasty is performed to decompress the renal pelvis either because of intrinsic (congenital) or extrinsic (major vessel) compression of the ureter. A distended renal pelvis is often diagnosed antenatally and, in some instances, decompressed in utero by percutaneous nephrostomy. The ureteric narrowing often occurs at the ureteropelvic junction, where the ureter exits the renal pelvis. Surgery involves disconnecting the ureter at the renal pelvis, reshaping it, and then reinserting it into the kidney. Such surgery is often performed in the lateral or prone position, with the table jackknifed. The duration of this surgery is approximately 2 hours, with a success rate of approximately 95%. Dissection is often retroperitoneal, but the incision is placed immediately below the rib cage. This procedure has been performed laparoscopically and with the use of robotics.105,215b
Anesthetic considerations include the prone or lateral decubitus position (complicated by placing the table in the “jackknife position”), postoperative pain, and adequate fluid resuscitation. IV access should be established in an upper extremity to maintain adequate atrial filling pressures. If the table is placed in the jackknife position, it is very important to measure the child’s blood pressure before and after the table is jackknifed, because venous return may become severely compromised, necessitating reducing the extent of the jackknife and resuscitating the child with fluid. The laparoscopic approach to pyeloplasty in children is new. In renal surgery, CO2 is insufflated in the retroperitoneal space to provide surgical access.216 Because of the anatomic differences between the retroperitoneal and intraperitoneal spaces, greater pressures may be required to provide adequate surgical visibility. A mean retroperitoneal pressure of 12 mm Hg increases Petco2 and peak inspiratory pressures, and decreases blood pressure.217 Early evidence indicates that laparoscopic surgery yields similar outcomes to the open approach, although the duration of surgery is approximately one-third greater.218,219 In part, this has been attributed to the learning curve of this technique. Evidence suggests that the laparoscopic approach decreases the length of hospital stay and may decrease postoperative pain105,219 (see previous discussion of pain management after laparoscopic surgery).
Nephrectomy
Indications for nephrectomy and partial nephrectomy in children include nonfunctioning kidney, dysplastic kidney, urolithiasis, Wilms or other tumor, end-stage renal failure (pretransplantation or to control hypertension), hemolytic uremic syndrome, and polycystic disease. Children who require a nephrectomy will require a thorough preoperative assessment in terms of history, physical examination, and laboratory testing, depending on their underlying pathology. These children are often anemic because of chronic disease and possibly decreased erythropoietin concentrations. If the child is being staged for kidney transplant, the nephrologists may prefer to avoid blood transfusions at this time. If time is available and the patient is anemic, oral FeSO4 and vitamin C should be commenced 3 to 6 weeks before surgery (vitamin C increases gut absorption of FeSO4) to increase the hemoglobin concentration, particularly if erythropoietin supplementation is planned.220,221 These children are often small in size and this should be considered when preparing the anesthetic equipment.
Nephrectomies are commonly performed using a retroperitoneal approach with the child in the lateral decubitus position and the table jackknifed. The incision is usually large and located just subcostal to the twelfth rib. If an open approach is planned, a low thoracic epidural catheter may be placed before positioning the child. If, however, a laparoscopic or robotic-assisted approach is planned,89 then no neuraxial block is necessary but the same surgical approach occurs. Outcomes after laparoscopic nephrectomy in children are similar to those after an open procedure, according to the experience from one center,222 although recent evidence points to less pain and earlier discharge from the hospital.89
Neuroblastoma
Neuroblastoma is the most common extracranial solid tumor that presents in childhood, comprising 10% of all tumors and 15% of all deaths from tumors.223 It is the second most common abdominal tumor, after Wilms tumor. Its incidence is approximately 1 in 100,000 children in the United States.224,225 Neuroblastomas arise in the abdomen in 75% of cases and along the sympathetic chain anywhere from the neck to the pelvis in 25%. Only one-third of those that arise in the abdomen arise in the adrenal glands. The median age at presentation is 2 years, with up to 90% occurring in children under 5 years of age. In some series, up to 50% of the cases appear in the first month of life, with some diagnosed antenatally. This tumor occurs equally in boys and girls. Reports of familial neuroblastoma are rare. However, these tumors may be associated with other disorders, including Beckwith-Wiedemann syndrome, neurofibromatosis, Hirschsprung disease, and central hypoventilation syndrome.223
Most neuroblastomas are first detected as palpable masses in the abdomen. Symptomatic presentation may present as local pressure on adjacent organs or structures (liver, kidney, or spine), as metastases (lymph nodes, bone marrow, liver, and skin), or with manifestations of excess neurohumoral production (i.e., from catecholamine [e.g., systemic hypertension] or vasoactive intestine polypeptides [e.g., diarrhea]).223 Urinary catecholamines are increased in greater than 90% of children (more than 1 year of age) with neuroblastoma. In a minority of instances, the diagnosis is made through incidental examination, either by radiography or ultrasonography.
Staging of these tumors follows two protocols.224 The International Neuroblastoma Staging System depends on tumor resectability, lymph node involvement, and metastases. However, if patients are not surgical candidates, the staging score holds little relevance. To further stage these tumors, a second scoring system was developed, the International Neuroblastoma Risk Group classification system, which is based on the preoperative radiologic findings only. Survival likelihood depends on a low score, extraabdominal (as opposed to intraabdominal) primary tumor, and younger age. Therapeutic intervention is tailored to the staging of the tumor at presentation and the size of the tumor. Those with favorable tumor biology, no distant metastases, and younger than 18 months of age, are often curable with surgical resection alone. Those with less favorable tumor biology, metastases, and a large tumor size that may present a challenge surgically, are ideally treated with a combination of chemotherapy, radiation, and/or bone marrow transplantation to reduce the tumor size first, and then with surgical resection. Patients with less favorable biology present a challenge for surgical resection because of local infiltration, large tumor size, and vascular extension. Debulking the tumor with incomplete resection has been met with mixed reviews. The most promising treatments for neuroblastomas with poor prognoses include molecular profiling of the tumor.224 The long-term survival after treating these tumors is 88% for infants and children less than 18 months of age, 49% for children 18 months to 12 years of age, and 10% for those 12 years old or older.226 Preoperative assessment requires a general systems review, with particular focus on the organ systems involved with the tumor. These tumors may present as a large intra-abdominal mass that compromises respiration, most commonly manifested as tachypnea. Those with catecholamine-secreting tumors may also present with chronic hypertension. This is mitigated to a large extent if the tumor is shrunk preoperatively, but intraoperative hypertensive episodes still occur in 25% of the children (with urinary catecholamines) during surgery. Children with catecholamine-secreting neuroblastomas should have both α-adrenergic and β-adrenergic blockades established preoperatively (see previous discussion of pheochromocytoma) to avoid swings in blood pressure during manipulation of the tumor.227 Less frequently, chronic diarrhea from vasoactive intestine polypeptides may cause chronic dehydration and electrolyte derangements that require correction.
Although the blood pressure may be controlled with established α-adrenergic blockade, manipulation and squeezing of the tumor during its removal may cause a surge in catecholamine release, necessitating the use of antihypertensive medications intraoperatively (see previous discussion of pheochromocytoma).227 Labetalol may be effective in children with hypertension during resection of neuroblastomas227; however, it may cause paroxysmal hypertension and heart failure in children who have only β-adrenergic blockade established, because of the predominant β-adrenergic blocking action of labetalol.
Wilms Tumor
Renal tumors comprise 2.5% to 7% of tumors in children. Wilms tumor is the most common abdominal tumor in children, with an incidence of 8 in 1,000,000 children less than 15 years old, and the most common solid renal tumor beyond the first year of life.225,228 These tumors arise from persistent immature parenchymal renal tissue (referred to as Wilms tumorlet cells) often in the periphery of the kidney (as opposed to the collecting ducts), enclosed by a pseudocapsule (Fig. 27-11). They may achieve a large size before detection, often compressing adjacent renal parenchyma. Histologically, the tumor often includes up to three distinct tissue cell lines: epithelial, blastemal, and stromal cells.228 The presence of anaplastic cells (in 4% of Wilms tumors) and, more specifically, whether the cells are focal or diffuse in the tumor, and older age at the time of presentation suggests a less favorable response to chemotherapy and less favorable long-term prognosis.228 With tailored multimodal therapy, the survival from Wilms tumor in the past several decades has increased dramatically, from 30% to 90%.
Eighty percent of the children with of Wilms tumor present between 1 and 5 years of age (peaking at 3 to 4 years of age), with no gender or racial bias.225,228 Presentation of Wilms tumor is similar to that of other intraabdominal tumors in the form of an incidental mass on physical examination; approximately 6% are bilateral. Congenital anomalies coexist with Wilms tumors in 12% of children, notably in genitourinary anomalies (5%), hemihypertrophy (2.5%), and aniridia (1%).228 A number of genetic syndromes predispose to Wilms tumors, including Beckwith Wiedemann syndrome.225 Wilms tumors occur twice as frequently in children with horseshoe kidneys than in those with normal kidneys, and is also more frequent in those with multicystic dysplastic kidneys.
Preoperatively, most children with Wilms tumors appear well, presenting with constitutional findings, including weight loss, loss of appetite, and malaise. Occasionally, they have an associated syndrome that should be detailed. Physical examination includes a palpable abdominal mass in 75% to 90% of children. Laboratory investigations before nephrectomy include routine complete blood count, electrolytes, renal function, as well as coagulation indices. Polycythemia may be present from tumor-induced excess erythropoietin production. Acquired Von Willebrand disease is present in less than 10% of these children.225 Blood should be cross-matched for the surgery. Microscopic hematuria occurs in 25% of children whereas gross hematuria is rare,228 suggesting tumor invasion of the collecting ducts. Systemic hypertension is present in 25% of children, presumably as a result of hyperreninemia. Preoperative investigations should include radiography and ultrasonography, the latter highlighting hyperechogenic structures within the kidney. Tumor extension into the ipsilateral renal vein and inferior vena cava must be examined before embarking on surgery, as chemotherapy may lead to involution of these tumors (Fig. 27-12).225 Magnetic resonance imaging and computed tomography are valuable tools for delimiting the tumor borders within the kidney, as well as metastases in the lungs or other organs.225 Most recent summaries of the radiologic investigations may determine the need for additional interventions, including echocardiogram and lung scan to determine the presence of tumor in the heart and lungs, respectively. An echocardiogram may be specifically required to evaluate myocardial function if doxorubicin and other anthracycline chemotherapeutic agents had been administered.225
Anesthetic management of children with Wilms tumor is similar to that with neuroblastoma. No specific anesthetic regimen is preferred. Invasive monitoring and large bore IV access (upper extremities to avoid interference from tumor extension into the inferior vena cava) with adequate blood warming capability is mandatory. Anesthesia may be further complicated by hypertension (precipitated by tumor handling), coagulopathy (acquired von Willebrand disease), extension of the tumor into the proximal inferior vena cava or right atrium, pulmonary tumor emboli, acute right heart failure, and considerations concerning preoperative or previous treatment with chemotherapeutic drugs.225 These drugs may impair hepatic or hematopoietic function (actinomycin D), cause inappropriate antidiuretic hormone release (vincristine), or myocardial damage (doxorubicin).225 Pain may be controlled using either IV or regional anesthesia, though the risk of a coagulopathy must be ruled out before administering an epidural block.
Bladder and Cloacal Exstrophy
Bladder exstrophy is a rare congenital anomaly of the genitourinary tract occurring in 1 in 50,000 births with a 2 : 1 male to female ratio.229 It occurs as a failure of the abdominal wall to close during fetal development and results in defects of the anterior wall of the bladder and overlying midline abdominal wall. Bladder exstrophy may present with a spectrum of anomalies, including widening of the symphysis pubis, and genital anomalies, such as epispadias, bifid clitoris, and undescended testes (Fig. 27-13). Some regard bladder and cloacal exstrophies as distinct entities, whereas others consider them both extremes in a continuum of antenatal defects. Cloacal exstrophy includes features of bladder exstrophy plus an omphalocele and spinal defects, and always includes imperforate anus. Because the antenatal diagnosis of bladder exstrophy using ultrasound may be technically difficult, and thus dependent on indirect signs (absence of bladder filling, a low-set umbilicus, widening of the pubic ramus, small external genitalia, or a lower abdominal mass), the diagnosis is confirmed at birth when the anterior abdominal wall defect with the exposed bladder mucosa are evident.
Therapy is aimed at the surgical reconstruction of the bladder with preservation of renal function while achieving urinary continence and satisfactory appearance of the external genitalia. Surgical repair is usually achieved via a planned surgical approach.230,231 In a very carefully selected group of infants, surgical management may be carried out as a single-stage procedure.232 Alternatively, a planned staged surgical repair may be chosen with management directed at closure of the bladder, posterior urethra, and abdominal wall. In addition, a bilateral iliac osteotomy is often performed to facilitate surgical closure, decrease the stress on the midline soft tissues, and reduce the risk of postoperative wound dehiscence.
Wound dehiscence, bladder prolapse, and multiple attempts at bladder closure are among the risk factors for decreased bladder growth and the inability for the later development of continence.233 In addition to the immediate postoperative complications, children who have undergone surgical repair of bladder exstrophy carry an increased risk for the development of renal, bladder, and colon adenocarcinoma.234 Children who require frequent bladder catheterizations or repeat surgeries are at risk for developing latex allergy if latex products are used.56 Today, most bladder catheters exclude latex.
Surgical management of the epispadias is usually performed at 6 to 12 months of age, and reconstruction of the bladder neck by 5 years, the latter to allow for bladder training. After the initial surgical repair, children are immobilized for 4 to 6 weeks,235 with a successful outcome likely when a modified Buck traction with an external fixator or a modified Bryant traction236 and adequate postoperative analgesia are used.237
A general anesthetic in combination with an epidural or caudal continuous infusion technique is often used if there are no associated spine abnormalities.238 Opioids (other than remifentanil) have had a limited role in neonates because of their potential to cause postoperative respiratory depression, although pediatric anesthesiologists today are much more aggressive in anticipating pain and administering opioids to neonates and infants, even if it delays extubation.
Maintenance of anesthesia may be achieved using an inhalation anesthetic, small doses of opioids, and intermittent boluses (0.5 to 1 mL/kg) or continuous infusions of bupivacaine (0.125% to 0.25%) or ropivacaine (0.2%) with or without epinephrine through a lumbar or caudal epidural catheter. Caudal or epidural analgesia may be provided by advancing the catheter to the appropriate surgical dermatomal level after induction of anesthesia. The catheter is either secured directly to the skin or tunneled using the epidural insertion needle.239 A continuous infusion of a local anesthetic may be used and maintained for up to 3 days to provide analgesia and a mild motor blockade that favors immobility. However, care must be taken to avoid systemic toxicity. Neonates are at increased risk for developing local anesthetic toxicity because of their low serum protein concentration and metabolism. Albumin and α1-acid glycoprotein are reduced in this age group,240 which reduces protein binding and increases the free-fraction of bupivacaine. This free form is metabolized to a lesser extent in the neonate because of immature metabolic pathways, and contributes to an increased risk of cardiac and systemic toxicity.241,242 This is especially true of the amide local anesthetics. If the epidural infusion is maintained beyond 48 to 72 hours, serum concentrations of local anesthetic should be sampled once or twice a day to guide therapy and minimize the risk of complications. Because serum concentrations of bupivacaine increase after only 48 hours of a continuous epidural infusion,241 and serum concentrations are difficult to assay, lidocaine 0.1% (0.8 mg/kg/hr) may be preferable. Ropivacaine is also currently being used for infusions that last less than 72 hours.243
The outcome of this complex surgery is determined primarily by the surgical expertise. Urologic incontinence, bladder prolapse, and epispadias revisions will require additional urologic surgery, as well as possible osteotomies, for satisfactory closure and surgical outcomes. Renal function is usually well-maintained throughout. Complications of complete repair are reported to be similar to those of the staged repair, but usually involve additional soft tissue defects.244
Acknowledgment
The authors thank P.A. Lonnqvist for his contributions to this chapter in the previous edition.
Kim PH, Patil MB, Kim SS, et al. Early comparison of nephrectomy options in children (open, transperitoneal laparoscopic, laparo-endoscopic single site (LESS), and robotic surgery). BJU Int. 2012;109:910–915.
Lasersohn L. Anaesthetic considerations for paediatric laparoscopy. S Afr J Surg. 2011;49:22–26.
Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675.
Ure BM, Suempelmann R, Metzelder MM, Kuebler J. Physiological responses to endoscopic surgery in children. Semin Pediatr Surg. 2007;16:217–223.
Walker RW, Ravi R, Haylett K. Effect of cricoid force on airway calibre in children: a bronchoscopic assessment. Br J Anaesth. 2010;104:71–74.
1 Chandra V, Dutta S, Albanese CT. Robot-assisted pediatric surgery. In: Saxena AK, Hollwarth ME, eds. Essentials of pediatric endoscopic surgery. Berlin: Springer-Verlag; 2009:87–90.
2 Bricker SRW, McLuckie A, Nightingale DA. Gastric aspirates after trauma in children. Anaesthesia. 1989;44:721–724.
3 Marcus RJ, Thompson JP. Anaesthesia for manipulation of forearm fractures in children: a survey of current practice. Paediatr Anaesth. 2000;10:273–277.
4 Schmitz A, Thomas S, Melanie F, et al. Ultrasonographic gastric antral area and gastric contents volume in children. Paediatr Anaesth. 2012;22:144–149.
5 Bouvet L, Mazoit JX, Chassard D, et al. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114:1086–1092.
6 Lerman J. On cricoid pressure: “may the force be with you.”. Anesth Analg. 2009;109:1363–1366.
7 Ahmed Z, Zestos M, Chidiac E, Lerman J. A survey of cricoid pressure use among pediatric anesthesiologists. Paediatr Anaesth. 2009;19:183–187.
8 Weir PS. Anaesthesia for appendicectomy in childhood: a survey of practice in Northern Ireland. Ulster Med J. 1997;66:34–37.
9 Morris J, Cook TM. Rapid sequence induction: a national survey of practice. Anaesthesia. 2001;56:1090–1097.
10 Walker RW, Ravi R, Haylett K. Effect of cricoid force on airway calibre in children: a bronchoscopic assessment. Br J Anaesth. 2010;104:71–74.
11 Meakin GH. Role of muscle relaxants in pediatric anesthesia. Curr Opin Anaesthesiol. 2007;20:227–231.
12 Rawicz M, Brandom BW, Wolf A. The place of suxamethonium in pediatric anesthesia. Paediatr Anaesth. 2009;19:561–570.
13 Plaud B, Meretoja O, Hofmockel R, et al. Reversal of rocuronium-induced neuromuscular blockade with sugammadex in pediatric and adult surgical patients. Anesthesiology. 2009;110:284–294.
14 Kinouchi K, Fukumitsu K, Tashiro C, et al. Duration of apnoea in anaesthetized children required for desaturation of haemoglobin to 95%: comparison of three different breathing gases. Pediatr Anaesth. 1995;5:115–119.
15 Kinouchi K, Tanigami H, Tashiro C, et al. Duration of apnea in anesthetized infants and children required for desaturation of hemoglobin to 95%. The influence of upper respiratory infection. Anesthesiology. 1992;77:1105–1107.
16 Herman NL, Carter B, Van Decar TK. Cricoid pressure: teaching the recommended level. Anesth Analg. 1996;83:859–863.
17 Moynihan RJ, Brock-Utne JG, Archer JH, Feld LH, Kreitzman TR. The effect of cricoid pressure on preventing gastric insufflation in infants and children. Anesthesiology. 1993;78:652–656.
18 Manning BJ, Winter DC, McGreal G, Kirwan WO, Redmond HP. Nasogastric intubation causes gastroesophageal reflux in patients undergoing elective laparotomy. Surgery. 2001;130:788–791.
19 Huggins PS, Tuomi SK, Young C. Effects of nasogastric tubes on the young, normal swallowing mechanism. Dysphagia. 1999;14:157–161.
20 Bailey AG, McNaull PP, Jooste E, Tuchman JB. Perioperative crystalloid and colloid fluid management in children: where are we and how did we get here? Anesth Analg. 2010;110:375–390.
21 Carlotti AP, Carvalho WB. Abdominal compartment syndrome: a review. Pediatr Crit Care Med. 2009;10:115–120.
22 De DJ, Ramet J. ACS in paediatrics. Acta Clin Belg Suppl. 2007:149–151.
23 Fenton SJ, Dodgion CM, Meyers RL, Nichol PF, Scaife ER. Temporary abdominal vacuum-packing closure in the neonatal intensive care unit. J Pediatr Surg. 2007;42:957–960.
24 Kidd JN, Jr., Jackson RJ, Smith SD, Wagner CW. Evolution of staged versus primary closure of gastroschisis. Ann Surg. 2003;237:759–764.
25 Morrell BJ, Vinden C, Singh RN, Kornecki A, Fraser DD. Secondary abdominal compartment syndrome in a case of pediatric trauma shock resuscitation. Pediatr Crit Care Med. 2007;8:67–70.
26 Perks DH, Grewal H. Abdominal compartment syndrome in the pediatric patient with blunt trauma. J Trauma Nurs. 2005;12:50–54.
27 Beck R, Halberthal M, Zonis Z, et al. Abdominal compartment syndrome in children. Pediatr Crit Care Med. 2001;2:51–56.
28 Shear W, Rosner MH. Acute kidney dysfunction secondary to the abdominal compartment syndrome. J Nephrol. 2006;19:556–565.
29 Neville HL, Lally KP, Cox CS, Jr. Emergent abdominal decompression with patch abdominoplasty in the pediatric patient. J Pediatr Surg. 2000;35:705–708.
30 Kuhls E, Gauntlett IS, Lau M, et al. Effect of increased intra-abdominal pressure on hepatic extraction and clearance of fentanyl in neonatal lambs. J Pharmacol Exp Ther. 1995;274:115–119.
31 Gauntlett IS, Fisher DM, Hertzka RE, et al. Pharmacokinetics of fentanyl in neonatal humans and lambs: effects of age. Anesthesiology. 1988;69:683–687.
32 Yaster M, Buck JR, Dudgeon DL, et al. Hemodynamic effects of primary closure of omphalocele/gastroschisis in human newborns. Anesthesiology. 1988;69:84–88.
33 Ejike JC, Humbert S, Bahjri K, Mathur M. Outcomes of children with abdominal compartment syndrome. Acta Clin Belg Suppl. 2007:141–148.
34 Diaz FJ, Fernandez SA, Gotay F. Identification and management of abdominal compartment syndrome in the pediatric intensive care unit. P R Health Sci J. 2006;25:17–22.
35 Suominen PK, Pakarinen MP, Rautiainen P, Mattila I, Sairanen H. Comparison of direct and intravesical measurement of intraabdominal pressure in children. J Pediatr Surg. 2006;41:1381–1385.
36 DeCou JM, Abrams RS, Miller RS, Gauderer MW. Abdominal compartment syndrome in children: experience with three cases. J Pediatr Surg. 2000;35:840–842.
37 Kawar B, Siplovich L. Abdominal compartment syndrome in children: the dilemma of treatment. Eur J Pediatr Surg. 2003;13:330–333.
38 Wheeler M, Coté CJ. Preoperative pregnancy testing in a tertiary care children’s hospital: a medico-legal conundrum. J Clin Anesth. 1999;11:56–63.
39 Sigaut S, Skhiri A, Stany I, et al. Ultrasound guided internal jugular vein access in children and infant: a meta-analysis of published studies. Paediatr Anaesth. 2009;19:1199–1206.
40 Gueugniaud PY, Muchada R, Moussa M, Haro D, Petit P. Continuous oesophageal aortic blood flow echo-Doppler measurement during general anaesthesia in infants. Can J Anaesth. 1997;44:745–750.
41 Gueugniaud PY, David JS, Petit P. Early hemodynamic variations assessed by an echo-Doppler aortic blood flow device in a severely burned infant: correlation with the circulating cytokines. Pediatr Emerg Care. 1998;14:282–284.
42 Tibby SM, Hatherill M, Durward A, Murdoch IA. Are transoesophageal Doppler parameters a reliable guide to paediatric haemodynamic status and fluid management? Intensive Care Med. 2001;27:201–205.
43 Ungerleider RM, Greeley WJ, Sheikh KH, et al. Routine use of intraoperative epicardial echocardiography and Doppler color flow imaging to guide and evaluate repair of congenital heart lesions. A prospective study. J Thorac Cardiovasc Surg. 1990;100:297–309.
44 Ungerleider RM, Greeley WJ, Sheikh KH, et al. The use of intraoperative echo with Doppler color flow imaging to predict outcome after repair of congenital cardiac defects. Ann Surg. 1989;210:526–533.
45 Scohy TV, Gommers D, Jan ten Harkel AD, et al. Intraoperative evaluation of micromultiplane transesophageal echocardiographic probe in surgery for congenital heart disease. Eur J Echocardiogr. 2007;8:241–246.
46 Monsel A, Salvat-Toussaint A, Durand P, et al. The transesophageal Doppler and hemodynamic effects of epidural anesthesia in infants anesthetized with sevoflurane and sufentanil. Anesth Analg. 2007;105:46–50.
47 Larousse E, Asehnoune K, Dartayet B, et al. The hemodynamic effects of pediatric caudal anesthesia assessed by esophageal Doppler. Anesth Analg. 2002;94:1165–1168.
48 Chew MS, Poelaert J. Accuracy and repeatability of pediatric cardiac output measurement using Doppler: 20-year review of the literature. Intensive Care Med. 2003;29:1889–1894.
49 Mohan UR, Britto J, Habibi P, de MC, Nadel S. Noninvasive measurement of cardiac output in critically ill children. Pediatr Cardiol. 2002;23:58–61.
50 Wodey E, Gai V, Carre F, Ecoffey C. Accuracy and limitations of continuous oesophageal aortic blood flow measurement during general anaesthesia for children: comparison with transcutaneous echography-Doppler. Paediatr Anaesth. 2001;11:309–317.
51 Phillips I, Jamieson CG. Pulmonary aspiration complicating intraoperative small-bowel decompression: a case report and literature review. Can J Surg. 1996;39:495–498.
52 Borkar J, Dave N. Analgesic efficacy of caudal block versus diclofenac suppository and local anesthetic infiltration following pediatric laparoscopy. J Laparoendosc Adv Surg Tech A. 2005;15:415–418.
53 Poterio GM, Braga AF, Santos RM, Gomes IF, Luchetta MI. Anaphylaxis during renal transplantation of live donor graft in a child with latex allergy: case report. Rev Bras Anestesiol. 2009;59:210–218.
54 Meric F, Teitelbaum DH, Geiger JD, Harmon CM, Groner JI. Latex sensitization in general pediatric surgical patients: a call for increased screening of patients. J Pediatr Surg. 1998;33:1108–1111.
55 Degenhardt P, Golla S, Wahn F, Niggemann B. Latex allergy in pediatric surgery is dependent on repeated operations in the first year of life. J Pediatr Surg. 2001;36:1535–1539.
56 Sparta G, Kemper MJ, Gerber AC, Goetschel P, Neuhaus TJ. Latex allergy in children with urological malformation and chronic renal failure. J Urol. 2004;171:1647–1649.
57 Ausili E, Tabacco F, Focarelli B, et al. Prevalence of latex allergy in spina bifida: genetic and environmental risk factors. Eur Rev Med Pharmacol Sci. 2007;11:149–153.
58 Eiwegger T, Dehlink E, Schwindt J, et al. Early exposure to latex products mediates latex sensitization in spina bifida but not in other diseases with comparable latex exposure rates. Clin Exp Allergy. 2006;36:1242–1246.
59 Mazon A, Nieto A, Pamies R, et al. Influence of the type of operations on the development of latex sensitization in children with myelomeningocele. J Pediatr Surg. 2005;40:688–692.
60 Pires G, Morais-Almeida M, Gaspar A, et al. Risk factors for latex sensitization in children with spina bifida. Allergol Immunopathol (Madr). 2002;30:5–13.
61 Cremer R, Lorbacher M, Hering F, Engelskirchen R. Natural rubber latex sensitisation and allergy in patients with spina bifida, urogenital disorders and oesophageal atresia compared with a normal paediatric population. Eur J Pediatr Surg. 2007;17:194–198.
62 Sampathi V, Lerman J. Case scenario: perioperative latex allergy in children. Anesthesiology. 2011;114:673–680.
63 Birmingham PK, Dsida RM, Grayhack JJ, et al. Do latex precautions in children with myelodysplasia reduce intraoperative allergic reactions? J Pediatr Orthop. 1996;16:799–802.
64 Blumchen K, Bayer P, Buck D, et al. Effects of latex avoidance on latex sensitization, atopy and allergic diseases in patients with spina bifida. Allergy. 2010;65:1585–1593.
65 De Queiroz M, Combet S, Berard J, et al. Latex allergy in children: modalities and prevention. Paediatr Anaesth. 2009;19:313–319.
66 Mitsnefes MM, Daniels SR, Schwartz SM, et al. Severe left ventricular hypertrophy in pediatric dialysis: prevalence and predictors. Pediatr Nephrol. 2000;14:898–902.
67 Dimitriu A, Nistor N, Condurache T, Brumariu O. The value of echocardiography for the assessment of the cardiac impairment in chronic renal failure in child. Rev Med Chir Soc Med Nat Iasi. 2000;104:63–66.
68 Atalay S, Ekim M, Tutar HE, et al. Systolic and diastolic function in children with chronic renal failure. Pediatr Int. 2002;44:18–23.
69 Mitsnefes MM, Daniels SR, Schwartz SM, Khoury P, Strife CF. Changes in left ventricular mass in children and adolescents during chronic dialysis. Pediatr Nephrol. 2001;16:318–323.
70 Scharer K, Schmidt KG, Soergel M. Cardiac function and structure in patients with chronic renal failure. Pediatr Nephrol. 1999;13:951–965.
71 Kuhn B, Peters J, Marx GR, Breitbart RE. Etiology, management, and outcome of pediatric pericardial effusions. Pediatr Cardiol. 2008;29:90–94.
72 Kabukcu M, Demircioglu F, Yanik E, Basarici I, Ersel F. Pericardial tamponade and large pericardial effusions: causal factors and efficacy of percutaneous catheter drainage in 50 patients. Tex Heart Inst J. 2004;31:398–403.
73 Sloand JA, Sloand EM. Studies on platelet membrane glycoproteins and platelet function during hemodialysis. J Am Soc Nephrol. 1997;8:799–803.
74 Galbusera M, Remuzzi G, Boccardo P. Treatment of bleeding in dialysis patients. Semin Dial. 2009;22:279–286.
75 Flynn JT. Cardiovascular disease in children with chronic renal failure. Growth Horm IGF Res. 2006;16:S84–S90.
76 Burnei G, Burnei A, Hodorogea D, et al. Diagnosis and complications of renovascular hypertension in children: literature data and clinical observations. J Med Life. 2009;2:18–28.
77 Chandar J, Zilleruelo G. Hypertensive crisis in children. Pediatr Nephrol. 2012;27:741–751.
78 VanDeVoorde RG, Mitsnefes MM. Hypertension and CKD. Adv Chronic Kidney Dis.. 2011;18:355–361.
79 Lilova M. Hypertension in children with chronic renal failure. Turk J Pediatr. 2005;47(Suppl):28–31.
80 Behnia R, Molteni A, Igic R. Angiotensin-converting enzyme inhibitors: mechanisms of action and implications in anesthesia practice. Curr Pharm Des. 2003;9:763–776.
81 Licker M, Schweizer A, Hohn L, Farinelli C, Morel DR. Cardiovascular responses to anesthetic induction in patients chronically treated with angiotensin-converting enzyme inhibitors. Can J Anaesth. 2000;47:433–440.
82 Smith I, Jackson I. Beta-blockers, calcium channel blockers, angiotensin converting enzyme inhibitors and angiotensin receptor blockers: should they be stopped or not before ambulatory anaesthesia? Curr Opin Anaesthesiol. 2010;23:687–690.
83 Lau ST, Lee YH, Caty MG. Current management of hernias and hydroceles. Semin Pediatr Surg. 2007;16:50–57.
84 Vernon AH, Georgeson KE, Harmon CM. Pediatric laparoscopic appendectomy for acute appendicitis. Surg Endosc. 2004;18:75–79.
85 Cordera F, Long KH, Nagorney DM, et al. Open versus laparoscopic splenectomy for idiopathic thrombocytopenic purpura: clinical and economic analysis. Surgery. 2003;134:45–52.
86 Reddy VS, Phan HH, O’Neill JA, et al. Laparoscopic versus open splenectomy in the pediatric population: a contemporary single-center experience. Am Surg. 2001;67:859–863.
87 Spurbeck WW, Prasad R, Lobe TE. Two-year experience with minimally invasive herniorrhaphy in children. Surg Endosc. 2005;19:551–553.
88 Eller MM, Duarte Lanna JC. Videolaparoscopy of the contralateral internal inguinal ring via the hernia sac in children with unilateral inguinal hernia-initial experience in Brazil, with a meta-analysis. Pediatr Surg Int. 2002;18:463–469.
89 Kim PH, Patil MB, Kim SS, et al. Early comparison of nephrectomy options in children (open, transperitoneal laparoscopic, laparo-endoscopic single site (LESS), and robotic surgery). BJU Int. 2012;109:910–915.
90 Margaron FC, Oiticica C, Lanning DA. Robotic-assisted laparoscopic Nissen fundoplication with gastrostomy preservation in neurologically impaired children. J Laparoendosc Adv Surg Tech A. 2010;20:489–492.
91 Esposito C, Montinaro L, Alicchio F, et al. Laparoscopic treatment of inguinal hernia in the first year of life. J Laparoendosc Adv Surg Tech A. 2010;20:473–476.
92 Rescorla FJ, West KW, Engum SA, Grosfeld JL. Laparoscopic splenic procedures in children: experience in 231 children. Ann Surg. 2007;246:683–687.
93 Wedgewood J, Doyle E. Anaesthesia and laparoscopic surgery in children. Paediatr Anaesth. 2001;11:391–399.
94 Rowney DA, Aldridge LM. Laparoscopic fundoplication in children: anaesthetic experience of 51 cases. Paediatr Anaesth. 2000;10:291–296.
95 Jona JZ, Cohen RD, Georgeson KE, Rothenberg SS. Laparoscopic pull-through procedure for Hirschsprung’s disease. Semin Pediatr Surg. 1998;7:228–231.
96 Kuebler JF, Ure BM. Minimally invasive surgery in the neonate. Semin Fetal Neonatal Med. 2011;16:151–156.
97 Tsao K, Lally PA, Lally KP. Minimally invasive repair of congenital diaphragmatic hernia. J Pediatr Surg. 2011;46:1158–1164.
98 Slater B, Rangel S, Ramamoorthy C, Abrajano C, Albanese CT. Outcomes after laparoscopic surgery in neonates with hypoplastic heart left heart syndrome. J Pediatr Surg. 2007;42:1118–1121.
99 Taylor KL, Holtby H, Macpherson B. Laparoscopic surgery in the pediatric patient post Fontan procedure. Paediatr Anaesth. 2006;16:591–595.
100 Koga H, Yamataka A, Kobayashi H, Lane GJ, Miyano T. Laparoscopy-assisted gastropexy for gastric volvulus in a child with situs inversus, asplenia, and major cardiac anomaly. J Laparoendosc Adv Surg Tech A. 2007;17:513–516.
101 Morray JP. Cardiac arrest in anesthetized children: recent advances and challenges for the future. Paediatr Anaesth. 2011;21:722–729.
102 Lee SL, Yaghoubian A, Kaji A. Laparoscopic vs open appendectomy in children: outcomes comparison based on age, sex, and perforation status. Arch Surg. 2011;146:1118–1121.
103 Fox D, Morrato E, Campagna EJ, et al. Outcomes of laparoscopic versus open fundoplication in children’s hospitals: 2005-2008. Pediatrics. 2011;127:872–880.
104 Masoomi H, Mills S, Dolich MO, et al. Comparison of outcomes of laparoscopic versus open appendectomy in adults: data from the Nationwide Inpatient Sample (NIS), 2006-2008. J Gastrointest Surg. 2011;15:2226–2231.
105 van der Toorn F, van den Hoek J, Wolffenbuttel KP, Scheepe JR. Laparoscopic transperitoneal pyeloplasty in children from age of 3 years: our clinical outcomes compared with open surgery. J Pediatr Urol. 2012. Feb 7 [Epub ahead of print]
106 Chandler NM, Danielson PD. Single-incision laparoscopic cholecystectomy in children: a retrospective comparison with traditional laparoscopic cholecystectomy. J Pediatr Surg. 2011;46:1695–1699.
107 Garcia-Henriquez N, Shah SR, Kane TD. Single-incision laparoscopic cholecystectomy in children using standard straight instruments: a surgeon’s early experience. J Laparoendosc Adv Surg Tech A. 2011;21:555–559.
108 Ostlie DJ. Single-site umbilical laparoscopic appendectomy. Semin Pediatr Surg. 2011;20:196–200.
109 Garey CL, Laituri CA, Ostlie DJ, et al. Single-incision laparoscopic surgery in children: initial single-center experience. J Pediatr Surg. 2011;46:904–907.
109a Rao PP, Rao PP, Bhagwat S. Single-incision laparoscopic surgery—current status and controversies. J Minimal Access Surgery. 2011;7:6–16.
110 Beebe DS, Zhu S, Kumar MV, et al. The effect of insufflation pressure on CO(2) pneumoperitoneum and embolism in piglets. Anesth Analg. 2002;94:1182–1187.
111 Nagao K, Reichert J, Beebe DS, Fowler JM, Belani KG. Carbon dioxide embolism during laparoscopy: effect of insufflation pressure in pigs. JSLS. 1999;3:91–96.
112 Baroncini S, Gentili A, Pigna A, et al. Anaesthesia for laparoscopic surgery in paediatrics. Minerva Anestesiol. 2002;68:406–413.
113 Yau P, Watson DI, Lafullarde T, Jamieson GG. Experimental study of effect of embolism of different laparoscopy insufflation gases. J Laparoendosc Adv Surg Tech A. 2000;10:211–216.
114 Tobias JD. Anaesthesia for minimally invasive surgery in children. Best Pract Res Clin Anaesthesiol. 2002;16:115–130.
115 Pennant JH. Anesthesia for laparoscopy in the pediatric patient. Anesthesiol Clin North America. 2001;19:69–88.
116 Hsing CH, Hseu SS, Tsai SK, et al. The physiological effect of CO2 pneumoperitoneum in pediatric laparoscopy. Acta Anaesthesiol Sin. 1995;33:1–6.
117 McHoney M, Corizia L, Eaton S, et al. Carbon dioxide elimination during laparoscopy in children is age dependent. J Pediatr Surg. 2003;38:105–110.
118 Pacilli M, Pierro A, Kingsley C, et al. Absorption of carbon dioxide during laparoscopy in children measured using a novel mass spectrometric technique. Br J Anaesth. 2006;97:215–219.
119 Laffon M, Gouchet A, Sitbon P, et al. Difference between arterial and end-tidal carbon dioxide pressures during laparoscopy in paediatric patients. Can J Anaesth. 1998;45:561–563.
120 Sanders JC, Gerstein N. Arterial to endtidal carbon dioxide gradient during pediatric laparoscopic fundoplication. Paediatr Anaesth. 2008;18:1096–1101.
121 Kalfa N, Allal H, Raux O, et al. Tolerance of laparoscopy and thoracoscopy in neonates. Pediatrics. 2005;116:e785–e791.
122 Wulkan ML, Vasudevan SA. Is end-tidal CO2 an accurate measure of arterial CO2 during laparoscopic procedures in children and neonates with cyanotic congenital heart disease? J Pediatr Surg. 2001;36:1234–1236.
123 Lasersohn L. Anaesthetic considerations for paediatric laparoscopy. S Afr J Surg. 2011;49:22–26.
124 Taylor SP, Hoffman GM. Gas embolus and cardiac arrest during laparoscopic pyloromyotomy in an infant. Can J Anaesth. 2010;57:774–778.
125 Fischler M. Occurrence of a carbon dioxide embolism during laparoscopic pyloromyotomy in a small child: several unresolved questions. Can J Anaesth. 2011;58:226–227.
126 Diemunsch PA, Van DT, Torp KD, Schaeffer R, Geny B. Calibrated pneumoperitoneal venting to prevent N2O accumulation in the CO2 pneumoperitoneum during laparoscopy with inhaled anesthesia: an experimental study in pigs. Anesth Analg. 2002;94:1014–1018.
127 Mann C, Boccara G, Grevy V, et al. Argon pneumoperitoneum is more dangerous than CO2 pneumoperitoneum during venous gas embolism. Anesth Analg. 1997;85:1367–1371.
128 Ure BM, Suempelmann R, Metzelder MM, Kuebler J. Physiological responses to endoscopic surgery in children. Semin Pediatr Surg. 2007;16:217–223.
129 Bergesio R, Habre W, Lanteri C, Sly P. Changes in respiratory mechanics during abdominal laparoscopic surgery in children. Anaesth Intensive Care. 1999;27:245–248.
130 Veyckemans F. Celioscopic surgery in infants and children: the anesthesiologist’s point of view. Paediatr Anaesth. 2004;14:424–432.
131 Manner T, Aantaa R, Alanen M. Lung compliance during laparoscopic surgery in paediatric patients. Paediatr Anaesth. 1998;8:25–29.
132 Bottcher-Haberzeth S, Dullenkopf A, Gitzelmann CA, Weiss M. Tracheal tube tip displacement during laparoscopy in children. Anaesthesia. 2007;62:131–134.
133 Rolf N, Coté CJ. Diagnosis of clinically unrecognized endobronchial intubation in paediatric anaesthesia: which is more sensitive, pulse oximetry or capnography? Paediatr Anaesth. 1992;2:31–35.
134 Sinha A, Sharma B, Sood J. ProSeal as an alternative to endotracheal intubation in pediatric laparoscopy. Paediatr Anaesth. 2007;17:327–332.
135 Kim JY, Shin CS, Lee KC, Chang YJ, Kwak HJ. Effect of pressure- versus volume-controlled ventilation on the ventilatory and hemodynamic parameters during laparoscopic appendectomy in children: a prospective, randomized study. J Laparoendosc Adv Surg Tech A. 2011;21:655–658.
136 Yokomori K, Terawaki K, Kamii Y, et al. A new technique applicable to pediatric laparoscopic surgery: abdominal wall ‘area lifting’ with subcutaneous wiring. J Pediatr Surg. 1998;33:1589–1592.
137 Kitano Y, Takata M, Sasaki N, et al. Influence of increased abdominal pressure on steady-state cardiac performance. J Appl Physiol. 1999;86:1651–1656.
138 Huettemann E, Sakka SG, Petrat G, Schier F, Reinhart K. Left ventricular regional wall motion abnormalities during pneumoperitoneum in children. Br J Anaesth. 2003;90:733–736.
139 Sakka SG, Huettemann E, Petrat G, et al. Transoesophageal echocardiographic assessment of haemodynamic changes during laparoscopic herniorrhaphy in small children. Br J Anaesth. 2000;84:330–334.
140 Kardos A, Vereczkey G, Pirot L, Nyirady P, Mekler R. Use of impedance cardiography to monitor haemodynamic changes during laparoscopy in children. Paediatr Anaesth. 2001;11:175–179.
141 Gueugniaud PY, Abisseror M, Moussa M, et al. The hemodynamic effects of pneumoperitoneum during laparoscopic surgery in healthy infants: assessment by continuous esophageal aortic blood flow echo-Doppler. Anesth Analg. 1998;86:290–293.
142 de Waal EE, Kalkman CJ. Haemodynamic changes during low-pressure carbon dioxide pneumoperitoneum in young children. Paediatr Anaesth. 2003;13:18–25.
143 Mattioli G, Montobbio G, Pini PA, et al. Anesthesiologic aspects of laparoscopic fundoplication for gastroesophageal reflux in children with chronic respiratory and gastroenterological symptoms. Surg Endosc. 2003;17:559–566.
144 Talamini MA, Mendoza-Sagaon M, Gitzelmann CA, et al. Increased mediastinal pressure and decreased cardiac output during laparoscopic Nissen fundoplication. Surgery. 1997;122:345–352.
145 Sfez M, Guerard A, Desruelle P. Cardiorespiratory changes during laparoscopic fundoplication in children. Paediatr Anaesth. 1995;5:89–95.
146 Joris JL, Noirot DP, Legrand MJ, Jacquet NJ, Lamy ML. Hemodynamic changes during laparoscopic cholecystectomy. Anesth Analg. 1993;76:1067–1071.
147 Terrier G. Anaesthesia for laparoscopic procedures in infants and children: indications, intra- and post-operative management, prevention and treatment of complications. Curr Opin Anaesthesiol. 1999;12:311–314.
148 Kalmar AF, Foubert L, Hendrickx JF, et al. Influence of steep Trendelenburg position and CO(2) pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br J Anaesth. 2010;104:433–439.
149 Awad H, Santilli S, Ohr M, et al. The effects of steep trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009;109:473–478.
150 Uzzo RG, Bilsky M, Mininberg DT, Poppas DP. Laparoscopic surgery in children with ventriculoperitoneal shunts: effect of pneumoperitoneum on intracranial pressure—preliminary experience. Urology. 1997;49:753–757.
151 Jackman SV, Weingart JD, Kinsman SL, Docimo SG. Laparoscopic surgery in patients with ventriculoperitoneal shunts: safety and monitoring. J Urol. 2000;164:1352–1354.
152 Esposito C, Colella G, Settimi A, et al. One-trocar laparoscopy: a valid procedure to treat abdominal complications in children with peritoneal shunt for hydrocephalus. Surg Endosc. 2003;17:828–830.
153 Fraser JD, Aguayo P, Sharp SW, et al. The safety of laparoscopy in pediatric patients with ventriculoperitoneal shunts. J Laparoendosc Adv Surg Tech A. 2009;19:675–678.
154 Demyttenaere S, Feldman LS, Fried GM. Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surg Endosc. 2007;21:152–160.
155 Gomez Dammeier BH, Karanik E, Gluer S, et al. Anuria during pneumoperitoneum in infants and children: a prospective study. J Pediatr Surg. 2005;40:1454–1458.
156 Abassi Z, Bishara B, Karram T, et al. Adverse effects of pneumoperitoneum on renal function: involvement of the endothelin and nitric oxide systems. Am J Physiol Regul Integr Comp Physiol. 2008;294:R842–R850.
157 Micali S, Silver RI, Kaufman HS, et al. Measurement of urinary N-acetyl-beta-D-glucosaminidase to assess renal ischemia during laparoscopic operations. Surg Endosc. 1999;13:503–506.
158 Mertens zur Borg IR, Di Biase M, Verbrugge S, Ijzermans JN, Gommers D. Comparison of three perioperative fluid regimes for laparoscopic donor nephrectomy: a prospective randomized dose-finding study. Surg Endosc. 2008;22:146–150.
159 Sauerland S, Lefering R, Neugebauer EA. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2004. CD001546
160 Chan KL, Hui WC, Tam PK. Prospective randomized single-center, single-blind comparison of laparoscopic vs open repair of pediatric inguinal hernia. Surg Endosc. 2005;19:927–932.
161 Lintula H, Kokki H, Vanamo K. Single-blind randomized clinical trial of laparoscopic versus open appendicectomy in children. Br J Surg. 2001;88:510–514.
162 Mayer S, Werner A, Wachowiak R, et al. Single-incision multiport laparoscopy does not cause more pain than conventional laparoscopy: a prospective evaluation in children undergoing appendectomy. J Laparoendosc Adv Surg Tech A. 2011;21:753–756.
163 Tomaszewski JJ, Casella DP, Turner RM, Casale P, Ost MC. Pediatric laparoscopic and robot-assisted laparoscopic surgery: technical considerations. J Endourol. 2012;26:602–613.
164 Lee RS, Sethi AS, Passerotti CC, Peters CA. Robot-assisted laparoscopic nephrectomy and contralateral ureteral reimplantation in children. J Endourol. 2010;24:123–128.
165 Chung DH, Georgeson KE. Fundoplication and gastrostomy. Semin Pediatr Surg. 1998;7:213–219.
166 Rothenberg SS. Experience with 220 consecutive laparoscopic Nissen fundoplications in infants and children. J Pediatr Surg. 1998;33:274–278.
167 Molik KA, Engum SA, Rescorla FJ, et al. Pectus excavatum repair: experience with standard and minimal invasive techniques. J Pediatr Surg. 2001;36:324–328.
168 Miller KA, Woods RK, Sharp RJ, et al. Minimally invasive repair of pectus excavatum: a single institution’s experience. Surgery. 2001;130:652–657.
169 Hoel TN, Rein KA, Svennevig JL. A life-threatening complication of the Nuss procedure for pectus excavatum. Ann Thorac Surg. 2006;81:370–372.
170 Marusch F, Gastinger I. [Life-threatening complication of the Nuss-procedure for funnel chest. A case report]. Zentralbl Chir. 2003;128:981–984.
171 Nuss D, Kelly RE, Jr., Croitoru DP, Katz ME. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33:545–552.
172 Palmer B, Yedlin S, Kim S. Decreased risk of complications with bilateral thoracoscopy and left-to-right mediastinal dissection during minimally invasive repair of pectus excavatum. Eur J Pediatr Surg. 2007;17:81–83.
173 Hebra A, Swoveland B, Egbert M, et al. Outcome analysis of minimally invasive repair of pectus excavatum: review of 251 cases. J Pediatr Surg. 2000;35:252–257.
174 Ohno K, Morotomi Y, Ueda M, et al. Comparison of the Nuss procedure for pectus excavatum by age and uncommon complications. Osaka City Med J. 2003;49:71–76.
175 Cucchiaro G, Farrar JT, Guite JW, Li Y. What postoperative outcomes matter to pediatric patients? Anesth Analg. 2006;102:1376–1382.
176 Cucchiaro G, Adzick SN, Rose JB, Maxwell L, Watcha M. A comparison of epidural bupivacaine-fentanyl and bupivacaine-clonidine in children undergoing the Nuss procedure. Anesth Analg. 2006;103:322–327.
177 Leonhardt J, Kubler JF, Feiter J, Ure BM, Petersen C. Complications of the minimally invasive repair of pectus excavatum. J Pediatr Surg. 2005;40:e7–e9.
178 Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675.
179 Havekes B, Romijn JA, Eisenhofer G, Adams K, Pacak K. Update on pediatric pheochromocytoma. Pediatr Nephrol. 2009;24:943–950.
180 Waguespack SG, Rich T, Grubbs E, et al. A current review of the etiology, diagnosis, and treatment of pediatric pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2010;95:2023–2037.
181 Pham TH, Moir C, Thompson GB, et al. Pheochromocytoma and paraganglioma in children: a review of medical and surgical management at a tertiary care center. Pediatrics. 2006;118:1109–1117.
182 Kantorovich V, Pacak K, Pheochromocytoma and paraganglioma. Progress in brain research. Martini L, ed. Progress in brain research, Philadelphia, Elsevier, 2010;Vol 182:343–373.
183 Hack HA. The perioperative management of children with phaeochromocytoma. Paediatr Anaesth. 2000;10:463–476.
184 Armstrong R, Sridhar M, Greenhalgh KL, et al. Phaeochromocytoma in children. Arch Dis Child. 2008;93:899–904.
185 Almeida MQ, Stratakis CA. Solid tumors associated with multiple endocrine neoplasias. Cancer Genet Cytogenet. 2010;203:30–36.
186 Sullivan J, Groshong T, Tobias JD. Presenting signs and symptoms of pheochromocytoma in pediatric-aged patients. Clin Pediatr (Phila). 2005;44:715–719.
187 Tarant NS, Dacanay RG, Mecklenburg BW, et al. Acute appendicitis in a patient with undiagnosed pheochromocytoma. Anesth Analg. 2006;102:642–643.
188 Goldstein RE, O’Neill JA, Jr., Holcomb GW, III., et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229:755–764.
189 Lord MS, Augoustides JG. Perioperative management of pheochromocytoma: focus on magnesium, clevidipine, and vasopressin. J Cardiothorac Vasc Anesth. 2012;26:526–531.
190 Treschan TA, Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology. 2006;105:599–612.
191 Hickman PE, Leong M, Chang J, Wilson SR, McWhinney B. Plasma free metanephrines are superior to urine and plasma catecholamines and urine catecholamine metabolites for the investigation of phaeochromocytoma. Pathology. 2009;41:173–177.
192 Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DS. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med. 2001;134:315–329.
193 Hull CJ. Phaeochromocytoma. Diagnosis, preoperative preparation and anaesthetic management. Br J Anaesth. 1986;58:1453–1468.
194 St. Peter SD, Valusek PA, Hill S, et al. Laparoscopic adrenalectomy in children: a multicenter experience. J Laparoendosc Adv Surg Tech A. 2011;21:647–649.
195 Serour F, Cohen A, Mandelberg A, Mori J, Ezra S. Dorsal penile nerve block in children undergoing circumcision in a day-care surgery. Can J Anaesth. 1996;43:954–958.
196 Holder KJ, Peutrell JM, Weir PM. Regional anaesthesia for circumcision. Subcutaneous ring block of the penis and subpubic penile block compared. Eur J Anaesthesiol. 1997;14:495–498.
197 Allan CY, Jacqueline PA, Shubhda JH. Caudal epidural block versus other methods of postoperative pain relief for circumcision in boys. Cochrane Database Syst Rev. 2003. CD003005
198 Fisher QA, McComiskey CM, Hill JL, et al. Postoperative voiding interval and duration of analgesia following peripheral or caudal nerve blocks in children. Anesth Analg. 1993;76:173–177.
199 El-Dawlatly AA, Turkistani A, Kettner SC, et al. Ultrasound-guided transversus abdominis plane block: description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. Br J Anaesth. 2009;102:763–767.
200 Makela E, Lahdes-Vasama T, Rajakorpi H, Wikstrom S. A 19-year review of paediatric patients with acute scrotum. Scand J Surg. 2007;96:62–66.
201 Becker A, Baum M. Obstructive uropathy. Early Hum Dev. 2006;82:15–22.
202 Salam MA. Posterior urethral valve: outcome of antenatal intervention. Int J Urol. 2006;13:1317–1322.
203 Ylinen E, la-Houhala M, Wikstrom S. Prognostic factors of posterior urethral valves and the role of antenatal detection. Pediatr Nephrol. 2004;19:874–879.
204 Ziylan O, Oktar T, Ander H, et al. The impact of late presentation of posterior urethral valves on bladder and renal function. J Urol. 2006;175:1894–1897.
205 Strand WR. Initial management of complex pediatric disorders: prunebelly syndrome, posterior urethral valves. Urol Clin North Am. 2004;31:399–415.
206 Ansari MS, Gulia A, Srivastava A, Kapoor R. Risk factors for progression to end-stage renal disease in children with posterior urethral valves. J Pediatr Urol. 2010;6:261–264.
207 Ger R, Coryllos EV. Management of the abdominal wall defect in the prune belly syndrome by muscle transposition: an 18-year follow-up. Clin Anat. 2000;13:341–346.
208 Lesavoy MA, Chang EI, Suliman A, et al. Long-term follow-up of total abdominal wall reconstruction for prune belly syndrome. Plast Reconstr Surg. 2012;129:104e–109e.
209 Franco I. Prune belly syndrome. http://emedicine.medscape.com. Accessed August 29, 2012
210 Wheatley JM, Stephens FD, Hutson JM. Prune-belly syndrome: ongoing controversies regarding pathogenesis and management. Semin Pediatr Surg. 1996;5:95–106.
211 Embleton ND, Wyllie JP, Wright MJ, Burn J, Hunter S. Natural history of trisomy 18. Arch Dis Child Fetal Neonatal Ed. 1996;75:F38–F41.
212 Nelson KE, Hexem KR, Feudtner C. Inpatient hospital care of children with trisomy 13 and trisomy 18 in the United States. Pediatrics. 2012. 129:869-76
213 Henderson AM, Vallis CJ, Sumner E. Anaesthesia in the prune-belly syndrome. A review of 36 cases. Anaesthesia. 1987;42:54–60.
214 Capozza N, Caione P. Vesicoureteral reflux: surgical and endoscopic treatment. Pediatr Nephrol. 2007;22:1261–1265.
215 Kutikov A, Guzzo TJ, Canter DJ, Casale P. Initial experience with laparoscopic transvesical ureteral reimplantation at the Children’s Hospital of Philadelphia. J Urol.. 2006;176:2222–2225.
215b Singh P, Dogra PN, Kumar R. Outcomes of robot-assisted laparoscopic pyeloplasty in children: a single center experience. J Endourol. 2012;26:249–253.
216 El-Ghoneimi A, Valla JS, Steyaert H, Aigrain Y. Laparoscopic renal surgery via a retroperitoneal approach in children. J Urol. 1998;160:1138–1141.
217 Lorenzo AJ, Karsli C, Halachmi S, et al. Hemodynamic and respiratory effects of pediatric urological retroperitoneal laparoscopic surgery: a prospective study. J Urol. 2006;175:1461–1465.
218 Canon SJ, Jayanthi VR, Lowe GJ. Which is better—retroperitoneoscopic or laparoscopic dismembered pyeloplasty in children? J Urol. 2007;178:1791–1795.
219 Ravish IR, Nerli RB, Reddy MN, Amarkhed SS. Laparoscopic pyeloplasty compared with open pyeloplasty in children. J Endourol. 2007;21:897–902.
220 Drueke T. Hyporesponsiveness to recombinant human erythropoietin. Nephrol Dial Transplant. 2001;16:25–28.
221 Espahbodi F, Kashi Z, Ala S, Hendoii N. Efficacy and safety of oral versus intravenous vitamin C in hemodialysis patients with functional iron deficiency. Int J Endocrinol Metab. 2007;3:129–134.
222 El-Ghoneimi A, Sauty L, Maintenant J, et al. Laparoscopic retroperitoneal nephrectomy in high risk children. J Urol. 2000;164:1076–1079.
223 Balassy C, Navarro OM, Daneman A. Adrenal masses in children. Radiol Clin North Am. 2011;49:711–727.
224 Davidoff AM. Neuroblastoma. Semin Pediatr Surg. 2012;21:2–14.
225 Whyte SD, Mark AJ. Anesthetic considerations in the management of Wilms’ tumor. Paediatr Anaesth. 2006;16:504–513.
226 Cheung NK, Zhang J, Lu C, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012;307:1062–1071.
227 Creagh-Barry P, Sumner E. Neuroblastoma and anaesthesia. Paediatr Anaesth. 1992;2:147–152.
228 Geller E, Kochan PS. Renal neoplasms of childhood. Radiol Clin North Am. 2011;49:689–709.
229 Siffel C, Correa A, Amar E, et al. Bladder exstrophy: an epidemiologic study from the International Clearinghouse for Birth Defects Surveillance and Research, and an overview of the literature. Am J Med Genet C Semin Med Genet. 2011;157C:321–332.
230 Grady RW, Mitchell ME. Complete primary repair of exstrophy. J Urol. 1999;162:1415–1420.
231 Husmann DA. Surgery insight: advantages and pitfalls of surgical techniques for the correction of bladder exstrophy. Nat Clin Pract Urol. 2006;3:95–100.
232 Hammouda HM, Kotb H. Complete primary repair of bladder exstrophy: initial experience with 33 cases. J Urol. 2004;172:1441–1444.
233 Oesterling JE, Jeffs RD. The importance of a successful initial bladder closure in the surgical management of classical bladder exstrophy: analysis of 144 patients treated at the Johns Hopkins Hospital between 1975 and 1985. J Urol. 1987;137:258–262.
234 Gargollo PC, Borer JG. Contemporary outcomes in bladder exstrophy. Curr Opin Urol. 2007;17:272–280.
235 Husmann DA, McLorie GA, Churchill BM. Closure of the exstrophic bladder: an evaluation of the factors leading to its success and its importance on urinary continence. J Urol. 1989;142:522–524.
236 Meldrum KK, Baird AD, Gearhart JP. Pelvic and extremity immobilization after bladder exstrophy closure: complications and impact on success. Urology. 2003;62:1109–1113.
237 Kost-Byerly S, Jackson EV, Yaster M, et al. Perioperative anesthetic and analgesic management of newborn bladder exstrophy repair. J Pediatr Urol. 2008;4:280–285.
238 Bosenberg AT. Epidural analgesia for major neonatal surgery. Paediatr Anaesth. 1998;8:479–483.
239 Aram L, Krane EJ, Kozloski LJ, Yaster M. Tunneled epidural catheters for prolonged analgesia in pediatric patients. Anesth Analg. 2001;92:1432–1438.
240 Lerman J, Strong HA, LeDez KM, et al. Effects of age on the serum concentration of alpha 1-acid glycoprotein and the binding of lidocaine in pediatric patients. Clin Pharmacol Ther. 1989;46:219–225.
241 Larsson BA, Lonnqvist PA, Olsson GL. Plasma concentrations of bupivacaine in neonates after continuous epidural infusion. Anesth Analg. 1997;84:501–505.
242 Mazoit JX, Denson DD, Samii K. Pharmacokinetics of bupivacaine following caudal anesthesia in infants. Anesthesiology. 1988;68:387–391.
243 Bosenberg AT, Thomas J, Cronje L, et al. Pharmacokinetics and efficacy of ropivacaine for continuous epidural infusion in neonates and infants. Paediatr Anaesth. 2005;15:739–749.
244 Gearhart JP, Baird AD. The failed complete repair of bladder exstrophy: insights and outcomes. J Urol. 2005;174:1669–1672.