Gastrointestinal Tract Endoscopic and Tissue Processing Techniques and Normal Histology
Douglas G. Adler
Francis A. Farraye
James M. Crawford
Introduction
Endoscopy provides a unique opportunity to visualize the mucosal surface of the gastrointestinal (GI) tract as well as a variety of extraluminal and extraintestinal organs and structures. When considered within the context of a specific clinical picture, endoscopic images may be all that is needed to establish a specific diagnosis or provide sound clinical management.1 However, endoscopists often need to sample tissue. Examination by a qualified pathologist of specimens obtained at endoscopy is a routine and critical part of managing disorders of the alimentary tract. The purpose of this chapter is to orient the pathologist to the clinical and technical considerations unique to specimens obtained endoscopically from the alimentary tract. This is followed by a discussion of the normal anatomy of the tubal gut.
Bowel Preparation
The effectiveness of endoscopy often depends on the quality of the bowel preparation.2 Preparation of the upper GI tract for endoscopy typically involves, at minimum, a 6-hour fast. Preparation for colonoscopy is achieved by use of oral purging agents, either with or without enemas. Most colonoscopy preparation regimens include the use of a clear liquid diet for 1 to 2 days, followed by cleansing with oral polyethylene glycol (PEG)-electrolyte solution or other oral laxatives (e.g., magnesium citrate, senna) and rectal enemas (Table 1.1). In general, vomiting is reported more frequently with oral PEG-based high-volume lavage regimens than with other agents.3,4 PEG lavage regimens reportedly provide more consistent cleansing.5,6
Table 1.1
Common Preparation Methods for Colonoscopy

PEG, Polyethylene glycol; PO, per os (by mouth).
Purgative- and laxative-based regimens are more likely to cause flattening of surface epithelial cells, goblet cell depletion, lamina propria edema, mucosal inflammation, and increased crypt cell proliferation, although these effects occur infrequently. Osmotic electrolyte solutions, such as PEG-based solutions, are better agents for preserving mucosal histology.7–10 In the most severe form of mucosal damage due to purgatives such as sodium phosphates, sloughing of the surface epithelium, neutrophilic infiltration of the lamina propria, and hemorrhage may be encountered, and the changes may even resemble pseudomembranous colitis, nonsteroidal antiinflammatory drug-induced injury, or inflammatory bowel disease.11,12 Oral sodium phosphate bowel preparations were removed from the market in 2008 after their use was associated with renal injury. Chemical-induced colitis, caused by inadequate cleaning of endoscopic instruments, also has been reported but is very rare. Sodium phosphate–based preparations may also cause endoscopically visible aphthoid-like erosions similar in appearance to Crohn’s disease.13 Mucosal changes in this situation may also resemble pseudomembranous colitis, both endoscopically and microscopically.14
Methods for Obtaining Tissue Specimens
There are a limited number of methods available for obtaining tissue via GI endoscopy. This section describes several of these methods and the common situations in which they are used.
Endoscopic Pinch Biopsy
Pinch biopsy, performed with the use of a biopsy forceps during endoscopy, is the most common form of tissue sampling; the biopsy site is usually fully visualized at the time of sampling. Suction capsule biopsy requires fluoroscopic guidance to position a long tube with the biopsy apparatus and is done separately from endoscopy without visualization. Suction capsule biopsy without bowel visualization is still performed in some centers, but it is less successful than endoscopy-guided biopsy in obtaining tissue and therefore has fallen out of favor.15 Pinch biopsies may be small or large (the latter are referred to as “jumbo” biopsies) and can be obtained with or without the use of electrocautery. Electrocautery has value for hemostasis and destruction of residual tissue but introduces burn artifact into the harvested tissue.
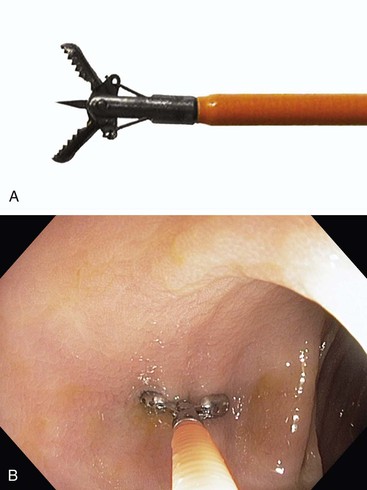
All standard biopsy forceps have a similar design (Fig. 1.1). The sampling portion consists of a pair of small cups that are in apposition when closed. In this manner, they can be passed through the channel of a gastroscope or colonoscope. Some biopsy forceps have a spike at the base of the cup or teeth to help seat the forceps against the mucosa. The spike also helps to impale multiple biopsy specimens before the forceps is removed from the endoscope.
After insertion into the endoscope and emergence from the distal end, routine biopsy forceps can be opened to a 4- to 8-mm width. The opened forceps is pressed against the mucosal surface for tissue sampling. Large-cup (jumbo) biopsy forceps have jaws that open to a width of 7 to 9 mm. The biopsy forceps is closed against the mucosal surface, and the endoscopist pulls the forceps away from the mucosa to remove the fragment of tissue. This method often yields samples that include muscularis mucosae, except in regions such as the gastric body, where the mucosal folds are quite thick.16 The submucosa is sampled occasionally with either standard or jumbo forceps.17
The sample size varies according to the amount of pressure the endoscopist applies to the forceps. In addition, application of a fully opened biopsy forceps flush against the mucosa before closure usually yields larger pieces of tissue, compared with tissue obtained by tangential sampling or incomplete opening of the forceps. In general, biopsy specimens are 4 to 8 mm in length.18,19 The forceps shape does not impart a significant difference in either size or adequacy of biopsy specimens.18 Single-use disposable biopsy forceps also have been shown to provide excellent samples.20 In essence, there are no differences in the quality of tissue samples obtained among the dozen or more biopsy forceps currently available, so the primary considerations in the selection of an endoscopic biopsy forceps are usually related to cost.21
After the biopsy specimens have been obtained and the forceps have been removed from the endoscope, an assistant dislodges the tissue fragments from the forceps with a toothpick or a similar small, sharp instrument. The tissue is then placed into a container containing appropriate fixative and labeled according to instructions provided by the endoscopist.
Specimens obtained with a jumbo forceps often exceed 6 mm in maximum diameter, but these are not necessarily deeper than standard biopsies. Rather, a jumbo forceps typically provides more mucosa for analysis. This is particularly useful during surveillance tissue sampling, such as in patients with Barrett’s esophagus or ulcerative colitis. Jumbo biopsy forceps are as safe as standard biopsy forceps.22 However, use of jumbo forceps is limited by their diameter because the instrument cannot fit through a standard endoscope accessory channel. Jumbo forceps require a 3.2-mm-diameter channel, characteristic of therapeutic endoscopes, which may be less comfortable for patients. In addition, although jumbo biopsy specimens are larger than standard biopsy specimens, this does not necessarily mean that they are of greater diagnostic value.23
Upper endoscopy or colonoscopy may be performed for clinical indications driven by symptomatology. Colonoscopy, in particular, may also be performed for screening purposes in clinically asymptomatic individuals. Selection of a biopsy site at the time of endoscopy is driven by the need to assess visible mucosal abnormalities. In addition, it is advantageous to establish the inflammatory status of the “background” mucosa by sampling normal-appearing mucosa during the evaluation of conditions such as gastroesophageal reflux disease (GERD), nonulcer dyspepsia, diarrhea, polyps, and nodules and for surveillance of premalignant conditions, including Barrett’s esophagus and inflammatory bowel disease. For example, in patients with inflammatory or dysplastic polyps of the stomach, it is essential to sample adjacent nonpolypoid mucosa to help determine the background disorder in the stomach in which the polyp has developed. As a second example, the ampulla of Vater may be biopsied to exclude adenomatous change in patients with familial adenomatous polyposis, because the lifetime incidence of ampullary adenomas in these patients exceeds 50%.24
Biopsy of biliary or pancreatic strictures may be carried out under fluoroscopic guidance during endoscopic retrograde cholangiopancreatography (ERCP) with the use of either standard or specially designed biopsy forceps.25 Even gallbladder lesions observed on ERCP may be amenable to endoscopic biopsy, although this is rarely performed clinically.25 Endoscopy-directed diagnostic biopsies are extremely safe. In one study of 50,833 consecutive patients who underwent upper endoscopy, none had any biopsy-associated complications.22 The risk of perforation after diagnostic or therapeutic colonoscopy (with polypectomy) is extremely low.26
Occasionally, an endoscopist uses a specialized insulated biopsy forceps to sample a small polyp (“hot biopsy”), after which remaining tissue is ablated in situ using electrocautery.27 Unfortunately, cautery artifact in such small tissue samples often makes histologic interpretation difficult (or impossible).16,28 In addition, the electrocautery technique carries an excessive risk of perforation resulting from deep tissue burn, particularly in the cecum and ascending colon.29,30 Finally, destruction of residual dysplastic tissue by electrocautery may be incomplete in as many as 17% of cases.31 For these reasons, hot biopsies have been largely abandoned by most endoscopists.
There is a limited literature available regarding the relatively new and controversial “resect and discard” concept for diminutive colorectal polyps found during screening colonoscopy. This concept suggests that one can safely perform endoscopic removal of diminutive colon polyps (usually by simple pinch biopsy removal using cold forceps or cold snare polypectomy) without the need for pathologic analysis of the polyp. The reasoning is that diminutive polyps have a very low likelihood of harboring either malignancy or advanced adenomatous features such as high-grade dysplasia or villiform change. Therefore, discarding these lesions without histologic evaluation should be cost-effective and clinically efficacious,32–34 with an endoscopic sensitivity for correctly classifying adenomas of 94% and specificity of 89%.32 Furthermore, these polyps may be assessed by in situ optical scanning techniques that can help predict polyp histology.35 For instance, narrow band imaging has a reported negative predictive value of 95%,36 which further increases physician confidence that the polyps can be discarded without pathologic examination.
Endoscopic Snare Polypectomy
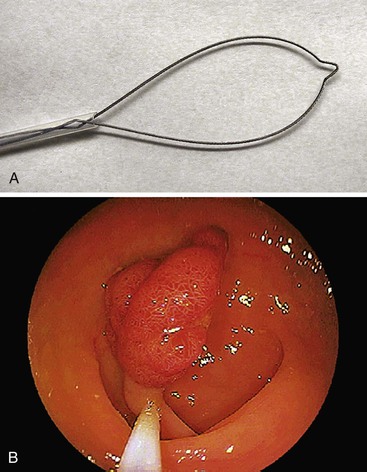
During endoscopy, a loop of wire may be placed around a polypoid lesion that protrudes into the lumen of the gut for the purpose of removing the polyp (Fig. 1.2). This technique is used primarily for colonic polyps, but polyps throughout the alimentary tract may be excised in this manner. Depending on their size, excised polyps are either retrieved through the suction channel of the endoscope or held by the snare after resection while the colonoscope is removed from the patient.
Many endoscopists have reported successful removal of diminutive polyps (<0.5 cm in diameter) during both “hot” (with electrocautery) and “cold” (without electrocautery) snare polypectomy.37,38 These endoscopists use small metal snares, termed mini-snares, that open to a size of either 1 to 2 cm or 2 to 3 cm. Polyps larger than 0.5 cm are amenable to snare polypectomy, although the size of the polyp that can be excised may be limited by the size of the loop placed around it (and the endoscopist’s estimation of perforation risk). Alternatively, large polyps can be removed in a piecemeal fashion and submitted to pathology in several parts. This technique usually requires multiple transections of the lesion until the entire polyp has been removed.27 One caveat with this technique is that identifiable tissue margins may be lost, so that the pathologist is often unable to determine the status of the resection margins.
A hot snare allows the endoscopist to apply modulated electrosurgical current to a metal wire that cuts through pedunculated polyps at the base. This assists tissue cutting and coagulation. Electrocautery also minimizes bleeding from larger blood vessels located in the stalk of the polyp. Cold polypectomy, without electrical current, avoids use of cautery, thereby limiting the amount of burn artifact in the specimen and minimizing the risk of perforation. In general, the risk of perforation from either mechanical or electrical injury is minimal but is greater in portions of the colon that are covered by a free serosal surface, such as the transverse colon. Information on the relative risk of clinically significant hemorrhage after hot polypectomy is limited, but the risk is generally considered to be low (0.4%).39,40 A large cross-sectional study from South Korea established that loop polypectomy is only rarely performed without electrical current (i.e., cold polypectomy), but this is usually inadvertent, resulting from failure of application of the current.41 Absence of electrical current is associated with an increased risk of clinically significant postpolypectomy hemorrhage. A higher risk of postpolypectomy hemorrhage also occurs in patients with pedunculated polyps larger than 1.7 cm or a stalk diameter larger than 0.5 cm, sessile polyps, or malignant lesions.42
For polyps excised in one piece by either hot or cold polypectomy, the polyp base constitutes the surgical margin of resection. This is true for both pedunculated and sessile polyps. For polyps removed by hot snare polypectomy, the cauterized portion of the specimen constitutes the surgical margin. An artificial stalk can be created when large sessile lesions are loop-excised. A true pedunculated polyp, with a stalk, has a narrow base that persists after removal; the base of a sessile polyp is typically as wide as the mucosal surface that is sampled.
Snares are available in a variety of shapes and sizes. Some snares can be rotated, which provides the endoscopist with greater control of snare placement. The choice of snare size is usually based on the size of the lesion being removed. The selection of a particular snare shape is a matter of personal choice.
Snare polypectomy is performed in a similar fashion regardless of whether colonic, esophageal, gastric, or small bowel lesions are being removed. The ampulla of Vater may be resected by standard snare techniques if an ampullary lesion is discovered.43 The risk of perforation during snare polypectomy is less than 0.1%,44,45 and perforation usually results from transmural burn secondary to cautery. One commonly used technique aimed at decreasing the risk of perforation is to pull the snared polyp away from the mucosa so that less cautery is applied to the underlying tissue.
Another commonly used method is saline-assisted polypectomy.46,47 A small needle is passed through the endoscope and inserted into the gut wall adjacent to the polyp. A bolus of normal saline is then injected. Fluid collects within the submucosal plane, lifting the mucosal-based polyp away from the muscularis propria. A standard snare polypectomy is then performed, but the cushion of saline insulates the deeper tissue layers from the electrical current. Saline-assisted polypectomy is usually reserved for large sessile polyps and ampullary polyps and, theoretically, results in a decreased rate of polypectomy-associated perforation.
Endoscopic Mucosal Resection
The use of a liquid cushion to expand the submucosa and minimize transmural cautery damage is a principal feature of endoscopic mucosal resection (EMR). This technique is commonly used to resect premalignant and malignant lesions confined to the mucosa.48 In general, EMR requires some measure of confidence that a lesion is, in fact, confined to the mucosa or submucosa. Many endoscopists now rely on endoscopic ultrasonography (EUS) to determine the depth of a particular lesion before EMR. The accuracy of high-frequency EUS (15 or 20 MHz) may be as great as 95% for determining whether a lesion is limited to the mucosa.48
Several variations of the EMR technique are currently used. Many rely on submucosal injection of liquid, but there is no agreement regarding the type or quantity of liquid that should be injected.49 Most endoscopists advocate the use of saline alone. Others add diluted epinephrine to saline in an attempt to constrict small blood vessels at the base of the lesion. Submucosal fluid collections are absorbed in a relatively short time period, which can limit their value. To lengthen the amount of time that the submucosal cushion may last, and thus maximize the time available for performing a safe resection, investigators have used hypertonic solutions of 3.5% saline or 50% dextrose. Others advocate use of sodium hyaluronate instead of saline. None of these agents is used more often than simple saline. The quantity of liquid injected also varies. There is general agreement that the selected lesion should appear, endoscopically, to be raised by the cushion of liquid before the EMR is performed. Failure to lift the lesion despite generous use of submucosal saline (the so-called nonlifting sign) may be a sensitive indicator that a lesion has spread deeper into the bowel wall.50
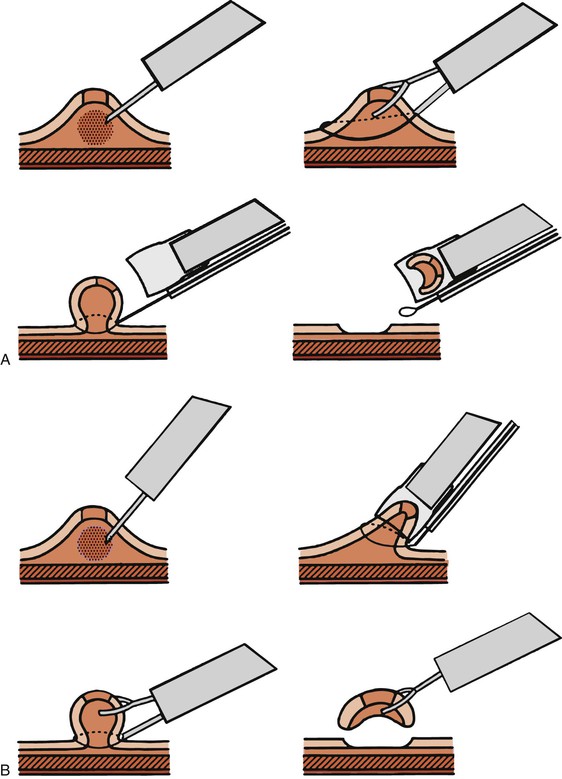
Two major types of resection techniques are used—those that do not use suction and those that do. When suction is not used, the endoscopist uses a dual-channel endoscope. A snare, passed through one instrument channel, is opened and placed around the lesion. A biopsy forceps is passed through the second channel and used to grab the lesion and pull the mucosa through the snare farther away from the muscularis propria. The snare is then closed around the base of the tented lesion, and electrocautery is applied (Fig. 1.3). This method is referred to as the lift-and-cut technique or a strip biopsy.48,51
Suction methods of EMR incorporate the use of a cap fitted onto the tip of an endoscope. The cap presents an open surface to the mucosa and creates a short chamber into which the selected lesion may be aspirated and held by suction, which is applied through a single-channel endoscope. A specialized snare is opened in the cap before aspiration of the lesion. Once the mucosa has been drawn into the cap, the snare may be closed around the lesion and cautery applied in the usual fashion.48 This technique, also called aspiration mucosectomy, has been widely successful for removing lesions throughout the GI tract.52
A newer EMR technique is similar to aspiration mucosectomy, but after the lesion has been suctioned into the cap, a tiny rubber ring is released around the base of the lesion, similar to the method used during endoscopic variceal ligation. Once suction is released, the lesion appears contained within a “pseudopolyp” that may be removed by snare cautery. This is known as band-ligation EMR (Fig. 1.4).
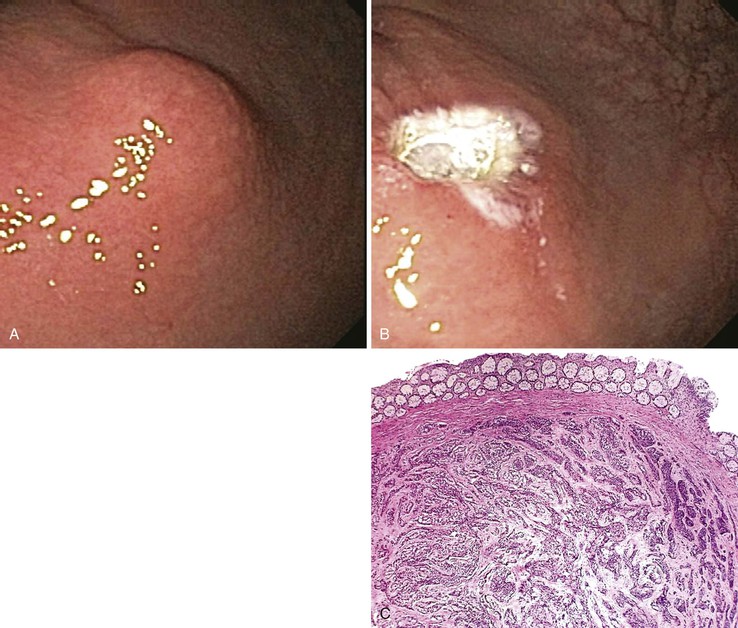
EMR allows the endoscopist to attempt an en bloc resection and thus, potentially, to completely resect an early malignant lesion. En bloc resection is limited, however, to small lesions (1.5 to 2 cm in largest diameter).51 If deep margins are positive for neoplasia, surgical resection of the affected region is advocated.53 Current indications for EMR include superficial carcinoma of the esophagus or stomach in patients who are not candidates for surgery; unifocal high-grade (or low-grade) dysplasia in Barrett’s esophagus; and large, flat colorectal adenomas (which might otherwise require piecemeal resection) regardless of the degree of dysplasia. EMR as a form of primary therapy for small, superficial cancer has gained increasing popularity in the United States but is even more widely used in Japan, where early gastric cancer is more common.48,51,53 EMR is also used as a form of primary therapy for small submucosal lesions such as rectal carcinoid tumors or leiomyomas. In many cases, the submucosal lesion can be completely resected54 (Fig. 1.5).
Major complications of EMR include bleeding and perforation. Bleeding occurs in fewer than 1% to as many as 20% of cases, depending on the size of the lesion and its location.48,51,53 Clinically significant bleeding is rare and usually is amenable to endoscopic hemostatic cauterization. Perforation rates are lower than 2% in the hands of experienced operators.55 A recent recommendation is that the endoscopist should immediately inspect the underside of the resected specimen for the presence of a “target sign”: a pale ring of muscularis mucosa resected with the mucosal lesion. Applying endoscopic clips to the corresponding defect in the resection site is purported to represent an effective method to mitigate against the risk of postprocedure bleeding.56 In the hands of experienced operators, EMR results in large specimens for pathologic analysis, even in the absence of complete resection. Success rates for en bloc resection of early gastric cancers by EMR range from 36% to 74%,51,53 greatly reducing the need for full-thickness surgical resection.
Methods of Processing Tissue for Pathologic Evaluation
A general framework for processing biopsy specimens is provided in Table 1.2.
Table 1.2
Techniques of Processing Tissue Specimens Obtained by Endoscopy
| Technique | Comment |
| Formalin fixation | Routine processing of all alimentary tract biopsies; immediate immersion in fixative. Permits immunohistochemistry, molecular analysis. |
| Flow cytometry | Suspected hematologic malignancy; fresh tissue in sterile culture medium |
| Electron microscopy | Suspected poorly differentiated malignancy, infection (e.g., Whipple disease, microsporidiosis); immediate immersion in electron microscopy fixative |
| Electron microscopy fixative only | Suspected systemic mastocytosis, for which plastic-embedded thick sections with toluidine blue staining may be used for identification of mast cells |
| Microbial culture | Suspected viral, fungal, or parasitic infection; sterile tissue |
| Biochemical analysis | Suspected metabolic deficiency (e.g., disaccharidase deficiency); frozen tissue |
| Cytogenetics* | Suspected neoplasm (benign or malignant); fresh tissue in sterile culture medium |
| Cell culture* | Suspected neoplasm (benign or malignant); fresh tissue in sterile culture medium |
* Usually for investigational purposes only.
Formalin
Of the many types of fixatives used for human tissue, 10% buffered formalin remains the standard and is well suited for mucosal biopsies of the GI tract. It is inexpensive, is harmless to the tissue even after long periods, and is compatible with most of the stains commonly used for morphologic assessment. Hollende solution, B5, and Bouin fixative have been used for mucosal biopsies because of better preservation of nuclear morphology compared with formalin. However, the heavy metal content of these fixatives creates biohazard disposal problems that are greater than those of formaldehyde-based fixatives. These fixatives also interfere with isolation of nucleic acid from tissue; the search for substitute fixatives and new tissue processing techniques is an active area of scientific investigation.
On occasion, the formaldehyde in formalin may be irritating to the eyes and upper respiratory tract of personnel. The level at which formalin is considered carcinogenic is well above the level that causes sensory irritation, which has a threshold of 1.0 ppm.57 Accordingly, in pathology suites, proper ventilation should be used to maintain exposure below 1.0 ppm, the lowest concentration that may exert a cytotoxic effect in humans.58,59 A workplace surveillance program for formalin exposure is recommended.60 Typical occupational exposure in endoscopy suites is exceedingly brief, so special ventilation is not usually required in that hospital area.
Alimentary tract biopsy specimens should be placed in a volume of formalin fixative that is at least 10 times greater than that of the tissue, and the fixative should surround the specimen completely. These parameters are usually easily met with small endoscopic tissue samples and even with those obtained by EMR, using small tubes of fixative solution. For routine processing, it is a common mistake to place specimens on saline-soaked gauze for delivery to the pathology suite: Severe drying may occur. Complete immersion of these biopsies in formalin should always occur at the bedside. Formaldehyde diffuses into tissue at a rate of approximately 1.0 mm per hour at room temperature.61 Therefore, up to 1 hour is often needed to adequately fix a specimen with a diameter greater than 1.0 mm, and more time is needed for larger specimens. Controlled microwave fixation at 63° to 65° C can greatly speed the process and is useful for rapid processing of specimens.62
Orientation of Formalin-Fixed Tissue Obtained at Endoscopy
Esophageal, gastric, and colonic mucosal biopsies do not require precise orientation before tissue processing and embedding. Until the mid-1980s, most peroral small intestinal biopsies were obtained by either a Crosby suction capsule or a Quinton hydraulic assembly.63,64 These two methods were performed fluoroscopically and therefore did not permit direct visualization of the alimentary tract. Biopsies obtained by these methods were carefully oriented under a dissecting microscope before fixation and embedding. By the late 1980s, the fluoroscopy had been replaced by a suction capsule biopsy procedure in direct endoscopic biopsy of the small intestine.65,66 Biopsies obtained by this technique are not usually oriented before immersion in fixative, processing, and embedding. Rather, microscopic examination of multiple tissue sections usually permits identification of portions of the small intestinal mucosa that are well oriented and therefore can be assessed satisfactorily for tissue architecture.
In contrast, processing of an endoscopic polypectomy specimen in the pathology suite requires diligent effort.67 The size and surface configuration (bosselated or villiform) of the polyp should be noted, and the base of the polyp should be identified and described as to whether it is sessile or contains a cylindrical stalk. Regardless of the configuration of the stalk, the base of the polyp should always be inked. Ink and cautery artifact on a microscopic slide are valuable landmarks for locating the relevant resection margins. Small polyps (<1 cm in diameter) should be bisected along the vertical plane of the stalk so that the surgical margin is included. Both halves of the specimen can then be submitted in one cassette.
Section levels should be numbered consecutively; the first level is the one usually located closest to the middle of the polyp stalk. Large polyps (≥1 cm in diameter) may be sectioned differently if the polyp head is too wide to fit into a single cassette. First, the polyp should be bisected along its long axis and fixed overnight in formalin. Once fixed, the sides of the polyp may be trimmed away from the stalk on a vertical axis and submitted in separate cassettes that are labeled accordingly. The middle of the polyp, including the base, should be sectioned vertically and submitted in an appropriate number of cassettes. If a stalk is identified histologically, the status of the margins should always be noted in the surgical pathology report.
If the polyp has been excised in a piecemeal fashion, the size, color, surface configuration (bosselated or villiform), and aggregate dimensions of the tissue fragments should be noted. It is important to record the number of tissue fragments received in the pathology suite.
Flow Cytometry
GI lesions suspected of representing a lymphoproliferative process are usually submitted for histology but should also be processed for flow cytometry.68 Biopsy specimens intended for flow cytometric analysis, such as gastric biopsies of a mass lesion or EUS-guided fine-needle aspiration (FNA) samples from concerning lymph nodes, should be placed in sterile culture medium and delivered as rapidly as possible to the flow cytometry laboratory. Ideally, this should occur within several hours, but storage of specimens at 4° C overnight is an acceptable alternative.
On receipt in the laboratory, the tissue specimen is disaggregated, and a cell suspension is prepared. Cocktails of fluorescently labeled antibodies appropriate to the diagnostic question are applied to the cell suspension. Current flow cytometry machines can analyze 5000 to 10,000 cells per second, measuring multiple wavelengths of laser-induced fluorescence simultaneously and thus permitting rapid and highly efficient analysis of cell populations. This technique cannot be performed on fixed tissue. It is therefore incumbent on the endoscopist to consider the possibility of a lymphoproliferative disorder at the time of endoscopy in order to ensure that tissue is preserved in a fresh state. Communication between the endoscopist and the pathologist before or immediately after the procedure increases the likelihood that the flow cytometry sample will be received and processed in a timely fashion.
Electron Microscopy
For the rare instances in which electron microscopy of an alimentary tract biopsy is contemplated, tissue samples should be placed directly into the appropriate fixative, which usually consists of a mixture of paraformaldehyde and glutaraldehyde. Unlike formaldehyde-based fixatives, bifunctional glutaraldehyde fixatives penetrate only approximately 0.5 mm into the tissue. Therefore, tissue fragments to be placed in fixative for subsequent electron microscopy should, ideally, measure less than 1.0 mm in maximal dimension. Indications for electron microscopy of endoscopic biopsy specimens are now largely limited to examination of unusual tumors.69 However, this technique is also helpful in cases of unknown diarrhea in children and in patients with the acquired immunodeficiency syndrome (AIDS) for detection of parasitic organisms.
Endoscopy-Induced Artifacts
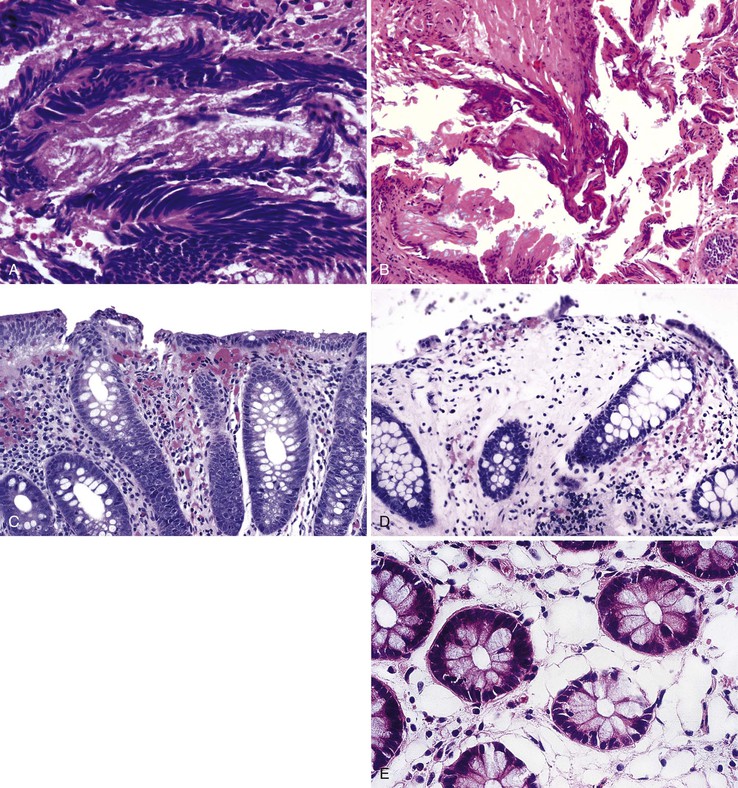
Many types of tissue artifacts may be introduced into tissues as a result of bowel preparation, endoscopic trauma, or tissue handling. Some of these are listed in Table 1.3. Histologic features of artifacts are provided in Table 1.4. The most common type of artifact (or effect) is lamina propria edema and intramucosal hemorrhage, known as scope trauma (Fig. 1.6). Other effects include aggregation and clumping of inflammatory cells in the lamina propria, surface flattening, mucin depletion, and even erosion and influx of air into the tissue (pseudolipomatosis).70–72 The most common histologic artifacts include cautery and crush artifacts occurring as a complication of tissue resection techniques. These may be difficult to avoid in clinical practice given the nature of current endoscopic resection technologies (Fig. 1.7). Cautery artifact as a result of hot biopsy is a normal and expected component of endoscopic polypectomy with electrocautery. Specifically, the region of cauterization may provide a useful landmark of the surgical margin.
Table 1.3
Endoscopic Events That May Affect Tissue Analysis
| Event | Comment |
| Trauma (tissue hemorrhage) | “Scope trauma” (caused by mechanical damage from the endoscope) or excessive mechanical manipulation for access before biopsy |
| Cautery artifact | Excessive use of electrical current during “hot” biopsy |
| Crush artifact | Excessive use of mechanical force during pinch biopsy |
| Inadequate sampling depth | Absence of submucosa (e.g., evaluate submucosal lesion, rule out amyloid) |
| Inadequate sampling location | Absence of muscularis mucosa (for evaluation of Hirschsprung disease) |
| Insufficient regional sampling (e.g., of “normal-appearing” mucosa) | |
| Chemical colitis | Inadequate rinsing of cleaning solution from the endoscope |
| Laxative-induced changes | Edema, damage to surface epithelium from exposure to oral and rectal laxatives |
| Air-drying | Failure to immerse specimen promptly in fixative |
| Postbiopsy healing | Sampling of a previous biopsy site during subsequent endoscopy |
| Wrong fixative | Formalin rather than fixative for electron microscopy (suboptimal but not irretrievable) |
| No fresh tissue | Failure to preserve fresh tissue; precludes flow cytometry, cytogenetics |
Table 1.4
Histologic Artifacts Related to Endoscopy
| Event | Feature |
| “Scope trauma” | Mucosal lamina propria hemorrhage or edema |
| Changes related to bowel preparation | Clumping of inflammatory cells, mucin depletion, epithelial degenerative changes, focal neutrophilic infiltration, hemorrhage, edema, air in mucosa (pseudolipomatosis) |
| Insufflation of air at endoscopy | Air spaces within mucosa or submucosa (pseudolipomatosis) |
| Cautery artifact | Coagulated, eosinophilic tissue without cellular or nuclear detail |
| Crush artifact | Compressed tissue with markedly elongated, wavy nuclear remnants and no identifiable architecture |
| Chemical colitis from inadequate cleaning of the endoscope | |
| Degenerative damage to or sloughing of surface epithelium, intraepithelial neutrophils and congestion, focal intramucosal hemorrhage | |
| Laxative-induced changes | Lamina propria edema and neutrophilic infiltration, flattening or sloughing of mucosal surface epithelium, decreased goblet cell numbers |
| Air-drying | Eosinophilic and compressed tissue and loss of nuclear detail at edge of tissue fragment |
| Postbiopsy healing | See Table 1.5 |
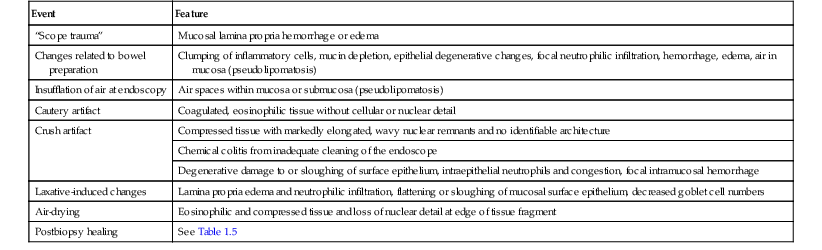
Pathologic Features of a Healing Biopsy Site
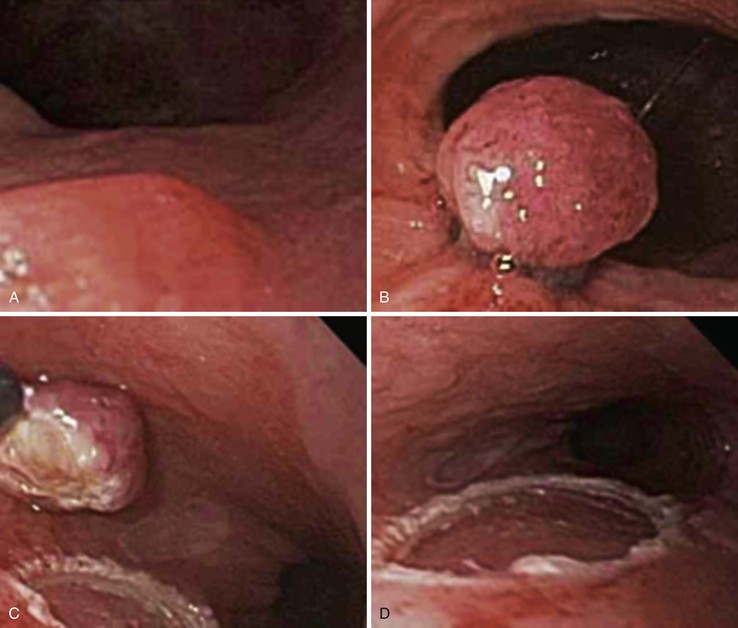
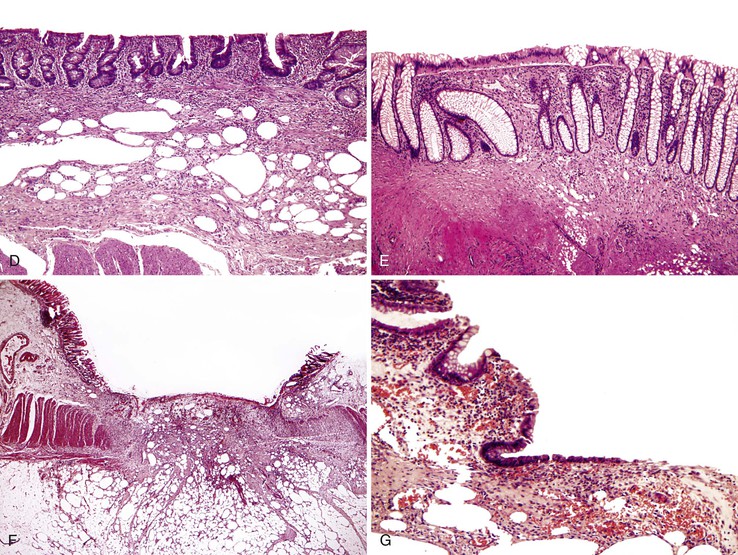
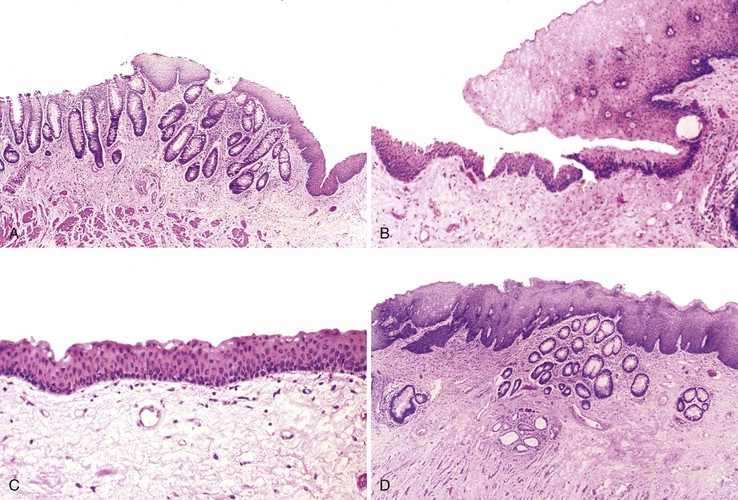
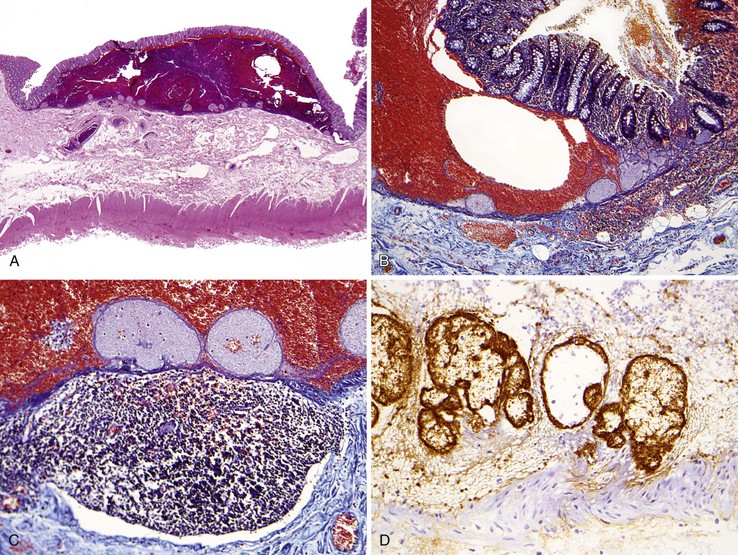
After endoscopic biopsy, the tissue healing process begins quickly (Table 1.5). After endoscopic polypectomy involving removal of both mucosa and a portion of submucosa, granulation tissue forms during the first days after biopsy73 (Fig. 1.8, A and B). Routine superficial biopsies that involve only mucosa reepithelialize within 48 hours (see Fig. 1.8, C) and heal completely within a few weeks with only mild residual architectural distortion (see Fig. 1.8, D). Ulcers that penetrate into the muscularis propria, such as those that form after aggressive endoscopic mucosal resection, often take 3 to 6 days to reepithelialize (see Fig. 1.8, F and G ) and as long as a month to heal completely.
Table 1.5
Pathologic Features of a Healing Mucosal Biopsy Site
| Time | Feature |
| Immediate | Blood clot with coagulum |
| Hours | Acute inflammation; granulation tissue reaction |
| 2 days* | Reepithelialization of inflamed biopsy site by ingrowth of epithelial cells from adjacent preserved epithelium; early formation of submucosal scar |
| 1-4 wk | Restoration of mucosa with rudimentary glandular architecture, maturation of submucosal scar |
| Months | Residual minimal mucosal architectural distortion, submucosal scar |
* Longer with deep biopsies that involve the muscularis propria.
After routine endoscopic mucosal biopsy during upper or lower endoscopy, there is no increased risk of perforation because of subsequent insufflation (as from repeat endoscopy or from barium enema), even immediately after the biopsy. The risk of perforation after a deep biopsy or endoscopic musocal resection that involves the muscularis propria returns to baseline within 3 to 6 days.73
Pathologists should be aware of the changes associated with colonic biopsy site repair and not misinterpret focal architectural distortion of the mucosa or focal submucosal scarring as evidence of inflammatory bowel disease. In addition, the finding of muscularis propria in a biopsy specimen in which this depth of sampling is not expected (e.g., in an esophageal biopsy specimen) should be reported to alert the treating physician of the potential risk of perforation.
Methods for Obtaining Cytology Specimens
See Chapters 3, 35, and 45.
Brush Cytology
Brush cytology is a method used for broad sampling of the mucosal surface.74,75 Cytology brushes all have a common design: Bristles, usually composed of nylon or metal fibers, branch off a thin metal shaft that runs lengthwise within a protective plastic sheath. The various brushes that are currently available do not seem to vary in terms of performance characteristics.76 The cytology brush is passed through an accessory channel of an endoscope. The end of the sheath is passed out of the tip of the endoscope, and the bristle portion of the brush is then extended from the sheath. The brush is rubbed back and forth several times along the surface of the lesion or stricture and is then pulled back into the sheath. The sheath is withdrawn from the endoscope, and the brush is pushed out of the sheath, thus exposing the bristles. The bristle portion of the brush may be cut off, placed into fixative, and sent in its entirety to the cytopathology laboratory. Alternatively, the bristles may be rolled against a glass slide in the endoscopy suite. The slides should be immediately sprayed with fixative or submerged within it and subsequently delivered to the cytopathologist. If smears are made in the endoscopy suite, little additional benefit is derived from inclusion of the bristles for cytopathologic analysis.77 Brush cytology is most often used in the pancreaticobiliary tree to sample strictures in the pancreaticobiliary tract. Another common use is sampling of esophageal plaques or lesions suspected to represent candidiasis.
Fluorescence in situ hybridization (FISH) is an increasingly used technique that can be applied to biliary brush cytology tissue specimens in patients with suspected pancreaticobiliary cancer and in those with primary sclerosing cholangitis (who are by definition at increased risk for cholangiocarcinoma). FISH relies on the fact that a very high percentage of pancreaticobiliary malignancies show chromosomal aneuloploidy, typically with chromosomal gains or additions. The presence of these polysomies is strongly associated with malignancy. Commonly used and commercially available probes are used to target the pericentromeric regions of chromosomes 3 (CEP 3), 7 (CEP 7), and 17 (CEP 17) and the chromosomal band 9p21 (LSI 9p21). FISH can be performed on biliary brush cytology specimens. A dedicated biliary brushing is often obtained for FISH testing. When FISH results are combined with those of routine biliary cytology, the diagnostic yield is much higher.78–81
Fine-Needle Aspiration
FNA is another widely used method for obtaining tissue for cytology.82–84 FNA needles may be used during standard endoscopy or EUS. EUS provides endoscopists with the ability to sample tissue from parenchymal lesions and lymph nodes and fluid from cystic lesions. EUS provides real-time imaging to ensure that the intended target is localized and sampled. The needles used for FNA during endoscopy are hollow 19- to 25-gauge needles and are often fitted with a central stylet to avoid gathering of intervening tissue. Some needles can also obtain a “core” of tissue (which may be analyzed histologically) in addition to samples for cytologic evaluation.
Once the lesion of interest has been identified, the sheath is pushed out of the endoscope, and the needle is advanced into the target tissue under ultrasonographic guidance (during EUS). If a stylet is present, it is removed, and suction is applied to a syringe at the proximal end of the needle. The endoscopist moves the needle forward and backward within the lesion, filling the distal needle lumen with tissue. Some endoscopists use suction during EUS FNA of solid lesions, whereas others do not. Suction is used to aspirate cysts85 for obvious reasons; there is some disagreement regarding the use of stylets in routine practice.86 The needle is then withdrawn into the sheath, and the entire apparatus is removed from the endoscope. Complications from FNA biopsy occur in fewer than 2% of cases; they include bleeding and, in the setting of pancreatic mass FNA, acute pancreatitis.
Optical Techniques
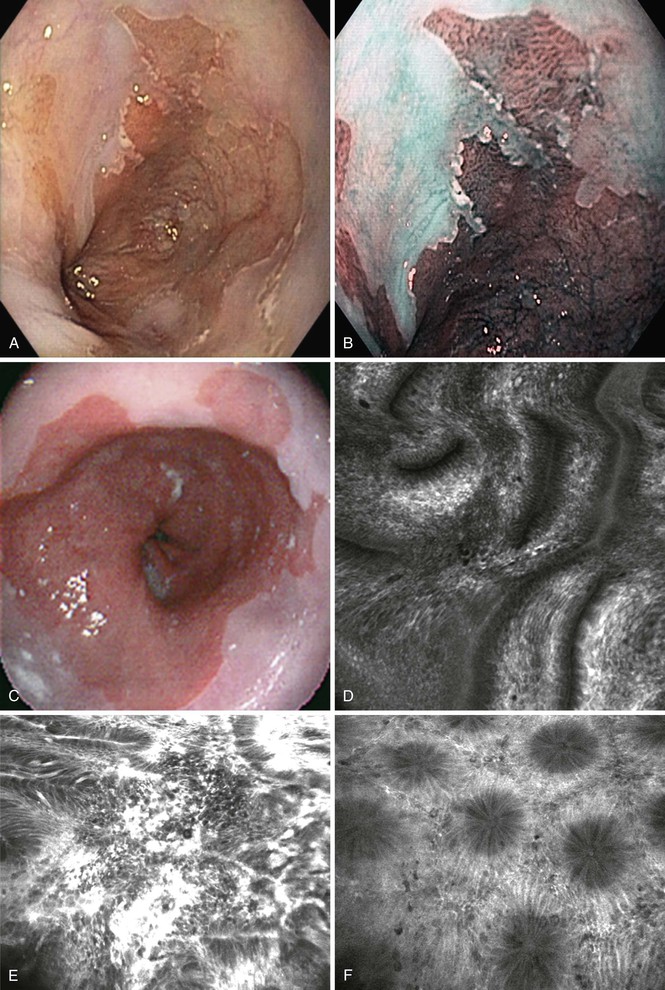
In recent years, there has been an increase in the use of optical techniques to assess in real time the pathologic status of patients with various disease states.87 Narrow band imaging (NBI) is a technique in which a high-definition videoendoscope is used to allow evaluation of the GI mucosal surface without the use of dyes. NBI is commercially available, and a high percentage of endoscopists have access to this technology. NBI uses different types of optical filters to apply specific wavelengths of light that can achieve deep penetration of the tissue. Specifically, use of red light results in visualization of deeper tissue layers, because red light penetrates more deeply into tissue than blue light does. NBI allows enhanced mucosal and vascular resolution, compared with white light endoscopy. NBI also allows the endoscopist to evaluate large areas of the GI mucosa without the use of vital dyes.
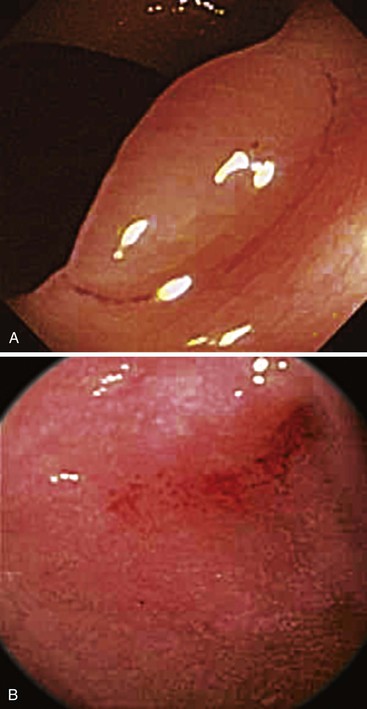
NBI has several clinical uses. In Barrett’s esophagus, evaluation with NBI in which the vascular pattern and the mucosal regularity are assessed can be used to identify patients with dysplastic mucosa (Fig. 1.9, A and B).88,89 NBI has also been used to evaluate gastric and colonic lesions, in attempts to increase the polyp detection rate during screening colonoscopy and to assess for dysplasia or malignancy in endoscopically visible lesions. NBI does not appear to increase the adenoma or polyp detection rate during screening colonoscopy,90,91 but it does allow real-time detection of adenomas during colonoscopy.92 This technology raises the possibility of performing an “optical biopsy” to identify adenomas during endoscopy. As stated earlier, whether diminutive, resected lesions optically identified as adenomas should still be sent for formal pathologic evaluation or simply discarded remains a highly controversial issue.
One other technique related to NBI is confocal laser endomicroscopy (CLE). This technique uses laser technology to illuminate tissue and detect reflected fluorescent light. This technique allows cellular resolution in vivo and in real time. The technique can be performed with special endoscopes (scope-based CLE) or with standard endoscopes using specialized mini-probes that can be passed through the working channel of the endoscope (probe-based CLE). Probes are available for use in the esophagus, stomach, colon, and biliary tree. This technique has not entered mainstream clinical practice but is currently an area of intense research because there is a wide range of potential clinical applications, including the evaluation of Barrett’s esophagus, gastric and colonic polypoid lesions, inflammatory bowel disease, and pancreaticobiliary strictures (see Fig. 1.9, C to F).93 Barrett’s esophagus may be the most promising area of interest given the desire to target dysplastic or malignant tissue endoscopically for biopsy and potential ablation to facilitate targeted biopsies. In this context the technique appears to be accurate and reproducible.94–96 However, it is unclear whether CLE will be used routinely in clinical practice in the future.
Normal Histology of the Tubal Gut
Esophagus
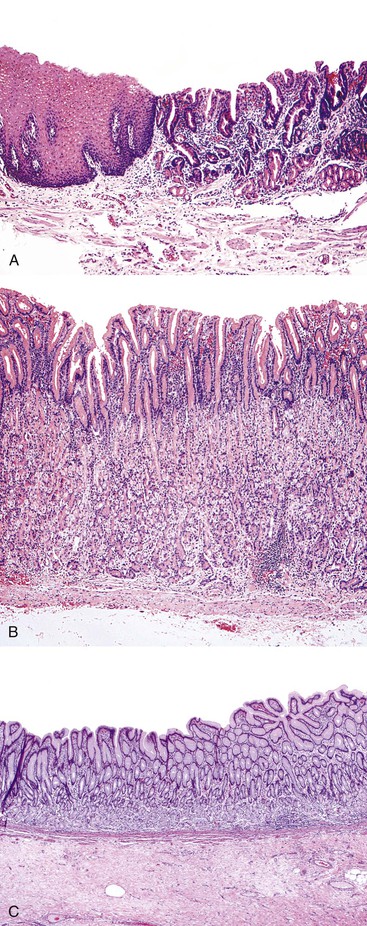
The adult human esophagus measures approximately 25 cm in length. For the endoscopist, the length of the esophagus is measured as the anatomic distance from the incisors. The esophagus usually begins at 15 cm, and the gastroesophageal junction (GEJ) is typically located at 40 cm. The 3-cm segment of proximal esophagus at the level of the cricopharyngeus muscle (15 to 18 cm from the incisors) is referred to as the upper esophageal sphincter. The 2- to 4-cm segment just proximal to the anatomic GEJ (at 36 to 40 cm from the incisors), at the level of the diaphragm, is referred to as the lower esophageal sphincter. Both “sphincters” are physiologic, because there are no universal anatomic landmarks that outline these high-pressure regions in relation to the underlying esophageal musculature, although they can often be recognized during endoscopy by experienced clinicians.
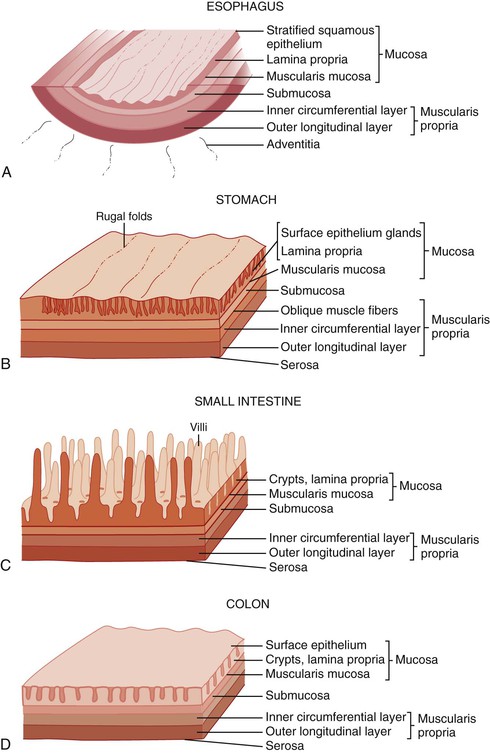
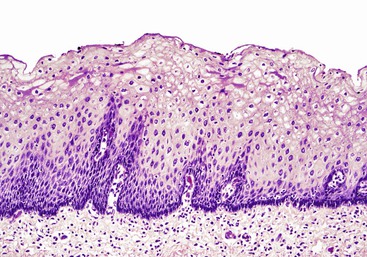
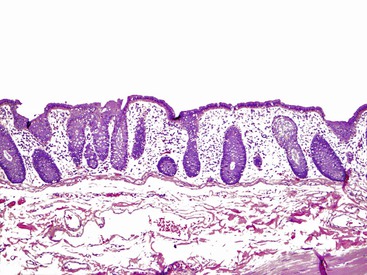
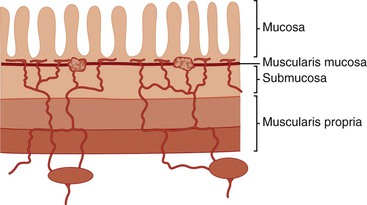
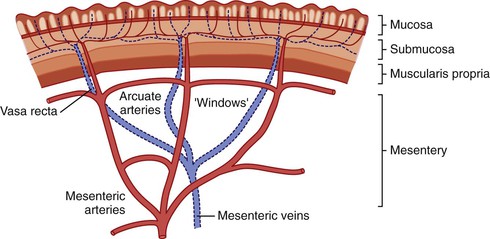
In keeping with the structural organization of the entire alimentary tract (Fig. 1.10), the wall of the esophagus consists of mucosa, submucosa, muscularis propria, and adventitia. The mucosa has a smooth, glistening, pink-tan surface. It has three components: a nonkeratinizing stratified squamous epithelial layer with an underlying lamina propria and muscularis mucosae (Fig. 1.11). The basal cell zone of the squamous epithelium occupies 10% to 15% of the total thickness of the epithelial layer. A small number of specialized cell types, such as endocrine cells, Langerhans cells, and lymphocytes, are typically present in the deeper portion of the squamous epithelium. The intraepithelial lymphocytes are mainly T cells.97 Melanocytes may be present in the esophagus in 3% to 8% of normal individuals.98,99 The lamina propria is the nonepithelial (mesenchymal) portion of the mucosa and is located above the muscularis mucosae. It consists of areolar connective tissue and contains vascular and neural structures and scattered inflammatory cells. Finger-like extensions of the lamina propria, termed papillae, extend into the epithelial layer, usually to between one third to one half of its thickness. In esophagitis (e.g., reflux esophagitis), the papillae extend into the upper third of the epithelial layer. The muscularis mucosae is a thick layer of longitudinally oriented smooth muscle bundles.
The submucosa consists of loose connective tissue containing blood vessels, a rich network of lymphatics, inflammatory cells, lymphoid follicles, nerve fibers (including the ganglia of Meissner plexus), and submucosal glands. Submucosal glands, which connect to the lumen of the esophagus by ducts lined with squamous epithelium, are scattered along the entire esophagus but are more concentrated in the upper and lower portions. Submucosal glands are suspended within the delicate mesenchyme of the submucosa. They have a simple acinar structure and resemble salivary glands in that they contain mucous cells surrounding a central lumen in a radial fashion. Their mucin-containing fluid secretions help lubricate the esophagus. Submucosal glands also secrete biologically active peptides such as those from the trefoil factor family 3 (TTF3)100; these peptides play a role in mucosal protection and repair. Identification of a squamous duct and submucosal mucous glands is considered a definitive anatomic landmark of the tubular esophagus. In the deep portion of the submucosa, the gland ducts contain two discrete layers of cuboidal cells, which become progressively more squamoid at higher levels of the submucosa and mucosa. A mild, concentric, chronic inflammatory infiltrate surrounding the gland ducts is commonly present.
Endoscopic biopsies of the esophagus usually yield squamous epithelium, lamina propria, and, occasionally, the prominent underlying muscularis mucosae. Sampling of the submucosa is variable. The anatomic landmarks change in patients with Barrett’s esophagus: The lamina propria no longer lies underneath the epithelial layer only but is also located between the glands. A newly developed and more delicate muscularis mucosae lies directly underneath the glands. This layer of muscularis mucosae represents the superficial layer of a “double muscularis” in patients with Barrett’s esophagus (see Chapter 14 for more details).101
Stomach
The stomach is a large, saccular organ with a volume of 1.2 to 1.5 L but a potential capacity of more than 3 L. It extends from just left of the midline superiorly, where it is joined to the esophagus, to just right of the midline inferiorly, where it connects to the duodenum. The stomach begins at the GEJ, which is generally considered to represent the most proximal point of the gastric folds. It ends at the pylorus, where the muscularis propria thickens to create the pyloric sphincter. The concavity of the right, inner curve of the stomach is termed the lesser curvature, and the convexity of the left, outer curve is called the greater curvature. The angle along the lesser curve, termed the incisura angularis, marks the approximate point at which the stomach narrows before its junction with the duodenum.
The stomach is divided into five anatomic regions. The cardia is a narrow (0.1 to 0.4 cm in length), conical portion of the stomach that is located immediately distal to the GEJ. It has no anatomic landmarks and therefore is defined by the presence of mucous glands or mixed mucous and oxyntic glands in the most proximal gastric mucosa. The fundus is the dome-shaped portion of the proximal stomach that extends superolateral to the GEJ and is composed exclusively of oxyntic glands. The body, or corpus, comprises the remainder of the stomach proximal to the incisura angularis. The stomach distal to the incisura, called the antrum, contains simple mucous glands and is demarcated from the duodenum by the pylorus and its associated sphincter.
The gastric wall consists of mucosa, submucosa, muscularis propria, and serosa. The interior surface of the stomach exhibits coarse rugae (“folds”). The rugal folds of mucosa and submucosa extend longitudinally and are most prominent in the proximal stomach. The rugae flatten when the stomach is distended. A finer, mosaic-like pattern is delineated by small furrows within the mucosa. Finally, the delicate texture of the mucosa is punctuated by millions of gastric foveolae, or “pits,” which lead to the mucosal glands.
The normal gastric mucosa has two main epithelial compartments: the superficial foveolar (“leaf-like”) compartment and the deeper glandular compartment. The foveolar compartment is relatively uniform throughout the stomach. In contrast, the glandular compartment exhibits major differences in thickness and composition in different regions of the stomach (Fig. 1.12). The foveolar compartment consists of mucous cells, which line the entire mucosal surface, and gastric pits ( foveolae). The tall, columnar, mucin-secreting foveolar cells contain basal nuclei and crowded, small, relatively clear, mucin-containing granules in the supranuclear region of the cytoplasm. Deep in the gastric pits are the so-called mucous neck cells, which have a lower content of mucin granules and are thought to represent the cell progenitors of both the surface epithelium and the gastric glands. Mitoses may be identified in this region, because the entire gastric mucosal surface is usually replaced completely every 2 to 6 days.
The glandular compartment consists of gastric glands, which vary among the different anatomic regions of the stomach.
• As noted earlier, in the cardia the mucosal glands contain either pure mucous cells or a mixture of mucous and oxyntic cells for a length of 0.1 to 0.4 cm in most individuals (see Chapter 15). In a small proportion of individuals, a portion of the circumference of the cardia may contain only pure oxyntic glands.
Gastric gland cell types include the following.
• Endocrine (or enteroendocrine) cells are scattered among the epithelial cells of the oxyntic and mucous glands (see Chapter 29 for details). The cytoplasm of these triangle-shaped cells contains small, brightly eosinophilic granules that are concentrated on the basal aspect of the cell. These cells can act in an “endocrine” fashion, by releasing their products into the circulation, or in a “paracrine” fashion, via secretion directed into the local tissue. In antral mucosa, most endocrine cells consist of gastrin-producing G cells. In the body, the endocrine cells produce histamine, which binds the H2 receptor on parietal cells and leads to increased acid production. These cells are also referred as enterochromaffin-like cells. Other enterochromaffin-like cells in the oxyntic glands include D cells (which produce somatostatin) and X cells (which produce endothelin). These cells play an important role in modulating acid production.
The Gastric Cardia
The stomach begins at the most proximal aspect of the gastric folds. The gastric cardia is viewed as an anatomic region of the stomach, approximately 0.1 to 0.4 cm in length, which is located at the proximal cone of the gastric cavity, just distal to the squamocolumnar mucosal boundary (Z-line) in normal individuals. Traditionally, the gastric cardia is viewed as having “cardiac” mucosa, which is a mucinous, glandular mucosa typically lacking the oxyntic glands that contain chief and parietal cells (see Fig. 1.12, A). However, some individuals show a mixture of both types of glands (mucous and oxyntic) (see later discussion and Chapters 14 and 15).
The strict (physiologic) definition of the GEJ is actually manometric, in that the high-pressure zone of the lower esophageal sphincter defines the true distal end of the esophagus. Because manometry is not a normal part of routine endoscopy and the GEJ passes through the diaphragmatic orifice, the performance of endoscopy on a live, breathing patient makes it difficult to identify precisely the true anatomic location of the GEJ region. The point of flaring of the gastric cavity identified by retroflexion of the endoscope is considered to be a reliable indicator of the beginning of the stomach. However, an axial hiatal hernia or proximal migration of the squamocolumnar mucosal junction in the setting of gastroesophageal reflux (whether physiologic or pathologic) can makes it very difficult to identify the anatomic site of the most proximal stomach at the time of endoscopy.
The origin and nature of epithelium in the cardia region of the stomach is controversial. In 1997, Öberg and colleagues102 found that 26% of endoscopic biopsies obtained at and below the GEJ in 334 patients showed absence of cardia-type mucinous glands. Patients who had cardiac mucosa were also significantly more likely to have GERD. Chandrasoma and coworkers103 reported that the presence of cardia-type gastric mucosa or “oxyntocardiac mucosa” (combined oxyntic and mucous glands) in the GEJ correlated with acid reflux. They concluded that all cardia-type mucosa in the GEJ region represents metaplastic transformation of the squamous epithelium as a result of reflux. In another autopsy study by the same group,104 the entire circumference of the GEJ was examined histologically in 18 patients, and cardia-type mucosa was completely absent in 10 (56%). These findings were contradicted by Kilgore and associates,105 who found cardia-type mucosa at the GEJ in all autopsies of 30 pediatric patients, a population considered to be at low risk for GERD. Other investigators have found either mucous glands or mixed mucous glands in most patients at the GEJ, even in those without any history of GERD (Table 1.6).
Table 1.6
The Gastric Cardiac Mucosa: Key Publications
| Author | Study Design | Findings | Conclusions |
| Öberg et al, 1997102 | Endoscopic biopsies; 334 patients | No cardiac mucosa was detectable in 88 (26%). | Cardiac mucosa is the result of GERD. |
| Chandrasoma et al, 2000103 | Endoscopic biopsies with acid reflux measurement | Cardiac/oxyntocardiac mucosa was related to GERD in all 71 patients. | Cardiac mucosa is not a normal anatomic structure. |
| Chandrasoma et al, 2000104 | Autopsy study, adults | Cardiac mucosa was absent in 10 (56%) of 18 cases. | Cardiac mucosa is not a normal anatomic structure. |
| Kilgore et al, 2000105 | Autopsy study, pediatric | Cardiac mucosa was present in all 30 cases. | Cardiac mucosa is a normal anatomic structure. |
| Sarbia et al, 2002106 | Esophagogastric resection specimens | Cardiac mucosa or oxyntocardiac mucosa is present in all 20 cases. | Cardiac and oxyntocardiac mucosa is a dynamic structure. |
| Park et al, 2003107 | Autopsy study, fetal and pediatric | Transitional zone was present in the proximal fetal stomach | Cardiac mucosa composed of pure mucous cells is not a normal developmental structure. |
| Glickman et al, 2002108 | Biopsy study, 74 pediatric patients | Pure mucous or mixed mucous/oxyntic glands were present in 100% of patients | Cardiac mucosa is present in most pediatric patients and may increase in length with GERD. |
| Chandrasoma et al, 2003109 | Endoscopic biopsies, 959 patients | Abnormal columnar epithelium was present in 811 (84.6%). | A histologic system for classifying columnar mucosa of the cardiac region is proposed. |
| Marsman et al, 2004110 | Endoscopic biopsies, 198 patients | Cardiac mucosa was present in 62% of patients, oxyntocardiac mucosa in 38%. | Cardiac mucosa is uniformly present adjacent to the squamous epithelium of the esophagogastric junction. |
| De Hertogh et al, 2005111 | Autopsy study, fetal | Simple columnar epithelium was identified in distal esophagus of 48 fetal autopsy specimens. | At least a part of the adult cardiac mucosa has a congenital origin. |
| Lord et al, 2004112 | Endoscopic biopsies, after esophageal resection | Cardiac mucosa was identified in 10 of 20 patients in cervical esophagus. | Cardiac mucosa can be acquired, likely related to reflux of acid into remnant esophagus. |
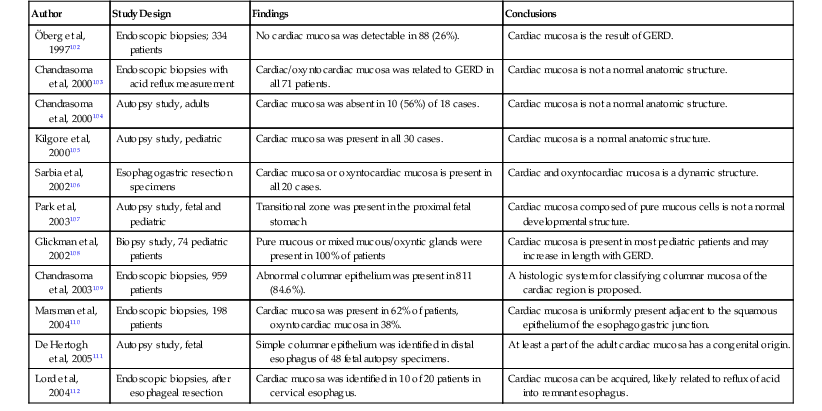
GERD, Gastroesophageal reflux disease.
Data from Odze RD. Unraveling the mystery of the gastroesophageal junction: a pathologist’s perspective. Am J Gastroenterol. 2005;100:1853-1867.
A summary of the objective evidence and the controversies surrounding the nature of the cardia was reported by Odze in 2005.113 In that evidence-based review, the preponderance of data indicates that the true gastric cardia is an extremely short segment (<0.4 cm) of mucosa that is typically composed of pure mucous glands or mixed mucous/oxyntic glands. Notably, these glands are histologically indistinguishable from metaplastic mucinous columnar epithelium of the distal esophagus characteristic of Barrett’s esophagus. In patients with GERD, the length of cardia-type mucosa increases and extends proximally above the level of the anatomic GEJ into the distal esophagus. Thus, intestinal metaplasia of either the true gastric cardia or esophageal metaplastic columnar epithelium may occur. For a more detailed discussion of the gastric cardia and intestinal metaplasia of the GEJ region, the reader is referred to Chapter 15.
Small Intestine
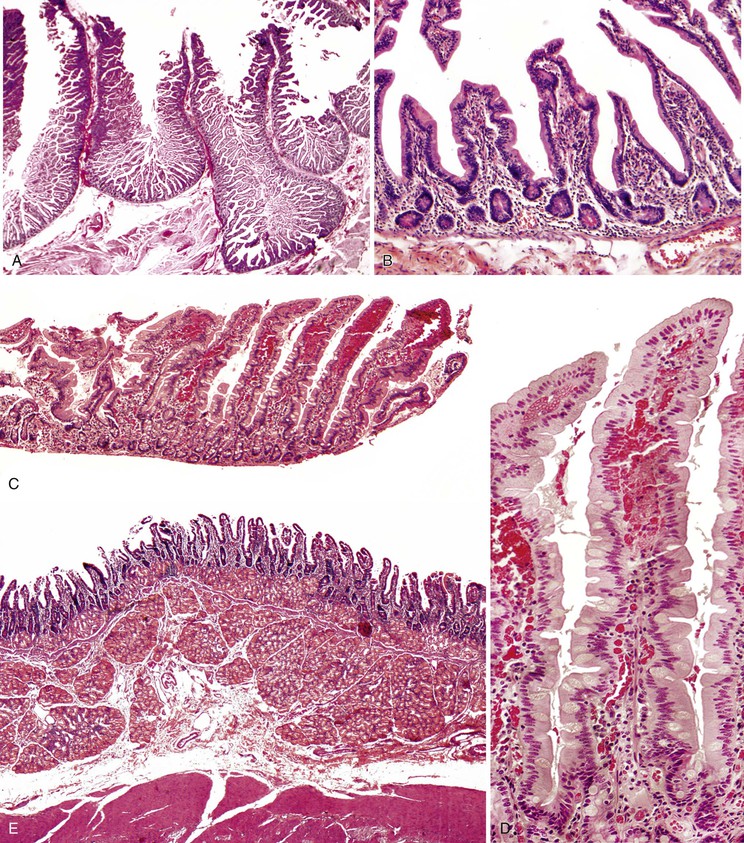
The adult small intestine is approximately 6 m in length. The colon (large intestine) is approximately 1.5 m in length. The first 25 cm of small intestine, the duodenum, is retroperitoneal; the jejunum marks the entry of the small intestine into the peritoneal cavity. The remainder of the small intestine is intraperitoneal until the point at which it enters the colon at the ileocecal valve. The demarcation between the jejunum and ileum is not a clearly defined landmark; the jejunum arbitrarily constitutes the proximal third of the intraperitoneal portion, and the ileum the remainder.
The most distinctive feature of the small intestine is its mucosal lining, which is designed to provide maximal surface area for the purpose of food absorption. The luminal area is enhanced by the presence of circumferentially oriented plica circulares, which protrude into the lumen to impart a corrugated texture to the intestinal surface. When cut in longitudinal section and examined histologically, the plica circulares are seen to provide an undulating substrata for the mucosal lining (Fig. 1.13, A). At medium-power magnification, the mucosa consists of innumerable villi, which extend into the lumen as finger-like projections covered by epithelial lining cells (see Fig. 1.13, B). The central core of lamina propria contains blood vessels, lymphatics, a small population of lymphocytes, eosinophils, mast cells, and scattered fibroblasts and vertically oriented smooth muscle cells. Between the bases of the villi are the pitlike crypts of Lieberkühn, which contain stem cells that replenish and regenerate the epithelium. The crypts extend down to the muscularis mucosae. The muscularis mucosae is a smooth, continuous sheet that serves to anchor the configuration of villi and crypts alike. Villus height is greatest in the duodenum (except in the first portion) and in the proximal jejunum. Figure 1.13, C and D, represents a tissue sample obtained by a fluoroscopic suction-capsule biopsy technique. In normal individuals, the villus-to-crypt height ratio is between 4 : 1 and 5 : 1, but this is variable. In the proximal duodenum, which is exposed to gastric peptic juices to the highest degree, the villus-to-crypt height ratio may reach only 2 : 1 to 3 : 1. Within the duodenum are abundant submucosal mucous glands, termed Brunner glands (see Fig. 1.13, E). They are present immediately distal to the pyloric channel and extend into the second portion of the duodenum. These glands secrete bicarbonate ions (which help neutralize peptic juice as it enters the small intestine), glycoproteins, and pepsinogen II. Except for their submucosal location, Brunner glands are virtually indistinguishable from the mucous glands of the distal stomach.
The surface epithelium of the small intestinal villi contains three principal cell types. Columnar absorptive cells are recognized by the dense array of microvilli on their luminal surface (the “brush border”) and by an underlying mat of microfilaments (the “terminal web”) (see Fig. 1.13, D). Interspersed regularly between absorptive cells are mucin-secreting goblet cells and a few endocrine cells, described later. Goblet cells in the small intestine contain mainly acidic sialated mucins, identifiable by the Alcian blue stain performed at pH 2.5 (acidic). Within the crypts reside stem cells, goblet cells, more abundant endocrine cells, and scattered Paneth cells. Paneth cells contain apically oriented, bright eosinophilic granules and help maintain intestinal homeostasis through secretion of growth factors and a variety of antimicrobial proteins (e.g., defensins) that play a role in mucosal innate immunity against bacterial infection.114,115 Paneth cells are located throughout the small intestine and in the proximal portion of the colon, including the cecum, ascending colon, and proximal portion of the transverse colon. They normally are absent from the distal transverse, descending, and sigmoid colon and the rectum.
Endocrine Cells
A diverse population of endocrine cells are scattered among the epithelial cells that line the small intestinal villi and small and large intestinal crypts (see also Chapter 29). Comparable cells are present in the epithelium lining the pancreas, biliary tract, lung, thyroid, and urethra. Gut endocrine cells exhibit characteristic morphologic features. In most cells, the cytoplasm contains abundant fine eosinophilic granules that harbor secretory products. The main portion of the cell is located at the base of the epithelium, and the nucleus resides on the luminal side of the cytoplasmic granules. The number of endocrine cells in the small intestine is greater than in the colon. The greatest diversity of endocrine cell types is in the duodenum and jejunum, and they become less diverse distally.116 5-Hydroxytryptamine–containing endocrine cells are present in all regions of the small and large intestine and comprise the single largest endocrine cell population. A minor proportion of these cells contain substance P. The second largest cell population is that of glicentin cells, which are more numerous in the ileum and colon. Somatostatin cells occur throughout the alimentary tract. Cells that store cholecystokinin, motilin, secretin, or gastric inhibitory polypeptide are more numerous in the duodenum and jejunum compared with the ileum. Gastrin cells are few and occur exclusively in the proximal duodenum. Many other peptides and bioactive compounds are released by endocrine cells in the small intestine and colon, including β-endorphin, pro–γ-melanocyte-stimulating hormone (pro–γ-MSH), β-lipotropin, neurotensin, glicentin, glucagon, and pancreatic polypeptide (see Chapter 29 for details).
Histologic distinction between endocrine cells and Paneth cells is based on the size and color of the eosinophilic cytoplasmic granules. Although both cell types are pyramidal in shape, with broad bases that narrow toward the crypt lumen, endocrine cells are small (approximately 8 µm in height), do not extend to the surface of the epithelial layer, and contain abundant small, deeply eosinophilic granules. These granules may have a basal orientation, with the nucleus displaced apically. Paneth cells are larger (about 20 µm in vertical height), with a luminal apical plasma membrane, and contain a population of larger, coarse, and brightly eosinophilic granules. Paneth cell granules are always apical relative to the basally located cell nucleus.
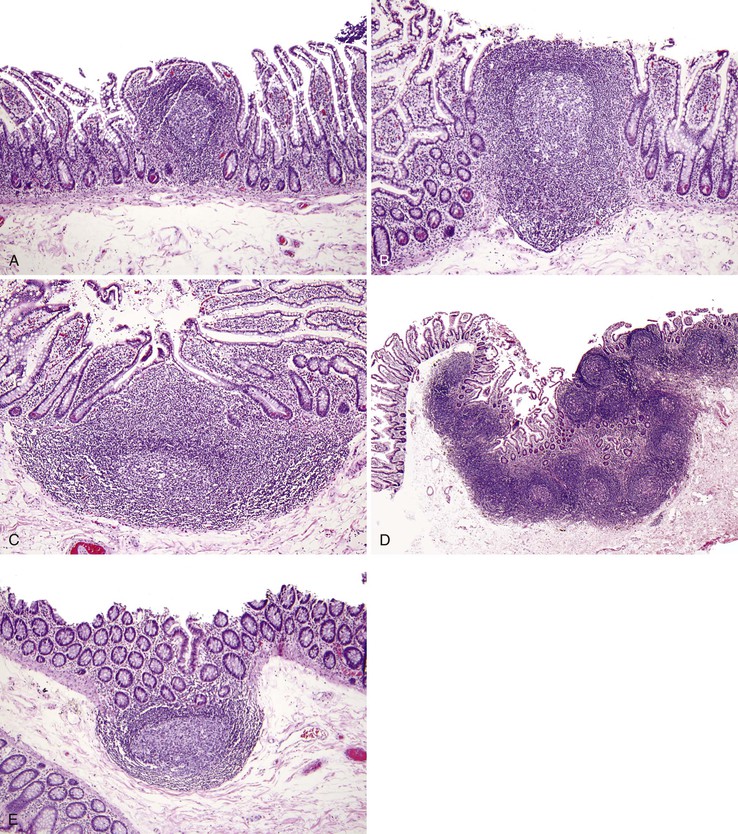
The Intestinal Mucosal Immune System
Humans are exposed to an enormous load of environmental antigens through the GI tract, and the ultrastructural surface area of the GI tract exposed to environmental antigens far exceeds that of the skin and pulmonary tract. The immune system must balance antigenic tolerance against immune defense. The function of the intestinal immune system is best addressed on the basis of its anatomy, almost all features of which can be identified by routine light microscopy (Fig. 1.14; see also Chapter 31). Throughout the small intestine and colon are nodules of lymphoid tissue, which lie either within the mucosa or within both the mucosa and the submucosa. Lymphoid nodules distort the surface epithelium to produce broad domes rather than villi; within the distal ileum, confluent areas of dense lymphoid tissue become macroscopically visible as Peyer patches. The surface epithelium overlying lymphoid nodules contains both columnar absorptive cells and M (membranous) cells, the latter found only in the small and large intestinal lymphoid sites. These cells cannot be readily identified by light microscopy. M cells are capable of transporting antigenic macromolecules, intact, from the lumen to the underlying lymphocytes, thus serving as an important afferent limb of the intestinal immune system.
Throughout the intestines, T lymphocytes are scattered within the surface epithelium, usually at the base of the epithelial layer. These T cells are referred to as intraepithelial lymphocytes (IELs), and are generally of the cytotoxic CD8+ phenotype. However, there is remarkable diversity of T-cell subtypes, some unique to the intestine.117 In normal small intestinal villi, IELs usually decrease in number from the base toward the tip. CD3 immunohistochemistry can aid in detection of IELs, particularly because some lymphocytes have irregular nuclear borders, which makes their identification on H&E stain more difficult.118 In healthy individuals, the duodenum usually contains less than 26 to 29 IELs per 100 epithelial cell nuclei (mean, 11 per 100 in H&E–stained sections, 13 per 100 in CD3-stained sections).119 The range of IEL counts among healthy individuals can vary widely, from 1.8 to 26 per 100 epithelial nuclei, and there is no correlation between IEL counts and the villus-to-crypt height ratios.120 The mean number of IELs decreases progressively in the distal small intestine and colon.121,122 Normal villus IEL counts in the terminal ileum are in the range of 2 IELs per 100 epithelial nuclei.123 A normal IEL count in the ileum does not preclude abnormality in the duodenum.124 A modest elevation in IEL counts accompanies many types of inflammatory conditions of the colon.125
The lamina propria contains helper T cells (CD4+), educated B cells, and plasma cells. The lamina propria plasma cells secrete dimeric immunoglobulin A (IgA), IgG, and IgM, which enter into the splanchnic circulation. IgA is transcytosed directly across enterocytes, or across hepatocytes, for secretion into bile; both are mechanisms for delivering IgA into the intestinal lumen. Finally, other antigen-presenting cells located in the lamina propria include macrophages and dendritic cells. The intestinal lymphoid nodules and mucosal lymphocytes, together with isolated lymphoid follicles in the appendix and mesenteric lymph nodes, constitute the mucosa-associated lymphoid tissue (MALT). Although MALT is most prominent in the small intestine, the concept has relevance to both the stomach (as an acquired anatomic compartment) and the colon (in which it also is normally present; see Chapter 31 for details).
Colon
The colon is subdivided into the cecum and the ascending, transverse, and descending colon. Unlike the jejunum and ileum, whose anatomic location and mechanical attachment to the posterior abdomen are entirely dictated by the mesentery, the anatomic locations of the colonic segments are established by other means. The bulbous cecum and the ascending colon constitute the entire portion of the colon on the right side of the abdomen and are fixed in location. Although peritoneal membrane covers their ventral surfaces, the dorsal aspect of both the cecum and the ascending colon adheres directly to the posterior abdominal wall. (The appendix, which inserts into the cecum just below the insertion of the ileum into the cecum, is an intraabdominal viscus, being entirely covered with peritoneum.) The transverse colon begins at the hepatic flexure and swings across the most ventral aspect of the abdominal cavity to reach the splenic flexure. The transverse colon is suspended by the lesser omentum, which reflects off the greater curvature of the stomach. In turn, the greater omentum hangs from the transverse colon. The descending colon is adherent to the left posterior abdominal wall, similar to its counterpart (the ascending colon) on the right side of the peritoneal cavity. The sigmoid colon begins at the pelvic brim and loops ventrally into the peritoneal cavity. The sigmoid colon is the only portion of the colon that is suspended entirely by mesentery. Therefore, it is subject to redundancy that may, rarely, lead to volvulus. Distally, the colon is adherent to the posterior wall of the pelvis beginning at the rectum, at approximately the level of the third sacral vertebra. Halfway along its 15-cm length, the rectum passes between the crura of the peroneal muscles to exit the abdominal cavity.
In normal adults, the length of the colon is quite variable but usually measures in the range of 0.8 to 1.1 m. From the endoscopist’s perspective, the rectal canal is approximately 15 cm in length, beginning at the anal verge. The variable length of the sigmoid colon makes identification of further landmarks less reliable, but the splenic flexure is located about 0.4 m proximal to the anal verge, and the hepatic flexure about 0.7 m proximal.
The anatomy of the wall of the colon is unique in that the external layer of the muscularis propria is discontinuous. Instead, three longitudinal strips of smooth muscle lie on top of the inner continuous circumferential smooth muscle layer of the muscularis propria. These longitudinal strips are termed the tinea coli. One strip is located at the attachment of the mesentery to the colon. The second and third strips are located equidistant at approximately 120 and 240 degrees around the circumference of the colon. Each strip is approximately 0.5 cm in width, and they become more prominent distally. The tinea coli begin at the cecum, so that the bulbous end of the cecum is creased by the outer two tinea coli as they arc to their respective locations on opposite sides of the cecal wall. Notably, throughout the entire length of colon, arteries and veins penetrate through the continuous inner muscle layer at the edges of the tinea coli. These blood vessels constitute the circumferential ramifications of the mesenteric vasculature. Hence, there are three double tracks of holes in the inner muscle coat, owing to the orifices created by the penetrating vasculature. It is through these holes that diverticula usually protrude (see Chapter 7). Small tags of adipose tissue, the epiploic appendages, also are attached to the colon, at the edges of the nonmesenteric tinea coli 120 and 240 degrees around the circumference of the colon. In this way, two double tracks of intermittent epiploic appendages are created along the entire length of the colon. Protruding diverticula can be difficult to identify because they are in the same circumferential location as the epiploic appendages and may, in fact, protrude into epiploic appendages.
The cecum has the widest diameter of the colon, as well as the highest wall tension. Nevertheless, the mural thickness of the normal cecum is only approximately 0.2 cm. The mural thickness increases gradually over the length of the colon and reaches approximately 0.4 cm in the sigmoid colon, which corresponds to the increasingly solid nature of the luminal contents. The lack of a continuous outer longitudinal muscle layer in the muscularis propria implies that the circumferential inner smooth muscle layer dictates the real diameter of the colon. The diameter varies irregularly from mildly pinched constrictions to intervening dilated segments, each approximately 2 to 4 cm in length. From the luminal aspect, the constrictions are termed haustral folds, and they are prominent anatomic features notable during endoscopy.
The ileum inserts into the cecum at the ileocecal valve. This is a prominent circumferential lip of mucosa and fatty submucosa that extends approximately 0.5 to 1 cm into the cecal lumen. The luminal opening may be slit-shaped or oval. The thickness of the “lip” is approximately 0.3 cm, but it may be thicker in some individuals. The proximal aspect of the ileocecal valve contains small intestinal mucosa, and the distal aspect has colonic mucosa. The mucosal transition occurs at the level of the abrupt luminal convexity of the valve. This structure represents the mechanism that minimizes reflux of cecal contents into the ileum. Whether the “valve” restricts flow of ileal contents into the cecum has never been established; it does not constitute a real muscular sphincter.
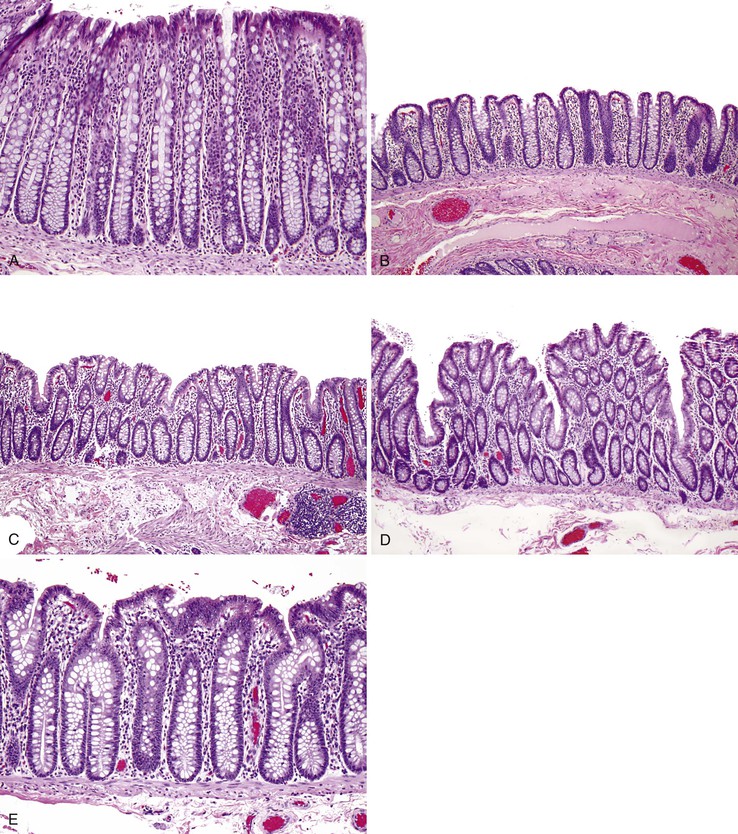
The function of the colon is to reclaim luminal water and electrolytes. Unlike the mucosa of the small intestine, the colonic mucosa has no villi and is flat. The mucosa is punctuated by numerous straight, nonbranching, tubular crypts that extend down and touch the muscularis mucosae (Fig. 1.15, A). The surface epithelium is composed of columnar absorptive cells, which have shorter and less abundant microvilli than those in the small intestine, and goblet cells. The crypts contain abundant goblet cells, endocrine cells (see the earlier discussion of small intestine), and undifferentiated crypt cells. Paneth cells are occasionally present at the base of crypts in the cecum and the ascending and proximal transverse colon. IELs are present throughout the colonic mucosal epithelium. Normal counts are less than 5 IELs per 100 epithelial nuclei.122
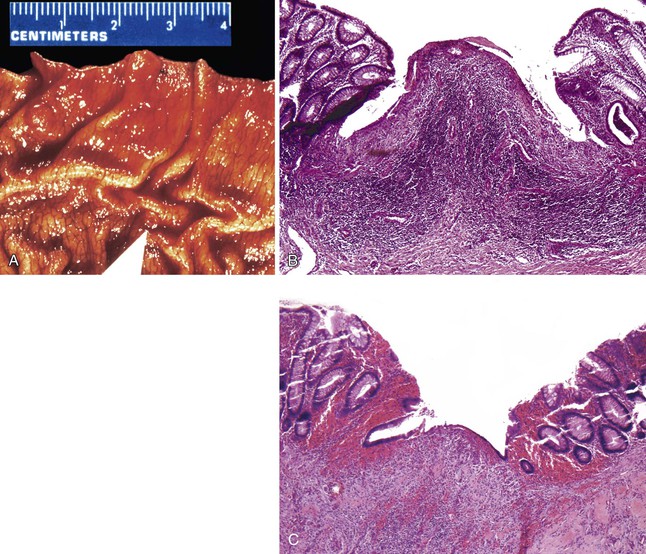
Two sources of potential diagnostic error arise from the normal variation in colonic mucosal microanatomy. First, on occasion, the colonic mucosa exhibits undulation of the surface as a normal anatomic variant (see Fig. 1.15, B to D). A particular feature of this variant is that crypts that are located at the base of undulations appear to branch in the upper third of the mucosa (see Fig. 1.15, E), akin to the type II crypt branching evident on scanning electron microscopy.125 Confusion arises when these normal crypts are interpreted as evidence of architectural distortion characteristic of chronic colitis. Crypt branching is considered abnormal only when it occurs in the lower two thirds of the mucosal layer. Second, in the immediate vicinity of a mucosal lymphoid nodule, the crypts are typically distorted.126 Although this may be obvious if the tissue section transects a lymphoid nodule, a tissue section that passes near but not through a lymphoid nodule will reveal only disorganized crypts. Scanning of multiple serial sections helps identify the lymphoid nodule.
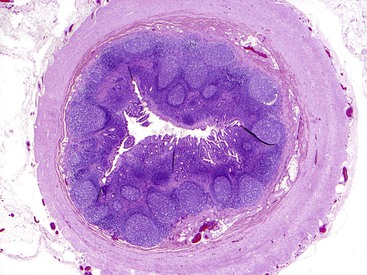
Appendix
The vermiform appendix is a narrow, worm-shaped structure that protrudes from the posteromedial aspect of the cecum, 2 cm (or less) below the insertion of the ileum into the cecum. The appendix is located at the proximal root of the outer tinea coli of the cecum. Because the anterior tinea coli of the cecum is usually prominent, it serves as a guide to locate the appendix. The length of the normal appendix is quite variable, from 2 to 20 cm in length. Its diameter is consistent and uniform along its length, approximately 0.3 to 0.5 cm. It has a rudimentary mesentery only on a portion of its length. The intraperitoneal location of the appendix also is variable. The appendix may lie behind the cecum, hang over the brim of the pelvis, or lie in front or behind the ileum. However, in any individual, the location is relatively fixed.
The appendix is completely invested by peritoneum, and has both an inner circumferential layer and a fully circumferential outer longitudinal muscle layer of the muscularis propria. The mucosa of the appendix is colonic in type. However, the most prominent feature is the abundance of lymphoid tissue that lies within both the lamina propria and the submucosa (Fig. 1.16). The lymphoid tissue is particularly prominent in younger individuals and dissipates gradually over a person’s lifetime. The concept that the appendix undergoes normal “fibrous obliteration” late in life has long been postulated. An alternative proposal is that the fibrotically obliterated lumen in resected appendices result from stromal proliferation in response to clinically silent mucosal or axial neuromas caused by repeated subclinical attacks of inflammation.127
Rectum and Anus
The rectum begins within the abdominal cavity and tapers rapidly to the base of the pelvis. The discontinuous tinea coli converge and unite, again constituting a complete outer longitudinal smooth muscle layer of the muscularis propria. Where the rectum exits the peritoneal cavity to enter the anal canal, it is completely invested by both inner and outer smooth muscle coats of the muscularis propria and acquires an adventitia rather than a serosal covering.
There are subtle differences in the normal histology of the distal rectal mucosa.128 Compared with nonrectal colonic mucosa, distal rectal mucosa exhibits crypts that are not as closely spaced and are slightly shorter (Fig. 1.17). Unlike the crypts in the rest of the colon, those in the rectum do not extend directly down to the muscularis mucosae. The crypts may be slightly dilated or tortuous and are somewhat less numerous. The surface epithelium may be slightly cuboidal rather than tall columnar. The intervening lamina propria contains a moderate number of lymphocytes, plasma cells, macrophages, and occasional neutrophils. Scattered muciphages are common in the lamina propria of the rectum, particularly in older adults. Presumably, they represent the vestiges of previous mucosal injury. It is important to recognize the simplified and somewhat distorted mucosal architecture of the distal rectal columnar mucosa as normal and not as indicating true architectural distortion characteristic of chronic inflammatory bowel disease.
The anal canal is a complex anatomic structure that shows considerable individual variation in mucosal histology128 (see Chapter 32). First, it is critical to understand the macroscopic anatomy of the anal canal (Fig. 1.18). The rectal vault descends into the muscular anal canal, which is composed of the muscularis propria of the anal canal (the internal anal sphincter) and the anorectal skeletal musculature (the external anal sphincter). The external anal sphincter is a complex arrangement of perineal muscle fibers, the most proximal of which is the puborectalis muscle (sling). The puborectalis muscle loops from the pubis bone around the upper portion of the anal canal and back to the pubis, imparting a sharp mucosal angle to the posterior aspect of the rectal vault. As the rectum enters the anal canal, the transverse folds of the colorectal mucosa end, and the mucosa aligns along the long axis into 6 to 10 vertical anal columns. The anal columns terminate about halfway down the anal canal, with interconnecting semicircular anal valves that delineate discrete mucosal recesses termed the anal sinuses. Anal mucin-producing glands empty into the anal sinuses. These anal valves and sinuses are particularly prominent in children but become less pronounced with age. The anal columns may actually protrude into the lumen, earning the name anal papillae. The circumferential ring of anal valves and sinuses is termed the dentate line. Immediately below the dentate line is a zone of smooth mucosa, which flares at the anal verge to become anal skin, which is visible on external examination. The overall distance of the anal canal, in vivo, averages 4.2 cm in normal adults.
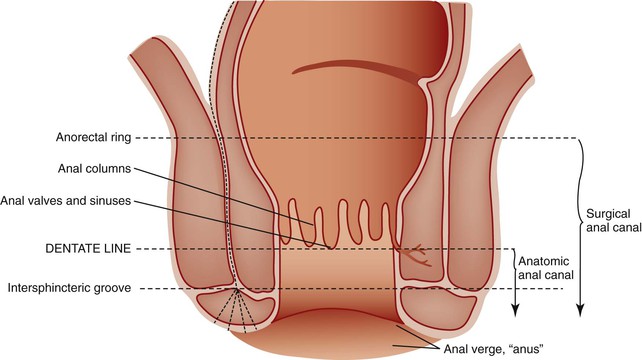
The mucosa of the anal canal is divided into three zones according to the type of epithelial lining. The upper third, above the anal columns, is rectal columnar mucosa. Next is the anal transitional zone, which spans the distance of the anal columns down to the dentate line, approximately 1 cm. Distal to the dentate line is a nonkeratinizing stratified squamous mucosa; at the anal verge, this becomes keratinized skin and contains adnexal structures typical of perineal skin.
The mucosa of the anal transitional zone is the most variable (Fig. 1.19). In some instances, nonkeratinizing anal squamous mucosa extends up the anal columns and transitions directly into the columnar rectal mucosa at its most proximal extent. However, in many individuals, a transitional mucosa is present that consists of four to nine cell layers that are neither squamous nor columnar but rather stratified cuboidal or polygonal and overlie a basal cell layer. Occasional mucin goblet cells may be present as well. Transitional mucosa may be present, especially in the anal sinuses, extending proximal from the nonkeratinizing squamous mucosa of the lower anal canal and transitioning to rectal columnar mucosa proximally. Regardless of whether the anal canal mucosa is columnar, transitional, or nonkeratinizing squamous, this region retains the designation of the anal transitional zone.
Lymph Node Drainage and Lymphatics of the Tubal Gut
General principles of lymphatic drainage are straightforward.129: Lymphatics in the mucosa or submucosa drain through the muscularis propria, then enter either into larger lymphatic channels located in the perivisceral adventitia or into a pedicle or mesentery. However, there are key anatomic features in each segment of the tubal gut, which have become increasingly important to understand because of the intraoperative use of sentinel lymph node biopsy to identify lymph nodes that drain invasive carcinomas of the gut and potentially harbor metastatic cancer.130–132
Esophagus
The mucosal anatomy of the esophagus bears one key difference from the remainder of the tubal gut: The squamous mucosa overlies a definitive layer of lamina propria, which is supported by the muscularis mucosae and submucosa. In the stomach, small intestine, and colon, the lamina propria is intimately interdigitated with the epithelium, so that the bases of epithelial glands or crypts lie directly on the muscularis mucosae. Hence, unlike elsewhere, in the esophagus there is a rich mucosal plexus of lymphatics in the lamina propria oriented predominantly in a longitudinal direction.133 This plexus connects with less extensive plexuses in the submucosa and muscularis propria and eventually drains to regional lymph nodes. Because of this arrangement, esophageal cancers can display early and extensive intramucosal, submucosal, and mural spread along the axis of the esophagus, well beyond the margins of grossly visible tumor.
Stomach
In the stomach, lymphatic channels are absent from the superficial lamina propria but are present in the interglandular region of the deeper portions of the mucosa.134 They converge into thicker channels that pierce the muscularis mucosae and enter a submucosal plexus. From there, they drain into the lymphatic plexus between the circular and longitudinal layers of the muscularis propria, which runs along the muscle fibers to form a polygonal meshwork. Valves are present in this intramural network. From there, larger lymphatic channels track along the major arteries and veins into the gastric and colonic mesenteries.
Small Intestine
The lymphatic drainage of the small intestine is distinct.135 In the lamina propria of each villus are three or more lymphatic channels that run parallel to one another along the long axis. Given the heavy flow of chylomicrons and fatty droplets from the absorptive epithelium to the lymphatic space, the endothelial lining typically contains numerous gaps. These lymphatic channels collect into central lacteals located within the deeper part of the villi, which have a continuous endothelial lining and a reticulin fiber sheath to which smooth muscle fibers attach. The smooth muscle fibers are also oriented longitudinally in the villi, and they intermittently contract to force lymph along the channels. The lacteals anastomose with each other at the base of each villus and form an expanded sinus network, the intravillous lymphatic sinus. Penetrating lymphatic channels then traverse the muscularis mucosae to enter an extensive submucosal lymphatic plexus. This latter plexus drains through lymphatics in the muscularis propria to large conducting lymphatics in the mesentery and, thence, to the major lymphatic ducts located mainly parallel to the larger vascular structures and at the mesenteric root.
Colon
In the colon, a lymphatic plexus lies just underneath the muscularis mucosae. This plexus sends small branches into the deep mucosa at the level of the bases of the colonic crypts, to form a narrow lymphatic zone located immediately above the muscularis mucosae.136 The submucosal plexus drains to an intramural lymphatic plexus located between the inner circular and outer discontinuous longitudinal layers of the muscularis propria (Fig. 1.20). As in the small intestine, lymphatic channels that exit the colonic wall enter the mesocolon in a radial pattern of drainage. Intramucosal, submucosal, and mural lymphatic channels may be sites for microscopic metastasis. However, in contrast to the esophagus, although there may be intramural lateral spread of invasive tumor as much as 2 cm from the primary tumor focus,137 microscopic evidence of colonic cancer more than 2 cm proximal or distal to the macroscopic tumor mass is an exceedingly rare occurence.138
The existence of lymphatic channels in the colorectal mucosa located immediately above the muscularis mucosae is often overlooked by pathologists, particularly in light of the fact that lymphatic channels are very difficult to identify on routine H&E-stained tissue sections (Fig. 1.21).
The number of intramucosal lymphatics does not increase in inflammatory conditions (e.g., ulcerative colitis) but does increase in association with some pathologic changes such as widening of the muscularis mucosae, hyperplasia of the muscle fibers, filiform changes in the mucosa, and hyperplasia of the MALT.139 In colonic specimens with epithelial dysplasia (adenomatous change), an association between dysplastic epithelium and ectatic, and quantitatively increased, lymphatics may be present. However, in cases with carcinoma, no relationship between malignant tumor and quantity of intramucosal lymphatics has been identified. Deposits of carcinoma within intramucosal lymphatics typically occur only within the immediate vicinity of the primary tumor mass. Whether intramucosal lymphatics are involved in clinically significant colorectal tumor metastasis remains to be determined. Abundant data suggest that carcinomas confined to the mucosa (intramucosal) are not at significant risk of lymph node metastasis.140 Invasion of carcinoma into the submucosa remains a biologic requisite for regional or distant cancer metastasis.141
Lymph Nodes
The esophagus drains into numerous lymph node groups, including five directly adjacent to the esophagus in paratracheal, parabronchial, paraesophageal, carinal, and posteriormediastinal locations; supraclavicular lymph nodes may also exhibit drainage from the esophageal region (Fig. 1.22). The cervical esophagus also drains into the internal jugular and cervical lymph nodes, the upper tracheal lymph nodes, and, potentially, the supraclavicular lymph nodes. The infradiaphragmatic portion of the esophagus drains into the left gastric nodes along the lesser curvature and into the ring of lymph nodes surrounding the cardia.
Lymphatics from the gastric wall drain into numerous lymph nodes distributed in chains along the greater and lesser curvatures, in the cardia region, and in the splenic hilum (Fig. 1.23). As detailed by Fenoglio-Preiser and colleagues,133 the drainage patterns are as follows:
• Lesser curvature and lower esophagus: left gastric lymph nodes
• Pylorus: right gastric and hepatic lymph nodes along the course of the hepatic artery
• Cardia: pericardial lymph nodes surrounding the GEJ and left gastric lymph nodes
• Proximal portion of the greater curvature: pancreaticosplenic lymph nodes in the hilum of the spleen
Effluents from all lymph node groups ultimately pass to the celiac nodes surrounding the main celiac axis.
There are approximately 200 mesenteric lymph nodes in the small and large intestinal mesentery. Small mesenteric lymph nodes lie along the radial and arcuate ramifications of the distal mesenteric vasculature subjacent to the bowel wall (Fig. 1.24). Larger ones lie along the primary arcades and major intestinal arteries, especially near the bifurcation of major vessels. The major lymph node groups are located at the root of the superior and inferior mesenteric arteries. These lymphatics converge in lymph nodes located at the mesenteric root. Lymph fluid passes from there to the cisterna chyli, a lymphatic sac that lies in the retroperitoneum behind the aorta and immediately below the diaphragm (Fig. 1.25). The cisterna chyli gives rise to the thoracic duct, which tracks alongside the aorta into the thorax. From there, it runs between the aorta and the azygos vein and receives lymphatic branches from the posterior mediastinal structures, the intercostals, and the jugular, subclavian, and bronchomediastinal ducts before emptying into the angle between the left internal jugular and left subclavian veins.
Distal rectal lymphatics drain laterally along the course of the inferior hemorrhoidal vessels, and from there into paraaortic lymph nodes to end in the hypogastric, obturator, and internal iliac nodes. Alternatively, they follow the superior rectal artery to drain into lymph nodes in the sigmoid mesocolon near the origin of the inferior mesenteric artery. Lymphatic fluid drains from the anus into the endopelvic fascia along the lateral aspect of the ischiorectal space, to the genital femoral sulcus on either side, and ultimately to the inferomedial group of superficial inguinal lymph nodes. Some anal canal lymphatics connect with the rectal lymphatics, whereas others may drain to the common iliac, middle and lateral sacral, lower gluteal, external iliac, or deep inguinal lymph nodes.