CHAPTER 33 Gastrointestinal Consequences of Infection with Human Immunodeficiency Virus
For the 15 years of the acquired immunodeficiency syndrome (AIDS) epidemic that antedated effective antiviral therapy for the human immunodeficiency virus (HIV), the world witnessed an explosion of cases typically manifested by opportunistic infections (e.g., Pneumocystis jiroveci pneumonia) and neoplasms (e.g., Kaposi’s sarcoma). Early in the epidemic, the focus of attention was on characterizing these disorders, and when effective, using prophylactic antimicrobial therapy. In 1995 the concept of highly active antiretroviral therapy (HAART) was born, and the face of the epidemic changed overnight. HAART decreases viral replication and, consequently, circulating HIV. In some patients HIV becomes undetectable in the blood. Associated with a reduction in viral load, there is substantive improvement in immune function that can be assessed by objective measures such as an increase in the CD4 lymphocyte count and clinically by a decrease in opportunistic infections (OIs), as well as improved survival.1–3 With immune reconstitution provided by HAART, both primary and secondary prophylaxis against a variety of OIs also may be discontinued.4 The current focus of management thus centers around viral control rather than prevention and treatment of opportunistic infections. Although dramatic changes have been witnessed as a result of HAART, the global epidemic continues, ensuring that this disease will be present for years to come.5
With the immune reconstitution associated with HAART, there also has been a shift to the management of chronic diseases, as well as drug side effects. Hepatitis C virus (HCV) infection is highly prevalent in HIV-infected patients. Because of HAART, chronic liver disease has assumed increasing importance, as evidenced by reports demonstrating that end-stage liver disease, most often a result of HCV, is a leading cause of hospitalization and death.6 Similarly, HIV-infected patients responding to HAART who have gastrointestinal (GI) complaints are more likely to have drug-induced side effects or nonopportunistic gastrointestinal infections, shifting management strategies back to disorders prevalent in normal hosts.7
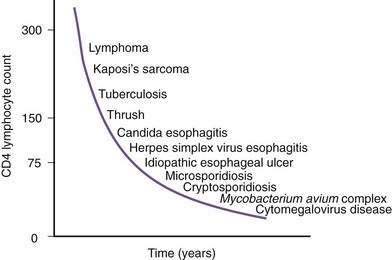
Figure 33-1. Time line of opportunistic disorders based on CD4 lymphocyte count.
(From Wilcox CM, Saag MS. Gastrointestinal complications of HIV infection: Changing priorities in the HAART era. Gut 2008; 57: 861-70.)
ODYNOPHAGIA AND DYSPHAGIA
Before the era of HAART, esophageal complaints (dysphagia and odynophagia) were commonly reported to occur in at least one third of patients during the course of HIV disease. Because of HAART, the incidence of esophageal disease has fallen, and the number of patients with diseases not unique to AIDS, such as gastroesophageal reflux, has risen.7
Candida albicans, the most frequent esophageal infection in AIDS, frequently coexists with other disorders in this setting. Although most cases of Candida occur in the setting of AIDS, Candida esophagitis may occur during primary HIV infection as a result of transient immunosuppression.8 Oral thrush often predicts concurrent esophagitis; however, the absence of thrush does not exclude the possibility of esophageal candidiasis. Overall, the positive and negative predictive values of thrush for Candida esophagitis for patients naïve to HAART are 90% and 82%, respectively.9
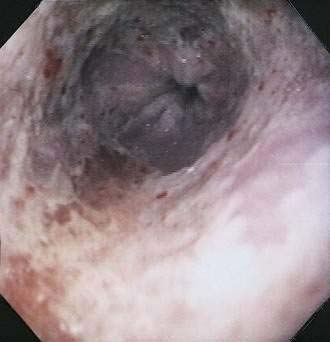
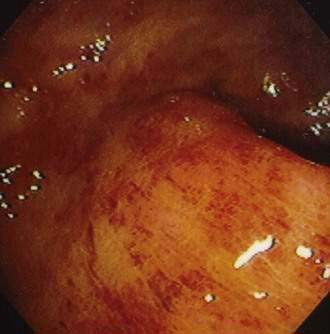
Although CMV is the most commonly identified pathogen in AIDS, its association with esophageal disease is less frequent than Candida. CMV causes mucosal ulceration; thus patients with CMV esophagitis complain of odynophagia or substernal chest pain, characteristically severe.10 Dysphagia is much less common than in patients with Candida esophagitis and is rarely the primary complaint. Fever is rare. Generally, upper endoscopy reveals extensive ulcerations that are large and deep, although the endoscopic pattern is variable (Fig. 33-2).11 Candidal coinfection is common. Mucosal biopsies characteristically demonstrate viral cytopathic effect in mesenchymal and/or endothelial cells in the granulation tissue. As is typical for gut involvement with CMV, characteristic inclusions may be absent, necessitating confirmation by immunohistochemical stains. Biopsy of granulation tissue in the ulcer base provides the highest yield for viral cytopathic effect, whereas viral culture is less sensitive and cytologic brushings are unhelpful.12
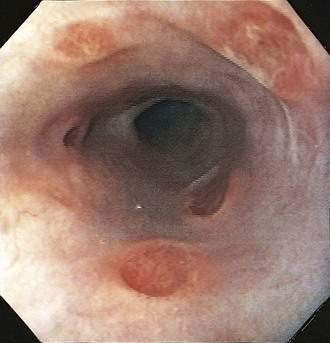
A syndrome of nonspecific (idiopathic, aphthous) esophageal ulceration is common (Fig. 33-3).10 The clinical presentation and endoscopic appearance are indistinguishable from CMV. Criteria for diagnosis of idiopathic ulcers include the following: (1) endoscopic and histopathologic ulcer; (2) no evidence of viral cytopathic effect by both routine histology and immunohistochemical studies; and (3) no clinical or endoscopic evidence of reflux disease or pill-induced esophagitis. As with CMV, these ulcers occur in late-stage disease, with most patients having a CD4 count less than 50/mm3. However, they have also been described in patients with the acute HIV seroconversion syndrome. The pathogenesis of these ulcers remains unknown, but mucosal HIV infection does not appear to be causative.
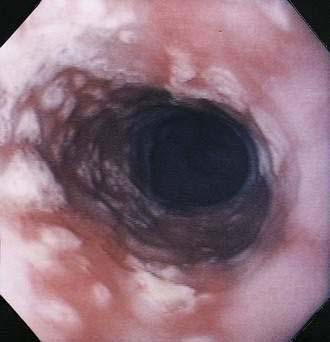
In contrast with other immunocompromised hosts, herpes simplex virus (HSV) esophagitis is infrequent in AIDS.10 In immunocompetent patients, esophagitis is usually due to HSV type 1; however, AIDS patients may have esophagitis due to either type 1 or type 2 herpes. The disease is similar to herpetic infections of other mucous membranes in that the pathogenetic features follow a predictable sequence: discrete vesicles form, then shallow ulcers, which finally coalesce into regions of diffuse shallow ulceration. It is during this late stage of diffuse esophagitis that most patients with herpes are evaluated. In contrast with CMV esophagitis and idiopathic ulcer, these ulcers tend to be shallow; large, deep ulcers are rare (Fig. 33-4). Biopsies and cytologic brushings taken from the margin of the ulcers (the sites of active viral replication) are most likely to show epithelial cell invasion and nuclear changes typical of herpes infections. Viral cultures of biopsy specimens are usually positive.12
EVALUATION AND MANAGEMENT
A specific cause of esophageal complaints in the AIDS patient cannot be made on the basis of symptoms or physical examination alone (Table 33-1). Nevertheless, a few generalizations may be made. The presence of oral thrush associated with mild to moderate dysphagia without odynophagia is likely caused by Candida esophagitis. In contrast, the patient with severe odynophagia without dysphagia or thrush is more likely to have ulcerative esophagitis (viral, idiopathic). The patient complaining of substernal burning and regurgitation is most likely to have gastroesophageal reflux disease. Intermittent solid and liquid dysphagia may be related to a motility disturbance.13
Table 33-1 Differential Diagnosis of Dysphagia and Odynophagia in Patients with AIDS
AIDS, acquired immunodeficiency syndrome.
Endoscopy with biopsy is the only means of establishing a specific etiology for the cause of dysphagia and odynophagia. Conventional barium swallow radiography in the patient with esophageal complaints is not worthwhile, although it may reveal typical features of Candida, CMV, or herpes. In addition, the radiographic appearance of an esophageal ulcer cannot adequately distinguish etiology, thus mandating subsequent endoscopy and biopsy for a definitive diagnosis. Multiple mucosal biopsies are preferred over brush cytology for ulcerated lesions.12
Given the preponderance of Candida infection, an empirical approach to the management of esophageal symptoms is reasonable in most patients with AIDS. Patients with dysphagia and/or odynophagia who also have oral thrush should be treated empirically with fluconazole 100 mg/day after a 200-mg loading dose.14 Itraconazole or fluconazole suspensions are effective alternatives. If symptoms persist despite a 1-week empirical trial, endoscopy with biopsy should be performed in preference to the initiation of other empirical trials or escalation of the dose of fluconazole. Narcotics are appropriate for the patient with severe pain until specific treatment for the underlying cause can be initiated. Relapse of Candida esophagitis is invariable unless immune function is improved with antiretroviral treatment. Despite chronic prophylaxis, relapse frequently occurs often due to antifungal resistance. This is much less of a problem now that patients are receiving HAART. In the absence of HAART, caspofungin and posaconazole may be effective for patients with resistance to antifungal drugs.15
CMV and HSV infection should be treated similarly to other gut involvement with these viruses (see following). Idiopathic ulcers respond in more than 90% of patients to oral glucocorticoids (e.g., 40 mg prednisone per day initially, tapered over 4 weeks).16 The basis for glucocorticoid efficacy is unknown; infectious causes should be assiduously excluded before administering gluocorticoids in this setting. Thalidomide is also highly effective and may be curative when prednisone fails.16 The main side effects of thalidomide are somnolence, rash, and neuropathy. The devastating teratogenic effects mandate its use to be limited to men.
DIARRHEA
Before HAART, diarrhea occurred in up to 90% of patients during the course of HIV disease, especially those from developing countries. In the era of HAART, diarrhea is a less frequent complaint and etiologically is now most often drug-induced (antiretroviral therapy) or is caused by disorders unrelated to HIV infection.17 Alterations in the mucosal immune system in AIDS predispose to intestinal infections, may lead to untreatable chronic infection by organisms that typically cause self-limited infection in healthy hosts (e.g., Cryptosporidium), and may contribute to a more virulent clinical course of otherwise common enteric infections (e.g., Salmonella, Shigella, Campylobacter). Despite the vast spectrum of protozoal, viral, bacterial, and fungal organisms that cause diarrhea in the patient with AIDS, a differential diagnosis can be developed on the basis of the clinical presentation and degree of immunodeficiency (Table 33-2).
Table 33-2 Differential Diagnosis of Diarrhea in Patients with AIDS
| Protozoa |
AIDS, acquired immunodeficiency syndrome; CMV, cytomegalovirus; HIV, human immunodeficiency virus.
EVALUATION AND MANAGEMENT
Protozoa account for the most prevalent class of diarrheal pathogens in most series,18 largely because many of these infections can lead to chronic diarrhea and are refractory to treatment. Cryptosporidium, a cause of self-limited diarrhea in healthy hosts, remains the most frequent protozoa identified in HIV-infected patients worldwide.18 Clinical presentation and outcome are related to the degree of immunocompromise and the subtype of organism.19 Stool testing of asymptomatic individuals shows a high carriage rate because some subtypes appear to be less pathogenic.20 The small bowel is the most common site of infection, although the organisms can be recovered in all regions of the gut, as well as in biliary and respiratory epithelium. Diarrhea is typically severe, with stool volumes of several liters per day not uncommon. Borborygmi, nausea, and weight loss are frequently associated symptoms; right upper quadrant pain suggests biliary tract involvement (see later). The pathogenesis of this infection is uncertain. The diagnosis of intestinal cryptosporidiosis is most often made by acid-fast stain of the stool, where the organisms appear as bright red spherules, similar in size to red blood cells. The sensitivity of stool testing varies and depends on the burden of organisms, character of the stool (formed versus liquid), and primary site of infection. Stool antigen detection and polymerase chain reaction (PCR) markedly increase sensitivity of stool testing. Cryptosporidia may be identified in small bowel or rectal biopsies even when the stool examination is negative.21
Specific antimicrobial treatment of cryptosporidial infection remains disappointing. Numerous antimicrobial agents have been tested, most without significant effect (Table 33-3). Nitazoxanide and azithromycin have been most recently evaluated with mixed results, and combination therapies have also been used. Nitazoxanide can lead to cryptosporidial oocyte clearance in HIV-seronegative patients but has not shown efficacy in HIV-infected patients.22 Although not pathogen-specific, currently the most effective therapy for cryptosporidia is HAART, in which improvement of immune function results in a clinical remission of diarrhea and clearance of cryptosporidia from the stool and on small bowel biopsy.23 For patients failing HAART and/or in whom antimicrobial therapy is ineffective, symptomatic treatment should include fluid support, antidiarrheal agents, and occasionally narcotics such as tincture of opium to control the diarrhea.
Table 33-3 Treatment of Infectious Causes of Diarrhea in Patients with AIDS
| PATHOGEN | TREATMENT | DURATION (DAYS) |
|---|---|---|
| Protozoa | ||
| Cryptosporidia | Paromomycin, azithromycin, nitazoxanide | 14-28 |
| Cyclospora spp. | Trimethoprim-sulfamethoxazole or ciprofloxacin | 14-28 |
| Isospora belli | Trimethoprim-sulfamethoxazole or ciprofloxacin or pyrimethamine | 14-28 |
| Microsporidia | Albendazole (Encephalitozoon intestinalis) | 14-28 |
| Metronidazole, atovaquone, fumagillin (not available in United States)† | ||
| Viruses | ||
| Cytomegalovirus | Ganciclovir | 14-28* |
| Foscarnet | 14-28* | |
| Cidofovir | 14-28* | |
| Herpes simplex | Acyclovir | 5-10* |
| Bacteria | ||
| Salmonella, Shigella, Campylobacter spp. | Fluoroquinolone (e.g., ciprofloxacin) | 10-14* |
| Clostridium difficile | Vancomycin, metronidazole | 10-14 |
| Small intestinal bacterial overgrowth | Metronidazole, ciprofloxacin | 10-14 |
| Mycobacterium tuberculosis | Isoniazid, rifampin, pyrazinamide, ethambutol | 9-12 mo |
| Mycobacterium avium complex | Multidrug regimens for symptomatic infection (see text) | 9-12 mo |
| Fungi | ||
| Histoplasmosis | Amphotericin B; then itraconazole | 28 |
| Coccidioidomycosis | Amphotericin B; then fluconazole | 28 |
| Cryptococcosis | Amphotericin B; then fluconazole | 28 |
AIDS, acquired immunodeficiency syndrome.
* Duration of therapy dictated by immune reconstitution with highly active antiretroviral therapy.
† Molina JM, Tourneur M, Sarfati C, et al. Fumagillin treatment of intestinal microsporidiosis. N Engl J Med 2002; 346:1963.
Isospora belli is a sporozoan, which, like Cryptosporidium, is a cause of chronic diarrhea in untreated patients with HIV infection. The disease is rare in the United States, but it is more frequent and endemic in developing countries such as Haiti. The organism may be identified by acid-fast stain of the stool or duodenal secretions or on mucosal biopsy. This infection can be effectively treated with antibiotics, specifically trimethoprim-sulfamethoxazole and ciprofloxacin.24
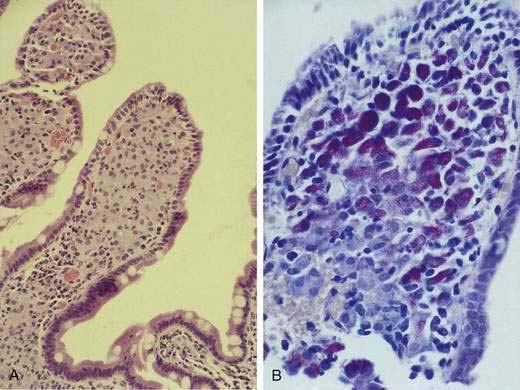
Microsporidia emerged as common intestinal infections in AIDS, but their prevalence has markedly fallen in the HAART era.25 Intestinal and hepatobiliary disease may be caused by two species of microsporidia: Enterocytozoon bieneusi and Encephalitozoon intestinalis. The reported prevalence of microsporidia without HAART varied from 15% to 39%.18,21,26 Typical symptoms include watery, nonbloody diarrhea of mild to moderate severity usually without associated crampy abdominal pain. Weight loss is common, although not to the degree observed with Cryptosporidium. Infection is associated with severe immunodeficiency with median CD4 counts of infected individuals of less than 100/mm3.26 As with infection from cryptosporidia, the pathogenesis of disease remains poorly defined. The organism incites little tissue inflammation and is rarely associated with villous atrophy and cell degeneration. Microsporidia can be discerned by light microscopy when tissue is embedded in plastic or paraffin (Fig. 33-5). Staining of embedded mucosal biopsies with Brown-Brenn, Gram stain, or modified Masson trichrome stain is superior to routine hematoxylin and eosin staining.27 E. intestinalis can usually be differentiated from E. bieneusi by its larger size and infection of lamina propria macrophages; electron microscopy is definitive. Stool staining techniques are only moderately sensitive, while small bowel biopsies are generally positive. No effective therapy is available for E. bieneusi, whereas albendazole is effective for E. intestinalis.28 As with the treatment of cryptosporidia, HAART is the best therapy, resulting in resolution of diarrhea with loss of this pathogen from stool and on small bowel biopsy.23
For unclear reasons, infections by the protozoa Giardia lamblia and Entamoeba histolytica are not consistently seen with increased frequency or virulence in AIDS.29 However, in a study from Taiwan, where E. histolytica is endemic, amebic colitis was identified as a common cause of diarrhea.30 The nonpathogenic Entamoeba dispar is morphologically similar to E. histolytica and can only be distinguished by more specific stool or enzyme-linked immunosorbent assay tests.29 Blastocystis hominis, Endolimax nana, and Entamoeba coli are nonpathogenic protozoa that are seen more commonly in men who have sex with men and are often found in association with other protozoal parasites. Rare cases of enteric leishmaniasis, Pneumocystis jiroveci infection, and toxoplasmosis have been reported.
Helminths, particularly Strongyloides stercoralis and Ascaris lumbricoides, are uncommon pathogens.31 Patients may present with abdominal pain, diarrhea, and eosinophilia. The clinical syndrome and recurrence rate associated with these parasites do not appear to be altered in the setting of HIV infection.
Viral infection of the large bowel, and rarely the small bowel, is an important cause of diarrhea in HIV infection. CMV is the most common viral cause of diarrhea and the most frequent cause of chronic diarrhea in patients with AIDS and multiple negative stool tests.32 This infection characteristically occurs late in the course of HIV infection when the CD4 lymphocyte count falls below 100/L (see Fig. 33-1). Infection is most common in the colon (Fig. 33-6), but concomitant disease in the esophagus, stomach, or small bowel may be observed. Isolated small bowel disease typically results in abdominal pain rather than diarrheal illness. The pathogenesis has not been totally elucidated. Infection of vascular endothelial cells is common, suggesting a role for mucosal ischemia; true histopathologic evidence of vasculitis is rare. An important role for local proinflammatory cytokine activation has been suggested.33
The clinical manifestations of enteric CMV infection vary greatly and include asymptomatic carriage, nonspecific symptoms of weight loss and fevers, and focal enteritis/colitis including appendicitis or diffuse ulcerating hemorrhagic involvement with bleeding or perforation. As a result, patients can present with one of several constellations of symptoms, including abdominal pain; peritonitis; watery, nonbloody diarrhea; or hematochezia.34 The most common presentation, however, is abdominal pain associated with chronic diarrhea. Although the endoscopic spectrum is variable, the hallmark of CMV enteritis/colitis is subepithelial hemorrhage and mucosal ulceration (see Fig. 33-6).34
The diagnosis of GI CMV infection is best established by demonstrating viral cytopathic effect in tissue specimens.35 The inclusions may be atypical in appearance or few in number, requiring immunostaining and/or in situ hybridization for confirmation.35 Cultures for CMV are usually positive when inclusions are present, but they are less sensitive and specific for CMV infection than histopathologic identification. If inclusions are few in number and are demonstrable in tissue that appears macroscopically normal, the patient should be considered to have CMV colonization rather than true CMV infection.
A number of effective therapies are available for the treatment of CMV (see Table 33-3). The most commonly used agent is ganciclovir, an acyclovir derivative, which is effective in approximately 75% of cases.36 Ganciclovir requires daily intravenous administration for several weeks, depending on the location and severity of disease. Valganciclovir, an oral analog of ganciclovir, has excellent GI absorption and efficacy for CMV retinitis, but has not been well studied for induction therapy in GI disease. Valganciclovir has become widely used as preemptive therapy in the transplant setting and has shown promise as first-line therapy.37 An alternative of equivalent efficacy to ganciclovir is foscarnet, a pyrophosphate analog that inhibits viral replication. In contrast to ganciclovir, it has the advantage of being less marrow suppressive, although renal insufficiency and disturbances in mineral metabolism (hypocalcemia, hypomagnesemia, hypophosphatemia) are frequent. Foscarnet tends to be less well tolerated than ganciclovir. Although rarely used, cidofovir is the newest agent for CMV. Like ganciclovir and foscarnet, it must be given intravenously, and similar efficacy rates have been reported for retinal disease. Anecdotal experience suggests it to be effective for GI disease caused by CMV. Because of its long half-life (2 weeks), cidofovir can be given once weekly, which may be particularly advantageous for some patients. The main side effect is nephrotoxicity that may be irreversible. Because of the severe immunodeficiency required for the development of CMV disease, recurrences are common following withdrawal of therapy; however, immune reconstitution with HAART will negate the need for long-term suppressive therapy.3 At the time of diagnosis of GI CMV infection, all patients should have an ophthalmologic examination to exclude CMV retinitis because this site of infection requires close follow-up to ensure remission, thereby preventing blindness. Although widely used in the transplant setting, the role of CMV antigenemia or DNA concentrations by PCR to predict subsequent disease and guide the use of preemptive therapy remains less well defined.38
A number of other viruses (e.g., Norwalk, adenovirus), as well as novel enteric viruses (astrovirus, picobirnavirus) have been identified in symptomatic and asymptomatic patients, but their overall contribution to diarrheal disease in AIDS is small.39
The role of HIV itself as a diarrheal pathogen is limited. Although HIV can be identified within gut tissue in some patients with AIDS, the virus has been confined to lamina propria macrophages and enterochromaffin cells, and not epithelial cells. An idiopathic AIDS enteropathy has been proposed to account for the diarrhea in AIDS patients who lack an identifiable pathogen and may reflect indirect effects of HIV on enteric homeostasis. With improvements in diagnostic techniques, greater awareness of the spectrum of diarrheal pathogens in AIDS, recognition of the importance of drugs as additional causes and use of panendoscopy with biopsy for patients with negative stool tests, a diminishing fraction of patients have truly “idiopathic diarrhea.” Although a variety of morphologic and functional abnormalities of the small bowel have been shown in HIV-infected patients, their role in causing or contributing to GI symptoms is likely small.40 Institution of protease inhibitors has been shown to improve chronic unexplained diarrhea.41
Infections by enteric bacteria are more frequent and more virulent in HIV-infected individuals compared with healthy hosts. Salmonella, Shigella, and Campylobacter have higher rates of bacteremia and antibiotic resistance. Diagnosis is straightforward because the organisms usually can be grown from stool samples (see Chapter 107). These enteric infections typically present with high fever, abdominal pain, and diarrhea that may be bloody. Abdominal pain can be severe, mimicking an acute abdomen. As noted, bacteremia is common, and parenteral antibiotics should be administered empirically in severely ill patients when these infections are suspected pending results of stool and blood cultures; a fluoroquinolone such as ciprofloxacin may be a particularly attractive choice for empirical therapy and if organisms are multiply resistant.
Diarrhea due to Clostridium difficile has emerged as the most common bacterial pathogen, not because it is an OI, but rather because antibiotic use is far greater and hospitalization more frequent in this population than in healthy hosts.42 The clinical presentation, response to therapy, and relapse rate are no different than in immunocompetent patients.43 Diagnosis rests on standard assays of stool for C. difficile enterotoxin. Treatment with metronidazole or vancomycin is generally effective (see Chapter 108).
Small bowel bacterial overgrowth (see Chapter 102) is uncommon in AIDS patients,44 and its role in causing diarrhea appears limited.
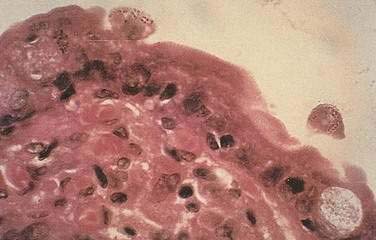
Diagnosis of GI MAC infection is best made by endoscopic biopsy; fecal acid-fast smear is much less sensitive than culture. The organism is readily seen on biopsy specimens with acid-fast staining, and the number of organisms is often striking (Fig. 33-7). Blood culture positivity may suggest the diagnosis. Affected patients have severe malabsorption and weight loss in association with blunting of villi and suffusion of macrophages with mycobacteria. As is typical of MAC infection, in AIDS there is a poorly formed inflammatory response and granulomas are rarely present. Response to antibiotic therapy is variable and depends in part on the extent of immunocompromise; however, eradication is rarely achieved. Multidrug regimens are required for therapy including combinations of amikacin, ethambutol, rifampin, clarithromycin, and ciprofloxacin, which can reduce, but not eradicate, MAC organisms. As with other OIs, institution of HAART in these patients may improve immune function, hasten clinical resolution of the infection, prevent relapse such that long-term antimicrobial therapy will be unnecessary, and enhance survival.3,45
Although extrapulmonary M. tuberculosis is characteristic of AIDS, luminal GI tract involvement remains infrequent but when present usually involves the ileocecal region or colon.46 Fistula formation, intussusception, and perforation, as well as peritoneal and rectal involvement, also have been reported. Tuberculous involvement of the gut in HIV is most commonly found in developing countries. In contrast with MAC, M. tuberculosis infections in AIDS generally respond to multidrug antituberculous therapy with clinical and microbiologic cure, although treatment may be difficult due to drug interactions.47 Infections caused by mycobacteria (e.g., MAC lymphadenitis) and viruses (e.g., CMV uveitis) have been described following institution of HAART.48 This immune reconstitution syndrome results in an exuberant inflammatory response directed toward previously quiescent or incubating pathogens, resulting in paradoxical exacerbations of these infections.
Fungal infections of the gut have been recognized in AIDS. GI histoplasmosis has been most commonly described and occurs in the setting of disseminated infection, often in association with pulmonary and hepatic histoplasmosis. It may manifest as a diffuse colitis with large ulcerations and diarrhea, as a mass, or as serosal disease in association with peritonitis.49 The diagnosis of disseminated histoplasmosis may be suspected in a patient with high fever and markedly elevated serum LDH.50 The diagnosis is established by fungal smear and culture of urine, infected tissue or blood; histoplasmosis antigen assay may provide supportive evidence. The infection is often managed initially by liposomal amphotericin B administration. Long-term suppressive therapy with itraconazole has been used successfully, whereas a response to HAART mitigates the need for long-term suppressive therapy. Rare cases of systemic cryptococcosis and coccidioidomycosis with gut involvement also have been described. A peculiar fungal infection due to Penicillium marneffei has been reported from Southeast Asia that can cause colitis and chronic diarrhea.51
With the advent of HAART, drug-induced diarrhea has become an increasingly important and a frequent cause of antiretroviral drug discontinuation.52 The most common agents associated with diarrhea are the protease inhibitors, with nelfinavir having the highest rate.53 Generally the diarrhea is mild to moderate in severity and is not associated with weight loss. The mechanism(s) for diarrhea due to these agents is poorly understood. Symptomatic therapies are generally effective. A suggested approach to the evaluation of diarrhea is outlined in Table 33-4.
Table 33-4 Evaluation of Diarrhea in Patients with AIDS
| In all patients |
| Stool specimen for bacterial culture: For Salmonella, Shigella, and Campylobacter spp.; Clostridium difficile toxin |
| Stool smear for fecal leukocytes, ova and parasite examination (at least 3-6 specimens), and acid-fast stain |
| If patient has rectal bleeding, tenesmus, or fecal leukocytes |
| Flexible sigmoidoscopy or colonoscopy with biopsy of mucosa for histopathology, viruses, protozoa |
| Cultures of rectal tissue for bacteria (especially for Campylobacter spp.); viruses (optional) |
| If diarrhea and weight loss persist and above evaluation is negative |
| Upper endoscopy with small bowel mucosal biopsy |
AIDS, acquired immunodeficiency syndrome.
ABDOMINAL PAIN
The exact frequency of abdominal pain in patients with AIDS is unknown, but like other GI complications of AIDS, the prevalence and etiology have been altered by HAART. In most patients with AIDS, abdominal pain, when severe, is directly related to HIV and its consequences. However, the physician must consider not only the manifestations of OIs and neoplasms but also the more common causes of abdominal pain in the general population.54
The differential diagnosis of abdominal pain in AIDS, presented in Table 33-5, is organized by the site of origin of the pain. For each organ system, a list of potential complications with their likely causes is offered. In some instances causes are listed because of their known ability to produce symptoms by involving a particular organ. Table 33-5 does not include non–AIDS-specific diagnoses that have assumed more importance in the era of HAART. Table 33-6 defines abdominal pain in terms of the four most common pain syndromes, their most likely causes, and the diagnostic methods indicated. Generally the duration and severity of symptoms dictate the urgency of evaluation.
Table 33-5 Differential Diagnosis of Abdominal Pain in Patients with AIDS*
| ORGAN | CAUSES |
|---|---|
| Stomach | |
| Gastritis | CMV,† Cryptosporidia |
| Focal ulcer | CMV,† PUD |
| Outlet obstruction | Cryptosporidia, CMV, lymphoma, PUD |
| Mass | Lymphoma, KS, CMV |
| Small Bowel | |
| Enteritis | Cryptosporidia,† CMV, MAC |
| Obstruction | Lymphoma,† KS |
| Perforation | CMV,† lymphoma |
| Colon | |
| Colitis | CMV, enteric bacteria,† HSV |
| Obstruction | Lymphoma,† KS, intussusception |
| Perforation | CMV,† lymphoma, HSV |
| Appendicitis | KS,† Cryptosporidia, CMV |
| Liver, Spleen | |
| Infiltration | Lymphoma,† CMV, MAC |
| Biliary tract | |
| Cholecystitis | CMV,† Cryptosporidia,† Microsporidia |
| Papillary stenosis | CMV,† Cryptosporidia,† KS |
| Cholangitis | CMV† |
| Pancreas | |
| Pancreatitis | CMV,† KS, pentamidine, ddI |
| Tumor | Lymphoma, KS |
| Mesentery, Peritoneum | |
| Infiltration | MAC,† Cryptococcus spp., KS, lymphoma, histoplasmosis, tuberculosis, coccidioidomycosis, toxoplasmosis |
AIDS, acquired immunodeficiency syndrome; CMV, cytomegalovirus; ddI, didanosine; HSV, herpes simplex virus; KS, Kaposi’s sarcoma; MAC, Mycobacterium avium complex; PUD, peptic ulcer disease.
* The differential diagnosis does not include non–AIDS-specific conditions.
Table 33-6 Evaluation of Abdominal Pain Syndromes in Patients with AIDS
| SYNDROME | SUSPECTED DIAGNOSIS | DIAGNOSTIC APPROACH |
|---|---|---|
| Dull pain, diarrhea, mild nausea, vomiting | Infectious enteritis | Stool culture, O&P; sigmoidoscopy |
| Acute, severe pain, with peritoneal irritation | Perforation, infectious peritonitis | Abdominal plain films, surgical consultation, US or CT, paracentesis if ascites is present, laparoscopy |
| Right upper quadrant pain, abnormal liver biochemical tests | Cholecystitis, cholangitis, hepatic infiltrates, cholangiopathy | US or CT, MRC, ERCP, liver biopsy |
| Subacute pain, severe nausea and vomiting | Intestinal obstruction | Abdominal plain films, CT, small bowel series, endoscopy, barium enema |
AIDS, acquired immunodeficiency syndrome; CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; MRC, magnetic resonance cholangiography; O&P, ova and parasites; US, ultrasonography.
EVALUATION AND MANAGEMENT (see Table 33-6)
As with any patient, the history is helpful in localizing the origin of abdominal pain. Associated symptoms and signs should suggest the particular organ involved, and the quality and duration of the abdominal pain may implicate specific diseases. Generally, the same workup as for a patient without AIDS should be initiated. Abdominal ultrasonography and CT scanning are useful early in the assessment of abdominal pain and may highlight regions of disease not suspected clinically. In the patient with acute pancreatitis, drug-induced causes must be considered.55 Management of abdominal pain falls broadly into surgical versus nonsurgical options. Indications for surgical intervention in AIDS patients are the same as for patients without AIDS. All tissue specimens must be submitted for viral and fungal culture and for pathologic examination, and enlarged mesenteric nodes should undergo biopsy. Laparoscopic surgery will provide a less invasive alternative to laparotomy in selected patients.56 The nonsurgical management of abdominal pain is determined by the clinical evaluation.
ANORECTAL DISEASE
The frequency of anorectal disease among homosexual AIDS patients is higher than in other AIDS patients. Common findings in HIV-infected patients include perirectal abscesses, anal fistulas, perianal HSV, idiopathic ulcerations, and infectious proctitis, but lymphoma, ulcerations due to CMV, tuberculosis, and histoplasmosis may also be seen (Table 33-7).
Table 33-7 Differential Diagnosis of Anorectal Disease in Patients with AIDS
| Infections |
| Bacteria |
AIDS, acquired immunodeficiency syndrome.
The frequency of anorectal squamous cell carcinomas is strikingly higher in homosexual men than in other members of the population, and the risk increases as HIV disease advances. These neoplasms result from human papillomavirus (HPV) infections acquired through sexual contact, particularly HPV types 16 and 18. Morphologic studies have documented histologic progression, often in the same lesion, from a benign lesion, condyloma acuminatum, to marked anal dysplasia or squamous cell carcinoma. Cytologic specimens of the anal canal, similar to Papanicolaou smears, are increasingly used for screening and have high predictive value for dysplasia.57
EVALUATION AND MANAGEMENT
In HIV-infected patients and patients with AIDS, physical examination should include careful inspection of the skin and mucous membranes, as well as palpation of the lymph nodes. Visual inspection of the anus for ulcers, fissures, and masses should precede digital examination. Palpation of the perianal area and buttocks for abscess should be performed. The presence of severe pain on rectal examination strongly suggests ulcerative disease, hemorrhoids, or neoplasms. Palpation of the anal canal may reveal masses or fissures not otherwise evident. All patients with anorectal symptoms should have anoscopy and sigmoidoscopy (rigid or flexible) with mucosal biopsy. Evaluation under general anesthesia may be necessary when pain is severe. Specimens should be evaluated for evidence of neoplasm or infection; when appropriate, they should be examined with bacterial (including gonococcal and chlamydial), viral, and fungal cultures. CT scan may define the extent of disease if a neoplasm is identified. Healing of anorectal disease following surgical or medical therapy will largely be determined by the stage of HIV infection. HIV-positive patients without AIDS have favorable outcomes following anorectal surgery, with acceptable wound healing, whereas patients with AIDS are more likely to have a poor outcome. The survival of patients with squamous cell cancer has improved in the HAART era.58
GASTROINTESTINAL BLEEDING
GI bleeding in AIDS is as likely to arise from sources not unique to AIDS as from OIs or neoplasms. Infections and neoplasms seen exclusively with AIDS can rarely cause GI bleeding (Table 33-8). Studies have found that the causes of upper GI bleeding are most frequently due to disorders not linked to AIDS including peptic ulcer, whereas in contrast, the most common cause of lower GI bleeding is CMV colitis.59 As in any other patient, ulceration, from any cause, is the most common pathologic lesion; thus, disorders causing ulcer (e.g., CMV, HSV) are the most common etiologies. Enteric pathogens including Campylobacter, Shigella, Salmonella, E. histolytica, and Chlamydia may cause rectal bleeding from ulceration or colitis. Other pathogens such as MAC, microsporidia, and cryptosporidia almost never cause bleeding because mucosal infection does not typically result in ulceration. Enteric lymphoma (e.g., Burkitt’s) or Kaposi’s sarcoma lesions may ulcerate and bleed spontaneously, although most enteric Kaposi’s sarcoma lesions are asymptomatic.
Table 33-8 Differential Diagnosis of Gastrointestinal Bleeding in Patients with AIDS (Excluding Non–AIDS-Specific Diagnoses)
| Esophagus |
AIDS, acquired immunodeficiency syndrome.
EVALUATION AND MANAGEMENT
The evaluation of GI bleeding in a patient with AIDS parallels the approach taken in otherwise healthy patients (see Chapter 19). Endoscopy is preferred in all patients, especially those with severe immunodeficiency, given the likelihood of opportunistic diseases that generally require mucosal biopsy for diagnosis and because endoscopic therapy for hemostasis can be performed.
Appropriate initial management of severe GI bleeding due to AIDS-related diseases does not require a specific diagnosis, and treatment parallels any other patient (see Chapter 19). Specific therapies for the underlying disease necessarily depend on the results of mucosal biopsy and/or microbiologic studies.
HEPATOMEGALY AND ABNORMAL BIOCHEMICAL LIVER TESTS
Hepatomegaly, a frequent finding in AIDS, is usually associated with one or more liver chemistry test abnormalities. As with other organ systems, the spectrum and clinical manifestations of hepatobiliary disease in patients with HIV evolves as immunocompromise advances, and HAART has altered the frequency, manifestation, and outcome of a number of these diseases (Table 33-9). Hepatobiliary disease can be broadly classified into either hepatic parenchymal abnormalities, biliary abnormalities, or a combination of the two. Currently, parenchymal abnormalities are most often related to viral hepatitis and drug-induced disease. In the era of HAART, liver disease has assumed much greater importance as a cause of morbidity and mortality6 and now represents one of the most frequent non–HIV-related causes of death.60
Table 33-9 Differential Diagnosis of Hepatomegaly and Abnormal Biochemical Liver Tests in Patients with AIDS
| Hepatic Parenchymal Disease |
AIDS, acquired immunodeficiency syndrome.
* Especially sulfonamides, protease inhibitors.
Drug-induced liver injury has emerged as the most prevalent cause of liver test abnormalities and is related to the increasing array of antiretroviral medications. Use of other prescription (or nonprescription) drugs, as well as herbal remedies, should also be considered a cause of abnormal liver test results in the HIV-infected patient.61 Before HAART, drug hepatotoxicity was most commonly due to sulfonamides, and the increased frequency of adverse reactions to these medications is well recognized in AIDS.62 The protease inhibitors are the most common causes of abnormal liver tests, with ritonavir cited most often.63 The mechanisms of liver injury include drug allergy or idiosyncratic reactions to drugs and exacerbation of underlying viral hepatitis. The major risk factors for drug-induced hepatotoxicity include coexistent viral hepatitis, older age, and greater rise in CD4 cells after HAART.64–66 The liver test abnormalities usually follow a hepatocellular pattern; jaundice (primarily indirect hyperbilirubinemia) is uncommon but has been observed most frequently with indinavir.
The lactic acidosis syndrome, characterized by marked hepatomegaly, steatosis, metabolic lactic acidosis, and liver failure, is now well recognized. The pathogenesis of the syndrome is due to impaired mitochondrial DNA synthesis from the nucleoside reverse transcriptase inhibitors such as zidovudine, dideoxyinosine (ddI), and stavudine.67 An associated myopathy, peripheral neuropathy, and pancreatitis may occur as well. Risk factors for the syndrome are unknown. The liver tests typically show a hepatocellular pattern but can be normal or minimally increased. Hepatic steatosis is evident on imaging of the liver. Although reversal has occurred in some patients following drug withdrawal, most patients have worsening disease and death. Liver transplantation is curative.
MAC is consistently the most frequent specific hepatic finding in AIDS in late-stage HIV disease.68 The pathologic hallmark of the infection is the presence of poorly formed granulomas containing acid-fast bacilli within foamy histiocytes. Organisms may be observed in the absence of granulomas and can be cultured from liver biopsy in the absence of infected histiocytes. In developing countries, M. tuberculosis is the most common OI involving the liver. M. tuberculosis, in contrast to MAC, may occur before HIV-infected patients are profoundly immunocompromised. Tuberculosis is commonly extrapulmonary (≈80%) in patients with HIV infection.69 Hepatic disease as part of miliary tuberculosis has been noted. Rarer manifestations include tuberculous abscesses and bile duct tuberculomas.46 The diagnosis of hepatic tuberculosis is made by culture of the organism from liver tissue obtained by percutaneous or laparoscopic biopsy. PCR may allow earlier diagnosis. As with MAC, typical-appearing mycobacteria can be observed by appropriate staining of biopsy specimens.
Clinical and autopsy studies in AIDS patients have reported up to a 90% seroprevalence of hepatitis B markers indicating past or present infection.70,71 More recent studies suggest lower rates, perhaps partly due to use of HBV vaccines.72 Concurrent HIV and HBV infections lead to alterations of HBV antigen-antibody display, viral replication, and clinical consequences. Several reports have described reappearance of hepatitis B surface antigen (HBsAg) in HIV-infected patients previously thought to be immune to HBV, as indicated by the presence of anti-HBs. Recurrence of HBsAg may arise from either reinfection or reactivation with advanced immunodeficiency. In addition, there is an accelerated loss of naturally acquired anti-HBs even in those patients who remain HBsAg negative. With loss or reduction in immunity to HBV, there is an increased prevalence of hepatitis B e antigen (HBeAg) expression, elevated mean levels of deoxyribonucleic acid (DNA) polymerase, and increased titers of antihepatitis B core antigen.73 Acquisition of the chronic carrier state is also much more likely in the HIV-infected patient, especially if infection occurs when immunodeficiency is more advanced. Thus a larger proportion of patients with HIV and HBV infections have a chronic carrier state, with highly infectious serum and body fluids, compared with those who are HIV negative.
Although HIV infection leads to more prevalent chronic HBV carriage, it appears to attenuate the severity of biochemical and histologic liver disease. The mechanism for reduced hepatitis B virus-related liver injury following HIV infection is not certain but has been attributed to a diminution in lymphocyte-mediated hepatocellular injury as a result of HIV effects on lymphocytes. In those patients without serologic evidence of past or present HBV and HIV infection, the efficacy of vaccination is related to the stage of immunocompromise.71 HBV has no independent effect on survival for patients with HIV.74
Conversely, the institution of HAART in a chronic carrier of HBV can have catastrophic consequences following immune reconstitution. Patients may develop an acute flare of hepatitis that can be severe, leading to fulminant hepatic failure. However, the proportion of coinfected patients who develop an acute hepatitis B flare following use of HAART appears to be low.75 It is believed that reconstitution of immune function with HAART leads to production of antibody that is directed to infected hepatocytes as in the normal host. Seroconversion to anti-HBe and/or anti–hepatitis B surface antigen (HBs) may also be observed. Inclusion of lamivudine, which has potent antiviral effects on HBV, in the HAART regimen may reduce the likelihood of an acute flare of hepatitis B. Also the development of escape mutants during long-term lamivudine therapy may precipitate acute hepatitis. Current recommendations are to include two drugs that are active against HBV to prevent emergence of resistant variants. These observations suggest that all patients who are to receive HAART therapy should be screened for active or past HBV infection. Vaccination should be considered in all eligible patients but is less effective, especially in those most immunosuppressed. Treatment options for HBV infection in the setting of HIV have been summarized.76
The consequences of HIV infection on delta hepatitis (hepatitis D) appear similar to those of HBV, although far fewer patients have been studied.77
Case reports and small case series have reported higher serum titers of HAV ribonucleic acid (RNA), as well as more prolonged viremia and higher serum aminotransferase levels among HIV-infected patients. Despite these observations there are no data to suggest that acute HAV infection in HIV-infected patients leads to more severe hepatic disease or worse outcomes compared with non–HIV-infected patients. Hepatitis A vaccine is safe in HIV-positive patients, although less immunogenic.71
The prevalence of HCV infection in those with HIV infection depends in large part on the risk group evaluated. Prevalence is highest in injection drug users (52% to 89%) and hemophiliac patients with HIV,78 whereas in homosexual men and non-drug users, the prevalence is much lower, ranging from 1% to 11%.79 Unlike HBV, the clinical course of HCV worsens as HIV-related immunocompromise advances. Studies in large cohorts of hemophiliac patients have demonstrated dramatic increases in HCV RNA levels with progressive HIV disease, associated with aspartate aminotransferase (AST) elevations and hepatomegaly.80–82 Coinfected patients also have a higher rate of active cirrhosis on biopsy and an accelerated course to clinical cirrhosis and liver failure. Factors that predict fibrosis and progression to cirrhosis in coinfected patients include older age at infection, higher serum alanine aminotransferase (ALT) levels, higher inflammatory activity, alcohol consumption of more than 50 g/day and CD4 count less than 500 cells/mm3.83,84 Steatohepatitis also may play a role.85 The mechanism for this more rapid disease course is unknown but has been similarly recognized in other immunocompromised patients. However, as HIV-infected patients are now living longer as a result of HAART, HCV-induced liver disease and its consequences (e.g., hepatocellular cancer) are assuming significant clinical relevance. Recent studies show HCV-related cirrhosis and its complications to be a common cause of hospitalization and cause of death.6,60 Like hepatitis B, HCV does not cause progression of HIV disease.
The effect of HAART on hepatitis C viral dynamics and liver injury is emerging. Some studies have found attenuation of disease,86 whereas others documented exacerbations reflected by increases in serum aminotransferases.87 Hepatitis C viral load has also been variably affected. The effect of HAART on the natural history of HCV-coinfected patients has been contradictory.88,89
Interferon therapy for HIV/HCV coinfected patients is less effective than in otherwise healthy individuals, particularly if the CD4 count is very low (<200/mm3). Combination therapy of peginterferon plus ribavirin results in sustained virologic response rates of 14% to 36% for HCV genotype 1 infection and 43% to 73% for HCV genotypes 2 and 3.90 Guidelines for therapy have been proposed.76
Fungal infections of the liver are not unusual when immunocompromise is advanced. Histoplasmosis, cryptococcosis, and coccidioidomycosis of the liver may be observed in patients with disseminated fungal disease, predominantly but not exclusively in regions of high prevalence of the organism.91 Candida infection of the liver is rare, in contrast to its high prevalence in mucosal sites.
Hepatic involvement by non-Hodgkin’s lymphoma may be the index manifestation of AIDS and the primary site of the neoplasm. This tumor in the AIDS patient tends to be more aggressive histologically and clinically, spreading rapidly to extranodal sites, making liver involvement more likely.92 The lesions are typically focal and may be large.93 The prognosis is determined largely by the extent of underlying immunocompromise and Karnofsky performance score rather than the lymphoma itself. Improvements in survival have been demonstrated in those receiving HAART.94
Isolated cases of P. jiroveci hepatitis have been described and are attributable to the use of inhaled pentamidine, which fails to protect extrapulmonic sites from this opportunistic pathogen. The liver may also be the site of infection by the protozoa Cryptosporidium, Microsporidium, or Dicrocoelium dentriticum or by other multicellular organisms including leishmania.95
Bacillary peliosis hepatis, caused by either Bartonella henselae or Bartonella quintana, is a systemic infection that may be associated with fever, skin lesions, abdominal pain, and lytic bone lesions.96 Liver tests usually show a disproportionate elevation of the serum alkaline phosphatase. Liver biopsies demonstrate regions of a myxoid stroma in association with granular purple material, which with Warthin-Starry stain or electron microscopy reveal clumps of organisms. Treatment with either erythromycin (orally, or in severe cases, intravenously), tetracycline, minocycline, or a cephalosporin are reportedly effective, although prolonged or lifelong therapy is necessary.
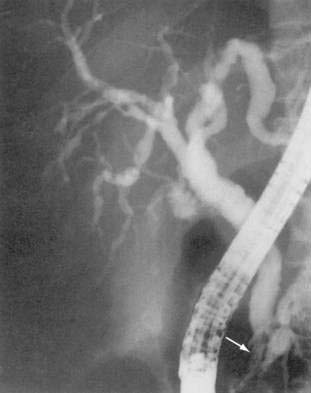
Ductular changes consist of papillary stenosis alone, sclerosing cholangitis-like lesions alone, a combination of the two, or long extrahepatic strictures. Most series have found papillary stenosis with intrahepatic disease as the most common findings (Fig. 33-8). Ultrasonography or CT detects ductular abnormalities, usually dilatation, in most of those with cholangiographically proven disease, implying that a negative imaging study does not definitively exclude the diagnosis. The etiology in most cases is due to infection of the duodenal and biliary epithelium with Cryptosporidium, CMV, or Microsporidium.97 For patients with predominantly papillary stenosis, sphincterotomy results in a symptomatic improvement in most patients; serum alkaline phosphatase may continue to rise, however, probably reflecting progression of associated intrahepatic disease. In some patients eradication of the infecting pathogen results in improvement of the radiographic abnormalities.98 Survival in AIDS cholangiopathy is linked to severity of immunodeficiency.99
Acalculous cholecystitis has also been described in AIDS patients, presenting as severe abdominal pain and, occasionally, peritonitis. This syndrome is usually caused by a specific infection, most frequently CMV, but also from microsporidia, cryptosporidia, and I. belli.100 Laparoscopic cholecystectomy is the treatment of choice.
EVALUATION AND MANAGEMENT
Because the clinical history and the finding of symptomatic hepatomegaly are nonspecific, further evaluation is always necessary. Elevations of serum ALT and/or AST are common, but neither the pattern nor the extent of elevation of these tests appears to correlate with specific findings in the liver. Nevertheless, some generalizations can be made. Significant elevation of the aminotransferases favors a drug-induced or viral cause. In contrast, marked elevation of alkaline phosphatase correlates statistically with the presence of MAC infection in the liver in AIDS when extrahepatic obstruction is absent. Ultrasonography, CT, and MR cholangiography (MRC) should be used early because they are especially useful in identifying ductal dilation, gallbladder pathology, and focal hepatic lesions.101
The indications for liver biopsy for the patient with suspected intrahepatic disease are limited. Biopsy is appropriate when symptomatic, treatable disease of the liver is anticipated, and when a specific diagnosis of hepatic disease is necessary. Although a specific diagnosis is likely in most patients, liver biopsy rarely identifies a previously undiagnosed infection, suggesting that the liver is rarely the site of disease not manifest elsewhere. This observation underscores the importance of reserving liver biopsy for those circumstances in which less invasive diagnostic methods such as blood cultures and bone marrow biopsy have not yielded a diagnosis.102 Biopsy may play a role in the treatment decision for HCV therapy as in the normal host. Focal lesions identified by abdominal imaging can be sampled under ultrasonography or CT guidance. Use of transjugular liver biopsy may be indicated in selected settings such as hemophilia. Specific infections or neoplasms are usually evident on tissue sections of appropriately stained biopsy material.
Call SA, Heudebert G, Saag M, et al. The changing etiology of chronic diarrhea in HIV-infected patients with CD4 cell counts less than 200 cells/mm3. Am J Gastroenterol. 2000;95:3142-7. (Ref 17.)
Ko WF, Cello JP, Rogers SJ, et al. Prognostic factors for the survival of patients with AIDS cholangiopathy. Am J Gastroenterol. 2003;98:2176-81. (Ref 99.)
Monkemuller KE, Call SA, Lazenby AJ, Wilcox CM. Declining prevalence of opportunistic gastrointestinal disease in the era of combination antiretroviral therapy. Am J Gastroenterol. 2000;95:457-62. (Ref 7.)
Novoa AM, de Olalla PG, Clos R, et al. Increase in the non-HIV-related deaths among AIDS cases in the HAART era. Curr HIV Res. 2008;6:77-81. (Ref 60.)
Puoti M, Torti C, Ripamonti D, et al. Severe hepatotoxicity during combination antiretroviral treatment: Incidence, liver histology, and outcome. J Acquir Immune Defic Syndr. 2003;32:259-67. (Ref 66.)
Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Medicine. 2008;9:82-8. (Ref 76.)
San-Andrés FJ, Rubio R, Castilla J, et al. Incidence of acquired immunodeficiency syndrome–associated opportunistic diseases and the effect of treatment on a cohort of 1115 patients infected with human immunodeficiency virus, 1989-1997. Clin Infect Dis. 2003;36:1177-85. (Ref 2.)
Sanchez TH, Brooks JT, Sullivan PS, et al. Bacterial diarrhea in persons with HIV infection, United States, 1992-2002. Clin Infect Dis. 2005;41:1621-7. (Ref 42.)
Tanaka PY, Hadad DJ, Barletti SC, et al. Bone marrow biopsy in the diagnoses of infectious and non-infectious causes in patients with advanced HIV infection. J Infect. 2007;54:362-6. (Ref 102.)
Weber R, Sabin CA, Friis-Müller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632-41. (Ref 6.)
Wilcox CM. Etiology and evaluation of diarrhea in AIDS: A global perspective at the millennium. World J Gastroenterol. 2000;6:177-186. (Ref 32.)
Wilcox CM, Alexander LN, Clark WS, et al. Fluconazole compared with endoscopy for human immunodeficiency virus-infected patients with esophageal symptoms. Gastroenterology. 1996;110:1803-9. (Ref 14.)
Wilcox CM, Schwartz DA, Clark WS. Esophageal ulceration in human immunodeficiency virus infection: Etiology, response to therapy, and long-term outcome. Ann Intern Med. 1995;123:143-9. (Ref 10.)
1. Dragsted UB, Mocroft A, Vella S, et al. Predictors of immunological failure after initial response to highly active antiretroviral therapy in HIV-1-infected adults: A EuroSIDA Study. J Infect Dis. 2004;190:148-55.
2. San-Andrés FJ, Rubio R, Castilla J, et al. Incidence of acquired immunodeficiency syndrome–associated opportunistic diseases and the effect of treatment on a cohort of 1115 patients infected with human immunodeficiency virus, 1989-1997. Clin Infect Dis. 2003;36:1177-85.
3. The Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet. 2008;372:293-99.
4. Kirk O, Reiss P, Uberti-Foppa C, et al. Safe interruption of maintenance therapy against previous infection with four common HIV-associated opportunistic pathogens during potent antiretroviral therapy. Ann Intern Med. 2002;37:239-50.
5. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442.
6. Weber R, Sabin CA, Friis-Müller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632-41.
7. Monkemuller KE, Call SA, Lazenby AJ, Wilcox CM. Declining prevalence of opportunistic gastrointestinal disease in the era of combination antiretroviral therapy. Am J Gastroenterol. 2000;95:457-62.
8. Kassutto S, Rosenberg ES. Primary HIV type 1 infection. Clin Infect Dis. 2004;38:1447-53.
9. Wilcox CM, Straub RF, Clark WS. Prospective evaluation of oropharyngeal findings in human immunodeficiency virus–infected patients with esophageal ulceration. Am J Gastroenterol. 1995;90:1938-41.
10. Wilcox CM, Schwartz DA, Clark WS. Esophageal ulceration in human immunodeficiency virus infection: Etiology, response to therapy, and long-term outcome. Ann Intern Med. 1995;123:143-9.
11. Wilcox CM, Straub RF, Schwartz DA. Prospective endoscopic characterization of cytomegalovirus esophageal ulceration in patients with AIDS. Gastrointest Endosc. 1994;40:481-4.
12. Wilcox CM, Rodgers W, Lazenby A. Prospective comparison of brush cytology, viral culture, and histology for the diagnosis of ulcerative esophagitis in AIDS. Clin Gastroenterol Hepatol. 2004;2:564-7.
13. Zalar AE, Olmos MA, Piskorz EL, et al. Esophageal motility disorders in HIV patients. Dig Dis Sci. 2003;48:962-7.
14. Wilcox CM, Alexander LN, Clark WS, et al. Fluconazole compared with endoscopy for human immunodeficiency virus-infected patients with esophageal symptoms. Gastroenterology. 1996;110:1803-9.
15. Vazquez JA, Skiest DJ, Tissot-Dupont H, et al. Safety and efficacy of posaconazole in the long-term treatment of azole-refractory oropharyngeal and esophageal candidiasis in patients with HIV infection. HIV Clin Trials. 2007;8:86-97.
16. Alexander LN, Wilcox CM. A prospective trial of thalidomide for the treatment of HIV-associated idiopathic esophageal ulcers. AIDS Res Hum Retrovirus. 1997;13:301-4.
17. Call SA, Heudebert G, Saag M, et al. The changing etiology of chronic diarrhea in HIV-infected patients with CD4 cell counts less than 200 cells/mm3. Am J Gastroenterol. 2000;95:3142-7.
18. Datta D, Gazzard B, Stebbing J. The diagnostic yield of stool analysis in 525 HIV-1 infected individuals. AIDS. 2003;17:1711-13.
19. Manabe YC, Clark DP, Moore RD, et al. Cryptosporidiosis in patients with AIDS: Correlates of disease and survival. Clin Infect Dis. 1998;27:536-42.
20. Cama VA, Ross J, Crawford S, et al. Differences in clinical manifestations among Cryptosporidium species and subtypes in HIV-infected persons. J Infect Dis. 2007;196:684-91.
21. Blanshard C, Francis N, Gazzard BG. Investigation of chronic diarrhea in acquired immunodeficiency syndrome. A prospective study of 155 patients. Gut. 1996;39:824-32.
22. Abubakar I, Aliyu SH, Arumugam C, et al. Treatment of cryptosporidiosis in immunocompromised individuals. Systematic review and meta-analysis. Br J Clin Pharmacol. 2007;63:387-93.
23. Carr A, Marriott D, Field A, et al. Treatment of HIV-1 associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256-61.
24. Verdier RI, Fitzgerald DW, Johnson WD, et al. Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. Ann Intern Med. 2000;132:885-8.
25. Van Hal SJ, Muthiah K, Matthews G, et al. Declining incidence of intestinal microsporidiosis and reduction in AIDS-related mortality following introduction of HAART in Sydney, Australia. Trans R Soc Trop Med Hyg. 2007;101:1096-100.
26. Weber R, Ledergerber B, Zbinden R, et al. Enteric infections and diarrhea in human immunodeficiency virus-infected persons. Prospective community-based cohort study. Arch Intern Med. 1999;159:1473-80.
27. Orenstein JM. Diagnostic pathology of microsporidiosis. Ultrastruct Pathol. 2003;27:141-9.
28. Gross U. Treatment of microsporidiosis including albendazole. Parasitol Res. 2003;90(Suppl 1):S14-18.
29. Lowther SA, Dworkin MS, Hanson DL, et al. Entamoeba histolytica-Entamoeba dispar infections in human immunodeficiency virus-infected patients in the United States. Clin Infect Dis. 2000;30:955-9.
30. Wei SC, Hung CC, Chen MY, et al. Endoscopy in acquired immunodeficiency syndrome patients with diarrhea and negative stool studies. Gastrointest Endosc. 2000;51:427-32.
31. Pinlaor S, Mootsikapun P, Pinlaor P, et al. Detection of opportunistic and non-opportunistic intestinal parasites and liver flukes in HIV-positive and HIV-negative subjects. Southeast Asian J Trop Med Public Health. 2005;36:841-5.
32. Wilcox CM. Etiology and evaluation of diarrhea in AIDS. A global perspective at the millennium. World J Gastroenterol. 2000;6:177-86.
33. Redman TK, Britt WJ, Wilcox CM, et al. Human cytomegalovirus enhances chemokine production by lipopolysaccharide-stimulated lamina propria macrophages. J Infect Dis. 2002;185:584-90.
34. Wilcox CM, Chalasani N, Lazenby A, et al. Cytomegalovirus colitis in AIDS. An endoscopic and clinical study. Gastrointest Endosc. 1998;48:39-43.
35. Monkemuller KE, Bussian AH, Lazenby AJ, et al. Special histologic stains are rarely beneficial for the evaluation of HIV-related gastrointestinal infections. Am J Clin Pathol. 2000;114:387-94.
36. Parente F, Bianchi Porro G. Treatment of cytomegalovirus esophagitis in patients with acquired immune deficiency syndrome. A randomized controlled study of foscarnet versus ganciclovir. The Italian Cytomegalovirus Study Group. Am J Gastroenterol. 1998;93:317-22.
37. Len O, Gavaldà J, Aguado JM, et al. Valganciclovir as treatment for cytomegalovirus disease in solid organ transplant recipients. Clin Infect Dis. 2008;46:20-7.
38. Mhiri L, Kaabi B, Houimel M, et al. Comparison of pp65 antigenemia, quantitative PCR and DNA hybrid capture for detection of cytomegalovirus in transplant recipients and AIDS patients. J Virol Methods. 2007;143:23-8.
39. Thomas PD, Pollok RC, Gazzard BG. Enteric viral infections as a cause of diarrhea in the acquired immunodeficiency syndrome. HIV Med. 1999;1:19-24.
40. Knox TA, Spiegelman D, Skinner SC, et al. Diarrhea and abnormalities of gastrointestinal function in a cohort of men and women with HIV infection. Am J Gastroenterol. 2000;95:3482-9.
41. Foudraine NA, Weverling GJ, van Gool T, et al. Improvement of chronic diarrhea in patients with advanced HIV-1 infection during potent antiretroviral therapy. AIDS. 1998;12:35-41.
42. Sanchez TH, Brooks JT, Sullivan PS, et al. Bacterial diarrhea in persons with HIV infection, United States, 1992-2002. Clin Infect Dis. 2005;41:1621-7.
43. Pulvirenti JJ, Mehra T, Hafiz I, et al. Epidemiology and outcome of Clostridium difficile infection and diarrhea in HIV infected inpatients. Diagn Microbiol Infect Dis. 2002;44:325-30.
44. Wilcox CM, Waites KB, Smith PD. No relationship between gastric pH, small bowel bacterial colonization, and diarrhea in HIV-1 infected patients. Gut. 1999;44:101-5.
45. Horsburgh CRJr, Gettings J, Alexander LN, et al. Disseminated Mycobacterium avium complex disease among patients infected with human immunodeficiency virus, 1986-2000. Clin Infect Dis. 2001;33:1938-43.
46. Smith MB, Boyard MC, Veasey S, et al. Generalized tuberculosis in the acquired immune deficiency syndrome. A clinicopathologic analysis based on autopsy findings. Arch Pathol Lab Med. 2000;124:1267-74.
47. Aaron L, Saadoun D, Calatroni I, et al. Tuberculosis in HIV-infected patients. A comprehensive review. Clin Microbiol Infect. 2004;10:388-98.
48. Shelburne SAIII, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS Rev. 2003;5:67-79.
49. Assi M, McKinsey DS, Driks MR, et al. Gastrointestinal histoplasmosis in the acquired immunodeficiency syndrome. Report of 18 cases and literature review. Diagn Microbiol Infect Dis. 2006;55:195-201.
50. Graviss EA, Vanden Heuvel EA, Lacke CE, et al. Clinical predication model for differentiation of disseminated Histoplasma capsulatum and Mycobacterium avium complex infections in febrile patients with AIDS. J Acquir Immune Defic Syndr. 2000;24:30-6.
51. Wu TC, Chan JW, NG CK, et al. Clinical presentations and outcomes of Penicillium marneffei infections. A series from 1994 to 2004. Hong Kong Med J. 2008;14:103-9.
52. McMahon DK, Dinubile MJ, Meibohm AR, et al. Efficacy, safety, and tolerability of long-term combination antiretroviral therapy in asymptomatic treatment-naïve adults with early HIV infection. HIV Clin Trials. 2007;8:269-81.
53. Guest JL, Ruffin C, Tschampa JM, et al. Differences in rates of diarrhea in patients with human immunodeficiency virus receiving lopinavir-ritonavir or nelfinavir. Pharmacotherapy. 2004;24:727-35.
54. Smits SJ, Du Toit RS. The acute AIDS abdomen—a prospective clinical and pathological study. S Afr J Surg. 2005;43:88.
55. Trindade AJ, Juysman A, Huprikar SS, et al. A case study and review of pancreatitis in the AIDS population. Dig Dis Sci. 2008;53:2616-20.
56. Sauerland S, Agresta F, Bergamaschi R, et al. Laparoscopy for abdominal emergencies. Evidence-based guidelines of the European Association for Endoscopic Surgery. Surg Endosc. 2006;20:14-29.
57. Cranston RD, Hart SD, Gornbein JA, et al. The prevalence, and predictive value of abnormal anal cytology to diagnose anal dysplasia in a population of HIV-positive men who have sex with men. Int J STD AIDS. 2007;18:77-80.
58. Oehler-Jänne C, Huguet F, Provencher S, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal. A multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26:2550-7.
59. Chalasani N, Wilcox CM. Etiology and outcome of lower gastrointestinal bleeding in patients with AIDS. Am J Gastroenterol. 1998;93:175-8.
60. Novoa AM, de Olalla PG, Clos R, et al. Increase in the non–HIV-related deaths among AIDS cases in the HAART era. Curr HIV Res. 2008;6:77-81.
61. Estes JD, Stolpman D, Olyaei A, et al. High prevalence of potentially hepatotoxic herbal supplement use in patients with fulminant hepatic failure. Arch Surg. 2003;138:852-8.
62. Floris-Moore MA, Amodio-Groton MI, Catalano MT. Adverse reactions to trimethoprim/sulfamethoxazole in AIDS. Ann Pharmacother. 2003;37:1810-13.
63. Becker S. Liver toxicity in epidemiological cohorts. Clin Infect Dis. 2004;38(Suppl 2):S49-55.
64. Sulkowski MS, Thomas DL, Chaisson RE, et al. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74-80.
65. Wit FW, Weverling GJ, Weel J, et al. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186:23-31.
66. Puoti M, Torti C, Ripamonti D, et al. Severe hepatotoxicity during combination antiretroviral treatment. Incidence, liver histology, and outcome. J Acquir Immune Defic Syndr. 2003;32:259-67.
67. Ogedegbe AE, Thomas DL, Diehl AM. Hyperlactataemia syndromes associated with HIV therapy. Lancet Infect Dis. 2003;3:609-10.
68. Amarapurkar AD, Sangle NA. Histological spectrum of liver in HIV—autopsy study. Ann Hepatol. 2005;4:47-51.
69. Aaron L, Saadoun D, Calatroni I, et al. Tuberculosis in HIV-infected patients. A comprehensive review. Clin Microbiol Infect. 2004;10:388-98.
70. Rodriguez-Mendez ML, Gonzalez-Quintela A, Aguilera A, et al. Association of HCV and HBV markers in Spanish HIV-seropositive patients in relation to risk practices. Hepatogastroenterology. Nov-Dec 2003;50:2093-7.
71. Kellerman SE, Hanson DL, McNaghten AD, et al. Prevalence of chronic hepatitis-B and incidence of acute hepatitis-B infection in human immunodeficiency virus-infected subjects. J Infect Dis. 2003;188:571-7.
72. Lugoboni F, Migliozzi S, Mezzelani P, et al. Progressive decrease of hepatitis B in a cohort of drug users followed over a period of 15 years. The impact of anti-HBV vaccination. Scand J Infect Dis. 2004;36:131-3.
73. Sheng WH, Chen MY, Hsieh SM, et al. Impact of chronic hepatitis B virus (HBV) infection on outcomes of patients infected with HIV in an area where HBV infection is hyperendemic. Clin Infect Dis. 2004;38:1471-7.
74. Puoti M, Airoldi M, Bruno R, et al. Hepatitis B virus coinfection in human immunodeficiency virus–infected subjects. AIDS Rev. 2002;4:27-35.
75. Piroth L, Grappin M, Buisson M, et al. Hepatitis B virus seroconversion in HIV/HBV coinfected patients treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;23:356-7.
76. Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Medicine. 2008;9:82-8.
77. Sheng WH, Hung CC, Kao JH, et al. Impact of hepatitis D virus infection on the long-term outcomes of patients with hepatitis B virus and HIV coinfection in the era of highly active antiretroviral therapy. A matched cohort study. Clin Infect Dis. 2007;44:988-95.
78. Amon JJ, Garfein RS, Ahdieh-Grant L, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994-2004. Clin Infect Dis. 2008;46:1852-8.
79. Verucchi G, Calza L, Manfredi R, et al. Human immunodeficiency virus and hepatitis C virus coinfection. Epidemiology, natural history, therapeutic options and clinical management. Infection. 2004;32:33-46.
80. Darby SC, Kan SW, Spooner RJ, et al. The impact of HIV on mortality rates in the complete UK hemophilia population. AIDS. 2004;18:525-33.
81. Yee TT, Griffioen A, Sabin CA, et al. The natural history of HCV in a cohort of haemophilic patients infected between 1961 and 1985. Gut. 2000;47:845-51.
82. Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52:1035-40.
83. Poynard T, Mathurin P, Lai CL, et al. A comparison of fibrosis progression in chronic liver disease. J Hepatol. 2003;38:257-65.
84. Martin-Carbonero L, Benhamou Y, Puoti M, et al. Incidence and predictors of severe liver fibrosis in human immunodeficiency virus-infected patients with chronic hepatitis C. A European collaborative study. Clin Infect Dis. 2004;38:128-33.
85. Sterling RK, Contos MJ, Smith PG, et al. Steatohepatitis. Risk factors and impact on disease severity in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology. 2008;47:1118-27.
86. Pouti M, Gargiulo F, Roldan EQ, et al. Liver damage and kinetics of hepatitis C virus and human immunodeficiency virus replication during the early phases of combination antiretroviral treatment. J Infect Dis. 2000;181:2033-6.
87. Gavazzi G, Bouchard O, Leclercq P, et al. Change in aminotransferases in hepatitis C virus and HIV coinfected patients after highly active antiretroviral therapy. Differences between complete and partial virologic responders? AIDS Res Hum Retrovir. 2000;16:1021-3.
88. Tural C, Fuster D, Tor J, et al. Time on antiretroviral therapy is a protective factor for liver fibrosis in HIV and hepatitis C virus (HCV) co-infected patients. J Viral Hepat. 2003;10:118-25.
89. Qurishi N, Kreuzberg C, Luchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708-13.
90. Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected patients. N Engl J Med. 2004;351:451-9.
91. Viriyavejakul P, Rojanasunan P, Viriyavejakul A, et al. Opportunistic infections in the liver of HIV-infected patients in Thailand. A necropsy study. Southeast Asian J Trop Med Public Health. 2000;31:663-7.
92. Dal Maso L, Franceschi S. Epidemiology of non-Hodgkin lymphomas and other haemolymphopoietic neoplasms in people with AIDS. Lancet. 2003;4:110-19.
93. Rizzi EB, Schinina V, Cristofaro M, et al. Non-Hodgkin’s lymphoma of the liver in patients with AIDS. Sonographic, CT, and MRI findings. J Clin Ultrasound. 2001;29:125-9.
94. Gerard L, Galicier L, Maillard A, et al. Systemic non-Hodgkin lymphoma in HIV-infected patients with effective suppression of HIV replication. Persistent occurrence but improved survival. J Acquir Immune Defic Syndr. 2002;30:478-84.
95. Singh S, Sivakumar R. Recent advances in the diagnosis of leishmaniasis. J Postgrad Med. 2003;49:55-60.
96. Plettenberg A, Lorenzen T, Burtsche BT, et al. Bacillary angiomatosis in HIV-infected patients-an epidemiological and clinical study. Dermatology. 2001;201:326-31.
97. Sheikh RA, Prindiville TP, Yenamandra S, et al. Microsporidial AIDS cholangiopathy due to Encephalitozoon intestinalis. Case report and review. Am J Gastroenterol. 2000;95:2364-71.
98. Velásquez J, Gancedo E, Fainboim H, et al. Strategies for the treatment of AIDS-associated sclerosing cholangitis. Am J Med. 2004;116:569-70.
99. Ko WF, Cello JP, Rogers SJ, et al. Prognostic factors for the survival of patients with AIDS cholangiopathy. Am J Gastroenterol. 2003;98:2176-81.
100. Cacciarelli AG, Naddaf SY, el-Zeftawy HA, et al. Acute cholecystitis in AIDS patients. Correlation of Tc-99m hepatobiliary scintigraphy with histopathologic laboratory findings and CD4 counts. Clin Nucl Med. 1998;23:226-8.
101. Bilgin M, Balci NC, Erdogan A, et al. Hepatobiliary and pancreatic MRI and MRCP findings in patients with HIV infection. AJR. 2008;191:228-32.
102. Tanaka PY, Hadad DJ, Barletti SC, et al. Bone marrow biopsy in the diagnoses of infectious and non-infectious causes in patients with advanced HIV infection. J Infect. 2007;54:362-6.