CHAPTER 38 Gastrointestinal and Hepatic Disorders in the Pregnant Patient
GASTROINTESTINAL AND HEPATIC FUNCTION IN NORMAL PREGANCY
GASTROINTESTINAL FUNCTION
The amplitude and duration of esophageal muscle contractions in pregnant and nonpregnant women are similar.1 In the distal esophagus, the velocity of peristaltic waves has been found to decrease by approximately one third during pregnancy, but remains within the normal range.2 In contrast, resting lower esophageal sphincter tone progressively declines during gestation, most likely as consequence of inhibition of smooth muscle contraction by progesterone.2–4 This effect coupled with increased abdominal pressure during gestation is responsible for the gastroesophageal reflux symptoms that occur in 70% of pregnant women.5
The effects of pregnancy on gastric motility are unclear. Delayed gastric empting has been demonstrated by some authors, especially during delivery,6 whereas no effect on gastric emptying has been found by others.7 Pregnant women appear to have normal basal and stimulated gastric secretion.8 Transit time of intestinal contents is prolonged during gestation; delayed small-bowel transit is most pronounced during the third trimester and is associated with slowing of the migratory motor complex.9,10 Colonic transit time is prolonged in pregnant animals. Progesterone is thought to have a direct inhibitory effect on smooth muscle cells that slows motility.11 A role for endogenous opioids also has been suggested.12 Together, these changes often result in mild physiologic constipation.
The absorptive capacity of the small intestine increases during pregnancy to meet the metabolic demands of the fetus; increased absorption of calcium, amino acids, and vitamins has been demonstrated.13–16 Animal experiments have revealed pregnancy-induced increases in small-intestinal weight and villous height in conjunction with mucosal hypertrophy.17,18 The activity of some brush border enzymes increases during lactation and then decreases after weaning.19,20
During pregnancy the maternal immune system must adapt to the presence of the fetus. Adaptive changes can influence the response to infection and modulate the course of underlying autoimmune disease. Although many pregnancy-induced immunologic changes remain obscure, it has been proven that during pregnancy there is a shift from cellular to humoral responses with down-regulation of Th1 and up-regulation of Th2 cytokines. Pregnancy modulates natural killer (NK) cell cytotoxicity and induces T-regulatory cells that affect the maternal immune response.21,22 Unfortunately, we still do not understand the affects of pregnancy on the mechanisms responsible for autoimmune diseases such as Crohn’s disease and autoimmune hepatitis well enough to allow us to predict clinical events.
Pregnancy causes an alteration in bile composition, including cholesterol supersaturation, decreased chenodeoxycholic acid and increased cholic acid concentrations, and an increase in the size of the bile acid pool.23 These changes are associated with greater residual gallbladder volumes in the fasting as well as fed states. Sex-steroid hormones may inhibit gallbladder contraction in pregnant women, promoting precipitation of cholesterol crystals and stone formation.24,25
HEPATIC FUNCTION AND LIVER BIOCHEMICAL TESTS
During pregnancy, maternal blood volume increases progressively until, by the thirtieth week of gestation, it is 50% greater than normal, remaining so until confinement.26 This volume expansion, attributed to the effects of steroid hormones and elevated plasma levels of aldosterone and renin, is responsible for dilution of some blood constituents, such as red blood cells (physiologic anemia). Total serum protein concentration diminishes 20% by mid-pregnancy, largely as a result of a reduced serum albumin level. A reciprocal relationship between falling serum albumin and rising serum alpha-fetoprotein concentrations in pregnant women has been proposed.27
DRUG SAFETY IN PREGNANT PATIENTS
Patients and physicians tend to withhold pharmacologic treatment with medications during pregnancy because they fear harming the fetus. Nevertheless, avoidance of medical interventions may adversely affect the mother’s health and the pregnancy outcome. Having stated this, no medication or other therapeutic intervention can be considered definitely safe during pregnancy. Indeed, the placenta is not a reliable barrier to the passage of most drugs, the distribution of a drug within the fetal compartment cannot be accurately predicted, and data on long-term effects of in utero fetal drug exposure are practically impossible to collect. The necessity of any proposed therapy should be discussed with the patient, and known and unknown risks of treatments must be carefully evaluated. The U.S. Food and Drug Administration (FDA) categorizes drugs based on their potential fetal toxicity during pregnancy (Tables 38-1 and 38-2). The FDA classification, however, is of limited practical value because it is based on very few data. A recent publication of the American College of Physicians28 makes recommendations concerning drug therapy in pregnant women.
Table 38-1 Food and Drug Administration Categories of Fetal Risk from Medicines*
| CATEGORY | CRITERIA |
|---|---|
| A | Adequate well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities. |
| B | Animal studies have revealed no evidence of harm to the fetus; however, there are no adequate and well-controlled studies in pregnant women. |
| Or | |
| Animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus. | |
| C | Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women. |
| Or | |
| No animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women. | |
| D | Adequate well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus. The benefits of therapy, however, may outweigh its potential risk. |
| X | Adequate well-controlled or observational studies in animals or pregnant women have demonstrated evidence of fetal abnormalities. The use of the product is contraindicated in women who are or may become pregnant. |
* U.S. Food and Drug Administration. FDA Consumer Magazine. May-Jun 2001; 35:3.
Table 38-2 Food and Drug Administration Categories of Fetal Risk for Some Medications Used to Treat Gastrointestinal and Hepatic Diseases
| MEDICATION | INDICATION | FDA CATEGORY |
|---|---|---|
| Adalimumab | IBD | B |
| Adefovir | Hepatitis B | C |
| Amoxicillin | Infection | B |
| Azathioprine | IBD; autoimmune hepatitis | D |
| Balsalazide | IBD | C |
| Benzodiazepines | Sedation | D |
| Bismuth | Helicobacter pylori infection | C |
| Budesonide | IBD | B |
| Certolizumab pegol | IBD | B |
| Cimetidine | GERD; PUD | B |
| Clarithromycin | Infection | C |
| Cyclosporine | Transplantation | C |
| Entecavir | Hepatitis B | C |
| Esomeprazole | GERD; PUD | B |
| Famotidine | GERD; PUD | B |
| Fentanyl | Sedation, analgesia | C |
| Infliximab | IBD | B |
| Interferon alpha | Hepatitis B, C | C |
| Lamivudine | Hepatitis B | C |
| Lansoprazole | GERD; PUD | B |
| Meperidine | Sedation, analgesia | B |
| Mesalamine | IBD | B |
| Methotrexate | IBD | X |
| Metoclopramide | Nausea; GERD | B |
| Metronidazole | Infection | B* |
| Nizatidine | GERD; PUD | B |
| Olsalazine | IBD | C |
| Omeprazole | GERD; PUD | C |
| Ondansetron | Nausea, vomiting | B |
| Pantoprazole | GERD; PUD | B |
| Penicillin | Infection | B |
| Prednisone | IBD; autoimmune hepatitis | B |
| Prednisolone | IBD; autoimmune hepatitis | C |
| Propofol | Sedation | B |
| Rabeprazole | GERD; PUD | B |
| Ranitidine | GERD; PUD | B |
| Ribavirin | Hepatitis C | X |
| Sucralfate | PUD | B |
| Sulfasalazine | IBD | B |
| Telbivudine | Hepatitis B | B |
| Tenofovir | Hepatitis B | B |
| Tetracycline | Antibiotic | D |
| Thalidomide | Sedative; myeloma | X |
B, no evidence of risk in humans; C, possible risk; D, evidence of fetal risk; X, definite fetal risk; GERD, gastroesophageal reflux disease; IBD, inflammatory bowel disease; PUD, peptic ulcer disease.
ENDOSCOPY DURING PREGNANCY
It is estimated that 20,000 pregnant women undergo endoscopy each year.29 Recommendations concerning endoscopy in this setting are largely based on expert opinion and case reports. Although the safety of endoscopy during pregnancy has not been completely established, it is performed routinely if there is a clear indication.30 Pregnant women have undergone upper gastrointestinal tract endoscopy, colonoscopy, sigmoidoscopy, endoscopic retrograde colangiopancreatograpy (ERCP), and percutaneous gastroscopy safely.31 In addition to general contraindications to endoscopic procedures, specific contraindications during pregnancy include imminent or threatened delivery, ruptured membranes, placental abruption, and pregnancy-induced hypertension.32
Several precautions should be observed to avoid complications when endoscoping a pregnant patient.32 Given the extreme sensitivity of the fetus to maternal hypoxia, pregnant women should receive supplemental oxygen with continuous blood-saturation monitoring. When the fetus is capable of surviving outside the uterus, usually around 24 weeks of gestation, external fetal heart monitoring before, during, and after invasive procedures is advisable to enable prompt delivery if fetal distress occurs. In the second and third trimesters, the supine position and external abdominal pressure should be avoided because resulting compression of the vena cava and aorta may cause hypotension and placental hypoperfusion. ERCP should be performed only with therapeutic intent and by expert endoscopists; every effort should be made to avoid fetal radiation.31 Sedation with meperidine (pregnancy category B), which crosses the blood-brain barrier more slowly than fentanyl (pregnancy category C) and morphine (pregnancy category C), is preferred, although fentanyl may be superior during lactation because it is poorly excreted in breast milk. Sedation with benzodiazepines (pregnancy category D) should be avoided, especially during the first trimester, because diazepam has been reported to cause fetal malformations.33,34 Extensive experience with propofol (pregnancy category B) is lacking, and its high lipid solubility is a reason for concern.35 Patients are advised to avoid breast-feeding and to discard breast milk for 24 hours after a procedure requiring sedation.32
IMAGING AND RADIATION EXPOSURE DURING PREGNANCY
The National Commission on Radiation Protection recommends limiting exposure to ionizing radiation during pregnancy to less than 5 cGy.36,37 The potential for radiation damage to the fetus is determined by dose and gestational age at the time of exposure (Table 38-3).
| GESTATIONAL AGE (DAYS) | EFFECTS OF RADIATION |
|---|---|
| 0-9 | Death |
| 13-50 | Teratogenesis |
| Growth restriction | |
| 51-280 | Growth restriction |
| CNS abnormalities | |
| Possible cancer risk |
CNS, central nervous system.
Computed tomography (CT) should be performed only when its potential benefits clearly outweigh its risks and should be done, if possible, after completion of organogenesis.38 Helical CT may be associated with less fetal radiation exposure than plain CT. Magnetic resonance imaging (MRI) often is a superior alternative to CT; MRI without contrast has not been associated with adverse pregnancy outcomes and magnetic fields are not considered harmful to living organisms.39 There is a theoretical risk of thermal injury to the fetus from MRI in early pregnancy, and MRI is not recommended during the first 12 weeks of gestation. Gadolinium crosses the placenta and its safety in pregnant women has not been formally assessed. Ultrasonography is widely used and safe during pregnancy.
GASTROINTESTINAL DISORDERS AND PREGNANCY
NAUSEA, VOMITING, AND HYPEREMESIS GRAVIDARUM (see Chapter 14)
Sixty percent to 70% of pregnant women report having some nausea in their first trimester, and more than 40% report vomiting.40 Onset of these symptoms typically is in the fourth to sixth week of gestation, with a peak occurrence in the eighth to twelfth week and resolution by week 20. Although nausea and vomiting may vary from mild to severe, most affected individuals still are able to obtain adequate oral nutrition and hydration, in some cases by eating frequent small meals of dry starchy foods.
Severe persistent vomiting demanding medical intervention, or hyperemesis gravidarum, is less common, occurring in 2% or less of all pregnancies.41,42 Hyperemesis is associated with fluid, electrolyte, and acid-base imbalances; nutritional deficiency; and weight loss, and is defined by the presence of ketonuria and a 5% decrease from prepregnancy weight. Although the prognosis of hyperemesis gravidarum is generally favorable, severe untreated disease may lead to significant maternal and fetal morbidity. Symptoms usually begin at weeks 4 to 5 and improve by weeks 14 to 16 of gestation. In up to 20% of affected patients, however, vomiting persists until delivery.43 Reported risk factors for hyperemesis include a personal or family history of the disorder, a female fetus or multiple gestation, gestational trophoblastic disease, fetal trisomy 21, hydrops fetalis, and maternal Helicobacter pylori infection.44
The etiology of hyperemesis gravidarum is likely multifactorial, including contributions by hormonal changes, gastrointestinal dysmotility, H. pylori infection, and psychosocial factors. Pregnancy-related hormones, specifically human chorionic gonadotropin (HCG) and estrogen, have been implicated as important causes of hyperemesis.45 Symptoms worsen during periods of peak HCG concentrations, and conditions associated with higher serum HCG levels, for example, multiple gestation, trophoblastic disease, and trisomy 21, are associated with an increased incidence of hyperemesis.46 Elevated serum estrogen concentrations, as seen in obese patients, also have been associated with this disorder.47 Estrogen and progesterone are thought to cause nausea and vomiting by altering gastric motility and slowing gastrointestinal transit time.48 Other hormones implicated in the pathogenesis of hyperemesis include the thyroid hormones and leptin.49 Abnormal thyroid function test results are found in two thirds of patients with hyperemesis gravidarum.50 The alpha-subunit of HCG has thyroid-stimulating hormone (TSH)–like activity that suppresses TSH release and causes a slight rise in free thyroxine (T4) levels51 but, despite these findings, this gestational transient thyrotoxicosis is not associated with unfavorable pregnancy outcomes and usually does not require treatment. The role of H. pylori as an etiology of hyperemesis is controversial. Several studies have found H. pylori infection to be significantly associated with the disorder,44,52 whereas others could not establish any relationship between the two conditions.53 A small study of the effect of H. pylori eradication in pregnant patients with vomiting showed symptomatic improvement.54
Vomiting in patients with hyperemesis gravidarum often is triggered by olfactory, and even auditory and visual stimuli. A pregnancy-unique quantification of nausea and emesis (PUQE score) can be used to evaluate the number of hours of nausea and the number of episodes of emesis and retching per day in affected women, and is helpful in tailoring therapy.55 Hospital admission for intravenous fluid and electrolyte replacement and, sometimes, nutritional support is indicated when affected individuals develop hypotension, tachycardia, ketosis, weight loss, or muscle wasting. Abnormal laboratory test results in such patients include hypokalemia, hyponatremia, and ketonuria. Hyperemesis is associated with slight increases in serum aminotransferase and bilirubin levels in 25% to 40% of cases. Hyperamylasemia is seen in a quarter of affected patients due to excessive salivary gland production stimulated by prolonged vomiting.56
Severe hyperemesis gravidarum is associated with poor maternal and fetal outcomes. In a study of more than 150,000 singleton pregnancies, infants born to women with hyperemesis who had gained less than 7 kg of weight during pregnancy were more likely to have low birth weights, be premature and small for gestational age, and to have low Apgar scores.41 Severe, albeit rare, maternal complications of hyperemesis include Mallory-Weiss tears with upper gastrointestinal bleeding, Boerhaave’s syndrome, Wernicke’s encephalopathy with or without Korsakoff’s psychosis, central pontine myelinolysis, retinal hemorrhage, and spontaneous pneumomediastinum.57 Lastly, severe depression after elective termination of pregnancy has been reported.58
Given the potential for morbidity and mortality in hyperemesis gravidarum, affected individuals should be treated aggressively. Obstetric management should be overseen, if possible, by physicians qualified in maternal-fetal medicine. The goals of therapy are maintenance of adequate maternal fluid intake and nutrition as well as symptom control. Patients should be advised to eat multiple small meals as tolerated and to avoid an empty stomach, which may trigger nausea. Also avoidance of offensive odors, separation of solid and liquid foods, and consumption of a high-carbohydrate diet may be helpful.59 Antiemetics and antireflux medications are first-line pharmacologic therapy for outpatients who have failed dietary modifications (see Table 38-2). Phenothiazines (chlorpromazine, prochlorperazine), the dopamine antagonist metoclopramide, and pyridoxine (vitamin B6) have proven beneficial in this setting.60 Extensive data show lack of teratogenesis and good fetal safety for many of these drugs.61,62 Treatment with ondansetron (pregnancy category B), a 5-hydroxytryptamine-3 (5-HT3) receptor antagonist, should be considered in patients who do not respond to the above measures. The safety of ondansetron therapy during pregnancy is supported by a recent controlled trial,63 case reports, and widespread clinical experience. Failure of oral medical therapy can be managed in the home setting with intravenous fluid replacement, medications, and multivitamins. As many as 50% of pregnant patients treated through central intravenous catheters, including those inserted peripherally, have catheter-related complications,64 most likely as result of the relative hypercoagulable state and increased susceptibility to infections seen in pregnant women. Enteral feeding through a nasoenteric tube or even total parenteral nutrition is sometimes required to maintain maternal nutrition.65
GASTROESOPHAGEAL REFLUX DISEASE (see Chapter 43)
At least as many women experience pyrosis as nausea during pregnancy; by the end of the third trimester, 50% to 80% of pregnant patients have had new, or an exacerbation of preexisting, heartburn.66,67 Pyrosis, however, rarely is accompanied by overt esophagitis or its complications.68 Pregnant women with heartburn also may have regurgitation and, as already mentioned, nausea and vomiting, as well as atypical symptoms, such as persistent cough and wheezing. Symptoms usually develop toward the end of the second trimester, persist until delivery, and may be predictive of recurrent gastroesophageal reflux disease (GERD) later in life.66 Risk factors for reflux include multiparity, older maternal age, excessive weight gain, and reflux complicating a prior pregnancy.5,66,69
Esophagogastroduodenoscopy (EGD) is rarely required for the assessment of pregnant women with symptoms of GERD.70 There are no data assessing the use of 24-hour ambulatory pH monitoring in this setting, and use of a barium esophagram is undesirable because it entails fetal x-ray exposure. Thus evaluation of suspected GERD in a pregnant woman depends on the clinical experience and judgment of the physician and requires due consideration of the patient’s history and all potential, reasonable causes for the patient’s present symptoms.
Mild reflux symptoms often can be controlled by modifications of diet and lifestyle. Liquid antacids and sucralfate (FDA category B) often are prescribed as first-line pharmacologic therapy.71 Alginic acid (FDA category B) also is effective.72 Magnesium-containing antacids should be avoided during the late third trimester because they theoretically may impair labor. Ranitidine (FDA category B) remains the treatment of choice for patients who have persistent heartburn despite liquid antacid therapy.73 Proton pump inhibitors should be reserved for the most refractory cases, given their more recent introduction to the market. A recent meta-analysis found no significant risk of fetal malformations in babies exposed to proton pump inhibitors in utero.74 Omeprazole is a pregnancy class C drug because it has caused fetal toxicity in animals; all other available proton pump inhibitors are pregnancy category B drugs. The promotility agent, metoclopramide, has not been used extensively to treat GERD during gestation, although it is used during obstetric anesthesia.
GASTRIC AND DUODENAL ULCER DISEASE (see Chapters 52 and 53)
Case studies and retrospective series suggest that the incidence of peptic ulcer disease (PUD) is lower in pregnant women than in nonpregnant individuals.75,76 This impression, if it is valid, may be related to decreased use of nonsteroidal anti-inflammatory drugs (NSAIDs) by cautious patients or possibly to increased use of antacid medications to treat nausea or heartburn. It is conceivable, but equally unproven, that gestational steroids promote gastrointestinal mucosal cytoprotection. PUD is likely underdiagnosed during pregnancy, given the reluctance of physicians to perform diagnostic tests on pregnant women. Gastric acid secretion and the natural history of H. pylori infection, as far as we know, are not altered by gestation.
The dyspeptic symptoms that often accompany pregnancy, especially nausea, vomiting, and heartburn, may make diagnosis of PUD in this setting difficult. Because PUD is exceedingly common in the population as a whole, physicians who care for pregnant women should be vigilant for its occurrence in their patients. A trial of empirical acid suppression may be useful in women with suspected PUD, both as a diagnostic and a therapeutic maneuver, and is thought to be safe.77–80 First-line therapies include ranitidine and sucralfate. In confusing cases, diagnostic EGD is indicated (see earlier). Patients with H. pylori infection may be given antibiotics during pregnancy or after delivery.
INFLAMMATORY BOWEL DISEASE (see Chapters 111 and 112)
Physicians who treat patients with inflammatory bowel disease (IBD) are likely to encounter the disorder in pregnant women. The majority of cases of IBD first present in women younger than age 30 years, the years of peak fertility.81,82 Ulcerative colitis and Crohn’s disease may be more common in women than in men; some authors report women to have an approximately 30% greater risk than men of developing IBD.82
There is controversy regarding the effects of IBD on fertility. Pregnancy rates in IBD patients may be spuriously low because of self-image problems that result in sexual avoidance and voluntary childlessness.83 Fear of IBD in offspring and fear of fetal malformation secondary to maternal therapy are often cited as major causes of childlessness by affected women.84 Female fertility itself, however, does not appear to be impaired by uncomplicated IBD.85,86 A notable exception is fertility in ulcerative colitis patients treated with total colectomy and ileoanal J-pouch anastomosis.87,88 A recent meta-analysis found a three-fold increase in the risk of infertility in IBD patients who had undergone this procedure.88 Infertility in these individuals most likely is caused by pelvic adhesions and fallopian tube scarring. Potential infertility should be discussed with patients of childbearing age who are considering this operation. Male fertility is impaired by sulfasalazine treatment, which causes decreased sperm counts that usually return to normal within six months of discontinuing the drug.89
An initial presentation of IBD during pregnancy is unusual; when IBD first develops in a pregnant woman, it most often does so during the first trimester.90,91 Cases of this type are no more severe than those in nonpregnant individuals. Likewise, pregnancy does not appear to increase the severity of, or morbidity due to, preexisting IBD; disease activity prior to conception seems to be the most important factor determining the cause of the illness during gestation.92 Some authors have suggested that pregnancy might even have a beneficial effect on the disease.93 Disease activity does appear to determine the effect of IBD on pregnancy outcome, although pregnancy is not contraindicated in patients with even the most severe disease.
The goals of the treating physician are thus to minimize IBD symptoms and morbidity prior to conception. Most experts agree that during gestation affected patients should continue optimized prepregnancy therapy to avoid possible flares resulting from medication withdrawal. Exacerbations of IBD that do occur during pregnancy should be managed aggressively because they may result in fulminant colitis and have serious consequences, including hemorrhage, perforation, sepsis, fetal demise, and premature labor. Treatment of fulminant colitis is the same as in nonpregnant individuals, namely high-dose glucocorticoids, intravenous antibiotics, cyclosporine, and salvage biological therapies (see Table 38-2). The indications for bowel surgery likewise are the same as in nonpregnant patients, although bowel surgery is associated with premature labor as well as maternal and fetal mortality.94,95 A Turnbull-Blowhole colostomy to achieve colonic decompression and fecal diversion may be safer than total colectomy.96 Synchronous cesarean section and subtotal colectomy have been advocated for patients with fulminant colitis after 28 weeks of gestation.97 IBD patients are at risk for poor pregnancy outcomes, even if they have mild or inactive disease.98 Major complications include premature birth, low-birth-weight and small-for-gestational-age infants, and increased cesarean section rates.99 The risk of fetal malformations in this setting is unclear.100
The majority of IBD patients require several medications to remain symptom-free. Limited reliable safety data are available on most commonly used IBD drugs, thus it is important to carefully review the possible risks and known benefits of therapy with patients before conception. Potentially teratogenic drugs should be discontinued before conception, if at all possible. Methotrexate and thalidomide (pregnancy category X) are known teratogens and abortifacients, and should be used with caution in patients of childbearing age. The optimum period of abstinence from these medications before conception is unknown; a minimum of six months is recommended. The 5-aminosalicylates (all pregnancy category B except osalazine, which is pregnancy category C) are widely used during pregnancy to treat mild IBD. A prospective study of pregnant patients treated with mesalamine, as well as a large case series, did not show any increased risk of teratogenicity from this therapy.101,102 Azathioprine and its metabolite, 6-mercaptopurine (pregnancy category D), also are commonly used as maintenance treatments in patients with mild to moderate IBD. They both cross the placenta and are excreted in breast milk; however, data concerning use of these agents in the transplant setting have failed to confirm the teratogenicity that had been seen in animal studies.103 A study of pregnant IBD patients treated with 6-mercaptopurine failed to demonstrate an increase in preterm delivery, spontaneous abortion, congenital abnormalities, or childhood neoplasia.104 Based on these data and extensive experience with this drug and its metabolites in pregnant women, experts concur that their discontinuation before or during pregnancy is not advisable. Instead, dose reduction and careful monitoring of metabolite levels in the mother are recommended.105
Glucocorticoids (pregnancy category C) have been used for decades to treat pregnant patients with moderate to severe IBD as well as other more common glucocorticoid-responsive diseases, such as asthma. Early reports suggested an increased risk of congenital malformations in the infants of treated mothers.102 Subsequent prospective studies and substantial experience with drugs in this class confirm that the risk of malformations secondary to their use is extremely low. Glucocorticoid treatment during pregnancy is, however, associated with other complications including maternal glucose intolerance, macrosomia, and fetal adrenal suppression.106 Prednisolone (pregnancy category C) is more efficiently metabolized by the placenta than other glucocorticoids and may pose less risk of adrenal suppression.107 Adverse outcomes have not been reported after use of oral budesonide (pregnancy category C) during pregnancy.108
Many organ transplant recipients have been treated with cyclosporine (pregnancy category C) without reports of significant teratogenicity. At present, there are very few data concerning the safety of treatment with other immunomodulatory agents in pregnant women. These compounds are immunoglobulins and as such are capable of reaching the fetal compartment, especially during the third trimester, when they are actively transported across the placenta. High levels of infliximab (pregnancy category B) were detected in infants exposed to the drug in utero.109 Post-marketing registries of safety data and small case series have not identified an increased incidence of fetal malformations or miscarriage in women treated during pregnancy with infliximab or adalimumab.110 Experts have suggested that therapy with antibodies against tumor necrosis factor-α (TNF-α) be discontinued early in the third trimester to avoid significant fetal exposure until better data on the safety of these agents are available; when necessary glucocorticoids may be substituted.87
APPENDICITIS (see Chapter 116)
Suspected acute appendicitis is the most common nonobstetric indication for exploratory laparotomy in pregnant women.111,112 Appendicitis complicates approximately 1 in 1500 pregnancies, and may develop at any time during the course of gestation.112 Diagnosis may be difficult because the enlarging uterus displaces the cecum cephalad, altering the location of pain caused by appendiceal inflammation, and resulting in increasingly delayed detection as pregnancy progresses.113 Late diagnosis of an inflamed appendix is responsible for complications that are associated with excess maternal and fetal morbidity and mortality.114 During all three trimesters of pregnancy, right lower quadrant pain is the most common presenting symptom of appendicitis.115 In addition to pain, affected individuals frequently complain of nausea, but this symptom often is difficult to interpret during gestation. Graded-compression ultrasonography is the diagnostic test of choice for pregnant patients suspected of having appendicitis.115 Helical CT also has been reported to be helpful in this setting.112 Pregnant patients with appendicitis during any trimester may be treated with laparoscopic appendectomy,116 although potential interference by the gravid uterus may be a relative contraindication to this procedure during the third trimester.117 Appropriate supportive care can prevent fetal loss associated with appendiceal perforation.118
BILIARY AND PANCREATIC DISORDERS AND PREGNANCY
GALLSTONE DISEASE (see Chapter 65)
Pregnant women tend to form gallstones because of changes in gallbladder function and bile composition (see earlier). Gallstones frequently are noted during gestation when ultrasonographic examination is performed to evaluate the fetus119; the prevalence of gallstones in asymptomatic pregnant women is reported to be between 2.5% and 12%. Despite this high prevalence, the incidence of acute cholecystitis is not increased by pregnancy. Cholecystitis is probably more common in the postpartum period than during gestation.120 Other complications of cholelithiasis, including choledocholithiasis and pancreatitis (discussed later), also are rare in pregnant women.
Initial conservative management of suspected gallstone-related disease with intravenous fluids, analgesia, and antibiotics has been recommended to reduce maternal and fetal morbidity incident to surgery.121 A more aggressive operative approach, however, may be associated with superior outcomes. Open cholecystectomy in the first trimester of pregnancy can precipitate abortion, whereas in the third trimester it can induce premature labor. Many experts believe that laparoscopic cholecystectomy is the preferred approach when surgery is indicated in cases of acute cholecystitis, even near term when the uterus is very large.122–125 Endoscopic extraction of bile duct stones with minimal use of fluoroscopy and appropriate maternal shielding is acceptable when necessary to treat choledocholithiasis in pregnant women.125
PANCREATITIS (see Chapter 58)
Acute pancreatitis is uncommon during gestation, occurring once in every 1066 to 3300 pregnancies.126,127 Most cases are due to gallstones and present during the third trimester or the puerperium. The hypertriglyceridemia normally seen in pregnant women may be more severe in persons with familial hyperlipidemia, predisposing them to develop pancreatitis on this basis.128 The clinical characteristics of acute pancreatitis during gestation are similar to those in nonpregnant women.
HEPATIC DISORDERS UNIQUE TO PREGNANCY
CHOLESTASIS OF PREGNANCY
Cholestasis of pregnancy is a form of intrahepatic cholestasis associated with pruritus, elevated serum bile acid levels, and the findings of bland cholestasis on liver biopsy.129 This disorder may have a variable course, making it difficult to diagnose.130 It nevertheless has serious implications for fetal well-being, and cases must be identified as promptly as possible.131
Cholestasis of pregnancy usually presents in the third trimester, but may be seen earlier in gestation, even in the first trimester. Its first and most characteristic symptom is pruritus, and as a result patients may be referred to a dermatologist for initial evaluation. As in other forms of cholestasis, the pruritus of cholestasis of pregnancy is most severe in the skin of the palms and soles and experienced most intensely at night. Only 10% to 25% of affected individuals later develop jaundice. Elevated serum bile acid levels (>10 µmol/L) confirm the presence of cholestasis; some patients with the disorder also have bilirubinuria and even mild hyperbilirubinemia.132 Serum alkaline phosphatase concentrations are modestly increased, but gamma glutamyl transpeptidase (GGTP) levels are normal or only marginally elevated.132 The latter pattern of test results is atypical of adult cholestasis, but is seen in pediatric patients with progressive familial intrahepatic cholestasis, as in Byler’s syndrome.133 Serum aminotransferase levels are elevated in affected women, sometimes to values of 1000 U/L or higher, making it difficult, on occasion, to distinguish cholestasis of pregnancy from hepatitis.134 Symptoms and laboratory test abnormalities of patients may wax and wane. Intense cholestasis is associated with steatorrhea that usually is subclinical but can cause fat-soluble vitamin deficiencies, most notably deficiency of vitamin K.
Improvement of symptoms and laboratory test results begins with delivery of the infant, and usually, although not invariably, is prompt and complete. Rare patients experience prolonged cholestasis that may be indicative of underlying biliary tract disease, such as primary biliary cirrhosis or sclerosing cholangitis.135,136 Women with ordinary cholestasis of pregnancy have no residual hepatic defect after resolution of the disorder, but they are at increased risk for development of gallstones, cholecystitis, and pancreatitis.137 In addition, 60% to 70% of affected individuals develop cholestasis during subsequent pregnancies (although recurrent episodes may be less severe than the initial one) or with use of oral contraceptives. The risk of recurrence with subsequent pregnancy is increased by interval cholecystectomy.138
Cholestasis of pregnancy has serious implications for fetal well-being. There are many reports of increased frequencies of fetal distress, unexplained stillbirth, and need for premature delivery in the babies of women with this disorder.139 Fetal hypoxia and meconium staining have been reported at delivery in 19% of Swedish women with cholestasis of pregnancy.140 These complications were shown to correlate with maternal bile acid levels greater than 40 µmol/L. Although the risk to the fetus may be reduced by close monitoring of affected mothers, it cannot be eliminated completely.141–144 Planned early elective delivery as soon as the fetal lungs have matured has been recommended for this reason.
As discussed in Chapter 64, a number of the molecular mechanisms of bile formation have been elucidated in recent years, resulting in a more sophisticated understanding of many cholestatic disorders.145 Mutations of the MDR3 (ABCB4) gene are likely responsible for approximately 15% of cases of cholestasis of pregnancy.146–148 The MDR3 gene product is a phospholipid flippase that translocates phosphatidylcholine from the inner to the outer leaflet of the canalicular hepatocyte membrane where it is solubilized by bile acids to form mixed micelles. There is, however, no relationship of cholestasis of pregnancy to human leukocyte antigen (HLA) type.149
Environmental and hormonal factors likely also contribute to development of cholestasis in pregnant women. In Chile and Scandinavia, where cholestasis of pregnancy is common, the disorder occurs most often during colder months. The incidence of cholestasis of pregnancy in Chile has declined, possibly due to a fall in mean plasma selenium levels.150 An increased sensitivity to the cholestatic effects of exogenous estrogen has been demonstrated in family members, including male relatives, of patients who develop cholestasis while pregnant.151 Therapeutic or experimental administration of estrogen compounds to susceptible women can precipitate the disorder.152,153 Similarly, progesterone therapy during gestation is associated with development of cholestasis.154 The finding that ursodeoxycholic acid alters the metabolism of progesterone may explain its therapeutic effect in this setting.155 It is possible that some women with cholestasis of pregnancy have inherited an enhanced sensitivity to estrogen or a variation in the metabolism of progesterone that causes cholestasis in response to a variety of stimuli, including some medications and dietary factors.
The differential diagnosis of cholestasis of pregnancy includes other cholestatic disorders such as primary biliary cirrhosis, primary sclerosing cholangitis, benign recurrent intrahepatic cholestasis, viral hepatitis, toxic liver injury, and bile duct obstruction. Liver biopsy specimens of affected individuals reveal bland changes typical of cholestasis due to a variety of etiologies, but biopsy usually is not necessary to make the diagnosis. It is important to remember that pregnancy may exacerbate a preexisting subclinical cholestatic disorder. For example, a family of sisters with progressive liver disease who also developed recurrent severe cholestasis of pregnancy was described in 1997.135
Management of cholestasis of pregnancy is primarily palliative. Ursodeoxycholic acid is helpful in relieving symptoms, may reduce fetal complication rates,146 and is well tolerated by mother and fetus.131,156,157 Studies of treated individuals have demonstrated a change in the bile acid content of maternal serum and amniotic fluid, as well as increased placental bile acid transport.158–160 Most investigators have prescribed a conventional dose (15 mg/kg/day), although one report suggests that a higher dose (20 to 25 mg/kg/day) is more effective.153 Treatment with bile-acid binders such as cholestyramine157 and guar gum also may relieve symptoms,161 but it is important to keep in mind that therapy with these agents worsens steatorrhea and resultant fat-soluble vitamin deficiencies.162 Administration of S-adenosyl-l-methionine (SAMe) to patients with cholestasis of pregnancy has had mixed therapeutic results131,163–165; use in combination with ursodeoxycholic acid may increase its benefit.166 A short course of oral dexamethasone (12 mg/day for 7 days) has been reported to reduce itching and serum bile acid levels in persons with this disorder167 but also was associated with clinical deterioration in one case.168 Sedatives, such as phenobarbital, may relieve itching in cholestasis patients, but may adversely affect the fetus. Exposure to ultraviolet B light has been suggested as therapy in this setting. As in other cholestatic syndromes, no treatment is always and completely effective in women with cholestasis of pregnancy, with the usual exception of delivery.
LIVER DISEASE OF PREECLAMPSIA
Preeclampsia is a disease of unclear etiology that remains difficult to define and, on occasion, to diagnose.169 In broad terms, preeclampsia is a form of pregnancy-related hypertension that is associated with damage and dysfunction of one or more maternal organs, possibly including the liver, that may produce severe, even life-threatening, complications and affect pregnancy outcome. Preeclampsia complicates 3% to 10% of pregnancies, occurring in the second half of pregnancy or the puerperium and most commonly, but not exclusively, in primiparous women or women with multiple gestations.170 Usual criteria for making the diagnosis include a sustained blood pressure of 140/90 mm Hg or greater after the twentieth week of pregnancy in a previously normotensive woman, accompanied by proteinuria (≥300 mg/24 hr), which is approximately equivalent to a urine protein concentration of 30 mg/dL (“1+ dipstick”) on random testing.169 Many patients also are hyperreflexic and have edema.
Liver disease is recognized as a common and potentially ominous complication of preeclampsia. The HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count), first described by Weinstein in 1982,171 is the most usual form of preeclamptic liver disease and is presumed to underlie development of hepatic hematoma, rupture, and infarction in some women with this disorder.172–174 Recent evidence, however, suggests that there are different preeclampsia phenotypes and that HELLP syndrome may be a distinct genetic and clinical entity.175 Although preeclampsia is common in patients with acute fatty liver of pregnancy (AFLP) and may play a role in the pathogenesis of this disorder, AFLP usually is not classified as a preeclamptic liver disease.176
HEMOLYSIS, ELEVATED LIVER ENZYMES, AND LOW PLATELET COUNT (HELLP) SYNDROME
HELLP syndrome is seen in up to 12% of women with severe preeclampsia.177–179 In addition to the diagnostic abnormalities of hemolysis, elevated serum aminotransferase levels, and thrombocytopenia in conjunction with hypertension and proteinuria, patients with typical HELLP syndrome frequently have complaints of chest, epigastric, and right upper quadrant abdominal pain (Table 38-4). These symptoms often are accompanied by nausea, vomiting, headache, and blurred vision in varying combinations. Some pregnant patients, however, may present with an asymptomatic fall in the platelet count during observation for preeclampsia, or initially have no hypertension or proteinuria.180 Other women may complain of malaise, suggesting the diagnosis of a viral syndrome.181 Most affected individuals seek treatment after the twenty-seventh week of gestation, but up to 11% may do so earlier. It is important to note that presentation of HELLP syndrome after delivery, despite absence of signs of preeclampsia at delivery, occurs in up to 30% of cases.177,182
Table 38-4 Clinical Characteristics of Hemolysis, Elevated Liver Enzymes, and Low Platelet Count (HELLP) Syndrome
| Presenting Symptom | Percent Affected |
| Abdominal pain (right upper quadrant; epigastric) | 65 |
| Nausea or vomiting | 36 |
| Headache | 31 |
| Bleeding | 9 |
| Jaundice | 5 |
| Laboratory Test Level (Normal Value) | Median (Range) |
| Serum aspartate aminotransferase (<40 U/L) | 249 (70-633) |
| Serum bilirubin (<1 mg/dL) | 1.5 (0.5-25) |
| Platelets (>125 × 103/mm3) | 57 (7-99) |
| Maternal Complications | Percent Affected |
| Disseminated intravascular coagulation | 21 |
| Abruptio placentae | 16 |
| Acute kidney injury | 8 |
| Hepatic subcapsular hematoma | 1 |
| Death | 1 |
From Sibai BH, Ramadan MK, Usta I, et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. Am J Obstet Gynecol 1993; 169:100-6.
The diagnosis of HELLP syndrome is based on an assessment of the clinical circumstances and features of the illness at the time of presentation; there is no single diagnostic test that confirms the presence of the disorder.179,183 Hemolysis in patients with HELLP is mild. Fragmented red-blood cells (schistocytes) are seen on smears, and the serum lactate dehydrogenase (LDH) level is elevated. Serum aminotransferase levels also are elevated, sometimes minimally and other times greater than 1000 U/L in association with laboratory signs of cholestasis.177,184 Serum bilirubin levels often are elevated, but in most patients to low levels compatible with the finding of hemolysis. Elevated serum levels of glutathione S-transferase alpha,185 d-dimer,186 tissue polypeptide antigen (TPA),187 and fibronectin188 have been described in persons with HELLP syndrome, and these tests may have some use in predicting the presence or severity of liver disease.
Cross-sectional abdominal imaging, especially CT and MRI, may be useful in making the diagnosis of HELLP syndrome and detecting cases of intrahepatic hemorrhage and infarction. Imaging should be performed in patients with complaints of severe abdominal pain, neck or shoulder pain, or a sudden drop in blood pressure. One report documented abnormal findings in 45% of such patients.172
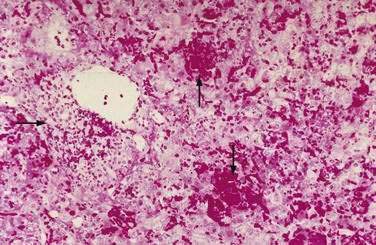
Liver biopsy specimens demonstrate periportal hemorrhage, intrasinusoidal fibrin deposition and irregular areas of liver cell necrosis with mild reactive hepatitis, findings characteristic of preeclampsia (Fig. 38-1). If steatosis is present, it is modest and does not have the appearance of the extensive pericentral microvesicular fat accumulation that occurs in patients with AFLP (discussed later). There is little if any correlation between the severity of liver biopsy lesions and laboratory test abnormalities in patients with HELLP syndrome; mild thrombocytopenia and mild increases in serum aminotransferase levels do not connote insignificant liver damage.189 Liver biopsy, however, rarely is necessary to make a diagnosis in these patients, and possibly may precipitate development of intraparenchymal hepatic hematoma or contained hepatic rupture.
Although most pregnant women with low platelet counts and preeclampsia have HELLP syndrome, the differential diagnosis includes other causes of thrombocytopenia, including immune thrombocytopenic purpura, thrombotic thrombocytopenic purpura,190 and the antiphospholipid antibody syndrome.191 Elevated serum aminotransferase levels in patients with preeclampsia are most frequently misdiagnosed as being caused by viral hepatitis.192 A diagnosis of AFLP also should be considered in patients with clinical findings of HELLP syndrome, but AFLP usually is associated with signs of more significant liver disease and possibly liver failure, albeit with lower serum aminotransferase levels, and is not necessarily associated with thrombocytopenia.
Any hypothesis concerning the pathophysiology of preeclampsia must explain the known characteristics of the disorder, in particular the inappropriately high systemic vascular resistance and low plasma volume seen in affected individuals. Preeclampsia follows, and presumably is a consequence of, an abnormality of placental formation in which a failure of both trophoblast invasion of the uterine lining and dilation of the spiral arteries result in the physiologic inability to increase uteroplacental perfusion appropriately as gestation progresses.193 Female relatives, including the mothers of patients with preeclampsia, often have a history of the disorder, and evidence exists in some populations for its inheritance as either an autosomal recessive trait or an autosomal dominant trait with variable penetrance.175,194,195 Women with a circulating procoagulant, for example factor V Leiden or anticardiolipin antibody, are at risk for developing early and severe preeclampsia.196,197 There is convincing evidence that development of this disorder is mediated by excessive production of soluble fms-like tyrosine kinase 1 (sFlt1), a potent antagonist of vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), and of soluble endoglin (sEng), an inhibitor of capillary formation.198,199 Pregnant rats that overexpress both sEng and sFlt1 develop proteinuria, severe hypertension, laboratory findings of HELLP syndrome, and intrauterine growth restriction.200 Women with preeclampsia similarly produce excessive amounts of sFlt1 and sEng.199 The events responsible for abnormal production of these factors in this setting have yet to be elucidated.
Preeclampsia frequently is diagnosed in patients with AFLP. Although the latter disorder is not formally classified as a hepatic complication of preeclampsia, these two disease entities may share a common pathogenesis. Women with long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency, a cause of defective intramitochondrial beta oxidation of fatty acids, have been reported to develop preeclampsia and HELLP syndrome while pregnant with an affected fetus.201,202 Abnormal mitochondrial fatty-acid metabolism also may play a role in the pathogenesis of AFLP (see following). Other studies, however, have not demonstrated LCHAD deficiency in women with HELLP syndrome.203 Additional hypotheses advanced to explain the pathogenesis of preeclampsia include lipid peroxidation and oxidative stress in response to an unknown primary insult, endothelial dysfunction,188,204 abnormal fluidity of the endothelial cell membrane, abnormal cell permeability to calcium, or inheritance of a molecular variant of angiotensin known to be associated with hypertension.205 Interestingly there is no animal model for the human syndrome of preeclampsia. Furthermore, the histologic characteristics of this condition are unique and are not similar to those of any other known liver disease in humans or animals.
The clinical abnormalities that characterize HELLP syndrome usually resolve rapidly after childbirth.206 Transient postpartum diabetes insipidus has been reported in women with the disorder.207 Rarely, HELLP syndrome becomes gradually worse prior to delivery with subsequent development of postpartum liver failure, sepsis, consumptive coagulopathy, and, rarely, even death.208 In the absence of appropriate supportive therapy and expedited delivery, affected patients may progress to renal failure, hepatic hematoma, and hepatic rupture. Neither serum aminotransferase levels nor platelet counts are predictive of outcome in women with HELLP syndrome.209 The disorder can recur during subsequent pregnancies but usually does not.210,211
Management of HELLP syndrome is primarily supportive; patients should be treated in an intensive care setting prior to delivery, preferably by an obstetrician qualified in the practice of maternal-fetal medicine. Some affected patients may have a decline in serum aminotransferase levels and a rise in platelet counts with supportive care.212 Under such circumstances, a delay in delivery may be appropriate in cases of fetal immaturity, but the fetus usually fails to grow in the setting of preeclampsia. Patients with severe preeclampsia and HELLP syndrome may require antepartum platelet transfusions and hemodialysis. Plasmapheresis after delivery has been advocated by some authorities but has not been proven to alter disease outcome.182,213 Glucocorticoid therapy also has been used in this setting. Many affected women receive glucocorticoids before delivery, not as disease treatment, but to promote fetal lung maturity214; glucocorticoid therapy of HELLP syndrome per se has not been evaluated in a controlled clinical trial.215–217 Orthotopic liver transplantation may be appropriate treatment for some HELLP-syndrome patients,218,219 but early diagnosis and prompt delivery almost always make this and other extreme therapeutic measures unnecessary in these individuals. Full recovery without sequelae is anticipated for the vast majority of affected patients.
HEPATIC RUPTURE, HEMATOMA, AND INFARCT
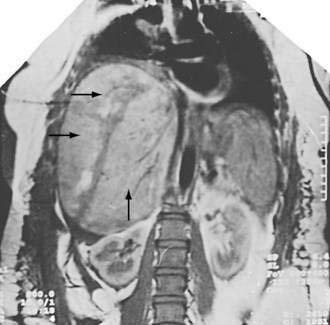
Spontaneous rupture of the liver may complicate preeclampsia and HELLP syndrome, usually in the third trimester of pregnancy close to term or in the early postpartum period. Patients with this often fatal disorder present with abdominal distention and pain, and cardiovascular collapse.220,221 In contrast to other patients with preeclampsia, women with spontaneous hepatic rupture tend to be older and to have had multiple previous pregnancies. Diagnosis is made by signs of liver rupture on ultrasonography, CT, or MRI in conjunction with aspiration of blood on paracentesis.172,222 Imaging studies often show that affected patients have a partially contained subcapsular hematoma (Fig. 38-2).223 Management must be aggressive, with rapid delivery of the fetus by the obstetrician and repair of the liver, preferably by an experienced liver surgeon. Postoperatively, patients have a protracted course that may include disseminated intravascular coagulation and hepatic failure. A few patients with hepatic rupture have undergone orthotopic liver transplantation after emergent hepatectomy and interval portosystemic shunt as a temporizing measure while a donor graft is sought.219,224,225 Rarely, a hemodynamically stable patient with a ruptured liver can be successfully treated without surgery.226 Survivors of hepatic rupture may have uneventful subsequent pregnancies,227 but recurrence of hematoma and rupture also have been reported.228
Some pregnant women with preeclampsia, HELLP syndrome, and abdominal pain have contained subcapsular hematoma. In this circumstance, patients can be observed with serial CT imaging and managed without surgery.172,228 Experts have recommended angiographic embolization of hepatic artery branches supplying blood to the affected portion of the liver in such cases.
Hepatic hematoma and rupture complicating preeclampsia presumably result from extravasation of blood from one or several microscopic areas of periportal hemorrhage under Glisson’s capsule. Periportal hemorrhage is a typical pathologic finding in the livers of patients with preeclampsia and HELLP syndrome.229 The capsule is believed to be stretched and torn away from the surface of the liver by the enlarging hematoma. Ultimately, the capsule ruptures, allowing the liver surface to bleed freely into the peritoneal cavity.
Necrotic hepatic infarcts also may complicate preeclampsia. Affected individuals present with fever, leukocytosis, anemia, and marked elevation of serum aminotransferase levels,172,174,226 and in the most severe cases develop multiorgan failure, including liver failure. Cross-sectional imaging demonstrates confluent hepatic infarcts. Needle aspiration of these areas yields blood and necrotic tissue; immediately adjacent liver parenchyma contains periportal hemorrhage and fibrin deposition typical of preeclampsia and HELLP syndrome. Hepatic infarction sometimes is associated with the presence of a hypercoagulable condition such as factor V Leiden or antiphospholipid antibody.230
ACUTE FATTY LIVER OF PREGNANCY
AFLP is a form of microvesicular fatty liver disease unique to human gestation that presents late in pregnancy, often as fulminant hepatic failure with sudden onset of coagulopathy and encephalopathy in a woman without a prior history of liver disease.231,232 AFLP is diagnosed on the basis of typical clinical and pathologic manifestations in approximately 1 of 6700 third-trimester pregnancies,233 but it also is recognized that subclinical cases exist.234 The pathophysiologic mechanisms underlying development of this disorder are unknown, although at least some patients with AFLP have an inherited LCHAD deficiency that also affects the fetus.235,236
AFLP presents late in pregnancy; in the majority of cases, symptoms develop between 34 and 37 weeks of gestation, although cases beginning as early as 19 to 20 weeks of gestation have been reported. Rarely, the onset of AFLP is after delivery. Initial symptoms usually include nausea and vomiting, often associated with abdominal pain. Pruritus may be an early complaint; overlap with cholestasis of pregnancy has occurred but is rare.237 Patients with AFLP frequently are confused, and have pregnancy-related complications, such as premature labor, vaginal bleeding, and decreased fetal movement. The disorder is most common in primiparous women and in women with multiple gestations.238 Affected individuals have a much greater than expected number of male fetuses (2.7 : 1).239 Of note is the fact that preeclampsia, an accompanying diagnosis in 21% to 64% of cases,233,240 also is associated with first pregnancies, twin pregnancies, and male fetuses.
The course of AFLP is quite variable. Hypoglycemia and hyperammonemia occur and should be suspected when at-risk patients exhibit signs of altered central nervous system function. Other complications of liver failure, including ascites, pleural effusion, acute pancreatitis, respiratory failure, renal failure, and infection may develop in patients with AFLP; vaginal bleeding or post–cesarean section bleeding is common in these individuals. Transient diabetes insipidus sometimes is seen241; more rarely, affected patients have myocardial infarction242 or pulmonary fat emboli.243
Diagnosis of AFLP almost always is based on the appearance of typical clinical features of the disorder, including laboratory test results, during the later stages of pregnancy. Hepatic imaging is not reliable in confirming the presence of AFLP,244 but plays a crucial role in identifying hepatic hematoma, rupture and infarction.
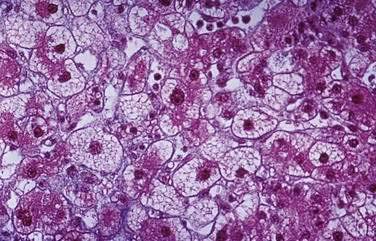
Liver biopsy usually is unnecessary to make the diagnosis, but histologic results may be pathognomonic and therefore useful if the obstetrician has reservations about delivery. However, transjugular sampling of liver tissue may be necessitated by coagulopathy. The histologic hallmark of AFLP is microvesicular fatty infiltration of the liver that is most prominent in hepatocytes surrounding central veins (zone three) and spares those surrounding portal areas (Fig. 38-3). Microvesicular steatosis of this type has a relatively homogeneous appearance on light microscopy and may be difficult to discern on examination of ordinary hematoxylin and eosin–stained specimens. To confirm the diagnosis, special techniques must be used; frozen tissue may be stained for fat with oil-red O, or electron microscopy can be used to examine a glutaraldehyde-fixed specimen. Plans must be made prior to the biopsy for appropriate handling of the liver tissue. Other histologic findings in affected patients can be misleading; they may include lobular disarray suggestive of viral hepatitis and biliary ductular proliferation and inflammation suggestive of cholangitis.234,245 Patients with AFLP do not have the periportal hemorrhage and fibrin deposition seen in the livers of individuals with preeclampsia and HELLP syndrome.
The differential diagnosis in suspected cases of AFLP includes those causes of acute hepatic failure not associated with pregnancy, especially viral hepatitis and toxic liver injury. Uncommon types of viral hepatitis, such as hepatitis E and herpes simplex hepatitis, may be more severe in pregnant than in nonpregnant individuals.246,247 These viral agents can be identified by appropriate serologic tests. A more difficult problem is distinguishing AFLP from other liver diseases that complicate pregnancy, particularly the preeclamptic liver diseases, namely HELLP syndrome and hemorrhagic or ischemic liver injury. For example, patients with AFLP may develop preeclampsia and disseminated intravascular coagulation with attendant thrombocytopenia, thereby meeting the diagnostic criteria for HELLP syndrome. Fortunately it is not usually necessary to distinguish among these various diagnoses because AFLP, HELLP syndrome, and preeclampsia are treated by expedited delivery of the infant. It is, however, of crucial importance to recognize hepatic hematoma and rupture rapidly.
The pathogenesis of AFLP, like that of preeclampsia, has not been elucidated. Initially AFLP was thought to be caused by exposure to a toxin; microvesicular steatosis of the liver is known, for example, to be caused by treatment with sodium valproate or intravenous tetracycline. Despite an intensive search, however, no toxin that might be responsible for development of AFLP has been identified. Because of the coincidental occurrence of preeclampsia and AFLP in many patients, the disorder has been considered by some experts to be a severe form of preeclamptic liver disease.234,248,249 Arguing against this conclusion are the absence of the usual histologic features of preeclampsia in liver biopsy specimens from patients with AFLP and the absence of the usual clinical features of preeclampsia in many patients with AFLP.
There is a well-established association between AFLP and inherited defects in beta oxidation of fatty acids.202,236,250 This connection is empirically supported by similar clinical and histologic findings in patients with AFLP and those with Jamaican vomiting sickness, a liver disease caused by a toxin in unripe akee fruit that disables intramitochondrial beta oxidation of fatty acids. Maternal liver disease (HELLP or AFLP) has been reported in 62% of the mothers of infants with defects of fatty-acid oxidation.236 AFLP may develop regardless of maternal genotype if the fetus is deficient in LCHAD and carries at least one allele for the G1528C LCHAD mutation.235 Another beta oxidation defect, carnitine palmitoyltransferase I deficiency, also has been associated with AFLP.251 Prenatal genetic diagnosis based on chorionic villus sampling has proved to be feasible and accurate in pregnant members of affected families.252,253 Not every investigator, however, has been able to confirm the association between AFLP and beta oxidation defects,254 and other as yet unknown mechanisms may play a role in the pathogenesis of this disorder.
Patients with AFLP should be managed in an intensive care setting, preferably by obstetricians qualified in the practice of maternal-fetal medicine in cooperation with other appropriate specialists. Early diagnosis and prompt delivery of the infant are imperative to minimize maternal and fetal morbidity and mortality. Affected individuals may be very ill post-partum until the physiologic defects responsible for their clinical abnormalities resolve and the livers recover. Supportive care may include infusion of blood products, mechanical ventilation, hemodialysis, and antibiotic therapy. Hepatic encephalopathy is treated as indicated by measures intended to evacuate feces and bacteria from the colon. Infusion of concentrated glucose solution may be required to treat or prevent hypoglycemia. Although many patients with AFLP have disseminated intravascular coagulation and depressed antithrombin III levels, treatment with heparin or antithrombin III is not recommended.255 Patients with diabetes insipidus may be managed with 1-deamino-8-d-arginine-vasopressin (DDAVP).241 Some individuals with liver failure secondary to AFLP require emergency orthotopic liver transplantation as a potentially life-saving measure.256,257 Most affected women, however, recover completely with appropriate supportive care. Persistent or even increasing hyperbilirubinemia and multiple complications after delivery do not necessarily indicate the need for liver transplantation.
Survival of patients with AFLP has been reported to be 100% with prompt diagnosis, delivery of the infant, and intensive care.233,240,258 Infants of affected women have perinatal mortality rates of less than 7%; the surviving baby may have LCHAD deficiency and develop nonketotic hypoglycemia and obtundation. Recurrence of AFLP has been documented, particularly in women with LCHAD deficiency.259,260 In cases of AFLP the mother, father, and child should be tested for the G1528C LCHAD mutation.235
USUAL HEPATIC DISORDERS AND PREGNANCY
VIRAL HEPATITIS
Hepatitis E Virus (see Chapter 80)
Hepatitis E virus infection occurs in nonindustrialized nations, usually as an epidemic disease during the monsoon season in central and south Asia and India. Hepatitis E is rare in the West. Cases have been reported in travelers returning to the United States from endemic areas, particularly Mexico. Acute hepatitis E during the third trimester of pregnancy is a cause of fulminant hepatic failure and has a mortality rate of up to 20%.181 Maternal hepatitis E virus infection also has been associated with intrauterine fetal death.261,262 The risks of intrauterine death and abortion in any trimester are greater in pregnant women with hepatitis E than they are in their uninfected counterparts. Maternal-fetal transmission of hepatitis E resulting in symptomatic neonatal hepatitis has occurred263; no known therapy prevents vertical transmission of this virus. Pregnant women should avoid traveling to endemic areas during monsoon season and outbreaks of the disease.
Herpes Simplex Virus (see Chapter 81)
Subclinical hepatitis associated with primary herpes simplex virus infection is common. In pregnant or immunosuppressed individuals, this virus may cause severe liver disease. Infection during pregnancy, particularly the third trimester, can result in fulminant hepatic failure.247 Affected individuals are obtunded and usually anicteric with elevated serum aminotransferase levels and coagulopathy. They may have subtle oropharyngeal or genital herpetic lesions. Encephalopathy may result from herpes encephalitis. The diagnosis of herpes simplex virus infection can be confirmed by serologic testing. Liver biopsy specimens from affected patients usually demonstrate characteristic intracytoplasmic inclusion bodies and areas of focal hemorrhage. Treatment with acyclovir is effective and appears to prevent viral transmission to the fetus.247
Hepatitis B and D Viruses (see Chapter 78)
As a rule, maternal hepatitis B virus infection does not influence the course of pregnancy, nor does pregnancy alter the natural history of maternal hepatitis B infection. Most women of childbearing age with chronic hepatitis B are healthy virus carriers with a very low risk of developing complications of their disease. The importance of hepatitis B during pregnancy is related to its role in the perpetuation of chronic infection through vertical transmission: maternal-fetal transmission of hepatitis B virus is responsible for most cases of chronic hepatitis B worldwide, especially in Southeast Asia and Africa.264 Mothers with a reactive serum test for hepatitis B e antigen have more circulating virus and higher rates of perinatal transmission than do mothers who have undetectable serum hepatitis B e antigen and a reactive serum test for hepatitis B e antibody,265 although the latter individuals can still be a source of neonatal infection.266 Without treatment, 90% of infants born to hepatitis B e antigen–positive mothers and 10% of infants born to hepatitis B e antigen–negative mothers develop hepatitis B virus infection. The infants of mothers with a reactive serum test for hepatitis B surface antigen should receive hepatitis B immunoglobulin at birth and hepatitis B vaccine during the first day of life and at ages one and six months.267
Women with chronic hepatitis B are not treated with interferon during pregnancy.268 There is a paucity of data on the use of oral agents in this setting; treatment of hepatitis B with nucleotide and nucleoside analogs generally is guided by experience in pregnant patients with human immunodeficiency virus (HIV) infection.269 Telbivudine and tenofovir are pregnancy category B drugs and are therefore preferred. Lamivudine is a pregnancy category C drug, but is thought to be associated with a low risk of complications270 and has been reported to reduce the incidence of neonatal vaccination failure.271 Data concerning the safety of entecavir and adefovir in pregnant women classified as pregnancy category C drugs are insufficient to allow any conclusions.
Hepatitis D virus infection requires simultaneous acute or chronic hepatitis B virus infection. There is no evidence that pregnancy changes the natural course of hepatitis D. Prevention of vertical transmission of hepatitis D is best accomplished by vaccination of the mother against infection with hepatitis B virus, or appropriate therapy of existing maternal hepatitis B prior to pregnancy in conjunction with vaccination and administration of immunoglobulin to the infant. A case report has documented prevention of vertical transmission of hepatitis B and D viruses by this management.272
Hepatitis C Virus (see Chapter 79)
Chronic hepatitis C virus infection does not appear to affect the outcome of pregnancy,273 and there are no convincing data to suggest that pregnancy alters the natural history of hepatitis C infection. Vertical transmission of hepatitis C virus is uncommon274,275 unless maternal serum virus titers are unusually high, as sometimes occurs in patients with HIV coinfection.276 Serum levels of hepatitis C virus ribonucleic acid (RNA) greater than or equal to 1019 copies per mL have been associated with vertical transmission in as many as 36% of cases.277 The incidence of perinatal infection does not seem to be related to whether the baby is delivered vaginally or by cesarean section.277 Although hepatitis C viral RNA can be detected in breast milk,278 breast-feeding is not considered to be a risk factor for neonatal infection.279 Vertical transmission of hepatitis C is not prevented by treatment of the infant with immunoglobulin.272 Women with hepatitis C are not treated with interferon and ribavirin during pregnancy; ribavirin is a well established teratogen (pregnancy category X).
WILSON DISEASE (see Chapter 75)
Wilson disease in women of childbearing age is associated with amenorrhea and infertility. Treatment of affected individuals to remove excess copper may result in resumption of ovulatory cycles and a subsequent pregnancy. Pregnant patients must remain on medication to treat Wilson disease because discontinuation of therapy can cause sudden copper release, hemolysis, acute liver failure, and death.280 D-penicillamine is potentially teratogenic in humans,281 but has been used safely during pregnancy at doses necessary for copper chelation.282 Similarly, trientine is teratogenic in animals but appears to be safe in humans as treatment for copper overload. Zinc is not teratogenic, and some experts favor its use during pregnancy as therapy for Wilson disease for this reason.283
AUTOIMMUNE LIVER DISEASES (see Chapters 88 and 89)
Primary biliary cirrhosis is much more common in postmenopausal women than it is in their fertile counterparts. Pregnant women with primary biliary cirrhosis may experience an exacerbation of pruritus284 that can be ameliorated by treatment with ursodeoxycholic acid,285 although the safety of this therapy during pregnancy has not been formally proven.
HEPATIC NEOPLASIA AND MASS LESIONS (see Chapter 94)
Hepatocellular carcinoma occurs almost exclusively in persons with chronic liver disease and may present in the absence of cirrhosis in young people with chronic hepatitis B virus infection. At-risk patients should have standard screening for liver cancer during pregnancy. It must be borne in mind that maternal serum alpha-fetoprotein levels always are modestly elevated during normal pregnancy,286 and can further increase in cases of fetal Down syndrome, neural tube defects, and hydatidiform mole, thus limiting the positive predictive value for diagnosing hepatocellular carcinoma.
Hepatic fibrolamellar carcinoma has been reported to occur in pregnant women.287 Fibrolamellar carcinoma is a slow-growing cancer usually found in young adults; the median age of affected persons is 25 years.288 Unlike typical primary liver cancer, this neoplasm has no known association with cirrhosis or chronic liver disease and is not a cause of increased serum alpha-fetoprotein levels.
HEPATIC VEIN THROMBOSIS (BUDD-CHIARI SYNDROME) (see Chapter 83)
Pregnancy is a predisposing factor for the development of venous thrombosis. Hepatic vein thrombosis may occur in association with HELLP syndrome289 and with preeclampsia in women who have antiphospholipid antibody.290 Pregnant women who develop hepatic vein thrombosis should be evaluated for the presence of antiphospholipid antibody and other circulating procoagulants, for example factor V Leiden.291
PREGNANCY AFTER LIVER TRANSPLANTATION
After successful orthotopic liver transplantation, women of childbearing age may become pregnant and deliver normal infants.103,292 Delaying pregnancy until the second post-transplant year may be associated with a lower risk of prematurity. Transplant patients should continue immunosuppressive therapy during gestation; treatment regimens used to prevent graft rejection have not been associated with teratogenicity. Adverse effects of these medications, however, including hypertension and hyperglycemia, may increase the incidence of fetal distress and preeclampsia in liver transplant recipients who are pregnant. In rare instances, pregnancy has been complicated by organ rejection.
ACOG Committee on Obstetric Practice. Guidelines for diagnostic imaging during pregnancy. Committee opinion no: 299. Obstet Gynecol. 2004;104:647-51. (Ref 37.)
ACOG Committee on Practice Bulletins—Obstetrics. Diagnosis and management of preeclampsia and eclampsia. No.: 33. Obstet Gynecol. 2002;99:159-67. (Ref 169.)
Francella A, Dyan A, Bodian C, et al. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: A retrospective cohort study. Gastroenterology. 2003;124:9-17. (Ref 104.)
Kanal E. Pregnancy and safety of magnetic resonance imaging. Magn Reson Imaging Clin N Am. 1994;2:309-17. (Ref 39.)
Keely E, Barbour LA, Lee RV, editors. Medical care of the pregnant patient, 2nd ed, Philadelphia: ACP Press, 2008. Chapter 3. (Ref 28.)
Lammert F, Marschall HU, Glantz A, et al. Intrahepatic cholestasis of pregnancy: Molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33:1012-21. (Ref 129.)
Mahadevan U. American Gastroenterological Association Institute technical review on the use of gastrointestinal medication in pregnancy. Gastroenterology. 2006;131:283-311. (Ref 268.)
Mahadevan U, Sandborn WJ, Li DK, et al. Pregnancy outcomes in women with inflammatory bowel disease: A large community-based study from Northern California. Gastroenterology. 2007;133:1106-12. (Ref 98.)
McKay DB, Josephson MA. Pregnancy in recipients of solid organs—Effects on mother and child. N Engl J Med. 2006;354:1281-93. (Ref 103.)
Medical radiation exposure of pregnant and potentially pregnant women. Bethesda (MD): National Council on Radiation Protection & Measurements; 1977. Report No: 54. (Ref 36.)
Mofenson LM, Centers for Disease Control and PreventionU.S. Public Health Service Task Force. Recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. MMWR Recom Rep. 2002;51(RR-18):1-38. (Ref 269.)
Nikfar S, Abdollahi M, Moretti ME, et al. Use of proton pump inhibitors during pregnancy and rates of major malformations: A meta-analysis. Dig Dis Sci. 2002;47:1526-9. (Ref 74.)
Qureshi WA, Rajan E, Adler DG, et al. ASGE guideline: Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc. 2005;61:357-362. (Ref 30.)
1. Ulmsten U, Sundstrom G. Esophageal manometry in pregnant and non-pregnant women. Am J Obstet Gynecol. 1978;132:260-4.
2. Van Thiel DH, Gavalier JS, Joshi SN, et al. Heartburn of pregnancy. Gastroenterology. 1977;72:666-8.
3. Fisher RS, Robert GS, Grabowoski CJ, et al. Altered lower esophageal sphincter function during early pregnancy. Gastroenterology. 1978;74:1233-7.
4. Fisher RS, Robert GS, Grabowski CJ, Cohen S. Inhibition of lower esophageal sphincter circular smooth muscle by female sex hormone. Am J Physiol. 1978;234:243-7.
5. Marrero JM, Goggin PM, de Caestecker JS, et al. Determinants of pregnancy heartburn. Br J Obstet Gynaecol. 1992;99:731-4.
6. Davison JS, Davison MC, Hay DM. Gastric emptying time in late pregnancy and labour. J Obstet Gynaecol Br Commonw. 1970;77:37-41.
7. Chiloiro M, Darconza G, Piccioli E, et al. Gastric empting and orocecal transit time in pregnancy. J Gastroenterol. 2001;36:538-43.
8. Waldum HL, Straume BK, Lundgren R. Serum group I pepsinogens during pregnancy. Scand J. Gastroenterology. 1980;15:61-3.
9. Lawson M, Kern FJr, Everson GT. Gastrointestinal transit time in human pregnancy: prolongation in the second and third trimesters followed by postpartum normalization. Gastroenterology. 1986;89:996-9.
10. Wald A, Van Thiel DH, Hoechstetter L, et al. Effect of pregnancy on gastrointestinal transit. Dig Dis Sci. 1982;27:1015-18.
11. Xiao L, Pricolo V, Biancani P, Behar J. Role of progesterone signaling in the regulation of G-protein levels in female chronic constipation. Gastroentrology. 2005;128:667-75.
12. Iwasaki H, Collins JG, Saito Y, et al. Naloxone-sensitive, pregnancy-induced changes in behavioral responses to colorectal distension: pregnancy-induced analgesia to visceral stimulation. Anesthesiology. 1991;74:927-33.
13. Harvey J, Dainty JR, Hollands WJ, et al. Effect of high dose iron supplements on fractionate zinc absorption and status in pregnant women. Am J Clin Nutr. 2007;85:131-6.
14. Millard KN, Frazer DM, Wilkins SJ, Anderson GJ. Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat. Gut. 2004;53:655-60.
15. Brown J, Robertson J, Gallagher N. Humoral regulation of vitamin B12 absorption by pregnant mouse small intestine. Gastroenterology. 1977;72:881-8.
16. Dugas MC, Hazelwood RC, Lawrence AL. Influence of pregnancy and-or exercise on intestinal transport of amino acids in rats. Proc Soc Exp Biol Med. 1970;135:127-31.
17. Burdett K, Reek C. Adaptation of the small intestine during pregnancy and lactation in the rat. Biochem J. 1979;184:245-51.
18. Prieto RM, Ferrer M, Fe JM, et al. Morphological adaptive changes of small intestinal tract regions due to pregnancy and lactation in rats. Ann Nutr Metab. 1994;38:295-300.
19. Cripps AW, Williams VJ. The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. Br J Nutr. 1975;33:17-32.
20. Elias E, Dowling RH. The mechanism for small bowel adaptation in lactating rats. Clin Sci Mol Med. 1976;51:427-33.
21. Koch CA, Platt JL. T cell recognition and immunity in the fetus and mother. Cell Immunol. 2007;248:7-12.
22. Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol. 2008;77:14-22.
23. Valdivieso V, Covarrubias C, Siegel F, Cruz F. Pregnancy and cholelithiasis: pathogenesis and natural course of gallstones diagnosed in early puerperium. Hepatology. 1993;17:1-4.
24. Kline LW, Karpinski E. Progesterone inhibits gallbladder motility through multiple signaling pathways. Steroids. 2005;70:673-9.
25. Ko CW, Beresford SA, Schulte SJ, et al. Incidence, natural history, and risk factors for biliary sludge and stone during pregnancy. Hepatology. 2005;41:359-65.
26. Ueland K, Novy MJ, Metcalfe J. Cardiorespiratory responses to pregnancy and exercise in normal women and patients with heart disease. Am J Obstet Gynecol. 1973;115:4-10.
27. Maher JE, Goldenberg RL, Tamura T, et al. Albumin levels in pregnancy: A hypothesis—decreased levels of albumin are related to increased levels of alpha-fetoprotein. Early Hum Devel. 1993;34:209-15.
28. Powrie RO. Principles for drug prescribing in pregnancy. In Rosene-Montella K, Keely E, Barbour LA, Lee RV, editors: Medical care of the pregnant patient, 2nd ed, Philadelphia: ACP Press, 2008.
29. Cappell MS. The fetal safety and clinical efficacy of gastrointestinal endoscopy during pregnancy. Gastroenterol Clin North Am. 2003;32:123-79.
30. Qureshi WA, Rajan E, Adler DG, et al. ASGE guideline: Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc. 2005;61:357-62.
31. Jamidar PA, Beck GJ, Hofmann BJ, et al. Endoscopic retrograde cholangiopancreatography in pregnancy. Am J Gastroenterol. 1995;90:1263-7.
32. Cappell MS. Sedation and analgesia for gastrointestinal endoscopy during pregnancy. Gastrointest Endosc Clin North Am. 2006;16:1-31.
33. Safra MJ, Oakley GP. Association between cleft lip with or without cleft palate and prenatal exposure to diazepam. Lancet. 1975;2:478-80.
34. Dolovich LR, Addis A, Vaillancourt JM, et al. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ. 1998;317:839-43.
35. Cox PB, Marcus MA, Bos H. Pharmacological considerations during pregnancy. Curr Opin Anaesthesiol. 2001;14:311-16.
36. Medical radiation exposure of pregnant and potentially pregnant women. Bethesda (MD): National Council on Radiation Protection & Measurements; 1977. Report No: 54
37. ACOG Committee on Obstetric Practice. Guidelines for diagnostic imaging during pregnancy. Committee opinion no: 299. Obstet Gynecol. 2004;104:647-51.
38. Felmlee JP, Gray JE, Leetzow ML, Price JC. Estimated fetal radiation dose from multislice CT studies. AJR Am J Roentgenol. 1990;154:185-90.
39. Kanal E. Pregnacy and safety of magnetic resonance imaging. Magn Reson Imaging Clin North Am. 1994;2:309-17.
40. Gadsby R, Barnie-Adshead AM, Jagger C. A prospective study of nausea and vomiting during pregnancy. Br J Gen Pract. 1993;43:245-8.
41. Dodds L, Fell DB, Joseph KS, et al. Outcomes of pregnancies complicated by hyperemesis gravidarum. Obset Gynecol. 2006;107:285-92.
42. Attard CL, Kholi MA, Coleman S, et al. The burden of illness of severe nausea and vomiting of pregnancy in the United States. Am J Obstet Gynecol. 2002;186:220-7.
43. Bashiri A, Neumann L, Maymon E, et al. Hyperemesis gravidarum: Epidemiologic features, complications and outcome. Eur J Obstet Gynecol Reprod Biol. 1995;63:135-8.
44. Frigo P, Lang C, Reisenberger K, et al. Hyperemesis gravidarum associated with Helicobacter pylori seropositivity. Obstet Gynecol. 1998;91:615-7.
45. Goodwin TM. Nausea and vomiting of pregnancy: An obstetric syndrome. Am J Obstet Gynecol. 2002;186:184-9.
46. Verberg MFG, Gillott DJ, Al-Fardan N, et al. Hyperemesis gravidarum, a literature review. Human Reprod Update. 2005;11:527-39.
47. Depue RH, Bernstein L, Ross RK, et al. Hyperemesis gravidarum in relation to estradiol levels, pregnancy outcome, and other maternal factors: A seroepidemiologic study. Am J Obstet Gynecol. 1987;156:1137-41.
48. Walsh JW, Hasler WL, Nugent CE, Owyang C. Progesterone and estrogen are potential mediators of gastric slow wave dysrhythmias in nausea of pregnancy. Am J Physiol. 1996;270:G506-14.
49. Aka N, Atalay S, Sayharman S, et al. Leptin and leptin receptor levels in pregnant women with hyperemesis gravidarum. Aust N Z J Obstet Gynecol. 2006;46:274-7.
50. Goowin TM, Montero M, Mestman JH. Transient hyperthyroidism and hyperemesis gravidarum: clinical aspects. Am J Obstet Gynecol. 1992;167:638-52.
51. Pekary AE, Jackson IM, Goodwin TM, et al. Increased in vitro thyrotropic activity of partially sialated human chorionic gonadotropin extracted from hydatidiform moles of patients with hyperthyroidism. J Clin Endocrinol Metab. 1993;76:70-4.
52. Kocak I, Akcan Y, Ustun C, et al. Helicobacter pylori seropositivity in patients with hyperemesis gravidarum. Int J Gynecol Obstet. 1999;66:251-4.
53. Karadeniz RS, Ozdegirmenci O, Altay MM, et al. Helicobacter pylori seropositivity and stool antigen in patients with hyperemesis gravidarum. Infect Dis Obstet Gynecol. 2006;2:1-3.
54. Jacoby EB, Porter KB. Helicobacter pylori infection and persistent hyperemesis gravidarum. Am J Perinatol. 1999;16:85-8.
55. Koren G, Piwko C, Ahn E, et al. Validation studies of the pregnancy unique quantification of emesis (PUQE) scores. J Obstet Gynaecol. 2005;25:241-4.
56. Robertson C, Miller H. Hyperamylasemia in bulimia nervosa and hyperemesis gravidarum. Int J Eat Disord. 1999;26:223-7.
57. Kuscu NK, Koyuncu F. Hyperemesis gravidarum: Current concepts and management. Postgrad Med J. 2002;78:76-9.
58. Mazzotta P, Stewart DE, Koren G, et al. Factors associated with elective termination of pregnancy among Canadian and American women with nausea and vomiting of pregnancy. J Psychosom Obstet Gynaecol. 2001;22:7-12.
59. Kaiser LL, Allen L. Position of the American Dietetic Association: Nutrition and lifestyle for a healthy pregnancy outcome. J Am Diet Assoc. 2002;102:1479-90.
60. Bsat FA, Hoffman DE, Seubert DE. Comparison of three outpatient regimens in the management of nausea and vomiting in pregnancy. J Perinatol. 2003;23:531-5.
61. Milkovich L, van den Berg BJ. An evaluation of the teratogenicity of certain antinauseant drugs. Am J Obstet Gynecol. 1976;125:244-8.
62. Seto A, Einarson T, Koren G. Pregnancy outcome following first trimester exposure to antihistamines—a meta-analysis. Am J Perinatol. 1997;14:119-24.
63. Einarson A, Malatepe C, Navioz Y. The safety of ondansetron for nausea and vomiting of pregnancy: A prospective comparative study. Br J Obstet Gynecol. 2004;111:940-3.
64. Russo-Stieglitz KE, Levine AB, Wagner BA, et al. Pregnancy outcome in patients requiring parenteral nutrition. J Matern Fetal Med. 1999;8:164-7.
65. Serrano P, Velloso A, Garcia-Luna PP, et al. Enteral nutrition by percutaneous endoscopic gastrojejunostomy in severe hyperemesis gravidarum: A report of two cases. Clin Nutr. 1998;17:135-9.
66. Rey E, Rodriguez-Artalejo F, Herraiz MA, et al. Gastroesophageal reflux symptoms during and after pregnancy: A longitudinal study. Am J Gastroenterol. 2007;102:2395-400.
67. Richter JE. Gastroesphageal reflux during pregnancy. Gastroenterol Clin North Am. 2003;32:235-61.
68. Cappell MS. Clinical presentation, diagnosis, and management of gastroesophageal reflux disease. Med Clin North Am. 2005;89:243-91.
69. Bainbridge ET, Temple JG, Nicholas SP, et al. Symptomatic gastro-esophageal reflux in pregnancy. A comparative study of white European and Asian in Birmingham. Br J Clin Pract. 1983;37:53-7.
70. Cappell MS, Colon V, Sidhom OA. A study of eight medical centers of the safety and clinical efficacy of esophagogastroduodenoscopy in 83 pregnant females with follow-up of fetal outcome with comparison to control groups. Am J Gastroenterol. 1996;91:348-54.
71. Ranchet G, Gangemi O, Petrone M. Sucralfate in the treatment of gravid pyrosis. G Ital Obstet Ginecol. 1990;12:1-16.
72. Lindow SW, Regnell P, Sykes J, et al. An open-label multicenter study to assess the safety and efficacy of a novel reflux supplement (Gaviscon advance) in the treatment of heartburn in pregnancy. Int J Clin Pract. 2003;57:175-9.
73. Larson JD, Patatanian E, Miner PB, et al. Double-blind, placebo-controlled study of ranitidine for gastroesophageal reflux symptoms during pregnancy. Obstet Gynecol. 1997;90:83-7.
74. Nikfar S, Abdollahi M, Moretti ME, et al. Use of proton pump inhibitors during pregnancy and rates of major malformations: A meta-analysis. Dig Dis Sci. 2002;47:1526-9.
75. Clark DH. Peptic ulcer in women. BMJ. 1953;1:1254-7.
76. Cappell MS. Gastric and duodenal ulcers during pregnancy. Gastroenterol Clin North Am. 2003;32:263-8.
77. Dicke JM, Johnson RF, Henderson GI, et al. A comparative evaluation of the transport of H2-receptor antagonists by the human and baboon placenta. Am J Med Sci. 1988;295:198-206.
78. Rayburn W, Liles E, Christensen H, et al. Antacids vs. antacids plus non-prescription ranitidine for heartburn during pregnancy. Int J Gynaecol Obstet. 1999;66:35-7.
79. Larson JD, Patatanian E, Miner PBJr, et al. Double-blind, placebo-controlled study of ranitidine for gastroesophageal reflux symptoms during pregnancy. Obstet Gynecol. 1997;90:83-7.
80. Magee LA, Inocencion G, Kamboj L, et al. Safety of first trimester exposure to histamine H2 blockers. A prospective cohort study. Dig Dis Sci. 1996;41:1145-9.
81. Garland CF, Lilienfeld AM, Mendeloff AI, et al. Incidence rates of ulcerative colitis and Crohn’s disease in fifteen areas of the United States. Gastroenterology. 1981;81:1115-24.
82. Calkins BM, Mendeloff AI. Epidemiology of inflammatory bowel disease. Epidemiol Rev. 1986;80:60-91.
83. Mayberry JF, Waterman IT. European survey of fertility and pregnancy in women with Crohn’s disease: A case control study by European collaborative group. Gut. 1986;27:821-5.
84. Dubinsky M, Abraham B, Mahadevan U. Management of the pregnant IBD patient. Inflamm Bowel Dis. 2008;14:1736-50.
85. Mahadevan U. Fertility and pregnancy in the patient with inflammatory bowel disease. Gut. 2006;55:1198-206.
86. Hudson M, Flett G, Sinclair TS, et al. Fertility and pregnancy in inflammatory bowel disease. Int J Gynaecol Obstet. 1997;58:229-37.
87. Olsen KO, Joelsson M, Laurberg S, et al. Fertility after ileal pouch–anal anastomosis in women with ulcerative colitis. Br J Surgery. 1999;86:493-5.
88. Waljee A, Waljee J, Morris AM, Higgins PD. Threefold increased risk of infertility: A meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis. Gut. 2006;55:1575-80.
89. Heetun ZS, Byrnes C, Neary P, O’Morain C. Review article: reproduction in the patient with inflammatory bowel disease. Aliment Pharmacol Ther. 2007;26:513-33.
90. Hanan IM. Inflammatory bowel disease in the pregnant woman. Compr Ther. 1998;24:409-14.
91. Korelitz BI. Pregnancy, fertility and inflammatory bowel disease. Am J Gastroenterol. 1985;80:365-70.
92. Alstead EM. Inflammatory bowel disease in pregnancy. Postgrad Med J. 2002;78:23-6.
93. Calkins BM, Mendeloff AI. Epidemiology of inflammatory bowel disease. Epidemiol Rev. 1986;80:60-91.
94. Fedorkow DM, Persaud D, Nimrod CA. Inflammatory bowel disease: a controlled study of late pregnancy outcome. Am J Obstet Gynecol. 1989;160:998-1001.
95. Hill JA, Clark A, Scott NA. Surgical treatment of acute manifestations of Crohn’s disease during pregnancy. J R Soc Med. 1997;90:64-6.
96. Ooi BS, Remzi FH, Fazio VW. Turnbull-Blowhole colostomy for toxic ulcerative colitis in pregnancy: Report of two cases. Dis Colon Rectum. 2003;46:111-15.
97. Haq AI, Sahai A, Hallwoth S, et al. Synchronous colectomy and cesarean section for fulminant ulcerative colitis: Case report and review of literature. Int J Colorectal Dis. 2006;21:465-9.
98. Mahadevan U, Sandborn WJ, Li DK, et al. Pregnancy outcomes in women with inflammatory bowel disease: A large community-based study from Northern California. Gastroenterology. 2007;133:1106-12.
99. Cornish C, Tan E, Teare J, Teoh TG. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2007;56:830-7.
100. Dominitz JA, Young JC, Boyko EJ. Outcomes of infants born to mothers with inflammatory bowel disease: A population-based cohort study. Am J Gastroenterol. 2002;97:641-8.
101. Diav-Citrin O, Park YH, Veerasuntharam G, et al. The safety of mesalamine in human pregnancy: A prospective controlled cohort study. Gastroenterology. 1998;114:23-8.
102. Mogadam M, Dobbins WO, Korelitz BI, Ahmed SW. Pregnancy in inflammatory bowel disease: effect of sulfasalazine and corticosteroids on fetal outcome. Gastroenterolgy. 1981;80:72-6.
103. McKay DB, Josephson MA. Pregnancy in recipients of solid organs—Effects on mother and child. N Engl J Med. 2006;354:1281-93.
104. Francella A, Dyan A, Bodian C, et al. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: A retrospective cohort study. Gastroenterology. 2003;124:9-17.
105. Kane S. Inflammatory bowel disease in pregnancy. Gastroenterol Clin North Am. 2003;32:323-40.
106. Muirhead N, Sabharwal AR, Rieder MJ, et al. The outcome of pregnancy following renal transplantation—The experience of a single center. Transplantation. 1992;54:429-32.
107. Anderson GG, Rotchell Y, Kaiser DG. Placental transfer of methylprednisolone following maternal intravenous administration. Am J Obstet Gynecol. 1981;140:699-701.
108. Beaulieu DB, Ananthakrishnan AN, Issa M, et al. Budesonide induction and maintenance therapy for Crohn’s disease during pregnancy. Inflamm Bowel Dis. 2009;15:25-8.
109. Vasiliauskas EA, Church JA, Silverman N, et al. Case report: evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol. 2006;4:1255-8.
110. Katz JA, Antoni C, Keenan GF, et al. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn’s disease and rheumatoid arthritis. Am J Gastroenterol. 2004;99:2385-92.
111. Horowitz MD, Gomez GA, Santiesteban R, et al. Acute appendicitis during pregnancy. Diagnosis and management. Arch Surg. 1985;120:1362-7.
112. Castro AM, Shipp TD, Castro EE, et al. The use of helical computed tomography in pregnancy for the diagnosis of acute appendicitis. Am J Obstet Gynecol. 2001;184:954-7.
113. Cunningham FG, McCubbin JH. Appendicitis complicating pregnancy. Obstet Gynecol. 1975;45:415-20.
114. Hoshino T, Ihara Y, Suzuki T. Appendicitis during pregnancy. Int J Gynaecol Obstet. 2000;69:271-3.
115. Barloon TJ, Brown BP, Abu-Yousef MM, et al. Sonography of acute appendicitis in pregnancy. Abdom Imaging. 1995;20:149-51.
116. Lyass S, Pikarsky A, Eisenbert VH, et al. Is laparoscopic appendectomy safe in pregnant women? Surg Endosc. 2001;15:377-9.
117. de Perrot M, Jenny A, Morales M, et al. Laparoscopic appendectomy during pregnancy. Surg Laparosc Endosc Percutan Tech. 2000;10:368-71.
118. Tracey M, Fletcher HS. Appendicitis in pregnancy. Am Surg. 2000;66:555-9.
119. Valdivieso V, Covarrubias C, Siegel F, et al. Pregnancy and cholelithiasis: pathogenesis and natural course of gallstones diagnosed in early puerperium. Hepatology. 1993;17:1-4.
120. Dixon NP, Faddis DH, Silberman H. Aggressive management of cholecystitis during pregnancy. Am J Surg. 1987;154:292-4.
121. Daradkeh S, Sumrein I, Daoud F, et al. Management of gallbladder stones during pregnancy: Conservative treatment or laparoscopic cholecystectomy? Hepatogastroenterology. 1999;46:3074-6.
122. Sungler P, Heinerman PM, Steiner H, et al. Laparoscopic cholecystectomy and interventional endoscopy for gallstone complications during pregnancy. Surg Endosc. 2000;14:267-71.
123. Graham G, Baxi L, Tharakan T. Laparoscopic cholecystectomy during pregnancy: a case series and review of the literature. Obstet Gynecol Surg. 1998;53:566-74.
124. Glasgow RE, Visser BC, Harris HW, et al. Changing management of gallstone disease during pregnancy. Surg Endosc. 1998;12:241-46.
125. Nesbitt TH, Kay HH, McCoy MC, et al. Endoscopic management of biliary disease during pregnancy. Obstet Gynecol. 1996;87:806-9.
126. Wilkinson EJ. Acute pancreatitis in pregnancy: A review of 98 cases and a report of 8 new cases. Obstet Gynecol Surg. 1973;28:281-303.
127. Ramin KD, Ramin SM, Richey SD, et al. Acute pancreatitis in pregnancy. Am J Obstet Gynecol. 1995;173:187-91.
128. Achard JM, Westeel PF, Moriniere P, et al. Pancreatitis related to severe acute hypertriglyceridemia during pregnancy: Treatment with lipoprotein apheresis. Intensive Care Med. 1991;17:236-7.
129. Lammert F, Marschall HU, Glantz A, et al. Intrahepatic cholestasis of pregnancy: molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33:1012-21.
130. Reyes H. The enigma of intrahepatic cholestasis of pregnancy: Lessons from Chile. Hepatology. 1982;2:87-96.
131. Davies M, da Silva RC, Jones SR, et al. Fetal mortality associated with cholestasis of pregnancy and the potential benefit of therapy with ursodeoxycholic acid. Gut. 1995;37:580-4.
132. Bacq Y, Myara A, Brechot MC, et al. Serum conjugated bile acid profile during intrahepatic cholestasis of pregnancy. J Hepatol. 1995;22:66-70.
133. Shneider BL. Genetic cholestasis syndromes. J Pediatr Gastroenterol Nutr. 1999;28:124-31.
134. Wilson JA. Intrahepatic cholestasis of pregnancy with marked elevation of transaminases in a black American. Dig Dis Sci. 1997;32:665-8.
135. Leevy CB, Koneru B, Klein KM. Recurrent familial prolonged intrahepatic cholestasis of pregnancy associated with chronic liver disease. Gastroenterology. 1997;113:966-72.
136. Olsson R, Tysk C, Aldenborg F, et al. Prolonged postpartum course of intrahepatic cholestasis of pregnancy. Gastroenterology. 1993;105:267-71.
137. Ropponen A, Sund R, Riikonen S, et al. Intrahepatic cholestasis of pregnancy as an indicator of liver and biliary diseases: A population-based study. Hepatology. 2006;43:723-8.
138. Glasinovic J, Marinovic I, Mege R, et al. Intrahepatic cholestasis of pregnancy in cholecystectomized women: An epidemiological study. In: Reyes H, Leuschner U, Arias I, editors. Pregnancy, sex hormones, and the liver: Proceedings of the 89th Falk Symposium. Santiago, Chile. Hingham, Mass: Kluwer Academic; 1995 Nov 10-11:248-9.
139. Heinonen S, Kirkinen P. Pregnancy outcome with intrahepatic cholestasis. Obstet Gynecol. 1999;94:189-93.
140. Glantz A, Marschall H-U, Mattsson L-A. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467-74.
141. Rioseco A, Ivankovic MB, Manzur A, et al. Intrahepatic cholestasis of pregnancy: a retrospective case-control study of perinatal outcome. Am J Obstet Gynecol. 1994;170:890-5.
142. Alsulyman OM, Ouzounian JG, Ames-Castro M, et al. Intrahepatic cholestasis of pregnancy: perinatal outcome associated with expectant management. Am J Obstet Gynecol. 1996;175:957-60.
143. Lee RH, Kwok KM, Ingles S, et al. Pregnancy outcomes during an era of aggressive management for intrahepatic cholestasis of pregnancy. Am J Perinatol. 2008;25:341-5.
144. Fagan EA. Intrahepatic cholestasis of pregnancy. BMJ. 1994;309:1243-4.
145. Jacquemin E, De Vree JM, Cresteil D, et al. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology. 2001;120:1448-58.
146. Wasmuth HE, Glantz A, Keppler H, et al. Intrahepatic cholestasis of pregnancy: The severe form is associated with common variants of the hepatobiliary phospholipid transporter ABCB4. Gut. 2007;56:265-70.
147. Pauli-Magnus C, Lang T, Meier Y, et al. Sequence analysis of bile salt export pump (ABCB11) and multidrug resistant p-glycoprotein 3 (ABCB4, MDR3) in patients with intrahepatic cholestasis of pregnancy. Pharmacogenetics. 2004;14:91-102.
148. Keitel V, Vogt C, Haussinger D, Kubitz R. Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology. 2006;131:624-9.
149. Mella JG, Roschmann E, Glasinovic JC, et al. Exploring the genetic role of the HLA-DPB1 locus in Chileans with intrahepatic cholestasis of pregnancy. J Hepatol. 1966;24:320-3.
150. Reyes H, Baez MF, Gonzalez MC, et al. Selenium, zinc and copper plasma levels in intrahepatic cholestasis of pregnancy, in normal pregnancies and in healthy individuals, in Chile. J Hepatol. 2000;32:542-9.
151. Reyes H, Ribalta J, Gonzalez MC, et al. Sulfobromophthalein clearance tests before and after ethinyl estradiol administration, in women and men with familial history of intrahepatic cholestasis of pregnancy. Gastroenterology. 1981;81:226-31.
152. Kreek MJ, Weser E, Sleisenger MH, et al. Idiopathic cholestasis of pregnancy. The response to challenge with the synthetic estrogen, ethinyl estradiol. N Engl J Med. 1967;277:1391-5.
153. Vore M. Estrogen cholestasis. Membranes, metabolites, or receptors? Gastroenterology. 1987;93:643-9.
154. Bacq Y, Sapey T, Brechot MC, et al. Intrahepatic cholestasis of pregnancy: A French prospective study. Hepatology. 1997;26:358-64.
155. Meng LJ, Reyes H, Axelson M, et al. Progesterone metabolites and bile acids in serum of patients with intrahepatic cholestasis of pregnancy: Effect of ursodeoxycholic acid therapy. Hepatology. 1997;26:1573-9.
156. Palma J, Reyes H, Ribalta J, et al. Ursodeoxycholic acid in the treatment of cholestasis of pregnancy: A randomized, double-blind study controlled with placebo. J Hepatol. 1997;27:1022-8.
157. Kondrackiene J, Beuers U, Kupcinskas L. Efficacy and safety of ursodeoxycholic acid versus cholestyramine in intrahepatic cholestasis of pregnancy. Gastroenterology. 2005;129:894-901.
158. Brites D, Rodrigues CM, Oliveira N, et al. Correction of maternal serum bile acid profile during ursodeoxycholic acid therapy in cholestasis of pregnancy. J Hepatol. 1998;28:91-8.
159. Mazzella G, Nicola R, Francesco A, et al. Ursodeoxycholic acid administration in patients with cholestasis of pregnancy: effects on primary bile acids in babies and mothers. Hepatology. 2001;33:504-8.
160. Serrano MA, Brites D, Larena MG, et al. Beneficial effect of ursodeoxycholic acid on alterations induced by cholestasis of pregnancy in bile acid transport across the human placenta. J Hepatol. 1998;28:829-39.
161. Riikonen S, Savonius H, Gylling H, et al. Oral guar gum, a gel-forming dietary fiber, relieves pruritus in intrahepatic cholestasis of pregnancy. Acta Obstet Gynecol Scand. 2000;79:260-4.
162. Sadler LC, Lane M, North R. Severe fetal intracranial haemorrhage during treatment with cholestyramine for intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1995;102:169-70.
163. Frezza M, Centini G, Cammareri G, et al. S-adenosylmethionine for the treatment of intrahepatic cholestasis of pregnancy. Results of a controlled clinical trial. Hepatogastroenterology. 1990;37:122-5.
164. Ribalta J, Reyes H, Gonzalez MC, et al. S-adenosy-L-methionine in the treatment of patients with intrahepatic cholestasis of pregnancy: A randomized, double-blind, placebo-controlled study with negative results. Hepatology. 1991;13:1084-9.
165. Binder T, Salaj P, Zima T, Vitek L. Randomized prospective comparative study of ursodeoxycholic acid and S-adenosyl-L-methionine in the treatment of intrahepatic cholestasis of pregnancy. J Perinat Med. 2006;34:383-91.
166. Nicastri PL, Diaferia A, Tartagni M, et al. A randomized placebo-controlled trial of ursodeoxycholic acid and S-adenosylmethionine in the treatment of intrahepatic cholestasis of pregnancy. Br J Obstet Gynaecol. 1998;105:1205-7.
167. Glantz A, Marschall H-U, Lammert F, Mattsson L-A. Intrahepatic cholestasis of pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42:1399-405.
168. Kretowicz E, McIntyre HD. Intrahepatic cholestasis of pregnancy, worsening after dexamethasone. Aust N Z J Obstet Gynaecol. 1994;34:211-13.
169. ACOG Committee on Practice Bulletins—Obstetrics. Diagnosis and management of preeclampsia and eclampsia. No: 33. Obstet Gynecol. 2002;99:159-67.
170. Sibai BM, Hauth J, Caritis S, et al. Hypertensive disorders in twin versus singleton gestations: National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938-42.
171. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159-67.
172. Barton JR, Sibai BM. Hepatic imaging in HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count). Am J Obstet Gynecol. 1996;174:1820-5.
173. Manas KJ, Welsh JD, Rankin RA, et al. Hepatic hemorrhage without rupture in Preeclampsia. N Engl J Med. 1985;312:424-6.
174. Krueger KJ, Hoffman BJ, Lee WM. Hepatic infarction associated with eclampsia. Am J Gastroenterology. 1990;85:588-92.
175. Lachmeijer AM, Arngrimsson R, Bastiaans EJ, et al. A genome-wide scan for preeclampsia in the Netherlands. Eur J Hum Genet. 2001;9:758-64.
176. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;46:7397-404.
177. Sibai BM, Ramadan MK, Usta I, et al. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169:1000-6.
178. Baxter JK, Weinstein L. HELLP syndrome: The state of the art. Obstet Gynecol Surg. 2004;59:838-45.
179. Barton JR, Sibai BM. Diagnosis and management of hemolysis, elevated liver enzymes, and low platelets syndrome. Clin Perinatol. 2004;32:807-33.
180. Aarnoudse JG, Houthoff HJ, Weits J, et al. A syndrome of liver damage and intravascular coagulation in the last trimester of normotensive pregnancy. A clinical and histopathological study. Br J Obstet Gynecol. 1986;93:145-55.
181. Tomsen TR. HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) presenting as generalized malaise. Am J Obstet Gynecol. 1995;172:1878-80.
182. Julius CJ, Dunn ZL, Blazina JF. HELLP syndrome: laboratory parameters and clinical course in four patients treated with plasma exchange. J Clin Apheresis. 1994;9:228-35.
183. Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 2004;103:981-91.
184. Catanzarite VA, Steinberg SM, Mosley CA, et al. Severe preeclampsia and fulminant and extreme elevation of aspartate aminotransferase and lactate dehydrogenase levels: High risk for maternal death. Am J Perinatol. 1995;12:310-13.
185. Steegers EA, Mulder TP, Bisseling JG, et al. Glutathione S-transferase alpha as marker for hepatocellular damage in pre-eclampsia and HELLP syndrome. Lancet. 1995;345:1571-72.
186. Neiger R, Trofatter MO, Trofatter KFJr. D-Dimer test for early detection of HELLP syndrome. South Med. 1995;88:416-19.
187. Schrocksnadel H, Daxenbichler G, Artner E, et al. Tumor markers in hypertensive disorders of pregnancy. Gynecol Obstet Invest. 1993;35:204-8.
188. Paternoster DM, Stella A, Simioni P, et al. Coagulation and plasma fibronectin parameters in HELLP syndrome. Int J Gynaecol Obstet. 1995;50:263-8.
189. Barton JR, Riely CA, Adamec TA, et al. Hepatic histopathologic condition does not correlate with laboratory abnormalities in HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count). Am J Obstet Gynecol. 1992;167:1538-43.
190. Hsu HW, Belfort MA, Vernino S, et al. Postpartum thrombotic thrombocytopenic purpura complicated by Budd-Chiari syndrome. Obstet Gynecol. 1995;85:839-43.
191. Ilbery M, Jones AR, Sampson J. Lupus anticoagulant and HELLP syndrome complicated by placental abruption, hepatic, dermal and adrenal infarction. Aust N Z J Obstet Gynaecol. 1995;35:215-17.
192. Mizutani S, Nomura S, Hirose R, et al. Intra-uterine fetal death due to pre-eclampsia which was misdiagnosed to be complicating with hepatitis. Horm Metab Res. 1993;25:187-9.
193. Pijnenborg R, Anthony J, Davey DD, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648-55.
194. Arngrimsson R, Bjornsson S, Geirsson RT, et al. Genetic and familial predisposition to eclampsia and pre-eclampsia in a defined population. Br J Obstet Gynaecol. 1990;97:762-9.
195. Chesley LC, Cooper DW. Genetics of hypertension in pregnancy: possible single gene control of pre-eclampsia and eclampsia in the descendants of eclamptic women. Br J Obstet Gynaecol. 1986;93:898-908.
196. Dizon-Townson DS, Nelson LM, Easton K, et al. The factor V Leiden mutation may predispose women to severe preeclampsia. Am J Obstet Gynecol. 1996;175:902-5.
197. van Pampus MG, Dekker GA, Wolf H, et al. High prevalence of hemostatic abnormalities in women with a history of severe preeclampsia. Am J Obstet Gynecol. 1999;180:1146-50.
198. Maynard SE, Min J-Y, Merchan J, et al. Excess placental soluble fms-like tyrosine kinsase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension and proteinuria in preeclampsia. J Clin Invest. 2003;111:649-58.
199. Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992-1005.
200. Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642-62.
201. Tyni T, Ekholm E, Pihko H. Pregnancy complications are frequent in long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. Am J Obstet Gynecol. 1998;178:603-8.
202. Borwning MF, Levy HL, Wilkins-Haug L, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107:115-20.
203. den Boer ME, Ijlst L, Wijburg FA, et al. Heterozygosity for the common LCHAD mutation (1528G>C) is not a major cause of HELLP syndrome and the prevalence of the mutation in the Dutch population is low. Pediatr Res. 2000;48:151-4.
204. Friedman SA, Lubarsky SL, Ahokas RA, et al. Preeclampsia and related disorders. Clinical aspects and relevance of endothelin and nitric oxide. Clin Perinatol. 1995;22:343-55.
205. Ward K, Hata A, Jeunemaitre X, et al. A molecular variant of angiotensinogen associated with preeclampsia. Nat Genet. 1993;4:59-61.
206. Makkonen N, Harju M, Kirkinen P. Postpartum recovery after severe pre-eclampsia and HELLP syndrome. J Perinat Med. 1996;24:641-9.
207. Ferrara JM, Malatesta R, Kemmann E. Transient nephrogenic diabetes insipidus during toxemia in pregnancy. Diagn Gynecol Obstet. 1980;2:227-30.
208. Isler CM, Rinehart BK, Terrone DA, et al. Maternal mortality associated with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 1999;181:924-8.
209. Haddad B, Barton JR, Livingston JC, et al. Risk factors for adverse maternal outcomes among women with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183:444-8.
210. Sibai BM, Ramadan MK, Chari RS, et al. Pregnancies complicated by HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): Subsequent pregnancy outcome and long-term prognosis. Am J Obstet Gynecol. 1995;172:125-9.
211. Sullivan CA, Magaan EF, Perry KJJr, et al. The recurrence risk of the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP) in subsequent gestations. Am J Obstet Gynecol. 1994;171:930-43.
212. Visser W, Wallenburg HC. Maternal and perinatal outcome of temporizing management in 254 consecutive patients with severe pre-eclampsia remote from term. Eur J Obstet Gynecol Reprod Biol. 1995;63:147-54.
213. Martin JJJr, Files JC, Blake PG, et al. Postpartum plasma exchange for atypical preeclampsia-eclampsia as HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1995;172:1107-25.
214. Magann EF, Bass D, Chauhan SP, et al. Antepartum corticosteroids: Disease stabilization in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol. 1994;171:1148-53.
215. Martin JJJr, Perry KGJr, Blake PG, et al. Better maternal outcomes are achieved with dexamethasone therapy for postpartum HELLP (hemolysis, elevated liver enzymes, and thrombocytopenia) syndrome. Am J Obstet Gynecol. 1997;177:1011-17.
216. O’Brien JN, Milligan DA, Barton JR. Impact of high-dose corticosteroid therapy for patients with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183:921-4.
217. Tompkins MJ, Thiagarajah S. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome: the benefit of corticosteroids. Am J Obstet Gynecol. 1999;181:304-9.
218. Strate T, Broering DC, Bloechle C, et al. Orthotopic liver transplantation for complicated HELLP syndrome. Case report and review of the literature. Arch Gynecol Obstet. 2000;264:108-11.
219. Shames BD, Gernandez LA, Sollinger HW, et al. Liver transplantation for HELLP syndrome. Liver Transpl. 2005;11:224-8.
220. Risseeuw JJ, de Vries JE, van Eyck J, et al. Liver rupture postpartum associated with preeclampsia and HELLP syndrome. J Matern Fetal Med. 1999;8:32-5.
221. Sheikh RA, Yasmeen S, Pauly MP, et al. Spontaneous intrahepatic hemorrhage and hepatic rupture in the HELLP syndrome: four cases and a review. J Clin Gastroenterol. 1999;28:323-8.
222. Zissin R, Yaffe D, Fejgin M, et al. Hepatic infarction in preeclampsia as part of the HELLP syndrome: CT appearance. Abdom Imaging. 1999;24:594-6.
223. Chan AD, Gerscovich EO. Imaging of subcapsular hepatic and renal hematomas in pregnancy complicated by preeclampsia and the HELLP syndrome. J Clin Ultrasound. 1999;27:35-40.
224. Erhard J, Lange R, Niebel W, et al. Acute liver necrosis in the HELLP syndrome: successful outcome after orthotopic liver transplantation. A case report. Transpl Int. 1993;6:179-81.
225. Hunter SK, Martin M, Benda JA, et al. Liver transplant after massive spontaneous hepatic rupture in pregnancy complicated by preeclampsia. Obstet Gynecol. 1995;85:819-22.
226. Chiang KS, Athey PA, Lamki N. Massive hepatic necrosis in the HELLP syndrome: CT correlation. J Comput Assist Tomogr. 1991;15:845-7.
227. Alleman JS, Delarue MW, Hasaart TH. Successful delivery after hepatic rupture in previous pre-eclamptic pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992;47:76-9.
228. Greenstein D, Henderson JM, Boyer TD. Liver hemorrhage: Recurrent episodes during pregnancy complicated by preeclampsia. Gastroenterology. 1994;106:1668-71.
229. Wilson RH, Marshall BM. Postpartum rupture of a subcapsular hematoma of the liver. Acta Obstet Gynecol Scand. 1992;71:394-7.
230. Seige M, Schweigart U, Moessmer G, et al. Extensive hepatic infarction caused by thrombosis of right portal vein branches and arterial vasospasm in HELLP syndrome associated with homozygous factor V Leiden. Am J Gastroenterol. 1998;93:473-4.
231. Sheehan HL. The pathology of hyperemesis and vomiting of late pregnancy. J Obstet Gynaecol. 1940;46:658-99.
232. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192:1416-19.
233. Castro MA, Fassett MJ, Reynolds TB, et al. Reversible peripartum liver failure: a new perspective on the diagnosis, treatment, and cause of acute fatty liver of pregnancy, based on 28 consecutive cases. Am J Obstet Gynecol. 1999;181:389-95.
234. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;46:7397-404.
235. Ibdah JA, Bennett MJ, Rinaldo P, et al. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N Engl J Med. 1999;340:1723-31.
236. Sibai BM. Imitators of severe preeclampsia. Obstet Gynecol. 2007;109:956-66.
237. Vanjak D, Moreau R, Roche-Sicot J, et al. Intrahepatic cholestasis of pregnancy and acute fatty liver of pregnancy. An unusual but favorable association? Gastroenterology. 1991;100:1123-5.
238. Malone FD, Kaufman GE, Chelmow D, et al. Maternal morbidity associated with triplet pregnancy. Am J Perinatol. 1998;15:73-7.
239. James WH. Sex ratios of offspring and the causes of placental pathology. Hum Reprod. 1995;10:1403-6.
240. Usta IM, Barton JR, Amon EA, et al. Acute fatty liver of pregnancy: an experience in the diagnosis and management of fourteen cases. Am J Obstet Gynecol. 1994;171:1342-7.
241. Kennedy S, Hall PM, Seymour AC, et al. Transient diabetes insipidus and acute fatty liver of pregnancy. Br J Obstet Gynaecol. 1994;101:387-91.
242. Coulson CC, Kuller JA, Bowes WAJr. Myocardial infarction and coronary artery dissection in pregnancy. Am J Perinatol. 1995;12:328-30.
243. Jones MB. Pulmonary fat emboli associated with acute fatty liver of pregnancy. Am J Gastroenterol. 1993;88:791-2.
244. Castro M, Ouzounian J, Colletti P, et al. Radiologic studies in acute fatty liver of pregnancy. A review of the literature and 19 new cases. J Reprod Med. 1996;41:839-43.
245. Rolfes DB, Ishak KG. Acute fatty liver of pregnancy: a clinicopathologic study of 35 cases. Hepatology. 1985;5:1149-58.
246. Hamid SS, Jafri SM, Khan H, et al. Fulminant hepatic failure in pregnant women: acute fatty liver or acute viral hepatitis? J Hepatol. 1996;25:20-7.
247. Klein NA, Mabie WC, Shaver DC, et al. Herpes simplex virus hepatitis in pregnancy. Two patients successfully treated with acyclovir. Gastroenterology. 1991;100:239-44.
248. Dani R, Mendes GS, Medeiros J de L, et al. Study of the liver changes occurring in preeclampsia and their possible pathogenetic connection with acute fatty liver of pregnancy. Am J Gastroenterol. 1996;91:292-4.
249. Minakami H, Oka N, Sato T, et al. Preeclampsia: a microvesicular fat disease of the liver? Am J Obstet Gynecol. 1988;159:1043-7.
250. Bellig LL. Maternal acute fatty liver of pregnancy and the associated risk for long-chain 3-hydroxyacyl-coenzyme A dehydrogenase (LCHAD) deficiency in infants. Adv Neonatal Care. 2004;4:26-32.
251. Innes AM, Seargeant LE, Balachandra K, et al. Hepatic carnitine palmitoyltransferase I deficiency presenting as maternal illness in pregnancy. Pediatr Res. 2000;47:43-5.
252. Ibdah JA, Zhao Y, Viola J, et al. Molecular prenatal diagnosis in families with fetal mitochondrial trifunctional protein mutations. J Pediatr. 2001;138:396-9.
253. Ijlst L, Mandel H, Oostheim W, et al. Molecular basis of hepatic carnitine palmitoyltransferase I deficiency. J Clin Invest. 1998;102:527-31.
254. Mansouri A, Fromenty B, Durand F, et al. Assessment of the prevalence of genetic metabolic defects in acute fatty liver of pregnancy. J Hepatol. 1996;25:781.
255. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174:211-16.
256. Franco J, Newcomer J, Adams M, et al. Auxiliary liver transplant in acute fatty liver of pregnancy. Obstet Gynecol. 2000;95:1042.
257. Ockner SA, Brunt EM, Cohn SM, et al. Fulminant hepatic failure caused by acute fatty liver of pregnancy treated by orthotopic liver transplantation. Hepatology. 1990;11:59-64.
258. Reyes H, Sandoval L, Wainstein A, et al. Acute fatty liver of pregnancy: A clinical study of 12 episodes in 11 patients. Gut. 1994;35:101-6.
259. MacLean MA, Cameron AD, Cumming GP, et al. Recurrence of acute fatty liver of pregnancy. Br J Obstet Gynaecol. 1994;101:453-4.
260. Wilcken B, Leung KC, Hammond J, et al. Pregnancy and fetal long-chain 3-hydroxyacyl coenzyme A dehydrogenase deficiency. Lancet. 1993;341:407-8.
261. Khuroo MS, Kamili S, Jameel S. Vertical transmission of hepatitis E virus. Lancet. 1995;345:1025-6.
262. Nanda SK, Ansari IH, Acharya SK, et al. Protracted viremia during acute sporadic hepatitis E virus infection. Gastroenterology. 1995;108:225-30.
263. Rab MA, Bile MK, Mubarik MM, et al. Water-borne hepatitis E virus epidemic in Islamabad, Pakistan: a common source outbreak traced to the malfunction of a modern water treatment plant. Am J Trop Med Hyg. 1997;57:151-7.
264. Lok AS. Natural history and control of perinatally acquired hepatitis B virus infection. Dig Dis. 1992;10:46-52.
265. Beasley RP, Trepo C, Stevens CE, et al. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977;105:94-8.
266. Beath SV, Boxall EH, Watson RM, et al. Fulminant hepatitis B in infants born to anti-HBe hepatitis B carrier mothers. BMJ. 1992;304:1169-70.
267. American Academy of Pediatrics Committee on Infectious Diseases. Universal hepatitis B immunization. Pediatrics. 1992;89:795-800.
268. Mahadevan U. American Gastroenterological Association Institute technical review on the use of gastrointestinal medication in pregnancy. Gastroenterology. 2006;131:283-311.
269. Mofenson LM, Centers for Disease Control and PreventionU.S. Public Health Service Task Force. Recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. MMWR Recom Rep. 2002;51(RR-18):1-38.
270. Su GG, Pan KH, Zhao NF, et al. Efficacy and safety of lamivudine treatment for chronic hepatitis B in pregnancy. World J Gastroenterol. 2004;10:910-12.
271. van Zonneveld M, van Nunen AB, Niesters HG, et al. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294-7.
272. Omata M, Ito Y, Imazeki F, et al. Infection with delta agent in Japan. Hepatogastroenterology. 1985;32:220-3.
273. Silverman NS, Jenkin BK, Wu C, et al. Hepatitis C virus in pregnancy: Seroprevalence and risk factors for infection. Am J Obstet Gynecol. 1993;169:583-7.
274. Reinus JF, Leikin EL, Alter HJ, et al. Failure to detect vertical transmission of hepatitis C virus. Ann Intern Med. 1992;117:881-6.
275. Zanetti AR, Tanzi E, Paccagnini S, et al. Mother-to-infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission. Lancet. 1995;345:289-91.
276. Eyster ME, Alter HJ, Aledort LM, et al. Heterosexual co-transmission of hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Ann Intern Med. 1991;115:764-8.
277. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med. 1994;330:744-50.
278. Ruiz-Extremera A, Salmeron J, Torres C, et al. Follow-up of transmission of hepatitis C to babies of human immunodeficiency virus-negative women: the role of breast-feeding in transmission. Pediatr Infect Dis J. 2000;19:511-16.
279. Polywka S, Schroter M, Feucht HH, et al. Low risk of vertical transmission of hepatitis C virus by breast milk. Clin Infect Dis. 1999;29:1327-9.
280. Shimono N, Ishihashi H, Ikematsu H, et al. Fulminant hepatic failure during perinatal period in a pregnant woman with Wilson’s disease. Gastroenterol Jpn. 1991;26:69-73.
281. Solomon L, Abrams G, Dinner M, et al. Neonatal abnormalities associated with D-penicillamine treatment during pregnancy. N Engl J Med. 1977;296:54-5.
282. Scheinberg IH, Sternlieb I. Pregnancy in penicillamine-treated patients with Wilson’s disease. N Engl J Med. 1975;293:1300-2.
283. Brewer GJ, Johnson VD, Dick RD, et al. Treatment of Wilson’s disease with zinc. XVII: Treatment during pregnancy. Hepatology. 2000;31:364-70.
284. Olsson R, Loof L, Wallerstedt S. Pregnancy in patients with primary biliary cirrhosis—A case for dissuasion? The Swedish Internal Medicine Liver Club. Liver. 1993;13:316-18.
285. Ruo J, Schonig T, Stremmel W. Therapy with ursodeoxycholic acid in primary biliary cirrhosis in pregnancy. Z Gastroenterol. 1996;34:188-91.
286. Lau WY, Leung WT, Ho S, et al. Hepatocellular carcinoma during pregnancy and its comparison with other pregnancy-associated malignancies. Cancer. 1995;75:2669-76.
287. Kroll D, Mazor M, Zirkin H, et al. Fibrolamellar carcinoma of the liver in pregnancy. A case report. J Reprod Med. 1991;36:823-7.
288. Reinus JF, Yantiss RK. A 22-year-old man with night sweats, weight loss, and a hepatic mass. N Engl J Med. 2000;343:1533-60.
289. Gordon SC, Polson DJ, Shirkhoda A. Budd-Chiari syndrome complicating pre-eclampsia: diagnosis by magnetic resonance imaging. J Clin Gastroenterol. 1991;13:460-2.
290. Segal S, Shenhav S, Segal O, et al. Budd-Chiari syndrome complicating severe preeclampsia in a parturient with primary antiphospholipid syndrome. Eur J Obstet Gynecol Reprod Biol. 1996;68:227-9.
291. Fickert P, Ramschak H, Kenner L, et al. Acute Budd-Chiari syndrome with fulminant hepatic failure in a pregnant woman with factor V Leiden mutation. Gastroenterology. 1996;111:1670-3.
292. Christopher V, Al-Chalabi T, Richardson P, et al. Pregnancy outcome after liver transplantation: A single-center experience of 71 pregnancies in 45 recipients. Liver Transpl. 2006;12:1037-9.