Gastroesophageal Reflux
Gastroesophageal reflux (GER) is a disease that is commonly encountered in infants and children. In general, the episodes of GER that are seen in infants and children are not clinically significant and will have no identifiable etiology. In addition, 60–65% of children with GER will undergo spontaneous symptom resolution by 2 years of life, regardless of any medical treatment.1 However, some children will have pathologic gastroesophageal reflux disease (GERD) that will result in either failure to grow appropriately, respiratory complications, or apparent life-threatening events (ALTE). This is the population that will require medical and/or surgical intervention.
History
The effects of GERD in pediatric patients have been reported for over a century.2–6 Before the introduction of proton pump inhibitors (PPIs) in the 1990s, the medical management of GERD in children and adults was relatively ineffective and based on antacids and histamine antagonists. Due to the limited spectrum of medical management in the 1950s and 1960s, several surgeons developed operative approaches for GERD management. Lortat–Jacob, Hill, Belsey, Nissen, Rosetti, and Thal all contributed greatly to the surgical management of GERD.7–14
These antireflux procedures were initially developed in adults and were effective in controlling GERD. Subsequently, they were applied to infants and children with good success.7,14,15 The overall management of GERD has progressed significantly over the past two decades with more effective PPIs and refinement in the surgical technique. One of the most important surgical advances occurred in 1991 when Dallemagne reported his experience with laparoscopic fundoplication.16 An expected evolutionary cascade led to the use of laparoscopy in children by Georgeson17 and Lobe.18 It is due to these pioneers, and the many researchers that followed, that we now know that laparoscopic fundoplication is safe and effective in treating GERD with acceptable morbidity and excellent control of symptoms in infants and children. The most daunting task currently is how to identify those children who will benefit from a fundoplication. As our diagnostic tools are being further evaluated and newer methods are being introduced, there has been renewed skepticism about who should have a fundoplication. At the same time, ongoing refinement of the operative approach has yielded even better outcomes.
Pathophysiology
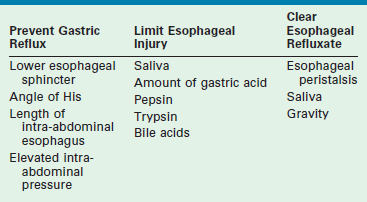
GERD is defined as the pathologic effects of involuntary passage of gastric contents into the esophagus. Ultimately, the pathophysiologic alteration that is responsible for the development of GERD is incompetence of the antireflux barriers that exist between the lower esophagus and the stomach. The result of this incompetence is the presence of gastric refluxate in direct contact with the esophageal mucosa. While initially felt to be purely acidic, research in the utility of the pH probe as a diagnostic tool has shown this is not true.19 In fact, adult literature has implicated alkaline bile reflux as a causative factor in the development of Barrett esophageal metaplasia.20 The pathologic events that occur because of GERD are due to one or multiple failures of the normal physiologic barriers that exist to prevent gastric contents from entering the esophagus, or to limit injury to the esophagus as a result of gastric refluxate, or to clear the refluxate that enters the esophagus (Table 28-1).
TABLE 28-1
Mechanisms That Either Prevent Gastroesophageal Reflux, Limit the Esophageal Injury, or Clear the Refluxate
In adults, the consequence of this refluxate in the esophagus is primarily limited to erosive esophagitis, esophageal stricture, and Barrett esophagitis. In children, its detrimental effects are much broader. Associated physiologic, anatomic, and developmental abnormalities coexist in children that make GERD and its consequences much more complex. Many children with GERD have significant neurologic impairment. These children can have increased spasticity with retching and related increased abdominal pressures. Poor swallowing mechanisms lead to gagging and choking, which add to this intermittent increased abdominal pressure. Sometimes, a hiatal hernia develops (Fig. 28-1), further predisposing to GERD. Congenital anomalies such as esophageal atresia with or without tracheoesophageal fistula (EA/TEF), duodenal and proximal small bowel atresias, congenital diaphragmatic hernia (CDH), and gastroschisis/omphalocele all predispose to the development of GERD. The consequences of GERD in children lead to the same complications seen in adults (erosive esophagitis, stricture, and Barrett esophagitis), but also include pulmonary effects (reactive airway disease and pneumonia), potential malnutrition secondary to the inability to maintain adequate caloric intake, and apneic episodes leading to ALTE spells.
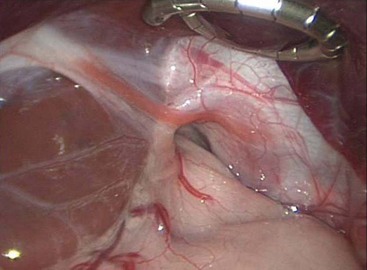
FIGURE 28-1 This intraoperative photograph depicts a de novo hiatal hernia in a 13-month-old infant. Note the enlarged hiatus. A portion of the upper stomach has herniated into the chest.
Barriers against GERD
The most important factor for preventing reflux of gastric contents into the esophagus is the lower esophageal sphincter (LES). Embryologically, the LES arises from the inner circular muscle layer of the esophagus, which is asymmetrically thickened in the distal esophagus. This thickened muscle layer creates a high-pressure zone that can be measured manometrically. In addition, this muscular thickening extends onto the stomach more prominently on the greater than lesser curvature.21 The phrenoesophageal membrane, arising from the septum transversum of the diaphragm and the collar of Helvetius, holds the LES in position. The result is an LES that lies partially in the chest and partially in the abdomen. This positioning is important for the normal barrier function against GER. Esophageal manometry can identify this transition (which is known as the respiratory inversion point) from the thoracic to the abdominal esophagus.
The LES is an imperfect valve that creates a pressure gradient in the distal esophagus. The ability to prevent GER is directly proportional to the LES pressure and its length, provided that LES relaxation is normal. In an adult study, LES pressures greater than 30 mmHg prevented GER, as documented by 24-hour pH study, whereas pressures between 0–5 mmHg correlated with abnormal pH studies in more than 80% of patients.22 Also, GER is statistically significantly more likely to develop in adults if the LES pressure falls below 6 mmHg at the respiratory inversion point or if the overall LES length is 2 cm or less.23 As noted previously, the LES is relatively fixed across the esophageal hiatus by its surrounding attachments. Malposition of the LES, which can occur with a hiatal hernia or abnormal development, causes loss of the protective function of the LES, resulting in GER. Finally, LES relaxation occurs with esophageal peristalsis initiated by the swallowing mechanism. This relaxation is normal and must occur. Inappropriate LES relaxations, referred to as transient LES relaxations, have been shown to occur sporadically, unassociated with the swallowing mechanism. Interestingly, when children with symptoms of GER were studied with pH and manometry simultaneously, reflux episodes rarely correlated with decreased LES pressures. Rather, the majority of reflux episodes occurred during transient LES relaxations, and no reflux episodes were identified during LES relaxation after swallowing with normal peristaltic sequence.24,25 There continues to be growing support that these transient LES relaxations are the primary mechanism for GER.
Another barrier to the development of symptomatic GER is the intra-abdominal length of the esophagus.26 Although no absolute effective intra-abdominal esophageal length has been identified that prevents GER, correlation between several lengths and GER have been identified. In one report, an intra-abdominal length of 3–4.5 cm in adults with normal abdominal pressure provided LES competency 100% of the time.22 A length of 3 cm was sufficient in preventing reflux in 64% of individuals, whereas less than 1 cm of intra-abdominal esophagus resulted in reflux in 81% of patients. It is believed that failure to mobilize adequate esophageal length for intra-abdominal positioning during antireflux operations can lead to less than successful results or recurrent GER in adults. However, we now know these data are not applicable in infants and children and that complete mobilization of the esophagus, in the absence of a hiatal hernia, is detrimental in infants and children through the results of a multicenter, prospective, randomized trial.27
A third barrier to reflux is the angle of His, which is the angle at which the esophagus enters the stomach. The usual orientation is that of an acute angle, which creates a flap valve at the gastroesophageal junction. Although the actual functional component of the angle of His is not well known, it has been shown to provide resistance to GER. Experimentally, when this angle is more obtuse, GER is more prone to develop. Conversely, accentuation of the angle inhibits GER.28
The ability of the angle of His to prevent GER may be diminished as a result of abnormal development or may be iatrogenic, as occurs after gastrostomy placement. When a normal angle of His is present, there is a convoluted fold of mucosa present at the gastroesophageal junction. This mucosa creates a rosette-like configuration that collapses on itself with increases in intragastric pressure or negative pressure in the thoracic esophagus, thus acting as an additional weak antireflux valve.29,30
Patients with increased abdominal pressure as a result of neurologically related retching, physiologic effects (obesity, ascites, peritoneal dialysis), or anatomic abnormalities (gastroschisis, omphalocele, CDH) are at increased risk for developing GERD owing to the effects of chronic pressure from the abdomen into the thorax.31–37 Finally, certain congenital defects such as congenital short esophagus, congenital hiatal hernia, and EA/TEF predispose to GERD. In patients with EA/TEF, the esophagus has abnormal peristalsis and the LES is incompetent. It has been reported that up to 30% of these patients will require antireflux surgery after repair of their EA/TEF.38–40 Regarding CDH, anatomic abnormalities of the esophageal hiatus and the esophagus predispose to GERD, with 15–20% of surviving patients undergoing an antireflux operation for GERD.35–37
Once the barrier to GER has been overcome (or failed), mechanisms for esophageal clearance become important in preventing damage associated with exposure of the esophageal mucosa to the gastric refluxate. The primary mechanism for esophageal clearance remains esophageal motility. However, gravity and saliva contribute to the ability of the esophagus to clear the refluxate.41,42 There are three types of esophageal contractions: primary, secondary, and tertiary. Primary contraction waves are initiated with swallowing and are responsible for the clearance of refluxed contents in 80–90% of reflux episodes. Secondary waves occur when material is refluxed into the esophagus and clearance is required, especially when the reflux occurs during sleep.43,44 Tertiary waves have nothing to do with esophageal clearance and are sporadic, non-propagating contractions. When impaired esophageal motility is present as a result of abnormal smooth muscle function, impaired vagal stimulation, or obstruction, refluxed gastric contents are not moved caudad into the stomach in a timely manner. This prolonged exposure can lead to esophageal mucosal injury and can potentiate the motility disturbance due to vagal and/or smooth muscle inflammation or injury. Saliva neutralizes refluxed material, and patients with GERD have been found to have decreased salivary function. It has also been shown that positional effects of GERD treatment may be related to gravity assisting in the clearance of esophageal refluxate.45–48
The final element for prevention of esophageal injury related to GERD is the ability to limit injury once refluxed contents have reached the esophagus. In addition to functioning as a neutralizing agent, saliva also aids in lubricating the esophageal contents, thus making it easier to clear any retained refluxate. Acid exposure has traditionally been postulated to cause the most significant injury, but more recent data has also implicated alkaline bile reflux as well.20,49 Some pediatric patients with documented GERD have been shown to have increased acid secretion.50,51 To this end, the role of PPIs in controlling GERD in this population is very important because they have the dual effect of increasing the gastric pH while simultaneously decreasing the acid volume.52–54 However, it is now recognized that some children with GERD have normal pH probe studies and acid reflux with esophageal injury is not as big an issue for this subset of patients as is poor nutrition and pulmonary complications. Other substances that increase esophageal mucosal injury include bile salts, pepsin, and trypsin. When combined with acid, bile salts are injurious to the esophageal mucosa by increasing the permeability of the esophageal mucosa to existing acid, thus further potentiating injury.55,56 Pepsin and trypsin are both proteolytic enzymes that can injure the esophageal mucosa. Both of these enzymes are more toxic at lower pH levels and, hence, are more injurious in the presence of acid.57,58
Clinical Manifestations
The presentation of GERD in infants and children is variable and depends on the patient’s age and overall medical condition. The surgeon must consider both this variability and the patient characteristics when evaluating a child with symptoms for possible GERD. Although the symptoms of GERD are variable for each patient, the actual frequency of symptoms seen in infants who have required surgical intervention for GERD has been reported (Table 28-2).59
TABLE 28-2
Frequency of Symptoms in Patients with Gastroesophageal Reflux
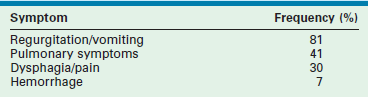
Adapted from Tovar JA, Olivares P, Diaz M, et al. Functional results of laparoscopic fundoplication in children. J Pediatr Gastroenterol Nutr 1998;26:429–31.
When considering the symptoms associated with GERD, persistent regurgitation is the most common complaint reported by parents of children with GERD.60 However, in infants, vomiting is often physiologic and can be ‘normal.’ This type of vomiting is termed chalasia of infancy and is seen early in life, usually during burping, after feeding, or when placed in the recumbent position.61 Chalasia does not interfere with normal growth or development and rarely leads to other complications. It is a self-limited process with most infants transitioning to being asymptomatic by 2 years of age or near the time of initiating solid foods.1 No treatment is necessary in patients who have chalasia, and no diagnostic evaluation should be pursued. However, when persistent regurgitation is the result of GER, it can lead to complications, including significant malnutrition and growth failure due to insufficient caloric intake.
In infants, another presenting symptom is irritability due to pain. Painful esophagitis can be the result of the acid refluxate. Discomfort leads to crying despite consoling measures.62,63 Occasionally, small volumes of feeds briefly assist in alleviating pain. However, this is generally not a lasting effect.24,25 In contrast to infants, children with GERD more often present with complaints of pain. As in adults, the pain is retrosternal in nature, and often described as heartburn. Long-standing GERD with esophagitis can lead to chronic inflammation or even ulcer formation with eventual scarring and stricture. Dysphagia develops as a result of a narrowed esophageal lumen, as well as possible esophageal dysmotility secondary to long-standing mucosal inflammation. Obstructive symptoms and pain are the two most common associated complaints when an esophageal stricture is present.64,65
Barrett esophagitis is a premalignant condition that is associated with prolonged GERD. It occurs when metaplasia develops in the esophageal squamous epithelium that is replaced with columnar epithelium. In adults, it is thought to be the result of chronic esophageal injury. Whether it develops from gastric acid injury or exposure to alkaline bile reflux is currently a controversial topic.66–68 Although uncommon in infants and children, when it does develop, serious complications often result. In addition to the increased risk for adenocarcinoma, approximately 50% of these patients will develop stricture and many patients will develop ulcers.69,70 Aggressive GERD management, along with vigilant long-term surveillance via yearly esophagogastroscopy, must be pursued to minimize these often difficult and possibly fatal complications.
Respiratory symptoms are commonly seen in infants and children. Delineating the role of GER as an etiologic agent for ongoing respiratory complaints can be difficult because of the similarity of the symptoms that are seen with other pulmonary diseases and the fact that primary aspiration from oropharyngeal dysmotility may be the inciting factor not GER. Chronic cough, wheezing, choking, apnea, or near sudden infant death syndrome (SIDS) can all be symptoms attributable to GER. Recurrent bronchitis or pneumonia can occur from aspiration of the refluxate.71 Esophageal stimulation via acidification of the esophageal mucosa causes vagally mediated laryngospasm and bronchospasm, which clinically presents as apnea or choking or mistakenly as asthma.72,73 Esophageal inflammation, as seen with esophagitis, likely enhances this mechanism.74,75 The effects of GER on premature infants with respiratory problems have been studied.76 Most of these infants were intubated for varying periods owing to respiratory distress syndrome or bronchopulmonary dysplasia. In the former group, GERD was responsible for a deteriorating pulmonary status requiring intubation. In the latter, deterioration of pulmonary status plus failure to thrive and anorexia led to the diagnosis of GERD. All improved with correction of the GERD.77
Although uncommon, hemorrhage can be a presenting symptom of GERD. Esophagitis, gastritis, and ulcer formation can lead to hematochezia or melena in a small percentage of infants or children.59
Diagnostic Evaluation
Upper gastrointestinal radiography is the most frequent initial study employed. Evidence for reflux is seen in up to 50% of the examinations.78,79 However, the absence of reflux is an extremely poor indicator of GERD as a cause of the patient’s symptoms. Furthermore, the presence of reflux on an upper gastrointestinal series does not necessarily indicate pathologic GER. The contrast study is most useful for delineating the anatomy of the esophagus and esophagogastric junction. It also evaluates esophageal clearance and assesses esophageal and gastric motility. The contrast study can identify the presence of esophageal strictures, webs, or distal obstructions, such as duodenal obstruction, antral web, or malrotation, as the cause of the reflux symptoms—which may be the case in 4% of patients.80 These correctable, anatomic causes for GER are critical to rule out before pursuing further studies.
Twenty-four hour pH probe monitoring has been considered the gold standard in diagnosing GERD since the 1980s when DeMeester established scores that correlated with the presence or absence of GERD.81–83 Boix-Ochoa later proposed a revised score that was applicable to pediatric patients aged 2 months to 3 years old that is still used today.84 Currently, the 24-hour pH probe monitoring study is recommended in infants with respiratory symptoms, especially ALTE spells or apneic events; infants who are irritable with intractable crying and anorexia; infants and children with reactive airway disease or recurrent pneumonia; and children unresponsive to medical measures in whom GER is suspected. Special considerations should be given to those children who become symptomatic after fundoplication. Conversely, the study generally is not useful or necessary for infants with uncomplicated regurgitation, children with esophagitis already diagnosed by endoscopy and biopsy, and children with dysphagia or heartburn thought to be caused by GER.
The pH study is performed by placing an electrode 2–3 cm proximal to the gastroesophageal junction and measuring the pH in the distal esophagus. The accuracy of the pH examination is dependent on the cessation of all antireflux medication: PPIs should be withheld for seven days, and histamine receptor blockers are stopped 48 hours before the study. A reflux episode is considered to have occurred if the esophageal pH is recorded as less than 4. Ideally, the examination should occur over an uninterrupted 24-hour period. The pH is continuously monitored via the esophageal electrode while the patient’s position (upright, supine, prone) and activities (awake, asleep, eating) are simultaneously recorded. The final score is calculated based on the percent of total time that the pH was less than 4, the total number of reflux episodes, the number of episodes lasting longer than 5 minutes, and the longest reflux episode.85,86
Data from the pH probe monitoring has increased our understanding of GER and taught us that not all GER is acidic. Three patterns of reflux have been described in symptomatic infants, as determined by extended esophageal pH monitoring: continuous, discontinuous, and mixed.87 Those infants with the discontinuous type rarely required an antireflux operation, whereas approximately half of those with the other two types did. One should keep in mind that medical treatment at the time of this study was much less effective than it is currently. Nonetheless, this study indicates that pH monitoring can be useful in sorting out infants with GER who may or may not require an antireflux procedure.88,89 Incidentally, all of the infants in this study, including the ‘normal controls,’ experienced reflux frequently in the first two hours after being given apple juice.
The etiology for this non-acid reflux is likely to be due to the infant diet and buffering. The infant is not capable of acidifying their gastric secretions to the extent that older children and adults are.90,91 Probe data from infants showed that gastric pH was <4 for 15% of the time in infants compared to a pH <4 for 42% of the time in older children.92 Furthermore, the infant’s milk-based diet serves as a buffer altering the pH of the gastric contents. This effect was investigated by measuring gastric pH after feeds whereby the gastric pH was >4 for a mean time of 130 minutes post-feeding.93
By using one ‘old’ diagnostic technique and one ‘new’ one, some of the disparities between pH probe observations and ‘events’ may be better understood. This is based on the observation that some reflux of acid into the lower esophagus occurs while the intraesophageal pH is still less than 4 due to a traditional acid reflux episode. This is called ‘acid re-reflux’ (ARR) and will be missed by using only pH-monitoring techniques.94 ARR is most likely to occur in patients with severe esophagitis, postprandially, and in the recumbent posture. It is now thought to be a common cause of prolonged acid contact. Detecting ARR provides a better estimation of the incompetence of the antireflux barrier than does traditional pH probe evaluations.
Two methods may be used to evaluate ARR. The first is scintigraphy, which directly measures radiolabeled liquid gastric contents flowing into the esophagus, independent of the pH of the refluxate or the esophageal lumen. The second is multichannel intraluminal impedance (MII), a method that recognizes the flow of gastric contents into the esophagus by detecting decreases in impedance from high (the esophagus) to low (the stomach) values across electrode pairs placed throughout the esophagus and in the stomach. The presence of acid, therefore, is also completely irrelevant with this diagnostic tool which can be performed at the same time as pH probe monitoring. Furthermore, MII also can distinguish liquid from gas refluxate.95–97
Recent studies using MII support the concept that measuring acid reflux (pH study) may not be the best method of evaluating GERD.98–101 These studies reaffirm that the pH probe does not simultaneously detect the majority of reflux events as defined by impedance monitoring, presumably because the re-reflux boluses are not acidic. When MII and pH monitoring are used simultaneously, there are significant reflux episodes that are not identified with pH monitoring alone because the episodes are actually non-acid reflux episodes with a pH greater than 4.99 These nonacid reflux episodes are less common in untreated GERD patients than in normal patients. MII has shown that GERD patients more commonly have liquid-type reflux events, whereas non-GERD patients generally have more gas-type reflux events.100 Additionally, MII data suggest that treatment with PPIs does not decrease the amount of reflux but rather converts the reflux to non-acid or weakly acidic in nature.101
Although essential in adults, esophageal manometry is infrequently utilized in the pediatric population. When employed, the study measures the motility of the esophagus and the pressure at the LES via a multiple-port pressure transducer placed in the esophagus and traversing the LES. The clinical data accumulated in adult patients have revealed several important points that are likely applicable to infants and children with GERD. First, it has been shown that pharyngeal swallowing and primary peristaltic contractions are responsible for the majority of the esophageal clearance of refluxed gastric contents, rather than by secondary and tertiary peristalsis as previously believed.102 Additionally, through the use of a concomitant 24-hour pH study and esophageal manometry, it has been shown that there is a direct relationship between worsening esophagitis secondary to GERD and deterioration of esophageal motility. Manometric evaluation has been particularly useful in documenting abnormal distal esophageal motility in infants after repair of EA/TEF.103 It is hoped that, as technology continues to provide more appropriately sized instruments for sophisticated manometric studies in infants and children, the usefulness and feasibility of such studies will increase our knowledge of the physiology and abnormalities associated with GERD in this population.
Endoscopic evaluation of the esophagus and stomach is occasionally needed in the diagnosis of GERD in infants and children. Hematemesis, dysphagia, irritability in infants, or dysphagia with or without heartburn in children, should prompt esophagogastroscopy to determine if esophagitis is present. Other complications, such as ulcer formation, esophageal stricture, and Barrett esophagus, can also be diagnosed during the endoscopic examination. Mucosal biopsy should be performed to stage the severity of esophagitis or to histologically exclude dysplasia or malignancy in Barrett esophagus.104,105
The relationship between delayed gastric emptying (DGE) and GERD in infants and children has been extensively studied and continues to be one of the more controversial aspects of antireflux surgery. The evaluation for DGE is undertaken using radionuclide scanning via a technetium-99-labeled meal. When documented preoperatively, DGE has not been shown to significantly improve when an emptying procedure is performed at the time of an antireflux procedure.106 In fact, one study evaluating patients with DGE undergoing fundoplication showed significantly improved gastric emptying for both solids and liquids after fundoplication alone.107 Neurologically impaired children with GERD have been shown to have DGE more often than neurologically normal children. Conflicting data regarding the benefit and complication rates for these patients undergoing emptying procedures at the time of their fundoplication have been reported as well.108 Based on these data, it is not recommended that an emptying procedure be performed for a patient with DGE and GERD unless a second operative intervention would place the patient at significant morbidity or mortality.
At our institution, the evaluation for GERD usually includes an upper gastrointestinal contrast study to evaluate for normal anatomy and no evidence of malrotation. We are moving away from using the pH probe in favor of clinical symptoms of GER, even utilizing nasogastric feeding trials with close clinical observation. If there is still uncertainty about the diagnosis, a pH probe/impedance study is performed. The main exception is the baby with significant underlying airway disease in the neonatal intensive care unit who needs a gastrostomy for feeding and for whom the intensivists request a fundoplication to protect the airway from aspiration. The neurologically impaired infant requiring a gastrostomy may be another exception. Esophagogastroscopy and esophageal manometry are employed only when circumstances suggest that the information they will provide will dictate changes in the operative management. An example of this situation is the patient with symptoms of GER but a normal pH study. When esophagitis or other complications of GERD are found after esophagogastroscopy or manometry, surgical intervention is usually recommended. Preoperative gastric emptying studies are not performed on a routine basis, primarily owing to the improvement that has been seen and reported in gastric emptying after fundoplication.106,107 If symptoms of DGE persist after antireflux surgery, gastric emptying studies can be performed with a subsequent emptying procedure, if necessary. However, all patients requiring a second fundoplication undergo an emptying study to be sure their recurrent symptoms are not exacerbated by DGE.
Treatment
Once GERD has been diagnosed, the question becomes: should medical or surgical treatment be applied?15 This decision needs to be individualized based on the patient’s age, anatomic information, disease severity, and social environment (which will affect compliance with a treatment regimen). In the majority of cases, nonoperative treatment is the initial therapy of choice.
Medical Management
Position and Feeding
Nonoperative therapy for GERD in infants and children has been based on postural changes and dietary modification for many years. It is important to know the caloric needs of the patient so that a reduction in feeding volume in an attempt to limit reflux does not result in caloric deprivation. Postural and dietary modifications alone will result in clinical improvement in the vast majority of infants with GERD.109,110 In older children, dietary alterations should include a diet low in fat and the elimination of chocolate, coffee, tea, carbonated drinks, and spicy foods.
The seated semi-upright position (approximately 45°) for an infant with reflux has been recommended since the 1950s. In the 1960s, Carré showed that 60% of children with GERD treated in this way improved by approximately 2 years of age and an additional 30% improved by age 4 years.1,111
Failure of postural therapy may be related to social problems, chronic infections, or impaired gastric clearance. In older patients, postural treatment is impractical because of the virtual impossibility of maintaining the desired semi-sitting posture for sleep. Close attention to the details of postural therapy by the family members is most important to its success.112
Prokinetic Agents
Historically, prokinetic agents have been utilized in an attempt to increase LES pressure, enhance esophageal peristalsis, and accelerate gastric emptying. The use of cisapride and, more recently, metoclopramide has been questioned with regard to their safety.113–115 In fact, cisapride is no longer available due to safety concerns. Both randomized controlled trials and meta-analyses have shown no clinically relevant improvement in children receiving cisapride or metoclopramide.113,114,116,117 Therefore, the current recommendation regarding prokinetic agents in the management of GERD is that there is no beneficial effect and their use is not advantageous.
Acid Alteration
Measures to reduce gastric acidity should be undertaken for patients with complicated acid reflux, especially with esophagitis.118 Alterations in gastric acid may be accomplished by neutralization with antacids, by competition with histamine-2 (H2)-receptor antagonists, or by PPIs. Because of the superiority of PPIs in controlling acid production, H2-receptor antagonists or antacids are being utilized less frequently.
PPIs inhibit the final step of gastric acid secretion by blocking proton production by bonding and deactivating H+, K+-ATPase (or proton pump) by traversing the parietal cell membrane and accumulating in the secretory canaliculi.119 The PPI omeprazole has been demonstrated to reduce gastric acid production to zero.120–122 It is a very powerful medicine that affects gastric acid production for 72 hours after cessation of administration. A prospective study determined that, within the therapeutic dose range (0.7–3.5 mg/kg/day), omeprazole was both efficacious and safe for children.121 In this study, omeprazole was found to be highly effective in severe (grade IV) esophagitis and patients refractory to other medical therapy. A dosage of 0.7 mg/kg/day healed 45% of patients, and 1.4 mg/kg/day healed another 30%. On a body weight basis, the dosages required in children are generally higher than those in adults.122 For children unable to swallow the whole capsule, it is suggested to open the capsule and give the granular contents in a weakly acidic vehicle such as orange juice, yogurt, or cranberry juice. The granules are stable in acid but are degraded in a neutral or alkaline pH. Multiple PPIs are now available. However, the use of PPIs is limited to acid reflux and will have no benefit for the patient with non-acid reflux.
Operative Management
Another scenario is the infant who presents with an ALTE spell and GER is documented but no other etiology is identified. This patient may be best served with a fundoplication as the initial therapy. In a review from our institution involving 81 infants presenting with ALTE, their symptoms resolved with fundoplication in 78.123 The median follow-up in this study was 1,738 days. Two required a second fundoplication when their symptoms recurred, and one needed a pyloromyotomy. Interestingly, 96.3% of these patients had been treated with antireflux mediation and 87.7% were taking antireflux medications at the time of their ALTE. Therefore, medical management may not be effective in this population.
Laparoscopic Nissen Fundoplication
The patient is placed at the end of the operating table so that the surgeon can stand at the foot of the bed and the assistant to his or her right. The scrub nurse stands to the surgeon’s left (Fig. 28-2). For infants, the legs should be placed in a frog-leg position. For older children, the lithotomy position can be used with stirrups. Neurologically impaired children may have contractures that preclude lithotomy, and careful consideration should be given to ensure they have appropriate padding of their pressure points. Although a single monitor placed over the patient’s head is usually sufficient, two monitors, placed to the right and the left of the patient’s head, can be used as well. An orogastric tube is introduced by the anesthesiologist to decompress the stomach. The bladder is usually emptied using a Credé maneuver.
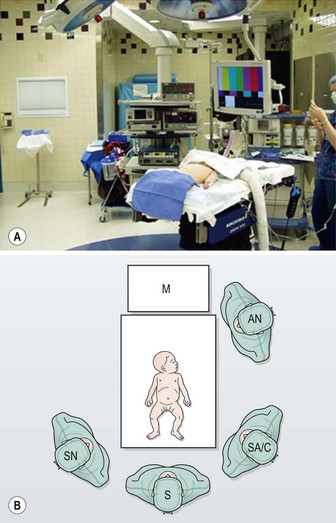
FIGURE 28-2 For laparoscopic fundoplication, the patient is placed supine on the operating table. Infants and young children are positioned at the foot of the bed in a frog-leg position and the foot of the bed is dropped. The surgeon (S) and surgical assistant/camera holder (SA/C) stand next to the patient at the end of the bed. The scrub nurse (SN) is to the surgeon’s left. A single monitor (M) is placed over the patient’s head. AN, anesthesiologist. (Adapted from Holcomb GW III. Laparoscopic Nissen fundoplication. In: Holcomb GW, Georgeson KE, Rothenberg SS, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008. p. 15–20.)
After prepping and draping, a 5 mm vertical incision is made in the center of the umbilicus and carried down through the umbilical fascia. A Step sheath (Covidien, Mansfield, MA) is gently introduced into the abdominal cavity, followed by introduction of a cannula with a blunt-tipped trocar through the sheath. By using this open technique, injury to the underlying viscera should be extremely rare. The sheath can be secured to the umbilical skin for stabilization should the surgeon desire. A pneumoperitoneum is created to a pressure of 12–15 mmHg, and diagnostic laparoscopy is performed with a 5 mm, 45°-angled telescope. Four stab incisions are then placed in infants, and three stab incisions and a 5 mm port for the ultrasonic scalpel are utilized in children older than 5 years of age. The arrangement of these cannulas is seen in Figure 28-3. A liver retractor is introduced through the lateral right port. The two main working sites are the instruments positioned on either side of the midline. The assistant’s instrument is in the patient’s left lateral abdomen.
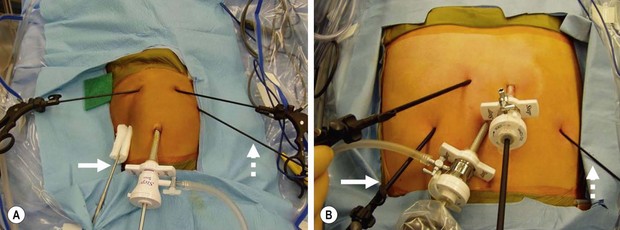
FIGURE 28-3 There are a number of ways to orient the instruments when performing a laparoscopic fundoplication. With our technique, a 45°-angled, 5 mm telescope is introduced after insertion of the 5 mm umbilical cannula. The liver retractor is introduced in the patient’s right subcostal region (solid arrow). The two main working ports are in the left and right epigastrium. The main working port for the surgeon is the one in the patient’s left epigastric region. It is through this incision that dissecting instruments, needle holder, and suture are introduced. The instrument utilized by the surgical assistant is in the patient’s left subcostal region (dotted arrow). The stab incision technique can be utilized for both (A) infants and (B) adolescents. (From Holcomb GW III. Laparoscopic Nissen fundoplication. In: Holcomb GW, Georgeson KE, Rothenberg SS, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008. p. 15–20.)
We have standardized our technique and have utilized it for the past 12 years.124–129 Initially, the superior short gastric vessels are ligated and divided. Electrocautery connected to a Maryland dissecting instrument is used in the younger patients. As previously mentioned, the ultrasonic scalpel is used in older children. The retroesophageal window is initially made from the patient’s left side because it is easy to accomplish after mobilization and ligation of the superior short gastric vessels. We do not mobilize the esophagus very much to help reduce postoperative transmigration of the fundoplication wrap. (This will be discussed later.) Once the left side of the patient’s gastroesophageal junction has been identified, the stomach is flipped to the patient’s left and attention is turned toward the right aspect of the esophagus and upper stomach. The gastrohepatic ligament is incised to expose the esophagus and stomach on the right side. Great care must be taken to always know the location of the left gastric artery. It is imperative that the fundoplication wrap is positioned above the left gastric artery rather than inferior to it. The opening in the retroesophageal window is then completed from the right side so that the fundus can be brought through posteriorly for the Nissen fundoplication. Again, as little esophageal mobilization as possible is performed at the gastroesophageal junction (Fig. 28-4).

FIGURE 28-4 If an adequate length of intra-abdominal esophagus is present, then as little dissection as possible is performed to help prevent migration of the fundoplication wrap through an enlarged esophageal hiatus. The phrenoesophageal ligament is kept intact on both the patient’s (A) right side and (B) left side of the esophagus. Note creation of the retroesophageal window has been initiated (arrows). (From Holcomb GW III. Laparoscopic Nissen fundoplication. In: Holcomb GW, Georgeson KE, Rothenberg SS, editors. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008. p. 15–20.)
At this point, usually a single suture is placed posterior to the esophagus to close a small hiatal hernia that may have either been present initially or created during the dissection. This is usually accomplished with a 2-0 silk suture. After placement of this suture and tying it, a small bite of the esophagus at the 7 o’clock position is then taken with the same needle and tied to help obliterate the space between the posterior esophagus and the posterior crural closure. Next, esophagocrural sutures are then created with 3-0 silk at the 8, 11, 1, and 5 o’clock positions to further obliterate the space between the esophagus and the crura to prevent transmigration of the fundoplication wrap (Fig. 28-5).127 However, the utility of these stitches is not known if the phrenoesophageal membrane is preserved. After placement of these crural sutures, the bougie is then introduced. A table describing the appropriate bougie size for neonates weighing less than 15 kg has been developed and validated (Table 28-3).124 The fundoplication is then performed using a standard Nissen technique. Usually, three 2-0 sutures are utilized to perform the fundoplication. The most superior suture also incorporates a small portion of the anterior esophagus to anchor the wrap around the intra-abdominal esophagus. The length of the fundoplication is measured. Usually a length of approximately 2 cm is desired.124 For older children, 2.5–3.0 cm may be appropriate.
TABLE 28-3
Recommended Bougie Size for Esophageal Calibration in Patients Weighing Less Than 15 kg
| Weight (kg) | Bougie Size |
| 2.5–4.0 | 20–24 |
| 4.0–5.5 | 24–28 |
| 5.5–7.0 | 28–32 |
| 7.0–8.5 | 32–34 |
| 8.5–10.0 | 34–36 |
| 10.0–15.0 | 36–40 |
From Ostlie DJ, Miller KA, Holcomb GW III. Effective Nissen fundoplication length and bougie diameter size in young children undergoing laparoscopic Nissen fundoplication. J Pediatr Surg 2002;37:1664–6.
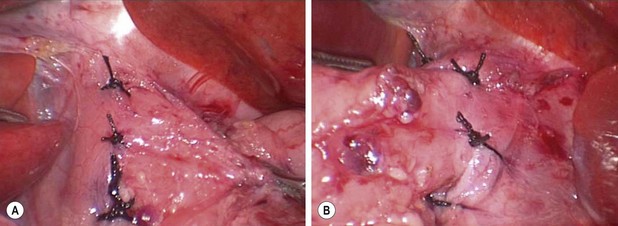
FIGURE 28-5 Following closure of the esophageal hiatus with a 2-0 silk suture placed posterior to the esophagus, esophagocrural sutures are placed at the 8, 11, 1, and 5 o’clock positions around the esophagus. These photographs show the (A) right and (B) left sides of the patient’s esophagus. The purpose of these sutures is to secure the esophagus in the intra-abdominal position to reduce the incidence of postoperative reflux and also to obliterate the space between the esophagus and crura in an effort to prevent transmigration of the fundoplication wrap.
Gastrostomy
A red rubber catheter is introduced by the anesthesiologist into the stomach which is then insufflated with 30–60 mL of air to prevent incorporating the back wall of the stomach with the suture utilized to secure the stomach to the anterior abdominal wall. The anterior wall of the stomach is grasped with a locking grasper and brought toward the anterior abdominal wall. The technique for laparoscopic gastrostomy is seen in Figure 28-6. Two 2-0 PDS sutures (Ethicon, Inc., Somerville, NJ) are placed through the anterior abdominal wall cephalad to the grasper, through the stomach, and out through the anterior abdominal wall inferior to the instrument that has been used to grasp the stomach. Next, a needle followed by a guide wire is introduced through the abdominal wall and stomach in the center of the square formed by the two PDS sutures. Dilators from a Cook Vascular Dilator Set (Cook, Inc., Bloomington, IN) are used to serially dilate the anterior abdominal wall and gastrotomy. In infants, a 16 French dilator is usually the largest needed. In older children, the 20 French dilator may be required. The gastrostomy button is then placed over the guide wire and into the stomach. Under visualization, the balloon on the Mic-Key (Ballard Medical Products, Draper, UT) gastrostomy button is inflated. Attention must be paid to be sure that the button is, in fact, in the stomach and not external to the stomach (Fig. 28-7). This can also be confirmed with the angled telescope by looking around each side of the stomach with the button in place. The PDS sutures are then secured over the button to prevent its dislodgement. Our protocol is to cut these sutures in five days. Others may cut them sooner. This technique was initially described by Georgeson and Owings, and details about complications have been published.130,131
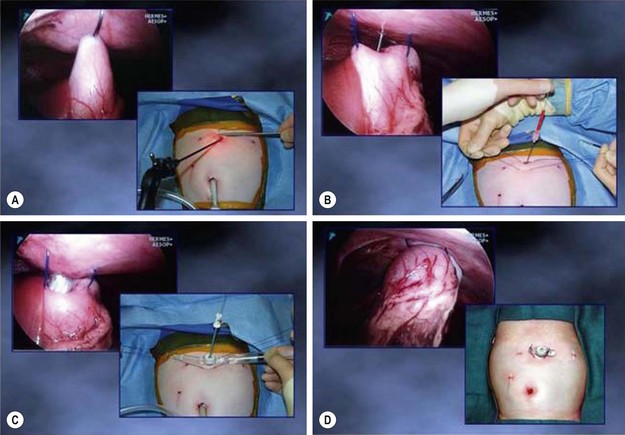
FIGURE 28-6 (A) After approximation of the stomach to the anterior abdominal wall, two sutures of 2-0 or 0 PDS (depending on the patient’s age) are placed extracorporeally through the abdominal wall, through the stomach, and out through the abdominal wall inferior to the gastrostomy. (B) After placing the extracorporeal sutures, an 18 gauge needle is introduced through the left epigastric incision and into the stomach under direct visualization. Following a rush of air through the needle, a guide wire is inserted through the needle and the needle is removed. With the guide wire in place, the tract is serially dilated using the Cook Vascular Dilator Set (Cook, Inc., Bloomington IN). These dilators come in 8, 12, 16, and 20 French sizes. (C) After dilating the tract and gastrostomy with the 20 French dilator, the 8 French dilator is placed through the Mic-Key gastrostomy button and is introduced over the guide wire and into the stomach. (D) After placement of the button within the stomach, the balloon on the button is inflated, the guide wire and dilator are removed, and the extracorporeal sutures are tied over the button to secure it to the anterior abdominal wall. (From Holcomb GW III. Gastroesophageal reflux in infants and children. In: Fischer JE, editor. Mastery of Surgery. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 650–1.)

FIGURE 28-7 After the button is introduced into the stomach and inflated, it is very important to insure that the button is, in fact, in the gastric lumen. Often, it is helpful to take an angled telescope (70°) to look around the portion of the stomach that is adherent to the anterior abdominal wall so that one can feel secure that the button is not outside the stomach. In this patient, the button was deflated and removed, and then reinserted correctly.
Postoperative Care
If the patient did not need a gastrostomy, then liquids are allowed several hours after the procedure. It is very important to mention to the family that there is initial edema around the fundoplication. Therefore, for the first three weeks, especially in older children, the diet should be a mechanical soft diet that has the consistency of pudding, apple sauce, mashed potatoes, and so on. Essentially, meats and pizza should not be allowed because these food substances can become lodged above the fundoplication wrap. After three weeks, the edema usually resolves and small portions of meats and pizza can be added to the diet.
Outcomes
Our group has been interested in the efficacy of laparoscopic fundoplication for the past 12 years. A number of articles have been published from our institution detailing our thoughts about indications, complications, the operative technique, and ways to improve our results.123-129,132–137 Also, there have been several articles published in the last few years looking at long-term outcomes.138–142
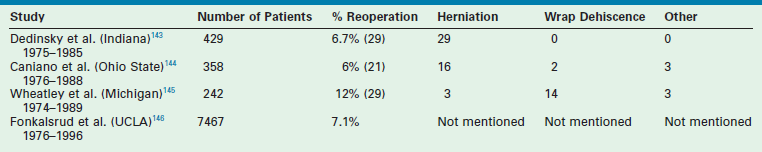
In early 2002, in looking at our outcomes from January 2000, through March 2002, we believed that the need for repeat fundoplication was higher than desired.127 In 130 patients undergoing laparoscopic Nissen fundoplication during that time, the incidence of repeat fundoplication was 12%. All patients who required a repeat operation had transmigration of the fundoplication wrap. During that time period, the esophagus was being extensively mobilized to try to create at least a 2 cm length of intra-abdominal esophagus. Moreover, there was no attempt to obliterate the space between the esophagus and the crura. These principles derived from prior training as well as literature reports in adults.22,23 Although the operations proceeded nicely and no conversions were needed, this 12% incidence of re-do procedures seemed high. However, historical reports for the open operation have also documented a relatively high incidence of repeat fundoplications from 6–12% (Table 28-4).60,143–146
In an attempt to reduce the incidence of postoperative transmigration of the wrap, two modifications were made in our operative technique beginning in April, 2002. First, there was minimal mobilization of the esophagus. It was believed that the main reason for wrap transmigration was that the esophagus was being mobilized and a space was being created between the esophagus and crura to allow for the transmigration to occur. Therefore, the phrenoesophageal membrane was kept intact to obliterate this space. Second, to further reinforce this space, sutures were placed between the esophagus and crura. Initially, only two sutures were used, but eventually four sutures have come to be placed for the purpose of further obliterating this space (see Fig. 28-5). No other modifications in the operative technique were made. In looking at the results from April, 2002, through December, 2004, the incidence of transmigration was reduced to 5%.121 This was actually reduced even further when looking at the patients in whom four esophageal crural sutures were utilized rather than two or three.
In 2005, conversations with Georgeson and colleagues at the University of Alabama–Birmingham, prompted a prospective, randomized trial looking at the operative technique.27 It was believed that the efficacy of esophageal mobilization should be evaluated. The primary endpoint was transmigration of the wrap. A power analysis based on the difference between the 12% and 5% repeat fundoplication rate previously mentioned was made, and the study was powered at 360 patients. The patients were randomized on the day of the surgery. One group was randomized to receive minimal esophageal mobilization with placement of four esophagocrural sutures. The other group was randomized to extensive esophageal mobilization to create a 2 cm length of intra-abdominal esophagus along with the four esophagocrural sutures. Also, patients were randomized according to neurological status. In addition, all patients received an upper gastrointestinal series at one-year postoperatively to evaluate for transmigration of the fundoplication wrap.27
The study was stopped early after 177 patients had been entered because the primary outcome overwhelmingly favored minimal esophageal mobilization with an 8% transmigration rate in the minimal dissection group compared to a 30% rate in the maximal dissection group (p = 0.002). Neurological status did not impact these outcomes. Furthermore, reoperation rates were higher in the maximal dissection group compared to the minimal dissection group (18% versus 3%, p = 0.006).27 It is clear that minimal dissection in the pediatric patient without a hiatal hernia is important to prevent postoperative transmigration of the fundoplication wrap. The need for the four reinforcing esophagocrural stitches is currently being investigated in a prospective, randomized trial at our center.
Repeat Fundoplication
As mentioned previously, the goal of the initial fundoplication is to control GERD but also to prevent the need for a second operation.147 In 2006, our group looked at our experience with re-do fundoplications.148,149 Of 273 patients who underwent laparoscopic fundoplication by the senior surgeon (GWH) between January 2000 and April 2006, 21 required a re-do fundoplication (Fig. 28-8).148 The re-do operative technique generally fell into two groups. In one group, it was performed laparoscopically without the use of mesh to reinforce the large hiatal closure that had developed after transmigration of the wrap. In the other group, acellular small intestinal submucosa (Surgisis [SIS], Cook, Inc, Bloomington IN) was used to reinforce the hiatal closure because it was believed that a great deal of tension was needed to close the large muscular defect (Fig. 28-9). Initially four-ply SIS was used, but eight-ply SIS was employed when it became available. In the patients undergoing the second operation without Surgisis, three required a second re-do or third overall fundoplication. To date, no patient has required another repeat operation in which SIS was placed at the time of the initial repeat procedure.148
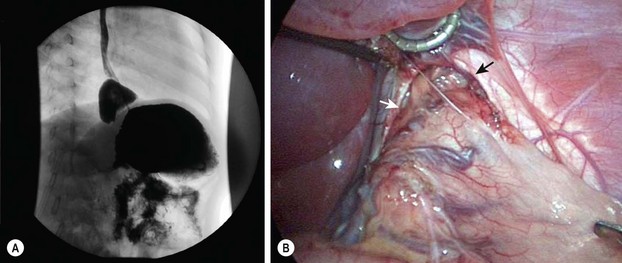
FIGURE 28-8 In 5–10% of patients undergoing laparoscopic fundoplication, reoperation becomes necessary. (A) In our experience, almost all reoperations are due to transmigration of the fundoplication wrap, which is seen on this upper gastrointestinal study. (B) The intraoperative photograph shows a large esophageal hiatus (arrows) with transmigration of the fundoplication wrap and upper stomach into the lower mediastinum. Note the significant lack of adhesions after the initial laparoscopic fundoplication. (From Ostlie DJ, Holcomb GW III. Reiterative surgery for gastroesophageal reflux. Semin Pediatr Surg 2007;16:252–8.)
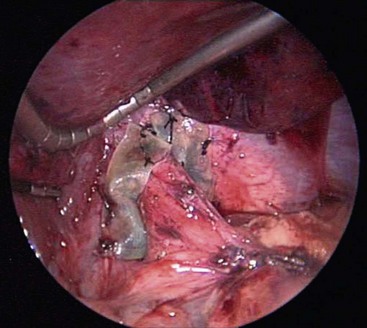
FIGURE 28-9 This intraoperative photograph shows the eight-ply Surgisis that has been wrapped around the esophagus and is overlapped anterior to the esophagus. The eight-ply Surgisis is secured to the esophagus medially and the diaphragm laterally with interrupted 3-0 silk sutures. It is employed to help reinforce the closure of the crura at the time of repeat fundoplication.
At the same time as our concept developed about reinforcing the hiatal closure with Surgisis, a multi-institution, prospective randomized trial was being performed in adults.149 The investigators were looking at the efficacy of placing 4-ply Surgisis at the time of initial repair of a large paraesophageal hernia defect to help prevent recurrence. This trial also closed early because of the marked disparity in results favoring the use of Surgisis to help close the large diaphragmatic defect. The primary outcome variable was recurrence of the paraesophageal hernia. The study closed at 108 total patients because, at the time of interim review, 12 patients (24%) had developed a recurrence in the arm in which Surgisis was not used, and only four patients (9%) had a recurrent paraesophageal hernia in the Surgisis arm.
Laparoscopic fundoplication has evolved into the preferred technique for surgical management of GERD. Although the Nissen operation is generally performed, similar results have been noted with the Thal operation.150–153 It is only through critical evaluation of one’s experience that advances are made in improving the results. There is no doubt that patients have less discomfort and earlier discharge from the hospital after the laparoscopic operation.136 Moreover, there is a faster return to regular activities as well. However, the operative technique continues to need ongoing evaluation with proper data collection and critical analysis to improve these results.
References
1. Carré, IJ. The natural history of the partial thoracic stomach in children. Arch Dis Child. 1959; 34:344–353.
2. Fonkalsrud, EW, Foglia, RP, Ament, ME, et al. Operative treatment for the gastroesophageal syndrome in children. J Pediatr Surg. 1989; 24:525–529.
3. Billard, P, Maladie des Enfants Nouveau-Nés París, 1828.
4. Allison, PR, Johnston, AS, Royce, GB. Short esophagus with simple peptic ulceration. J Thorac Cardiovasc Surg. 1943; 12:432.
5. Tileston, W. Peptic ulcer of the esophagus. Am J Med Sci. 1906; 132:240.
6. Winkelstein, A. Peptic esophagitis: A new clinical entity. JAMA. 1935; 104:906.
7. Thal, AP. A unified approach to surgical problems of the esophagogastric junction. Ann Surg. 1968; 168:542–549.
8. Lortat-Jacob, JL. Le traitment chirurgical des maladies du reflux gastroesophagienne. Presse Med. 1957; 65:457.
9. Hill, LD. An effective operation for hiatal hernia: An eight-year appraisal. Ann Surg. 1967; 166:681.
10. Hill, LD. Surgery and gastroesophageal reflux. Gastroenterology. 1972; 63:183.
11. Belsey, R. Surgery of the diaphragm. In: Brown JM, ed. Surgery of Children. Baltimore: Williams & Wilkins; 1963:762.
12. Belsey, R. Gastroesophageal Reflux and Hiatal Hernia. Boston: Little, Brown; 1972.
13. Nissen, R, Rossetti, M. Die Behandlung von Hiatushernie und Reflux-oesophagitis mit Gastropexie und Fundoplication. Stuttgart: Georg Thieme Verlag; 1959.
14. Nissen, R. Gastropexy and fundoplication in surgical treatment of hiatal hernia. Am J Dig Dis. 1961; 6:954–961.
15. Boix-Ochoa, J. The physiologic approach to the management of gastric esophageal reflux. J Pediatr Surg. 1986; 21:1032–1039.
16. Dallemagne, B, Weerts, JM, Jehaes, C, et al. Laparoscopic Nissen fundoplication: Preliminary report. Surg Laparosc En dosc. 1991; 1:138–143.
17. Georgeson, KE. Laparoscopic gastrostomy and fundoplication. Pediatr Ann. 1993; 92:675–677.
18. Lobe, TE, Schropp, KP, Lunsford, K. Laparoscopic Nissen fundoplication in childhood. J Pediatr Surg. 1993; 28:358–361.
19. Fike, FB, Mortellaro, VE, Pettiford, JN, et al. Diagnosis of gastroesophageal reflux disease in infants. Pediatr Surg Int. 2011; 27:791–797.
20. Yen, CJ, Izzo, JG, Lee, DF, et al. Bile acid exposure up-regulates tuberous sclerosis complex 1/mammalian target of rapamycin pathway in Barrett’s-associated esophageal adenocarcinoma. Cancer Res. 2008; 68:2632–2640.
21. Liebermann-Meffert, D, Allgower, M, Schmid, P, et al. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979; 76:31–38.
22. DeMeester, TR, Wernly, JA, Bryant, GH, et al. Clinical and in vitro analysis of determinants of gastroesophageal competence. Am J Surg. 1979; 137:39–46.
23. Branton, SA, Hinder, RA, Floch, NR, et al. Surgical treatment of gastroesophageal reflux disease. In: Castell DO, Richter JE, eds. The Esophagus. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 1999:511–525.
24. Werlin, SL, Dodds, WJ, Hogan, WJ, et al. Mechanisms of GER in children. J Pediatr. 1980; 97:244–249.
25. Cucchiara, S, Bartolotti, M, Minella, R, et al. Fasting and postprandial mechanisms of GER in children with GERD. Dig Dis Sci. 1993; 38:86–92.
26. Winans, CS, Harris, LD. Quantitation of lower esophageal sphincter competence. Gastroenterology. 1967; 52:773–778.
27. StPeter, SD, Barnhart, DC, Ostlie, DJ, et al. Minimal vs extensive esophageal mobilization during laparoscopic fundoplication: A prospective randomized trial. J Pediatr Surg. 2011; 46:163–168.
28. Thor, KB, Hill, LD, Mercer, DD, et al. Reappraisal of the flap valve mechanism in the gastroesophageal junction. Acta Chir Scand. 1987; 153:25–28.
29. Altschuler, SM, Boyle, JT, Nixon, TE, et al. Simultaneous reflex inhibition of lower esophageal sphincter and crural diaphragm in cats. Am J Physiol. 1985; 249:586–591.
30. Roussos, C, Macklem, PT. The respiratory muscles. N Engl J Med. 1982; 307:786–797.
31. Barak, N, Ehrenpreis, ED, Harrison, JR, et al. Gastro-oesophageal reflux disease in obesity: Pathophysiological and therapeutic considerations. Obes Rev. 2002; 3:9–15.
32. Min, F, Tarlo, SM, Bargman, J, et al. Prevalence and causes of cough in chronic dialysis patients. Adv Perit Dial. 2000; 16:129–133.
33. Navarro-Rodriguez, T, Hashimoto, CL, Carrilho, FJ, et al. Reduction of abdominal pressure in patients with ascites reduces gastroesophageal reflux. Dis Esophagus. 2003; 16:77–82.
34. Koivusalo, A, Rintala, R, Lindahl, H. Gastroesophageal reflux in children with a congenital abdominal wall defect. J Pediatr Surg. 1999; 34:1127–1129.
35. Jaillard, SM, Pierrat, V, Dubois, A, et al. Outcome at 2 years of infants with congenital diaphragmatic hernia: A population-based study. Ann Thorac Surg. 2003; 75:250–256.
36. Kamiyama, M, Kawahara, H, Okuyama, H, et al. Gastroesophageal reflux after repair of congenital diaphragmatic hernia. J Pediatr Surg. 2002; 37:1681–1684.
37. Fasching, G, Huber, A, Uray, E, et al. Gastroesophageal reflux and diaphragmatic motility after repair of congenital diaphragmatic hernia. Eur J Pediatr Surg. 2000; 10:360–364.
38. Bergmeijer, JH, Tibboel, D, Hazebroek, FW. Nissen fundoplication in the management of gastroesophageal reflux occurring after repair of esophageal atresia. J Pediatr Surg. 2000; 35:573–576.
39. Kubiak, R, Spitz, L, Kiely, EM, et al. Effectiveness of fundoplication in early infancy. J Pediatr Surg. 1999; 34:295–299.
40. Schalamon, J, Lindahl, H, Saarikoski, H, et al. Endoscopic follow-up in esophageal atresia—for how long is it necessary? J Pediatr Surg. 2003; 38:702–704.
41. Cadiot, G, Bruhat, A, Rigaud, D, et al. Multivariate analysis of pathophysiological factors in reflux oesophagitis. Gut. 1997; 40:167–174.
42. Ho, SC, Chang, CS, Wu, CY, et al. Ineffective esophageal motility is a primary motility disorder in gastroesophageal reflux disease. Dig Dis Sci. 2002; 47:652–656.
43. Holloway, RH. Esophageal body motor response to reflux events: Secondary peristalsis. Am J Med. 2000; 108(Suppl):205–265.
44. Jeffery, HE, Ius, D, Page, M. The role of swallowing during active sleep in the clearance of reflux in term and preterm infants. J Pediatr. 2000; 137:545–558.
45. Allen, ML, Zamani, S, Dimarino, AJ, Jr. The effect of gravity on esophageal peristalsis in humans. Neurogastroenterol Motil. 1997; 9:71–76.
46. Orenstein, SR. Effects on behavior state of prone versus seated positioning for infants with gastroesophageal reflux disease. Pediatrics. 1990; 85:765–767.
47. Vandenplas, Y, Hassall, E. Mechanisms of gastroesophageal reflux and gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2002; 35:119–136.
48. Vandenplas, Y, Sacre-Smits, L. Seventeen-hour continuous esophageal pH monitoring in the newborn: Evaluation of the influence of position in asymptomatic and symptomatic babies. J Pediatr Gastroenterol Nutr. 1985; 4:356–361.
49. Richter, JE. Importance of bile reflux in Barrett’s esophagus. Dig Dis Sci. 2001; 18:208–216.
50. Collen, MJ, Ciarleglio, CA, Stanczak, VJ, et al. Basal gastric acid secretion in children with atypical epigastric pain. Am J Gastroenterol. 1988; 83:923–926.
51. Kalach, N, Badran, AM, Jaffray, P, et al. Correlation between gastric acid secretion and severity of acid reflux in children. Turk J Pediatr. 2003; 45:6–10.
52. Boyle, JT. Acid secretion from birth to adulthood. J Pediatr Gastroenterol Nutr. 2003; 37:S12–S16.
53. Gibbons, TE, Gold, BD. The use of proton pump inhibitors in children: A comprehensive review. Paediatr Drugs. 2003; 5:25–40.
54. Gold, BD, Freston, JW. Gastroesophageal reflux in children: Pathogenesis, prevalence, diagnosis, and role of proton pump inhibitors in treatment. Paediatr Drugs. 2002; 4:673–685.
55. Penagini, R. Bile reflux and oesophagitis. Eur J Gastroenterol Hepatol. 2001; 13:1–3.
56. Todd, JAQ, de Caestecker, J, Jankowski, J. Gastro-esophageal reflux disease and bile acids. J Pediatr Gastroenterol Nutr. 2003; 36:172–174.
57. Richter, JE. Duodenogastric reflux-induced (alkaline) esophagitis. Curr Treat Options Gastroenterol. 2004; 7:53–58.
58. Vaezi, MF, Singh, S, Richter, JE. Role of acid and duodenogastric reflux in esophageal mucosal injury: A review of animal and human studies. Gastroenterology. 1995; 108:1897–1907.
59. Tovar, JA, Olivares, P, Diaz, M, et al. Functional results of laparoscopic fundoplication in children. J Pediatr Gastroenterol Nutr. 1998; 26:429–431.
60. Fonkalsrud, EW, Ashcraft, KW, Coran, AG, et al. Surgical treatment of gastroesophageal reflux in children: A combined hospital study of 7,467 patients. Pediatrics. 1998; 101:419–422.
61. Neuhauser, EBD, Berenberg, W. Cardio-esophageal relaxation as cause of vomiting in infants. Radiology. 1947; 48:480–483.
62. Luostarinen, M. Nissen fundoplication for reflux esophagitis: Long-term clinical and endoscopic results in 109 of 127 consecutive patients. Ann Surg. 1993; 217:329–337.
63. Richardson, JD, Kuhns, JG, Richardson, RL, et al. Properly conducted fundoplication reverses histologic evidence of esophagitis. Ann Surg. 1983; 197:763–770.
64. O’Neill, JA, Jr., Betts, J, Ziegler, MM, et al. Surgical management of reflux strictures of the esophagus in childhood. Ann Surg. 1982; 196:453–460.
65. Hyman, PE. Gastroesophageal reflux: One reason why baby won’t eat. J Pediatr. 1994; 125(Suppl):S103–S109.
66. Kountourakis, P, Ajani, JA, Davila, M, et al. Barrett’s esophagus: A review of biology and therapeutic approaches. Gastrointest Cancer Res. 2012; 5:49–57.
67. Wiseman, EF, Ang, YS. Risk factors for neoplastic progression in Barrett’s esophagus. World J Gastroenterol. 2011; 17:3672–3683.
68. Quante, M, Bhagat, G, Abrams, JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012; 21:36–51.
69. Hassall, E, Weinstein, WM, Ament, ME. Barrett’s esophagus in childhood. Gastroenterology. 1985; 89:1331–1337.
70. Othersen, HB, Jr., Ocampo, RJ, Parker, EF, et al. Barrett’s esophagus in children. Ann Surg. 1993; 217:676–681.
71. Lundell, L, Myers, JC, Jamieson, GG. The effect of antireflux operations on lower oesophageal sphincter tone and postprandial symptoms. Scand J Gastroenterol. 1993; 28:725–731.
72. Halper, LM, Jolley, SG, Tunnell, WP, et al. The mean duration of gastroesophageal reflux during sleep as an indicator of respiratory symptoms from gastroesophageal reflux in children. J Pediatr Surg. 1991; 26:686–690.
73. Foglia, RP, Fonkalsrud, EW, Ament, ME, et al. Gastroesophageal fundoplication for the management of chronic pulmonary disease in children. Am J Surg. 1980; 140:72–79.
74. del Rosario, JF, Orenstein, SR. Evaluation and management of gastroesophageal reflux and pulmonary disease. Curr Opin Pediatr. 1996; 8:209–215.
75. Jolley, SG, Herbst, JJ, Johnson, DG, et al. Esophageal pH monitoring during sleep identifies children with respiratory symptoms from gastroesophageal reflux. Gastroenterology. 1981; 80:1501–1506.
76. Hrabovsky, EE, Mullett, MD. Gastroesophageal reflux and the premature infant. J Pediatr Surg. 1986; 21:583–587.
77. Orenstein, SA. An overview of reflux-associated disorders in infants: Apnea, laryngospasm, and aspiration. Am J Med. 2001; 111:60S–63S.
78. Suwandhi, E, Ton, MN, Schwarz, TS. Gastroesophageal reflux in infancy and childhood. Pediatr Ann. 2008; 35:259–266.
79. Ramenofsky, ML, Powell, RW, Curreri, PW. Gastroesophageal reflux: pH probe-directed therapy. Ann Surg. 1986; 203:531–535.
80. Valusek, PA, St. Peter, SD, Keckler, SJ, et al. Does an upper gastrointestinal study change operative management for gastroesophageal reflux? J Pediatr Surg. 2010; 45:1169–1172.
81. Spencer, J. Prolonged pH recording in the study of gastroesophageal reflux. Br J Surg. 1969; 56:912–914.
82. Johnson, LF, DeMeester, TR. Twenty-four hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol. 1974; 62:325–332.
83. DeMeester, TR, Johnson, LF, Joseph, GJ, et al. Patterns of gastroesophageal reflux in health and disease. Ann Surg. 1976; 184:459–469.
84. Boix-Ochoa, J, Lafuente, JM, Gil-Vernet, JM. Twenty-four hour esophageal pH monitoring in gastroesophageal reflux. J Pediatr Surg. 1980; 15:74–78.
85. Koch, A, Gass, R. Continuous 20–24 hour esophageal pH monitoring in infancy. J Pediatr Surg. 1981; 16:109–113.
86. Stein, HJ, DeMeester, TR. Indications, technique, and clinical use of ambulatory 24-hour esophageal motility monitoring in a surgical practice. Ann Surg. 1993; 217:128–137.
87. Jolley, SG, Herbst, JJ, Johnson, DG, et al. Patterns of postcibal gastroesophageal reflux in symptomatic infants. Am J Surg. 1979; 138:946–950.
88. Jamieson, JR, Stein, HJ, DeMeester, TR, et al. Ambulatory 24-hour esophageal pH monitoring: Normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol. 1992; 87:1102–1111.
89. Colletti, RB, Christie, DL, Orenstein, SR. Indications for pediatric esophageal pH monitoring. J Pediatr Gastroenterol Nutr. 1995; 21:253–262.
90. Harada, T, Hyman, PE, Everett, S, et al. Meal-stimulated gastric acid secretion in infants. J Pediatr. 1984; 104:534–538.
91. Kelly, EJ, Newell, SJ, Brownlee, KG, et al. Gastric acid secretion in preterm infants. Early Hum Dev. 1993; 35:215–220.
92. Sondheimer, JM, Clark, DA, Gervaise, EP. Continuous gastric pH measurement in young and older healthy preterm infants receiving formula and clear liquid feedings. J Pediatr Gastroenterol Nutr. 1985; 4:352–355.
93. Mitchell, DJ, McClure, BG, Tubman, TRJ. Simultaneous monitoring of gastric and oesophageal pH reveals limitations of conventional oesophageal pH monitoring in milk fed infants. Arch Dis Child. 2001; 84:273–276.
94. Shay, SS, Johnson, LF, Richter, JE. Acid rereflux. Dig Dis Sci. 2003; 48:1–9.
95. Wenzl, TC, Moroder, C, Trachterna, M, et al. Esophageal pH monitoring and impedance measurement: A comparison of two diagnostic tests for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2002; 34:519–523.
96. Vela, MF, Camacho-Lobato, L, Srinivasan, R, et al. Simultaneous intraesophageal impedance and pH measurement of acid and non-acid gastroesophageal reflux: Effect of omeprazole. Gastroenterology. 2001; 120:1599–1606.
97. Sifrim, D, Holloway, RH, Silny, J, et al. Acid, non-acid and gas reflux in patients with gastroesophageal reflux disease during 24-hr ambulatory pH-impedance recordings. Gastroenterology. 2001; 120:1588–1598.
98. Kahrilas, P. Will impedance testing rewrite the book on GERD? Gastroenterology. 2001; 120:1862–1864.
99. Vela, MF. Multichannel intraluminal impedance and pH monitoring in gastroesophageal reflux disease. Expert Rev Gastroenterol Hepatol. 2008; 2:665–672.
100. Lopez-Alonso, M, Moya, MJ, Cabo, JA, et al. Twenty-four-hour esophageal impedance-pH monitoring in healthy preterm neonates: Rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics. 2006; 118:e299–e308.
101. Wise, JL, Murray, JA. Utilising multichannel intraluminal impedance for diagnosing GERD: A review. Dis Esophagus. 2007; 20:83–88.
102. Bremner, RM, Hoeft, SF, Costantini, MD, et al. Pharyngeal swallowing. Ann Surg. 1993; 218:364–370.
103. Shepard, R, Fenn, S, Seiber, WK. Evaluation of esophageal function in postoperative esophageal atresia and tracheoesophageal fistula. Surgery. 1966; 59:608–617.
104. Biller, JA, Winter, HS, Grand, RJ, et al. Are endoscopic changes predictive of histologic esophagitis in children? J Pediatr. 1983; 103:215–218.
105. Meyers, WF, Roberts, CC, Johnson, DG, et al. Value of tests for evaluation of gastroesophageal reflux in children. J Pediatr Surg. 1985; 20:515–520.
106. Brown, RA, Wynchank, S, Rode, H, et al. Is a gastric drainage procedure necessary at the time of antireflux surgery? J Pediatr Gastroenterol Nutr. 1997; 25:377–380.
107. Maddern, GJ, Jamieson, GG. Fundoplication enhances gastric emptying. Ann Surg. 1985; 201:296–299.
108. Maxson, RT, Harp, S, Jackson, RL, et al. Delayed gastric emptying in neurologically impaired children with gastroesophageal reflux: The role of pyloroplasty. J Pediatr Surg. 1994; 29:726–729.
109. Katz, PO. Treatment of gastroesophageal reflux disease: Use of algorithms to aid in management. Am J Gastroenterol. 1999; 94:3–10.
110. Orenstein, SR, Whitington, PF, Orestein, DM. The infant seat as a treatment for gastroesophageal reflux. N Engl J Med. 1983; 309:760–763.
111. Carré, IJ. Postural treatment of children with a partial thoracic stomach (hiatus hernia). Arch Dis Child. 1960; 35:569–580.
112. Ponsonby, AL, Dwyer, T, Gibbons, LE, et al. Factors potentiating the risk of sudden infant death syndrome associated with the prone position. N Engl J Med. 1993; 329:377–382.
113. Augood, C, MacLennan, S, Gilbert, R, et al. Cisapride treatment for gastro-oesophageal reflux in children. Cochrane Database Syst Rev. (4):2003.
114. Dalby-Payne, JR, Morris, AM, Craig, JC. Meta-analysis of randomized controlled trials on the benefits and risks of using cisapride for the treatment of gastroesophageal reflux in children. J Gastroenterol Hepatol. 2003; 18:196–202.
115. Enger, C, Cali, C, Walker, AM. Serious ventricular arrhythmias among users of cisapride and other QT-prolonging agents in the United States. Pharmacoepidemiol Drug Saf. 2002; 11:477–486.
116. Machida, HM, Forbes, DA, Gall, DG, et al. Metoclopramide in gastroesophageal reflux of infancy. J Pediatr. 1988; 112:483–487.
117. Rudolph, CD, Mazur, LJ, Liptak, GS, et al. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children. Recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001; 32:S1–31.
118. Dimand, RJ. Use of H2-receptor antagonists in children. Ann Pharmacother. 1990; 24(Suppl):42–46.
119. Wolfe, MM, Sachs, G. Acid suppression: Optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology. 2000; 118:S9–31.
120. Zimmermann, AE, Walters, JK, Katoma, BG, et al. A review of omeprazole use in the treatment of acid-related disorders in children. Clin Ther. 2001; 2385:660–679.
121. Hassall, E, Israel, D, Shepherd, R, et al. Omeprazole for treatment of chronic erosive esophagitis in children: A multicenter study of efficacy, safety, tolerability and dose requirements. International Pediatric Omeprazole Study Group. J Pediatr. 2000; 137:800–807.
122. Andersson, T, Hassall, E, Lundborg, P, et al. Pharmacokinetics of orally administered omeprazole in children. International Pediatric Omeprazole Pharmacokinetic Group. Am J Gastroenterol. 2000; 95:3101–3106.
123. Valusek, PA, St. Peter, SD, Tsao, K, et al. The use of fundoplication for prevention of apparent life-threatening events. J Pediatr Surg. 2007; 42:1022–1025.
124. Ostlie, DJ, Miller, KA, Holcomb, GW, III. Effective Nissen fundoplication length and bougie diameter size in young children undergoing laparoscopic Nissen fundoplication. J Pediatr Surg. 2002; 37:1664–1666.
125. Ostlie, DJ, Miller, KA, Woods, RK, et al. Single cannula technique and robotic telescopic assistance in infants and children who require laparoscopic Nissen fundoplication. J Pediatr Surg. 2003; 38:111–115.
126. Ostlie, DJ, Holcomb, GW, III. Laparoscopic fundoplication in infants and children. In: Langer JC, Albanese CT, eds. Pediatric Minimal Access Surgery: A Principle and Evidence Based Approach. New York: Marcel Dekker; 2005:167–190.
127. St. Peter, SD, Valusek, PA, Calkins, CM, et al. Use of esophagocrural sutures and minimal esophageal dissection reduces the incidence of postoperative transmigration of laparoscopic Nissen fundoplication wrap. J Pediatr Surg. 2007; 42:25–30.
128. Holcomb, GW, III. Gastroesophageal reflux in infants and children. In: Fischer JE, ed. Mastery of Surgery. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2012:769–780.
129. Holcomb, GW, III. Laparoscopic Nissen fundoplication. In: Holcomb GW, III., Rothenberg SS, Georgeson KW, III., eds. Atlas of Pediatric Laparoscopy and Thoracoscopy. Philadelphia: Elsevier; 2008:15–20.
130. Aprahamian, CJ, Morgan, TL, Harmon, CM, et al. U-stitch laparoscopic gastrostomy technique has a low rate of complications and allows primary button placement: Experience with 461 pediatric procedures. J Laparoendosc Adv Surg Tech A. 2006; 16:643–649.
131. Georgeson, K, Owings, E. Surgical and laparoscopic techniques for feeding tube placement. Gastrointest Endosc Clin North Am. 1998; 8:581–592.
132. Ostlie, DJ, Holcomb, GW, III., et al. Clinical principles of abdominal surgery. In: Oldham KT, Colombani PM, Foglia RP, eds. Surgery of Infants and Children. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2005:1067–1086.
133. Ostlie, DJ, Holcomb, GW, III. Laparoscopic fundoplication with gastrostomy. Semin Pediatr Surg. 2002; 11:196–204.
134. Holcomb, GW, III. Laparoscopic fundoplication in an infant. Surg Endosc. 2003; 17:1319.
135. St. Peter, SD, Holcomb, GW, III. Gastroesophageal reflux disease and fundoplication in infants and children. Ann Pediatr Surg. 2007; 3:1–10.
136. Ostlie, DJ, St. Peter, SD, Snyder, CL, et al. A financial analysis of pediatric laparoscopic versus open fundoplication. J Laparoendosc Adv Surg Tech. 2007; 17:493–496.
137. Barsness, KA, St. Peter, SD, Holcomb, GW, 3rd., et al. Laparoscopic fundoplication after previous open abdominal operations in infants and children. J Laparoendosc Adv Surg Tech A. 2009; 1:S47–S49.
138. Davis, CS, Baldea, A, Johns, JR, et al. The evolution and long-term results of laparoscopic antireflux surgery for the treatment of gastroesophageal reflux disease. JSLS. 2010; 14:332–341.
139. Rhee, D, Zhang, Y, Chang, DC, et al. Population-based comparison of open vs laparoscopic esophagogastric fundoplication in children: Application of the Agency for Healthcare Research and Quality pediatric quality indicators. J Pediatr Surg. 2011; 46:648–654.
140. Mauritz, FA, van Herwaarden-Lindeboom, MY, Stomp, W, et al. The effects and efficacy of antireflux surgery in children with gastroesophageal reflux disease: A systematic review. J Gastrointest Surg. 2011; 15:1872–1878.
141. Esposito, C, De Luca, C, Alicchio, F, et al. Long-term outcome of laparoscopic Nissen Procedure in pediatric patients with gastroesophageal reflux disease measured using the modified QOSG Roma III European Society for Pediatric Gastroenterology and Hepatology and Nutrition’s Questionnaire. J Laparoendosc Adv Surg Tech. 2012; 22:937–940.
142. Kubiak, R, Andrews, J, Grant, HW. Long-term outcome of laparoscopic Nissen fundoplication compared with laparoscopic Thal fundoplication in children: A prospective, randomized study. Ann Surg. 2011; 253:44–49.
143. Dedinsky, GK, Vane, DW, Black, T, et al. Complications and reoperation after Nissen fundoplication in childhood. Am J Surg. 1987; 153:177–183.
144. Caniano, DA, Ginn-Pease, ME, King, DR. The failed antireflux procedure: Analysis of risk factors and morbidity. J Pediatr Surg. 1990; 25:1022–1025.
145. Wheatley, MJ, Coran, AG, Wesley, JR, et al. Redo fundoplication in infants and children with recurrent gastroesophageal reflux. J Pediatr Surg. 1991; 26:758–761.
146. Fonkalsrud, WE, Ashcraft, KW, Coran, AG, et al. Surgical treatment of gastroesophageal reflux in children: A combined hospital study of 7467 patients. Pediatrics. 1998; 101:419–422.
147. Ostlie, DJ, Holcomb, GW, III. Reiterative laparoscopic surgery for recurrent gastroesophageal reflux. Semin Pediatr Surg. 2007; 16:252–258.
148. St. Peter, SD, Ostlie, DJ, Holcomb, GW, III. The use of biosynthetic mesh to enhance hiatal repair at the time of redo Nissen fundoplication. J Pediatr Surg. 2007; 42:1298–1301.
149. Oelschlager, BK, Pellegrini, CA, Hunter, J, et al. Biologic prosthesis reduces recurrence after laparoscopic paraesophageal hernia repair. Ann Surg. 2006; 244:481–490.
150. Esposito, C, Montupet, P, van der Zee, D, et al. Long-term outcome of laparoscopic Nissen, Toupet, and Thal antireflux procedures for neurologically normal children with gastroesophageal reflux disease. Surg Endosc. 2006; 20:855–858.
151. van der Zee, DC, Bax, KN, Ure, BM, et al. Long-term results after laparoscopic Thal procedure in children. Semin Laparosc Surg. 2002; 9:168–171.
152. Esposito, C, Becmeur, F, Centonze, A, et al. Laparoscopic reoperation following unsuccessful antireflux surgery in childhood. Semin Laparosc Surg. 2002; 9:177–179.
153. Esposito, C, van der Zee, DC, Settimi, A, et al. Risks and benefits of surgical management of gastroesophageal reflux in neurologically impaired children. Surg Endosc. 2003; 17:708–710.