Chapter 54 Gastroenterologic Perspectives on Natural Orifice Transluminal Endoscopic Surgery (NOTES)
Introduction
Through opposite trends of invasiveness, gastrointestinal (GI) endoscopy and laparoscopic surgery have converged to produce an intriguing but still largely experimental field termed natural orifice transluminal endoscopic surgery (NOTES). Since the publication in 2004 by Kalloo and colleagues1 on transgastric peritoneoscopy in the porcine model, NOTES continues to develop as a minimally invasive, “scar-less” technique for intraperitoneal and more recently intrathoracic surgeries. The theoretical advantages of NOTES include cosmesis; decreased anesthesia time; shortened postoperative recovery; and elimination of postsurgical complications associated with transabdominal and transthoracic incisions, such as skin infections, wound dehiscence, and hernias. Early questionnaire studies indicated that patients would prefer a NOTES approach over a traditional laparoscopic approach primarily because of issues surrounding pain and visible scars.2
Current State of Natural Orifice Transluminal Endoscopic Surgery (NOTES) Research
Since the first NOTES publication by Kalloo and colleagues in 2004,1 there has been progress toward human NOTES in numerous international centers but only a handful of U.S. centers. As of July 2009, the Natural Orifice Surgery Consortium for Assessment and Research NOTES registry had 166 human cases from eight U.S. sites and two international sites, 162 of which were approved by an institutional review board.3 SILS cases were not included in this registry. Most of these natural orifice cases were performed via the transvaginal route because of the historical precedent of culdoscopy performed by gynecologists, a reduced concern regarding sterility, and a decreased need for “secure” closure of the posterior fornix access point compared with enteral closure. Most of these cases also required laparoscopic assistance and would be more accurately categorized as hybrid NOTES procedures. Of the few pure NOTES procedures, Swanstrom and coworkers4 succeeded in performing transgastric cholecystectomy. Other pure NOTES procedures on closer examination have been hybrid transgastric procedures. There have been two small published case series of transgastric cholecystectomies assisted by the use of one to three transabdominal trocars.5,6 There has also been a series of 10 diagnostic transgastric peritoneoscopies performed after laparoscopic exploration before Whipple’s surgery,7 but the challenge of access closure was not directly addressed. Generally, most pure NOTES publications to date have been animal and human cadaveric studies.
Of the eight fundamental obstacles to NOTES identified in the original 2005 White Paper (Box 54.1),8 the most challenging obstacles to pure NOTES human studies have been the ones heavily dependent on device development.9 Two specific areas of technical need are highlighted here. First, current closure devices remain in various stages of development with none emerging as the clear favorite. Viscerotomy closure must be 100% secure and ideally be easy to perform and reproducible for any chance of clinical adoption of NOTES. No device has yet fulfilled all of these requirements. The second and probably the most rate-limiting step in the clinical adoption of NOTES is the commercial development of a robust, flexible, versatile multitasking platform. Complex surgical maneuvers, even in surgeries as commonplace as cholecystectomies, have technical requirements that cannot be met by a double-channel endoscope, such as triangulation of instruments, aggressive traction and countertraction of tissue, position fixation, and reliable force transmission over instruments. The NOTES platform should be versatile enough to manage iatrogenic intraperitoneal complications, such as hemorrhage, perforation, or organ injury. It is not surprising that there has been such a preponderance of hybrid NOTES studies and a general tendency to retain the laparoscopic paradigm insofar as SILS is concerned.
Future Indications for the Gastroenterologist
This chapter elaborates on five areas we believe are potential NOTES procedures that will be performed by gastroenterologists in the future (Box 54.2). These procedures share one or more of the following characteristics: (1) surgical sites more easily reached using a flexible endoscope or flexible NOTES platform compared with a laparoscope or via open access, (2) surgical procedures of low to medium complexity, and (3) probable improved outcomes from adjunct use of EUS for access or directed biopsies or resections.
Endoscopic Full-Thickness Resection
EFTR represents the next step in the natural evolution of endoscopic procedures aimed at removing GI cancers and cancerous precursor lesions. EFTR follows in the line of polypectomy, EMR, and ESD. For early stage gastric cancers, ESD cures certain T1, N0 adenocarcinomas. EFTR may find a role in the treatment of T2, N0 adenocarcinoma and small GI stromal tumors.10 In 2006, Kaehler and associates11 reported EFTR in two patients (one with early gastric cancer and the other with carcinoid tumor) using a flexible stapler. There have been other case reports of gastric and duodenal EFTR but with laparoscopic assistance.12,13 In these cases, resection was performed endoscopically, but closure was performed laparoscopically. A full-thickness suturing device has been used to close comparably smaller gastric defects during or after EMR of gastric tumors14 and a perforation resulting from GI stromal tumor resection using ESD.15
Although experience of EFTR in other organs has been reported mostly in animal studies, human studies are likely to be performed in the near future. Successful EFTR has been reported in an animal survival study.16 Colonic full-thickness resection has also been successfully shown in the animal model using various closure strategies, including but not limited to T-tags,17 clips,18 nitinol compression clamps,19 and staplers.20
Diagnostic Peritoneoscopy and Lymph Node Harvesting
NOTES peritoneoscopy with lymph node harvesting has potential as a minimally invasive and accurate means of intraabdominal cancer staging. In patients in whom operability is uncertain, NOTES peritoneoscopy and lymph node mapping may be preferable to staging laparoscopy. In pancreatic, biliary, or gynecologic cancers, transgastric peritoneoscopy could be used to assess quickly for peritoneal and liver implants.21 Diagnostic transgastric endoscopic peritoneoscopy has already been performed in humans for staging of pancreatic head masses. In this particular study, transgastric peritoneoscopy corroborated laparoscopic findings for surgical decision making in 19 of 20 patients.22
NOTES locoregional sentinel node sampling could potentially be combined with EFTR in the same procedure.9 In early stage gastric cancer, gastric EFTR could be performed with perigastric lymph node harvesting. In early stage colon cancer, direct NOTES sentinel node sampling could potentially save a patient from radical mesenteric lymphadenectomy.23 Cahill and colleagues24,25 reported the technical feasibility of lymphatic mapping and node biopsy by NOTES in a porcine model. They performed transvaginal endoscopic peritoneoscopy for gastric lymphatic mapping24 and transgastric endoscopic peritoneoscopy for lymphatic mapping in the sigmoid colonic mesentery.25 Methylene blue was injected into the submucosal layer for identification of lymphatic channels and lymph nodes. Excisional biopsy was performed using a coagulating electrode and an endoscopic grasper.
Finally, lymph node harvesting is of particular interest for staging of neoplasias of the chest and mediastinum as an alternative to thoracoscopy and mediastinoscopy, which can be quite painful.26 The adjunctive use of EUS is likely to be important for safe mediastinal entry. In addition to lymph node harvesting, more recent human cadaveric studies have shown that other procedures may be possible, such as pleural biopsy, vagotomy, thymectomy, thoracic duct ligation, thymectomy, and pericardial window formation.27
Liver Biopsies
When directed core liver biopsies are required, the transgastric approach may play a role. Steele and coworkers28 showed transgastric liver biopsy in three patients undergoing laparoscopic gastric bypass surgery, adding only 4 to 6 minutes to the primary procedure. Segments II, III, IVb, V, and VI (partial) were able to be visualized. Self-limited minor bleeding was encountered after obtaining liver biopsy specimens using standard endoscopic biopsy forceps. Because this study protocol was added to gastric bypass surgeries, gastrostomy closure was not specifically addressed. In another study evaluating liver biopsies in a porcine model, animals were randomly assigned to liver biopsy by laparotomy, laparoscopy, and transgastric NOTES. Both the incidence and the severity of adhesions were lowest with transgastric NOTES.29
Bariatric Surgery
Natural orifice bariatric procedures may not be transluminal per se but are likely to represent a significant opportunity for therapeutic gastroenterologists in the future given both the obesity pandemic and the need for a safe and durable alternative to bariatric surgery. Ultimately, avoidance of the transabdominal route makes intuitive sense in morbidly obese patients. Numerous strategies are available for endoluminal treatment of obesity, including suturing, stapling, implantable polymers and prostheses (e.g., sleeves), electrical stimulation, and mucosal ablation.30 These strategies have been developed to replicate the understood mechanisms of bariatric surgery, classically described as restrictive, malabsorptive, or both.31 Preclinical studies have shown promising early data regarding safety and efficacy (percent excess weight loss 24% to 58% depending on the device).32,33 Understanding of the pathophysiology of obesity and the mechanisms of bariatric surgery is in its infancy. The complex interplay between anatomic alterations and neurohormonal modulation remains to be worked out, but the elucidation of critical mechanisms promises to yield bariatric devices and procedures for the gastroenterologist’s armamentarium.32
Anastomosis
Creation of an endoscopic anastomosis has been a goal of NOTES since its inception. Endoscopic gastrojejunostomy for palliation of malignant gastric outlet obstruction would theoretically be a superior alternative to surgical gastrojejunostomy at the end of life. Similarly, endoscopic creation of a cholecystogastrostomy could enable direct endoscopic treatment of gallbladder disease or allow for prophylactic gallstone removal and mucosal ablation. Early feasibility work in endoscopic anastomosis creation has featured the use of T-tags or endoscopic suturing devices.34,35 Additional work is under way in our laboratory featuring injectable, self-assembling magnets, which could significantly simplify endoscopic anastomotic creation (unpublished data).
Equipment
The evolution of NOTES depends on technical development. The most pressing need is a safe and reproducible means of closing perforations. Several strategies are available for perforation closure—suturing, tissue anchors, staples, clips, occluding devices.36 Requirements would differ depending on the tissue involved (e.g., gastric vs. colonic vs. esophageal). Similarly, there is still a need for robust, flexible, multitasking platforms. Promising prototypes of direct drive systems and multitasking flexible endoscopes are now being tested, and it is hoped that these new tools would enable more complex surgical maneuvers.37
Anatomic Considerations
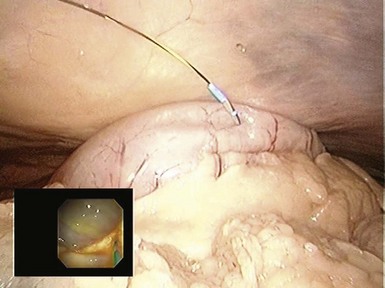
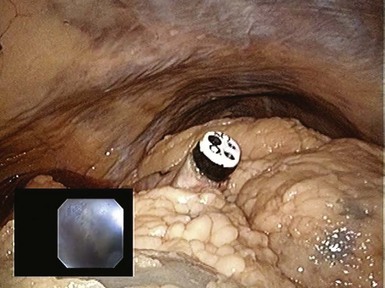
The transgastric approach provides excellent access to the lower abdomen and pelvis (Table 54.1). To view the upper abdomen from a transgastric access point, the endoscope must be retroflexed, which can limit maneuverability and reach. Peritoneal access is targeted along the anterior gastric body guided by transabdominal palpation. When creating the gastrostomy, the endoscopist should be mindful of the gastroepiploic arteries running along the greater curvature, the left lobe of the liver, and the anterior abdominal wall. The most frequently used technique for access involves using a needle-knife attached to cautery. A wire is passed through the needle-knife into the peritoneal cavity. An endoscopic dilating balloon is advanced over the wire to dilate the gastrostomy tract. The endoscope is advanced into the peritoneal cavity. Alternatively, the gastrostomy can be created using the percutaneous endoscopic gastrostomy tube technique whereby a transabdominal needle delivers the wire over which a balloon is advanced to dilate the gastrostomy. Another option is to use the tunneling method whereby a 5- to 15-cm submucosal tunnel is created before serosal exit to create a gastric flap that self-approximates and seals more easily (Figs. 54.1, 54.2, and 54.3).
Table 54.1 Natural Orifice Transluminal Endoscopic Surgery (NOTES) Access Points: Relative Advantages and Disadvantages
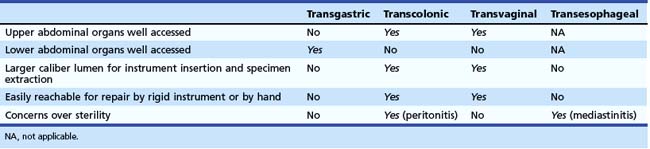
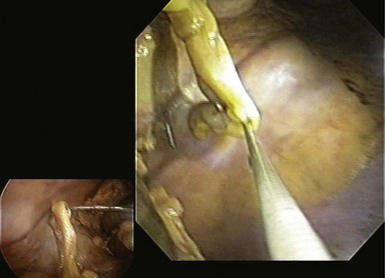
Fig. 54.3 Transgastric appendectomy. Endoscopic view with laparoscopic view (inset) in human cadaver.
In contrast to the transgastric approach, the transcolonic approach provides excellent access to the upper abdominal organs, but it requires retroflexion to visualize and engage the lower abdominal organs (see Table 54.1). Usually an entry point is chosen in the proximal anterior rectum, just proximal to the superior valve of Houston, because the proximal third of the rectum is covered by visceral peritoneum.38 The peritoneal reflection is generally 7 to 9 cm from the anal verge in men and 5 to 7.5 cm from the anal verge in women. This anterior peritonealized space is the rectovesicle pouch in men and the rectouterine pouch in women. As with transgastric access, the needle-knife is used to create the initial transmural cut. However, there is usually no need to balloon dilate as in transgastric access because of the comparatively thinner colonic wall. Other sites of inferior access include transvaginal and transvesicular, which are briefly mentioned here. As described previously, most recent NOTES literature has used the transvaginal route given a historical precedent in the form of culdoscopy, decreased concerns of sterility, and the adequacy of hand-sewn sutures for closure of the posterior fornix. However, this technique is available only to female patients, and gastroenterologists would likely not be involved in this approach. The transvesicular approach has not seen much clinical use.
The transesophageal approach (see Table 54.1) is usually guided by the use of EUS to identify an area of the mediastinum beyond the esophageal wall that appears suitable for entry, with no vessels. For viscerotomy creation, most published studies (all in animals or cadavers) use either the direct needle-knife technique or a modified tunneling method for “flap” access. As with the transcolonic approach, the transesophageal approach is associated with concerns of infection (i.e., mediastinitis). Closure methods for transesophageal access in animal studies have included clips and a T-tag suturing system, but a more fail-safe closure method is likely required before embarking on human trials.
Peritoneal and mediastinal anatomy would initially be foreign to the endoscopist accustomed to endoluminal navigation. To “recalibrate” the endoscopist to new anatomic perspectives, part of the answer may lie in a formal NOTES training fellowship.39 Although such a fellowship would incorporate fundamentals of surgical training (e.g., surgical exposure, understanding tissue planes during dissection), NOTES would cover new ground even for the surgeon. NOTES allows for full prone positioning of the patient, which in the porcine model has shown full exposure of the retroperitoneum, a view not seen even by laparoscopic surgeons.
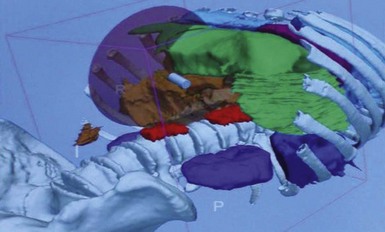
Additionally, part of the answer may lie with complementary, real-time image-guided navigational systems to compensate for difficulties with spatial positioning and orientation, identification of anatomy, and localization of pathology.40 A real-time three-dimensional CT guidance system has been employed for navigational assistance during colonoscopy, ERCP and EUS, and NOTES procedures in human cadaveric studies.41 This system can track endoscope position with a three-dimensional volumetric reconstruction of a preoperative computed tomography scan (Fig. 54.4).
Precautions
The concern regarding infection is paramount in NOTES. More recent studies in patients have shown that intravenous antibiotics are sufficient in preventing peritonitis after transgastric peritoneoscopy.42,43 However, the mean intraperitoneal time in these studies was less than 21 minutes. Transmural breach involving longer durations, organs with greater microbial load (e.g., transcolonic or even transesophageal), and prosthetic implantation may require additional mucosal antiseptics, such as chlorhexidine or povidone-iodine, which have been shown to reduce mucosal microbial burden to near zero when used in conjunction with intravenous antibiotics.44
1 Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
2 Swanstrom LL, Volckmann E, Hungness E, et al. Patient attitudes and expectations regarding natural orifice transluminal endoscopic surgery. Surg Endosc. 2009;23:1519-1525.
3 2009 Conference/4th International NOSACAR Conference on NOTES Working Group Presentations. Registry Development. Available at http://www.noscar.org/presentations09.html Accessed March 28, 2010
4 Ujiki MB. Biliary—human NOTES transgastric cholecystectomy. The DAVE Project. 2008. Available at http://daveproject.org/viewfilms.cfm?filmid=758 Accessed May 5, 2009
5 Soper N. Analysis and future of NOTES in humans. Available at http://www.easts.fr/doi-lt01ensoper003.htm Accessed November 2008
6 Swanstrom LL. Transgastric NOTES cholecystectomy: Phase I efficacy and outcomes trial. Available at http://www.eats.fr/doi-lt01enswanstrom006.htm Accessed November 2007
7 Hazey JW, Narula VK, Renton DB, et al. Natural orifice transgastric endoscopic peritoneoscopy in humans: Initial clinical trial. Surg Endosc. 2008;22:16-20.
8 ASGE/SAGES Working Group on Natural Orifice Transluminal Endoscopic Surgery. White Paper. Gastrointest Endosc. 2006;63:199-203.
9 SAGES/ASGE Joint Committee on NOTES. NOTES: Where have we been and where are we going? Surg Endosc. 2008;22:1143-1145.
10 Asakuma M, Nomura E, Lee SW, et al. Ancillary N.O.T.E.S. procedures for early stage gastric cancer. Surg Oncol. 2009;18:157-161.
11 Kaehler G, Grobholz R, Langner C, et al. A new technique of endoscopic full-thickness resection using a flexible stapler. Endoscopy. 2006;38:86-89.
12 Abe N, Mori T, Takeuchi H, et al. Successful treatment of early stage gastric cancer by laparoscopy-assisted endoscopic full-thickness resection with lymphadenectomy. Gastrointest Endosc. 2008;68:1220-1224.
13 Tsujimoto H, Ichikura T, Nagao S, et al. Minimally invasive surgery for resection of duodenal carcinoid tumors: Endoscopic full-thickness resection under laparoscopic observation. Surg Endosc. 2010;24:471-475.
14 Von Renteln D, Schmidt A, Riecken B, et al. Gastric full-thickness suturing during EMR and for treatment of gastric-wall defects (with video). Gastrointest Endosc. 2008;67:738-744.
15 Von Renteln D, Riecken B, Walz B, et al. Endoscopic GIST resection using Flushknife ESD and subsequent perforation closure by means of endoscopic full-thickness suturing. Endoscopy. 2008;40(Suppl 2):E224-E225.
16 Fritscher-Ravens A, Cuming T, Jacobsen B, et al. Feasibility and safety of endoscopic full-thickness esophageal wall resection and defect closure: A prospective long-term survival animal study. Gastrointest Endosc. 2009;69:1314-1320.
17 Raju GS, Malhotra A, Ahmed I. Colonoscopic full-thickness resection of the colon in a porcine model as a prelude to endoscopic surgery of difficult colon polyps: A novel technique (with videos). Gastrointest Endosc. 2009;70:159-165.
18 Raju GS, Ahmed I, Shibukawa G, et al. Endoluminal clip closure of a circular full-thickness colon resection in a porcine model (with videos). Gastrointest Endosc. 2007;65:503-509.
19 Kopelman Y, Siersema PD, Nir Y, et al. Endoluminal compression clip: Full-thickness resection of the mesenteric bowel wall in a porcine model. Gastrointest Endosc. 2009;70:1146-1157.
20 Rajan E, Gostout CJ, Burgart LJ, et al. First endoluminal system for transmural resection of colorectal tissue with a prototype full-thickness resection device in a porcine model. Gastrointest Endosc. 2002;55:915-920.
21 Nassif J, Zacharopoulou C, Wattiez A. Staging of gynaecological malignancies by natural orifice transluminal endoscopic surgery (N.O.T.E.S.). Surg Oncol. 2009;18:147-152.
22 Nau P, Anderson J, Yuh B, et al. Diagnostic transgastric endoscopic peritoneoscopy: Extension of the initial human trial for staging of pancreatic head masses. Surg Endosc. 2010;24:1440-1446.
23 Cahill RA. Regional nodal staging for early stage colon cancer in the era of endoscopic resection and N.O.T.E.S. Surg Oncol. 2009;18:169-175.
24 Cahill RA, Asakuma M, Perretta S, et al. Gastric lymphatic mapping for sentinel node biopsy by natural orifice transluminal endoscopic surgery (NOTES). Surg Endosc. 2009;23:1110-1116.
25 Cahill RA, Perretta S, Leroy J, et al. Lymphatic mapping and sentinel node biopsy in the colonic mesentery by natural orifice transluminal endoscopic surgery (NOTES). Ann Surg Oncol. 2008;15:2677-2683.
26 Perretta S, Allemann P, Dallemagne B, et al. Natural orifice transluminal endoscopic surgery (N.O.T.E.S.) for neoplasia of the chest and mediastinum. Surg Oncol. 2009;18:177-180.
27 Ryou M, Shaikh S, Fernandez-Esparrach G, et al. NOTES thoracic surgery in a human cadaveric model: Transesophageal exploration of the mediastinal, pericardial, and pleural spaces followed by pleural biopsy, lymph node sampling, thoracic duct ligation, vagotomy, thymectomy, and pericardial window. Gastrointest Endosc. 2008;67:AB111.
28 Steele K, Schweitzer MA, Lyn-Sue J, et al. Flexible transgastric peritoneoscopy and liver biopsy: A feasibility study in human beings (with videos). Gastrointest Endosc. 2008;68:61-66.
29 Dubcenco E, Assumpcao L, Dray X, et al. Adhesion formation after peritoneoscopy with liver biopsy in a survival porcine model: Comparison of laparotomy, laparoscopy, and transgastric natural orifice transluminal endoscopic surgery (NOTES). Endoscopy. 2009;41:971-978.
30 Schauer P, Chand B, Brethauer S. New applications for endoscopy: The emerging field of endoluminal and transgastric surgery. Surg Endosc. 2007;21:347-356.
31 Stylopoulos N, Aguirre V. Mechanisms of bariatric surgery and implications for the development of endoluminal therapies for obesity. Gastrointest Endosc. 2009;70:1167-1175.
32 Cote GA, Edmundowicz SA. Emerging technologies: Endoluminal treatment of obesity. Gastrointest Endosc. 2009;70:991-999.
33 Ellsmere JC, Thompson CC, Brugge WR, et al. Endoscopic interventions for weight loss surgery. Obesity. 2009;17:929-933.
34 Kantsevoy SV, Jagannath SB, Niiyama H, et al. Endoscopic gastrojejunostomy with survival in a porcine model. Gastrointest Endosc. 2005;62:287-292.
35 Bergstrom M, Ikeda K, Swain P, et al. Transgastric anastomosis by using flexible endoscopy in a porcine model (with video). Gastrointest Endosc. 2006;63:307-312.
36 Sumiyama K, Gostout CJ, Gettman MT. Status of access and closure techniques for NOTES. J Endourol. 2009;23:765-771.
37 Thompson CC, Ryou M, Soper NJ, et al. Evaluation of a manually driven, multitasking platform for complex endoluminal and natural orifice transluminal endoscopic surgery. Gastrointest Endosc. 2009;70:121-125.
38 Moran EA, Gostout CJ. Anatomical considerations for natural orifice transluminal endoscopic surgery. Clin Anat. 2009;22:627-632.
39 Gamboa AJ, Box GN, Preminger GM, et al. NOTES: Education and training. J Endourol. 2009;23:813-819.
40 Coughlin G, Samavedi S, Palmer KJ, et al. Role of image-guidance systems during NOTES. J Endourol. 2009;23:803-812.
41 Vosburgh KG, Stylopoulos N, Estepar RS, et al. EUS with CT improves efficiency and structure identification over conventional EUS. Gastrointest Endosc. 2007;65:866-870.
42 Narula VK, Happel LC, Volt K, et al. Transgastric endoscopic peritoneoscopy does not require decontamination of the stomach in humans. Surg Endosc. 2009;23:1331-1336.
43 Nau P, Anderson J, Yuh B, et al. Diagnostic transgastric endoscopic peritoneoscopy: Extension of the initial human trial for staging of pancreatic head masses. Surg Endosc. 2010;24:1440-1446.
44 Ryou M, Hazan R, Rahme L, et al. The effectiveness of current sterility techniques in natural orifice transluminal endoscopic surgery (NOTES). Gastrointest Endosc. 2007;65:AB290.