Chapter 57 Gastroduodenal and Colonic Endoprostheses
Technical Review
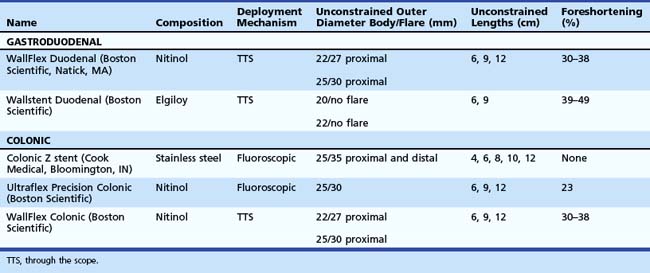
Stent designs and deployment systems are evolving rapidly worldwide, with new devices arriving frequently on the market. Three SEMS for gastroduodenal use and two for colonic obstruction have been approved by the U.S. Food and Drug Administration (FDA) and are available in the United States (Table 57.1). These are all uncovered to reduce the risk of migration. Over time, uncovered SEMS are expected to be incorporated into the surrounding tissues.1 These devices become permanent shortly after placement and require surgical resection for removal once there is significant tissue ingrowth through the mesh. No uncovered SEMS are approved by the FDA for benign disease at this time. Endoscopists should become familiar with several important characteristics of enteral stents, including their composition, lattice pattern and width, flexibility, expandability, deployment mechanism, and degree of foreshortening after placement in the bowel.2
The flexibility and expandability of a stent are directly related to its primary composition.3 Most SEMS are made from shape-memory alloys such as nitinol (nickel-titanium) and Elgiloy (cobalt-chromium-nickel), which balance the need for strong radial forces to expand a malignant stricture while maintaining enough flexibility to minimize the risk of bowel perforation and allow for deployment in tortuous segments of the bowel. SEMS are made in one of two general designs. Stents with large delivery systems that cannot be passed through a therapeutic endoscope are often referred to as over the wire enteral SEMS. These are typically designed for obstructions in the rectum and sigmoid colon and have a larger internal diameter when fully deployed. Most newer models are designed for through the scope (TTS) deployment, in which the stent is contained within a sheath that can be fitted into the working channel of a therapeutic endoscope (3.7-mm).
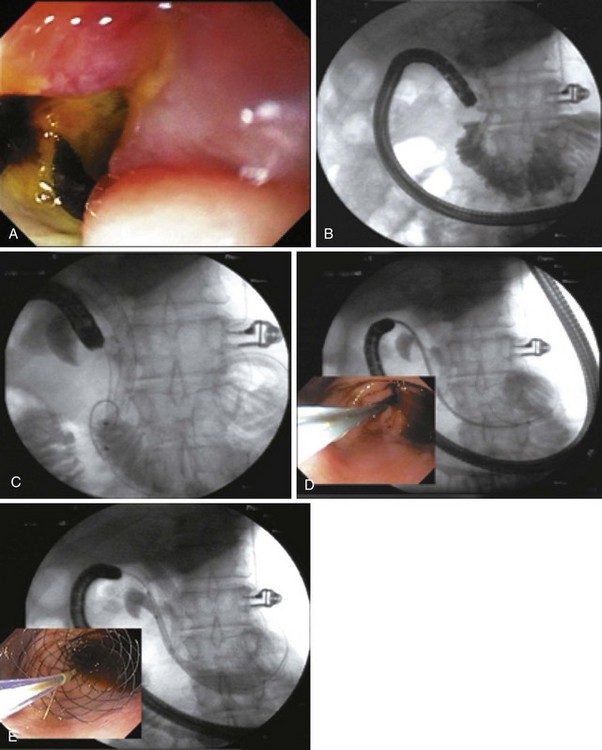
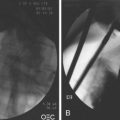
As additional stent designs from various manufacturers become commercially available, it is convenient to describe all stents by their characteristics: stent type (TTS or over the wire), design (woven or laser cut), covering, deployed length and diameter, shortening percentage at deployment, potential for reconstrainability during deployment, and size of the endoscope channel required to accommodate the deployment system. SEMS design allows stent placement under endoscopic and fluoroscopic visualization even in cases where the endoscope cannot traverse the stricture, provided that a guidewire and stent deployment system can be advanced into the correct position (Fig. 57.1). In this setting, a guidewire is advanced across the stricture, which may be facilitated by using a standard endoscopic retrograde cholangiopancreatography (ERCP) catheter or a multilumen biliary extraction balloon catheter. Contrast agent injection and fluoroscopy can be helpful in delineating the anatomy. Once the stenosis is engaged and passed with the guidewire, the balloon catheter can be advanced beyond the stenosis, and the length of the stricture can be measured using the inflated balloon and fluoroscopic image. The guidewire is left in place, and the initial catheter is exchanged for an appropriately sized SEMS system. The stent and sheath mechanism can be advanced over the guidewire without having to remove the endoscope. Once correctly positioned across the stricture, the stent can be released by an assistant as the stent position is carefully maintained by the endoscopist. Several stent deployment systems allow recapturing of the partially deployed stent if the position is suboptimal. Currently available TTS enteral stents continue to expand and foreshorten over several days after being released until the full external diameter is achieved. The endoscopist should account for this in choosing the length and position of the stent before its deployment.
Malignant Gastroduodenal Obstruction
Tumors of the periampullary region, distal stomach, and proximal duodenum often manifest with symptoms of nausea and vomiting secondary to gastric outlet obstruction (GOO). Because most of these patients have unresectable disease, symptom palliation is typically the primary goal. Before the advent of SEMS, surgical gastrojejunostomy had been used to bypass the obstruction. Despite limiting surgical decompression to good surgical candidates, this option would still be associated with significant morbidity and mortality.4
Among patients with GOO, clinical success is typically defined as resolution of symptoms (nausea, vomiting) and tolerance of a soft diet. Comparative trials have shown the superiority of SEMS compared with surgical bypass in palliation of symptoms, length of hospitalization, and cost-effectiveness.5–9 A systematic review of 606 cases of GOO reported technical success and clinical response rates to enteral stent placement of 97% and 87%.10 Newer stent designs such as the WallFlex Enteral stent (Boston Scientific) and the Niti-S Enteral Colonic stent (Taewoong Medical Co., Ltd., Kyonggi-Do, South Korea) are promoted for their greater flexibility while maintaining sufficient radial forces to open strictures.11–13 These stents have blunt woven ends theoretically to reduce the risk of wire penetration and subsequent perforation. As a result of its woven design, the Niti-S stent has less foreshortening (23%) compared with other TTS enteral stents, but the clinical significance of this characteristic is unclear.12 Although there are no comparative trials available, case series using these stents suggest their equivalence to older devices. A multicenter, randomized study comparing the Enteral WallFlex stent with surgical gastrojejunostomy suggested better short-term (<2 months) clinical improvement after stent placement but more favorable long-term results with surgical bypass.14 Although stent placement was associated with lower initial inpatient costs, the differences in long-term costs and incremental cost-effectiveness of either approach are minimal.15 When deciding between endoprosthetic placement and surgery, the patient’s comorbidities and anticipated long-term prognosis are the most important considerations.
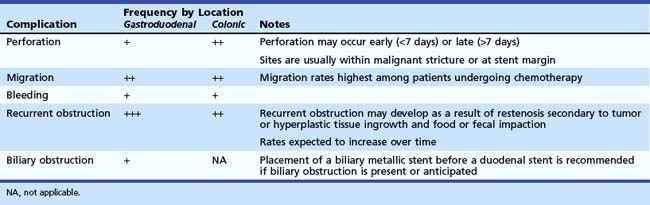
Complications of stent placement for GOO include perforation, bleeding, stent migration, restenosis, biliary obstruction, and failure to expand fully, despite technically successful placement (Table 57.2).10,16 Perforation (0.7%) and bleeding (0.5%) rates are low, but these are potentially life-threatening complications that warrant discussion with the patient. Newer stent designs advertise greater flexibility and blunt ends to reduce the rate of perforation, but there are no data to support these claims.11,12
Stent migration and restenosis secondary to tumor ingrowth or granulation tissue hyperplasia between the lattices of uncovered SEMS are more commonly encountered than perforation. As expected, restenosis rates steadily increase over time, with an incidence of 18% reported in the largest series.10 Given the limited life expectancy of many patients with GOO secondary to malignant obstruction, stent restenosis is more likely to occur in patients who are likely to survive beyond the limited life expectancy of patients with malignant GOO. Stent migration may be more likely in patients who are treatment-naïve and expected to undergo systemic chemotherapy. Both stent migration and restenosis can often be managed with placement of additional endoprostheses.
Covered SEMS have been considered to address the issue of tumor ingrowth. However, migration rates of 10% to 20% have limited their use.17,18 Kim and associates19 published their series of 213 patients with GOO who underwent placement of a layered dual nitinol endoprosthesis composed of a partially covered outer stent overlying an inner, uncovered stent. The reported migration rate of 4% was markedly lower than rates reported in other series of covered SEMS, and the mean patency period was 324 days. Although the rate of biliary obstruction in this series was low (2%), biliary obstruction may occur more frequently with covered SEMS, and access to the biliary tree is significantly limited after deployment. Generally, because access to the papilla is limited by duodenal stents, placement of a biliary SEMS before a duodenal SEMS is recommended for patients with impending or concurrent biliary obstruction.3 In a series of 176 patients who underwent enteral stent placement, the rate of cholangitis after stent placement was 6% (9 patients); all patients with cholangitis had received an enteral stent that traversed the major papilla.20 Endoscopic drainage of the bile duct was technically successful in only two of six patients who underwent attempted ERCP.
Colonic Obstruction
Malignant obstruction of the large bowel was traditionally a surgical emergency that required urgent decompression. Historically, surgeons performed a Hartmann’s procedure, in which the primary tumor was resected and a diverting colostomy was created to decompress the proximal colon.21 Patients had to wait at least 8 weeks for colostomy reversal, although many had to wait longer or never underwent the procedure because of age and underlying comorbidities.22 The presence of a colostomy is unquestionably associated with a significantly lower quality of life.23 The management of these cases has significantly changed with the advent of endoscopic devices for colonic decompression. Stents placed for malignant colonic obstruction can serve as a bridge to a single-stage surgical resection or as a palliative measure in patients with advanced disease.
With successful placement of a stent, patients can receive a full bowel preparation before undergoing a single-stage resection with reanastomosis. In addition to avoiding a colostomy, a prepared colon may be inspected to rule out a synchronous tumor at the time of surgery. No randomized, controlled trials have compared emergency surgery with endoscopic placement of a stent as a bridge to surgery in acute malignant colonic obstruction. However, in a meta-analysis of the published literature comparing stent placement with open surgery, stent placement was associated with a shorter hospital stay, lower mortality, fewer medical complications, and reduced rate of stoma formation.24 In a pooled analysis of 1198 patients who underwent placement of a colonic stent for acute malignant obstruction, the technical and clinical success rates were 94% and 91%.25 Comparable success rates were reported in multiple case series using newer stent designs.26–31 In a multicenter series of 36 patients, 94% were successfully bridged to a single-stage resection within 2 weeks of stent placement.27
In the largest published series of colonic stents, technical and clinical failure rates were highest in patients with right-sided colonic lesions and patients with large bowel obstruction secondary to extrinsic compression.25 Subsequent publications reporting on the use of colonic stents for obstruction secondary to extrinsic lesions confirmed a significantly lower success rate.32,33 Stent placement in the proximal colon has become more feasible with the TTS design, provided that the endoscope can be straightened before advancing the stent sheath through the working channel.29 Distal rectal strictures within several centimeters of the anal verge are also problematic because the stent is more likely to cause significant perianal discomfort.34 Decision models based on available data suggest colonic stent placement is a dominant strategy compared with emergency surgery because it reduces the number of operations, the need for a stoma, and costs.35,36 An ongoing clinical trial is comparing a surgery-first with stent-first approach to acute, left-sided malignant colonic obstruction.37
Although the feasibility of colonic stents as a bridge to surgery is well established, data are conflicting on the use of stents for long-term palliation. A randomized trial comparing surgical palliation with endoscopic stent placement (WallFlex Colonic) was closed after only 21 patients were enrolled because of a high rate of perforation in the stent group.38 Although no perforations occurred during stent placement, six of nine patients returned with perforations during the follow-up period. The authors speculated the perforations might be related to the particular stent used in this trial because three of six patients developed perforations related to erosions through normal colonic wall. However, other open-label and registry studies have not shown such a dramatic perforation rate.39–42 A case series of 50 patients who underwent placement of a different colonic stent (Ultraflex Precision Colonic) for palliation led to durable relief in symptoms in 81% after 6 months of follow-up.28 No perforations were reported in this series.
Perforation is observed more frequently with colonic compared with gastroduodenal stents, with a reported rate of 3.8% in the largest compilation.25 The risk seems to be highest in patients who undergo balloon dilation of the malignant stricture before stent deployment.25,26 Delayed perforations have been reported several months after deployment.38 Stent migration most commonly occurs within the first week after placement and is observed more frequently with right-sided lesions. In addition, recurrent obstruction may occur 1 year after decompression, often related to tumor ingrowth or fecal impaction. Newer designs have incorporated a wider proximal flare to facilitate passage of stool, although data supporting this theory are lacking.26 Tumor ingrowth does not seem to occur more frequently with stents that have a wider mesh.26
Covered stents have a lower rate of recurrent obstruction (4.7% vs. 7.8%), but migration rates of 30% compared with approximately 11% with uncoated stents have precluded their widespread adoption.25 A dual nitinol stent was designed to address the issue of migration associated with covered devices. This stent combines an inner, bare nitinol stent with an outer device that is bare nitinol at its margins but nylon in the middle (S&G Biotech, Seongnam, Korea). Song and colleagues43 published their experience with this device in 147 patients with malignant colon obstruction. Overall, technical and clinical success rates were greater than 90%, comparable with other series. Although the rates of tumor ingrowth and stent migration were favorable, the perforation rate was 22% in the bridge to surgery group and 5% in the palliative group. Further developments in covered stent technology are necessary before their widespread use for long-term palliation of colonic obstruction becomes standard practice.
Alternative Indications
As SEMS have become widely available, physicians have investigated their role in the management of benign diseases. The absence of a durable, safe, and removable enteral stent precludes the use of stents in benign disease at the present time. Potential applications for SEMS in the colon may include strictures related to radiation, inflammatory bowel disease, or previous diverticulitis and ischemic colitis.44–46 In the largest series of 23 patients, delayed (defined as >7 days after deployment) complications occurred more frequently.44 Five patients in this series had colonic fistulas complicating their benign strictures, all of which responded to stent therapy.
Covered stent technology also offers the potential for treating anastomotic leaks. Initial experience was limited to esophageal pathology such as fistulas, anastomotic leaks, and perforations.47–51 Most reports described off-label use of self-expanding plastic stents designed and approved by the FDA for subsequent removal from the esophagus. As expected, these stents have a significantly higher risk of migration.47 Nevertheless, gastroenterologists have emerged in an area that was previously dominated by interventional radiologists and thoracic surgeons.52 More recently, coated stents have also been applied successfully in the management of anastomotic leaks after Roux-en-Y gastric bypass surgery, with success rates approaching 80%.50,53–55 To facilitate removal of a partially covered metallic stent, Eisendrath and coworkers54 inserted a self-expanding plastic stent through the SEMS to induce necrosis of the granulation tissue that had developed along the margins of the SEMS. Eleven patients returned several weeks later for removal of both devices. No rigorous clinical trials comparing SEMS with surgical alternatives for the management of benign diseases have been reported to date. The widespread application of SEMS for benign indications depends on the development of a stent that is effective (having adequate flexibility and expandability), durable (low migration rate with prolonged follow-up), and removable (associated with minimal tumor and granulation tissue ingrowth).
1 Ell C, Hochberger J, May A, et al. Coated and uncoated self-expanding metal stents for malignant stenosis in the upper GI tract: Preliminary clinical experiences with Wallstents. Am J Gastroenterol. 1994;89:1496-1500.
2 Baron TH. Expandable gastrointestinal stents. Gastroenterology. 2007;133:1407-1411.
3 Tierney W, Chuttani R, Croffie J, et al. Enteral stents. Gastrointest Endosc. 2006;63:920-926.
4 van der Schelling GP, van den Bosch RP, Klinkenbij JH, et al. Is there a place for gastroenterostomy in patients with advanced cancer of the head of the pancreas? World J Surg. 1993;17:128-132.
5 Mehta S, Hindmarsh A, Cheong E, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006;20:239-242.
6 Del Piano M, Ballare M, Montino F, et al. Endoscopy or surgery for malignant GI outlet obstruction? Gastrointest Endosc. 2005;61:421-426.
7 Johnsson E, Thune A, Liedman B. Palliation of malignant gastroduodenal obstruction with open surgical bypass or endoscopic stenting: Clinical outcome and health economic evaluation. World J Surg. 2004;28:812-817.
8 Espinel J, Sanz O, Vivas S, et al. Malignant gastrointestinal obstruction: Endoscopic stenting versus surgical palliation. Surg Endosc. 2006;20:1083-1087.
9 Fiori E, Lamazza A, Volpino P, et al. Palliative management of malignant antro-pyloric strictures: Gastroenterostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:269-271.
10 Dormann A, Meisner S, Verin N, et al. Self-expanding metal stents for gastroduodenal malignancies: Systematic review of their clinical effectiveness. Endoscopy. 2004;36:543-550.
11 van Hooft JE, Uitdehaag MJ, Bruno MJ, et al. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): A prospective multicenter study. Gastrointest Endosc. 2009;69:1059-1066.
12 Maetani I, Isayama H, Mizumoto Y. Palliation in patients with malignant gastric outlet obstruction with a newly designed enteral stent: A multicenter study. Gastrointest Endosc. 2007;66:355-360.
13 Piesman M, Kozarek RA, Brandabur JJ, et al. Improved oral intake after palliative duodenal stenting for malignant obstruction: A prospective multicenter clinical trial. Am J Gastroenterol. 2009;104:2404-2411.
14 Jeurnink SM, Steyerberg EW, Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): A multicenter randomized trial. Gastrointest Endosc. 2010;71:490-499.
15 Jeurnink SM, Polinder S, Steyerberg EW, et al. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. J Gastroenterol. 2010;45:537-543.
16 Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: Experience in 36 patients. Am J Gastroenterol. 2002;97:72-78.
17 Park KB, Do YS, Kang WK, et al. Malignant obstruction of gastric outlet and duodenum: Palliation with flexible covered metallic stents. Radiology. 2001;219:679-683.
18 Seo EH, Jung MK, Park MJ, et al. Covered expandable nitinol stents for malignant gastroduodenal obstructions. J Gastroenterol Hepatol. 2008;23:1056-1062.
19 Kim JH, Song HY, Shin JH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: Prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc. 2007;66:256-264.
20 Telford JJ, Carr-Locke DL, Baron TH, et al. Palliation of patients with malignant gastric outlet obstruction with the enteral Wallstent: Outcomes from a multicenter study. Gastrointest Endosc. 2004;60:916-920.
21 Mitry E, Barthod F, Penna C, et al. Surgery for colon and rectal cancer. Best Pract Res Clin Gastroenterol. 2002;16:253-265.
22 Wong RW, Rappaport WD, Witzke DB, et al. Factors influencing the safety of colostomy closure in the elderly. J Surg Res. 1994;57:289-292.
23 Nugent KP, Daniels P, Stewart B, et al. Quality of life in stoma patients. Dis Colon Rectum. 1999;42:1569-1574.
24 Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc. 2007;21:225-233.
25 Sebastian S, Johnston S, Geoghegan T, et al. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057.
26 Small AJ, Baron TH. Comparison of Wallstent and Ultraflex stents for palliation of malignant left-sided colon obstruction: A retrospective, case-matched analysis. Gastrointest Endosc. 2008;67:478-488.
27 Fregonese D, Naspetti R, Ferrer S, et al. Ultraflex precision colonic stent placement as a bridge to surgery in patients with malignant colon obstruction. Gastrointest Endosc. 2008;67:68-73.
28 Repici A, Fregonese D, Costamagna G, et al. Ultraflex precision colonic stent placement for palliation of malignant colonic obstruction: A prospective multicenter study. Gastrointest Endosc. 2007;66:920-927.
29 Repici A, Adler DG, Gibbs CM, et al. Stenting of the proximal colon in patients with malignant large bowel obstruction: Techniques and outcomes. Gastrointest Endosc. 2007;66:940-944.
30 Soto S, Lopez-Roses L, Gonzalez-Ramirez A, et al. Endoscopic treatment of acute colorectal obstruction with self-expandable metallic stents: Experience in a community hospital. Surg Endosc. 2006;20:1072-1076.
31 Garcia-Cano J, Gonzalez-Huix F, Juzgado D, et al. Use of self-expanding metal stents to treat malignant colorectal obstruction in general endoscopic practice (with videos). Gastrointest Endosc. 2006;64:914-920.
32 Keswani RN, Azar RR, Edmundowicz SA, et al. Stenting for malignant colonic obstruction: A comparison of efficacy and complications in colonic versus extracolonic malignancy. Gastrointest Endosc. 2009;69:675-680.
33 Shin SJ, Kim TI, Kim BC, et al. Clinical application of self-expandable metallic stent for treatment of colorectal obstruction caused by extrinsic invasive tumors. Dis Colon Rectum. 2008;51:578-583.
34 Song HY, Kim JH, Kim KR, et al. Malignant rectal obstruction within 5 cm of the anal verge: Is there a role for expandable metallic stent placement? Gastrointest Endosc. 2008;68:713-720.
35 Targownik LE, Spiegel BM, Sack J, et al. Colonic stent vs. emergency surgery for management of acute left-sided malignant colonic obstruction: A decision analysis. Gastrointest Endosc. 2004;60:865-874.
36 Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T, et al. Stenting or stoma creation for patients with inoperable malignant colonic obstructions? Results of a study and cost-effectiveness analysis. Surg Endosc. 2004;18:421-426.
37 van Hooft JE, Bemelman WA, Breumelhof R, et al. Colonic stenting as bridge to surgery versus emergency surgery for management of acute left-sided malignant colonic obstruction: A multicenter randomized trial (Stent-in 2 study). BMC Surg. 2007;7:12.
38 van Hooft JE, Fockens P, Marinelli AW, et al. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40:184-191.
39 Repici A, De Caro G, Luigiano C, et al. WallFlex colonic stent placement for management of malignant colonic obstruction: A prospective study at two centers. Gastrointest Endosc. 2008;67:77-84.
40 Jiménez-Pérez J, Casellas JA, Garcia-Canco J, et al. Bridge to surgery stenting in patients with malignant colonic obstruction using the WallFlex colonic stent: Report of a prospective multicenter registry. Gastrointest Endosc. 2008;67:AB307.
41 De Caro G, Repici A, Comunale S, et al. Treatment of acute malignant colorectal obstruction with Wallflex colonic stents: A prospective study. Gastrointest Endosc. 2007;65:AB283.
42 Gonzalez-Huix F, Goldberg P, Vandervoort JG, et al. Clinical experience with a nitinol self-expanding colonic stent: Data from the WallFlex-eR colonic international registry. Gastrointest Endosc. 2008;67:AB304.
43 Song HY, Kim JH, Shin JH, et al. A dual-design expandable colorectal stent for malignant colorectal obstruction: Results of a multicenter study. Endoscopy. 2007;39:448-454.
44 Small AJ, Young-Fadok TM, Baron TH. Expandable metal stent placement for benign colorectal obstruction: Outcomes for 23 cases. Surg Endosc. 2008;22:454-462.
45 Law WL, Choi HK, Chu KW, et al. Radiation stricture of rectosigmoid treated with self-expanding metallic stent. Surg Endosc. 2002;16:1106-1107.
46 Davidson R, Sweeney WB. Endoluminal stenting for benign colonic obstruction. Surg Endosc. 1998;12:353-354.
47 Ott C, Ratiu N, Endlicher E, et al. Self-expanding Polyflex plastic stents in esophageal disease: Various indications, complications, and outcomes. Surg Endosc. 2007;21:889-896.
48 Langer FB, Wenzl E, Prager G, et al. Management of postoperative esophageal leaks with the Polyflex self-expanding covered plastic stent. Ann Thorac Surg. 2005;79:398-403.
49 Hunerbein M, Stroszczynski C, Moesta KT, et al. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg. 2004;240:801-807.
50 Babor R, Talbot M, Tyndal A. Treatment of upper gastrointestinal leaks with a removable, covered, self-expanding metallic stent. Surg Laparosc Endosc Percutan Tech. 2009;19:e1-e4.
51 Schubert D, Scheidbach H, Kuhn R, et al. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc. 2005;61:891-896.
52 Siersema PD. Treatment of esophageal perforations and anastomotic leaks: The endoscopist is stepping into the arena. Gastrointest Endosc. 2005;61:897-900.
53 Kriwanek S, Ott N, Ali-Abdullah S, et al. Treatment of gastro-jejunal leakage and fistulization after gastric bypass with coated self-expanding stents. Obes Surg. 2006;16:1669-1674.
54 Eisendrath P, Cremer M, Himpens J, et al. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39:625-630.
55 Merrifield BF, Lautz D, Thompson CC. Endoscopic repair of gastric leaks after Roux-en-Y gastric bypass: A less invasive approach. Gastrointest Endosc. 2006;63:710-714.