Chapter 5 Gamma Knife Surgery for Cerebral Vascular Malformations and Tumors
Recently it has been shown that the gamma knife can palliate some ocular tumors. In a more limited application of the concept, the treatment of extracranial tumors in abdominal and thoracic locations has evolved with the use of the various stereotactic body radiation therapy machines.1–4 Obviously, lesions that lie outside the central nervous system will not be treated by a neurosurgeon. However, when it is used for neurosurgical pathology, no one is more qualified to apply it. It is the operator and the pathology that define the use of a technology. When Walter Dandy placed a cystoscope in a ventricle for the first time, he was not performing a urologic procedure.5 The microscope when used by the neurosurgeon, ophthalmologist, or otolaryngologist is a neurosurgical, ophthalmologic, or otolaryngologic instrument, respectively.
1. Lack of historic perspective makes it difficult for some neurosurgeons to realize that Leksell’s concept was rooted in the philosophy of the founders of neurosurgery, that is, recognition of technological advances and their application to neurosurgical practice. The early adoption by Cushing of the x-ray machine, his use of the “radium bomb” in glioma treatment,6 and the introduction, also by Cushing, of radiofrequency treatment of lesions are just a few examples of “technology transfer.”
2. The difficulty of accepting a neurosurgical procedure without opening the skull, despite the fact that every neurosurgeon knows that trephination is itself a minor part of the neurosurgical act. The laser beam, the bipolar coagulator, and the ultrasound probe are accepted without resistance because they are used after trephining the skull. The recently introduced “photon radiosurgery” with its limited scope compared to gamma surgery is accepted widely as “neurosurgery” because it reaches the target through a small burr hole.
3. The loss of the thrill and glamor provided by open surgery.
4. The deeply rooted acceptance of the dogma that where ionizing beams are involved a radiotherapist is needed. The last 40 years have demonstrated that a neurosurgeon can acquire the necessary knowledge of radiophysics and radiobiology to handle ionizing beams. This is much easier than for a radiation oncologist to master neuroanatomy and management of neurosurgical lesions, and thus to exclude bias when deciding whether to use the microscope or the gamma knife in each particular case.
There is no substitute at this time for the physical extirpation of a mass lesion in terms of cure or control of either vascular or oncologic pathologies. The attractiveness of radiosurgery is not that it supplants open neurosurgical procedures, but that it allows treatment of pathologies only treated earlier with unacceptable morbidity or mortality. There is, and likely always will be a gray area where the benefits of various modalities are debated. It will only be through evaluation of long-term results of these various therapies as well as their availability, cost, experience of the operators and individual patient preferences that the “best” therapy in any given case is decided.
Table 5-1 lists all the cases treated with the gamma knife worldwide through 2006. It should be kept in mind that many of the indications listed are not universally accepted as appropriate for gamma surgery. In this chapter we will give our version of the facts for each indication.
TABLE 5-1 Cases Treated with Gamma Knife Worldwide through December 2008
| Diagnosis | n | Percentage |
|---|---|---|
| Vascular | 65,084 | 12.95 |
| Arteriovenous malformation | 57,136 | 11.37 |
| Cavernous angioma | 3,258 | 0.65 |
| Other vascular | 4690 | 0.93 |
| Tumor | 397,215 | 79.01 |
| Benign | 176,319 | 35.07 |
| Vestibular schwannoma | 46,835 | 9.32 |
| Trigeminal schwannoma | 2,822 | 0.56 |
| Other schwannoma | 1,590 | 0.32 |
| Meningioma | 64,115 | 12.75 |
| Pituitary tumor | 38,553 | 7.67 |
| Pineal tumor | 3,540 | 0.70 |
| Craniopharyngioma | 4,053 | 0.81 |
| Hemangioblastoma | 2,056 | 0.41 |
| Chordoma | 1,911 | 0.38 |
| Hemaniopericytoma | 1,151 | 0.23 |
| Benign glial tumors | 3,594 | 0.71 |
| Other benign tumors | 6,099 | 1.21 |
| Malignant | 220,896 | 43.94 |
| Metastasis | 185,070 | 36.81 |
| Malignant glial tumors | 26,437 | 5.26 |
| Chondrosarcoma | 775 | 0.15 |
| Nasopharyngeal carcinoma | 1,454 | 0.29 |
| Other malignant tumors | 7,160 | 1.42 |
| Ocular Disorder | 1,966 | 0.39 |
| Functional | 38,461 | 7.65 |
| Intractable pain targets | 622 | 0.12 |
| Trigeminal neuralgia | 32,798 | 6.52 |
| Parkinson’s disease | 1,473 | 0.29 |
| Obsessive compulsive disorder | 154 | 0.03 |
| Epilepsy | 2,399 | 0.48 |
| Other functional targets | 1,015 | 0.20 |
| Total indications | 502,726 | 100.0 |
Note: These figures include cases treated at gamma knife sites throughout the world from 1968 through December 2008.
Source: Elekta Radiosurgery Inc.
History
Clarke and Horsley developed the first stereotactic system,7 and the method was first applied clinically by Spiegel et al.8 This system allowed for the localization of intracranial structures by their spatial relationship to Cartesian coordinates relative to a ring rigidly affixed to the skull. This was a prerequisite to the development of radiosurgery by Lars Leksell. His ambition was to develop a method of destroying localized structures deep within the brain without the degree of coincident brain trauma associated with open procedures. The convergence of multiple ionizing beams at one stereotactically defined point was the result. A nominal dose is delivered to the paths of each incident beam. However, at the point of intersection of the beams, a dose proportional to the number of individual beams is delivered. The physical specifications of the device would be designed to ensure steep drop-off of delivered radiation at the edge of the intersection point. This would allow precise selection of the targeted lesion and minimization of trauma to surrounding tissue. He named this concept radiosurgery in 1951.9
Various sources of ionizing radiation were tried. Leksell first used an orthovoltage x-ray tube coupled to a stereotactic frame in the treatment of trigeminal neuralgia and for cingulotomy in obsessive compulsive disorders.9 A cyclotron was then used as an accelerated proton source and used to treat various pathologies.10,11 The cyclotron was too cumbersome and expensive for widespread application. A linear accelerator was evaluated but found at that time to lack the inherent precision necessary for this work. Fixed gamma sources of cobalt-60 and a fixed stereotactic target fulfilled the requirements of precision and compactness. The first gamma knife was built between 1965 and 1968.
Pathophysiology
Normal Tissue
The relative radioresistance of normal brain relates to its low mitotic activity. Also, the rate at which a total dose of radiation is applied affects the damage caused by the dose. This is due to the ability of the cell to effect repairs during the actual time of irradiation. A higher dose rate (same total dose applied over a shorter period of time) consequently increases the lethality of the dose. The normal tissue surrounding the stereotactically targeted pathologic tissue receives a markedly lower dose but over the same time. Therefore, not only is the total dose lower, but the dose rate is lower as well. The radiation effect is seen most clearly at doses above and below 1 Gy/min.12 This radiobiological phenomenon explains part of the relative safety of single dose radiation with steep gradients at the edge of targeted tissue on the surrounding structures. There are likely additional mechanisms of such sparing.
In order to understand the radiobiology of a single high-dose of radiation on normal brain the parietal lobe of rats treated by a gamma knife was studied at our center. It was found that a dose of 50 Gy caused astrocytic swelling without changes in neuronal morphology or breakdown of the blood–brain barrier at 12 months. There was fibrin deposition in the walls of capillaries. At 75 Gy, necrosis was seen at 4 months as was breakdown of the blood–brain barrier. More vigorous morphological changes were seen in astrocytes and hemispheric swelling coincident with the necrosis occurred at 4 months. With the dose increased to 120 Gy, necrosis was seen at 4 weeks but not associated with hemispheric swelling. Astrocytic swelling occurred at only 1 week postirradiation.13 These findings are consistent with earlier reports on the effective dose to produce well-defined lesions in the thalamus in patients treated with the gamma knife.14,15
Tumor Response
Little is known about the pathophysiologic changes induced by gamma surgery at the cellular level in tumors. Division of tumor cells is presumably inhibited by radiation induced damage to DNA. Also it has been shown that the microvascular supply to tumors is inhibited by changes resulting from gamma surgery. In meningiomas studied after this treatment there was reduction of blood flow over time.16 Tumors responding early showed the greatest reduction in blood flow. Other authors have proposed that the induction of apoptosis by gamma radiation to proliferating cells may be responsible for at least a portion of the effect of gamma surgery on tumors.17,18 Although such contentions may be premature they may point the direction to future research.
In order to conformally cover the target more than one isocenter is nearly always utilized. When multiple radiation fields are made to overlap the radiation dose distribution becomes inhomogeneous. The resulting areas of local maxima are called “hot spots.” Controversy exists as to whether the presence of “hot spots” in gamma surgery is beneficial or detrimental. An even dose distribution is an essential and basic concept in radiotherapy. There is some evidence that these areas may be of benefit in gamma surgery. The factors to keep in mind to understand this line of reasoning are as follows: due to radiation geometry hot spots are usually located in the deep portions of the target. In tumors this is usually the area that receives the poorest blood supply and is therefore relatively hypoxic and radioresistant. Furthermore the ability of a cell to respond to otherwise sublethal dosages of radiation can be affected by its own condition as well as the state of the cells near it. Cells that are sublethally injured and are in the vicinity of similar cells recover more often than cells that are in the vicinity of lethally injured cells. The hot spots therefore create islands of lethally injured cells that will enhance the cell kill in the sublethal injury zone.19 Oxygen is a radiosensitizer, and the relatively high-dose rate of the hot spots will act to offset any loss of efficacy in the hypoxic core of the target. This position is supported by the work of other authors.20
Cranial Nerves
The optic and acoustic nerves are the most sensitive to radiation of the cranial nerves. Being central nervous system tracts, containing oligodendrocytes, and carrying complex information is thought to be the source of their vulnerability. These tracts are unable to regenerate following injury. Optic neuropathy has been reported as a complication following single doses greater than 8 Gy.21 The tolerable level of radiation to the optic apparatus is still a subject of debate. Some advocate that the optic apparatus can tolerate doses as high as 12 to 14 Gy.22–24 Others recommend an upper limit of 8 Gy.21,25 Small volumes of the optic apparatus exposed to doses of 10 Gy or less may be acceptable in some cases.26,27 Both the tolerable absolute dose and volume undoubtedly vary from patient to patient. This degree of variability likely depends upon the extent of damage to the optic apparatus by pituitary adenoma compression, ischemic changes, type and timing of previous interventions (e.g., fractionated radiation therapy and surgery), the patient’s age, and the presence or absence of other co-morbidities (e.g., diabetes).
The cranial nerves in the cavernous sinus are relatively robust. Low incidence of neuropathies has been reported with doses up to 40 Gy.21 We have not observed any neuropathies of CN IX through XII in the treatment of glomus jugulare tumors.
Normal Cerebral Vasculature
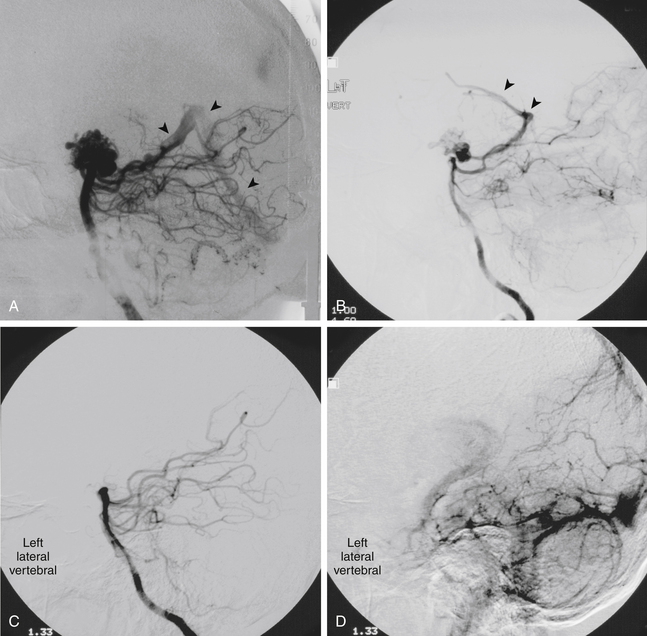
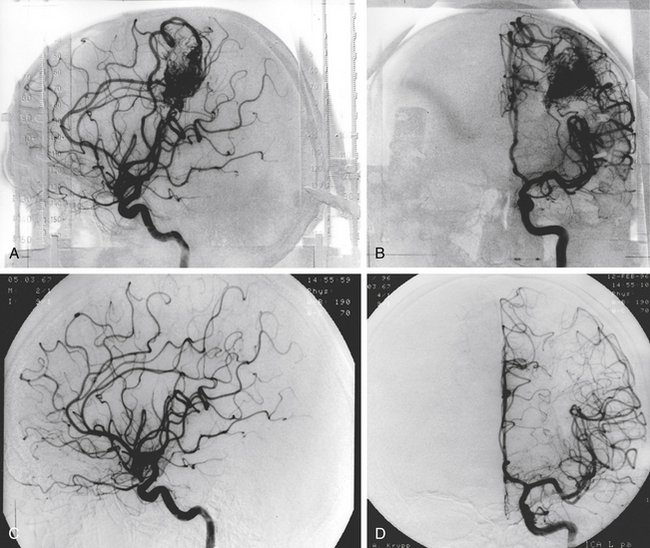
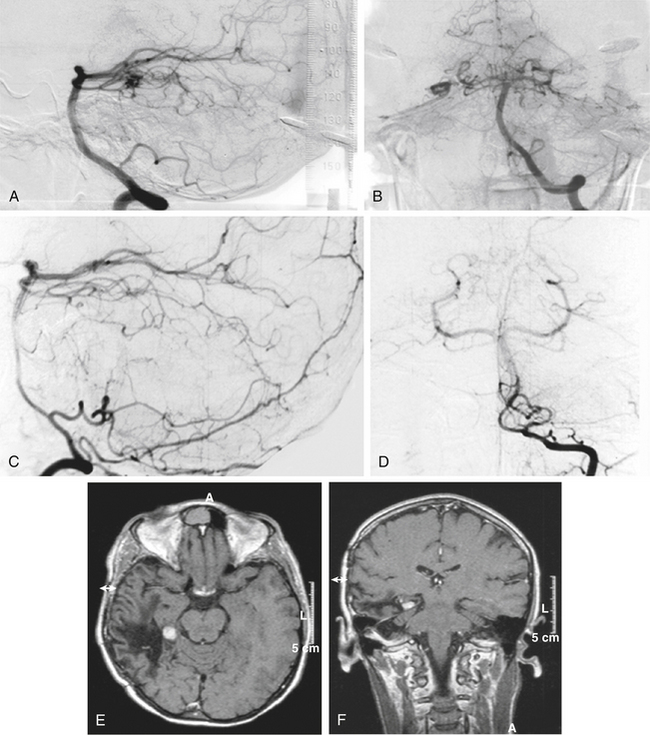
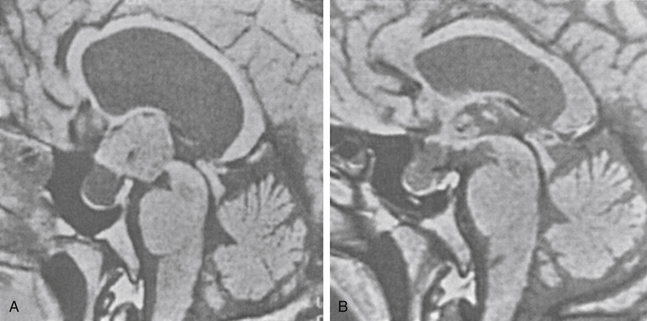
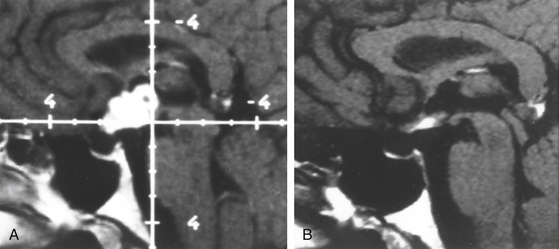
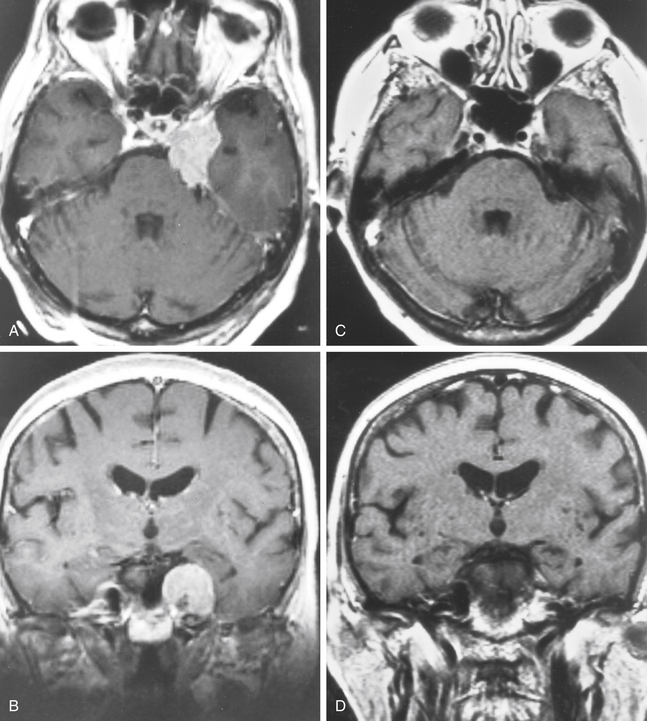
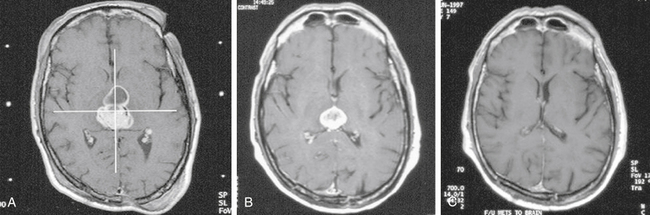
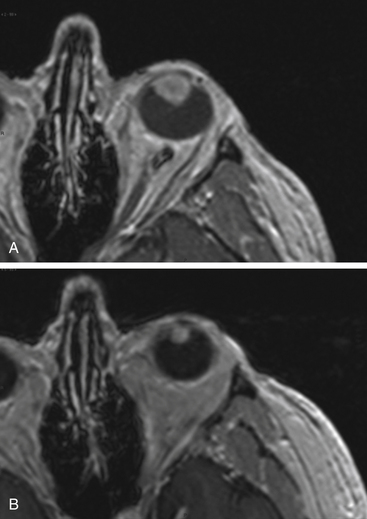
There is both clinical and experimental data regarding the effect of single high-dose gamma irradiation of normal cerebral vasculature. In treating 1,917 arteriovenous malformations we have seen only two incidences of clinical syndromes possibly associated with the stenosis of normal vessels. This low incidence has occurred even though occasionally normal vessels are included in the treatment field. One case was reported after treating a glioma with 90 Gy gamma surgery followed by 40 Gy of fractionated whole brain irradiation of a middle cerebral artery occlusion. Steiner et al. described two cases in which disproportionate white matter changes might have been ascribed to venous stenosis and occlusion.28 Another case, of a diencephalic AVM, demonstrated marked edema associated with venous outflow occlusion. This patient suffered visual and cognitive deficits but over the course of months his neurologic status returned to baseline (Fig. 5-1).
In our treatment of pituitary adenomas with cavernous sinus extension or parasellar meningiomas, we have not observed occlusion of normal vasculature. This absence of stenosis is noted even though the internal carotid artery or portions of the circle of Willis, or its proximal branches, are often included in the treatment field. The only incidence of treating an intracranial aneurysm by us with a gamma knife did lead to narrowing and eventual occlusion of the adjacent small posterior communicating artery segment.29 Whether this was associated with the obliteration of the aneurysm neck or primary changes in the artery is unknown. It is possible that the incidence of occlusion of smaller vessels is more common than recognized as the occlusion would occur slowly and compensatory changes could take place preventing clinical syndromes from occurring. Regardless, the clinical impact is minimal. Others have noted injury to the cavernous segment of the carotid artery following radiosurgery for pituitary adenomas. A total of four cases have been reported and in only two of these cases were the patients symptomatic from carotid artery stenosis.30–32 Pollock et al. have recommended that the prescription dose should be limited to less than 50% of the intracavernous carotid artery vessel diameter.32 Shin et al. recommended restricting the dose to the internal carotid artery to less than 30 Gy.33
Experimental studies done on normal vasculature in the brains of rats and cats showed similar findings.34,35 The primary injury was endothelial necrosis and desquamation, muscular coat hypertrophy and fibrosis at lower doses (25 to 100 Gy). At doses up to 300 Gy necrosis of the muscular layer was seen in cats. In only one instance, a rat anterior cerebral artery treated with 100 Gy was occlusion of a vessel seen. Similar studies on hypercholesterolemic rabbits treated with 10 to 100 Gy showed no histologic changes in the basilar arteries and no instances of occlusion after 2 to 24 months.36
Arteriovenous Malformations
The minimal clinical and only moderate histologic change in the normal cerebral vasculature after high doses of gamma radiation is in sharp contrast to the response of the vessels of an arteriovenous malformation (AVM). Complete radiographic obliteration can be achieved after appropriate gamma surgery. The effects of ionizing radiation and a role in the management of AVMs was first reported in 1928 by Cushing and Bailey.37 During craniotomy for an AVM he had to interrupt surgery due to major hemorrhage from the lesion. He then treated the patient with fractionated radiation. At reoperation 5 years later only an obliterated avascular mass was discovered. This early success was overshadowed by numerous series of failures.38–40 In this early period, Johnson was the only one to report reasonable results with a 45% angiographic obliteration.41 The introduction of the gamma knife rekindled interest in the treatment of AVMs with radiation.42
The pathologic changes in AVMs treated with the gamma knife have been described by several authors.43–45 The earliest change is damage to the endothelium with swelling of the endothelial cells and subsequent denudation or separation of the endothelium from the underlying vessel wall. The most important changes are seen later in the intima with the appearance of loosely organized spindle cells (myofibroblasts) and an extracellular matrix containing collagen type IV, not seen in the intima of untreated vessels. Expansion of the extracellular matrix and cellular degeneration define the final stage prior to luminal obliteration. The occlusion of the vessels is not a thrombotic process but rather the culmination of concentric narrowing of the vessel by an expanding vessel wall.
Gamma Surgery Procedure
Perioperative Management
Patients are routinely evaluated the day prior to gamma surgery. Preoperative consults are obtained as necessary including evaluation by the neuroradiology service. The patients are loaded with anti-seizure medications and levels drawn prior to therapy. Patients already on medication for seizures also have their levels evaluated. Although we have never had a patient have a seizure during therapy, the small but serious risk of a generalized seizure while the patient is secured within the gamma unit makes every precaution reasonable. Patients are also started on systemic dexamethasone the evening prior to therapy and this is continued until the following evening. The use of high-dose peri-operative dexamethasone is empiric. Although we have used steroids throughout our experience with the gamma knife, their original purpose, to minimize vasogenic edema at the time of therapy, has never been documented as a problem. Hence its prophylactic use is debatable.
Imaging and Dose Planning
The accuracy of a gamma surgery is ultimately dependent on the neurosurgeon’s ability to visualize the intended target. Thus, the technique would be impossible without imaging studies that allow three-dimensional views of anatomic structures in the brain. Magnetic resonance image (MRI) is the most used imaging modality because of its superior visualization of soft tissue structures and solid tumors. Typical MRI protocols include T1-weighted pre- and postcontrast (Gadolinium-enhanced) images through the entire volume of the head. Sequences may be a collection of 2D image slices, or a true 3D acquisition such as the MP-RAGE or its successors. Specialized sequences such as constructive interference in steady state (CISS) protocols may be used for circumstances such as visualization of the internal auditory canals, the cerebellopontine angle, and parasellar regions.
Indications
Vascular Malformation
Arteriovenous Malformations
The indications for gamma surgery of arteriovenous malformations (AVMs) versus other treatment options are in many cases unclear at best. Small asymptomatic inoperable AVMs are clearly best treated with the gamma knife while AVMs with a large symptomatic hemorrhage in noneloquent superficial brain are best treated with open surgery. The reason for this is that the risk-benefit value is clear in both of these situations. In other situations it is more ambiguous. Knowledge of the capabilities of various treatments to effect cure, the associated morbidity and mortality associated with the treatment and the natural history of the disease following various treatments must be known to accurately prescribe the most efficacious treatment plan. Unfortunately these are in most instances not known. The natural history of AVMs is not fully understood. Some authors believe that size matters, with smaller AVMs bleeding at a higher rate than larger ones or at a lower rate.46–49 There is also evidence that size is independent of the hemorrhage rate.50–52 Similarly the rate of hemorrhage of an AVM following a previous hemorrhage is thought to be higher than the rate in unruptured AVMs by some authors46,52,53 but not by others.47,54,55 The effects of age, gender, pregnancy and AVM location also confound the question of risk of rupture.50–52,56–58
Early Experience
Since the first AVM case treated by Steiner et al. in 1972,42 we have treated over 2500 AVMs with the gamma knife. As experience with this tool grows the capabilities and limitations of the gamma knife are being defined.
As in microsurgery, also when performing gamma surgery, feeding arteries or draining veins should be left alone and only the nidus should be treated. In very large AVMs, only partially treated due to the excessive dose necessary to treat optimally, occasional cures have been achieved. This is thought to be due to fortuitous inclusion of all the pathologic shunts within the higher dose treatment field. Targeting only the feeding vessels to the AVM have had very limited success because of recruitment of small angiographically occult feeding arteries. Interestingly, the first patient ever treated had only the feeding vessels targeted and a cure was obtained.42 The early success with this strategy has not been reproduced.
Imaging Outcomes
Following gamma surgery, angiography reveals hemodynamic changes occur before changes in the size and shape of an AVM.59 First, the flow rate decreases progressively. This may be related to the changes in the sizes of the feeding arteries and outflow veins. The outcome of an AVM following radiosurgery may be a total, subtotal, or partial obliteration of the nidus.
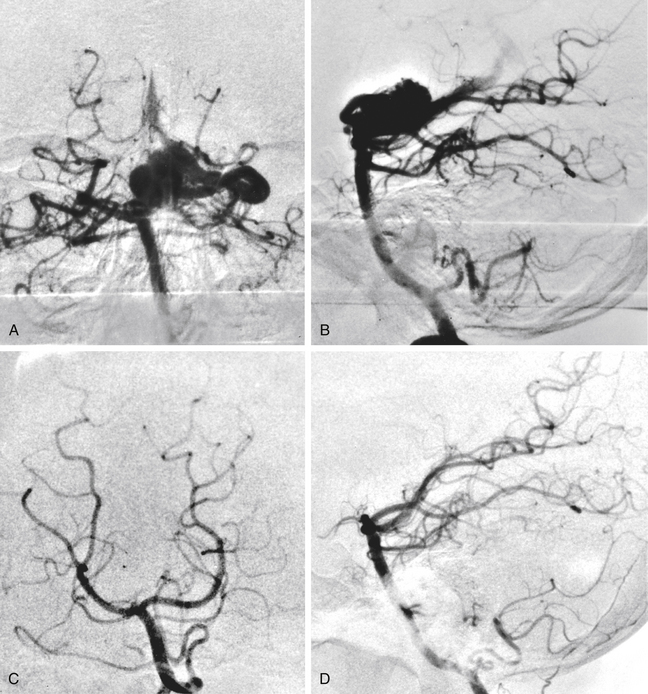
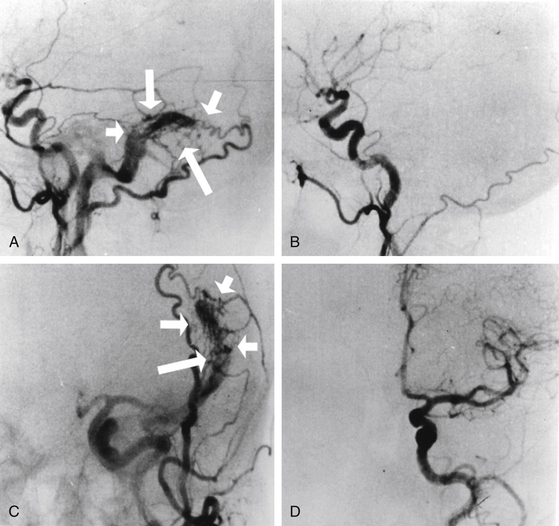
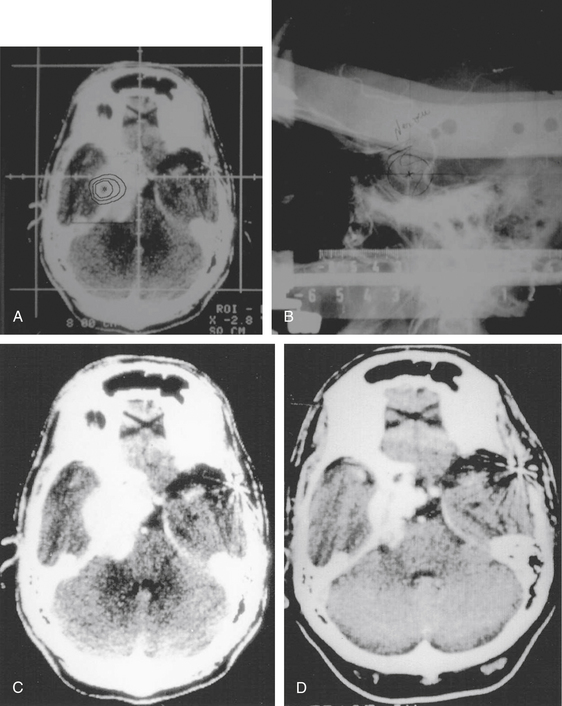
Total obliteration of the AVM after radiosurgery was defined as “complete absence of former nidus, normalization of afferent and efferent vessels, and a normal circulation time on high-quality rapid serial subtracted angiography”59 (Fig. 5-2). Any remaining nidus, regardless of its size, is considered partial obliteration (Fig. 5-3). Subtotal obliteration of an AVM means the angiographic persistence of an early filling draining veins without demonstrable nidus (Fig. 5-4).60
Clinical Outcomes
A review of the long-term clinical outcomes following gamma surgery was carried out on 247 patients we treated between 1970 and 1983.61 The presenting symptoms widely varied and 94% of the patients had hemorrhaged prior to therapy. Ninety-eight of these patients had chronic headaches and 66% had complete relief following gamma surgery. An additional 9% improved. Twenty-six percent had seizures prior to therapy and 19% of these became seizure free and 51% improved. Eleven patients (5%) without prior seizures had at least one seizure following therapy. Resolution or significant improvements were also seen in 53 of 74 patients with motor deficits (72%), 19 out of 46 with a sensory deficit (41%), 23 out of 44 with memory disturbance (52%), and 26 out of 35 with language dysfunction (74%).
Outcomes of Gamma Surgery for Arteriovenous Malformations after 1989
Since 1989, a total of 1350 AVM patients were treated with gamma surgery at the Lars Leksell center for Gamma Surgery, University of Virginia, Charlottesville. Excluding 82 patients completely lost to follow-up, 139 patients with follow-up of less than 2 years and additional 106 patients with large AVMs undergoing only partial treatment, we analyze the outcome of 1023 AVMs. There were 523 males and 500 females with a mean age of 34 years (range 4–82 years). The presenting symptoms leading to the diagnosis of AVMs was hemorrhage in 529 (52%), seizure in 237 (23%), headache in 133 (13%), and neurologic deficits in 94 (9%). In 30 patients (3%), the AVMs were incidental findings. The locations of the AVMs were in the cerebral hemispheres in 630 (62%), basal ganglion in 96 (9%), thalamus in 82 (8%), corpus callosum in 38 (4%), brain stem in 84 (8%), cerebellum in 68 (7%), and insula in 25 patients (2%). The Spetzler-Martin grading of the AVMs were Grade I in 174 (17%) patients, grade II in 328 (32.1%), Grade III in 440 (43%), Grade IV in 78 (7.6%) and grade V in three (0.3%). One hundred and twenty-two patients (12%) had previous partial resection of the nidi, and 244 patients (24%) underwent preradiosurgical embolization. The nidus volume ranged from 0.1 to 33 cm3 (mean 3.5 cm3). The mean prescription dose was 21.1 Gy (range 5–36 Gy), and the mean maximum dose was 39.0 Gy (range 10–60 Gy). The mean number of isocenters was 2.7 (range 1–22).
The reported obliteration rate following radiosurgery varied greatly.62–65 One should be cautious when interpreting the results owning to the biases injected from different cut-off time and imaging modality used to conclude total obliteration. Studies only including patients with long follow-up, reporting only patients undergoing angiography or including MRI as imaging study to conclude obliteration tend to overestimate the success rate of radiosurgery.63,66,67
Gamma Surgery for AVMs in Pediatric Patients
Following gamma surgery, a total obliteration was confirmed in 109 (59%) and subtotal obliteration in 9 (5%). Forty-nine (26%) patients still had patent residual nidus. In 19 (10%) patients, obliteration was confirmed on MRI only. The actuarial angiographic obliteration rate was 34% at 2 years, 46% at 3 years, and 51% at 5 years. In general, the imaging outcome of pediatric patients is similar to that observed in adult population. A negative history of preradiosurgical embolization and a high prescription dose were significantly associated with increased rate of obliteration.
Some studies had proposed that in pediatric patients the response to radiosurgery seems to be less favorable.66 Hypotheses such as the immature vessels in pediatric cases more likely to recover from radiation induced damage and neovascularization in response to radiation have been proposed. Our experience show comparable result in children compared to adults. Additionally, we observe that adverse radiation induced damage seems to be more tolerable for kids, which proves that radiosurgery has a favorable benefit risk profile in the management of pediatric AVMs. However, the risk of hemorrhage remained in pediatric patients and the development of secondary tumor cannot be overlooked.
Gamma Surgery following Embolization of Arteriovenous Malformations
The effectiveness of partial embolization followed by gamma surgery in the management of relatively large AVMs still remains controversial. When comparing the outcome in patients treated with gamma knife alone to those with combined embolization and gamma knife treatment, recent studies reported less favorable outcome in patients with preradiosurgical embolization.65,68
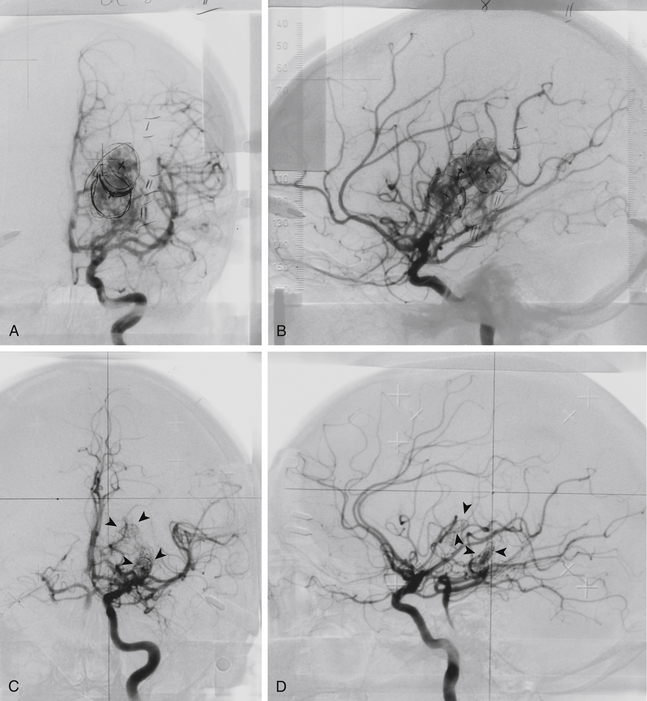
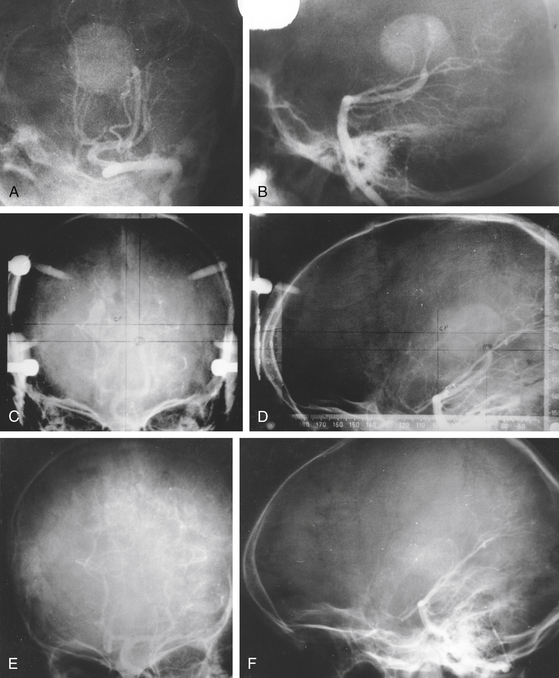
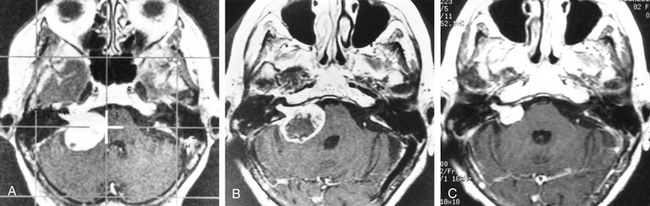
After gamma surgery an angiographically confirmed total obliteration of the AVMs was achieved in 71 patients (27%) (Fig. 5-5). A total obliteration on MRI was observed in 26 patients (10%). In 157 patients (60%) only a partial obliteration could be obtained after a follow-up period of at least 2 years. Eight patients (3%) presented with a subtotal obliteration. The outcome after gamma surgery in embolized AVMs (obliteration rate 27%) is much less favorable than AVMs treated with gamma knife only (obliteration rate 72%).
Recanalization of previously embolized parts of the nidus,69 difficulty in nidus delineation following previous embolization,65 and attenuation of radiation dose by embolization materials70 have been proposed to explain the less favorable outcome in patients with preradiosurgical embolization. Theoretically, volume reduction following embolization affords a lower chance of radiosurgery-related adverse effect; however, our data do not show this. Additionally, the complications from embolization are not negligible. Therefore, the use of embolization before gamma knife treatment remains problematic and awaits further investigation.
Gamma Surgery for Large Arteriovenous Malformations
The following strategies are currently available to treat large AVMs with radiosurgery.
1. Embolization of a portion of the AVMs then performing radiosurgery if the nidus shrinks to a size manageable with radiosurgery. However, embolization should effectively shrink the nidus for radiosurgery to achieve good results; otherwise fragmentation of the nidus into a number of segments will make the radiosurgical planning difficult and increasing the probability of radiosurgery failure.
2. Staged radiosurgery to selected volumes of the AVMs. Sirin et al.71 used staged volumetric radiosurgery in 28 large AVMs. Out of the 21 patients, seven underwent repeat radiosurgery and were eliminated from outcome analysis. Of the remaining 14 patients, three had total obliteration on angiograms, and 4 had no flow voids on MRI but had no follow-up angiography. Four patients had hemorrhages after radiosurgery resulting in two deaths. Worsened neurologic deficits occurred in one patient.
3. Treating the whole nidus in one session with low-dose radiosurgery. Pan et al.72 reported an obliteration rate of 25% for AVMs with volume larger than 15 cm3. The obliteration rate increased to 50% at 50 months follow-up. The morbidity was 3.3%. Post-treatment hemorrhage occurred in 9.2% of cases.
4. At Lars Leksell center, we evaluated a protocol using combined radiosurgery and microsurgery for the management of large AVMs. Gamma surgery was performed for the deep medullary portion of the AVMs as a first step. The second step was planned as microsurgical extirpation of the superficial segment if the goal of the first step, obliteration of the deep segment of the AVM, was achieved. However, in less than 5% of the patients, this goal was achieved.
Hemorrhage Risk in the Treatment–Response Interval
Whether gamma surgery without obliteration of the nidus provides partial protection from hemorrhage is still controversial. It has been demonstrated by some authors that there may be some degree of protective effect.73–75 Because the incidence of hemorrhage in a matched group of untreated patients will likely never be known and the timing of obliteration is not known except as being between diagnostic scans, it is a difficult position to support.
The incidence of hemorrhage following gamma surgery during the first 2 years was studied in 1604 of our patients and reported by Karlsson et al.76 There were 49 hemorrhages for an annual incidence of 1.4%. This is slightly lower than the generally accepted rate of 2% to 4% per year but includes all 1604 patients, consisting of those known and not known to have obliterated AVMs. Of these hemorrhages, 14 were fatal (annual rate of 0.4%) and 9 had permanent neurologic deficits (annual rate of 0.3%).
Repeat Gamma Surgery for Incompletely Obliterated Arteriovenous Malformations
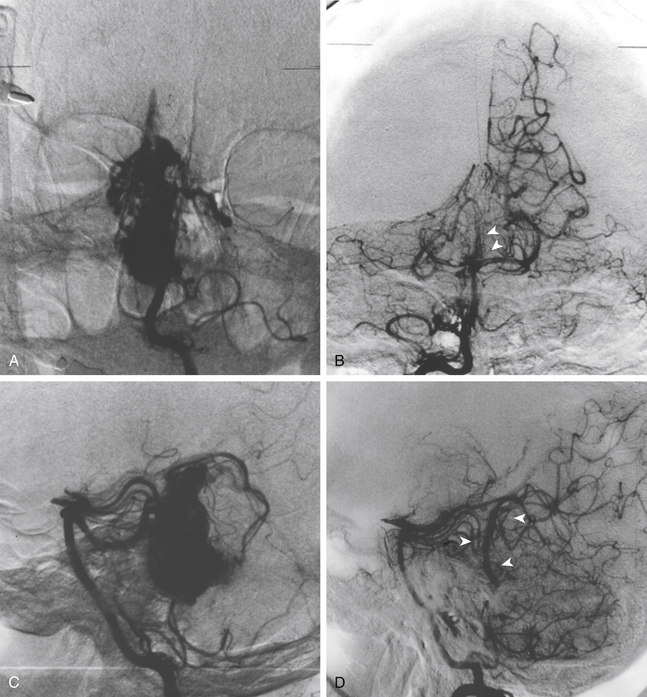
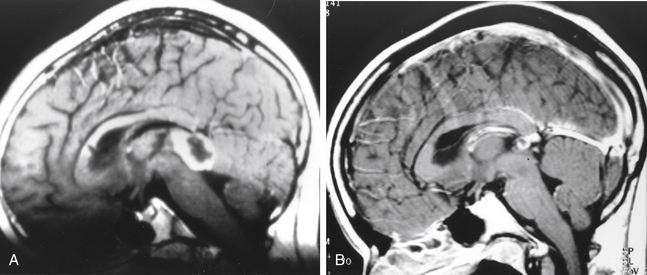
Repeat treatment yielded a total angiographic obliteration in 77 (55%) (Fig. 5-6) and subtotal obliteration in 9 (6.4%) patients. In 38 (27.1%) patients, the AVMs remained patent. In 16 patients (11.4%) no flow voids were observed on the MRI. High prescription dose, small nidus volume, nidi with only superficial venous drainage, and a negative history of prior embolization were significantly associated with increased rate of AVM obliteration. Clinically, 126 patients improved or remained stable and 14 experienced deterioration (8 due to a rebleed, 2 caused by persistent arteriovenous shunting, and 4 related to radiation induced changes).
We advise repeat gamma surgery in cases with still patent nidi 3 to 4 years after initial gamma surgery when open surgery or endovascular procedures were expected to yield higher risk of complications than gamma surgery. Our experience showed that when repeating gamma knife treatment a dose of at least 20 Gy led to a higher chance of subsequent nidus obliteration (77% vs. 47% with prescription dose less than 20 Gy).
Hemorrhage from Angiographically Confirmed Obliterated Arteriovenous Malformations
Some studies have noted AVM reappearance after apparent gamma knife surgery obliteration.77 In our histopathologic analysis, gamma surgery of AVMs caused endothelial damage, proliferation of smooth muscle cells, and the elaboration of extracellular collagen by these cells, which led to progressive stenosis and obliteration of the AVM nidus.43 In this same report, there was evidence of small trapped vessels that would have very little blood flow. It is unclear what histopathologic process would permit the formation of new vessels following radiosurgical-induced obliteration of the AVM nidus. However, it is clear that the infrequent and small trapped vessels observed by Schneider et al.43 could not explain the angiographic findings reported by Linqvist et al.78 Such inconsistent findings following radiosurgical treatment of AVMs suggest the need for further clinical and histopathologic investigation and the continued follow-up of patients, particularly pediatric ones, following gamma surgery.
In our series of AVMs treated with gamma surgery, we observed no recurrent hemorrhage after angiographically confirmed obliteration of the AVMs. In one case reported by Guo et al., a rebleed occurred after angiographic documentation of nidus obliteration.79 The MRI findings suggested that hemorrhage possibly resulted from radiation-induced tissue damage. Furthermore, the histologic examination of the suspected recanalized AVM revealed channels that were one-fiftieth the size of the smallest vascular channels in AVMs, making it unlikely that these were vessels with significant blood flow. This view has been further confirmed by the fact that a repeat angiogram revealed no evidence of residual malformation or recanalization. Rebleeding, in spite of post-treatment angiograms interpreted as normal, may be explained by unsatisfactory quality of the neuroimaging studies or inadequate interpretation leading to the misdiagnosis of angiographic cure.
Subtotal Obliteration of Arteriovenous Malformations
Subtotal obliteration of an AVM following gamma surgery has been reported sporadically. This angiographic phenomenon implies a complete disappearance of AVM nidus but persistence of early filling drainage veins (see Fig. 5-4). Theoretically, the early filling venous drainage suggests that some shunting still persists.
We reported a series of 159 patients with subtotal obliteration of AVMs (SOAVMs).60 The incidence of SOAVMs was 7.6% from a total of 2093 AVM patients who were treated with gamma surgery and had angiographic follow-up available. The diagnosis was made after a mean of 29.4 months (range 4–178 months) following gamma surgery.
Our series shows that subtotal obliteration of the AVMs did not necessarily prove to be a premature stage of an ongoing obliteration, and instead might be the end point of the obliteration process. Earlier in our series, we repeated gamma surgery for SOAVMs, targeting the proximal segment of the early filling vein. After repeat treatment, 79% SOAVMs were obliterated. However, the necessity of retreatment remains to be determined given the fact that in the whole group no hemorrhage occurred and that 73% of SOAVMs obliterated spontaneously.
Complications
Radiation-Induced Changes
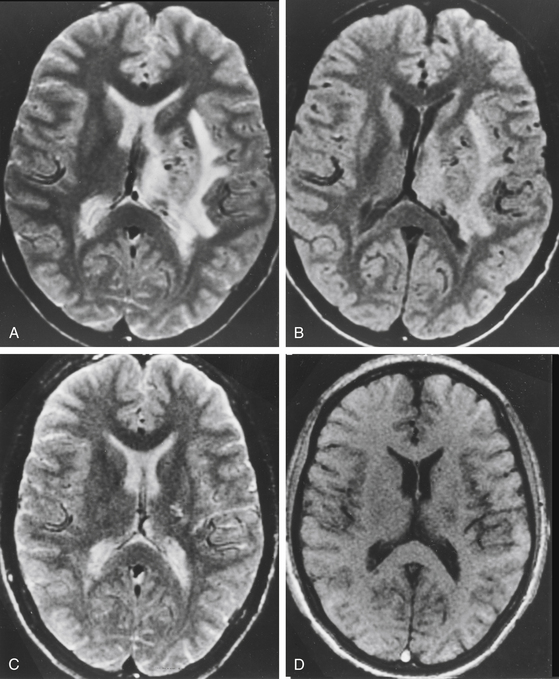
Radiation-induced change is an increased T2 signal around the AVMs on MRI following radiosurgery (Fig. 5-7). Radiation damage of glial cells, endothelial cells damage followed by breakdown of blood–brain barrier, excessive generation of free radicals or release of vascular endothelial growth factors have been proposed to explain this imaging finding. The severity of radiation-induced changes on images and associated neurologic deficits varied ranging from asymptomatic, a few millimeters increased T2 signal surrounding the treated nidus to massive brain edema with symptoms and signs of increased intracranial pressure. From our 1500 gamma knife procedures performed for AVM patients with follow-up MRI available for analysis, 34% of patients developed radiation-induced changes. Among them, 60% had mild (a few millimeters of increased T2 signal surrounding the nidus), 33% had moderate (compression of ventricle and effacement of sulci) and 7% had severe (midline shift) radiation-induced changes. The mean time to the development of radiation-induced changes was 13 months after gamma surgery. Resolution of these changes was the usual course and the mean duration of the changes was 22 months. Large nidus volumes, high prescription doses, history with preradiosurgical embolization, and nidus without previous hemorrhage were associated with higher risk of radiation-induced changes.
Cyst Formation
A rare occurrence following gamma surgery for AVMs is the development of an expansive cyst at or adjacent to an obliterated AVM (Fig. 5-8). Cyst developed after resolution of previous hemorrhages or fluid cavities from encephalomalacia after surgeries should not be considered as complications related to gamma surgery. First reported in 199245 in two patients out of a series of forty, we found a total of 20 patients (1.6%) developing a cyst after a mean of 8.1 years postradiosurgery from our 1272 patients with follow-up MRI available.80 Six patients had large cysts and three of them were symptomatic requiring surgery. Two cases underwent craniotomy and drainage of the cyst. The cyst wall showed no evidence of neoplasia. Direct radiation injury to the perilesional brain tissue, increased permeability of the blood–brain barrier with accumulation of the exudative fluid, hemodynamic perturbations during gradual obliteration of the nidus with subsequent ischemic tissue damage, and tissue destruction due to subclinical perilesional hemorrhages have been proposed as the possible mechanisms of cyst formation.
Radiosurgery-Induced Neoplasia
We found two meningiomas from 1333 AVM patients treated with gamma surgery (Fig. 5-9); however, follow-up imaging was performed over a period of at least 10 years in only 288 of these patients.81 If we conservatively estimate that radiosurgery-induced lesions would be evident within a 10-year time interval, then our incidence of radiosurgery-induced neoplasia is 2 in 2880 person-years or 69 in 100,000 person-years. Thus, there is a 0.7% chance that a radiation-induced tumor may develop within 10 years following gamma surgery. The long latency and relative rarity of these lesions following radiosurgery may defy a conclusive determination of the true incidence.
Dural Arteriovenous Fistulas
Although dural arteriovenous fistulas (dAVFs) comprise approximately 15% of all intracranial vascular malformations, the precise mechanism of formation remains unknown. The leading theories include adjacent venous sinus stasis as well as alterations in local expression of vasogenic factors, such as vascular endothelial growth factor and fibroblastic growth factor.82,83
From a treatment standpoint, experience with dAVFs is distinct from AVMs. Studies have established that the flow dynamics of dAVFs are the most important indicator of the need to treat and modality to choose, be it embolization, open microsurgery, or radiosurgery. As the aggressive natural history of lesions with cortical venous reflux differs significantly from lesions without angiographic evidence of cortical venous reflux, early definitive therapy via endovascular procedures or open surgical resection appears to be preferable to radiosurgery as a first-line treatment. Although radiosurgery is thought to be an effective agent for decreasing neovascularization in dAVFs, the time interval needed for the desired effect is too great to justify radiosurgery as a first-line therapy.84
The long-term analysis of radiosurgery for dAVFs over 25 years at the Karolinska University Hospital in Stockholm, Sweden included 52 patients treated between 1978 and 2003. The obliteration rate reported in this study was 68% with 16 dAVFs presenting as less aggressive Borden I or Cognard I/IIa lesions.85 In a similar institutional experience at the Lars Leksell center between 1989 and 2005, 55 patients with dAVFs were treated with gamma surgery, primarily as an adjunct to surgery or embolization. Obliteration rates measured by angiography at 3 years ranged from 54% to 65%, with the 16 patients classified as Borden I lesions (Fig. 5-10). Unlike the Karolinska study, the majority of patients treated at the Lars Leksell center received gamma surgery as a secondary therapy, with 41 of the 54 patients receiving surgical or endovascular intervention prior to radiosurgery. Regardless of the difference in utilization of radiosurgery as a primary or secondary treatment modality, the results of these long-term studies indicate that gamma knife is an effective and safe treatment for intracranial dAVFs.
Vein of Galen Malformations
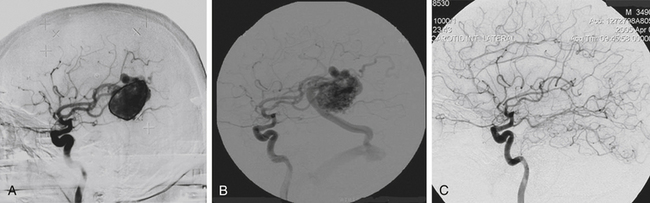
We have treated nine patients with vein of Galen malformations. The patients ranged in age from 4 to 72 years of age. Among these patients, there were three with Yasargil Type I, one with Type II, two with type III, and three with type IV malformations. Prior embolization had failed in four of the cases. Three of the vein of Galen malformations were treated twice with radiosurgery. Follow-up angiograms were obtained in eight of the patients treated.86 Four malformations were completely obliterated (Fig. 5-11). Another one seems to be obliterated but definitive confirmation could not be obtained as the patient refused a final angiogram. Another patient has some residual fistula not in the initial radiosurgical treatment field and has been retreated. Two other patients had marked reduction of flow through their malformations.
Cavernous Malformations
The success of treating AVMs prompted the treatment of cavernous malformations (CMs) with the gamma knife. Their tendency to be small in size, with the lack of intervening normal brain tissue and relatively low rate of clinically significant hemorrhage made CMs a natural target for the gamma knife. The rate of hemorrhage for cavernomas is widely disparate in the neurosurgical literature reported as between 0.1% and 32%.87–90 This is largely due to semantic differences in defining a hemorrhage with some authors counting the presence of a hemosiderin ring on MRI as evidence of a hemorrhage as well as in determining the date from which patients were at risk for hemorrhages.
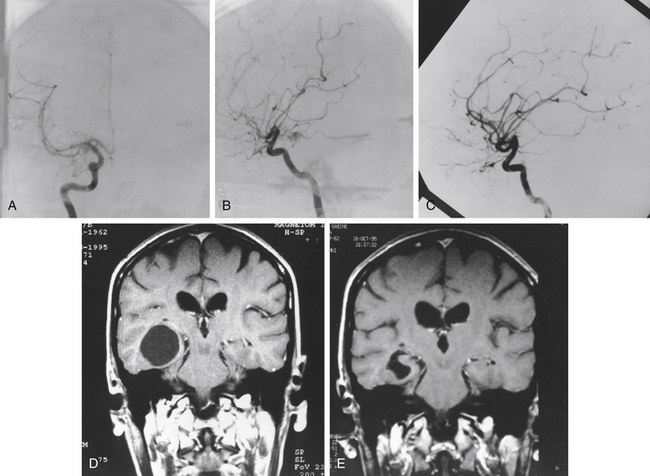
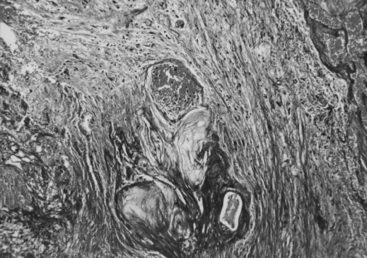
Gamma surgery of CMs appears to have a histologic effect on them, which is not evident on imaging studies. In a case reported earlier,29 a gamma surgery treated CM followed for 5 years showed no change on MRI studies. Histologic examination following surgical removal of the lesion showed it to be partially obliterated (Fig. 5-12).
A total of 23 patients have been treated by us for CMs between 1985 and 1996, 22 of which are available for follow-up evaluation.91 Maximum treatment dose varied from 11 to 60 Gy (mean 33 Gy). Peripheral dose varied from 9 to 35 Gy (mean 18 Gy). Nine symptomatic hemorrhages occurred in this group after therapy for an annual incidence of 8%. Four of these patients were subsequently operated upon. Six patients suffered neurologic decline secondary to radiation-induced changes, five of which were permanent. Two patients subsequently underwent surgery. Thus the permanent radiation-induced complication rate is 22%, nearly 12 times higher than expected for a similarly treated group of AVM patients. The high incidence of post-treatment hemorrhage and radiation-induced complications is greater than the expected morbidity from an untreated group. For this reason the routine use of the gamma knife in the treatment of CMs cannot be supported at this time.
There have been observations in literature that demonstrate a protective effect of gamma surgery on the rate of hemorrhage in these lesions. Based on 38 cases, Kondziolka et al.92 maintain that radiosurgery offers benefit to these patients. They reported that 6 patients (15%) had significant reduction in size with a 13% hemorrhage rate; 10 of the patients (26%) developed neurologic deficits, 2 of these underwent surgery and succumbed to the illness. The rate of complications reported by them in the face of the fact that only 15% of the patients in this series had any reduction in the size of the lesion does not, in our opinion, constitute grounds for justifying radiosurgery for CMs. Furthermore, their contention that the complications reported by us may in part be due to the high doses used in treatment does not get support from their statistics with a lower dose. These 38 patients were a part of a later report on 47 patients from the same authors detailing the outcome. They found a postradiosurgery annual hemorrhage rate of 8.8%, which is high when compared to the risk reported by the same authors at 0.6% to 4.5%, even if the difference is not statistically significant. They chose, however, to compare their postradiosurgery hemorrhage rates with the preradiosurgery rate in the same subjects, making the assumption that the rate could be based on an epoch starting from first observation or first hemorrhage. This is fallacious since the malformation was present before the presenting hemorrhage and most likely from birth. Recomputed on this basis the preradiosurgery annual bleed rate comes to 5.9%, which is more congruent with expected natural history. Once again the incidence of hemorrhages postradiosurgery seems spuriously higher.
During the past decade, gamma knife has been widely used in some centers to treat CMs. Since the lesions cannot be precisely visualized by any imaging studies, the outcome of radiosurgery was universally evaluated based on the hemorrhage rate before and after radiosurgery using the same group of patients as control. There are methodological flaws in such study design. As been demonstrated in publications,93 patients with previous hemorrhages tend to bleed more often. By calculating the clustering number of hemorrhages in the short period of follow-up time, which was prematurely terminated by radiosurgery prompted by the hemorrhage, the preradiosurgical hemorrhage rate was erroneously high ranging from 17% to 36%.64,88,94–96 The hemorrhage rate after gamma surgery is these series ranged from 2% to 4%, which seems to be significantly low compared to the preradiosurgical bleeding rate but actually is not much different while comparing to the number reported in series studying natural course of CMs. Of note, the complications in these series remained to be high.
Developmental Venous Anomalies
The natural history of developmental venous anomalies (previously named venous angiomas) is benign97 and clearly not a surgical lesion. Prior to clear elucidation of this prognosis, 19 patients were treated for this entity by us.98 One patient was cured and three were partially obliterated. Three patients suffered radionecrosis, and one had symptomatic edema. One patient with radionecrosis underwent subsequent debridement. A 5% cure incidence with a 30% complication incidence for a benign entity is unacceptable.
Tumors
In an attempt to eliminate some of the subjectivity of naked eye observations and the obvious fallacy resulting from the estimation of a three dimensional object on the basis of three linear measurements in orthogonal planes, we have developed software that allows estimation of lesion volume based on MRI or CT.99 The procedure involves scanning the study into a computer program and outlining the pathology in each slice. The computer then measures the area within the contour and calculates a volume based on slice thickness, the process is repeated for each slice and the total volume calculated by integrating the individual slice volumes. The error of this method was determined by comparing various hand-partitioned volumes with volumes estimated using polyhedrons to approximate the regions of interest on each slice. The average relative error is strongly dependent on the number of axial slices obtained through the object of interest and is fairly independent of the size of the object itself. For objects varying in volume from 0.1 to 10 cc, the average relative errors per number of slices through the object have been computed as follows: 3% for 7 slices, 4% for 6 slices, 6% for 5 slices, 11% for 4 slices, and 21% for 3 slices. Volumetric estimation based on 1 or 2 slices through the region of interest produces unacceptable average relative errors of more than 40%. We now require all follow-up studies to be performed with a slice thickness of 3 mm with zero overlap or gap between adjacent slices. Such a protocol generally helps ensure the acquisition of 3 or more slices through the region of interest. Even though the technique of volume estimation has an acceptable level of accuracy, we prefer to ignore changes of less than 15%.
Pituitary Adenomas
The efficacy of radiation in the treatment of pituitary adenomas was well-documented before gamma surgery was used for this disease.100,101 Reduced fractionation techniques had been shown to have effectiveness in the treatment of Cushing’s disease and were the impetus for the use of radiosurgery. MRI has replaced less exact invasive localization procedures such as cisternography and CT in the planning of gamma surgery in patients with pituitary adenomas. There still remain difficulties with the use of gamma surgery. The peripheral dose that can be delivered for macroadenomas is limited if the optic apparatus is in proximity with the tumor. Localization of microadenomas can be difficult with even the best MRI examinations (e.g., a fat suppression MRI protocol) and amelioration of hypersecretory syndromes is delayed.
Another indication for gamma surgery is persistence or recurrence of elevated hormone levels after microsurgery. In the presence of residual or recurrent tumor that is not readily amenable to further extirpation, either because of its location or the inability to localize the tumor within the sella, gamma surgery can be applied. Tumor within the cavernous sinus can be treated. Difficulty in localizing the tumor usually requires radiosurgical targeting of all the contents within the sella, and such an approach carries a fair risk of postradiosurgical hormonal insufficiency.102 If the patient has a secretory microadenoma but the symptomatology is not urgent and microsurgery is for some reason not considered, then gamma surgery can be used as the primary therapy.
In preparation for treatment with high-dose, narrow beam radiation, many centers have recommended a temporary cessation of antisecretory medications in the peritreatment time period. In 2000, Landolt et al. first reported a significantly lower hormone normalization rate in acromegalic patients who were receiving antisecretory medications at the time of radiosurgery.103 Since then, this same group as well as others has documented a counterproductive effect of antisecretory medications on the rate of hormonal normalization following gamma surgery.32,104 The degree to which and the mechanism by which antisecretory medications lower hormonal normalization rates is unknown, but Landolt et al. have hypothesized that these drugs lower the tumor’s metabolic rate and decrease their radiosensitivity.105,106 Moreover, the optimal time period to hold antisecretory medications in conjunction with gamma surgery is not clear. Landolt and Lomax recommend that dopamine agonists be withheld 2 months prior to the procedure.104 For acromegalics, they recommend altering antisecretory medication administration as early as 4 months prior to radiosurgery and completely halting all antisecretory medications 2 weeks prior to radiosurgery.103 Although many centers have incorporated such methodology into their treatment regimen, the potential risk and benefits of halting antisecretory medication administration should be weighed. The functional adenoma may be more likely to respond to gamma surgery. However, in the absence of antisecretory medication control, it may also enlarge thereby risking adjacent structures (e.g., the optic apparatus), necessitating a lower prescription dose, and making effective treatment more difficult.
Most centers have observed effective growth control of pituitary adenomas following gamma surgery. However, there have been a wide range of outcomes with regard to hormonal normalization of secretory adenomas. The varied outcome results for hormonal normalization may arise from the following reasons:105 early studies utilized CT rather than more precise MRI for dose planning;106 different criteria for defining an endocrinologic cure have been applied in various studies and there is little consensus even within the neuro-endocrinologic community for precise defining criteria107; and many studies had short or intermediate follow-up periods and may not have been long enough to observe patients with an endocrinologic recurrence following an initial remission.
Nonsecretory Tumors
We have treated 100 patients with nonsecretory pituitary tumors, 90 of which have imaging and endocrinologic follow-up of a minimum of 6 months and an average of 45 months108 (Tables 5-2 and 5-3). Of these, 59 (65.6%) had a decrease in the volume of their tumors (Fig. 5-13) and 24 (26.7%) had no change in the size. In seven (7.8%) patients, the tumors increased in size. Of note, among 61 tumors involving the cavernous sinus, 39 shrank, 17 remained unchanged, and five increased in size. The minimal effective peripheral dose was 12 Gy; peripheral doses greater than 20 Gy did not seem to provide additional benefit. In 61 patients with residual pituitary function at the time of gamma surgery, new hormone deficiency occurred in 12 patients (20%).
Growth Hormone–Secreting Tumors (Table 5-4)
We have performed gamma knife procedures on 137 patients with growth hormone secreting adenomas (Table 5-4). Follow-up of at least 18 months was available for 95 of these patients. There was normalization of IGF-1 in 53% of cases at an average time of 30 months after radiosurgery. Three patients developed recurrence of their acromegaly after initial remission, with a mean time to recurrence of 42 months. New endocrinologic deficiencies developed in 34% of patients, with hypothyroidism and low testosterone levels being the most common new endocrinopathies.
Prolactin-Secreting Tumors
Prolactinomas are usually well-controlled by dopamine agonists (Table 5-5). Nonetheless, this medication occasionally fails to achieve remission and is not tolerated by all patients. Alternative for these patients not responding to medical therapy is surgery. Patients who are refractory to medical and/or surgical therapy may be treated with gamma surgery. Of the 37 prolactin secreting tumors treated by us at the Lars Leksell gamma knife center,109 28 have imaging follow-up of 1 year or more. Thirteen (46%) had a decrease in the size, 12 (43%) were unchanged and three (11%) were increased. Excluding patients with normal prolactin level before gamma surgery and those with normal level at the last follow-up while still receiving dopamine agonists, endocrine follow-up was available for 23 patients. There was remission (serum prolactin level <20 ng/ml) in 26% of cases. New onset endocrine deficiency developed in 29% of patients. Two patients had new onset extraocular movement problems; one developed an oculomotor and the other an abducens nerve palsy. In both cases, the cavernous sinus was involved and both cases received a prescription dose of 25 Gy.
ACTH-Secreting Tumors
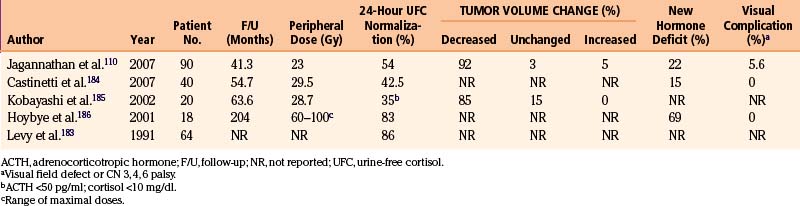
A total of 107 patients with Cushing’s disease underwent gamma surgery at our institution (Table 5-6). Seventeen patients who had less than 12 months follow-up were excluded, leaving 90 patients evaluable.110 All but one patient had undergone previous trans-sphenoidal operations. Of note, in 23 patients in which no tumor can be identified on planning MRI, the entire sellar content and adjacent cavernous sinuses were targeted. The mean prescription dose of gamma surgery was 23 Gy (range 8–30 Gy). Of 67 patients with visible tumors, imaging follow-up demonstrated a decrease in the size of the tumor in 62 cases (92%), no change in 2 (3%), and an increase in size in 3 (5%). However since the hypercortisolism defines the dangerous character of the ACTH-secreting tumor, the control of endocrine abnormalities is the true measure of tumor control. Normal 24-hour, urine-free cortisol levels were achieved in 49 patients (44%), at an average time of 13 months post-treatment (range 2–67 months). Ten patients who achieved remission after gamma surgery suffered a recurrence. Seven of these patients had repeat gamma knife procedures, with three patients achieving another remission. New endocrine deficiencies developed in 20 patients (22%), with hypothyroidism being the most commonly found new endocrinopathy. Five patients developed new-onset ophthalmoplegia (four of them with visual acuity deficits). One of them had received prior conventional fractionated radiation therapy, three had two gamma knife surgeries, and one had fractionated radiotherapy and subsequently two gamma procedures. Evidence of radiation-induced changes presenting as increased enhancement of parasellar area was seen in three patients but only one had symptoms attributable to these changes.
The results of 35 patients treated at the Karolinska Institute have been reported.111 Of the 29 patients that had follow-up of up to 9 years, 22 (76%) had normalization of their endocrine abnormalities, 10 within 1 year and the remainder within 3.
Nelson’s Syndrome
Patients with an ACTH-secreting pituitary tumor may require a bilateral adrenalectomy to treat their Cushing’s disease when surgical extirpation and radiosurgery for the pituitary tumor failed to normalize hormonal production (Table 5-7). Approximately one third of these patients will experience Nelson’s syndrome, namely, enlargement of residual pituitary adenoma, developing hyperpigmentation and/or having an elevated level of serum ACTH. At the Lars Leksell center, we have performed gamma surgery on 23 Nelson’s patients. Five patients had previously received conventional fractionated radiation therapy, and two patients had received prior gamma surgery for Cushing’s disease. Median prescription dose to the tumor margin was 25 Gy (range 4–30 Gy). Twenty two patients had imaging follow-up and the mean imaging follow-up was 20 months (range 125–124 months). Fifteen patients had elevated ACTH level before gamma surgery and follow-up ACTH level was available. The mean endocrine follow-up was 50 months (range 13–166 months). Tumors decreased in 12 (55%) patients, remained unchanged in 8 (36%), and increased in 2 (9%). ACTH levels decreased in 10 patients (67%) with a median decrease of 75% (range 29%–93%). Three patients (31%) achieved normal ACTH levels with a mean time to remission of 9.4 months postradiosurgery. New endocrinopathies were seen in 4 out of 10 patients with residual pituitary function before gamma knife procedures, growth hormone deficiency being the most common new hormonal deficit. One patient suffered from a permanent oculomotor nerve palsy.
Craniopharyngiomas
Craniopharyngiomas are very difficult tumors to treat. Their benign histology is misleading. The near impossibility to resect completely and usual location in and about the hypothalamus make them difficult to cure. Microsurgery, intracystic instillation of radioisotopes, radiation therapy, and now radiosurgery have all been used. Long-term evaluation of patients with craniopharyngiomas is available after various treatment protocols.112,113 The general consensus is that as complete a surgical resection as possible, without creating significant morbidity, should be performed; this is followed by radiation therapy and gives reasonable long-term survival. The ill effects on children after receiving fractionated brain irradiation are well-known.114,115 Good results with resection alone have been achieved but only in the hands of a few neurosurgeons. Even so, long-term results of children with subtotal resection followed by radiation therapy has been shown to be superior to complete resection alone116 and the deficits incurred with aggressive surgery can be considerable.
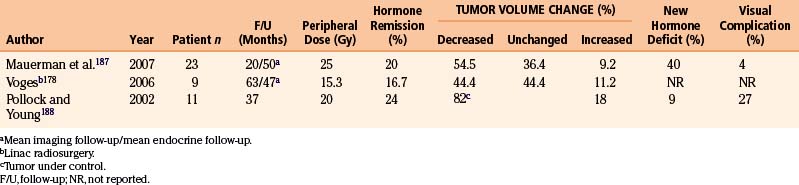
We treated 37 craniopharyngiomas in 35 patients. The prescription doses ranged from 6 to 25 Gy (mean 13.3 Gy). The follow-up ranged between 8 and 212 months with a mean of 62.5 months. Four tumors increased in size. A decrease in the solid component of the tumor was seen in 29 (Fig. 5-14) and no change was seen in 4. However, of the patients whose solid tumors decreased or remained unchanged, 10 developed new or enlarged cystic component with 4 of them requiring further surgical resection and 4 receiving intracavitary P32 instillation. In total, 23 patients improved or remained stable clinically. Twelve deteriorated and 10 of them died from complications of disease or surgeries. The mean 5-year survival rate was 71%.
Several other series have been reported in the use of gamma surgery in the treatment of craniopharyngiomas117,118 with results similar to ours. As larger series with longer follow-up become available it is likely that gamma surgery will either take the place of less discriminate radiation therapy, or be a useful adjunct to it.
Meningiomas
Meningiomas are usually benign, circumscribed tumors that arise from the coverings of the central nervous system and therefore tend to be superficial. Because of these attributes microsurgical extirpation of the entire tumor as well as any involved meninges is the treatment of choice. Unfortunately many meningiomas do not have one or more of the mentioned attributes. Aggressive, locally invasive tumors, especially those invading or involving critical or difficult to control neural or vascular structures and those at the skull base can be problematic in their complete removal. The use of radiation to lower the recurrence rate following microsurgical removal of meningiomas was shown to be beneficial.118–120 Recurrence rates were found to be dramatically decreased, and for patients with residual tumor following surgery the progression of tumor growth was significantly decreased.
Tumors within the parasellar compartment, which is a part of the extradural neural axis compartment, can be difficult to remove with microsurgery without significant morbidity.121,122 Residual tumor attached to still patent vascular or critical neural structures can be targeted with gamma surgery and allows less radical microsurgical resection and a lower incidence of morbidity.
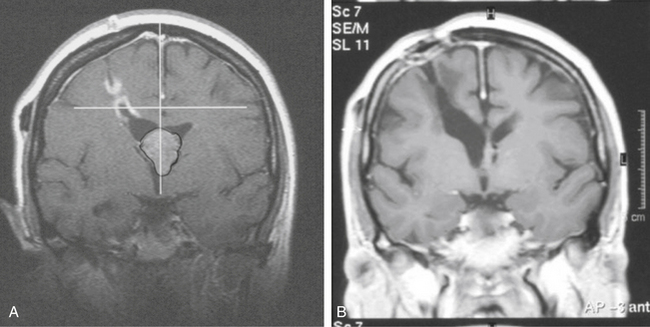
We have recently reviewed the central skull base meningiomas involving the sellar-parasellar space in 138 patients. There were 107 females (78%) and 31 (22%) males. The mean age was 54 years (range 19–85). Mean tumor volume at the time of radiosurgery was 7.5 cm3 (range 0.2–55 cm3). Eighty-four patients had prior microsurgery with partial resection of the tumor. Fifty-four had an upfront gamma procedure. In our assessment, the gamma treatment was optimal in 109 (79%) and nonoptimal in 29 cases (21%). We defined nonoptimal treatment as follows: the part of the tumor close to the optic apparatus did not receive the desired prescription dose, typically because there was no distance between the tumor and the visual pathways. The mean prescription dose used in this series was 13.7 Gy (range 4.8–30 Gy). The mean maximum dose was 34.2 Gy (range 16–60 Gy). The mean MRI follow-up was 82.4 months (range 24–216 months). Sixty-six tumors (48%) shrank (Fig. 5-15), 52 (38%) remained unchanged and 20 (14%) increased in size. Fourteen patients developed new or deteriorated cranial nerve deficits; 11 due to tumor progress and 3 in spite of good tumor control. There was no mortality. Young age, optimal treatment, and smaller tumor size were correlated with better outcome. The tumor progression–free survival at 5 years was 95.4% and at 10 years was 71%.
We have long-term follow-up of 10 to 21 years for 31 meningiomas treated with the gamma knife. Two thirds of these tumors have either shrunk significantly or remained stable, and among these were cases where only the vascular supply for the tumor was targeted (Fig. 5-16). This has resulted in significant tumor shrinkage and lasting effect even in the long term. Our practice has been to obtain a stereotactic angiogram prior to gamma surgery for large tumors. This allows treatment to include the vascular supply when ideal treatment is not possible because of radiation dose constraints imposed by the treatment volume or proximity of the tumor to the optic apparatus. Recently we have used MRA source images instead of angiograms to conduct treatment planning. Using MRI, the group from Heidelberg proved that radiation occluded small nutrient vessels of meningiomas providing the rationale for the treatment we have used since 1976.
Vestibular Schwannomas
Historically and incorrectly referred to as an acoustic neuroma, we prefer the designation of vestibular schwannoma, which recognizes the anatomic and histologic origins of these tumors.123 There may be no other intracranial neuropathology about which the proper treatment arouses as much controversy as the vestibular schwannoma. Neurosurgeons cite the series of surgeons with enormous experience removing these tumors to justify suboccipital removal, while otolaryngologists sacrifice the inner ear during the translabyrinthine approach in an attempt to better expose and preserve the facial nerve. Radiosurgery’s proponents cite excellent tumor control and low morbidity but must acknowledge that although the tumor often shrinks, it is still there. Therefore it is in the best interest of our patients that long-term outcome in patients treated with these three modalities be thoroughly evaluated.
The first vestibular schwannomas treated with the gamma knife were by Leksell and Steiner in 1969.124 Since then more than 36,843 have been treated around the world through 2006. The indications for gamma surgery for this tumor vary. Some physicians advocate gamma surgery in medically high risk patients, patients who refuse microsurgery, and in patients with postoperative residual tumors. However, others advocate gamma surgery as the treatment of choice in nearly all cases of vestibular schwannomas. The usefulness of irradiation in the postoperative period was shown by Wallner in 1987 where external beam irradiation lowered the recurrence rate from 46 to 6% in Boldrey’s surgical series at the University of California at San Francisco.125 By then, gamma surgery was already being widely applied to this disease under many circumstances. The fact is that for a number of reasons few neurosurgeons acquire the necessary competency to satisfactorily extirpate these tumors. This situation may change if a method to improve the acquisition of skills required for extirpation of these lesions is found.
The advent of MRI has made planning for gamma procedures much more exact. With a high-quality MRI scan and a relatively small tumor, the seventh cranial nerve can occasionally be visualized and carefully excluded from the treatment field. The trigeminal nerve can nearly always be identified except with the largest tumors, which in most cases should not be treated primarily with gamma surgery.
At the Lars Leksell center, we have treated 470 patients with vestibular schwannomas. A total of 153 of these patients with more than 12 months of follow-up have been reported.126 Radiosurgery was the primary treatment for 96 of such patients and was the adjutant (following microsurgery) in 57. The volume of the treated tumors ranged from 0.02 to 18.3 cm3.
Of the patients treated primarily with gamma surgery a decrease in tumor size was seen in 81% (78 patients), no change in 12%, and an increase in size in 6%. Among those 78 patients with a decrease in the size of their tumors, the decrease was greater than 50% in 20 patients. It is our policy to not consider decreases in volume of less than 15% as significant. This is true of all tumors that we treat. Imaging follow-up for these patients ranged from 1 to 10 years.
Other centers report similar rates of tumor control (i.e., with no change or decrease in the size of the tumor) seen in 89% to 100% of patients.127–129
Evaluation of the material from the Karolinska group included evaluation of radiographic changes besides size.130 The most common change was loss of central enhancement within the tumor on either contrasted MRI or CT studies. This occurred in 70% of patients and typically was observed within 6 to 12 months of treatment. However, these changes were reversible. Another change that was observed and that we have often seen is a transient increase in the size of the tumor during the first 6 months after gamma surgery. This is commonly seen in tumors that then regress to their original size or smaller (Fig. 5-17).
We have not seen an instance of cerebellar edema or hydrocephalus requiring spinal fluid diversion following gamma surgery for vestibular schwannomas, but both of these have been reported elsewhere.130,131
Astrocytomas
Low-Grade Astrocytomas
Yen et al. reported on a series of 20 patients with brain-stem gliomas presenting with clinical or imaging progression treated with gamma surgery.132 Sixteen tumors were located in the midbrain, four in the pons and one in the medulla oblongata. The mean tumor volume at the time of gamma surgery was 2.5 cm3. Tissue diagnosis was available in only 10 cases (50%). The cases without histology were treated based upon appearance on imaging as well-defined small tumors. Mean prescription dose was 15 Gy (range 10–18 Gy). The tumor disappeared in 4 patients (20%) (Fig. 5-18) and shrank in 12 patients (60%) after a minimal of 12 months of follow-up (mean follow-up 78 months). Transitory extrapyramidal symptoms and fluctuating times of consciousness occurred in one patient. Tumor progression occurred in four patients. One of these four patients required a ventriculoperitoneal shunt for hydrocephalus, two experienced neurologic deterioration, and one died of tumor progression.
High-Grade Astrocytomas
We have treated 56 malignant astrocytomas. Our experience has been similar to other reported series133–135 with the majority of patients showing initial decrease in size or remaining stable for a period of time (Fig. 5-19); however, recurrence and progression is the rule with these tumors and no therapy is curative. Because of the differences in histology and the wide variety of therapies and protocols available for these tumors it is difficult to judge the benefit of gamma surgery. Although our group and Nwokedi et al.136 have observed a statistically significant prolongation of life expectancy in the group of patients undergoing aggressive multi-modality treatment (e.g., including some or all of the following: radical tumor debulking, radiation therapy, chemotherapy, and gamma surgery), it remains to be seen if these findings will be borne out in larger, better controlled studies. The limit of the benefit that radiation can contribute to the treatment of these lesions seems to have been reached, hence it may be stated that the dose escalation with the gamma knife in the treatment protocol of this disease will change only marginally the clinical outcome. It is also conceivable that targeting tumor angiogenesis and finding ways to induce apoptosis will have some impact on the management of these cases.
Neurocytomas
Central neurocytomas were described by Hassoun et al. in 1982.137 According to Brandes et al., 210 cases were reported in the literature.138 The histology, biological behavior and clinical course of central neurocytomas may vary from benign to more aggressive patterns.139 Surgical resection is the first choice of treatment. Rades and Fehlhauer compared 108 and 74 patients who underwent complete or incomplete resection.140 At 5 years tumor control rates were 85% and 46%, respectively. If the surgical resection is not total, the residual tumor should be irradiated. The 5-year tumor control rate with postoperative radiotherapy increased from 46% to 83% after incomplete resection but radiotherapy did not seem to improve survival rate.140
With the advent of radiosurgery, it was used as upfront or adjunct therapy following surgery. We used the gamma knife in 7 patients with a total of 9 neurocytomas.141 The mean tumor volume at the time of the gamma procedures ranged from 1.4 to 19.8 cm3 (mean 6.0 cm3). A mean prescription dose of 16 Gy (range 13–20 Gy) was used. After a mean follow-up period of 60 months, 4 tumors disappeared and 5 shrank significantly (Fig. 5-20). One patient had a hemorrhage in the tumor 1 year after gamma surgery and the tumor had decreased significantly at the last follow-up. This patient had a new tumor at some distance of the successfully treated one. Four patients were asymptomatic during the follow-up period and a patient with hemiparesis caused by a previous transcortical resection was stable. A patient died of sepsis due to a shunt infection.
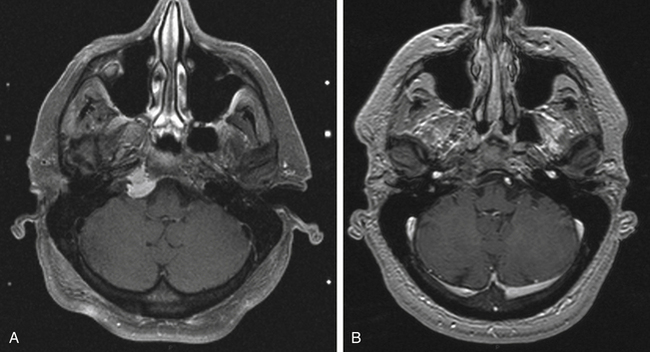
Chordoma
Skull base chordomas are a rare neoplasm arising from the remnants of notochord. Although histologically benign, these tumors are locally invasive and present significant management challenges. There is consensus that surgical debulking should be performed. Proton radiotherapy has been the mainstay of treatment for recurrent or residual tumors.142,143
Imaging follow-up was available for all patients with a median time of 88 months (range 8–167), and clinical follow-up was available for 11 patients with a median of 70 months (range 8–132). At the last follow-up, tumor control was achieved in five out of 15 patients (33.3%) after initial GKS (Fig. 5-21). Actuarial 5- and 10-year tumor control rates after one gamma surgery was 42.6% and 34%, respectively. Three patients who underwent a second gamma surgery achieved good tumor control. Actuarial 5- and 10-year tumor control rates after one or more gamma procedures improved to 50.3%. Symptomatic progression was seen in 75% of the patients.
For the management of intracranial chordoma, surgery as radical as possible is the main treatment alternative—a goal frequently not achieved and some forms of radiation have to be used as an adjunct. Chordoma is relatively radioresistant and respond best to high doses of fractionated proton radiotherapy. The physical and biological properties of the proton—it carries higher kinetic energy and as they slow down a higher release of energy (Bragg peak)—make it an excellent source of radiation for chordomas. Amichetti et al. in a review article presented the current data on the treatment of skull base chordomas with proton beam and compared the outcomes to those obtained with fractionated photon radiation, ion therapy, fractionated stereotactic photon therapy, and radiosurgery.142 With proton therapy, doses above 70 cobalt Gray equivalent can be applied safely, achieving local control rates at 3 years of 67.4% to 87.5%, at 5 years of 46% to 73%, and at 10 years of 54%. The estimated overall survival rates are 66.7% to 80.5% at 5 years and 54% at 10 years.
Chondromas and Chondrosarcomas
Muthukumar et al. treated 15 patients (nine with chordomas and six with chondrosarcomas) with gamma surgery and reported their results with an average follow-up of 4 years. Four of their patients had died; only two deaths were related to progression of disease and both of these had progression outside of the treated area. Only one of the surviving 11 had tumor progression, and five had shrunk.144 Gamma surgery seems to be a reasonable treatment alternative for these tumors, but longer follow-up and larger series are required before definitive statements can be made.
Hemangiopericytomas
Hemangiopericytomas are tumors of mesenchymal origin. They are recognized for their aggressive clinical behavior, high recurrence rates, and tendency for distant metastases even after a gross total resection. Initial treatment is usually resection. Upon recurrence, adjuvant treatment is frequently used. Chemotherapy has provided only marginal benefit.145 Radiosurgery or fractionated radiotherapy has been used as alternatives.
Between 1989 and 2008, we treated 28 recurrent or residual hemangiopericytomas in 21 patients with gamma surgery. The median age was 47 years (range 31–61 years). Eight patients had prior fractionated radiotherapy. The mean prescription and maximum radiosurgical doses to the tumors were 17 and 40 Gy, respectively. Thirteen tumors had undergone repeat gamma surgery. The median follow-up period was 68 months (range 2–138 months). At the last follow-up, local tumor control was demonstrated in 10 of 21 patients (47.6%). Of the 28 tumors treated, 8 decreased in size on follow-up imaging (28.6%), 5 remained unchanged (17.9%), and 15 ultimately progressed (53.6%). The progression free survival rates were 90%, 60%, and 29% at 1, 3, and 5 years after initial gamma surgery. The progression free survival rate improved to 95%, 72%, and 72% at 1, 3, and 5 years after one or multiple gamma surgery. The 5-year survival rate after radiosurgery was 81%. In 4 (19%) of 21 patients, extracranial metastases developed.
Metastatic Tumors
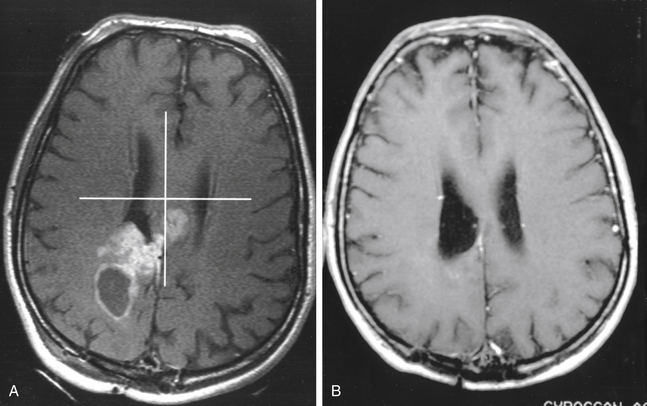
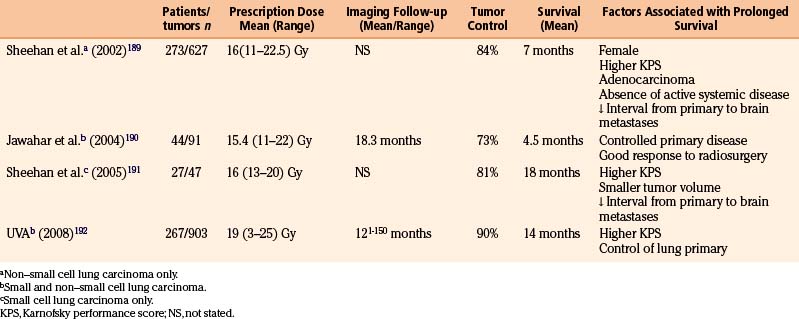
Most reports regarding gamma surgery for metastatic tumors report a 7- to 15-month survival following treatment. Local tumor control rates range from 71% to 98.5%. The histology, dosages, and previous treatments vary considerably through the literature. Most centers are using a peripheral dose of 18 to 22 Gy. These doses are adjusted down if whole brain irradiation has been given previously. The reduction of dose in the instance of tumors that appear after whole brain irradiation is possibly not necessary or desirable. In a study comparing the efficacy of surgery plus whole brain radiotherapy with radiosurgery alone in the treatment of solitary brain metastases less than or equal to 3.5 cm in diameter, local tumor control and 1-year death rates did not statistically differ between the two groups.146 In a large, multi-institutional study, the omission of upfront fractionated radiation therapy did not compromise the overall length of survival in brain metastasis patients who had undergone radiosurgery.147 Radiosurgery even appears to be efficacious for treating traditionally relatively “radioresistant” brain metastases such as melanoma and renal cell carcinoma.
Lung Carcinoma
Lung carcinoma is the leading cause of death from cancer and the most common source of brain metastases (Table 5-8). Depending on the actual histologic subtypes, lung carcinoma metastasizes intracranially between 13% and 54% of the time. The overall frequency of brain metastasis in patients with lung carcinoma is approximately 32% and between 54% and 64% of patients with lung carcinoma metastatic to the brain harbor or eventually develop multiple lesions.148,149 Treatment options include symptomatic medical management with corticosteroids and whole-brain radiation therapy, which lead to a median survival of 3 to 6 months. Patient with small cell lung cancer developed metastases quite early and adjuvant chemotherapy has also become well-accepted for the treatment of extracranial disease in small cell lung cancer. We have treated 903 metastases in 262 patients with lung carcinoma. The median survival was 15.4 months. Age of less than 65 years, a Karnofsky performance score of greater than 70, and controlled extracranial disease were associated with increased survival.
Breast Carcinoma
While improved neuroimaging enable early diagnosis of brain metastases, better treatment of systemic disease increased the frequency of the diagnosis of intracranial metastases as well. Breast cancer is a paradigm in this regard (Table 5-9). The advent of an effective targeted therapy, such monoclonal antibody against HER-2, allows patients to survive much longer than before, thus increased likelihood of central nervous system relapse.
Breast cancer is the second most common cause of cerebral metastases. Incidences of metastasis to the central nervous system ranging from 10% to 20% have been reported in clinical series and 30% in autopsy series.150,151 Breast cancer is considered to be a relatively radiosensitive tumor and radiosurgery in the treatment of breast carcinoma that has metastasized to the brain has shown its effectiveness in prolonging overall survival.
We have treated 43 patients with a total of 84 metastatic lesions from breast carcinoma. Overall median survival was 13 months after gamma surgery.152 Analysis revealed that a high Karnofsky performance score and a single lesion correlated with increased survival. Overall median time to local tumor control failure was 10 months. The tumor control rate from the literature ranged from 81% to 94% and the median survival ranged from 13 to 19 months.105,152,153
Melanoma
The incidence of melanoma has progressively risen over the decades (Table 5-10). Melanoma is now the third most common primary tumor associated with central nervous system metastasis. Brain metastases were found in up to 75% of melanoma cases at autopsy and involvement of the central nervous system is the cause of the death in approximately one third of all patients.106 Radiosurgery seems to overcome the problem of radioresistance observed in melanoma. With a single high radiosurgical dose, the tumor control rate following radiosurgery for cerebral metastatic melanoma seems favorable ranging from 61 to 90%. However, the prolongation of survival is not promising with most series reporting median survival in the range of 5.7 to 7.1 months.154–157
Renal Cell Carcinoma
Renal cell carcinomas are responsible for approximately 2% of cancer deaths in the United States annually, and they have a 10% incidence of developing into brain metastases158 (Table 5-11). The resistance to fractionated radiation therapy and a tendency for limited numbers of metastases make radiosurgery a rational alternative in the management of renal cell carcinoma metastases to the brain. The reported tumor control rate following radiosurgery for metastatic renal cell carcinoma ranged from 83% to 96%.151,159–161
A series of 40 patients with 65 renal cell carcinoma brain metastases was treated by us. The average survival was 9.2 months after radiosurgery. A total of 41 tumors decreased in volume, 6 tumors disappeared, and 16 tumors remained unchanged in size (Fig. 5-22). Only 2 tumors increased in size following treatment. An unmatched control group of 119 patients that received external brain radiation therapy had an average survival of 4.4 months.162 Factors associated with longer survival in the group treated with gamma surgery included a higher Karnofsky performance status, absence of extracranial metastases, adjutant whole brain radiation therapy and prior surgical resection. The size and number of metastases did not have a significant effect on survival, although in cases of single metastasis with controlled local disease long-term survival could be achieved.
Uveal Melanomas
The most common surgical treatment for uveal melanomas is enucleation, but several centers have relatively large series in the treatment of these tumors with gamma surgery.163–166 Other therapeutic options include radium plaque therapy, and proton beam therapy. The first uveal melanoma treated with gamma surgery was in Buenos Aires167 and gamma surgery has become a more frequently used procedure for this unusual pathology (Fig. 5-23). The use of gamma surgery and its stereotactic technique requires that the eyeball be fixated relative to the stereotactic frame. This is accomplished with retrobulbar blocks and external fixative sutures that are attached to the frame.
The Sheffield group reported a series of 29 patients treated and followed for an average of 14 months. The average peripheral dose was 73 Gy, corresponding to the 50% isodose line.163 The dose was delivered in two sessions not more than 8 days apart. All but two patients had good local control. The two failures required later enucleation. Three patients died of metastatic disease. More recent work suggests that a lower margin dose of 41.5 Gy may be just as effective in terms of tumor control but be associated with a lower risk of neovascular glaucoma.168 In a series of 75 patients with uveal melanoma followed for a minimum of 10 months, Simonova et al. reported 84% local tumor control and secondary glaucoma in 25%.169
Amichetti M., Cianchetti M., Amelio D., et al. Proton therapy in chordoma of the base of the skull: a systematic review. Neurosurg Rev. 2009;32:403-416.
Andrade-Souza Y.M., Ramani M., Scora D., et al. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery. 2007;60:443-451. discussion 451-442
Jagannathan J., Sheehan J.P., Pouratian N., et al. gamma knife surgery for Cushing’s disease. J Neurosurg. 2007;106:980-987.
Johnson R. Radiotherapy of cerebral angiomas with a note on some problems in diagnosis Berlin. Springer-Verlag; 1975.
Karlsson B., Kihlstrom L., Lindquist C., et al. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88:293-297.
Kobayashi T., Kida Y., Mori Y., Hasegawa T. Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg. 2005;103:482-488.
Kondziolka D., Lunsford L.D., Kestle J.R. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
Lindquist C., Guo W.Y., Karlsson B., Steiner L. Radiosurgery for venous angiomas. J Neurosurg. 1993;78:531-536.
Mingione V., Yen C.P., Vance M.L., et al. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg. 2006;104:876-883.
Pan D.H., Guo W.Y., Chung W.Y., et al. Gamma knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg. 2000;93(Suppl 3):113-119.
Pan L., Zhang N., Wang E.M., et al. Gamma knife radiosurgery as a primary treatment for prolactinomas. J Neurosurg. 2000;93(Suppl 3):10-13.
Prasad D., Steiner M., Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:745-759.
Schneider B.F., Eberhard D.A., Steiner L.E. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997;87:352-357.
Sheehan J., Yen C.P., Steiner L. Gamma knife surgery-induced meningioma. Report of two cases and review of the literature. J Neurosurg. 2006;105:325-329.
Sirin S., Kondziolka D., Niranjan A., et al. Prospective staged volume radiosurgery for large arteriovenous malformations: indications and outcomes in otherwise untreatable patients. Neurosurgery. 2006;58:17-27. discussion 17-27
Snell J.W., Sheehan J., Stroila M., Steiner L. Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. Technical note. J Neurosurg. 2006;104:157-162.
Stafford S.L., Pollock B.E., Leavitt J.A., et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177-1181.
Steiner L., Forster D., Leksell L., et al. Gammathalamotomy in intractable pain. Acta Neurochir (Wien). 1980;52:173-184.
Steiner L., Leksell L., Greitz T., et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Report of a case. Acta Chir Scand. 1972;138:459-464.
Steiner L., Yen C.P., Jagannathan J., et al. Gamma Knife: clinical aspect. In: Lozano A.M., Gildenberg P.L., Tasker R.R. Textbook of Stereotactic and Functional Neurosurgery. New York. Springer; 2009:1037-1085.
Tishler R.B., Loeffler J.S., Lunsford L.D., et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
Tsuzuki T., Tsunoda S., Sakaki T., et al. Tumor cell proliferation and apoptosis associated with the gamma knife effect. Stereotact Funct Neurosurg. 1996;66(Suppl 1):39-48.
Yen C.P., Sheehan J., Steiner M., et al. Gamma knife surgery for focal brainstem gliomas. J Neurosurg. 2007;106:8-17.
Yen C.P., Varady P., Sheehan J., et al. Subtotal obliteration of cerebral arteriovenous malformations after gamma knife surgery. J Neurosurg. 2007;106:361-369.
1. Chang S.D., Adler J.R. Robotics and radiosurgery—the cyberknife. Stereotact Funct Neurosurg. 2001;76:204-208.
2. Gerszten P.C., Ozhasoglu C., Burton S.A., et al. Evaluation of CyberKnife frameless real-time image-guided stereotactic radiosurgery for spinal lesions. Stereotact Funct Neurosurg. 2003;81:84-89.
3. Hamilton A.J., Lulu B.A., Fosmire H., Gossett L. LINAC-based spinal stereotactic radiosurgery. Stereotact Funct Neurosurg. 1996;66:1-9.
4. Lax I., Blomgren H., Naslund I., Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994;33:677-683.
5. Dandy W.E. Cerebral Ventriculoscopy. Bull John Hopkins Hosp. 33, 1922.
6. Schulder M., Loeffler J.S., et al. Historical vignette: the radium bomb: Harvey Cushing and the interstitial irradiation of gliomas. J Neurosurg. 1996;84:530-532.
7. Clarke R., Horsley V. One method of investigating the deep ganglia and tracts of the central nervous system (cerebellum). BMJ. 1906;2:1799-1800.
8. Spiegel E.A., Wycis H.T., Marks M., Lee A.J. Stereotaxic apparatus for operations on the human brain. Science. 1947;106:349-350.
9. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
10. Larsson B., Leksell L., Rexed B. The use of high-energy protons for cerebral surgery in man. Acta Chir Scand. 1963;125:1-7.
11. Leksell L., Larsson B., Andersson B., et al. Lesions in the depth of the brain produced by a beam of high energy protons. Acta radiol. 1960;54:251-264.
12. Hall E.J., Marchese M., Hei T.K., Zaider M. Radiation response characteristics of human cells in vitro. Radiat Res. 1988;114:415-424.
13. Kamiryo T., Kassell N.F., Thai Q.A., et al. Histological changes in the normal rat brain after gamma irradiation. Acta Neurochir (Wien). 1996;138:451-459.
14. Flickinger J.C., Lunsford L.D., Kondziolka D. Dose-volume considerations in radiosurgery. Stereotact Funct Neurosurg. 1991;57:99-105.
15. Steiner L., Forster D., Leksell L., Meyerson B.A., Boethius J. Gammathalamotomy in intractable pain. Acta Neurochir (Wien). 1980;52:173-184.
16. Hawighorst H., Engenhart R., Knopp M.V., et al. Intracranial meningeomas: time- and dose-dependent effects of irradiation on tumor microcirculation monitored by dynamic MR imaging. Magn Reson Imaging. 1997;15:423-432.
17. Marekova M., Cap J., Vokurkova D., Vavrova J., Cerman J. Effect of therapeutic doses of ionising radiation on the somatomammotroph pituitary cell line, GH3. Endocr J. 2003;50:621-628.
18. Tsuzuki T., Tsunoda S., Sakaki T., et al. Tumor cell proliferation and apoptosis associated with the gamma knife effect. Stereotact Funct Neurosurg. 1996;66(Suppl 1):39-48.
19. Hopewell J.W., Wright E.A. The nature of latent cerebral irradiation damage and its modification by hypertension. Br J Radiol. 1970;43:161-167.
20. Verhey L.J., Smith V., Serago C.F. Comparison of radiosurgery treatment modalities based on physical dose distributions. Int J Radiat Oncol Biol Phys. 1998;40:497-505.
21. Tishler R.B., Loeffler J.S., Lunsford L.D., et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
22. Chen J.C., Giannotta S.L., Yu C., et al. Radiosurgical management of benign cavernous sinus tumors: dose profiles and acute complications. Neurosurgery. 2001;48:1022-1030. discussion 1030-1022
23. Ove R., Kelman S., Amin P.P., Chin L.S. Preservation of visual fields after peri-sellar gamma-knife radiosurgery. Int J Cancer. 2000;90:343-350.
24. Stafford S.L., Pollock B.E., Leavitt J.A., et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177-1181.
25. Girkin C.A., Comey C.H., Lunsford L.D., Goodman M.L., Kline L.B. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104:1634-1643.
26. Leber K.A., Bergloff J., Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43-50.
27. Lundstrom M., Frisen L. Atrophy of optic nerve fibres in compression of the chiasm: degree and distribution of ophthalmoscopic changes. Acta Ophthalmol (Copenh). 1976;54:623-640.
28. Steiner L, Greitz T, Backlund E: Radiosurgery in arterio-venous malformations of the brain undue effects. Presented at INSERM Symposium on Stereotactic Irradiations, Paris, 1979.
29. Steiner L., Yen C.P., Jagannathan J., et al. Gamma knife: clinical aspect. In: Lozano A.M., Gildenberg P.L., Tasker R.R. Textbook of Stereotactic and Functional Neurosurgery. New York: Springer; 2009:1037-1085.
30. Lim Y.L., Leem W., Kim T.S., et al. Four years’ experiences in the treatment of pituitary adenomas with gamma knife radiosurgery. Stereotact Funct Neurosurg. 1998;70(Suppl 1):95-109.
31. Muramatsu J., Yoshida M., Shioura H., et al. [Clinical results of LINAC-based stereotactic radiosurgery for pituitary adenoma]. Nippon Igaku Hoshasen Gakkai Zasshi. 2003;63:225-230.
32. Pollock B.E., Nippoldt T.B., Stafford S.L., et al. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas: factors associated with endocrine normalization. J Neurosurg. 2002;97:525-530.
33. Shin M., Kurita H., Sasaki T., et al. Stereotactic radiosurgery for pituitary adenoma invading the cavernous sinus. J Neurosurg. 2000;93(Suppl 3):2-5.
34. Kamiryo T., Lopes M.B., Berr S.S., et al. Occlusion of the anterior cerebral artery after gamma knife irradiation in a rat. Acta Neurochir (Wien). 1996;138:983-990. discussion 990-981
35. Nilsson A., Wennerstrand J., Leksell D., Backlund E.O. Stereotactic gamma irradiation of basilar artery in cat: preliminary experiences. Acta Radiol Oncol Radiat Phys Biol. 1978;17:150-160.
36. Kihlström L., Lindquist C., Adler J.R. Histological studies of gamma knife lesions in normal and hypercholesterolemic rabbits. In: Steiner L., editor. Baseline and Trends. New York: Raven Press, 1992. Radiosurgery
37. Cushing H., Bailey P. Tumors Arising from the Blood Vessels of the Brain. Springfield, IL: Charles C Thomas; 1928.
38. French L.A., Chou S.N. Conventional methods of treating intracranial arteriovenous malformations. Prog Neurol Surg. 1969;3:74-319.
39. Olivecrona H., Riives J. Arteriovenous aneurysms of the brain: their diagnosis and treatment. Arch Neurol Psychiatry. 1948;59:567-602.
40. Ray B.S. Cerebral arteriovenous aneurisms. Surg Gynecol Obstet 615-648. 1941.
41. Johnson R. Radiotherapy of cerebral angiomas with a note on some problems in diagnosis Berlin. Springer-Verlag; 1975.
42. Steiner L., Leksell L., Greitz T., et al. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Report of a case. Acta Chir Scand. 1972;138:459-464.
43. Schneider B.F., Eberhard D.A., Steiner L.E. Histopathology of arteriovenous malformations after gamma knife radiosurgery. J Neurosurg. 1997;87:352-357.
44. Szeifert G.T., Kemeny A.A., Timperley W.R., Forster D.M. The potential role of myofibroblasts in the obliteration of arteriovenous malformations after radiosurgery. Neurosurgery. 1997;40:61-65. discussion 65-66
45. Yamamoto M., Jimbo M., Kobayashi M., et al. Long-term results of radiosurgery for arteriovenous malformation: neurodiagnostic imaging and histologic studies of angiographically confirmed nidus obliteration. Surg Neurol. 1992;37:219-230.
46. Graf C.J., Perret G.E., Torner J.C. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
47. Itoyama Y., Uemura S., Ushio Y., et al. Natural course of unoperated intracranial arteriovenous malformations: study of 50 cases. J Neurosurg. 1989;71:805-809.
48. Jomin M., Lesoin F., Lozes G. Prognosis for arteriovenous malformations of the brain in adults based on 150 cases. Surg Neurol. 1985;23:362-366.
49. Spetzler R.F., Hargraves R.W., McCormick P.W., et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:918-923.
50. Brown R.D.Jr., Wiebers D.O., Forbes G., et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352-357.
51. Crawford P.M., West C.R., Chadwick D.W., Shaw M.D. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1-10.
52. Fults D., Kelly D.L.Jr. Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery. 1984;15:658-662.
53. Forster D.M., Steiner L., Hakanson S. Arteriovenous malformations of the brain. A long-term clinical study. J Neurosurg. 1972;37:562-570.
54. Ondra S.L., Troupp H., George E.D., Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
55. Sadasivan B., Malik G.M., Lee C., Ausman J.I. Vascular malformations and pregnancy. Surg Neurol. 1990;33:305-313.
56. Forster D.M., Kunkler I.H., Hartland P. Risk of cerebral bleeding from arteriovenous malformations in pregnancy: the Sheffield experience. Stereotact Funct Neurosurg. 1993;61(Suppl 1):20-22.
57. Harbaugh K.S., Harbaugh R.E. Arteriovenous malformations in elderly patients. Neurosurgery. 1994;35:579-584.
58. Robinson J.L., Hall C.S., Sedzimir C.B. Arteriovenous malformations, aneurysms, and pregnancy. J Neurosurg. 1974;41:63-70.
59. Lindquist C., Steiner L. Stereotactic radiosurgical treatment of arteriovenous malformations. In: Lunsford L.D., editor. Modern Stereotactic Neurosurgery. Boston: Martinus Nijhoff; 1988:491-505.
60. Yen C.P., Varady P., Sheehan J., Steiner M., Steiner L. Subtotal obliteration of cerebral arteriovenous malformations after gamma knife surgery. J Neurosurg. 2007;106:361-369.
61. Steiner L., Lindquist C., Adler J.R., et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1-8.
62. Karlsson B., Lindquist C., Steiner L. Prediction of obliteration after gamma knife surgery for cerebral arteriovenous malformations. Neurosurgery. 1997;40:425-430. discussion 430-421
63. Liscak R., Vladyka V., Simonova G., et al. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery. 2007;60:1005-1014. discussion 1015-1006
64. Pollock B.E., Garces Y.I., Stafford S.L., et al. Stereotactic radiosurgery for cavernous malformations. J Neurosurg. 2000;93:987-991.
65. Schlienger M., Atlan D., Lefkopoulos D., et al. Linac radiosurgery for cerebral arteriovenous malformations: results in 169 patients. Int J Radiat Oncol Biol Phys. 2000;46:1135-1142.
66. Pan D.H., Kuo Y.H., Guo W.Y., et al. Gamma knife surgery for cerebral arteriovenous malformations in children: a 13-year experience. J Neurosurg Pediatr. 2008;1:296-304.
67. Shin M., Maruyama K., Kurita H., et al. Analysis of nidus obliteration rates after gamma knife surgery for arteriovenous malformations based on long-term follow-up data: the University of Tokyo experience. J Neurosurg. 2004;101:18-24.
68. Andrade-Souza Y.M., Ramani M., Scora D., et al. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery. 2007;60:443-451. discussion 451-442
69. Gobin Y.P., Laurent A., Merienne L., et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 1996;85:19-28.
70. Andrade-Souza Y.M., Ramani M., Beachey D.J., et al. Liquid embolisation material reduces the delivered radiation dose: a physical experiment. Acta Neurochir (Wien). 2008;150:161-164. discussion 164
71. Sirin S., Kondziolka D., Niranjan A., et al. Prospective staged volume radiosurgery for large arteriovenous malformations: indications and outcomes in otherwise untreatable patients. Neurosurgery. 2006;58:17-27. discussion 17-27
72. Pan D.H., Guo W.Y., Chung W.Y., et al. Gamma knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg. 2000;93(Suppl 3):113-119.
73. Kjellberg R.N., Davis K.R., Lyons S., et al. Bragg peak proton beam therapy for arteriovenous malformation of the brain. Clin Neurosurg. 1983;31:248-290.
74. Maruyama K., Kondziolka D., Niranjan A., et al. Stereotactic radiosurgery for brainstem arteriovenous malformations: factors affecting outcome. J Neurosurg. 2004;100:407-413.
75. Maruyama K., Shin M., Tago M., et al. Radiosurgery to reduce the risk of first hemorrhage from brain arteriovenous malformations. Neurosurgery. 2007;60:453-458. discussion 458-459
76. Karlsson B., Lindquist C., Steiner L. Effect of gamma knife surgery on the risk of rupture prior to AVM obliteration. Minim Invasive Neurosurg. 1996;39:21-27.
77. Matsumoto H., Takeda T., Kohno K., et al. Delayed hemorrhage from completely obliterated arteriovenous malformation after gamma knife radiosurgery. Neurol Med Chir (Tokyo). 2006;46:186-190.
78. Lindqvist M., Karlsson B., Guo W.Y., et al. Angiographic long-term follow-up data for arteriovenous malformations previously proven to be obliterated after gamma knife radiosurgery. Neurosurgery. 2000;46:803-808. discussion 809-810
79. Guo W.Y., Karlsson B., Ericson K., Lindqvist M. Even the smallest remnant of an AVM constitutes a risk of further bleeding: case report. Acta Neurochir (Wien). 1993;121:212-215.
80. Pan H.C., Sheehan J., Stroila M., et al. Late cyst formation following gamma knife surgery of arteriovenous malformations. J Neurosurg. 2005;102(Suppl):124-127.
81. Sheehan J., Yen C.P., Steiner L. Gamma knife surgery-induced meningioma. Report of two cases and review of the literature. J Neurosurg. 2006;105:325-329.
82. Kojima T., Miyachi S., Sahara Y., et al. The relationship between venous hypertension and expression of vascular endothelial growth factor: hemodynamic and immunohistochemical examinations in a rat venous hypertension model. Surg Neurol. 2007;68:277-284. discussion 284
83. Terada T., Higashida R.T., Halbach V.V., et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884-889.
84. Kilic K., Konya D., Kurtkaya O., et al. Inhibition of angiogenesis induced by cerebral arteriovenous malformations using gamma knife irradiation. J Neurosurg. 2007;106:463-469.
85. Borden J.A., Wu J.K., Shucart W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166-179.
86. Payne B.R., Prasad D., Steiner M., et al. Gamma surgery for vein of Galen malformations. J Neurosurg. 2000;93:229-236.
87. Del Curling O.Jr., Kelly D.L.Jr., Elster A.D., Craven T.E. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702-708.
88. Hasegawa T., McInerney J., Kondziolka D., et al. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery. 2002;50:1190-1197. discussion 1197-1198
89. Kondziolka D., Lunsford L.D., Kestle J.R. The natural history of cerebral cavernous malformations. J Neurosurg. 1995;83:820-824.
90. Robinson J.R., Awad I.A., Little J.R. Natural history of the cavernous angioma. J Neurosurg. 1991;75:709-714.
91. Karlsson B., Kihlstrom L., Lindquist C., et al. Radiosurgery for cavernous malformations. J Neurosurg. 1998;88:293-297.
92. Kondziolka D., Lunsford L.D., Flickinger J.C., Kestle J.R. Reduction of hemorrhage risk after stereotactic radiosurgery for cavernous malformations. J Neurosurg. 1995;83:825-831.
93. Barker F.G.2nd, Amin-Hanjani S., Butler W.E., et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery. 2001;49:15-24. discussion 24-15
94. Amin-Hanjani S., Ogilvy C.S., Candia G.J., et al. Stereotactic radiosurgery for cavernous malformations: Kjellberg’s experience with proton beam therapy in 98 cases at the Harvard Cyclotron. Neurosurgery. 1998;42:1229-1236. discussion 1236-1228
95. Liu K.D., Chung W.Y., Wu H.M., et al. Gamma knife surgery for cavernous hemangiomas: an analysis of 125 patients. J Neurosurg. 2005;102(Suppl):81-86.
96. Tsien C., Souhami L., Sadikot A., et al. Stereotactic radiosurgery in the management of angiographically occult vascular malformations. Int J Radiat Oncol Biol Phys. 2001;50:133-138.
97. Garner T.B., Del Curling O.Jr., Kelly D.L.Jr., Laster D.W. The natural history of intracranial venous angiomas. J Neurosurg. 1991;75:715-722.
98. Lindquist C., Guo W.Y., Karlsson B., Steiner L. Radiosurgery for venous angiomas. J Neurosurg. 1993;78:531-536.
99. Snell J.W., Sheehan J., Stroila M., Steiner L. Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. Technical note. J Neurosurg. 2006;104:157-162.
100. Kjellberg R.N., Shintani A., Frantz A.G., Kliman B. Proton-beam therapy in acromegaly. N Engl J Med. 1968;278:689-695.
101. Levy R.P., Fabrikant J.I., Frankel K.A., et al. Charged-particle radiosurgery of the brain. Neurosurg Clin North Am. 1990;1:955-990.
102. Sheehan J.M., Vance M.L., Sheehan J.P., et al. Radiosurgery for Cushing’s disease after failed trans-sphenoidal surgery. J Neurosurg. 2000;93:738-742.
103. Landolt A.M., Haller D., Lomax N., et al. Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab. 2000;85:1287-1289.
104. Landolt A.M., Lomax N. Gamma knife radiosurgery for prolactinomas. J Neurosurg. 2000;93(Suppl 3):14-18.
105. Akyurek S., Chang E.L., Mahajan A., et al. Stereotactic radiosurgical treatment of cerebral metastases arising from breast cancer. Am J Clin Oncol. 2007;30:310-314.
106. Amendola B.E., Wolf A.L., Coy S.R., et al. Gamma knife radiosurgery in the treatment of patients with single and multiple brain metastases from carcinoma of the breast. Cancer J. 2000;6:88-92.
107. Amer M.H., Al-Sarraf M., Baker L.H., Vaitkevicius V.K. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42:660-668.
108. Mingione V., Yen C.P., Vance M.L., et al. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg. 2006;104:876-883.
109. Pouratian N., Sheehan J., Jagannathan J., et al. Gamma knife radiosurgery for medically and surgically refractory prolactinomas. Neurosurgery. 2006;59:255-266. discussion 255-266
110. Jagannathan J., Sheehan J.P., Pouratian N., et al. Gamma knife surgery for Cushing’s disease. J Neurosurg. 2007;106:980-987.
111. Rahn T., Thoren M., Hall K., Backlund E- O. Stereotactic radiosurgery in the treatment of MB Cushing. Amsterdam: Presented at INSERM Symposium; 1979.
112. Hetelekidis S., Barnes P.D., Tao M.L., et al. 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 1993;27:189-195.
113. Rajan B., Ashley S., Gorman C., et al. Craniopharyngioma—a long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26:1-10.
114. Chadderton R.D., West C.G., Schuller S., et al. Radiotherapy in the treatment of low-grade astrocytomas. II. The physical and cognitive sequelae. Childs Nerv Syst. 1995;11:443-448.
115. Mulhern R.K., Kepner J.L., Thomas P.R., et al. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1723-1728.
116. Kobayashi T., Kida Y., Mori Y., Hasegawa T. Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg. 2005;103:482-488.
117. Niranjan A., Kano H., Mathieu D., et al. Radiosurgery for craniopharyngioma. Int J Radiat Oncol Biol Phys. 2010;78:164-171.
118. Barbaro N.M., Gutin P.H., Wilson C.B., et al. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20:525-528.
119. Goldsmith B.J., Wara W.M., Wilson C.B., Larson D.A. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80:195-201.
120. Taylor B.W.Jr., Marcus R.B.Jr., Friedman W.A., Ballinger W.E.Jr., Million R.R. The meningioma controversy: postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299-304.
121. Parkinson D. Lateral sellar compartment O.T. (cavernous sinus): history, anatomy, terminology. Anat Rec. 1998;251:486-490.
122. Parkinson D. Extradural neural axis compartment. J Neurosurg. 2000;92:585-588.
123. Henschen F. Über Geschwülste der hinteren Schädelgrube insbesondere des Kleinhirnbrückenwinkels: Klinische und anatomische Studien. Jena, Germany: Verlag von Gustav Fischer; 1910.
124. Leksell L. A note on the treatment of acoustic tumours. Acta Chir Scand. 1971;137:763-765.
125. Wallner K.E., Sheline G.E., Pitts L.H., et al. Efficacy of irradiation for incompletely excised acoustic neurilemomas. J Neurosurg. 1987;67:858-863.
126. Prasad D., Steiner M., Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:745-759.
127. Bertalanffy A., Dietrich W., Aichholzer M., et al. Gamma knife radiosurgery of acoustic neurinomas. Acta Neurochir (Wien). 2001;143:689-695.
128. Flickinger J.C., Kondziolka D., Niranjan A., Lunsford L.D. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001;94:1-6.
129. Iwai Y., Yamanaka K., Shiotani M., Uyama T. Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery. 2003;53:282-287. discussion 287-288
130. Noren G., Greitz D., Hirsch A., Lax I. Gamma knife surgery in acoustic tumours. Acta Neurochir Suppl (Wien). 1993;58:104-107.
131. Flickinger J.C., Lunsford L.D., Linskey M.E., et al. Gamma knife radiosurgery for acoustic tumors: multivariate analysis of four year results. Radiother Oncol. 1993;27:91-98.
132. Yen C.P., Sheehan J., Steiner M., et al. Gamma knife surgery for focal brainstem gliomas. J Neurosurg. 2007;106:8-17.
133. Gutin P.H., Wilson C.B. Radiosurgery for malignant brain tumors. J Clin Oncol. 1990;8:571-573.
134. Larson D.A., Prados M., Lamborn K.R., et al. Phase II study of high central dose gamma knife radiosurgery and marimastat in patients with recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 2002;54:1397-1404.
135. Loeffler J.S., Alexander E.3rd, Shea W.M., et al. Radiosurgery as part of the initial management of patients with malignant gliomas. J Clin Oncol. 1992;10:1379-1385.
136. Nwokedi E.C., DiBiase S.J., Jabbour S., et al. Gamma knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery. 2002;50:41-46. discussion 46-47
137. Hassoun J., Gambarelli D., Grisoli F., et al. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982;56:151-156.
138. Brandes A.A., Amista P., Gardiman M., et al. Chemotherapy in patients with recurrent and progressive central neurocytoma. Cancer. 2000;88:169-174.
139. Tomura N., Hirano H., Watanabe O., et al. Central neurocytoma with clinically malignant behavior. AJNR Am J Neuroradiol. 1997;18:1175-1178.
140. Rades D., Fehlauer F. Treatment options for central neurocytoma. Neurology. 2002;59:1268-1270.
141. Yen C.P., Sheehan J., Patterson G., Steiner L. Gamma knife surgery for neurocytoma. J Neurosurg. 2007;107:7-12.
142. Amichetti M., Cianchetti M., Amelio D., et al. Proton therapy in chordoma of the base of the skull: a systematic review. Neurosurg Rev. 2009;32:403-416.
143. Austin-Seymour M., Munzenrider J., Goitein M., et al. Fractionated proton radiation therapy of chordoma and low-grade chondrosarcoma of the base of the skull. J Neurosurg. 1989;70:13-17.
144. Muthukumar N., Kondziolka D., Lunsford L.D., Flickinger J.C. Stereotactic radiosurgery for chordoma and chondrosarcoma: further experiences. Int J Radiat Oncol Biol Phys. 1998;41:387-392.
145. Galanis E., Buckner J.C., Scheithauer B.W., et al. Management of recurrent meningeal hemangiopericytoma. Cancer. 1998;82:1915-1920.
146. Muacevic A., Kreth F.W., Horstmann G.A., et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91:35-43.
147. Sneed P.K., Suh J.H., Goetsch S.J., et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53:519-526.
148. Boring C.C., Squires T.S., Tong T. Cancer statistics. CA Cancer J Clin. 1991;41:19-36. 1991
149. Delattre J.Y., Krol G., Thaler H.T., Posner J.B. Distribution of brain metastases. Arch Neurol. 1988;45:741-744.
150. DiStefano A., Yong Yap Y., Hortobagyi G.N. Blumenschein GR: The natural history of breast cancer patients with brain metastases. Cancer. 1979;44:1913-1918.
151. Muacevic A., Siebels M., Tonn J.C., Wowra B. Treatment of brain metastases in renal cell carcinoma: radiotherapy, radiosurgery, or surgery? World J Urol. 2005;23:180-184.
152. Goyal S., Prasad D., Harrell F.Jr., et al. Gamma knife surgery for the treatment of intracranial metastases from breast cancer. J Neurosurg. 2005;103:218-223.
153. Firlik K.S., Kondziolka D., Flickinger J.C., Lunsford L.D. Stereotactic radiosurgery for brain metastases from breast cancer. Ann Surg Oncol. 2000;7:333-338.
154. Mathieu D., Kondziolka D., Cooper P.B., et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Clin Neurosurg. 2007;54:241-247.
155. Radbill A.E., Fiveash J.F., Falkenberg E.T., et al. Initial treatment of melanoma brain metastases using gamma knife radiosurgery: an evaluation of efficacy and toxicity. Cancer. 2004;101:825-833.
156. Selek U., Chang E.L., Hassenbusch S.J.3rd, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys. 2004;59:1097-1106.
157. Yu C., Chen J.C., Apuzzo M.L., et al. Metastatic melanoma to the brain: prognostic factors after gamma knife radiosurgery. Int J Radiat Oncol Biol Phys. 2002;52:1277-1287.
158. Halperin E.C., Harisiadis L. The role of radiation therapy in the management of metastatic renal cell carcinoma. Cancer. 1983;51:614-617.
159. Payne B.R., Prasad D., Szeifert G., et al. Gamma surgery for intracranial metastases from renal cell carcinoma. J Neurosurg. 2000;92:760-765.
160. Sheehan J.P., Sun M.H., Kondziolka D., et al. Radiosurgery in patients with renal cell carcinoma metastasis to the brain: long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg. 2003;98:342-349.
161. Shuto T., Inomori S., Fujino H., Nagano H. Gamma knife surgery for metastatic brain tumors from renal cell carcinoma. J Neurosurg. 2006;105:555-560.
162. Wronski M., Maor M.H., Davis B.J., et al. External radiation of brain metastases from renal carcinoma: a retrospective study of 119 patients from the M.D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 1997;37:753-759.
163. Langmann G., Pendl G., Klaus M., Papaefthymiou G., Guss H. Gamma knife radiosurgery for uveal melanomas: an 8-year experience. J Neurosurg. 2000;93(Suppl 3):184-188.
164. Marchini G., Gerosa M., Piovan E., et al. Gamma knife stereotactic radiosurgery for uveal melanoma: clinical results after 2 years. Stereotact Funct Neurosurg. 1996;66(Suppl 1):208-213.
165. Mueller A.J., Talies S., Schaller U.C., et al. Stereotactic radiosurgery of large uveal melanomas with the gamma-knife. Ophthalmology. 2000;107:1381-1387. discussion 1387-1388
166. Rennie I., Forster D., Kemeny A., Walton L., Kunkler I. The use of single fraction Leksell stereotactic radiosurgery in the treatment of uveal melanoma. Acta Ophthalmol Scand. 1996;74:558-562.
167. Chinela A., Zambrano A., Bunge H. Gamma knife surgery in uveal melanomas. In: Steiner L., editor. Radiosurgery Baselines and Trends. New York: Raven Press; 1992:161-169.
168. Langmann G., Pendl G., Mullner K., et al. High- compared with low-dose radiosurgery for uveal melanomas. J Neurosurg. 2002;97:640-643.
169. Simonova G., Novotny J.Jr., Liscak R., Pilbauer J. Leksell gamma knife treatment of uveal melanoma. J Neurosurg. 2002;97:635-639.
170. Pollock B.E., Cochran J., Natt N., et al. Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int J Radiat Oncol Biol Phys. 2008;70:1325-1329.
171. Iwai Y., Yamanaka K., Yoshioka K. Radiosurgery for nonfunctioning pituitary adenomas. Neurosurgery. 2005;56:699-705. discussion 699-705
172. Losa M., Valle M., Mortini P., et al. Gamma knife surgery for treatment of residual nonfunctioning pituitary adenomas after surgical debulking. J Neurosurg. 2004;100:438-444.
173. Sheehan J.P., Kondziolka D., Flickinger J., Lunsford L.D. Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J Neurosurg. 2002;97:408-414.
174. Losa M., Gioia L., Picozzi P., et al. The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J Clin Endocrinol Metab. 2008;93:2546-2552.
175. Vik-Mo E.O., Oksnes M., Pedersen P.H., et al. Gamma knife stereotactic radiosurgery for acromegaly. Eur J Endocrinol. 2007;157:255-263.
176. Pollock B.E., Jacob J.T., Brown P.D., Nippoldt T.B. Radiosurgery of growth hormone-producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg. 2007;106:833-838.
177. Jezkova J., Marek J., Hana V., et al. Gamma knife radiosurgery for acromegaly—long-term experience. Clin Endocrinol (Oxf). 2006;64:588-595.
178. Voges J., Kocher M., Runge M., et al. Linear accelerator radiosurgery for pituitary macroadenomas: a 7-year follow-up study. Cancer. 2006;107:1355-1364.
179. Castinetti F., Taieb D., Kuhn J.M., et al. Outcome of gamma knife radiosurgery in 82 patients with acromegaly: correlation with initial hypersecretion. J Clin Endocrinol Metab. 2005;90:4483-4488.
180. Choi J.Y., Chang J.H., Chang J.W., et al. Radiological and hormonal responses of functioning pituitary adenomas after gamma knife radiosurgery. Yonsei Med J. 2003;44:602-607.
181. Pan L., Zhang N., Wang E.M., et al. Gamma knife radiosurgery as a primary treatment for prolactinomas. J Neurosurg. 2000;93(Suppl 3):10-13.
182. Mokry M., Ramschak-Schwarzer S., Simbrunner J., et al. A six-year experience with the postoperative radiosurgical management of pituitary adenomas. Stereotact Funct Neurosurg. 1999;72(Suppl 1):88-100.
183. Levy R.P., Fabrikant J.I., Frankel K.A., et al. Heavy-charged-particle radiosurgery of the pituitary gland: clinical results of 840 patients. Stereotact Funct Neurosurg. 1991;57:22-35.
184. Castinetti F., Nagai M., Dufour H., et al. Gamma knife radiosurgery is a successful adjunctive treatment in Cushing’s disease. Eur J Endocrinol. 2007;156:91-98.
185. Kobayashi T., Kida Y., Mori Y. Gamma knife radiosurgery in the treatment of Cushing disease: long-term results. J Neurosurg. 2002;97:422-428.
186. Hoybye C., Grenback E., Rahn T., et al. Adrenocorticotropic hormone-producing pituitary tumors: 12- to 22-year follow-up after treatment with stereotactic radiosurgery. Neurosurgery. 2001;49:284-291. discussion 291-282
187. Mauermann W.J., Sheehan J.P., Chernavvsky D.R., et al. Gamma knife surgery for adrenocorticotropic hormone–producing pituitary adenomas after bilateral adrenalectomy. J Neurosurg. 2007;106:988-993.
188. Pollock B.E., Young W.F.Jr. Stereotactic radiosurgery for patients with ACTH-producing pituitary adenomas after prior adrenalectomy. Int J Radiat Oncol Biol Phys. 2002;54:839-841.
189. Sheehan J.P., Sun M.H., Kondziolka D., et al. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg. 2002;97:1276-1281.
190. Jawahar A., Matthew R.E., Minagar A., et al. Gamma knife surgery in the management of brain metastases from lung carcinoma: a retrospective analysis of survival, local tumor control, and freedom from new brain metastasis. J Neurosurg. 2004;100:842-847.
191. Sheehan J., Kondziolka D., Flickinger J., Lunsford L.D. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain: outcomes and prognostic factors. J Neurosurg. 2005;102(Suppl):247-254.
192. Pan H.C., Sheehan J., Stroila M., et al. Gamma knife surgery for brain metastases from lung cancer. J Neurosurg. 2005;102(Suppl):128-133.
193. Lederman G., Wronski M., Fine M. Fractionated radiosurgery for brain metastases in 43 patients with breast carcinoma. Breast Cancer Res Treat. 2001;65:145-154.
194. Christopoulou A., Retsas S., Kingsley D., et al. Integration of gamma knife surgery in the management of cerebral metastases from melanoma. Melanoma Res. 2006;16:51-57.
195. Mingione V., Oliveira M., Prasad D., et al. Gamma surgery for melanoma metastases in the brain. J Neurosurg. 2002;96:544-551.
196. Muacevic A., Kreth F.W., Mack A., et al. Stereotactic radiosurgery without radiation therapy providing high local tumor control of multiple brain metastases from renal cell carcinoma. Minim Invasive Neurosurg. 2004;47:203-208.
197. Hoshi S., Jokura H., Nakamura H., et al. Gamma-knife radiosurgery for brain metastasis of renal cell carcinoma: results in 42 patients. Int J Urol. 2002;9:618-625. discussion 626; author reply 627
198. Goyal L.K., Suh J.H., Reddy C.A., Barnett G.H. The role of whole brain radiotherapy and stereotactic radiosurgery on brain metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2000;47:1007-1012.