Chapter 5 Gait Analysis
Technology and Clinical Applications
Since the later part of the twentieth century, gait analysis has become a useful clinical tool in the management of walking and movement problems for patients with neurologic and orthopedic conditions. Technology related to gait analysis and our understanding of the role of gait analysis in clinical assessment and management have improved significantly in recent years. Gait analysis was initially used in the last decade of the nineteenth century by the Weber brothers. Muybridge21 contributed to the understanding of movement with his famous sequential photographs, first of horses and later of walking and running men. Composited animations of some of Muybridge’s original work can be seen online (http://photo.ucr.edu/photographers/muybridge/contents.html). Later, Marey19 used light-colored marking strips on dark-clad subjects for the analysis of body movements. Bernstein2 initiated the formal study of kinematics with his detailed photographic studies of normal human locomotion movement. In 1947 Schwartz et al.24 made the first quantitative studies of the forces generated at the floor-foot interface during walking. Later, electromyography (EMG) recordings were possible. Inman’s group13 at the University of California Biomechanics Laboratory refined the simultaneous recording of multiple muscle group activity during normal ambulation.
Gait analysis has evolved into a recognized objective medical evaluation technique that is important in surgical planning10 and in the planning of other therapeutic interventions, such as botulinum toxin injection in the management of spasticity and the prescription and optimization of lower extremity orthotic and prosthetic devices.8 Other applications include sport movement analysis, analysis of other musculoskeletal conditions, and outcomes measurement. The most important contribution of gait analysis might be as a quantitative assessment tool for movement generally and walking specifically. In some centers, computer models of walking are used to drive simulation models that are then modified with the proposed interventions to determine whether the treatment will achieve the desired goal.
These criteria require that the clinician be familiar with the complex physiologic interactions of normal gait biomechanics, with normal and abnormal patterns of motor control, and with the technology used for its assessment. In addition, the clinician must possess the ability to relate these features to the pathologic motion that is observed during walking to effectively diagnose and address the problems of abnormal gait. To properly identify and evaluate the gait problems of the patient, the clinician must be able to produce a hypothesis and then attempt to understand what the problem is, where and when it is present, and why it occurs. Knowledge of appropriate available interventions, as well as a thorough medical history and examination, is needed to determine the most appropriate treatment interventions.7
Normal Locomotion
Walking requires significant motor coordination, yet most people can perform this complicated task without even thinking about it. The fundamental objective of bipedal human locomotion is to move safely and efficiently from one point to another.3 Humans are the only animals who characteristically have upright walking. Gait can be described as an interplay between the two lower limbs, one in touch with the ground, producing sequential restraint and propulsion, while the other swings freely and carries with it the forward momentum of the body. Most healthy individuals accomplish walking in a similar manner between the ages of 4 and 8 years because everyone has the same basic anatomic and physiologic makeup. Gait patterns are highly repeatable both within a subject and between subjects, but clearly each person has a unique walking style.
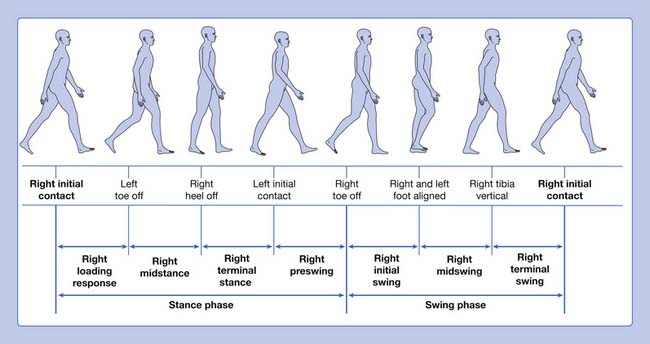
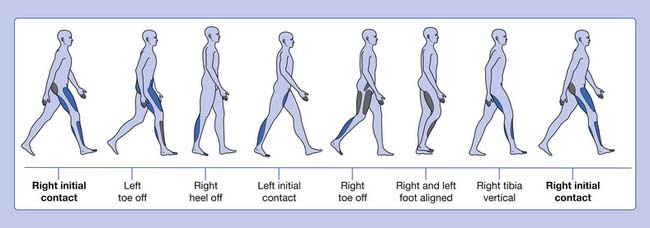
Gait is cyclic and can be characterized by the timing of foot contact with the ground; an entire sequence of functions by one limb is identified as a gait cycle (Figure 5-1).3,13 Each gait cycle has two basic components: stance phase, which designates the duration of foot contact with the ground, and swing phase, the period during which the foot is in the air for the purpose of limb advancement. The swing phase can be further divided into three functional subphases: initial swing, midswing, and terminal swing. In the same manner the stance phase can be partitioned into one event and four subphases: initial contact, loading response, midstance, terminal stance, and preswing.1,6
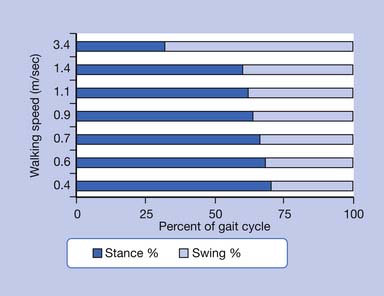
The stance phase can alternatively be subdivided into three periods according to foot-floor contact patterns. The beginning and the end of the stance phase mark the period of double support, during which both feet are in contact with the floor, allowing the weight of the body to be transferred from one limb to the other. When double support is absent, the motion is, by one definition, running. Single limb support begins when the opposite foot is lifted from the ground for the swing phase. For normal subjects walking at self-selected comfortable speeds, the normal distribution of the floor contact period during the gait cycle is broadly divided into 60% for the stance phase and 40% for the swing phase, with approximately 10% overlap for each double support time. These ratios vary greatly with changes in walking velocity (Figure 5-2).
The step period is the time measured from an event in one foot to the subsequent occurrence of the same event in the other foot. There are two steps in each stride or gait cycle. The step period is useful for identifying and measuring asymmetry between the two sides of the body in pathologic conditions. Step length is the distance between the feet in the direction of progression during one step. The stride period is defined as the time from an event of one foot until the recurrence of the same event for the same foot; initial contact to initial contact is used to define the stride period. Stride length is the distance between the same foot in the direction of progression during one stride. Left and right strides are equal in normal ambulation, but this might not be the case in pathology. The stride period is often time-normalized for the purpose of averaging gait parameters over several strides both between and within subjects (i.e., the absolute time is transformed to 100%). Cadence refers to the number of steps in a period of time (commonly expressed as steps per minute). The step length, step time, and cadence are fairly symmetric for both legs in normal individuals. These are all useful parameters when evaluating pathologic gait. The base of support refers to the lateral distance between the feet. This is usually measured as the perpendicular distance between the medial borders or centerlines of the left and right feet.
Gait Dysfunction
Because of the complex relationship of multiple body segments, it is difficult to clearly identify the primary cause and compensation (substitution) in a gait deviation. One approach is to look at the different phases of locomotion and identify factors that affect the particular expected functional component when attempting to understand pathologic gait. Following this functional approach, the stance phase dysfunctions can be categorized into three groups, as shown in Box 5-1.
Ankle-Foot Instability
The foot interaction with the ground is inadequate, interfering with its inherent weight-bearing function. This can be exemplified as an abnormal posture of the foot present in the form of equinus, equinovarus, ankle valgus with or without equinus, toe flexion, hallux extension (hitchhiker’s great toe),20 and/or excessive ankle dorsiflexion as seen with insufficient plantar flexor strength. Ankle-foot instability problems are commonly seen in the patient with neurologic sequelae after central nervous system injuries.
Quantitative Gait Analysis
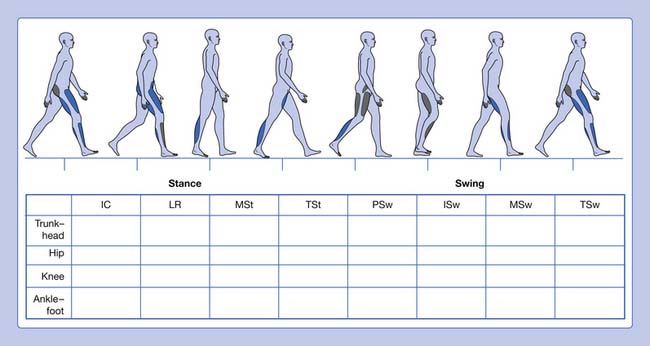
Informal visual analysis of gait is routinely performed by clinicians and used as the basis to develop the initial questioning and examination of a patient (Table 5-1). This sometimes casual observation can be more useful, albeit with many limitations, if performed in a careful, systematic manner. This can be done using a simple form that guides the clinician on documenting the findings (Figure 5-3). This type of analysis can yield good descriptive information, especially when slow-motion video technology is used to supplement it. The complexity and speed of events that occur during walking, coupled with deviations and possible compensations that occur in pathologic gait, define the limitations of a visual-based qualitative analysis of locomotion.3 Fortunately there are a great many tools available to increase our ability to observe and quantify gait.
| Phase of Gait Cycle | Description |
|---|---|
| Stance Phase | |
| Initial contact | The instant the foot contacts the ground |
| Loading response | From flat foot position until the opposite foot is off the ground for swing |
| Midstance | From the time the opposite foot is lifted until the ipsilateral tibia is vertical |
| Terminal stance | From heel rise until the opposite foot contacts the ground (contralateral initial contact) |
| Preswing | From initial contact of the opposite foot and ends with ipsilateral toe-off |
| Swing Phase | |
| Initial swing | Begins with lift-off of the foot from the floor and ends when the foot is aligned with the opposite foot |
| Midswing | Begins when the foot is aligned with the opposite foot and ends when the tibia is vertical |
| Terminal swing | Begins when the tibia is vertical and ends when the foot contacts the ground (initial contact) |
In the laboratory, gait can be studied through the collection of a wide range of information. Four primary components of quantitative gait analysis (Box 5-2) can be recorded:
Kinematics
Temporal and Spatial Descriptive Measures
Available techniques include the simple use of ink and paper, foot switches, and instrumented walkways to the most sophisticated systems that require the patient to be instrumented (which can provide considerable additional data). One example of a system that requires no patient instrumentation is the Electronic Gait Mat II. This instrumented walkway measures 3.8 m in length and contains approximately 10,000 electronic switches, scanned at 100 Hz. Patients can use gait aids or shoes and braces, if necessary, as they walk over the mat, which ideally is mounted flush with the floor. A recording of foot contact generates a timed “electronic footprint.” A printout that provides calculated data about walking speed, cadence, stance, and swing times for each foot, as well as stride lengths, step lengths, and the width of the base of support, is generated.6,25 The data can be easily stored for future reference or to perform other data analysis.9 Comparing left- and right-side data from one subject can be used to determine the extent of unilateral impairment. Comparisons can also be made with normative gender, age, and walking speed–matched data. This allows inference of the level of dysfunction.
Motion Analysis
Video and passive optoelectronic systems use retroreflective markers applied to the subject. The markers are “illuminated” by an external power source and are tracked by the detectors (camera). Near-automatic marker identification and digitization are reliable if marker paths do not cross, as can usually be expected for standard marker placements in normal walking. However, conversion into quantitative data might require some manual intervention for marker identification in pathologic gait, where increased limb rotation, sudden motions, or crossover of segment paths can occur. Manual digitization and tracking of the raw data can be in some instances time-consuming and error-prone.4,6,22 With active optoelectronic systems, each marker is self-illuminated (hence the designation “active”). No postcollection marker identification is needed because time sequencing between marker illumination and detector reception uniquely identifies each light-emitting diode.6 Each marker is activated at a slightly different (in the order of microseconds) instant in time. Telemetry (via infrared transmitters) in newer active systems such as the CODA CX1 (Charnwood Dynamics Ltd, Rothely, England) has eliminated the use of “umbilical cords” to power each marker. Not having to manually identify or track markers, and the real-time nature of these systems are advantages over the passive marker systems.
Kinetics
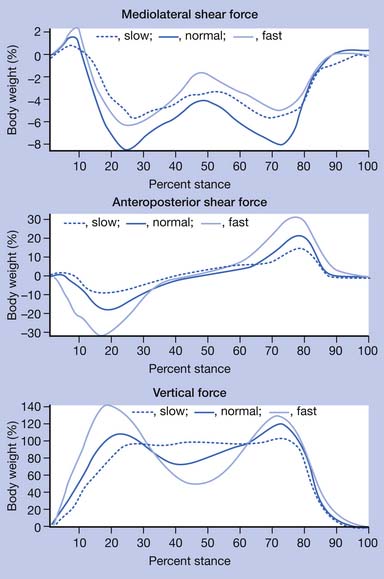
Kinetic analysis deals with the forces that are produced during walking. Sir Isaac Newton described basic but critical concepts that are useful in understanding the effect of gravity on gait. He stated in his third law of motion that “for every action there is an equal and opposite reaction.” This concept indicates that, as long as gravity is present, there is a reaction force where the body interacts with the ground. The ground reaction force is a reflection of the body weight and acceleration. This force can be resolved into a convenient set of directions, such as vertical, anteroposterior, and mediolateral (Figure 5-4). The anteroposterior shear forces are sometimes referred to as propulsion and breaking forces, respectively. Friction is responsible for the generation of shear forces. A force plate is a “sophisticated scale” that can measure vertical (downward force similar to the body weight registered on a scale) as well as shear forces, which are those acting in the plane of the floor secondary to friction. Triaxial force plates measure the total force (a vector summation of all three components) acting on the center of pressure (a focal point under the foot at which the force is idealized to be concentrated). Preferably two platforms placed adjacent to each other are used, so that the total force under each foot can be recorded independently and simultaneously. In most instances the force platforms are placed in the midpoint of the walkway and concealed in the floor so that steady-state, natural walking parameters are measured. Together the forces in all three directions measured by the force plates comprise the total force.
An innovation, however, is that the force is superimposed in real-time as a visible line on a video image of the walking subject at the location at which the force acts. This is accomplished using laser optics5 or computer processing in a specialized system (Digital force, Bertec, Columbus, Ohio). This force line visualization system has a significant clinical utility because it provides visual information regarding the effects of gravity on joint rotation without the need to instrument the patient. In addition, it is simple to setup and has slow-motion video playback capabilities.
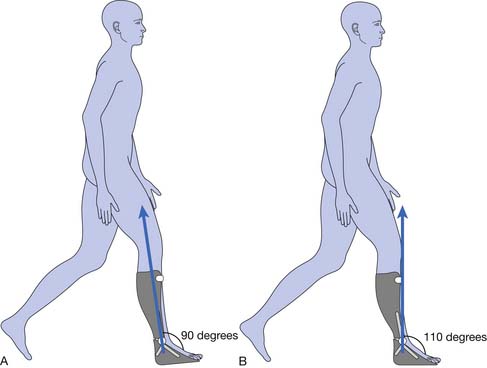
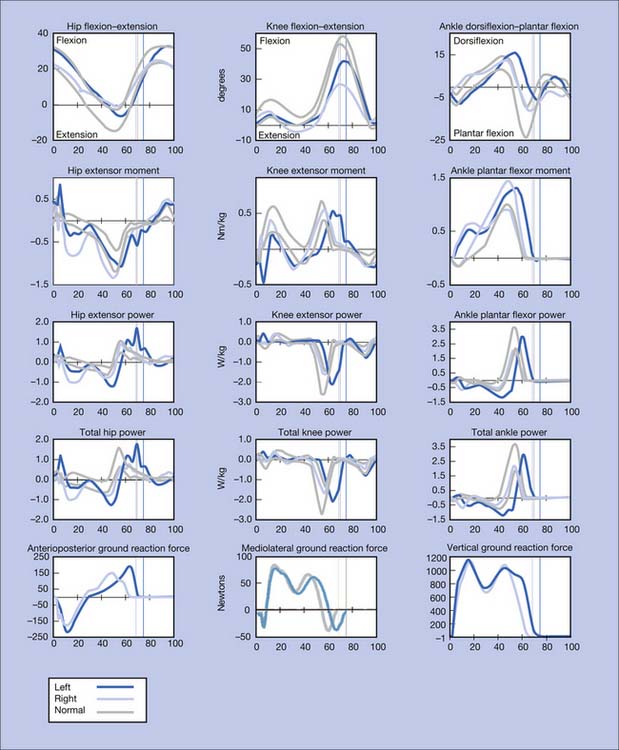
A force is transmitted from the floor to the foot, and it is literally “passed on up” to all other body segments. The product of the magnitude of the ground reaction force under each foot and its location with respect to a given joint center (ankle, knee, hip, etc.) are major factors that determine the torque or moments produced by the external force about that joint. This moment is a measure of the joint rotational tendency (flexion or extension, abduction or adduction, internal or external rotation) produced by the external force. Internal forces—generated primarily by muscles, ligaments, and the geometry of the joint articulation (bony contact)—act to control the rotation of the joints caused by this external force. For example, the ground reaction force, when positioned anterior to the knee (Figure 5-5), produces a moment that tends to drive the knee into extension, and must be countered and controlled by muscle force (knee flexors, extensors, etc.).
The relative motion of body segments produces forces that affect the motion of the entire body. This brings to light an important but not commonly considered concept (which is an area of research in a few laboratories): that the acceleration of each body segment affects the acceleration of all other segments in the body.27 A fairly involved engineering analysis is necessary to understand these interactions, but these effects should further our understanding of whole-body mechanics and ultimately have the potential to reshape some of the traditional lines of thinking in gait biomechanics.16
While force plates measure the sum or total force acting under the entire foot, it is sometimes useful to measure discrete components of that force acting over specific areas of the foot, or the distribution of pressure. Mathematically, pressure = force/area. A given force acting over an area produces larger pressures than the same force distributed over a large area. The pressure-time characteristics of the contact surface may have profound effects on the gait pattern. The forces generated at the point of contact with the floor can be measured with force platforms, as described above. Measuring the force distribution, for example, as it occurs inside the shoe, necessitates the use of devices that can be placed inside the footwear and in direct contact with the foot without disturbing the foot-shoe interface. Ultrathin Mylar pressure-resistive sensors and specialized software permit collection of multiple gait cycles. Analysis of these data is done by calibrated color pressure grids. Software allows evaluation of force and pressure, as well as integrals of these measures. These systems are produced by Tekscan in the United States and others in Europe and Japan, and are useful for this purpose. Floor-embedded pressure sensor mats are also available to measure discrete pressures (Figure 5-6). One disadvantage is that most systems allow the capture of only one step at a time, and frequent guidance to capture a complete step might be necessary because of the size of the mat sensor. Pressure measurement devices have clinical value particularly in the assessment of the deformed, insensate, or painful foot, and in the evaluation and fitting of customized foot or ankle-foot orthoses.
Dynamic Polyelectromyography
In normal locomotion (Figure 5-7), forces are elicited from 28 muscles in each lower limb and muscles in the trunk and arms to carefully control the gravitational forces, yielding a smooth, coordinated, and energy-efficient movement pattern. Redundancy exists in the relationship between muscles and the joints on which they act; in other words, the association between a particular movement and the muscle forces producing the movement is not unique. The cause of a particular movement cannot be specifically assigned to a muscle based on the observed movement. Persons with spastic paraparesis secondary to brain or spinal cord injuries present the greater diagnostic challenge, because muscle function is disrupted at many levels and the overlay of spasticity or other phenomena common to the upper motor neuron syndrome often causes the clinical evaluation during an examination to differ significantly from the muscle pattern used during walking and standing.
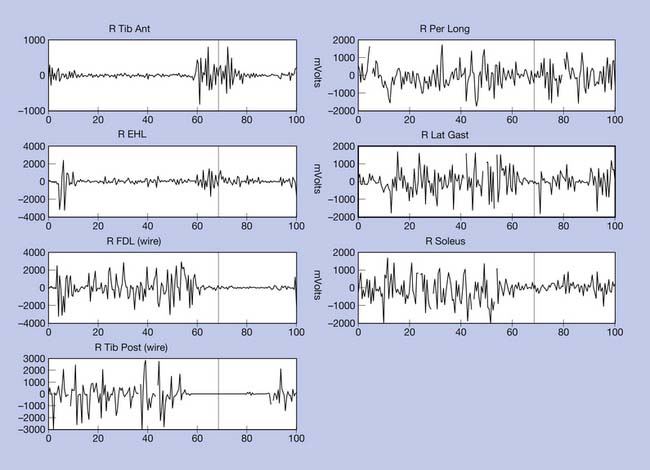
The electrical activity of all the muscles (EMG) that are capable of producing the target movement—which is not limited to a muscle directly spanning a particular segment or joint—needs to be evaluated. EMG recordings provide information about the timing and duration of muscle activation, and under certain conditions, relative strength can also be ascertained. The EMG signal is an accurate indicator of muscle activation and can be used to infer neurologic control information. Superficial muscles are preferentially studied using surface bipolar electrodes secured to the skin with double-sided tape after the skin has been prepared. For deep muscles, or to differentiate between adjacent muscles when cross talk can be of concern, a pair of indwelling fine wire electrodes (Figure 5-8) are inserted through a 25-gauge hypodermic needle, which is immediately removed, leaving only the flexible wires behind. The thin wires measure 50 μm and are coated with Teflon or nylon except at the tips, where the muscle electrical potentials are recorded.
Patient EMG profiles can be compared with the mean and standard deviations of tabulated normative data, if speed-matched, to identify how the timing deviates from the normal. The timing classification scheme for EMG activity shown in Table 5-2 was devised in an attempt to standardize terminology.15
Table 5-2 Classification of Dynamic Electromyographic Activity
| Class | Definition |
|---|---|
| 1 | Premature |
| 2 | Premature prolonged |
| 3 | Out of phase |
| 4 | Normal |
Energetics
Normal walking requires a relatively low level of metabolic energy consumption during steady state at comfortable walking speeds. Normal gait on level surfaces is most efficient at a walking speed of 1 to 1.3 m/s, which is equivalent to 60 to 80 m/min or 3 mph. Comfortable walking speed for an individual usually corresponds to minimum energy cost per unit distance. The CoM is a point where all the mass of the body is idealized to be concentrated. In a homogeneous object the CoM is simply the geometric center of the object. For a symmetric object, like a sphere or cube, the CoM is the center of the object. For the human body the CoM has been experimentally found to be located 2 cm in front of the second sacral vertebra (in anatomic position). It has a dynamic nature (meaning that its location changes as the orientation of the body changes) and under certain conditions may even be located outside the body. The position of the CoM is intimately related to the location of the ground reaction force; simply put, they move in tandem. During walking the CoM moves in a sinusoidal path with an average of 5 cm vertical and horizontal displacement. This displacement of the CoM requires work, which in turn has an energy cost. In fact, the six determinants of gait, as described by Saunders and Inman et al.22 (Box 5-3), were identified as the strategies necessary to produce forward progression with the least energy expenditure by minimizing the excursion of the CoM. While regarded as true and classic for many years, the effect of the determinants on energy expenditure during gait has come under closer scrutiny, and researchers have begun to challenge some of the original precepts.11,12,17,18
Box 5-3 Inman’s Six Determinants of Gait
There is a link between motion of the CoM and energy expended during walking. Sudden acceleration or deceleration of the CoM will increase energy consumption. The three main events that consume energy during walking are controlled deceleration toward the end of swing phase, shock absorption at heel strike, and forward propulsion of the CoM at push-off. Running is more efficient than walking faster than 2 m/s. Walking on a 10% to 12% incline will double energy expenditure. Willis et al.26 proposed that human preferred walking velocity is determined in part by the metabolic control of skeletal muscle and coincides with the lower level at which carbohydrate oxidation occurs.
Pathologic Gait
Abnormal Base of Support
Equinus Foot Deformity
A similar abnormal gait pattern can be seen in a patient using a prosthesis set in excessive plantar flexion or set anterior to the trochanter-knee-ankle line, or in a patient with articulated foot-limited dorsiflexion. An ankle-foot orthosis that limits dorsiflexion beyond 5 degrees of equinus can impose the same gait deviation (Figure 5-9).
Following is a clinical case presentation to exemplify the use of the described methodology and technology for the evaluation of gait disorders and formulation of a treatment plan.
Clinical Case Presentation
Diagnostic Workup
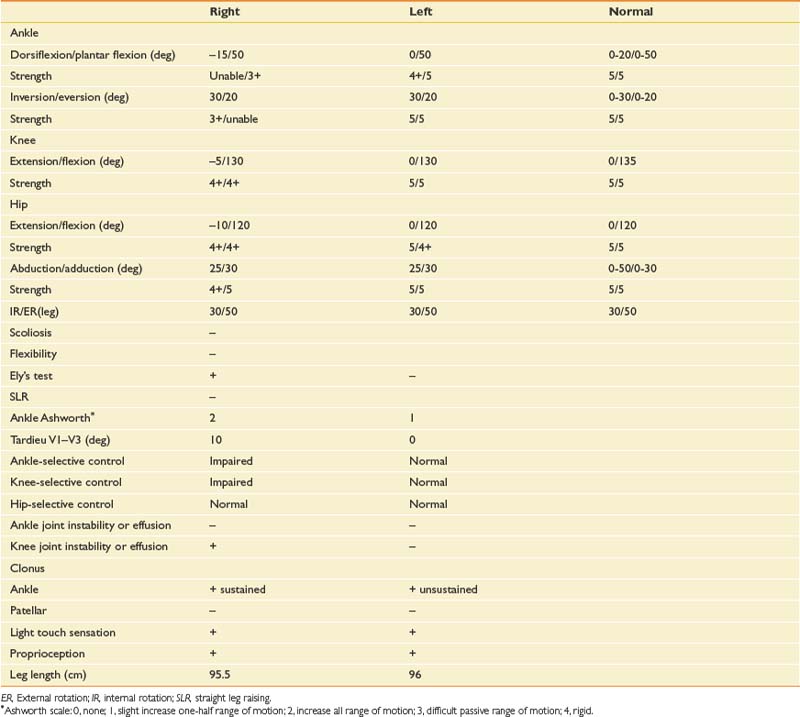
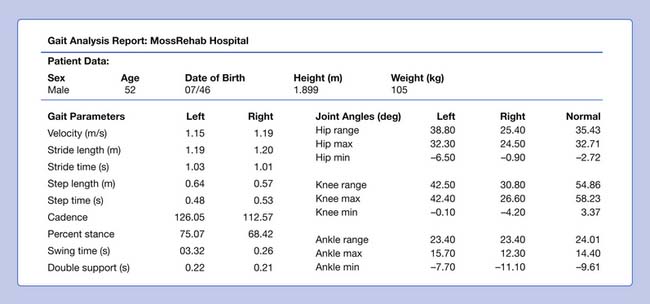
In the examination the patient is an alert, pleasant, cooperative, moderately obese man who is in no acute distress. His body weight is 105 kg. Passive range of motion and manual muscle testing are as shown in Table 5-3.
Expected Functional Penalties
Instrumented Gait Analysis
Analysis includes the following:
Findings
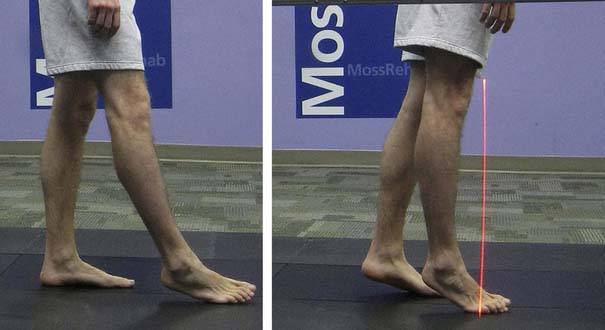
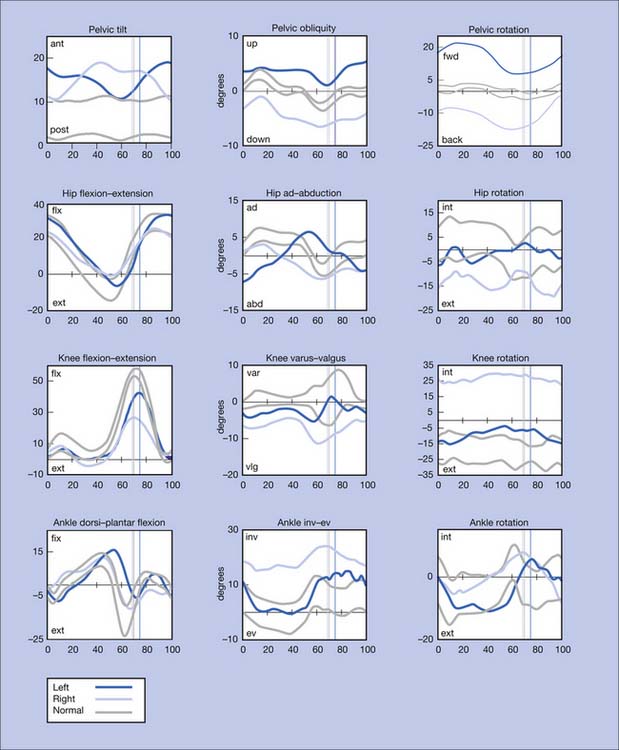
Figure 5-10 summarizes the findings. Video frame-by-frame analysis demonstrates evidence of abnormal right ankle–foot posture, with equinus, varus, and toe flexion in swing phase. Ankle equinus and varus as well as toe curling are evident in stance phase. Abnormal force line location in front of the right knee is noted.
Impression
The right equinus posture appears to be caused by overactivation of the gastrocnemius more than the soleus. The ankle varus results from out-of-phase activation of the extensor hallucis longus and tibialis anterior. No abnormal activation of the tibialis posterior is evident in the swing phase of this evaluation. The reduction in right hip, knee, and ankle power is probably related to the abnormal ankle-foot posture. Spastic right “stiff knee” cannot be ruled out. There is no evidence of right knee hyperextension on the kinematic data, but this may be related to mechanical joint limitation in extension (see earlier discussion). Stretching of hamstrings during stance might be the cause of his knee pain, while high force concentration over the forefoot might be the cause of his foot-ankle pain.
Possible Treatment Interventions
The clinician should obtain radiographs of the right knee to rule out bony block or joint problems.
Because botulinum toxin type A requires repeated injections and the patient is more than 2 years postinjury, surgical intervention in the form of Achilles tendon lengthening, split tibialis anterior tendon transfer, and myotendinous lengthening of the extensor hallucis longus can be considered. To supplement the weak ankle plantar flexors and avoid toe curling when the ankle dorsiflexion range of motion is increased, a release and transfer of the long toe flexors to the os calcis can be considered.14
Flexion Deformity of the Toes
The toes may be held in flexion during the swing and stance phases. When wearing shoes, the patient complains of pain at the tip of the toes and also over the dorsum of the phalangeal joints, which is worsened by weight-bearing. Callus formation in these areas is frequently seen. The gait pattern will demonstrate gradual loading of the affected limb and shortening of the step length and stance time. Likely causes are neurologic injuries, complex regional pain syndrome prolonged immobilization, and contractures. Clinical examination combined with kinetics and dynamic EMG recordings can be helpful in sorting out the cause of the deformity. In patients with spasticity, the recordings likely will demonstrate prolonged or out-of-phase activation of the flexor digitorum longus and flexor hallucis longus, and can demonstrate abnormal coactivation of the gastrocnemius-soleus or lack of activation of the toe extensors.
Limb Clearance and Advancement
Limb clearance and advancement occur during the swing phase of gait and are vital precursors for proper limb positioning in order for the leg to accept the body weight during the ensuing stance. When limb clearance is inadequate, limb advancement is usually compromised. Impaired limb clearance may cause a patient to trip and fall, particularly when walking on uneven, inclined, or carpeted surfaces or when transitions in flooring surface take place. Reduction of limb advancement produces shortening of step length and reduction in walking speed.
1. Bampton S. A guide to the visual examination of pathological gait. Philadelphia: Temple University–Moss Rehabilitation Hospital; 1979.
2. Bernstein N: The technique of the study of movements. In: Slonim A, editor: Textbook of the physiology of work, Moscow, 1934.
3. Cappozzo A. Gait analysis methodology. Hum Mov Sci. 1984;3:27-50.
4. Chiari L., Della Croce U., Leardini A. et al: Human movement analysis using stereophotogrammety. II. Instrumental errors. Gait Posture. 2005;21(2):197-211.
5. Cook T.M., Cozzens B.A., Kenosian H. A technique for force-line visualization. Philadelphia: Moss Rehabilitation Hospital; 1979.
6. Esquenazi A., Hirai B. Assessment of gait and orthotic prescription. Phys Med Rehabil Clin N Am. 1991;2:473-485.
7. Esquenazi A., Keenan M. Gait analysis. In Gans B., editor: Rehabilitation medicine: principles and practice, ed 2, Philadelphia: Lippincott, 1993.
8. Esquenazi A., Mayer N. Instrumented assessment of muscle overactivity and spasticity with dynamic polyelectromyographic and motion analysis for treatment planning. Am J Phys Med Rehabil. 2004;83(suppl 10):S19-S29.
9. Esquenazi A., Talaty M. Normal and pathological gait analysis. In: Lehmkuhl L.D., editor. Physical medicine and rehabilitation: the complete approach. Malden: Blackwell Science, 2000.
10. Fuller D.A., Keenan M.A., Esquenazi A., et al. The impact of instrumented gait analysis on surgical planning: treatment of spastic equinovarus deformity of the foot and ankle. Foot Ankle Int. 2002;22(8):738-743.
11. Gard S., Childress D. The effect of pelvic list on the vertical displacement of the trunk during normal walking. Gait Posture. 1997;5:233-238.
12. Gard S., Childress D. The influence of stance-phase knee flexion on the vertical displacement of the trunk during normal walking. Arch Phys Med Rehabil. 1999;80:26-32.
13. Inman V., Ralston H., Todd F. Human walking. Baltimore: Williams & Wilkins; 1981.
14. Keenan M.A., Lee G.A., Tuckman S.A., et al. Improving calf muscle strength in patients with spastic equinovarus deformity by transfer of the long toe flexors to the os calcis. J Head Trauma Rehabil. 1999;14(2):163-175.
15. Keenan M.A.E., Haider T., Stone L.R. Dynamic electromyography to assess elbow spasticity. J Hand Surg [AM]. 1990;15:607-614.
16. Kepple T.M., Siegel K.L., Stanhope S.J. Relative contributions of the lower extremity joint moments to forward progression and support during gait. Gait Posture. 1997;6:1-8.
17. Kerrigan D., Della Croce U., Marciello M., et al. A refined view of the determinants of gait: significance of heel rise. Arch Phys Med Rehabil. 2000;81:1077-1080.
18. Kerrigan D., Riley P., Lelas J., et al. Quantification of pelvic rotation as a determinant of gait. Arch Phys Med Rehabil. 2001;82:217-220.
19. Marey E: La methode graphique dans les sciences experimentales et particularierement en physiologie et en medicine. In: Masson G, editor: Deuxieme tirage augmente d’un supplement sur le development de le methode graphique par l’emploi de la photographie, Paris, 1885.
20. Mayer N., Esquenazi A., Keenan M.A.E. Assessing and treating muscle overactivity in the upper motor neuron syndrome. In: Zasler N., Katz D., Zafonte R., editors. Brain injury medicine principles and practice. New York: Demos, 2006.
21. Muybridge E. Animal locomotion: an electro-photographic investigation of consecutive phases of animal movements. Philadelphia: University of Pennsylvania; 1887.
22. Rowell D., Mann R. Human movement analysis. Soma. 1989;3:13-20.
23. Saunders J.B., Inman V.T., Eberhart H.D. The major determinants in normal and pathological gait. J Bone Joint Surg Am. 1953;35:544-553.
24. Schwartz R., Heath A., Misiek W., et al. Kinetics of human gait: the making and interpretation of electrobasographic records of gait. J Bone Joint Surg. 1934;16:343-350.
25. Taylor D: An instrumented gait mat. The International Conference on Rehabilitation Engineering, Toronto; 1980.
26. Willis W., Ganley K., Herman R. Fuel oxidation during human walking. Metabolism. 2005;54(6):793-799.
27. Zajac F.E., Gordon M.E. Determining muscle’s force and action in multi-articular movement. Exerc Sport Sci Rev. 1989;17:187-230.