CHAPTER 102 Fusion Surgery
INTRODUCTION
Fusion has been used for decades to manage a variety of spinal disorders. However, the incidence of spinal fusion surgery varies among countries and among regions within a country. During its infancy, spinal fusion surgery typically involved extensive muscle dissection, copious amounts of autogenous bone graft, bracing, and prolonged bed rest. The introduction of spinal instrumentation provided an opportunity to increase the rate of successful fusion, decrease the recovery period, minimize the cardiopulmonary and musculoskeletal deconditioning resulting from immobility, and allow surgeons to perform more complex spinal reconstructive surgeries. Initial fusion techniques were primarily posterior, but anterior column support was subsequently developed to minimize the failure rates associated with the use of posterior instrumentation. Anterior column fusion can be achieved with bone graft material secured within the disc space from either an anterior or posterior approach. Anterior column fusion has inherent advantages as it occurs along the weight bearing portion of the lumbar spine (80% anterior versus 20% posterior), has superior blood supply, and a superior ability to maintain sagittal alignment.
At present, controversy still exists concerning the indications for spinal fusion, the type of procedure to perform, the choice of graft material, and the use of instrumentation. The most widely accepted indication for spinal fusion is instability, which may arise from trauma, tumor, infection, and degenerative disease. Instability may also arise iatrogenically from the surgical treatment of these aforementioned conditions. With the improvement of surgical techniques, along with the development and marketing of spinal instrumentation, there has been a noticeable trend toward the increasing use of spinal instrumentation.1
INDICATIONS
Although there is agreement regarding instability as an indication for fusion, there is a lack of consensus on the precise definition of instability. The clinical manifestations and assessment of instability of the spinal column are discussed in detail in the previous chapter. In general, the various methods for determining spinal instability are either for trauma, degenerative conditions, or tumor. The White and Panjabi ‘checklist approach’ and the Denis classification system remain the most recognized methods of determining instability in the setting of trauma.2 Recently, Gertzbein and coworkers have developed a detailed description of thoracolumbar fracture patterns that can also be helpful in determining instability.3,4 Gaines and coworkers utilize a ‘load-sharing’ system to determine the stability of the anterior column in the setting of burst fractures.5,6 This system is useful for determining when anterior reconstruction is necessary or when short-segment posterior instrumented fusion may be helpful to treat burst fractures. Whichever system is used, instability due to trauma must take into consideration the mechanism of injury, the degree of deformity, and the location of injured ligamentous and bony structures.
For destructive lesions, particularly tumors, the degree of vertebral body and/or pedicle involvement and the degree of deformity enter into in the determination of instability.5–7 At present, no method of systematically assessing impending instability exists for infectious processes. Other forms of instability, such as inflammatory, congenital, degenerative, and postoperative instability, are not well assessed with grading systems such as those described by Gaines and coworkers.
TECHNIQUE
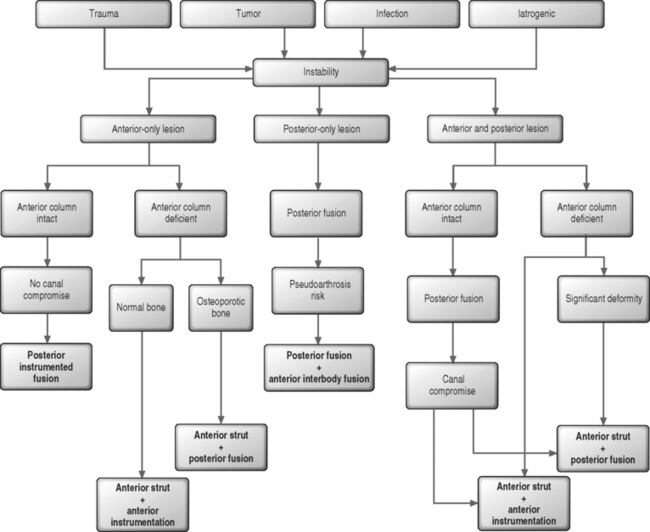
The method by which surgery is performed depends on the cause and character of the instability. The available methods of treatment include anterior, posterior, or combined anterior–posterior surgical approaches. General guidelines have some degree of support either in the literature or by consensus (Fig. 102.1).
Anterior lesions
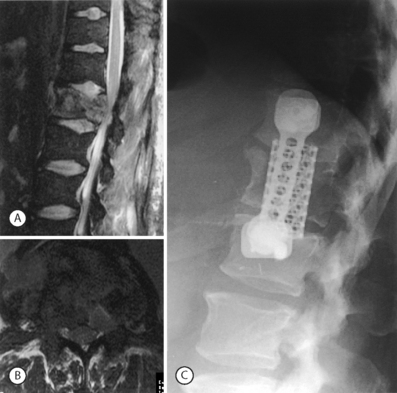
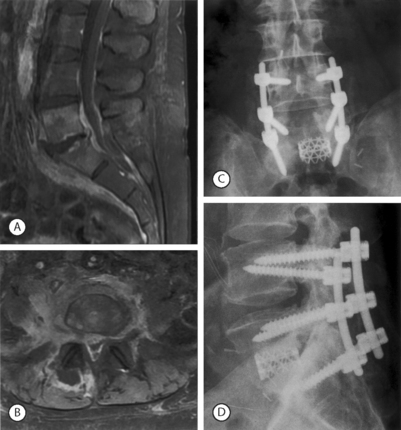
When instability is due to a lesion that is primarily anterior, surgical stabilization can be performed from an anterior approach. This anterior approach generally involves discectomy, perhaps corpectomy, structural grafting and anterior instrumentation. Anterior surgery is indicated if there is canal compromise due to the anterior lesion, if anterior column stability is deficient, or a previous posterior procedure has failed to stabilize the segment(s). The load-sharing classification system of Gaines and coworkers is one method of determining the integrity of the anterior column.5,6 The load-sharing classification system describes the integrity of the anterior column in the setting of trauma. However, this concept can be applied to other lesions of the anterior column. If there is no significant osteoporosis or deformity that requires correction, then anterior strut grafting with anterior instrumentation will provide sufficient stabilization (Fig. 102.2).
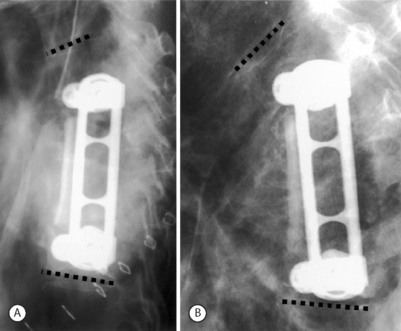
However, certain conditions warrant posterior surgery, either together with the anterior surgery or posterior surgery alone. Posterior surgery alone can be used to treat lesions that are otherwise anterior if the anterior column has sufficient stability and there is no need for decompression. In some cases, such as when there is no neurologic deficit, posterior-only surgery can be used to indirectly reduce retropulsed fragments of bone. Posterior surgery may be further entertained if there are anteriorly exposure risks, such as previous abdominal surgery or severe obesity. Posterior fusion may also be necessary if there is significant osteoporosis. In the setting of osteoporosis, anterior surgery alone may be inadequate in achieving spinal stability (Fig. 102.3).
Posterior lesions
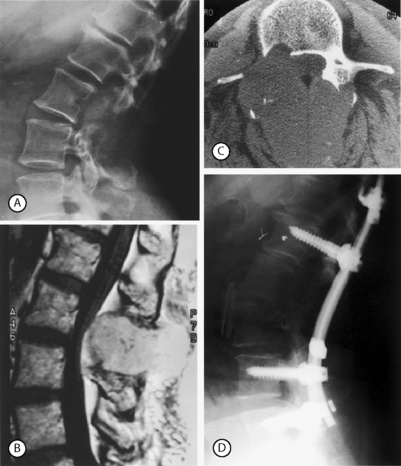
When instability stems from a posterior lesion, posterior surgery via instrumented fusion is usually the best option (Fig. 102.4). Unfortunately, instability due to a purely posterior lesion is relatively uncommon. The most common such lesion is the Chance fracture involving a flexion–distraction injury. If there is severe osteoporosis, additional levels may be added to the construct. The length of posterior instrumentation can be extended as necessary to achieve stability without much added difficulty. If necessary, pedicle screws can also be reinforced with bone cement to achieve better fixation in osteoporotic bone. Interbody fusion can be performed posteriorly via transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) to improve fusion rates, especially in long fusions or in fusions to the sacrum.
Combined anterior–posterior lesions
In combined lesions of both the anterior and posterior columns, the surgical approach is dictated by several factors. The first issue is whether anterior surgery is necessary. Anterior surgery is necessary if there is isolated or predominantly anterior canal compromise requiring decompression or if anterior column stability is compromised. Anterior surgery is usually in the form of strut or structural grafting. If there is good bone stock and no significant deformity, then anterior instrumentation may be all that is needed. If there is no canal compromise and the anterior column is stable, posterior instrumented fusion alone is sufficient to obtain stability. If there is osteoporosis, the levels of posterior fusion can be increased for improved fixation. When the anterior column is deficient and it is combined with severe deformity, osteoporosis, or infection, then anterior strut graft fusion combined with posterior instrumentation is necessary (Fig. 102.5).
FACTORS AFFECTING THE OUTCOME OF FUSION SURGERY
Biologic factors
Nutrition
Malnutrition is associated with reduced cognitive function, poor wound healing, impaired muscle function, decreased bone mass, immune dysfunction, anemia, delayed recovery from surgery, and ultimately increased morbidity and mortality.7 Nutritional deficiencies can be confirmed using various studies such as total white blood cell count, serum albumin and total protein, transferrin levels, nitrogen balance, and anthropometric measurements.
Hormones
Thyroid hormones have a direct stimulatory effect on cartilage growth and maturation, and promote bone healing.8 These hormones are required for the synthesis of somatomedins by the liver.9 Growth hormone promotes bone healing by increasing calcium absorption from the intestine, and promoting bone formation and mineralization.8 Androgens and estrogens are considered important for skeletal development and preventing age-related bone loss. Estrogens may increase bone mineralization through their effect on increasing serum parathyroid hormone (PTH).10 Excess corticosteroids can negatively affect bone healing by decreasing synthesis of the major components of the bone matrix. These steroids have also been shown to inhibit the differentiation of osteoblasts from mesenchymal cells.11
Smoking
The pathophysiological effects of smoking are multidimensional and include arteriolar vasoconstriction, cellular hypoxia, demineralization of bone, and delayed revascularization. Cigarette smoking also inhibits osteoblastic activity resulting in decreased rate of successful unions, with slowed bone healing and prolonged treatment.12,13 It appears that cigarette smoke has a greater impact upon cancellous bone than cortical bone, and causes decreased bone healing around implants. The constituents of cigarette smoke, and not only nicotine, seem to be more important.14,15
Complication rates after surgery and the rate of nonunion in smokers has been shown to be consistently higher than in nonsmokers.16,17 In a recent prospective clinical study designed to determine the effects of smoking on the healing of tibial deformities, half of the smokers and only one-fifth of nonsmokers developed complications.18 Smokers required an average of 16 days more treatment. Delayed healing and pseudoarthroses were more common in smokers than nonsmokers. The risk ratio for smokers to develop complications as compared to nonsmokers was 2.5.18 For spinal fusion, an increased rate of pseudoarthrosis has been observed following posterolateral lumbar spine grafting or anterior cervical interbody fusion in smokers.19 Smoking had a significant negative impact on healing and clinical recovery after these procedures.
Drugs
Many drugs such as methotrexate and Adriamycin can inhibit bone formation if administered in the early postoperative period. Nonsteroidal antiinflammatory drugs (NSAIDs) act by inhibiting action of cyclooxygenase (COX) enzymes and decreasing production of prostaglandins (PGs). This can result in diminished bone formation, healing, and remodeling. These effects seem to be greater with nonselective COX inhibitors, such as ketorolac, than with selective COX-2 inhibitors at therapeutic levels.20 Ketorolac also decreases bone mineralization and endochondral ossification in a dose-dependent manner.21 Other NSAIDs, such as diclofenac, can also significantly delay fracture healing.22 The timing of administration of NSAIDs also appears important. In animal studies, the earlier indometacin was resumed postoperatively, the greater was its negative effect on intertransverse process arthrodesis.23 Recombinant bone morphogenetic protein-2 (BMP-2), however, seems to show promise for combating the inhibitory effect of ketorolac on bone formation during spinal arthrodesis.24
It has been observed that longer duration of administration of selective COX inhibitors decreases the rate of successful fusion.25 In a recent study, however, it was found that the perioperative (short-term) administration of celecoxib had no apparent effect on the rate of nonunuion at 1 year following surgery. In this study, patients had elective decompressive lumbar laminectomy and instrumented spinal fusion. In addition, the use of the selective COX-2-specific NSAID resulted in a significant reduction in postoperative pain and opioid use following surgery.26 As such, short-term administration of selective COX inhibitors for perioperative pain, unlike long-term use, appears to be a reasonable therapeutic option.
AGE The effects of aging on spinal fusion are multifactorial. With the elderly there is often a decrease in food intake secondary to reduction in appetite. If prolonged, this can lead to a decline in nutritional status. Bone healing, including spinal fusion is affected by the nutritional status of the individual.7 Common medical conditions, such as renal and cardiac insufficiency, may exist in the elderly and can affect bone healing. The clinical observation that incisional wounds also heal less well in the aged may be secondary to these coexisting pathologies.27
The number of osteogenic stem cells may be more deficient in the elderly than in younger individuals. In animal studies, the numbers of osteoprogenitor cells present in the bone marrow from aged rats were 65% lower than the amount found in young adults. These cells were also 10 times less likely to differentiate in vitro and to form bone in vivo.28 However, bone-stimulating growth factors may have a role in increasing the bone-forming capacity of aged bone in the future.29,30
During aging, bone mass loss, structural continuity, and strength gradually diminish. Osteoporotic bone is weak and difficult to stabilize with spinal instrumentation. Although gradual bone loss starts at 30 years of age, an accelerated rate of loss occurs after menopause in women. Men also experience bone loss, but in a more insidious fashion. The rate of successful fusion in elderly individuals of both sexes with osteoporotic bone tends to be lower.31
Surgical factors
Approaches
The surgical approach can affect the outcome of lumbar fusion. Fritzell et al. studied the outcomes of fusion surgery using three different, well-accepted techniques of lumbar fusion.32 In their randomized prospective study, they show that posterolateral fusion without instrumentation achieves arthrodesis in 72% of patients. With the addition of posterior instrumentation, the fusion rate increased to 87%. For patients treated with combined posterior instrumented fusion and interbody fusion (i.e. circumferential fusion) the fusion rate was 91%. Overall, the fusion rates for grafts used in circumferential, posterior interbody, anterior interbody, and posterolateral fusions methods are about 91%, 89%, 86%, and 85%, respectively.1
Instrumentation
It is well established that spinal instrumentation increases the likelihood of successful fusion. Fischgrund et al. demonstrated that the fusion rate with instrumentation was twice that obtained without instrumentation for patients with degenerative spondylolisthesis.33 Zdeblick observed statistically greater fusion rates in patients with rigid instrumentation than those without. In his study, the fusion success with semirigid instrumentation (plate/screw system) was not different from that obtained without instrumentation.34 In contrast, Thomsen et al. found that the fusion rates were not significantly different between instrumented (supplementary pedicle screw fixation) and noninstrumented groups for patients undergoing posterolateral lumbar spinal fusion.35
Perhaps the best perspective regarding the impact of instrumentation on the fusion rate was revealed in a meta-analysis of the outcomes of lumbar fusion surgeries that were reported in the literature from 1970 to 2000.1 In this study, the average instrumented fusion rate (89%) was significantly higher than the noninstrumented fusion rate (84%). The fusion rates with semirigid instrumentation and rigid instrumentation were not significantly different (91% and 88%, respectively). In addition, both semi-rigid and rigid stabilization resulted in significantly higher fusion rates than no instrumentation (p=0.019, p=0.022, respectively).
Number of levels fused
The number of levels fused is an important factor that can dramatically affect the outcome of spinal fusion. In a retrospective study, the length of hospital stay, operative time, amount of intraoperative blood loss, and transfusion requirements were closely related to the number of levels fused for patients having revision posterior lumbar spine decompression, fusion, and segmental instrumentation. The arthrodesis rate also decreased with number of levels fused.36 Exact figures of fusion rates with number of levels fused vary according to the disease or condition in question, the approach, and whether the fusion was augmented with bone growth enhancers. In a meta-analysis study which assessed data obtained from fusions done over the past 20 years, the mean fusion rates were 89% in one-level operations, 69% in two-level operations, and 71% when three or more levels were fused.1
Graft source
Allograft bone can be procured in greater quantities than autografts. Allografts are incorporated more slowly and to a lesser degree than autografts. Fresh-frozen grafts are stronger, more immunogenic, and more completely incorporated than freeze-dried grafts. In most studies, fusion using autograft bone leads to higher fusion rates than fusion, using allograft or synthetic bone substitutes alone. In a selected comparison, uninstrumented interbody fusion using autograft was compared with uninstrumented interbody fusion using autograft/allograft mixtures. The fusion rates were 88% and 82%, respectively, and were not statistically different.37 If used anteriorly, allografts are well suited for reconstructive procedures and have good fusion rates, especially if combined with posterior fusions. When used with BMP-2, the effectiveness of an allograft can be increased. In one study, patients undergoing anterior lumbar fusion surgery with structural threaded cortical allograft bone dowels showed higher rates of fusion, improved neurologic status, and lower back and leg pain when compared with the autologous control group.38 Considering the possible complications associated with harvesting iliac crest bone, the use of allogenic bone appears justified.39,40
Graft morphology
The size of a structural bone graft, its positioning, and elasticity should be considered when used for lumbar fusions. A larger graft with low stiffness should be favored from a mechanical point of view. Stiff bone grafts increase stresses on adjacent endplates.41 Placement of a bone graft in a more anterior location can better resist flexion moments and decreases the tensile forces acting on the posterior ligaments. Bone grafts placed at a posterior site are better in resisting torsional moments and decreasing contact force of the facet joint in the fused segment.42 The degree to which these aforementioned observations may influence the long-term clinical outcome of spinal fusion is unclear.
Bone morphogenic proteins
The osteoinductive potentials of bone morphogenic proteins have been well described. Recombinant human BMP-2 has been approved by the FDA for single-level interbody fusions of the lumbar spine. In the first prospective, multicenter study involving 279 patients with degenerative lumbar disc, the fusion rate at 24 months was higher for the BMP-treated group compared with those who received autogenous iliac-crest bone graft (94.5% versus 88.7%).43 Clinical trials have shown that rhBMP-2-filled fusion cages can eliminate the need for harvesting iliac crest graft.44,45 The benefits of BMPs in other applications of spinal fusion are currently under study.
Interbody cages
Intervertebral cages or spacers were developed to prevent the collapse and pseudoarthrosis seen with bone-only interbody fusions. Initially, the early types consisted of vertically placed titanium mesh cylinders and rectangular carbon fiber cages. Later, this was followed by the development of stand-alone threaded Bagby and Kuslich (BAK) intervertebral cages. Threaded intervertebral cages usually stabilize a segment through distraction and tensioning of the annular and ligamentous structures. By partially reaming the endplates, one may expose cancellous bone for arthrodesis, increasing the likelihood for successful fusion.46 Anteriorly placed threaded cages significantly stabilize the motion segment in all directions except extension. With posteriorly placed cages, there is less stability as a result of the facetectomy required for placement of the device. Interbody cages may be implanted via a variety of surgical approaches to the disc space, with or without supplemental posterior fixation.
For single-level degenerative disc disease, anteriorly placed intervertebral threaded cages have shown a high degree of clinical success.47 Both patient selection and technical expertise seem equally important in determining patient outcome.47 Patients with multilevel disease, normal (tall) looking disc on radiographs, or with osteoporotic bone, have a higher incidence of complication and failure. Posteriorly or transforaminally placed cages which are supplemented by pedicle screws seem successful for the treatment of spondylolisthesis.47 For the management of pure discogenic pain without collapse, multilevel disc disease, or disc degeneration in elderly patients, the use of cages is less certain.47
Electrical stimulation
Internal devices utilize DCES. Their mechanism of action is thought to involve the attraction of charged proteins, growth factors, and bone-forming cells to the fusion site. They may also alter the function of voltage-gated channels with activation of cyclic AMP and triggering of a second messenger cascade. Other proposed mechanisms include Faradic reactions at the cathode–bone graft interface with formation of OH and H2O2. This reduces the local oxygen tension (PO2) and slightly increases the pH. The end result of both the electric field effects and the Faradic products is a stimulation of calcium uptake.48–50 Increased pH stimulates osteoblastic bone formation and mineralization but inhibits osteoclastic bone resorption. The end result is an increased rate of new bone formation.51
In contrast to DCES, PEMF devices generate an electromagnetic field across the spinal fusion area and are worn externally, usually 3–8 hours per day for 3–6 months. As these braces can be removed by patients, noncompliance can potentially hinder treatment.52 Successful arthrodesis can also be obtained with shorter application times. In a prospective study by Linovitz and coworkers, successful outcomes for one-level or two-level fusions (between L3 and S1) without instrumentation, either with autograft alone or in combination with allograft, were obtained when the combined magnetic field device was worn for 30 minutes per day for 9 months.53 Such devices with shorter wearing times may have better patient acceptance.
The mechanism of action of PEMF is not well understood, but is thought to involve alterations in cell membrane potentials and parahormone receptors of bone cells. An increase in calcium influx into bone cells, promotion of calcification, and vascularization also occurs with PEMF. PEMF may also be capable of altering the production of cytokines, and increasing the expression of bone morphogenic protein (BMP)-2 and BMP-4 mRNA by cells. In ovariectomized rats, PEMF stimulation can decrease local production of tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6) and inhibit osteoclastogenesis.54 A possible effect on prostaglandin E2 (PGE2) levels is also suggested.55 PEMF seems to enhance cellular differentiation and does increase the number of bone-forming cells. This stimulatory effect is greatest during the early stages of bone healing.56 However, there may be some place for PEMF stimulation in the later stages of treatment in cases of nonunion. For example, in patients with symptomatic pseudoarthrosis after lumbar spinal fusion, pulsed electromagnetic field stimulation has been shown to be an effective nonoperative salvage approach to achieving fusion.57 In one study, 67% of patients who were treated with a pulsed electromagnetic field device worn consistently 2 hours a day for at least 90 days achieved fusion. Treatment was equally effective for posterolateral fusions (66%) as with interbody fusions (69%).57
Since 1985 when the first report of the clinical efficacy of PEMF on spinal fusion was published, numerous studies have demonstrated the benefits of electrical stimulation for improving the success rate of spinal fusion surgery.58,59 Not all modes of electrical stimulation are equally effective in promoting successful spinal arthrodesis. Clinical data reveal superiority of DCES to PEMF, particularly when used to enhance posterior spinal fusions. Capacitive coupling seems superior to PEMF, and perhaps DCES as an adjunct to posterior spinal fusion.60
Demineralized bone matrix
Demineralized bone matrix (DBM) is prepared by decalcification of cortical bone. It is less immunogenic than allograft bone graft. The active components consists of several glycoproteins including BMPs. Preclinical investigations on the use of DBM in the spine have shown promising results. However, clinical studies on the effects on DBM on spinal fusion are lacking. In one study, titanium mesh cages and coralline hydroxyapatite combined with DBM were effective for anterior interbody fusion of the lumbar spine when used as part of a rigidly instrumented circumferential fusion.61 DBM was also effective in reducing the amount of autologous bone graft required for successful lumbar spinal fusion.62 Further clinical studies are needed to clearly establish the usefulness of DBM in spinal fusion.
1 Bono CM, Lee CK. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine. 2004;29(4):455-463. discussion Z5
2 White AA, Panjabi M. Clinical biomechanics of the spine. Philadelphia: Lippincott, 1990.
3 Gertzbein SD, Seligman J, Holtby R, et al. Centrode patterns and segmental instability in degenerative disc disease. Spine. 1985;10(3):257-261.
4 Gertzbein SD, Seligman J, Holtby R, et al. Centrode characteristics of the lumbar spine as a function of segmental instability. Clin Orthop. 1986;208:48-51.
5 McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine. 1994;19(15):1741-1744.
6 Parker JW, Lane JR, Karaikovic EE, Gaines RW. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 4½-year series. Spine. 2000;25(9):1157-1170.
7 Einhorn TA, Bonnarens F, Burstein AH. The contributions of dietary protein and minerals to the healing of experimental fractures. A biomechanical study. J Bone Joint Surg [Am]. 1986;68(9):1389-1395.
8 Udupa KN, Gupta LP. The effect of growth hormone and thyroxine in healing of fractures. Indian J Med Res. 1965;53(7):623-628.
9 Schalch DS, Heinrich UE, Draznin B, et al. Role of the liver in regulating somatomedin activity: hormonal effects on the synthesis and release of insulin-like growth factor and its carrier protein by the isolated perfused rat liver. Endocrinology. 1979;104(4):1143-1151.
10 Gallagher JC, Riggs BL, DeLuca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1980;51(6):1359-1364.
11 Pereira RM, Delany AM, Canalis E. Cortisol inhibits the differentiation and apoptosis of osteoblasts in culture. Bone. 2001;28(5):484-490.
12 Hoogendoorn JM, Simmermacher RK, Schellekens PP, et al. [Adverse effects of smoking on healing of bones and soft tissues]. Unfallchirurg. 2002;105(1):76-81.
13 Harvey EJ, Agel J, Selznick HS, et al. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31(9):518-521.
14 Cesar-Neto JB, Duarte PM, Sallum EA, et al. A comparative study on the effect of nicotine administration and cigarette smoke inhalation on bone healing around titanium implants. J Periodontol. 2003;74(10):1454-1459.
15 Nociti FHJr, Cesar NJ, Carvalho MD, et al. Bone density around titanium implants may be influenced by intermittent cigarette smoke inhalation: a histometric study in rats. Int J Oral Maxillofac Implants. 2002;17(3):347-352.
16 Ishikawa SN, Murphy GA, Richardson EG. The effect of cigarette smoking on hindfoot fusions. Foot Ankle Int. 2002;23(11):996-998.
17 Adams CI, Keating JF, Court-Brown CM. Cigarette smoking and open tibial fractures. Injury. 2001;32(1):61-65.
18 Toksvig-Larsen S. Cigarette smoking delays bone healing: a prospective study of 200 patients operated on by the hemicallotasis technique. Acta Orthop Scand. 2004;75(3):347-351.
19 Hilibrand AS, Fye MA, Emery SE, et al. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg [Am]. 2001;83A(5):668-673.
20 Gerstenfeld LC, Thiede M, Seibert K, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21(4):670-675.
21 Ho ML, Chang JK, Wang GJ. Effects of ketorolac on bone repair: A radiographic study in modeled demineralized bone matrix grafted rabbits. Pharmacology. 1998;57(3):148-159.
22 Beck A, Krischak G, Sorg T, et al. Influence of diclofenac (group of nonsteroidal anti-inflammatory drugs) on fracture healing. Arch Orthop Trauma Surg. 2003;123(7):327-332.
23 Riew KD, Long J, Rhee J, et al. Time-dependent inhibitory effects of indomethacin on spinal fusion. J Bone Joint Surg [Am]. 2003;85A(4):632-634.
24 Martin GJJr, Boden SD, Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spinal fusion. Spine. 1999;24(21):2188-2193. discussion 2193–2194
25 Gerstenfeld LC, Einhorn TA. COX inhibitors and their effects on bone healing. Expert Opin Drug Saf. 2004;3(2):131-136.
26 Reuben SS, Ekman EF. The effect of cyclooxygenase-2 inhibition on analgesia and spinal fusion. J Bone Joint Surg [Am]. 2005;87A(3):536-542.
27 Viidik A, Mosekilde L, Quirinia A. [Research on aging: biological perspectives]. Ugeskr Laeger. 1992;154(42):2884-2889.
28 Quarto R, Thomas D, Liang CT. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int. 1995;56(2):123-129.
29 Blumenfeld I, Srouji S, Lanir Y, et al. Enhancement of bone defect healing in old rats by TGF-beta and IGF-1. Exp Gerontol. 2002;37(4):553-565.
30 Sumner DR, Turner TM, Cohen M, et al. Aging does not lessen the effectiveness of TGFbeta2-enhanced bone regeneration. J Bone Miner Res. 2003;18(4):730-736.
31 Tunturi T, Kataja M, Keski-Nisula L, et al. Posterior fusion of the lumbosacral spine. Evaluation of the operative results and the factors influencing them. Acta Orthop Scand. 1979;50(4):415-425.
32 Fritzell P, Hagg O, Wessberg P, et al. 2001 Volvo Award Winner in clinical studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26(23):2521-2532. discussion 2532–2534
33 Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22(24):2807-2812.
34 Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results. Spine. 1993;18(8):983-991.
35 Thomsen K, Christensen FB, Eiskjaer SP, et al. 1997 Volvo Award winner in clinical studies. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine. 1997;22(24):2813-2822.
36 Zheng F, Cammisa FPJr, Sandhu HS, et al. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine. 2002;27(8):818-824.
37 Urist MR, Dawson E. Intertransverse process fusion with the aid of chemosterilized autolyzed antigen-extracted allogeneic (AAA) bone. Clin Orthop. 1981;154:97-113.
38 Burkus JK, Transfeldt EE, Kitchel SH, et al. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27(21):2396-2408.
39 Ehrler DM, Vaccaro AR. The use of allograft bone in lumbar spine surgery. Clin Orthop. 2000;371:38-45.
40 Wimmer C, Krismer M, Gluch H, et al. Autogenic versus allogenic bone grafts in anterior lumbar interbody fusion. Clin Orthop. 1999;360:122-126.
41 Cheng CK, Chen CS, Liu CL. Biomechanical analysis of the lumbar spine with anterior interbody fusion on the different locations of the bone grafts. Biomed Mater Eng. 2002;12(4):367-674.
42 Zander T, Rohlmann A, Klockner C, et al. Effect of bone graft characteristics on the mechanical behavior of the lumbar spine. J Biomech. 2002;35(4):491-497.
43 Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15(5):337-349.
44 Haid RWJr, Branch CLJr, Alexander JT, et al. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4(5):527-538. discussion 538–539
45 Mummaneni PV, Pan J, Haid RW, et al. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1(1):19-23.
46 Sasso RC, Kitchel SH, Dawson EG. A prospective, randomized controlled clinical trial of anterior lumbar interbody fusion using a titanium cylindrical threaded fusion device. Spine. 2004;29(2):113-122. discussion 121–122
47 Zdeblick TA, Phillips FM. Interbody cage devices. Spine. 2003;28(15 Suppl):S2-S7.
48 Baranowski TJJr, Black J, Brighton CT, Friedenberg ZB. Electrical osteogenesis by low direct current. J Orthop Res. 1983;1(2):120-128.
49 Brighton CT, Friedenberg ZB, Black J, et al. Electrically induced osteogenesis: relationship between charge, current density, and the amount of bone formed: introduction of a new cathode concept. Clin Orthop. 1981;161:122-132.
50 Brighton CT, Friedenberg ZB. Electrical stimulation and oxygen tension. Ann NY Acad Sci. 1974;238:314-320.
51 Oishi M, Onesti ST. Electrical bone graft stimulation for spinal fusion: a review. Neurosurgery. 2000;47(5):1041-1055. discussion 1055–1056
52 Mooney ML, Carlson P, Szentpetery S, et al. A prospective study of the clinical utility of lymphocyte monitoring in the cardiac transplant recipient. Transplantation. 1990;50(6):951-954.
53 Linovitz RJ, Pathria M, Bernhardt M, et al. Combined magnetic fields accelerate and increase spinal fusion: a double-blind, randomized, placebo controlled study. Spine. 2002;27(13):1383-1389. discussion 1389
54 Chang K, Hong-Shong Chang W, Yu YH, et al. Pulsed electromagnetic field stimulation of bone marrow cells derived from ovariectomized rats affects osteoclast formation and local factor production. Bioelectromagnetics. 2004;25(2):134-141.
55 Chang K, Chang WH. Pulsed electromagnetic fields prevent osteoporosis in an ovariectomized female rat model: a prostaglandin E2-associated process. Bioelectromagnetics. 2003;24(3):189-198.
56 Diniz P, Shomura K, Soejima K, et al. Effects of pulsed electromagnetic field (PEMF) stimulation on bone tissue-like formation are dependent on the maturation stages of the osteoblasts. Bioelectromagnetics. 2002;23(5):398-405.
57 Simmons JWJr, Mooney V, Thacker I. Pseudoarthrosis after lumbar spine fusion: nonoperative salvage with pulsed electromagnetic fields. Am J Orthop. 2004;33(1):27-30.
58 Mooney V. A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine. 1990;15(7):708-712.
59 Mooney V, McDermott KL, Song J. Effects of smoking and maturation on long-term maintenance of lumbar spinal fusion success. J Spinal Disord. 1999;12(5):380-385.
60 Kahanovitz N. Electrical stimulation of spinal fusion: a scientific and clinical update. Spine J. 2002;2(2):145-150.
61 Thalgott JS, Giuffre JM, Klezl Z, et al. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J. 2002;2(1):63-69.
62 Girardi FP, Cammisa FPJr. The effect of bone graft extenders to enhance the performance of iliac crest bone grafts in instrumented lumbar spine fusion. Orthopedics. 2003;26(5 Suppl):s545-s548.