CHAPTER 17 Fusion for Degenerative Arthritis of the Ankle
Unlike hip and knee arthritis, primary ankle osteoarthritis is rare, and most degenerative ankle arthritis is post-traumatic in nature. Autopsy studies demonstrate that degenerative changes are about three times more prevalent in the knee than the ankle and that degenerative changes in both joints increase with increasing age.1,2 Radiographic studies that aim to quantify the prevalence of ankle osteoarthritis are of limited value because of the low correlation between the identification of osteophytes on plain films and the development of symptomatic osteoarthritis.3 Clinical studies suggest that knee and hip osteoarthritis is 8 to 10 times more common than ankle osteoarthritis.1,4
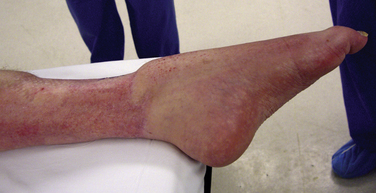
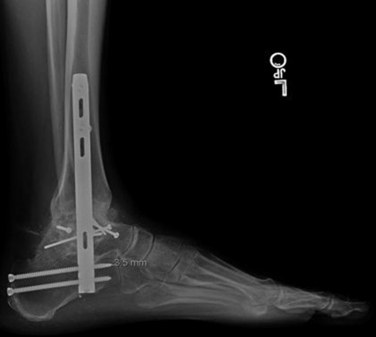
Because ankle arthritis is primarily post-traumatic, it affects a younger and more active patient population than hip and knee osteoarthritis. This fact demands a durable surgical option when nonoperative interventions have failed. The use of total ankle replacement is evolving, and new designs are being developed, but the gold standard for most cases of end-stage ankle arthritis has been open ankle arthrodesis. During the past 2 decades, arthroscopic ankle arthrodesis has become a viable alternative to the open procedure and has shown encouraging results.5–8 Proposed advantages of arthroscopic techniques are less postoperative pain and morbidity, decreased blood loss, and shorter hospital stay. One important advantage of arthroscopic ankle arthrodesis is that it can be performed in patients with a poor soft tissue envelope (Fig. 17-1).
ANATOMY AND PATHOGENESIS
The ankle is relatively resistant to primary degenerative osteoarthritis, possibly because of the properties of ankle cartilage, including relatively better retention of tensile fracture stress and tensile stiffness with age, as described by Kempson and colleagues.9 The cartilage in the ankle is metabolically different from that in the knee. Ankle cartilage appears to be less affected by the catabolic cytokine interleukin 1 (IL-1) and deleterious collagenases that are produced in response to IL-1.10
Secondary osteoarthritis of the ankle can develop after fracture or ligamentous injury. Rotational ankle fractures and ligamentous injury with recurrent instability are the most common causes.11–15 In Saltzman’s practice during 13 years, 445 (70%) of 639 patients with Kellgren-Lawrence grade 3 or 4 ankle arthritis were post-traumatic cases, and only 46 patients (7.2%) had primary oseteoarthritis.13 Other recorded causes in this study of ankle arthritis included neuropathic disease (e.g., Charcot neuroarthropathy), inflammatory arthropathies (e.g., rheumatoid arthritis), crystalline arthropathies (e.g., pseudogout), osteochondritis, osseous necrosis, and postinfectious arthropathy.
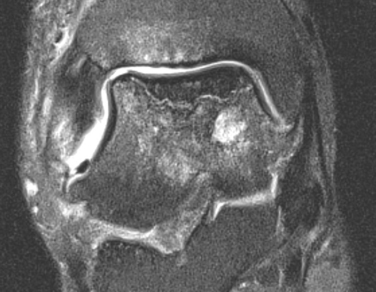
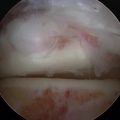
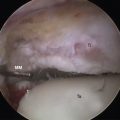
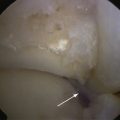
The relative resistance of the ankle joint to primary osteoarthritis is likely a combination of its congruency, which results in inherent stability and restrained motion; unique tensile properties; and distinctive metabolic characteristics. Unfortunately, the ankle is quite susceptible to post-traumatic arthritis, and this may be related to its thinner and stiffer articular cartilage not being able to accommodate articular step-offs or the stresses of improperly constrained motion. Step-offs lead to increased local contact stresses that the thin cartilage of the ankle may not be able to accommodate as well as the thicker cartilage in the hip and knee.16,17 These increased localized contact stresses likely contribute to the degeneration of articular cartilage that is seen after trauma. Other disease processes, such as Charcot arthropathy and osteochondritis, with large osteochondral defects can lead to step-offs or incongruity in the articular surface and result in increased contact stresses (Figs. 17-2 and 17-3).
PATIENT EVALUATION
History and Physical Examination
The first step in evaluating the patient with ankle pain is to obtain a proper history, particularly any history of trauma. One major ankle sprain can result in a significant injury to ankle joint cartilage or persistent instability, with the eventual development of degenerative changes.13 Inquiring about other joint pain is important because multiple joint involvement may indicate systemic causes, such as inflammatory arthropathy. Ankle arthritis is not usually the first manifestation of a systemic disease process. Ascertaining a diabetic history is important, because diabetes is a risk factor for Charcot neuroarthropathy and related to perioperative morbidity in those with end-stage organ disease.
The seated examination includes evaluation of range of motion in the ankle, hindfoot, midfoot, and forefoot. Ankle stability should be assessed by drawer testing, with the foot in plantar flexion and neutral position, which investigates the competence of the anterior talofibular ligament and calcaneofibular ligament, respectively. Talar tilt should be assessed. Foot alignment is important because deformity in the foot may cause secondary ankle disease. For example, pes planus with medial column instability may be associated with secondary ankle valgus and eventual degenerative change. Conversely, realignment of a deformed ankle can alter the foot position and adversely or positively affect function of other joints, particularly the subtalar joint. If compensatory foot deformities are identified on examination, their passive, manual correctability has to be assessed.
Diagnostic Imaging
Radiographs should be done weight bearing if possible. The four radiographic views we use in our clinic to evaluate ankle pain include the anteroposterior, lateral, mortise, and hindfoot alignment views. Radiographs of the degenerative ankle can show joint space narrowing, osteophyte formation, subchondral sclerosis, and subchondral cysts. The hindfoot alignment view is important in the evaluation hindfoot varus or valgus and ankle deformity in the coronal plane.18 It is taken with the patient standing on a platform facing a collector that angles away from the platform at 20 degrees. The x-ray tube is posterior to the ankle, with the beam perpendicular to the plane of the film at the level of the ankle. The source-to-collector distance is 40 inches. On average, the most inferior aspect of the calcaneus lies just medial to the longitudinal midaxis of the tibia (Fig. 17-4). Magnetic resonance imaging (MRI) has limited usefulness unless the examiner suspects an osteochondral lesion of the talus, osseous necrosis, or ligamentous abnormality that will alter patient care. In such cases, MR arthrography may be advantageous. Computed tomography (CT) is the better choice for three-dimensional bony imaging, and it allows visualization near hardware, unlike MRI. Noninvasive joint distraction plus air-contrast arthrography enhances visualization of the ankle’s articular features.19
Selective fluoroscopically guided injections can be helpful in diagnosing patients who have clinical or radiographic findings that suggest more than one source of pain. It is reasonable to expect 75% pain relief in an area that is injected.20 It is important to identify the patients’ ankle pain as global (i.e., affecting most of the joint) or focal (i.e., affecting a specific region), because this distinction may guide the treatment options. It is essential to identify patients with coexisting subtalar pain, because that population needs to be counseled more intensely about the risks of residual pain and progression of adjacent joint arthritis.
TREATMENT
Nonoperative and operative treatments can help reduce symptoms and improve the function of painful ankle arthritis. There are no well-designed retrospective or prospective clinical trials reporting on nonoperative treatment of diffuse ankle arthritis. Our experience is that nonsteroidal anti-inflammatory drugs have variable efficacy in addressing the pain of ankle arthritis. The judicious use of corticosteroid injections may provide temporary relief and be beneficial in acute exacerbations in someone who has tolerable steady-state pain. Clinical trials suggest that hyaluronate-based injectables may diminish ankle pain and improve function in patients with ankle osteoarthritis, and additional trials exploring this treatment modality are being conducted.21,22
Nonoperative interventions primarily focus on mechanical unloading and immobilization. Devices that address this goal include the cane, ankle foot orthoses (AFOs), and the leather ankle lacer with an embedded polypropylene shell.23 To fully unload the ankle joint requires a rigid linkage between the acceptance of force on the sole of the foot and the leg above the ankle. The prototypic device, a weight-bearing patellar brace (PTB), is poorly tolerated because load transfer irritates the soft tissues in front of the knee. Adding a rocker-bottom sole to a shoe or the use of a solid ankle-cushioned heel (SACH) may also provide relief by reducing ankle excursion with gait.
Operative intervention should be considered only after failure of nonoperative treatment methods. The surgeon should identify the cause of the patient’s problem and to what extent ligamentous instability, malalignment, or other foot deformity contributes to the perceived pain from ankle arthritis. When planning surgical interventions, recreating normal foot alignment can encourage improved foot function, regardless of the chosen surgical technique. Surgical options for end-stage degenerative ankle arthritis include osteotomies about the ankle, débridement, distraction, ankle arthroplasty, and ankle arthrodesis. Ankle arthrodesis can be performed in a variety of ways, including open, mini-open, and arthroscopic techniques.
In terms of arthroscopic ankle arthrodesis, the indications remain the same, with the exception of a failed total ankle replacement. Well-aligned ankles and those that are easily realigned are excellent candidates for arthroscopic fusion. Patients with soft tissue compromise (e.g., those with prior trauma, burn victims, patients with skin grafts) or vasculopathy are strongly considered for an arthroscopic approach. In the past, it was thought that ankle varus or valgus greater than 5 degrees was an absolute contraindication to arthroscopic arthrodesis. However, later reports suggested that substantial ankle varus or valgus is a relative contraindication rather than an absolute one.24,25 The investigators consider any ankle that can be re-aligned properly after arthroscopic débridement appropriate but acknowledge that patients should be counseled that conversion to an open approach is prudent if an extensive capsulotomy is required to achieve correct alignment. Additional contraindications for the arthroscopic procedure are significant focal bone loss and deformity and extremely rigid ankles. In general, the desired position of the arthrodesis is neutral dorsiflexion, 5 degrees of ankle valgus, equal or slightly greater external rotation compared with the contralateral leg, and placement of the anterior aspect of the talar dome at the level of the anterior aspect of the tibia.
Arthroscopic ankle arthrodesis was first developed in the mid-1980s. Myerson and Quill reported the first comparative series, with 17 arthroscopic procedures compared with 16 open procedures done with a medial malleolar osteotomy.7 They found an average fusion time of 8.7 weeks in the arthroscopic group and 14.5 weeks in the open group, with similar fusion rates for both groups. Their criteria for clinical union included an absence of tibiotalar motion, crepitus, or pain with ambulation or on examination. Radiographic union was defined as the appearance of osseous trabeculae across the tibiotalar arthrodesis site and incorporation of bone graft into the fusion mass when bone graft was used.
One of the first long-term series was published in 1996 by Glick and colleagues.6 They reported 34 cases with an average follow-up of 8 years. Fusion rates were 97%, and good or excellent results were reported for 86% of patients. Three ankles in this series were rated poor because of subtalar pain and a nonunion, and a malunion accounted for the other poor results.
O’Brien and colleagues added support to arthroscopic arthrodesis by showing comparable fusion rates between 19 arthroscopically treated patients and 17 patients undergoing open arthrodesis using flat cuts.8 The arthroscopic group had decreased operative times, decreased tourniquet times, less blood loss, and decreased hospital stays.
Case 1
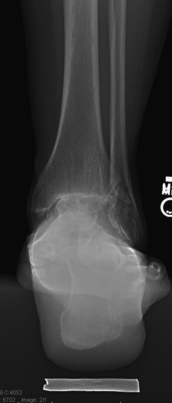
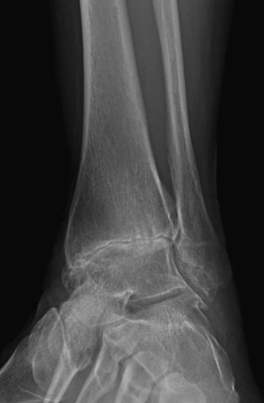
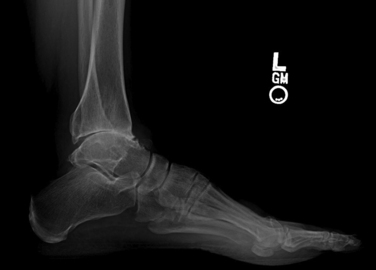
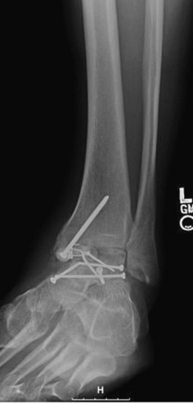
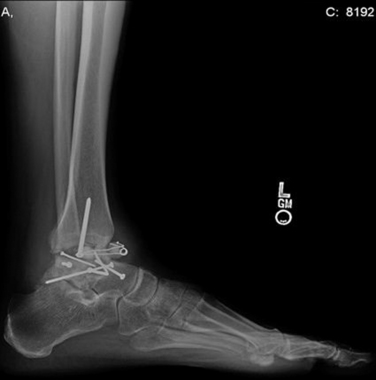
On physical examination she had mild hindfoot varus and an ankle arc of motion of 15 degrees. She had a small ankle effusion, and palpation elicited tenderness along the medial, anterior, and lateral joint lines. The subtalar joint was not tender to palpation and had normal motion. The result of the anterior drawer test was negative. She was neurovascularly intact. A hindfoot alignment radiograph showed minimal varus (see Fig. 17-4). Anteroposterior, lateral, and mortise views showed loss of tibiotalar joint space and osteophyte formation (Fig. 17-5). The subtalar joint was preserved (Fig. 17-6).
Anterior Arthroscopic Ankle Arthrodesis
Room Setup
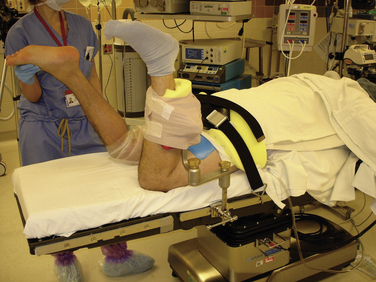
The bed is placed in a beach chair position and then in slight Trendelenburg so the operative leg is nearly parallel to the floor. The heel is positioned just off the edge of the table. The break in the bed should be positioned at the knee, so that when the end of the table is dropped, traction can be applied to the limb without moving the patient. This reflexed position provides sufficient resistance against the thigh to allow for distraction when traction is applied (Fig. 17-7). Preparing above the knee allows for assessment of rotational alignment. An alternative is to use a well-leg holder against the posterior thigh of the operative limb. We mark landmarks, including malleoli, branches of the superficial peroneal nerve, and the expected level of the tibiotalar joint space.
Joint Preparation
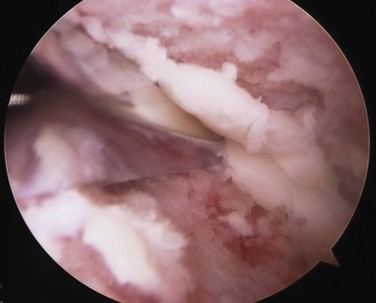
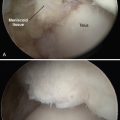
Any residual cartilage on the tibia and talus are removed with the shaver or curettes (Video 1). Having a set of thin-handled, angled curettes is helpful. If the lateral gutter does not show significant wear, the cartilage can be left in this location, and the fibula is excluded from the fusion. However, if there are degenerative changes in this region, we remove all residual cartilage and incorporate the fibula into the fusion.
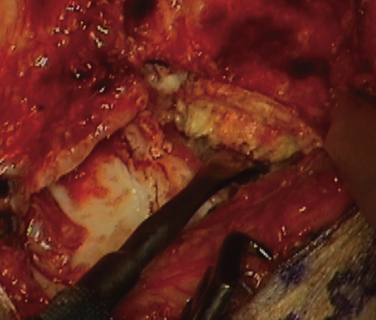
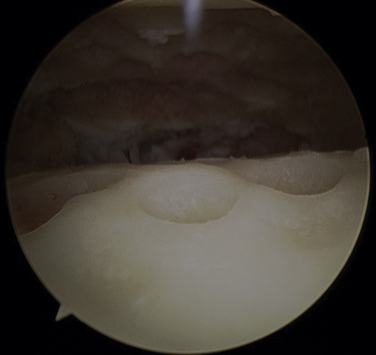
After all the cartilage is removed, a 3- to 4-mm burr is used to pockmark the subchondral surface so it looks like the surface of a golf ball (Fig. 17-8). It is crucial to do this across the entire undersurface of the tibia and the anterior two thirds of the talar dome. Distraction helps us to preserve the natural contours of the ankle joint. If the joint is unable to be débrided because it is too tight, the incision can be extended and a laminar spreader placed into the joint to facilitate cartilage removal to perform a mini-open procedure.26 Adequacy of débridement is then determined by deflating the tourniquet, turning off the pump, and inspecting the joint for sufficient punctate bleeding.
Fixation
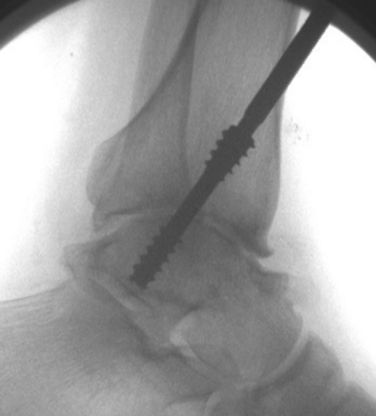
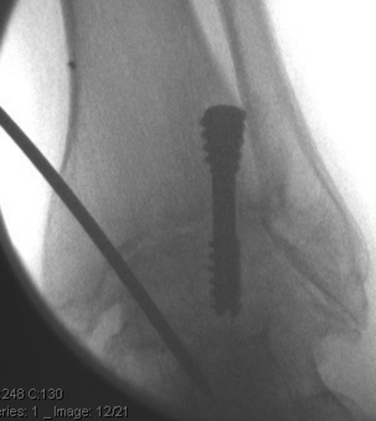
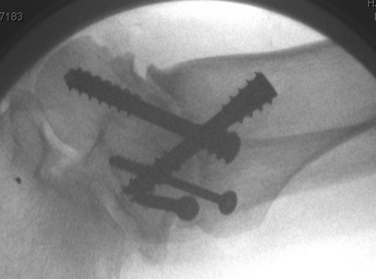
The first pin is placed through a 2-cm incision placed on the anterior leg about 5 cm above the joint. This incision needs to be long enough to ensure that neither the superficial nor deep peroneal structures are injured by screw placement. The pin is placed on the anterolateral part of the tibia starting 3 cm above the joint, just superior and medial to Chaput’s tubercle, and it is directed 10 to 20 degrees posterior from vertical into the posterior half of the talar dome. Lateral and anteroposterior views are needed to confirm pin placement before a large, cannulated, partially threaded compression screw (usually 6.5 mm) is used to pull the talus up into the mortise (Fig. 17-9).
A second screw is placed from a posteromedial position in the tibia to an anterocentral position in the talus (Fig. 17-10). The leg is placed in a figure-of-four position, and a 1-cm incision is made along the posteromedial edge of the tibia 3 cm above the joint line. Blunt dissection and retraction get the pin down to the bone. The pin should start at least 4 mm anterior to the sheath of the posterior tibial tendon and traverse the posteromedial corner of the ankle, coursing into the center of the neck of the talus (Fig. 17-11). Direct visualization of the posterior tibial tendon sheath is recommended to prevent injury to the tendon. After the guide pin is overdrilled, a partially threaded, large, cannulated screw is placed, which may slightly compress the construct back into the posteromedial corner.
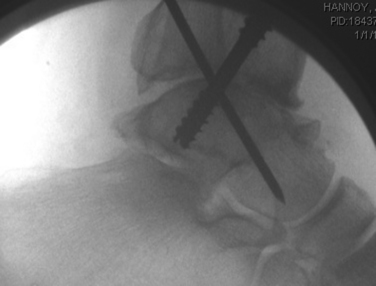
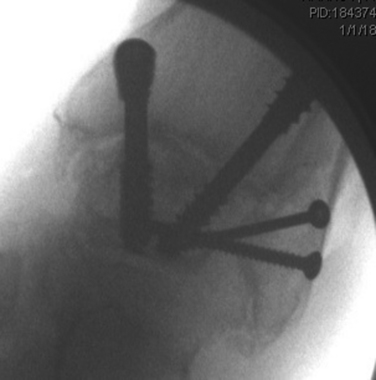
If the lateral gutter (fibulotalar articulation) has no significant arthritis, it does not need to be débrided or fixed. However, if the fibulotalar joint is arthritic, a third screw is placed from a posterolateral position in the fibula to an inferocentral position in the talar neck and body. We often downsize the width of this screw to avoid cracking the fibula and add accessory screws directed from the fibula to the talar dome (Figs. 17-12 and 17-13). Another consideration is to perform a transverse osteotomy of the fibula a few centimeters proximal to the joint line to decouple forces from the proximal fibula to the distal fibular fragment. This may enhance fusion of the syndesmotic region, and the distal fibular fragment can be secured with the same screw arrangement described earlier.
Small gaps usually exist between the bony surfaces after fixation. If the lateral malleolus is included in the fusion, there is often a small gap laterally because the medial screw may pull the talus medially. We inject about 5 to 10 cc of demineralized bone matrix into these small gaps through our portals. Alternatively, the demineralized bone matrix can be placed after the guide pins are placed, but it should be done before final screw placement.
Postoperative Care
The ankle is placed in a well-padded posterior splint. The splint and sutures are removed after 10 to 14 days, and a below-knee cast is placed. We allow patients 5 to 10 pounds of heel weight bearing so they can maintain their balance. Others allow full weight bearing at this point.27 Our group has no experience with early weight bearing, and we remain concerned about increasing the rate of nonunion.
Case 2
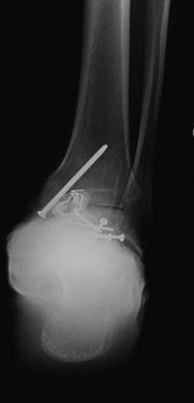
The patient had much ankle pain and hindfoot pain. Examination revealed that he had three healed incisions about the ankle. It was diffusely tender to palpation, and the ankle and subtalar range of motion was limited. Radiographs and CT scans demonstrated osseous necrosis of the medial half of the talus, including the dome and the body, and arthrosis of the tibiotalar and subtalar joints (Figs. 17-14 and 17-15). The patient’s overall alignment was well preserved (Fig. 17-16). Because the patient had numerous prior incisions, there was concern that his soft tissue envelope might be compromised. He was considered for a combined prone arthroscopic ankle and subtalar arthrodesis.
Combined Posterior Arthroscopic and Subtalar Arthrodesis
Room Setup
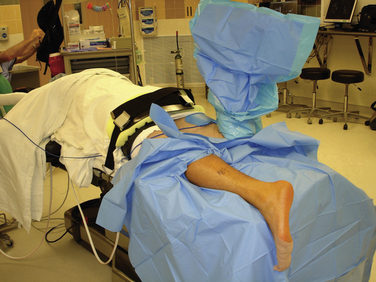
The patient is positioned in the prone position, with the involved ankle placed approximately 10 cm distal to end of the table. We sling the safety strap posterior and inferior to the buttocks to resist the force of distraction. General anesthesia and a regional nerve block are used. Instruments include a custom distraction device; a 2.7-mm, 30-degree angled arthroscope; a larger, 4.0-mm arthroscope; thin periosteal elevators; long and thin-handled curettes; aggressive shavers; and a 4.0 mm burr. Before standard preparation and draping, the nonoperative limb is flexed to about 90 degrees at the knee and held in this position for the duration of the surgery (Figs. 17-17 and 17-18). This allows full access to the operative limb. On the operative side, the Achilles tendon, the medial and lateral malleoli, the sural nerve, the posteromedial neurovascular bundle, and the estimated levels of the tibiotalar and subtalar joints are marked with a surgical pen.
Joint Preparation
If necessary, a third portal is established approximately 1 cm proximal and 1 cm posterior to the tip of the lateral malleolus. The sural nerve is at risk, and caution is required when inserting instruments. This portal gives greater access to the posterior facet of the subtalar joint. Most of the procedure is done with the arthroscope in the posterolateral portal and the instruments in the posteromedial portal, although both portals are used in an alternating fashion for viewing and for instrumentation. Arthroscopic cannulas may be used.
Synovectomy is performed first with the shaver to improve visualization. The residual articular cartilage of the entire posterior facet is then removed with a thin, curved periosteal elevator, curettes, and an aggressive shaver (Fig. 17-19). Visualization of the interosseous ligament signifies the anterior extent of the débridement, and by staying posterior to this structure, the surgeon avoids the sinus tarsi vasculature. The bone surfaces are then pockmarked with the burr, as described for the ankle arthrodesis. It is best to preserve the overall congruency of the joint during the débridement and pockmarking to get good opposition of the joint during fixation.
Fixation
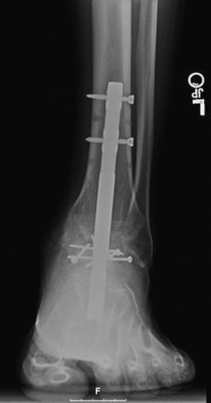
Another option for fixation is a tibiotalocalcaneal fusion nail. After the joints are débrided and alignment verified, the nail can be placed with fluoroscopic guidance. We prefer a transverse incision in the heel, and care is taken to avoid the plantar nerves. Typically, the incision is placed mostly on the lateral half of the heel fat pad at the junction between the middle and distal thirds. Fluoroscopic confirmation is essential. The dissection down to the calcaneus is done bluntly, and retractors are used to prevent injury to the local neurovascular structures. The nail is inserted with reaming. The construct is compressed before final interlocking screws are placed (Figs. 17-20 and 17-21). Postoperative instructions are the same as for the ankle fusion described previously.
CLINICAL RESULTS
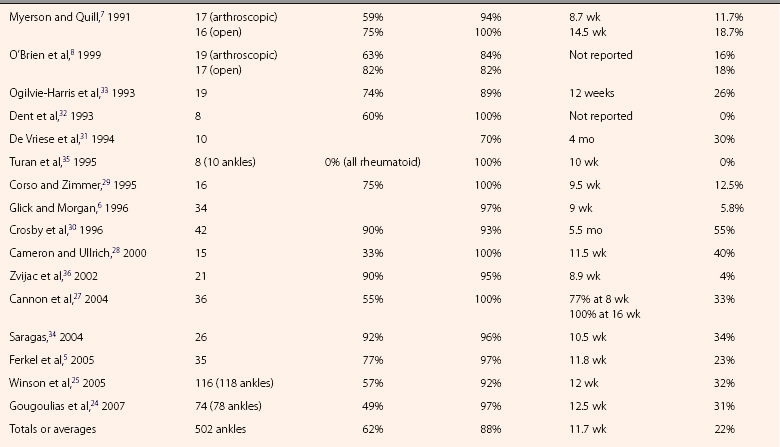
Many clinical series published since 1990 have reported the outcomes of arthroscopic ankle arthrodesis. All the studies are retrospective in nature, and only a few have compared the outcomes of arthrodesis with those of open treatment. Myerson and Quill published the first comparison of arthroscopic with open techniques.7 Seventeen patients were treated with arthroscopic ankle arthrodesis, and 16 patients were treated with an open technique (i.e., medial malleolar osteotomy). The investigators found that the arthroscopic group had a shorter average time to fusion (8.7 vs. 14.5 weeks) and a much shorter hospital stay (1.5 vs. 4 days). The arthroscopic group had one pseudoarthrosis, for a fusion rate of 94.1%,compared with 100% in the open group. However, this study was not controlled, and the two groups were dissimilar, with the open group having patients with greater deformity and bone loss.
O’Brien and colleagues attempted to devise a better study by having two comparable groups in regard to the amount of deformity.8 Nineteen patients were treated arthroscopically, and 17 were treated with open arthrodesis with flat cuts. The investigators reported an 84% fusion rate for the arthroscopic group and an 82% rate for the open group. They found the arthroscopic group had a shorter operative time by 12 minutes (166 vs. 184 minutes) and a significantly shorter hospital stay (1.6 vs. 3.4 days). The complication rate was similar for both groups.
The following results are compiled from an additional 13 clinical series (Table 17-1). Reported fusion rates range from 70% to 100%.5,6,24,25,27-36 Five studies reported 100% fusion rates, and the other eight studies had rates ranging from 89% to 97%, with only one study reporting a fusion rate below 89%.36 A common definition for fusion is an ankle that is clinically stable on examination and pain free on weight bearing, and it has radiographic signs of bridging trabeculae. Five studies reported mean fusion times of less than 10.5 weeks, with the fastest mean time to fusion reported at 8.9 weeks.6,29,34–36 The other studies reported mean fusion times ranging from 11 to 16 weeks.
Five of the series reported the clinical outcomes of patients, with good or excellent results for 80% to 95% of patients and with follow-up ranging from 14 months to 18 years.5,6,25,33,36 In the largest of the studies, Winson and colleagues reported good or excellent results for 83 (80%) of 104 patients at an average follow-up of 5.4 years.25 Other studies have reported satisfaction rates of 95% to 100%.34,35
No studies have reported on arthroscopic tibiotalocalcaneal fusions. However, Amendola and colleagues described the technique and early results of posterior arthroscopic subtalar arthrodesis (PASTA).37 They reported 11 feet in 10 patients that underwent PASTA. Ten of the 11 joints fused by 10 weeks, with one patient going on to nonunion. Eight patients were very satisfied with the results, one was satisfied, and one patient was not satisfied with the results of subtalar fusion. The patients demonstrated a 50-point improvement in their modified American Orthopaedic Foot and Ankle Society (AOFAS) score (i.e., 36 to 86 points). Other than the one nonunion, there were no postoperative complications.
COMPLICATIONS
Complications with the arthroscopic procedure usually compare favorably with those reported with open techniques. Complications rates in the literature range from 0% to 55%. However, prominent or painful hardware is responsible for many of the reported complications that eventually result in additional procedures. In an early study that focused on complications in arthroscopic ankle fusion, Crosby and associates reported an overall complication rate of 55% in a series of 42 patients. There were three (7%) nonunions, two (5.1%) delayed unions, two stress fractures at the tibial pin sites used for distraction, five infections (i.e., four superficial infections at the sites pins were used and one deep infection), and six (14%) cases of painful hardware (four patients elected to have the screws removed), and four patients went on to have painful subtalar arthritis.30 The amount of subsequent subtalar arthritis is similar to that reported in other studies.24,25,28 Other reported complications include cutaneous nerve injury,29,33 deep peroneal nerve palsy,27 malunion, dorsalis pedis pseudoaneurysm,6 and deep venous thrombosis with nonfatal pulmonary embolism.25
CONCLUSIONS
Reported series of arthroscopic ankle arthrodesis usually have comparable or improved rates of fusion, patient satisfaction, and adverse outcomes compared with the results of traditional open fusion techniques. It is an excellent option for patients with minimal deformity in the coronal plane and those with a compromised soft tissue envelope. Postoperative morbidity is less than with an open approach, patients spend less time in the hospital after treatment, and patient satisfaction is high. For surgeons with the proper skills, arthroscopic ankle arthrodesis is a good option for treating patients with end-stage ankle degenerative disease. Arthroscopic tibiotalocalcaneal fusion from a posterior approach is a promising procedure for patients with ankle and subtalar degenerative disease, but additional investigation is required before firm conclusions can be drawn regarding its efficacy.
1. Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667-674.
2. Muehleman C, Bareither D, Huch K, et al. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis Cartilage. 1997;5:23-37.
3. van der Schoot DK, Den Outer AJ, Bode PJ, et al. Degenerative changes at the knee and ankle related to malunion of tibial fractures. 15-year follow-up of 88 patients, J Bone Joint Surg Br. 781996 722-725.
4. Cushnaghan J, Dieppe P. Study of 500 patients with limb joint osteoarthritis. I. Analysis by age, sex, and distribution of symptomatic joint sites. Ann Rheum Dis. 1991;50:8-13.
5. Ferkel RD, Hewitt M. Long-term results of arthroscopic ankle arthrodesis. Foot Ankle Int. 2005;26:275-280.
6. Glick JM, Morgan CD, Myerson MS, et al. Ankle arthrodesis using an arthroscopic method. long-term follow-up of 34 cases, Arthroscopy. 121996 428-434.
7. Myerson MS, Quill G. Ankle arthrodesis. A comparison of an arthroscopic and an open method of treatment. Clin Orthop Relat Res. 1991;268:84-95.
8. O’Brien TS, Hart TS, Shereff MJ, et al. Open versus arthroscopic ankle arthrodesis. a comparative study, Foot Ankle Int. 201999 368-374.
9. Kempson GE. Age-related changes in the tensile properties of human articular cartilage. a comparative study between the femoral head of the hip joint and the talus of the ankle joint, Biochim Biophys Acta. 10751991 223-230.
10. Chubinskaya S, Huch K, Mikecz K, et al. Chondrocyte matrix metalloproteinase-8. up-regulation of neutrophil collagenase by interleukin-1 beta in human cartilage from knee and ankle joints, Lab Invest. 741996 232-240.
11. Demetriades L, Strauss E, Gallina J. Osteoarthritis of the ankle. Clin Orthop Relat Res. 1998;349:28-42.
12. Harrington KD. Degenerative arthritis of the ankle secondary to long-standing lateral ligament instability. J Bone Joint Surg Am. 1979;61:354-361.
13. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis. report of a consecutive series of 639 patients from a tertiary orthopaedic center, Iowa Orthop J. 252005 44-46.
14. Schafer D, Hintermann B. Arthroscopic assessment of the chronic unstable ankle joint. Knee Surg Sports Traumatol Arthrosc. 1996;41:48-52.
15. Wyss C, Zollinger H. The causes of subsequent arthrodesis of the ankle joint. Acta Orthop Belg. 1991;57(suppl 1):22-27.
16. Ateshian GA, Soslowsky LJ, Mow VC. Quantitation of articular surface topography and cartilage thickness in knee joints using stereophotogrammetry. J Biomech. 1991;24:761-776.
17. Athanasiou KA, Niederauer GG, Schenck RCJr. Biomechanical topography of human ankle cartilage. Ann Biomed Eng. 1995;23:697-704.
18. Saltzman CL, el-Khoury GY. The hindfoot alignment view. Foot Ankle Int. 1995;16:572-576.
19. El-Khoury GY, Alliman KJ, Lundberg HJ, et al. Cartilage thickness in cadaveric ankles. measurement with double-contrast multi-detector row CT arthrography versus MR imaging, Radiology. 2332004 768-773.
20. Khoury NJ, el-Khoury GY, Saltzman CL, Brandser EA. Intraarticular foot and ankle injections to identify source of pain before arthrodesis. AJR Am J Roentgenol. 1996;167:669-673.
21. Carpenter B, Motley T. The role of viscosupplementation in the ankle using hylan G-F 20. J Foot Ankle Surg. 2008;47:377-384.
22. Luciani D, Cadossi M, Tesei F, et al. Viscosupplementation for grade II osteoarthritis of the ankle. a prospective study at 18 months’ follow-up, Chir Organi Mov. 922008 155-160.
23. Saltzman CL, Shurr D, Kamp J, Cook TA. The leather ankle lacer. Iowa Orthop J. 1995;15:204-208.
24. Gougoulias NE, Agathangelidis FG, Parsons SW. Arthroscopic ankle arthrodesis. Foot Ankle Int. 2007;28:695-706.
25. Winson IG, Robinson DE, Allen PE. Arthroscopic ankle arthrodesis. J Bone Joint Surg Br. 2005;87:343-347.
26. Paremain GD, Miller SD, Myerson MS. Ankle arthrodesis. results after the miniarthrotomy technique, Foot Ankle Int. 171996 247-252.
27. Cannon L. Early weight bearing is safe following arthroscopic ankle arthrodesis. Foot Ankle Surg. 2004;10:135-139.
28. Cameron SE, Ullrich P. Arthroscopic arthrodesis of the ankle joint. Arthroscopy. 2000;16:21-26.
29. Corso SJ, Zimmer TJ. Technique and clinical evaluation of arthroscopic ankle arthrodesis. Arthroscopy. 1995;11:585-590.
30. Crosby LA, Yee TC, Formanek TS, Fitzgibbons TC. Complications following arthroscopic ankle arthrodesis. Foot Ankle Int. 1996;17:340-342.
31. De Vriese L, Dereymaeker G, Fabry G. Arthroscopic ankle arthrodesis. Preliminary report. Acta Orthop Belg. 1994;60:389-392.
32. Dent CM, Patil M, Fairclough JA. Arthroscopic ankle arthrodesis. J Bone Joint Surg Br. 1993;75:830-832.
33. Ogilvie-Harris DJ, Lieberman I, Fitsialos D. Arthroscopically assisted arthrodesis for osteoarthritic ankles. J Bone Joint Surg Am. 1993;75:1167-1174.
34. Saragas N. Results of arthroscopic arthrodesis of the ankle. Foot Ankle Surg. 2004;10:141-143.
35. Turan I, Wredmark T, Fellander-Tsai L. Arthroscopic ankle arthrodesis in rheumatoid arthritis. Clin Orthop Relat Res. 1995;320:110-114.
36. Zvijac JE, Lemak L, Schurhoff MR, et al. Analysis of arthroscopically assisted ankle arthrodesis. Arthroscopy. 2002;18:70-75.
37. Amendola A, Lee KB, Saltzman CL, Suh JS. Technique and early experience with posterior arthroscopic subtalar arthrodesis. Foot Ankle Int. 2007;28:298-302.