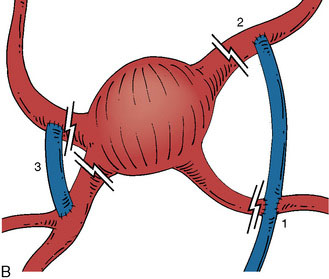
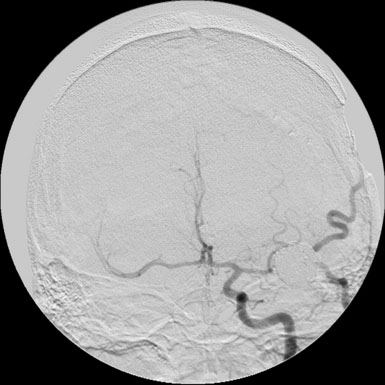
30 Fusiform Intracranial Aneurysms
Management Strategies
Introduction
In reviewing treatises of the past decade1,2 on this topic, it is clear that neuroimaging represents major progress in the understanding of these lesions. The contributions of rotational digital subtraction angiography (R-DSA), computed tomographic angiography (CT-A), and MRI have refined the preoperative understanding of this vascular lesion and further clarified the interaction with the surrounding parenchyma. While diagnostic radiology provides an improved ability to formulate a surgical plan, the rapid evolution of endovascular techniques has changed the role of the cerebrovascular surgeon in the management of fusiform intracranial aneurysms. Endovascular vessel reconstruction and flow diversion techniques have, in many cases, provided a viable alternative in the management of fusiform aneurysms.3–6 Despite these improvements, many such lesions are “beyond the reach” of endovascular devices and encourage cooperation between surgical and endovascular specialists to execute the best possible management of this complex disease.7–11 To be part of this multidisciplinary team, the modern cerebrovascular surgeon must be able to demonstrate proficiency in the use of intracranial bypass techniques.
Preoperative understanding
Potential Causes
Although many factors may contribute to their development, fusiform aneurysms are generally suspicious for a dissecting etiology that may or may not have an atherosclerotic component.12–14 The presence of intramural hemorrhage has been observed in both acute and chronic lesions.14 The contribution of infection, heritable arteriopathy, neoplasia, radiation, or previous cerebrovascular surgery should be considered when formulating a treatment strategy.1,12,15–18 The location of the aneurysm may also suggest the etiology of the aneurysm and modify the management strategy. For example, fusiform aneurysms of the distal anterior circulation aneurysms are more likely to be related to trauma or infection, while a fusiform aneurysm of the vertebrobasilar junction frequently has an atherosclerotic component.
Trial Balloon Occlusion (TBO)
The use of trial balloon occlusion (TBO) should be a routine consideration in the management of all fusiform aneurysms. The substantial pathology of the arterial wall increases the possibility of permanent wall injury and need for parent vessel occlusion as a salvage during the procedure. In many institutions, the necessity and type of bypass (high-, mid-, low-flow) are determined by information obtained from the results of a protocol including this technique.19 Although 80% of patients may tolerate ICA or nondominant vertebral artery occlusion,20–23 a 20% chance of morbidity is unacceptable. Our technique of utilizing clinical examination and single photon emission computed tomography (SPECT) has been previously published.24 Since the introduction of the balloon occlusion technique by Matas,25 significant changes in endovascular technology have allowed safer and more precise placement of a compliant balloon in an area that more closely mimics clip placement. This precise placement has produced more reliable results. Anecdotal experiences of balloon occlusion of the inflow to fusiform aneurysms have been discussed (personal communication) but not reported. The use of provocative measures such as EEG and hypotension in an attempt to further improve predictive values has been reported with up to a 95% correlation with successful vessel sacrifice.19,26–28
Surgical considerations by location
Middle Cerebral Artery
Fusiform aneurysms in the MCA have an uncertain natural history. In addition to being a common location for rupture, a concurrent risk for ischemia and symptoms attributed to mass effect also exists. Although data from the International Study of Unruptured Intracranial Aneurysms (ISUIA) noted a 5-year cumulative risk of 40% in giant lesions of the anterior circulation,29 as an isolated group, the relative risk of rupture for the middle cerebral artery appears to be inversely proportional to growth. In a retrospective meta-analysis of spontaneous fusiform aneurysms of the middle cerebral artery, Day et al. emphasized that the risk of ischemia and mass effect was higher in the larger lesions than the risk of rupture.15 As a group these lesions are exposed more superficially, are less likely to be endovascular candidates, and are frequently treated by primary reconstruction.30 When bypass options are entertained, the strategy should minimize the number of vascular territories at risk even if a staged procedure is necessary. Reliance on collateral circulation in this location is a gamble for the patient.
Case 1: Calcified lesion of proximal M2
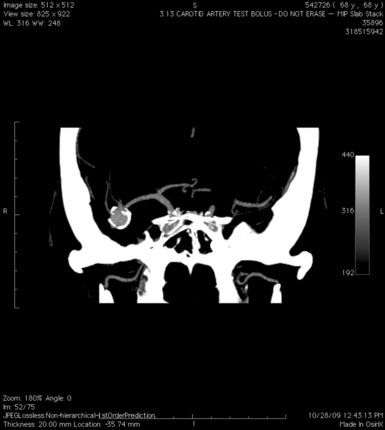
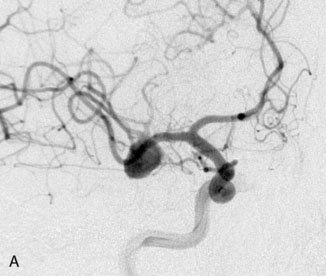
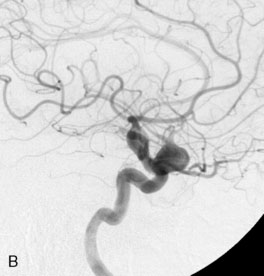
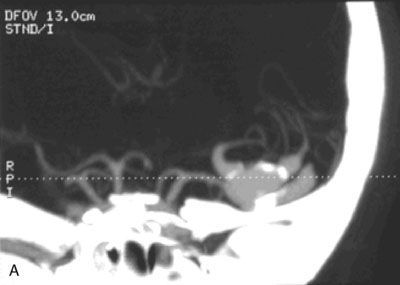
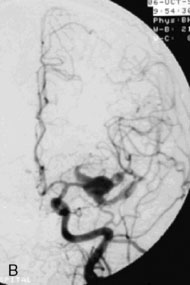
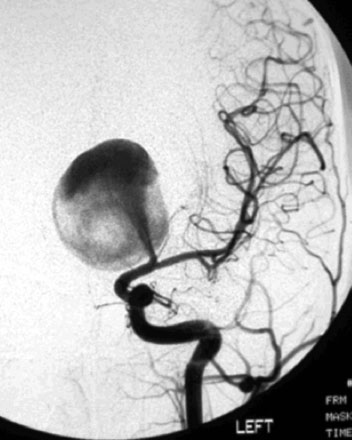
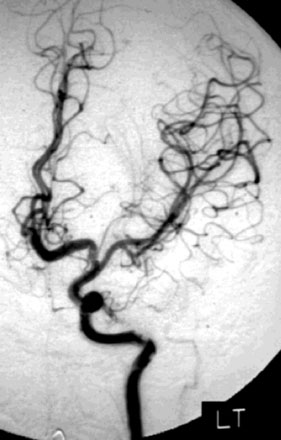
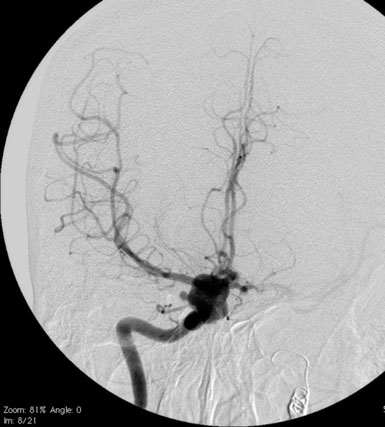
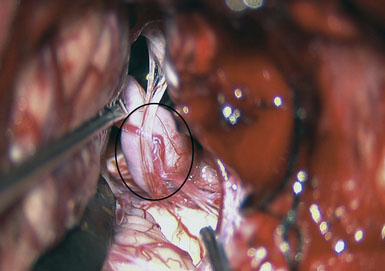
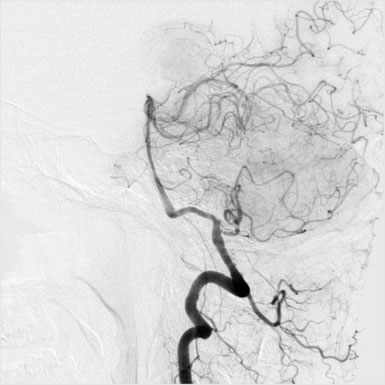
A 55-year-old male presented with a 3-month history of progressive left hand numbness. MRI and MRA suggested an 18 × 14 × 11 mm fusiform, right middle cerebral artery aneurysm that involved the origin of the insular M2 branch as well as subsequent exiting M3 branches. These findings were confirmed by CTA, which further clarified significant mural calcifications (Figure 30–1). Both CTA and R-DSA (Figure 30–2) made it clear that the multiple M3 vessels originating from the dome of the aneurysm would require the use of at least two bypass conduits to maintain the patency of these vessels if the aneurysm was to be obliterated.
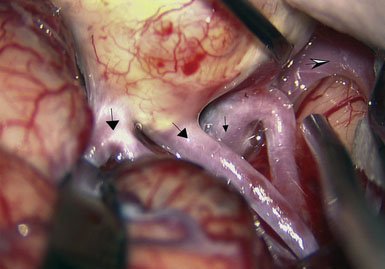
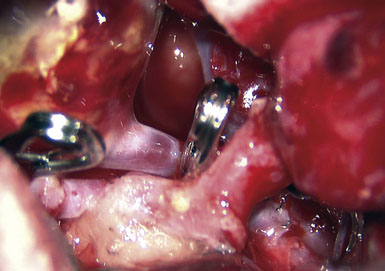
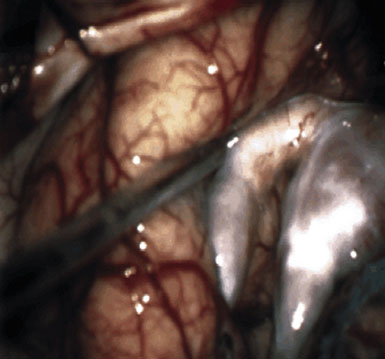
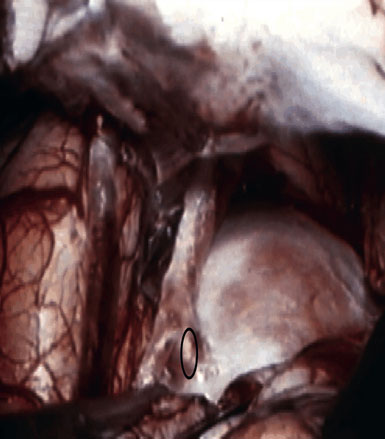
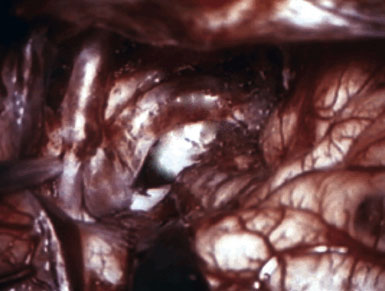
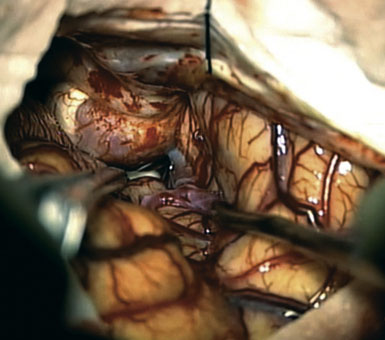
Preoperatively, a plan was made for the use of the ipsilateral superficial temporal artery (STA) and lesser saphenous vein as possible bypass conduits. The use of both STA branches was also a consideration. Intraoperative evaluation of the aneurysm confirmed the radiographic anatomy (Figure 30–3). The close proximity of a large anterior temporal artery made it possible to include an in situ, side-to-side anastomosis to an M3 vessel with the treatment options. A cut-flow of 20 cc/min was measured in the frontal branch of the STA. This was thought to be more than adequate to supplement ultrasonic flow measurements of 13 cc/min (Charbel microflow probe, Transonic Systems) noted at the medial M3 trunk. The side-to-side anastomosis was chosen for the lateral M3 branch (Figure 30–4) due to the inadequate length of the parietal STA branch.
After establishing patency of the bypassed segments, primary clipping of the aneurysm was prevented by the mural calcifications. The aneurysm was opened and tandem clips were placed for complete occlusion that was confirmed by postoperative angiography (Figure 30–5).
Case 2: Metastatic cardiac myxoma of the M1 bifurcation
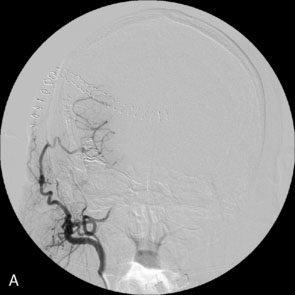
A 34-year-old, right-handed woman presented with episodic left hemisphere transient ischemic attacks (TIAs). A previous “stroke” at age 4 was followed by an operation on her heart for treatment of atrial myxoma. Evidence of previous ischemia and calcifications were visualized in the left sylvian fissure (Figure 30–6). Coronal CT reconstructions revealed the partially calcified wall of the lesion (Figure 30–7A). Angiography demonstrated a large, left-sided fusiform aneurysm involving a distal M1 segment and all exiting M2 segments. The proximal M1 segment containing the origins of the lateral lenticulostriate vessels was unaffected (Figure 30–7B).
With the likelihood of metastatic cardiac myxoma, a complete excision of the distal middle cerebral artery and proximal M2 vessels was planned via a left frontotemporal craniotomy. A right radial artery was harvested to serve as the conduit for the EC-IC bypass from the external carotid artery. After complete exposure of the left MCA from the origin of lateral lenticulostriate vessels to distal normal M2 branches (Figure 30–8 and Figure 30–9A), one M2 was amputated from the aneurysm and sutured, end to side, to the radial artery graft 2 cm from its distal tip (Figure 30–9B, 1). A temporary clip was placed on the radial graft distal to this anastomosis and the second M2 insular branch was amputated and sutured to the distal tip of the radial artery graft (Figure 30–9B, 2). Then temporary clips were placed on the M1 segment distal to the origin of the lenticulostriate vessels and a second clip on the third M2, just distal to its origin. The aneurysm was then excised and removed from the field. A short radial artery interposition graft was placed (end to end) between the M1 and remaining M2 (Figure 30–9B, 3).
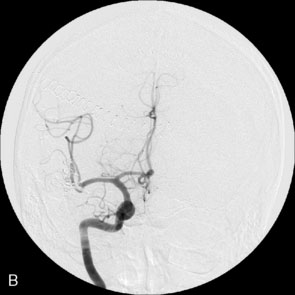
Immediate postoperative angiography demonstrated opacification of the two insular M2 segments via the EC-IC bypass (Figure 30–10). Significant spasm of the radial graft was clear. The more proximal M2 segment filled in continuity with the native M1. Clinically, the patient had a brief exacerbation of her mild expressive aphasia, but had returned to her preoperative level of functioning with 3 days. No clinical changes were noted at a 5-year follow-up examination.
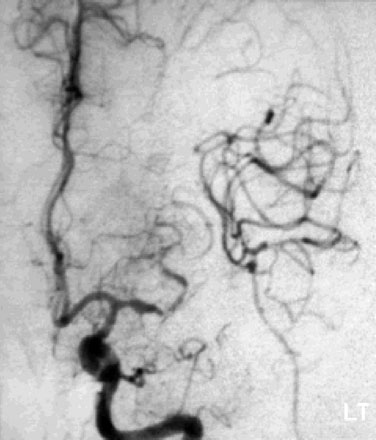
Figure 30–10 Intraoperative angiography of completed bypass with exclusion of the fusiform aneurysm.
Learning points
The MCA lesions presented here represent cases where disease pathology required complete trapping and exclusion of a necessary arterial segment. Thorough preoperative evaluation of the imaging allowed for multiple reconstructive options to be available in surgery. In Case 1, the intraoperative evaluation and blood-flow measurements (Transonic Systems) suggested robust collateral flow during proximal occlusion of the aneurysm. The ability to use a single STA to supply two exiting branches was also suggested by these flow measurements. Although the “double-barrel bypass”30 would have, similar to Case 2, put less vascular territory at risk, the orientation of the vessels exiting the calcified aneurysm would not allow for a tension-free anastomosis of the second STA. Although an in situ anastomosis was performed, preparation of the radial artery for interposition grafting might have minimized vascular risk and provided a conduit with a better size match than the saphenous vein that was available.
In Case 2, it is important to emphasize the utility of addressing vascular beds individually. The use of the cervical radial artery bypass allowed for “tying in” each bed serially such that no more than 20 minutes of occlusion was permitted per arterial territory. Previous studies have confirmed a significant increase in operative morbidity when temporary occlusion persists for longer than 19 minutes.31 The postoperative spasm of the radial artery, noted in Case 2, is a reported liability of the radial artery bypass. This occurred despite use of the pressure distension technique that has been described by Sekhar, et al.32 Endovascular angioplasty should be considered should medical blood pressure manipulation not succeed in improving the clinical exam.
Anterior Cerebral Artery
Similar to their proximal counterparts, distal aneurysms of the anterior circulation commonly involve the origins of the exiting vessels. Detailed analysis by Lehecka et al.33 revealed a broad base, wider than the parent artery, in 68% of patients, and 94% of patients had a branch origin at the base. The same group revealed that 52% of these lesions were associated with another aneurysm.34 More distally located lesions commonly supply well-collateralized vascular beds. TBO testing, in an elective situation, may reveal that vessel sacrifice is the less morbid therapy for the more distal anterior circulation aneurysm. When this is not feasible, the anatomic arrangement of the distal anterior cerebral vascular is favorable for side-to-side, in situ reconstruction.
Case 3: Fusiform aneurysm of a singular anterior cerebral artery
A 15-year-old female was found to harbor a 3.7-cm fusiform aneurysm in the parasellar region following MRI work-up of precocious puberty and right hemianopsia (Figure 30–11). CT imaging suggested no mural calcifications. Initial angiography suggested a giant left ICA bifurcation aneurysm with bilateral anterior cerebral arteries filling only from a left carotid injection (Figure 30–12). Based on these findings, direct clipping of this lesion was planned via a left frontotemporal craniotomy. No bypass was planned.
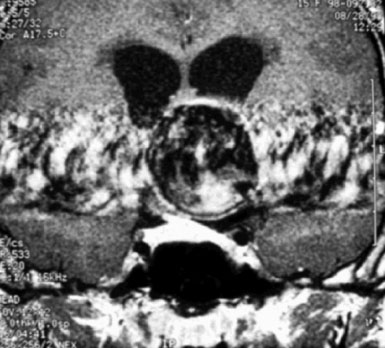
Figure 30–11 Coronal image of a large pulsatile lesion above the left cavernous internal carotid artery.
Upon exposure, a fusiform aneurysm of the entire left A1 segment was identified (Figure 30–13). The ICA bifurcation and left A1-A2 junction were nonpathologic. Preparations were made to harvest a portion of lesser saphenous vein. The aneurysm was excised and a short saphenous vein graft was used as an interposition graft (Figure 30–14). Postoperative angiography demonstrated a widely patent graft with good filling of both A2 segments (Figure 30–15). There was no neurological deficit postoperatively.
Proximal Internal Carotid Artery
Involvement of the proximal ICA is common in discussions of the subgroup of fusiform aneurysms.1,2,7,11,16 Because of the relationship to the anterior clinoid process, close association with the optic apparatus, and common mural calcifications, fusiform ophthalmic aneurysms represent a unique challenge to the basic concepts of proximal control, adequate visualization, and clip application. For these reasons, carotid occlusion following TBO evaluation should remain a part of the treatment plan despite the best of intentions. Patient age, visual complaints, and marginal TBO results are relevant in the decision to selectively include bypass in the surgical treatment of fusiform aneurysms of this region.
Case 4: Enlarging ophthalmic aneurysm
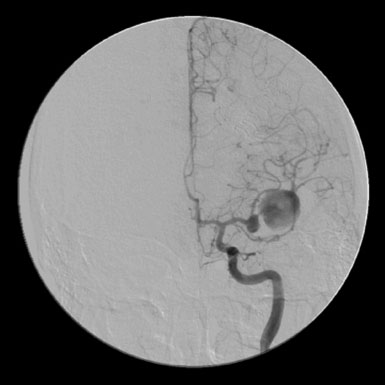
A 57-year-old female presented with progressive visual loss in her right eye and enlargement of a known right ophthalmic aneurysm over the preceding year. A large left ophthalmic aneurysm had been treated by carotid sacrifice 5 years earlier. Angiographic evaluation revealed a 2-cm multilobulated, partially thrombosed, fusiform aneurysm of the proximal ICA (Figure 30–16). Prompt clinical failure of a TBO confirmed the need for creation of a high-flow bypass. In an attempt to minimize the carotid occlusion time, the Excimer Laser Assisted Non-Occlusive Anastomosis (ELANA) technique35,36 was used to perform a saphenous vein bypass graft from the proximal external carotid to the ipsilateral M2 artery (Figure 30–17 and Figure 30–18). No carotid occlusion was necessary to create the intracranial anastomosis. The ICA was sacrificed, intraoperatively, proximal to the posterior communicating artery (distal) and at the cervical ICA (proximal). Intraoperative angiography confirmed the absence of aneurysm opacification (Figure 30–19). The patient noted improved right eye vision and is functioning at her preoperative baseline at 1.5 years follow-up.
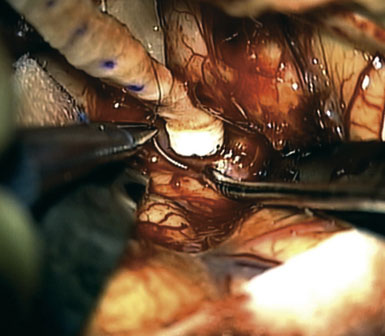
Figure 30–18 Anastomosis of the saphenous vein is being performed. A platinum ring has been placed within the everted vein cuff.
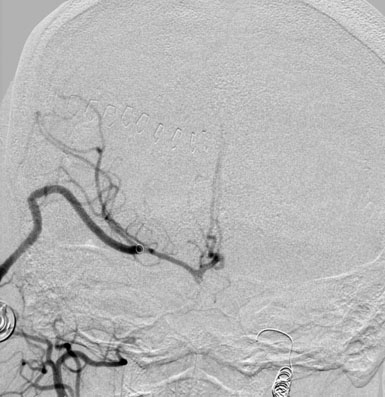
Figure 30–19 Intraoperative angiography verifies EC-IC graft patency and without filling of the ophthalmic aneurysm.
Learning points
In the case of a growing fusiform aneurysm of the proximal ICA, a component of progressive visual loss strongly suggests that surgical decompression be part of the treatment strategy. Despite the existence of some literature and clinical experience suggesting equivalence between surgical and endovascular therapies in similar circumstances,11,37,38 the size of the lesion and presence of a pre-existing visual field deficit minimize our enthusiasm for endovascular approaches to this problem. The previous contralateral ICA sacrifice and the significant clinical changes during TBO eliminated the option of Hunterian ligation from the treatment strategy. The presence of STA collaterals to the ophthalmic artery suggested that carotid sacrifice that involved this vessel would not further endanger the patient’s vision. Although clip reconstruction should be the treatment of choice, the significant vessel wall involvement clarified by R-DSA and optic nerve displacement (see Figure 30–17) support a deconstructive option with bypass.
The ELANA technique has been well described in the literature35,36,39,40 and is slowly becoming available to more cerebrovascular surgeons. The adequate caliber of the M2 vessel (∼2.3 mm) and the demonstrated need for carotid replacement increase the likelihood of success with the procedure. The use of a nonocclusive technique was ideal based on this patient’s blood flow needs.
Posterior Circulation
When discussing fusiform aneurysms of the posterior circulation, the giant and highly morbid transitional41 or dolichoectatic lesions involving the vertebral and basilar arteries are a common focus. Treatment of these lesions is often deconstructive and no consensus exists in the cerebrovascular community regarding an appropriate treatment strategy with or without the addition of bypass. Aneurysms of the proximal vertebral artery are more commonly managed by bypass techniques and will be the focus of discussion for this section.
There is little disagreement that the proximal intracranial vertebral artery (VA) is prone to traumatic dissection and frequently affected by atherosclerotic changes. The classification of nonatherosclerotic and fusiform aneurysms by Mizutani and colleagues heavily concentrated on the vertebrobasilar region and provides a good clinicopathological discussion on these lesions.42 Heightened awareness of acute stroke attributed to pathology in this region has increased the number of these lesions that present for neurosurgical consultation.
Case 5: Aneurysm of the proximal PICA
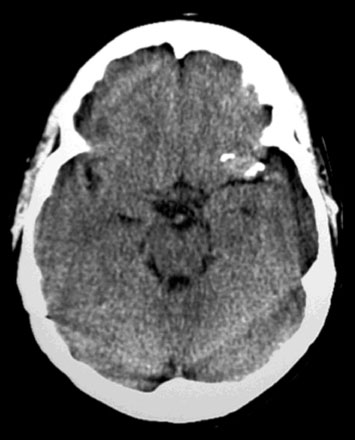
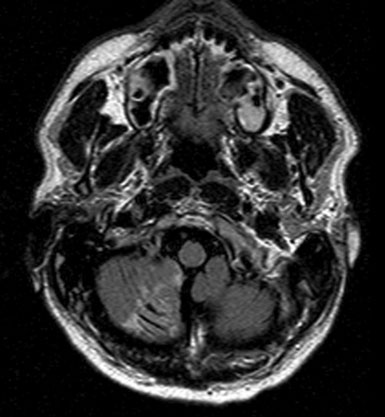
A 46-year-old male presented with an MRI showing symptomatic infarct of the right cerebellum (Figure 30–20). Subsequent angiographic workup revealed a fusiform aneurysm of the right posterior inferior cerebellar (PICA) artery that demonstrated enlargement following a 6-week period of convalescence on antiplatelet therapy (Figure 30–21). Although conservative medical management was discussed, surgical repair of the vessel was recommended and accepted as the next course of therapy.
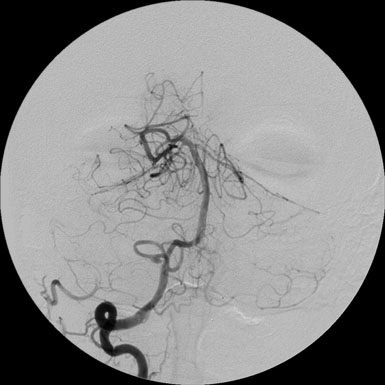
Figure 30–21 AP injection of the right vertebral artery revealed PICA aneurysm distal to the origin from the vertebral artery.
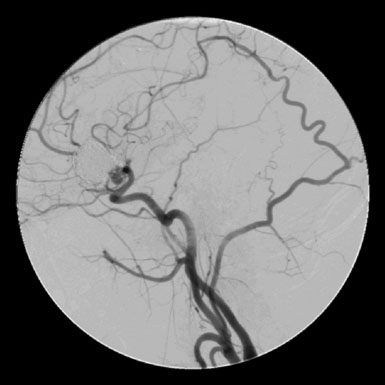
Based on the angiographic anatomy, surgical options included direct clip reconstruction, aneurysm excision with primary anastomosis, OA-PICA bypass, or PICA-PICA bypass. The OA was harvested before a far lateral suboccipital craniectomy was performed. Intraoperative evaluation revealed redundancy of the PICA distal to the fusiform aneurysm that was located on the first PICA segment proximal to lateral medullary perforators (Figure 30–22). This redundancy allowed for the reimplantation of the PICA at a more proximal location on the ipsilateral VA. A previously unseen perforator just distal to the aneurysm made the primary anastomosis a difficult exercise. Doppler flow and indocyanine green angiography (ICG-A) findings of graft patency were confirmed by subsequent angiography (Figure 30–23).
Learning points
The preceding case demonstrates a viable option for the management of a proximal PICA lesion that was of dissecting etiology. Intraoperative visualization clearly revealed arterial dissection, as the etiology for both lesions involved the PICA proximal to the telovelotonsillar, or fourth segment. As established literature exists concerning the stroke risk involved when PICA is occluded proximal to this segment,30,43,44 the need for bypass techniques should be expected when addressing aneurysms at this level. In Case 5, the redundancy of the PICA distal to the aneurysm allowed for reimplantation of the PICA on the proximal VA and placed only the ipsilateral PICA territory at ischemic risk. It was fortunate that the involved perforators had a length to allow for mobilizing the PICA vessel proximally. Similar ischemic risks would have resulted from the use of OA-PICA anastomosis but the concern for a tension-free repair limited this as an option.
Unpublished experience from this institution includes radiographic documentation of continued dissection and aneurysm formation beyond a “repaired” segment. This argues the case for complete exclusion of injured vascular tissue where possible as well as close postoperative, and delayed, imaging follow-up of arterial dissections. IC-IC bypass techniques with the use of radial artery grafting can facilitate reaching this goal.45 Although the use of a PICA-PICA bypass30,46,47 would be appropriate in this case, the potential exposure of two vascular territories to ischemic risk generally places this as a third bypass option for pathologies of the VA that involve the PICA.
Combined Microvascular and Endovascular Approaches
In developing a management strategy for the fusiform aneurysm, treatment options should include the discussion of both endovascular and microsurgical techniques. In their discussion of combined operative and endovascular approaches from 1998, Hacein-Bey and colleagues appropriately emphasize the conviction that a “prospective evaluation” of lesion complexities and appreciation of the complementary nature of surgical and endovascular techniques offers the best chance for reducing treatment morbidity and improving long-term outcomes.7 While both of these techniques provide reconstructive and deconstructive options, current endovascular technology is more limited by vessel size, while surgical techniques are limited by vessel location. As the notion of becoming a complete cerebrovascular surgeon increasingly involves endovascular training, it is likely that combined approaches to complex aneurysms will only increase in frequency.
Case 6: Traumatic aneurysm of the middle cerebral artery
A 26-year-old male with a history of multiple concussive injuries related to football was found to harbor a fusiform aneurysm of the left MCA at an outside institution. “Clip reconstruction was not possible” at the time of the surgery, and wrapping and acrylic application was performed. The patient developed a seizure disorder and recovered from a right hemiparesis following this surgery. He presented to our institution, 2 years later, with refractory seizures, an intermittent aphasia, and growth of the known aneurysm (Figure 30–24).
A complex surgical approach was further complicated by the failure of the initial left STA-M2 bypass. This required a return to the operating room for harvest of the occipital artery that was tunneled subcutaneously in a second attempt at bypass of the temporal M2. Angiographic confirmation of OA-M2 bypass patency allowed for initial embolization of the distal aneurysm (Figure 30–25) that was followed 6 weeks later by the stent-assisted endovascular reconstruction of the insular M2 artery and complete embolization of the aneurysm (Figure 30–26). A transient postoperative hemiparesis resolved and the patient returned to his preoperative baseline at 3 months of follow-up.
Conclusion
In his 1944 publication, Intracranial Arterial Aneurysms, Walter Dandy proclaimed that “intracranial aneurysms…are now added to the lengthening line of lesions that are curable by surgery.”48 Since that time, the methods by which the cerebrovascular surgeon excludes an aneurysm from the circulation while maintaining parent vessel flow have been refined by evolutions in microvascular technique, radiological imaging, and a better comprehension of cerebral hemodynamics. The relatively infrequent occurrence of fusiform aneurysms in the aneurysm population accounts for less standardized management of these lesions. This does not change the need for adherence to established principles of surgical exposure, microvascular technique, and maintenance of proximal and distal control. The fusiform aneurysm increases the importance of preoperative evaluation and thorough intraoperative inspection while increasing the probability that bypass will contribute to a favorable surgical outcome.
When a bypass becomes part of the treatment strategy of fusiform aneurysms, it is helpful to understand the benefits and disadvantages of the EC-IC, IC-IC, interposition, and reimplantation alternatives that define “the bypass” and are described in this text. The ability to intraoperatively evaluate and quantitate blood flow as a final determinant of bypass conduit should not be underestimated in its significance.49 It is essential for the modern cerebrovascular surgeon to realize that bypass alternatives are not exclusionary of one another in the management of fusiform aneurysms. These options should be creatively considered, along with endovascular techniques, to provide an elegant solution to the problem of the fusiform aneurysm.
1 Drake C.G., Peerless S.J. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141-162.
2 Sundt T.M., et al. Giant intracranial aneurysms. Clin Neurosurg. 1991;37:116-154.
3 Fiorella D., et al. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64:212-217. discussion 217
4 Hauck E.F., et al. Stent/coil treatment of very large and giant unruptured ophthalmic and cavernous aneurysms. Surg Neurol. 2009;71:19-24. discussion 24
5 Van Rooij W.J., Sluzewski M. Endovascular treatment of large and giant aneurysms. Am J Neuroradiol. 2008;30:12-18.
6 Lylyk P., et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64:632-642. discussion 642–643; quiz N6
7 Hacein-Bey L., et al. Complex intracranial aneurysms: combined operative and endovascular approaches. Neurosurgery. 1998;43:1304-1312. discussion 1312–1313
8 Lawton M.T., et al. Combined microsurgical and endovascular management of complex intracranial aneurysms. Neurosurgery. 2003;52:263-274. discussion 274–275
9 Lawton M.T., et al. Combined microsurgical and endovascular management of complex intracranial aneurysms. Neurosurgery. 2008;62(Suppl 3):1503-1515.
10 Hoh B.L., et al. Combined surgical and endovascular techniques of flow alteration to treat fusiform and complex wide-necked intracranial aneurysms that are unsuitable for clipping or coil embolization. J Neurosurg. 2001;95:24-35.
11 Hoh B.L., et al. Results after surgical and endovascular treatment of paraclinoid aneurysms by a combined neurovascular team. Neurosurgery. 2001;48:78-89. discussion 89–90
12 Anson J.A., Lawton M.T., Spetzler R.F. Characteristics and surgical treatment of dolichoectatic and fusiform aneurysms. J Neurosurg. 1996;84:185-193.
13 Moffie D. Fusiform aneurysm of intracranial arteries. Psychiatr Neurol Neurochir. 1968;71:85-91.
14 Nakatomi H., et al. Clinicopathological study of intracranial fusiform and dolichoectatic aneurysms: insight on the mechanism of growth. Stroke. 2000;31:896-900.
15 Day A.L., et al. Spontaneous fusiform middle cerebral artery aneurysms: characteristics and a proposed mechanism of formation. J Neurosurg. 2003;99:228-240.
16 Park S., et al. Intracranial fusiform aneurysms: its pathogenesis, clinical characteristics and management. J Kor Neurosurg Soc. 2008;44:116.
17 Morris B., et al. Cerebrovascular disease in childhood cancer survivors: a children’s oncology group report. Neurology. 2009;73:1906-1913.
18 Horowitz M.B., et al. Multidisciplinary approach to traumatic intracranial aneurysms secondary to shotgun and handgun wounds. Surg Neurol. 1999;51:31-41. discussion 41–42
19 Surdell D.L., et al. Revascularization for complex intracranial aneurysms. Neurosurg Focus. 2008;24:E21.
20 Abud D.G., et al. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. 2005;26:2602-2609.
21 Dare A.O., et al. Failure of the hypotensive provocative test during temporary balloon test occlusion of the internal carotid artery to predict delayed hemodynamic ischemia after therapeutic carotid occlusion. Surg Neurol. 1998;50:147-155. discussion 155–156
22 Vazquez Anon V., et al. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: technical aspects, cerebral monitoring, and results. Neuroradiology. 1992;34:245-251.
23 Miller J.D., Jawad K., Jennet B. Safety of carotid ligation and its role in the management of intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1977;40:64-72.
24 Eckard D.A., Purdy P.D., Bonte F.J. Temporary balloon occlusion of the carotid artery combined with brain blood flow imaging as a test to predict tolerance prior to permanent carotid sacrifice. AJNR Am J Neuroradiol. 1992;13:1565-1569.
25 Matas R.I. Testing the Efficiency of the Collateral Circulation as a Preliminary to the Occlusion of the Great Surgical Arteries. Ann Surg. 1911;53:1-43.
26 Graves V.B., et al. Endovascular occlusion of the carotid or vertebral artery with temporary proximal flow arrest and microcoils: clinical results. AJNR Am J Neuroradiol. 1997;18:1201-1206.
27 Mathis J.M., et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol. 1995;16:749-754.
28 Standard S.C., et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol. 1995;16:1453-1458.
29 The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risk of surgical and endovascular treatment. Lancet. 2003;362:103-110.
30 Lawton M.T., et al. Revascularization and aneurysm surgery: current techniques, indications, and outcome. Neurosurgery. 1996;38:83-92. discussion 92–94
31 Samson D., et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34:22-28. discussion 28–9
32 Sekhar L.N., et al. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms: techniques and outcomes for 17 patients. Neurosurgery. 2001;49:646-658. discussion 658–659
33 Lehecka M., et al. Anatomic features of distal anterior cerebral artery aneurysms: a detailed angiographic analysis of 101 patients. Neurosurgery. 2008;63:219-228. discussion 228–229
34 Lehecka M., et al. Distal anterior cerebral artery aneurysms: treatment and outcome analysis of 501 patients. Neurosurgery. 2008;62:590-601. discussion 590–601
35 Langer D.J., et al. Excimer laser-assisted nonocclusive anastomosis. An emerging technology for use in the creation of intracranial-intracranial and extracranial-intracranial cerebral bypass. Neurosurg Focus. 2008;24:E6.
36 Streefkerk H.J., et al. The excimer laser-assisted nonocclusive anastomosis practice model: development and application of a tool for practicing microvascular anastomosis techniques. Neurosurgery. 2006;58(Suppl 1):ONS148-ONS156. discussion ONS148–ONS156
37 Day A.L. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990;72:677-691.
38 Ferguson G.G., Drake C.G. Carotid-ophthalmic aneurysms: visual abnormalities in 32 patients and the results of treatment. Surg Neurol. 1981;16:1-8.
39 Streefkerk H.J., Bremmer J.P., Tulleken C.A. The ELANA technique: high flow revascularization of the brain. Acta Neurochir Suppl. 2005;94:143-148.
40 Streefkerk H.J., et al. The ELANA technique: constructing a high flow bypass using a non-occlusive anastomosis on the ICA and a conventional anastomosis on the SCA in the treatment of a fusiform giant basilar trunk aneurysm. Acta Neurochir (Wien). 2004;146:1009-1019. discussion 1019
41 Flemming K., et al. History of radiographically defined vertebrobasilar nonsaccular intracranial aneurysms. Cerebrovasc Dis. 2005;20:270-279.
42 Mizutani T., et al. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery. 1999;45:253-259. discussion 259–260
43 Ali M.J., et al. Trapping and revascularization for a dissecting aneurysm of the proximal posteroinferior cerebellar artery: technical case report and review of the literature. Neurosurgery. 2002;51:258-262. discussion 262–263
44 Hudgins R.J., et al. Aneurysms of the posterior inferior cerebellar artery. A clinical and anatomical analysis. J Neurosurg. 1983;58:381-387.
45 Sanai N., Zador Z., Lawton M.T. Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass. Neurosurgery. 2009;65:670-683. discussion 683
46 Ausman J.I., et al. Posterior inferior to posterior inferior cerebellar artery anastomosis combined with trapping for vertebral artery aneurysm. Case report. J Neurosurg. 1990;73:462-465.
47 Lemole G.M., et al. Cerebral revascularization performed using posterior inferior cerebellar artery–posterior inferior cerebellar artery bypass. Report of four cases and literature review. J Neurosurg. 2002;97:219-223.
48 Dandy W.E. Intracranial Arterial Aneurysms. Ithaca, NY: Comstock, 1944.
49 Amin-Hanjani S., et al. The utility of intraoperative blood flow measurement during aneurysm surgery using an ultrasonic perivascular flow probe. Neurosurgery. 2008;62(Suppl 3):1346-1353.